
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Microbiol., 23 December 2022
Sec. Infectious Agents and Disease
Volume 13 - 2022 | https://doi.org/10.3389/fmicb.2022.1079390
This article is part of the Research TopicAnimal Emerging and Reemerging DiseasesView all 24 articles
 Qing Yao1
Qing Yao1 Tingting Xie1
Tingting Xie1 Yu Fu1
Yu Fu1 Jiajia Wan1
Jiajia Wan1 Wendie Zhang1
Wendie Zhang1 Xuejun Gao1
Xuejun Gao1 Jing Huang2
Jing Huang2 Diangang Sun1
Diangang Sun1 Fuxian Zhang1
Fuxian Zhang1 Weicheng Bei3
Weicheng Bei3 Liancheng Lei1,4*
Liancheng Lei1,4* Feng Liu1*
Feng Liu1*Introduction: To survive in various hostile environments, two-component system is an adaptive mechanism for diverse bacteria. Activity of the CpxA/CpxR two-component system contributes to coping with different stimuli, such as pH, osmotic and heat stress.
Methods: However, the role of the CpxA/CpxR system in cold resistance is little-known. In this study, we showed that CpxA/CpxRwas critical for A. pleuropneumoniae growth under cold stress.
Results: β-Galactosidaseanalysis showed that CpxA/CpxR positively regulated the predicted cold stress gene cspC. The mutant for cold stress gene cspC was impaired in the optimal growth of A. pleuropneumoniae under cold stress. Furthermore, electrophoretic mobility shift assays demonstrated that CpxR-P could directly regulate the transcription of the cold stress gene cspC.
Discussion: These results presented in this study illustrated that the CpxA/CpxR system plays an important role in cold resistance by upregulating expression of CspC. The data give new insights into how A. pleuropneumoniae survives in cold stress.
Actinobacillus pleuropneumoniae is an important swine pathogen responsible for respiratory infectious disease, porcine contagious pleuropneumonia (PCP). PCP is characterized by fibrinous, hemorrhagic, and necrotic lung lesions, and causes substantial losses in the swine industry worldwide (Guitart-Matas et al., 2022; Zhang et al., 2022). To date, 19 reference serovars have been identified based on the composition of the capsular polysaccharide (CPS; Stringer et al., 2021; Scherrer et al., 2022). Previous studies have reported that five putative two-component systems (TCS) were found in the genome of A. pleuropneumoniae, such as CpxR/CpxA, ArcA/ArcB, QseB/QseC, NarP/NarQ, and PhoB/PhoR (Xu et al., 2008).
The CpxR/CpxA system is commonly used by Gram-negative bacteria to regulate many bacterial processes, mainly triggered by a wide range of environmental conditions, such as pH, osmolarity and temperature (Nakayama and Watanabe, 1995; Tschauner et al., 2014; Liu et al., 2022a). The CpxR/CpxA system is composed of the transmembrane sensor kinase CpxA and the cytoplasmic response regulator CpxR (Danese et al., 1995; Raivio and Silhavy, 1997). When bacteria are exposed to extracytoplasmic stresses, the histidine kinase CpxA autophoshorylates and then transfers a phosphoryl group to the response regulator CpxR (Price and Raivio, 2009; Raivio, 2014). Phosphorylation enables CpxR to bind to the promoter region of multiple genes, and alters the transcription of these genes (Raivio et al., 2013).
Bacteria have evolved various complicated networks to cope with different stress factors, such as temperature, pH and mosmotic (Telhig et al., 2020). When subjected to a sudden drop in temperatures, microbes undergo severe unfavorable disturbances such as decreased membrane fluidity and ribosome efficiency, and increase formation of stable secondary structures in nucleic acids (Goto et al., 2015). To adapt to the extreme environments, bacteria activate the expression of cold shock proteins (Csp) that function as general RNA or DNA chaperones to eliminate their secondary structures (Kloska et al., 2020). Csp protein are ubiquitous in a broad variety of bacteria, and multiple variants of this protein have been found, such as CspA, CspB, CspC, CspD, CspE, CspF, CspG, CspH and CspI in E. coli (Derzelle et al., 2000).
Porcine pleuropneumonia caused by A. pleuropneumoniae leads to economic losses to affected pig farmers. Before causing infection in the host, A. pleuropneumoniae must well cope with different environmental cues in vitro. However, the cold adaptation mechanism of A. pleuropneumoniae is poorly understood. In the present study, we found that the CpxA/CpxR system plays an important role in APP resistance to cold stress. Furthermore, we investigated the mechanism of the Cpx-mediated cold resistance, and showed that the CpxA/CpxR-CspC pathway contribute to the cold stress response in A. pleuropneumoniae.
The bacterial strains and plasmids, as well as primers used in this study, are listed in Tables 1,2. A. pleuropneumoniae cells were grown in tryptic soy broth (TSB; Solarbio, China) or on TSB agar plates supplemented with 10 μg/ml NAD (Sigma-Aldrich, United States) and 10% fetal bovine serum (FBS; Every Green, China). E. coli cells were grown in LB medium supplemented with 50 μg/ml diaminopimelic acid (Sigma-Aldrich, United States) or relevant antibiotics (chloramphenicol, 5 μg/ml; kanamycin, 50 μg/ml). The ΔcspC, ΔcspD and CΔcspC strains were generated using the suicide plasmid pEMOC2 and the shuttle plasmid pJFF224-XN as described earlier (Liu et al., 2022a). In order to induce gene expression, plasmid pJFF224-PcspC which expressed the cspC gene under the control of IPTG-inducible promoter was constructed, and electrically transferred to the mutant strain ΔcpxAR.
Bacterial cells grown overnight in TSB medium at 37°C were subcultured 1:1000 in fresh TSB broth. Cells grown up to the exponential phase (OD600; 0.6) were exposed to 4°C for a series of times. Total RNA was extracted from bacterial cultures using the Bacteria Total RNA Isolation Kit (Sangon Biotech, China). The synthesis of complementary DNA (cDNA) was achieved using the HiScript II Q RT SuperMix for qRT-PCR (Vazyme, China). For quantitative RT-PCR, the reactions were performed using SYBR qPCR Mix (Vazyme, China) and run in the CFX96 Real-Time System (Bio-Red, United States). Data were normalized using the 16S rRNA as internal control, and calculated using the 2−ΔΔCt method.
A cspC/cspD-lacZ fusions containing the promoter region of cspC/cspD and the lacZ gene, and cloned into the Xho I and Not I sites of the plasmid pJFF224-XN. Then, the recombinant plasmid was introduced into the wild-type and the ∆cpxAR mutant strain. All strains were grown overnight in TSB broth at 37°C, diluted 1:1,000 in fresh TSB broth, and grown at 20°C for a series of times. The collected culture was assayed for β-Galactosidase activity by using a β-galactosidase (β-GAL) Activity Assay Kit (Micromethod; Sangon Biotech) according to the manufacturer’s specification.
The His6-CpxR protein was expressed using E. coli BL21 (DE3)-containing pET30a-CpxR and purified by using a Ni-nitrilotriacetic acid (Ni-NTA) resin affinity chromatography (Qiagen, Germany). The purified protein was phosphorylated by acetyl phosphate (Sigma, United States) according to previously described procedures (Li et al., 2018). DNA probes were amplified and purified and then labeled using a Biotin Labeling Kit (Beyotime, China). Then, the phosphorylated CpxR and labeled probes were used for protein-DNA EMSAs as described previously (Cheng et al., 2021). Labeled DNA probe (1 μM) and various concentrations of phosphorylated CpxR protein (0–4 pmol) were incubated at 24°C for 20 min in reaction buffer (50 mM Tris–HCl, pH 8.0, 2.5 mM MgCl2, 100 mM KCl, 0.2 mM DTT, 10% glycerol, 2 μg salmon sperm DNA). For competition experiments with unlabeled DNA probes, a 100-fold molar excess was preincubated with phosphorylated CpxR protein. The reaction mixtures were loaded on a 4% non-denaturing polyacrylamide electrophoresis in 0.5 × Tris-borate-EDTA (TBE) buffer. The bands of labeled probes were subsequently transferred to nylon membrane (Beyotime, China), and detected using the Chemiluminescent EMSA Kit (Beyotime, China).
To measure cold growth, the A. pleuropneumoniae strain S4074 and its mutant derivatives were cultured in TSB medium overnight at 37°C, then diluted 1:100 (~1 × 107 CFU/ml) into fresh TSB broth and incubated at 20°C for 48 h. Every 4 h for 48 h, samples were serially diluted and plated on TSB agar, and the OD600 was measured.
The −10 and − 35 promoter regions, and transcriptional atart site (TSS) of the cold shock gene cspC were, respectively, predicated by BPROM (Prediction of bacterial promoters)1 and Berkeley Drosophila Genome Project (BDGP).2 All data were analyzed using two-tailed Student’s t-tests (GraphPad Prism version 7.0, GraphPad Software, inc., San Diego, United States), and presented as mean ± standard deviation (SD). p < 0.05 was considered statistically significant.
To investigate whether CpxAR is involved in the cold adaptation of A. pleuropneumoniae, we tested the growth rates of the WT, ∆cpxAR and C∆cpxAR strains when exposed to cold stress (20°C). Under cold stress, the growth rate of the mutant strain ∆cpxAR was significantly lower than that of the WT and C∆cpxAR strains (Figures 1A,B). Our previous study found that the growth rate of ∆cpxAR was also lower than that of the WT at 37°C, and the difference was obviously much smaller than that at 20°C (Li et al., 2018). The qRT-PCR analysis showed that the mRNA level of the cpxA and cpxR genes were upregulated under cold stress in the WT strain (Figures 1C,D). These results suggest that CpxA/CpxR plays an important role in cold adaptation of A. pleuropneumoniae.

Figure 1. CpxA/CpxR is required for APP cold growth. The growth rates of the WT, ∆cpxAR and C∆cpxAR strains at 20°C were monitored by measurement of OD600 (A) and viable cell counts (B). qRT-PCR analysis of cpxA (C) and cpxR (D) genes in A. pleuropneumoniae S4074 under 20°C. *p < 0.05.
Analysis of the A. pleuropneumoniae genome revealed that it contains two Csp proteins (CspC and CspD) which have a high amino acid identity with E.coli CspA (Figure 2A). Each protein possesses a conserved cold shock domain (CSD) that harbors two nucleic acid-binding motifs RNAP1 and RNAP2.
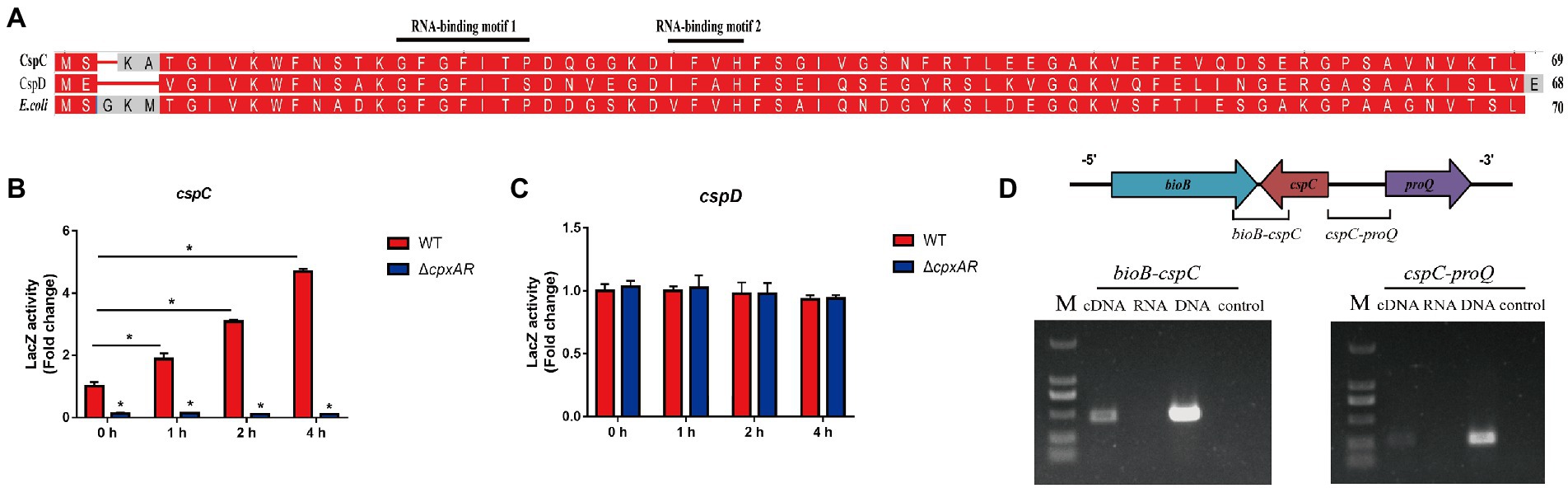
Figure 2. cspC transcription is activated by CpxR. (A) Alignment of cold shock proteins homologs showing the conserved cold shock domain (CSD), which harbors two nucleic acid-binding motifs RNAP1 and RNAP2. The A. pleuropneumoniae CspC and CspD, and E. coli CspA are included for comparison. Promoter activity of cspC (B) and cspD (C) genes in the WT, ∆cpxAR and C∆cpxAR strains under 20°C. *p < 0.05. (D) Transcriptional characteristics of cspC gene as determined by RT-PCR at 20°C.
To elucidate the mechanism of CpxA/CpxR affecting APP cold adaptability, β-Galactosidase assay was performed to examine the link between CpxR and the cold shock genes cspC and cspD. As shown in Figures 2B,C, the promoter activity of cspC-lacZ under cold stress were significantly elevated in the WT strain, and decreased in the ∆cpxAR mutant compared with the former, but the the cspD gene was not. These findings indicated that CpxA/CpxR regulates the expression of the cold shock gene cspC which is cold inducible in A. pleuropneumoniae.
The cold shock gene cspC is adjacent to the gene bioB and proQ in the A. pleuropneumoniae chromosome (Figure 2D). In order to describe the cspC gene, we performed RT-PCR across the bioB-cspC and cspC-proQ junctions. The RT-PCR analysis indicated that the cspC gene is transcribed separately from the proQ gene.
To investigate whether CspC and CspD are involved in cold stress, we generated the ∆cspC and ∆cspD mutant strains, and the C∆cspC complemented strain, and tested the growth rates of the WT, ∆cspC, ∆cspD and C∆cspC strains at 20°C and 37°C. Under cold stress (20°C), the ∆cspC mutant exhibited growth defects, whereas the WT, ∆cspD and C∆cspC strains displayed normal growth (Figures 3A,B). When the bacteria were cultured at 37°C, the growth property of ∆cspC mutant was similar to those of the WT and C∆cspC strains (Figures 3C,D). The growth of ∆cpxAR/PcspC in liquid media was then assayed. Clearly, when CspC was produced with IPTG at 0.1 mM and above, the growth defect of the ∆cpxAR mutant strain was significantly rescued (Figures 3E,F). These results suggested that CspC is essential for cold growth in A. pleuropneumoniae.
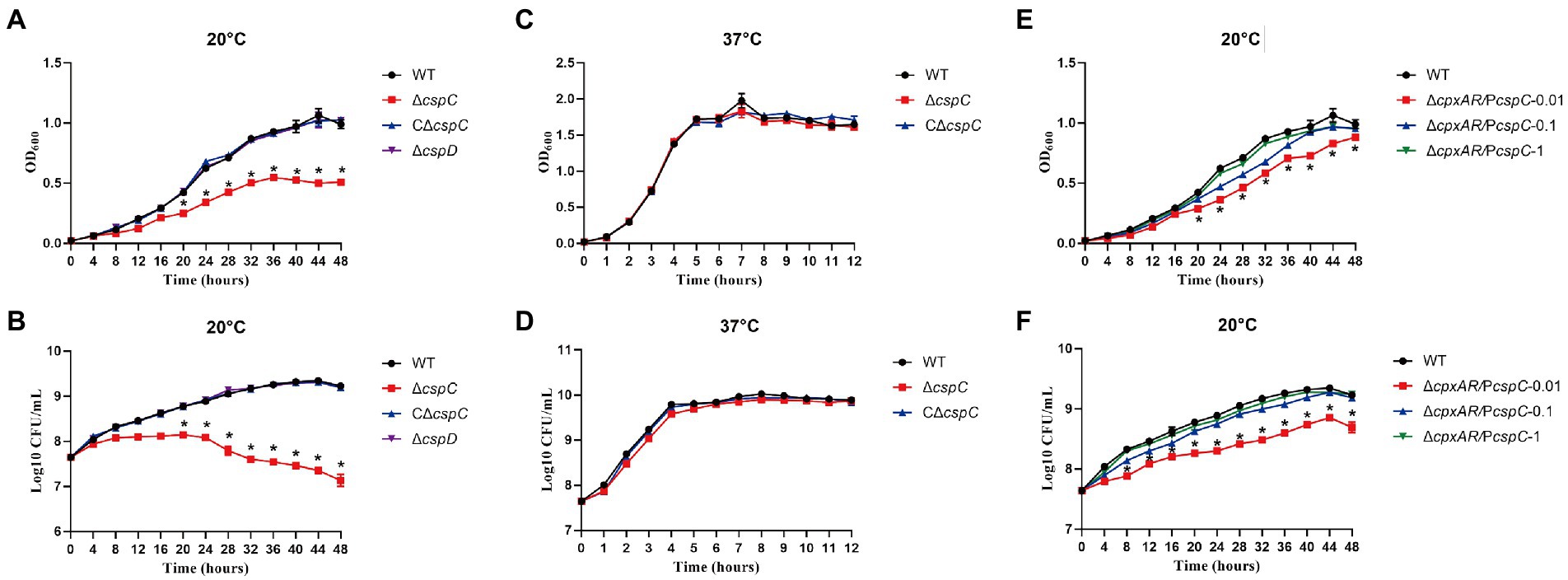
Figure 3. CspC is critical for APP cold growth. The growth rates of the WT, ∆cspC, C∆cspC and ∆cspD strains at 20°C were monitored by measurement of OD600 (A) and viable cell counts (B). The growth rates of the WT, ∆cspC and C∆cspC strains at 37°C were monitored by measurement of OD600 (C) and viable cell counts (D). The growth rates of strains expressing cspC with IPTG at indicated concentrations were monitored by measurement of OD600 (E) and viable cell counts (F). *p < 0.05.
Since CpxR is a response regulator, we examined the interaction between the recombinant CpxR protein and the putative cspC promoter region using EMSA. As shown in Figure 4A, incubation of biotin-labeled cspC with CpxR protein led to the formation of DNA-protein complexes in a protein concentration-dependent manner. Meanwhile, no CpxR-DNA complex was observed with adding excess amounts of unlabeled probes, suggesting that the CpxR could bind specifically to the cspC promoter region (Figure 4B).
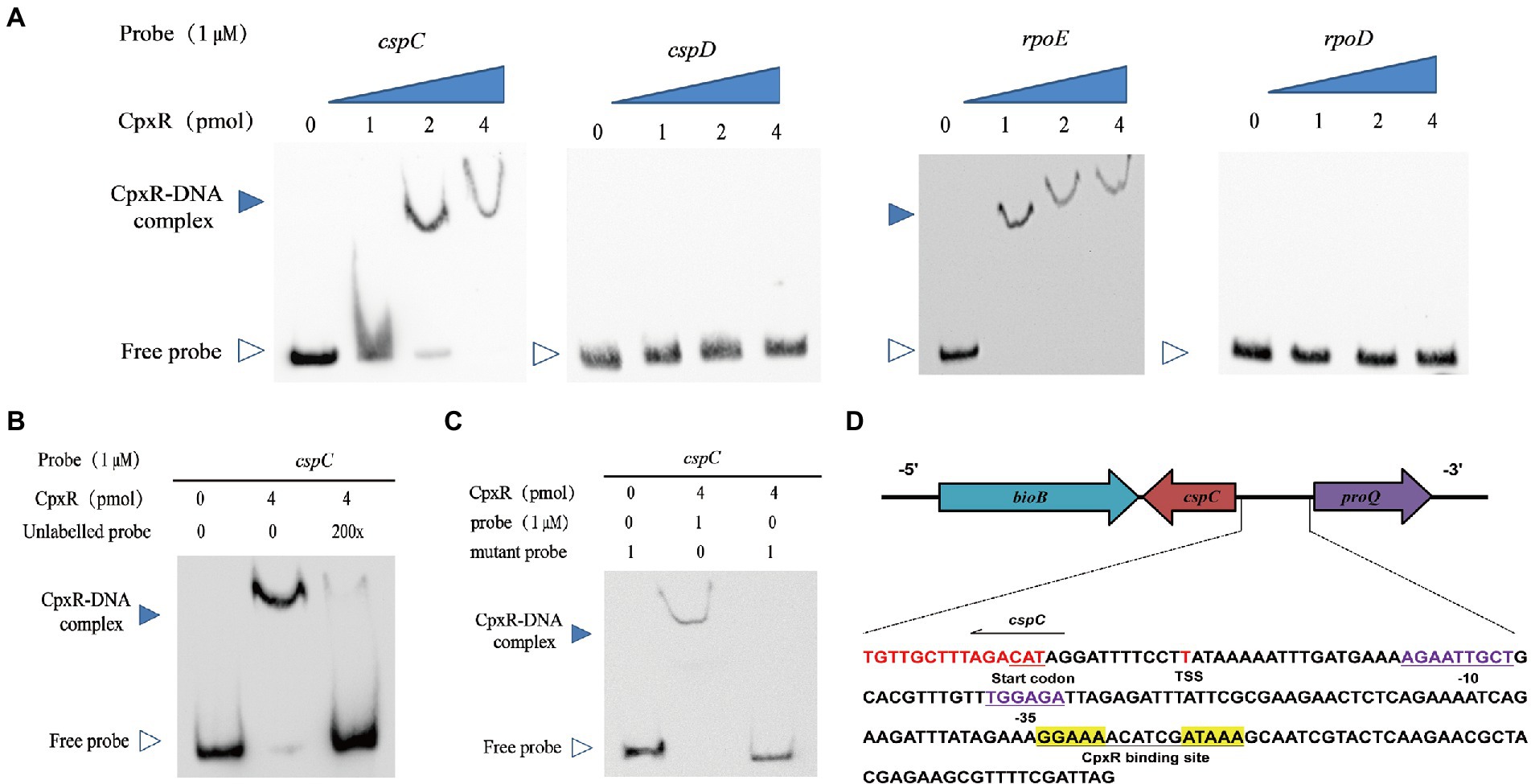
Figure 4. CpxR binds promoter of cspC but not that of cspD. (A) EMSAs analysis of the binding affinity of various amounts of CpxR-P with the cspC, cspD, rpoE (positive control), rpoD (negative control) promoter regions. (B) EMSAs analysis of the binding of CpxR-P with the cspC promoter region under adding excess amounts of unlabeled probes. (C) Labeled mutant cspC promoter region were incubated with various concentrations of CpxR-P. (D) Characterizing the CpxR binding site. The putative CpxR binding site is underlined and shown in yellow background. The −35 and − 10 box are underlined and shown in purple nucleotides. The transcription start site (TSS) is shown in red.
Previous studies found that the CpxR binding site has a conserved sequence GTAAA-(N)4–8-GTAAA (Bruna et al., 2018; Jia et al., 2022). In this study, we found that a 16-nt region (5’-GGAAAACATCGATAAA-3′) was relatively consistent with the characteristics of conserved sequence. As shown in Figure 4C, CpxR-P was unable to bind to a mutant cspC putative promoter region in which the 16-nt region was deleted from the putative CpxR-P binding box. In order to describe the CpxR binding site in detail, the cspC promoter region and transcription start site were, respectively, analyzed by BPROM and BDGP. As shown in Figure 4D, a putative −10 AGAATTGC box, −35 TGGAGA box and TSS were detected, and, respectively, located 30 bp, 50 bp and 12 bp upstream of the start codon.
Two-component system (TCS) is a key signal transduction mechanism that controls many aspects of bacterial physiology, sense a broad range of stimuli and make an appropriate response to adapt and survive in changing environmental conditions. The CpxA/CpxR system identified as a pleiotropic TCS in many Gram-negative bacteria, regulates many virulence factors, such as lipopolysaccharide, polysaccharide capsule, and type IV pilus and responds to a variety of extracellular stimuli including salt, heat, metals, and pH (Jubelin et al., 2005; Hunke et al., 2012; Yan et al., 2020; Liu et al., 2022a,b). However, the link between CpxA/CpxR and cold stress is still unknown. In this study, in-frame mutation of the cpxA and cpxR genes exhibited growth defects, and the mRNA level of the cpxA and cpxR genes elevated under cold stress, suggesting that CpxA/CpxR plays a crucial role in cold adaptation of A. pleuropneumoniae. Furthermore, we provided the first insights into how CpxA/CpxR contributes to cold growth in A. pleuropneumoniae.
Cold is an adverse environment for bacteria, altering secondary structures of nucleic acids that render cells nonviable (Balhesteros et al., 2010). To stabilize secondary structures of the RNA, bacteria activate the expression of multiple variants of Csp proteins under cold stress, such as the nine Csp proteins (CspA to CspI) in E. coli (Graumann and Marahiel, 1999; Li et al., 2021). In the present study, we found that the genome of A. pleuropneumoniae encodes two Csp proteins (CspC and CspD), which are constituted of a single cold shock domain (CSD). Here, β-Galactosidase analysis showed that CspC is cold inducible, and positively regulated by CpxA/CpxR. In addition, these cold growth tests revealed that CspC was vital for APP cold adaptability. These results indicated that CspC is involved in the mechanism of CpxA/CpxR-mediated cold stress.
To response to the adverse condition, activated CpxR binds to the promoter region of target genes, and regulates these genes expression. Previous studies showed that the CpxR binding consensus sequence is identified as GTAAA-(N)4-8-GTAAA, or TTTAC-(N)4-8-TTTAC in many other bacteria (De Wulf et al., 2002; Srinivasan et al., 2012; Feldheim et al., 2016). According to the consensus sequence, we found a potential CpxR binding site (GGAAA-N6-ATAAA) located 52 bp upstream of the promoter −35 region, and 95 bp upstream of the transcription start site. Previous studies showed that the CpxR-binding site is generally located upstream of the promoter region and within 100 bp upstream of the transcription start site, primarily functions as a class I factor and activates their transcription (De Wulf and Lin, 2000; Raffa and Raivio, 2002; Yamamoto and Ishihama, 2006). In addition, we verified that CpxR could directly bind to the promoter region of the cspC gene, and the CpxR-cspC interaction was specific by EMSA. These results revealed that CpxR positively regulates the cspC gene transcription.
In conclusion, this study illuminates the mechanism of A. pleuropneumoniae cold stress and demonstrate the involvement of CpxA/CpxR for the first time. These findings indicated that CpxA/CpxR contributes to cold growth by positively regulating the expression of cspC gene (Figure 5). Since the CpxA/CpxR and CspC are conserved in many bacteria, the revelation of the CpxA/CpxR-CspC pathway will provide important implications for elucidating cold stress in other bacteria.
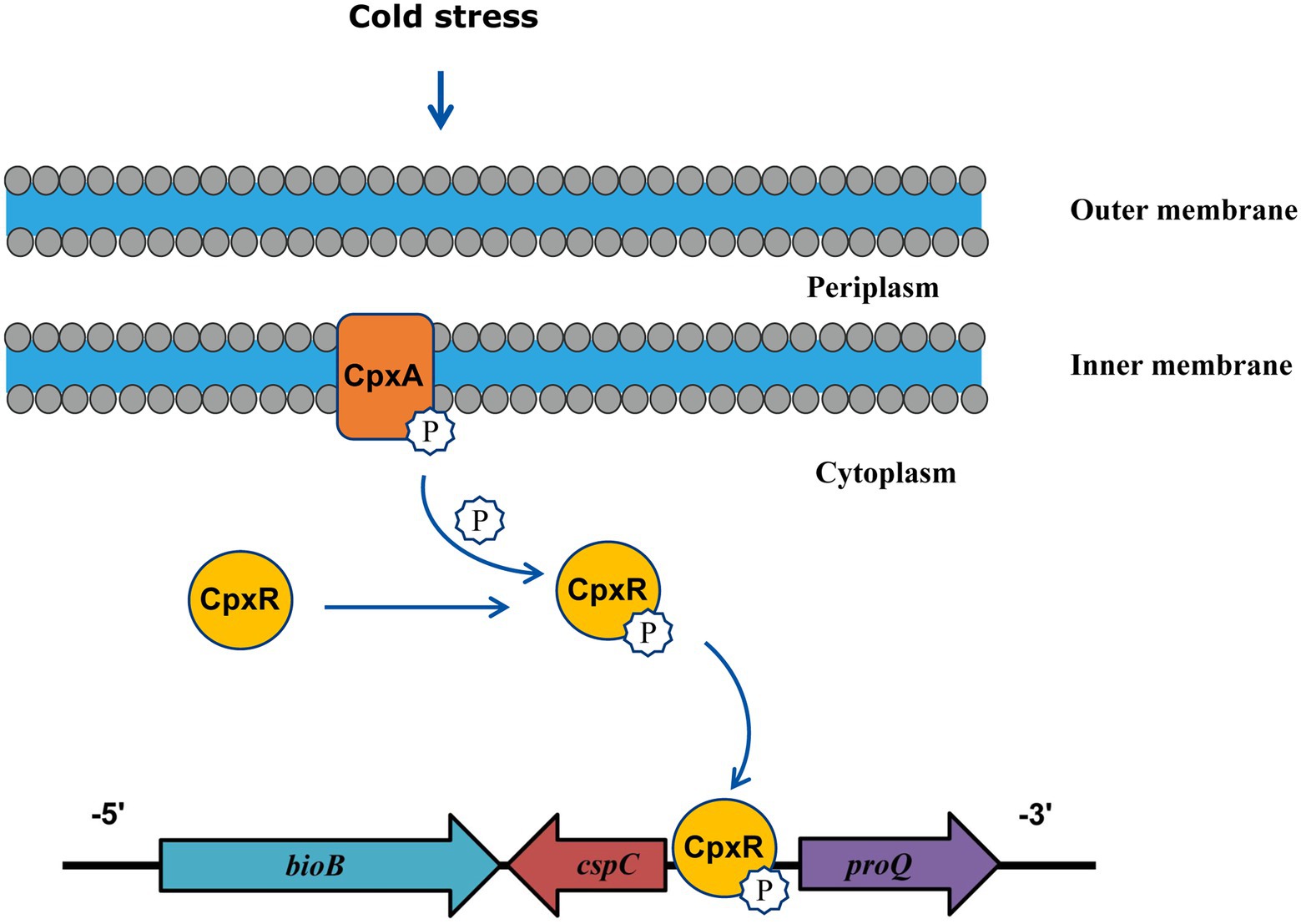
Figure 5. Model illustrating that CpxA/CpxR mediates cold stress in A. pleuropneumoniae. Under cold stress, phosphorylated form of CpxA transfers the phosphate group to CpxR, then the active CpxR binds the promoter of the cspC gene to upregulate its transcription.
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/Supplementary material.
FL and LL conceived and designed the experiments. QY, YF, JW, WZ and TX performed the experiments. FL and QY analyzed the data. XG,WB, DS, and FZ contributed reagents, materials, and analysis tools. JH polished the language. All authors contributed to the article and approved the submitted version.
This research was supported by the National Natural Science Foundation of China (32002252).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmicb.2022.1079390/full#supplementary-material
Balhesteros, H., Mazzon, R. R., da Silva, C. A., Lang, E. A., and Marques, M. V. (2010). CspC and CspD are essential for Caulobacter crescentus stationary phase survival. Arch. Microbiol. 192, 747–758. doi: 10.1007/s00203-010-0602-8
Bruna, R. E., Molino, M. V., Lazzaro, M., Mariscotti, J. F., and Garcia Vescovi, E. (2018). CpxR-dependent thermoregulation of Serratia marcescens PrtA metalloprotease expression and its contribution to bacterial biofilm formation. J. Bacteriol. 200, e00006–18. doi: 10.1128/JB.00006-18
Cheng, C., Liu, F., Jin, H., Xu, X., Xu, J., Deng, S., et al. (2021). The DegU orphan response regulator contributes to heat stress resistance in listeria monocytogenes. Front. Cell. Infect. Microbiol. 11:761335. doi: 10.3389/fcimb.2021.761335
Danese, P. N., Snyder, W. B., Cosma, C. L., Davis, L. J., and Silhavy, T. J. (1995). The Cpx two-component signal transduction pathway of Escherichia coli regulates transcription of the gene specifying the stress-inducible periplasmic protease. DegP. Genes Dev 9, 387–398. doi: 10.1101/gad.9.4.387
De Wulf, P., and Lin, E. C. (2000). Cpx two-component signal transduction in Escherichia coli: excessive CpxR-P levels underlie CpxA* phenotypes. J. Bacteriol. 182, 1423–1426. doi: 10.1128/JB.182.5.1423-1426.2000
De Wulf, P., McGuire, A. M., Liu, X., and Lin, E. C. (2002). Genome-wide profiling of promoter recognition by the two-component response regulator CpxR-P in Escherichia coli. J. Biol. Chem. 277, 26652–26661. doi: 10.1074/jbc.M203487200
Derzelle, S., Hallet, B., Francis, K. P., Ferain, T., Delcour, J., and Hols, P. (2000). Changes in cspL, cspP, and cspC mRNA abundance as a function of cold shock and growth phase in lactobacillus plantarum. J. Bacteriol. 182, 5105–5113. doi: 10.1128/JB.182.18.5105-5113.2000
Feldheim, Y. S., Zusman, T., Speiser, Y., and Segal, G. (2016). The legionella pneumophila CpxRA two-component regulatory system: new insights into CpxR's function as a dual regulator and its connection to the effectors regulatory network. Mol. Microbiol. 99, 1059–1079. doi: 10.1111/mmi.13290
Goto, S., Kawamoto, J., Sato, S. B., Iki, T., Watanabe, I., Kudo, K., et al. (2015). Alkyl hydroperoxide reductase enhances the growth of Leuconostoc mesenteroides lactic acid bacteria at low temperatures. AMB Express 5:11. doi: 10.1186/s13568-015-0098-3
Graumann, P. L., and Marahiel, M. A. (1999). Cold shock proteins CspB and CspC are major stationary-phase-induced proteins in Bacillus subtilis. Arch. Microbiol. 171, 135–138. doi: 10.1007/s002030050690
Guitart-Matas, J., Gonzalez-Escalona, N., Maguire, M., Vilaro, A., Martinez-Urtaza, J., Fraile, L., et al. (2022). Revealing genomic insights of the unexplored porcine pathogen Actinobacillus pleuropneumoniae using whole genome sequencing. Microbiol Spectr 10:e0118522. doi: 10.1128/spectrum.01185-22
Hunke, S., Keller, R., and Muller, V. S. (2012). Signal integration by the Cpx-envelope stress system. FEMS Microbiol. Lett. 326, 12–22. doi: 10.1111/j.1574-6968.2011.02436.x
Jia, Y., Hu, H., Zhai, Y., Zhao, B., Sun, H., Hu, G., et al. (2022). CpxR negatively regulates IncFII-replicon plasmid pEC011 conjugation by directly binding to multi-promoter regions. Res. Vet. Sci. 150, 98–106. doi: 10.1016/j.rvsc.2022.05.016
Jubelin, G., Vianney, A., Beloin, C., Ghigo, J. M., Lazzaroni, J. C., Lejeune, P., et al. (2005). CpxR/OmpR interplay regulates curli gene expression in response to osmolarity in Escherichia coli. J. Bacteriol. 187, 2038–2049. doi: 10.1128/JB.187.6.2038-2049.2005
Kloska, A., Cech, G. M., Sadowska, M., Krause, K., Szalewska-Palasz, A., and Olszewski, P. (2020). Adaptation of the marine bacterium Shewanella baltica to low temperature stress. Int. J. Mol. Sci. 21:338. doi: 10.3390/ijms21124338
Li, H., Liu, F., Peng, W., Yan, K., Zhao, H., Liu, T., et al. (2018). The CpxA/CpxR two-component system affects biofilm formation and virulence in Actinobacillus pleuropneumoniae. Front. Cell. Infect. Microbiol. 8:72. doi: 10.3389/fcimb.2018.00072
Li, H., Yang, R., Hao, L., Wang, C., and Li, M. (2021). CspB and CspC are induced upon cold shock in Bacillus cereus strain D2. Can. J. Microbiol. 67, 703–712. doi: 10.1139/cjm-2021-0025
Liu, F., Peng, W., Yan, K., Huang, J., Yuan, F., He, Q., et al. (2022a). CpxAR of Actinobacillus pleuropneumoniae contributes to heat stress response by repressing expression of type IV pilus gene apfA. Microbiol. Spectr. e02523–22. doi: 10.1128/spectrum.02523-22
Liu, F., Yao, Q., Huang, J., Wan, J., Xie, T., Gao, X., et al. (2022b). The two-component system CpxA/CpxR is critical for full virulence in Actinobacillus pleuropneumoniae. Front. Microbiol. 13:1029426. doi: 10.3389/fmicb.2022.1029426
Nakayama, S., and Watanabe, H. (1995). Involvement of cpxA, a sensor of a two-component regulatory system, in the pH-dependent regulation of expression of Shigella sonnei virF gene. J. Bacteriol. 177, 5062–5069. doi: 10.1128/jb.177.17.5062-5069.1995
Price, N. L., and Raivio, T. L. (2009). Characterization of the Cpx regulon in Escherichia coli strain MC4100. J. Bacteriol. 191, 1798–1815. doi: 10.1128/JB.00798-08
Raffa, R. G., and Raivio, T. L. (2002). A third envelope stress signal transduction pathway in Escherichia coli. Mol. Microbiol. 45, 1599–1611. doi: 10.1046/j.1365-2958.2002.03112.x
Raivio, T. L. (2014). Everything old is new again: an update on current research on the Cpx envelope stress response. Biochim. Biophys. Acta 1843, 1529–1541. doi: 10.1016/j.bbamcr.2013.10.018
Raivio, T. L., Leblanc, S. K., and Price, N. L. (2013). The Escherichia coli Cpx envelope stress response regulates genes of diverse function that impact antibiotic resistance and membrane integrity. J. Bacteriol. 195, 2755–2767. doi: 10.1128/JB.00105-13
Raivio, T. L., and Silhavy, T. J. (1997). Transduction of envelope stress in Escherichia coli by the Cpx two-component system. J. Bacteriol. 179, 7724–7733. doi: 10.1128/jb.179.24.7724-7733.1997
Scherrer, S., Peterhans, S., Neupert, C., Rademacher, F., Bartolomei, G., Sidler, X., et al. (2022). Development of a novel high resolution melting assay for identification and differentiation of all known 19 serovars of Actinobacillus pleuropneumoniae. Microbiology 11:e1272. doi: 10.1002/mbo3.1272
Srinivasan, V. B., Vaidyanathan, V., Mondal, A., and Rajamohan, G. (2012). Role of the two component signal transduction system CpxAR in conferring cefepime and chloramphenicol resistance in Klebsiella pneumoniae NTUH-K2044. PLoS One 7:e33777. doi: 10.1371/journal.pone.0033777
Stringer, O. W., Bosse, J. T., Lacouture, S., Gottschalk, M., Fodor, L., Angen, O., et al. (2021). Proposal of Actinobacillus pleuropneumoniae serovar 19, and reformulation of previous multiplex PCRs for capsule-specific typing of all known serovars. Vet. Microbiol. 255:109021. doi: 10.1016/j.vetmic.2021.109021
Telhig, S., Ben Said, L., Zirah, S., Fliss, I., and Rebuffat, S. (2020). Bacteriocins to thwart bacterial resistance in gram negative bacteria. Front. Microbiol. 11:586433. doi: 10.3389/fmicb.2020.586433
Tschauner, K., Hornschemeyer, P., Muller, V. S., and Hunke, S. (2014). Dynamic interaction between the CpxA sensor kinase and the periplasmic accessory protein CpxP mediates signal recognition in E. coli. PLoS One 9:e107383. doi: 10.1371/journal.pone.0107383
Xu, Z., Zhou, Y., Li, L., Zhou, R., Xiao, S., Wan, Y., et al. (2008). Genome biology of Actinobacillus pleuropneumoniae JL03, an isolate of serotype 3 prevalent in China. PLoS One 3:e1450. doi: 10.1371/journal.pone.0001450
Yamamoto, K., and Ishihama, A. (2006). Characterization of copper-inducible promoters regulated by CpxA/CpxR in Escherichia coli. Biosci. Biotechnol. Biochem. 70, 1688–1695. doi: 10.1271/bbb.60024
Yan, K., Liu, T., Duan, B., Liu, F., Cao, M., Peng, W., et al. (2020). The CpxAR two-component system contributes to growth, stress resistance, and virulence of Actinobacillus pleuropneumoniae by upregulating wecA transcription. Front. Microbiol. 11:1026. doi: 10.3389/fmicb.2020.01026
Keywords: Actinobacillus pleuropneumoniae, two-component system, CpxA/CpxR, cold stress, cspC
Citation: Yao Q, Xie T, Fu Y, Wan J, Zhang W, Gao X, Huang J, Sun D, Zhang F, Bei W, Lei L and Liu F (2022) The CpxA/CpxR two-component system mediates regulation of Actinobacillus pleuropneumoniae cold growth. Front. Microbiol. 13:1079390. doi: 10.3389/fmicb.2022.1079390
Received: 25 October 2022; Accepted: 09 December 2022;
Published: 23 December 2022.
Edited by:
Qing Pan, Qingdao Agricultural University, ChinaReviewed by:
Santosh Kumar, University of Wisconsin-Madison, United StatesCopyright © 2022 Yao, Xie, Fu, Wan, Zhang, Gao, Huang, Sun, Zhang, Bei, Lei and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Feng Liu,  bGl1ZmVuZzY4NDMxQDE2My5jb20=; Liancheng Lei,
bGl1ZmVuZzY4NDMxQDE2My5jb20=; Liancheng Lei,  bGVpbGlhbmNoZW5nQDE2My5jb20=
bGVpbGlhbmNoZW5nQDE2My5jb20=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.