
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
BRIEF RESEARCH REPORT article
Front. Aging Neurosci. , 07 September 2022
Sec. Neurocognitive Aging and Behavior
Volume 14 - 2022 | https://doi.org/10.3389/fnagi.2022.927295
Background: Early identification of people at risk for cognitive decline is an important step in delaying the occurrence of cognitive impairment. This study investigated whether multimodal signals assessed using electroencephalogram (EEG) and gait kinematic parameters could be used to identify individuals at risk of cognitive impairment.
Methods: The survey was conducted at the Veterans Medical Research Institute in the Veterans Health Service Medical Center. A total of 220 individuals volunteered for this study and provided informed consent at enrollment. A cap-type wireless EEG device was used for EEG recording, with a linked-ear references based on a standard international 10/20 system. Three-dimensional motion capture equipment was used to collect kinematic gait parameters. Mild cognitive impairment (MCI) was evaluated by Seoul Neuropsychological Screening Battery-Core (SNSB-C).
Results: The mean age of the study participants was 73.5 years, and 54.7% were male. We found that specific EEG and gait parameters were significantly associated with cognitive status. Individuals with decreases in high-frequency EEG activity in high beta (25–30 Hz) and gamma (30–40 Hz) bands increased the odds ratio of MCI. There was an association between the pelvic obliquity angle and cognitive status, assessed by MCI or SNSB-C scores. Results from the ROC analysis revealed that multimodal signals combining high beta or gamma and pelvic obliquity improved the ability to discriminate MCI individuals from normal controls.
Conclusion: These findings support prior work on the association between cognitive status and EEG or gait, and offer new insights into the applicability of multimodal signals to distinguish cognitive impairment.
With the growing proportion of the elderly population worldwide, the prevalence of dementia is rapidly increasing and is projected to reach 75 million by 2030 and 132 million by 2050 (Dua et al., 2017). Dementia is a clinical syndrome characterized by the loss of cognitive function, behavioral deterioration, and an impaired ability to perform everyday activities (McKhann et al., 2011). As the most common form of dementia, Alzheimer’s disease (AD) is thought to begin at least 10–20 years before the appearance of AD symptoms; however, there is a lack of modification therapy to delay the onset or alter its progressive course (Holtzman et al., 2011).
Early detection of cognitive decline is an important step in delaying the occurrence of mild cognitive impairment (MCI) and AD and maximizing illness control and improving the quality of life (Solomon et al., 2014). MCI is an intermediate stage between healthy aging and AD and is characterized by an objective cognitive decline in one or more cognitive domains without any significant impairment in activities daily living (Petersen et al., 2014). If MCI and AD are suspected, the clinical diagnosis mainly relies on neuropsychological tests, laboratory assessments, and brain imaging. However, their use has several limitations related to the low accuracy of cognitive tests, the limited validation and invasiveness of laboratory tests, and the high cost and low availability of the equipment. Recent advances in measuring biomedical signals have offered promising solutions to these limitations (Chen et al., 2020). Physiological signals (i.e., electrophysiological, biophysical, and biochemical signals) have been employed to assist in the clinical diagnosis or detection of impaired cognitive function (Chen et al., 2020). The present study focused on whether multimodal physiological signals can be used to assess the odds of cognitive impairment. To narrow the scope of multimodal physiological signals, we chose the specific signals of an electroencephalogram (EEG) and motor activity, with reference to a review of existing literature (Jeong, 2004; Bahureksa et al., 2017).
Electroencephalogram is a recording of electrical activity taken from the scalp, and reveals synaptic activity that is correlated with the brain state (Dauwels and Kannan, 2012). EEG is one of the most commonly used methods for monitoring and diagnosing abnormal brain function during a normal aging process (Cassani et al., 2018). Studies have shown that MCI and AD are associated with increased delta and theta activities, decreased alpha and beta activities, and a lesser mean peak frequency, although EEG dynamics are somewhat disputable (Huang et al., 2000; Jelic et al., 2000; Jeong, 2004). Further studies have proposed novel EEG-based methodologies for the diagnosis of patients with MCI or AD and normal individuals (Ahmadlou et al., 2011; Sankari et al., 2012; Amezquita-Sanchez et al., 2019). A high accuracy ranging from 83 to 99.3% distinguishing patients with AD from normal participants was reported using novel methodologies, such as the Katz fractal dimension, epoch-based entropy, and the integrated multiple signal classification and empirical wavelet transform (Ahmadlou et al., 2011; Houmani et al., 2015; Amezquita-Sanchez et al., 2019). Thus, these findings suggest that a specific pattern in EEG signals may be helpful in detecting individuals with cognitive impairment.
In addition, body movements are associated with several brain regions. A conventional measure is gait, which is an automatic motor activity regulated by subcortical and spinal regions. Although MCI criteria require relatively preserved daily activity functioning, individuals with MCI can experience gait dysfunction (Morris et al., 2016; Bahureksa et al., 2017). Under single-task conditions, several gait parameters (i.e., velocity, stride length, and stride time) were significantly discriminated for patients with MCI or AD in comparison with cognitively healthy individuals (Morris et al., 2016; Bahureksa et al., 2017). Furthermore, dual-task assessment (i.e., the concurrent performance of motor-cognitive tasks) revealed significant increases in stride time, step length, and step width variabilities in MCI vs. healthy controls (Boripuntakul et al., 2014; Bahureksa et al., 2017). Although these gait differences do not reflect a drastic decline in every function, gait assessment may be relevant for predicting cognitive decline and worsening of dementia (Allali et al., 2016; Lin et al., 2016; Bahureksa et al., 2017).
In this study, we investigated whether multimodal signals assessed using EEG and gait kinematic parameters could be used to identify individuals at risk of cognitive impairment. The rationale for the combined use of EEG and gait parameters is based on different approaches but consistent information that can help detect cognitive impairment. The EEG is an important physiological signal that reflects the activity of the cerebral cortex by collecting changes in the scalp electrode measurement (Jeong, 2004). Gait is a measure of motor abilities that reflects progressive brain dysfunction in aging and neurodegenerative diseases (Bahureksa et al., 2017). Both EEG and gait abnormalities are associated with the risk of cognitive decline (Huang et al., 2000; Jelic et al., 2000; Jeong, 2004; Morris et al., 2016; Bahureksa et al., 2017) and are considered useful for early identification of the presence of cognitive impairment (Ahmadlou et al., 2011; Sankari et al., 2012; Allali et al., 2016; Lin et al., 2016; Bahureksa et al., 2017; Amezquita-Sanchez et al., 2019). Modeling both EEG and gait parameters together might strongly predict cognitive impairment. To achieve this aim, we: (1) measured the EEG and gait among study participants; (2) compared the differences in the two measured signal types according to cognitive status (normal cognition vs. MCI); and (3) evaluated the ability of these multimodal signals (combined EEG and gait kinematic parameters) to distinguish individuals with MCI from those with normal cognition.
Individuals aged ≥60 years who visited the Department of Neurology at Veterans Health Service Medical Center (Seoul, South Korea) between March and December 2021 were the target population for the current study. The inclusion criteria were as follows: (1) those who complained of cognitive decline, (2) those who could independently complete clinical tests and questionnaires, and (3) those who agreed to participate in this study. The exclusion criteria were as follows: (1) a diagnosis of dementia (ICD-10: F00-F09, G30), (2) diagnosed with brain infarction, cerebral hemorrhage, or Parkinson’s disease, and (3) suffering from another serious disease (e.g., cancer or mental illness). The inclusion and exclusion criteria were evaluated by experienced neurological clinicians. The study protocols were approved by the Institutional Ethical Review Board of the Veterans Health Service Medical Center (IRB No. BOHUN 2021-02-024).
The participants underwent a health survey consisting of EEG and gait measurements, cognitive examinations, and questionnaires. The survey was conducted at the Veterans Medical Research Institute in the Veterans Health Service Medical Center. A total of 235 individuals volunteered for this study and provided informed consent at enrollment. Participants were requested to take part in the follow-up study. Of these, 15 participants subsequently dropped out of the study due to incomplete answers on disease history (n = 1), refusal to undergo EEG (n = 10), and abnormal gait (n = 4). After these exclusions, 220 individuals (93.6%) remained eligible for the current study.
A cap-type wireless EEG device (MINDD SCAN, Ybrain Inc., South Korea) was used for EEG recording. The EEGs were recorded from 19 electrode sites (Fp1, Fp2, F7, F3, Fz, F4, F8, T7, C3, Cz, C4, T8, P7, P3, Pz, P4, P8, O1, and O2) with a linked-ear references based on a standard international 10/20 system. Participants underwent EEG recording at resting-state with eyes closed to measure endogenous pattern of brain activity of the target population. The sampling frequency and resolutions were 500 Hz and 24 bits, respectively.
For EEG signal processing, a 1 Hz high-pass filter and a 60 Hz notch filter were applied to remove eye and power noises. Independent component analysis was also used to eliminate eye-blink and muscle artifacts. For the analysis, over 3 min of artifact-free EEGs were selected from total 5 min of recorded resting-state EEG data. The relative power of six frequency bands, namely delta (1–4 Hz), theta (4–8 Hz), alpha (8–12 Hz), beta (12–25 Hz), high beta (25–30 Hz), and gamma (30–40 Hz) bands, was defined for further analysis of the target population.
Three-dimensional motion capture equipment was used to collect kinematic gait parameters using the NORAXON myoMOTION sensor (Scottsdale, AZ, United States), which is a wireless inertial measurement unit (IMC) system. The IMU sensor transmits human motion capture directly to the myoMOTION receiver to compute the angular changes of selected body segments. Using “Fusion algorithms,” the information from the accelerometer, gyroscope, and magnetometer was used to measure the three-dimensional rotation angles of each sensor in absolute space. The algorithm utilizes gyroscope and acceleration data from the foot-mounted IMU to identify the period when the foot is in the stance and swing phases via an on/off signal. The IMU was recorded at sampling rates of 100–200 Hz. The IMU data are mathematically combined, filtered, and processed using the myoMOTION software to quantify angular changes for joints, and the output is exported in Excel files (Alothmany et al., 2014).
For this study, the seven IMUl sensors were placed on the participants’ shoes (on the top of the upper foot), shins (frontal, on the tibia bone), thighs (frontal attachment to the lower quadrant of the quadriceps), and bony area of the sacrum, on both the left and right. Calibration was performed using the upright position to determine the value of the 0° angle in the studied joints. Participants were instructed to walk at their usual pace for 6 and 10 m. The kinematic parameters included the averaged signals for each stance phase and swing phase. The parameters of the stance and swing phases were the angles of hip abduction, hip flexion, hip external rotation, knee flexion, ankle abduction, ankle dorsiflexion, ankle inversion, pelvic tilt, pelvic obliquity, and pelvic rotation.
Participants completed the brief version of the Seoul Neuropsychological Screening Battery (SNSB), named the SNSB-Core (SNSB-C; Kang et al., 2015). The SNSB-C is a comprehensive test that evaluates the level of cognitive function or impairment in the five following cognitive domains: attention, language and related functions, visuospatial functions, memory, and frontal/executive functions (Jahng et al., 2015). The SNSB-C is composed of 14 sub-tests, including the Digit Span Test, a shortened version of the Korean-Boston Naming Test, Rey Complex Figure Test, Seoul Verbal Learning Test-Elderly’s version, a shortened version of the Korean-Color Word Stroop Test, Controlled Oral Word Association Test, Korean-Trail Making Test-Elderly’s version, and Digit Symbol Coding (Jahng et al., 2015).
The composite scores of the SNSB-C were expressed as z-scores standardized for age, sex, and education. The score provides an index of overall cognitive functioning, and is an alternative to the Korean Mini-Mental State Examination (which is a brief global instrument used to assess cognitive abilities) for screening patients with cognitive impairment (Jahng et al., 2015). Participants were divided into normal cognition and MCI groups (Kang and Na, 2003). Individuals with normal cognition were defined as those who had a percentile >16th in all sub-tests of the SNSB-C, while individuals with MCI were defined as those with a percentile ≤16th in one or more sub-tests of the SNSB-C.
Demographic variables included age, sex (male or female), and years of education. Medical conditions were assessed based on the presence of hypertension and dyslipidemia. To measure functional disability, a Korean version of the Instrumental Activities of Daily Living (K-IADL) was administered to all participants.
Continuous variables (i.e., age, education years, SNSB scores, and EEG and gait parameters) were compared between the normal cognition and MCI groups using t-tests. Between-group comparisons in categorical variables (i.e., sex and the presence of hypertension and/or dyslipidemia) were made using the Chi-squared test. To determine the effect of EEG and gait parameters on cognitive status, we applied logistic regression analysis to estimate the odds ratio (OR) and its 95% confidence interval (CI) for MCI. Linear regression analysis was conducted to estimate beta coefficients and standard error (SE) of the percentile of total SNSB-C scores associated with EEG and gait parameters. These regression models were adjusted for age, sex, education, and medical history of hypertension and dyslipidemia.
To evaluate the diagnostic usefulness of multimodal signals in discriminating between normal cognition and MCI groups, we conducted receiver operating characteristic (ROC) curve analysis. The ROC curve is a plot of sensitivity against 1-specificity for a given diagnostic test. We calculated the area under the ROC curve (AUC), which is a popular indicator of the overall performance of a diagnostic test. The AUC varies from 0.5 (no discrimination, that is, no ability to diagnose individuals with normal and mildly impaired cognition) to 1 (perfect discrimination). We considered three different ROC curves, as follows: (1) including the EEG parameter only, (2) including the gait parameter only, and (3) including both EEG and gait parameters, and provided the AUC value of each.
All analyses were performed using the Statistical Analysis System version 9.2 (SAS Institute, Cary, NC, United States), and statistical significance was set at p ≤ 0.05.
Of the 220 study population, 151 (68.7%) had MCI and 69 had normal cognition at the time of enrollment (Table 1). The mean age of the study participants was 74.2 years, and 54.5% were male. The proportion of individuals with dyslipidemia was the highest at 57.3%, followed by those with hypertension (54.1%) and diabetes mellitus (36.4%). We compared the characteristics between individuals without MCI and those with MCI. There were no significant differences in all characteristics: age (73.1 vs. 74.7 years); period of education (10.3 vs. 10.3 years); proportion of men (52.2 vs. 55.6%); proportion with a history of hypertension (47.8 vs. 56.9%), dyslipidemia (63.8 vs. 54.3%), and diabetes mellitus (44.9 vs. 32.5%); and the K-IADL score (0.1 vs. 0.1). To evaluate the association of EEG and gait parameters with cognitive status, logistic regression (in which MCI was an event) and linear regression (in which the z-score percentile of the total SNSB-C score was a continuous variable) analyses were conducted. The regression models were adjusted for age, sex, years of education, and history of hypertension and/or dyslipidemia. The results are presented in Tables 2–4.
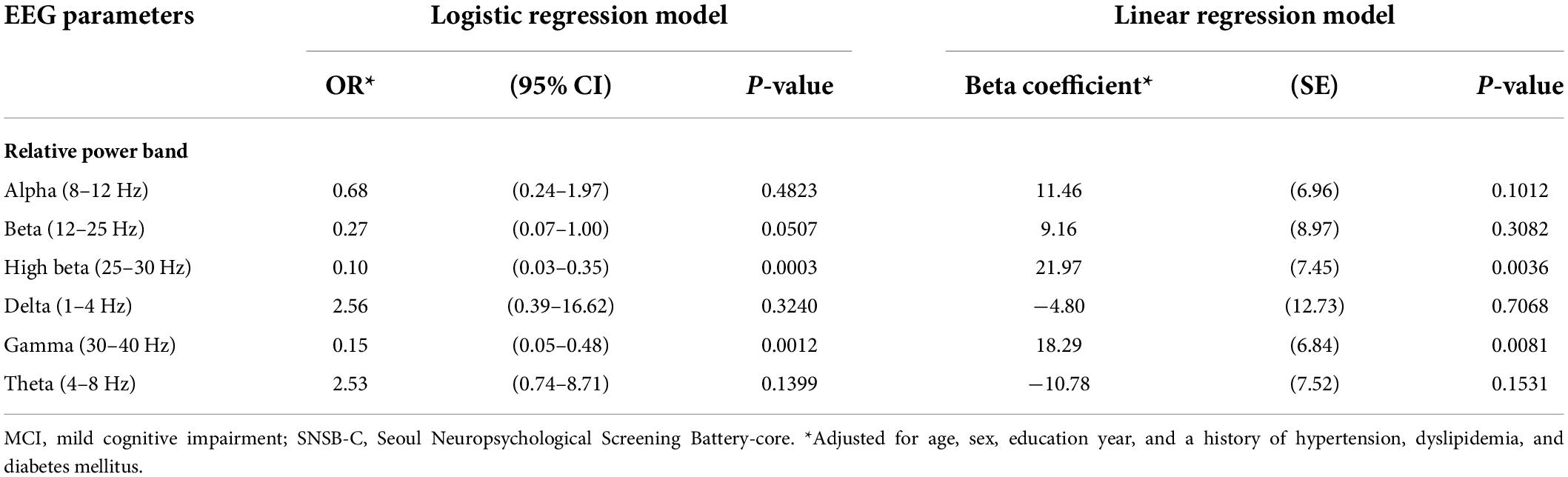
Table 2. Results from the logistic and linear regression models: OR (95% CI) for MCI and beta coefficients (SE) for SNSB-C score percentile, associated with EEG parameters in relative power.
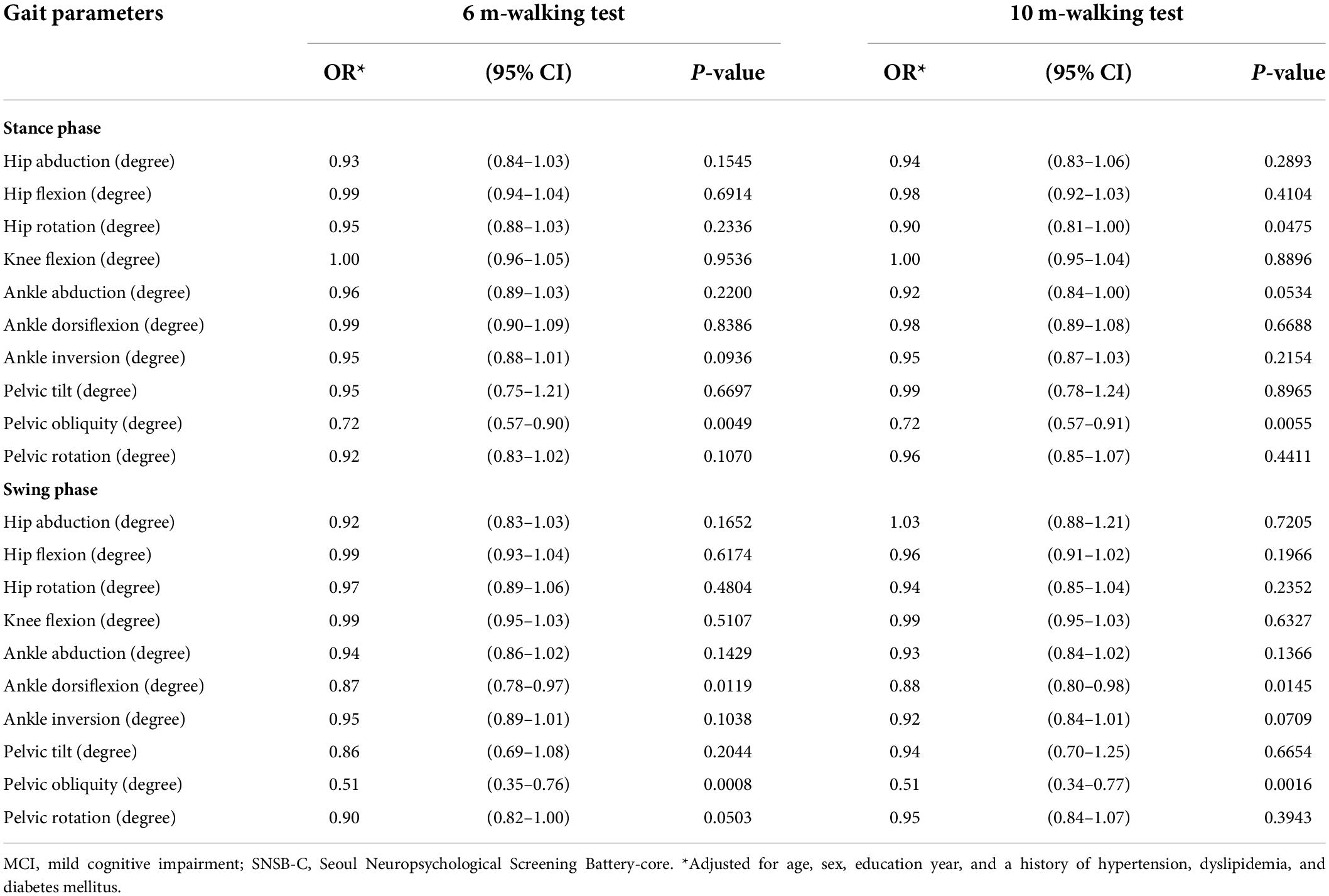
Table 3. Results from the logistic regression models: OR (95% CI)* for MCI, associated with gait parameters.
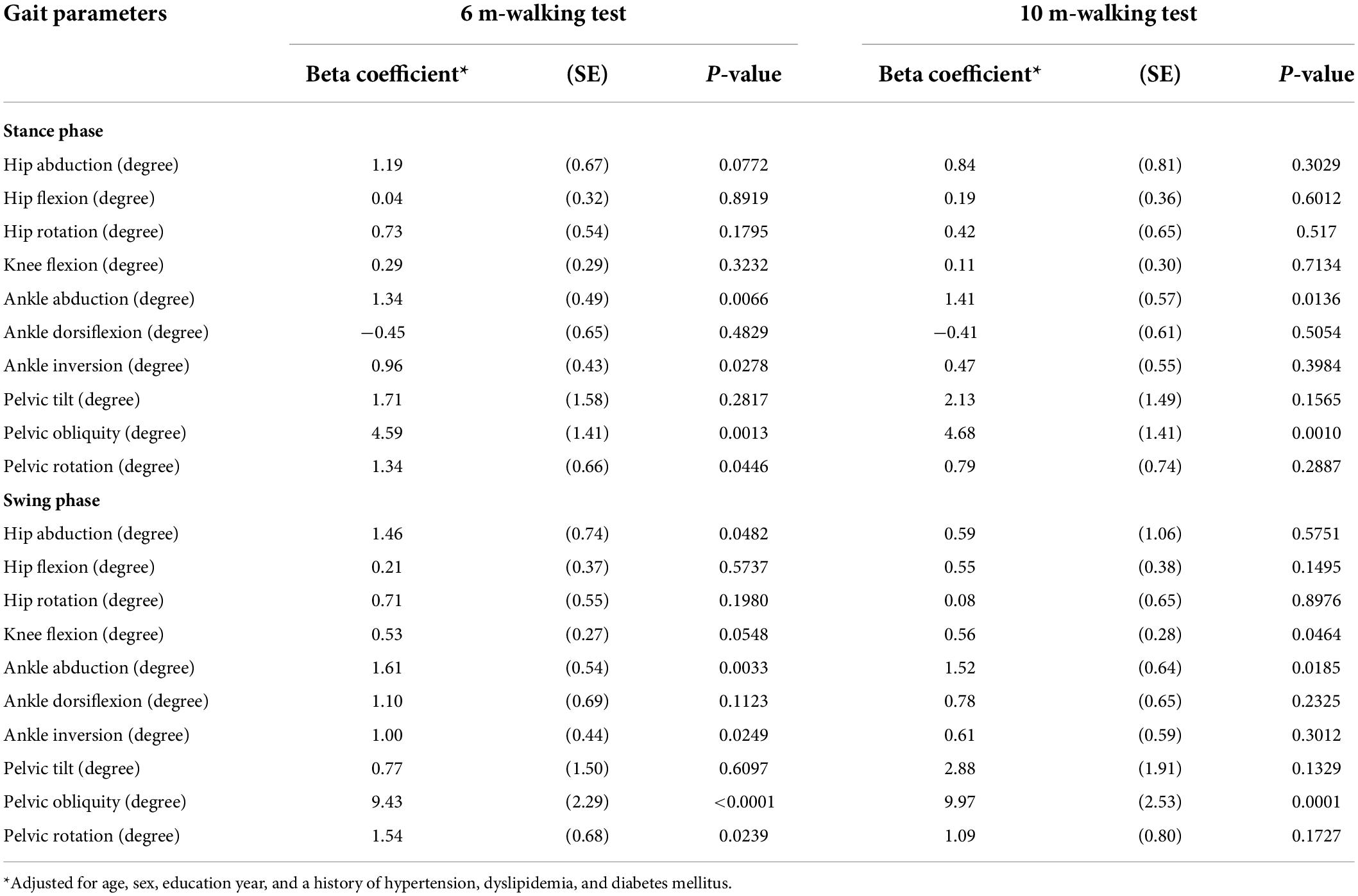
Table 4. Results from the linear regression models: beta coefficients (SE)* for SNSB-C score percentile, associated with gait parameters.
For the relative power of EEG parameters (Table 2), ORs (95% CI) for MCI were significantly associated with decreases in high beta (adjusted OR = 0.10; 95% CI = 0.03–0.35, p = 0.0003) and gamma (adjusted OR = 0.15; 95% CI = 0.05–0.48, p = 0.0012). In the linear regression, beta coefficients (SE) for the SNSB-C score were significantly associated with increases in high beta (adjusted beta = 21.97; SE = 7.45, p = 0.0036) and gamma bands (adjusted beta = 18.29; SE = 6.84, p = 0.0081) in relative power.
For gait parameters (Table 3), a decrease in pelvic obliquity angle (6-m walking: adjusted OR = 0.72; 95% CI = 0.57–0.90, p = 0.0049; 10-m walking: adjusted OR = 0.72; 95% CI = 0.57–0.91, p = 0.0055) during the stance phase and decreases in ankle dorsiflexion angle (6-m walking: adjusted OR = 0.87; 95% CI = 0.78–0.97, p = 0.0119; 10-m walking: adjusted OR = 0.88; 95% CI = 0.80–0.98, p = 0.0145) and pelvic obliquity angle (6-m walking: adjusted OR = 0.51; 95% CI = 0.35–0.76, p = 0.0008; 10-m walking: adjusted OR = 0.51; 95% CI = 0.34–0.77, p = 0.0016) during the swing phase consistently and significantly increased the likelihood for developing MCI in both the 6- and 10-m walking tests.
The linear regression (Table 4) of kinematic gait parameters revealed that beta coefficients (SE) for the SNSB-C score consistently increased in terms of increases in ankle abduction angle and pelvic obliquity angle during both the 6- and 10-m walking tests. In the stance phase, adjusted beta coefficients (SE) during the 6- and 10-m walking tests were 1.34 (0.49; p = 0.0066) and 1.41 (0.57; p = 0.0136) for ankle abduction angle, and 4.59 (1.41; p = 0.0013) and 4.68 (1.41; p = 0.0010) for pelvic obliquity angle. In the swing phase, adjusted beta coefficients (SE) during the 6- and 10-m walking tests were 1.61 (0.54; p = 0.0033) and 1.52 (0.64; p = 0.0185) for ankle abduction angle, and 9.43 (2.29; p < 0.0001) and 9.97 (2.53; p = 0.0001) for pelvic obliquity angle.
Based on the chosen significance level of α, high-frequency EEG activity in high beta (25–30 Hz) and gamma (30–40 Hz) bands was significantly associated with MCI and cognitive performance. Among the gait parameters, the pelvic obliquity angle in kinematics was significantly associated with MCI and cognitive performance, and this association was consistent in both the 6- and 10-m walking tests.
To assess the diagnostic utility of multimodal signals (i.e., the combination of EEG and gait parameters) for distinguishing normal and mildly impaired cognition, ROC curves and AUC values were used (Figures 1, 2). The three following ROC curves were created and calculated: (A) model with EEG parameters (high beta or gamma), (B) model with gait parameters (pelvic obliquity angle), and (C) model with EEG and gait parameters (high beta with pelvic obliquity or gamma with pelvic obliquity).
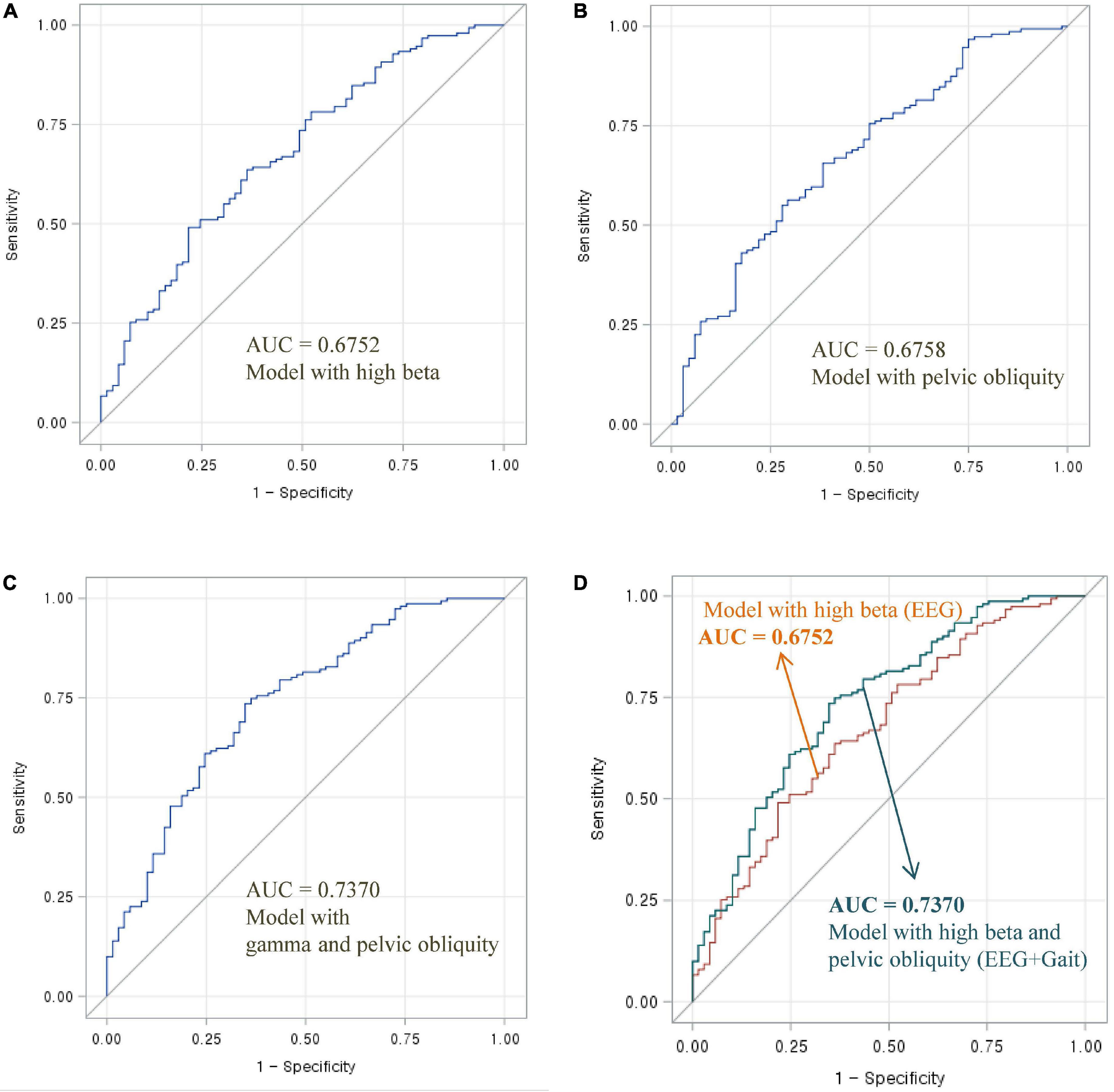
Figure 1. Area under the ROC curve of EEG and gait parameters for classifying MCI and normal cognition: (A) Model with high beta power, (B) model with pelvic oblique, (C) combined model with high beta power and pelvic oblique, and (D) comparison of AUC curves.
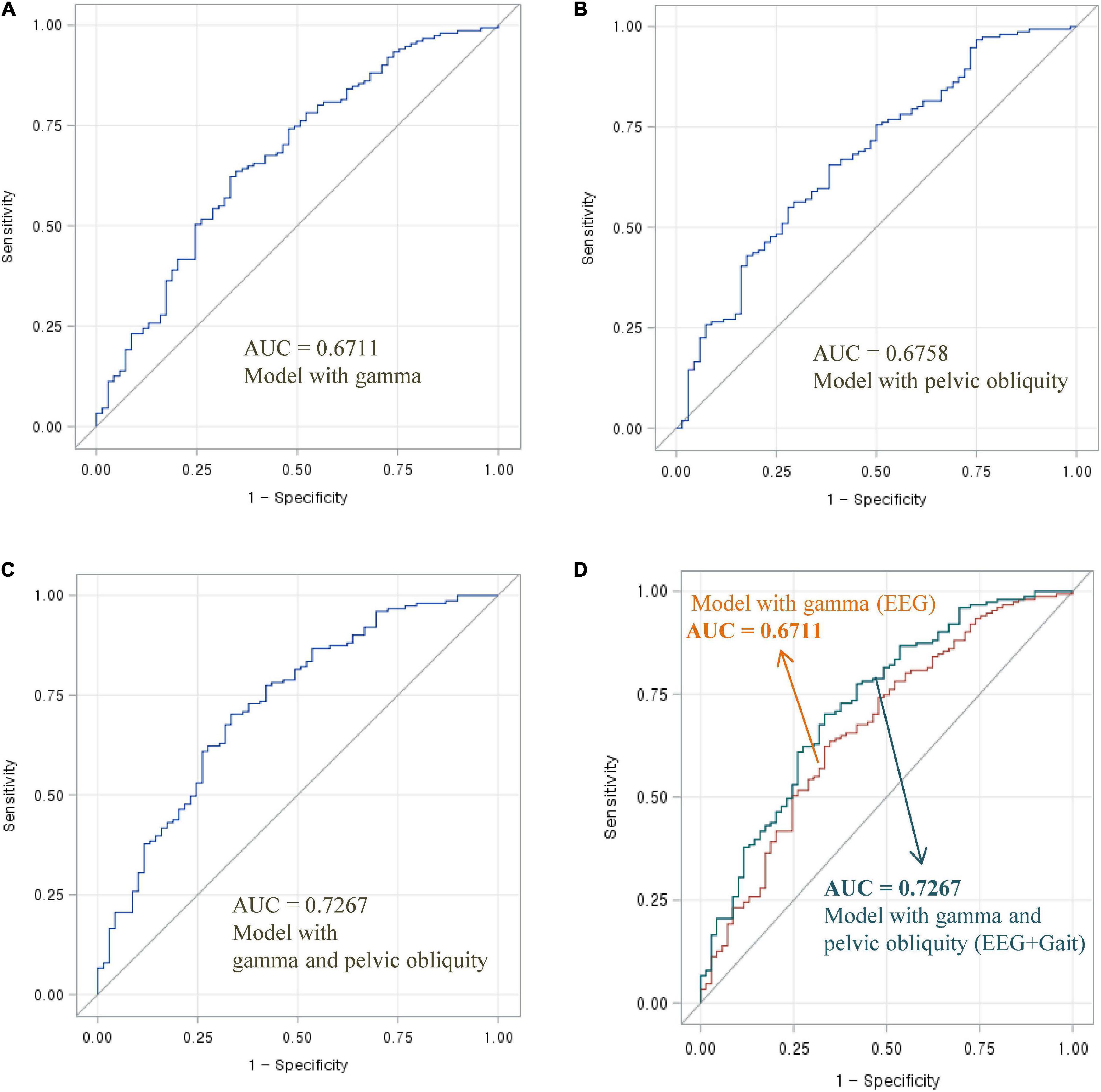
Figure 2. Area under the ROC curve of EEG and gait parameters for classifying MCI and normal cognition: (A) Model with gamma power, (B) model with pelvic oblique, (C) combined model with gamma power and pelvic oblique, and (D) comparison of AUC curves.
As shown in Figure 1, the AUC value was 0.6752 for high beta activity (A) and 0.6758 for pelvic obliquity angle (B). Calculation of the AUC demonstrated better performance (AUC = 0.7370) by the combination of the two parameters (D) than by a single parameter (either high beta or pelvic obliquity). As shown in Figure 2, the AUC value was 0.6711 for gamma activity (A) and 0.6758 for pelvic obliquity angle (B). Calculation of the AUC demonstrated better performance (AUC = 0.7267) by the combination of the two parameters (D) than by a single parameter (either gamma or pelvic obliquity).
Our results confirmed that specific EEG and gait parameters were significantly associated with cognitive status. Individuals with decreases in high-frequency EEG activity in high beta (25–30 Hz) and gamma (30–40 Hz) bands increased the probability of MCI. We also found an association between the pelvic obliquity angle and cognitive status, assessed by MCI or SNSB-C scores. The main aim of this study was to determine whether multimodal signals with EEG and gait parameters are meaningful in distinguishing cognitive impairment. Results from the ROC analysis revealed that multimodal signals combining high beta or gamma and pelvic obliquity improved the ability to discriminate MCI individuals from normal controls. These findings support prior work on the association between cognitive status and EEG or gait, and offer new insights into the applicability of multimodal signals to distinguish cognitive impairment.
Neural oscillations are rhythmic or repetitive electrical discharges in the brain (Başar, 2013). EEG generates time-series data on neural oscillations in the brain, even though voltage oscillations detected on the scalp are small (Jeong, 2004). We found significant decreases in high beta (25–30 Hz) and gamma (30–40 Hz) band oscillations in the MCI group, as assessed by the SNSB-C. Conversely, increased high beta and gamma activities were significantly associated with better cognitive performance.
Beta activity is typically observed in motor activity (Honaga et al., 2010). Although voltage oscillations detected on the scalp are small, EEG generates time-series data on neural oscillations at different frequency bands (i.e., delta, theta, alpha, beta, and gamma) in the brain (Jeong, 2004). Delta oscillations are prominent in the early developmental stages and during slow-wave sleep (Knyazev, 2012). Functionally, delta oscillations are implicated in the synchronization of brain activity with autonomic functions, motivational processes associated with both reward and atavistic defensive mechanisms, and in cognitive processes mostly related to attention and the detection of motivationally salient stimuli in the environment (Knyazev, 2012). Theta oscillations are low-frequency oscillations in the local field potential within the hippocampus, amygdala, and neocortex (Hutchison and Rathore, 2015). Theta activity is dominant feature synchronized across brain regions during both wake and rapid-eye movement sleep (Hutchison and Rathore, 2015). Alpha oscillations are the most prominent EEG features during wakefulness; the more an individual is stable and relaxed, the greater the amplitude (Halgren et al., 2019). Beta oscillations are typically observed during motor activity (Honaga et al., 2010). The modulation of beta band oscillations plays a key role in action planning and execution, wherein the beta power decreases during action planning and decreases further during action execution. Gamma oscillations are the fastest and subtlest brain waves. Gamma power modulates perception and consciousness (Missonnier et al., 2010). Delta, theta, and alpha oscillations are global processing modes that cover relatively large cortical regions and serve integration across diverse cortical sites by synchronizing coherent activity and phase coupling across widely spatially distributed neural assemblies. Oscillations of beta and gamma ranges or local EEG modes are distributed in higher frequencies, lower amplitudes, and a more limited topographic area (Knyazev, 2012). Although the direction of increase or decrease in each oscillation is not consistent owing to different experimental protocols and participant’ characteristics, a substantial evidence suggests that oscillations in all frequency bands are linked to cognitive processes such as attention, perception, and memory (Missonnier et al., 2007, 2010; Ray and Maunsell, 2015; Spitzer and Haegens, 2017). Our data displayed the association of high beta/gamma oscillations with cognitive performance. Using the SNSB-C, we found significant decreases in high beta (25–30 Hz) and gamma (30–40 Hz) band oscillations in the MCI group. Many studies have shown that a decrease in beta or gamma power is a characteristic of AD progression (Başar and Düzgün, 2016; Wang et al., 2017; Smailovic and Jelic, 2019). There is scarce evidence of any such spectral changes in patients with MCI. Güntekin et al. (2013) found that patients with MCI tended to have a lower beta response than healthy controls during the visual oddball paradigm. Michels et al. (2017) investigated the association between amyloid deposition and ApoE and EEG measurements in 17 healthy controls and 17 patients with MCI. The authors revealed that beta power was lower in patients with MCI relative to healthy controls, and was negatively associated with ApoE4-status and global amyloid deposition (r = 0.51, p = 0.02) (Michels et al., 2017). In a recent case-control study, Murty et al. (2021) measured patterns of brain activity in elderly people classified as healthy, with MCI, and with AD. They found significantly reduced stimulus-induced gamma rhythms in the patients with MCI and AD compared to age- and sex-matched controls (Murty et al., 2021). Taken together, these studies indicate that high beta and gamma levels are associated with cognitive impairment. While our results can be interpreted in a similar way, the current data does not provide information about the mechanism underlying the association of higher frequency bands, especially high beta and gamma bands, with cognitive performance.
Gait and cognition share anatomical structures and brain control processes (Cohen et al., 2016). Close associations between gait abnormalities and cognitive deficits have been reported in the elderly (Pieruccini-Faria et al., 2021). Consistent with prior work, we also observed a significant difference in certain kinematic gait variables between individuals with normal cognition and those with MCI. There was a strong and consistent association between lower pelvic obliquity angle and cognitive status (assessed by MCI or SNSB-C scores) in both 6- and 10-m walking tests. This indicates that kinematic gait disturbance and cognitive decline may be correlated. Most studies have stressed the importance of spatio-temporal gait features (i.e., slow gait velocity and shorter stride length and time) that can distinguish cognitive impairment (Toots et al., 2019; Xie et al., 2019; Sebastiani et al., 2020). There has been little interest in gait kinematics according to cognitive status; two recent studies have provided evidence of an association between gait kinematics and cognitive status (Rucco et al., 2017; Zhong et al., 2021). Rucco et al. (2017) examined gait patterns in patients with AD and those with behavioral variants of frontotemporal dementia. A significant increase in dorsiflexion and a decrease in plantar flexion under cognitive dual-task conditions were observed in patients with AD relative to the normal controls (Rucco et al., 2017). More recently, Zhong et al. (2021) found that older adults with MCI had larger knee peak extension angles and smaller knee heel strike angles than those with normal cognition. These previous studies suggest that cognitive impairment may affect gait kinematic parameters, along with spatio-temporal parameters. Regarding the relationship between pelvic obliquity and cognitive impairment, the results of Martín-Gonzalo et al. (2019) seem to support our own, showing the discrimination of pelvic movement according to the status of cognitive impairment. The authors compared gait kinematic metrics (complexity and irreversibility) in patients with MCI and mild AD relative to healthy controls. The amount of irreversibility of the pelvic tilt kinematic signal was decreased in patients with MCI and mild AD, whereas complexity in the hip flexion and pelvic obliquity kinematic signals was decreased in patients with mild AD. These results suggest that kinematic disorganization is present at an early stage of cognitive impairment. Further studies are needed to understand how individuals with cognitive decline adapt to the progressive deterioration of motor functions, specifically gait kinematics.
The primary aim of this study was to examine whether combined EEG and gait parameters are useful in distinguishing between cognitive impairment and normal cognition. Previous studies have analyzed the usefulness of multimodal techniques, such as using machine learning methods and combining neuroimaging data, biological tests, or clinical records, in the early diagnosis of cognitive decline and AD. To the best of our knowledge, the present study is the first to use multimodal signals that incorporate EEG and gait to classify MCI risk. As shown in Figures 1, 2, the combination of EEG and gait kinematic parameters (high beta power with pelvic obliquity AUC = 0.7370 and gamma power with pelvic obliquity AUC = 0.7267) had additional value compared to high beta power (AUC = 0.6752), gamma power (AUC = 0.6300), and pelvic obliquity (AUC = 0.6758) alone. This is encouraging, as it indicates that multimodal signals significantly improved MCI prediction compared with predictions made by the models using EEG or gait data only. Given the benefits of EEG and gait as being non-invasive, relatively inexpensive, and easily available measures, our multimodal approach may be effective for screening of cognitive decline and monitoring AD risk. However, this study has several limitations, such as its cross-sectional design, unmeasured confounders, and the possibility of misclassified MCI. Thus, as with any diagnostic test, its clinical utility should be confirmed in follow-up studies.
In conclusion, we found that high-frequency EEG activity and pelvic obliquity angle were significantly associated with cognitive status. This finding supports the proposal that multimodal signals could be used to identify individuals at risk of cognitive impairment. However, further studies to verify the reliability of the combined use of EEG and kinetic gait measures for identifying MCI individuals are needed because these measures need to be contrasted with other, more easily obtained, and reliable predictors of MCI (e.g., gait speed). Additional studies are also required to develop guidelines for using EEG and kinetic gait measures for MCI prediction that can be applied in clinical settings. If our follow-up study reveals that multimodal signals combining EEG and gait are able to reasonably predict MCI risk, this might be a good strategy to improve the performance of MCI diagnostic tests.
The original contributions presented in this study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
The studies involving human participants were reviewed and approved by the Institutional Ethical Review Board of the Veterans Health Service Medical Center (IRB no. BOHUN 2021-02-024-002). The patients/participants provided their written informed consent to participate in this study.
J-YM planned the study, performed the statistical analyses, and wrote the manuscript. S-WH supervised the data collection and analysis and revised the manuscript. KL reviewed the collected data and revised the manuscript. K-BM received funding, planned the study, supervised the data collection and analysis, and contributed to manuscript. All authors contributed to the article and approved the submitted version.
This research was supported by the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI) and Korea Dementia Research Center (KDRC), funded by the Ministry of Health and Welfare and Ministry of Science and ICT, South Korea (grant number: HU20C0487). This work was supported by VHS Medical Center Research Grant, South Korea (VHSMC 22026). The sponsors had no role in the design, methods, subject recruitment, data collection, analyses, and preparation of the manuscript.
KL was CEO at Ybrain Inc.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Ahmadlou, M., Adeli, H., and Adeli, A. (2011). Fractality and a wavelet-chaos-methodology for EEG-based diagnosis of Alzheimer disease. Alzheimer Dis. Assoc. Disord 25, 85–92. doi: 10.1097/WAD.0b013e3181ed1160
Allali, G., Annweiler, C., Blumen, H. M., Callisaya, M. L., De Cock, A. M., Kressig, R. W., et al. (2016). Gait phenotype from mild cognitive impairment to moderate dementia: Results from the GOOD initiative. Eur. J. Neurol. 23, 527–541. doi: 10.1111/ene.12882
Alothmany, N., Khan, M., Alnowaimi, A., and Morfeq, E. (2014). Accuracy of joint angles tracking using markerless motion system. J. Biomech. 47, 587–591.
Amezquita-Sanchez, J. P., Mammone, N., Morabito, F. C., Marino, S., and Adeli, H. (2019). A novel methodology for automated differential diagnosis of mild cognitive impairment and the Alzheimer’s disease using EEG signals. J. Neurosci. Methods 322, 88–95. doi: 10.1016/j.jneumeth.2019.04.013
Bahureksa, L., Najafi, B., Saleh, A., Sabbagh, M., Coon, D., Mohler, M. J., et al. (2017). The impact of mild cognitive impairment on gait and balance: A systematic review and meta-analysis of studies using instrumented assessment. Gerontology 63, 67–83. doi: 10.1159/000445831
Başar, E. (2013). Brain oscillations in neuropsychiatric disease. Dialogues Clin. Neurosci. 15, 291–300.
Başar, E., and Düzgün, A. (2016). How is the brain working?: Research on brain oscillations and connectivities in a new “Take-Off” state. Int. J. Psychophysiol. 103, 3–11. doi: 10.1016/j.ijpsycho.2015.02.007
Boripuntakul, S., Lord, S. R., Brodie, M. A., Smith, S. T., Methapatara, P., Wongpakaran, N., et al. (2014). Spatial variability during gait initiation while dual tasking is increased in individuals with mild cognitive impairment. J. Nutr. Health Aging 18, 307–312.
Cassani, R., Estarellas, M., San-Martin, R., Fraga, F. J., and Falk, T. H. (2018). Systematic review on resting-state EEG for Alzheimer’s Disease diagnosis and progression assessment. Dis. Markers 2018, 5174815. doi: 10.1155/2018/5174815
Chen, Y., Zhang, Y., Liang, Z., Cao, Y., Han, Z., and Feng, X. (2020). Flexible inorganic bioelectronics. npj Flexible Electr. 4:2.
Cohen, J. A., Verghese, J., and Zwerling, J. L. (2016). Cognition and gait in older people. Maturitas 93, 73–77.
Dauwels, J., and Kannan, S. (2012). Diagnosis of Alzheime’s disease using electric signals of the brain. A grand challenge. Asia Pac. Biotech. News 16, 22–38.
Dua, T., Seeher, K. M., Sivananthan, S., Chowdhary, N., Pot, A. M., and Saxena, S. (2017). World Health Organization’s Global action plan on the public health response to dementia 2017-2025. Alzheimers Dement 13, 1450–1451.
Güntekin, B., Emek-Savaş, D. D., Kurt, P., Yener, G. G., and Başar, E. (2013). Beta oscillatory responses in healthy subjects and subjects with mild cognitive impairment. Neuroimage Clin. 3, 39–46.
Halgren, M., Ulbert, I., Bastuji, H., Fabo, D., Rey, M., Devinsky, O., et al. (2019). The generation and propagation of the human alpha rhythm. Proc. Natl. Acad. Sci. U.S.A. 116, 23772–23782.
Holtzman, D. M., Morris, J. C., and Goate, A. M. (2011). Alzheimer’s disease: The challenge of the second century. Sci. Transl. Med. 3:77sr71.
Honaga, E., Ishii, R., Kurimoto, R., Canuet, L., Ikezawa, K., Takahashi, H., et al. (2010). Post-movement beta rebound abnormality as indicator of mirror neuron system dysfunction in autistic spectrum disorder: An MEG study. Neurosci. Lett. 478, 141–145. doi: 10.1016/j.neulet.2010.05.004
Houmani, N., Dreyfus, G., and Vialatte, F.B. (2015). Epoch-based entropy for early screening of Alzheimer’s Disease. Int. J. Neural Syst. 25:1550032.
Huang, C., Wahlund, L., Dierks, T., Julin, P., Winblad, B., and Jelic, V. (2000). Discrimination of Alzheimer’s disease and mild cognitive impairment by equivalent EEG sources: A cross-sectional and longitudinal study. Clin. Neurophysiol. 111, 1961–1967. doi: 10.1016/s1388-2457(00)00454-5
Hutchison, I. C., and Rathore, S. (2015). The role of REM sleep theta activity in emotional memory. Front. Psychol. 6:1439. doi: 10.3389/fpsyg.2015.01439
Jahng, S., Na, D. L., and Kang, Y. (2015). Constructing a composite score for the Seoul neuropsychological screening battery-core. Dement. Neurocogn. Disorder. 14, 137–142. doi: 10.1212/WNL.0000000000010347
Jelic, V., Johansson, S. E., Almkvist, O., Shigeta, M., Julin, P., Nordberg, A., et al. (2000). Quantitative electroencephalography in mild cognitive impairment: Longitudinal changes and possible prediction of Alzheimer’s disease. Neurobiol. Aging 21, 533–540.
Jeong, J. (2004). EEG dynamics in patients with Alzheimer’s disease. Clin. Neurophysiol 115, 1490–1505.
Kang, Y., and Na, D. L. (2003). Seoul neuropsychological screening battery (SNSB). Seoul: Human Brain Research & Consulting Co.
Kang, Y., Jahng, S., and Na, D. L. (2015). Seoul neuropsychological screening battery-core (SNSB-C). Seoul: Human Brain Research & Consulting Co.
Knyazev, G. G. (2012). EEG delta oscillations as a correlate of basic homeostatic and motivational processes. Neurosci. Biobehav. Rev. 36, 677–695. doi: 10.1016/j.neubiorev.2011.10.002
Lin, Y. C., Hsu, W. C., Wu, C. K., Chang, W. H., Wu, K. P., and Wong, A. M. (2016). Comparison of motor performance of upper and lower extremities in dual-task tests in patients with mild Alzheimer’s dementia. Aging Clin. Exp. Res. 28, 491–496. doi: 10.1007/s40520-015-0441-1
Martín-Gonzalo, J. A., Pulido-Valdeolivas, I., Wang, Y., Wang, T., Chiclana-Actis, G., and Algarra-Lucas, M. C. (2019). Permutation entropy and irreversibility in gait kinematic time series from patients with mild cognitive decline and early Alzheimer’s dementia. Entropy 21:868.
McKhann, G. M., Knopman, D. S., Chertkow, H., Hyman, B. T., Jack, C. R. Jr., Kawas, C. H., et al. (2011). The diagnosis of dementia due to Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement 7, 263–269.
Michels, L., Muthuraman, M., Anwar, A. R., Kollias, S., Leh, S. E., Riese, F., et al. (2017). Changes of functional and directed resting-state connectivity are associated with neuronal oscillations, apoe genotype and amyloid deposition in mild cognitive impairment. Front. Aging Neurosci. 9:304. doi: 10.3389/fnagi.2017.00304
Missonnier, P., Deiber, M. P., Gold, G., Herrmann, F. R., Millet, P., Michon, A., et al. (2007). Working memory load-related electroencephalographic parameters can differentiate progressive from stable mild cognitive impairment. Neuroscience 150, 346–356. doi: 10.1016/j.neuroscience.2007.09.009
Missonnier, P., Herrmann, F. R., Michon, A., Fazio-Costa, L., Gold, G., and Giannakopoulos, P. (2010). Early disturbances of gamma band dynamics in mild cognitive impairment. J. Neural. Transm (Vienna) 117, 489–498. doi: 10.1007/s00702-010-0384-9
Morris, R., Lord, S., Bunce, J., Burn, D., and Rochester, L. (2016). Gait and cognition: Mapping the global and discrete relationships in ageing and neurodegenerative disease. Neurosci. Biobehav. Rev. 64, 326–345. doi: 10.1016/j.neubiorev.2016.02.012
Murty, D. V., Manikandan, K., Kumar, W. S., Ramesh, R. G., Purokayastha, S., Nagendra, B., et al. (2021). Stimulus-induced gamma rhythms are weaker in human elderly with mild cognitive impairment and Alzheimer’s disease. Elife 10:e61666. doi: 10.7554/eLife.61666
Petersen, R. C., Caracciolo, B., Brayne, C., Gauthier, S., Jelic, V., and Fratiglioni, L. (2014). Mild cognitive impairment: A concept in evolution. J. Intern. Med. 275, 214–228.
Pieruccini-Faria, F., Black, S. E., Masellis, M., Smith, E. E., Almeida, Q. J., Li, K. Z. H., et al. (2021). Gait variability across neurodegenerative and cognitive disorders: Results from the Canadian consortium of neurodegeneration in aging (CCNA) and the gait and brain study. Alzheimers Dement 17, 1317–1328. doi: 10.1002/alz.12298
Ray, S., and Maunsell, J. H. (2015). Do gamma oscillations play a role in cerebral cortex? Trends Cogn. Sci. 19, 78–85.
Rucco, R., Agosti, V., Jacini, F., Sorrentino, P., Varriale, P., De Stefano, M., et al. (2017). Spatio-temporal and kinematic gait analysis in patients with Frontotemporal dementia and Alzheimer’s disease through 3D motion capture. Gait Posture 52, 312–317. doi: 10.1016/j.gaitpost.2016.12.021
Sankari, Z., Adeli, H., and Adeli, A. (2012). Wavelet coherence model for diagnosis of Alzheimer disease. Clin. EEG Neurosci. 43, 268–278.
Sebastiani, P., Andersen, S. L., Sweigart, B., Du, M., Cosentino, S., Thyagarajan, B., et al. (2020). Patterns of multi-domain cognitive aging in participants of the Long Life Family Study. Geroscience 42, 1335–1350. doi: 10.1007/s11357-020-00202-3
Smailovic, U., and Jelic, V. (2019). Neurophysiological markers of Alzheimer’s Disease: Quantitative EEG approach. Neurol. Ther. 8, 37–55.
Solomon, A., Mangialasche, F., Richard, E., Andrieu, S., Bennett, D. A., Breteler, M., et al. (2014). Advances in the prevention of Alzheimer’s disease and dementia. J. Intern. Med. 275, 229–250.
Spitzer, B., and Haegens, S. (2017). Beyond the Status Quo: A role for beta oscillations in endogenous content (re)activation. eNeuro 4. doi: 10.1523/ENEURO.0170-17.2017
Toots, A. T. M., Taylor, M. E., Lord, S. R., and Close, J. C. T. (2019). Associations Between Gait Speed and Cognitive Domains in Older People with Cognitive Impairment. J. Alzheimers Dis. 71, S15–S21.
Wang, J., Fang, Y., Wang, X., Yang, H., Yu, X., and Wang, H. (2017). Enhanced gamma activity and cross-frequency interaction of resting-state electroencephalographic oscillations in patients with Alzheimer’s Disease. Front. Aging Neurosci. 9:243. doi: 10.3389/fnagi.2017.00243
Xie, H., Wang, Y., Tao, S., Huang, S., Zhang, C., and Lv, Z. (2019). Wearable sensor-based daily life walking assessment of gait for distinguishing individuals with amnestic mild cognitive impairment. Front Aging Neurosci. 11:285. doi: 10.3389/fnagi.2019.00285
Keywords: diagnosis, cognitive impairment, multimodal signals, EEG, kinematic gait analysis
Citation: Min J-Y, Ha S-W, Lee K and Min K-B (2022) Use of electroencephalogram, gait, and their combined signals for classifying cognitive impairment and normal cognition. Front. Aging Neurosci. 14:927295. doi: 10.3389/fnagi.2022.927295
Received: 24 April 2022; Accepted: 08 August 2022;
Published: 07 September 2022.
Edited by:
Jeannette R. Mahoney, Albert Einstein College of Medicine, United StatesReviewed by:
Manuel Enrique Hernandez, University of Illinois Urbana-Champaign, United StatesCopyright © 2022 Min, Ha, Lee and Min. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Kyoung-Bok Min, bWlua2JAc251LmFjLmty
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.