- 1Department of Neurology and Centre of Clinical Neuroscience, First Faculty of Medicine and General University Hospital in Prague, Charles University, Prague, Czechia
- 2Soteria Biotech Co, Ltd., New Taipei City, Taiwan
Obstructive sleep apnea (OSA) and Alzheimer's disease (AD) are common in the elderly population. Obstructive sleep apnea that may cause significant changes in the cerebrospinal fluid β-amyloid and T-tau and/or P-tau protein levels is often identified as a risk factor for development of AD. Although the underlying mechanisms of AD are still not fully understood, a hypothesis associating OSA with AD has been already proposed. In this systematic mini-review, we first discuss the recent findings supporting the association of OSA with an increased risk of AD and then provide evidence suggesting the positive effect of OSA treatment on a reduced risk of AD.
Introduction
Alzheimer's disease (AD) and sleep disorders are both known to have a significant impact on global health. Sleep disorders such as insomnia, obstructive sleep apnea (OSA), or narcolepsy cause changes in the sleeping pattern of individuals and, in turn, strongly affect their life quality and health. OSA affects almost 50% of individuals and is considered the most common form of breathing-related sleep disorders, with a prevalence around 85% (Benjafield et al., 2019). Other types of breathing-related sleep disorders, such as central sleep apnea and complex/mixed form sleep apnea, are much less common (Morgenthaler et al., 2006); therefore, they are not included in the present review. We remind the reader that OSA is characterized by repetitive episodes of complete or partial blockage/collapse of the airways. Hence, the majority of OSA patients usually have anatomical abnormalities, which cause upper airway dilator muscle dysfunction and instability of breathing control and lung volume. However, the pharyngeal dilator muscle maintaining airway patency and obstruction only occur during sleep once the muscles are relaxed (White, 2005). It was observed that moderate OSA affects at least 1 in 15 adults, whereas 1 in 5 adults have mild OSA (Young et al., 2002). Nonetheless, due to a lack of awareness by both the public and health professionals, almost 90% of OSA patients are yet undiagnosed (Young et al., 1997). Similarly, a more recent finding from a prospective observational study concluded that almost 81% of study subjects were undiagnosed with OSA (Finkel et al., 2009). Obstructive sleep apnea is associated not only with daytime somnolence, metabolic syndrome, cardiovascular diseases, hypertension, and other chronic issues, but also with a cognitive decline, especially of attention, memory, and executive functions (Nair et al., 2011).
Alzheimer's disease is an irreversible, progressive brain disorder that deteriorates cognitive abilities, negatively impacting the ability for the individual to carry out the simplest of tasks and, consequently, disrupts day-to-day life functions (Kuo et al., 2020). Alzheimer's disease is the most common type of dementia and accounts for more than 60% of dementia cases (Kalaria et al., 2008). Despite the fact that underlying mechanisms and causes for AD are still not completely understood, researchers generally agree that extracellular β-amyloid build-up (plaques) and intraneuronal neurofibrillary (tau) tangles accumulated throughout the brain are the hallmarks of AD (Querfurth and LaFerla, 2010). The amyloid plaques were also found in individuals with OSA, supporting a possible connection between OSA and an increased risk for development of AD (Bubu et al., 2020). The purpose of this study is to (i) assess this association between OSA and AD, (ii) identify the population that is more susceptible to OSA and AD, and (iii) find if treatment of OSA may affect the progress of AD.
Procedure
A systematic review of the English language literature regarding the topic was performed using the main electronic databases via PubMed, the Cochrane Library, and Google Scholar, which account for the majority of the biomedical journals published worldwide. The literature search was conducted in February 2020 and the combination of the following key terms were used: “Alzheimer's disease” as a medical subject heading term (MeSH), or “Alzheimer's dementia” (MeSH), or “Alzheimer” (MeSH), or “AD” and “Obstructive Sleep Apnea” (MeSH), or “OSA.” Then, the relevant studies were identified by reviewing articles satisfying the key terms and also by tracing the references of these retrieved articles. The exclusion criteria were as follows: (a) animal studies; (b) case reports; (c) reviews including mini-reviews and systematic reviews, meta-analyses, and case studies; (d) non-OSA studies, that is, studies focused on intermittent hypoxia, sleep fragmentation, central sleep apnea, or mixed sleep apnea; (e) non-AD studies, that is, studies focusing just on patients with either mild cognitive impairment (MCI) or the other types of dementia like vascular dementia and Lewy body dementia; and (f) duplicate or non-English materials. In addition to inclusion and exclusion criteria, we limited our search to only the most recent studies in the considered topic.
We initially identified 72 related articles that were published between 2010 and 2020 (see Figure 1A). Then, after we applied both the inclusion and exclusion criteria, a total of only 12 articles satisfied the search criteria. Among them, just seven articles discussed an association between OSA and AD, and could be potentially used to find a population that is more susceptible to OSA and AD. Another five articles were used to evaluate whether the treatment of OSA would possibly affect the course of AD. The flowchart of the study search, including selection strategy, is given in Figure 1B.
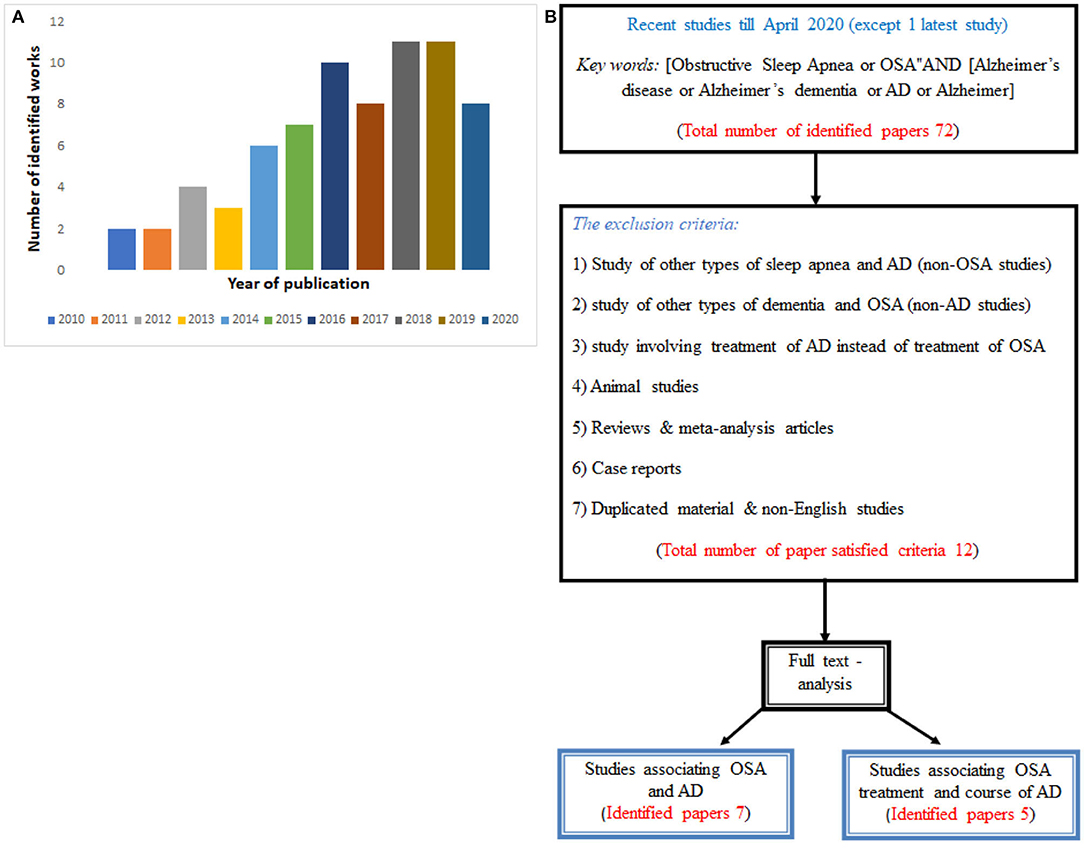
Figure 1. (A) Histogram of the number of publications per year of publication used in the present review, and (B) Flowchart diagram showing the procedure with papers satisfying required criteria.
Results
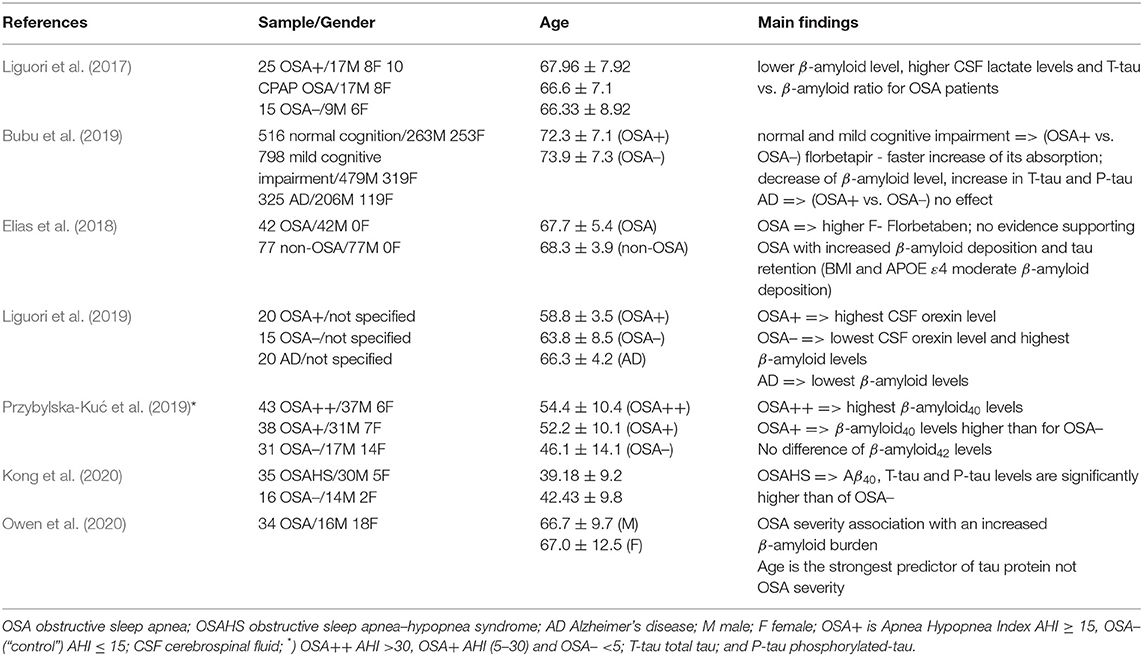
First, we evaluated studies that have examined the relationship between OSA and AD and, afterwards, we identified the population that is perhaps more susceptible to both OSA and AD. The main findings of the considered studies are, for reader's convenience, summarized in Table 1. It has been already demonstrated by several studies (Bubu et al., 2020; Kuo et al., 2020) that OSA may induce an alteration in cerebrospinal fluid (CSF) β-amyloid, particularly Aβ40 and Aβ42 and T-tau and/or P-tau protein levels, which are also the known pathological hallmarks of AD. Intermittent hypoxia and sleep fragmentation, that is, the common OSA processes, have been identified as the fundamental processes that can generate neurodegenerative changes. Intermittent hypoxia and sleep fragmentation in OSA patients may significantly affect the brain structure and cause problems with β-amyloid metabolism. Individuals with severe OSA compared to individuals with or without moderate OSA have significantly higher β-Amyloid isoform 40 (Aβ40) plasma concentrations (Przybylska-Kuć et al., 2019). These plasma concentrations could be connected to hypoxia severity and, consequently, may also indicate an increased risk for the development of AD.
In general, intermittent hypoxia may be responsible for the alteration in blood pressure and, correspondingly, hypertension in OSA individuals (Bubu et al., 2020). It has also been suggested that it is usually hypoxia which is responsible for increasing oxidative stress and altering the CSF and T-tau protein levels, that is, lowering in Aβ42 concentration and increasing CSF lactate level and Aβ42/T-tau ratio for AD individuals (Liguori et al., 2017). Importantly, according to findings of recent longitudinal studies (Bubu et al., 2019; Kong et al., 2020), hypoxia may, in addition to creating higher CSF T-tau and P-tau levels, accelerate an increase in β-amyloid deposition.
Short wave sleep and REM sleep, which are associated with CSF β-amyloid dynamics, are strongly affected by sleep fragmentation (Bubu et al., 2020). They may cause problems with metabolism and the removal of neurotoxic β-amyloid and, as such, they may impair the individual‘s cognitive functions, such as memory, and induce early signs of AD neuropathological changes (Liguori et al., 2017). It has also been suggested that β-amyloid deposition can be moderated by the apolipoprotein E ε4 (APOE ε4) and the body mass index (BMI), that is, OSA individuals have more APOE ε4 carriers and a higher BMI than those without OSA (Elias et al., 2018).
Some researchers believe that OSA may be an early trigger of AD neuropathological processes (Gagnon et al., 2014). Evidence supporting this hypothesis has recently been presented by Liguori et al. (2019). In their study, they observed connections between CSF orexin levels and β-amyloid with sleep pattern in an OSA patient.
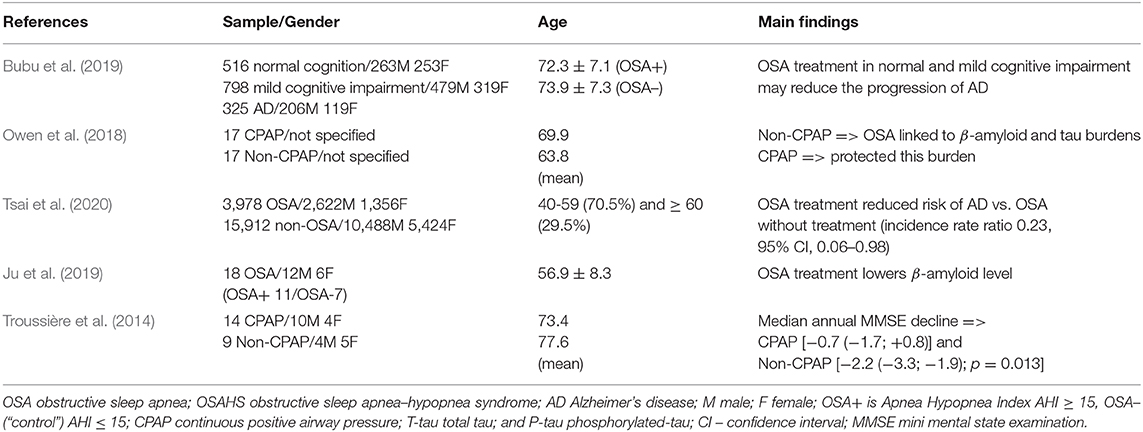
We provide the most recent studies that were identified to assess whether the treatment of OSA may modify the process of AD in Table 2. As we have discussed previously, OSA has been identified as a risk factor for the development of AD, therefore it was suggested that treatment of OSA by, for instance, surgery or continuous positive airway pressure (CPAP) might help to reduce the risk for the development of AD. The positive effect of CPAP treatment on OSA patients with AD has been observed by Owen et al. (2018). They found that CPAP treatment can help to moderate AD neuropathology. More recently, the association between OSA and a higher risk for development of AD has been highlighted by Tsai et al. (2020). In this population-based cohort study, where a relatively large sample size of 19,890 individuals (3,978 OSA patients and 15,912 non-OSA patients) was investigated, the OSA patients were found to have a higher risk for AD than the non-OSA ones. They also observed that OSA treatment significantly reduced the progress of AD. Nonetheless, this study does not account for other modifiable risk factors of AD such as alcohol misuse, smoking, social engagement, and BMI. In addition, in this study the diagnoses of AD and OSA were not recorded by the standard clinical assessments but only by insurance claims. The detailed clinical trial studies with clear medical assessments and brain imagining are required to verify conjecture that treatment of OSA may help to either delay the prodromes or change the course of AD.
Sleep disruption (fragmentation) in mild-to-severe OSA patients significantly decreases slow wave activity, which in time can lead to cognitive impairment. The lower slow wave activities are, the higher the neurotoxic β-Amyloid levels can be found, causing plaques and complicating its removal and, correspondingly, potentially increasing the risk for AD, as discussed above. Ju et al. (2019) provide evidence supporting that CPAP treatment may help to reduce sleep fragmentation, increases slow wave activity, and notably decreases the β-Amyloid and tau levels. They have also suggested that an early diagnosis and moderation of OSA by CPAP might possibly reduce the risk for development of AD in later life. Sleep disturbance is common for AD patients, especially for those diagnosed with mild-to-moderate stage of AD. Some researchers suggested that CPAP treatment of OSA during mild-to-moderate stages of AD could potentially slow the progression of cognitive impairment (Chong et al., 2006; Canessa et al., 2011). Findings of a 3-year follow-up study by Troussière et al. (2014) provide evidence supporting this hypothesis. In their study, and also in the systematic study performed by Bubu et al. (2019), a significantly slower cognitive decline was observed for patients undergoing CPAP treatment.
Discussion
In the first part of this mini-review, we assess studies (see Table 1) that are trying to: (i) find an association between OSA and AD; and (ii) identify a population that is predisposed to OSA and AD by focusing on biomarkers β-amyloid and T-tau (P-tau) protein, of which the accumulation in brain (complication with their removal) are the typical signs of AD. These studies mainly focus on how intermittent hypoxia and sleep fragmentation, which are both common in OSA, are linked to AD. All of the considered studies (i.e., seven studies) provide clear evidence supporting the association between OSA and AD. However, among them, only three studies (Gagnon et al., 2014; Liguori et al., 2017; Bubu et al., 2019) suggested that the OSA patients experience lower CSF Aβ42 levels. Moreover, OSA has been found to affect CSF biomarkers for AD. The neuropathology of AD in OSA individuals has been recently discussed by Owen et al. (2020). They show that the burden of β-amyloid increases in the hippocampus with an increase of OSA severity, whereas for tau proteins age, and not severity of OSA, has been found as the strongest predictor.
Unfortunately, none of the included studies present conclusive findings or comments on the population more predisposed to OSA and AD. Hence, we account for findings of earlier epidemiological studies, which linked a higher risk of OSA with an increased age of individuals and their gender. We remind the reader that OSA is prevalent in elderly populations and it affects male individuals more than female ones (see Young et al., 2002). Furthermore, the chance of being diagnosed with dementia also increases exponentially with age. For example, according to Qiu et al. (2009), more than one third of the oldest adults (85+) are being diagnosed with at least one type of dementia. However, extensive investigations on a large population cohort are still required to confirm the widely accepted hypothesis that elderly individuals are more susceptible to both OSA and AD.
The second goal of this review was to clarify whether treatment of OSA may change the progress of AD. As we have discussed in section Results, sleep fragmentation caused by OSA may cause a significant increase of oxidative stress levels and inflammation that, in turn, can promote the pathogenesis of AD. Results given in Table 2 provide clear evidence supporting the suggestion that OSA may increase the risk of AD and that treatment of OSA (e.g., CPAP treatment) could possibly slow the progression of cognitive decline in individuals diagnosed with AD. For example, CPAP treatment can help to decrease sleep fragmentation, to stabilize levels of biomarkers β-amyloid and T-tau protein (the accumulation if which is a typical sign of AD), and, correspondingly, to improve a patient's cognitive and daytime functioning. It is noteworthy that in a large population-based cohort study by Tsai et al. (2020), the prevalence of AD in OSA patients (0.8%) was much higher than in non-OSA patients (0.3%). These findings also support results of an earlier preliminary study by Cooke et al. (2009), where authors suggested that a long-term CPAP treatment may possibly slow the rate of cognitive deterioration.
Conclusions
Life expectancy has notably increased in past century, but this brings new challenges to public health, as elderly individuals are more vulnerable to cardiovascular diseases, depression, AD, and OSA (Kuo et al., 2020). In this mini-review, we first assess if OSA may be associated with AD, and then we discuss recent studies on the effect of OSA treatment for improving cognition in AD patients. Our research provides clear evidence supporting an association between OSA and AD. Sleep fragmentation and intermittent hypoxia are common for OSA patients and have been shown to cause neurodegenerative changes in brain structure, and to alter β-amyloid and T-tau protein levels, which are also known as the biomarkers of AD. CPAP treatment of OSA reduces sleep fragmentation and improves slow wave sleep. As a result, treatment of OSA can help to stabilize β-amyloid and T-tau protein levels and, correspondingly, improve an individual's cognition. Findings of recent studies also support the hypothesis that treatment of OSA may not only reduce the risk of AD but also, for AD patients, it could perhaps help to slow cognitive impairment. Although assessed studies did not provide a definite conclusion on what population is more vulnerable to both OSA and AD, based on the included studies and current epidemiology data, it is widely accepted that elderly individuals (65+) are more susceptible to both OSA and AD.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary materials, further inquiries can be directed to the corresponding author.
Author Contributions
C-YK, H-TH, and I-HL carried out the bibliographic search and wrote the first draft of the manuscript. The conceptualization and design were performed by C-YK and TN. The final version of the manuscript was prepared by C-YK and TN. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by Progres Q27/LF1.
Conflict of Interest
H-TH and I-HL were employed by the company Soteria Biotech Co, Ltd.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
Benjafield, A. V., Ayas, N. T., Eastwood, P. R., Heinzer, R., Ip, M. S. M., Morrell, M. J., et al. (2019). Estimation of the global prevalence and burden of obstructive sleep apnoea: a literature-based analysis. Lancet Respir. Med. 7, 687–698. doi: 10.1016/S2213-2600(19)30198-5
Bubu, O. M., Andrage, A. G., Umasabor-Bubu, O. Q., Hogan, M. M., Turner, A. D., de Leon, M. J., et al. (2020). Obstructive sleep apnea, cognition and Alzheimer's disease: a systematic review integrating three decades of multidisciplinary research. Sleep Med. Rev. 50:101205. doi: 10.1016/j.smrv.2019.101250
Bubu, O. M., Pirraglia, E., Andrade, A. G., Sharma, R. A., Gimenez-Badia, S., Umasabor-Bubu, O. Q., et al. (2019). Obstructive sleep apnea and longitudinal Alzheimer's disease biomarker changes. Sleep 42:zsz048. doi: 10.1093/sleep/zsz048
Canessa, N., Castronovo, V., Cappa, S. F., Aloia, M. S., Marelli, S., Falini, A., et al. (2011). Obstructive sleep apnea: brain structural changes and neurocognitive function before and after treatment. Am. J. Respir. Crit. 183, 1419–1426. doi: 10.1164/rccm.201005-0693OC
Chong, M. S., Ayalon, L., Marler, M., Loredo, J. S., Corey-Bloom, J., Palmer, B. W., et al. (2006). Continuous positive airway pressure reduces subjective daytime sleepiness in patients with mild to moderate Alzheimer's disease with sleep disordered breathing. J. Am. Geriatr. Soc. 54, 2076–2081. doi: 10.1111/j.1532-5415.2006.00694.x
Cooke, J. R., Ayalon, L., Palmer, B. W., Loredo, J. S., Corey-Bloom, J., Natarajan, L., et al. (2009). Sustained use of CPAP slows deterioration of cognition, sleep, and mood in patients with Alzheimer's disease and obstructive sleep apnea: a preliminary study J. Clin. Sleep Med. 15, 305–309. doi: 10.5664/jcsm.27538
Elias, A., Cummins, T., Tyrrell, R., Lamb, F., Dore, V., Williams, R., et al. (2018). Risk of Alzheimer's disease in obstructive sleep apnea syndrome: amyloid-β and tau imaging. J. Alzheimers Dis. 66, 733–741. doi: 10.3233/JAD-180640
Finkel, K. J., Searleman, A. C., Tymkew, H., Tanaka, C. Y., Saager, L., Safer-Zadeh, E., et al. (2009). Prevalence of undiagnosed obstructive sleep apnea among adult surgical patients in an academic medical center. Sleep Med. 10, 753–758. doi: 10.1016/j.sleep.2008.08.007
Gagnon, K., Baril, A. A., Gagnon, J. F., Fotin, M., Décary, A., Lafond, C., et al. (2014). Cognitive impairment in obstructive sleep apnea. Pathol. Biol. 62, 233–240. doi: 10.1016/j.patbio.2014.05.015
Ju, Y. S., Zangrilli, M. A., Finn, M. B., Fagan, A. M., and Holtzman, D. M. (2019). Obstructive sleep apnea treatment, slow wave activity, and amyloid-β. Ann. Neurol. 85, 291–295. doi: 10.1002/ana.25408
Kalaria, R. N., Maestre, G. E., Arizaga, R., Friedland, R. P., Galasko, D., Hall, K., et al. (2008). Alzheimer's disease and vascular dementia in developing countries: prevalence, management, and risk factors. Lancet Neurol. 7, 812–826. doi: 10.1016/S1474-4422(08)70169-8
Kong, W., Zheng, Y., Xu, W., Gu, H., and Wu, J. (2020). Biomarkers of Alzheimer's disease in severe obstructive sleep apnea–hypopnea syndrome in the Chinese population. Eur. Arch. Oto-Rhino-Laryngol. doi: 10.1007/s00405-020-05948-2
Kuo, C. Y., Stachiv, I., and Nikolai, T. (2020). Association of late life depression, (non-) modifiable risk and protective factors with dementia and Alzheimer's disease: literature review on current evidences, preventive interventions and possible future trends in prevention and treatment of dementia. Int. J. Environ. Res. Public Health 17:7475. doi: 10.3390/ijerph17207475
Liguori, C., Mercuri, N. B., Nuccetelli, M., Izzi, F., Cordella, A., Bernardini, S., et al. (2019). Obstructive sleep apnea may induce orexinergic system and cerebral β-amyloid metabolism dysregulation: is it a further proof for Alzheimer's disease risk? Sleep Med. 56, 171–176. doi: 10.1016/j.sleep.2019.01.003
Liguori, C., Romigi, A., Nuccetelli, M., Zannino, S., Sancesario, G., Martorana, A., et al. (2017). Obstructive sleep apnea is associated with early but possibly modifiable Alzheimer's disease biomarkers changes. Sleep 40:zsx011. doi: 10.1093/sleep/zsx011
Morgenthaler, T., Kagramanov, V., Hanak, V., and Decker, P. (2006). Complex sleep apnea syndrome: is it a unique clinical syndrome? Sleep 29, 1203–1209. doi: 10.1093/sleep/29.9.1203
Nair, D., Dayyat, E. A., Zhang, S. X., Wang, Y., and Gozal, D. (2011). Intermittent hypoxia-induced cognitive deficits are mediated by NADPH oxidase activity in a murine model of sleep apnea. PLoS ONE 6:e19847. doi: 10.1371/journal.pone.0019847
Owen, J. E., Benediktsdottir, B., Cook, E., Olafsson, I., Gislasson, T., and Robinson, S. R. (2020). Alzheimer's disease neuropathology in the hippocampus and brainstem of people with obstructive sleep apnea. Sleep 21:zsaa195. doi: 10.1093/sleep/zsaa195
Owen, J. E., Gislasson, T., Benediktsdottir, B., and Robinson, S. R. (2018). Continuous positive airway pressure use is associated with a lower burden of Alzheimer's disease neuropathology in obstructive sleep apnea patients compared to non-use. Sleep 41:A114. doi: 10.1093/sleep/zsy061.295
Przybylska-Kuć, S., Zakrzewski, M., Dybała, A., Kiciński, P., Dzida, G., Myśliński, W., et al. (2019). Obstructive sleep apnea may increase the risk of Alzheimer's disease. PLoS ONE 14:e0221255. doi: 10.1371/journal.pone.0221255
Qiu, C., Kivipelto, M., and von Strauss, E. (2009). Epidemiology of Alzheimer's disease: occurrence, determinants, and strategies toward intervention. Dialogues Clin. Neurosci. 11, 111–128. doi: 10.31887/DCNS.2009.11.2/cqiu
Querfurth, H. W., and LaFerla, F. M. (2010). Alzheimer's disease. N. Engl. J. Med. 362, 329–344. doi: 10.1056/NEJMra0909142
Troussière, A. C., Charley, C. M., Salleron, J., Richard, F., Delbeuck, X., Derambure, P., et al. (2014). Treatment of sleep apnoea syndrome decreases cognitive decline in patients with Alzheimer's disease. J. Neurol. Neurosurg. Psychiatr. 85, 1405–1408. doi: 10.1136/jnnp-2013-307544
Tsai, M. S., Li, H. Y., Huang, C. G., Wang, R. Y. L., Chuang, L. P., Chen, N. H., et al. (2020). Risk of Alzheimer's disease in obstructive sleep apnea patients with or without treatment: real-world evidence. Laryngoscope 130, 2292–2298. doi: 10.1002/lary.28558
White, D. P. (2005). Pathogenesis of obstructive and central sleep apnea. Am. J. Resp. Crit. Care Med. 172, 1363–1370. doi: 10.1164/rccm.200412-1631SO
Young, T., Evans, L., Finn, L., and Palta, M. (1997). Estimation of the clinically diagnosed proportion of sleep apnea syndrome in middle-aged men and women. Sleep 20, 705–706. doi: 10.1093/sleep/20.9.705
Keywords: obstructive sleep apnea (OSA), Alzheimer's disease, sleep disturbance and sleep disordered breathing, continuous positive air pressure, OSA treatment, cerebrospinal fluid-CSF
Citation: Kuo C-Y, Hsiao H-T, Lo I-H and Nikolai T (2021) Association Between Obstructive Sleep Apnea, Its Treatment, and Alzheimer's Disease: Systematic Mini-Review. Front. Aging Neurosci. 12:591737. doi: 10.3389/fnagi.2020.591737
Received: 05 August 2020; Accepted: 04 December 2020;
Published: 06 January 2021.
Edited by:
Dennis Qing Wang, Southern Medical University, ChinaReviewed by:
Arash Tadjalli, University of Florida, United StatesYinxia Chao, National Neuroscience Institute (NNI), Singapore
Copyright © 2021 Kuo, Hsiao, Lo and Nikolai. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chih-Yun Kuo, Z2lubnkmI3gwMDA0MDtkcmF6bmlyZXZpemUuY3o=
 Chih-Yun Kuo
Chih-Yun Kuo Hung-Ta Hsiao
Hung-Ta Hsiao Ing-Hsien Lo2
Ing-Hsien Lo2