- 1Hubei Key Laboratory of Natural Products Research and Development, College of Biological and Pharmaceutical Sciences, China Three Gorges University, Yichang, China
- 2Institute of Pharmaceutical Biology and Biotechnology, Heinrich-Heine-University Duesseldorf, Duesseldorf, Germany
- 3Institute of Physiological Chemistry, Universitätsmedizin der Johannes Gutenberg-Universität Mainz, Mainz, Germany
- 4Key Laboratory of Study and Discovery of Small Targeted Molecules of Hunan Province, School of Medicine, Hunan Normal University, Changsha, China
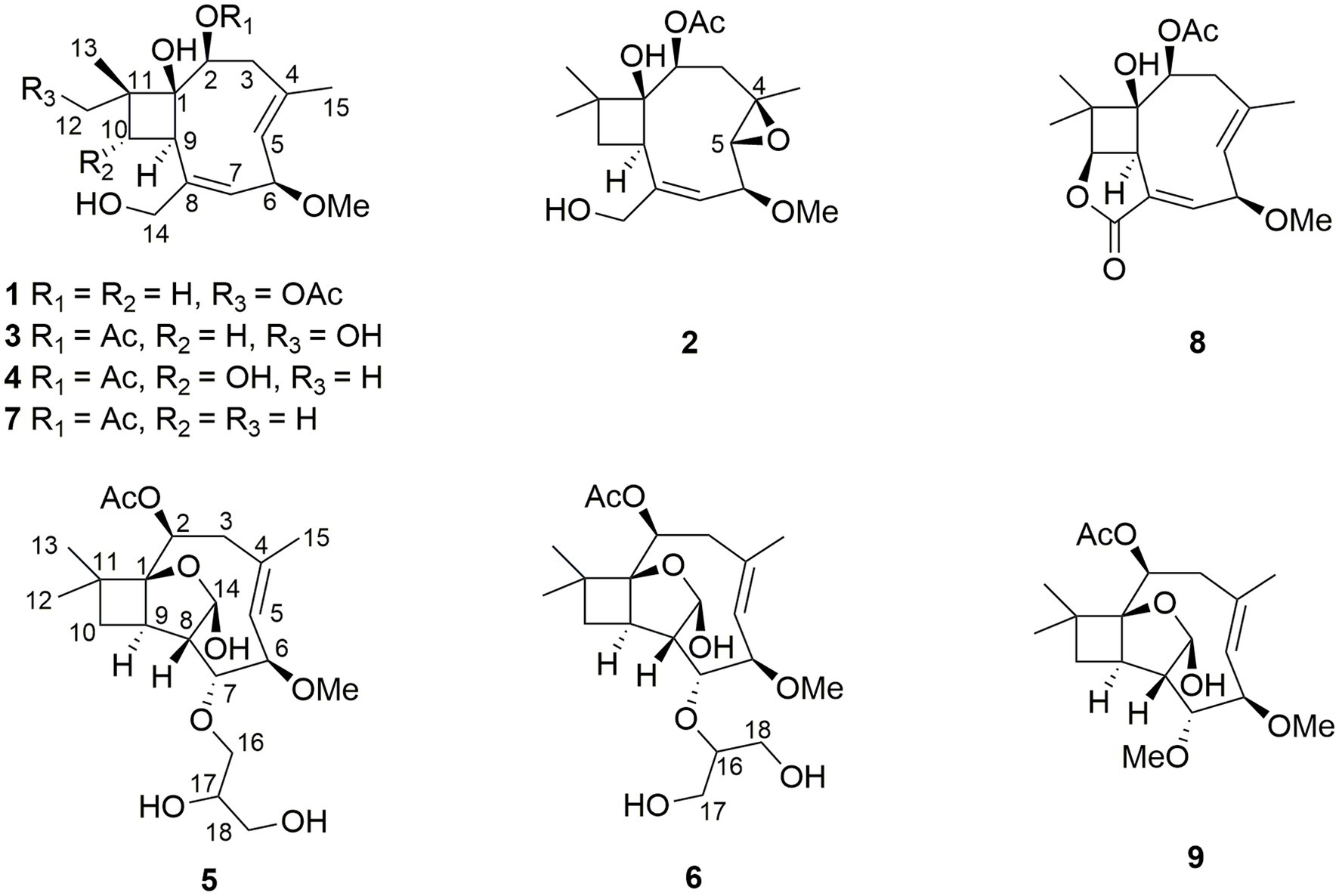
Two new caryophyllene-type sesquiterpenes pestalotiopsins U and V (1 and 2) and three known compounds pestalotiopsin B (7), pestaloporinate B (8), and pestalotiopsin C (9) were isolated by the cultivation of the endophytic fungus Pestalotiopsis lespedezae on solid rice medium, while four additional new caryophyllene pestalotiopsins W–Z (3–6) were obtained when 3.5% NaI was added to the fungal culture medium. The structures of the new compounds were determined by HRESIMS and 1D/2D nuclear magnetic resonance data. Compounds 1–9 were tested for cytotoxicity against the mouse lymphoma cell line L5178Y, but only 6 displayed significant activity with an IC50 value of 2.4 μM.
Introduction
Fungi from the genus Pestalotiopsis are widely distributed in diverse substrata such as plants, soil, alga, polluted steam water, and extreme environments in temperate and tropical regions (Maharachchikumbura et al., 2011, 2014). They are famous not only as phytopathogens but also as endophytes living in a well-balanced equilibrium with their host plants (Hopkins and McQuilken, 2000). The discovery of new bioactive natural products from this genus has established it as a promising candidate for bioprospecting (Xu et al., 2010; Deshmukh et al., 2017). Examples of recently discovered new compounds from Pestalotiopsis include benzophenones from the marine-derived fungus P. neglecta, which were able to suppress cell proliferation and induce apoptosis of the pancreatic cancer cell line PANC-1 at a low micromolar dosage by targeting the MEK/ERK pathway (Wang et al., 2019). Cuautepestalorin, which was isolated from a bioactive extract of Pestalotiopsis sp., was found to be active when evaluated in vitro as an α-glucosidase inhibitor (Rivera-Chavez et al., 2019).
Caryophyllene is a sesquiterpene consisting of a four-membered ring and a flexible nine-membered ring with an E-configurated double bond. Caryophyllene is known as a constituent of many essential oils from plants (Rampogu et al., 2018), but it also occurs as a natural product of plant-associated fungi such as Cytospora spp. (Li et al., 2017), Seiridium spp. (Tayone et al., 2020), Trichoderma spp. (Yu et al., 2015), and Pestalotiopsis spp. (Liu S. et al., 2016; Liu Y. et al., 2016). Some caryophyllene derivatives have also been reported from marine sources. For instance, the caryophyllene-type sesquiterpenes punctaporonins H–M were obtained from the fermentation broth of the sponge-associated fungus Hansfordia sinuosae, while punctaporonin H was reported as a new natural product capable of reducing triglycerides and total cholesterol content (Wu et al., 2014). Pestalotiopsins A and B are highly oxygenated caryophyllene derivatives from Pestalotiopsis sp. isolated from Taxus brevifolia (Pulici et al., 1996). (+)-Pestalotiopsin A bears an unprecedented oxatricyclic core structure with seven stereogenic centers, and its absolute stereochemistry was established based on an enantioselective total synthesis of both enantiomers (Takao et al., 2008, 2009).
The fungus P. lespedezae was isolated from the underground part of the medicinal plant Youngia japonica (L.) DC (Asteraceae) gathered in China. In a previous study, 10 new polyketide-terpenes, including a new iodized compound, were obtained following the addition of halogen salts such as NaCl, NaBr, and NaI to the solid rice medium (Yu et al., 2021). In the present study, we report two new caryophyllene-type sesquiterpenes pestalotiopsins U and V (1 and 2) and three known compounds pestalotiopsin B (7), pestaloporinate B (8), and pestalotiopsin C (9) from the solid rice medium culture of P. lespedezae, whereas four additional new caryophyllene pestalotiopsins W–Z (3–6) were obtained from the same fungus when cultured on solid rice medium with the addition of 3.5% NaI. All compounds were evaluated for their cytotoxicity against the mouse lymphoma cell line L5178Y, with only 6 showing significant cytotoxicity with an IC50 value of 2.4 μM (Figure 1).
Materials and methods
General experimental procedures
A Jasco P-2000 polarimeter was used for the measurement of optical rotations. Mass spectra (ESI) and HRESIMS data were obtained from an LC–MS HP1100 Agilent Finnigan LCQ Deca XP ThermoQuest spectrometer and a UHR-QTOF maXis 4G (Bruker Daltonics) mass spectrometer, respectively. NMR spectra were recorded with Bruker ARX 400 or 600 NMR spectrometers, with chemical shifts referring to the residual solvent peaks. Melting points were measured on an SGW X-4 micro-melting point instrument (Shanghai Jingke, China). A Dionex UltiMate-3400SD system (Thermo Scientific) coupled with an LPG-3400SD pump and a photodiode array detector (DAD 300RS) was employed for HPLC analysis. The analytical column (125 × 4 mm) was prefilled with Eurosphere-10 C18 (Knauer, Germany), and a gradient of MeOH and 0.1% formic acid in H2O was used: 0 min, 10% MeOH; 5 min, 10% MeOH; 35 min, 100% MeOH; 45 min, 100% MeOH. Semi-preparative HPLC was conducted using a Merck Hitachi HPLC System (UV detector L-7400; pump L-7100; Eurosphere-100 C18, 300 × 8 mm), and the mobile phase was a mixture of MeOH-H2O. Merck MN silica gel 60 M (0.04–0.063 mm) and Sephadex LH-20 were included in column chromatography. Thin-layer chromatography plates precoated with silica gel F254 were applied for monitoring and collecting fractions with detection under 254 and 365 nm or by spraying the plates with anise aldehyde reagent. The solvents used for column chromatography and spectroscopic measurements were distilled and spectral grade, respectively.
Fungal material and identification
The fungus investigated in this study was obtained from the underground part of Youngia japonica (L.) DC (Asteraceae) collected by Prof. Han Xiao (Guizhou Nationalities University) from Guiyang, Guizhou, P.R. China, in April 2018. Based on the DNA amplification results and sequencing of its ITS region, this fungus was identified as Pestalotiopsis lespedezae (GenBank accession number MT521347). Details of the protocol are the same as described before (Kjer et al., 2010). A deep-frozen sample of this fungal strain (No. 8G-C) is deposited in the refrigerator at −80°C at the Institute of Pharmaceutical Biology and Biotechnology, Heinrich-Heine-University Duesseldorf.
Fermentation, extraction, and isolation
Cultivation of P. lespedezae was conducted on solid rice medium in 10 Erlenmeyer flasks (1 L each). Each flask contained 100 g rice and 110 mL of demineralized water. The flasks were autoclaved at 121°C for 20 min before inoculation. After fermentation for 14 days at 20°C, 500 mL of EtOAc was added to each flask, followed by shaking the flasks at 140 rpm for 8 h. The crude extracts were obtained by filtration and removal of the solvent under reduced pressure.
In the OSMAC experiments, either 3.5 g NaCl, 3.5 g NaBr, or 3.5 g NaI were added to each flask containing 100 g rice and 110 mL of demineralized water. After fermentation and extraction, the chromatographic profiles of the respective extracts were obtained by HPLC. Based on the results, a large-scale fermentation was conducted by growing the fungus on the solid rice medium with the addition of 3.5 g NaI in 20 flasks.
The crude extract (8.5 g) from the rice fungal culture was dealt with a liquid–liquid partition between n-hexane and MeOH. The MeOH layer (4.6 g) was then subjected to vacuum liquid silica gel column chromatography with a gradient of n-hexane and EtOAc (100,1 to 0:100, v/v) as the mobile phase to give five fractions (Fr.1–5). Fr.2 (0.8 g) was then divided into three subfractions (Fr.2.1–2.3) by Sephadex LH-20 column chromatography (60 × 3 cm) using MeOH as eluent. Compounds 7 (10.0 mg, tR = 20.6 min) and 8 (5.9 mg, tR = 23.2 min) were yielded from Fr.2.2 (50 mg) through semi-preparative HPLC (MeOH-H2O: 0–3 min, 55%; 3–28 min, from 55 to 70%; 28–33 min, 100%), while compounds 1 (3.5 mg, tR = 20.8 min), 2 (4.8 mg, tR = 21.5 min), and 9 (32.5 mg, tR = 24.0 min) were obtained from Fr.2.3 (100 mg) by semi-preparative HPLC (MeCN-H2O: 0–3 min, 15%; 3–23 min, from 15 to 25%; 23–31 min, 100%).
The large-scale fermentation of P. lespedezae on rice medium with the addition of 3.5 g NaI yielded 10.1 g of crude extract. In a similar manner, including liquid–liquid partition and vacuum liquid silica gel column chromatography as described above, four subfractions (Fr.1–4) were obtained. Fr.3 (2.0 g) was subjected to ODS column (60 × 200 mm) chromatography using MeOH to give five subfractions (Fr.3.1–3.5). Fr.3.3 (500 mg) was then submitted to a Sephadex LH-20 column (60 × 3 cm) with MeOH as eluent, followed by separation through semi-preparative HPLC (MeCN-H2O: 0–2 min, 15%; 2–25 min, from 15 to 20%; 25–30 min, 100%) to yield compounds 3 (4.5 mg, tR = 14.4 min), 4 (3.7 mg, tR = 16.2 min), 5 (2.0 mg, tR = 23.3 min), and 6 (2.1 mg, tR = 22.7 min).
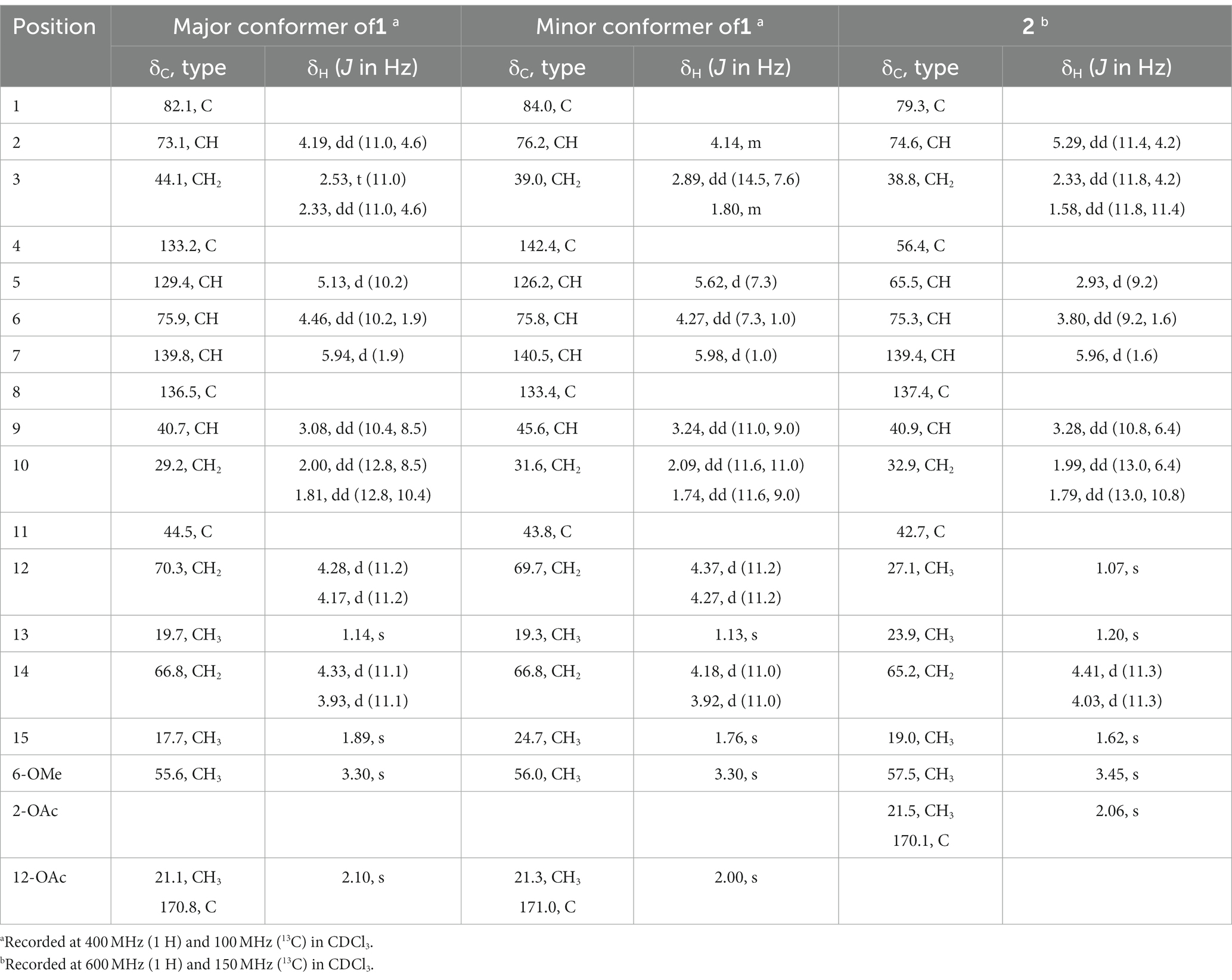
Pestalotiopsin U (1): colorless oil, [α]20 D–55 (c 0.10, MeOH); UV (MeOH) λmax 207 nm; 1H and 13C NMR data, see Table 1; HRESIMS m/z 363.1780 [M + Na] + (C18H28O6Na, calcd. 363.1778).
Pestalotiopsin V (2): needle crystals, m.p. 102–103°C, [α]20 D –64 (c 0.10, MeOH); UV (MeOH) λmax 207 nm; 1H and 13C NMR data, see Table 1; HRESIMS m/z 363.1782 [M + Na] + (C18H28O6Na, calcd. 363.1778).
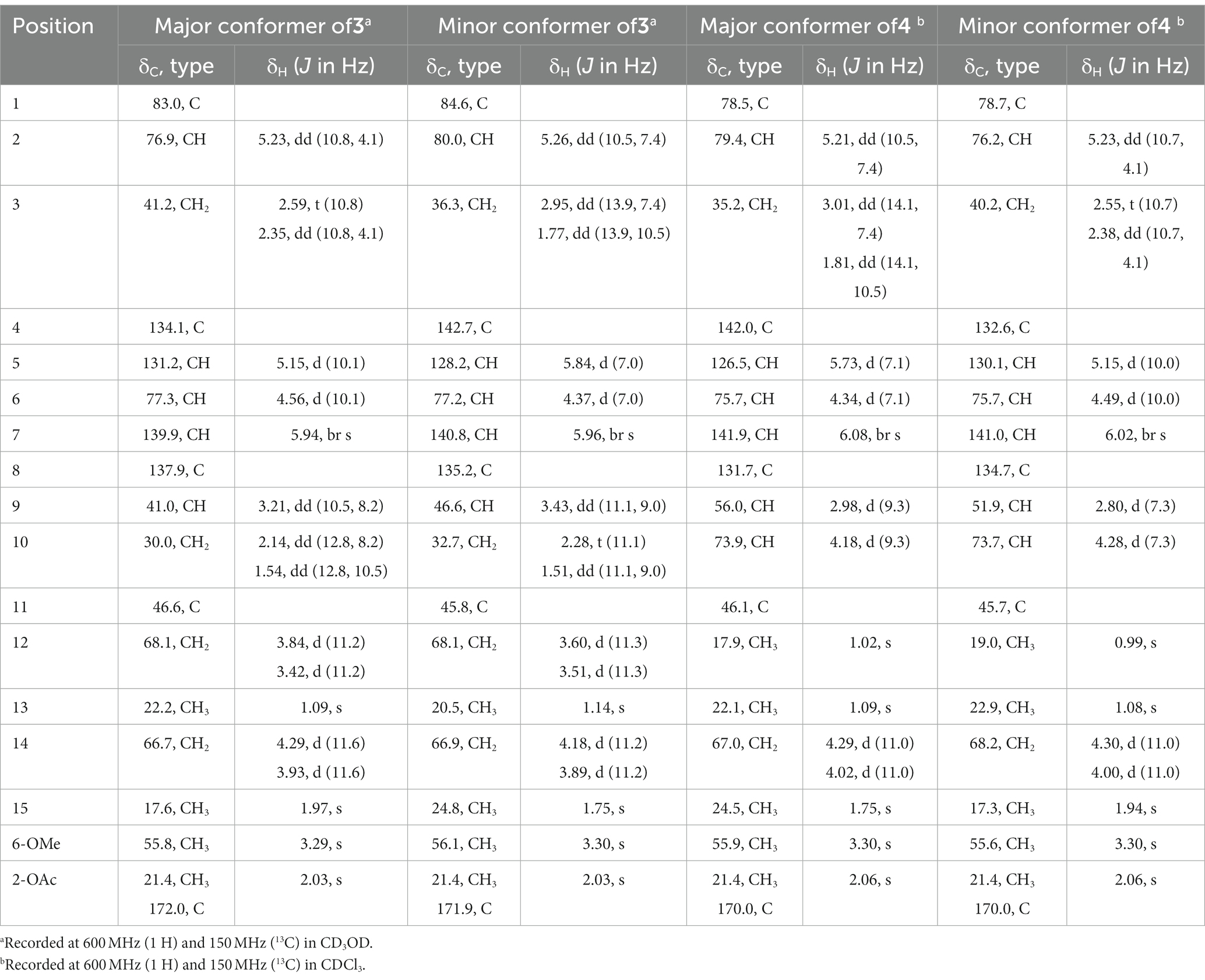
Pestalotiopsin W (3): colorless oil, [α]20 D–108 (c 0.30, MeOH); UV (MeOH) λmax 207 nm; 1H and 13C NMR data, see Table 2; HRESIMS m/z 363.1780 [M + Na] + (C18H28O6Na, calcd. 363.1778).
Pestalotiopsin X (4): colorless oil, [α]20 D–49 (c 0.10, MeOH); UV (MeOH) λmax 207 nm; 1H and 13C NMR data, see Table 2; HRESIMS m/z 363.1781 [M + Na] + (C18H28O6Na, calcd. 363.1778).
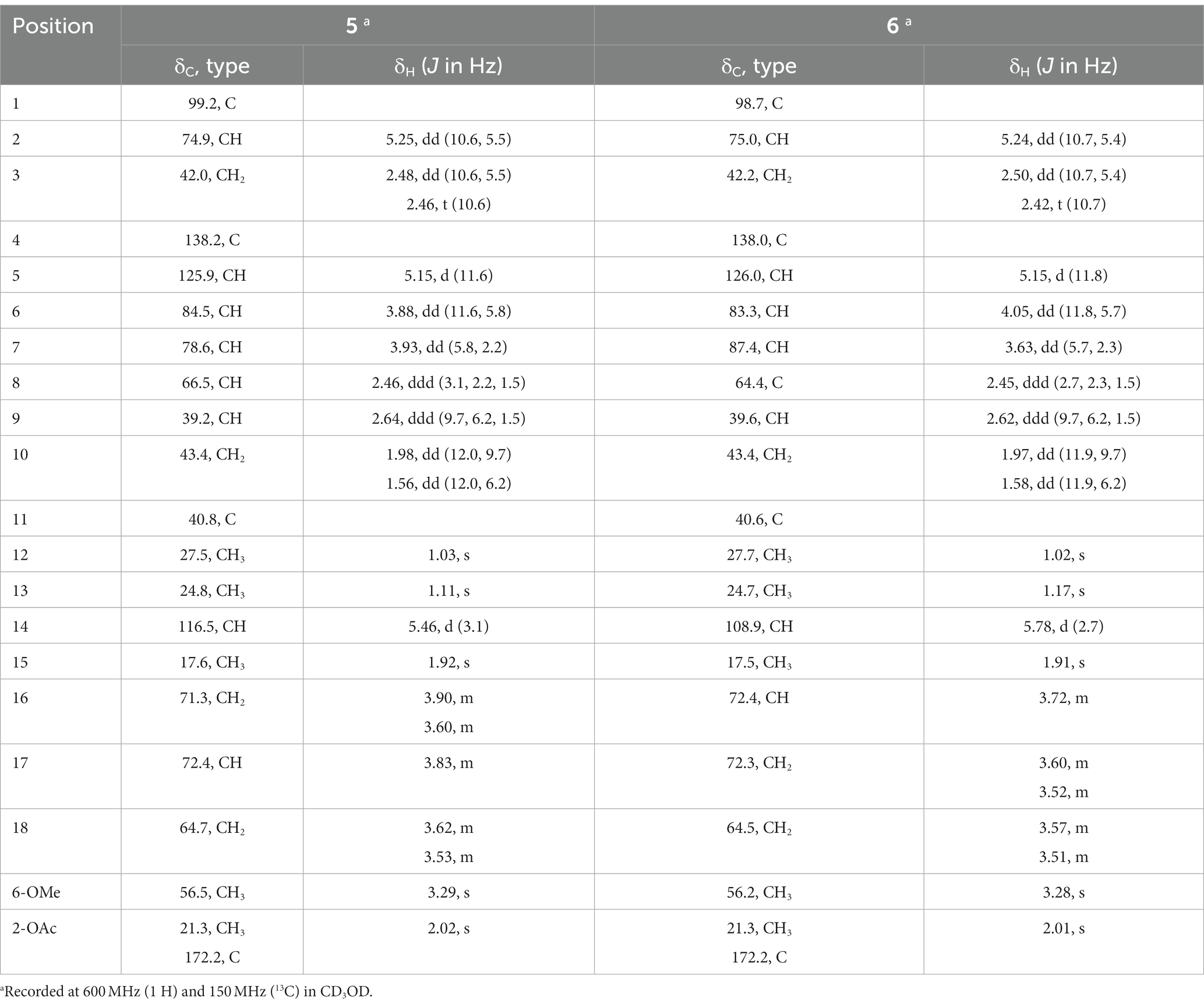
Pestalotiopsin Y (5): colorless oil, [α]20 D–30 (c 0.15, MeOH); UV (MeOH) λmax 207 nm; 1H and 13C NMR data, see Table 3; HRESIMS m/z 437.2137 [M + Na] + (C21H34O8Na, calcd. 437.2146).
Pestalotiopsin Z (6): colorless oil, [α]20 D–18 (c 0.18, MeOH); UV (MeOH) λmax 207 nm; 1H and 13C NMR data, see Table 3; HRESIMS m/z 437.2142 [M + Na] + (C21H34O8Na, calcd. 437.2146).
Cytotoxicity assay
MTT method was applied to evaluate the cytotoxicity of all isolated compounds against the mouse lymphoma cell line L5178Y. Kahalalide F and 0.1% EGMME/DMSO were used as positive and negative controls, respectively (Ashour et al., 2006). The cells were grown in RPMI medium and 10% fetal calf serum (FCS) (Biochrom/Merck). Thiazolyl blue tetrazolium bromide (MTT; # M2128 Sigma) was used as an indicator for cell viability. The viability assay reaction is based on the oxidation of the yellowish MTT solution to solid formazan via mitochondrial dehydrogenases in living cells. The crystals formed were solubilized with acidified isopropanol, and the intensity was determined colorimetrically at 570 nm (Chuenjitkuntaworn et al., 2016).
Results and discussion
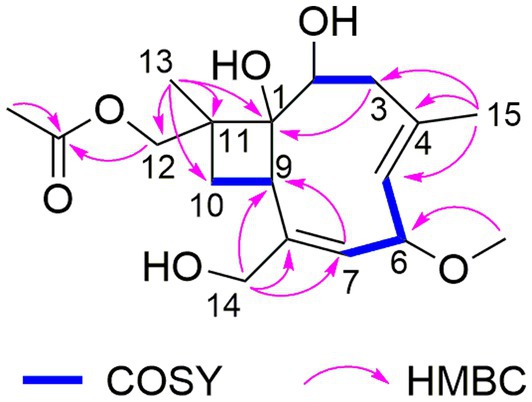
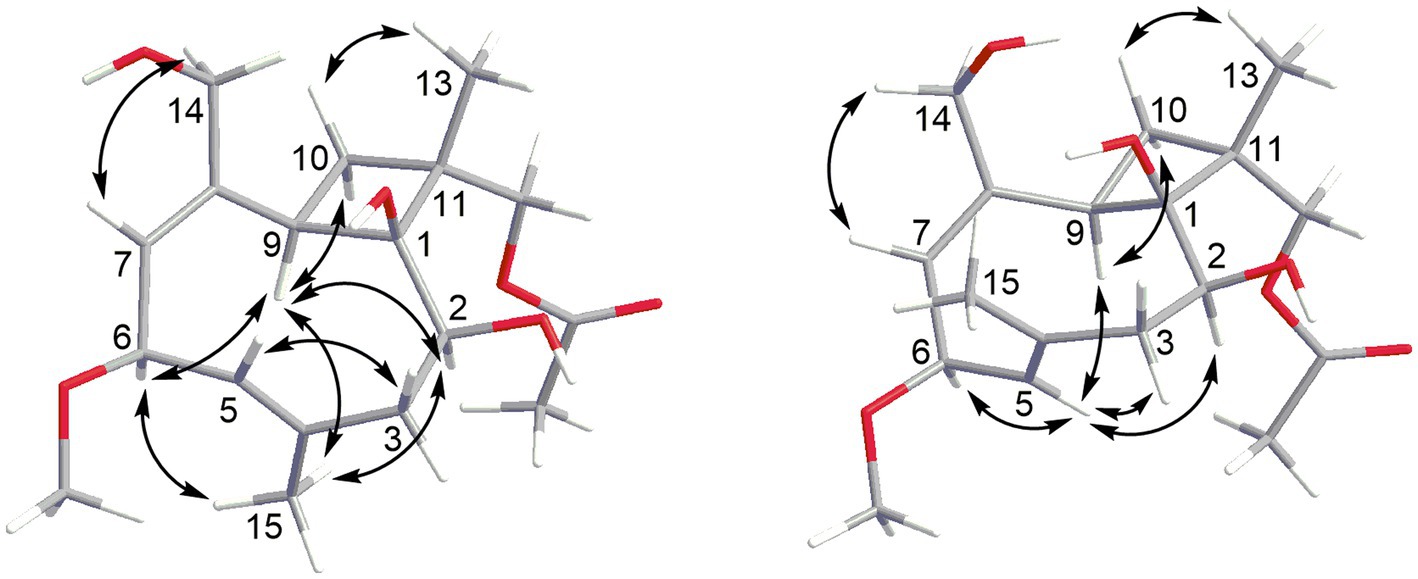
The HRESIMS data of 1 showed the pseudomolecular ion peaks at m/z 341.1960 [M + H]+, 358.2225 [M + NH4]+, and 363.1780 [M + Na]+, which indicated the molecular formula C18H28O6 with five degrees of unsaturation, containing one additional oxygen atom by comparison with that of the co-isolated known compound pestalotiopsin B (7) (Pulici et al., 1996). In the 1H NMR spectrum, compound 1 was observed as a mixture of two conformational isomers with a ratio of approximately 80:20, which was also found for pestalotiopsin B (7) and other previously reported caryophyllene-type sesquiterpenes such as pestalotiopsins C1 (Yu et al., 2015), M, and N (Tayone et al., 2020). The 1H and 13C NMR data of 1 (Table 1) resembled those of pestalotiopsin B (7). However, signals of the one methyl group were replaced by signals of an oxygenated methylene at δC 70.3, δH 4.28 and 4.17 (CH2-12, major conformer) in 1. The HMBC correlations from Me-13 (δH 1.14) to C-1 (δC 82.1), C-10 (δC 29.2), C-11 (δC 44.5), and C-12, as well as the HMBC correlations from H2-12 and protons of the acetoxy group (δH 2.10) to the carbonyl carbon (δC 170.8) of the acetoxy group, confirmed the location of the acetoxy group at C-12 in 1 rather than location at C-2 in pestalotiopsin B (7). The remaining structure of 1 was found to be identical to that of 7 after the detailed analysis of the 2D NMR spectra of 1 (Figure 2). 4E and 7E geometries were assigned by NOE correlations of H-5/H-3a, H-7/H2-14 in both conformers of 1 (Figure 3). However, characteristic differences between the two conformers were found for the ROESY correlations associated with H-5 and Me-15. In the major conformer of 1, the ROESY correlations between Me-15/H-2, Me-15/H-6, Me-15/H-9, H-9/H-2, H-9/H-6, H-9/H-10b (δH 1.81), and between Me-13/H-10a (δH 2.00) established that H-2, H-6, H-9, and Me-15 were on the same face of the ring, whereas OH-1 and Me-13 were on the opposite side. In the minor conformer, the ROESY correlations from H-5 to H-2, H-6 and H-9, and between H-9/H-10b (δH 1.74), Me-13/H-10a (δH 2.09) indicated α-orientation of H-2, H-5, H-6, and H-9, whereas OH-1 and Me-13 were β-oriented. Thus, the relative configuration of 1 was assigned as shown, and the obvious difference in the two conformational isomers was the orientation of H-5 and Me-15 caused by coupled hindered rotations regarding the C-3/C-4 and C-5/C-6 bonds. Thus, compound 1 was elucidated as a new caryophyllene derivative, for which the trivial name pestalotiopsin U is proposed.
Compound 2 had the molecular formula C18H28O6 as established by the sodiated-molecular peak at m/z 363.1782 in the HRESIMS data, containing one additional oxygen atom when compared to that of pestalotiopsin B (7) (Pulici et al., 1996). The 1H and 13C NMR data (Table 1) of 2 were similar to those of 7, except for the fact that two olefinic carbons were replaced by two oxygenated carbons at δC 65.5 (C-5) and 56.4 (C-4) in 2. An epoxy substituent at C-4/C-5 was evident from the COSY correlations between H-5 (δH 2.93)/H-6 (δH 3.80)/H-7 (δH 5.96), together with the HMBC correlations from Me-15 (δH 1.62) to C-3 (δC 38.8), C-4, and C-5. Detailed analysis of the 2D NMR spectra for 2 revealed that the remaining planar structure of 2 was identical to that of pestalotiopsin B (7). The double bond of C-7/C-8 was E-configurated as determined by the NOE correlation between H-7 and H-14b (δH 4.03). Moreover, the ROESY correlations between H-2 (δH 5.29)/Me-15, Me-15/H-6, H-6/H-9 (δH 3.28), H-9/H-2, H-2/H-3a (δH 2.33), H-3a/Me-15, and Me-15/H-9 indicated the cofacial orientation of these protons, and thus the relative configuration of 2 was determined as shown.
Pestalotiopsin W (3) possessed the same molecular formula as 1, according to the sodiated-molecular peak at m/z 363.1780 in the HRESIMS data. The 1H NMR spectrum of 3 (Table 2) suggested an approximately 56:44 mixture of two conformers. Comparison of the 1H and 13C NMR data of 3 and 1 suggested that their structures were closely related. The major differences found were a downfield shifted oxygenated methine (H-2, δH 5.23 in major conf. and 5.26 in minor conf. in 3 compared to δH 4.19 in major conf. and 4.14 in minor conf. in 1) together with an upfield shifted oxygenated methylene (Hab-12, δH 3.84, 3.42 in major conf. and 3.60, 3.51 in minor conf. in 3 compared to δH 4.28, 4.17 in major conf. and 4.37, 4.27 in minor conf. in 1). These findings suggested the attachment of the acetoxy group at C-2 in 3 rather than at C-12 in 1, which was further confirmed by the COSY correlation between Hab-3 and the above-mentioned downfield-shifted oxygenated methine together with the HMBC correlation from the latter and a methyl group at δH 2.03 to the carbonyl carbon δC 172.0 or 171.9 in both conformers of 3. Detailed analysis of the 2D NMR spectra of 3 revealed that the remaining substructure and relative configuration of 3 were identical to those of 1.
Based on the HRESIMS data, the molecular formula of 4 was deduced as C18H28O6, which was identical to that of 3. Two sets of signals with a ratio of approximately 73:27 were observed in the 1H NMR spectrum of 4 (Table 2). Analysis of the 1H and 13C NMR data (Table 3) revealed structural similarity between 4 and 3. However, the replacement of signals for a hydroxymethyl group by those of an additional methyl group (Me-12) at δH 1.02 and δC 17.9 (major conf.) or at δH 0.99 and δC 19.0 (minor conf.) in 4 indicated a different location of the hydroxy group in 4. The COSY correlation between H-10 (δH 4.18 and 4.28 for major and minor conformers, respectively) and H-9, together with the HMBC correlations from the additional methyl group Me-12 to C-1, C-10, C-11, and C-13, confirmed the location of a hydroxy group at C-10 in 4 rather than at C-12 in 3. The remaining structure of 4 was determined to be identical to that of 3 after the detailed analysis of the 2D NMR spectra of 4. The olefinic double bonds at C-4/C-5 and C-7/C-8 were shown to be E-configurated, as indicated by the NOE correlations between H-5/H-3a and H-7/H2-14 in both conformers of 4, respectively. In the major conformer of 4, the NOE correlations between H-6/H-5, H-5/H-9, H-9/Me-12, Me-12/H-2, H-2/H-5, and between Me-13 and H-10 indicated α-orientation of H-2, H-5, H-6, H-9, and Me-12, whereas OH-1, Me-13, and H-10 were β-orientated. Meanwhile, in the minor conformer of 4, the NOE correlations between H-6/Me-15, Me-15/H-9, H-9/Me-12, Me-12/H-2, H-2/Me-15, and between Me-13 and H-10 indicated the α-orientation of H-2, H-6, H-9, Me-12, and Me-15, whereas OH-1, Me-13, and H-10 were β-orientated. Thus, the relative configuration at C-1, C-2, C-6, and C-9 of 4 was assigned to be identical as 1 and 3, while the additional OH-10 was α-oriented in 4. It is worth mentioning that in 1 and 3, H-5 and H-9 were aligned on the opposite and the same side of the cyclononadiene ring in the major and minor conformers, respectively, whereas in 4, H-5 and H-9 were aligned on the same and the opposite side of the cyclononadiene ring in the major and minor conformers, respectively.
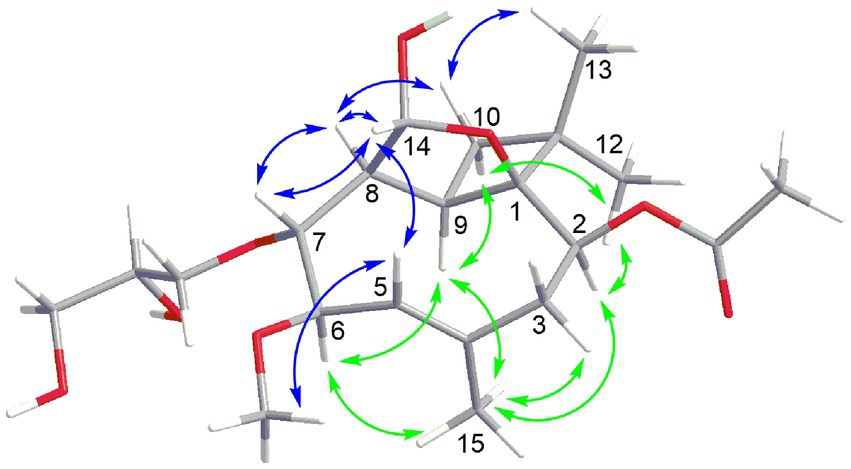
Compound 5 had the molecular formula C21H34O8 as deduced by HRESIMS, indicating 5 degrees of unsaturation. The 13C NMR data of 5 (Table 3) displayed a carbonyl carbon at δC 172.2 and carbon signals of a double bond at δC 138.2 and 125.9, accounting for two of the five degrees of unsaturation, thus implying the presence of a tricyclic ring system. The 1H and 13C NMR data of 5 were almost identical to those of co-isolated known tricyclic caryophyllene pestalotiopsin C (9) (Pulici et al., 1997), except for the absence of signals for a methoxy group and appearance of two additional oxygenated methylene (CH2-16 at δC 71.3, δH 3.90 and 3.60; CH2-18 at δC 64.7, δH 3.62 and 3.53) and an additional oxygenated methine (CH-17 at δC 72.4, δH 3.83) in 5. The COSY correlations between H-5/H-6/H-7/H-8/H-14 and between Hab-16/H-17/Hab-18, together with the HMBC correlations from Hab-16 to C-7, indicated the attachment of an additional 2,3-dihydroxypropoxy group at C-7. The remaining structure of 5 was determined to be identical to that of 9 after detailed analysis of the 2D NMR data of 5. In the ROESY spectrum (Figure 4), the cross-peaks between Me-13/H-10b (δH 1.56), H-10b/H-8, H-8/H-7, H-7/H-14, H-14/H-8, H-14/H-5, H-5/OMe-6, H-5/H-3b (δH 2.46), and between H-9/H-10a (δH 1.98), H-10a/Me-12, Me-12/H-2, H-2/Me-15, Me-15/H-6, H-6/H-9, H-9/Me-15, and Me-15/H-3a (δH 2.48) indicated the relative configuration of 5 as shown, which is identical to that of structurally related pestalotiopsins A (Pulici et al., 1996), C (9) (Pulici et al., 1997), E (Putra et al., 2019) and J (Xiao et al., 2017).
The molecular formula of pestalotiopsin Z (6) was determined to be identical to that of 5 based on the HRESIMS data. Comparison of their 1H and 13C NMR data (Table 2) and analysis of the 2D NMR spectra indicated that both compounds shared the same tricyclic caryophyllene core in addition to the presence of a glycerol subunit. However, the HMBC correlation from the oxygenated methine at δH 3.72 (H-16) in 6 instead of an oxygenated methylene in 5 to C-7 confirmed that the substituent at C-7 was (1,3-dihydroxypropan-2-yl)oxy in 6 rather than 2,3-dihydroxypropoxy in 5. The relative configuration of 6 was assigned to be identical to that of 5 by the comparison of their coupling constants and NOE relationships.
Sequencing data of the fungal genomes indicated that a large part of secondary metabolic gene clusters are silent under standard laboratory culture conditions (Debbab et al., 2013; Rutledge and Challis, 2015). In our current research, more new analogs were isolated when 3.5% NaI was added. These results, together with other reported successful examples (Wang et al., 2015; Yamazaki et al., 2015), suggested that OSMAC could be an effective approach that may result in a steep accumulation of target compounds or enhancement of diverse secondary metabolites from fungi by activating their silent gene clusters (Wiemann and Keller, 2014).
Pestalotiopsins A-T have been reported from different fungi so far (Pulici et al., 1996, 1997; Baker et al., 2008; Xiao et al., 2017; Putra et al., 2019; Tayone et al., 2020; Kang et al., 2023); however, they seldom showed cytotoxicity. In our study, all compounds were tested for cytotoxicity against the mouse lymphoma cell line L5178Y, but only compound 6 exhibited potent cytotoxicity with an IC50 value of 2.4 μM compared to the positive control of 4.3 μM. The other tested compounds did not show any inhibitory effect at a concentration of 10 μM. The presence of a (1,3-dihydroxypropan-2-yl)oxy moiety in 6 rather than 2,3-dihydroxypropoxy in 5 or a methoxy group in 9 might contribute to the cytotoxicity.
Conclusion
In summary, six new caryophyllene-type sesquiterpenes pestalotiopsins U–Z (1–6) and three known derivatives pestalotiopsin B (7), pestaloporinate B (8), and pestalotiopsin C (9) were obtained through the fermentation of the endophytic fungus P. lespedezae on solid rice medium. Pestalotiopsins W–Z (3–6) were not detected when the fungus was grown on rice medium without the addition of 3.5% NaI. Cytotoxicity against the mouse lymphoma cell line L5178Y of all isolated compounds was evaluated, but only pestalotiopsin Z (6) showed significant activity with an IC50 value of 2.4 μM.
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/Supplementary material.
Author contributions
XY contributed to extraction, isolation, and manuscript preparation. WM carried out cytotoxicity assay. MF and YG contributed to part of fungal fermentation. ZG and KZ contributed to part of structure elucidation. PP and ZL supervised the research work and revised the manuscript. All authors contributed to the article and approved the submitted version.
Funding
ZL wants to thank Intra Institutional Open Fund of School of Medicine, Hunan Normal University (KF2022001) for support.
Acknowledgments
XY thanks Prof. Han Xiao for the help during plant sample collection and Prof. Chengxiong Liu from China Three Gorges University for collecting the NMR data.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmicb.2023.1248896/full#supplementary-material
References
Ashour, M., Edrada, R., Ebel, R., Wray, V., Watjen, W., Padmakumar, K., et al. (2006). Kahalalide derivatives from the Indian sacoglossan mollusk Elysia grandifolia. J. Nat. Prod. 69, 1547–1553. doi: 10.1021/np060172v
Baker, T. M., Edmonds, D. J., Hamilton, D., O'Brien, C. J., and Procter, D. J. (2008). Synthesis and reactions of the pestalotiopsin skeleton. Angew. Chem. Int. Ed. Engl. 47, 5631–5633. doi: 10.1002/anie.200801900
Chuenjitkuntaworn, B., Osathanon, T., Nowwarote, N., Supaphol, P., and Pavasant, P. (2016). The efficacy of polycaprolactone/hydroxyapatite scaffold in combination with mesenchymal stem cells for bone tissue engineering. J. Biomed. Mater. Res. A 104, 264–271. doi: 10.1002/jbm.a.35558
Debbab, A., Aly, A. H., and Proksch, P. (2013). Mangrove derived fungal endophytes–a chemical and biological perception. Fungal Divers. 61, 1–27. doi: 10.1007/s13225-013-0243-8
Deshmukh, S. K., Prakash, V., and Ranjan, N. (2017). Recent advances in the discovery of bioactive metabolites from Pestalotiopsis. Phytochem. Rev. 16, 883–920. doi: 10.1007/s11101-017-9495-3
Hopkins, K. E., and McQuilken, M. P. (2000). Characteristics of Pestalotiopsis associated with hardy ornamental plants in the UK. Eur. J. Plant Pathol. 106, 77–85. doi: 10.1023/a:1008776611306
Kang, X., Yang, W., Zheng, Y., Zheng, M., Xiao, Y., Wang, J., et al. (2023). Caryophyllene sesquiterpenoids with various ring systems from the fungus Pestalotiopsis chamaeropis. Phytochemistry 207:113569. doi: 10.1016/j.phytochem.2022.113569
Kjer, J., Debbab, A., Aly, A. H., and Proksch, P. (2010). Methods for isolation of marine-derived endophytic fungi and their bioactive secondary products. Nat. Protoc. 5, 479–490. doi: 10.1038/nprot.2009.233
Li, Y., Wang, Q., Liu, X., and Che, Y. (2017). Punctaporonins N-S, new caryophyllene sesquiterpenoids from Cytospora sp. Biomed. Res. Int. 2017:7871459. doi: 10.1155/2017/7871459
Liu, S., Dai, H., Makhloufi, G., Heering, C., Janiak, C., Hartmann, R., et al. (2016). Cytotoxic 14-membered macrolides from a mangrove-derived endophytic fungus, Pestalotiopsis microspora. J. Nat. Prod. 79, 2332–2340. doi: 10.1021/acs.jnatprod.6b00473
Liu, Y., Yang, M. H., Wang, X. B., Li, T. X., and Kong, L. Y. (2016). Caryophyllene sesquiterpenoids from the endophytic fungus, Pestalotiopsis sp. Fitoterapia 109, 119–124. doi: 10.1016/j.fitote.2015.12.003
Maharachchikumbura, S. S. N., Guo, L. D., Chukeatirote, E., Bahkali, A. H., and Hyde, K. D. (2011). Pestalotiopsis—morphology, phylogeny, biochemistry and diversity. Fungal Divers. 50, 167–187. doi: 10.1007/s13225-011-0125-x
Maharachchikumbura, S. S. N., Hyde, K. D., Groenewald, J. Z., Xu, J., and Crous, P. W. (2014). Pestalotiopsis revisited. Stud. Mycol. 79, 121–186. doi: 10.1016/j.simyco.2014.09.005
Pulici, M., Sugawara, F., Koshino, H., Okada, G., Esumi, Y., Uzawa, J., et al. (1997). Metabolites of Pestalotiopsis spp., endophytic fungi of Taxus brevifolia. Phytochemistry 46, 313–319. doi: 10.1016/s0031-9422(97)00285-9
Pulici, M., Sugawara, F., Koshino, H., Uzawa, J., Yoshida, S., Lobkovsky, E., et al. (1996). Pestalotiopsins a and B: new caryophyllenes from an endophytic fungus of Taxus brevifolia. J. Org. Chem. 61, 2122–2124. doi: 10.1021/jo951736v
Putra, H. N., Rukachaisirikul, V., Saithong, S., Phongpaichit, S., Preedanon, S., Sakayaroj, J., et al. (2019). Caryophyllene sesquiterpenes, chromones and 10-membered macrolides from the marine-derived fungus Pseudopestalotiopsis sp. PSU-AMF45. Tetrahedron 75:130530. doi: 10.1016/j.tet.2019.130530
Rampogu, S., Zeb, A., Baek, A., Park, C., Son, M., and Lee, K. W. (2018). Discovery of potential plant-derived peptide deformylase (PDF) inhibitors for multidrug-resistant bacteria using computational studies. J. Clin. Med. 7:563. doi: 10.3390/jcm7120563
Rivera-Chavez, J., Zacatenco-Abarca, J., Morales-Jimenez, J., Martinez-Avina, B., Hernandez-Ortega, S., and Aguilar-Ramirez, E. (2019). Cuautepestalorin, a 7,8-dihydrochromene-oxoisochromane adduct bearing a hexacyclic scaffold from Pestalotiopsis sp. IQ-011. Org. Lett. 21, 3558–3562. doi: 10.1021/acs.orglett.9b00962
Rutledge, P. J., and Challis, G. L. (2015). Discovery of microbial natural products by activation of silent biosynthetic gene clusters. Nat. Rev. Microbiol. 13, 509–523. doi: 10.1038/nrmicro3496
Takao, K., Hayakawa, N., Yamada, R., Yamaguchi, T., Morita, U., Kawasaki, S., et al. (2008). Total synthesis of (−)-pestalotiopsin a. Angew. Chem. Int. Ed. Engl. 47, 3426–3429. doi: 10.1002/anie.200800253
Takao, K., Hayakawa, N., Yamada, R., Yamaguchi, T., Saegusa, H., Uchida, M., et al. (2009). Total syntheses of (+)- and (−)-pestalotiopsin a. J. Org. Chem. 74, 6452–6461. doi: 10.1021/jo9012546
Tayone, W. C., Nishiyama, M., Tanaka, K., Hayato, M., Enomoto, M., and Hashimoto, M. (2020). DFT supported structural elucidations of seiridiasteriscane a, unique 15-nor-asteriscane and novel pestalotiopsin congeners from Seiridium sp. KT3957. Tetrahedron 76:131197. doi: 10.1016/j.tet.2020.131197
Wang, B., Park, E. M., King, J. B., Mattes, A. O., Nimmo, S. L., Clendinen, C., et al. (2015). Transferring fungi to a deuterium enriched medium results in assorted, conditional changes in secondary metabolite production. J. Nat. Prod. 78, 1415–1421. doi: 10.1021/acs.jnatprod.5b00337
Wang, W., Park, C., Oh, E., Sung, Y., Lee, J., Park, K. H., et al. (2019). Benzophenone compounds, from a marine-derived strain of the fungus Pestalotiopsis neglecta, inhibit proliferation of pancreatic cancer cells by targeting the MEK/ERK pathway. J. Nat. Prod. 82, 3357–3365. doi: 10.1021/acs.jnatprod.9b00646
Wiemann, P., and Keller, N. P. (2014). Strategies for mining fungal natural products. J. Ind. MIicrobiol. Biotechnol. 41, 301–313. doi: 10.1007/s10295-013-1366-3
Wu, Z., Liu, D., Proksch, P., Guo, P., and Lin, W. (2014). Punctaporonins H-M: caryophyllene-type sesquiterpenoids from the sponge-associated fungus Hansfordia sinuosae. Mar. Drugs 12, 3904–3916. doi: 10.3390/md12073904
Xiao, J., Lin, L., Hu, J., Jiao, F., Duan, D., Zhang, Q., et al. (2017). Highly oxygenated caryophyllene-type and drimane-type sesquiterpenes from Pestalotiopsis adusta, an endophytic fungus of Sinopodophyllum hexandrum. RSC Adv. 7, 29071–29079. doi: 10.1039/c7ra04267a
Xu, J., Ebada, S. S., and Proksch, P. (2010). Pestalotiopsis a highly creative genus: chemistry and bioactivity of secondary metabolites. Fungal Divers. 44, 15–31. doi: 10.1007/s13225-010-0055-z
Yamazaki, H., Rotinsulu, H., Narita, R., Takahashi, R., and Namikoshi, M. (2015). Induced production of halogenated epidithiodiketopiperazines by a marine derived Trichoderma cf. brevicompactum with sodium halides. J. Nat. Prod. 78, 2319–2321. doi: 10.1021/acs.jnatprod.5b00669
Yu, X., Gao, Y., Frank, M., Mándi, A., Kurtán, T., Müller, W. E., et al. (2021). Induction of ambuic acid derivatives by the endophytic fungus Pestalotiopsis lespedezae through an OSMAC approach. Tetrahedron 79:131876. doi: 10.1016/j.tet.2020.131876
Keywords: Pestalotiopsis lespedezae , endophytic fungus, caryophyllene-type sesquiterpenes, pestalotiopsins, OSMAC approach, cytotoxicity
Citation: Yu X, Müller WEG, Frank M, Gao Y, Guo Z, Zou K, Proksch P and Liu Z (2024) Caryophyllene-type sesquiterpenes from the endophytic fungus Pestalotiopsis lespedezae through an OSMAC approach. Front. Microbiol. 14:1248896. doi: 10.3389/fmicb.2023.1248896
Edited by:
Tünde Pusztahelyi, University of Debrecen, HungaryReviewed by:
Sunil Kumar Deshmukh, The Energy and Resources Institute (TERI), IndiaWen-Bo Han, Northwest A&F University, China
Gang Li, Qingdao University, China
Copyright © 2024 Yu, Müller, Frank, Gao, Guo, Zou, Proksch and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Peter Proksch, cHJva3NjaEB1bmktZHVlc3NlbGRvcmY=; Zhen Liu, bGl1emhlbjIwMjBAaHVubnUuZWR1LmNu; emhlbmZlaXppMEBzaW5hLmNvbQ==
 Xiaoqin Yu
Xiaoqin Yu Werner E. G. Müller
Werner E. G. Müller Marian Frank2
Marian Frank2 Peter Proksch
Peter Proksch Zhen Liu
Zhen Liu