- 1Guangdong Provincial Key Laboratory of Gastroenterology, Department of Gastroenterology, Nanfang Hospital, Southern Medical University, Guangzhou, China
- 2Hepatology Unit, Department of Infectious Diseases, Nanfang Hospital, Southern Medical University, Guangzhou, China
- 3Department of Gastroenterology, Beijing Friendship Hospital, Capital Medical University, Beijing, China
- 4Integrative Microecology Center, Department of Gastroenterology, Shenzhen Hospital, Southern Medical University, Shenzhen, China
Background: Akkermansia muciniphila is a member of the gut microbiome, using mucin as sources of carbon, nitrogen, and energy. Since the first discovery of this unique bacterium in 2004, A. muciniphila has been extensively studied. It is considered a promising “next-generation beneficial microbe.” The purpose of this paper is to sort out the research status and summarize the hotspots through bibliometric analysis of the publications of A. muciniphila.
Methods: The publications about A. muciniphila from January 2004 to February 2022 were obtained from the Web of Science Core Collection. Visualization analyses were performed using three bibliometric tools and GraphPad Prism.
Results: A total of 1,478 published documents were analyzed. Annual publication number grew from 1 in 2004 to 336 in 2021, with China being the leading producer (33.36%). De Vos, Willem M was the most productive author with the highest H-index (documents = 56, H-index = 37), followed by Cani, Patrice D (documents = 35, H-index = 25). And Scientific Reports published the most papers. PNAS was the keystone taxa in this field, with high betweenness centrality (0.11) and high frequency. The keywords with high frequency in recent years include: oxidative stress, diet, metformin, fecal microbiota transplantation, short-chain fatty acids, polyphenols, microbiota metabolites and so on. The keyword “oxidative stress” was observed to be increasing in frequency recently.
Conclusion: Over time, the scope of the research on the clinical uses of A. muciniphila has gradually increased, and was gradually deepened and developed toward a more precise level. A. muciniphila is likely to remain a research hotspot in the foreseeable future and may contribute to human health.
Introduction
Akkermansia muciniphila, discovered in 2004, is a Gram-negative, non-motile, ovoid intestinal anaerobe that lacks endospores (Derrien et al., 2004). It belongs to the phylum Verrucomicrobia and is the only species of this phylum found in human stools. A. muciniphila, which lives in the mucus layer of the intestine, degrades and uses mucin as its sole source of nitrogen, carbon, and energy (Derrien et al., 2004, 2008).
Researchers have investigated “new weapons” at the microbial level to combat disease, and A. muciniphila has attracted significant interest in the fields of biological and biomedical research since its discovery. In addition to its relationship with many metabolic diseases (Everard et al., 2013; Depommier et al., 2019; Yan et al., 2021), A. muciniphila is negatively associated with numerous conditions including inflammatory bowel disease, amyotrophic lateral sclerosis, autism, epilepsy, and hypertension (Li et al., 2017; Olson et al., 2018; Bárcena et al., 2019; Blacher et al., 2019; Cheng and Xie, 2021; Ke et al., 2021). A. muciniphila was implicated in patient responsiveness to programmed cell death protein 1 (PD-1) blockers in cancer immunotherapy studies (Gopalakrishnan et al., 2018; Matson et al., 2018; Routy et al., 2018). Over the past decade, A. muciniphila has attracted significant attention in academic circles due to its “probiotic” effect in many diseases; therefore, it is considered a promising “next-generation beneficial microbe” (Cani and de Vos, 2017). An increasing number of studies revealed that A. muciniphila plays important roles in various biological aspects; however, the mechanisms underlying its functions remain unclear.
The global trends and hotspots of A. muciniphila research have not been studied systematically on a temporal scale despite intensive research interest in recent years. Journal citations and publications can be tracked with bibliometrics through quantitative and qualitative analyses of scientific production and research status (Chen et al., 2014). Therefore, this study aimed to identify the foci and frontiers in A. muciniphila research using bibliometric analyses to facilitate further in-depth research at the clinical and basic research levels.
Materials and methods
Data collection
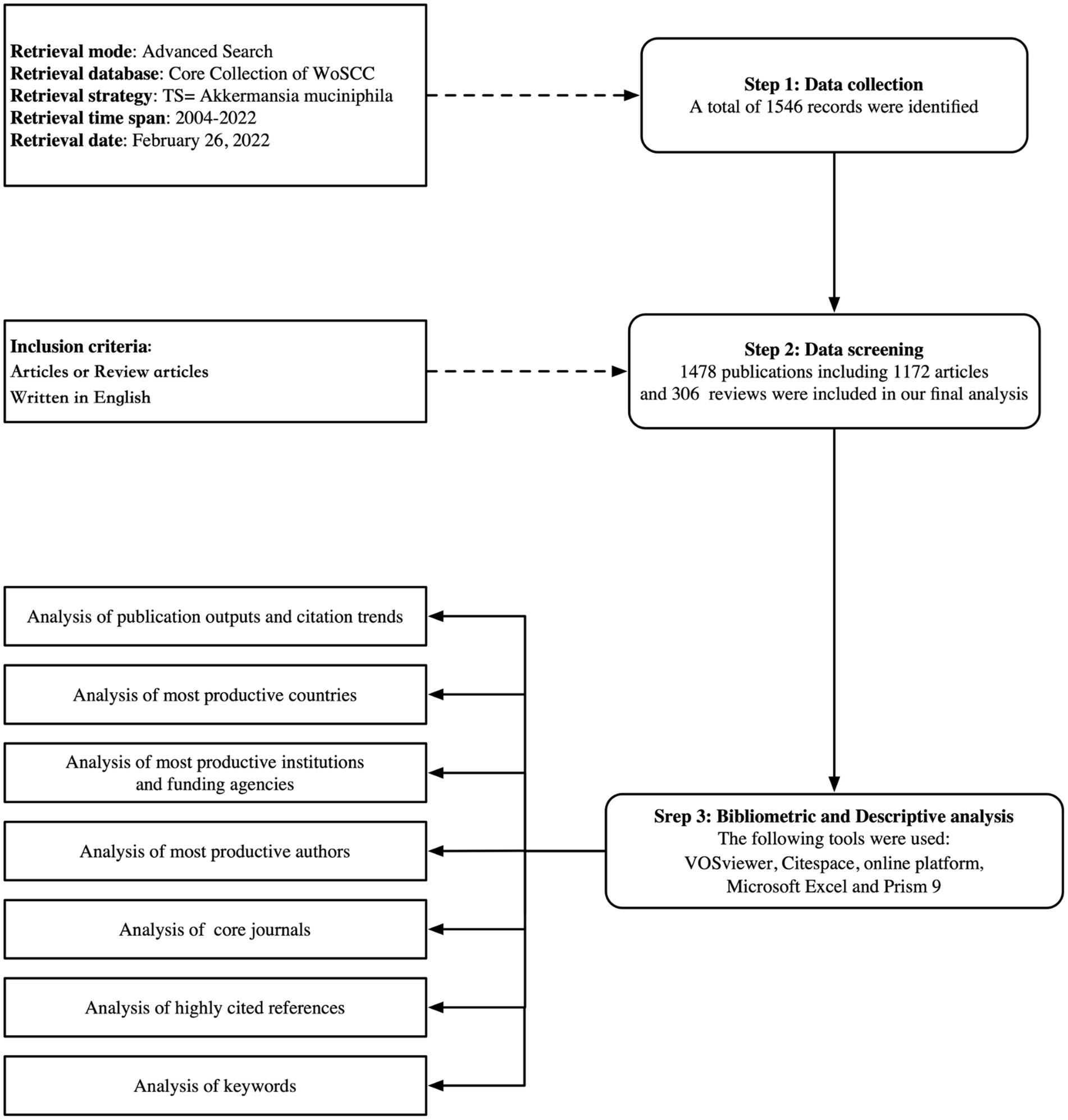
We obtained bibliometric analysis data from the Web of Science Core Collection database (WoSCC), a popular multidisciplinary database in the field of scientometrics (Kokol and Vošner, 2018; Cheng et al., 2022a,b,c). To avoid bias caused by daily database updates, all WoSCC searches were conducted on February 26, 2022. The search formula used was TS = Akkermansia muciniphila. In total, 1,546 publications were retrieved, but only 1,478 publications remained after 68 publications were excluded (meeting abstracts, early access, editorial materials, proceedings papers, book chapters, corrections, news items, letters, and/or non-English literature). A plain-text file was exported with all the full records and cited references for further analysis (Figure 1).
Deduplication of the obtained data was performed using the CiteSpace software (version 5.8. R3). Two researchers independently extracted the publications, countries, institutions, funding agencies, authors, journals, citations, keywords, highly cited references, Hirsch index (H-index; Engqvist and Frommen, 2008), and average citations per item (ACI). To ensure data accuracy and reliability, discrepancies were reconciled via discussions and negotiations. The 2021 Journal Citation Report (Clarivate Analytics, Philadelphia, PA, United States) was used to obtain journal information.
Data analyses
The CiteSpace (version 5.8. R3; Chen, 2004; Chen et al., 2014), VOSviewer (van Eck and Waltman, 2010), an online bibliometric platform,1 and GraphPad Prism (version 8.4.3) were used for bibliometric and visual analyses. The relevant information was summarized in a table using Microsoft Excel (version 16.58). Figure 2 is drawn with Figdraw.2
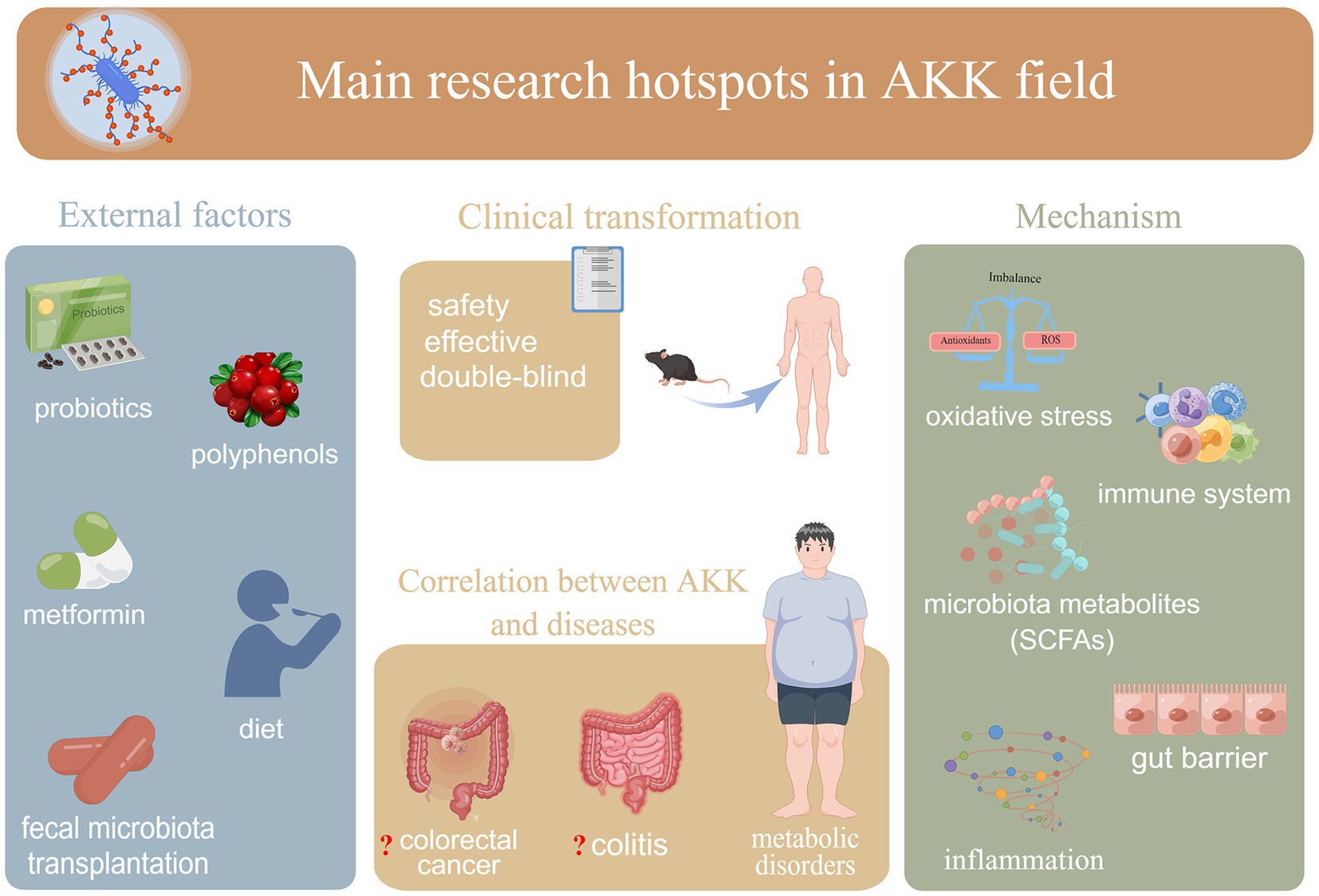
Figure 2. Recent hotspot directions of A. muciniphila research: (i) external factors affecting A. muciniphila; (ii) mechanisms underlying the association between A. muciniphila and hosts (including bacteria); (iii) correlations between A. muciniphila and different diseases; (iv) safety and efficacy of clinical use of A. muciniphila. AKK:A. muciniphila.
Results
Publication and citation trends
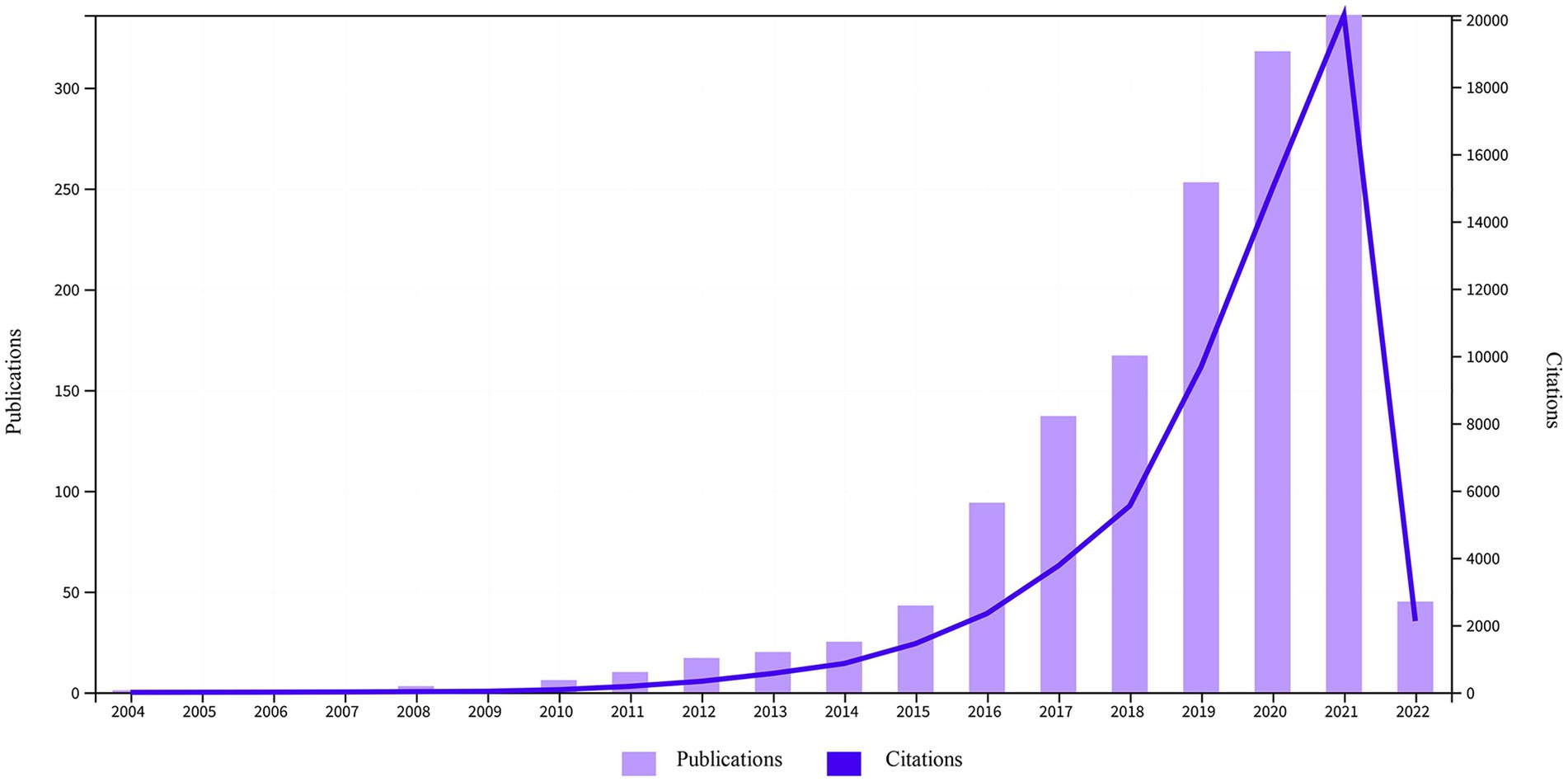
In total, 1,478 papers (1,172 original articles and 306 reviews) were analyzed (Figure 1). Figure 3 shows the upward trend in publications and citations over the past 18 years. The number of publications rose from one to 336 from 2004 to 2021. Approximately 88.30% of the articles were published between 2016 and 2021, and the number of publications in the first 2 months of 2022 exceeded that in all of 2015. The total number of citations was 62,095 (51,188 if excluding self-citations).
Analysis of the countries/regions
Figure 4 shows the distribution of A. muciniphila-related publications worldwide. East Asia, North America, Western Europe, and South Europe were the most productive countries/regions (Figure 4A). Figure 4B and Figure 4C demonstrate the basic information and trends in annual publication output, respectively, among the top ten countries (2004–2022). Seventy-three countries/regions produced publications on A. muciniphila. China ranked first with 493/1,478 publications (33.36%), followed by the United States (387/1,478; 26.18%). The United States had the highest H-index (63), whereas Finland (138.88) and the Netherlands (130.13) had the highest ACI (Figure 4B). In addition, network analysis was used to identify cooperative relationships between countries. As shown in Figure 4D, the closest cooperation occurred between China and the US, followed by that between Finland and the Netherlands.
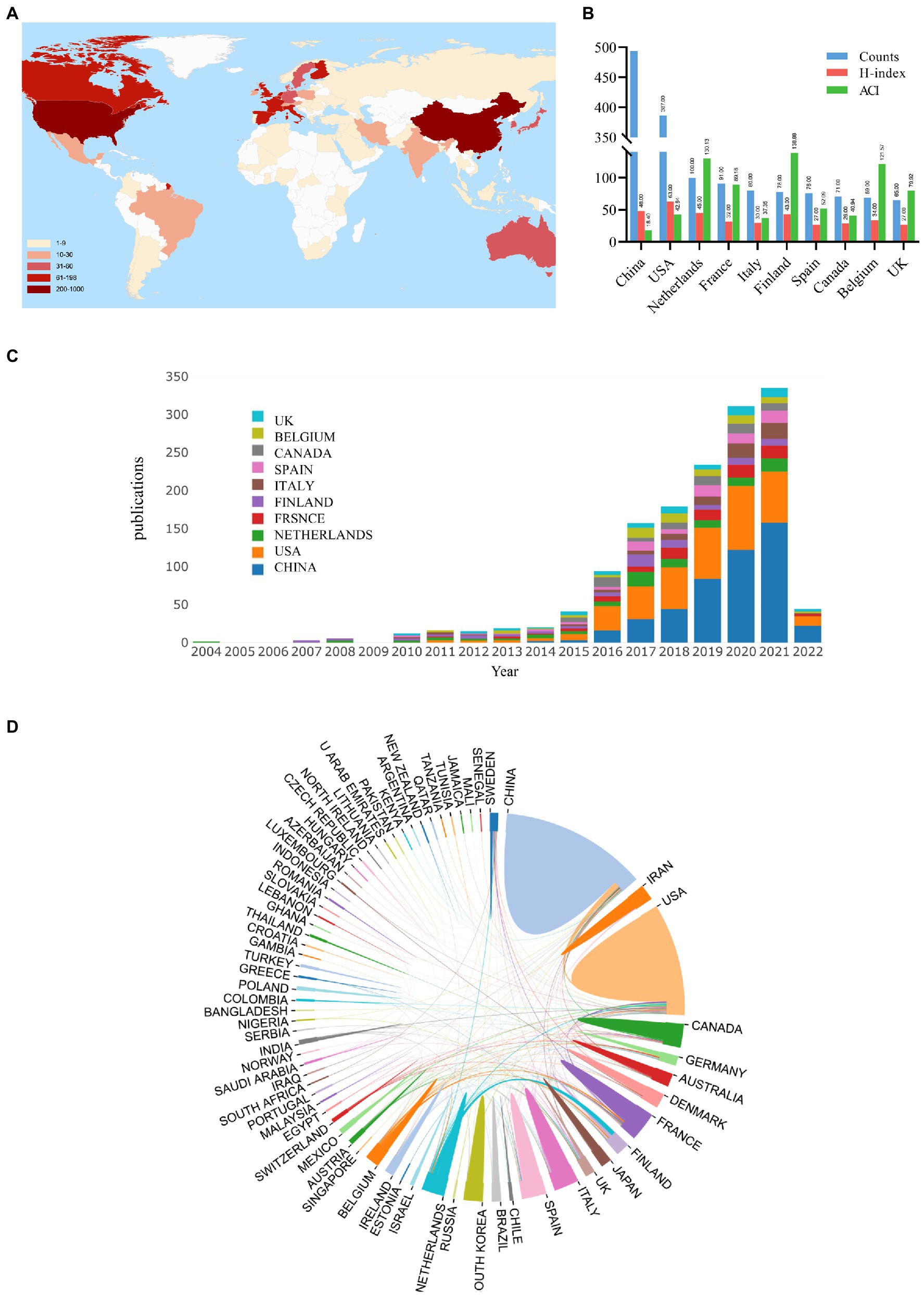
Figure 4. Analysis of the contribution of different countries/regions to A. muciniphila research. (A) Geovisualization of A. muciniphila research distribution. Color shades correlate with the number of articles published. England, Northern Ireland, Scotland, and Wales were reclassified together as the United Kingdom; Taiwan was merged into China. (B) The publication counts, H-index, and ACI of the top 10 most productive countries/regions. (C) Trends in A. muciniphila publications from the top 10 countries/regions from 2004 to 2022. The colors represent different countries/regions. (D) Cooperation of countries/regions involved in A. muciniphila research. The proportion of the area correlates to the number of national publications, and the thickness of the line reflects the strength of cooperation between countries.
Analysis of the institutions and funding agencies
Of the top 10 institutions, Wageningen University & Research in the Netherlands had the high H-index and was the most productive institution (H-index = 39, publications = 69; Figure 5A). It was followed by two institutions each from France, Belgium, and China, and one each from the United States, Finland, and Denmark (Figure 5A). Walloon Excellence in Life Sciences and Biotechnology (WELBIO) had the highest ACI (211.70). Figure 5B illustrates the collaborations between institutions with a minimum number of eight published articles (associated institutions only). The node size indicates the degree of cooperation of an institution with other institutions (weighted by the total link strength [TLS]; the higher the TLS value, the stronger the cooperation strength; Li et al., 2022). A total of 201 lines and 70 nodes were present on the institutional network map, and the University of Helsinki (TLS = 79) and Wageningen University Research (TLS = 72) had the highest TLS (Figure 5B).
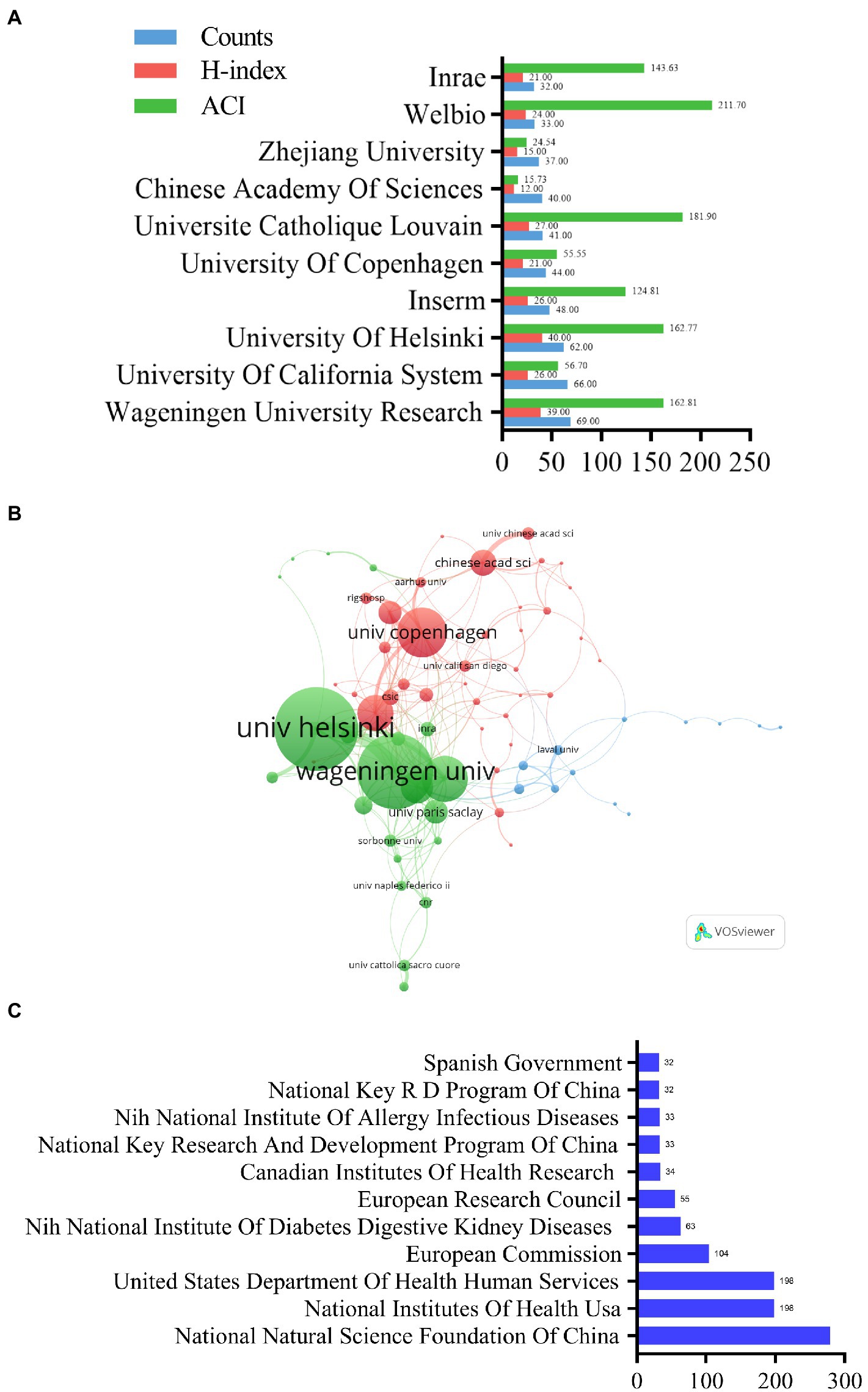
Figure 5. Analysis of the contribution of different institutions and funding agencies to A. muciniphila research. (A) The publication counts, H-index, and ACI of the top 10 most productive institutions. (B) Co-authorship analysis of the institutions. Each node represents a different institution. Node size reflects the strength of cooperation. (C) Top 10 related funding agencies which support A. muciniphila research (Spanish Government and National Key RD Program of China tied for 10th).
The funding agencies’ contributions showed similar trends as that of the countries/region (Figure 5C). Four agencies in the US and three in China were included. The National Natural Science Foundation of China was the largest sponsor (279 studies; Figure 5C), followed by the National Institutes of Health (United States) and the United States Department of Health and Human Services.
Analysis of the most productive authors
The most productive authors (those with at least 15 publications) are listed in Table 1. De Vos, Willem M. from the University of Helsinki and Wageningen University was the most prolific author with the highest H-index (publications = 56, H-index = 37), followed by Cani, Patrice D. from the Universite Catholique de Louvain (publications = 35, H-index = 25). Everard, Amandine from the Universite Catholique de Louvain had the highest ACI (344.25). Figure 6A illustrates the collaboration network of authors who had at least six publications (weighted using TLS). The network consisted of 61 nodes and 174 lines (six nodes not shown). De Vos and Willem had the highest TLS (111), followed by Cani and Patrice (100) and Delzenne and Nathalie (77). The co-citation analysis included 101 nodes and 4,999 lines for authors with 70 citations or more. Derrien, Muriel (citations = 1,153), Everard, Amadine (citations = 1,126), and Cani and Patrice (citations = 1,096) were the most-cited authors (Figure 6B).
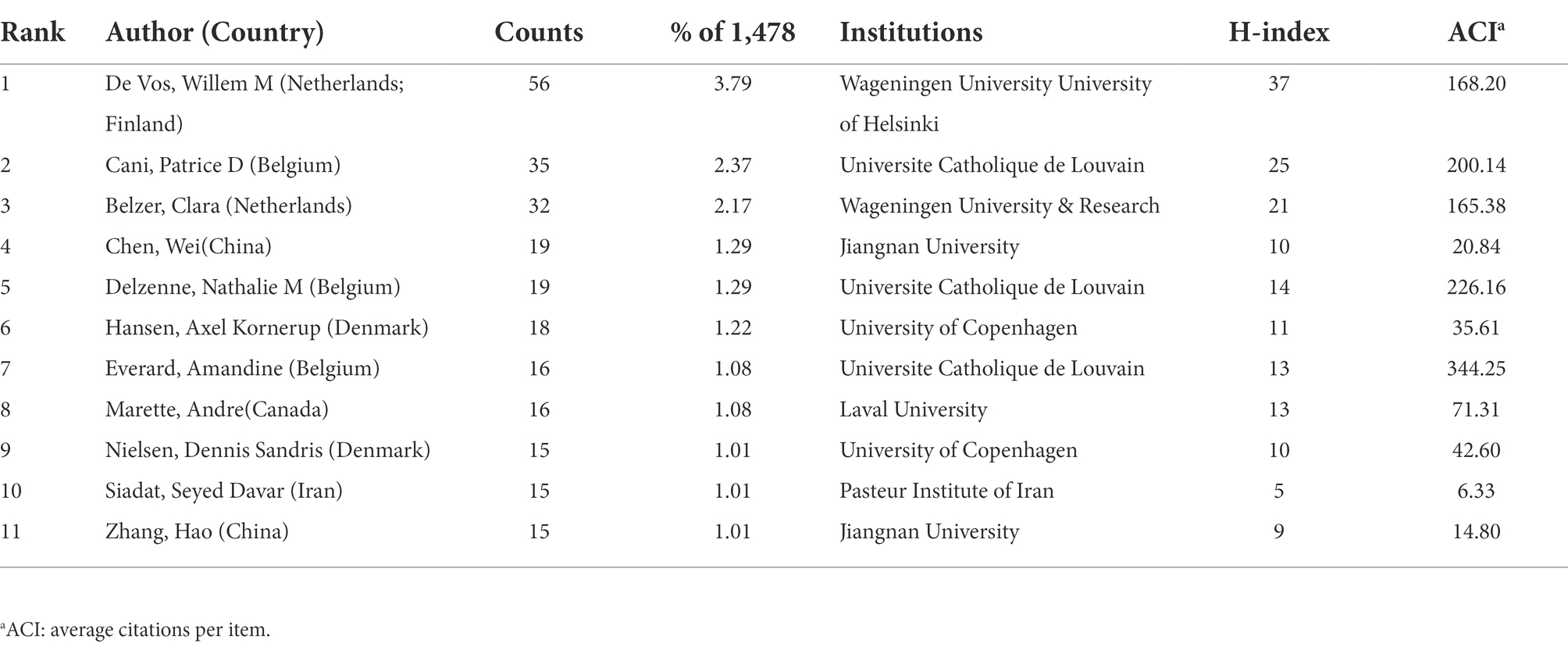
Table 1. The most productive authors (those with at least 15 publications) in the field of A. muciniphila research.
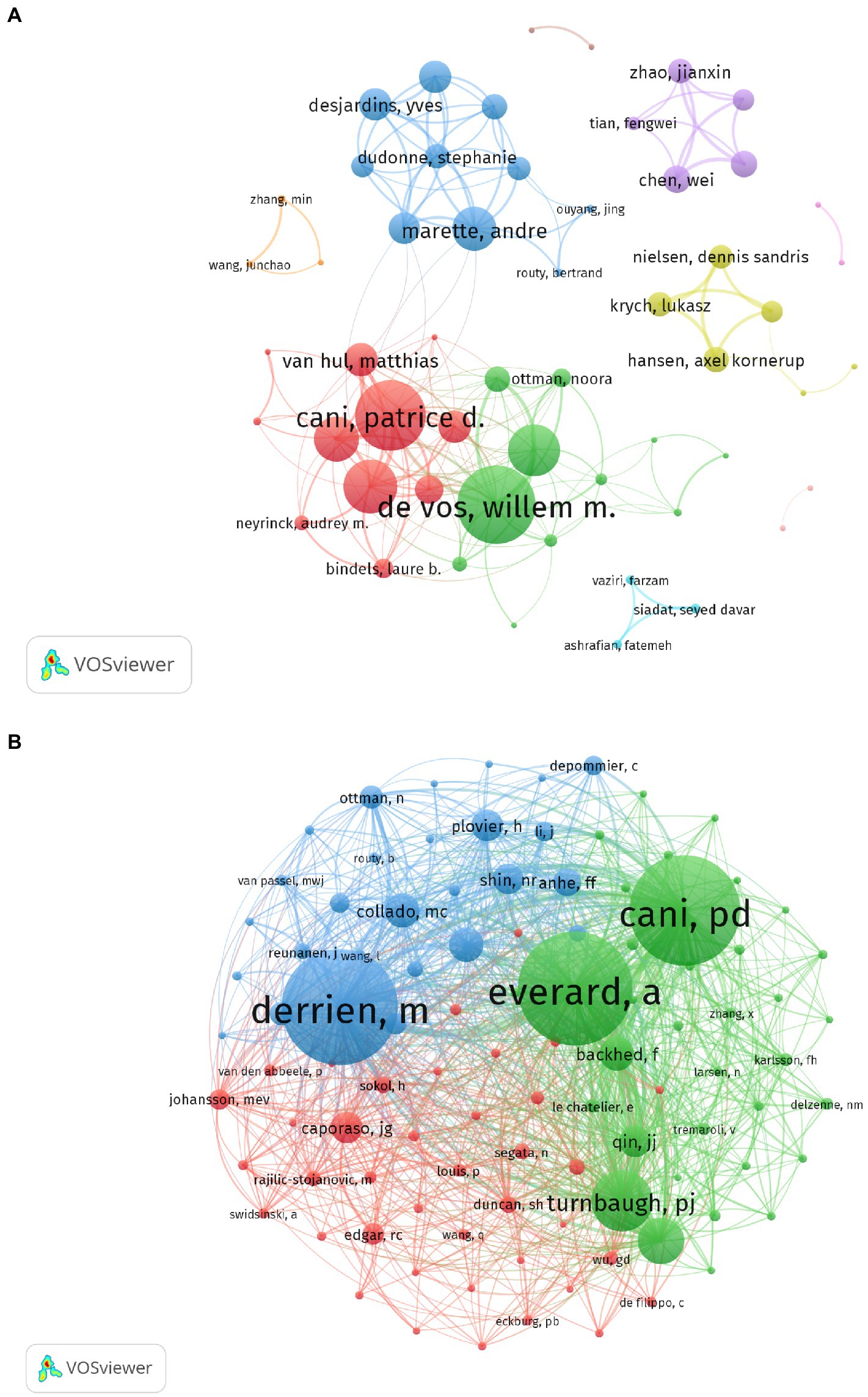
Figure 6. Contribution analysis of authorship in A. muciniphila research. (A) Co-authorship analysis of authors. Each node represents a different author. After the network was generated, cooperation between authors was shown as same-colored clusters. The size of the nodes reflects the strength of their cooperation. (B) Co-citation analysis of cited authors generated by VOS viewer. Each node represents a different cited author. The size of the nodes is weighted by citations.
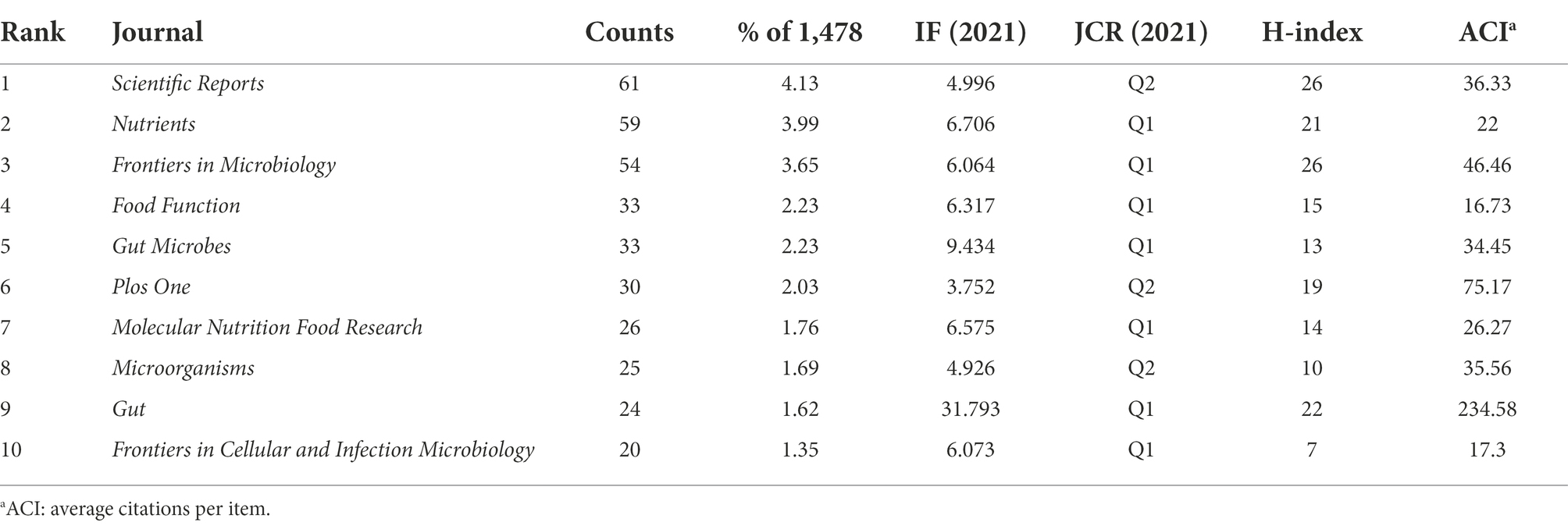
Analysis of the journals
The top 10 most productive journals in A. muciniphila research are listed in Table 2, and accounted for approximately 24.70% of all publications (365/1,478). Scientific Reports published the highest number of papers (61/1,478), followed by Nutrients (59/1,478) and Frontiers in Microbiology (54/1,478). A co-citation analysis was also conducted to investigate the influence of the journals. The top four most-cited journals were Proceedings of the National Academy of Sciences of the United States of America (PNAS; 1,181), PLOS One (1,129), Nature (1,092), and Gut (1,092; Figure 7A). Notably, PNAS had a central value of 0.11, indicating a high betweenness centrality.
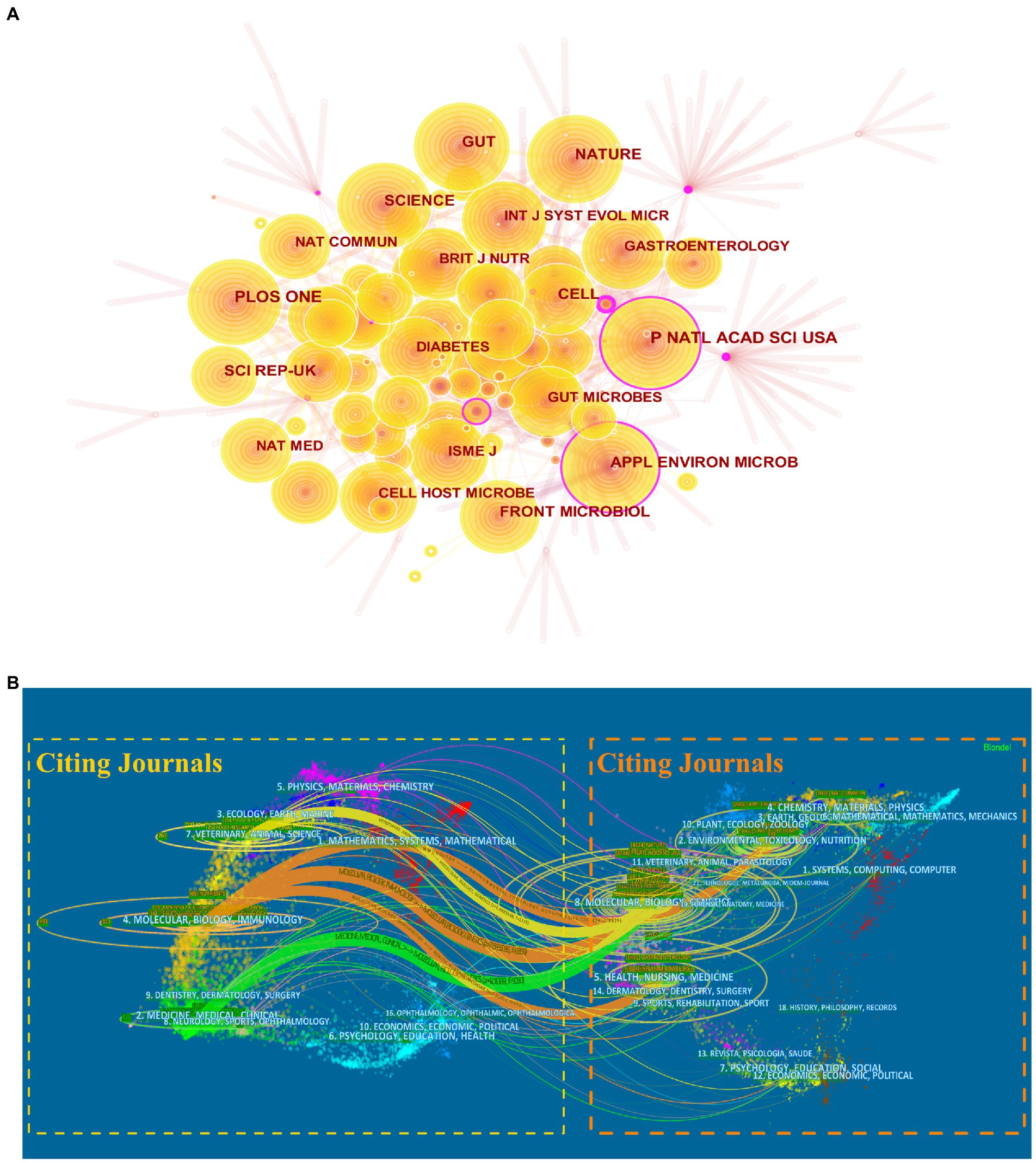
Figure 7. Analysis of core journals of A. muciniphila research. (A) Co-citation analysis of journals. Each node represents a different journal. The size of the nodes is weighted by the number of citations. The purple outer circle highlights nodes with intermediary centrality greater than 0.1. (B) Dual-map overlay of the journals publishing A. muciniphila articles generated by CiteSpace. Each label indicates a separate research subject covered by the journal. On the map, the left side represents the citing journals, while the right side represents the cited journals. There are different colored lines for the different reference paths, which begin with the citing map and end at the cited map.
Figure 7B shows the dual-map overlay depicting the flow from the citing to cited subject categories, mainly including three orange pathways (from “molecular, biology, immunology” to “molecular, biology, genetics,” “environmental, toxicology, nutrition,” and “health, nursing, medicine”), one green pathway (from “medicine, medical, clinical” to “molecular, biology, genetics”), and one yellow pathway (from “veterinary, animal, science” to “molecular, biology, genetics”).
Analysis of highly cited references
Supplementary Table S1 lists the top 10 co-cited articles on A. muciniphila research, which are generally viewed as the ‘classics’ (Li et al., 2022). The most-cited paper was published by Everard et al. (2013) in PNAS, and was cited 319 times in this field. Dao et al. (2016) and Derrien et al. (2017) published the second and third most-cited papers, respectively.
Research hotspots were traced using co-citation analysis of the references. The co-citations were visualized and clustered to analyze the research focus. The modularity value (Q-value) and the mean silhouette value (S-value) were calculated to evaluate the clustering quality, where Q > 0.3 and S > 0.7 indicate that the clustering structure is significant and convincing (Wu et al., 2021a). Figure 8A illustrates the top 10 largest clusters with good homogeneity (S = 0.9419, Q = 0.7849). Citation bursts were mainly concentrated in cluster #0 (A. muciniphila) and cluster #1 (metformin). As shown in Figure 8B, the reference with the highest citation burst strength was by Everard et al. (2013). Notably, bursts in several studies have been increasing recently (Callahan et al., 2016; Desai et al., 2016; Koh et al., 2016; Cani and de Vos, 2017; Derrien et al., 2017; Plovier et al., 2017; Chelakkot et al., 2018; Grander et al., 2018; Depommier et al., 2019; Zhang et al., 2019).
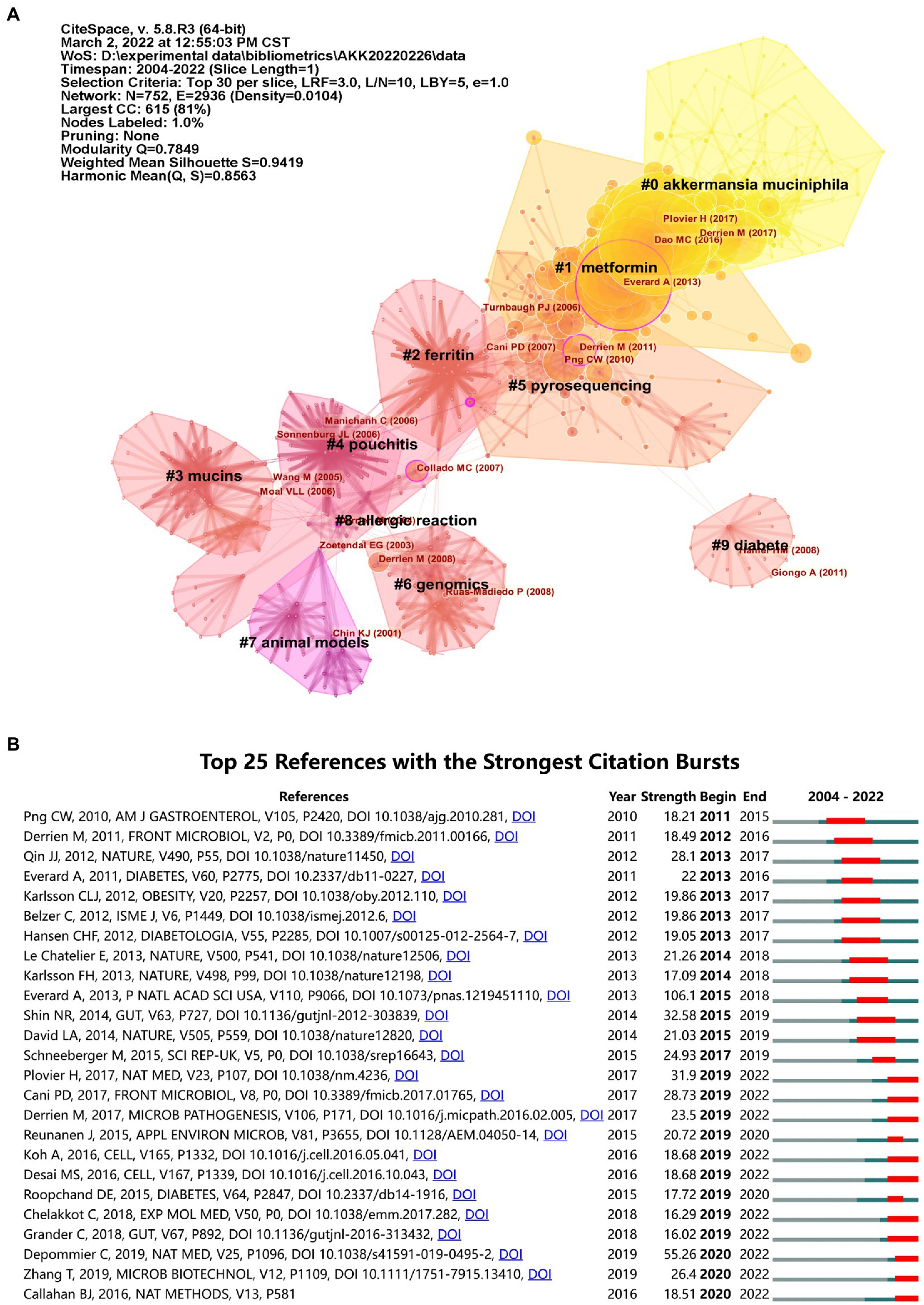
Figure 8. Analysis of references in A. muciniphila research. (A) The network map of co-cited references. Each node represents a different reference. The cited references form several natural clusters, which are closely related. The purple outer circle highlights nodes with intermediary centrality greater than 0.1. (B) Top 25 A. muciniphila-related references with the strongest citation bursts (2004–2022).
Analysis of the keyword research knowledge
After synonym merging, 2,389 author keywords were obtained from 1,478 articles. A heatmap was generated for author keywords that occurred at least 30 times from 2004 to 2022 in the A. muciniphila research field (Figure 9A). The heatmap is colored and sized according to the frequency of the keywords. The top 10 keywords were “A. muciniphila,” “gut microbiota,” “obesity,” “inflammation,” “prebiotics,” “chain fatty acids,” “diet,” “insulin resistance,” “metabolism,” and “diet-induced obesity.” Additionally, Figure 9B color-codes the keywords based on the year in which they appeared. The keywords with an average appearance year after 2019 (more recent appearance) include “microbiota metabolites,” “metformin,” “fecal microbiota transplantation,” “oxidative stress,” “immune system,” “short-chain fatty acids,” “diet,” “colorectal cancer,” “diabetes,” “gut barrier function,” “double-blind,” “polyphenols,” etc. (Figure 9B). Furthermore, among the top 25 keywords with the strongest citation bursts, “oxidative stress” was observed to be increasing in frequency recently (Figure 9C). There may be a continued focus on these emerging keywords in the future.
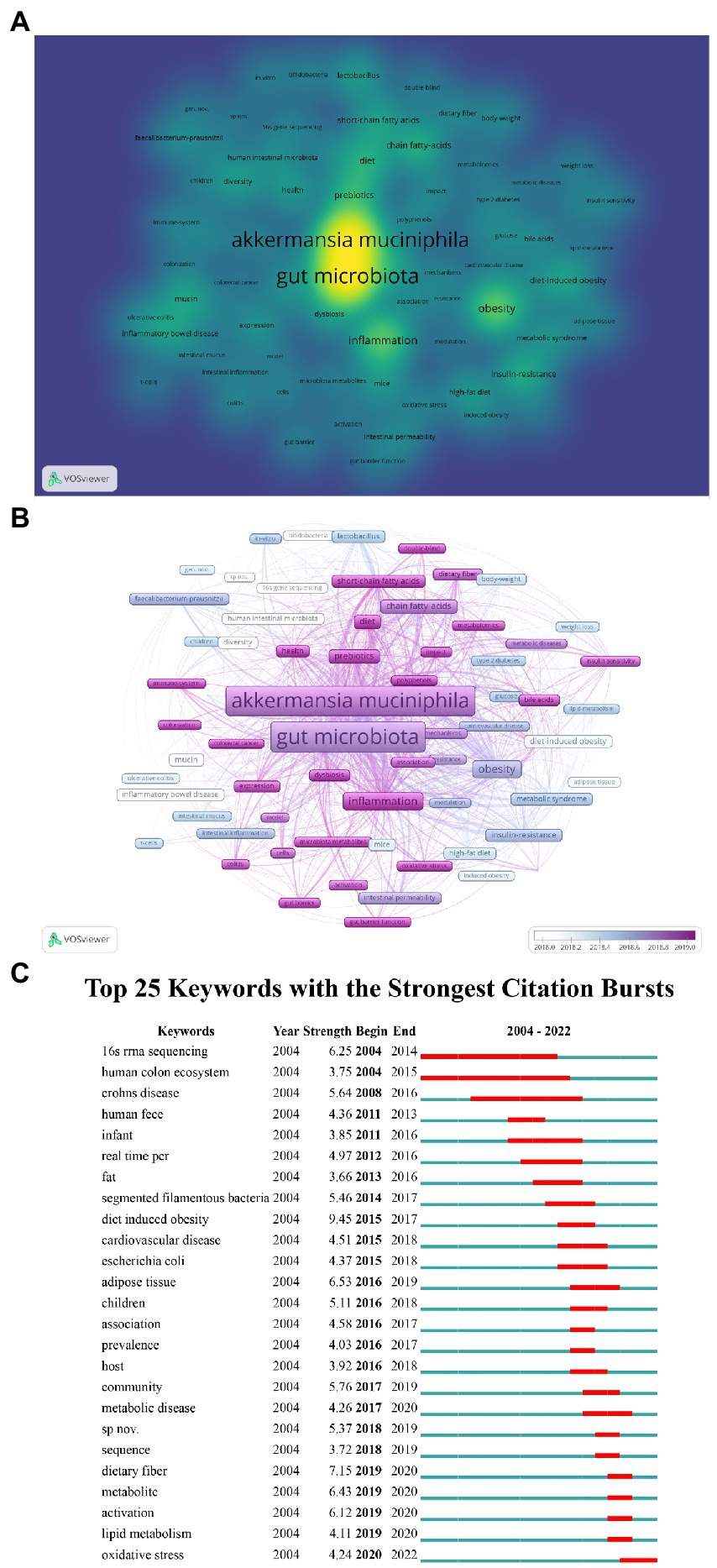
Figure 9. Author keywords analysis. (A) Keywords co-occurrence heatmap. The heatmap color and size reflect the frequency of keywords. (B) Overlay visualization of A. muciniphila-related keywords. Each node represents a different author keyword. The color of the nodes corresponds to the average year in which the keyword appeared according to the color gradient shown at the bottom right. (C) Top 25 A. muciniphila-related keywords with the strongest citation bursts (2004–2022).
Discussion
The frequency of A. muciniphila publications has shown exponential growth curve over the last 18 years, possibly due to growing treatment needs and advances in microbiome technology (e.g., 16S rRNA sequencing, metagenomics, metabolomics, etc.). The remarkable efficacy of A. muciniphila on obesity and diabetes has promoted its exploration in various fields. The cliff-like shape of the citation curve shown in Figure 3 indicates that this field is a research hotspot, and its popularity will continuously increase and become a hot research topic in the future.
The most productive countries were China and the United States. Initially, the United States was the topmost productive country; however, with increasing interest in the field among Chinese researchers, this gap gradually narrowed as publications from China increased in frequency. Combined with the institutional and funding agencies analyses, the high output of China and the United States is likely related to human investments and financial resources. The H-index is a crucial parameter for assessing the publication quality and academic influence of countries, institutions, journals, or researchers (Engqvist and Frommen, 2008). As with the H-index, the ACI can also represent the scientific output and academic status of publications. Based on the ACI, Finland played a crucial role in this field. Although China had the highest number of publications, the two institutions from China had the lowest average citation rate and H-index among the top ten institutions. Thus, the quality of the publications requires improvement. Cooperation between countries is essential, as strong cooperative relationships were observed among the countries with the most publications and highest ACI. Many countries/institutions have low influence levels, and inter-agency cooperation should be prioritized.
Analysis of the cooperative relationships between authors revealed inter-author connection networks. De Vos and Willem was the most productive author with the highest H-index. The author and the institutions and/or country the author belongs to can exert a significant influence on the emerging A. muciniphila research field. Furthermore, De Vos and Willem is a leading expert in gut microbiota research and is at the forefront of exploring microorganisms through molecular (meta-) genomics and systems approaches, focusing on the human gut (Belzer and de Vos, 2012). Another highly influential author is Cani and Patrice. Their research interests include interactions among gut microbes, the host, and specific biological systems, such as the endocannabinoid and the innate immune system, and their associations with metabolic disorders. Interestingly, De Vos and Willem and Patrice and Cani are co-founders of A-Mansia Biotech SA, the Akkermansia company.3 They facilitate the transformation of basic research into clinical applications (Depommier et al., 2019). It is evident that their academic collaboration has contributed to their current success in this field. Both of these pioneering researchers make significant contributions to this field, and many highly cited references and citation burst references were published by their teams (Everard et al., 2011, 2013; Belzer and de Vos, 2012; Schneeberger et al., 2015; Cani and de Vos, 2017; Derrien et al., 2017; Plovier et al., 2017; Depommier et al., 2019).
Journal statistics help researchers select suitable journals for publishing their research. Scientific Reports, Nutrients, and Frontiers in Microbiology were the major journals that published A. muciniphila-related articles. The betweenness centrality of nodes in a network is a vital centrality indicator (Wu et al., 2021b), indicating co-citation relationships between multiple nodes and which journals are “transportation hubs.” PNAS had high betweenness centrality and frequency, and is considered a keystone taxon in this field. As article carriers, journals reflect the position of published articles in the field. The key position of PNAS is the result of the articles published in the field, such as the study published by Everard et al. (Everard et al., 2013), which is discussed in the next paragraph. These journals are predicted to publish more high-quality research. A dual-journal overlay shows how topics and journals are interrelated (Li et al., 2022). Although this research draws on the distribution of the cited articles, citing articles on A. muciniphila were more active in the “veterinary, animal, science,” “molecular, biological, immunology,” and “medicine, medical, genetics” fields.
Within A. muciniphila research, highly cited references or references with strong citation bursts are important nodes through which A. muciniphila has distinguished itself from other probiotics and has become a “next generation probiotic.” Citations are a simple and effective indicator of the impact and quality of research. The article published in PNAS by Everard et al. (2013) has the highest citation frequency as well as a high mediation centrality, indicating that its content provides information on a currently relevant topic. This article proposes that live A. muciniphila can reverse high fat diet-induced metabolic disorder in mice by restoring mucus secretion and improving intestinal permeability, while heat-killed A. muciniphila lacks this effect. The article details an important milestone in our knowledge on the interaction between microbiota and the intestinal epithelium. The second most-cited article is a clinical research study published by Dao et al. (2016). The authors described the relationship between A. muciniphila and metabolism in overweight/obese adults after clinical dietary intervention. The article stated that A. muciniphila may be a potential prognostic tool for predicting the success of dietary interventions. The third most-cited paper was published by Plovier et al. (2017). This study identified a component of the A. muciniphila outer membrane protein-AMUC_1100 (which interacts with TLR2) that plays a role in reducing fat development, insulin resistance, and dyslipidemia in mice after pasteurization. This study proposed a solution for the unknown safety of A. muciniphila growth medium substances when ingested by humans. The most logical explanation for a sudden increase in the citation frequency of an article is that it addresses a specific lack of information in currently available literature (2003). The article with the strongest citation burst was the article published by Everard et al. (2013). The second-strongest citation burst was the randomized double-blind controlled study published by Depommier et al. (2019), which detailed the first human experimental results for A. muciniphila supplementation. This study indicated that A. muciniphila is safe and well-tolerated by patients; it also suggested that dead A. muciniphila bacteria may be more beneficial than live bacteria. The third strongest citation burst was that of an article published by Shin et al. (2014), which determined whether the antidiabetic effects of metformin were associated with changes in gut microbiota composition. It is evident from the changes in citation burst trends that the research hotspot had transitioned from the correlation between A. muciniphila and disease to the causal relationships between them, and from animal experiments to human studies of safety and efficacy.
Since keywords represent a publication’s core content, keyword co-occurrence analysis (a method developed through bibliometric research and data visualization) can be applied to identify popular research topics in a particular field at a certain time. Our results showed the following four main research directions in the A. muciniphila field (Figure 2):
i. External factors affecting A. muciniphila (e.g., “diet,” “polyphenols,” “metformin,” and “fecal microbiota transplantation”). In recent years, dietary strategies for improving gut A. muciniphila abundance have attracted research and development interest (Zhou, 2017). Although these promotion strategies are not necessarily applicable to the general population, these results strongly suggest the potential efficacy of certain foods or supplements for increasing intestinal A. muciniphila levels.
ii. The correlation between A. muciniphila and different diseases. The associations between A. muciniphila and metabolic disorders, including obesity, type 2 diabetes, nonalcoholic fatty liver disease, and cardiovascular diseases, are key to explaining many existing questions in A. muciniphila research. Highly cited references or references with strong citation bursts with respect to A. muciniphila research have all been related to metabolic disorders. For specific developmental milestones in research on A. muciniphila and metabolic disorders, please refer to the earlier discussion on references. In recent years, colitis and colorectal cancers have also attracted much attention. This could be attributed to the still-unclear relationship between A. muciniphila and colitis or colorectal cancers. Ring et al. (2019) demonstrated that A. muciniphila colonization does not affect colitis. This is somewhat different from the conclusions of an earlier study by Seregin et al., which reported that A. muciniphila can promote the occurrence of colitis in mouse models. Some studies have found that the abundance of A. muciniphila is increased in patients with colorectal cancer (Sanapareddy et al., 2012; Weir et al., 2013; Zackular et al., 2013; Dingemanse et al., 2015; Wang et al., 2022), whereas others have suggested that A. muciniphila is unrelated to colon tumors (Lopez-Siles et al., 2018) or even prevents colitis-associated colorectal cancer (Wang et al., 2020). Thus, the relationship between A. muciniphila and both diseases remains controversial. However, it is worth noting that most of the current studies have not distinguished between these microorganisms to the species level. To clarify the relationships between A. muciniphila and various diseases, this nuance should not be ignored in future clinical studies.
iii. Mechanisms underlying A. muciniphila–host (including bacteria) associations. Basic research focuses more on the biological mechanisms and potential therapeutic targets of A. muciniphila. The active components of A. muciniphila are still being clarified. Interestingly however, pasteurized A. muciniphila, live A. muciniphila, and even secreted proteins (e.g., Amuc_1100) and extracellular vesicles can regulate gut barrier function and/or the immune system by acting on different molecules. Regarding the effects of A. muciniphila on human health (e.g., “microbiota metabolites,” “immune system,” and “gut barrier function”), interested readers may wish to refer to previous detailed explorations of such topics (Yan et al., 2021; Rodrigues et al., 2022). The molecular mechanisms underlying these effects are an ongoing hot topic in the field. Recently, Bae et al. (2022) revealed that A. muciniphila induces immune cells to secrete specific cytokines via cell membrane phospholipids and resetting the activation threshold of dendritic cells, clarifying the molecular mechanism underlying A. muciniphila-mediated immune regulation in vitro. However, the molecular mechanisms underlying A. muciniphila–host interactions still require further research.
iv. The safety and efficacy of the clinical use of A. muciniphila (e.g., “double-blind”) (Depommier et al., 2019).
Among the top 25 keywords with the strongest citation bursts, “oxidative stress” showed increasing strength. Oxidative stress is caused by imbalances between intracellular reactive oxygen species and antioxidant defense systems (Papadia et al., 2008), and is considered an important risk factor for cardiovascular diseases, diabetes, and other diseases. Research on oxidative stress mainly focuses on three aspects: promoting oxidative stress, fighting oxidative stress, and balancing the oxidative and antioxidant systems. Several studies suggested that A. muciniphila may be associated with oxidative stress regulation (Yassour et al., 2016; Roshanravan et al., 2017; Mitsou et al., 2019; Wu et al., 2020; Zhang et al., 2020; Deng et al., 2021; Mesnage et al., 2021; Chen et al., 2022) and may promote oxidative stress resistance in various diseases (Cerro et al., 2022; Qian et al., 2022; Xia et al., 2022). Polyphenols decrease intestinal oxidative stress by inducing A. muciniphila growth (Anhê et al., 2015). The relationship between oxidative stress and A. muciniphila is an important topic for future research.
Our study has some limitations. Firstly, since it takes time for an article to achieve a certain number of citations, recent high-quality articles may not have been included, causing biased results. Secondly, there may be a time delay when exploring the research frontier. Finally, our analysis can only show the influence of the research content in the A. muciniphila research field, and cannot represent influences outside this field.
Conclusion
We evaluated and quantified articles on A. muciniphila and visualized the hotspots and global research trends in this field. Over the past 19 years, publications on A. muciniphila have increased significantly in frequency, with China having the highest number of publications. De Vos and Willem was the most productive author and had the highest H-index, followed by Cani and Patrice. “Oxidative stress,” “diet,” “metformin,” “fecal microbiota transplantation,” “short-chain fatty acids,” “polyphenols,” and “microbiota metabolites” are some of the frequently used keywords in recent years. These keywords are potential hotspots for future research and require further exploration. Although studies consider A. muciniphila to be a beneficial probiotic and has potential in the treatment of many diseases, providing an in-depth analysis of the mechanisms underlying its role in promoting human health with respect to high-frequency diseases may improve the research status of A. muciniphila.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding authors.
Author contributions
YC, ZL, QL, and PW: conceptualization. HK, YW, XL, and SC: data curation. HK and ZL: writing—original draft preparation. ZL, HK, YW, SC, and YC: writing—review and editing. YC, QL, and PW: supervision. YC: funding acquisition. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the Special Scientific Research Fund for National Natural Science Foundation, grant numbers 82070543 and 8177031240, and the National High Technology Research and Development Program of China, grant number 2021YFA0717001.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmicb.2022.1037708/full#supplementary-material
Abbreviations
ACI, Average citations per item; H-index, The Hirsch Index; IFs, Impact factors; JCR, Journal citation reports; MeSH, Medical subject headings; PNAS, Proceedings of the National Academy of Sciences of the United States of America; TLS, Total link strength; WoSCC, Web of Science Core Collection database.
Footnotes
References
Anhê, F. F., Roy, D., Pilon, G., Dudonné, S., Matamoros, S., Varin, T. V., et al. (2015). A polyphenol-rich cranberry extract protects from diet-induced obesity, insulin resistance and intestinal inflammation in association with increased Akkermansia spp. population in the gut microbiota of mice. Gut 64, 872–883. doi: 10.1136/gutjnl-2014-307142
Bae, M., Cassilly, C. D., Liu, X., Park, S.-M., Tusi, B. K., Chen, X., et al. (2022). Akkermansia muciniphila phospholipid induces homeostatic immune responses. Nature 608, 168–173. doi: 10.1038/s41586-022-04985-7
Bárcena, C., Valdés-Mas, R., Mayoral, P., Garabaya, C., Durand, S., Rodríguez, F., et al. (2019). Healthspan and lifespan extension by fecal microbiota transplantation into progeroid mice. Nat. Med. 25, 1234–1242. doi: 10.1038/s41591-019-0504-5
Belzer, C., and de Vos, W. M. (2012). Microbes inside—from diversity to function: the case of Akkermansia. ISME J. 6, 1449–1458. doi: 10.1038/ismej.2012.6
Blacher, E., Bashiardes, S., Shapiro, H., Rothschild, D., Mor, U., Dori-Bachash, M., et al. (2019). Potential roles of gut microbiome and metabolites in modulating ALS in mice. Nature 572, 474–480. doi: 10.1038/s41586-019-1443-5
Callahan, B. J., McMurdie, P. J., Rosen, M. J., Han, A. W., Johnson, A. J. A., and Holmes, S. P. (2016). DADA2: high-resolution sample inference from Illumina amplicon data. Nat. Methods 13, 581–583. doi: 10.1038/nmeth.3869
Cani, P. D., and de Vos, W. M. (2017). Next-generation beneficial microbes: the case of Akkermansia muciniphila. Front. Microbiol. 8:1765. doi: 10.3389/fmicb.2017.01765
Cerro, E. D.-D., Lambea, M., Félix, J., Salazar, N., Gueimonde, M., and De la Fuente, M. (2022). Daily ingestion of Akkermansia mucciniphila for one month promotes healthy aging and increases lifespan in old female mice. Biogerontology 23, 35–52. doi: 10.1007/s10522-021-09943-w
Chelakkot, C., Choi, Y., Kim, D.-K., Park, H. T., Ghim, J., Kwon, Y., et al. (2018). Akkermansia muciniphila-derived extracellular vesicles influence gut permeability through the regulation of tight junctions. Exp. Mol. Med. 50:e450. doi: 10.1038/emm.2017.282
Chen, C. (2004). Searching for intellectual turning points: progressive knowledge domain visualization. Proc. Natl. Acad. Sci. U. S. A. 101, 5303–5310. doi: 10.1073/pnas.0307513100
Chen, C., Dubin, R., and Kim, M. C. (2014). Emerging trends and new developments in regenerative medicine: a scientometric update (2000 – 2014). Expert. Opin. Biol. Ther. 14, 1295–1317. doi: 10.1517/14712598.2014.920813
Chen, J., Wang, M., Zhang, P., Li, H., Qu, K., Xu, R., et al. (2022). Cordycepin alleviated metabolic inflammation in Western diet-fed mice by targeting intestinal barrier integrity and intestinal flora. Pharmacol. Res. 178:106191. doi: 10.1016/j.phrs.2022.106191
Cheng, K., Guo, Q., Shen, Z., Yang, W., Wang, Y., Sun, Z., et al. (2022a). Bibliometric analysis of global research on cancer photodynamic therapy: focus on Nano-related research. Front. Pharmacol. 13:927219. doi: 10.3389/fphar.2022.927219
Cheng, K., Guo, Q., Yang, W., Wang, Y., Sun, Z., and Wu, H. (2022b). Mapping knowledge landscapes and emerging trends of the links between bone metabolism and diabetes mellitus: a Bibliometric analysis from 2000 to 2021. Front. Public Health 10:918483. doi: 10.3389/fpubh.2022.918483
Cheng, D., and Xie, M. Z. (2021). A review of a potential and promising probiotic candidate— Akkermansia muciniphila. J. Appl. Microbiol. 130, 1813–1822. doi: 10.1111/jam.14911
Cheng, K., Zhou, Y., and Wu, H. (2022c). Bibliometric analysis of global research trends on monkeypox: are we ready to face this challenge? J. Med. Virol. doi: 10.1002/jmv.27892 [Epub ahead of print].
Dao, M. C., Everard, A., Aron-Wisnewsky, J., Sokolovska, N., Prifti, E., Verger, E. O., et al. (2016). Akkermansia muciniphila and improved metabolic health during a dietary intervention in obesity: relationship with gut microbiome richness and ecology. Gut 65, 426–436. doi: 10.1136/gutjnl-2014-308778
Deng, Z., Wu, N., Wang, J., Geng, L., Yue, Y., Wang, F., et al. (2021). Low molecular weight fucoidan fraction LF2 improves metabolic syndrome via up-regulating PI3K-AKT-mTOR axis and increasing the abundance of Akkermansia muciniphila in the gut microbiota. Int. J. Biol. Macromol. 193, 789–798. doi: 10.1016/j.ijbiomac.2021.10.188
Depommier, C., Everard, A., Druart, C., Plovier, H., Van Hul, M., Vieira-Silva, S., et al. (2019). Supplementation with Akkermansia muciniphila in overweight and obese human volunteers: a proof-of-concept exploratory study. Nat. Med. 25, 1096–1103. doi: 10.1038/s41591-019-0495-2
Derrien, M., Belzer, C., and de Vos, W. M. (2017). Akkermansia muciniphila and its role in regulating host functions. Microb. Pathog. 106, 171–181. doi: 10.1016/j.micpath.2016.02.005
Derrien, M., Collado, M. C., Ben-Amor, K., Salminen, S., and de Vos, W. M. (2008). The Mucin degrader Akkermansia muciniphila is an abundant resident of the human intestinal tract. Appl. Environ. Microbiol. 74, 1646–1648. doi: 10.1128/AEM.01226-07
Derrien, M., Vaughan, E. E., Plugge, C. M., and de Vos, W. M. (2004). Akkermansia muciniphila gen. Nov., sp. nov., a human intestinal mucin-degrading bacterium. Int. J. Syst. Evol. Microbiol. 54, 1469–1476. doi: 10.1099/ijs.0.02873-0
Desai, M. S., Seekatz, A. M., Koropatkin, N. M., Kamada, N., Hickey, C. A., Wolter, M., et al. (2016). A dietary fiber-deprived gut microbiota degrades the colonic mucus barrier and enhances pathogen susceptibility. Cells 167, 1339–1353. doi: 10.1016/j.cell.2016.10.043
Dingemanse, C., Belzer, C., van Hijum, S. A. F. T., Günthel, M., Salvatori, D., den Dunnen, J. T., et al. (2015). Akkermansia muciniphila and helicobacter typhlonius modulate intestinal tumor development in mice. Carcinogenesis 36, 1388–1396. doi: 10.1093/carcin/bgv120
Engqvist, L., and Frommen, J. G. (2008). The h-index and self-citations. Trends Ecol. Evol. 23, 250–252. doi: 10.1016/j.tree.2008.01.009
Everard, A., Belzer, C., Geurts, L., Ouwerkerk, J. P., Druart, C., Bindels, L. B., et al. (2013). Cross-talk between Akkermansia muciniphila and intestinal epithelium controls diet-induced obesity. Proc. Natl. Acad. Sci. U. S. A. 110, 9066–9071. doi: 10.1073/pnas.1219451110
Everard, A., Lazarevic, V., Derrien, M., Girard, M., Muccioli, G. G., Neyrinck, A. M., et al. (2011). Responses of gut microbiota and glucose and lipid metabolism to prebiotics in genetic obese and diet-induced Leptin-resistant mice. Diabetes 60, 2775–2786. doi: 10.2337/db11-0227
Gopalakrishnan, V., Spencer, C. N., Nezi, L., Reuben, A., Andrews, M. C., Karpinets, T. V., et al. (2018). Gut microbiome modulates response to anti–PD-1 immunotherapy in melanoma patients. Science 359, 97–103. doi: 10.1126/science.aan4236
Grander, C., Adolph, T. E., Wieser, V., Lowe, P., Wrzosek, L., Gyongyosi, B., et al. (2018). Recovery of ethanol-induced Akkermansia muciniphila depletion ameliorates alcoholic liver disease. Gut 67, 891–901. doi: 10.1136/gutjnl-2016-313432
Ke, H., Li, F., Deng, W., Li, Z., Wang, S., Lv, P., et al. (2021). Metformin exerts anti-inflammatory and mucus barrier protective effects by enriching Akkermansia muciniphila in mice with ulcerative colitis. Front. Pharmacol. 12:726707. doi: 10.3389/fphar.2021.726707
Koh, A., De Vadder, F., Kovatcheva-Datchary, P., and Bäckhed, F. (2016). From dietary fiber to host physiology: short-chain fatty acids as key bacterial metabolites. Cells 165, 1332–1345. doi: 10.1016/j.cell.2016.05.041
Kokol, P., and Vošner, H. B. (2018). Discrepancies among Scopus, web of science, and PubMed coverage of funding information in medical journal articles. J. Med. Libr. Assoc. 106, 81–86. doi: 10.5195/jmla.2018.181
Li, Z., Ke, H., Lin, Q., Shen, Z., and Chen, Y. (2022). Global trends in gut microbiota and clostridioides difficile infection research: a visualized study. J. Infect. Public Health 15, 806–815. doi: 10.1016/j.jiph.2022.06.011
Li, J., Zhao, F., Wang, Y., Chen, J., Tao, J., Tian, G., et al. (2017). Gut microbiota dysbiosis contributes to the development of hypertension. Microbiome 5:14. doi: 10.1186/s40168-016-0222-x
Lopez-Siles, M., Enrich-Capó, N., Aldeguer, X., Sabat-Mir, M., Duncan, S. H., Garcia-Gil, L. J., et al. (2018). Alterations in the abundance and co-occurrence of Akkermansia muciniphila and Faecalibacterium prausnitzii in the colonic mucosa of inflammatory bowel disease subjects. Front. Cell. Infect. Microbiol. 8:281. doi: 10.3389/fcimb.2018.00281
Matson, V., Fessler, J., Bao, R., Chongsuwat, T., Zha, Y., Alegre, M.-L., et al. (2018). The commensal microbiome is associated with anti–PD-1 efficacy in metastatic melanoma patients. Science 359, 104–108. doi: 10.1126/science.aao3290
Mesnage, R., Teixeira, M., Mandrioli, D., Falcioni, L., Ducarmon, Q. R., Zwittink, R. D., et al. (2021). Use of shotgun Metagenomics and metabolomics to evaluate the impact of glyphosate or roundup MON 52276 on the gut microbiota and serum Metabolome of Sprague-Dawley rats. Environ. Health Perspect. 129:017005. doi: 10.1289/EHP6990
Mitsou, E. K., Detopoulou, M., Kakali, A., Fragopoulou, E., Nomikos, T., Antonopoulou, S., et al. (2019). Mining possible associations of faecal a. muciniphila colonisation patterns with host adiposity and cardiometabolic markers in an adult population. Benefic. Microbes 10, 741–749. doi: 10.3920/BM2019.0033
Olson, C. A., Vuong, H. E., Yano, J. M., Liang, Q. Y., Nusbaum, D. J., and Hsiao, E. Y. (2018). The gut microbiota mediates the anti-seizure effects of the Ketogenic diet. Cells 173, 1728.e13–1741.e13. doi: 10.1016/j.cell.2018.04.027
Papadia, S., Soriano, F. X., Léveillé, F., Martel, M.-A., Dakin, K. A., Hansen, H. H., et al. (2008). Synaptic NMDA receptor activity boosts intrinsic antioxidant defenses. Nat. Neurosci. 11, 476–487. doi: 10.1038/nn2071
Plovier, H., Everard, A., Druart, C., Depommier, C., Van Hul, M., Geurts, L., et al. (2017). A purified membrane protein from Akkermansia muciniphila or the pasteurized bacterium improves metabolism in obese and diabetic mice. Nat. Med. 23, 107–113. doi: 10.1038/nm.4236
Qian, K., Chen, S., Wang, J., Sheng, K., Wang, Y., and Zhang, M. (2022). A β-N-acetylhexosaminidase Amuc_2109 from Akkermansia muciniphila protects against dextran sulfate sodium-induced colitis in mice by enhancing intestinal barrier and modulating gut microbiota. Food Funct. 13, 2216–2227. doi: 10.1039/D1FO04094D
Ring, C., Klopfleisch, R., Dahlke, K., Basic, M., Bleich, A., and Blaut, M. (2019). Akkermansia muciniphila strain ATCC BAA-835 does not promote short-term intestinal inflammation in gnotobiotic interleukin-10-deficient mice. Gut Microbes 10, 188–203. doi: 10.1080/19490976.2018.1511663
Rodrigues, V. F., Elias-Oliveira, J., Pereira, Í. S., Pereira, J. A., Barbosa, S. C., Machado, M. S. G., et al. (2022). Akkermansia muciniphila and gut immune system: a good friendship that attenuates inflammatory bowel disease, obesity, and diabetes. Front. Immunol. 13:934695. doi: 10.3389/fimmu.2022.934695
Roshanravan, N., Mahdavi, R., Alizadeh, E., Ghavami, A., Rahbar Saadat, Y., Mesri Alamdari, N., et al. (2017). The effects of sodium butyrate and inulin supplementation on angiotensin signaling pathway via promotion of Akkermansia muciniphila abundance in type 2 diabetes; a randomized, double-blind, placebo-controlled trial. J Cardiovasc Thorac Res 9, 183–190. doi: 10.15171/jcvtr.2017.32
Routy, B., Le Chatelier, E., Derosa, L., Duong, C. P. M., Alou, M. T., Daillère, R., et al. (2018). Gut microbiome influences efficacy of PD-1–based immunotherapy against epithelial tumors. Science 359, 91–97. doi: 10.1126/science.aan3706
Sanapareddy, N., Legge, R. M., Jovov, B., McCoy, A., Burcal, L., Araujo-Perez, F., et al. (2012). Increased rectal microbial richness is associated with the presence of colorectal adenomas in humans. ISME J. 6, 1858–1868. doi: 10.1038/ismej.2012.43
Schneeberger, M., Everard, A., Gómez-Valadés, A. G., Matamoros, S., Ramírez, S., Delzenne, N. M., et al. (2015). Akkermansia muciniphila inversely correlates with the onset of inflammation, altered adipose tissue metabolism and metabolic disorders during obesity in mice. Sci. Rep. 5:16643. doi: 10.1038/srep16643
Shin, N.-R., Lee, J.-C., Lee, H.-Y., Kim, M.-S., Whon, T. W., Lee, M.-S., et al. (2014). An increase in the Akkermansia spp. population induced by metformin treatment improves glucose homeostasis in diet-induced obese mice. Gut 63, 727–735. doi: 10.1136/gutjnl-2012-303839
van Eck, N. J., and Waltman, L. (2010). Software survey: VOSviewer, a computer program for bibliometric mapping. Scientometrics 84, 523–538. doi: 10.1007/s11192-009-0146-3
Wang, F., Cai, K., Xiao, Q., He, L., Xie, L., and Liu, Z. (2022). Akkermansia muciniphila administration exacerbated the development of colitis-associated colorectal cancer in mice. J. Cancer 13, 124–133. doi: 10.7150/jca.63578
Wang, L., Tang, L., Feng, Y., Zhao, S., Han, M., Zhang, C., et al. (2020). A purified membrane protein from Akkermansia muciniphila or the pasteurised bacterium blunts colitis associated tumourigenesis by modulation of CD8+ T cells in mice. Gut 69, 1988–1997. doi: 10.1136/gutjnl-2019-320105
Weir, T. L., Manter, D. K., Sheflin, A. M., Barnett, B. A., Heuberger, A. L., and Ryan, E. P. (2013). Stool microbiome and metabolome differences between colorectal cancer patients and healthy adults. PLoS One 8:e70803. doi: 10.1371/journal.pone.0070803
Wu, H., Cheng, K., Guo, Q., Yang, W., Tong, L., Wang, Y., et al. (2021a). Mapping knowledge structure and themes trends of osteoporosis in rheumatoid arthritis: a Bibliometric analysis. Front Med (Lausanne) 8:787228. doi: 10.3389/fmed.2021.787228
Wu, L., Lyu, Y., Srinivasagan, R., Wu, J., Ojo, B., Tang, M., et al. (2020). Astaxanthin-shifted gut microbiota is associated with inflammation and metabolic homeostasis in mice. J. Nutr. 150, 2687–2698. doi: 10.1093/jn/nxaa222
Wu, H., Tong, L., Wang, Y., Yan, H., and Sun, Z. (2021b). Bibliometric analysis of global research trends on ultrasound microbubble: a quickly developing field. Front. Pharmacol. 12:646626. doi: 10.3389/fphar.2021.646626
Xia, J., Lv, L., Liu, B., Wang, S., Zhang, S., Wu, Z., et al. (2022). Akkermansia muciniphila ameliorates acetaminophen-induced liver injury by regulating gut microbial composition and metabolism. Microbiol Spectr 10:e0159621. doi: 10.1128/spectrum.01596-21
Yan, J., Sheng, L., and Li, H. (2021). Akkermansia muciniphila: is it the holy grail for ameliorating metabolic diseases? Gut Microbes 13:1984104. doi: 10.1080/19490976.2021.1984104
Yassour, M., Lim, M. Y., Yun, H. S., Tickle, T. L., Sung, J., Song, Y.-M., et al. (2016). Sub-clinical detection of gut microbial biomarkers of obesity and type 2 diabetes. Genome Med. 8:17. doi: 10.1186/s13073-016-0271-6
Zackular, J. P., Baxter, N. T., Iverson, K. D., Sadler, W. D., Petrosino, J. F., Chen, G. Y., et al. (2013). The gut microbiome modulates colon tumorigenesis. MBio 4, e00692–e00613. doi: 10.1128/mBio.00692-13
Zhang, T., Li, Q., Cheng, L., Buch, H., and Zhang, F. (2019). Akkermansia muciniphila is a promising probiotic. Microb. Biotechnol. 12, 1109–1125. doi: 10.1111/1751-7915.13410
Zhang, M., Zou, X., Zhao, D., Zhao, F., and Li, C. (2020). Pork meat proteins Alter gut microbiota and lipid metabolism genes in the colon of adaptive immune-deficient mice. Mol. Nutr. Food Res. 64:1901105. doi: 10.1002/mnfr.201901105
Keywords: Akkermansia muciniphila, gut microbiota, bibliometrics, trends, visualization
Citation: Li Z, Ke H, Wang Y, Chen S, Liu X, Lin Q, Wang P and Chen Y (2022) Global trends in Akkermansia muciniphila research: A bibliometric visualization. Front. Microbiol. 13:1037708. doi: 10.3389/fmicb.2022.1037708
Edited by:
Muhammad Shahid Riaz Rajoka, Tohoku University, JapanCopyright © 2022 Li, Ke, Wang, Chen, Liu, Lin, Wang and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ye Chen, eWVjaGVuQHNtdS5lZHUuY24=; Pu Wang, NTY0MDM1NDQ4QHFxLmNvbQ==; Qianyun Lin, bGlucWlhbnl1bkBmb3htYWlsLmNvbQ==
†These authors have contributed equally to this work and share first authorship
 Zitong Li
Zitong Li Haoran Ke
Haoran Ke Ying Wang
Ying Wang Shuze Chen
Shuze Chen Xiuying Liu1
Xiuying Liu1 Pu Wang
Pu Wang Ye Chen
Ye Chen