
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Microbiol. , 24 September 2021
Sec. Antimicrobials, Resistance and Chemotherapy
Volume 12 - 2021 | https://doi.org/10.3389/fmicb.2021.737626
This article is part of the Research Topic Biofilms: Multi-species Community Interactions View all 6 articles
Quorum sensing (QS) and biofilm formation inhibition activity of esculetin on Aeromonas hydrophila SHAe 115 were evaluated. Exposure to esculetin at 25, 50, and 100μg/ml significantly inhibited the production of protease and hemolysin, the formation of biofilms and attenuated the swarming motility of A. hydrophila SHAe 115. Biofilm forming inhibition was also observed through confocal laser scanning microscopy and scanning electron microscope. Quantitative real-time PCR analysis indicated that genes positively related to QS and biofilm formation were downregulated to varying degrees, while gene (litR) negatively related to biofilm formation was significantly upregulated. The phenotypic results were in good agreement with gene expression levels. These results indicated that esculetin would be a potential QS inhibitor for A. hydrophila.
In the past few decades, indiscriminate use of antibiotics has led to the emergence of multiple drug-resistant bacteria, which has become a major problem threatening the global medical health and public health system (Tanwar et al., 2014). In most cases, due to the way antibiotics kill bacteria or inhibit bacterial growth, selective pressure leads to the emergence of resistant strains. Over the past decade, the development of new antibiotics has declined sharply, while drug-resistant strains have become tougher (Gutiérrez-Barranquero et al., 2015). Therefore, there is an urgent need to develop alternative therapies. These therapies need to target these multi-drug resistant strains in a biofilm state and will not exert selective pressure on resistant strains. The discovery of the bacterial quorum sensing (QS) system provides us with such a promising strategy to prevent and control microbial infections. QS is a cell-to-cell signaling communication system, it involves the production, release, and subsequent detection of chemical signaling molecules, called autoinducers, by a population-density-dependent intercellular communication system allowing bacteria to control the expression of genes related to virulence and pathogenesis. In general, these autoinducers include N-acyl homoserine lactones (AHL) and oligopeptides in gram-negative and gram-positive bacteria, respectively. QS controls the virulence behavior of a broad spectrum of bacterial pathogens and participate in the biofilm formation, a key driver of antibiotic resistance in many infections (Miller and Bassler, 2000; Waters and Bassler, 2005). Hence, QS systems have been proposed as an effective target for antimicrobial therapy, cause it can be blocked by ways of inhibiting the AHL molecule biosynthesis, degrading the synthesized AHL molecules and/or inactivating the AHL receptor protein (Belapurkar et al., 2014).
Aeromonas hydrophila is a conditional pathogen that is common to humans, livestock, and aquatic animals (Zhou and Zhou, 2012). A. hydrophila can infect molluscs (Grizzle and Brunner, 2009), crustaceans (Ma et al., 2012), fish (Liang and Xie, 2013; Zhao and Wang, 2015), amphibians (Meng et al., 2009), reptiles (Chen et al., 2003), and poultry (Pan et al., 2003). Humans can suffer from diarrhea, food poisoning, and secondary infections due to pathogenic A. hydrophila infection (Yang and Wang, 2006). A. hydrophila not only poses a threat to human health, but also cause huge economic losses to the aquaculture industry, which has attracted great attention over the world.
Many natural compounds have been reported as QS inhibitors (Jakobsen et al., 2012; Figueroa et al., 2014; Venkadesaperumal et al., 2015), and some of the most effective QS inhibition molecules derived from plants are coumarins, a large structurally diverse family of plant phenolic compounds characterized by their pharmacological properties (Venugopala et al., 2013). Biological studies on coumarins demonstrated that these compounds have potential activities, such as antitumor (Vanamala et al., 2006; Tehsina et al., 2011; Jamier et al., 2014), anti-inflammatory (Fylaktakidou et al., 2004; Bucolo et al., 2009), anticoagulant (Payá et al., 1992; Fereshteh et al., 2014), and antibacterial (Ojala et al., 2000; Souza et al., 2005). Among coumarins, hydroxylated coumarins, such as umbelliferone, daphnetin, and esculetin, showed stronger bioactivities. Wang et al. (2017) demonstrated that esculetin has obvious antibacterial effect on KPC-producing Klebsiella pneumoniae. Esculetin also has superior antibacterial activity against the phytopathogen Ralstonia solanacearum and can inhibit its biofilm formation (Yang et al., 2016). Thomas et al. (2017) showed that esculetin has a good inhibitory effect on the QS-regulated transcription factor SdiA of Salmonella typhi through molecular docking. Recent reports on coumarins inhibiting biofilm formation and reducing virulence factors of Escherichia coli and Pseudomonas aeruginosa (Lee et al., 2014) have drawn the attention of researchers to the potential of coumarins as QS inhibitors and anti-biofilm agents. According to Duncan et al. (1998), esculetin and umbelliferone could inhibit the growth of E. coli O157:H7. Dürig et al. (2010) reported that esculetin was able to prevent biofilm formation of Staphylococcus aureus without affecting its cell growth. D’Almeida et al. (2017) conducted a comparison of seven structurally related coumarins (coumarin and different hydroxylated derivatives) on the QS inhibitory and anti-biofilm activities against P. aeruginosa and Chromobacterium violaceum. The results showed that molecules with hydroxyl groups on the aromatic ring have higher activity on virulence factors inhibition and biofilm formation. Besides, research of Lee et al. (2014) showed that hydroxylation in position C-4 dramatically diminishes the anti-biofilm activity on E. coli O157:H7 while hydroxylation in position C-7 enhances it.
According to the above introduction, esculetin has good inhibitory activity against many bacteria, and many plants contain this compound. Our previous phytochemical work also isolated this compound. However, no study on the inhibitory effect of esculetin against A. hydrophila has been reported. Hereby, we investigated the influence of esculetin on QS-related virulence factors and biofilm formation of A. hydrophila SHAe115. We hope that esculetin can mitigate human disease caused by A. hydrophila and/or reduce the loss of aquaculture caused by A. hydrophila.
Aeromonas hydrophila SHAe 115 used in this study was obtained from China General Microbiological Culture Collection Center. All experiments were conducted at 37°C in Luria-Bertani (LB) medium.
Esculetin was isolated from Onosma bracteatum Wall. in our previous study (Sun et al., 2021). It was dissolved in DMSO to prepare a stock solution of 50mg/ml.
Esculetin was tested against A. hydrophila SHAe 115 to determine the minimum inhibitory concentration (MIC) according to Clinical and Laboratory Standards Institute (2015) (Zhou et al., 2017) and with an inoculum of 1–5×105CFUml−1. The OD620 value of bacterial cultures of A. hydrophila SHAe 115 at this concentration was approximately 0.1. Two-fold dilution method was used and a series of diluted esculetin solution (25, 50, 100, 200, 400, and 800μg/ml) was performed in LB broth. The experiment was carried out in 96-well polystyrene microtiter plate, with 10 wells for each concentration. MIC is defined as the minimum concentration of esculetin which inhibited the visible growth of A. hydrophila SHAe 115 and sub-MICs were selected for the assessment of anti-virulence and anti-biofilm activity.
For growth measurement, overnight cultures of A. hydrophila SHAe 115 were inoculated into fresh LB medium and the optical density (OD) value was adjusted to 0.1 at 620nm. The cultures were transferred to 96-well polystyrene microtiter plate and supplemented with esculetin of different final concentrations (25, 50, and 100μg/ml), and then incubated continuously at 37°C for 24h with shaking (180rpm). DMSO was set as the negative control, and each concentration was set for 10 wells. The bacterial growth was monitored at 1h intervals, and the OD620 was recorded by a microplate reader (Biotek, United States).
The hemolysin assay was performed with a few changes based on the method of Zhou et al. (2019). 1% overnight culture of A. hydrophila SHAe 115 was added to fresh LB medium (OD620 ≈0.1) and cultivated in 24-well polystyrene microtiter plate with or without esculetin at 37°C for 24h with shaking (180rpm). The final concentrations of esculetin were 25, 50, and 100μg/ml. DMSO was set as the negative control, and three parallel groups were set for each concentration. After the cultivation, the cultures were centrifuged at 10000rpm for 10min (4°C), and the cell free supernatants were collected. 100μl of cell free supernatants of esculetin treated and untreated cultures were mixed with 900μl of 2% washed sheep blood (in PBS, pH 7.2). The mixture was incubated for 1h at 37°C followed by 10min of centrifugation at 3000rpm. Absorbance of the supernatant was measured at 530nm.
Azocasein assay was conducted to evaluated the total proteolytic activity of A. hydrophila SHAe 115, and the activity was determined by the method of Ding et al. (2018). 75μl of the abovementioned cell free supernatant obtained from each group was added to 125μl of 0.3% azocasein solution (in 50mM Tris-HC1 and 0.5mM CaCl2) and incubated at 30°C for 15min. The reaction was ended by adding 600μl of 10% trichloroacetic acid. After centrifugation for 10min at 10000rpm, 700μl of NaOH (1M) was mixed with the supernatant and the OD440 was recorded by a microplate reader (Biotek, United States).
The swarming agar was freshly prepared with 0.8% nutrient broth (NB) medium, 0.5% glucose, and 0.3% agar (pH 7.2). 2μl overnight culture of A. hydrophila SHAe 115 was inoculated at the center of the agar plate containing a series of esculetin (25, 50, and 100μg/ml). DMSO was set as the control, and three parallel groups were set for each concentration. The plates were cultured at 37°C for 24h, and the swarming migration diameters were recorded (Zhou et al., 2019).
The effect of esculetin at sub-MICs on biofilm formation was measured according to Ding et al. (2018) with some modifications. Briefly, 1% overnight culture of A. hydrophila SHAe 115 was added to fresh LB medium (OD620 ≈ 0.1) and transferred to a 96-well polystyrene microtiter plate then incubated in the presence and absence of esculetin for 24h at 37°C without shaking. DMSO served as the negative control with 10 wells for each concentration. After the incubation, planktonic cells and spent media were discarded and the biofilms were washed with PBS (pH 7.2) for three times. After being fixed with methanol for 15min, the biofilms were stained with 0.05% crystal violet (CV). Further, excess stain was removed and the biofilms were rinsed three times with PBS (pH 7.2) and bound CV was dissolved with 95% ethanol. Biofilm biomass was quantified by measuring the absorbance of crystal violet-ethanol solutions at 570nm (Biotek, United States).
One percent overnight culture of A. hydrophila SHAe 115 was added to fresh LB medium (OD620 ≈ 0.1), and cultivated in 24-well polystyrene microtiter plate containing glass slides (d=14mm) with and without esculetin. Culture was incubated without agitation at 37°C for 24h. DMSO was set as the negative control, and three parallel groups were set for each concentration. After the incubation, planktonic cells and spent media were removed and the glass slides were gently rinsed three times with PBS (pH 7.2).
For scanning electron microscopy (SEM) observation, samples were prepared with the method described by Zhou et al. (2017). Biofilms on the glass slides were fixed with 2.5% glutaraldehyde and dehydrated with graded ethanol (50, 70, 80, 90, and 100%). Subsequently the slides were freeze-dried, gold-coated, and then observed under SEM (Thermoscientific, Verios G4 UC).
Method used in confocal laser scanning microscopy (CLSM) observation was according to Zhou et al. (2018). Briefly, the dried samples were stained with acridine orange (0.1%) for 15min and excess dye was discarded. After being washed with PBS (pH 7.2), the slides were then fixed with paraformaldehyde (4%) for 15min in the dark and subsequently subjected to CLSM (Nikon, A1+ SIM-S). For each group, we randomly selected five areas for image analysis.
The quantitative real-time PCR (qRT-PCR) assay was carried out under the guidance of Zhou et al. (2018) with slight modification. A. hydrophila SHAe 115 was grown in LB medium supplemented with or without esculetin (100μg/ml) at 37°C at 180rpm for 24h. After incubation, cells were washed with sterile PBS (pH 7.2) three times and collected after 10min centrifugation at 4°C. Total RNA was extracted from the bacterial cells using an RNA extraction kit (Biofit Biotechnologies, Chengdu, China) following the manufacturer’s instruction. Reverse transcript reaction was performed with a commercial reverse-transcription enzyme (Tsingke Biotechnology, Beijing, China) according to the manufacturer’s instruction. Quantitative real-time PCR was carried out with an ABI 7300 Plus real-time PCR system. The amplification was carried out in a 20μl reaction volume containing 2×T5 Fast qPCR Mix (SYBR Green I; 10μl, Tsingke Biotechnology, Beijing, China), primers (0.8μl of each), diluted cDNA (1μl), and ddH2O (7.4μl). The thermocycling conditions were as follows: incubation for 10min at 95°C, followed by denaturation for 15s at 95°C, annealing and extension at 60°C for 60s. PCR amplification consisting of 45cycles was conducted. All samples were run in triplicate. The primers used in this study were listed in Table 1 (Kozlova et al., 2011). 16S rRNA served as an internal control (Patel et al., 2017). The relative expression of target genes was calculated by the conventional 2−ΔΔCT method proposed by Pfaffl (2001).
All of the experiments were performed in triplicate, and the data were presented as mean±standard deviation (SD). Statistical analysis was carried out with GraphPad Prism 8 software (San Diego, CA, United States). One-way ANOVA plus post-hoc Tukey test or two-tail paired t-test was used to evaluate the statistical significance between groups. The following terminology is used to denote the statistical significance: *p <0.05, **p <0.01, ***p <0.001.
The MIC of esculetin evaluated by two-fold dilution was 200μg/ml. All the following experiments were conducted at sub-MICs (25, 50, and 100μg/ml). The bacterial cultures treated with esculetin at sub-MICs did not show any significant inhibitory effect on growth of A. hydrophila SHAe 115 compared with the control (Figure 1).
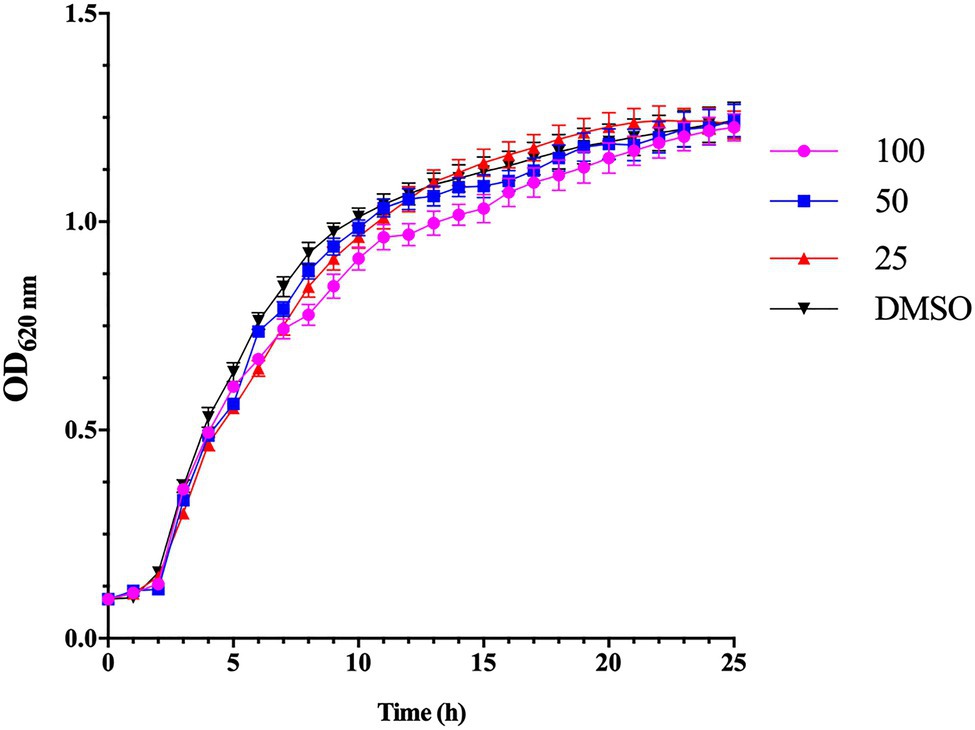
Figure 1. Effect of esculetin at sub-MICs (25, 50, and 100μg/ml) on growth of Aeromonas hydrophila SHAe 115. The data represent the mean values of experiments performed in triplicates. Data are presented as the absorbance of mean±SD.
As shown in Figure 2, esculetin has a significant inhibitory effect on the production of hemolysin of A. hydrophila SHAe 115 at sub-MICs. Compared to the DMSO control group, treatment with esculetin at 25, 50, and 100μg/ml caused reduction in hemolysin production of approximately 77, 86, and 87%, respectively.
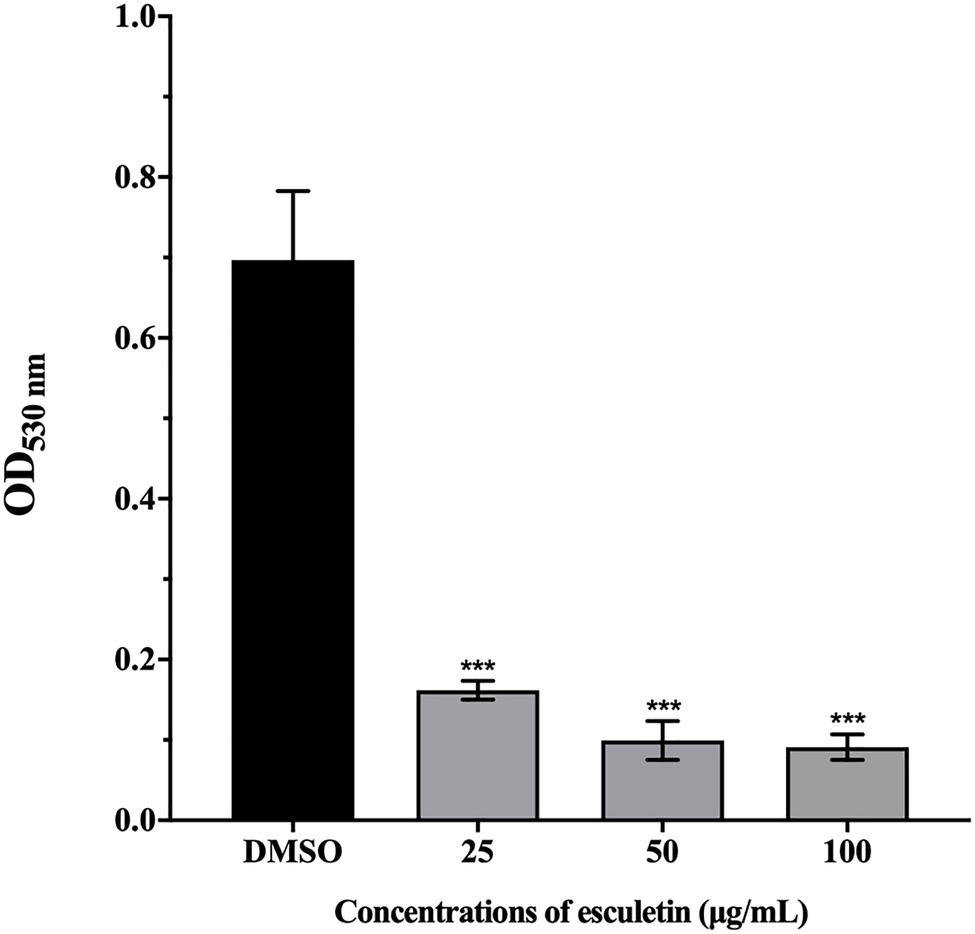
Figure 2. Effect of esculetin at sub-MICs (25, 50, and 100μg/ml) on hemolysin production of A. hydrophila SHAe 115. Data are presented as the absorbance of mean±SD of three independent experiments. ***p<0.001 compared to the DMSO control group by one-way ANOVA.
Bleeding is a common phenomenon that A. hydrophila infects animals, and the hemolytic activity can be detected in vivo and in vitro. Hemolysin is considered to be the main virulence factor of A. hydrophila, so the role of hemolysin is highly valued (James and Trevor, 1988). The hemolysin is a single polypeptide molecule, it is one of the exotoxins (also known as aerolysin) produced by A. hydrophila. Allan and Stevenson (1981) injected the exotoxin of A. hydrophila on rainbow trout and speckled trout, which caused disease in these fish. Thune et al. (1986) isolated β-hemolysin from a protease-deficient strain of A. hydrophila that can kill catfish. Most studies proved that aerolysin genes are highly conserved in Aeromonas spp. (Chacón et al., 2003; Lu et al., 2004; Nam and Joh, 2007), indicating that they play an important role in pathogenicity. Our results showed that esculetin significantly inhibited the hemolysin production of A. hydrophila SHAe 115, which proved that esculetin can effectively attenuate the pathogenicity of this bacteria.
This assay was carried out to analyze the potential of esculetin in inhibiting the production of protease in A. hydrophila SHAe 115. Azocasein was used as the substrate. The obtained results indicated that the production of protease was significantly reduced. And the inhibitory ability of esculetin on protease increased with concentration. Protease production was inhibited in the level of 31, 41, and 46%, respectively, in groups supplied with esculetin at concentrations of 25, 50, and 100μg/ml.
Extracellular proteases (Chu and Lu, 2000) are one of the virulence factors of A. hydrophila. At present, metalloproteases and serine proteases are widely studied. They are widely present in A. hydrophila. Some extracellular proteases have direct pathogenicity, and some can activate other pathogenic factors. For example, the exotoxins secreted by A. hydrophila are in the form of an inactive precursor and require extracellular proteases to activate them (Chacón et al., 2003). In our study, as concentration increased, the inhibitory ability of esculetin on proteases gradually increased, as shown in Figure 3. On one hand, the reduction of protease activity can reduce the pathogenicity of A. hydrophila SHAe 115; on the other hand, the lack of protease activation of exotoxins (such as hemolysin) will also reduce the pathogenicity of the bacteria.
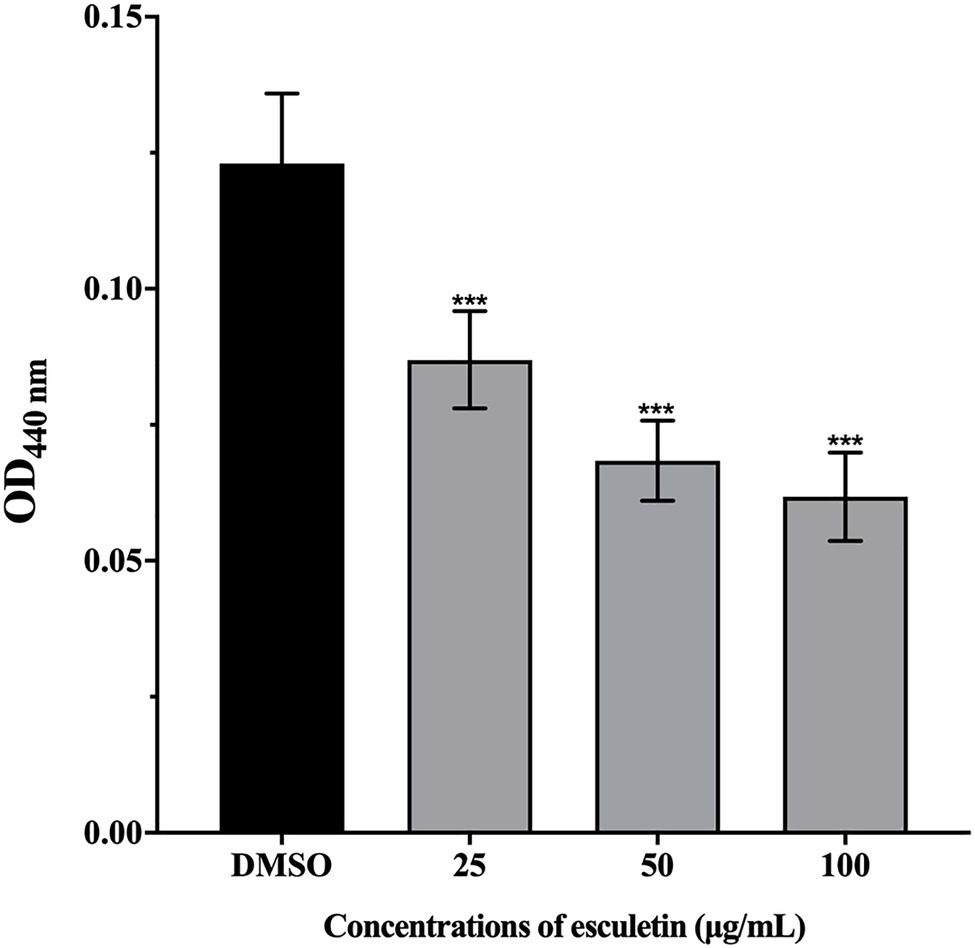
Figure 3. Effect of esculetin at sub-MICs (25, 50, and 100μg/ml) on protease activity of A. hydrophila SHAe 115. Data are presented as the absorbance of mean±SD of three independent experiments. ***p<0.001 compared to the DMSO control group by one-way ANOVA.
The swarming motility of A. hydrophila SHAe 115 was clearly visible in DMSO control (Figure 4A). However, on esculetin supplements at concentrations from 25 to 100μg/ml, the swarming motility of A. hydrophila SHAe 115 was repressed (Figures 4B–D). And, the diameter of swarming zone decreased as the concentration increased. The bacteria exhibited a total swarming diameter of 24mm. When treated with esculetin at sub-MICs, the swarming diameter decreased to 16, 12, and 8mm, respectively, with an inhibition of 32, 48, and 66% (Figure 4E).
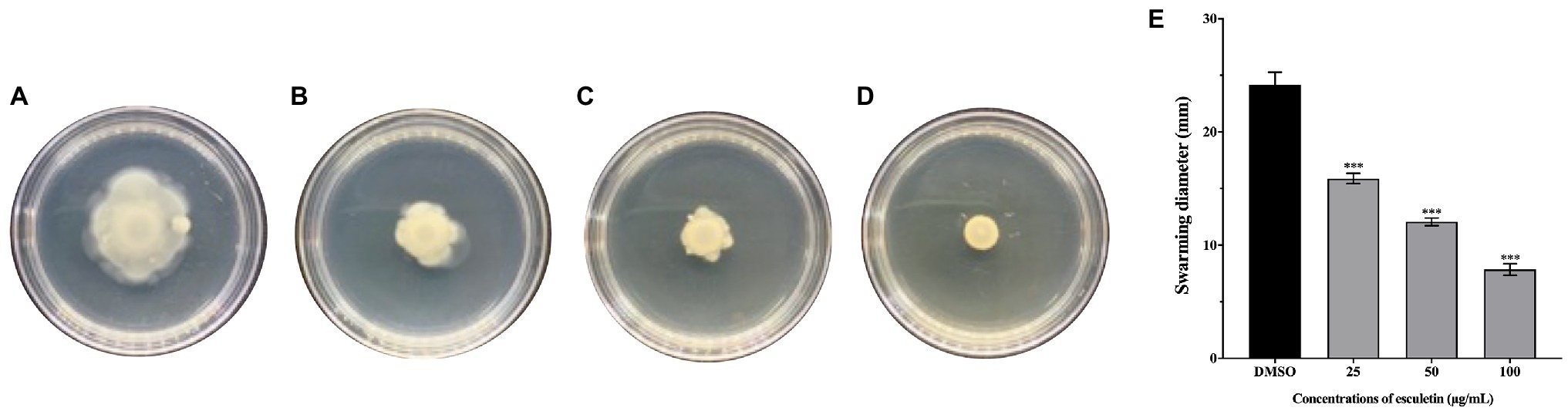
Figure 4. Effect of esculetin at sub-MICs (25, 50, and 100μg/ml) on swarming motility of A. hydrophila SHAe 115. (A) DMSO, (B) 25 μg/ml, (C) 50 μg/ml, (D) 100μg/ml, (E) Swarming zone diameter. Data are presented as the swarming diameter of mean±SD of three independent experiments. ***p<0.001 compared to the DMSO control group by one-way ANOVA.
The swarming motility of bacteria has been characterized as flagellar-mediated motility which is regulated by QS system (Köhler et al., 2000; Déziel et al., 2003). And the motility driven by flagellum played an import part in the pathogenicity of the bacteria. Previous studies have suggested that swarming motility can contribute to biofilm formation (Shrout et al., 2010). The swarming motility of A. hydrophila SHAe 115 was significantly inhibited by esculetin at sub-MICs (Figure 4). As shown in the figure, the diameters of swarming zone of the bacteria treated with esculetin were much smaller than that of the control, suggesting that esculetin had some inhibitory effects on the swarming motility of the bacteria. And the reason might be the interference of QS system in the pathogen caused by esculetin.
As shown in Figure 5A, esculetin significantly inhibited the biofilm formation. As the concentration increased, the inhibitory activity of esculetin acted in a concentration-dependent manner. The biofilm inhibition rate was approximately 38, 60, and 79%, respectively, as the bacteria treated with esculetin at 25, 50, and 100μg/ml.
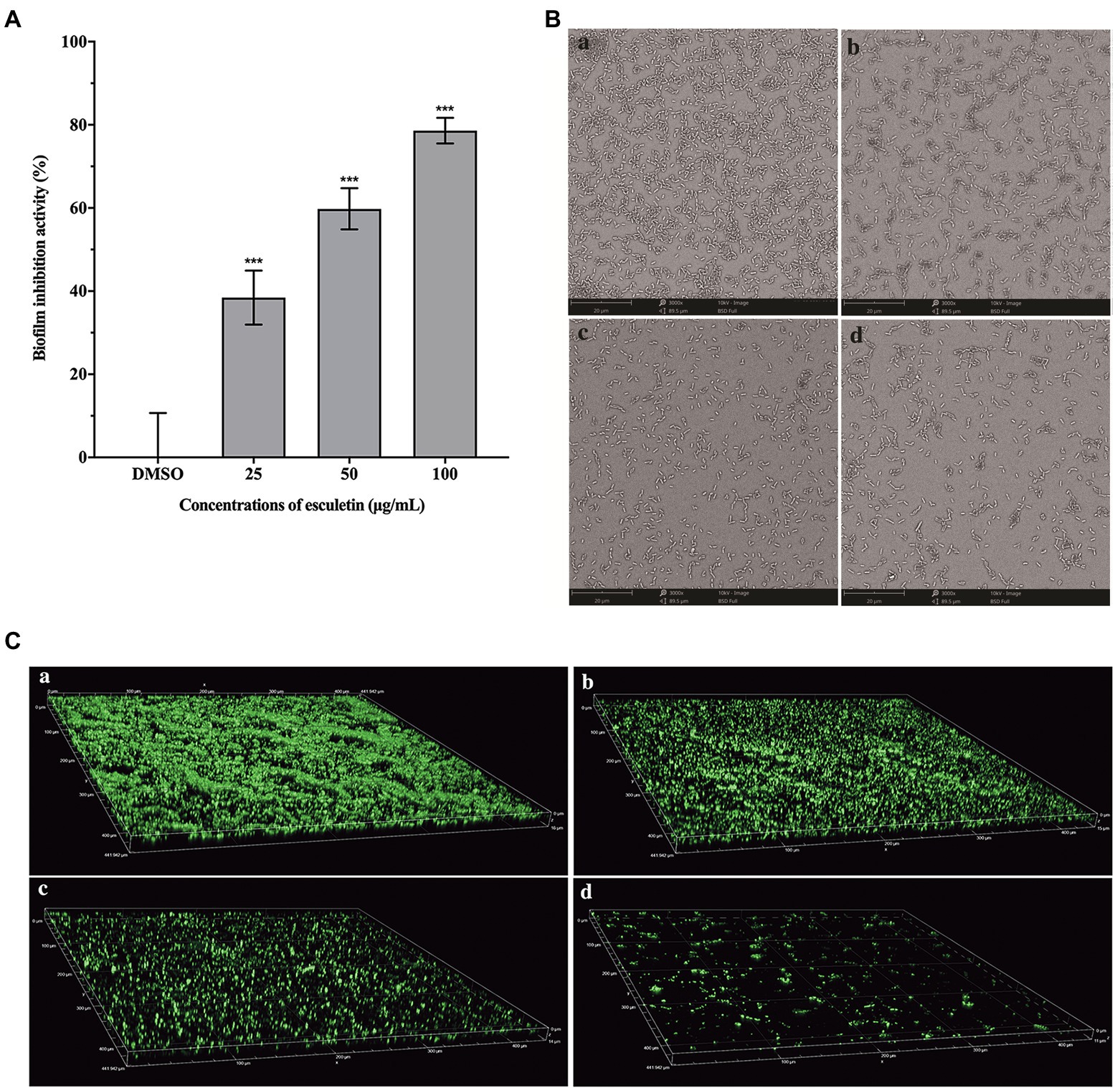
Figure 5. Effect of esculetin on biofilm formation. (A) Quantitative analysis of biofilm biomass, (B) SEM images, and (C) CLSM images of A. hydrophila SHAe 115 biofilms treated with (a) DMSO, (b) 25μg/ml, (c) 50μg/ml, and (d) 100μg/ml of esculetin. Data are presented as the inhibition rate of mean±SD of three independent experiments. ***p<0.001 compared to the DMSO control group by one-way ANOVA.
In addition to the quantitative analysis of the biofilm biomass with CV staining method, we also observed the development of biofilm structure through SEM and CLSM after incubation in the presence and absence of esculetin. SEM images showed that the biofilm was thick and dense in the DMSO control group (Figure 5Ba), while with esculetin at sub-MICs treatment, the biofilm was hindered and finally turned sparse as the concentration increased (Figure 5Bb–d). CLSM images also demonstrated similar results. After incubated in the presence of esculetin, the biofilm got sparser with the increased concentration and its thickness reduced from 16 to 11μm (Figures 5Ca–d).
Biofilms are microbial communities, in which bacterial cells are embedded in a self-generated matrix of lipids, exopolysaccharides (EPS), proteins, and nucleic acids that can block the entry of antimicrobial agents into cells (Ramanathan et al., 2017; Zhou et al., 2017). Biofilms are closely related to the multi-drug resistance of many bacteria (Morohoshi et al., 2007). Therefore, destroying or inhibiting the formation of biofilm can be an effective way to attenuate the pathogenicity and drug resistance of bacteria. Our results suggested that esculetin significantly inhibited the biofilm formation of A. hydrophila SHAe 115, making it loose and sparse. The possible reason was that esculetin affected the synthesis of the matrix (such as EPS) composing the biofilm.
The qRT-PCR assay was carried out to examine the effect of esculetin at 100μg/ml on changes in the gene expression of motility and biofilm formation. The results showed that ahyI, ahyR, luxS, csgAB, and fleQ were significantly downregulated and their expression levels were reduced by 32, 29, 39, 12, and 65%, respectively. While litR was upregulated, the expression level increased by 40%. Esculetin had no obvious effect on fleN (Figure 6).
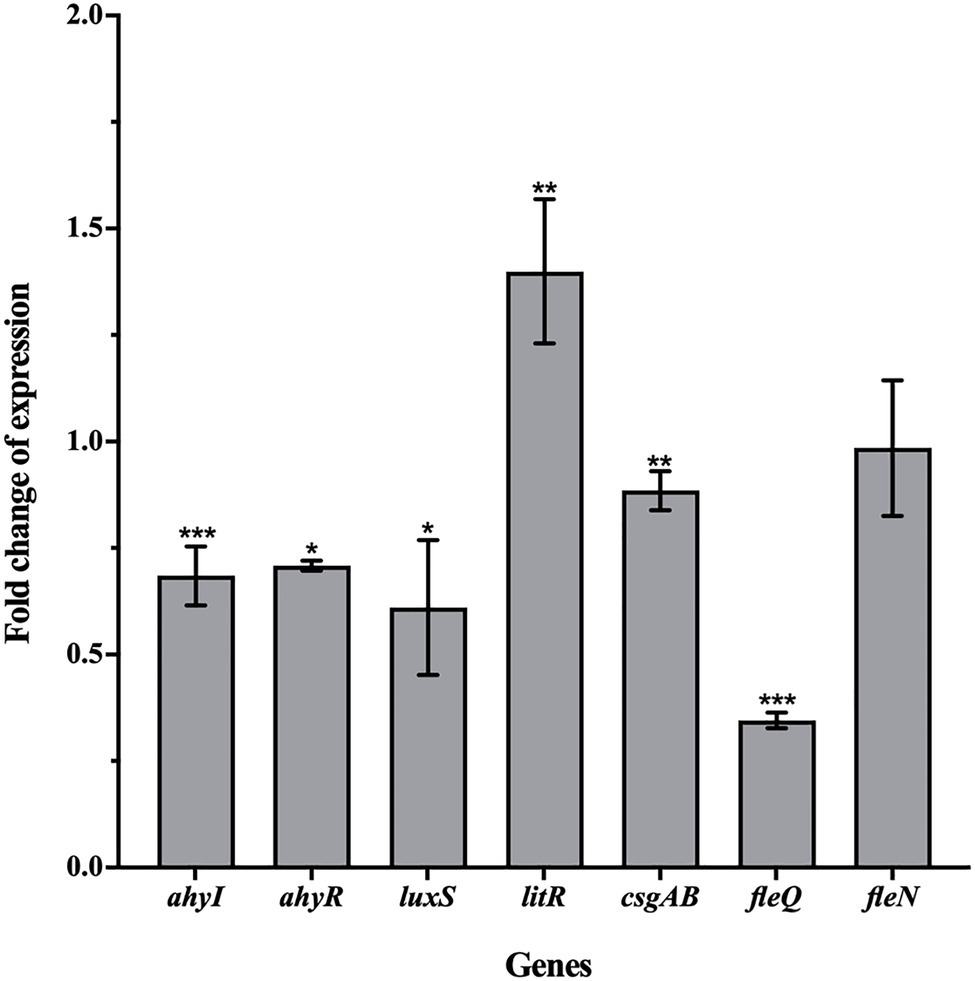
Figure 6. Effect of esculetin at 100μg/ml on the expression of QS-related genes in A. hydrophila SHAe 115. Data are presented as the expression fold changes of mean±SD of three independent experiments. *p<0.05, **p<0.01, and ***p<0.001 compared to the DMSO control group by t-test.
According to the reports of Kozlova et al. (2008 and 2011) and Khajanchi et al. (2009), the ahyI/R genes in A. hydrophila are homologs of lasI/R that are responsible for regulating the AHL-mediated AI-1 QS system. They demonstrated that the ahyI/R system was mainly responsible for the QS-related virulence production and biofilm formation in A. hydrophila. After treatment with esculetin, the expression levels of ahyI and ahyR decreased, which interfered the AHL-mediated AI-1 QS system. As a result, the production of virulence factors related to this system and the biofilm formation were inhibited. litR gene in A. hydrophila is a homolog of hapR gene. This gene in Vibrio cholerae encodes HapR protein that can negatively regulate bacterial biofilm formation and EPS biosynthesis (Hammer and Bassler, 2003) by lowering the intracellular level of c-di-GMP (Waters et al., 2008). Further, HapR can also negatively affect the transcription of vpsT, and the product of vpsT (VpsT) is a transcriptional activator required for the expression of EPS biosynthesis operon in V. cholerae (Yildiz et al., 2001; Casperlindley and Yildiz, 2004). While in A. hydrophila, the homologous gene of vpsT is csgAB (Kozlova et al., 2011). The increase of litR and decrease of csgAB gene may affect the biosynthesis of EPS together, thereby affecting the formation of biofilm. FleQ, encoded by fleQ, is a master regulator of flagellar gene expression in P. aeruginosa (Hickman and Harwood, 2010). FleN, encoded by fleN, is an anti-activator of FleQ which downregulates FleQ activity through direct interactions. Kozlova et al. (2011) reported that they found fleQ and fleN genes in A. hydrophila. Hence, the transcription of fleQ and fleN and interactions between FleQ and FleN play a crucial role in the motility of A. hydrophila. Another study of Kozlova et al. (2008) demonstrated that the luxS system also existed in the bacteria and it was mainly responsible for the motility. On one hand, the expression level of fleQ reduced, which affected the development of bacterial flagella. On the other hand, the expression level of luxS also reduced, and the inhibitory effect of esculetin on these two genes may jointly affect the motility of the bacteria.
The present study explored the inhibitory effect of esculetin on QS-related virulence factors and biofilm formation of A. hydrophila SHAe 115. The results showed that esculetin can significantly inhibit the production of hemolysin and protease, affect the swarming motility of A. hydrophila SHAe 115 at sub-MICs. They are all the main virulence factors of the bacteria. The formation of biofilm was also inhibited, the biofilm biomass decreased, and the biofilm structure turned thinner and sparser compared to the control group. qRT-PCR analysis indicated that genes positively related to motility and biofilm formation were downregulated to varying degrees, while gene (litR) negatively related to biofilm formation was significantly upregulated. The results of swarming motility and biofilm formation were in good agreement with gene expression analysis. Therefore, in combination with the activities against other pathogenic bacteria, esculetin has the potential to be a QS inhibitor.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
BS conceived and designed the experiments and also wrote the paper. BS and HL performed the experiments. BS, HL, HJ, and ZW analyzed the data. All authors contributed to the article and approved the submitted version.
This work was supported by grants from the National Natural Science Foundation of China (41766006).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmicb.2021.737626/full#supplementary-material
Allan, B., and Stevenson, R. (1981). Extracellular virulence factors of Aeromonas hydrophila in fish infections. Can. J. Microbiol. 27, 1114–1122. doi: 10.1139/m81-174
Belapurkar, R., Tale, V. S., and Madkaikar, R. (2014). Exploiting quorum sensing to inhibit the bacterial pathogens. Int. J. Curr. Microbiol. Appl. Sci. 3, 453–458.
Bucolo, C., Ward, K. W., Mazzon, E., Cuzzocrea, S., and Drago, F. (2009). Protective effects of a coumarin derivative in diabetic rats. Invest. Ophth. Vis. Sci. 50, 3846–3852. doi: 10.1167/iovs.08-3328
Casperlindley, C., and Yildiz, F. H. (2004). VpsT is a transcriptional regulator required for expression of vps biosynthesis genes and the development of rugose colonial morphology in Vibrio cholerae O1 El Tor. J. Bacteriol. 186, 1574–1578. doi: 10.1128/JB.186.5.1574-1578.2004
Chacón, M. R., Figueras, M. J., Castro-Escarpulli, G., Soler, L., and Guarro, J. (2003). Distribution of virulence genes in clinical and environmental isolates of Aeromonas spp. Anton. Leeuw 84, 269–278. doi: 10.1023/A:1026042125243
Chen, X., Gong, Y., Kong, F., and Su, Y. (2003). Study on Aeromanas hydrophila collected from the cultured Crocodilus porosus. J. Xiamen Univ. (Nat. Sci.) 42, 369–373. doi: 10.3321/j.issn:0438-0479.2003.03.023
Chu, W., and Lu, C. (2000). Pathogenicity of Aeromonas hydrophila extracellular protease. J. Nanjing Agricult. Univ. 23, 80–84. doi: 10.3321/j.issn:1000-2030.2000.02.020
D’Almeida, R. E., Molina, R. D. I., Viola, C. M., Luciardi, M. C., Peñalver, C. N., Bardón, A., et al. (2017). Comparison of seven structurally related coumarins on the inhibition of quorum sensing of Pseudomonas aeruginosa and Chromobacterium violaceum. Bioorg. Chem. 73, 37–42. doi: 10.1016/j.bioorg.2017.05.011
Déziel, E., Lépine, F., Milot, S., and Villemur, R. (2003). rhlA is required for the production of a novel biosurfactant promoting swarming motility in Pseudomonas aeruginosa: 3-(3-hydroxyalkanoyloxy)alkanoic acids (HAAs), the precursors of rhamnolipids. Microbiology 149, 2005–2013. doi: 10.1099/mic.0.26154-0
Ding, T., Li, T., and Li, J. (2018). Impact of curcumin liposomes with anti-quorum sensing properties against foodborne pathogens Aeromonas hydrophila and Serratia grimesii. Microb. Pathog. 122, 137–143. doi: 10.1016/j.micpath.2018.06.009
Duncan, S. H., Flint, H. J., and Stewart, C. S. (1998). Inhibitory activity of gut bacteria against Escherichia coli O157 mediated by dietary plant metabolites. FEMS Microbiol. Lett. 164, 283–288. doi: 10.1111/j.1574-6968.1998.tb13099.x
Dürig, A., Kouskoumvekaki, I., Vejborg, R. M., and Klemm, P. (2010). Chemoinformatics-assisted development of new anti-biofilm compounds. Appl. Microbiol. Biotechnol. 87, 309–317. doi: 10.1007/s00253-010-2471-0
Fereshteh, G., Mohammad, A., Mohammad, R. S. A., Soodabeh, S., Tahmineh, A., Amir, N. A., et al. (2014). Anticoagulant activity of isolated coumarins (suberosin and suberenol) and toxicity evaluation of Ferulago carduchorum in rats. Pharm. Biol. 52, 1–6. doi: 10.3109/13880209.2014.892140
Figueroa, M., Jarmusch, A. K., Raja, H. A., El-Elimat, T., and Oberlies, N. H. (2014). Polyhydroxyanthraquinones as quorum sensing inhibitors from the guttates of Penicillium restrictum and their analysis by desorption electrospray ionization mass spectrometry. J. Nat. Prod. 77, 1351–1358. doi: 10.1021/np5000704
Fylaktakidou, K. C., Hadjipavlou-Litina, D. J., Litinas, K. E., and Nicolaides, D. N. (2004). Natural and synthetic coumarin derivatives with anti-inflammatory/antioxidant activities. Curr. Pharm. Des. 10, 3813–3833. doi: 10.2174/1381612043382710
Grizzle, J. M., and Brunner, C. J. (2009). Infectious diseases of freshwater mussels and other freshwater bivalve mollusks. Rev. Fish. Sci. 17, 425–467. doi: 10.1080/10641260902879000
Gutiérrez-Barranquero, J. A., Reen, F. J., Mccarthy, R. R., and O’Gara, F. (2015). Deciphering the role of coumarin as a novel quorum sensing inhibitor suppressing virulence phenotypes in bacterial pathogens. Appl. Microbiol. Biotechnol. 99, 3303–3316. doi: 10.1007/s00253-015-6436-1
Hammer, B. K., and Bassler, B. L. (2003). Quorum sensing controls biofilm formation in Vibrio cholerae. Mol. Microbiol. 50, 101–104. doi: 10.1046/j.1365-2958.2003.03688.x
Hickman, J. W., and Harwood, C. S. (2010). Identification of FleQ from Pseudomonas aeruginosa as a c-di-GMP-responsive transcription factor. Mol. Microbiol. 69, 376–389. doi: 10.1111/j.1365-2958.2008.06281.x
Jakobsen, T. H., Gennip, M. V., Phipps, R. K., Shanmugham, M. S., and Givskov, M. (2012). Ajoene, a sulfur-rich molecule from garlic, inhibits genes controlled by quorum sensing. Antimicrob. Agents Chemother. 56, 2314–2325. doi: 10.1128/AAC.05919-11
James, D., and Trevor, T. (1988). Surface protein composition of Aeromonas hydrophila virulent for fish: identification of a surface array protein. J. Bacteriol. 170, 499–506. doi: 10.1128/jb.170.2.499-506.1988
Jamier, V., Marut, W., Valente, S., Chéreau, C., Chouzenoux, S., Nicco, C., et al. (2014). Chalcone-Coumarin derivatives as potential anti-cancer drugs: an in vitro and in vivo investigation. Anticancer Agent Med. Chem. 14, 963–974. doi: 10.2174/1871520613666131224124445
Khajanchi, B. K., Sha, J., Kozlova, E. V., Erova, T. E., Suarez, G., Sierra, J. C., et al. (2009). N-Acylhomoserine lactones involved in quorum sensing control the type VI secretion system, biofilm formation, protease production, and in vivo virulence in a clinical isolate of Aeromonas hydrophila. Microbiology 155, 3518–3531. doi: 10.1099/mic.0.031575-0
Köhler, T., Curty, L. K., Barja, F., Van Delden, C., and Pechère, J.-C. (2000). Swarming of Pseudomonas aeruginosa is dependent on cell-to-cell signaling and requires flagella and pili. J. Bacteriol. 182, 5990–5996. doi: 10.1128/JB.182.21.5990-5996.2000
Kozlova, E. V., Khajanchi, B. K., Sha, J., and Chopra, A. K. (2011). Quorum sensing and c-di-GMP-dependent alterations in gene transcripts and virulence-associated phenotypes in a clinical isolate of Aeromonas hydrophila. Microb. Pathog. 50, 213–223. doi: 10.1016/j.micpath.2011.01.007
Kozlova, E. V., Popov, V. L., Sha, J., Foltz, S. M., and Chopra, A. K. (2008). Mutation in the S-ribosylhomocysteinase (luxS) gene involved in quorum sensing affects biofilm formation and virulence in a clinical isolate of Aeromonas hydrophila. Microb. Pathog. 45, 343–354. doi: 10.1016/j.micpath.2008.08.007
Lee, J.-H., Kim, Y.-G., Cho, H. S., Ryu, S. Y., and Lee, J. (2014). Coumarins reduce biofilm formation and the virulence of Escherichia coli O157:H7. Phytomedicine 21, 1037–1042. doi: 10.1016/j.phymed.2014.04.008
Liang, L., and Xie, J. (2013). Isolation and identification, virulence factor detection, and susceptibility test of pathogen Aeromonas hydrophila isolated from Mylopharyngod piceus. Chinese J. Ecol. 32, 3236–3242. doi: 10.13292/j.1000-4890.2013.0495
Lu, Q., Li, L., and Liu, M. (2004). Cloning and expression of Aerolysin gene of pathogenic Aeromonas hydrophil. Chinese J. Vet. Sci. 24, 21–23. doi: 10.3969/j.issn.1005-4545.2004.01.009
Ma, X., Xue, H., and Tang, J. (2012). Isolation, identification and drug susceptibility test of pathogenic Aeromonas hydrophila from Procambarus clarkia. J. Aquac. 33, 45–47. doi: 10.3969/j.issn.1004-2091.2012.08.021
Meng, Y., Zeng, L., Yang, Y., and Xiao, H. (2009). Isolation and identification of the ascitesosis disease pathogen of giant salamander, Andrias davidiamus. J. Northwest A & F Univ. 37, 77–81. doi: 10.13207/j.cnki.jnwafu.2009.03.038
Miller, M. B., and Bassler, B. L. (2000). Quorum sensing in bacteria. Annu. Rev. Microbiol. 55, 165–199. doi: 10.1146/annurev.micro.55.1.165
Morohoshi, T., Shiono, T., Takidouchi, K., Kato, M., Kato, N., Kato, J., et al. (2007). Inhibition of quorum sensing in Serratia marcescens AS-1 by synthetic analogs of N-acylhomoserine lactone. Appl. Environ. Microbiol. 73, 6339–6344. doi: 10.1128/AEM.00593-07
Nam, I.-Y., and Joh, K. (2007). Rapid detection of virulence factors of Aeromonas isolated from a trout farm by hexaplex-PCR. J. Microbiol. 45, 297–304.
Ojala, T., Remes, S., Haansuu, P., Vuorela, H., and Vuorela, P. (2000). Antimicrobial activity of some coumarin containing herbal plants growing in Finland. J. Ethnopharmacol. 73, 299–305. doi: 10.1016/S0378-8741(00)00279-8
Pan, X., Ling, Z., Zhang, Q., Liu, Y., Zhou, B., and Zhao, J. (2003). Isolation and identification of Aeromonas hydrophila from chickens. China Anim. Quarantine 20, 19–21. doi: 10.3969/j.issn.1005-944x.2003.09.013
Patel, B., Kumari, S., Banerjee, R., Samanta, M., and Das, S. (2017). Disruption of the quorum sensing regulated pathogenic traits of the biofilm-forming fish pathogen Aeromonas hydrophila by tannic acid, a potent quorum quencher. Biofouling 33, 1–11. doi: 10.1080/08927014.2017.1336619
Payá, M., Halliwell, B., and Hoult, J. R. S. (1992). Interactions of a series of coumarins with reactive oxygen species. Scavenging of superoxide, hypochlorous acid and hydroxyl radicals. Biochem. Pharmacol. 44, 205–214. doi: 10.1016/0006-2952(92)90002-Z
Pfaffl, M. W. (2001). A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 29:e45. doi: 10.1093/nar/29.9.e45
Ramanathan, S., Ravindran, D., Arunachalam, K., and Arumugam, V. R. (2017). Inhibition of quorum sensing-dependent biofilm and virulence genes expression in environmental pathogen Serratia marcescens by petroselinic acid. Antonie Van Leeuwenhoe 111, 501–515. doi: 10.1007/s10482-017-0971-y
Shrout, J. D., Chopp, D. L., Just, C. L., Hentzer, M., and Parsek, M. R. (2010). The impact of quorum sensing and swarming motility on Pseudomonas aeruginosa biofilm formation is nutritionally conditional. Mol. Microbiol. 62, 1264–1277. doi: 10.1111/j.1365-2958.2006.05421.x
Souza, S. M. D., Monache, F. D., and Smania, A. (2005). Antibacterial activity of Coumarins. Z. Naturforsch. C J. Biosci. 60, 693–700. doi: 10.1515/znc-2005-9-1006
Sun, B., Jiang, H., Wang, Z., Luo, H., and Jia, A. (2021). Phytochemical constituents of Onosma bracteatum Wall. Phytochem. Lett. 45, 1–5. doi: 10.1016/j.phytol.2021.07.001
Tanwar, J., Das, S., Fatima, Z., and Hameed, S. (2014). Multidrug resistance: An emerging crisis. Interdiscip. Perspect. Infect. Dis. 2014:541340. doi: 10.1155/2014/541340
Tehsina, D., Claire, R., Christina, W., Suresh, A., Shigetoshi, K., and Dora, C.-M. (2011). Pancreatic anticancer activity of a novel geranylgeranylated coumarin derivative. Bioorg. Med. Chem. Lett. 21, 5770–5773. doi: 10.1016/j.bmcl.2011.08.005
Thomas, J., Devaraj, S., and Narayanan, S. (2017). Moleculao docking of salmonella Typhi quorum sensing regulated transcription factor SdiA. Glob. J. Eng. Sci. Res. Manage. 4, 59–65. doi: 10.5281/zenodo.569970
Thune, R. L., Johnson, M. C., Graham, T. E., and Amborski, R. L. (1986). Aeromonas hydrophila B-haemolysin: purification and examination of its role in virulence in 0-group channel catfish, Ictalurus punctatus (Rafinesque). J. Fish Dis. 9, 55–61. doi: 10.1111/j.1365-2761.1986.tb00979.x
Vanamala, J., Leonardi, T., Patil, B. S., Taddeo, S. S., Murphy, M. E., Pike, L. M., et al. (2006). Suppression of colon carcinogenesis by bioactive compounds in grapefruit. Carcinogenesis 27, 1257–1265. doi: 10.1093/carcin/bgi318
Venkadesaperumal, G., Kumar, M. C., and Halady, S. P. (2015). Quercetin influences quorum sensing in food borne bacteria: in-vitro and in-Silico evidence. PLoS One 10:e0134684. doi: 10.1371/journal.pone.0134684
Venugopala, K. N., Rashmi, V., and Odhav, B. (2013). Review on natural coumarin lead compounds for their pharmacological activity. Biomed. Res. Int. 2013:963248. doi: 10.1155/2013/963248
Wang, L., Liu, Z., Pan, J., Zhang, T., Qi, J., and Zhang, G. (2017). Effect of Esculetin inhibiting for KPC-producing Klebsiella pneumoniae in vitro. Lab. Med. Clin. 14, 3483–3486. doi: 10.3969/j.issn.1672-9455.2017.23.021
Waters, C. M., and Bassler, B. L. (2005). Quorum sensing: cell-to-cell communication in bacteria. Annu. Rev. Cell Dev. Biol. 21, 319–346. doi: 10.1146/annurev.cellbio.21.012704.131001
Waters, C. M., Lu, W., Rabinowitz, J. D., and Bassler, B. L. (2008). Quorum sensing controls biofilm formation in vibrio cholerae through modulation of cyclic Di-GMP levels and repression of vpsT. J. Bacteriol. 190, 2527–2536. doi: 10.1128/JB.01756-07
Yang, L., Ding, W., Xu, Y., Wu, D., Li, S., and Guo, B. (2016). New insights into the antibacterial activity of Hydroxycoumarins against Ralstonia solanacearum. Molecules 21:468. doi: 10.3390/molecules21040468
Yang, S., and Wang, M. (2006). Aeromonas hydrophila and its pathogensis to humans. Chin. J. Dis. Contr. Prev. 10, 511–514. doi: 10.3969/j.issn.1674-3679.2006.05.021
Yildiz, F. H., Dolganov, N. A., and Schoolnik, G. K. (2001). VpsR, a member of the response regulators of the two-component regulatory systems, is required for expression of vps biosynthesis genes and EPS(ETr)-associated phenotypes in vibrio cholerae O1 El Tor. J. Bacteriol. 183, 1716–1726. doi: 10.1128/JB.183.5.1716-1726.2001
Zhao, J., and Wang, L. (2015). Changes in serum indices of Schizothorax prenanti challenged with Aeromonas hydrophila. Fish. Sci. 34, 178–181. doi: 10.16378/j.cnki.1003-1111.2015.03.009
Zhou, J., Bi, S., Chen, H., Chen, T., Yang, R., Li, M., et al. (2017). Anti-biofilm and antivirulence activities of metabolites from Plectosphaerella cucumerina against Pseudomonas aeruginosa. Front. Microbiol. 8:769. doi: 10.3389/fmicb.2017.00769
Zhou, J., Luo, H., Jiang, H., Jian, T., Chen, Z., and Jia, A. (2018). Hordenine: a novel quorum sensing inhibitor and anti-biofilm agent against Pseudomonas aeruginosa. J. Agric. Food Chem. 66, 1620–1628. doi: 10.1021/acs.jafc.7b05035
Zhou, J. W., Ruan, L. Y., Chen, H. J., Luo, H. Z., Jiang, H., Wang, J., et al. (2019). Inhibition of quorum sensing and virulence in Serratia marcescens by hordenine. J. Agric. Food Chem. 67, 784–795. doi: 10.1021/acs.jafc.8b05922
Keywords: quorum sensing, biofilm, esculetin, Aeromonas hydrophila SHAe 115, quantitative real-time PCR
Citation: Sun B, Luo H, Jiang H, Wang Z and Jia A (2021) Inhibition of Quorum Sensing and Biofilm Formation of Esculetin on Aeromonas Hydrophila. Front. Microbiol. 12:737626. doi: 10.3389/fmicb.2021.737626
Received: 07 July 2021; Accepted: 25 August 2021;
Published: 24 September 2021.
Edited by:
Krassimira Hristova, Marquette University, United StatesReviewed by:
Khristina Judan Cruz, Central Luzon State University, PhilippinesCopyright © 2021 Sun, Luo, Jiang, Wang and Jia. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Aiqun Jia, YWppYUBoYWluYW51LmVkdS5jbg==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.