
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Microbiol. , 22 December 2023
Sec. Infectious Agents and Disease
Volume 14 - 2023 | https://doi.org/10.3389/fmicb.2023.1313838
This article is part of the Research Topic Reviews in Microbial Pathogenesis View all 17 articles
Kawasaki disease (KD) is a systematic vasculitis that is often complicated by coronary artery lesions and is a leading cause of acquired heart disease in developed countries. Previous studies have suggested that genetic susceptibility, together with an inducing infectious agent, could be involved in KD pathogenesis; however, the precise causative agent of this disease remains unknown. Moreover, there are still debates concerning whether KD is an infectious disease or an autoimmune disease, although many studies have begun to show that various pathogens functioning as critical inducers could activate different kinds of immune cells, consequently leading to the dysfunction of endothelial cells and systematic vasculitis. Here in this review, we attempt to summarize all the available evidence concerning pathogen infections associated with KD pathogenesis. We also discuss the related mechanisms, present a future perspective, and identify the open questions that remain to be investigated, thereby providing a comprehensive description of pathogen infections and their correlations with the host immune system in leading to KD.
Pathogen infectious diseases have posed a great challenge to human health worldwide (Baldari et al., 2023). Currently, various pathogens have been suggested as critical triggers in inducing systematic vasculitis in children with Kawasaki disease (KD), which is a leading cause of acquired heart disease in developed countries (McCrindle et al., 2017). High-dose intravenous immunoglobulin (IVIG) infusion and aspirin can subdue KD symptoms and partially reduce the occurrence of coronary artery lesions (CALs); however, approximately 10%–20% of affected children develop recrudescent or persistent fever even after IVIG infusion, and those patients have a higher risk of CAL (Li et al., 2018; Nadig et al., 2023). Critically, if this disease is not untreated in a timely manner, sudden death may occur due to coronary artery aneurysms (Shulman and Rowley, 2015; McCrindle et al., 2017; Sosa et al., 2019). Although genetic background (Chang et al., 2014), urban industrialization, environmental factors (Chang et al., 2020; Corinaldesi et al., 2020), and regional winds together with large-scale atmospheric circulation (Rodo et al., 2011, 2014), have been suggested to correlate with KD, these theories fail to explain the seasonal epidemics of this illness, and also fail to explain why Kawasaki disease does not broadly recur. Nevertheless, an increasing number of epidemiological and clinical data all point to KD having an infectious etiology. For example, epidemiological data from multiple centers worldwide demonstrate that KD has a significant seasonal epidemic (Valtuille et al., 2023), frequent occurrence, and low recurrence characteristics in young children (Nakamura et al., 2008, 2012; Burns et al., 2013; Lin et al., 2015; Ozeki et al., 2017, 2018; Kido et al., 2019; Kim et al., 2020; Xie et al., 2020). Notably, several studies have shown that both the immune repertoire (Kuo et al., 2019) and the heterogeneous host immune response including the autoantibody responses in KD children resemble those observed in patients with bacterial or viral infections (Lindquist and Hicar, 2019; Jackson et al., 2021; Ghosh et al., 2022), lending further support of an infectious disease cause of KD.
Additionally, serum KD-specific molecules which were mostly derived from biofilms possessed molecular structures common to MAMPs (microbe-associated molecular pattern) from Bacillus cereus, B. subtilis, Yersinia pseudotuberculosis (Y. pstb), and Staphylococcus aureus (Kusuda et al., 2014), implicating a possible relationship between MAMPs and the etiological mechanism of KD vasculitis. Recently, at least 14 types of viruses have been suggested to correlate with KD based on serological and polymerase chain reaction (PCR) analysis of clinical samples (Principi et al., 2013). However, another study showed that at least 15 types of viruses were related to KD because the isolation rates of various viruses in KD patients were significantly higher than those in the control group (Huang et al., 2015; Jackson et al., 2021). Viral infections can cause vascular damage either through direct invasion of the vascular endothelium or provoking a rapid cell-damaging event (Hara et al., 2021). This in turn results in a larger release of proinflammatory cellular components from damaged endothelial cells, pyroptosis, or proinflammatory cell death (Mohandas et al., 2023), hence making various kinds of innate immune cells infiltrate the coronary arteries of KD subjects (Kuijpers et al., 1999; Takahashi et al., 2010a). These data thus suggest that different kinds of microbes are implicated in the pathogenesis of KD, but which microbes are the key inducers and the underlying mechanisms remain unclear. In this review, to better understand the comprehensive profiles between microbial infection and KD pathogenesis, we summarized the major features of our current understanding with respect to various pathogens related to KD. We also discuss the state of this field in KD with respect to the relationship and/or mechanisms concerning the abnormal immune response triggered by various infectious agents, and the open questions that remain to be investigated.
Several DNA viruses, including Epstein–Barr virus (EBV), human adenovirus, human parvovirus B19, torque teno virus, herpes family virus, varicella zoster virus, bocaparvo virus, and cytomegalovirus have been identified to be associated with KD pathogenesis.
Adenovirus type 2 was first isolated from a patient with fatal Kawasaki disease (Embil et al., 1985), while another case report showed that human adenovirus infection can be found in monozygotic twin boys who developed KD (Fukuda et al., 2017). Among the adenovirus-infected cohort, the overall incidence of KD was 5.29 times higher than that of the non-adenovirus-infected control subjects (adjusted HR 5.29, 95% CI: 2.48–11.3), as shown by a population-based cohort study (Huang et al., 2020), suggesting a correlation between adenovirus infection and KD pathogenesis. Notably, there are also studies showing a lack of association between adenovirus infection and KD, suggesting that more intense research is needed to explore the relationships between adenovirus infections and KD (Okano et al., 1990; Shike et al., 2005).
Human parvovirus B19 (HPV-B19) is a single-stranded DNA virus that may have a pathogenic role in the development of KD with other predisposing factors because it can cause symptoms resembling those observed in KD patients (Nigro et al., 1994; Holm et al., 1995). Importantly, HPV-B19 infection should be considered in the differential diagnosis of KD patients who show atypical clinical symptoms during the erythema infectiosum epidemic stage (Oura et al., 2022).
The torque teno virus (TTV), which is a single-stranded circular DNA virus, was first found in the lymph node of a KD patient (Katano et al., 2012). For instance, a high viral load of torque teno virus 7 (TTV7) was identified in KD patients (Thissen et al., 2018; Spezia et al., 2023a), and the viral load of TTV positively correlated with the level of total bilirubin and aspartate aminotransferase in KD patients (Spezia et al., 2023b), suggesting that TTV might play a critical role in the pathophysiology of patients with KD.
Herpes simplex virus (HSV) consists of multiple subtypes (Rowley et al., 2011), and its family members, including EBV, HHV-6 and varicella-zoster, were all found to be involved in KD. For instance, a previous study showed that the DNA sequence of EBV can be detected in KD patients (Kikuta et al., 1988), and there are many cases of KD-like lesions, specifically coronary artery aneurysms (CAAs), that were suggested to be caused by EBV infection (Kikuta et al., 1993; Rosenfeld et al., 2020; Xiao et al., 2020). However, EBV might not be the direct causative agent of KD, as shown by another study (Kikuta et al., 1990). Notably, a case of Kawasaki disease triggered by EBV virus infection was found to be complicated with familial Mediterranean fever (Maggio et al., 2019). Moreover, the prevalence of EBV in KD children was significantly lower during the early stage (van Stijn et al., 2020), and deoxyuridine 5′-triphosphate nucleotide hydrolase (dUTPase), a pathogen nonstructural protein encoded by EBV, can stimulate monocyte-derived macrophages through Toll-like receptor 2-dependent signaling transduction (Ariza et al., 2009), suggesting that DUTPase could be used as a potential target for drug development against EBV infection and KD treatment.
In addition to EBV, certain KD patients also have concomitant varicella zoster virus or coxsackievirus A4 infection (Turkay et al., 2006; Toprak et al., 2015). Given that the features of HHV6-infected patients resemble those symptoms observed in KD children (Kakisaka et al., 2012; Alramadhan et al., 2020), HHV-6B was thus suggested to be a critical mediator during the pathogenesis of KD, and HHV-6B infection was also suggested to be responsible for the increased number of KD patients during the SARS-CoV-2 pandemic (Dursun and Temiz, 2020).
Human bocavirus (HboV) is a single-stranded DNA etiologic agent that has been suggested as a cause of acute respiratory tract infection in children (Schildgen et al., 2008). This virus was first identified in nasopharyngeal, serum or stool samples, and was thus suggested to play a pathogenic role in some cases of Kawasaki disease (Catalano-Pons et al., 2007). Late, this work was verified by the results from another group showing that HboV can indeed be detected in nasopharyngeal secretions of KD patients, demonstrating a coincidental or possible etiological association between HboV infection and KD pathogenesis (Santos et al., 2011). Furthermore, a significant correlation between HboV infection and KD incidence was identified based on epidemiological data (Kim et al., 2014; Lim et al., 2021), whereas some investigators have proposed that there is little correlation between HboV infection and KD based on the serological test (Lehmann et al., 2009). Cytomegalovirus was also suggested to be involved in the development of atypical KD and coronary aneurysms (Catalano-Pons et al., 2005; Guc et al., 2008). Taken together, more intense researches is needed to elucidate the precise mechanism concerning DNA viruses associated with KD pathogenesis.
Apart from the DNA viruses mentioned above, a total of nine types of RNA viruses have been suggested to correlate with KD pathogenesis, including coxsackie virus, enterovirus, human coronavirus NL63 (HCoV-NL63), influenza virus, measles virus, SARS-CoV-2 (severe acute respiratory syndrome coronavirus-2), feline virus, influenza A virus H1N1 and human immunodeficiency virus, as discussed below.
The coxsackie virus, which belongs to enteroviruses of small RNA viridine, has been identified as the main cause of viral myocarditis in humans since 1955 (Dalldorf, 1955). Both coxsackie virus B3 (CVB3) and coxsackie virus A4 were identified to correlate with KD (Rigante et al., 2012; Ueda et al., 2015), and this type of virus can induce neonatal symptoms similar to viral myocarditis observed in KD (Verma et al., 2009).
It has been demonstrated that the KD incidence in the enterovirus (EV)-infected cohort was significantly higher than that in the non-EV-infected cohort (Weng et al., 2018), thereby indicating a high correlation between EV infection and KD. In addition, a decreased incidence of severe enterovirus infection cases is simultaneously correlated with decreased KD hospitalizations during the SARS-CoV-2 epidemic (Guo et al., 2022), thus suggesting that enterovirus might function as a critical mediator during the pathogenesis of KD.
Although HCoV-NL63 was once identified in several KD patients (Dominguez et al., 2006), most data later do not support an association between HCoV-NL63 infection and KD (Baker et al., 2006; Chang et al., 2006; Lehmann et al., 2009). In fact, only 1 (2%) of 48 patients with KD was found to be positive for HCoV-NL63/NH (Shimizu et al., 2005), although HCoV-229E was also suggested to be involved in KD (Lehmann et al., 2009; Shirato et al., 2014).
Influenza viruses have been revealed to positively correlate with the monthly KD incidence (Kim et al., 2014). For instance, influenza A H1N1/09 virus has been shown to be associated with the pathogenesis of KD by several groups (Joshi et al., 2011; Wang et al., 2019; Banday et al., 2021). Additionally, Parainfluenza type 3 virus (PIV-3) was also found to correlate with KD (Schnaar and Bell, 1982; Karron et al., 1993), suggesting that influenza virus infection has etiological importance in the development of KD. However, given that concomitant influenza infection affects the clinical manifestations of KD and impacts the laboratory test results of the disease (Huang et al., 2015), it remains to be determined regarding influenza infection and KD pathogenesis.
The measles virus (MeV), which is an enveloped RNA virus, frequently causes acute febrile illness accompanied by a rash (Takemoto et al., 2022). This virus can be isolated from KD children, and the symptoms caused by MeV infection partially resemble those observed in KD patients (Whitby et al., 1991; Kuijpers et al., 2000).
The RNA respiratory virus SARS-CoV-2 (severe acute respiratory syndrome coronavirus 2) can induce multisystem inflammatory syndrome in children (also called MIS-C), including multifocal endovascular dermatitis, thrombosis, and systemic thrombotic microangiopathy, which resemble certain features observed in KD (Ackermann et al., 2020; Consiglio et al., 2020; Loomba et al., 2020; Bukulmez, 2021; Cherqaoui et al., 2021; Sancho-Shimizu et al., 2021; Sokolovsky et al., 2021; Zhang et al., 2021).
Moreover, SARS-CoV-2 can be detected in certain KD patients, and a host of SARS-CoV-2-positive patients exhibit KD-like syndrome (Consiglio et al., 2020; Jones et al., 2020; Toubiana et al., 2020; Sharma et al., 2021). However, although high titers of anti-SARS-CoV-2 antibodies have been detected both in KD and multisystem inflammatory syndrome patients (Kabeerdoss et al., 2021), the two diseases are different because of the differential T-cell subsets, interleukin (IL)-17A, and biomarkers associated with arterial damage (Consiglio et al., 2020). On the other hand, global studies have reported that the incidence of KD declined during the COVID-19 pandemic, suggesting a potential KD pathogenesis involving transmission among children (Ae et al., 2022). However, several earlier studies showed that the KD incidence has increased during the pandemic (Ouldali et al., 2020; Roe, 2020; Stower, 2020; Viner and Whittaker, 2020), supporting the hypothesis that KD might be caused by an unknown RNA virus that may function as the main trigger in inducing abnormal immune responses in genetically susceptible individuals.
In addition to the RNA viruses mentioned above, several other types of RNA viruses were also found to be involved in KD. For example, both a novel feline virus (Moynahan, 1987) and the influenza A virus (Wang et al., 2019) were suggested to be related to KD symptoms. Notably, HIV patients also show symptoms similar to those observed in KD patients (Johnson et al., 2016). The intracytoplasmic inclusion bodies induced by viruses can be isolated from KD patients, suggesting that the infectious etiologic agent of KD might be associated with an unknown novel RNA virus (Rowley et al., 2011). In addition, dengue virus was also identified in the serum of certain KD patients in southern Thailand, and mosquitoes were hypothesized to work with the dengue virus to spread the KD pathogen, thus inducing cell proliferation and morphological changes in endothelial cells and coronary arteritis lesions in KD patients (Sopontammarak et al., 2008). Moreover, regions with the highest reported arboviral infections in Venezuela simultaneously have the highest incidence of KD (Paniz-Mondolfi et al., 2020), demonstrating the critical roles of viral infections in mediating the pathogenesis of KD.
Regarding bacterial infection, the superantigens produced by gut bacteria may be involved in the onset of KD. Until recently, there were five Streptococcus spp. (S. pneumonia, pseudopneumoniae, oralis, gordonii, and sanguinis) were found to increase during the acute phase in KD patients based on metagenomic sequencing, indicating that Streptococci are involved in the pathogenesis of KD disease (Kinumaki et al., 2015). Furthermore, the stool of KD patients contains higher numbers of gram-positive bacteria, including Streptococcus, Staphylococcus, Eubacterium, and Peptostreptococcus genera, Hsp60-producing gram-negative bacteria, and a lower number of lactobacilli, when compared with those from healthy control children (Yamashiro et al., 1996; Takeshita et al., 2002; Nagata et al., 2009). Specifically, three pathogens, S. pyogenes (Leahy et al., 2012), S. mitis Nm-65 (Tabata et al., 2021), and S. sanguis (Tsurumizu et al., 1991), have been identified in the pleural fluid, tooth surface or blood of KD patients. Additionally, serum IgM antibodies against superantigens of S. aureus and S. pyogenes have been identified in KD patients (Matsubara et al., 2006), and these two pathogens together can produce 19 different superantigens (Llewelyn and Cohen, 2002). Mechanistically, S. aureus isolated from the rectum or pharynx of KD patients can secrete toxic shock syndrome toxin 1 (TSST-1) and staphylococcal protein A, which in turn stimulate Vβ2+ lymphocyte amplification and are thus involved in the abnormal immune responses of KD patients (Leung et al., 1993; Wann et al., 1999; Leung et al., 2002).
Regarding Yersinia pseudotuberculosis (Konishi et al., 1997), the Propionibacterium acnes strain and its products cytopathogenic proteins (CPPs; Kato et al., 1983; Tomita et al., 1987) can all be isolated from KD patients, suggesting a causative role of bacterial infection in mediating the pathogenesis of KD. Moreover, several recent studies suggest that Y. pstb infection is closely related to KD pathogenesis (Kato et al., 2019; Kamura et al., 2020; Miyata et al., 2022; Ohnishi et al., 2022), and the antibody titers of Y. pstb were significantly elevated in both Chinese and Japanese KD patients (Chou et al., 2005; Tahara et al., 2006). In contrast, a recent study showed that the positive rate of Y. pstb infection is much lower in KD patients (Horinouchi et al., 2015; Hayashi et al., 2023), but when the population is exposed to a higher risk of Y. pstb infection, the incidence of KD is much higher (Vincent et al., 2007).
In addition to the microbes mentioned above, M. pneumoniae infection was identified in an important proportion of KD patients (Umezawa et al., 1989; Ebrahim et al., 2011; Lee et al., 2011; Tang et al., 2016; Wang et al., 2021; Huang et al., 2022). For instance, the M. pneumoniae infection-positive rate in KD patients was significantly higher than that in non-KD patients during the SARS-CoV-2 epidemic (Ding et al., 2021), and certain KD patients were found to be coinfected with M. pneumoniae and Epstein–Barr virus (Huang et al., 2012).
Additionally, the positive rate of serum Chlamydia pneumoniae IgM antibody in KD children was significantly higher than that in the control group (Numazaki and Chiba, 1996); however, another study showed that the link between C. pneumoniae infection and KD pathogenesis or coronary artery lesions remains to be clarified (Chua et al., 2000; Strigl et al., 2000), suggesting that more intense research is needed to confirm the correlations between M. pneumoniae or C. pneumoniae infection and KD pathogenesis.
Rickettsia-like organisms were also found in biopsies of the skin and lymph nodes of KD patients (Tasaka and Hamashima, 1978). However, in most cases, only Coxiella burnetiid but not Rickettsia conorii, R. typhi, Coxiella burnetii or Ehrlichia phagocytophila was suggested to cause KD-like symptoms in young children (Kafetzis et al., 2001), suggesting its specific causative roles in KD pathogenesis.
Given that the fungus Candida albicans can be isolated from KD patients, and its extract, the Candida albicans water soluble fraction (CAWS) intraperitoneally injected in mice could induce symptoms resembling those observed in KD patients (Murata, 1979; Martinez et al., 2012; Yoshikane et al., 2015; Stock et al., 2016; Noval Rivas and Arditi, 2020). Furthermore, β-glucan, which is the major component of CAWS, is also increased in KD patients (Ishibashi et al., 2014). Mechanistically, the mannoprotein-β-glucan complex of C. albicans can affect the functions of leukocytes, endothelial cells, and platelets in vitro (Kurihara et al., 2003). The systematic vasculitis induced by CAWS in mice can be alleviated after administration of human immunoglobulin or etanercept (Takahashi et al., 2010b; Ohashi et al., 2013). Together, these findings imply that infectious agents might play critical roles in triggering this disease.
Another major KD-like murine coronary arteritis model involves induction by L. casei cell wall extract (LCWE), which is widely used to mimic systematic vasculitis in KD patients (Lehman et al., 1988; Abe et al., 2020). In the LCWE-induced mouse model, the TLR2 and ILβ-dependent signaling pathways were suggested to play important roles during its pathogenesis (Rosenkranz et al., 2005; Lee et al., 2012; Matundan et al., 2019). Additionally, the dectin-1/Syk signaling pathway in macrophages (Lin et al., 2013) and the Notch4 signaling pathway in endothelial progenitor cells are also involved in LCWE-induced coronary artery disease, thereby contributing to the development of KD pathogenesis (Wang et al., 2016). Moreover, LCWE was likewise suggested to function as immunogenic for proinflammatory T helper (Th) 1, Th17, and CD8+ T cells and inducible regulatory T cells (iTreg) (Noval Rivas et al., 2017; Hsieh et al., 2021). Taken together, the systematic vasculitis induced by CAWS or LCWE in mice resembles pathological features observed in KD patients, demonstrating the causative roles of etiological agent infection and related PAMP/MAMP signaling activation in inducing KD vasculitis (Table 1).
Taken together, various pathogens identified in KD were all suggested to be the critical triggers in causing systematic vasculitis, and these pathogens were demonstrated to work independently or synergistically to potentiate abnormal immune responses by inducing pyroptosis and/or proinflammatory cell death, hence leading to systematic vasculitis in KD (Figure 1). However, whether these pathogens are direct causes or merely the accompanying pathogens after KD induction remains elusive. Additionally, the causative agent of KD remains ambiguous, and several questions remain to be clarified. First, those pathogens suggested to be involved in the pathogenesis of KD largely rely on PCR and serological methods using a relatively small sample size. Second, the differences in timing of obtaining the blood sample and constraints of the study design used to measure pathogens in KD patients by different investigators could make pathogen identification inconsistent. Third, whether KD is caused by a single pathogen or is the combined result of more than one agent remains to be investigated. Consequently, the relationship between pathogen infection and KD vasculitis is far more complex than currently appreciated. Caution should be exercised in the clinic when considering the possible agents merely based on the symptom similarities between KD and other infectious diseases. Importantly, given that the recognition of the infectious origin of KD is a critical prerequisite to understanding its pathogenetic mechanism, more intense research using artificial intelligence, metagenomic sequencing and culturing specific pathogens isolated from KD patients from multiple centers and then verifying each of them in animal models could help uncover the underlying mechanisms of pathogen infections involved and thus facilitate the development of novel intervention strategies for Kawasaki disease.
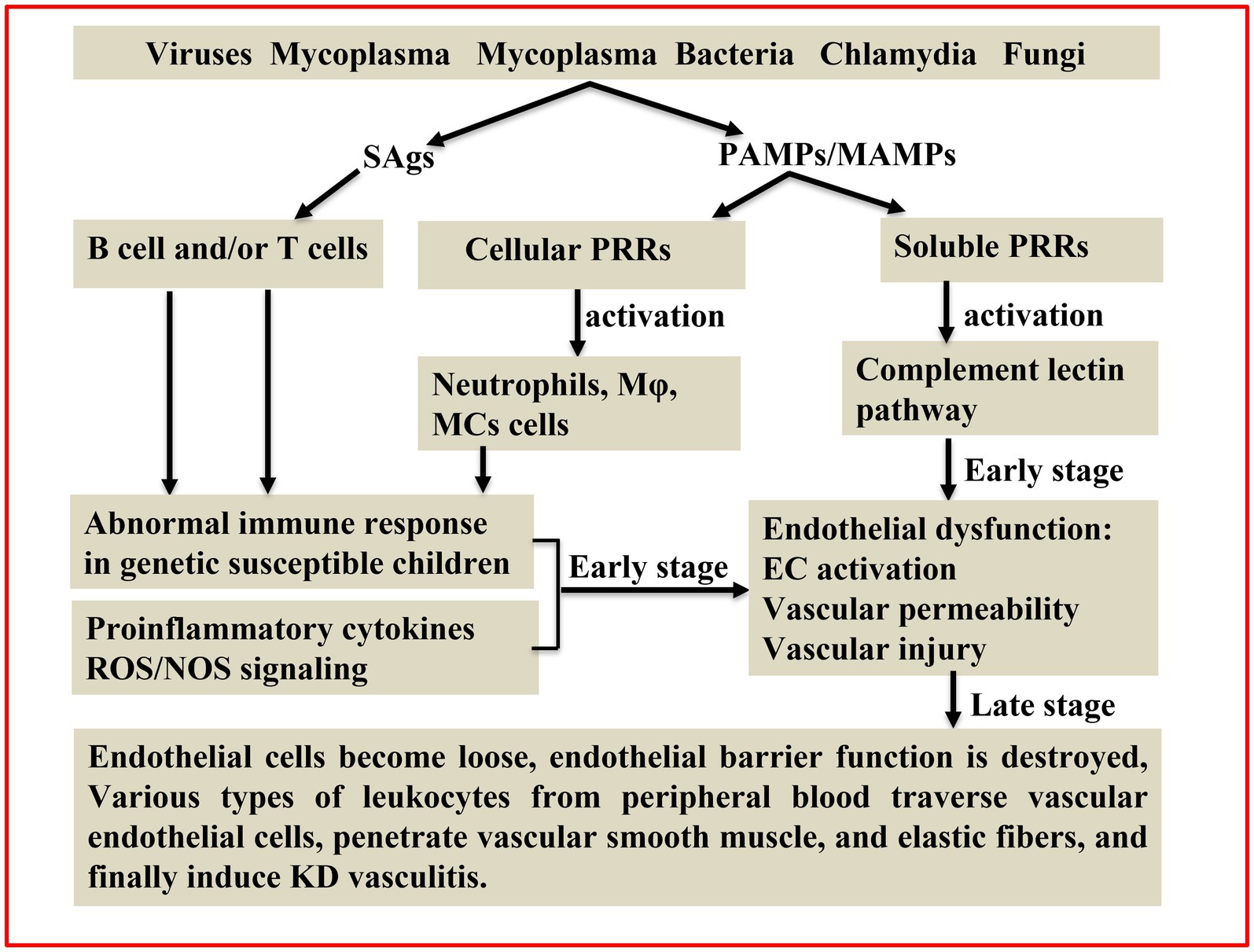
Figure 1. Schematic illustrating the pathogenic mechanisms of KD. The superantigens (SAgs) hypothesis and different infectious agents produce pathogen/microbe-associated molecular patterns (PAMPs/MAMPs) were all proposed to be involved in KD pathogenesis. SAgs non-specifically activate T cells and/or B cells. PAMPs/MAMPs also stimulate immune cells [e.g., macrophages (Mφ), dendritic cells (DCs), monocytes (MCs)] and endothelial cells (ECs) through cellular pattern recognition receptors (PRRs; e.g., TLRs, NOD1, and Dectin-1/-2). Additionally, PAMPs/MAMPs can activate the complement lectin pathway through soluble PRRs (e.g., ficolin-1 and mannose binding lectin-2). Activated complement pathways can induce inflammatory vascular damage through recruitment of innate inflammatory cells and direct injury to ECs. This cross-talk among different cells augments the production of proinflammatory cytokines/chemokines and reactive oxygen/nitrogen species (ROS/NOS), hence leading to a systemic inflammatory reaction in KD.
WW: Writing – original draft, Data curation. LZ: Conceptualization, Funding acquisition, Investigation, Writing – original draft. XL: Writing – original draft, Data curation, Project administration. ZL: Data curation, Resources, Writing – original draft. HL: Conceptualization, Investigation, Supervision, Writing – review & editing. GQ: Conceptualization, Funding acquisition, Writing – review & editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. Work in our lab was funded by the National Natural Science Foundation of China (grant number nos. 82171797, 81971477, 82371806, 81870365, 82070512, and 82270529), the Jiangsu Provincial Social Development Project (grant number SBE2021750252), and the Gusu Health Talent Program (grant number GSWS2020038).
The authors thank the editor and reviewers for their constructive comments and suggestions on the paper. We apologize to colleagues in the field whose work could not be cited owing to space limitations.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The reviewer YD declared a shared affiliation with the authors to the handling editor at the time of review.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
BCG, Bacillus Calmette-Guérin; CAA, Coronary artery aneurysm; CAWS, Candida albicans water soluble fraction; DAMP, Damaged-associated molecular patterns; ELISA, Enzyme-linked immunosorbent assay; KD, Kawasaki disease; LCWE, Lactobacillus casei cell wall extract; MAMP, Microbe associated molecular pattern; PAMP, Pathogen associated molecular pattern; PCR, Polymerase chain reaction; PRR, Pattern recognition receptor; SEB, Staphylococcal enterotoxin B; SEC, Staphylococcal enterotoxin C; SPE, Streptococcal pyrogenic exotoxins; TCR, T-cell receptor; TSS, Toxic shock syndrome; TSST-1, Toxic shock syndrome toxin-1.
Abe, J., Onimaru, M., Matsumoto, S., Noma, S., Baba, K., Ito, Y., et al. (1997). Clinical role for a superantigen in Yersinia pseudotuberculosis infection. J. Clin. Investig. 99, 1823–1830. doi: 10.1172/jci119349
Abe, M., Rastelli, D. D., Gomez, A. C., Cingolani, E., Lee, Y., Soni, P. R., et al. (2020). IL-1-dependent electrophysiological changes and cardiac neural remodeling in a mouse model of Kawasaki disease vasculitis. Clin. Exp. Immunol. 199, 303–313. doi: 10.1111/cei.13401
Abe, J., Terai, M., Nogami, H., Toyoda, Y., Nakajima, H., Nakano, T., et al. (2003). Colonization of the superantigen-producing Staphylococcus aureus among patients with Kawasaki disease. Pediatr. Res. 53:168. doi: 10.1203/00006450-200301000-00088
Ackermann, M., Verleden, S. E., Kuehnel, M., Haverich, A., Welte, T., Laenger, F., et al. (2020). Pulmonary vascular Endothelialitis, thrombosis, and angiogenesis in Covid-19. N. Engl. J. Med. 383, 120–128. doi: 10.1056/NEJMoa2015432
Ae, R., Makino, N., Kuwabara, M., Matsubara, Y., Kosami, K., Sasahara, T., et al. (2022). Incidence of Kawasaki disease before and after the COVID-19 pandemic in Japan: results of the 26th Nationwide survey, 2019 to 2020. JAMA Pediatr. 176, 1217–1224. doi: 10.1001/jamapediatrics.2022.3756
Alramadhan, M. M., Kamdar, A. A., Lafferty-Prather, M., Aguilera, E. A., and Wootton, S. H. (2020). Incomplete Kawasaki disease associated with human herpes Virus-6 variant B infection and aseptic meningitis. Glob Pediatr Health 7:2333794X20939759. doi: 10.1177/2333794X20939759
Ariza, M. E., Glaser, R., Kaumaya, P. T., Jones, C., and Williams, M. V. (2009). The EBV-encoded dUTPase activates NF-kappa B through the TLR2 and MyD88-dependent signaling pathway. J. Immunol. 182, 851–859. doi: 10.4049/jimmunol.182.2.851
Baker, S. C., Shimizu, C., Shike, H., Garcia, F., van der Hoek, L., Kuijper, T. W., et al. (2006). Human coronavirus-NL63 infection is not associated with acute Kawasaki disease. Adv. Exp. Med. Biol. 581, 523–526. doi: 10.1007/978-0-387-33012-9_94
Baldari, C. T., Onnis, A., Andreano, E., Del Giudice, G., and Rappuoli, R. (2023). Emerging roles of SARS-CoV-2 spike-ACE2 in immune evasion and pathogenesis. Trends Immunol. 44, 424–434. doi: 10.1016/j.it.2023.04.001
Banday, A. Z., Arul, A., Vignesh, P., Singh, M. P., Goyal, K., and Singh, S. (2021). Kawasaki disease and influenza-new lessons from old associations. Clin. Rheumatol. 40, 2991–2999. doi: 10.1007/s10067-020-05534-1
Bukulmez, H. (2021). Current understanding of multisystem inflammatory syndrome (MIS-C) following COVID-19 and its distinction from Kawasaki disease. Curr. Rheumatol. Rep. 23:58. doi: 10.1007/s11926-021-01028-4
Burns, J. C., Herzog, L., Fabri, O., Tremoulet, A. H., Rodo, X., Uehara, R., et al. (2013). Seasonality of Kawasaki disease: a global perspective. PloS One 8:e74529. doi: 10.1371/journal.pone.0074529
Catalano-Pons, C., Giraud, C., Rozenberg, F., Meritet, J. F., Lebon, P., and Gendrel, D. (2007). Detection of human bocavirus in children with Kawasaki disease. Clin. Microbiol. Infect. 13, 1220–1222. doi: 10.1111/j.1469-0691.2007.01827.x
Catalano-Pons, C., Quartier, P., Leruez-Ville, M., Kaguelidou, F., Gendrel, D., Lenoir, G., et al. (2005). Primary cytomegalovirus infection, atypical Kawasaki disease, and coronary aneurysms in 2 infants. Clin. Infect. Dis. 41, E53–E56. doi: 10.1086/432578
Chang, L. Y., Chiang, B. L., Kao, C. L., Wu, M. H., Chen, P. J., Berkhout, B., et al. (2006). Lack of association between infection with a novel human coronavirus (HCoV), HCoV-NH, and Kawasaki disease in Taiwan. J Infect Dis 193, 283–286. doi: 10.1086/498875
Chang, L.-Y., Lu, C.-Y., Shao, P.-L., Lee, P.-I., Lin, M.-T., Fan, T.-Y., et al. (2014). Viral infections associated with Kawasaki disease. J. Formos. Med. Assoc. 113, 148–154. doi: 10.1016/j.jfma.2013.12.008
Chang, L. S., Yan, J. H., Li, J. Y., Yeter, D. D., Huang, Y. H., Guo, M. M., et al. (2020). Blood mercury levels in children with Kawasaki disease and disease outcome. Int. J. Environ. Res. Public Health 17:3726. doi: 10.3390/ijerph17103726
Cherqaoui, B., Kone-Paut, I., Yager, H., Bourgeois, F. L., and Piram, M. (2021). Delineating phenotypes of Kawasaki disease and SARS-CoV-2-related inflammatory multisystem syndrome: a French study and literature review. Rheumatology (Oxford) 60, 4530–4537. doi: 10.1093/rheumatology/keab026
Choi, J.-W. (2020). Can we get a clue for the etiology of Kawasaki disease in the COVID-19 pandemic? Clin Experiment Pediatr 63, 335–336. doi: 10.3345/cep.2020.00955
Chou, C. T., Chang, J. S., Ooi, S. E., Huo, A. P., Chang, S. J., Chang, H. N., et al. (2005). Serum anti-Yersinia antibody in Chinese patients with Kawasaki disease. Arch. Med. Res. 36, 14–18. doi: 10.1016/j.arcmed.2004.09.004
Chua, P. K., Nerurkar, V. R., Yu, Q. G., Woodward, C. L., Melish, M. E., and Yanagihara, R. (2000). Lack of association between Kawasaki syndrome and infection with parvovirus B19, human herpesvirus 8, TT virus, GB virus C/hepatitis G virus or Chlamydia pneumoniae. Pediatr. Infect. Dis. J. 19, 477–479. doi: 10.1097/00006454-200005000-00019
Consiglio, C. R., Cotugno, N., Sardh, F., Pou, C., Amodio, D., Rodriguez, L., et al. (2020). The immunology of multisystem inflammatory syndrome in children with COVID-19. Cells 183, 968–981.e7. doi: 10.1016/j.cell.2020.09.016
Corinaldesi, E., Pavan, V., Andreozzi, L., Fabi, M., Selvini, A., Frabboni, I., et al. (2020). Environmental factors and Kawasaki disease onset in Emilia-Romagna, Italy. Int. J. Environ. Res. Public Health 17:1529. doi: 10.3390/ijerph17051529
Dalldorf, G. (1955). COXSACKIE VIRUSES. Annu. Rev. Microbiol. 9, 277–296. doi: 10.1146/annurev.mi.09.100155.001425
Ding, Y.-Y., Ren, Y., Qin, J., Qian, G.-H., Tang, Y.-J., Chen, Y., et al. (2021). Clinical characteristics of Kawasaki disease and concurrent pathogens during isolation in COVID-19 pandemic. World J. Pediatr. 17, 263–271. doi: 10.1007/s12519-021-00431-2
Dominguez, S. R., Anderson, M. S., Glode, M. P., Robinson, C. C., and Holmes, K. V. (2006). Blinded case-control study of the relationship between human coronavirus NL63 and Kawasaki syndrome. J Infect Dis 194, 1697–1701. doi: 10.1086/509509
Dursun, R., and Temiz, S. A. (2020). The clinics of HHV-6 infection in COVID-19 pandemic: Pityriasis rosea and Kawasaki disease. Dermatol. Ther. 33:e13730. doi: 10.1111/dth.13730
Ebrahim, M., Gabay, M., and Rivas-Chacon, R. F. (2011). Evidence of acute Mycoplasma infection in a patient with incomplete and atypical Kawasaki disease: a case report. Case Rep. Med. 2011:606920. doi: 10.1155/2011/606920
Embil, J. A., McFarlane, E. S., Murphy, D. M., Krause, V. W., and Stewart, H. B. (1985). Adenovirus type 2 isolated from a patient with fatal Kawasaki disease. Can. Med. Assoc. J. 132:1400.
Flossdorf, S., Schiwy-Bochat, K. H., Teifel, D., Fries, J. W. U., and Rothschild, M. A. (2020). Sudden death of a young adult with coronary artery vasculitis, coronary aneurysms, parvovirus B19 infection and Kawasaki disease. Forensic Sci. Med. Pathol. 16, 498–503. doi: 10.1007/s12024-020-00263-y
Fukuda, S., Ito, S., Fujiwara, M., Abe, J., Hanaoka, N., Fujimoto, T., et al. (2017). Simultaneous development of Kawasaki disease following acute human adenovirus infection in monozygotic twins: a case report. Pediatr. Rheumatol. Online J. 15:39. doi: 10.1186/s12969-017-0169-x
Ghosh, P., Katkar, G. D., Shimizu, C., Kim, J., Khandelwal, S., Tremoulet, A. H., et al. (2022). An artificial intelligence-guided signature reveals the shared host immune response in MIS-C and Kawasaki disease. Nat. Commun. 13:2687. doi: 10.1038/s41467-022-30357-w
Guc, B. U., Cengiz, N., Yildirim, S. V., and Uslu, Y. (2008). Cytomegalovirus infection in a patient with atypical Kawasaki disease. Rheumatol. Int. 28, 387–389. doi: 10.1007/s00296-007-0440-4
Guo, M. M.-H., Yang, K. D., Liu, S.-F., and Kuo, H.-C. (2022). Number of Kawasaki disease admissions is associated with number of domestic COVID-19 and severe enterovirus case numbers in Taiwan. Children 9:149. doi: 10.3390/children9020149
Hara, T., Yamamura, K., and Sakai, Y. (2021). The up-to-date pathophysiology of Kawasaki disease. Clin Transl Immunol 10:e1284. doi: 10.1002/cti2.1284
Hattori, T., Matsukawa, Y., Takei, M., Yamaguchi, K., Yamazaki, T., Sawada, U., et al. (2005). Adult Kawasaki disease unrelated to Epstein-Barr virus and group a Streptococcus. Intern. Med. 44, 1182–1184. doi: 10.2169/internalmedicine.44.1182
Hayashi, H., Uda, K., Araki, Y., Akahoshi, S., Tanaka, M., Miyata, K., et al. (2023). Association of Yersinia Infection with Kawasaki Disease: a prospective multicenter cohort study. Pediatr. Infect. Dis. J. 42, 1041–1044. doi: 10.1097/INF.0000000000004084
Hirata, N., Ishibashi, K.-I., Ohta, S., Hata, S., Shinohara, H., Kitamura, M., et al. (2006). Histopathological examination and analysis of mortality in DBA/2 mouse vasculitis induced with CAWS, a water-soluble extracellular polysaccharide fraction obtained from Candida albicans. Yakugaku Zasshi 126, 643–650. doi: 10.1248/yakushi.126.643
Holm, J. M., Hansen, L. K., and Oxhoj, H. (1995). Kawasaki disease associated with parvovirus B19 infection. Eur. J. Pediatr. 154, 633–634. doi: 10.1007/BF02079066
Horinouchi, T., Nozu, K., Hamahira, K., Inaguma, Y., Abe, J., Nakajima, H., et al. (2015). Yersinia pseudotuberculosis infection in Kawasaki disease and its clinical characteristics. BMC Pediatr. 15:177. doi: 10.1186/s12887-015-0497-2
Hsieh, L.-E., Tremoulet, A. H., Burns, J. C., Noval Rivas, M., Arditi, M., and Franco, A. (2021). Characterization of the T cell response to Lactobacillus casei Cell Wall extract in children with Kawasaki disease and its potential role in vascular inflammation. Front. Pediatr. 9:633244. doi: 10.3389/fped.2021.633244
Huang, F.-L., Chang, T.-K., Jan, S.-L., Tsai, C.-R., Wang, L.-C., Lai, M.-C., et al. (2012). Co-morbidity of Kawasaki disease. Indian J. Pediatr. 79, 815–817. doi: 10.1007/s12098-011-0589-4
Huang, S. H., Chen, C. Y., Weng, K. P., Chien, K. J., Hung, Y. M., Hsieh, K. S., et al. (2020). Adenovirus infection and subsequent risk of Kawasaki disease: a population-based cohort study. J. Chin. Med. Assoc. 83, 302–306. doi: 10.1097/JCMA.0000000000000266
Huang, X., Huang, P., Zhang, L., Xie, X., Xia, S., Gong, F., et al. (2015). Influenza infection and Kawasaki disease. Rev. Soc. Bras. Med. Trop. 48, 243–248. doi: 10.1590/0037-8682-0091-2015
Huang, S.-W., Lin, S.-C., Chen, S.-Y., and Hsieh, K.-S. (2022). Kawasaki disease with combined Cholestatic hepatitis and Mycoplasma pneumoniae infection: a case report and literature review. Front. Pediatr. 9:8215. doi: 10.3389/fped.2021.738215
Ishibashi, K., Fukazawa, R., Miura, N. N., Adachi, Y., Ogawa, S., and Ohno, N. (2014). Diagnostic potential of antibody titres against Candida cell wall beta-glucan in Kawasaki disease. Clin. Exp. Immunol. 177, 161–167. doi: 10.1111/cei.12328
Jackson, H., Menikou, S., Hamilton, S., McArdle, A., Shimizu, C., Galassini, R., et al. (2021). Kawasaki disease patient stratification and pathway analysis based on host transcriptomic and proteomic profiles. Int. J. Mol. Sci. 22:655. doi: 10.3390/ijms22115655
Johnson, D., and Azimi, P. (1985). Kawasaki disease associated with klebsiella-pneumoniae bacteremia and Para-influenza type-3 virus-infection. Pediatr. Infect. Dis. J. 4:100. doi: 10.1097/00006454-198501000-00024
Johnson, R. M., Bergmann, K. R., Manaloor, J. J., Yu, X., Slaven, J. E., and Kharbanda, A. B. (2016). Pediatric Kawasaki disease and adult human immunodeficiency virus Kawasaki-like syndrome are likely the same malady. Open Forum Infect. Dis. 3:ofw160. doi: 10.1093/ofid/ofw160
Jones, V. G., Mills, M., Suarez, D., Hogan, C. A., Yeh, D., Segal, J. B., et al. (2020). COVID-19 and Kawasaki disease: novel virus and novel case. Hosp. Pediatr. 10, 537–540. doi: 10.1542/hpeds.2020-0123
Joshi, A. V., Jones, K. D., Buckley, A. M., Coren, M. E., and Kampmann, B. (2011). Kawasaki disease coincident with influenza a H1N1/09 infection. Pediatr. Int. 53, e1–e2. doi: 10.1111/j.1442-200X.2010.03280.x
Kabeerdoss, J., Pilania, R. K., Karkhele, R., Kumar, T. S., Danda, D., and Singh, S. (2021). Severe COVID-19, multisystem inflammatory syndrome in children, and Kawasaki disease: immunological mechanisms, clinical manifestations and management. Rheumatol. Int. 41, 19–32. doi: 10.1007/s00296-020-04749-4
Kafetzis, D. A., Maltezou, H. C., Constantopoulou, I., Antonaki, G., Liapi, G., and Mathioudakis, I. (2001). Lack of association between Kawasaki syndrome and infection with Rickettsia conorii, Rickettsia typhi, Coxiella burnetii or Ehrlichia phagocytophila group. Pediatr. Infect. Dis. J. 20, 703–706. doi: 10.1097/00006454-200107000-00012
Kakisaka, Y., Ohara, T., Katayama, S., Suzuki, T., Sasai, S., Hino-Fukuyo, N., et al. (2012). Human herpes virus type 6 can cause skin lesions at the BCG inoculation site similar to Kawasaki disease. Tohoku J. Exp. Med. 228, 351–353. doi: 10.1620/tjem.228.351
Kam, K.-Q., Ong, J. S. M., and Lee, J. H. (2020). Kawasaki disease in the COVID-19 era: a distinct clinical phenotype? Lancet Child Adolesc Health 4, 642–643. doi: 10.1016/s2352-4642(20)30207-8
Kamura, T., Tanaka, Y., Tsumura, N., Ohya, T., and Okamatsu, Y. (2020). Yersinia pseudotuberculosis infection complicated with bacteremia in a 10-month-old boy. Case Rep Pediatr 2020:8846511. doi: 10.1155/2020/8846511
Karron, R. A., O'Brien, K. L., Froehlich, J. L., and Brown, V. A. (1993). Molecular epidemiology of a parainfluenza type 3 virus outbreak on a pediatric ward. J Infect Dis 167, 1441–1445. doi: 10.1093/infdis/167.6.1441
Katano, H., Sato, S., Sekizuka, T., Kinumaki, A., Fukumoto, H., Sato, Y., et al. (2012). Pathogenic characterization of a cervical lymph node derived from a patient with Kawasaki disease. Int. J. Clin. Exp. Pathol. 5, 814–823.
Kato, H., Fujimoto, T., Inoue, O., Kondo, M., Koga, Y., Yamamoto, S., et al. (1983). Variant strain of Propionibacterium acnes: a clue to the aetiology of Kawasaki disease. Lancet 2, 1383–1388. doi: 10.1016/s0140-6736(83)90921-2
Kato, A., Miyata, I., Tanaka, Y., Oishi, T., Teranishi, H., Akaike, H., et al. (2019). LAMP-based assay can rectify the diagnosis of Yersinia pseudotuberculosis infections otherwise missed by serology. J. Med. Microbiol. 68, 143–147. doi: 10.1099/jmm.0.000868
Kido, S., Ae, R., Kosami, K., Matsubara, Y., Makino, N., Sasahara, T., et al. (2019). Seasonality of i.v. immunoglobulin responsiveness in Kawasaki disease. Pediatr. Int. 61, 539–543. doi: 10.1111/ped.13863
Kikuta, H., Matsumoto, S., Yanase, Y., Kawasaki, T., Mizuno, F., and Osato, T. (1990). Recurrence of Kawasaki-disease and epstein-barr-virus infection. J Infect Dis 162:1215. doi: 10.1093/infdis/162.5.1215
Kikuta, H., Sakiyama, Y., Matsumoto, S., Hamada, I., Yazaki, M., Iwaki, T., et al. (1993). Detection of epstein-barr-virus dna in cardiac and aortic tissues from chronic, active epstein-barr-virus infection associated with Kawasaki disease-like coronary-artery aneurysms. J. Pediatr. 123, 90–92. doi: 10.1016/s0022-3476(05)81546-x
Kikuta, H., Taguchi, Y., Tomizawa, K., Kojima, K., Kawamura, N., Ishizaka, A., et al. (1988). Epstein-Barr virus genome-positive T lymphocytes in a boy with chronic active EBV infection associated with Kawasaki-like disease. Nature 333, 455–457. doi: 10.1038/333455a0
Kim, G. B., Park, S., Kwon, B. S., Han, J. W., Park, Y. W., and Hong, Y. M. (2014). Evaluation of the temporal association between Kawasaki disease and viral infections in South Korea. Korean Circ J 44, 250–254. doi: 10.4070/kcj.2014.44.4.250
Kim, H. S., Shin, S. W., Choi, B. G., and Choi, H. J. (2020). Differences over 10 years in epidemiologic and clinical features of Kawasaki disease at a single tertiary center. Clin Exp Pediatr 63, 157–158. doi: 10.3345/cep.2019.01109
Kinumaki, A., Sekizuka, T., Hamada, H., Kato, K., Yamashita, A., and Kuroda, M. (2015). Characterization of the gut microbiota of Kawasaki disease patients by metagenomic analysis. Front. Microbiol. 6:824. doi: 10.3389/fmicb.2015.00824
Konishi, N., Baba, K., Abe, J., Maruko, T., Waki, K., Takeda, N., et al. (1997). A case of Kawasaki disease with coronary artery aneurysms documenting Yersinia pseudotuberculosis infection. Acta Paediatr. 86, 661–664. doi: 10.1111/j.1651-2227.1997.tb08952.x
Kuijpers, T. W., Herweijer, T. J., Scholvinck, L., Wertheim-Van Dillen, P. M., and Van de Veer, E. M. A. (2000). Kawasaki disease associated with measles virus infection in a monozygotic twin. Pediatr. Infect. Dis. J. 19, 350–353. doi: 10.1097/00006454-200004000-00018
Kuijpers, T. W., Wiegman, A., van Lier, R. A., Roos, M. T., Wertheim-van Dillen, P. M., Pinedo, S., et al. (1999). Kawasaki disease: a maturational defect in immune responsiveness. J Infect Dis 180, 1869–1877. doi: 10.1086/315111
Kuo, H. C., Pan, C. T., Huang, Y. H., Huang, F. C., Lin, Y. S., Li, S. C., et al. (2019). Global investigation of immune repertoire suggests Kawasaki disease has infectious cause. Circ. J. 83, 2070–2078. doi: 10.1253/circj.CJ-19-0206
Kurihara, K., Shingo, Y., Miura, N. N., Horie, S., Usui, Y., Adachi, Y., et al. (2003). Effect of CAWS, a mannoprotein-beta-glucan complex of Candida albicans, on leukocyte, endothelial cell, and platelet functions in vitro. Biol. Pharm. Bull. 26, 233–240. doi: 10.1248/bpb.26.233
Kusuda, T., Nakashima, Y., Murata, K., Kanno, S., Nishio, H., Saito, M., et al. (2014). Kawasaki disease-specific molecules in the sera are linked to microbe-associated molecular patterns in the biofilms. PloS One 9:e113054. doi: 10.1371/journal.pone.0113054
Leahy, T. R., Cohen, E., and Allen, U. D. (2012). Incomplete Kawasaki disease associated with complicated Streptococcus pyogenes pneumonia: a case report. Can J Infect Dis Med Microbiol 23, 137–139. doi: 10.1155/2012/638357
Lee, M. N., Cha, J. H., Ahn, H. M., Yoo, J. H., Kim, H. S., Sohn, S., et al. (2011). Mycoplasma pneumoniae infection in patients with Kawasaki disease. Korean J. Pediatr. 54, 123–127. doi: 10.3345/kjp.2011.54.3.123
Lee, Y., Schulte, D. J., Shimada, K., Chen, S., Crother, T. R., Chiba, N., et al. (2012). Interleukin-1beta is crucial for the induction of coronary artery inflammation in a mouse model of Kawasaki disease. Circulation 125, 1542–1550. doi: 10.1161/CIRCULATIONAHA.111.072769
Lehman, T. J. A., Warren, R., Gietl, D., Mahnovski, V., and Prescott, M. (1988). Variable expression of lactobacillus-casei cell wall-induced coronary arteritis—an animal-model of kawasakis disease in selected inbred mouse strains. Clin. Immunol. Immunopathol. 48, 108–118. doi: 10.1016/0090-1229(88)90161-4
Lehmann, C., Klar, R., Lindner, J., Lindner, P., Wolf, H., and Gerling, S. (2009). Kawasaki disease lacks association with human coronavirus NL63 and human bocavirus. Pediatr. Infect. Dis. J. 28, 553–554. doi: 10.1097/inf.0b013e31819f41b6
Leung, D. Y., Meissner, H. C., Fulton, D. R., Murray, D. L., Kotzin, B. L., and Schlievert, P. M. (1993). Toxic shock syndrome toxin-secreting Staphylococcus aureus in Kawasaki syndrome. Lancet 342, 1385–1388. doi: 10.1016/0140-6736(93)92752-f
Leung, D. Y. M., Meissner, H. C., Shulman, S. T., Mason, W. H., Gerber, M. A., Glode, M. P., et al. (2002). Prevalence of superantigen-secreting bacteria in patients with Kawasaki disease. J. Pediatr. 140, 742–746. doi: 10.1067/mpd.2002.123664
Li, X., Chen, Y., Tang, Y., Ding, Y., Xu, Q., Sun, L., et al. (2018). Predictors of intravenous immunoglobulin-resistant Kawasaki disease in children: a meta-analysis of 4442 cases. Eur. J. Pediatr. 177, 1279–1292. doi: 10.1007/s00431-018-3182-2
Lim, J. H., Kim, Y. K., Min, S. H., Kim, S. W., Lee, Y. H., and Lee, J. M. (2021). Seasonal trends of viral prevalence and incidence of Kawasaki disease: a Korea public health data analysis. J. Clin. Med. 10:3301. doi: 10.3390/jcm10153301
Lin, C. Y., Chen, I. C., Cheng, T. I., Liu, W. T., Hwang, B., and Chiang, B. N. (1992). VIRUS-LIKE PARTICLES WITH REVERSE-TRANSCRIPTASE ACTIVITY ASSOCIATED WITH KAWASAKI-DISEASE. J. Med. Virol. 38, 175–182. doi: 10.1002/jmv.1890380305
Lin, M. C., Lai, M. S., Jan, S. L., and Fu, Y. C. (2015). Epidemiologic features of Kawasaki disease in acute stages in Taiwan, 1997-2010: effect of different case definitions in claims data analysis. J. Chin. Med. Assoc. 78, 121–126. doi: 10.1016/j.jcma.2014.03.009
Lin, I. C., Suen, J.-L., Huang, S.-K., Huang, S.-C., Huang, H.-C., Kuo, H.-C., et al. (2013). Dectin-1/Syk signaling is involved in Lactobacillus casei cell wall extract-induced mouse model of Kawasaki disease. Immunobiology 218, 201–212. doi: 10.1016/j.imbio.2012.04.004
Lindquist, M. E., and Hicar, M. D. (2019). B cells and antibodies in Kawasaki disease. Int. J. Mol. Sci. 20:1834. doi: 10.3390/ijms20081834
Llewelyn, M., and Cohen, J. (2002). Superantigens: microbial agents that corrupt immunity. Lancet Infect. Dis. 2, 156–162. doi: 10.1016/s1473-3099(02)00222-0
Loomba, R. S., Villarreal, E. G., and Flores, S. (2020). COVID-19 and Hyperinflammatory syndrome in children: Kawasaki disease with macrophage activation syndrome in disguise? Cureus 12:e9515. doi: 10.7759/cureus.9515
Maggio, M. C., Fabiano, C., and Corsello, G. (2019). Kawasaki disease triggered by EBV virus in a child with familial Mediterranean fever. Ital. J. Pediatr. 45:129. doi: 10.1186/s13052-019-0717-8
Martinez, H. G., Quinones, M. P., Jimenez, F., Estrada, C., Clark, K. M., Suzuki, K., et al. (2012). Important role of CCR2 in a murine model of coronary vasculitis. BMC Immunol. 13:56. doi: 10.1186/1471-2172-13-56
Matsubara, K., and Fukaya, T. (2007). The role of superantigens of group a Streptococcus and Staphylococcus aureus in Kawasaki disease. Curr. Opin. Infect. Dis. 20, 298–303. doi: 10.1097/QCO.0b013e3280964d8c
Matsubara, K., Fukaya, T., Miwa, K., Shibayama, N., Nigami, H., Harigaya, H., et al. (2006). Development of serum IgM antibodies against superantigens of Staphylococcus aureus and Streptococcus pyogenes in Kawasaki disease. Clin. Exp. Immunol. 143, 427–434. doi: 10.1111/j.1365-2249.2006.03015.x
Matundan, H. H., Sin, J., Rivas, M. N., Fishbein, M. C., Lehman, T. J., Chen, S., et al. (2019). Myocardial fibrosis after adrenergic stimulation as a long-term sequela in a mouse model of Kawasaki disease vasculitis. JCI Insight 4:279. doi: 10.1172/jci.insight.126279
McCrindle, B. W., Rowley, A. H., Newburger, J. W., Burns, J. C., Bolger, A. F., Gewitz, M., et al. (2017). Diagnosis, treatment, and long-term Management of Kawasaki Disease: a scientific statement for health professionals from the American Heart Association. Circulation 135, e927–e999. doi: 10.1161/CIR.0000000000000484
Miyata, I., Kato, A., and Ouchi, K. (2022). Evaluation of anti-Yersinia psueudotuberculosis-derived mitogen antibody in intravenous immunoglobulin products. J. Infect. Chemother. 28, 1582–1583. doi: 10.1016/j.jiac.2022.07.020
Mohandas, S., Jagannathan, P., Henrich, T. J., Sherif, Z. A., Bime, C., Quinlan, E., et al. (2023). Immune mechanisms underlying COVID-19 pathology and post-acute sequelae of SARS-CoV-2 infection (PASC). Elife 12:14. doi: 10.7554/eLife.86014
Murata, H. (1979). Experimental candida-induced arteritis in mice-relation to arteritis in the mucocutaneous lymph-node syndrome. Microbiol. Immunol. 23, 825–831. doi: 10.1111/j.1348-0421.1979.tb02815.x
Nadig, P. L., Joshi, V., Pilania, R. K., Kumrah, R., Kabeerdoss, J., Sharma, S., et al. (2023). Intravenous immunoglobulin in Kawasaki disease-evolution and pathogenic mechanisms. Diagnostics 13:338. doi: 10.3390/diagnostics13142338
Nagata, S., Yamashiro, Y., Ohtsuka, Y., Shimizu, T., Sakurai, Y., Misawa, S., et al. (2009). Heat shock proteins and superantigenic properties of bacteria from the gastrointestinal tract of patients with Kawasaki disease. Immunology 128, 511–520. doi: 10.1111/j.1365-2567.2009.03135.x
Nagi-Miura, N., Adachi, Y., and Ohno, N. (2008). Coronary arteritis induced by CAWS (Candida albicans water-soluble fraction) in various strains of mice. Nippon Ishinkin Gakkai Zasshi 49, 287–292. doi: 10.3314/jjmm.49.287
Nagi-Miura, N., Shingo, Y., Adachi, Y., Ishida-Okawara, A., Oharaseki, T., Takahashi, K., et al. (2004). Induction of coronary arteritis with administration of CAWS (Candida albicans water-soluble fraction) depending on mouse strains. Immunopharmacol. Immunotoxicol. 26, 527–543. doi: 10.1081/iph-200042295
Nakamura, T., Yamamura, J.-I., Sato, H., Kakinuma, H., and Takahashi, H. (2007). Vasculitis induced by immunization with Bacillus Calmette-Guerin followed by atypical mycobacterium antigen: a new mouse model for Kawasaki disease. FEMS Immunol. Med. Microbiol. 49, 391–397. doi: 10.1111/j.1574-695X.2007.00217.x
Nakamura, J., Watanabe, S., Kimura, H., Kobayashi, M., Karasawa, T., Kamata, R., et al. (2018). Adeno-associated virus vector-mediated Interleukin-10 induction prevents vascular inflammation in a murine model of Kawasaki disease. Sci. Rep. 8:7601. doi: 10.1038/s41598-018-25856-0
Nakamura, Y., Yashiro, M., Uehara, R., Oki, I., Watanabe, M., and Yanagawa, H. (2008). Epidemiologic features of Kawasaki disease in Japan: results from the nationwide survey in 2005-2006. J. Epidemiol. 18, 167–172. doi: 10.2188/jea.je2008001
Nakamura, Y., Yashiro, M., Uehara, R., Sadakane, A., Tsuboi, S., Aoyama, Y., et al. (2012). Epidemiologic features of Kawasaki disease in Japan: results of the 2009-2010 nationwide survey. J. Epidemiol. 22, 216–221. doi: 10.2188/jea.je20110126
Nigro, G., Zerbini, M., Krzysztofiak, A., Gentilomi, G., Porcaro, M. A., Mango, T., et al. (1994). Active or recent parvovirus B19 infection in children with Kawasaki disease. Lancet 343, 1260–1261. doi: 10.1016/s0140-6736(94)92154-7
Noval Rivas, M., Lee, Y., Wakita, D., Chiba, N., Dagvadorj, J., Shimada, K., et al. (2017). CD8+ T cells contribute to the development of coronary arteritis in the Lactobacillus casei Cell Wall extract-induced murine model of Kawasaki disease. Arthritis Rheumatol. 69, 410–421. doi: 10.1002/art.39939
Noval Rivas, M., and Arditi, M. (2020). Kawasaki disease: pathophysiology and insights from mouse models. Nat. Rev. Rheumatol. 16, 391–405. doi: 10.1038/s41584-020-0426-0
Numazaki, K., and Chiba, S. (1996). Kawasaki disease and Chlamydia Pneumoniae infection. J. Infect. Chemother. 2, 264–265. doi: 10.1007/bf02355125
Ocho, K., Iwamuro, M., Hasegawa, K., Hagiya, H., Rai, K., Yumoto, T., et al. (2018). Far East scarlet-like fever masquerading as adult-onset Kawasaki disease. Intern. Med. 57, 437–440. doi: 10.2169/internalmedicine.9250-17
Oharaseki, T., Yokouchi, Y., Enomoto, Y., Sato, W., Ishibashi, K., Miura, N., et al. (2020). Recognition of alpha-mannan by dectin 2 is essential for onset of Kawasaki disease-like murine vasculitis induced by Candida albicans cell-wall polysaccharide. Mod. Rheumatol. 30, 350–357. doi: 10.1080/14397595.2019.1601852
Ohashi, R., Fukazawa, R., Watanabe, M., Tajima, H., Nagi-Miura, N., Ohno, N., et al. (2013). Etanercept suppresses arteritis in a murine model of Kawasaki disease: a comparative study involving different biological agents. J. Vasc. Med. 2013:543141. doi: 10.1155/2013/543141
Ohnishi, T., Nakazawa, M., Wada, N., Abe, J., and Kamimaki, I. (2022). Yersinia pseudotuberculosis infection accompanied by intussusception and incomplete Kawasaki disease in a 7-year-old girl. Keio J. Med. 71, 50–52. doi: 10.2302/kjm.2021-0002-CR
Okano, M., Thiele, G. M., Sakiyama, Y., Matsumoto, S., and Purtilo, D. T. (1990). Adenovirus infection in patients with Kawasaki disease. J. Med. Virol. 32, 53–57. doi: 10.1002/jmv.1890320109
Ouldali, N., Pouletty, M., Mariani, P., Beyler, C., Blachier, A., Bonacorsi, S., et al. (2020). Emergence of Kawasaki disease related to SARS-CoV-2 infection in an epicentre of the French COVID-19 epidemic: a time-series analysis. Lancet Child Adolesc Health 4, 662–668. doi: 10.1016/S2352-4642(20)30175-9
Oura, K., Ishikawa, S., Shiraishi, H., Maruo, Y., Sato, N., Suganuma, T., et al. (2022). A one-year-old girl with human parvovirus B19 infection and Hypocomplementemia mimicking incomplete Kawasaki disease. J Med Cases 13, 229–234. doi: 10.14740/jmc3917
Ozeki, Y., Yamada, F., Kishimoto, T., Yashiro, M., and Nakamura, Y. (2017). Epidemiologic features of Kawasaki disease: winter versus summer. Pediatr. Int. 59, 821–825. doi: 10.1111/ped.13293
Ozeki, Y., Yamada, F., Saito, A., Kishimoto, T., Yashiro, M., Makino, N., et al. (2018). Epidemiologic features of Kawasaki disease distinguished by seasonal variation: an age-specific analysis. Ann. Epidemiol. 28, 796–800. doi: 10.1016/j.annepidem.2018.08.004
Paniz-Mondolfi, A. E., van den Akker, T., Marquez-Colmenarez, M. C., Delgado-Noguera, L. A., Valderrama, O., and Sordillo, E. M. (2020). Kawasaki disease seasonality in Venezuela supports an arbovirus infection trigger. J. Med. Virol. 92, 2903–2910. doi: 10.1002/jmv.26381
Principi, N., Rigante, D., and Esposito, S. (2013). The role of infection in Kawasaki syndrome. J. Infect. 67, 1–10. doi: 10.1016/j.jinf.2013.04.004
Raut, S., Roychowdhoury, S., Bhakta, S., Sarkar, M., and Nandi, M. (2021). Incomplete Kawasaki disease as presentation of COVID-19 infection in an infant: a case report. J. Trop. Pediatr. 67:47. doi: 10.1093/tropej/fmaa047
Rehman, S., Majeed, T., Ansari, M. A., and Al-Suhaimi, E. A. (2020). Syndrome resembling Kawasaki disease in COVID-19 asymptomatic children. J. Infect. Public Health 13, 1830–1832. doi: 10.1016/j.jiph.2020.08.003
Rigante, D., Cantarini, L., Piastra, M., Angelone, D. F., Valentini, P., Pardeo, M., et al. (2012). Kawasaki syndrome and concurrent Coxsackie virus B3 infection. Rheumatol. Int. 32, 4037–4040. doi: 10.1007/s00296-010-1613-0
Rivera-Figueroa, E. I., Santos, R., Simpson, S., and Garg, P. (2020). Incomplete Kawasaki disease in a child with COVID-19. Indian Pediatr. 57, 680–681. doi: 10.1007/s13312-020-1900-0
Rodo, X., Ballester, J., Cayan, D., Melish, M. E., Nakamura, Y., Uehara, R., et al. (2011). Association of Kawasaki disease with tropospheric wind patterns. Sci. Rep. 1:152. doi: 10.1038/srep00152
Rodo, X., Curcoll, R., Robinson, M., Ballester, J., Burns, J. C., Cayan, D. R., et al. (2014). Tropospheric winds from northeastern China carry the etiologic agent of Kawasaki disease from its source to Japan. Proc. Natl. Acad. Sci. U. S. A. 111, 7952–7957. doi: 10.1073/pnas.1400380111
Roe, K. (2020). A viral infection explanation for Kawasaki disease in general and for COVID-19 virus-related Kawasaki disease symptoms. Inflammopharmacology 28, 1219–1222. doi: 10.1007/s10787-020-00739-x
Rosenfeld, N., Tasher, D., Ovadia, A., Abiri, S., and Dalal, I. (2020). Kawasaki disease with a concomitant primary Epstein—Barr virus infection. Pediatr. Rheumatol. Online J. 18:65. doi: 10.1186/s12969-020-00459-0
Rosenkranz, M. E., Schulte, D. J., Agle, L. M., Wong, M. H., Zhang, W., Ivashkiv, L., et al. (2005). TLR2 and MyD88 contribute to Lactobacillus casei extract-induced focal coronary arteritis in a mouse model of Kawasaki disease. Circulation 112, 2966–2973. doi: 10.1161/CIRCULATIONAHA.105.537530
Rowley, A. H., Baker, S. C., Shulman, S. T., Rand, K. H., Tretiakova, M. S., Perlman, E. J., et al. (2011). Ultrastructural, immunofluorescence, and RNA evidence support the hypothesis of a "new" virus associated with Kawasaki disease. J Infect Dis 203, 1021–1030. doi: 10.1093/infdis/jiq136
Sancho-Shimizu, V., Brodin, P., Cobat, A., Biggs, C. M., Toubiana, J., Lucas, C. L., et al. (2021). SARS-CoV-2-related MIS-C: a key to the viral and genetic causes of Kawasaki disease? J. Exp. Med. 218:446. doi: 10.1084/jem.20210446
Sandhaus, H., Crosby, D., Sharma, A., and Gregory, S. R. (2020). Association between COVID-19 and Kawasaki disease: vigilance required from otolaryngologists. Otolaryngol. Head Neck Surg. 163, 316–317. doi: 10.1177/0194599820930238
Santos, R. A., Nogueira, C. S., Granja, S., Baptista, J. B., Ribeiro, M. L., and Rocha, M. G. (2011). Kawasaki disease and human bocavirus--potential association? J. Microbiol. Immunol. Infect. 44, 235–237. doi: 10.1016/j.jmii.2011.01.016
Schildgen, O., Muller, A., Allander, T., Mackay, I. M., Volz, S., Kupfer, B., et al. (2008). Human bocavirus: passenger or pathogen in acute respiratory tract infections? Clin. Microbiol. Rev. 21:291-+. doi: 10.1128/cmr.00030-07
Schnaar, D. A., and Bell, D. M. (1982). Kawasaki syndrome in two cousins with parainfluenza virus infection. Am. J. Dis. Child. 136, 554–555. doi: 10.1001/archpedi.1982.03970420078019
Sharma, C., Ganigara, M., Galeotti, C., Burns, J., Berganza, F. M., Hayes, D. A., et al. (2021). Multisystem inflammatory syndrome in children and Kawasaki disease: a critical comparison. Nat. Rev. Rheumatol. 17, 731–748. doi: 10.1038/s41584-021-00709-9
Shike, H., Shimizu, C., Kanegaye, J. T., Foley, J. L., Schnurr, D. P., Wold, L. J., et al. (2005). Adenovirus, adeno-associated virus and Kawasaki disease. Pediatr. Infect. Dis. J. 24, 1011–1014. doi: 10.1097/01.inf.0000183769.31951.1e
Shimizu, C., Shike, H., Baker, S. C., Garcia, F., van der Hoek, L., Kuijpers, T. W., et al. (2005). Human coronavirus NL63 is not detected in the respiratory tracts of children with acute Kawasaki disease. J Infect Dis 192, 1767–1771. doi: 10.1086/497170
Shirato, K., Imada, Y., Kawase, M., Nakagaki, K., Matsuyama, S., and Taguchi, F. (2014). Possible involvement of infection with human coronavirus 229E, but not NL63, in Kawasaki disease. J. Med. Virol. 86, 2146–2153. doi: 10.1002/jmv.23950
Shulman, S. T., and Rowley, A. H. (2015). Kawasaki disease: insights into pathogenesis and approaches to treatment. Nat. Rev. Rheumatol. 11, 475–482. doi: 10.1038/nrrheum.2015.54
Sokolovsky, S., Soni, P., Hoffman, T., Kahn, P., and Scheers-Masters, J. (2021). COVID-19 associated Kawasaki-like multisystem inflammatory disease in an adult. Am. J. Emerg. Med. 39, 253.e1–253.e2. doi: 10.1016/j.ajem.2020.06.053
Sopontammarak, S., Promphan, W., Roymanee, S., and Phetpisan, S. (2008). Positive serology for dengue viral infection in pediatric patients with Kawasaki disease in southern Thailand. Circ. J. 72, 1492–1494. doi: 10.1253/circj.CJ-08-0158
Sosa, T., Brower, L., and Divanovic, A. (2019). Diagnosis and Management of Kawasaki Disease. JAMA Pediatr. 173, 278–279. doi: 10.1001/jamapediatrics.2018.3307
Spezia, P. G., Filippini, F., Nagao, Y., Sano, T., Ishida, T., and Maggi, F. (2023a). Identification of Torquetenovirus species in patients with Kawasaki disease using a newly developed species-specific PCR method. Int. J. Mol. Sci. 24:674. doi: 10.3390/ijms24108674
Spezia, P. G., Matsudaira, K., Filippini, F., Miyamura, T., Okada, K., Nagao, Y., et al. (2023b). Viral load of Torquetenovirus correlates with Sano's score and levels of total bilirubin and aspartate aminotransferase in Kawasaki disease. Sci. Rep. 13:18033. doi: 10.1038/s41598-023-45327-5
Stock, A. T., Hansen, J. A., Sleeman, M. A., McKenzie, B. S., and Wicks, I. P. (2016). GM-CSF primes cardiac inflammation in a mouse model of Kawasaki disease. J. Exp. Med. 213, 1983–1998. doi: 10.1084/jem.20151853
Stower, H. (2020). Kawasaki disease in a COVID-19-struck region. Nat. Med. 26:822. doi: 10.1038/s41591-020-0959-4
Strigl, S., Kutlin, A., Roblin, P. M., Shulman, S., and Hammerschlag, M. R. (2000). Is there an association between Kawasaki disease and Chlamydia pneumoniae? J Infect Dis 181, 2103–2105. doi: 10.1086/315526
Suganuma, E., Sato, S., Honda, S., and Nakazawa, A. (2020). A novel mouse model of coronary stenosis mimicking Kawasaki disease induced by Lactobacillus casei cell wall extract. Exp. Anim. 69, 233–241. doi: 10.1538/expanim.19-0124
Tabata, A., Ohkuni, H., Itoh, Y., Fukunaga, Y., Tomoyasu, T., and Nagamune, H. (2021). Complete genome sequence of Streptococcus mitis strain Nm-65, isolated from a patient with Kawasaki disease. Microbiol Resourc Announce 10:20. doi: 10.1128/mra.01239-20
Tada, R., Nagi-Miura, N., Adachi, Y., and Ohno, N. (2008). The influence of culture conditions on vasculitis and anaphylactoid shock induced by fungal pathogen Candida albicans cell wall extract in mice. Microb. Pathog. 44, 379–388. doi: 10.1016/j.micpath.2007.10.013
Tada, R., Yamanaka, D., Nagi-Miura, N., Adachi, Y., and Ohno, N. (2014). Vasculitis and Anaphylactoid shock induced in mice by Cell Wall extract of the fungus Candida metapsilosis. Pol. J. Microbiol. 63, 223–230. doi: 10.33073/pjm-2014-029
Tahara, M., Baba, K., Waki, K., and Arakaki, Y. (2006). Analysis of Kawasaki disease showing elevated antibody titres of Yersinia pseudotuberculosis. Acta Paediatr. 95, 1661–1664. doi: 10.1080/08035250600750080
Takahashi, K., Oharaseki, T., Wakayama, M., Yokouchi, Y., Naoe, S., and Murata, H. (2004). Histopathological features of murine systemic vasculitis caused by Candida albicans extract—an animal model of Kawasaki disease. Inflamm. Res. 53, 72–77. doi: 10.1007/s00011-003-1225-1
Takahashi, K., Oharaseki, T., Yokouchi, Y., Hiruta, N., and Naoe, S. (2010a). Kawasaki disease as a systemic vasculitis in childhood. Ann. Vasc. Dis. 3, 173–181. doi: 10.3400/avd.sasvp01003
Takahashi, K., Oharaseki, T., Yokouchi, Y., Miura, N. N., Ohno, N., Okawara, A. I., et al. (2010b). Administration of human immunoglobulin suppresses development of murine systemic vasculitis induced with Candida albicans water-soluble fraction: an animal model of Kawasaki disease. Mod. Rheumatol. 20, 160–167. doi: 10.1007/s10165-009-0250-5
Takemoto, R., Suzuki, T., Hashiguchi, T., Yanagi, Y., and Shirogane, Y. (2022). Short-stalk isoforms of CADM1 and CADM2 trigger Neuropathogenic measles virus-mediated membrane fusion by interacting with the viral hemagglutinin. J. Virol. 96:e0194921. doi: 10.1128/jvi.01949-21
Takeshita, S., Kobayashi, I., Kawamura, Y., Tokutomi, T., and Sekine, I. (2002). Characteristic profile of intestinal microflora in Kawasaki disease. Acta Paediatr. 91, 783–788. doi: 10.1080/08035250213221
Tanaka, H., Yanai, C., Miura, N. N., Ishibashi, K.-I., Yamanaka, D., Ohnishi, H., et al. (2020). Coronary Vasculitis induced in mice by Cell Wall Mannoprotein fractions of clinically isolated Candida species. Med Mycol J 61, 33–48. doi: 10.3314/mmj.20-00008
Tang, Y., Yan, W., Sun, L., Huang, J., Qian, W., Hou, M., et al. (2016). Kawasaki disease associated with Mycoplasma pneumoniae. Ital. J. Pediatr. 42:83. doi: 10.1186/s13052-016-0292-1
Tasaka, K., and Hamashima, Y. (1978). Studies on rickettsia-like body in Kawasaki disease. Attempts of the isolation and characterization. Acta Pathol. Jpn. 28, 235–245. doi: 10.1111/j.1440-1827.1978.tb00535.x
Thissen, J. B., Isshiki, M., Jaing, C., Nagao, Y., Lebron Aldea, D., Allen, J. E., et al. (2018). A novel variant of torque Teno virus 7 identified in patients with Kawasaki disease. PloS One 13:e0209683. doi: 10.1371/journal.pone.0209683
Tomita, S., Kato, H., Fujimoto, T., Inoue, O., Koga, Y., and Kuriya, N. (1987). Cytopathogenic protein in filtrates from cultures of propionibacterium acnes isolated from patients with Kawasaki-disease. Br. Med. J. 295, 1229–1232. doi: 10.1136/bmj.295.6608.1229
Toprak, D., Serce, O., Turel, O., Atici, S., Soysal, A., and Bakir, M. (2015). Is varicella zoster virus an etiologic factor in Kawasaki disease? A case report and review of the literature. Glob Pediatr Health 2, 2333794X14567194–12333794X14567194. doi: 10.1177/2333794x14567194
Toubiana, J., Poirault, C., Corsia, A., Bajolle, F., Fourgeaud, J., Angoulvant, F., et al. (2020). Kawasaki-like multisystem inflammatory syndrome in children during the covid-19 pandemic in Paris, France: prospective observational study. BMJ 369:m2094. doi: 10.1136/bmj.m2094
Tsurumizu, T., Okonogi, H., Shibusawa, T., Hashimoto, T., Makino, M., Ota, H., et al. (1991). A case of Kawasaki's disease combined with septicemia--isolation of Streptococcus sanguis (MCLS-1) and Streptococcus pyogenes from blood at the acute stage. Kansenshogaku zasshi. J Japan Assoc Infect Dis 65, 124–128.
Turkay, S., Odemis, E., and Karadag, A. (2006). Kawasaki disease onset during concomitant infections with varicella zoster and Epstein-Barr virus. J. Natl. Med. Assoc. 98, 1350–1352.
Ueda, Y., Kenzaka, T., Noda, A., Yamamoto, Y., and Matsumura, M. (2015). Adult-onset Kawasaki disease (mucocutaneous lymph node syndrome) and concurrent Coxsackievirus A4 infection: a case report. Int Med Case Rep J 8, 225–230. doi: 10.2147/IMCRJ.S90685
Umezawa, T., Saji, T., Matsuo, N., and Odagiri, K. (1989). Chest-x-ray findings in the acute phase of Kawasaki disease. Pediatr. Radiol. 20, 48–51. doi: 10.1007/bf02010633
Valtuille, Z., Lefevre-Utile, A., Ouldali, N., Beyler, C., Boizeau, P., Dumaine, C., et al. (2023). Calculating the fraction of Kawasaki disease potentially attributable to seasonal pathogens: a time series analysis. EClinicalMedicine 61:102078. doi: 10.1016/j.eclinm.2023.102078
van Stijn, D., Slegers, A., Zaaijer, H., and Kuijpers, T. (2020). Lower CMV and EBV exposure in children with Kawasaki disease suggests an under-challenged immune system. Front. Pediatr. 8:627957. doi: 10.3389/fped.2020.627957
Ventura, M. J., Guajardo, E., Clark, E. H., Bhairavarasu, K., Kherallah, R. Y., DiNardo, A. R., et al. (2020). Correspondence on 'Paediatric multisystem inflammatory syndrome temporally associated with SARS-CoV-2 mimicking Kawasaki disease (Kawa-COVID-19): a multicentre cohort' by Pouletty et al. Ann. Rheum. Dis. 81:e239. doi: 10.1136/annrheumdis-2020-218959
Verma, N. A., Zheng, X. T., Harris, M. U., Cadichon, S. B., Melin-Aldana, H., Khetsuriani, N., et al. (2009). Outbreak of life-threatening coxsackievirus B1 myocarditis in neonates. Clin. Infect. Dis. 49, 759–763. doi: 10.1086/605089
Vincent, P., Salo, E., Skurnik, M., Fukushima, H., and Simonet, M. (2007). Similarities of Kawasaki disease and Yersinia pseudotuberculosis infection epidemiology. Pediatr. Infect. Dis. J. 26, 629–631. doi: 10.1097/INF.0b013e3180616d3c
Viner, R. M., and Whittaker, E. (2020). Kawasaki-like disease: emerging complication during the COVID-19 pandemic. Lancet 395, 1741–1743. doi: 10.1016/S0140-6736(20)31129-6
Wang, C.-Y., Song, C.-M., Liu, G.-H., Zhang, H., Chen, F.-S., and Lin, H. (2021). Association between Mycoplasma pneumoniae infection and coronary artery aneurysm in children with Kawasaki disease. Iran. J. Pediatr. 31:737. doi: 10.5812/ijp.104737
Wang, J., Sun, F., Deng, H.-L., and Liu, R.-Q. (2019). Influenza a (H1N1) pdm09 virus infection in a patient with incomplete Kawasaki disease a case report. Medicine 98:e15009. doi: 10.1097/md.0000000000015009
Wang, H., Xia, Y., Fu, S., Wang, W., Xie, C., Zhang, Y., et al. (2016). Notch4 signaling pathway of endothelial progenitor cells in a Kawasaki disease model induced by Lactobacillus casei Cell Wall extract. J. Vasc. Res. 53, 340–348. doi: 10.1159/000449061
Wann, E. R., Fehringer, A. P., Ezepchuk, Y. V., Schlievert, P. M., Bina, P., Reiser, R. F., et al. (1999). Staphylococcus aureus isolates from patients with Kawasaki disease express high levels of protein a. Infect. Immun. 67, 4737–4743. doi: 10.1128/IAI.67.9.4737-4743.1999
Weng, K.-P., Wei, J. C.-C., Hung, Y.-M., Huang, S.-H., Chien, K.-J., Lin, C.-C., et al. (2018). Enterovirus infection and subsequent risk of Kawasaki disease: a population-based cohort study. Pediatr. Infect. Dis. J. 37, 310–315. doi: 10.1097/inf.0000000000001748
Whitby, D., Hoad, J. G., Tizard, E. J., Dillon, M. J., Weber, J. N., Weiss, R. A., et al. (1991). Isolation of measles-virus from child with Kawasaki-disease. Lancet 338:1215. doi: 10.1016/0140-6736(91)92085-g
Xiao, H., Hu, B., Luo, R., Hu, H., Zhang, J., Kuang, W., et al. (2020). Chronic active Epstein-Barr virus infection manifesting as coronary artery aneurysm and uveitis. Virol. J. 17:8. doi: 10.1186/s12985-020-01409-8
Xie, L. P., Yan, W. L., Huang, M., Huang, M. R., Chen, S., Huang, G. Y., et al. (2020). Epidemiologic features of Kawasaki disease in Shanghai from 2013 through 2017. J. Epidemiol. 30, 429–435. doi: 10.2188/jea.JE20190065
Yamada, H., Ohta, H., Hasegawa, S., Azuma, Y., Hasegawa, M., Kadoya, R., et al. (2016). Two infants with tuberculid associated with Kawasaki disease. Hum. Vaccin. Immunother. 12, 2772–2776. doi: 10.1080/21645515.2016.1208329
Yamashiro, Y., Nagata, S., Ohtsuka, Y., Oguchi, S., and Shimizu, T. (1996). Microbiologic studies on the small intestine in Kawasaki disease. Pediatr. Res. 39, 622–624. doi: 10.1203/00006450-199604000-00010
Yanai, C., Tanaka, H., Miura, N. N., Ishibashi, K.-I., Yamanaka, D., Ohnishi, H., et al. (2020). Coronary Vasculitis induced in mice by the Cell Wall Mannoprotein of Candida krusei. Biol. Pharm. Bull. 43, 848–858. doi: 10.1248/bpb.b19-01060
Yoshikane, Y., Koga, M., Imanaka-Yoshida, K., Cho, T., Yamamoto, Y., Yoshida, T., et al. (2015). JNK is critical for the development of Candida albicans-induced vascular lesions in a mouse model of Kawasaki disease. Cardiovasc. Pathol. 24, 33–40. doi: 10.1016/j.carpath.2014.08.005
Keywords: bacteria, fungi, Kawasaki disease, vasculitis, virus
Citation: Wang W, Zhu L, Li X, Liu Z, Lv H and Qian G (2023) Emerging evidence of microbial infection in causing systematic immune vasculitis in Kawasaki disease. Front. Microbiol. 14:1313838. doi: 10.3389/fmicb.2023.1313838
Received: 10 October 2023; Accepted: 07 December 2023;
Published: 22 December 2023.
Edited by:
Svetlana Khaiboullina, University of Nevada, Reno, United StatesReviewed by:
Yueyue Ding, Children's Hospital of Suzhou University, ChinaCopyright © 2023 Wang, Zhu, Li, Liu, Lv and Qian. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Haitao Lv, aGFpdGFvc3pAMTYzLmNvbQ==; Guanghui Qian, Z2hxaWFuQHN1ZGEuZWR1LmNu
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.