- 1College of Life Sciences, Shandong First Medical University & Shandong Academy of Medical Sciences, Tai’an, China
- 2Key Laboratory of Chemistry and Engineering of Forest Products, State Ethnic Affairs Commission, Guangxi Key Laboratory of Chemistry and Engineering of Forest Products, Guangxi Collaborative Innovation Center for Chemistry and Engineering of Forest Products, School of Chemistry and Chemical Engineering, Guangxi University for Nationalities, Nanning, China
- 3State Key Laboratory of Crop Biology, College of Agronomy, Shandong Agriculture University, Tai’an, China
- 4State Key Laboratory of Natural Medicines, China Pharmaceutical University, Nanjing, China
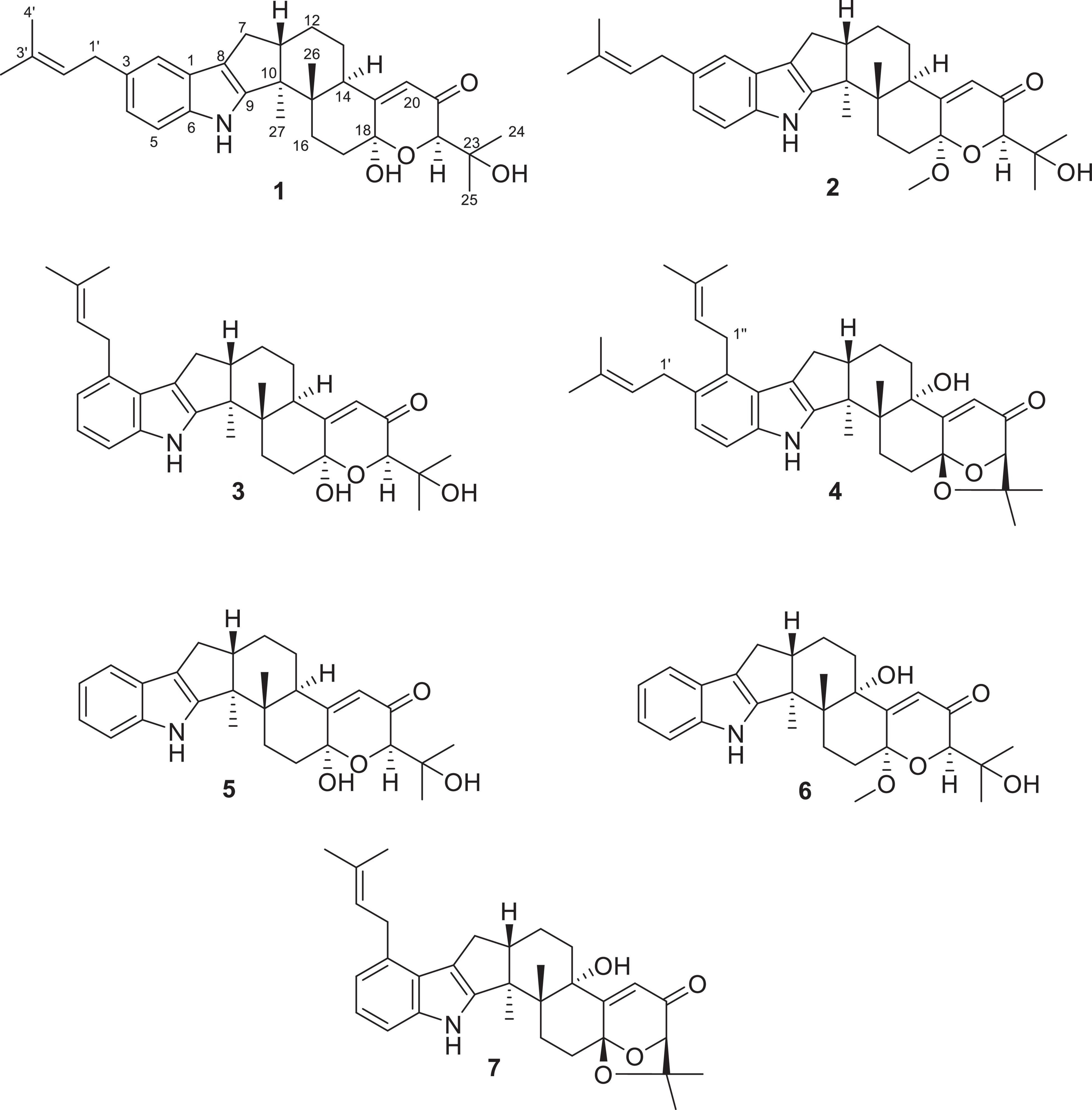
Four new indole-terpenoids (1–4) named encindolene A, 18-O-methyl-encindolene A, encindolene B, and encindolene C, as well as three known analogs (5–7), were isolated from the fungus Penicillium sp. HFF16 from the rhizosphere soil of Cynanchum bungei Decne. The structures of compounds including absolute configurations were elucidated by spectroscopic data and electronic circular dichroism (ECD) analysis. Anti-inflammatory activity evaluation revealed that compounds 1–7 inhibit the production of nitric oxide with IC50 values of 79.4, 49.7, 81.3, 40.2, 86.7, 90.1, and 54.4 μM, respectively, and decrease the levels of tumor necrosis factor-α, interleukin-6 contents in lipopolysaccharide-induced RAW264.7 macrophages.
Introduction
The paxilline-type indole-terpenoids are one of the largest classes of fungal indole-terpenoids with diverse structures. Typical representatives of such compounds include paxilline (Springer et al., 1975), thiersinines (Li et al., 2002), lolicines (Munday-Finch et al., 1998), shearinines (Belofsky and Gloer, 1995), and penerpenes (Kong et al., 2019). Many of these compounds have significant bioactivities, such as antibacterial, and anti-inflammatory activities. Inflammation, as a protective response of living tissues to injury and infection and stress, involves a wide variety of physiological and pathological processes (Medzhitov, 2008). During the process, if acute inflammatory response fails to eliminate stimuli, it will devolve a chronic inflammation response, which is associated with many diseases, including asthma, cancer, stroke, and obesity (Zhong and Shi, 2019). The chronic inflammation response is characterized by secretion of nitric oxide (NO) and proinflammatory cytokines such as tumor necrosis factor-α (TNF-α) and interleukin-6 (IL-6) (Medzhitov, 2008). Therefore, finding novel and effective anti-inflammatory compounds is urgently required.
In search of new compounds with anti-inflammatory activity, the secondary metabolites produced by Penicillium sp. HFF16 isolated from the rhizosphere soil of Cynanchum bungei Decne. in Mount Tai, East China, were investigated, which resulted in the isolation and identification of four new indole-terpenoids (1–4) named encindolene A, 18-O-methyl-encindolene A, encindolene B, and encindolene C, along with three known analogs including 7α-hydroxy-13-desoxy paxilline (5) (Peter and Christopher, 1994), 7-methoxypaxilline (6) (Ariantari et al., 2019), and paspalitrem C (7) (Dorner et al., 1984; Figure 1), which were isolated and identified. All of the compounds exhibited moderate inhibitory effects on the production of NO and proinflammatory cytokines (TNF-α and IL-6) in RAW264.7 macrophages stimulated by lipopolysaccharide (LPS). Herein, the isolation, structural elucidation, and bioactivities of these compounds were described.
Materials and Methods
General Experimental Procedures
Optical rotations were measured on a JASCO P-1020 digital polarimeter, and UV spectra were measured on a Beckman DU 640 spectrophotometer. Electronic circular dichroism (ECD) data were collected using a JASCO J-715 spectropolarimeter. NMR spectra were recorded on a Bruckmercury Plus-400 or a JNM-ECZR-500 spectrometers with TMS as an internal standard. High-resolution electrospray ionization mass spectrometry (HRESIMS) spectra were recorded with a Micromass Autospec-Uitima-TOF. Semipreparative high-performance liquid chromatography (HPLC) was carried out using an ODS column (YMC-pack ODS-A, 10 × 250 mm, 5 μm, 4 ml/min). Thin layer chromatography (TLC) and column chromatography (CC) were performed on plates precoated with silica gel GF254 (10–40 μm, Yantai Jiangyou Silicone Development Co., Ltd.).
Fungal Material and Fermentation
The fungus Penicillium sp. HFF16 was isolated from the rhizosphere soil of Cynanchum bungei Decne. in Mount Tai, China, in May 2020. After grinding, the sample (1.0 g) was diluted to 10–2 g/ml with sterile H2O, 100 μl of which was deposited on Bengal red medium (maltose 20 g, monosodium glutamate 10 g, glucose 10 g, yeast extract 3 g, corn pulp 1 g, mannitol 20 g, sodium chloride 0.3 g, potassium dihydrogen phosphate 0.5 g, agar 20 g per liter of tap water) plate containing chloramphenicol (200 μg/ml) as a bacterial inhibitor. A single colony was transferred onto another PDA plate and was identified according to its morphological characteristics and ITS gene sequences (Supplementary Material). The data presented in the study are deposited in the GenBank, accessionnumber (MZ165618). A reference culture of Penicillium sp. HFF16 maintained at –80°C was deposited in our laboratory. The isolate was cultured on plates of PDA medium at 28°C for 4 days. Plugs of agar supporting mycelium growth were cut and transferred aseptically to 7 × 250-ml Erlenmeyer flasks each containing 100 ml of liquid medium (potato 200 g, glucose 20 g per liter of tap water) and cultured at 28°C at 150 RPM for 3 days. The seed liquid was inoculated aseptically into 140 × 1,000-ml Erlenmeyer flasks each containing rice medium (80 g rice, 100 ml tap water) at 0.5% inoculation amount and incubated at room temperature under static conditions for 35 days.
Extraction and Isolation
The cultures (11.2 kg) were then extracted into EtOAc (40 L) by soaking overnight. The extraction was repeated three times. The combined EtOAc extracts were dried under vacuum to produce 38.2 g of extract. The EtOAc extract was subjected to a silica gel VLC column, eluting with a stepwise gradient of 0, 9, 11, 15, 20, 30, 50, and 100% EtOAc in petroleum ether (v/v) to give seven fractions (Fr. 1-7). Fraction 2 (2.3 g) was applied to ODS silica gel with gradient elution of MeOH-H2O (1:5, 2:3, 3:2, 4:1, and 1:0) to yield five subfractions (Fr. 2–1–Fr. 2–4). Fr. 2–4 (66 mg) was purified using semiprep HPLC (isocratic system 90% MeOH/H2O, v/v) to give compounds 7 (tR 9.86 min; 14 mg) and 4 (tR 16.41 min; 5.4 mg). Fraction 5 (7.3 g) was applied to ODS silica gel with gradient elution of MeOH-H2O (1:5, 2:3, 3:2, 4:1, and 1:0) to yield four subfractions (Fr. 5–1–Fr. 5–6). Fr. 5–1 (256 mg) was further purified using semiprep HPLC (isocratic system 90% MeOH/H2O, v/v) to give compound 5 (tR 6.0 min; 4.7 mg). Fr. 5–2 (306 mg) was further purified using semiprep HPLC (isocratic system 90% MeOH/H2O, v/v) to give compounds 6 (tR 8.4 min; 9.2 mg), 3 (tR 10.5 min; 6.2 mg), and 1 (tR 11.5 min; 5.3 mg). Fr. 5–3 (56 mg) was further purified using semiprep HPLC (isocratic system 90% MeOH/H2O, v/v) to give compound 2 (tR 16.0 min; 3.7 mg).
Encindolene A (1): white powder; [α]25 D–31 (c 0.1, MeOH); UV (MeOH) λmax (log ε): 289 (2.85) and 237 (3.46) nm; ECD (0.25 mM, MeOH) λmax 218 (–14.85), 242 (–8.04), 258 (+ 4.52), and 307 (+ 1.16) nm. 1H and 13C NMR data (Table 1); HRESIMS m/z 526.2902 [M + Na]+ (calcd for C32H41NO4Na, 526.2928).
18-O-methyl-encindolene A (2): white powder; [α]25 D–25 (c 0.1, MeOH); UV (MeOH) λmax (log ε): 284 (2.89) and 237 (3.51) nm; ECD (0.24 mM, MeOH) λmax 216 (–12.32), 243 (–9.41), 259 (+ 2.75), and 308 (+ 1.35) nm. 1H and 13C NMR d18-O-methyl-encindolene A (2): white powder; [α]25 D–25 (c 0.1, MeOH); UV (MeOH) λmax (log ε): 284 (2.89) and 237 (3.51) nm; ECD (0.24 mM, MeOH) λmax 216 (–12.32), 243 (–9.41), 259 (+ 2.75), and 308 (+ 1.35) nm. 1H and 13C NMR data (Table 1); 1H and 13C NMR data (Table 1); HRESIMS m/z 540.3078 [M + Na]+ (calcd for C33H43NO4Na, 540.3084).
Encindolene B (3): white powder; [α]25 D–16 (c 0.1, MeOH); UV (MeOH) λmax (log ε): 287 (2.73) and 235 (3.31) nm; ECD (1.2 mM, MeOH) λmax 2Encindolene B (3): white powder; [α]25 D–16 (c 0.1, MeOH); UV (MeOH) λmax (log ε): 287 (2.73) and 235 (3.31) nm; ECD (1.2 mM, MeOH) λmax 216 (–9.10), 239 (–11.19), 258 (+ 2.27), and 286 (+ 0.94) nm. 1H and 13C NMR data (Table 1); HRESIMS m/z 526.2903 [M + Na]+ (calcd for C32H41NO4Na, 526.2928).
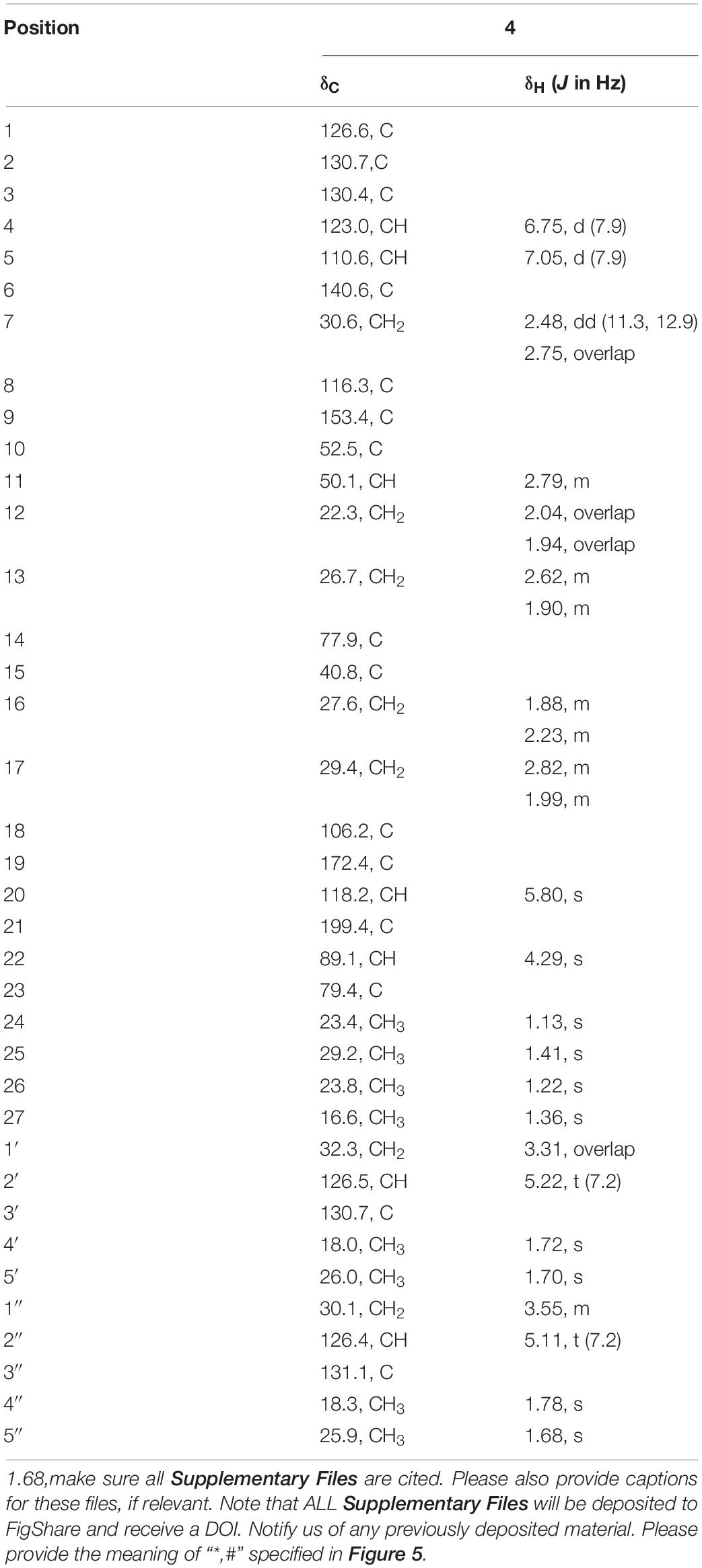
Encindolene C (4): white powder; [α]25 D + 98 (c 0.1, MeOH); UV (MeOH) λmax (log ε): 285Encindolene C (4): white powder; [α]25 D + 98 (c 0.1, MeOH); UV (MeOH) λmax (log ε): 285 (3.02) and 237 (3.54) nm; ECD (0.22 mM, MeOH) λmax 207 (+ 13.75), 245 (–31.6), 274 (+ 9.23), and 357 (+ 7.98) nm. 1H and 13C NMR data (Table 2); HRESIMS m/z 570.3581 [M + H]+ (calcd for C37H48NO4Na, 570.3578).
Cell viability of the test compounds were detected using MTT assay (Pan et al., 2021). RAW264.7 cells (Type Culture Collection of the Chinese Academy of Sciences, Shanghai, China) were cultured in DMEM supplemented with 10% fetal bovine serum (Gibco, United States) at 37°C in a 5% CO2 incubator. Cells were seeded in a 96-well plate at a concentration of 8 × 105 cells/well and treated with LPS (5 μg/ml) and various concentrations of test compounds (1–200 μM) for 24 h. After that, MTT solution (10 μl) was added and incubated at 37°C for 4 h. The purple crystals dissolved with dimethylsulfoxide (150 μl) were added, and the absorbance value was measured by a microplate reader at 570 nm.
Measurement of NO, TNF-α, and IL-6 Production
RAW264.7 cells were seeded in a 96-well plate at a concentration of 8 × 105 cells/well. After incubation, cells were pretreated with the test compounds with different dose (20–160 μM) and then stimulated with LPS (5 μg/ml) for 24 h. The NO concentration in culture medium was calculated by the commercial kit (Jiancheng, Nanjing, China) according to the manufacturer’s instruction. The TNF-α and IL-6 levels were determined using ELISA (Xiao et al., 2017). In brief, the cells were incubated with the test compounds (50 μM) in the presence or absence of LPS (5 μg/ml). After incubation for 24 h, the supernatant was detected for TNF-α and IL–6 at 450 nm. All data were expressed as the mean ± SD from at least three independent experiments.
Results and Discussion
Structure Elucidation of Compounds
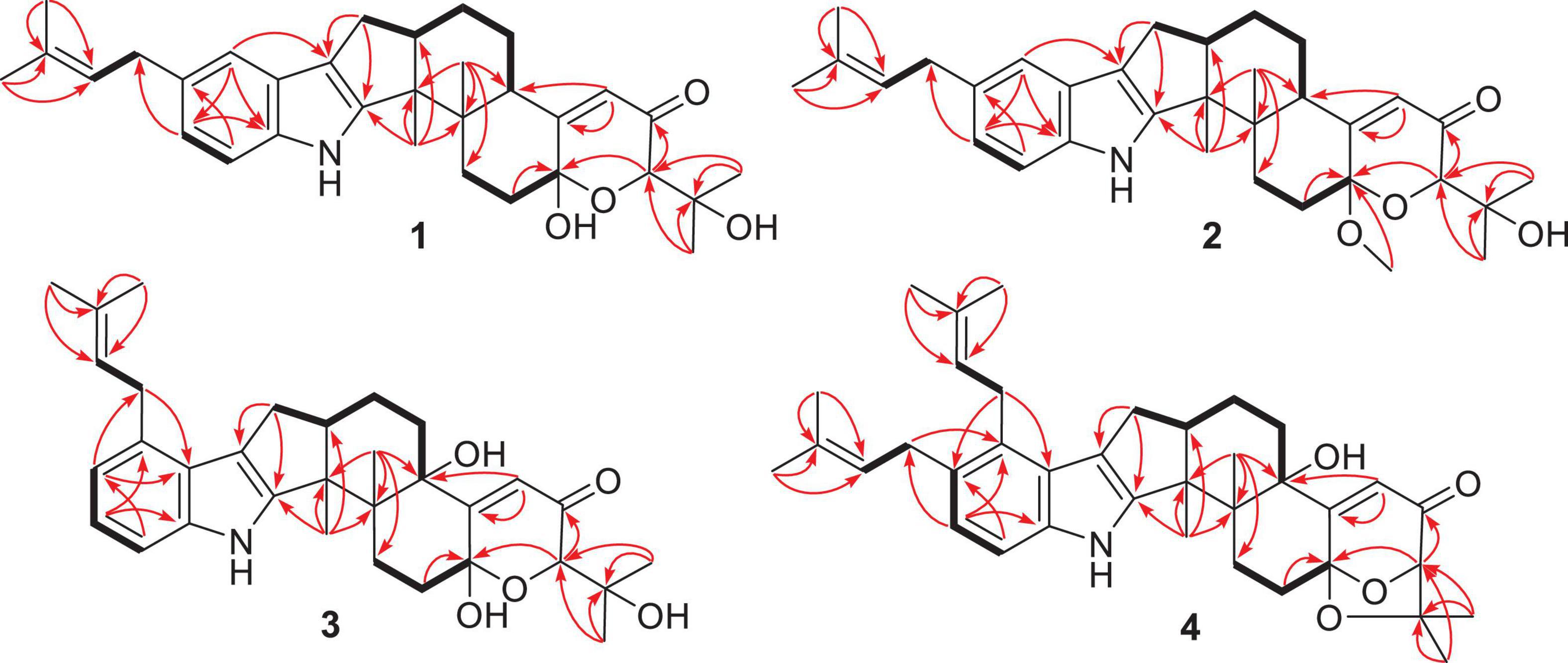
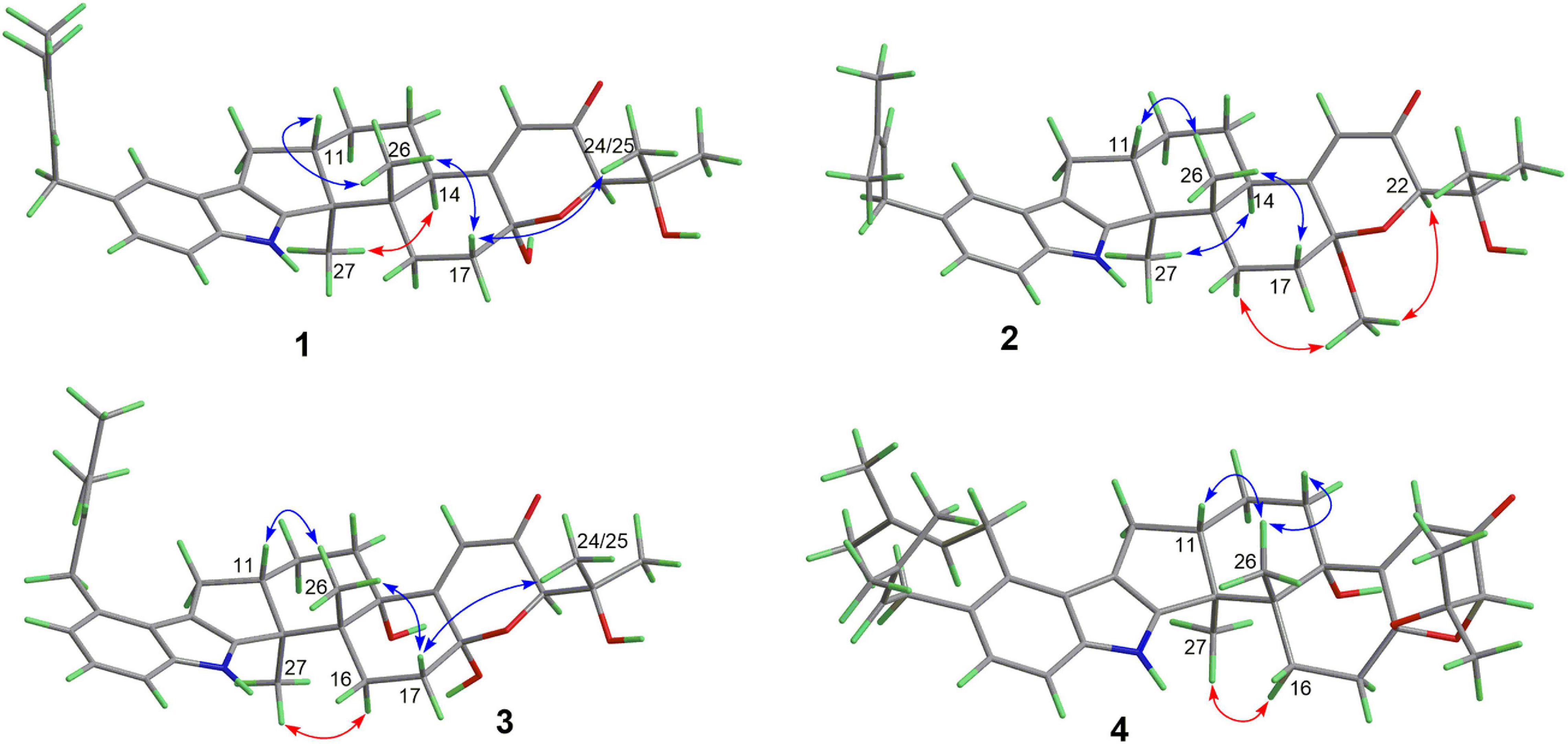
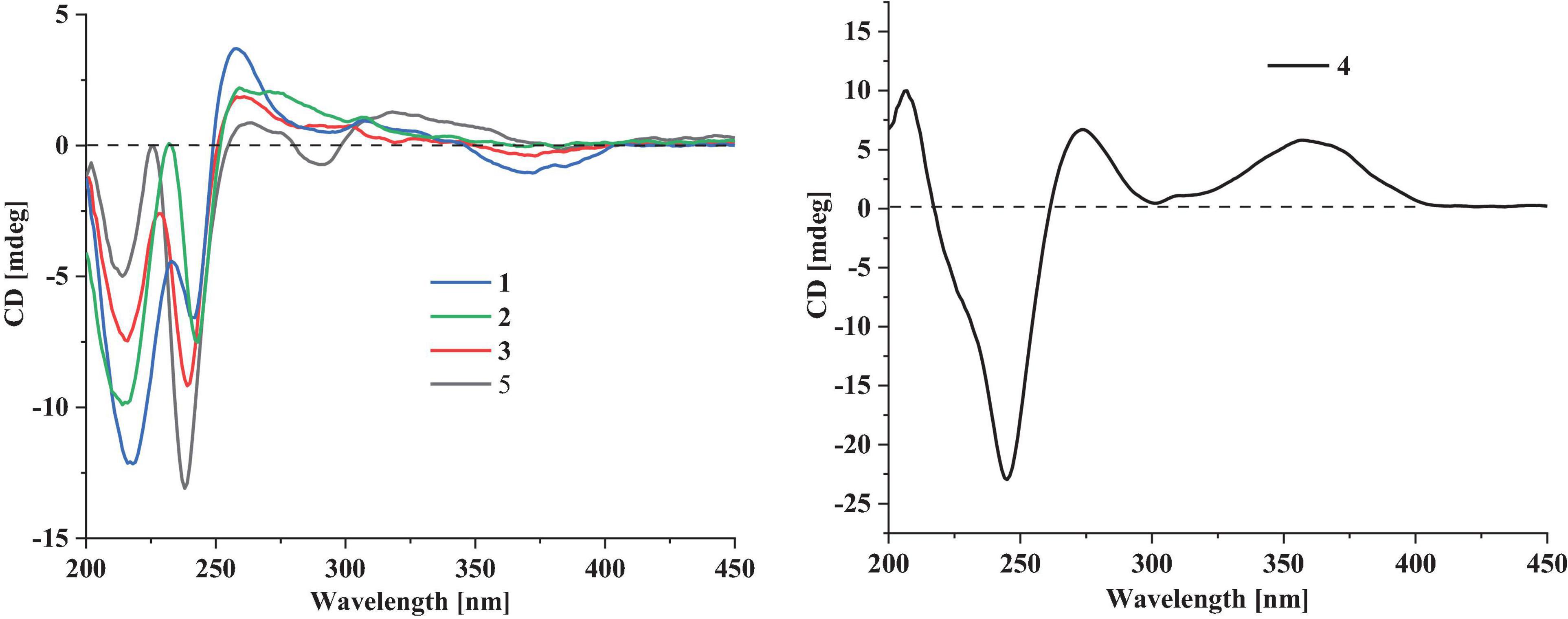
Compound 1 has a molecular formula C32H41NO4 as established from its HRESIMS and 13C NMR data (Supplementary Table 1). The 1H and 13C NMR data of 1 (Table 1), with the aid of a heteronuclear single quantum coherence (HSQC) spectrum, showed a total of 32 carbon signals comprising 1 ketone carbonyl, 12 olefinic or aromatic carbons with 5 protonated, 6 sp3 methylenes, 3 sp3 methines with 1 oxygenated, 4 sp3 non-protonated carbons with 2 oxygenated, and 6 methyls. These data are quite similar to those of 7α-hydroxy-13-desoxy paxilline (5) with the main differences being the presence of additional signals (δC/H 35.3/3.36, 126.2/5.36, 131.8, 17.9/1.74, and 26.0/1.74) corresponding to an isopentene group in the NMR data of 1. Besides, unlike that of 5, only three aromatic protons resonating into an ABX system were observed in the 1H NMR data of 1, indicating that the C–3 or C–4 of 1 was substituted. The above data suggested that 1 was a prenylated derivative of 5. COZY correlations (Figure 2) of H2-1′/H-2′ as well as HMBC correlations (Figure 2) from H3-4′ and H3-5′ to C-3′ and C-2′ and from H2–1′ to C–2, C–3, and C–4 confirmed the presence of an isopentene group at C–3. The remaining substructure was deduced to be the same as that of 5 by their similar NMR chemical shifts, which was further corroborated by detailed analysis of the two-dimensional NMR data (Figure 2) of 1. NOESY correlations (Figure 3) of H3–24(25)/H–17/H3–26/H–11 suggested the same face of these protons, while NOESY correlation of H3–27/H–14 indicated that they were on the face opposite to H3–26. The absolute configuration of 1 was determined to be the same as that of 5 by their similar ECD curves (Figure 4). Accordingly, compound 1 was assigned as a new indole-terpenoid and named as encindolene A.
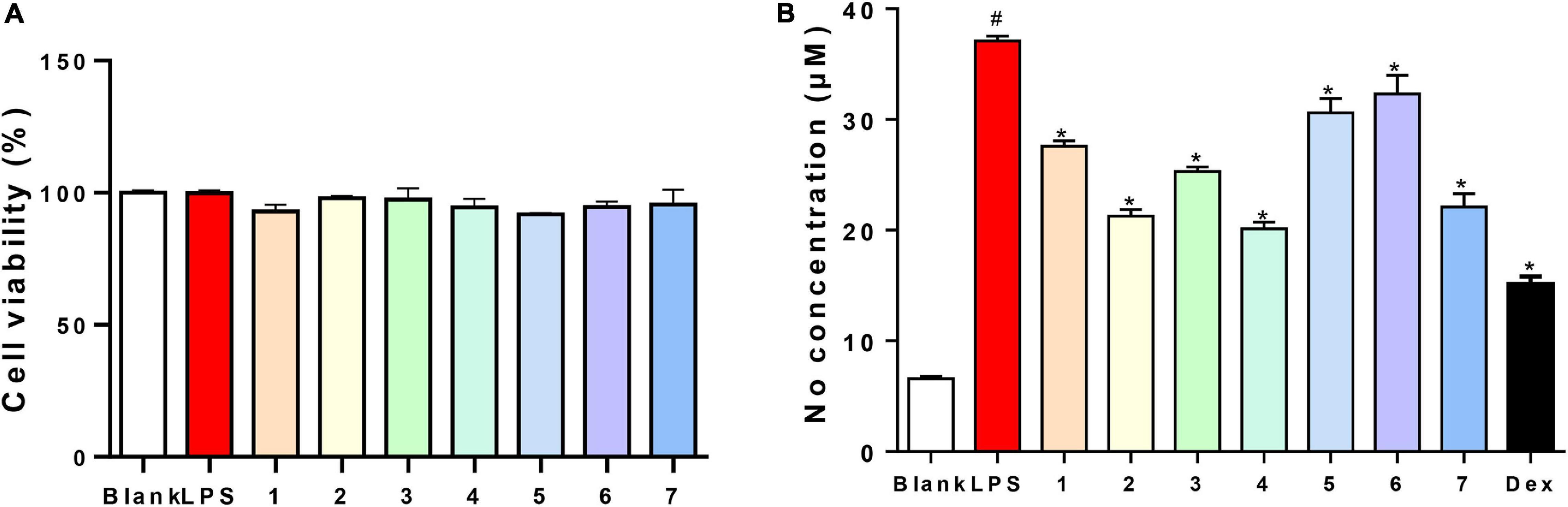
Figure 5. Viability effects and NO inhibitory activities of compounds 1–7 (A): 200 μM; (B): 40 μM) on RAW264.7 cells.
Compound 2 was determined to have the molecular formula C33H43NO4 based on the positive HRESIMS data, containing an additional methyl substituent in comparison with 1. The NMR spectra of 2 were closely related to those of 1 except for the appearance of an additional methoxy group at δC/H 49.6/3.41. The location of this methoxy group at C-18 in 2 was confirmed by the HMBC correlation (Figure 2) from its protons to C-18 (δC 98.1). Thus, compound 2 was elucidated as 18-O-methyl-sperindolene A according to compound 1. The relative and absolute configurations of 2 were determined to be the same as 1 by NOESY correlations (Figure 3) of H-22/MeO-18/H-16, H3–27/H–14, and H–11/H3–26/H–17, as well as the comparison of the ECD curve of 1 with that of 2 (Figure 4).
Compound 3 possessed the molecular formula C32H41NO5 as determined by HRESIMS data. The 1H-NMR, 13C-NMR, and HSQC data of 3 were quite similar to those of 1. However, three continuous proton signals at δH 6.70, 6.88, and 7.12 instead of an ABX coupling system as in 1 were shown in the aromatic region of the 1H-NMR of 3, implying the position of the isopentene group at C-2 or C-5. HMBC correlations (Figure 2) from H-1′ to C-1, C-2, and C-3 demonstrated the position of the isopentene group at C-2. The relative configuration of 3 was assigned to be the same as that of 1 by ROESY correlations (Figure 3) of H–24(25)/H–17/H3–26/H–11 and H3–27/H–14. The ECD curve (Figure 4) of 3 is very similar to those of 1 and 2, leading to the assignment of the absolute configuration of 3 as shown in Figure 1.
The HRESIMS data for 4 showed an ion peak at m/z 570.3581 [M + H]+, indicating the molecular formula C37H47NO4. The UV absorption at 237 and 285 nm suggested that 4 was also an indole-terpenoid. The 13C NMR data of 4 are very similar to that of paspalitrem C (7) (Dorner et al., 1984) except for the presence of five additional signals at δC/H 30.1/3.55, 126.4/5.11, 131.1, 18.3/1.78, and 25.9/1.68 corresponding to an isopentene group, as deduced from HMBC correlations from H3–4″ and H3–5″ to C-3″ and C-2″ and COZY correlations of H-2″ and H2–1″. Besides, unlike that of compound 7, the 1H NMR spectrum of 4 showed the presence of only two proton signals (δH 6.75 and 7.05) in the aromatic region, which are coupled to each other. This suggested the location of the above isopentene group at C-2. HMBC correlations (Figure 2) from H2–1″ to C-1, C-2, and C-3 further corroborated this deduction. The remaining substructure was determined to be the same as that of 7 by detailed analysis of the HMBC and COSY data (Figure 2). The relative configuration of compound 4 was also deduced to be the same as that of 7 by their nearly identical 1D NMR chemical shifts of C-7 to C-27 and was further confirmed by NOE correlations (Figure 3) of H–11/H3–26/H–13 and H3–27/H–16. The ECD curve of 4 showed strong positive Cotton effects (CEs) around 210, 275, and 325 nm (Figure 4). These data were very similar to those of shearilicine (Ariantari et al., 2019), a previously described analog bearing similar carbon skeleton as that of 4, thus leading to the assignment of the absolute configuration of 4.
Anti-inflammatory and Antibacterial Activities Assay
The antibacterial activities of all of the isolated compounds against Staphylococcus aureus and Escherichia coli were evaluated using the twofold dilution assay (Song et al., 2021). The results showed that all of the compounds were inactive. Compounds 1–7 were also non-cytotoxic to RAW264.7 cells at the concentration of 200 μM by MTT assay (Figure 5A). NO production was used as an indicator to evaluate the anti-inflammatory activity of 1–7. All of the compounds showed varying degrees of inhibitory activities on the production of NO in LPS-stimulated RAW264.7 cells, with IC50 values of 79.4, 49.7, 81.3, 40.2, 86.7, 90.1, and 54.4 μM, respectively, while 13.30 μM for dexamethasone, a positive control. Based on this, compounds 1–7 (40 μM) could suppress NO overproduction in cells (Figure 5B). To further confirm the anti-inflammatory activities, the effects of compounds 1–7 on the production of proinflammatory cytokines (TNF-α and IL-6) in LPS-induced RAW264.7 cells were evaluated. All the compounds showed moderate inhibitory effects on the production of TNF-α and IL-6 production at the concentration of 50 μM, with compound 4 showing the strongest effect (Figure 6).
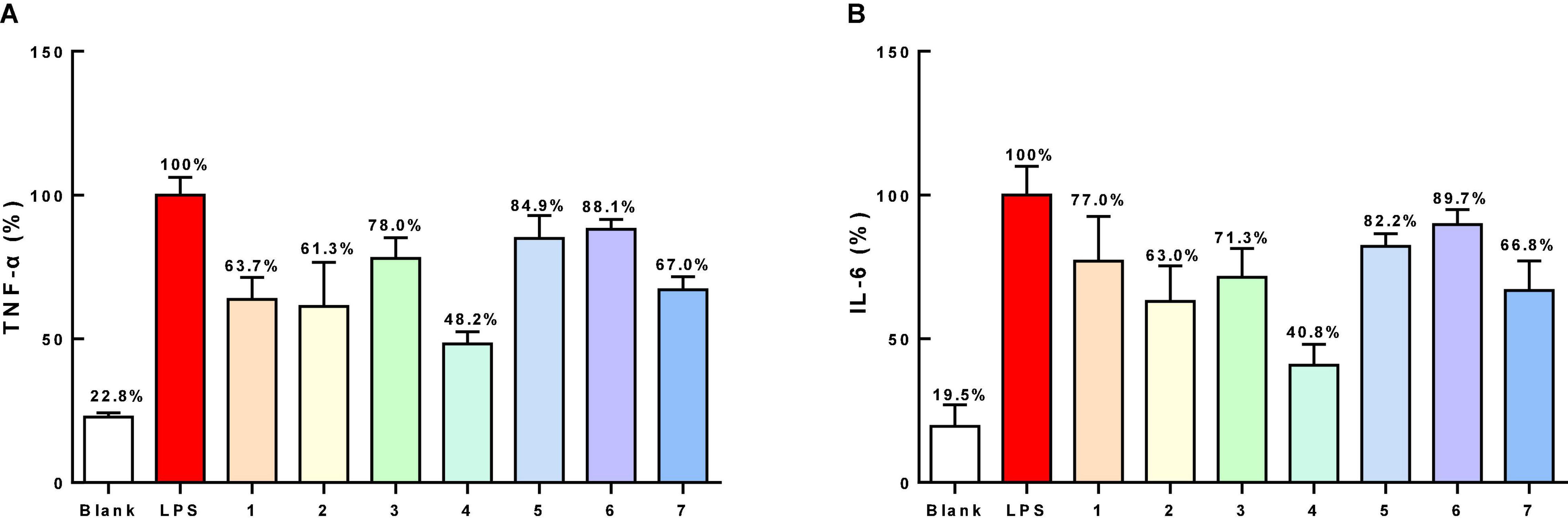
Figure 6. The inhibitory effects on TNF-α (A) and IL-6 (B) production of compounds 1–7 (50 μM) on RAW264.7 cells.
Conclusion
In summary, from the fungus Penicillium sp. HFF16, seven indole-terpenoids including four new were isolated and identified. These compounds could inhibit NO, TNF-α, and IL-6 production without affecting the cell viability in LPS-stimulated RAW264.7 macrophages. These results further demonstrated that fungi from medicinal plants are an abundant source of new bioactive products with medicinal use.
Data Availability Statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found below: GenBank, MZ165618.
Author Contributions
GP conceived and designed the experiments and was involved in isolation of compounds. YZ, SR, FL, QX, and XZ contributed to isolation of compounds. WP contributed to the collection of physical data of compounds. MY, CP, and GH performed genetic manipulation, strain fermentation, and extraction. TY contributed to the collection of the NMR data of compounds. FK revised the manuscript. LZ supervised the work and prepared the manuscript. NX contributed to bioactivity assay and revised the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This work was financially supported by the Natural Science Foundation of Shandong Province (No. ZR2019BH080), the National Natural Science Foundation of China (No. 82004014), the Open Project of State Key Laboratory of Natural Medicines (No. SKLNMKF202001), the Medical and Health Project of Shndong Province (No. 202001060294), the Science and Technology Innovation Development Project of Tai’an City (No. 2020NS059), and the Specific Research Project of Guangxi for Research Bases and Talents (No. AD18126005).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmicb.2021.710364/full#supplementary-material
References
Ariantari, N. P., Ancheeva, E., Wang, C., Mándi, A., Knedel, T. O., Kurtán, T., et al. (2019). Indole diterpenoids from an endophytic Penicillium sp. J. Nat. Prod. 82, 1412–1423. doi: 10.1021/acs.jnatprod.8b00723
Belofsky, G. N., and Gloer, J. B. (1995). Antiinsectan alkaloids: shearinines A-C and a new paxilline derivative from the ascostromata of Eupenicillium shearii. Tetrahedron 51, 3959–3968. doi: 10.1016/0040-4020(95)00138-X
Dorner, J. W., Cole, R. J., Cox, R. H., and Cunfer, B. M. (1984). Paspalitrem C, a new metabolite from sclerotia of Claviceps paspali. J. Agric. Food Chem. 32, 1069–1071. doi: 10.1021/jf00125a033
Kong, F. D., Fan, P., Zhou, L. M., Ma, Q. Y., Xie, Q. Y., Zheng, H. Z., et al. (2019). Penerpenes A-D, Four indole terpenoids with potent protein tyrosine phosphatase inhibitory activity from the marine-derived fungus Penicillium sp. KFD28. Org. Lett. 21, 4864–4867. doi: 10.1021/acs.orglett.9b01751
Li, C., Gloer, J. B., Wicklow, D. T., and Dowd, P. F. (2002). Thiersinines A and B: novel antiinsectan indole diterpenoids from a new fungicolous Penicillium Species (NRRL 28147). Org. Lett. 4, 3095–3098. doi: 10.1021/ol026424a
Medzhitov, R. (2008). Origin and physiological roles of inflammation. Nature 454, 428–435. doi: 10.1038/nature07201
Munday-Finch, S. C., Wilkins, A. L., and Miles, C. O. J. (1998). Isolation of Lolicine A, Lolicine B, Lolitriol, and Lolitrem N from loliumperenne infected with Neotyphodium lolii and evidence for the natural occurrence of 31-Epilolitrem N and 31-Epilolitrem F. Agric. Food Chem. 46, 590–598. doi: 10.1021/jf9706787
Pan, G. J., Li, Y. L., Che, X. Y., Tian, D., Han, W. J., Wang, Z. M., et al. (2021). New Thio-compounds and monoterpenes with anti-inflammatory activities from the fungus Aspergillus sp. CYH26. Front. Microbiol. 12:668938. doi: 10.3389/fmicb.2021.668938
Peter, G. M., and Christopher, M. W. (1994). Biosynthesis and transformation of tremorgenic indolediterpenoids by Penicillium paxilli and Acremonium lolii. Phytochemistry 36, 1209–1217. doi: 10.1016/S0031-9422(00)89639-9
Song, M., Liu, Y., Li, T., Liu, X., Hao, Z., Ding, S., et al. (2021). Plant natural flavonoids against multidrug resistant pathogens. Adv. Sci. (Weinh) e2100749. doi: 10.1002/advs.202100749
Springer, J. P., Clardy, J., Wells, J. M., Cole, R. J., and Kirksey, J. W. (1975). The structure of paxilline, a tremorgenic metabolite of Penicillium paxilli bainier. Tetrahedron Lett. 16, 2531–2534. doi: 10.1016/S0040-4039(00)75170-7
Xiao, N., Yang, L. L., Yang, Y. L., Liu, L. W., Li, J., Liu, B. L., et al. (2017). Ginsenoside Rg5 inhibits succinate-associated lipolysis in adipose tissue and prevents muscle insulin resistance. Front. Pharmacol. 8:43. doi: 10.3389/fphar.2017.00043
Keywords: fungus, Penicillium sp. HFF16, indole-terpenoids, anti-inflammatory activity, Cynanchum bungei Decne
Citation: Pan G, Zhao Y, Ren S, Liu F, Xu Q, Pan W, Yang T, Yang M, Zhang X, Peng C, Hao G, Kong F, Zhou L and Xiao N (2021) Indole-Terpenoids With Anti-inflammatory Activities From Penicillium sp. HFF16 Associated With the Rhizosphere Soil of Cynanchum bungei Decne. Front. Microbiol. 12:710364. doi: 10.3389/fmicb.2021.710364
Received: 18 May 2021; Accepted: 11 June 2021;
Published: 09 July 2021.
Edited by:
Paola Angelini, University of Perugia, ItalyReviewed by:
Deli Chen, Chinese Academy of Medical Sciences and Peking Union Medical College, ChinaXin Li, Zhejiang University, China
Duqiang Luo, Hebei University, China
Copyright © 2021 Pan, Zhao, Ren, Liu, Xu, Pan, Yang, Yang, Zhang, Peng, Hao, Kong, Zhou and Xiao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Liman Zhou, emhvdWxpbWFuODhAMTI2LmNvbQ==; Na Xiao, eGlhb25hMTk4NzA3QDEyNi5jb20=
 Guojun Pan1
Guojun Pan1 Fandong Kong
Fandong Kong Na Xiao
Na Xiao