
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Microbiol. , 18 June 2021
Sec. Systems Microbiology
Volume 12 - 2021 | https://doi.org/10.3389/fmicb.2021.643422
This article is part of the Research Topic Multi-omics Study on Gut Microbiota Related to Faecal Microbiota Transplantation View all 13 articles
Vaginal microbiota dysbiosis, characterized by the loss of Lactobacillus dominance and increase of microbial diversity, is closely related to gynecological diseases; thus, intervention on microbiota composition is significant and promising in the treatment of gynecological diseases. Currently, antibiotics and/or probiotics are the mainstay of treatment, which show favorable therapeutic effects but also bring problems such as drug resistance and high recurrence. In this review, we discuss the role of vaginal microbiota dysbiosis in various gynecological infectious and non-infectious diseases, as well as the current and potential interventions.
The vagina is an important and complex ecosystem, dominated by Lactobacillus, but also containing a small number of fungi and parasites, and the balanced microbial communities are vital for female health (Bradford and Ravel, 2017; Gupta et al., 2019). However, the microbial balance can be disrupted and leads to various infectious diseases, characterized by overgrowth of anaerobic bacteria (agent of bacterial vaginitis and atrophic vaginitis, BV and AV) and Candida albicans (agent of vulvovaginal candidiasis, VVC), and infections of Trichomonas vaginalis (agent of trichomonal vaginitis), Neisseria gonorrhoeae (agent of gonorrhea), Mycoplasma genitalium (agent of cervicitis), Chlamydia trachomatis (agent of pelvic inflammatory disease, PID), and various viruses including human papillomavirus (HPV, agent of cervical cancer), herpes simplex virus-2 (HSV-2, agent of genital ulcers), and human immunodeficiency virus (HIV, agent of acquired immunodeficiency syndrome, AIDS) (Gupta et al., 2019). In addition, some non-infectious diseases, e.g., induced abortions (with BV microbiome, etc.), intrauterine adhesions (IUA, with reduced Lactobacillus and increased Gardnerella, Prevotella, etc.), miscarriage (with BV microbiome), preterm (with BV microbiome), infertility (with BV microbiome), polycystic ovarian syndrome (PCOS, with reduced Lactobacillus crispatus, and increased mycoplasma and Prevotella), uterine fibroid (with increased Lactobacillus iners), and menstrual disorders (with increased uterine Gardnerella, Prevotella, Sneathia, and Veillonella), also show associations with microbial dysbiosis (Hay, 2004; Chen et al., 2017; Pelzer et al., 2018; Liu et al., 2019; Hong et al., 2020a, b), posing a serious threat to women’s reproductive health.
At present, the most conventional treatment strategy for microbial disorders is antibiotics (metronidazole, clotrimazole, azithromycin, etc.), which has a good therapy effect while accompanied with various adverse effects and recurrence (Cudmore et al., 2004; Bradshaw et al., 2006; Xie et al., 2017; Khosropour et al., 2018; Paez-Canro et al., 2019). Recently, probiotics based on Lactobacillus have shown promise in treating not only infectious diseases (e.g., BV, fungal infection, and urinary tract infections) but also non-infectious diseases (e.g., preterm, infertility, and PCOS) (Hanson et al., 2016; Lopez-Moreno and Aguilera, 2020). However, the treatment outcome of probiotics is usually mixed, which may be due to the fact that these diseases are usually caused by multiple microbes rather than one. Excitingly in 2019, Ahinoam et al. conducted a clinical study of vaginal microbiota transplantation (VMT) in five patients with recurrent BV, finding that four of them achieved long-term remission and established a long-term vaginal microbiota dominated by Lactobacillus (Lev-Sagie et al., 2019). Therefore, intervention on vaginal microbiota is significant and promising in treating gynecological diseases. Thus, in this review, we elaborated the role of microbiota dysbiosis in various gynecological infectious and non-infectious diseases, and the current and potential interventions.
The vagina is a stretchable, muscular duct connecting the uterus and external genitalia, and is responsible for the physiological functions of female sexual intercourse, menstrual discharge, and delivery of the fetus (Farage and Maibach, 2006). Its mucosal system, consisted of a stratified squamous epithelium and cervicovaginal fluid (CVF), is vital in maintaining vaginal health by immune response, antimicrobial products (e.g., B-defensin), finely balanced microbial communities, etc. (Torcia, 2019). Among these, the vaginal microbiota is the most changeable and vulnerable one in response to internal and external stimulus (Gajer et al., 2012).
Recently, the detailed composition and relative abundance of vaginal microbiota has been determined by high-throughput 16s rRNA sequencing, characterizing five microbial community state types (CST) in asymptomatic women (Ravel et al., 2011). Four of them (CST-I, II, III, V) were dominated by Lactobacillus species, while CST-IV was heterogenous and polymicrobial, characterized by lower level of Lactobacillus and higher level of anaerobic bacteria including Gardnerella, Atopobium, Mobiluncus, Prevotella, Streptococcus, Mycoplasma, and Ureaplasma (Ravel et al., 2011). At present, over 140 Lactobacillus species have been identified, but the only species that normally dominate the vaginal microbiota are L. crispatus, Lactobacillus gasseri, Lactobacillus jensenii, and L. iners (Smith and Ravel, 2017). They are considered as keystones of vaginal health, as they can produce lactic acid, hydrogen peroxide, and bacteriocins, maintaining acidic environment and preventing pathogen growth (Gupta et al., 2019); adhere to epithelium, repelling other bacteria adhesion (Torcia, 2019); and regulate immune and inflammatory response, enhancing the resistance of vagina to diseases (Aldunate et al., 2015). Thus, Lactobacillus dominance is generally considered as a hallmark of healthy vagina (Ma et al., 2012).
It is generally known that vaginal microbiota disturbance is highly related to various gynecological diseases, especially BV, which is characterized by the alteration of vaginal microbiome from Lactobacillus dominance to anaerobic and facultative bacteria (Gardnerella, Atopobium, Prevotella, Megasphaera, Leptotrichia, Sneathia, etc.) dominance (Ling et al., 2010; Srinivasan et al., 2012; Nasioudis et al., 2017). BV has been shown to be associated with various other reproductive tract disorders, including infertility, preterm, cervical cancer, and HIV acquisition (Leitich and Kiss, 2007; van Oostrum et al., 2013; Torcia, 2019). It is also reported that many sextually transmitted infections (STI), such as infections of N. gonorrhoeae and C. trachomatis, are facilitated by vaginal microbiota dysbiosis and more prevalent in BV-positive women (Lewis et al., 2017). In addition, as the microbiota research progresses intensively, a growing number of studies have linked vaginal microbiota dysbiosis to various gynecological non-infectious diseases, among which Liu et al. found that compared with healthy people, IUA patients had lower percentage of Lactobacillus, and higher percentage of Gardnerella and Prevotella (Liu et al., 2019); Hong et al. found that patients with PCOS had lower Lactobacillus and higher Mycoplasma and Prevotella than controls (Hong et al., 2020b); and Chen et al. found that Lactobacillus were less abundant, while L. iners were more abundant in patients with uterine fibroid than individuals without (Chen et al., 2017).
Therefore, as the balanced vaginal microbiota plays a significant role in female health, interventions aimed at restoring the healthy microbiota composition can be a good and reasonable therapy for gynecological diseases.
Until now, infection is one of the leading causes of gynecological disease, which is usually characterized by vaginal dysbiosis (van de Wijgert and Jespers, 2017). Below, we introduce the common infectious diseases, including common vaginitis, viral infections, and other infections, and discuss the therapy effects of restoring Lactobacillus-dominated vaginal microbiota by antibiotic and probiotic interventions (Figure 1).
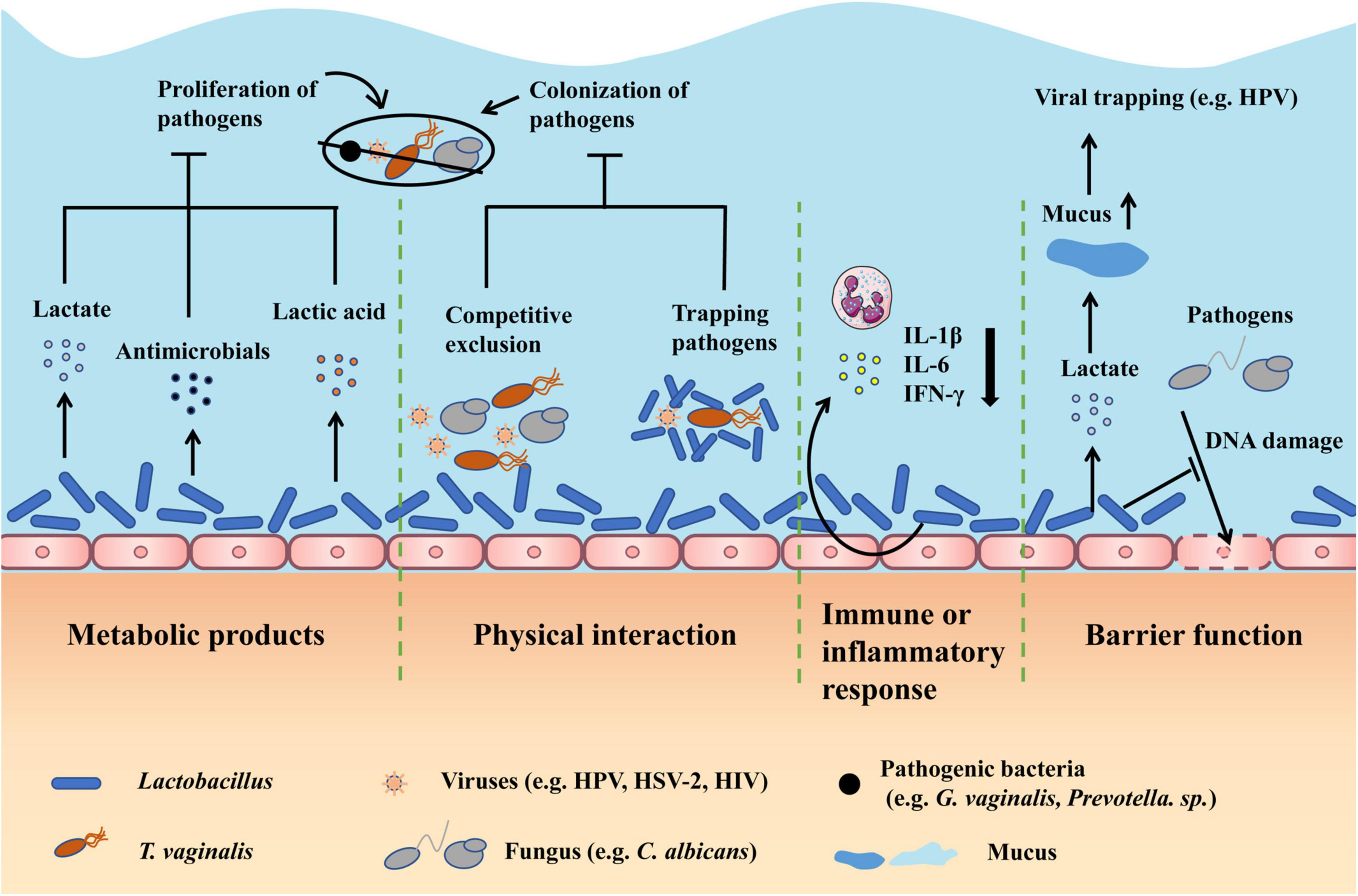
Figure 1. Therapy effects of restoring Lactobacillus-dominated vaginal microbiota in infectious diseases. First, Lactobacillus can produce metabolites including lactate, antimicrobials, and lactic acid to inhibit the proliferation of pathogens. Second, Lactobacillus can competitively exclude pathogens from adhering to epithelium and trap pathogens by direct physical contact to prevent the colonization of pathogens. Third, Lactobacillus can regulate the immune or inflammatory response, particularly relieving the inflammation by decreasing cytokines like IL-1β. Fourth, Lactobacillus can improve the barrier function by producing lactate, which can increase the mucus viscosity to facilitate viral trapping, and inhibiting pathogens from damaging the DNA of epithelial cells. DNA, deoxyribonucleic acid.
Bacterial vaginitis is the most common lower genital tract disease among fertile women and can predispose women to various STI and adverse birth outcome (Lewis et al., 2017). It mainly manifests as mucosal inflammation including abnormal vaginal discharge (increased, yellowish, and fishy odor) and sensation of itching and burning (Onderdonk et al., 2016). Currently, the routine treatment is oral and intravaginal antibiotics, usually clindamycin and metronidazole (Faught and Reyes, 2019). However, long-term use of antibiotics is likely to develop antimicrobial resistance and cause recurrent infections (Lev-Sagie et al., 2019).
Studies showed that BV is caused by replacement of Lactobacillus dominance by multiplication of over 10 anaerobic bacteria, such as Gardnerella, Atopobium, Prevotella, Megasphaera, Leptotrichia, and Sneathia, and probiotics based on Lactobacillus that used to regulate microbiota have been revealed beneficial in treating BV (Nasioudis et al., 2017; Wang et al., 2019). In vitro and clinical studies showed that Lactobacillus could reduce the pathogen colonization by preventing the pathogen adhering to epithelium (Ma et al., 2019), inhibit the pathogen growth by producing bacteriocins (Ma et al., 2019), maintain the acidic environment by generating lactic acid (Kim and Park, 2017), and relieve inflammatory response, in particular significantly reduce IL-1β and IL-6 cytokines (Hemalatha et al., 2012). Thus, antibiotics with probiotics can effectively cure BV by correcting the vaginal microbiota and improving the vaginal environment.
Vulvovaginal candidiasis is the most common vaginal fungal infection and typically manifests as mucosal inflammation, including cheese-like vaginal discharge, and vulvovaginal burning, itching, and redness (Sobel, 1992). The standard treatment for VVC is antifungal agents, including oral or intravaginal azole or triazole drugs, which can achieve over 80% cure rate (Nurbhai et al., 2007; Sobel and Sobel, 2018). However, the concomitant side effects (diarrhea, abnormal urination, and vaginal burning, itching, and irritation), drug resistance, and high recurrence rate hinder the recovery and pose a threat to health (Xie et al., 2017).
Studies showed that VVC primarily occurred during vaginal dysbiosis and immune deficiency, and was caused by overgrowth of C. albicans, which could cause epithelium destruction by destroying the intercellular linkage and intracellular mitochondrial structure, and elicit inflammation, in particular produce IL-6 and IL-8 cytokines (Niu et al., 2017; Li et al., 2019). As protective Lactobacillus can regulate the host immune response, inhibit the proliferation of C. albicans by producing metabolites such as lactate, and prevent the colonization of C. albicans, therapies aimed at adjusting microbiota can help in VVC recovery (Bradford and Ravel, 2017). In vivo study on VVC showed that Lactobacillus could regulate the immune response by decreasing T-helper 1 (Th1) cell/Th2 cell ratio and inhibiting the release of proinflammatory cytokines such as interleukin 17 (IL17) and interferon-γ (IFN-γ) (Li et al., 2019). Another in vitro study investigated the ability of L. crispatus to inhibit C. albicans infecting vaginal epithelial cells VK2/E6E7, and found that L. crispatus could significantly reduce the adherence of C. albicans to VK2/E6E7 cells (Niu et al., 2017). Besides, many studies suggested that Lactobacillus could exert direct antifungal effects by releasing antimicrobials, improving the epithelial barrier by decreasing DNA damage of epithelial cells, and improving the microbiota to prevent C. albicans overgrowth and VVC recurrence (Mc and Rosenstein, 2000; Reid et al., 2003; Strus et al., 2006; Yeh et al., 2007). Consequently, antibiotics with probiotics can treat VVC and prevent its onset by improving microbial, inflammatory, and epithelial status.
Trichomonal vaginitis, which manifests as painful and itching coitus, frothy discharge, and vaginal or cervical bleeding, is the most common STI worldwide and closely linked to PID and infertility (Pastorek et al., 1996; Petrin et al., 1998; Harp and Chowdhury, 2011; Edwards et al., 2016). Currently, the standard treatment for Trichomonal vaginitis is metronidazole or tinidazole, which not only are quite effective but also develop drug resistance (Muzny and Schwebke, 2013; Phukan et al., 2018).
The etiology of trichomonal vaginitis is T. vaginalis, which is a flagellated parasite of human genital tract that can cause severe damage to epithelial cells by mediating the lysis of epithelial cells, and elicit the inflammatory response, involving recruitment of neutrophils to infected tissues (Fichorova et al., 2006; Edwards et al., 2016). Studies showed that BV, especially with lack of Lactobacillus, could protect T. vaginalis from nucleic acid degradation through the production of polyamines and form biofilms to reduce drug sensitivity, thus facilitating T. vaginalis infection and increasing drug resistance (Figueroa-Angulo et al., 2012; Balkus et al., 2014; Jung et al., 2017). Therefore, it is suggested that restoring the vaginal microbiota can improve the anti-trichomoniasis treatment effect. A randomized clinical study showed that patients taking probiotics in addition to metronidazole showed earlier clinical resolution compared with the patients taking placebo. Besides, reduced leukocyte/epithelial cell ratio, decreased pH, and increased redox potential of the vaginal fluid were detected in the probiotic group, underlining the beneficial mechanisms of Lactobacillus to relieve inflammation, inhibit T. vaginalis growth, and damage T. vaginalis DNA, respectively (Sgibnev and Kremleva, 2020). Another in vitro study showed that the aggregation-promoting factor (APF)-2 of L. gasseri could significantly inhibit the adhering of T. vaginalis to human vaginal ectocervical cells (Phukan et al., 2018). Thus, antibiotics with probiotics can prevent and treat T. vaginalis infection via adjusting vaginal microbiota, inhibiting T. vaginalis growth, relieving inflammation, and preventing T. vaginalis colonization.
Atrophic vaginitis, caused by the reduction of estrogen and local immunity after menopause, is prevalent among postmenopausal women and is characterized by vulvovaginal dryness, dyspareunia, abnormal vaginal discharge, etc. (Stika, 2010). At present, the treatment principles of AV are giving estrogen to improve the vaginal immunity, and antibiotics (norfloxacin) to inhibit the pathogen growth (Paladine and Desai, 2018).
Estrogen deficiency in menopause leads to vaginal atrophy, resulting in reduced epithelial barrier function and facilitation of pathogen colonization (Jaisamrarn et al., 2013). Brotman et al. (2014) showed that women with AV had lower level of Lactobacillus and increased bacterial diversity, involving Anaerococcus, Peptoniphilus, Prevotella, and Streptococcus. Since Lactobacillus has been shown to improve the overall vaginal environment such as the vaginal immunity and epithelial barrier, probiotics have been used in combination with estrogen to treat AV. A randomized clinical study showed that long-term use of this combination was sufficient to improve the related clinical parameters, maintain the improved maturation of vaginal epithelium, and prevent symptomatic AV relapse (Jaisamrarn et al., 2013). Besides, it is suggested that Lactobacillus-dominated vaginal microbiota is significant in protecting postmenopausal women from AV and is considered to be a marker of successful AV treatment (Shen et al., 2016). Thus, antibiotics and probiotics can be used in combination with estrogen to prevent and treat AV by improving the vaginal microbiota and enhancing the epithelial barrier function and the overall vaginal environment.
Genital HPV, especially HPV-16 and 18 strains, are common sextually transmitted viruses and major causes of cervical cancer (Stanley, 2010). Though over 50% of HPV infections are cleared within a half year, persistent infections can cause symptoms including abnormal vaginal bleeding such as sextual intercourse bleeding, and abnormal vaginal discharge (Mitra et al., 2016; Kashyap et al., 2018). Conventional treatments for cervical cancer include surgery, chemotherapy, and radiotherapy, but they cannot prevent recurrence and have various side effects, such as menstrual change and vaginal pain (Waggoner, 2003).
Studies showed that vaginal microbiota disturbance with reduced Lactobacillus and increased microbial diversity was closely linked to HPV infection pathogenesis (Mitra et al., 2016). A cohort study of 32 sextually active American women showed that women with high level of Atopobium, Prevotella, and Gardnerella were most likely to be infected with HPV and had the slowest viral clearance (Brotman et al., 2014). Besides, CST-IV bacteria were shown to increase the severity of cervical lesions, promote the neoplastic progression by producing nitrosamines and ROS to induce DNA damage, and facilitate HPV infection by damaging the barrier and eliciting chronic inflammation (Mhatre et al., 2012; Borgdorff et al., 2016; Piyathilake et al., 2016). Thus, improving the microbiota composition is a feasible approach to prevent and treat HPV infection. A semi-randomized study of 54 HPV infected women showed that women treated with oral Lactobacillus caseii had greater clearance of HPV infection and cervical lesions than untreated patients (Verhoeven et al., 2013). Mitra et al. reviewed the currently accepted mechanisms of Lactobacillus-mediated protection to cervical health and showed that low vaginal pH (which could decrease around 10% risk of HPV positivity), lactate (which could increase the vaginal mucus viscosity and enhance the viral trapping), and bacteriocins (which could directly interfere the pathogen growth) were significant protective principles, and improvement in local immunity and inflammation was also suggested (Mitra et al., 2016). In addition, Palma et al. (2018) explored the effects of long- and short-term probiotic implementation in clearing HPV infection, and they found that patients with long-term probiotic treatment had significantly higher viral clearance rate, suggesting that long-term healthy vaginal microbiota was required to exert the optimal treatment effect. Therefore, antibiotics and probiotics can prevent and aid treatment of HPV infection by improving the microbial balance.
Other common viral agents that infect the reproductive tract are HSV-2 and HIV, which can cause genital ulcers and AIDS, respectively. Since these viruses cannot be completely eliminated by traditional antiviral therapy, prevention is particularly important for health maintenance. Studies showed that vaginal dysbiosis, especially BV, and lack of Lactobacillus could facilitate HSV-2 infection, and a protective vaginal microbiota could prevent and counteract HSV-2 infection by inhibiting HSV-2 replication, producing antimicrobials, and trapping HSV-2 particles (Cherpes et al., 2003; Evans et al., 2003; Conti et al., 2009; Mousavi et al., 2018; Torcia, 2019). As for HIV, many studies showed that women with CST-IV bacteria had a higher HIV infection rate than women with CST-I bacteria, and a Lactobacillus-dominated vaginal microbiota could protect the vagina from HIV infection by maintaining acidic environment, producing lactic acid, and reducing the viability of HIV particles (Gosmann et al., 2017; Nahui Palomino et al., 2017). Therefore, antibiotics with probiotics can prevent HIV and HSV-2 infection by correcting the microbial disturbance and creating an unfavorable vaginal environment for viruses.
Neisseria gonorrhoeae, the causative agent of gonorrhea, is continuously developing resistance to antimicrobial treatment (ceftriaxone and azithromycin) and impedes the recovery (Morgan and Decker, 2016; Unemo et al., 2019). An in vitro study showed that Lactobacillus could significantly reduce Neisseria gonococcus viability by creating acidic environment, producing bacteriocins, releasing biosurfactants, and co-aggregating with gonococci, and reduce gonococci adhering to epithelial cells (Foschi et al., 2017). Thus, probiotics can be an adjuvant therapy of antibiotics to treat N. gonorrhoeae infection by improving the vaginal microbiota.
Mycoplasma genitalium is a sextually transmitted pathogen that can lead to PID and cervicitis (Cazanave et al., 2012; Onderdonk et al., 2016), and its first line treatment with antibiotics (doxycycline and azithromycin) is compromised by drug resistance (Pinto-Sander and Soni, 2019). Studies showed that BV could favor M. genitalium infection (Brotman et al., 2010; Molenaar et al., 2018), and a protective vaginal microbiota dominated by Lactobacillus could counteract the infection by producing antimicrobials and maintaining acidic environment (Molenaar et al., 2018). Therefore, probiotics with antibiotics can prevent and treat M. genitalium infection by adjusting the microbial structure and improving the vaginal environment.
Chlamydia trachomatis is a common cause of PID (Haggerty et al., 2010; Mestrovic and Ljubin-Sternak, 2018), and its treatment with azithromycin or doxycycline also faces the challenge of antimicrobial resistance (O’Connell and Ferone, 2016; Zhang et al., 2017; Mestrovic and Ljubin-Sternak, 2018). In vitro studies showed that Lactobacillus could prevent Chlamydia colonization by maintaining acidic environment and consuming glucose (Nardini et al., 2016), inhibit Chlamydia multiplication at all infection stages, and inhibit the chronic infection by preventing the development of persistent trachomatis forms (Mastromarino et al., 2014). Therefore, antibiotics with probiotics can exert a conducive treatment effect on C. trachomatis infection by improving the vaginal microbiota.
The role of microbiota in non-infectious gynecological diseases was long underestimated, until recently when mounting evidence showed its significance. Herein, we introduce the common non-infectious diseases that were caused by physical injury, fertility problems, and endocrine disorders, and discuss the treatment effects of restoring Lactobacillus-dominated vaginal microbiota by antibiotic and probiotic interventions (Figure 2).
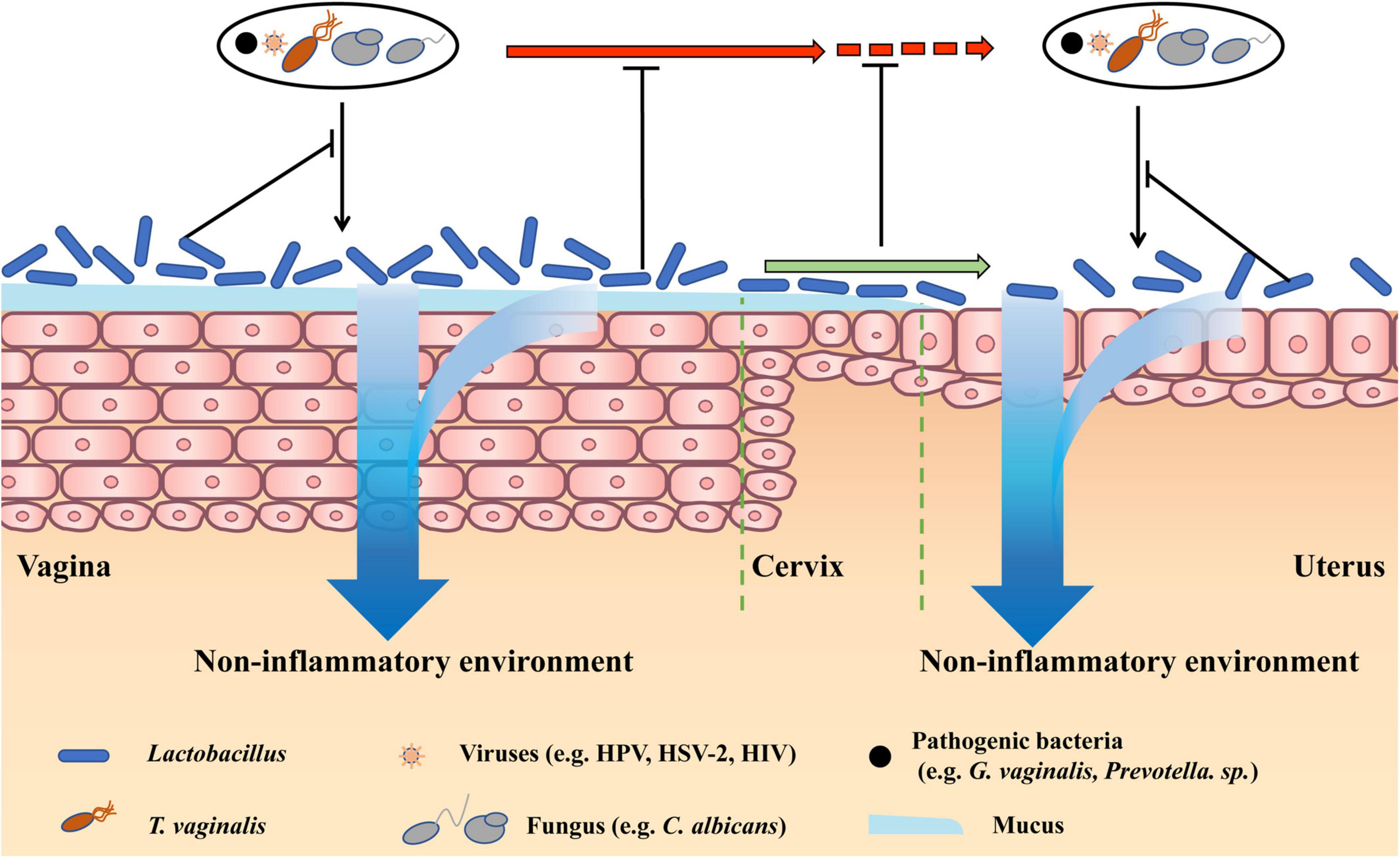
Figure 2. Therapy effects of restoring Lactobacillus-dominated vaginal microbiota in non-infectious diseases. Lactobacillus-dominated vaginal microbiota cannot only benefit vaginal health by preventing vaginal infections and creating a non-inflammatory environment but also prevent pathogens in the vagina moving to the cervix and uterus. Besides, vaginal Lactobacillus can move to the upper reproductive tract, preventing pathogens infecting the cervix and uterus, and creating a non-inflammatory cervical and uterine environment.
Induced abortion is a fairly common gynecological operation worldwide, and whether it is drug-induced or surgery-induced, it can cause a great damage to the female reproductive tract and predispose to serious complications such as incomplete abortion, heavy bleeding, infection, scarring of endometrium, adhesions of the uterine cavity and cervix, and endometriosis. Among these, upper genital tract infection is the most concerned and common clinical problem, which can further cause endometritis, salpingitis, and infertility (Mary and Mahmood, 2010; Carlsson et al., 2018).
Studies showed that postabortal infection was usually caused by pathogens from the lower genital tract, such as Chlamydia, N. gonorrhoeae, Mycoplasma, and BV-related bacteria, moving through the cervix to the uterus and even to fallopian tubes, causing infections and inflammation of the entire reproductive system (Bjartling et al., 2010). In this regard, World Health Organization (WHO) recommended the use of antibiotic prophylaxis to prevent the abortion-induced infection, and a meta-analysis of randomized clinical studies showed that perioperative antibiotics (metronidazole, nitroimidazoles, etc.) could effectively reduce the risk of postabortal infection by 50% (Sawaya et al., 1996; Carlsson et al., 2018). However, if the homeostasis of the uterus is not fully restored and is still conducive to microbial disorders, antibiotics cannot protect the women from being reinfected (Low et al., 2012). Therefore, as probiotics have a great potential in restoring the normal vaginal microbiota and improving the uterine environment, perioperative use of antibiotics and probiotics can prevent the postabortal infection.
Intrauterine adhesions is a condition in which the scar tissues build up inside the uterine cavity, which in many cases will cause adhesion of the opposing endometrium (Chi et al., 2018). Though the adhesions can be removed through the transcervical resection, the postoperative recurrence is high (Pabuccu et al., 2008). A clinical study showed that IUA patients had disturbed vaginal microbiota, characterized by obviously reduced probiotic Lactobacillus, and increased pathogenic Gardnerella and Prevotella, which may promote the uterine pathology and cause recurrence (Liu et al., 2019). Therefore, antibiotics and/or probiotics can promote the recovery and reduce the recurrence of IUA by correcting the vaginal microbiota.
Miscarriage, defined as a spontaneous pregnancy loss before the expected point, is the most prevalent pregnancy complication (Smith et al., 2019). The etiology is multifactorial and usually secondary to other disorders, such as uterine malformation, infections, chromosomal abnormalities, and hormone deficiency (progestogen) (Haas et al., 2019). In this regard, the preventive and curative measures are always aimed at addressing the primary causes (Haas et al., 2019).
Previous studies showed that pregnant women with BV had around two-fold higher risk of miscarriage than those without BV, which was thought to be caused by ascending of the BV-related bacteria from the vagina to the uterus, leading to endometritis, deciduitis, chorioamnionitis, and amniotic fluid infection (Ralph et al., 1999; Goldenberg et al., 2000). Studies exploring the specific mechanisms of BV favoring miscarriage showed that BV-related bacteria could produce lytic enzymes (proteases, phospholipases, etc.) to cause lysis of the fetal membranes, and induce the formation of prostaglandin, which could promote the uterine muscle contraction, decrease the cervical resistance, and induce the release of metalloproteinases (MMPs) to degrade the chorioamniotic membranes (Isik et al., 2016). Besides, the level of inflammatory cytokines IL-6 and IL-8 was also elevated in the amniotic fluid of pregnant women with BV (Keelan et al., 1997). A randomized controlled trial showed that pregnant women treated with clindamycin for BV were five times less likely to miscarry than those given placebo (Ugwumadu et al., 2003). This suggests that early screening and treatment of BV can prevent the infection-induced miscarriage. In addition, a clinical study suggested that hydrogen peroxide producing Lactobacillus in the vagina could not only benefit the vaginal health by improving the microbial and inflammatory status, but also create a favorable uterine environment for implantation and placentation (Eckert et al., 2003). Thus, antibiotics and probiotics could prevent and treat infection-induced miscarriage by restoring the vaginal microbiota and improving the intrauterine environment.
Plenty of evidence showed that BV could also increase the risk of preterm and infertility, mainly via facilitating STI and eliciting the intrauterine inflammation (Peelen et al., 2019; Hong et al., 2020a). Recent studies showed that Lactobacillus-dominated vaginal microbiota was negatively associated with preterm and infertility, and could prevent women from adverse fertility outcome by modulating vaginal microbiota and inflammatory cytokines IL-4 and IL-10 (Vitali et al., 2012; Hong et al., 2020a). Therefore, antibiotics and probiotics can prevent the preterm and infertility by improving the vaginal and uterine microbiota and eubiosis.
Polycystic ovarian syndrome is one of the most prevalent endocrine disorders in reproductive women, which usually manifests as menstrual disorder, hirsutism, and infertility (Hong et al., 2020b). Though current treatments are various, such as oral contraceptives to inhibit the maturation of ovarian follicles as a long-term PCOS management, and ovulation induction for PCOS patients with fertility requirement, they cannot cure it basically and lifestyle modification (e.g., loss weight) is still the first-line and mainstream treatment (Jin and Xie, 2018).
A case–control study of 39 PCOS patients and 40 healthy people showed that the vaginal microbiota of PCOS patients was significantly different from that of healthy people, characterized by increased diversity and increased relative abundance of Mycoplasma and Prevotella, and decreased relative abundance of L. crispatus (Hong et al., 2020b). This suggests that vaginal microbiota dysbiosis may participate or contribute to the PCOS pathology, and therapies targeted at improving the vaginal microbiota are promising. A systemic review involving 855 PCOS patients investigated the effects of probiotics (Lactobacillus) in treating PCOS, and the results showed that probiotic supplementation in PCOS women significantly improved their hormonal index by reducing free androgen index (FAI) and increasing sex hormone binding globulin (SHBG), and their inflammatory index by increasing plasma nitric oxide (NO) and reducing blood malondialdehyde (MDA) (Shamasbi et al., 2020). Besides, the authors also observed that patients given probiotics had increased total glutathione (GSH) and total antioxidant capacity (TAC) levels, and reduced testosterone, dehydroepiandrosterone sulfate (DHEAS, hormonal index), high sensitive C reactive protein (hsCRP, inflammatory index), and hirsutism score compared to those given placebo. As antibiotics and probiotics are aimed at restoring the normal vaginal microbiota and improving the environment of the reproductive system and beyond, they can be used to treat the PCOS symptoms and promote the recovery by improving the hormonal and inflammatory levels.
Recently, microbiota disturbance has also been implicated in the uterine fibroid (benign tumors in the uterus) and menstrual disorders (e.g., menorrhagia and dysmenorrhea), characterized by increased relative abundance of vaginal L. iners, and increased relative abundance of uterine Gardnerella, Prevotella, Sneathia, and Veillonella, respectively (Chen et al., 2017; Pelzer et al., 2018). Thus, antibiotics and/or probiotics are also promising in treating uterine fibroid and menstrual disorders.
Inspired by the similarity of the intestinal and vaginal microbiota, and the success of the fecal microbiota transplantation (FMT), VMT has also been proposed for the treatment of vaginal dysbacteriosis, which involves transplanting the entire vaginal microbiota of a healthy donor into the vagina of the patient to restore the overall diversity, stability, and normal composition of the microbiota (DeLong et al., 2019). The procedures of VMT are shown in Figure 3. The research of our group in 2017 showed that transplantation of healthy rat vaginal microbiota into the vagina of BV model rats restored the morphology of uterine tissue and reduced the serum inflammatory factors such as IL-6, IL-8, and TNF-α, showing obvious recovery effects on vaginal infections caused by dysregulation of vaginal microbiota. In 2019, the clinical study conducted by Lev-Sagie et al. (2019) further showed that VMT had a great effect on long-term recovery from recurrent, antibiotic unresponsive, and refractory BV. In this study, four of the five BV patients treated with VMT recovered effectively after 5–21 months of VMT treatment, showing significant improvements in symptoms, negative Amsel criteria, and Lactobacillus-dominated vaginal fluid under the microscopy, with a cure rate up to 80% and no observed adverse effect. Besides, the authors also found that patients with long-term resolution of BV had a dramatic change in microbial composition in the first month after VMT, which was dominated by increase of Lactobacillus and decrease of Bifidobacterium (closely related to Gardnerella), accompanied with reduced Fannyhessea and Prevotella. In 2021, our group verified the feasibility of VMT in animal models and explored the specific mechanisms (Chen et al., 2021). The results showed that vaginal secretions from healthy rats can be used to treat vaginal microbiota imbalance and prevent the recurrence in rats, which specifically manifested as the decrease of inflammatory cells, pro-inflammatory cytokines, and apoptotic factors in the uterine wall and the restoration of the diversity of vaginal microbiota.
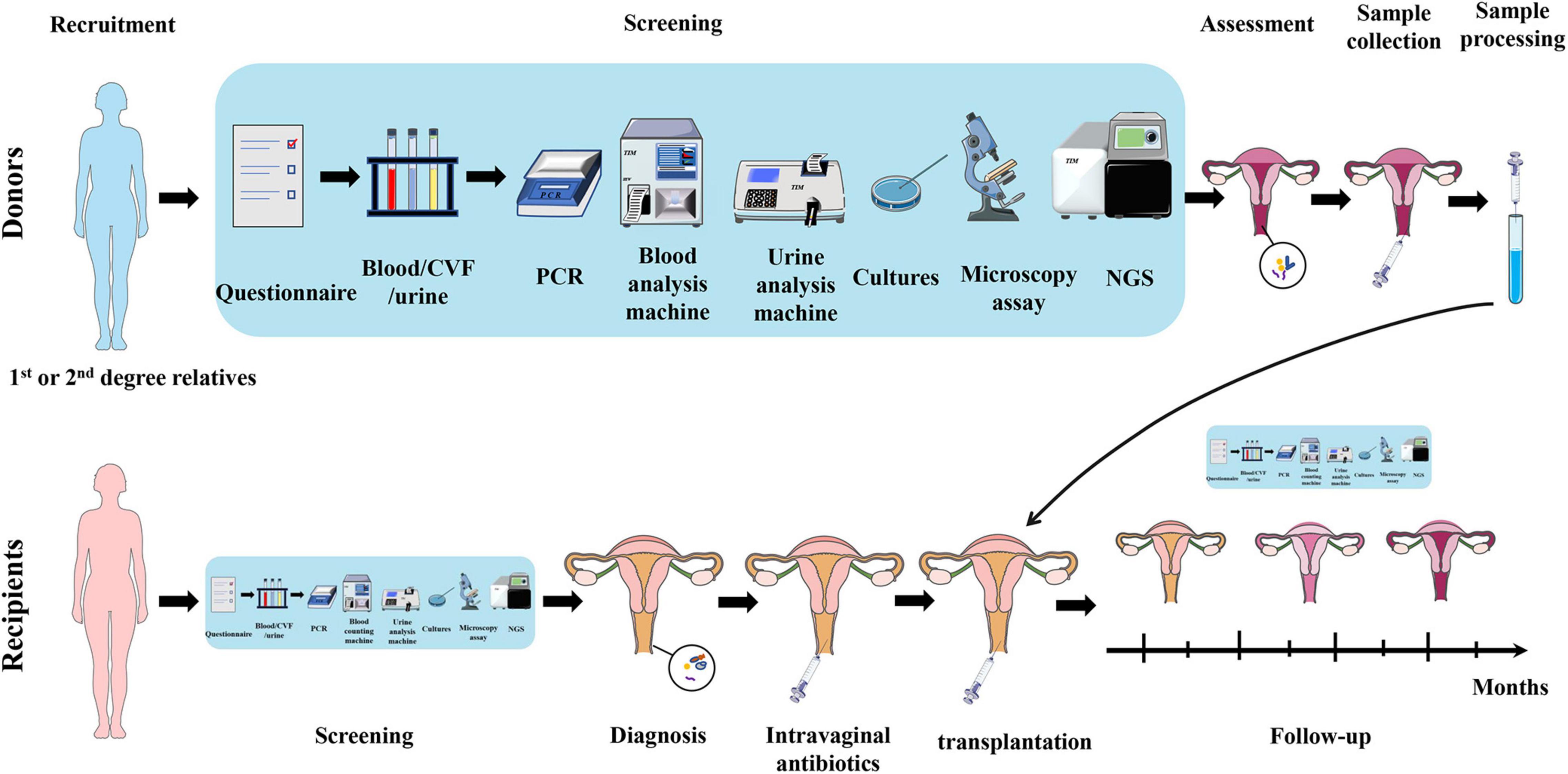
Figure 3. Schematic diagram of VMT operation. As for donors, first, donors are recruited, preferably from the recipients’ first and second relatives. Second, collect blood/CVF/urine samples of donors and conduct a series of screening by questionnaire, PCR, blood, and urine analysis machines, cultures, microscopy, and NGS. Third, collect CVF sample from qualified healthy donor and process it. It is then transplanted into the recipient’s vagina. As for recipients, first, recipients undergo the same screening process for diagnosis and basic health assessment. Second, recipients are given intravaginal antibiotics to prepare for transplantation. Third, transplant the prepared CVF solution from healthy donor into the recipient’s vagina. Fourth, follow-up studies are carried out on recipients to assess the treatment outcome and adverse effects. VMT, vaginal microbiota transplantation; CVF, cervicovaginal fluid; PCR, polymerase chain reaction; NGS, next-generation sequencing.
Preliminary studies of VMT have demonstrated the feasibility of VMT to treat BV, showing favorable therapeutic effects. Compared to other treatments for BV, VMT can completely restore the vaginal microbiota to a healthy state, thus showing better curative effects than conventional antibiotics and probiotics, while addressing the drug resistance, recurrence, and side effects associated with antibiotic treatment. Considering that besides BV, vaginal microbiota dysbiosis is also comprehensively involved in the progression of other gynecological diseases, and improving vaginal microbiota by antibiotics and probiotics shows good therapy effects, restoration of vaginal microbiota by VMT may also have favorable therapeutic effects in the treatment of various gynecological infectious and non-infectious diseases.
However, the clinical implementation of VMT still faces many problems, such as insufficient VMT clinical trial (only one research with five subjects), lacking standard protocol, transmission of unidentifiable and antimicrobial-resistance pathogens, unintended pregnancy, immune rejection, and unclear long-term effects. Therefore, the improvement of the VMT requires multi-disciplinary cooperation. Relevant personnel should formulate VMT screening guidelines as soon as possible, continue to explore the application potential of VMT in the treatment of BV and other gynecological diseases, develop a safe and effective new treatment regimen, and develop safety evaluation criteria. We have reasons to believe that safe, standard, and efficient VMT will bring new hope to patients with gynecological diseases and have a good prospect of application.
TC and ZL provided ideas of this review and designed its framework. YH conducted the research and wrote the manuscript. All authors edited the manuscript. All authors read and approved the final manuscript.
This study was supported by the National Natural Science Foundation of China (Grant No. 82060638), Academic and technical leaders of major disciplines in Jiangxi Province (Grant No. 20194BCJ22032), and Double thousand plan of Jiangxi Province (high end Talents Project of scientific and technological innovation).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Aldunate, M., Srbinovski, D., Hearps, A. C., Latham, C. F., Ramsland, P. A., Gugasyan, R., et al. (2015). Antimicrobial and immune modulatory effects of lactic acid and short chain fatty acids produced by vaginal microbiota associated with eubiosis and bacterial vaginosis. Front. Physiol. 6:164. doi: 10.3389/fphys.2015.00164
Balkus, J. E., Richardson, B. A., Rabe, L. K., Taha, T. E., Mgodi, N., Kasaro, M. P., et al. (2014). Bacterial vaginosis and the risk of trichomonas vaginalis acquisition among HIV-1-negative women. Sex. Transm. Dis. 41, 123–128. doi: 10.1097/olq.0000000000000075
Bjartling, C., Osser, S., and Persson, K. (2010). The association between Mycoplasma genitalium and pelvic inflammatory disease after termination of pregnancy. BJOG 117, 361–364. doi: 10.1111/j.1471-0528.2009.02455.x
Borgdorff, H., Gautam, R., Armstrong, S. D., Xia, D., Ndayisaba, G. F., van Teijlingen, N. H., et al. (2016). Cervicovaginal microbiome dysbiosis is associated with proteome changes related to alterations of the cervicovaginal mucosal barrier. Mucosal Immunol. 9, 621–633. doi: 10.1038/mi.2015.86
Bradford, L. L., and Ravel, J. (2017). The vaginal mycobiome: a contemporary perspective on fungi in women’s health and diseases. Virulence 8, 342–351. doi: 10.1080/21505594.2016.1237332
Bradshaw, C. S., Morton, A. N., Hocking, J., Garland, S. M., Morris, M. B., Moss, L. M., et al. (2006). High recurrence rates of bacterial vaginosis over the course of 12 months after oral metronidazole therapy and factors associated with recurrence. J. Infect. Dis. 193, 1478–1486. doi: 10.1086/503780
Brotman, R. M., Klebanoff, M. A., Nansel, T. R., Yu, K. F., Andrews, W. W., Zhang, J., et al. (2010). Bacterial vaginosis assessed by gram stain and diminished colonization resistance to incident gonococcal, chlamydial, and trichomonal genital infection. J. Infect. Dis. 202, 1907–1915. doi: 10.1086/657320
Brotman, R. M., Shardell, M. D., Gajer, P., Fadrosh, D., Chang, K., Silver, M. I., et al. (2014). Association between the vaginal microbiota, menopause status, and signs of vulvovaginal atrophy. Menopause 21, 450–458. doi: 10.1097/GME.0b013e3182a4690b
Carlsson, I., Breding, K., and Larsson, P. G. (2018). Complications related to induced abortion: a combined retrospective and longitudinal follow-up study. BMC Womens Health 18:158. doi: 10.1186/s12905-018-0645-6
Cazanave, C., Manhart, L. E., and Bebear, C. (2012). Mycoplasma genitalium, an emerging sexually transmitted pathogen. Med. Mal. Infect. 42, 381–392. doi: 10.1016/j.medmal.2012.05.006
Chen, C., Song, X., Wei, W., Zhong, H., Dai, J., Lan, Z., et al. (2017). The microbiota continuum along the female reproductive tract and its relation to uterine-related diseases. Nat. Commun. 8:875. doi: 10.1038/s41467-017-00901-0
Chen, T., Xia, C., Hu, H., Wang, H., Tan, B., Tian, P., et al. (2021). Dysbiosis of the rat vagina is efficiently rescued by vaginal microbiota transplantation or probiotic combination. Int. J. Antimicrob. Agents 57:106277. doi: 10.1016/j.ijantimicag.2021.106277
Cherpes, T. L., Meyn, L. A., Krohn, M. A., and Hillier, S. L. (2003). Risk factors for infection with herpes simplex virus type 2: role of smoking, douching, uncircumcised males, and vaginal flora. Sex. Transm. Dis. 30, 405–410. doi: 10.1097/00007435-200305000-00006
Chi, Y., He, P., Lei, L., Lan, Y., Hu, J., Meng, Y., et al. (2018). Transdermal estrogen gel and oral aspirin combination therapy improves fertility prognosis via the promotion of endometrial receptivity in moderate to severe intrauterine adhesion. Mol. Med. Rep. 17, 6337–6344. doi: 10.3892/mmr.2018.8685
Conti, C., Malacrino, C., and Mastromarino, P. (2009). Inhibition of herpes simplex virus type 2 by vaginal lactobacilli. J. Physiol. Pharmacol. 60, (Suppl. 6) 19–26.
Cudmore, S. L., Delgaty, K. L., Hayward-McClelland, S. F., Petrin, D. P., and Garber, G. E. (2004). Treatment of infections caused by metronidazole-resistant Trichomonas vaginalis. Clin. Microbiol. Rev. 17, 783–793, table of contents. doi: 10.1128/CMR.17.4.783-793.2004
DeLong, K., Zulfiqar, F., Hoffmann, D. E., Tarzian, A. J., and Ensign, L. M. (2019). Vaginal microbiota transplantation: the next frontier. J. Law Med. Ethics 47, 555–567. doi: 10.1177/1073110519897731
Eckert, L. O., Moore, D. E., Patton, D. L., Agnew, K. J., and Eschenbach, D. A. (2003). Relationship of vaginal bacteria and inflammation with conception and early pregnancy loss following in-vitro fertilization. Infect. Dis. Obstet. Gynecol. 11, 11–17. doi: 10.1155/S1064744903000024
Edwards, T., Burke, P., Smalley, H., and Hobbs, G. (2016). Trichomonas vaginalis: clinical relevance, pathogenicity and diagnosis. Crit. Rev. Microbiol. 42, 406–417. doi: 10.3109/1040841X.2014.958050
Evans, B. A., Kell, P. D., Bond, R. A., MacRae, K. D., Slomka, M. J., and Brown, D. W. (2003). Predictors of seropositivity to herpes simplex virus type 2 in women. Int. J. STD AIDS 14, 30–36. doi: 10.1258/095646203321043237
Farage, M., and Maibach, H. (2006). Lifetime changes in the vulva and vagina. Arch. Gynecol. Obstet. 273, 195–202. doi: 10.1007/s00404-005-0079-x
Faught, B. M., and Reyes, S. (2019). Characterization and treatment of recurrent bacterial vaginosis. J. Womens Health (Larchmt) 28, 1218–1226. doi: 10.1089/jwh.2018.7383
Fichorova, R. N., Trifonova, R. T., Gilbert, R. O., Costello, C. E., Hayes, G. R., Lucas, J. J., et al. (2006). Trichomonas vaginalis lipophosphoglycan triggers a selective upregulation of cytokines by human female reproductive tract epithelial cells. Infect. Immun. 74, 5773–5779. doi: 10.1128/IAI.00631-06
Figueroa-Angulo, E. E., Rendon-Gandarilla, F. J., Puente-Rivera, J., Calla-Choque, J. S., Cardenas-Guerra, R. E., Ortega-Lopez, J., et al. (2012). The effects of environmental factors on the virulence of Trichomonas vaginalis. Microbes Infect. 14, 1411–1427. doi: 10.1016/j.micinf.2012.09.004
Foschi, C., Salvo, M., Cevenini, R., Parolin, C., Vitali, B., and Marangoni, A. (2017). Vaginal lactobacilli reduce Neisseria gonorrhoeae viability through multiple strategies: an in vitro study. Front. Cell. Infect. Microbiol. 7:502. doi: 10.3389/fcimb.2017.00502
Gajer, P., Brotman, R. M., Bai, G., Sakamoto, J., Schutte, U. M., Zhong, X., et al. (2012). Temporal dynamics of the human vaginal microbiota. Sci. Transl. Med. 4:132ra152. doi: 10.1126/scitranslmed.3003605
Goldenberg, R. L., Hauth, J. C., and Andrews, W. W. (2000). Intrauterine infection and preterm delivery. N. Engl. J. Med. 342, 1500–1507. doi: 10.1056/NEJM200005183422007
Gosmann, C., Anahtar, M. N., Handley, S. A., Farcasanu, M., Abu-Ali, G., Bowman, B. A., et al. (2017). Lactobacillus-deficient cervicovaginal bacterial communities are associated with increased HIV acquisition in young South African women. Immunity 46, 29–37. doi: 10.1016/j.immuni.2016.12.013
Gupta, S., Kakkar, V., and Bhushan, I. (2019). Crosstalk between vaginal microbiome and female health: a review. Microb. Pathog. 136:103696. doi: 10.1016/j.micpath.2019.103696
Haas, D. M., Hathaway, T. J., and Ramsey, P. S. (2019). Progestogen for preventing miscarriage in women with recurrent miscarriage of unclear etiology. Cochrane Database Syst. Rev. 2019:CD003511. doi: 10.1002/14651858.CD003511.pub5
Haggerty, C. L., Gottlieb, S. L., Taylor, B. D., Low, N., Xu, F., and Ness, R. B. (2010). Risk of sequelae after Chlamydia trachomatis genital infection in women. J. Infect. Dis. 201, (Suppl. 2) S134–S155. doi: 10.1086/652395
Hanson, L., VandeVusse, L., Jerme, M., Abad, C. L., and Safdar, N. (2016). Probiotics for treatment and prevention of urogenital infections in women: a systematic review. J. Midwifery Womens Health 61, 339–355. doi: 10.1111/jmwh.12472
Harp, D. F., and Chowdhury, I. (2011). Trichomoniasis: evaluation to execution. Eur. J. Obstet. Gynecol. Reprod. Biol. 157, 3–9. doi: 10.1016/j.ejogrb.2011.02.024
Hay, P. E. (2004). Bacterial vaginosis and miscarriage. Curr. Opin. Infect. Dis. 17, 41–44. doi: 10.1097/00001432-200402000-00008
Hemalatha, R., Mastromarino, P., Ramalaxmi, B. A., Balakrishna, N. V., and Sesikeran, B. (2012). Effectiveness of vaginal tablets containing lactobacilli versus pH tablets on vaginal health and inflammatory cytokines: a randomized, double-blind study. Eur. J. Clin. Microbiol. Infect. Dis. 31, 3097–3105. doi: 10.1007/s10096-012-1671-1
Hong, X., Ma, J., Yin, J., Fang, S., Geng, J., Zhao, H., et al. (2020a). The association between vaginal microbiota and female infertility: a systematic review and meta-analysis. Arch. Gynecol. Obstet. 302, 569–578. doi: 10.1007/s00404-020-05675-3
Hong, X., Qin, P., Huang, K., Ding, X., Ma, J., Xuan, Y., et al. (2020b). Association between polycystic ovary syndrome and the vaginal microbiome: a case-control study. Clin. Endocrinol. 93, 52–60. doi: 10.1111/cen.14198
Isik, G., Demirezen, S., Donmez, H. G., and Beksac, M. S. (2016). Bacterial vaginosis in association with spontaneous abortion and recurrent pregnancy losses. J. Cytol. 33, 135–140. doi: 10.4103/0970-9371.188050
Jaisamrarn, U., Triratanachat, S., Chaikittisilpa, S., Grob, P., Prasauskas, V., and Taechakraichana, N. (2013). Ultra-low-dose estriol and lactobacilli in the local treatment of postmenopausal vaginal atrophy. Climacteric 16, 347–355. doi: 10.3109/13697137.2013.769097
Jin, P., and Xie, Y. (2018). Treatment strategies for women with polycystic ovary syndrome. Gynecol. Endocrinol. 34, 272–277. doi: 10.1080/09513590.2017.1395841
Jung, H. S., Ehlers, M. M., Lombaard, H., Redelinghuys, M. J., and Kock, M. M. (2017). Etiology of bacterial vaginosis and polymicrobial biofilm formation. Crit. Rev. Microbiol. 43, 651–667. doi: 10.1080/1040841X.2017.1291579
Kashyap, N., Krishnan, N., Kaur, S., and Ghai, S. (2018). Assessment of sign and symptoms of cervical cancer amongst the patient : a case control study. Nurs. Midwifery Res. J. 14, 19–25.
Keelan, J. A., Sato, T., and Mitchell, M. D. (1997). Interleukin (IL)-6 and IL-8 production by human amnion: regulation by cytokines, growth factors, glucocorticoids, phorbol esters, and bacterial lipopolysaccharide. Biol. Reprod. 57, 1438–1444. doi: 10.1095/biolreprod57.6.1438
Khosropour, C. M., Bell, T. R., Hughes, J. P., Manhart, L. E., and Golden, M. R. (2018). A population-based study to compare treatment outcomes among women with urogenital chlamydial infection in Washington State, 1992 to 2015. Sex. Transm. Dis. 45, 319–324. doi: 10.1097/OLQ.0000000000000764
Kim, J. M., and Park, Y. J. (2017). Probiotics in the prevention and treatment of postmenopausal vaginal infections: review article. J. Menopausal Med. 23, 139–145. doi: 10.6118/jmm.2017.23.3.139
Leitich, H., and Kiss, H. (2007). Asymptomatic bacterial vaginosis and intermediate flora as risk factors for adverse pregnancy outcome. Best Pract. Res. Clin. Obstet. Gynaecol. 21, 375–390. doi: 10.1016/j.bpobgyn.2006.12.005
Lev-Sagie, A., Goldman-Wohl, D., Cohen, Y., Dori-Bachash, M., Leshem, A., Mor, U., et al. (2019). Vaginal microbiome transplantation in women with intractable bacterial vaginosis. Nat. Med. 25, 1500–1504. doi: 10.1038/s41591-019-0600-6
Lewis, F. M., Bernstein, K. T., and Aral, S. O. (2017). Vaginal microbiome and its relationship to behavior, sexual health, and sexually transmitted diseases. Obstet. Gynecol. 129, 643–654. doi: 10.1097/AOG.0000000000001932
Li, T., Liu, Z., Zhang, X., Chen, X., and Wang, S. (2019). Local probiotic Lactobacillus crispatus and Lactobacillus delbrueckii exhibit strong antifungal effects against vulvovaginal candidiasis in a rat model. Front. Microbiol. 10:1033. doi: 10.3389/fmicb.2019.01033
Ling, Z., Kong, J., Liu, F., Zhu, H., Chen, X., Wang, Y., et al. (2010). Molecular analysis of the diversity of vaginal microbiota associated with bacterial vaginosis. BMC Genomics 11:488. doi: 10.1186/1471-2164-11-488
Liu, Z., Kong, Y., Gao, Y., Ren, Y., Zheng, C., Deng, X., et al. (2019). Revealing the interaction between intrauterine adhesion and vaginal microbiota using high-throughput sequencing. Mol. Med. Rep. 19, 4167–4174. doi: 10.3892/mmr.2019.10092
Lopez-Moreno, A., and Aguilera, M. (2020). Probiotics dietary supplementation for modulating endocrine and fertility microbiota dysbiosis. Nutrients 12:757. doi: 10.3390/nu12030757
Low, N., Mueller, M., Van Vliet, H. A., and Kapp, N. (2012). Perioperative antibiotics to prevent infection after first-trimester abortion. Cochrane Database Syst. Rev. 2012:CD005217. doi: 10.1002/14651858.CD005217.pub2
Ma, B., Forney, L. J., and Ravel, J. (2012). Vaginal microbiome: rethinking health and disease. Annu. Rev. Microbiol. 66, 371–389. doi: 10.1146/annurev-micro-092611-150157
Ma, D., Chen, Y., and Chen, T. (2019). Vaginal microbiota transplantation for the treatment of bacterial vaginosis: a conceptual analysis. FEMS Microbiol. Lett. 366:fnz025. doi: 10.1093/femsle/fnz025
Mary, N., and Mahmood, T. A. (2010). Preventing infective complications relating to induced abortion. Best Pract. Res. Clin. Obstet. Gynaecol. 24, 539–549. doi: 10.1016/j.bpobgyn.2010.03.005
Mastromarino, P., Di Pietro, M., Schiavoni, G., Nardis, C., Gentile, M., and Sessa, R. (2014). Effects of vaginal lactobacilli in Chlamydia trachomatis infection. Int. J. Med. Microbiol. 304, 654–661. doi: 10.1016/j.ijmm.2014.04.006
Mc, L. N., and Rosenstein, I. J. (2000). Characterisation and selection of a Lactobacillus species to re-colonise the vagina of women with recurrent bacterial vaginosis. J. Med. Microbiol. 49, 543–552. doi: 10.1099/0022-1317-49-6-543
Mestrovic, T., and Ljubin-Sternak, S. (2018). Molecular mechanisms of Chlamydia trachomatis resistance to antimicrobial drugs. Front. Biosci. (Landmark Ed.) 23:656–670. doi: 10.2741/4611
Mhatre, M., McAndrew, T., Carpenter, C., Burk, R. D., Einstein, M. H., and Herold, B. C. (2012). Cervical intraepithelial neoplasia is associated with genital tract mucosal inflammation. Sex. Transm. Dis. 39, 591–597. doi: 10.1097/OLQ.0b013e318255aeef
Mitra, A., MacIntyre, D. A., Marchesi, J. R., Lee, Y. S., Bennett, P. R., and Kyrgiou, M. (2016). The vaginal microbiota, human papillomavirus infection and cervical intraepithelial neoplasia: what do we know and where are we going next? Microbiome 4:58. doi: 10.1186/s40168-016-0203-0
Molenaar, M. C., Singer, M., and Ouburg, S. (2018). The two-sided role of the vaginal microbiome in Chlamydia trachomatis and Mycoplasma genitalium pathogenesis. J. Reprod. Immunol. 130, 11–17. doi: 10.1016/j.jri.2018.08.006
Morgan, M. K., and Decker, C. F. (2016). Gonorrhea. Dis. Mon. 62, 260–268. doi: 10.1016/j.disamonth.2016.03.009
Mousavi, E., Makvandi, M., Teimoori, A., Ataei, A., Ghafari, S., and Samarbaf-Zadeh, A. (2018). Antiviral effects of Lactobacillus crispatus against HSV-2 in mammalian cell lines. J. Chin. Med. Assoc. 81, 262–267. doi: 10.1016/j.jcma.2017.07.010
Muzny, C. A., and Schwebke, J. R. (2013). The clinical spectrum of Trichomonas vaginalis infection and challenges to management. Sex. Transm. Infect. 89, 423–425. doi: 10.1136/sextrans-2012-050893
Nahui Palomino, R. A., Zicari, S., Vanpouille, C., Vitali, B., and Margolis, L. (2017). Vaginal Lactobacillus inhibits HIV-1 replication in human tissues ex vivo. Front. Microbiol. 8:906. doi: 10.3389/fmicb.2017.00906
Nardini, P., Nahui Palomino, R. A., Parolin, C., Laghi, L., Foschi, C., Cevenini, R., et al. (2016). Lactobacillus crispatus inhibits the infectivity of Chlamydia trachomatis elementary bodies, in vitro study. Sci. Rep. 6:29024. doi: 10.1038/srep29024
Nasioudis, D., Linhares, I. M., Ledger, W. J., and Witkin, S. S. (2017). Bacterial vaginosis: a critical analysis of current knowledge. BJOG 124, 61–69. doi: 10.1111/1471-0528.14209
Niu, X. X., Li, T., Zhang, X., Wang, S. X., and Liu, Z. H. (2017). Lactobacillus crispatus modulates vaginal epithelial cell innate response to Candida albicans. Chin. Med. J. (Engl.) 130, 273–279. doi: 10.4103/0366-6999.198927
Nurbhai, M., Grimshaw, J., Watson, M., Bond, C., Mollison, J., and Ludbrook, A. (2007). Oral versus intra-vaginal imidazole and triazole anti-fungal treatment of uncomplicated vulvovaginal candidiasis (thrush). Cochrane Database Syst. Rev. 4:Cd002845. doi: 10.1002/14651858.CD002845.pub2
O’Connell, C. M., and Ferone, M. E. (2016). Chlamydia trachomatis genital infections. Microb. Cell 3, 390–403. doi: 10.15698/mic2016.09.525
Onderdonk, A. B., Delaney, M. L., and Fichorova, R. N. (2016). The human microbiome during bacterial vaginosis. Clin. Microbiol. Rev. 29, 223–238. doi: 10.1128/CMR.00075-15
Pabuccu, R., Onalan, G., Kaya, C., Selam, B., Ceyhan, T., Ornek, T., et al. (2008). Efficiency and pregnancy outcome of serial intrauterine device-guided hysteroscopic adhesiolysis of intrauterine synechiae. Fertil. Steril. 90, 1973–1977. doi: 10.1016/j.fertnstert.2007.06.074
Paez-Canro, C., Alzate, J. P., Gonzalez, L. M., Rubio-Romero, J. A., Lethaby, A., and Gaitan, H. G. (2019). Antibiotics for treating urogenital Chlamydia trachomatis infection in men and non-pregnant women. Cochrane Database Syst. Rev. 1:CD010871. doi: 10.1002/14651858.CD010871.pub2
Paladine, H. L., and Desai, U. A. (2018). Vaginitis: diagnosis and treatment. Am. Fam. Physician 97, 321–329.
Palma, E., Recine, N., Domenici, L., Giorgini, M., Pierangeli, A., and Panici, P. B. (2018). Long-term Lactobacillus rhamnosus BMX 54 application to restore a balanced vaginal ecosystem: a promising solution against HPV-infection. BMC Infect. Dis. 18:13. doi: 10.1186/s12879-017-2938-z
Pastorek, J. G. II, Cotch, M. F., Martin, D. H., and Eschenbach, D. A. (1996). Clinical and microbiological correlates of vaginal trichomoniasis during pregnancy. The vaginal infections and prematurity study group. Clin. Infect. Dis. 23, 1075–1080. doi: 10.1093/clinids/23.5.1075
Peelen, M. J., Luef, B. M., Lamont, R. F., de Milliano, I., Jensen, J. S., Limpens, J., et al. (2019). The influence of the vaginal microbiota on preterm birth: a systematic review and recommendations for a minimum dataset for future research. Placenta 79, 30–39. doi: 10.1016/j.placenta.2019.03.011
Pelzer, E. S., Willner, D., Buttini, M., and Huygens, F. (2018). A role for the endometrial microbiome in dysfunctional menstrual bleeding. Antonie van Leeuwenhoek 111, 933–943. doi: 10.1007/s10482-017-0992-6
Petrin, D., Delgaty, K., Bhatt, R., and Garber, G. (1998). Clinical and microbiological aspects of Trichomonas vaginalis. Clin. Microbiol. Rev. 11, 300–317.
Phukan, N., Brooks, A. E. S., and Simoes-Barbosa, A. (2018). A cell surface aggregation-promoting factor from Lactobacillus gasseri contributes to inhibition of Trichomonas vaginalis adhesion to human vaginal ectocervical cells. Infect. Immun. 86:e00907-17. doi: 10.1128/IAI.00907-17
Pinto-Sander, N., and Soni, S. (2019). Mycoplasma genitalium infection. BMJ 367, l5820. doi: 10.1136/bmj.l5820
Piyathilake, C. J., Ollberding, N. J., Kumar, R., Macaluso, M., Alvarez, R. D., and Morrow, C. D. (2016). Cervical microbiota associated with higher grade cervical intraepithelial neoplasia in women infected with high-risk human papillomaviruses. Cancer Prev. Res. (Phila) 9, 357–366. doi: 10.1158/1940-6207.CAPR-15-0350
Ralph, S. G., Rutherford, A. J., and Wilson, J. D. (1999). Influence of bacterial vaginosis on conception and miscarriage in the first trimester: cohort study. BMJ 319, 220–223. doi: 10.1136/bmj.319.7204.220
Ravel, J., Gajer, P., Abdo, Z., Schneider, G. M., Koenig, S. S., McCulle, S. L., et al. (2011). Vaginal microbiome of reproductive-age women. Proc. Natl. Acad. Sci. U.S.A. 108, (Suppl. 1) 4680–4687. doi: 10.1073/pnas.1002611107
Reid, G., Jass, J., Sebulsky, M. T., and McCormick, J. K. (2003). Potential uses of probiotics in clinical practice. Clin. Microbiol. Rev. 16, 658–672. doi: 10.1128/cmr.16.4.658-672.2003
Sawaya, G. F., Grady, D., Kerlikowske, K., and Grimes, D. A. (1996). Antibiotics at the time of induced abortion: the case for universal prophylaxis based on a meta-analysis. Obstet. Gynecol. 87(5 Pt 2), 884–890.
Sgibnev, A., and Kremleva, E. (2020). Probiotics in addition to metronidazole for treatment Trichomonas vaginalis in the presence of BV: a randomized, placebo-controlled, double-blind study. Eur. J. Clin. Microbiol. Infect. Dis. 39, 345–351. doi: 10.1007/s10096-019-03731-8
Shamasbi, S. G., Ghanbari-Homayi, S., and Mirghafourvand, M. (2020). The effect of probiotics, prebiotics, and synbiotics on hormonal and inflammatory indices in women with polycystic ovary syndrome: a systematic review and meta-analysis. Eur. J. Nutr. 59, 433–450. doi: 10.1007/s00394-019-02033-1
Shen, J., Song, N., Williams, C. J., Brown, C. J., Yan, Z., Xu, C., et al. (2016). Effects of low dose estrogen therapy on the vaginal microbiomes of women with atrophic vaginitis. Sci. Rep. 6:24380. doi: 10.1038/srep24380
Smith, P. P., Dhillon-Smith, R. K., O’Toole, E., Cooper, N., Coomarasamy, A., and Clark, T. J. (2019). Outcomes in prevention and management of miscarriage trials: a systematic review. BJOG 126, 176–189. doi: 10.1111/1471-0528.15528
Smith, S. B., and Ravel, J. (2017). The vaginal microbiota, host defence and reproductive physiology. J. Physiol. 595, 451–463. doi: 10.1113/JP271694
Sobel, J. D. (1992). Pathogenesis and treatment of recurrent vulvovaginal candidiasis. Clin. Infect. Dis. 14, (Suppl. 1) S148–S153. doi: 10.1093/clinids/14.supplement_1.s148
Sobel, J. D., and Sobel, R. (2018). Current treatment options for vulvovaginal candidiasis caused by azole-resistant Candida species. Expert Opin. Pharmacother. 19, 971–977. doi: 10.1080/14656566.2018.1476490
Srinivasan, S., Hoffman, N. G., Morgan, M. T., Matsen, F. A., Fiedler, T. L., Hall, R. W., et al. (2012). Bacterial communities in women with bacterial vaginosis: high resolution phylogenetic analyses reveal relationships of microbiota to clinical criteria. PLoS One 7:e37818. doi: 10.1371/journal.pone.0037818
Stanley, M. (2010). Pathology and epidemiology of HPV infection in females. Gynecol. Oncol. 117, (Suppl. 2) S5–S10. doi: 10.1016/j.ygyno.2010.01.024
Stika, C. S. (2010). Atrophic vaginitis. Dermatol. Ther. 23, 514–522. doi: 10.1111/j.1529-8019.2010.01354.x
Strus, M., Brzychczy-Włoch, M., Gosiewski, T., Kochan, P., and Heczko, P. B. (2006). The in vitro effect of hydrogen peroxide on vaginal microbial communities. FEMS Immunol. Med. Microbiol. 48, 56–63. doi: 10.1111/j.1574-695X.2006.00120.x
Torcia, M. G. (2019). Interplay among vaginal microbiome, immune response and sexually transmitted viral infections. Int. J. Mol. Sci. 20:266. doi: 10.3390/ijms20020266
Ugwumadu, A., Manyonda, I., Reid, F., and Hay, P. (2003). Effect of early oral clindamycin on late miscarriage and preterm delivery in asymptomatic women with abnormal vaginal flora and bacterial vaginosis: a randomised controlled trial. Lancet 361, 983–988. doi: 10.1016/S0140-6736(03)12823-1
Unemo, M., Golparian, D., and Eyre, D. W. (2019). Antimicrobial Resistance in Neisseria gonorrhoeae and treatment of gonorrhea. Methods Mol. Biol. 1997, 37–58. doi: 10.1007/978-1-4939-9496-0_3
van de Wijgert, J., and Jespers, V. (2017). The global health impact of vaginal dysbiosis. Res. Microbiol. 168, 859–864. doi: 10.1016/j.resmic.2017.02.003
van Oostrum, N., De Sutter, P., Meys, J., and Verstraelen, H. (2013). Risks associated with bacterial vaginosis in infertility patients: a systematic review and meta-analysis. Hum. Reprod. 28, 1809–1815. doi: 10.1093/humrep/det096
Verhoeven, V., Renard, N., Makar, A., Van Royen, P., Bogers, J. P., Lardon, F., et al. (2013). Probiotics enhance the clearance of human papillomavirus-related cervical lesions: a prospective controlled pilot study. Eur. J. Cancer Prev. 22, 46–51. doi: 10.1097/CEJ.0b013e328355ed23
Vitali, B., Cruciani, F., Baldassarre, M. E., Capursi, T., Spisni, E., Valerii, M. C., et al. (2012). Dietary supplementation with probiotics during late pregnancy: outcome on vaginal microbiota and cytokine secretion. BMC Microbiol. 12:236. doi: 10.1186/1471-2180-12-236
Wang, Z., He, Y., and Zheng, Y. (2019). Probiotics for the treatment of bacterial vaginosis: a meta-analysis. Int. J. Environ. Res. Public Health 16:3859. doi: 10.3390/ijerph16203859
Xie, H. Y., Feng, D., Wei, D. M., Mei, L., Chen, H., Wang, X., et al. (2017). Probiotics for vulvovaginal candidiasis in non-pregnant women. Cochrane Database Syst. Rev. 11:CD010496. doi: 10.1002/14651858.CD010496.pub2
Yeh, S. L., Lin, M. S., and Chen, H. L. (2007). Inhibitory effects of a soluble dietary fiber from Amorphophallus konjac on cytotoxicity and DNA damage induced by fecal water in Caco-2 cells. Planta Med. 73, 1384–1388. doi: 10.1055/s-2007-990228
Keywords: vaginal microbiota transplantation, bacterial vaginitis, antibiotics, Lactobacillus, gynecological diseases
Citation: Han Y, Liu Z and Chen T (2021) Role of Vaginal Microbiota Dysbiosis in Gynecological Diseases and the Potential Interventions. Front. Microbiol. 12:643422. doi: 10.3389/fmicb.2021.643422
Received: 24 February 2021; Accepted: 19 May 2021;
Published: 18 June 2021.
Edited by:
Yanling Wei, Army Medical University, ChinaReviewed by:
Valentina Taverniti, University of Milan, ItalyCopyright © 2021 Han, Liu and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Tingtao Chen, Y2hlbnRpbmd0YW8xOTg0QDE2My5jb20=; Zhaoxia Liu, bHp4aWE3N0AxNjMuY29t
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.