
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Microbiol. , 30 July 2021
Sec. Aquatic Microbiology
Volume 12 - 2021 | https://doi.org/10.3389/fmicb.2021.707954
This article is part of the Research Topic Ciliates: Key Organisms in Aquatic Environments View all 26 articles
Hypotrich ciliates with evolutionary novelties are continually being discovered, challenging the current taxonomic system and attracting increased attention. In the present work, two new urostylid ciliates, Heterobakuella bergeri gen. nov., sp. nov. and Anteholosticha perezuzae sp. nov., isolated from Chinese wetland samples, were identified based on morphology and 18S rRNA gene sequences. Heterobakuella gen. nov. is defined by three frontal cirri, single buccal cirrus, one parabuccal cirrus, midventral complex composed of cirral pairs and one cirral row, one left and two right marginal cirral rows, transverse and pretransverse cirri present, caudal and frontoterminal cirri absent. Heterobakuella can be easily distinguished from the morphologically most similar genus, Apobakuella, mainly by the single buccal cirrus (vs. one buccal cirral row) and one parabuccal cirrus (vs. several parabuccal cirral rows originated from different anlagen). Phylogenetic analyses show that H. bergeri branches within the clade formed by Bergeriella ovata, Monocoronella carnea, Anteholosticha gracilis, and Neourostylopsis spp., rather than the clade represented by Apobakuella. The other species, A. perezuzae, is mainly characterized by a distinctly slender body shape with an average length:width ratio about 7, distinctively shaped biconcave and greenish cortical granules, as well as one or two pretransverse cirri. Phylogenetic analyses indicate the genus Anteholosticha is non-monophyletic.
Hypotrichia Stein, 1859, is a large group of ciliated protists with extremely diverse morphologic and morphogenetic characters, with worldwide distribution in diverse habitats, including marine and fresh waters, and even desert soils (Berger, 1999, 2006, 2008, 2011; Foissner, 2016; Hu et al., 2019; Jung and Berger, 2019; Kaur et al., 2019; Kim and Min, 2019; Wang et al., 2020a,b; Chen et al., 2021; Luo et al., 2021; Ma et al., 2021; Song et al., 2021; Vd’ačný and Foissner, 2021; Wang et al., 2021). Urostylids, with more than 200 described species, are one of the largest groups in the subclass Hypotrichia (Berger, 2006). Studies from the past few decades have revealed that this group is even more diverse than previously thought, and many new species are likely awaiting discovery (Berger, 2006; Jiang et al., 2013; Jo et al., 2015; Kim et al., 2016; Song and Shao, 2017; Chen et al., 2018, 2020; Zhu et al., 2019; Shao et al., 2020; Xu et al., 2020; Zhang et al., 2020a). In addition, some classifications at the family and genus levels within this group (e.g., Bakuellidae Jankowski, 1979 and Anteholosticha Berger, 2003), and their systematic relationships, remain confusing (for reviews, see Berger, 2006; Huang et al., 2014; Lyu et al., 2015, 2018; Moon et al., 2020; Zhang et al., 2020b).
According to Berger (2006), some urostylid taxa characterized by having three frontal cirri and a midventral complex composed of cirral pairs in the anterior portion and at least one cirral row in the posterior portion were assigned to the family Bakuellidae. Accordingly, at least 10 genera, i.e., Apobakuella Li et al., 2011, Australothrix Blatterer and Foissner, 1988, Bakuella Agamaliev and Alekperov, 1976, Birojimia Berger and Foissner, 1989, Holostichides Foissner, 1987, Metaurostylopsis Song et al., 2001, Monourostylopsis Song et al., 2020, Neobakuella Li et al., 2011, Parabirojimia Hu et al., 2002, and Paragastrostyla Hemberger, 1985 could be assigned to this family (Berger, 2006; Li et al., 2011; Jiang et al., 2013; Song et al., 2020). However, molecular studies showed that the family Bakuellidae was not monophyletic, and some of its genera (e.g., Australothrix, Birojimia, and Parabirojimia) had phylogenetic incongruence between the morphological and molecular data, thus its classification scheme was questioned and some newly established families (e.g., Hemicycliostylidae Lyu et al., 2018 and Parabirojimidae Dai and Xu, 2011) were proposed later (Lynn, 2008; Dai and Xu, 2011; Foissner, 2016; Lyu et al., 2018). In addition, new bakuellid-like taxa with evolutionary novelties are regularly being discovered, which also renders the classification of the bakuellid-like taxa difficult in practice.
The genus Anteholosticha was established by Berger (2003) for some species previously assigned to Holosticha sensu Borror (1972), which lack distinct autapomorphies (e.g., anterior end of left marginal cirral row curved rightwards, adoral zone bipartite, buccal cirrus distinctly ahead of paroral membrane). Many studies have shown that Anteholosticha is extremely divergent (Berger, 2006, 2008; Park et al., 2012, 2013; Huang et al., 2014; Lyu et al., 2015; Zhao et al., 2015; Chen et al., 2018, 2020; Jung et al., 2021). Hence, Huang et al. (2014) assigned three species, Anteholosticha warreni (Song and Wilbert, 1997) Berger, 2003, A. scutellum (Cohn, 1866) Berger, 2003, and A. petzi Shao et al., 2011, each with a roughly U-shaped pattern of transverse cirri and forming a clade distinctly separated from other Anteholosticha groups in molecular trees, to the newly erected genus Arcuseries. To date, more than 40 nominal species of Anteholosticha have been reported, although many of them are poorly documented, with only simple morphologic descriptions and without molecular data. All these factors hamper our understanding of the systematics and taxonomy of this genus (Berger, 2006; Fan et al., 2016; Chen et al., 2018, 2020).
More studies, based on morphologic, molecular, and ecologic data, are thus urgently needed to tackle these problems. In this work, two novel wetland urostylid ciliates, Heterobakuella bergeri gen. nov., sp. nov. and Anteholosticha perezuzae sp. nov., are reported and their phylogenetic positions are discussed.
Heterobakuella bergeri gen. nov., sp. nov. was collected on November 12, 2019 from a small brook that flows into the Weishan Lake Wetland, administrated by the city of Jining, Shandong Province, China (34°46′14″N, 117°12′56″E) (Figure 1A), when the water temperature was 15°C. Anteholosticha perezuzae sp. nov. was isolated from the sample collected from a brackish water stream that originates from a small lake wetland near Tangdao Bay, Qingdao, China (35°56′18″N, 120°12′44″E) (Figure 1B) on March 27, 2017, where the water temperature was 15°C and salinity was 8‰. In each case, sampling water (up to 20 cm deep) was first stirred, then about 300 ml of water including sediments, mud, and rotten plants was collected and transferred to our laboratory. Next, the sample was divided into several aliquots that were used to establish raw cultures in Petri dishes (diameter = 9 cm). All raw-culture samples were maintained at room temperature (about 23°C). Because the two species we studied could be easily distinguished from other species present in the same Petri dish by body shape and color, the confusion with other species was avoided.
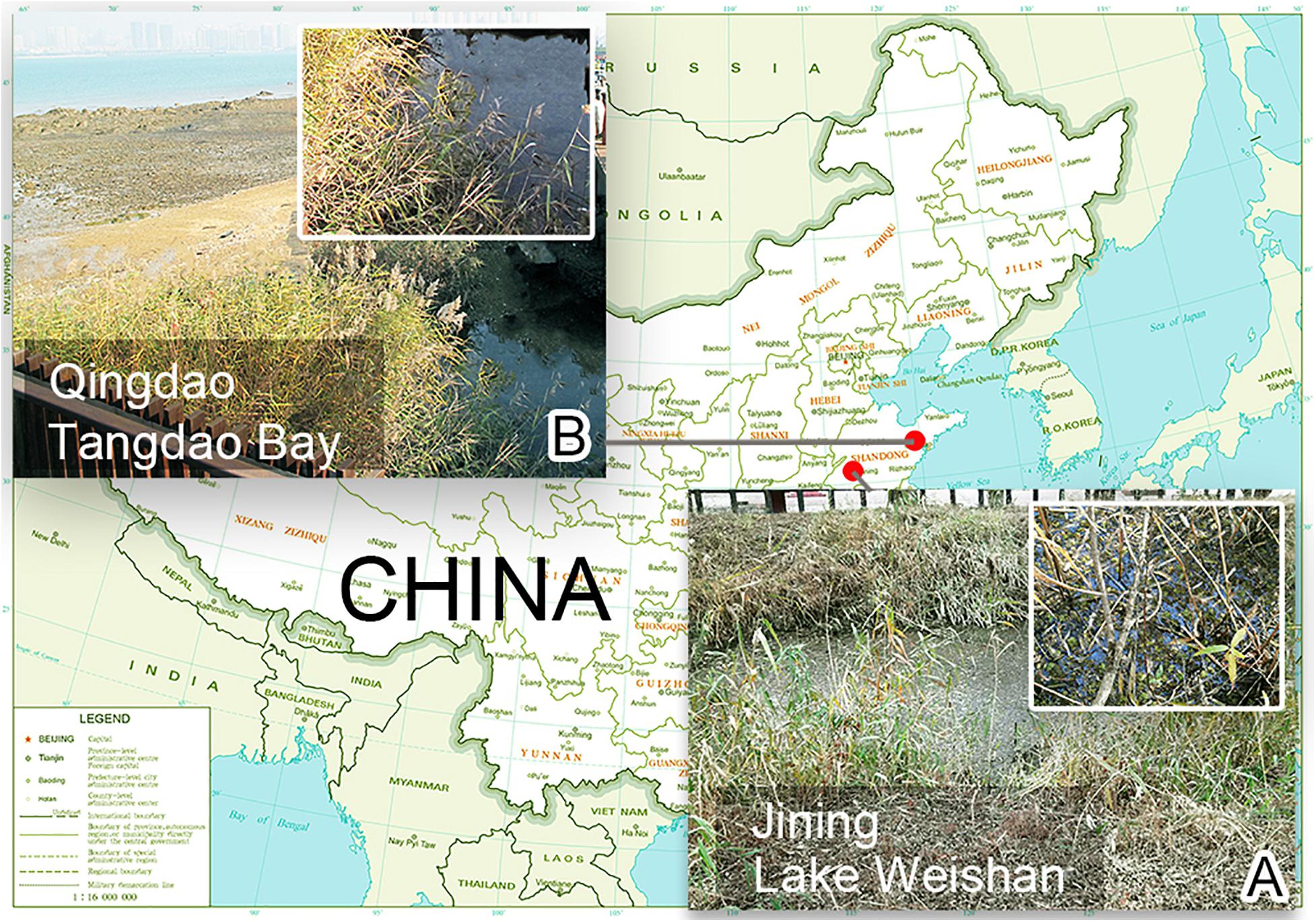
Figure 1. Locations of sampling sites. (A) Surroundings of the sampling site of Lake Weishan, the city of Jining, where Heterobakuella bergeri gen. nov., sp. nov. was collected. (B) Photograph of Tangdao Bay, the city of Qingdao, where Anteholosticha perezuzae sp. nov. was collected.
Live specimens were observed under the microscope (Olympus BX53; Olympus Corporation, Tokyo, Japan) with magnifications ranging from 100× to 1000×. Protargol impregnation was performed to reveal ciliary patterns and nuclear apparatus (Wilbert, 1975). Drawings were based on free-hand sketches with the help of drawing devices. Classification and terminology mainly follow Berger (2006).
Five cells of each species were isolated from Petri dishes and washed several times using filtered and sterilized site water. Using micropipettes, cells were transferred into three 1.5-ml eppendorf tubes, two tubes with one cell each and the third with three cells. Genomic DNA was extracted using the DNeasy Blood and Tissue Kit (Qiagen, Germantown, MD, United States) according to the manufacturer’s protocol with 25% of the suggested reagent volumes as described by Lu et al. (2020). The 18S rRNA genes of Heterobakuella bergeri gen. nov., sp. nov. and Anteholosticha perezuzae sp. nov. were amplified with the same forward primer 82F (5′-GAAACTGCGAATGGCTC-3′) (Jerome et al., 1996) but different reverse primers, 5.8SR (5′-TACTGATATGCTTAAGTTCAGCGG-3′) (Wang J. et al., 2017) for H. bergeri gen. nov., sp. nov. and 18SR (5′-TGATCCTTCTGCAGGTTCACCTAC-3′) (Medlin et al., 1988) for A. perezuzae sp. nov. To minimize amplification errors during PCR, we used Q5 Hot Start High-Fidelity DNA Polymerase (New England BioLabs, United States) as recommended by Wang C. et al. (2017). The thermocycler program was set according to Bai et al. (2020). The PCR products were sequenced bidirectionally in the Tsingke Biological Technology Company (Qingdao, China) using the PCR primers and three internal primers, i.e., Pro + B (5′-GGTTAAAAAGCTCGTAGT-3′), 900F (5′-CGATCAGATACCGTCCTAGT-3′), and 900R (5′-ACTAGGACGGTATCTGATCG-3′) for both species (Wang J. et al., 2017).
To infer the phylogenetic position of the two newly obtained species, we downloaded 18S rRNA gene sequences of 77 other ciliates from the National Center for Biotechnology Information (NCBI) database1, including 62 “core urostylids,” seven oxytrichids, three Arcuseries species, as well as the outgroup taxa composed of two Uncinata and three Holosticha species. GenBank accession numbers are shown in Figure 6. After combining all of the sequences, the alignment was carried out with the MUSCLE algorithm on the webserver GUIDANCE 22 with default parameters (Sela et al., 2015). The primer sequences were manually trimmed using BioEdit v.7.0 (Hall, 1999). The maximum likelihood (ML) analysis was done using RAxML-HPC2 (XSEDE v.8.2.12) on the CIPRES Science Gateway server3 with 1,000 bootstrap replicates and the GTRGAMMA model of nucleotide substitution (Stamatakis, 2014). Bayesian inference (BI) analysis was carried out using MrBayes (Ronquist et al., 2012) on CIPRES Science Gateway (XSEDE v.3.2.6), with the GTR + I + G nucleotide substitution model selected under Akaike Information Criterion (AIC) by jModelTest 2 (Darriba et al., 2012). Four Markov chain Monte Carlo (MCMC) simulations were run for 1,000,000 generations with the first 2,500 sampled trees discarded as burn-in. Convergence was assessed using RWTY (Warren et al., 2017). SeaView v.4.6.1 (Gouy et al., 2010) and MEGA 6.0 (Tamura et al., 2013) were used to visualize the tree topologies.
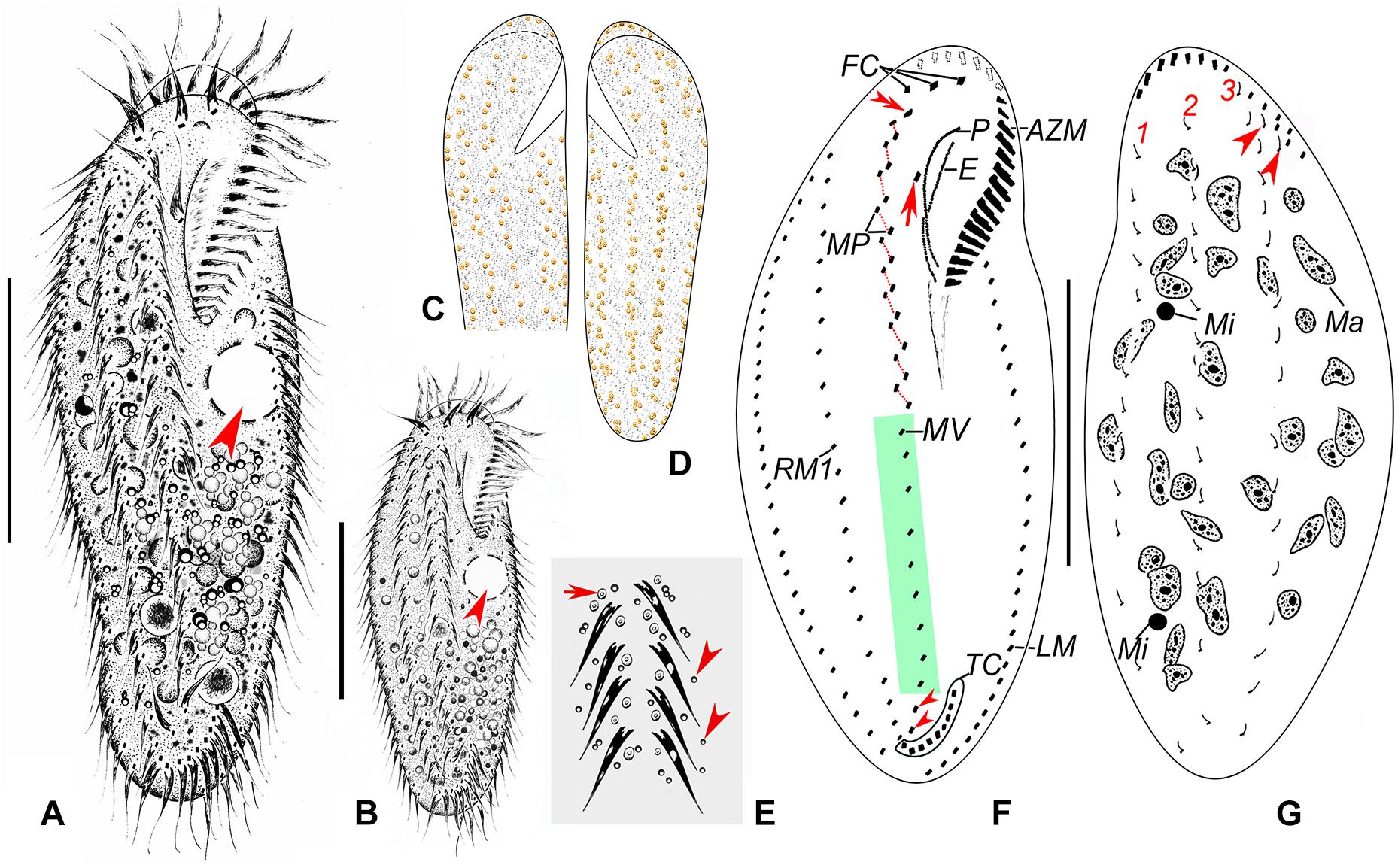
Figure 2. Morphology and infraciliature of Heterobakuella bergeri gen. nov., sp. nov. from life (A–E) and after protargol impregnation (F,G). (A,B) Ventral views of a typical individual (A) and a different cell (B), showing the body shape. Arrows depict the contractile vacuole. (C–E) Arrangement of cortical granules on ventral (C,E) and dorsal (D) sides, arrow and arrowheads in (E) indicate details of cortical granules and globules, respectively. (F,G) Ventral (F) and dorsal (G) views of the holotype specimen, showing infraciliature and nuclear apparatus. In (F), arrow shows buccal cirrus, arrowheads mark pretransverse cirri, and double-arrowhead indicates parabuccal cirrus. Arrowheads in (G) indicate two additional dorsal bristles. AZM, adoral zone of membranelles; E, endoral membrane; FC, frontal cirri; LM, left marginal cirral row; Ma, macronuclear nodules; Mi, micronuclei; MP, midventral cirral pairs; MV, midventral cirral row; P, paroral membrane; RM1, right marginal cirral row 1; TC, transverse cirri; 1–3, dorsal kineties 1–3. Scale bars = 50 μm.
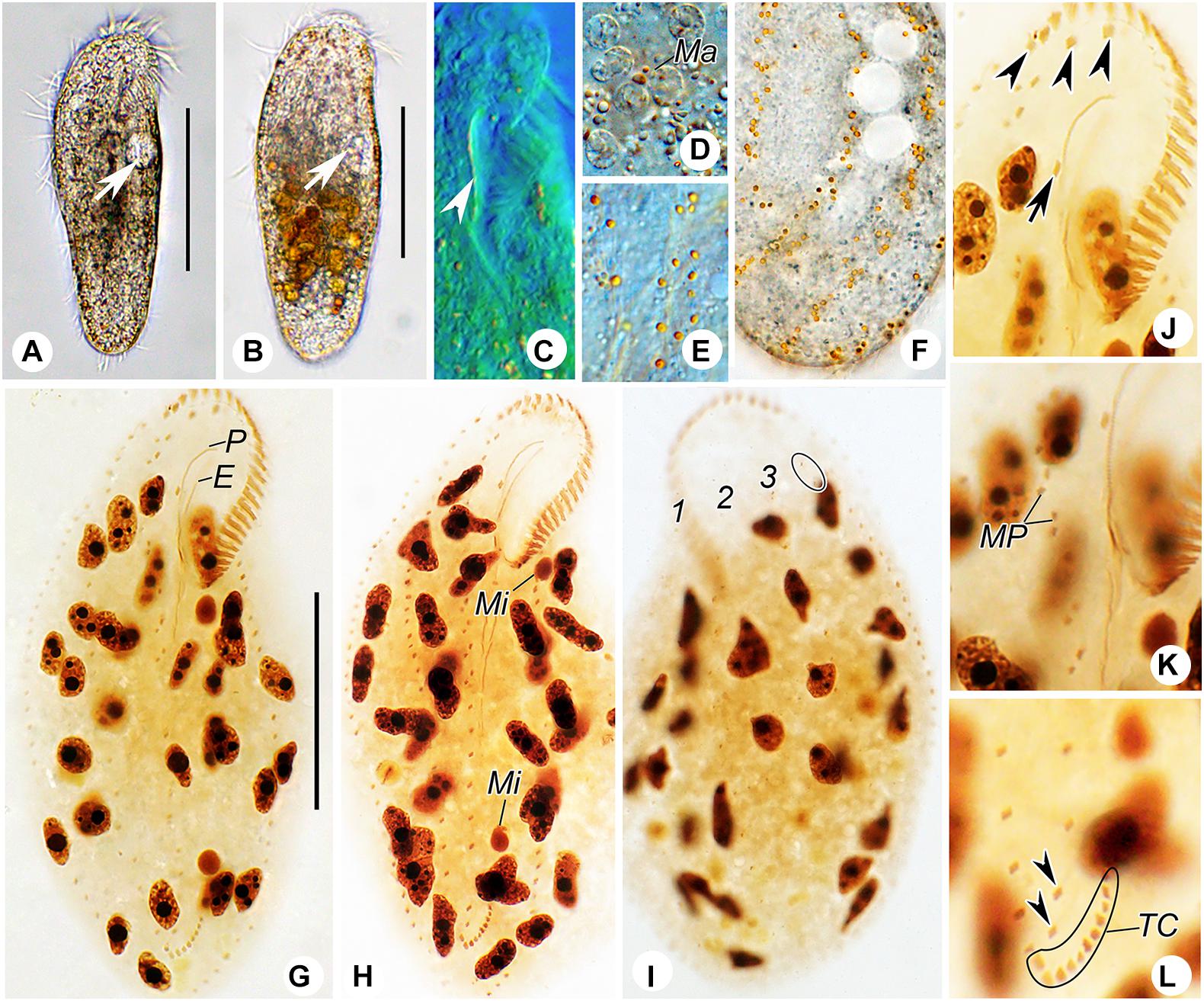
Figure 3. Photomicrographs of Heterobakuella bergeri gen. nov., sp. nov. from life (A–F) and after protargol impregnation (G–L). (A,B) Ventral views of two representative specimens; arrows indicate the contractile vacuole. (C) Ventral view to show the undulating membranes. (D) Macronuclear nodules. (E,F) Ventral (E) and dorsal (F) views to show the arrangement of cortical granules. (G,H) Ventral overviews of the holotype (G) and a paratype (H) specimen, showing the ciliary pattern. (I) Dorsal view of a paratype specimen, to demonstrate dorsal kineties and the nuclear apparatus. Circle marks two additional dorsal bristles. (J) Ventral view, to show three frontal cirri (arrowheads) and buccal cirrus (arrow). (K) Ventral view, showing the midventral cirral pairs. (L) Ventral view, to show two pretransverse cirri (arrowheads) and transverse cirri. E, endoral membrane; Ma, macronuclear nodules; Mi, micronuclei; MP, midventral cirral pairs; P, paroral membrane; TC, transverse cirri; 1–3, dorsal kineties 1–3. Scale bars = 50 μm.
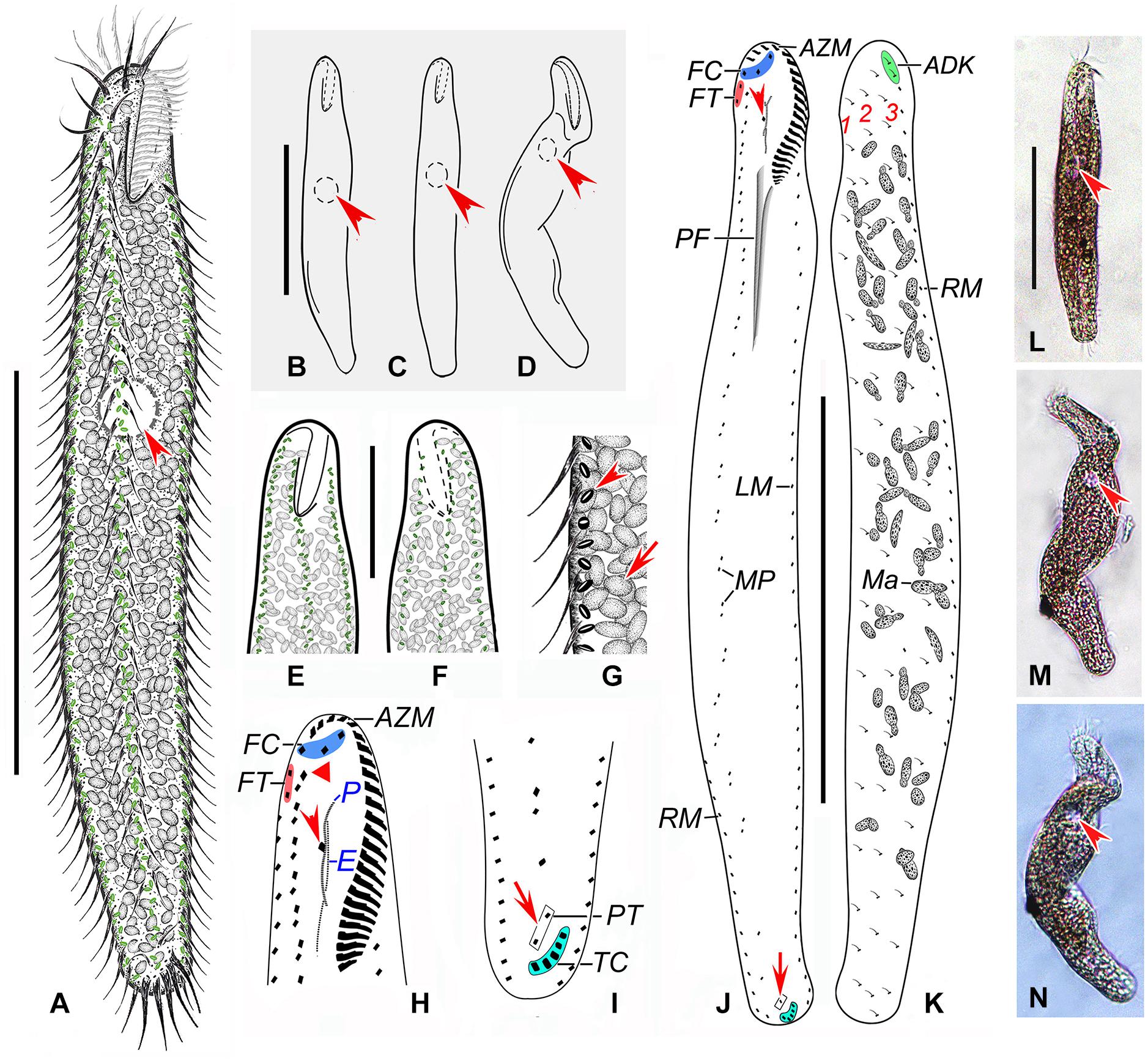
Figure 4. Anteholosticha perezuzae sp. nov. from life (A–G,L–N) and after protargol impregnation (H–K). (A–D) Overviews, showing the different body shapes and flexibility, arrowheads mark the contractile vacuole. (E–G) Ventral (E,G) and dorsal (F) views of the surface, showing the arrangement of slightly biconcave-shaped cortical granules (arrowhead) and packed ellipsoidal structures, likely mitochondria (arrow). (H) Ventral view of anterior part of cell, showing buccal cirrus (arrowhead) and parabuccal cirrus (red triangle). (I) Ventral posterior view, showing pretransverse cirri (arrow) and transverse cirri (light-blue part). (J,K) Ventral (J) and dorsal (K) views of the holotype specimen, showing the ciliary pattern and the nuclear apparatus; arrowhead marks buccal cirrus and arrow indicates the pretransverse cirrus. (L–N) Overviews of different specimens; arrows indicate contractile vacuole. ADK, additional dorsal bristles at anterior end of the right marginal cirral row; AZM, adoral zone of membranelles; E, endoral membrane; FC, frontal cirri; FT, frontoterminal cirri; LM, left marginal cirral row; Ma, macronuclear nodules; MP, midventral cirral pairs; P, paroral membrane; PF, pharyngeal fibers; PT, pretransverse cirri; RM, right marginal cirral row; TC, transverse cirri; 1–3, dorsal kineties 1–3. Scale bars = 110 μm (A–D,J–N), 70 μm (E,F).
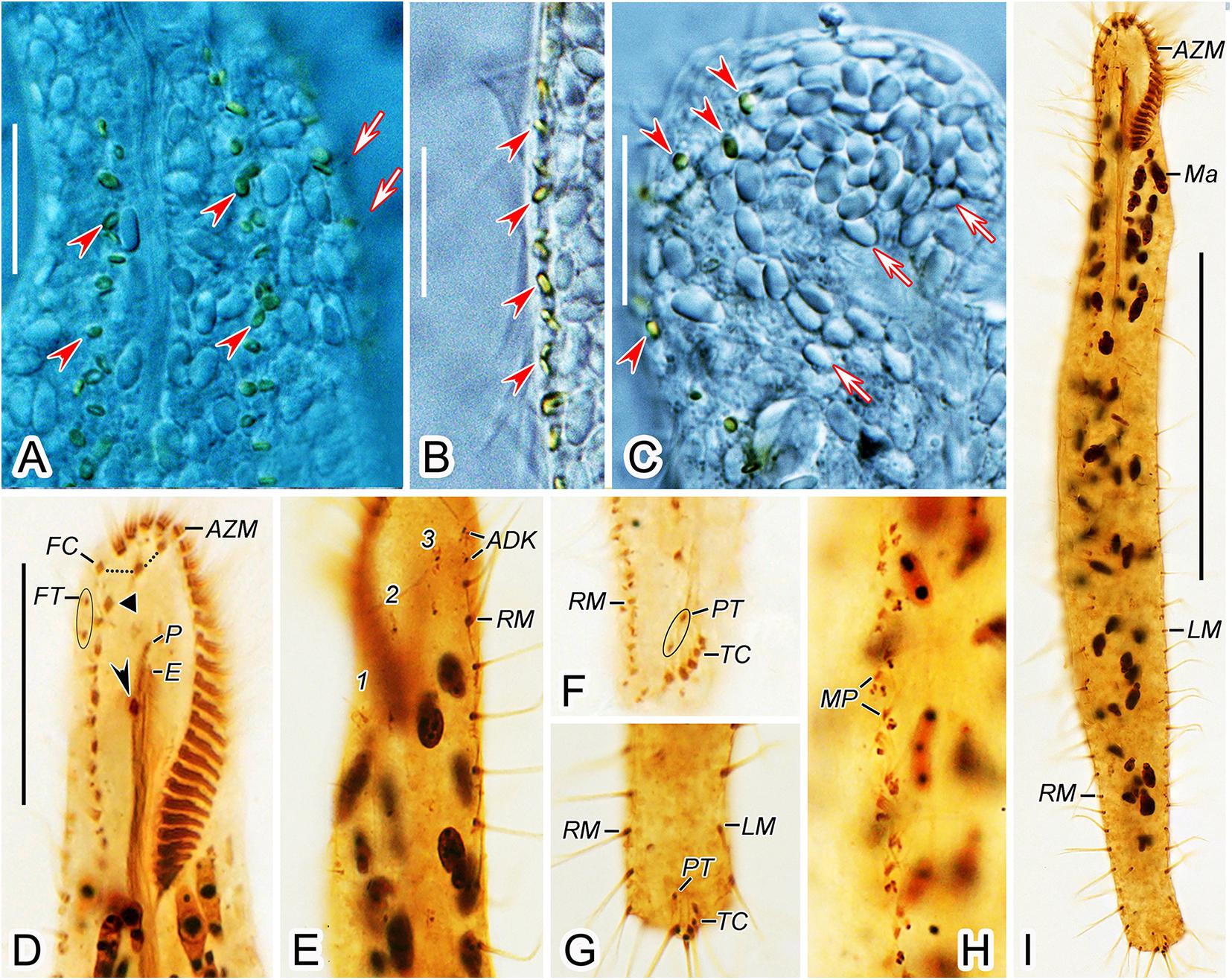
Figure 5. Anteholosticha perezuzae sp. nov. from life (A–C) and after protargol impregnation (D–I). (A–C) Ventral (A,B) and dorsal (C) views of the surface; arrowheads mark cortical granules. Arrows in (A) represent dorsal cilia and arrows in (C) point probable mitochondria. (D,E) Ventral (D) and dorsal (E) views of the anterior body regions; arrowhead shows the buccal cirrus, black triangle indicates the parabuccal cirrus. (F,G) Ventral views of the posterior body region, showing marginal cirral rows, pretransverse cirri, and transverse cirri. (H) Midventral cirral pairs. (I) Ventral view of the holotype specimen. ADK, additional dorsal bristles at anterior end of the right marginal cirral row; AZM, adoral zone of membranelles; E, endoral membrane; FC, frontal cirri; FT, frontoterminal cirri; LM, left marginal cirral row; Ma, macronuclear nodules; MP, midventral cirral pairs; P, paroral membrane; PT, pretransverse cirri; RM, right marginal cirral row; TC, transverse cirri; 1–3, dorsal kineties 1–3. Scale bars = 10 μm (A–C), 25 μm (D–H), 100 μm (I).
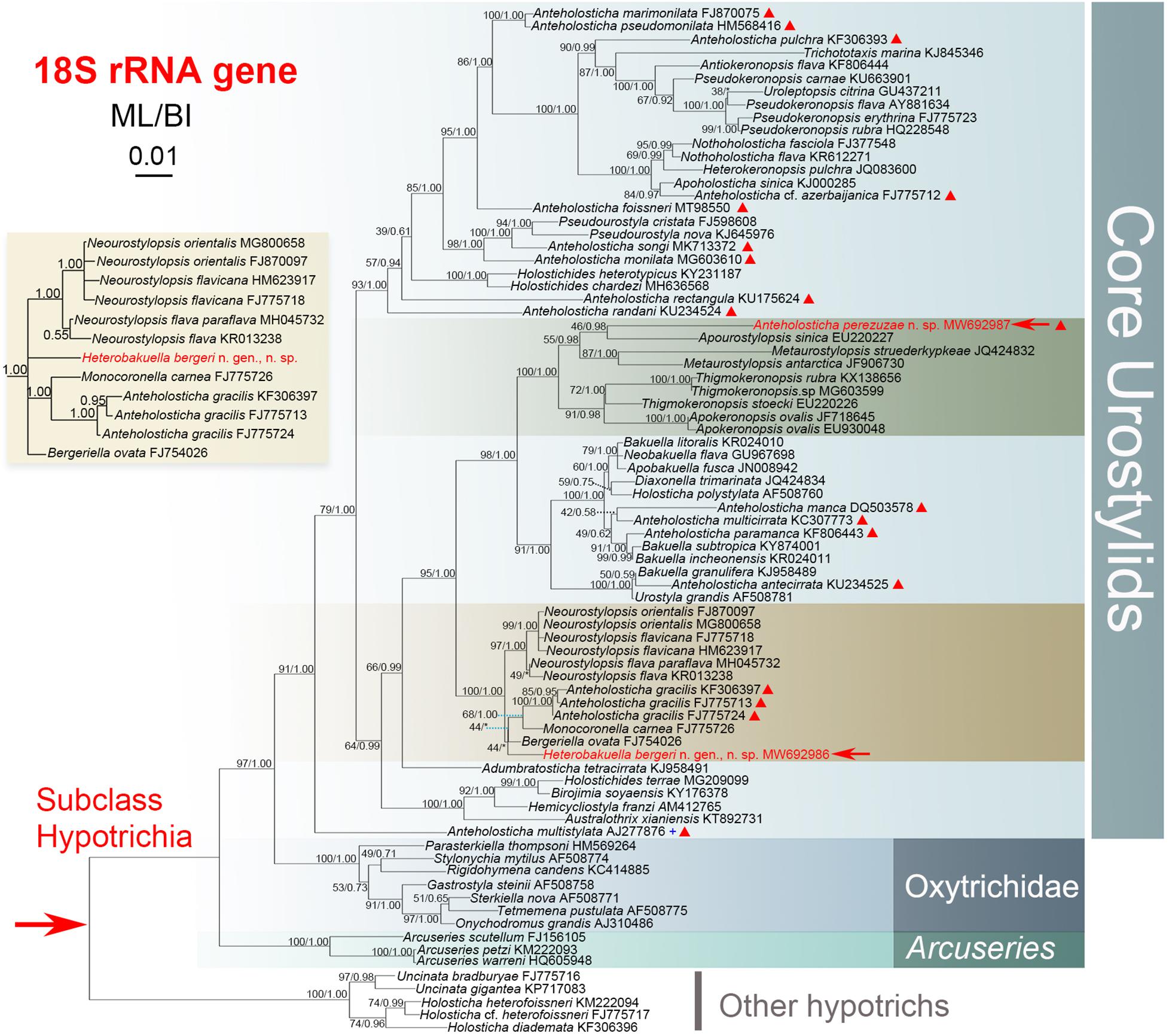
Figure 6. Maximum-likelihood (ML) tree inferred from 18S rRNA gene sequences, showing the systematic positions of Heterobakuella bergeri gen. nov., sp. nov. and Anteholosticha perezuzae sp. nov. (indicated in red). An inserted branch of Heterobakuella bergeri gen. nov., sp. nov. in Bayesian inference (BI), showing the systematic position of H. bergeri. Numbers near branches denote bootstrap values for ML and posterior probabilities for BI. Asterisks (*) indicate the disagreement between ML and BI trees and triangles indicate Anteholosticha species. The population of Anteholosticha multistylata marked with the plus (+) symbol was from NCBI without published morphological data. GenBank accession numbers are provided after species names. All branches are drawn to scale. The scale bar represents one substitution per 100 nucleotides.
For further comparison of the 18S rRNA gene sequences among Heterobakuella bergeri gen. nov., sp. nov. and other seven phylogenetically related species, the program BioEdit v.7.0 (Hall, 1999) was used to calculate the number of unmatched nucleotides and the pairwise identities, using “sequence difference count matrix” and “sequence identity matrix” options, respectively. After manually removing identical nucleotides, a nucleotide matrix with 84 unmatched sites is shown in Figure 7.
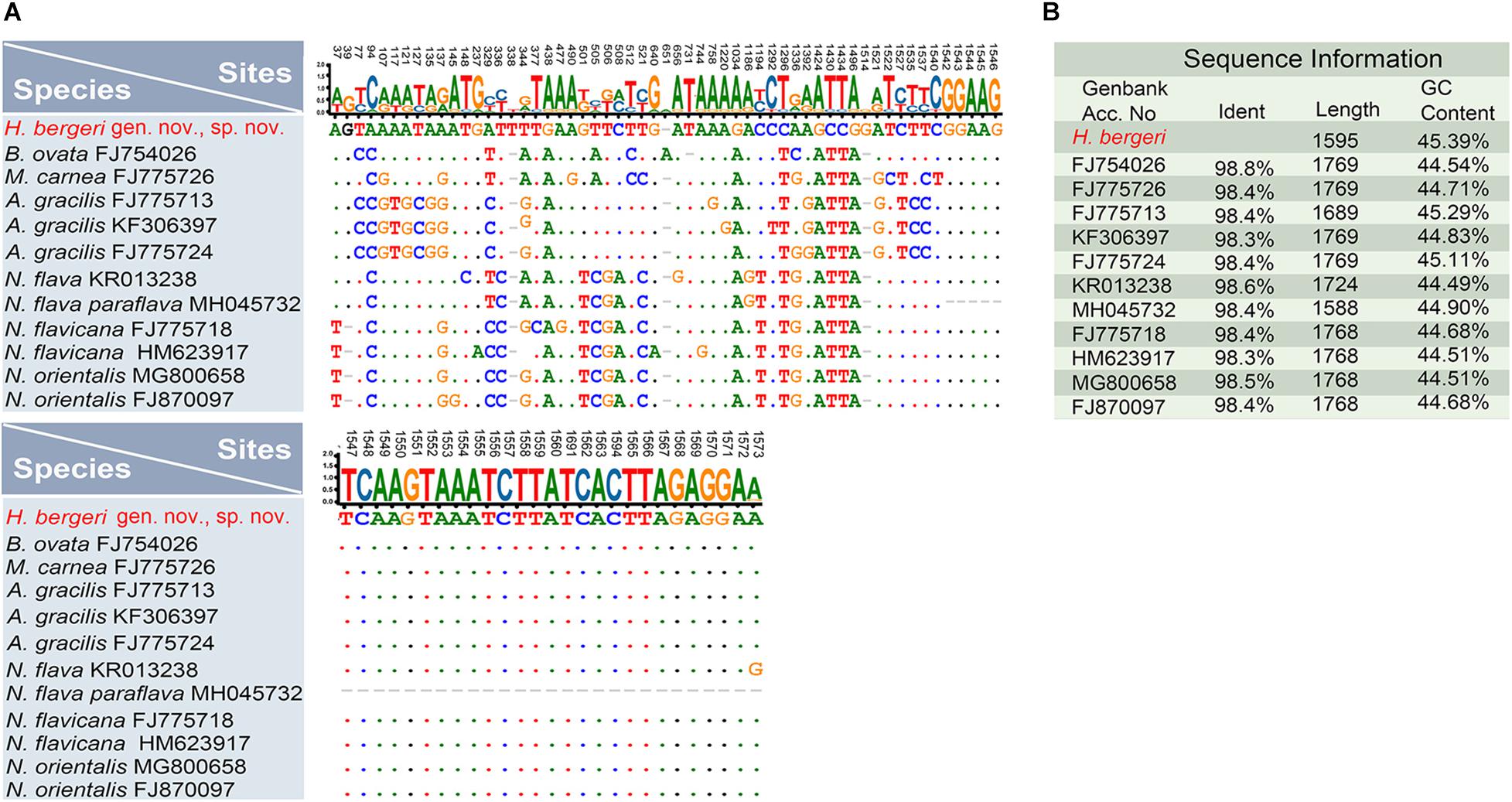
Figure 7. Nucleotide differences and similarities between Heterobakuella bergeri gen. nov., sp. nov. (indicated in red) and other related species based on 18S rRNA gene sequences. (A) Positions of unmatched nucleotides; short lines (–) indicate the missing nucleotides and solid dots represent matched sites. (B) Sequence information including identity, length, and GC content.
Present work: urn:lsid:zoobank.org:pub:9F118F25-20DD-4D16-99BA-558491E04EA4.
Heterobakuella gen. nov.: urn:lsid:zoobank.org:act:AD8318 E6-5248-442B-8FCF-01344F366E89.
Heterobakuella bergeri gen. nov., sp. nov.: urn:lsid:zoobank. org:act:51DBC371-A4C0-42A9-99DF-EDE5779A3F7.
Anteholosticha perezuzae sp. nov.: urn:lsid:zoobank.org: act:51DBC371-A4C0-42A9-99DF-EDE5779A3F71.
Subclass Hypotrichia Stein, 1859
Order Urostylida Jankowski, 1979
Genus Heterobakuella gen. nov.
Diagnosis. Urostylids with three clearly differentiated frontal cirri, one buccal and one parabuccal cirrus. Midventral complex composed of cirral pairs and one cirral row. One left and more than one right marginal cirral rows. Transverse and pretransverse cirri present. Caudal and frontoterminal cirri lacking.
Etymology. Composite of the Greek prefix hetero + (different) and the generic name Bakuella. This alludes to the fact that the new genus is different from the “core bakuellid genus” Bakuella.
Type species. Heterobakuella bergeri sp. nov.
Species assignable. The type species only.
Heterobakuella bergeri gen. nov., sp. nov.
Diagnosis. Size usually 90–130 × 30–45 μm in vivo. Body outline long-ellipsoidal and usually with the anterior part wider than the posterior one. Cortical granules yellow-brown, arranged along cirral rows and dorsal kineties. Adoral zone composed of about 25 membranelles. Seven to eleven transverse cirri, two pretransverse cirri, seven to twelve midventral cirral pairs, and a single cirral row composed of four to eleven cirri. One left and two right marginal cirral rows. Three complete dorsal kineties. About 29 macronuclear nodules. Freshwater habitat.
Dedication. We dedicate this new species to our eminent colleague, Prof. Helmut Berger (Consulting Engineering Office for Ecology, Salzburg, Austria) in recognition of his contributions to ciliatology.
Type locality. Lake Weishan (34°46′14″N, 117°12′56″E), Shandong Province, China.
Material deposited. A slide (No. SWY2019111201-1) with the protargol-impregnated holotype specimen and two slides (No. SWY2019111201-2, 3) with protargol-impregnated paratypes are deposited in the Laboratory of Protozoology, Ocean University of China.
Description. Cell size usually 90–130 × 30–45 μm, body length to width ratio about 2.5–3.5:1 in vivo, i.e., generally shorter and narrower than in protargol preparations (104–158 × 31–77 μm) due to the bleaching procedure (Table 1). Body rather flexible but not contractile, outline elongate elliptical to elongate ovoid, usually with anterior part wider than the posterior one (Figures 2A–D, 3A,B). Cytoplasm colorless, contains numerous colorless globules (about 0.4–0.8 μm in diameter) (Figures 2E, 3E,F), food vacuoles (about 5–25 μm across) in mid-body and posterior region, containing bacteria or algae (Figures 2A,B, 3B). Cortical granules yellow-brown, globular, about 0.5–1.3 μm in diameter, sparsely arranged along cirral rows and dorsal kineties (Figures 2C–E, 3E,F). Contractile vacuole about 11–15 μm across in diastole, located anterior to equator about two-fifths of body length near left margin (Figures 2A,B, 3A,B). Approximately 29 (22–46) irregularly ellipsoidal to ovoidal macronuclear nodules, 8–16 × 3–6 μm in size after protargol impregnation, scattered throughout cytoplasm (Figures 2G, 3D,G–I); one or two globular micronuclei, 2–5 μm in diameter, attached to macronuclear nodules (Figures 2G, 3H). Locomotion by slow to moderately rapid crawling on bottom of Petri dish, or by swimming while rotating about longitudinal axis.
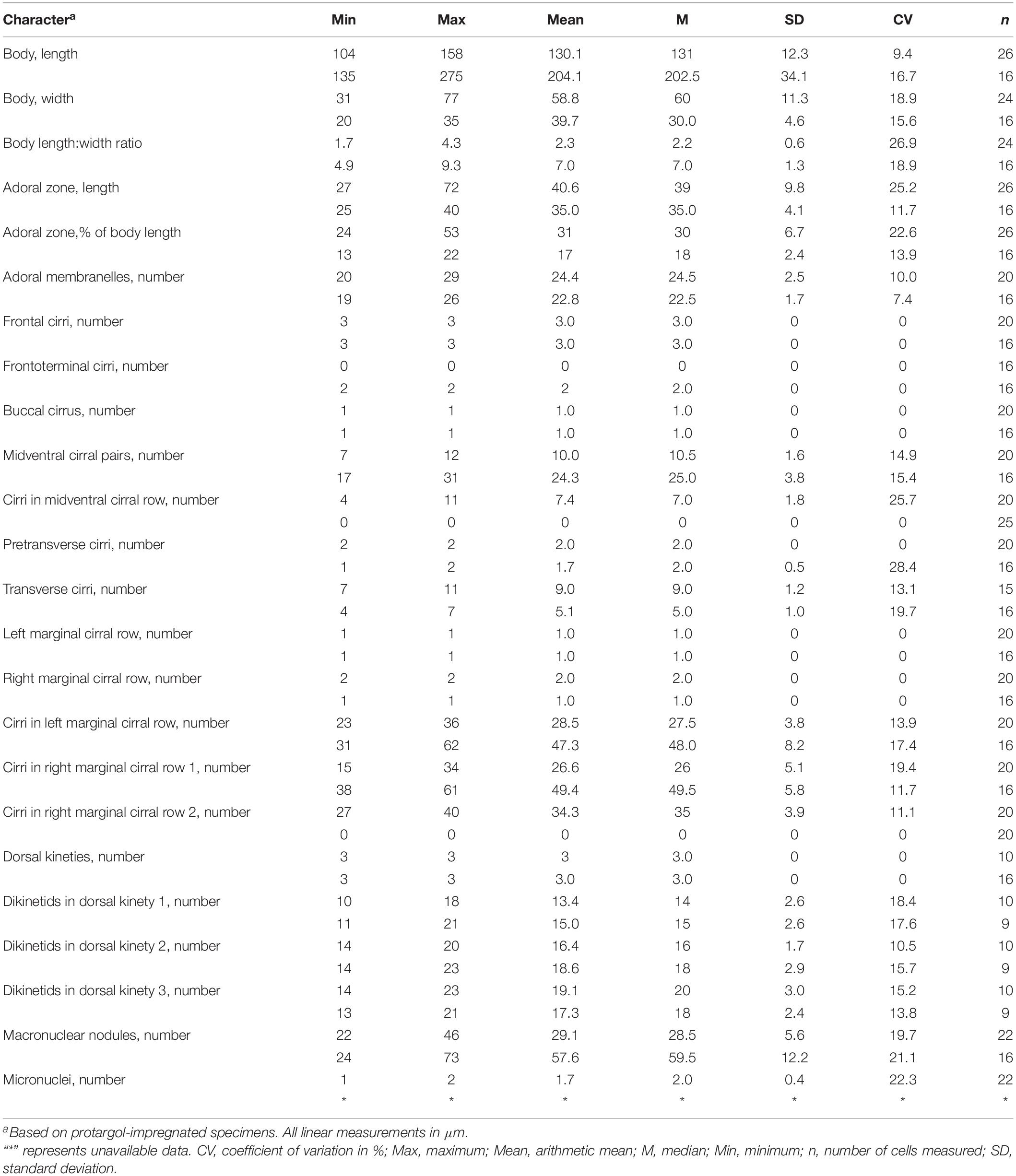
Table 1. Morphometric characterizations of Heterobakuella bergeri gen. nov., sp. nov. (upper line) and Anteholosticha perezuzae sp. nov. (lower line).
Adoral zone continuous, extending to about 33% (31–38%) of body length in vivo, 31% (24–43%) after protargol impregnation, composed of 20–29 membranelles, membranelle cilia 6–13 μm long. Paroral and endoral membranes nearly equal in length, both slightly curved, optically intersect at posterior 33% of paroral (Figures 2F, 3C,G). Most cirri about 10–12 μm long in vivo except frontal and transverse cirri which are 13–14 μm long, 14–17 μm long, respectively. Three clearly differentiated frontal cirri, rearmost one adjacent to distal end of adoral zone (Figures 2F, 3J). Single parabuccal cirrus behind rightmost frontal cirrus (Figure 2F). Single buccal cirrus situated at level of anterior 33% of paroral membrane (Figures 2F, 3J). Midventral complex extends nearly to pretransverse cirri, anterior part composed of seven to twelve midventral cirral pairs, arranged in typical zig-zag pattern, posterior part composed of four to eleven unpaired ventral cirri in a single row (Figures 2F, 3G,H,K). Seven to eleven transverse cirri in J-shaped pattern, two pretransverse cirri (Figures 2F, 3G,H,L). Consistently one left marginal cirral row (LMR) with 23–36 cirri, two right marginal cirral rows (RMR), rightmost with 27–40 cirri, the more medial one with 15–34 cirri (Figure 2F); anterior portions of both RMR usually extend onto dorsal side (Figures 2F,G). Three complete dorsal kineties, two or three additional dorsal bristles to right of anterior end of dorsal kinety 3 (Figures 2G, 3I). Caudal and frontoterminal cirri absent.
Genus Anteholosticha Berger, 2003
Anteholosticha perezuzae sp. nov.
Diagnosis. Body size 140–260 × 25–40 μm in vivo. Body outline vermiform, length:width ratio on average 7 (5–9):1 after protargol impregnation. Cortical granules biconcave, greenish, about 1.0–1.5 μm in diameter. Adoral zone continuous, with about 23 membranelles. Three frontal, two frontoterminal, three to six transverse, one or two pretransverse cirri. About 24 midventral cirral pairs. Three complete dorsal kineties. About 58 macronuclear nodules. Brackish water habitat.
Dedication. We dedicate this new species to our esteemed colleague, Prof. Blanca Pérez-Uz (Department of Genetics, Physiology and Microbiology, Faculty of Biological Sciences, Universidad Complutense de Madrid, Spain) in recognition of her contributions to protistology.
Type locality. A brackish stream near Tangdao Bay, Qingdao, China (35°56′18″N, 120°12′44″E).
Material deposited. A slide (No. ZTY2017032701-1) with the protargol-impregnated holotype and five slides (No. ZTY2017032701-2–6) with protargol-impregnated paratypes are deposited in the Laboratory of Protozoology, Ocean University of China.
Description. Size about 140–260 × 25–40 μm in vivo, usually 200 × 30 μm, after protargol impregnation. Body vermiform, length:width ratio about 5–9:1 (Figures 4A–D,L–N); dorsoventrally flatted about 3:2; flexible but not contractile. Contractile vacuole approximately 8–12 μm across in diastole, located at about anterior 33–43% of body length (Figures 4A–D,L–N, arrowheads). Body brownish at low magnification (Figures 4L–N). Cytoplasm colorless, packed with ellipsoidal structures, probably mitochondria (Figures 4G, 5C, about 3 or 4 μm in diameter). Food vacuoles not observed. Cortex flexible, contains biconcave shaped, greenish cortical granules, about 1.0–1.5 μm in diameter, evenly arranged in a line along every cirral row and dorsal kinety (Figures 4E–G, 5A–C). About 24–73 (on average 58) irregularly ellipsoidal macronuclear nodules scattered throughout cytoplasm, individual nodules approximately 5–10 × 3–5 μm in size after protargol impregnation; micronuclei difficult to determine because hardly distinguishable from similar-sized and similarly impregnated cytoplasmic inclusions (Figures 4K, 5I). Crawls moderately slowly on debris particles, sometimes swims by rotation about main body axis.
Buccal field distinctly narrow, extending from 13 to 22% of body length (Figures 4A–D,J,L–N, 5I). Adoral zone continuous, with 19–26 membranelles (Figures 4H,J, 5D,I); paroral and endoral membranes almost straight, partially overlap (Figures 4H,J, 5D); paroral membrane begins anterior to endoral, slightly shorter than endoral, 10–16 μm long in protargol preparations, endoral membrane about 15–19 μm long after protargol impregnation (Figures 4H, 5D). Three clearly differentiated frontal cirri (Figures 4H,J, 5D), approximately 15–18 μm long in vivo. Single parabuccal cirrus, about 15 μm long in vivo, behind rightmost frontal cirrus to left of frontoterminal cirri (Figures 4I, 5D). One buccal cirrus, about 10 μm long in vivo, situated right of mid-portion of paroral membrane (Figures 4H,J, 5D). Two frontoterminal cirri, located right of parabuccal cirrus (Figures 4H,J, 5D). Midventral complex consists of 17–31 midventral cirral pairs arranged in a zig-zag pattern (Figures 4K, 5H). One or two pretransverse cirrus/cirri anterior to transverse cirri (Figures 4I,J, 5F,G). Cilia of frontoterminal, midventral cirral, and pretransverse cirri about 8–13 μm long. Three to six transverse cirri, about 13–17 μm long in vivo, in oblique or slightly “J” shaped row (Figures 4I,J, 5F,G). One left and one right marginal cirral row, composed of 31–62, 38–61 cirri, respectively, cirri usually 10–14 μm long, sometimes up to 17 μm long after protargol preparations (Figures 4J,K, 5E–G,I). Dorsal bristles about 3–4 μm long in vivo, arranged in three bipolar rows; dorsal kinety 1 composed of 11–21 dikinetids, kinety 2 of 14–23 dikinetids, and kinety 3 of 13–21 dikinetids (Figures 4K, 5E; Table 1). Two additional dorsal bristles (dikinetids) at anterior end of the right marginal cirral row (Figures 4K, 5E). Caudal cirri absent.
The GenBank accession number, length, and G + C content of the 18S rRNA gene sequences of the two species are as follows: Heterobakuella bergeri gen. nov., sp. nov., MW692986, 1,595 bp, 45.39%; Anteholosticha perezuzae sp. nov., MW692987, 1,618 bp, 48.75%. Topologies of the maximum likelihood (ML) and Bayesian inference (BI) trees are nearly congruent; thus, only the ML tree is shown with support values from both algorithms (Figure 6). In Figure 6, Heterobakuella bergeri gen. nov., sp. nov. and Anteholosticha perezuzae sp. nov. are included in the “core urostylids” with strong statistical support.
In the 18S rRNA gene tree, Heterobakuella bergeri gen. nov., sp. nov. falls within a group including Bergeriella ovata, Monocoronella carnea, Anteholosticha gracilis, and Neourostylopsis spp. with full support. However, the position of H. bergeri gen. nov., sp. nov. within this well-supported clade is not robust, as without support in the ML tree (44%) and having an incongruent topology in the BI tree. In the ML tree, H. bergeri gen. nov., sp. nov. is sister to a cluster comprising B. ovata, M. carnea, and A. gracilis without support (44%). This poorly resolved cluster is then sister to the strongly supported clade (97%) including Neourostylopsis species with full support. While in the BI analysis, H. bergeri gen. nov., sp. nov. is placed as a polytomy with B. ovata, M. carnea, and a clade comprising A. gracilis, Neourostylopsis spp., respectively (insert in Figure 6).
Sequence comparisons between H. bergeri gen. nov., sp. nov. and these molecular related species show that H. bergeri gen. nov., sp. nov. differs from B. ovata by 18 nucleotide positions (98.8% sequence identity), from M. carnea by 25 nucleotide positions (98.4% sequence identity), from A. gracilis (three populations) by 26 or 27 nucleotide positions (98.3% or 98.4% sequence identity), from N. flava by 24 nucleotide positions (98.6% sequence identity), from N. flava paraflava by 21 nucleotide positions (98.4% sequence identity), from N. flavicana (two populations) by 26 or 27 nucleotide positions (98.4% or 98.3% sequence identity), and from N. orientalis (two populations) by 24 or 25 nucleotide positions (98.4% or 98.5% sequence identity), respectively (Figure 7).
Anteholosticha perezuzae sp. nov. does not cluster with any congeners in ML and BI analysis, and clusters with Apourostylopsis sinica (Shao et al., 2008) Chen et al., 2013 (EU220227) without support in ML analysis (46%) and with strong support in BI analysis (0.98). This sister cluster groups with a clade comprising two Metaurostylopsis species (M. struederkypkeae and M. antarctica) with weak support in ML analysis (55%) and high support in BI analysis (0.98). Then, the forming sister group among them clusters together with another clade comprising Thigmokeronopsis spp. and two populations of Apokeronopsis ovalis. Other Anteholosticha species are scattered throughout the tree as shown in Figure 6, again indicating the non-monophyly of Anteholosticha.
Seven urostylid genera with a continuous adoral zone, three frontal cirri, and midventral complex composed of cirral pairs and row(s) should be compared with Heterobakuella gen. nov., namely, Apobakuella, Bakuella, Holostichides, Metaurostylopsis, Monourostylopsis Song et al., 2020, Neobakuella, and Paragastrostyla (Berger, 2006; Li et al., 2011; Jiang et al., 2013; Song et al., 2020; Figure 8). Of these, Apobakuella is most similar to Heterobakuella gen. nov. in terms of the cirral pattern. Hitherto, Apobakuella, with the type species A. fusca, is monotypic (Jiang et al., 2013). Morphologically, Heterobakuella bergeri gen. nov., sp. nov. can be distinguished from A. fusca by the smaller body size (90–130 × 35–45 μm vs. 150–210 × 50–60 μm), cortical granules (one type vs. two types), buccal cirri (one vs. three to nine), parabuccal cirri (one vs. five to nine arranged in two or three rows), fewer midventral cirral rows with unpaired cirri in posterior portion(s) (one vs. four to nine), and habitat (freshwater vs. brackish). In addition, Heterobakuella gen. nov. can be easily separated from Holostichides and Paragastrostyla by transverse cirri (present vs. absent in Holostichides and Paragastrostyla) (Berger, 2006; Zhu et al., 2019), and from the other four genera by marginal cirral rows (a single left and two right rows vs. a single marginal cirral row on each side in Bakuella; two or more marginal cirral rows on each side in Metaurostylopsis; more than one left and single right marginal cirral rows in Monourostylopsis and Neobakuella), frontoterminal cirri (absent vs. present in Bakuella, Metaurostylopsis, Monourostylopsis, and Neobakuella) (Li et al., 2011; Jo et al., 2015; Lu et al., 2016; Moon et al., 2020; Song et al., 2020; Zhang et al., 2020b). Generic classification of hypotrichs is traditionally based on characteristics of the ciliature and nuclear apparatus in morphostatic and dividing cells (for reviews, see Berger, 1999, 2006, 2008, 2011). Our new species distinctly differs from the morphologically most similar genus, Apobakuella, as mentioned previously. In addition, the phylogenetic trees based on 18S rRNA gene sequences show our new species is distant from A. fusca, the sequences of these two species differing in 68 nucleotide positions. Thus, because our new species cannot be assigned to any existing genus, we erect the new genus, Heterobakuella.
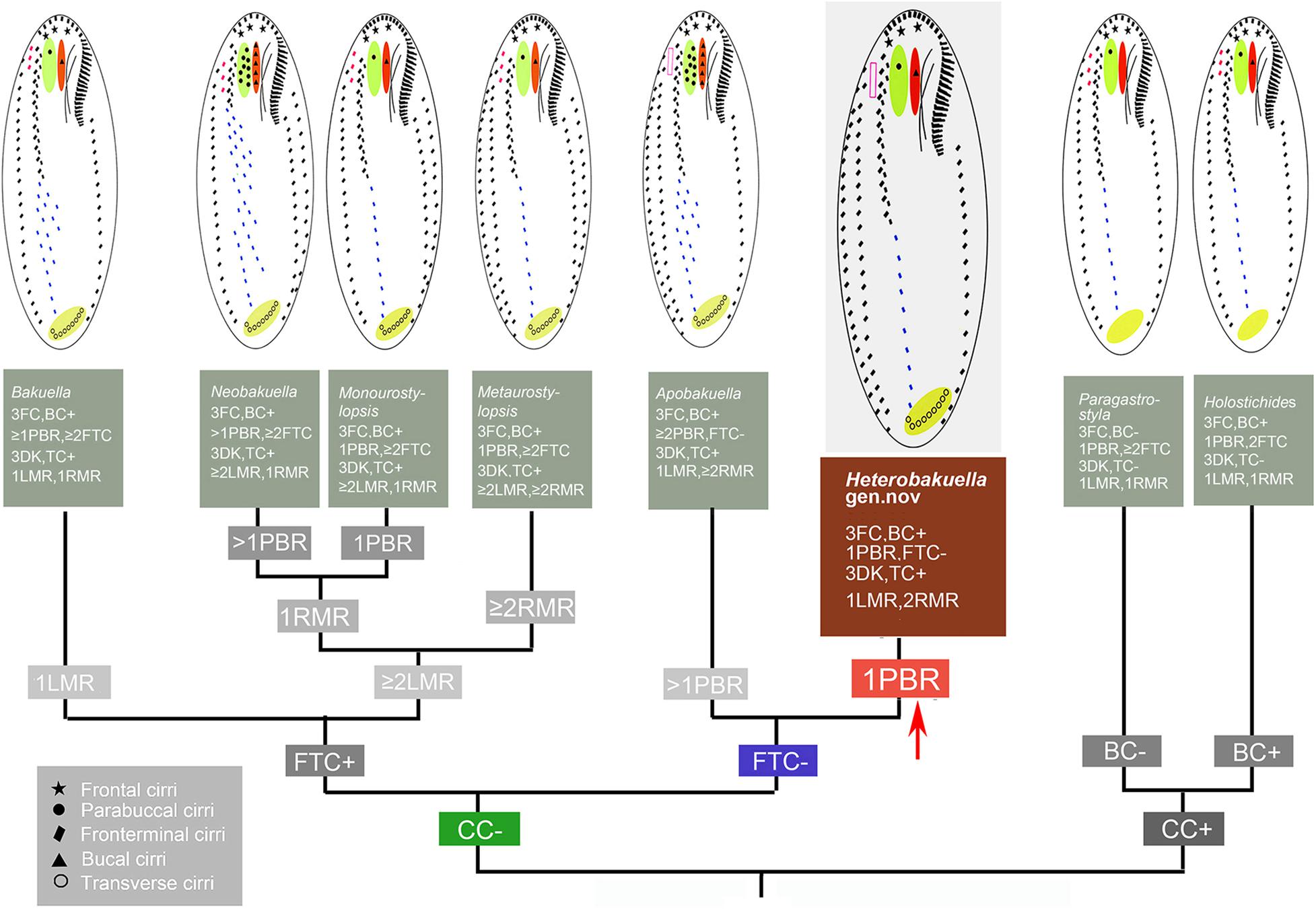
Figure 8. Comparisons of morphologically related genera. BC, buccal cirri; CC, caudal cirri; DK, dorsal kineties; FC, frontal cirri; FTC, frontoterminal cirri; LMR, left marginal cirral row; PBR, parabuccal row; RMR, right marginal cirral row; TC, transverse cirri.
Although Lynn (2008) merged all genera within Bakuellidae and most genera of two other families (e.g., Holostichidae and Urostylidae) from Berger’s (2006) classification into a single large family, Urostylidae, we prefer Berger’s (2006) classification since all genera assigned to Urostylidae sensu Lynn (2008) are paraphyletic and require further study and possibly further subdivision. The monophyly of Bakuellidae has recently been challenged in a number of reports (Lynn, 2008; Yi et al., 2008a,b; Chen et al., 2011; Dai and Xu, 2011; Foissner, 2016; Lyu et al., 2018), and additional data are needed to better define the family. Heterobakuella bergeri gen. nov., sp. nov. has morphological characteristics typical for Bakuellidae, including three frontal cirri and a midventral complex composed of pairs and row(s) of unpaired cirri (Berger, 2006). However, H. bergeri gen. nov., sp. nov. is distant from the well-known bakuellids including Apobakuella, Bakuella, Diaxonella, and Neobakuella in the 18S rRNA gene tree. Morphogenetic features of H. bergeri gen. nov., sp. nov. are, as yet, unknown. For these reasons, a confident familial assignment of Heterobakuella cannot be made at the current state of knowledge and we consider the genus as incertae sedis in Urostyloidea.
Anteholosticha perezuzae sp. nov. matches the generic definition of Anteholosticha given by Berger (2003). Hitherto, Anteholosticha is non-monophyletic and comprises more than 40 species (Berger, 2003, 2006, 2008; Li et al., 2011; Xu et al., 2011; Park et al., 2013; Fan et al., 2016; Chen et al., 2018, 2020; Jung et al., 2021). In terms of the vermiform shape (length:width ratio more than 5:1), the presence of many (more than 20) macronuclear nodules, three enlarged frontal cirri, three dorsal kineties, and a continuous adoral zone of membranelles, the following three species, namely A. fasciola (Kahl, 1932) Berger, 2003, A. grisea (Kahl, 1932) Berger, 2003, and A. violacea (Kahl, 1932) Berger, 2003, should be compared with Anteholosticha perezuzae sp. nov. (Berger, 2003, 2006).
Anteholosticha fasciola (Kahl, 1932) requires redescription (for a review, see Berger, 2006, page 441) but can be easily distinguished from A. perezuzae sp. nov. by shape (length:width ratio up to 10:1 vs. wider shape with the length:width ratio 5–9:1) and buccal cirri (two enlarged, possibly buccal cirri vs. one buccal cirrus). Li et al. (2009) classified a Chinese population as A. fasciola and transferred it to a new genus, Nothoholosticha Li et al., 2009, which is mainly diagnosed by an atypical bicorona in which the anterior corona is usually formed by four frontal cirri. We compared the Chinese population with the type population described by Kahl (1932) and found that they are likely two different species based on the body shape, the cirri along undulating membranes, and frontal cirri. Specifically, compared with the Chinese population, Kahl’s (1932) population displays a more slender shape with the length:width ratio 10:1 (vs. about 6:1), possesses two enlarged, possibly buccal cirri to the right of the undulating membranes (vs. one buccal cirrus), and three enlarged frontal cirri (vs. four anterior frontal cirri and two posterior cirri) (for a review, see Berger, 2006, page 441). Therefore, we consider the Chinese population of Li et al. (2009) to represent a different species from A. fasciola (Kahl, 1932) Berger, 2003. In any event, Notoholosticha spp. are easily distinguished from A. perezuzae by frontal cirri (six vs. three), atypical bicorona (present vs. absent), adoral zone of membranelles (bipartite vs. continuous).
Anteholosticha perezuzae sp. nov. can be distinguished from A. grisea (Kahl, 1932) Berger, 2003, because the new species possesses conspicuous greenish cortical granules (about 1.0–1.5 μm in diameter) along the marginal cirral rows, while cortical granules were not mentioned in any population of A. grisea (Berger, 2006, page 332). As noted by Berger (2006), Kahl was a very good observer, thus we prefer to accept cortical granules are lacking. Anteholosticha grisea invariably displays a blackish body color due to food vacuoles containing ingested rhodobacteria (Berger, 2006, page 332), while A. perezuzae sp. nov. shows a brownish body at low magnifications. In addition, A. grisea is more likely confined to the freshwater sapropel, while A. perezuzae sp. nov. is from a brackish stream.
According to Berger (2006), the taxonomy of Anteholosticha violacea is rather complicated. At least six populations were reported and the possibility that they belong to different species could not be excluded, thus Berger simply accepted Kahl’s data (Kahl, 1928, 1932; Berger, 2006). Anteholosticha perezuzae sp. nov. differs distinctly from Kahl’s (1928) population by frontal cirri (three enlarged frontal cirri vs. six to seven) and from Kahl’s (1932) second population by dorsal bristles (3–4 μm long vs. at least 8 μm long).
Considering the position and the specialized pattern of ventral cirri, viz., three frontal cirri and a midventral complex composed of cirral pairs and row(s), Heterobakuella might be closely related to the bakuellid taxa, especially to the morphologically similar genus Apobakuella. However, a close phylogenetic relationship between Heterobakuella and bakuellid taxa is not detected in the very conservative 18S rRNA gene tree, as these taxa do not form a monophyletic assemblage, and Heterobakuella branches off earlier than bakuellid taxa. Thus, their similar ventral cirral pattern likely represents a convergently evolved character, viz., the ventral ciliary pattern is analogous and not homologous. On the other hand, Heterobakuella groups together with Bergeriella, Monocoronella, Anteholosticha gracilis, and Neourostylopsis with the full support in 18S rRNA gene tree (Figure 6). Within this clade, Bergeriella, Heterobakuella, and Monocoronella are three monotypic genera with evolutionary novelties, and type species of which are B. ovata, H. bergeri, and M. carnea, respectively. Morphologically, H. bergeri can be easily distinguished from B. ovata by frontal cirri (three vs. 6–13) and postoral ventral rows (absent vs. present) (Liu et al., 2010), from M. carnea by frontal cirri arranged in monocorona (absent vs. present) (Chen et al., 2011), and from A. gracilis and Neourostylopsis spp. by midventral complex composed anteriorly of pairs and posteriorly of unpaired cirri (present vs. absent) (Berger, 2006; Chen et al., 2013). Thus, to understand their evolutionary relationship, more discoveries of further congeners and more genes must be awaited.
Because the genus Anteholosticha was established based only on a combination of plesiomorphies in the absence of obvious synapomorphies, Berger (2003, 2006) had hypothesized the non-monophyly of Anteholosticha. Subsequently, the non-monophyly of Anteholosticha has been reported in many previous studies (Park et al., 2013; Zhao et al., 2015; Fan et al., 2016; Chen et al., 2018, 2020; Paiva, 2020; Jung et al., 2021). Our analyses also confirm that Anteholosticha species are scattered among different branches inside the core urostylid assemblage. Morphological and molecular data for the newly discovered species A. perezuzae sp. nov. is highly incongruent. Anteholosticha perezuzae clusters with Apourostylopsis sinica (EU220227) without support in ML analysis and with strong support in BI analysis (0.98). However, morphologically, A. perezuzae sp. nov. can be easily separated from A. sinica by body size (140–260 × 25–40 μm vs. 100–120 × 30 um in vivo), one type of cortical granules (vs. both two), number of midventral cirral pairs (17–31 vs. 11–17), number of left marginal cirral rows (one vs. three), and number of left marginal cirral rows (one vs. two) (Shao et al., 2008; Chen et al., 2013). Likewise, if the “good” apomorphies for inferring their evolutionary relationship cannot be found, this polyphyly might be better resolved with future analysis of additional taxa. Regarding this problem of taxonomic and phylogenetic resolution, a new classification system proposed by Paiva (2020) might give us some inspirations. According to Paiva (2020), the newly established taxon Kentrurostylida corresponds to the “core urostylids”. Within the taxon, two secondary taxa Simplicitergida and Hispidotergida were established, based on three dorsal bristle rows and numerous dorsal bristles, respectively. Accordingly, A. antecirrata, A. gracilis, A. manca, A. multicirriata, and A. paramanca were assigned to Simplicitergida, while the other species, A. cf. azerbaijanica, A. foissneri, A. marimonilata, A. monilata, A. pseudomonilata, A. pulchra, A. songi, A. randani, and A. rectangula were placed in Hispidotergida. Under Paiva’s (2020) classification, Anteholosticha perezuzae would be assigned to Simplicitergida. From the current work, it is obvious that the taxonomy and phylogeny of both bakuellid-like taxa and those species currently included in Anteholosticha remains in a state of flux.
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/supplementary material.
YW designed and supervised the research study. WS, TZ, and JD drafted the manuscript. XL and WB revised and improved the manuscript. WS and TZ collected samples and performed staining. All authors contributed to the manuscript and approved the submitted version.
This work was financially supported by the National Natural Science Foundation of China (Nos. 32030015 and 31900319), and the China Postdoctoral Science Foundation Grant (Nos. BX20180348, 2018M642955, and 2021M692010).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
We would like to express our gratitude to Weibo Song, from Ocean University of China, for his kind help during drafting of the article; to Saleh A. Al-Farraj, King Saud University, for his suggestions during article revision; and to the “Weishan Wetland Station” for the institutional support.
Bai, Y., Wang, R., Liu, W., Warren, A., and Hu, X. (2020). Redescriptions of three tintinnine ciliates (Ciliophora: Tintinnina) from coastal waters in China based on lorica features, cell morphology, and rDNA sequence data. Eur. J. Protistol. 72:125659. doi: 10.1016/j.ejop.2019.125659
Berger, H. (1999). Monograph of the Oxytrichidae (Ciliophora, Hypotrichia). Monogr. Biol. 78, 1–1080. doi: 10.1007/978-94-011-4637-1
Berger, H. (2003). Redefinition of Holosticha Wrzesniowski, 1877 (Ciliophora, Hypotricha). Eur. J. Protistol. 39, 373–379. doi: 10.1078/0932-4739-00006
Berger, H. (2006). Monograph of the Urostyloidea (Ciliophora, Hypotricha). Monogr. Biol. 85, 1–1304. doi: 10.1007/1-4020-5273-1_1
Berger, H. (2008). Monograph of the Amphisiellidae and Trachelostylidae (Ciliophora, Hypotricha). Monogr. Biol. 88, 1–737. doi: 10.1007/978-1-4020-8917-6
Berger, H. (2011). Monograph of the Gonostomatidae and Kahliellidae (Ciliophora, Hypotricha). Monogr. Biol. 90, 1–741. doi: 10.1007/978-94-007-0455-8
Borror, A. C. (1972). Revision of the order Hypotrichida (Ciliophora, Protozoa). J. Protozool. 19, 1–23. doi: 10.1111/j.1550-7408.1972.tb03407.x
Chen, L., Dong, J., Wu, N., Xin, Y., Warren, A., Ning, Y., et al. (2020). Morphology and molecular phylogeny of a new hypotrich ciliate, Anteholosticha songi nov. spec., and an American population of Holosticha pullaster (Müller, 1773) Foissner et al., 1991 (Ciliophora, Hypotrichia). Eur. J. Protistol. 72:125646. doi: 10.1016/j.ejop.2019.125646
Chen, L., Liu, Y., Long, Y., Lyu, J., Feng, C., Ning, Y., et al. (2021). Morphology and molecular phylogeny of two new soil ciliates, Hemiurosomoida warreni nov. spec. and Hemiurosoma clampi nov. spec. (Ciliophora, Hypotrichia) from Tibet. Eur. J. Protistol. 77:125746. doi: 10.1016/j.ejop.2020.125746
Chen, L., Wu, W., El-Serehy, H. A., Hu, X., and Clamp, J. C. (2018). Morphology, morphogenesis, and phylogeny of an Anteholosticha intermedia (Ciliophora, Urostylida) population from the United States. Eur. J. Protistol. 65, 1–15. doi: 10.1016/j.ejop.2018.04.003
Chen, X., Dong, J., Lin, X., and Al-Rasheid, K. A. S. (2011). Morphology and phylogeny of a new urostylid ciliate, Monocoronella carnea n. g., n. sp. (Ciliophora, Hypotricha) from Daya Bay, Southern China. J. Eukaryot. Microbiol. 58, 497–503. doi: 10.1111/j.1550-7408.2011.00581.x
Chen, X., Shao, C., Liu, X., Huang, J., and Al-Rasheid, K. A. S. (2013). Morphology and phylogenies of two hypotrichous brackish-water ciliates from China, Neourostylopsis orientalis n. sp. and Protogastrostyla sterkii (Wallengren, 1900) n. comb., with establishment of a new genus Neourostylopsis n. gen. (Protista, Ciliophora, Hypotrichia). Int. J. Syst. Evol. Microbiol. 63, 1197–1209. doi: 10.1099/ijs.0.049403-0
Dai, R., and Xu, K. (2011). Taxonomy and phylogeny of Tunicothrix (Ciliophora, Stichotrichia), with the description of two novel species, Tunicothrix brachysticha n. sp. and Tunicothrix multinucleata n. sp., and the establishment of Parabirojimidae n. fam. Int. J. Syst. Evol. Microbiol. 61, 1487–1496. doi: 10.1099/ijs.0.024463-0
Darriba, D., Taboada, G. L., and Doallo, R. (2012). jModelTest 2: more models, new heuristics and parallel computing. Nat. Methods 9:772. doi: 10.1038/nmeth.2109
Fan, Y., Lu, X., Huang, J., Hu, X., and Warren, A. (2016). Redescription of two little-known urostyloid ciliates, Anteholosticha randani (Grolière, 1975) Berger, 2003 and A. antecirrata Berger, 2006 (Ciliophora, Urostylida). Eur. J. Protistol. 53, 96–108. doi: 10.1016/j.ejop.2016.01.001
Foissner, W. (2016). Terrestrial and semiterrestrial ciliates (Protozoa, Ciliophora) from Venezuela and Galápagos. Denisia 35, 1–912.
Gouy, M., Guindon, S., and Gascuel, O. (2010). SeaView version 4: a multiplatform graphical user interface for sequence alignment and phylogenetic tree building. Mol. Biol. Evol. 27, 221–224. doi: 10.1093/molbev/msp259
Hall, T. A. (1999). BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symp. Ser. 41, 95–98.
Hu, X., Lin, X., and Song, W. (2019). Ciliate Atlas: Species Found in the South China Sea. Beijing: Science Press.
Huang, J., Chen, Z., Song, W., and Berger, H. (2014). Three-gene based phylogeny of the Urostyloidea (Protista, Ciliophora, Hypotricha), with notes on classification of some core taxa. Mol. Phylogenet. Evol. 70, 337–347. doi: 10.1016/j.ympev.2013.10.005
Jerome, C. A., Simon, E. M., and Lynn, D. H. (1996). Description of Tetrahymena empidokyrea n. sp., a new species in the Tetrahymena pyriformis sibling species complex (Ciliophora, Oligohymenophorea), and an assessment of its phylogenetic position using small-subunit rRNA sequences. Can. J. Zool. 74, 1898–1906. doi: 10.1139/z96-214
Jiang, J., Huang, J., Li, L., Shao, C., Al-Rasheid, K. A. S., Al-Farraj, S. A., et al. (2013). Morphology, ontogeny, and molecular phylogeny of two novel bakuellid-like hypotrichs (Ciliophora: Hypotrichia), with establishment of two new genera. Eur. J. Protistol. 49, 78–92. doi: 10.1016/j.ejop.2012.05.003
Jo, E., Jung, J.-H., and Min, G.-S. (2015). Morphology and molecular phylogeny of two new brackish water ciliates of Bakuella (Ciliophora: Urostylida: Bakuellidae) from South Korea. J. Eukaryot. Microbiol. 62, 799–809. doi: 10.1111/jeu.12238
Jung, J.-H., and Berger, H. (2019). Monographic treatment of Paraholosticha muscicola (Ciliophora, Keronopsidae), including morphological and molecular biological characterization of a brackish water population from Korea. Eur. J. Protistol. 68, 48–67. doi: 10.1016/j.ejop.2018.12.004
Jung, J.-H., Omar, A., Park, M.-H., Nguyen, T. V., Jung, Y.-H., Yang, H.-M., et al. (2021). Anteholosticha foissneri n. sp. a marine hypotrich ciliate (Ciliophora: Spirotrichea) from Vietnam: morphology, morphogenesis, and molecular phylogeny. Eur. J. Protistol. 78:125768. doi: 10.1016/j.ejop.2021.125768
Kahl, A. (1928). Die infusorien (Ciliata) der oldesloer salzwasserstellen. Arch. Hydrobiol. 19, 50–123.
Kahl, A. (1932). Urtiere oder Protozoa I: wimpertiere oder Ciliata (Infusoria) 3. Spirotricha. Tierwelt Dtl. 25, 399–650.
Kaur, H., Shashi, Negi, R. K., and Kamra, K. (2019). Morphological and molecular characterization of Neogastrostyla aqua nov. gen., nov. spec. (Ciliophora, Hypotrichia) from River Yamuna, Delhi, comparison with Gastrostyla-like genera. Eur. J. Protistol. 68, 68–79. doi: 10.1016/j.ejop.2019.01.002
Kim, K.-S., Jung, J.-H., and Min, G.-S. (2016). A new soil ciliate, Birojimia soyaensis nov. spec. (Ciliophora: Urostylida) from South Korea. Acta Protozool. 55, 135–144. doi: 10.4467/16890027AP.16.013.5745
Kim, K.-S., and Min, G.-S. (2019). Morphology and molecular phylogeny of Oxytricha seokmoensis sp. nov. (Hypotrichia: Oxytrichidae), with notes on its morphogenesis. Eur. J. Protistol. 71:125641. doi: 10.1016/j.ejop.2019.125641
Li, L., Khan, S. N., Ji, D., Shin, M. K., and Berger, H. (2011). Morphology and small subunit (SSU) rRNA gene sequence of the new brackish water ciliate Neobakuella flava n. g., n. sp. (Ciliophora, Spirotricha, Bakuellidae) and SSU rRNA gene sequences of six additional hypotrichs from Korea. J. Eukaryot. Microbiol. 58, 339–351. doi: 10.1111/j.1550-7408.2011.00561.x
Li, L., Zhang, Q., Hu, X., Warren, A., Al-Rasheid, K. A. S., Al-Khedheiry, A. A., et al. (2009). A redescription of the marine hypotrichous ciliate, Nothoholosticha fasciola (Kahl, 1932) nov. gen., nov. comb. (Ciliophora: Urostylida) with brief notes on its cellular reorganization and SS rRNA gene sequence. Eur. J. Protistol. 45, 237–248. doi: 10.1016/j.ejop.2009.01.004
Liu, W., Shao, C., Gong, J., Li, J., Lin, X., and Song, W. (2010). Morphology, morphogenesis, and molecular phylogeny of a new marine urostylid ciliate (Ciliophora, Stichotrichia) from the South China Sea, and a brief overview of the convergent evolution of the midventral pattern within the Spirotrichea. Zool. J. Linn. Soc. 158, 697–710. doi: 10.1111/j.1096-3642.2009.00565.x
Lu, B., Wang, C., Huang, J., Shi, Y., and Chen, X. (2016). Morphology and SSU rDNA sequence analysis of two hypotrichous ciliates (Protozoa, Ciliophora, Hypotrichia) including the new species Metaurostylopsis parastruederkypkeae n. sp. J. Ocean Univ. China 15, 866–878. doi: 10.1007/s11802-016-3148-9
Lu, X., Wang, Y., Al-Farraj, S. A., El-Serehy, H. A., Huang, J., and Shao, C. (2020). The insights into the systematic relationship of Gastrostyla-affinitive genera, with report on a new saline soil ciliate genus and new species (Protozoa, Ciliophora). BMC Evol. Biol. 20:92. doi: 10.1186/s12862-020-01659-8
Luo, X., Huang, J., Bourland, W. A., El-Serehy, H. A., Al-Farraj, S. A., Chen, X., et al. (2021). Taxonomy of three oxytrichids (Protozoa, Ciliophora, Hypotrichia), with establishment of a new species Rubrioxytricha guangzhouensis spec. nov. Front. Mar. Sci. 7:623436. doi: 10.3389/fmars.2020.623436
Lynn, D. H. (2008). The Ciliated Protozoa: Characterization, Classification, and Guide to the Literature. Dordrecht: Springer.
Lyu, Z., Shao, C., Yi, Z., and Warren, A. (2015). A molecular phylogenetic investigation of Bakuella, Anteholosticha, and Caudiholosticha (Protista, Ciliophora, Hypotrichia) based on three-gene sequences. Zool. Scr. 62, 391–399. doi: 10.1111/jeu.12194
Lyu, Z., Wang, J., Huang, J., Warren, A., and Shao, C. (2018). Multigene-based phylogeny of Urostylida (Ciliophora, Hypotrichia), with establishment of a novel family. Zool. Scr. 47, 243–254. doi: 10.1111/zsc.12267
Ma, J., Zhao, Y., Zhang, T., Shao, C., Al-Rasheid, K. A. S., and Song, W. (2021). Cell division pattern and phylogenetic analyses of a new ciliate genus Parasincirra n. g. (Protista, Ciliophora, Hypotrichia), with a report of a new soil species, P. sinica n. sp. from northwest China. BMC Evol. Biol. 21:21. doi: 10.1186/s12862-020-01730-4
Medlin, L., Elwood, H. J., Stickel, S., and Sogin, M. L. (1988). The characterization of enzymatically amplified eukaryotic 16S-like rRNA-coding regions. Gene 71, 491–499. doi: 10.1016/0378-1119(88)90066-2
Moon, J. H., Kim, J. H., Quintela-Alonso, P., and Jung, J.-H. (2020). Morphology, morphogenesis, and molecular phylogeny of Neobakuella aenigmatica n. sp. (Ciliophora, Spirotrichea, Bakuellidae). J. Eukaryot. Microbiol. 67, 54–65. doi: 10.1111/jeu.12753
Paiva, T. da. S. (2020). Systematic redefinition of the Hypotricha (Alveolata, Ciliophora) based on combined analyses of morphological and molecular characters. Protist 171:125755. doi: 10.1016/j.protis.2020.125755
Park, K.-M., Jung, J.-H., and Min, G.-S. (2012). Redescription of two urostylid ciliates (Ciliophora: Urostylida), Anteholosticha pulchra and Metaurostylopsis struederkypkeae from Korea. Anim. Syst. Evol. Divers. 28, 20–28. doi: 10.5635/ASED.2012.28.1.020
Park, K.-M., Jung, J.-H., and Min, G.-S. (2013). Morphology, morphogenesis, and molecular phylogeny of Anteholosticha multicirrata n. sp. (Ciliophora, Spirotrichea) with a note on morphogenesis of A. pulchra (Kahl, 1932) Berger, 2003. J. Eukaryot. Microbiol. 60, 564–577. doi: 10.1111/jeu.12060
Ronquist, F., Teslenko, M., van der Mark, P., Ayres, D. L., Darling, A., Höhna, S., et al. (2012). MrBayes 3.2: efficient Bayesian phylogenetic inference and model choice across a large model space. Syst. Biol. 61, 539–542. doi: 10.1093/sysbio/sys029
Sela, I., Ashkenazy, H., Katoh, K., and Pupko, T. (2015). GUIDANCE2: accurate detection of unreliable alignment regions accounting for the uncertainty of multiple parameters. Nucleic Acids Res. 43, W7–W14. doi: 10.1093/nar/gkv318
Shao, C., Miao, M., Song, W., Warren, A., Al-Rasheid, K. A. S., Al-Quraishy, S. A., et al. (2008). Studies on two marine Metaurostylopsis spp. from China with notes on morphogenesis in M. sinica nov. spec. (Ciliophora, Urostylida). Acta Protozool. 47, 95–112.
Song, W., Qiao, Y., Dong, J., Bourland, W. A., Zhang, T., and Luo, X. (2020). Ontogeny and phylogeny of a new hypotrichous ciliate (Protista, Ciliophora), Metaurostylopsis alrasheidi n. sp., with establishment of a new genus Monourostylopsis n. gen. Front. Mar. Sci. 7:602317. doi: 10.3389/fmars.2020.602317
Song, W., Zhang, T., Zhang, X., Warren, A., Song, W., Zhao, Y., et al. (2021). Taxonomy, ontogenesis and evolutionary phylogeny of the algae-bearing ciliate Limnoholosticha viridis (Kahl, 1932), with establishment of a new genus and new family (Protista, Ciliophora, Hypotrichia). Front. Microbiol. 11:560915. doi: 10.3389/fmicb.2020.560915
Stamatakis, A. (2014). RAxML version 8: a tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics 30, 1312–1313. doi: 10.1093/bioinformatics/btu033
Tamura, K., Stecher, G., Peterson, D., Filipski, A., and Kumar, S. (2013). MEGA6: molecular evolutionary genetics analysis version 6.0. Mol. Biol. Evol. 30, 2725–2729. doi: 10.1093/molbev/mst197
Vd’ačný, P., and Foissner, W. (2021). Morphology and ontogenesis of two new Hemiholosticha species (Ciliophora, Hypotrichia, Hemiholostichidae nov. fam.). Eur. J. Protistol. 77:125763. doi: 10.1016/j.ejop.2020.125763
Wang, C., Zhang, T., Wang, Y., Katz, L. A., Gao, F., and Song, W. (2017). Disentangling sources of variation in SSU rDNA sequences from single cell analyses of ciliates: impacts of copy number variation and experimental error. Proc. R. Soc. B. 284:20170425. doi: 10.1098/rspb.2017.0425
Wang, J., Li, L., Warren, A., and Shao, C. (2017). Morphogenesis and molecular phylogeny of the soil ciliate Rigidohymena quadrinucleata (Dragesco and Njine, 1971) Berger, 2011 (Ciliophora, Hypotricha, Oxytrichidae). Eur. J. Protistol. 60, 1–12. doi: 10.1016/j.ejop.2017.04.006
Wang, J., Li, J., and Shao, C. (2020a). Morphology, morphogenesis, and molecular phylogeny of a novel saline soil ciliate, Heterourosomoida sinica n. sp. (Ciliophora, Hypotrichia). Eur. J. Protistol. 73:125666. doi: 10.1016/j.ejop.2019.125666
Wang, J., Zhao, Y., Lu, X., Lyu, Z., Warren, A., and Shao, C. (2020b). Does the Gonostomum-patterned oral apparatus in Hypotrichia carry a phylogenetic signal? Evidence from morphological and molecular data based on extended taxon sampling using three nuclear genes (Ciliophora, Spirotrichea). Sci. China Life Sci. 63, 1–12. doi: 10.1007/s11427-020-1667-3
Wang, J., Zhang, T., Li, F., Warren, A., Li, Y., and Shao, C. (2021). A new hypotrich ciliate, Oxytricha xianica sp. nov., with notes on the morphology and phylogeny of a Chinese population of Oxytricha auripunctata Blatterer & Foissner, 1988 (Ciliophora, Oxytrichidae). Mar. Life Sci. Technol. 3, 303–312. doi: 10.1007/s42995-020-00089-1
Warren, D. L., Geneva, A. J., and Lanfear, R. (2017). RWTY (R We There Yet): an R package for examining convergence of Bayesian phylogenetic analyses. Mol. Biol. Evol. 34, 1016–1020. doi: 10.1093/molbev/msw279
Wilbert, N. (1975). Eine verbesserte Technik der Protargolimprägnation für Ciliaten. Mikrokosmos 64, 171–179.
Xu, W., Wang, Y., Cheng, T., Yu, Y., El-Serehy, H., Al-Farraj, S. A., et al. (2020). Reevaluation of the ‘well-known’ Paraurostyla weissei complex, with notes on the ontogenesis of a new Paraurostyla species (Ciliophora, Hypotrichia). Eur. J. Protistol. 73:125672. doi: 10.1016/j.ejop.2020.125672
Xu, Y., Huang, J., Hu, X., Al-Rasheid, K. A. S., Song, W., and Warren, A. (2011). Taxonomy, ontogeny and molecular phylogeny of Anteholosticha marimonilata spec. nov. (Ciliophora, Hypotrichida) from the Yellow Sea, China. Int. J. Syst. Evol. Microbiol. 61, 2000–2014. doi: 10.1099/ijs.0.024638-0
Yi, Z., Song, W., Shao, C., Warren, A., Al-Rasheid, K. A. S., Roberts, D. M., et al. (2008a). Phylogeny of some systematically uncertain urostyloids Apokeronopsis, Metaurostylopsis, Thigmokeronopsis (Ciliophora, Stichotrichia) estimated with small subunit rRNA gene sequence information: discrepancies and agreements with morphological data. Eur. J. Protistol. 44, 254–262. doi: 10.1016/j.ejop.2007.12.002
Yi, Z., Song, W., Warren, A., Roberts, D. M., Al-Rasheid, K. A. S., Chen, Z., et al. (2008b). A molecular phylogenetic investigation of Pseudoamphisiella and Parabirojimia (Protozoa, Ciliophora, Spirotrichea), two genera with ambiguous systematic positions. Eur. J. Protistol. 44, 45–53. doi: 10.1016/j.ejop.2007.08.002
Zhang, T., Wang, Y., Cheng, T., Ma, J., Vd́ačný, P., Song, W., et al. (2020a). Systematics and multi-gene phylogeny of the subfamily Nothoholostichinae (Ciliophora, Hypotrichia), with integrative description of a new marine species Nothoholosticha luporinii n. sp. Front. Mar. Sci. 7:610886. doi: 10.3389/fmars.2020.610886
Zhang, T., Dong, J., Cheng, T., Duan, L., and Shao, C. (2020b). Reconsideration on taxonomy of the marine ciliate Neobakuella aenigmatica Moon et al., 2019 (Protozoa, Ciliophora, Hypotrichia). Mar. Life Sci. Technol. 2, 97–108. doi: 10.1007/s42995-020-00032-4
Zhao, X., Gao, S., Fan, Y., Strueder-Kypke, M., and Huang, J. (2015). Phylogenetic framework of the systematically confused Anteholosticha-Holosticha complex (Ciliophora, Hypotrichia) based on multigene analysis. Mol. Phylogenet. Evol. 91, 238–247. doi: 10.1016/j.ympev.2015.05.021
Zhu, E., Ba, S., Lyu, Z., Li, J., and Shao, C. (2019). Morphogenesis and molecular phylogeny of the soil ciliate Holostichides chardezi (Ciliophora, Hypotrichia, Bakuellidae), with redefinition of Holostichides Foissner, 1987 and establishment of a new genus Anteholostichides. J. Eukaryot. Microbiol. 66, 730–739. doi: 10.1111/jeu.12717
Keywords: ciliated protists, core urostylids, integrative taxonomy, phylogeny, 18S rRNA gene
Citation: Song W, Zhang T, Dong J, Luo X, Bourland WA and Wang Y (2021) Taxonomy and Molecular Phylogeny of Two New Urostylid Ciliates (Protozoa: Ciliophora) From Chinese Wetlands and Establishment of a New Genus. Front. Microbiol. 12:707954. doi: 10.3389/fmicb.2021.707954
Received: 11 May 2021; Accepted: 02 July 2021;
Published: 30 July 2021.
Edited by:
Jae-Ho Jung, Gangneung–Wonju National University, South KoreaReviewed by:
Santosh Kumar, Zoological Survey of India, IndiaCopyright © 2021 Song, Zhang, Dong, Luo, Bourland and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yurui Wang, d2FuZ3l1cnVpQHNubnUuZWR1LmNu
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.