- 1Department of Natural Medicinal Chemistry and Pharmacognosy, School of Pharmacy, Qingdao University, Qingdao, China
- 2Guangxi Key Laboratory of Chemistry and Engineering of Forest Products, Guangxi University for Nationalities, Nanning, China
- 3Hainan Key Laboratory of Research and Development of Natural Product From Li Folk Medicine, Hainan Institute for Tropical Agricultural Resources, Institute of Tropical Bioscience and Biotechnology, Chinese Academy of Tropical Agricultural Sciences, Haikou, China
- 4Key Laboratory of Chemical Biology of Ministry of Education, Department of Natural Product Chemistry, School of Pharmaceutical Sciences, Shandong University, Jinan, China
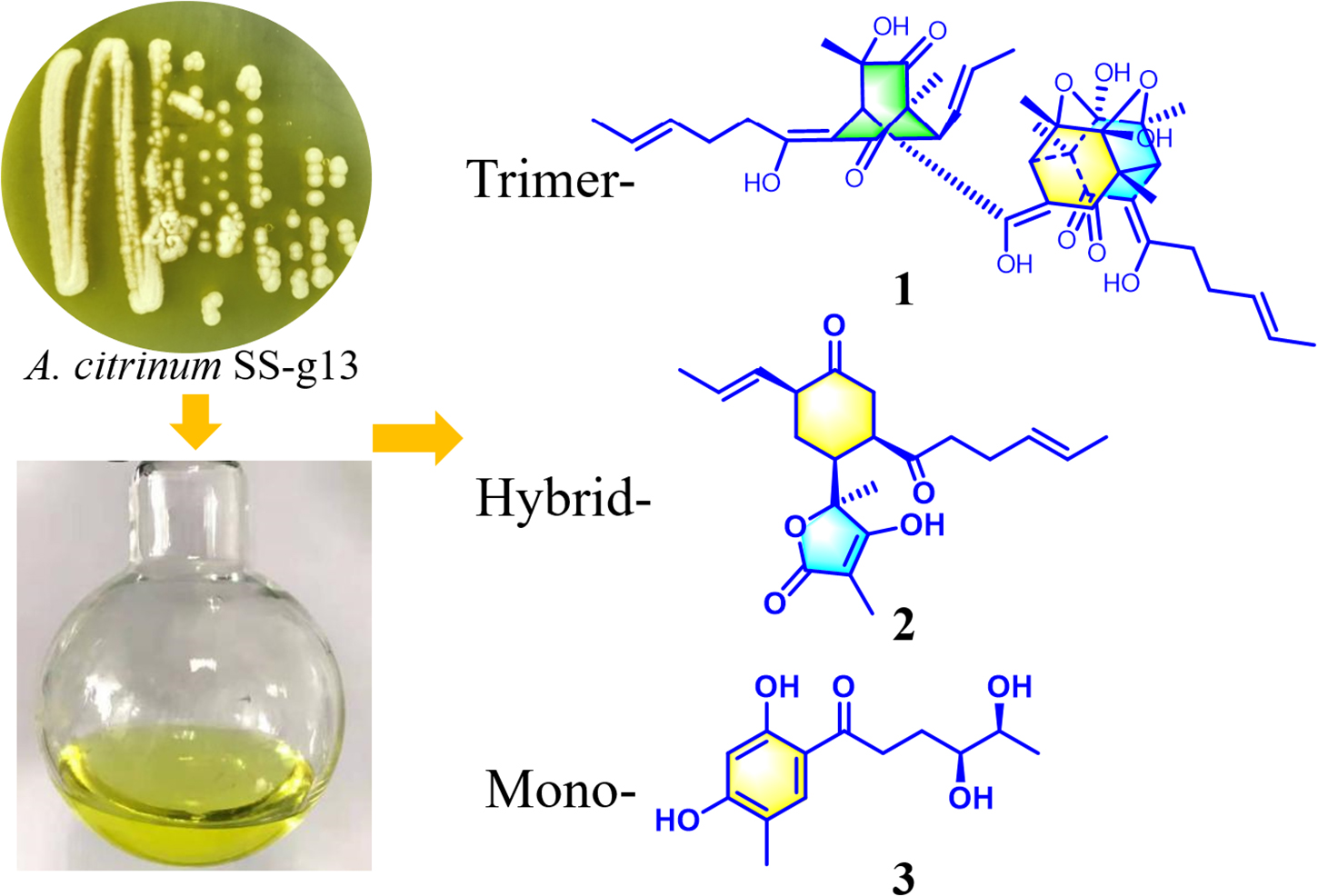
Three new sorbicillinoids, including trimer trisorbicillinone E (1), acremosorbicillinoids A and B (2 and 3), and a new alkaloid acremokaloid A (4), and a new natural product 2S,3S-acetyl-β-methyltryptophan (5), were isolated from an endophytic fungus Acremonium citrinum SS-g13, which is found in Fructus mori plant root. In addition, eight known sorbicillinoids (6–13) were also obtained. The new compound structures were established using NMR, HRESIMS spectra, and reported spectroscopic data. The absolute configurations of compounds 1–5, were determined by spectroscopic analysis, Snatzke’s method, and time-dependent density functional theory-electronic circular dichroism (TDDFT-ECD) calculations. Compound 11 exhibited significant cholesterol efflux enhancing activity. A plausible biosynthesis pathway for the sorbicillinoids is discussed.
Introduction
The sorbicillinoids family contains hexaketide metabolites with complex, highly oxygenated, bicyclic and tricyclic skeletons (Harned and Volp, 2011). Since the first report in 1948 (Cram, 1948; Cram and Tishler, 1948), there has been an increasing number of isolated sorbicillinoids, obtained from terrestrial and marine derived fungi. And these molecules were classified into the following groups according to the number of sorbicillinoid construction units: monomeric sorbicillinoids, bisorbicillinoids, trisorbicillinoids, and hybrid sorbicillinoids (Supplementary Figure 1; Meng et al., 2016). The carbon–carbon bond construction of sorbicillinoids in synthetic chemistry are Diels-Alder [4 + 2] (DA) and Michael [1 + 4] addition reactions. Due to their unique structures and diverse biological activities, sorbicillinoids have been subjects of interest in biosynthetic and synthetic studies in recent years (Meng et al., 2016; Kahlert et al., 2020; Chakrabarty et al., 2021).
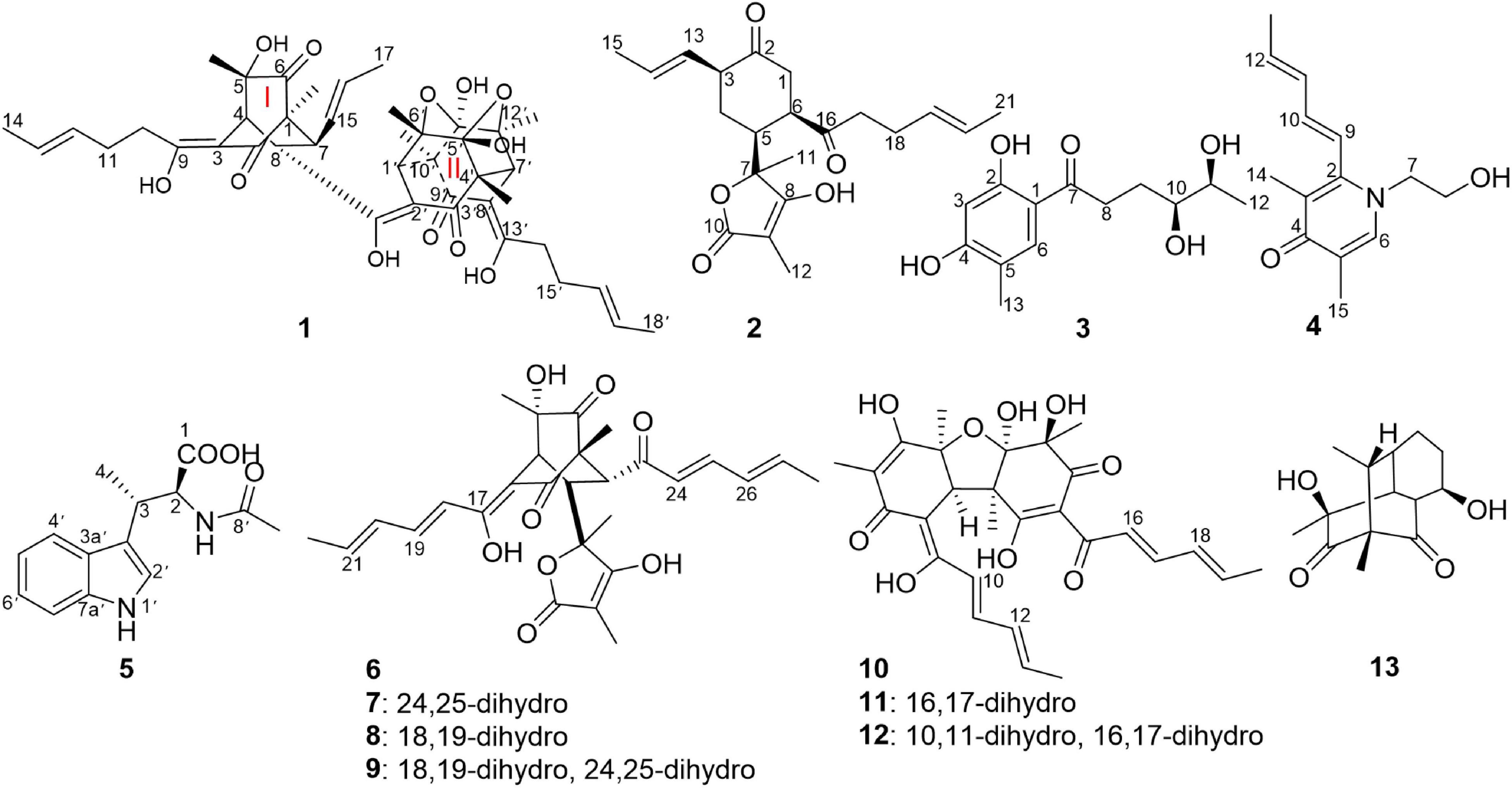
An investigation on the endophytic fungus Acremonium citrinum SS-g13 was previously carried out using 44 flasks containing rice culture media, resulting in three known sorbicillinoids (Peng et al., 2020). In our continuing search for novel sorbicillinoid-type secondary metabolites from this fungus, a large-scale culture strategy (Hu et al., 2021) was applied to explore its chemical diversity. In this study, 80 flasks were used to ferment this fungal strain. The fungal crude extract chemical investigation led to the isolation of three new sorbicillinoids, including trisorbicillinone E (1) and acremosorbicillinoids A (2) and B (3), one new alkaloid acremokaloid A (4), and the new natural product 2S,3S-acetyl-β-methyltryptophan (5). Among the products, trisorbicillinone E (1) was a novel trimeric sorbicillinoid. To the best of our knowledge, this kind of trimeric compound is rare in nature. Furthermore, only six trisorbicillinoids (Supplementary Figure 1; Li et al., 2007, 2010; Guo et al., 2013; Cao et al., 2020) have been discovered in nature, mainly from marine fungi. The other two sorbicillinoids, acremosorbicillinoids A and B (2 and 3), were hybrid and monomeric sorbicillinoids, respectively. In addition to the five new compounds isolated from the fungus A. citrinum SS-g13, seven known bisorbicillinoids, i.e., trichotetronine (6) (Shirota et al., 1997), dihydrotrichotetronine (7) (Shirota et al., 1997), 10,11-dihydrobislongiquinolide (8) (Yu et al., 2019), 10,11,16,17-tetrahydrobislongiquinolide (9) (Yu et al., 2019), bisvertinolone (10) (Andrade et al., 1992), dihydrobisvertinolone (11) (Liu et al., 2005b), tetrahydrobisvertinolone (12) (Liu et al., 2005b), and one known mono-sorbicillinoid penicillone B (13) (Liu et al., 2005c), were also discovered (Figure 1). The planar and spatial structures of these sorbicillinoids were determined. This study describes the isolation, structural elucidation, and biological activities of these isolates.
Materials and Methods
General Experimental Procedures
Optical rotations were measured on a Perkin-Elmer 241MC polarimeter (Perkin-Elmer Instruments, Norwalk, CT, United States) in MeOH at 20°C. Electronic circular dichroism (ECD) spectra were acquired on a Chirascan spectropolarimeter (Applied Photophysics, United Kingdom). Infrared (IR) spectra were recorded on a Nicolet iN10 (Thermo Fisher Scientific, Waltham, MA, United States). Nuclear magnetic resonance (NMR) spectra were obtained on JNM-ECP 600 (JEOL, Japan), DD2-500 (Agilent, United States), and AVANCE NEO 400 (Bruker, United States) operating at 600/500/400 (1H) and 150/125 (13C) MHz, using DMSO-d6 and CDCl3 as the solvent, with tetramethylsilane (TMS) as an internal standard. Low-resolution mass spectra (Applied Biosystems, United States) were obtained on a LTQ Orbitrap spectrometer equipped with an electrospray ionization (ESI) source. High-resolution electrospray ionization mass spectrometry (HRESIMS) data were determined on a Finnigan LC-QDECA mass spectrometer (Thermo Electron, San Jose, Calif., United States). The semi-preparative HPLC system (Agilent 1260 Infinity II, Agilent Technologies, Germany) was equipped with a 1260 Quat Pump VL, a 1260 Vialsampler, a 1260 multicolumn thermostat (MCT), a 1260 diode array detector (DAD) WR, and a ZORBAX SB-C18 column (5 μm, 9.4 × 250 mm).
Fungal Material
The endophytic fungus A. citrinum SS-g13 was isolated from the root of the terrestrial plant F. mori, collected in February 2017 from Dezhou, Shandong, People’s Republic of China. The fungus was identified according to its morphological characteristics and the analysis of the rDNA internal transcribed spacer (ITS) region (GenBank access number MK034752). This fungus was stored at −80°C at the School of Pharmacy, Qingdao University, People’s Republic of China.
Extraction and Isolation
The A. citrinum SS-g13 fungus was cultured on plates with potato dextrose agar medium at 28°C for 4 days. The fungal strain agar plugs were inoculated into 80 flasks (500 mL), each containing 80 g of rice, 0.24 g of peptone, and 120 mL of tap-water. The flasks were incubated under static conditions at 28°C. After 70 days of cultivation, the fermented cultures were extracted 3 times with ethyl acetate (EtOAc, 16 L × 3). The organic solvent was evaporated under reduced pressure to afford the crude extract (65.3 g).
Based on thin-layer chromatography (TLC) analysis, the crude extract was fractionated into six fractions (Fr.1–Fr.6) by column chromatography on silica gel, eluting with a gradient of CH2Cl2-MeOH (100–50%). Fr.2 (14.8 g) was fractionated by silica gel column chromatography with a gradient of EtOAc-petroleum ether (5–100%) to give eight subfractions (Fr.2.1–Fr.2.8). Fr.2.5 (2.9 g) was separated by a CombiFlash Rf 200 purification system (C18 spherical 20–35 μm 100A 80 g, 30 mL/min, eluting with a gradient of MeOH-H2O, from 60% MeOH to 80% MeOH over 50 min), obtaining six subfractions (Fr.2.5.1–Fr.2.5.6). Fr.2.5.6 (280.2 mg) was purified by semi-preparative HPLC (MeCN/H2O, 70:30, 2 mL/min, plus 0.1% FA) to obtain 1 (2.8 mg, tR 21.5 min), 10 (32.7 mg, tR 15.0 min), 11 (10.0 mg, tR 16.5 min), and 12 (7.5 mg, tR 18.0 min). Fr.2.6 (614.4 mg) was separated by a CombiFlash Rf 200 purification system (C18 spherical 20–35 μm 100A 80 g, 30 mL/min, eluting with MeOH-H2O, 75% MeOH for 25 min, and 100% MeOH for 10 min), affording four subfractions (Fr.2.6.1–Fr.2.6.4). Fr.2.6.1 (143.1 mg) was to obtain needle 13 (55.0 mg). Fr.2.6.2 (153.7 mg) was sequentially purified through semi-preparative HPLC (MeOH/H2O, 75:25, 2 mL/min, plus 0.1% FA) to give 9 (15.1 mg, tR 21.7 min). Fr.2.7 (851.5 mg) was purified by semi-preparative HPLC (MeOH/H2O, 75:25, 2 mL/min, plus 0.1% FA) to obtain six subfractions (Fr.2.7.1–Fr.2.7.6). Fr.2.7.1 (48.7 mg) was purified using semi-preparative HPLC (MeCN/H2O, 45:55, 2 mL/min, plus 0.1% FA) to yield 2 (1.3 mg, tR 27.0 min), and 6 (2.2 mg, tR 43.0 min). Fr.2.7.3 (173.6 mg) was purified by semi-preparative HPLC (MeCN/H2O, 48:52, 2 mL/min, plus 0.1% FA) to yield 8 (10.7 mg, tR 38.5 min). Fr.2.7.6 (92.1 mg) was separated by semi-preparative HPLC (MeCN/H2O, 50:50, 2 mL/min, plus 0.1% FA) to obtain 7 (15.6 mg, tR 39 min). Similarly, Fr.5 (2.2 g) was applied to a CombiFlash Rf 200 purification system (C18 spherical 20–35 μm 100A 80 g, 30 mL/min, eluting with MeOH-H2O, 5% MeOH for 5 min, a gradient of 5% MeOH to 100% MeOH over 40 min), which obtained three subfractions (Fr.5.1–Fr.5.3). Fr.5.3 (71.5 mg) was subsequently purified by semi-preparative HPLC (MeOH/H2O, 42:58, 2 mL/min, plus 0.1% FA) to afford 3 (4.6 mg, tR 21.0 min), 4 (5.3 mg, tR 14.4 min), and 5 (7.3 mg, tR 22.0 min), respectively.
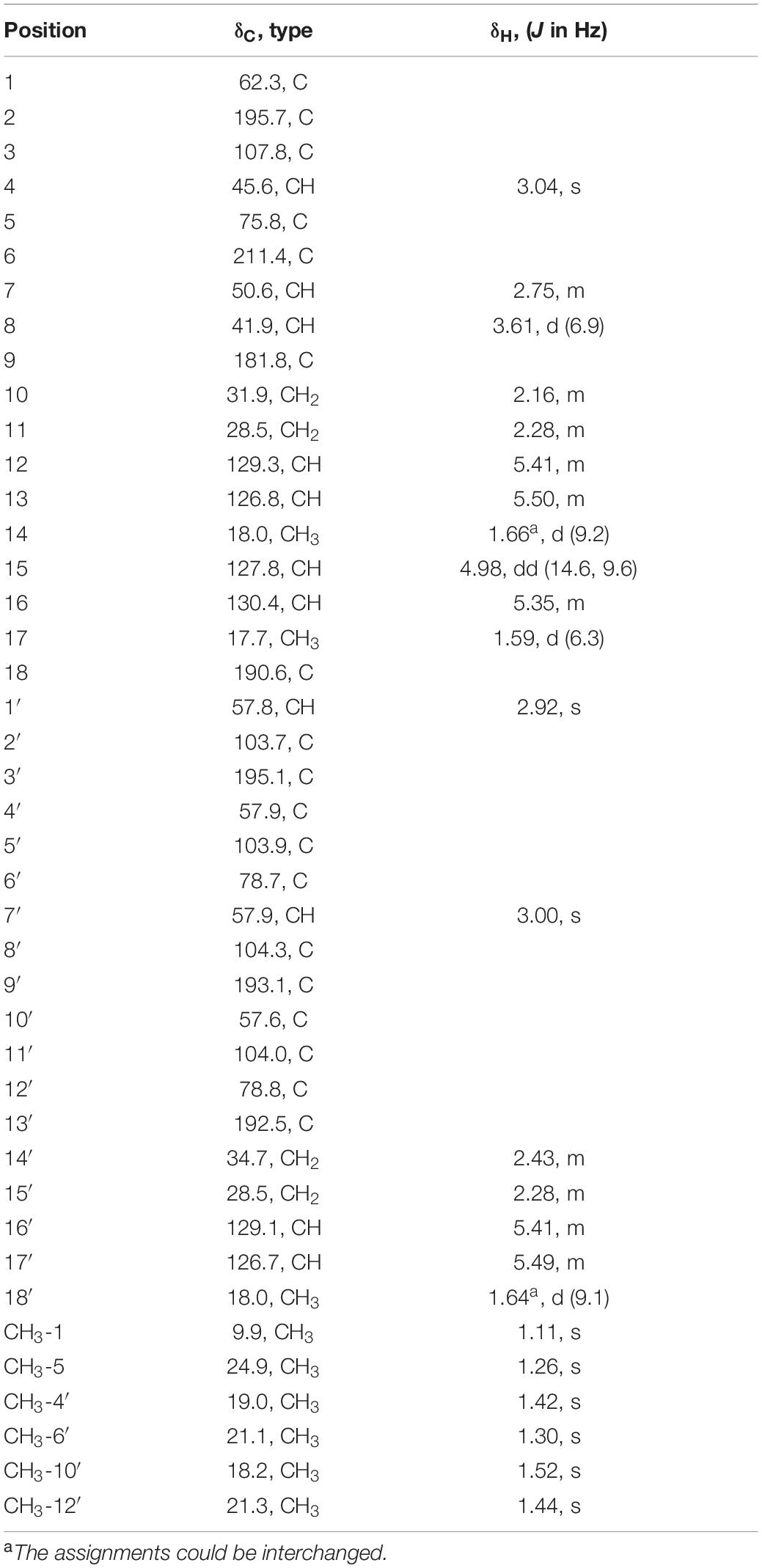
Trisorbicillinone E (1): yellow oil; [α]20D + 151.1 (c 0.080, MeOH); UV (MeOH) λmax 202, 296 nm; IR νmax 3,500, 2,922, 2,856, 1,732, 1,593, 1,448, 1,376, 1,264, 1,135, 1,007, 970 cm–1; 1H and 13C NMR data (see Table 1); HRESIMS m/z 749.3521 [M + H]+ (calcd. for C42H53O12 749.3532).
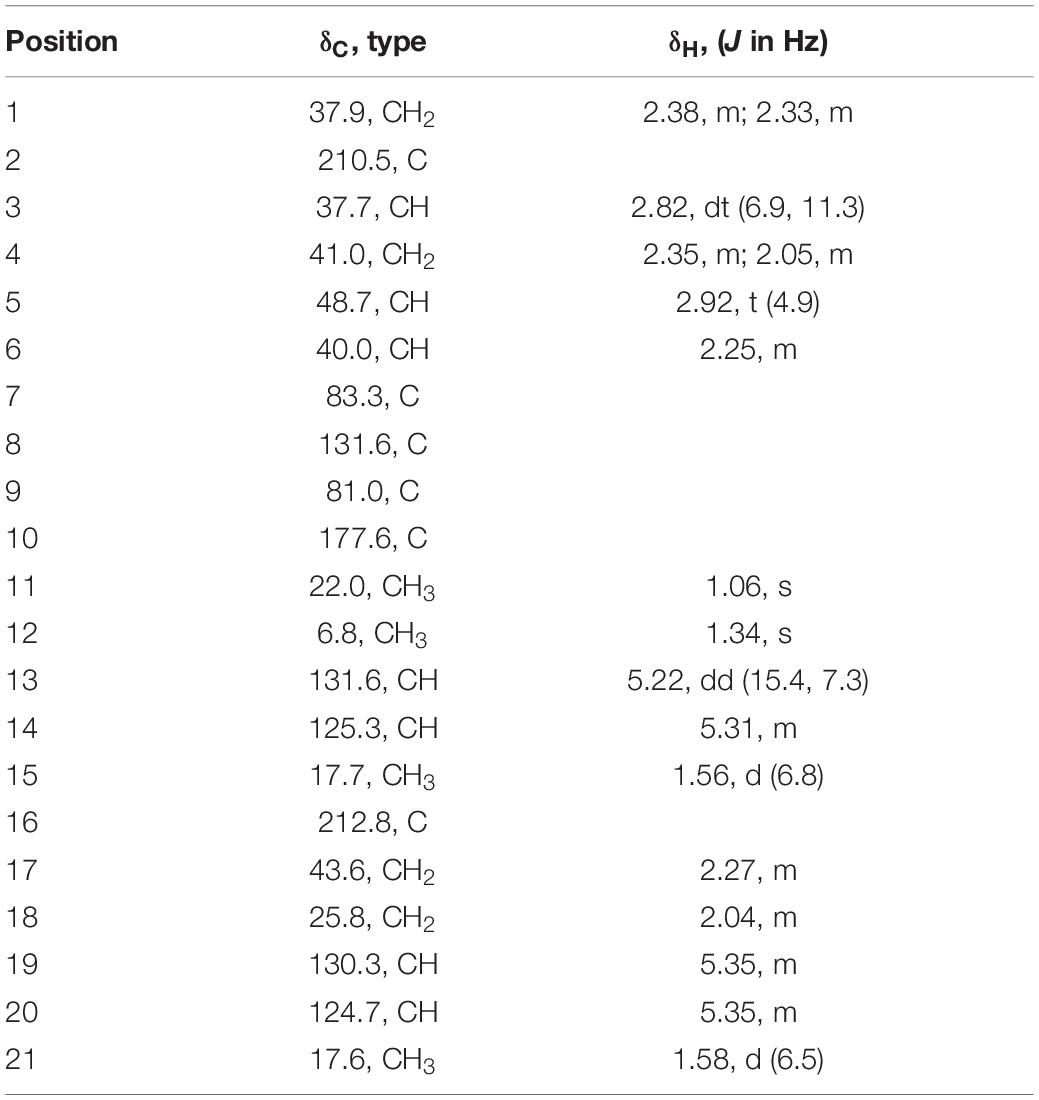
Acremosorbicillinoid A (2): yellow oil; [α]20D + 20.0 (c 0.020, MeOH); UV (MeOH) λmax 232 nm; IR νmax 3,495, 2,928, 2,856, 1,725, 1,593, 1,435, 1,372, 1,231, 1,056, 816 cm–1; 1H and 13C NMR data (see Table 2); HRESIMS m/z 383.1833 [M + Na]+ (calcd. for C21H28O5Na 383.1829).
Acremosorbicillinoid B (3): yellow oil; [α]20D + 7.0 (c 0.033, MeOH); UV (MeOH) λmax 214, 234, 278, 326 nm; IR νmax 3,495, 2,928, 1,724, 1,594, 1,425, 1,380, 1,230, 1,051, 822 cm–1; 1H and 13C NMR data (see Table 3); HRESIMS m/z 277.1053 [M + Na]+ (calcd. for C13H18O5Na 277.1046).
Acremokaloid A (4): yellow oil; [α]20D −0.9 (c 0.033, MeOH); UV (MeOH) λmax 200, 246, 280 nm; IR νmax 3,495, 1,632, 1,534, 1,497, 1,375, 1,285, 1,240, 1,076, 1,010, 682 cm–1; 1H and 13C NMR data (see Table 4); HRESIMS m/z 234.1485 [M + H]+ (calcd. for C14H20NO2 234.1494).
2S,3S-Acetyl-β-methyltryptophan (5): yellow oil; [α]20D + 33.6 (c 0.033, MeOH); UV (MeOH) λmax 196, 248, 290 nm; 1H and 13C NMR data (see Table 5); HRESIMS m/z 261.1234 [M + H]+ (calcd. for C14H17N2O3 261.1234).
Crystallographic data for penicillone B (13). Molecular formula, C14H20O4 (M = 252.30 g/mol), orthorhombic, space group = P212121; unit cell dimensions: a = 8.4852(4) Å, b = 10.2957(5) Å, c = 14.7986(8) Å, V = 1292.82(11) Å3, ρcalcd = 1.296 g/cm3, Z = 4, T = 296.00(2) K, μ(CuKα) = 0.770 mm–1, A total of 9,554 reflections were measured (10.466° ≤ 2Θ ≤ 133.170°) with 2,266 independent reflections (Rint = 0.0539, Rsigma = 0.0457). Final R indexes [I > = 2σ (I)]: R1 = 0.0453, wR2 = 0.1092. Final R indexes [all data]: R1 = 0.0510, wR2 = 0.1130. Largest diff. peak and hole = 0.225 and −0.275 eÅ–3. Flack parameter = −0.02 (15). Crystallographic data (excluding structure factors) for compound 13 in this paper have been deposited with the Cambridge Crystallographic Data Centre as supplementary publication number CCDC 2,081,490.
Biological Assays
The cytotoxicity of the five new compounds (1-5) was tested using the MTT assay (Xu et al., 2018) against the human cancer cell lines (A2870, HepG2, EC109, PC3, and A549) and the human bronchial epithelial cell line. Compounds 1-5 were also evaluated for the antibacterial activities against Gram-positive bacteria Staphylococcus aureus and Bacillus subtilis, and Gram-negative bacteria Pseudomonas aeruginosa and Escherichia coli by using the disk diffusion method (Pfaller and Jones, 2006). All the compounds (1-13) were tested for their quorum sensing (QS) inhibitory activity against Chromobacterium violaceum CV026, using a previously described method (Zhang et al., 2019). All the compounds were also tested against agricultural pathogenic fungi Colletotrichum musae (ACCC 31244), C. coccodes (ACCC 36067), Fusarium solanum, F. oxysporum f. sp. cubense, Cucumber fusarium wilt, Cowpea wilt, F. graminearum, Nectria sp., F. mangiferae, C. asianum, and Alternaria solani., using a disk diffusion and half dilution method (Zhang et al., 2019). Effect of know compounds 6, 7, 8, 11, and 12 on serum-mediated cholesterol efflux and on cell viability in J774A.1 macrophages were also tested (Sankaranarayanan et al., 2011; Wang et al., 2017; Hou et al., 2021).
Results and Discussion
Structural Elucidation of the Isolated Compounds
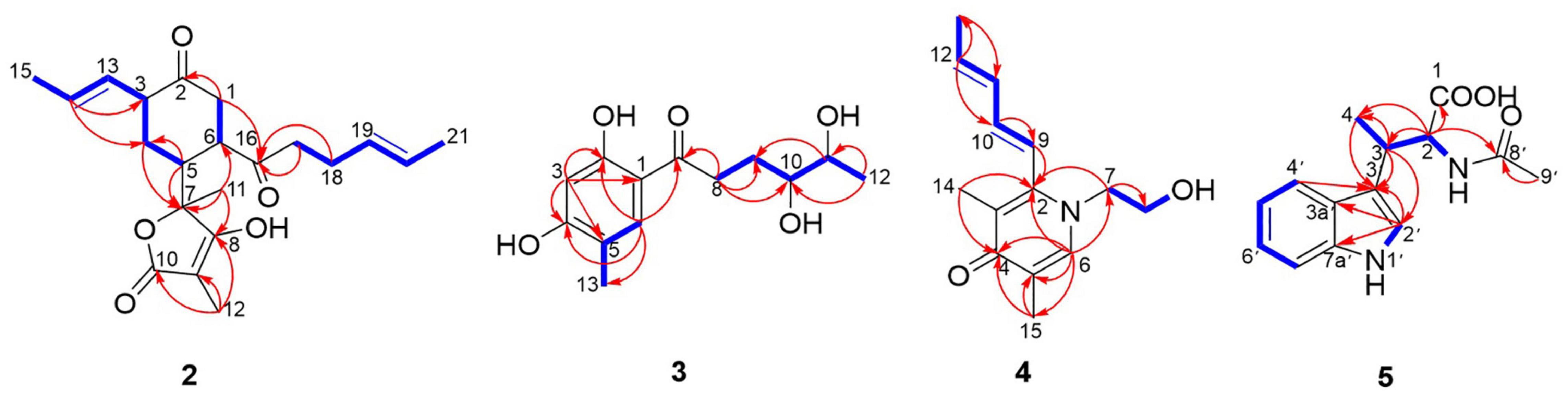
Trisorbicillinone E (1) was isolated as a yellow oil. The infrared (IR) spectrum of 1 (Supplementary Figure 11) indicated that hydroxy and carbonyl signals were present at 3,500 and 1,731 cm–1, respectively. The molecular formula of 1, C42H52O12 with seventeen unsaturation degrees, was established from a positive HRESIMS ion at 749.3521 [M + H]+ (calcd. for 749.3532 C42H53O12) (Supplementary Figure 10). Analysis of the 1D NMR spectroscopic data (Table 1) with the aid of the HSQC spectrum determined that 1 possessed six sp3 methyls (δC 9.9, 24.9, 19.0, 21.1, 18.2, and 21.3), three methyl groups attached to double bonds (δC 18.0, 17.7, and 18.0), five sp3 methines (δC 45.6, 50.6, 41.9, 57.8, and 57.9), and three 1,2-disubstituted double bonds (δC 129.3 and 126.8, 127.8 and 130.4, and 129.1 and 126.7). These structural features were similar to those of trisorbicillinone D (Li et al., 2010; Supplementary Figure 43), implying that 1 was also a sorbicillin trimer. By carefully comparing the 1D NMR data of compound 1 and trisorbicillinone D, two major differences were found. The first one was the absence of four olefinic carbon atoms in 1. This distinct feature indicated that in compound 1, two of the sorbyl side chains from C-9 to C-14 and from C-13′ to C-18′ might be partially hydrogenated. Another difference was the presence of a methyl group (17-CH3) at 1.59 ppm (H-17, 3H, d, J = 6.3 Hz) in 1 in contrast with the methyl group at 0.92 ppm (3H, d, J = 7.2 Hz) in trisorbicillinone D. Additionally, the 1H-1H COSY correlations (Figure 2) of H-4/H-8/H-7/H-15/H-16/3H-17 secured the presence of a prop-1-en-1-yl substituent at C-7 in 1. The different chemical shifts on the methyl mentioned above and the COSY and HMBC correlations (Supplementary Figure 44) suggested the structural changes proposed in compound 1 with respect to trisorbicillinone D.
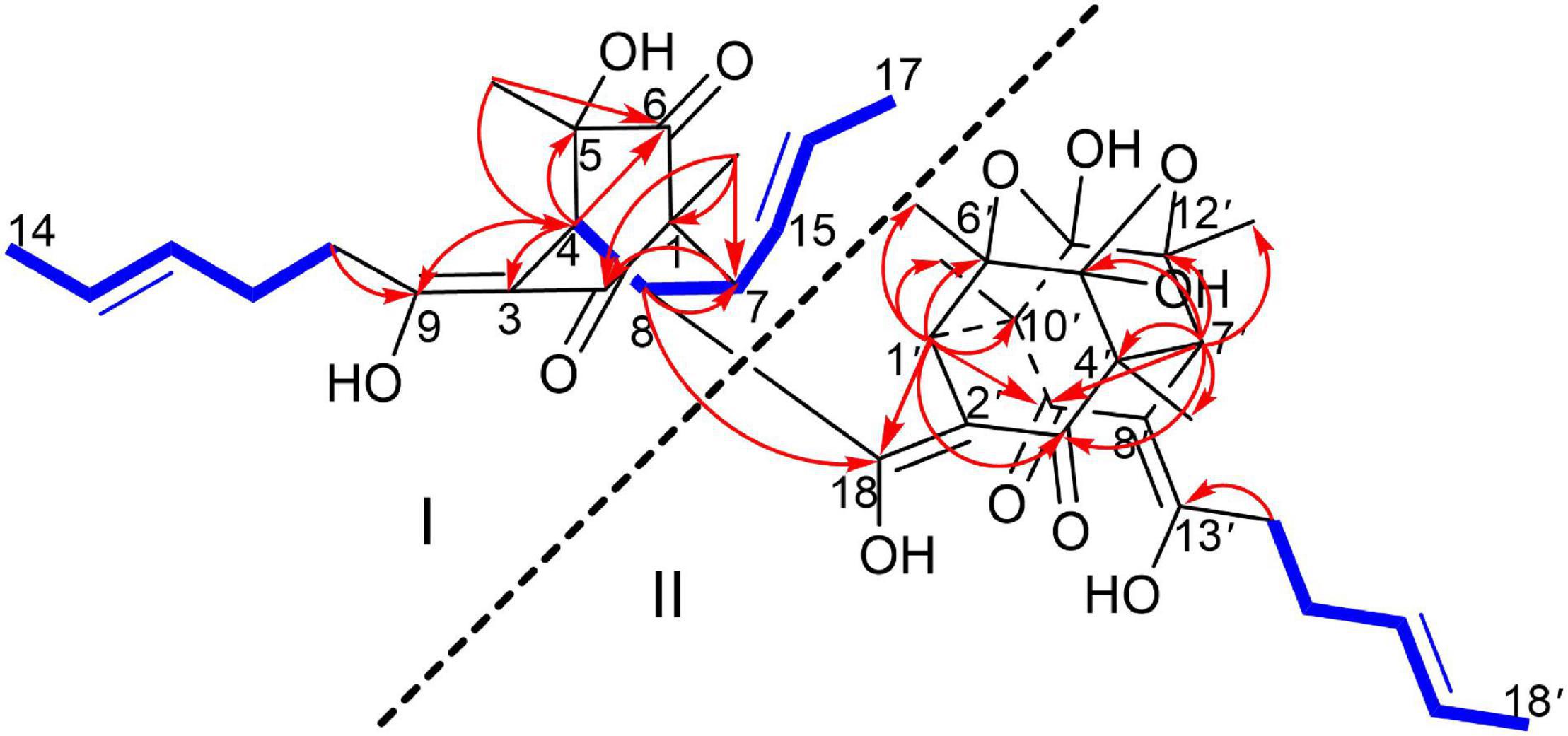
Figure 2. 1H-1H COSY (blue bold lines), Key HMBC (red arrows) correlations of trisorbicillinone E (1).
To unambiguously deduce the planar structure of 1, the HMBC spectrum was analyzed (Figure 2). In fragment I, the key HMBC correlations from CH3-5 (δH 1.26) to C-6 (δC 211.4) and C-4 (δC 45.6), from CH3-1 (δH 1.11) to C-1 (δC 62.3), C-2 (δC 195.7) and C-7 (δC 50.6), from H-7 (δH 2.75) to C-2 (δC 195.7), and from H-4 (δH 3.04) to C-3 (δC 107.8), C-5 (δC 75.8), C-6 and C-9 (δC 181.8) were assigned to an [2,2,2] octane group monomer. In addition, the 1H-1H COSY correlations between 2H-10 and 3H-14 indicated that a partially hydrogenated olefinic carbon chain was present. In fragment II, the HMBC correlations from H-1′ (δH 2.92) to C-3′ (δC 195.1), C-6′ (δC 78.7), C-9′ (δC 193.1), C-10′ (δC 57.6), CH3-6′ (δC 21.1), and CH3-10′ (δC 18.2), and from H-7′ (δH 3.00) to C-3′, C-4′ (δC 57.9), C-5′ (δC 103.9), C-12′ (δC 78.8), C-9′, CH3-4′ (δC 19.0), and CH3-12′ (δC 21.3), revealed that the sorbicillinoid monomer II had an open-ended cage structure (Gao et al., 1995). The 1H-1H COSY correlations between 2H-14′ and 3H-18′ also indicated that there was a partially hydrogenated olefinic carbon chain. The key HMBC correlations from H-8 (δH 3.61) to C-7 (δC 50.6) and C-18 (δC 190.6) and from H-1′ (δH 2.92) to C-18 verified the oxygen-bearing carbon atom C-18 linked between two sorbicillinone moieties (Supplementary Figure 44).
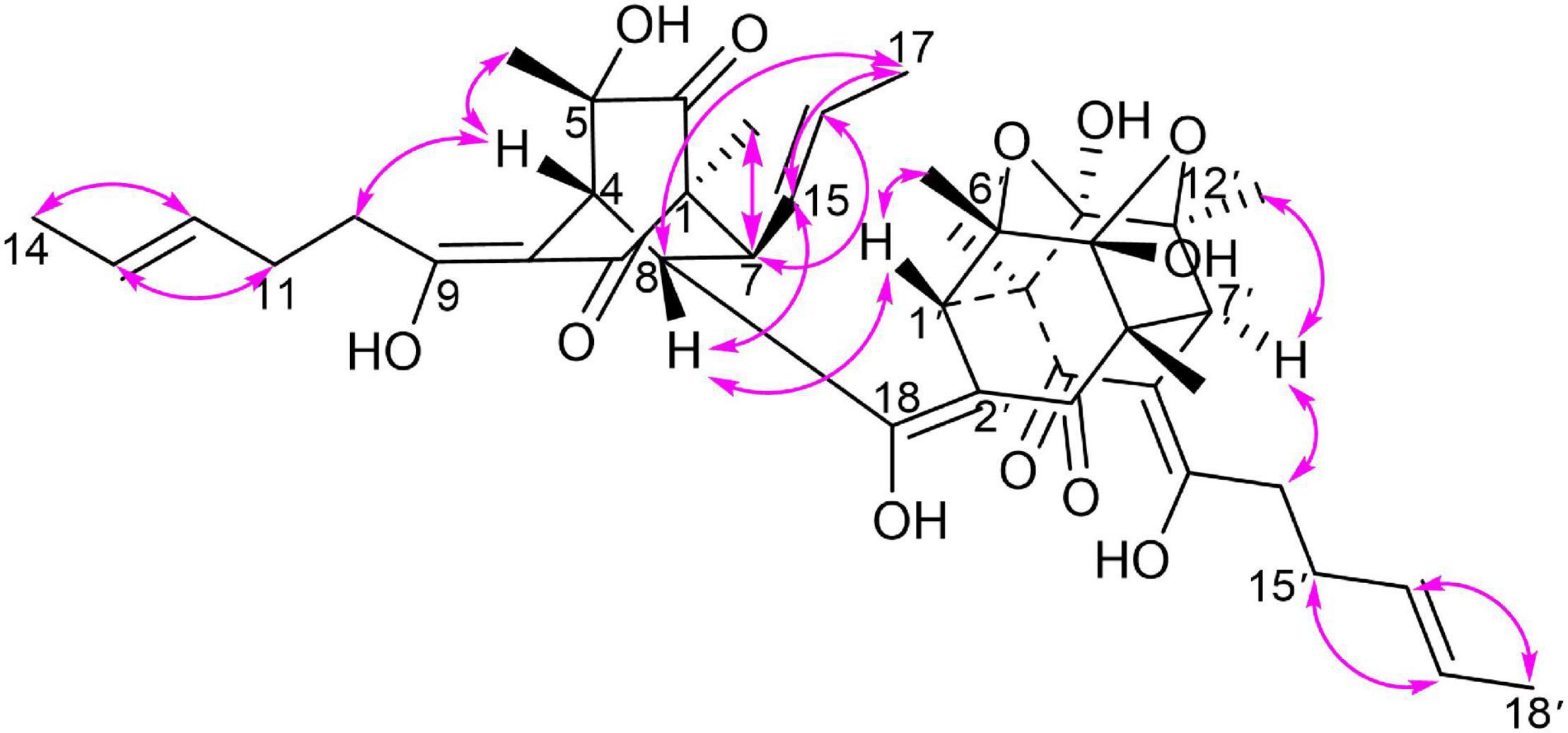
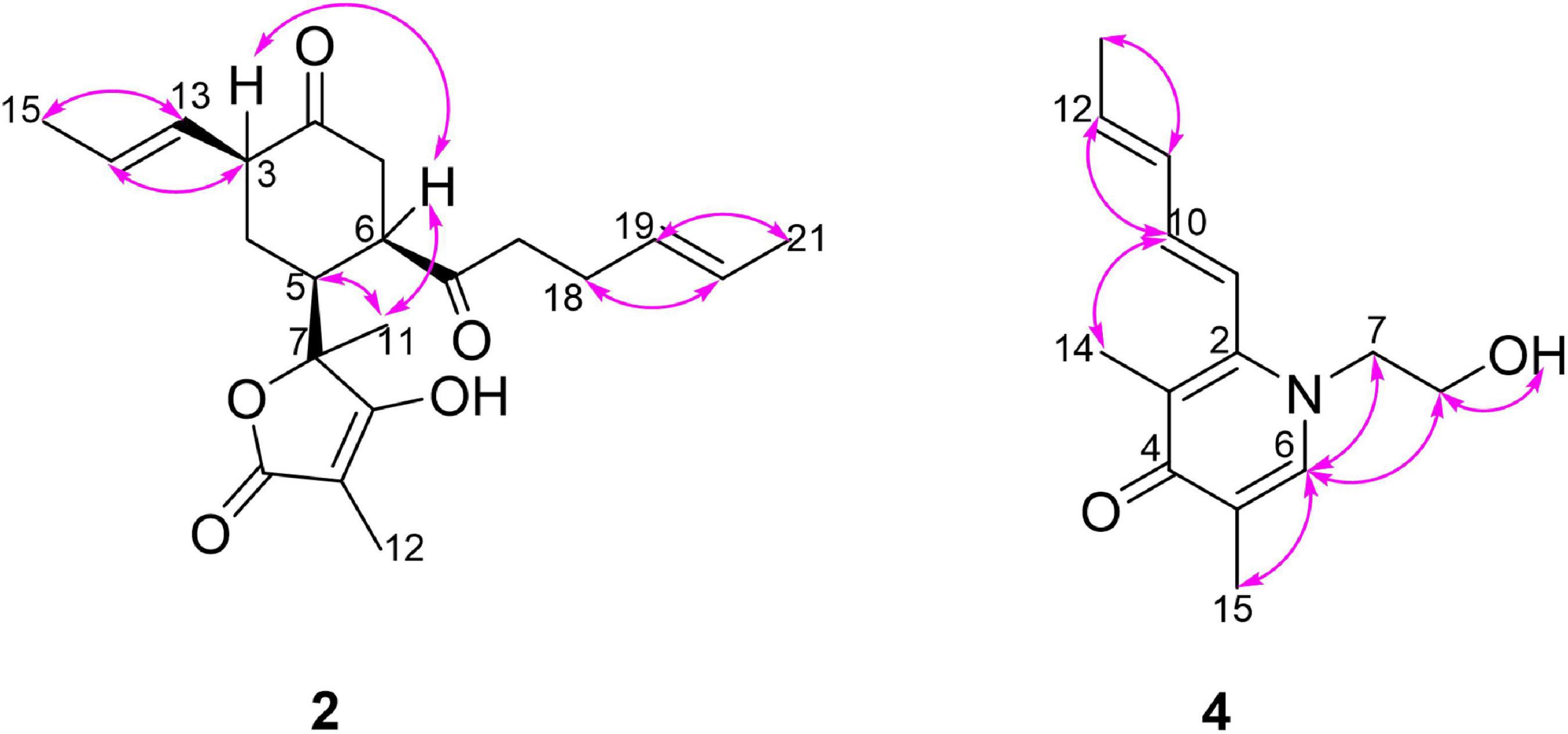
Monomer I was a [2,2,2] octane group, and its relative configuration was established by NOE interactions (Figure 3). On the basis of the NOESY analysis, the cross-peaks of H-8 with H-1′ and H-15 revealed that H-8, H-1′ and H-15 should be on the same side of the molecule, suggesting that Δ18 had a Z configuration. The key NOE correlation observed between H-7 and CH3-1 indicated that they existed in a cis relationship. NOESY correlations of H-4 with CH3-5 and 2H-10 indicated that they all existed on the same side of the bicyclo [2,2,2] octane moiety (Li et al., 2007). These comparisons allowed us to presume the relative configuration of monomer I in 1 as 1S*,4R*,5S*,7S*,8S*. In monomer II, The NOESY correlations were all matched with those of dihydrotrichodimerol (Liu et al., 2005a). In addition, the NOESY correlations of H-4 with 2H-10 and of H-7′ with H-14′ indicated that Δ3 and Δ8′ had Z configurations. The double bond E configurations in the three open carbon chains were deduced by the large coupling constant (3J15,16 = 14.6 Hz) and by the NOESY correlations between H-11 and H-13, H-15 and H-17, and H-15′ and H-17′.
Electronic circular dichroism (ECD) calculations were further employed to determine the absolute configuration of 1 (Supplementary Figure 2). The predicted ECD spectrum was obtained by the TDDFT [mPW1PW91/6–311G(d)] method and was subsequently compared with the experimental data. The calculated ECD spectrum agreed with the experimental curve, confirming the absolute configuration of compound 1 as 1S,4R,5S,7S,8S,1′S,4′R,5′R,6′S,7′S,10′R,11′R,12′S (Figure 4).
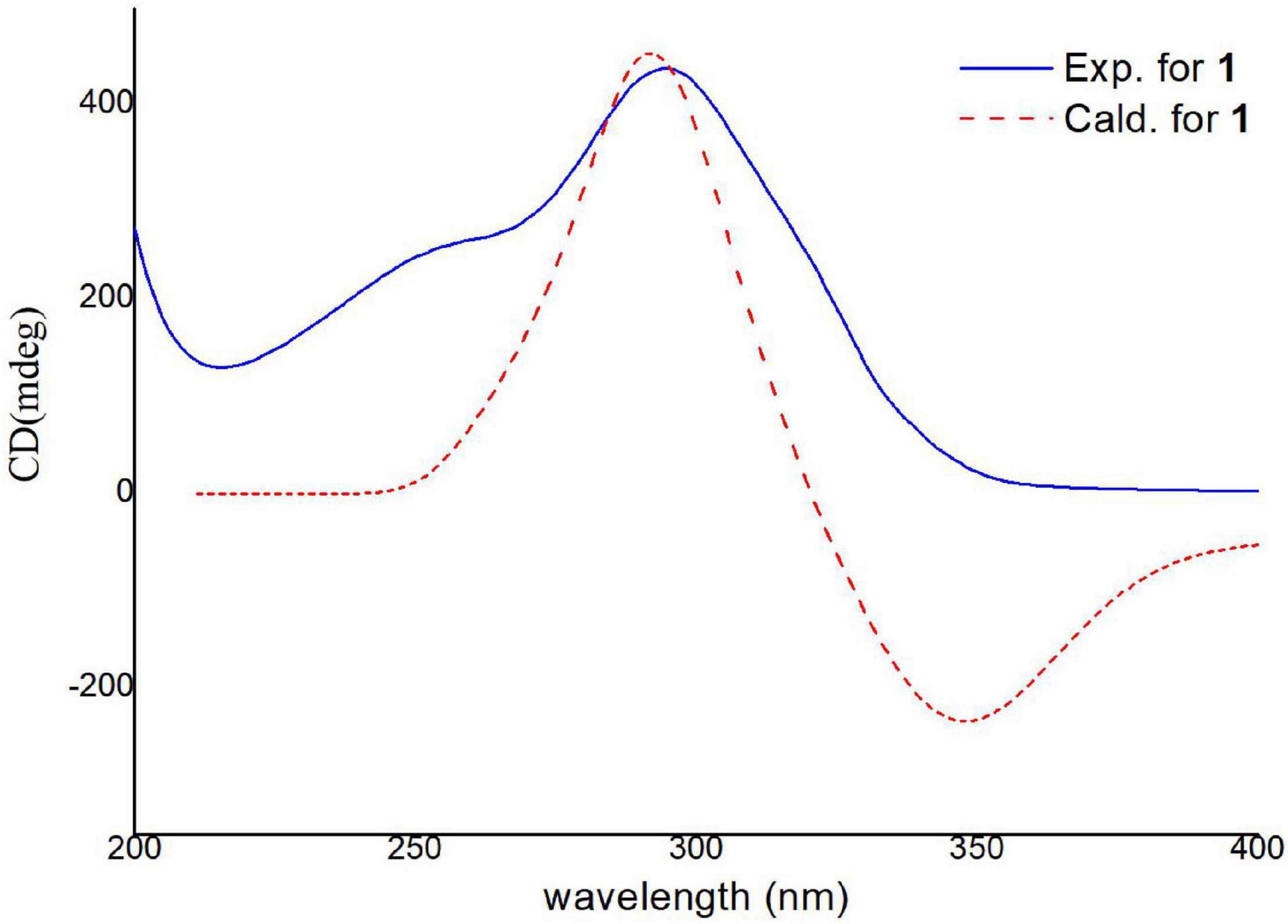
Figure 4. Experimental and calculated ECD spectra of compound 1 (calcd. ECD curves with a bathochromic shift for 20 nm).
For the six reported trisorbicillinoids (Li et al., 2007, 2010; Guo et al., 2013; Cao et al., 2020), trisorbicillinones A, B, C, and D, 10,11,27,28-tetrahydrotrisorbicillinone C, and sorbicillamine E (Supplementary Figure 1), it was not possible to deduce the absolute relations of the whole molecule. The reason was that the two sorbicillinone fragments had long distance and the complex chiral centers. Nevertheless, in compound 1, there was only one carbon atom between two sorbicillinone fragments, and NOESY correlations could be detected within the limits. Hence, combined with the ECD data, trisorbicillinone E (1) was the first trisorbicillinoid compound with the full absolute configuration determined.
Acremosorbicillinoid A (2) was isolated as a yellow oil and had a molecular formula of C21H28O5, as deduced from its positive HRESIMS ion at m/z 383.1833 [M + Na]+ (Supplementary Figure 20), indicating eight degrees of unsaturation. Its IR absorptions at 3,495 and 1,725 cm–1 (Supplementary Figure 21) indicated that hydroxyl and carbonyl groups were present. The 1H NMR spectrum of 2 (Table 2 and Supplementary Figure 12) revealed four methyl proton signals, four methylene proton signals, seven methine signals including four olefinic signals at 5.22–5.35 ppm, and three aliphatic signals at 2.25–2.92 ppm. In the 13C NMR spectrum (Table 2 and Supplementary Figure 13), there were six quaternary carbons. Based on the MS requirements, these data suggested that two rings were present in compound 2. The 1H-1H COSY correlations (Figure 5) between 3H-21/2H-17 and between H-13/3H-15 were assigned to two unsaturated chains. The key HMBC spectrum (Figure 5) of 3H-12 (δH 1.34) with C-10 (δC 177.6), C-9 (δC 81.0), and C-8 (δC 131.6) was expected to be an α,β-unsaturated lactone. The signals from 3H-11 (δH 1.06) to C-8, C-7 (δC 83.3), and C-6 (δC 40.0), from H-5 (δH 2.92) to C-7 and C-4 (δC 41.0), and from 2H-4 (δH 2.35, 2.05) to C-7 revealed that the α,β-unsaturated lactone was located at C-7. The HMBC correlations from 2H-1 (δH 2.38, 2.33) to C-2 (δC 210.5) and C-16 (δC 212.8), from 2H-17 (δH 2.27) and 2H-18 (δH 2.04) to C-16, and from H-14 (δH 5.31) to C-3 (δC 37.7) and C-4 were assigned to a six-membered ketone, with two sorbyl side chains.
The relative configuration of 2 was deduced by the coupling constants and NOESY spectrum (Figure 6). The large coupling constants (3J13,14 = 15.4 Hz) and NOESY correlations (Supplementary Figure 17) between H-13 and H-15, between H-18 and H-20, and between H-19 and H-21 indicated that the Δ13 and Δ19 double bonds had E configurations. The 1D NOE correlations (Supplementary Figure 18) of H-11 with H-5 and H-6 confirmed that they had the same orientation. In addition, in the 1H NMR spectrum, 3J5,6 = 0 Hz eliminated the pseudoaxial orientation of H-5 and H-6 (Isaka et al., 2001). The NOE cross-peak (Supplementary Figure 19) between H-3/H-6 revealed that H-3 and H-6 were on the same side. The relative configuration of the four stereocenters of 2 was determined to be 3S*,5S*,6R*,7S*. Furthermore, ECD calculations were employed to determine the absolute configuration (Supplementary Figure 2). The calculated ECD spectrum of 2 was obtained by the TDDFT [mPW1PW91/6–311G(d)] method and was subsequently compared with the experimental spectrum (Figure 7). The results revealed that the absolute configuration of compound 2 was 3S,5S,6R,7S.
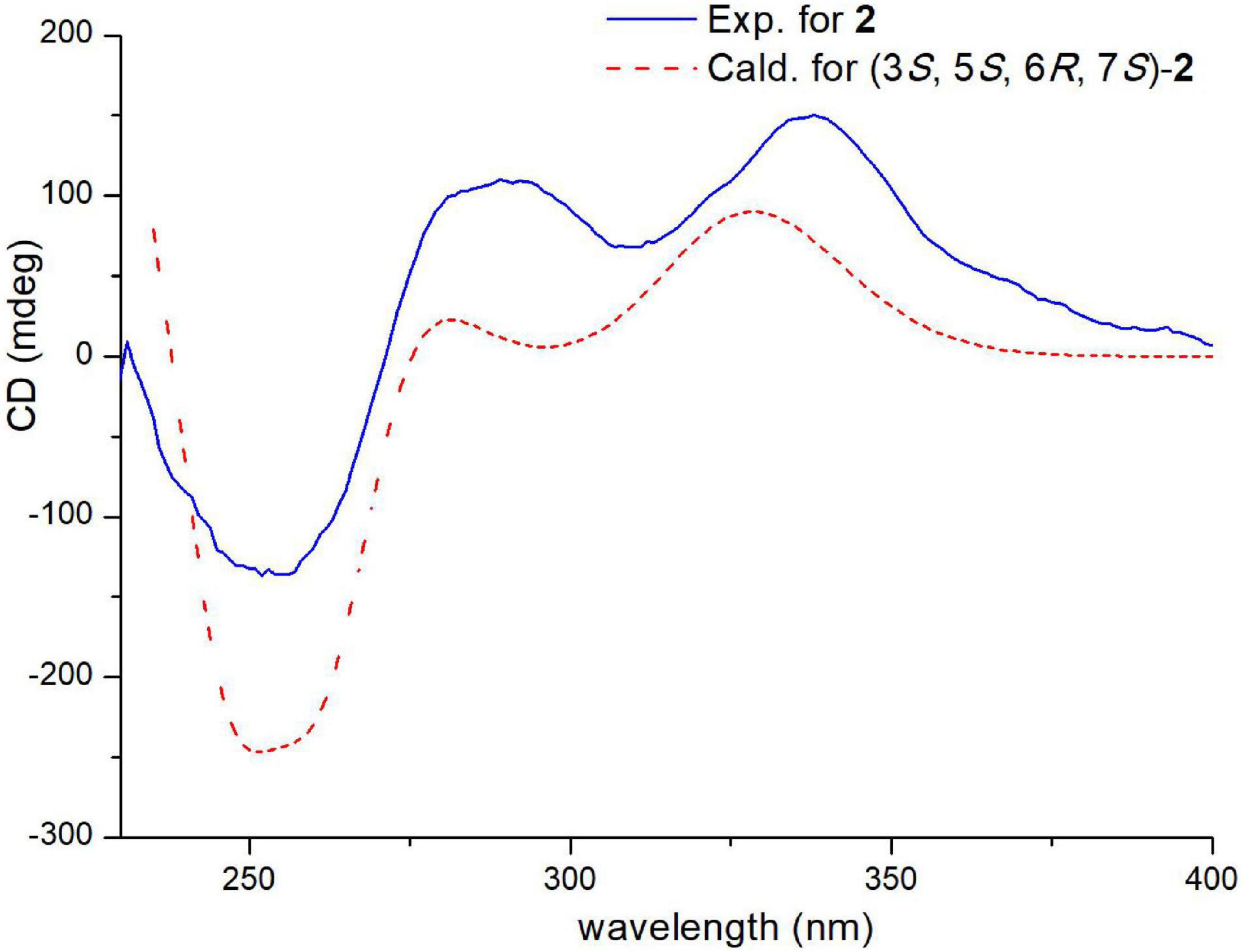
Figure 7. Experimental and calculated ECD spectra of compound 2 (calcd. ECD curves with a bathochromic shift for 45 nm).
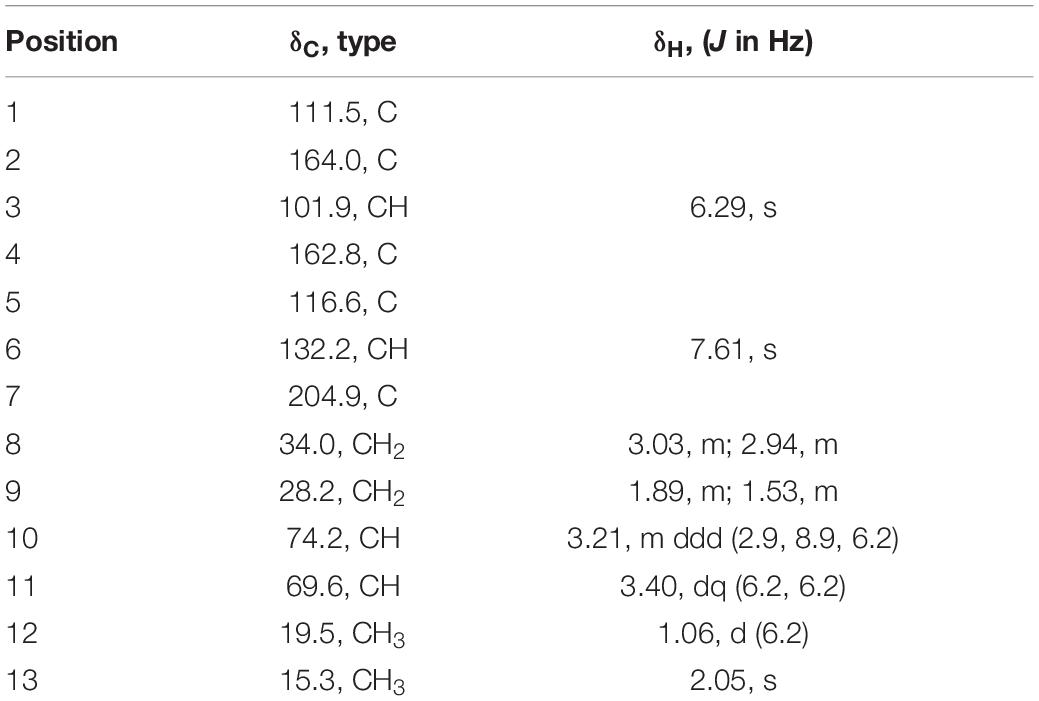
Acremosorbicillinoid B (3), a yellow oil, was determined to have a molecular formula of C13H18O5, with five indices of hydrogen deficiency based on HRESIMS analysis (ion peak at m/z 277.1053 [M + Na]+) (Supplementary Figure 27). Its IR spectrum displayed absorptions that were attributed to hydroxy (3,495 cm–1), carbonyl (1,724 cm–1), and aromatic (1,594, 1,425 cm–1) groups. The 1H NMR data (Table 3) displayed signals for two para-oriented aromatic singlet hydrogens (δH 6.29, 7.61), two methylene groups (δH 3.03, 2.94, 1.89, 1.53), two oxygenated methine groups (δH 3.21, 3.40), and two methyl signals (δH 1.06, 2.05). The 13C NMR and HSQC data resolved 13 carbon signals attributable to six aromatic carbons (δC 101.9-164.0), including two oxygenated aromatic carbon atoms (δC 164.0, 162.8). One carbonyl group (δC 204.9), two oxygenated secondary carbons (δC 74.2, 69.6), two methylene groups (δC 34.0, 28.2), and two methyl carbons (δC 19.5, 15.3) were also observed. A carbochain with two hydroxy groups, located at C-10 and C-11, was established according to the HMBC correlations (Figure 5) from 2H-8 (δH 3.03, 2.94) to C-7 (δC 204.9), C-9 (δC 28.2), and C-10 (δC 74.2), from 3H-12 (δH 1.06) to C-11 (δC 69.6), and C-10, and from H-11 (δH 3.40) to C-9, as well as the 2H-8/2H-9/H-10/H-11/3H-12 spin system observed in the 1H-1H COSY spectrum. Additionally, the HMBC signals from H-6 (δH 7.61) to C-2 (δC 164.0), C-4 (δC 162.8), C-7, and C-13 (δC 15.3) and from H-3 (δH 6.29) to C-1 (δC 111.5), C-2, C-4, and C-5 (δC 116.6) indicated the four replacement positions of the benzene derivatives.
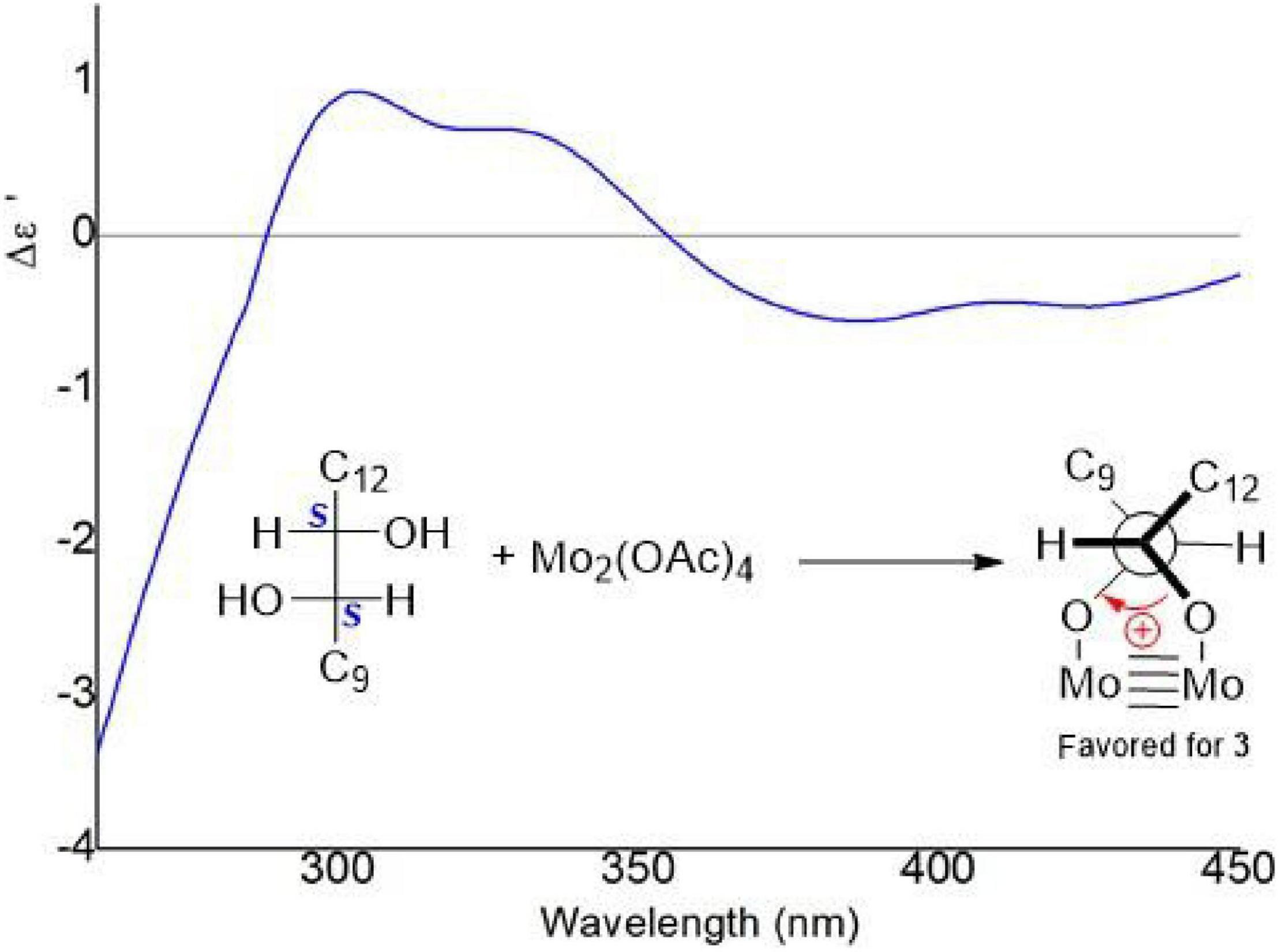
The vicinal diolsabsolute configurations in 3 were revealed by dimolybdenum tetraacetate [Mo2 (OAc)4]-induced CD (ICD, Snatzke’s method). In the ICD spectra, the Mo2 complex of 3 in DMSO gave a positive Cotton effect at approximately 310 nm (Figure 8). By the helicity rule of the Snatzke’s method, the torsional angle sign exhibited the signs of particular Cotton effects (Di Bari et al., 2001; Frelek et al., 2008), and the positive sign of the band was determined by the clockwise O–C–C–O torsional angle (the positive sign of the torsional angle). Since the coupling of H-10 and H-11 (3J = 6.18 Hz) indicated that the 10,11-diols in 3 had threo relative configuration (Jarvis et al., 1982, 1987), the conformation in the Mo2-complexe of 3 was preferred as determined by the ICD investigation (Figure 8). Thus, the absolute configurations of 3 at C-10 and C-11 could be assigned as 10S and 11S, respectively. Acremosorbicilliooid B (3) is a monomeric sorbicillinoid compound that is similar to sorbicillin (Supplementary Figure 43), and sorbicillin is the first member of the sorbicillinoid family (Cram, 1948; Cram and Tishler, 1948).
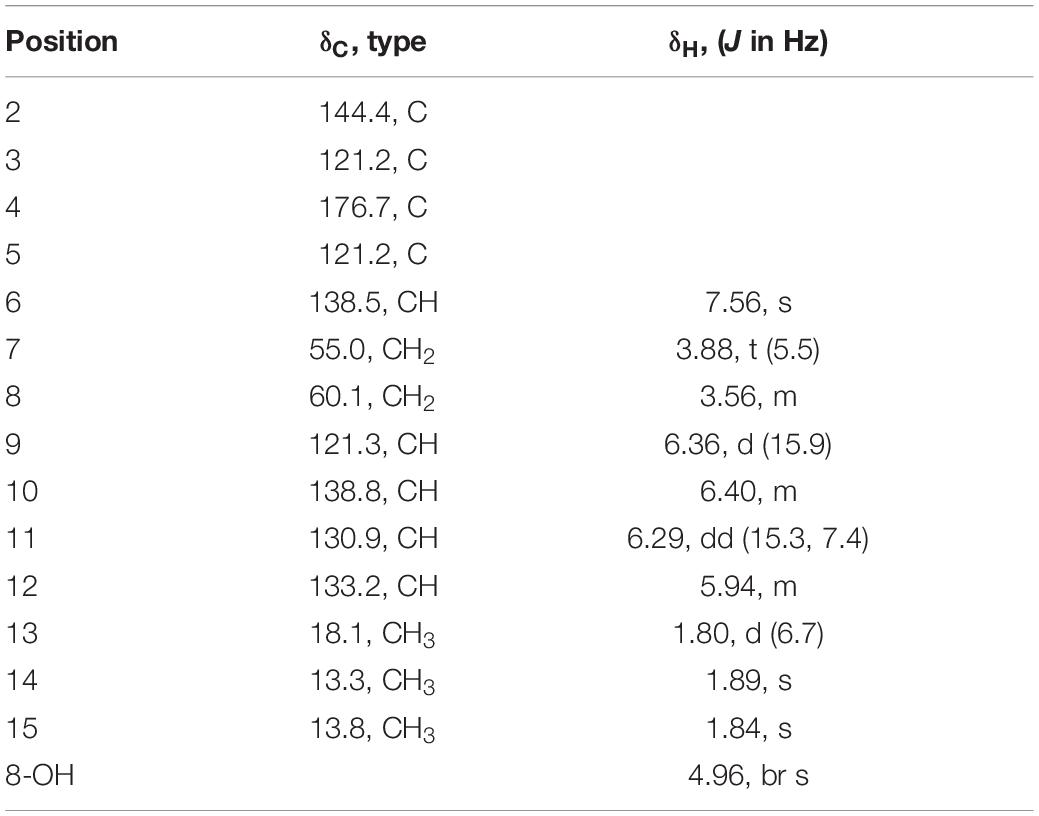
Acremosorbicillinoid C (4) was obtained as yellow oil. The molecular formula C14H19NO2 was established by the positive HRESIMS ion at m/z 234.1485 [M + H]+ (Supplementary Figure 35). The IR absorption at approximately 3,495 cm–1 was attributed to hydroxyl groups, while the peaks at 1,632 and 1,534 cm–1 implied the presence of ketones. Six degrees of unsaturation were indicated in addition to the 1H and 13C NMR data (Table 4). The 1H NMR spectrum of 4 displayed two singlet methyl signals, one doublet methyl signal, two oxygenated or nitrogenated methylene proton signals, five olefinic methine proton signals, and an exchangeable hydrogen proton. The 13C NMR spectrum showed fourteen carbons including one carbonyl signal, eight olefinic carbons, two methylenes, and three methyls. As one carbonyl and eight olephinic carbons accounted for five degrees of unsaturation, compound 4 was inferred to have a ring structure. Based on the 1H-1H COSY data, the correlative signal (Figure 5) between H-9 and 3H-13 suggested that a hydrogenated olefinic carbon chain was present. The key HMBC signal from H-9 (δH 6.36) to C-2 (δC 144.4) indicated that the pentadiene group fragment should be connected to C-2. The positions of two methyls, one carbonyl unit and one ethoxy, were established by the key HMBC connections of 3H-14/C-2 and C-4, of 3H-15/C-4, and C-5, of H-6/C-2, C-4, C-5, C-7 and C-15, and of 2H-7/C-2 and C-8. The coupling constants (3J9,10 = 15.9 Hz, 3J11,12 = 15.3 Hz), and the NOESY correlation (Figure 6) between H-11 and H-13 indicated that the two ethylenic bonds in the pentadiene group had E configurations.
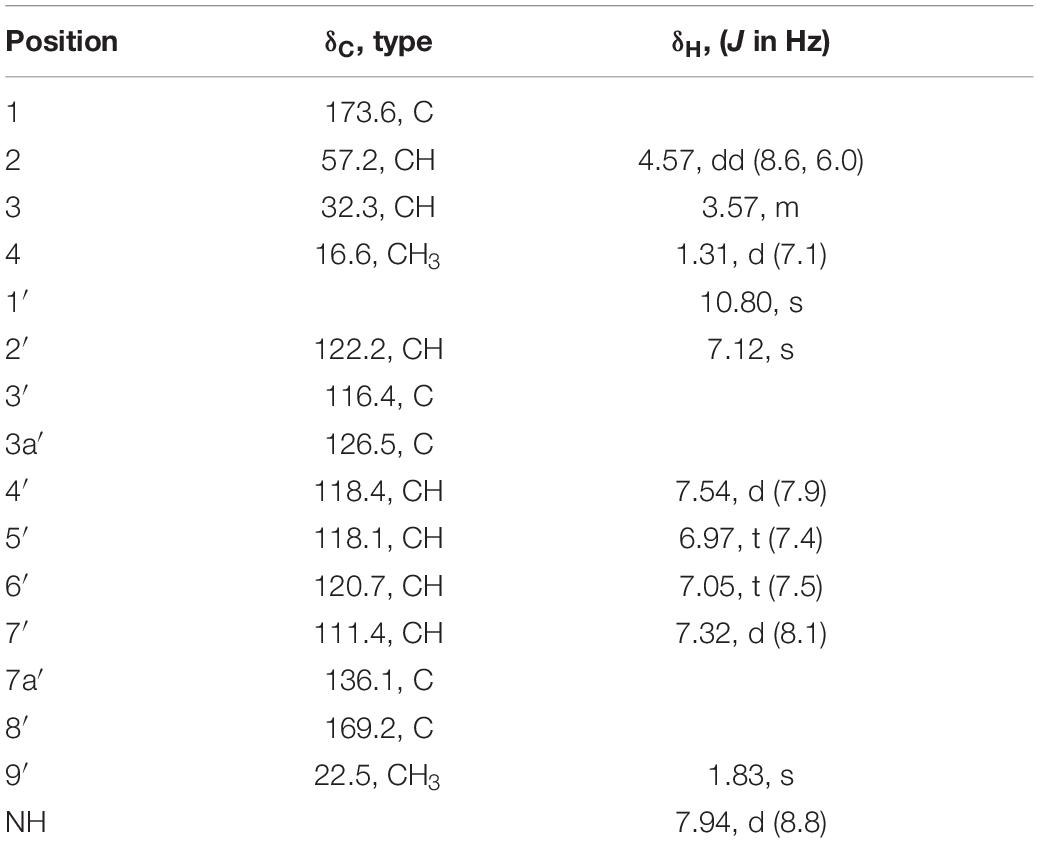
Acetyl-β-methyltryptophan (5) was obtained as a colorless needle crystal. Its molecular formula was determined to be C14H16N2O3 according to the HRESIMS peak at m/z 261.1234 [M + H]+ (Supplementary Figure 42). The combined 1D NMR spectroscopic data (Table 5) showed that compound 5 contained two carbonyl groups (C-1 and C-8′), two methyl groups (C-4 and C-9′), and an indol ring. The key HMBC connections (Figure 5) of H-2′/C-3′, H-2′/C-3a′, H-2′/C-7a′, and H-4′/C-3′ confirmed the existence of the indole moiety. The key HMBC correlations from H-2 (δH 4.57) and H-9′ (δH 1.83) to C-8′ (δC 169.2), from H-2 to C-1 (δC 173.6), C-3 (δC 32.3), and C-4 (δC 16.6), and from H-3 (δH 3.57) to C-4 indicated an amide carbon chain group was present in compound 5. The side chain connection point was C-3′, which was deduced by the HMBC correlations from H-3 to C-2′ (δC 122.2) and C-3′ (δC 116.4), and from H-4 to C-3′.
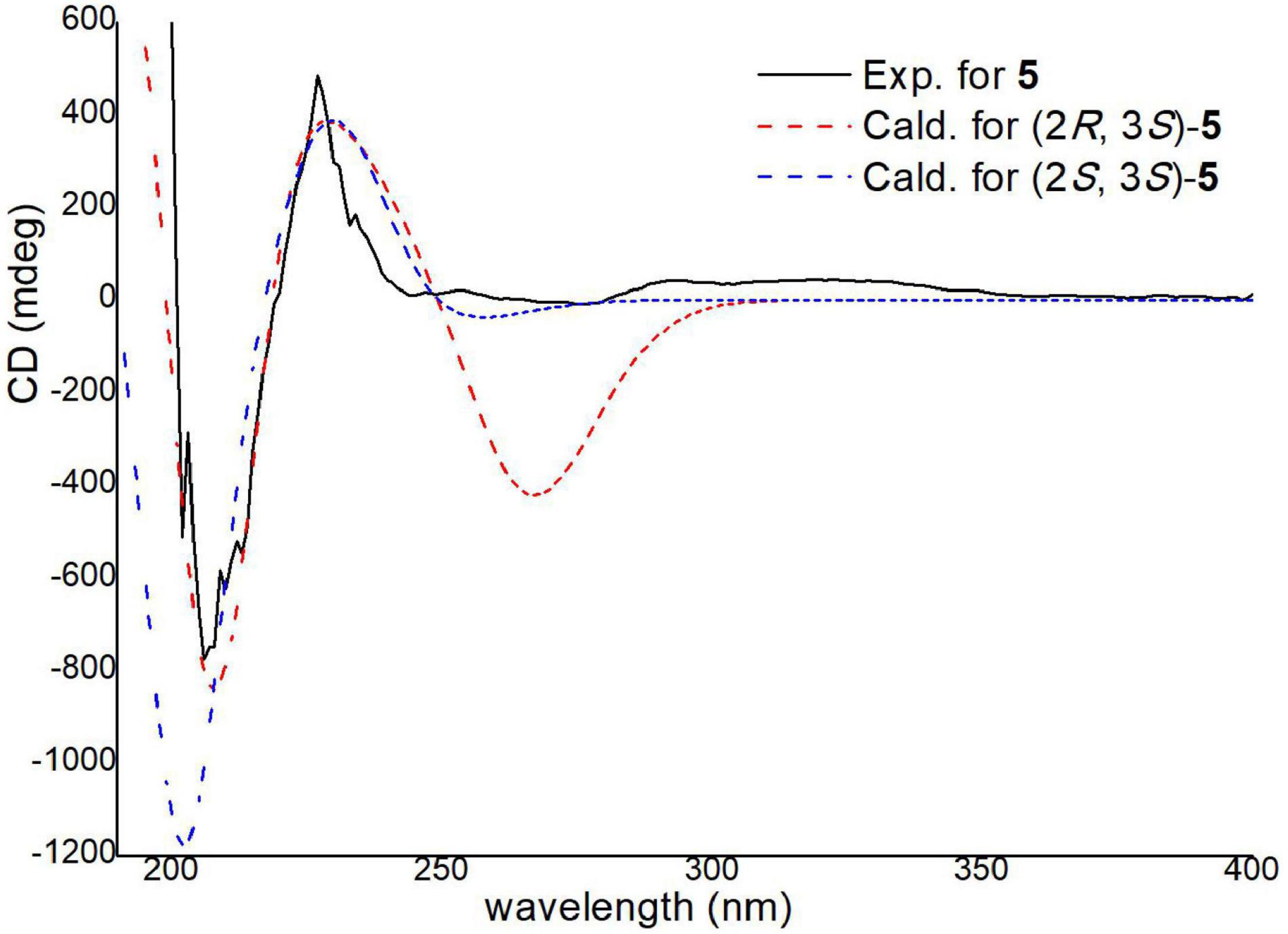
The absolute configurations of C-2 and C-3 were determined by ECD calculations (Supplementary Figure 2). The predicted ECD spectra of 2S,3R-5 and 2S,3S-5 were obtained by the TDDFT [mPW1PW91/6–311G(d)] method, and were compared with the experimental data (Figure 9). The 2S,3S calculated ECD spectrum agreed well with the experimental curve, confirming the absolute configuration of compound 5 as 2S,3S. Acetyl-β-methyltryptophan was recorded as an intermediate product to synthetize β-methyltryptophan (Snyder and Matteson, 1957). Moreover, 2S,3S-acetyl-β-methyltryptophan (5) was first obtained as a natural product from a natural source.
The structures of known compounds 6–13 were established by comparing their NMR data with previously reported data (Andrade et al., 1992; Shirota et al., 1997; Liu et al., 2005b,c; Yu et al., 2019). In addition, crystals of penicillones B (13) that were suitable for X-ray diffraction were obtained from the solvent CH3OH. A single-crystal X-ray crystallographic analysis of 13 conducted with Cu Kα radiation [Flack parameter of −0.02 (15)] (Supplementary Figure 45) first determined the challenging absolute configuration of 13.
Activity
None of the five new compounds (1-5) exhibited cytotoxic effects against the five human tumor cell lines and HBE cell line (IC50 > 40 μM), or any antimicrobial activities (MIC > 4 μg/well). None of the thirteen compounds (1-13) showed antifungal activities against agricultural pathogenic fungi (MIC > 40 μg/well), or QS inhibitory activity against C. violaceum CV026 (MIC > 40 μg/well). Know compound 11 exhibited cholesterol efflux enhancing activity (Supplementary Figure 46).
Conclusion
In this study, three new sorbicillinoids, trisorbicillinone E (1), acremosorbicillinoids A (2) and B (3), and two new compounds, acremokaloid A (4), and 2S,3S-acetyl-β-methyltryptophan (5), together with eight known sorbicillinoids (6-13), were isolated from the endophytic fungus A. citrinum SS-g13. Extensive NMR spectroscopic analysis and ECD calculations were used to elucidate the structures of the new molecules, including their absolute configurations. Compound 1, as the first trisorbicillinoid compound with an established absolute configuration, belongs to the trimeric sorbicillinoids family and is rare in nature. A plausible biosynthesis pathway with monomeric sorbicillinoid, bisorbicillinoid, trisorbicillinoid, and hybrid sorbicillinoid, four different structural types is proposed in Scheme 1. Sorbicillinol was hypothesized as a precursor of most sorbicillinoids. Sorbicillinoids were hypothesized as biosynthesized by polyketide synthases (PKs), and were proposed to be synthesized through intermolecular Diels-Alder, Michael dimerization reactions, and epoxidation. These theories are supported by literature precedence (Harned and Volp, 2011; Pang et al., 2021).
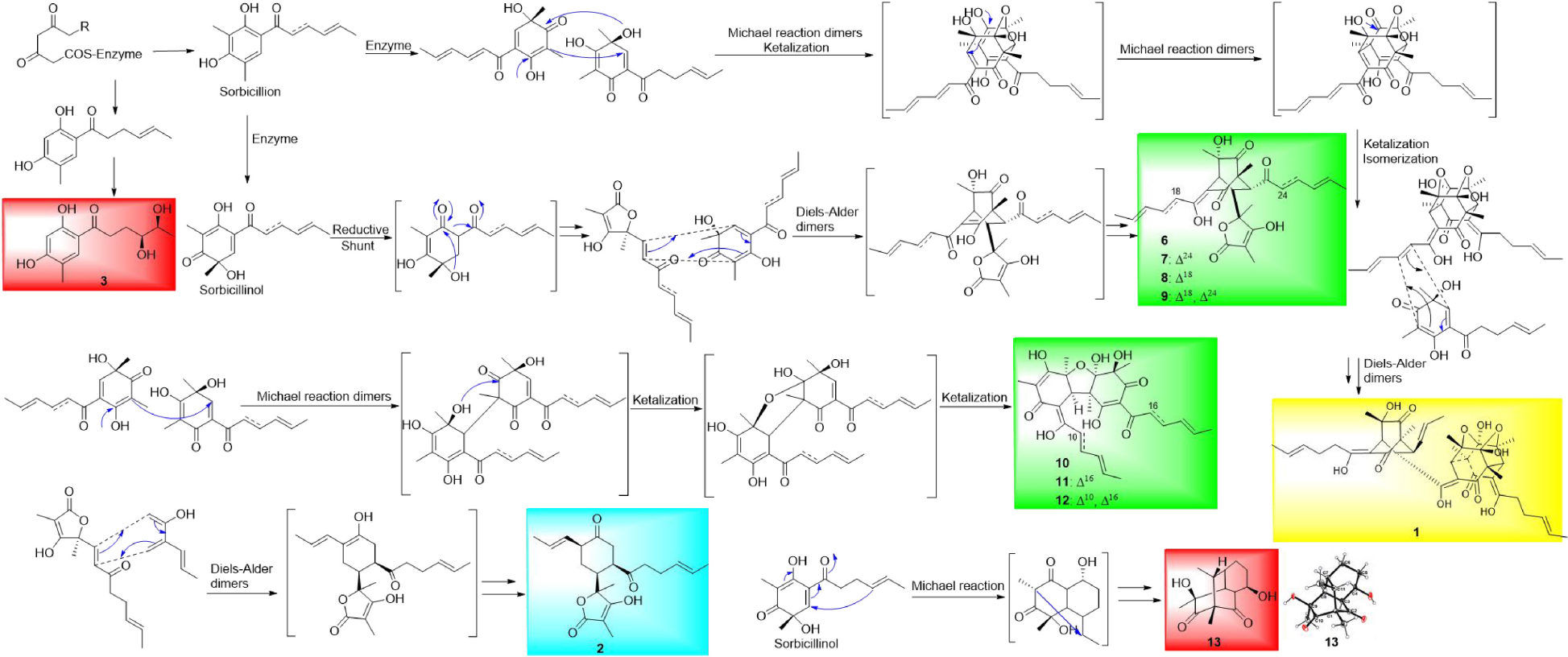
Scheme Plausible Biosynthetic Pathways of Monomeric Sorbicillinoids (red shaded), Bisorbicillinoids (green shaded), Trisorbicillinoid (yellow shaded), and Hybrid Sorbicillinoid (blue shaded).
Data Availability Statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/Supplementary Material.
Author Contributions
X-PP performed the isolation, purification, and structural characterization of all the compounds, and prepared the manuscript. GL and PW performed the ECD calculations. CW and QW contributed to the isolation of compounds and revised the manuscript. L-MW, Z-QH, and L-XJ contributed to the bioactivity evaluation. G-FL contributed to the physical constant determination of compounds. Z-YJ and C-XC contributed to the isolation and identification of the fungal strain. H-XL designed the research, and revised the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the National Natural Science Foundation of China (Grant Nos. 41706077, 81903494, and 41906030), China Postdoctoral Science Foundation (Grant No. 2019M652309), and the Specific Research Project of Guangxi for research bases and talents (Grant No. AD18126005).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmicb.2022.800626/full#supplementary-material
References
Andrade, R., Ayer, W. A., and Mebe, P. P. (1992). The metabolites of Trichoderma longibrachiatum. Part 1. Isolation of the metabolites and the structure of trichodimerol. Can. J. Chem. 70, 2526–2535. doi: 10.1139/v92-320
Cao, M.-J., Zhu, T., Liu, J.-T., Ouyang, L., Yang, F., and Lin, H. W. (2020). New sorbicillinoid derivatives with GLP-1R and eEF2K affinities from a sponge-derived fungus Penicillium chrysogenum 581F1. Nat. Prod. Res. 34, 2880–2886. doi: 10.1080/14786419.2019.1596099
Chakrabarty, S., Romero, E. O., Pyser, J. B., Yazarians, J. A., and Narayan, A. R. H. (2021). Chemoenzymatic total synthesis of natural products. Acc. Chem. Res. 54, 1374–1384. doi: 10.1021/acs.accounts.0c00810
Cram, D. J. (1948). Mold metabolites. II. The structure of sorbicillin, a pigment produced by the mold Penicillium notatum. J. Am. Chem. Soc. 70, 4240–4243. doi: 10.1021/ja01192a077
Cram, D. J., and Tishler, M. (1948). Mold metabolites. I. Isolation of several compounds from clinical penicillin. J. Am. Chem. Soc. 70, 4238–4239. doi: 10.1021/ja01192a076
Di Bari, L., Pescitelli, G., Pratelli, C., Pini, D., and Salvadori, P. (2001). Determination of absolute configuration of acyclic 1,2-diols with Mo2(OAc)4. 1. Snatzke’s method revisited. J. Org. Chem. 66, 4819–4825. doi: 10.1021/jo010136v
Frelek, J., Ruskowska, P., Suszczynska, A., Szewczyk, K., Osuch, A., Jarosz, S., et al. (2008). Configurational assignment of sugar erythro-1,2-diols from their electronic circular dichroism spectra with dimolybdenum tetraacetate. Tetrahedron: Asymmetry 19, 1709–1713. doi: 10.1016/j.tetasy.2008.06.029
Gao, Q., Leet, J. E., Thomas, S. T., Matson, J. A., and Bancroft, D. P. (1995). Crystal structure of trichodimerol. J. Nat. Prod. 58, 1817–1821. doi: 10.1021/np50126a003
Guo, W., Peng, J., Zhu, T., Gu, Q., Keyzers, R. A., and Li, D. (2013). Sorbicillamines A-E, nitrogen-containing sorbicillinoids from the deep-sea-derived fungus Penicillium sp. F23-2. J. Nat. Prod. 76, 2106–2112. doi: 10.1021/np4006647
Harned, A. M., and Volp, K. A. (2011). The sorbicillinoid family of natural products: isolation, biosynthesis, and synthetic studies. Nat. Prod. Rep. 28, 1790–1810. doi: 10.1039/c1np00039j
Hou, X., Zhang, C., Wang, L., and Wang, K. W. (2021). Natural piperine improves lipid metabolic profile of high-fat diet-fed mice by upregulating SR-B1 and ABCG8 transporters. J. Nat. Prod. 84, 373–381. doi: 10.1021/acs.jnatprod.0c01018
Hu, Z., Ye, Y., and Zhang, Y. (2021). Large-scale culture as a complementary and practical method for discovering natural products with novel skeletons. Nat. Prod. Rep. 38, 1775–1793. doi: 10.1039/d0np00069h
Isaka, M., Jaturapat, A., Rukseree, K., Danwisetkanjana, K., Tanticharoen, M., and Thebtaranonth, Y. (2001). Phomoxanthones A and B, novel xanthone dimers from the endophytic fungus Phomopsis species. J. Nat. Prod. 64, 1015–1018. doi: 10.1021/np010006h
Jarvis, B. B., Comezoglu, S. N., Rao, M. M., Pena, N. B., Boettner, F. E., Williams, T. M., et al. (1987). Isolation of macrocyclic trichothecenes from a large-scale extract of Baccharis megapotamica. J. Org. Chem. 52, 45–56. doi: 10.1021/jo00377a007
Jarvis, B. B., Stahly, G. P., Pavanasasivam, G., Midiwo, J. O., DeSilva, T., and Holmlund, C. E. (1982). Isolation and characterization of the trichoverroids and new roridins and verrucarins. J. Org. Chem. 47, 1117–1124. doi: 10.1021/jo00345a044
Kahlert, L., Bassiony, E. F., Cox, R. J., and Skellam, E. J. (2020). Diels-Alder reactions during the biosynthesis of sorbicillinoids. Angew. Chem. 132, 2–9. doi: 10.1002/anie.201915486
Li, D., Cai, S., Zhu, T., Wang, F., Xiao, X., and Gu, Q. (2010). Three new sorbicillin trimers, trisorbicillinones B, C, and D, from a deep ocean sediment derived fungus, Phialocephala sp. FL30r. Tetrahedron 66, 5101–5106. doi: 10.1016/j.tet.2010.04.111
Li, D., Wang, F., Xiao, X., Fang, Y., Zhu, T., Gu, Q., et al. (2007). Trisorbicillinone A, a novel sorbicillin trimer, from a deep sea fungus, Phialocephala sp. FL30r. Tetrahedron Lett. 48, 5235–5238. doi: 10.1016/j.tetlet.2007.05.134
Liu, W., Gu, Q., Zhu, W., Cui, C., and Fan, G. (2005b). Two new benzoquinone derivatives and two new bisorbicillinoids were isolated from a marine-derived fungus Penicillium terrestre. J. Antibiot. 58, 441–446. doi: 10.1038/ja.2005.57
Liu, W., Gu, Q., Zhu, W., Cui, C., and Fang, Y. (2005c). Penicillones A and B, two novel polyketides with tricyclo [5.3.1.03,8] undecane skeleton, from a marine-derived fungus Penicillium terrestre. Tetrahedron Lett. 46, 4993–4996. doi: 10.1016/j.tetlet.2005.05.087
Liu, W., Gu, Q., Zhu, W., Cui, C., and Fan, G. (2005a). Dihydrotrichodimerol and tetrahydrotrichodimerol, two new bisorbicillinoids, from a marine-derived Penicillium terrestre. J. Antibiot. 58, 621–624. doi: 10.1038/ja.2005.85
Meng, J., Wang, X., Xu, D., Fu, X., Zhang, X., Lai, D., et al. (2016). Sorbicillinoids from fungi and their bioactivities. Molecules 21:715. doi: 10.3390/molecules21060715
Pang, X., Zhou, X., Lin, X., Yang, B., Tian, X., Wang, J., et al. (2021). Structurally various sorbicillinoids from the deep-sea sediment derived fungus Penicillium sp. SCSIO06871. Bioorg. Chem. 107:104600. doi: 10.1016/j.bioorg.2020.104600
Peng, X.-P., Li, G., Ji, L.-X., Li, Y.-X., and Lou, H.-X. (2020). Acrepyrone A, a new γ-pyrone derivative from an endophytic fungus, Acremonium citrinum SS-g13. Nat. Prod. Res. 34, 1091–1096. doi: 10.1080/14786419.2018.1548462
Pfaller, M. A., and Jones, R. N. (2006). Performance accuracy of antibacterial and antifungal susceptibility test methods: report from the college of American Pathologists microbiology surveys program (2001-2003). Arch. Pathol. Lab. Med. 130, 767–778. doi: 10.5858/2006-130-767-PAOAAA
Sankaranarayanan, S., Kellner-Weibel, G., Llera-Moya, M., Phillips, M. C., Asztalos, B. F., Bittman, R., et al. (2011). A sensitive assay for ABCA1-mediated cholesterol efflux using BODIPY-cholesterol. J. Lipid. Res. 52, 2332–2340. doi: 10.1194/jlr.D018051
Shirota, O., Pathak, V., Hossain, C. F., Sekita, S., Takatori, K., and Satake, M. (1997). Structural elucidation of trichotetronines: polyketides possessing a bicyclo[2.2.2]octane skeleton with a tetronic acid moiety isolated from Trichoderma sp. J. Chem. Soc.-Perkin Trans. 1, 2961–2964. doi: 10.1039/a704963c
Snyder, H. R., and Matteson, D. S. (1957). The synthesis of the 2-Amino-3-(3-indolyl)-butyric acids (β-Methyltryptophans). J. Am. Chem. Soc. 79, 2217–2221. doi: 10.1021/ja01566a050
Wang, L., Wesemann, S., Krenn, L., Ladurner, A., Heiss, E. H., Dirsch, V. M., et al. (2017). Erythrodiol, an olive oil constituent, increases the half-life of ABCA1 and enhances cholesterol efflflux from THP-1-derived macrophages. Front. Pharmacol. 8:375. doi: 10.3389/fphar.2017.00375
Xu, K., Gao, Y., Li, Y.-L., Xie, F., Zhao, Z.-T., and Lou, H.-X. (2018). Cytotoxic p-terphenyls from the endolichenic fungus Floricola striata. J. Nat. Prod. 81, 2041–2049. doi: 10.1021/acs.jnatprod.8b00362
Yu, J., Han, H., Zhang, X., Ma, C., Sun, C., Che, Q., et al. (2019). Discovery of two new sorbicillinoids by overexpression of the Global Regulator LaeA in a marine-derived fungus Penicillium dipodomyis YJ-11. Mar. Drugs 17:446. doi: 10.3390/md17080446
Keywords: sorbicillinoid, trisorbicillinoid, Acremonium, endophyte, Fructus mori
Citation: Peng X-P, Li G, Wang L-M, Wang Q, Wang C, Ji L-X, Cao C-X, Lin G-F, Jiang Z-Y, He Z-q, Wang P and Lou H-X (2022) Structurally Various Sorbicillinoids From an Endophytic Fungus Acremonium citrinum SS-g13. Front. Microbiol. 13:800626. doi: 10.3389/fmicb.2022.800626
Received: 23 October 2021; Accepted: 15 February 2022;
Published: 23 March 2022.
Edited by:
Jose Ruben Tormo, Fundación MEDINA, SpainReviewed by:
Pramod B. Shinde, Central Salt and Marine Chemicals Research Institute (CSIR), IndiaZhengxi Hu, Huazhong University of Science and Technology, China
Fernando Reyes, Fundación MEDINA, Spain
Copyright © 2022 Peng, Li, Wang, Wang, Wang, Ji, Cao, Lin, Jiang, He, Wang and Lou. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hong-Xiang Lou, bG91aG9uZ3hpYW5nQHNkdS5lZHUuY24=
 Xiao-Ping Peng
Xiao-Ping Peng Gang Li
Gang Li Li-Mei Wang1
Li-Mei Wang1 Hong-Xiang Lou
Hong-Xiang Lou