
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Microbiol. , 26 September 2019
Sec. Fungi and Their Interactions
Volume 10 - 2019 | https://doi.org/10.3389/fmicb.2019.02167
This article is part of the Research Topic Fungal Molecular Systematics, Ranking, and Evolution Using Divergence Time View all 7 articles
 Jize Xu1,2,3*†
Jize Xu1,2,3*† Xiaodong Yu4†
Xiaodong Yu4† Mengzhao Lu4
Mengzhao Lu4 Jiajun Hu3
Jiajun Hu3 Odeshnee Moodley4,5
Odeshnee Moodley4,5 Chunlan Zhang2*
Chunlan Zhang2* Lei Gong3*
Lei Gong3* Yu Li3,4*
Yu Li3,4*Two new species (Melanoleuca galerina and M. subgrammopodia) and seven new recorded species from northern China are described here using morphological and molecular methods. Melanoleuca galerina is mainly characterized by its hygrophanous pileus, decurrent lamellae, fibrous stipe and spores with round warts. Key characteristics of M. subgrammopodia include its discolored pileus, fibrous stipe and urticiform cystidia. The divergence time of Melanoleuca fungi as well as the phylogenetic relationships within this genus were analyzed using DNA sequences of the internal transcribed spacer (ITS) and the nuclear large subunit rDNA (nrLSU) gene fragments. Analyses revealed that morphological identifications and phylogenetic relationships were consistent with the results of divergence time, thereby confirming that M. galerina and M. subgrammopodia are new species.
Individuals from Melanoleuca Pat. are widely distributed throughout the world and include some edible species (Singer, 1986). Currently, Index Fungorum1 lists 422 validly published species within Melanoleuca. Whereas, Kirk et al. (2008) only accepted 50 species from this genus. In respect of China, a total of 19 Melanoleuca species have been reported (Bau and Li, 1999; Zhang et al., 2001; Chen, 2007; Mao, 2009; Sun et al., 2012; Wang, 2013; He et al., 2014; Yu et al., 2014; Zhao et al., 2014; Wei et al., 2015; Du et al., 2016; Tian et al., 2018). Melanoleuca is a genus with limited morphological characteristics. It is mainly characterized by a collybioid to tricholomatoid habit, a rarely bright-colored pileus, with warty and strongly amyloid spores and having either absent or present cystidia (Singer, 1986). The divisions among Melanoleuca species are vague. Different taxonomists often have their own interpretations of morphological features, resulting in different classifications of the same species based on those differing opinions (Singer, 1986; Boekhout, 1988; Hyde et al., 2013). The following division views are currently widely approved. Singer (1962, 1986) divided Melanoleuca into four sections primarily based on the color of the pileus or stipe and the ornamentation of spores. Boekhout (1988) focused mainly on microscopic features, on the basis of presence and shape of cystidia and divided the genus into three subgenera namely: Subg. Melanoleuca Boekhout, Subg. Urocystis Boekhout and Subg. Macrocystis Boekhout. Bon (1991) introduced the spore Q value for delimiting subsections and divided the genus into three subgenera and eight sections (Vizzini et al., 2011). Before the advent of molecular phylogenetic tools, the taxonomical units of Boekhout (1988) were most frequently used for morphological identification of Melanoleuca. However, Vizzini et al. (2011) divided Melanoleuca into two subgenera (Subg. Urticocystis Vizzini and Subg. Melanoleuca Vizzini) by constructing phylogenetic relationships with the internal transcribed spacer (ITS) sequences. Then, Yu et al. (2014) and Kalmer et al. (2018) confirmed the results of Vizzini et al. (2011). Even now, the division views proposed by Vizzini et al. (2011) are widely supported by molecular systematics (Yu et al., 2014; Kalmer et al., 2018).
Some reports have established divergence times in the fungi. For example, Hennig (1966) first recommended the use of divergence time as a universal criterion for taxa ranking. Berbee and Taylor (2010), Oberwinkler (2012), and Hibbett (2014) estimated the divergence times of Basidiomycota and Ascomycota. Time-trees indicated that Basidiomycota are estimated to have diverged around 500 million years ago (Ma), and is a sister group to Ascomycota, being of similar age. Agaricomycetes diverged about 290 Ma as estimated by Floudas et al. (2012). Zhao et al. (2016) first attempted creating a taxonomic system of fungi based on divergence time which was a reconstruction of the taxonomic system used for Agaricus. Furthermore, Chen et al. (2017) utilized the taxonomic system based on divergence time for reconstruction of Agaricus subg. Minores, A. sect. Minores (Fr.) Henn. Additionally, Zhao et al. (2017) proposed that the divergence time of a lineage could be used as a universal criterion for ranking taxa and estimated the divergence time of Basidiomycota. Until now, divergence time has not been introduced into studies of Melanoleuca, moreover, its possible usage in taxonomic studies of the genus needs further verification.
Therefore, in the present study, we made use of universal sequences in fungi (ITS + nrLSU) to estimate the divergence time of Melanoleuca, and discussed the infrageneric classifications of 19 species from northern China based on morphological identifications and clarified their phylogenetic relationships in order to provide a theoretical basis for the study of Melanoleuca.
All samples were collected from 2011 to 2018 from northern China, and have been deposited in the Herbarium Mycology of Jilin Agricultural University (HMJAU) and Herbarium Mycology of Jinlin Agricultural Science and Technology University (HMJU). The specific details are shown in Table 1. Pictures of the habitats were taken by a Canon 80D camera. Macroscopic features were recorded using fresh collections. Color descriptions were based upon the classifications made by Kornerup and Wanscher (1978). Dried specimens were used for microscopic observations, using 5% KOH as the floating agent, Melzer’s reagent was used to examine the presence of amyloid or dextrinoid reactions. Slices of lamellae and pileipellis were observed under the Olympus BX 53 microscope. Free hand drawings were made from all microscopic observations. Shooting and measurements of anatomical features were presented in the Cellsens Standard. The data was recorded by (a) b–c × d–e (f), n was the number of examined basidiospores and Q (length: breadth ratios) was calculated from 30 mature basidiospores of 3 basidiocarps. Cystidial shapes were described as per Vizzini et al. (2011). In addition, basidiosopres were observed under the scanning electron microscope, using the following procedure: gills were attached to specimen holders by carbon tape, coated with platinum-palladium using a Hitachi MC 1000 Ion Sputter Coater and examined with a FEI Quanta 200 FE-SEM operated at 5–10 kV.
Genomic DNA was extracted from the dried specimens following the procedure described by Zhao et al. (2011). Polymerase chain reaction amplified sequences of the ITS and the nrLSU regions. Primers ITS1 and ITS4 (White et al., 1990) were used for the ITS region while primers LROR (Rehner and Samuels, 1994) and LR7 (Vilgalys and Hester, 1990) were used for the nrLSU region. The total volume of the PCR amplification reaction system was 50 μL containing of 10 μL of 5 × PCR buffer (Dingguo, Beijing, China), 4 μL of 200 μmol/L deoxynucleoside triphosphates, 1 μL of 200 μmol/L each primer, 5 U of Taq DNA polymerase and 10 μL of template DNA. The program parameters were set as follows, for ITS: initial denaturation at 94°C for 4 min; repeated for 30 cycles, denaturation at 94°C for 1 min, with annealing at 55°C for 1 min, extension at 72°C for 1 min, left at 72°C for 5 min and saved at 4°C; nrLSU: initial denaturation at 94°C for 4 min, repeated for 30 cycles, denaturation at 94°C for 90 s, with annealing at 55°C for 90 s, extension at 72°C for 90 s, left at 72°C for 5 min and saved at 4°C.
The products of PCR amplification were purified with the EasyPure Plasmid MiniPrep Kit (TransGen Biotech Co., Ltd., Beijing, China.), and resolved on a 1.0% agarose gel and subsequently submitted for sequencing (sequencing was completed by BGI Co., Ltd., Beijing, China).
A total of 110 sequences (ITS and nrLSU) representing 29 species were incorporated in the phylogenetic analyses, of which 26 sequences were retrieved from GenBank. Clitocybe subditopoda was used as the outgroup. Detailed specimen information appears on Table 1. All the sequences were aligned in Clustal X 2.1 (Lin et al., 2017). The conservative region was selected in Gblock2 and the vacancy gap in the data were treated as missing data (Talavera and Castresana, 2007). Saturation was tested using DAMBE 5.2 (Posada and Crandall, 1998) (model = test by Xia, 2013). MrModel Test 2.3 were used to select the fragment models (Wilgenbusch and Swofford, 2003; Nylander, 2004). The best model was used (ITS-nrLSU: GC) to construct a Maximum likelihood (ML) tree with PhyML (Guindon et al., 2009). The ML tree was evaluated by bootstrap analysis with 1000 replicates (Stamatakis, 2006). Bootstrap values greater than or equal to 60% were indicated along nodes.
Sequences incorporated in phylogenetic analyses were used to estimate the divergence time. All the sequences were aligned by MEGA v.7.03. Four species of Tricholomataceae were used as the outgroup. We used divergence times of Boletales (189 ± 20Ma), Archaeomarasmius leggetti (90 Ma), Quatsinoporises cranhanaii (125 Ma) and Mycena plumbea (90 ± 30 Ma) as calibration points (Hibbett et al., 1997; Smith et al., 2004; Feng, 2012). Divergence time was estimated in BEAST v.2.5.1. The best substitution model for each partition was inferred with the program MrModeltest 2.2 (Nylander, 2004): GTR + G + I for ITS and nrLSU. The number of substitution rate categories and Gamma shape parameters were 4.0362 and 4.0343, respectively. BEAUti v.2.5.14 was used to construct an XML file. The Relaxed clock model was selected according to the ESS value exceeding 200. Substitution models were independently estimated for each gene partition. The Yule speciation prior set was used to estimate the divergence time and the corresponding credibility intervals were constructed using treeModel. We ran an independent Monte Carlo Markov Chains (MCMC) of 10 million generations, logging states every 10,000 generations.
The checking for convergence and mixing of Log files were completed in Tracer v1.65. Tree files were summarized by the TreeAnnotator v.2.5.1., discarding 10% of states as burn-in and annotating clades with ≥0.8 posterior probability, and the maximum-clade-credibility tree (MCC) was generated. The resulting files were viewed using Figtree v.1.46.
Melanoleuca galerina YL and JX, sp. nov. (Figures 1, 2, 3A–C, 4A–C)
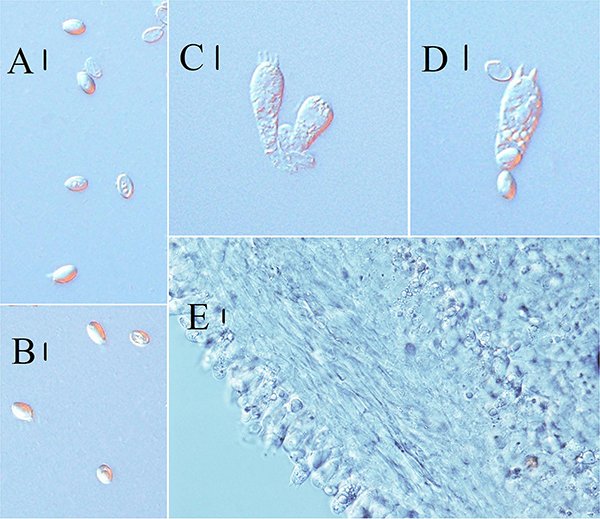
Figure 2. Microscopic characteristics of Melanoleuca galerina (Holotype, HMJAU48281). (A,B) Basidiospores; (C,D) Basidia; and (E) Trama (A,B = 5 μm; C–E = 10 μm).
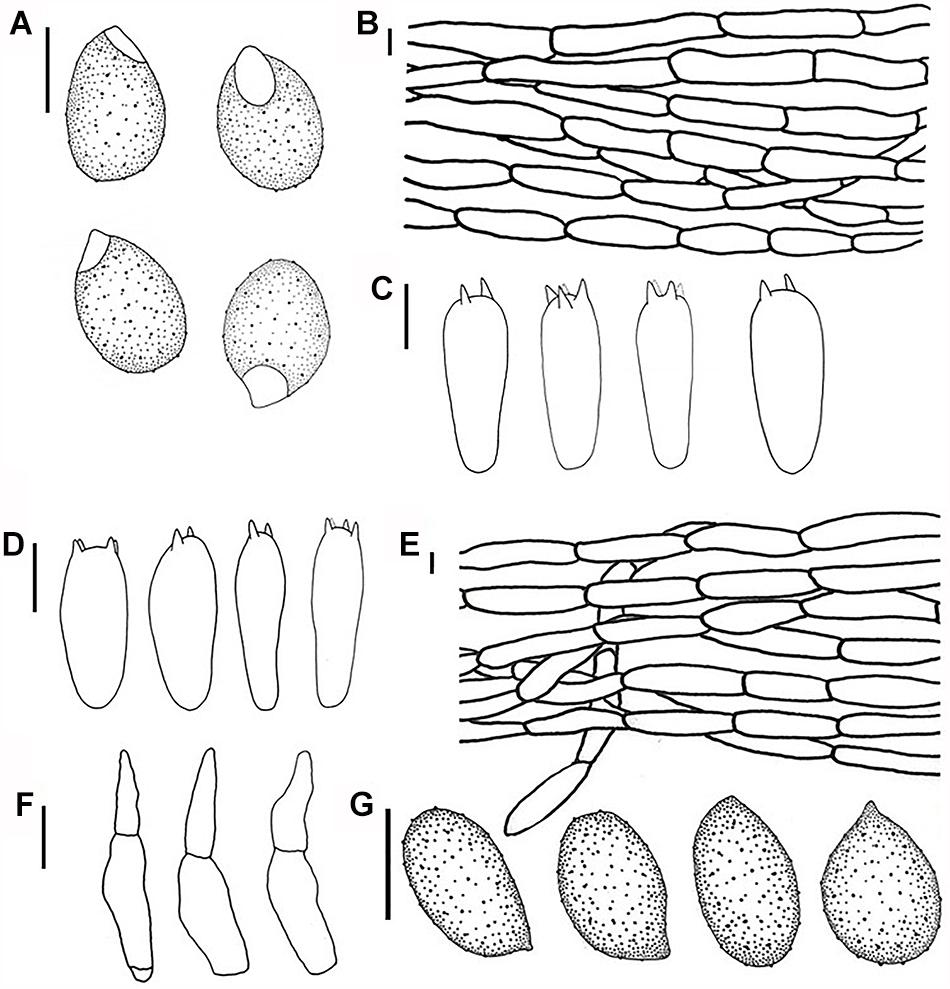
Figure 3. Line drawings: (A–C) Melanoleuca galerina (Holotype, HMJAU48281), (A) Basidiospores; (B) Pileipellis; (C) Basidia; (D–G) Melanoleuca subgrammopodia (Holotype, HMJAU48287), (D) Basidia; (E) Pileipellis; (F) Cheilocystidia; and (G) Basidiospores (A,G = 5 μm; B–E, F = 10 μm).
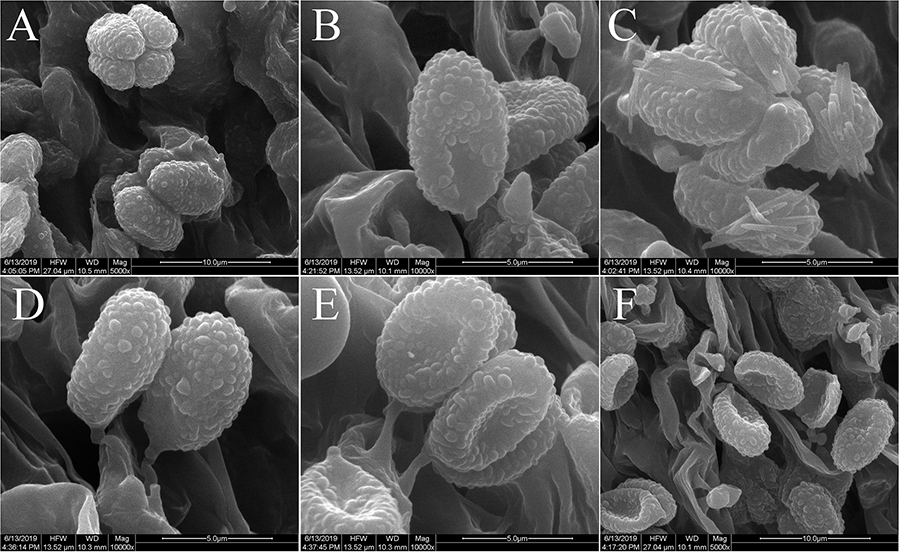
Figure 4. Scanning electron microscope images of basidiospores: (A–C) Melanoleuca galerina (Holotype, HMJAU48281); (D–F) M. subgrammopodia (Holotype, HMJAU48287).
MycoBank no.: MB 830397
Diagnosis: Pileus 8.5–9.8 cm, plane or slightly depressed at disc, pale when young, pale brown (5C4) to margin at the later stage, margin pale (1A2), surface hygrophanous, dehiscent in the center in the mature period. Lamellae white (1A2) when young, pale rose pink (4A2) in the mature period. Stipe 10–13 × 1.3–1.7 cm, longitudinally fibrous striate, slightly distorted, hollow. Cystidia absent. Clamp connections absent. Basidiospores (6.7) 7.1–8.4 × (3.5) 4.4–5.4 μm, amyloid.
Type: China: Jilin Province, Jilin City, Zuojia Town, 44°05′N, 126°05′E, 7 September 2016, Jize Xu, HMJAU48281 (Holotype).
Etymology: referring to the basidiospores, which are similar to Galerina.
Pileus 8.5–9.8 cm, plane or slightly depressed at disc, near round, light brown (5C4) in the center, slightly paling toward the margin, surface hygrophanous, smooth, not viscid when moist, margin pale (1A2), even, slightly cracked when matured. Lamellae 0.3–0.4 cm broad, decurrent, white (1A2) at first, pale rose pink (4A2) in the mature period, 19 lamellae/cm in the edge of the pileus. Stipe 10–13 × 1.3–1.7 cm, white (2A2) to creamy (29A2), pale brown (5C4) when moist, subcylindrical, longitudinally fibrous striate, slightly distorted, subequal, slightly attenuated in the middle, with poor pallid (1A1) tomentum toward the base, hollow when matured. Context 0.5 cm thick at pileus center, dirty white (28A2) to pale brown (5C4), with indistinct or slight fungoid smell and mild taste. Spore print white.
Basidiospores (6.7) 7.1–8.4 × (3.5) 4.4–5.4 μm, Q = (1.43) 1.51–1.89 (2.16), (n = 30), oblong-ellipsoid, hyaline, some with one oil drop, few with encrusted crystals on the surface, ornamented with round warts, warts up to 0.5 μm wide and 0.3–0.6 μm high, amyloid. Basidia 31–36 (39) × (7.4) 7.8–8.5 μm, clavate to subcylindrical, slightly broadened at apex, with two or four sterigmata, sterigmata up to 2.5–4.0 μm long. Cheilocystidia and pleurocystidia absent. Trama regular, hyphae 9.5–12 μm wide, cylindrical, thin-walled, hyaline. Pileipellis a cutis of radially parallel, thin-walled, dense hyphae, hyphae 10–14 μm wide, cylindrical, not or slightly constricted at the septa, hyaline. Clamp connections absent.
Habitat and distribution: Scattered on grass. Known from Jilin Province in China.
Additional specimens examined: China: Jilin Province: Jilin City, Zuojia Town, 44°05′N, 126°05′E, 7 September 2016, Jize Xu, HMJU00103.
Notes: Melanoleuca galerina is mainly characterized as having medium to large basidiomata, with a hygrophanous pileus, decurrent lamellae and a fibrous stipe which is hollow at maturity. It belongs to the subgenus Melanoleuca which has a key characteristic in that it lacks cystidia (Boekhout, 1988). In the subgenus Melanoleuca, M. galerina differs from M. ustaliformis Murrill, M. melaleuciformis Murrill, M. subfulvidisca Murrill and M. westiana Murrill in having a pale brown pileus and decurrent lamellae (Hesler, 2013b). Melanoleuca compressipes Murrill, M. albissima Murrill and M. watsonii Murrill are easily distinguished from M. galerina by their glabrous stipes (Murrill, 1940; Hesler, 2013b), apart from this, the spores in M. watsonii are much smaller than those of M. galerina (4–5 × 2.3–2.5 μm in M. watsonii, 7.2–8.3 × 4.3–4.9 μm in M. galerina). Melanoleuca compressipes differs from M. galerina in its sordid-white to dark-brown lamellae. In gross morphology, M. galerina is exceedingly similar to M. clelandii Grgur., but M. clelandii differs from M. galerina in having a larger basidiomata, with a pileus diameter up to 15.2 cm while the pileus diameter of M. galerina is approximately 9 cm (Grgurinovic, 1985).
Melanoleuca subgrammopodia YL and JX, sp. nov. (Figures 3D–G, 4D–F, 5, 6).
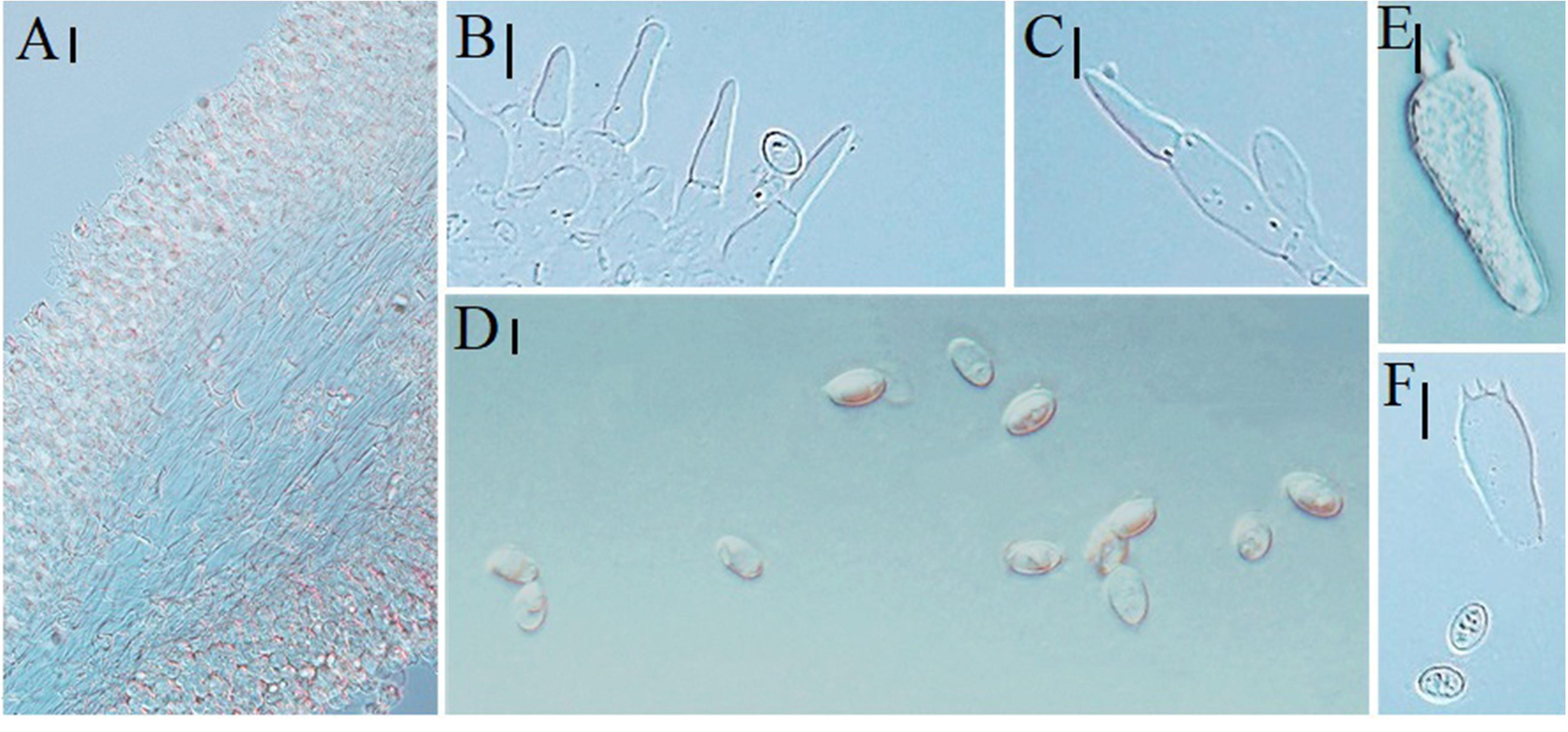
Figure 6. Microscopic characteristics of Melanoleuca subgrammopodia (Holotype, HMJAU48287). (A) Trama; (B,C) Cheilocystidia; (D) Basidiospores; and (E,F) Basidia (A–C = 10 μm; D = 5 μm; E,F = 10 μm).
MycoBank no.: MB830552
Diagnosis: Pileus 10–13 cm, depressed in the center, dark brown (9F6) at first, then becoming lighter, margin incurved when young, applanated after maturity. Lamellae 0.2–0.3 cm broad, white (1A2), separated from the edge of pileus after maturity. Stipe 6.5–7.5 × 0.7–0.9 cm near black (7F3) when young, light brown (6C6) when mature, longitudinally fibrous striate. Clamp connections absent. Cheilocystidia urticiform, of brevipes-type, septate, without crystals at the apex. Basidiospores (6.2) 7.2–8.3 (8.6) × 4.3–4.9 (5.3) μm, amyloid.
Type: China: Jilin Province, Jiaohe City, Hongyegu, 43°40′N, 127°04′E, 6 September 2018, Jize Xu, Jiajun Hu, HMJAU48287 (Holotype).
Etymology: refers to the longitudinally fibrous striate stipe.
Pileus 10–13 cm, depressed in the center, shallow funnel, dark brown (9F6) at first, then becoming lighter, pale brown (5B3) in the mature period, surface dry, smooth, not viscid when moist, margin even, slightly cracked, incurved when young, applanated after maturity. Lamellae 0.2–0.3 cm broad, adnate to decurrent, white (1A2), 9–11 lamellae/cm in the edge of the pileus, with intercalated lamellulae, separated from the edge of pileus after maturity. Stipe 6.5–7.5 × 0.7–0.9 cm, near black (7F3) when young, light brown (6C6) in the mature period, broadened at base, longitudinally fibrous striate. Context 0.2 cm thick at pileus center, dirty white (6B1), smell and taste indistinct. Spore print white.
Basidiospores (6.2) 7.2–8.3 (8.6) × 4.3–4.9 (5.3) μm, Q = (1.26) 1.46–1.76 (1.81), (n = 30), elliptical to subovoid, most with one oil drop, hyaline, ornamented with warts, warts round, up to 0.3 μm wide and 0.2–0.6 μm high, amyloid. Basidia 39–43 × 9.2–10.3 μm, clavate, slightly broadened at apex, with two or four sterigmata, sterigmata up to 1.5–4 μm long. Cheilocystidia urticiform, 38–50 × 6–10 μm, of brevipes-type, attenuated toward the apex, septate, without crystals at apex, hyaline. Pleurocystidia rare, similar to cheilocystidia. Trama regular, hyphae 11–13 μm cylindrical, thin-walled, hyaline. Pileipellis a cutis of radially parallel, thin-walled, dense hyphae, hyphae 11–14 μm wide, cylindrical, not or slightly constricted at the septa, hyaline. Clamp connections absent.
Habitat and distribution: Scattered on grass. Known from Jilin Province in China.
Additional specimens examined: China: Jilin Province, Jiaohe City, Hongyegu, 43°40′N, 127°04′E, 6 September 2018, Jize Xu, Jiajun Hu, HMJU00104.
Notes: The main characteristics of M. subgrammopodia are its larger basidiocarps, white lamellae, short stipe and urticiform cystidia. Melanoleuca subgrammopodia is related to members of the section Grammpodiae in the subgenus Urticocystis (Boekhout, 1988). Melanoleuca floridana Murrill is distinct from M. subgrammopodia in having a subtomentose pileus and stipe which are basal part clavate (Hesler, 2013a). Melanoleuca juliannae Rimóczi, Antonín, L. Nagy and Tomšovský is characterized by a violaceous-blue stipe and two types of cheilocystidia (the exscissa-type is most common while the brevipes-type is less frequent) (Vladimír et al., 2014). However, there is only one type of cheilocystidia in M. subgrammopodia (brevipes-type). Melanoleuca subgrammopodia is also close to M. subacris Murrill and M. subcylindrispora Murrill, M. subacris is distinct from M. subgrammopodia in having a uniform white pileus. The main characteristic of M. subcylindrispora are its subcylindrical spores, but the spores of M. subgrammopodia are elliptical to subovoid (Hesler, 2013b).
Melanoleuca griseobrunnea Antonín, Ďuriška and Tomšovský, M. pseudopaedida Bon, M. angelesiana A. H. Smith, M. microcephala (P. Karst.) Singer, M. communis Sánchez-García and J. Cifuentes, M. cinereifolia (Bon) Bon, M. nivea Métrod ex Boekhout are first recorded in China.
Melanoleuca angelesiana A. H. Smith, Mycologia 36: 252. 1944 (Figures 7A, 8G–I, 9G–I, 10C,D).

Figure 7. Basidiomata: (A) Melanoleuca angelesiana (HMJU00120); (B) M. cinereifolia (HMJU00124); (C) M. griseobrunnea (HMJU00134); (D) M. pseudopaedida (HMJU00155); (E,F) M. nivea (HMJU00148); (G,H) M. communis (HMJU00139); and (I) M. microcephala (HMJU00132).
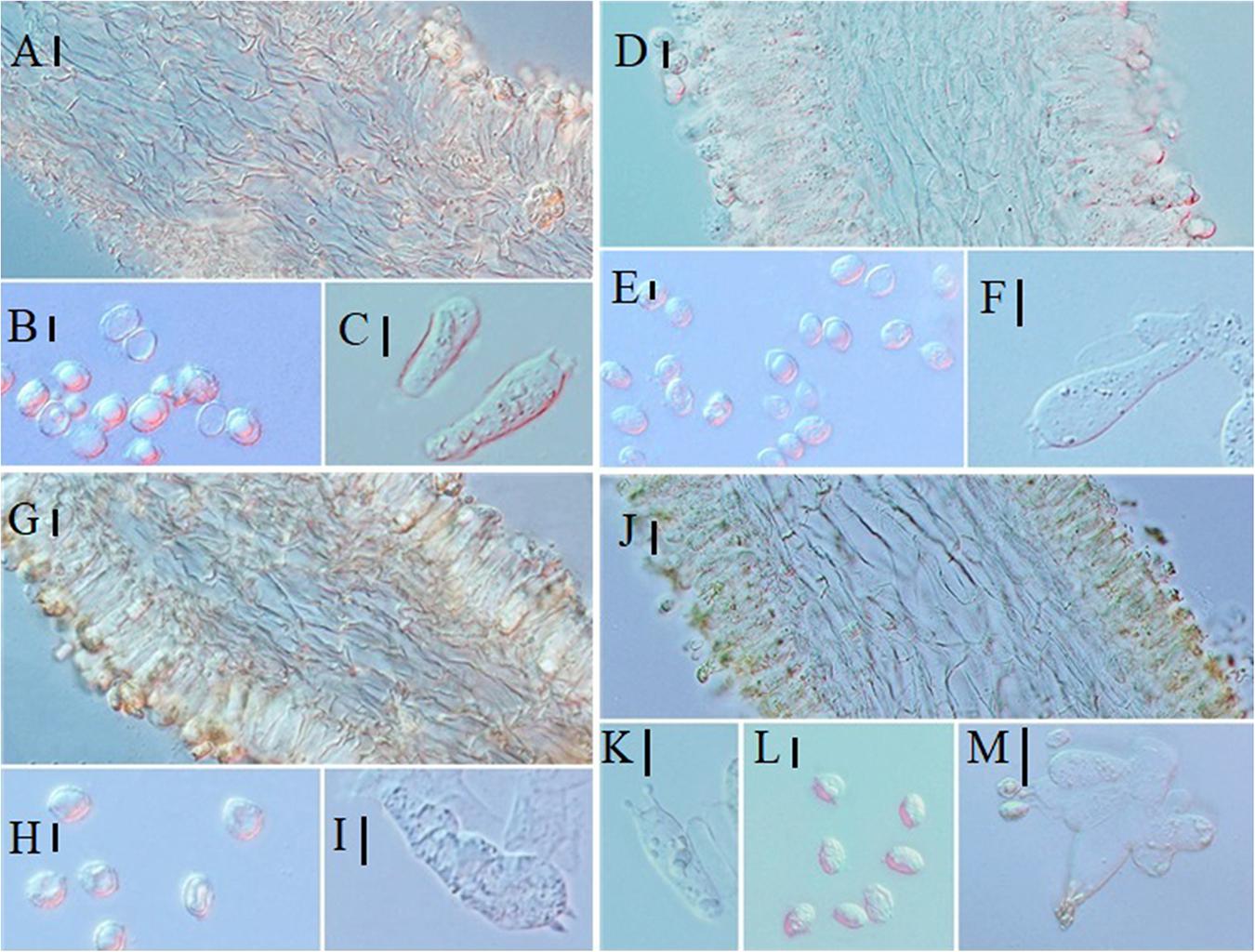
Figure 8. Microscopic characteristics: (A–C) Melanoleuca pseudopaedida (HMJU00155), (A) Trama; (B) Basidiospores; (C) Basidia; (D–F) M. microcephala (HMJU00132), (D) Trama; (E) Basidiospores; (F) Basidia; (G–I) M. angelesiana (HMJU00120), (G) Trama; (H) Basidiospores; (I) Basidia; (J–M) M. cinereifolia (HMJU00124), (J) Trama; (K) Basidia; (L) Basidiospores; (M) Cheilocystidia (A,C,D,F,G,I,M = 10 μm; B,E,H,J,K,L = 5 μm).
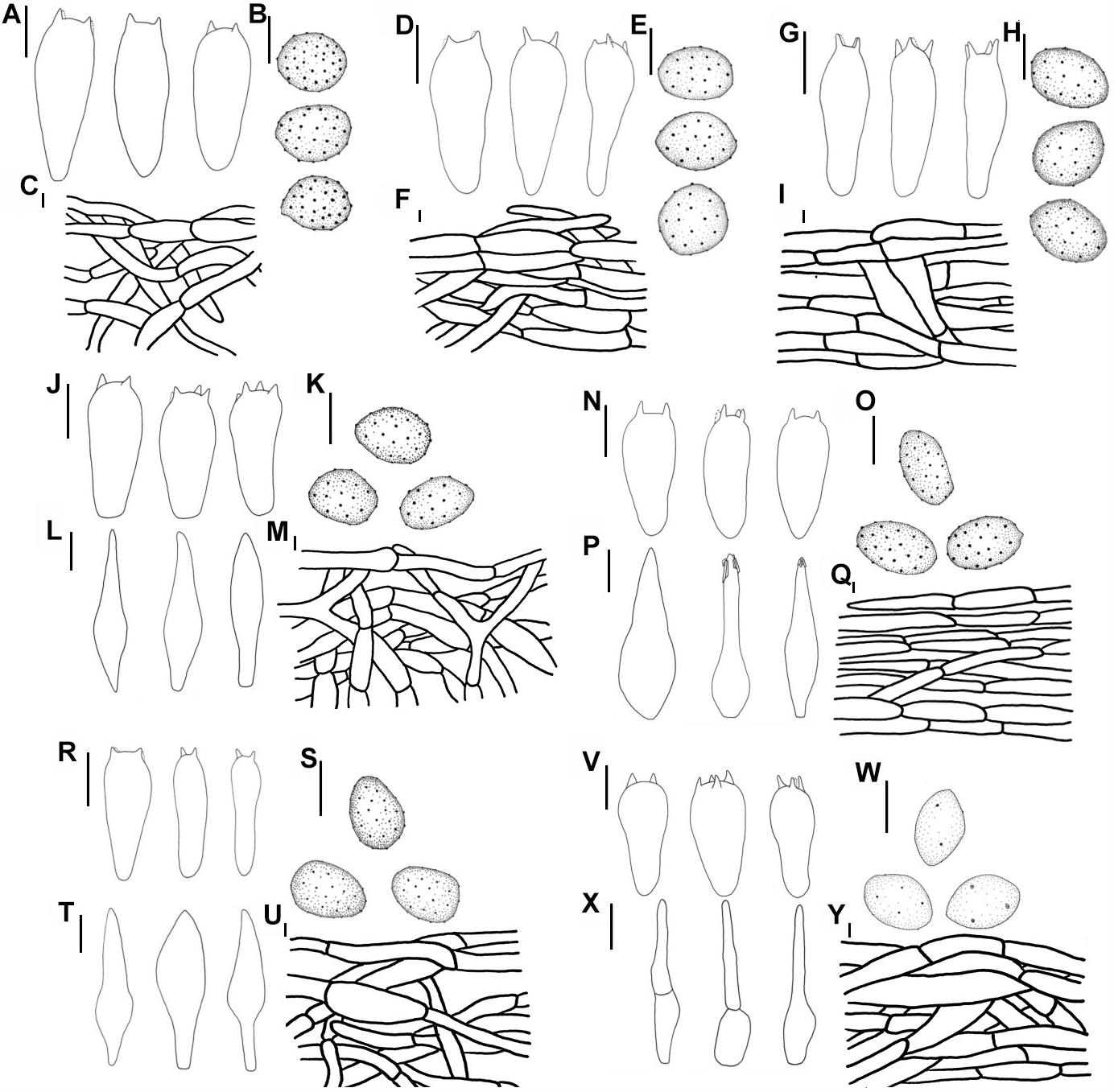
Figure 9. Line drawings: (A–C) M. pseudopaedida (HMJU00155), (A) Basidia; (B) Basidiospores; (C) Pileipellis; (D–F) M. microcephala (HMJU00132), (D) Basidia; (E) Basidiospores; (F) Pileipellis; (G–I) M. angelesiana (HMJU00120), (G) Basidia; (H) Basidiospores; (I) Pileipellis; (J–M) M. communis (HMJU00139), (J) Basidia; (K) Basidiospores; (L) Cheilocystidia; (M) Pileipellis; (N–Q) M. nivea (HMJU00148), (N) Basidia; (O) Basidiospores; (P) Cheilocystidia; (Q) Pileipellis; (R–U) M. cinereifolia (HMJU00124), (R) Basidia; (S) Basidiospores; (T) Cheilocystidia; (U) Pileipellis; (V–Y) M. griseobrunnea (HMJU00134), (V) Basidia; (W) Basidiospores; (X) Cheilocystidia; (Y) Pileipellis (A,D,G,J,L,N,P,R,T,V,X = 10 μm; B,C,E,F,H,I,K,M,O,Q,S,U,W,Y = 5 μm).
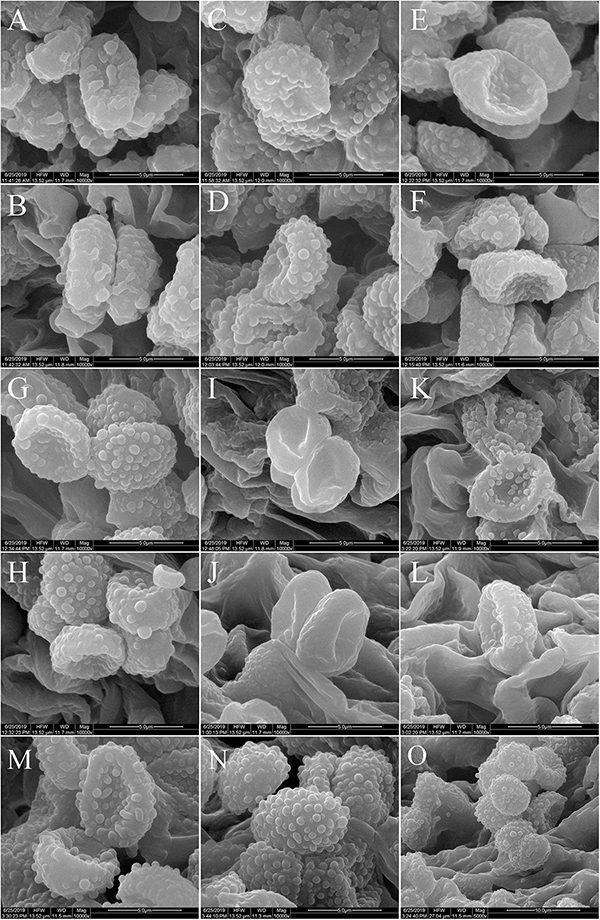
Figure 10. Scanning electron microscope images of basidiospores: (A,B) M. cinereifolia (HMJU00124); (C,D) M. angelesiana (HMJU00120); (E,F) M. communis (HMJU00139); (G,H) M. microcephala (HMJU00132); (I,J) M. griseobrunnea (HMJU00134); (K,L) M. nivea (HMJU00148); (M–O) M. pseudopaedida (HMJU00155).
Pileus 5–7 cm diameter, depressed at disc, dull olive brown, surface glabrous, dry, smooth, subviscid when moist, margin incurved, becoming plane or uplifted, paler grayish brown. Lamellae adnate, pale gray to pallid or white, moderately broad, narrowed toward the margin, 8–9 lamellae/cm in the edge of the pileus, with intercalated lamellulae, the edges even and staining brownish where bruised. Stipe 5–6 × 1–1.2 cm, surface concolorous with the pileus or paler, slightly flared at the base, longitudinally fibrous striate, glabrous, hollow when matured. Context 2–3 mm thick at pileus center, watery gray when moist, pallid when faded, odor none, taste mild. Spore print white.
Basidiospores 6.5–7.7 (7.9) × 4.5–5.5 (6.1) μm, Q = (1.27) 1.34–1.70 (1.79), (n = 30), elliptical, covered with strongly amyloid minute warts. Basidia 34–42 × 9.1–12 μm, clavate, with four sterigmata. Pleurocystidia and cheilocystidia absent. Trama interwoven, inamyloid. Pileipellis a cutis composed of interwoven to radial, thin-walled, homogeneous, dense hyphae, hyphae 7–13 μm wide, cylindrical, not or slightly constricted at the septa, hyaline.
Specimens examined: China: Heilongjiang Province, Yichun City, 12 September 2017, Jize Xu, HMJU00120; same location, 11 September 2017, Jize Xu, HMJU00114.
Habitat and distribution: Solitary, on ground of coniferous mixed forest. Known from China and America.
Notes: M. angelesiana is placed in clade A and belonging to subgenus Urticocystis. The Chinese collection shares very similar morphological features and DNA sequence (ITS and nrLSU). Differing from holotype (Smith, 1944), the material from Heilongjiang produces a dry pileus which is not umbonate.
Melanoleuca communis Sánchez-García & J. Cifuentes, Revista Mexicana de Micologia Suplemento-Micologia 116. 2013 (Figures 7G,H, 9J–M, 10E,F, 11A–D).
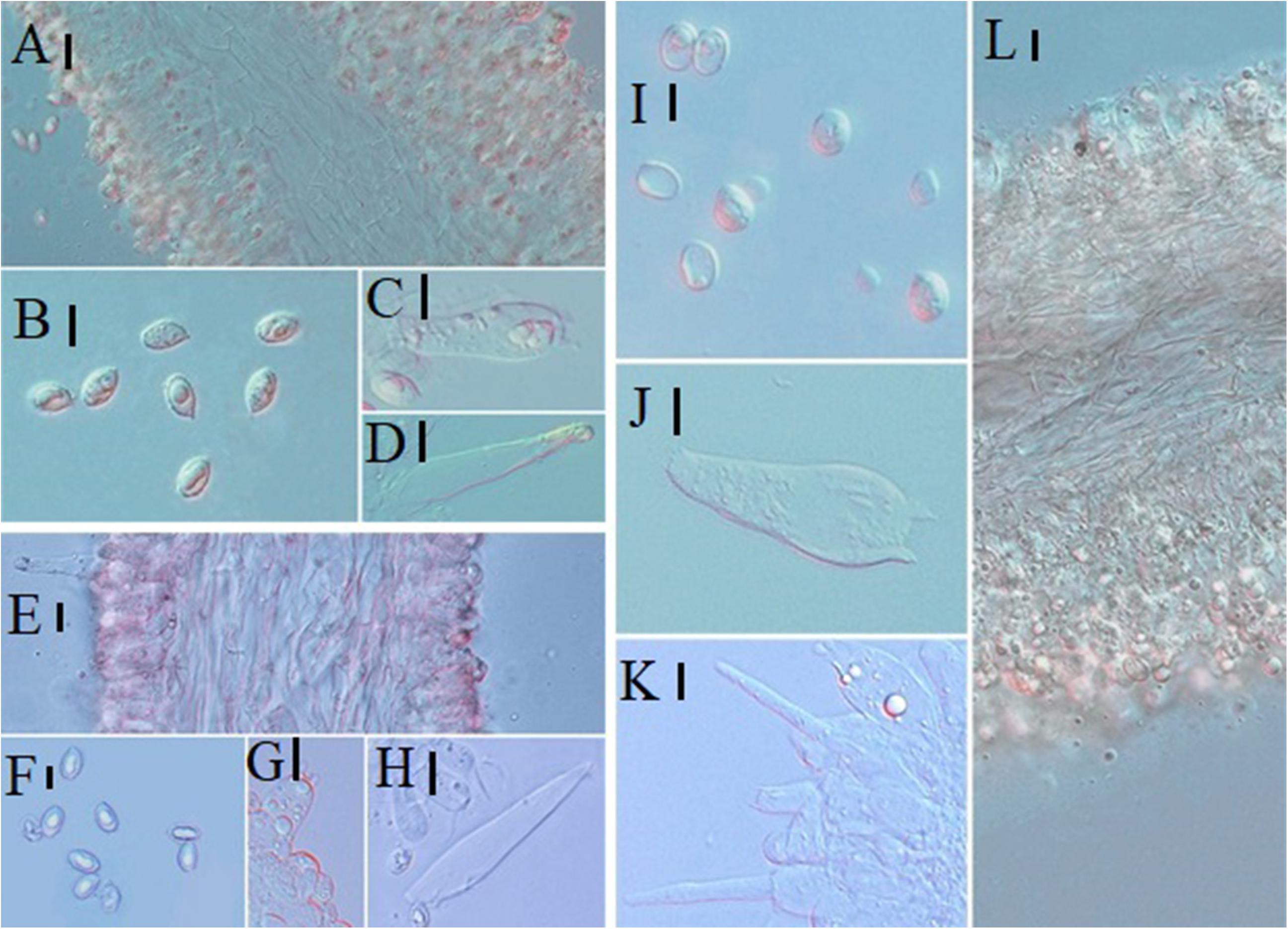
Figure 11. Microscopic characteristics: (A–D) Melanoleuca communis (HMJU00139), (A) Trama; (B) Basidiospores; (C) Basidia; (D) Cheilocystidia; (E–H) M. nivea (HMJU00148), (E) Trama; (F) Basidiospores; (G) Basidia; (H) Cheilocystidia; (I–L) M. griseobrunnea (HMJU00134), (I) Basidiospores; (J) Basidia; (K) Cheilocystidia; (L) Trama (A,C,D,E,G,H,J,K,L = 10 μm; B,F,I = 5 μm).
Pileus 2.8–16 cm diameter, plane to plane-convex, sometimes umbonate, brown slightly paler or yellowish-brown toward the margin, surface moist, not viscid, smooth, margin slightly incurved. Lamellae sinuate or adnate, white to yellowish, 6–8 lamellae/cm in the edge of the pileus, with intercalated lamellulae, edges jagged. Stipe 4–17 × 0.4–1.2 (1.8) cm, white to pale yellow, cylindrical to slightly attenuated toward the base, with yellowish longitudinal stripes, fleshy-fibrous, slightly whitish pruinose, solid. Context 1–2 mm thick at pileus center, white, sometimes brown-beige or grayish, smell sweetish, pleasant, sometimes absent, taste farinaceous, bitter, sometimes absent. Spore print white.
Basidiospores (6.5) 6.7–8.9 (9.4) × 4.1 (4.9)–5.6 μm, Q = 1.41–1.64 (1.68), (n = 30), elliptical to oblong, ornamented with amyloid, isolated warts, hyaline. Basidia (18) 21–31 (39) × (5) 8–10 μm, clavate, with four sterigmata. Cheilocystidia 51–67 × 11–16 μm, fusiform, sometimes lageniform usually with crystals at the apices. Pleurocystidia similar to cheilocystidia. Trama regular, inamyloid. Pileipellis a cutis constituted of interwoven, thin-walled, dense hyphae, hyphae 6–18 μm wide, cylindrical, not or slightly constricted at the septa, hyaline.
Specimens examined: China: Jilin Province, Jilin City, Zuojia Town, 5 September 2016, Jize Xu, HMJU00117; same location, 27 July 2016, Jize Xu, HMJU00146; same location, 27 July 2017, Jize Xu, HMJU00143; Liaoning Province, Fuxin City, Fumeng Town, Haitang Mountain, 6 August 2016, Jize Xu, HMJU00139, HMJU00144.
Habitat and distribution: Scattered on grass. Known from China and Mexico.
Notes: M. communis is included in clade B and belonging to subgenus Melanoleuca. Differing from holotype (Sánchez-García et al., 2013), the edges of lamellae for Chinese collections are jagged, and the stipes are slightly whitish pruinose.
Melanoleuca cinereifolia (Bon) Bon, Documents Mycologiques 9: 71. 1978 (Figures 7B, 8J–M, 9R–U, 10A,B).
Pileus 3.5–5.0 cm diameter, plane-convex to plane, cafe-gray, surface smooth, moist not viscid, margin straight. Lamellae sinuate, adnate, white, 12–13 lamellae/cm in the edge of the pileus, with intercalated lamellulae. Stipe 4 × 0.3 cm, surface concolorous with the pileus, cylindrical, longitudinally striate, fleshy-fibrous, pruinose or finely floccose at base, solid. Context 1.5–2.5 mm thick at pileus center, cafe-gray to chocolate color, odor none, taste absent. Spore print white.
Basidiospores 7.2–8.4 × 4.3–5.2 μm, Q = 1.42–2.00, (n = 30), elliptical to oblong, ornamented with warts, hyaline, amyloid; Basidia (20) 23–31 (33) × 8 (10) μm, clavate, with four sterigmata; Cheilocystidia (41) 49–59 (64) × (8) 10–12 (13) μm, fusiform to lageniform, with crystals at the apices. Pleurocystidia similar to cheilocystidia. Trama regular, inamyloid. Pileipellis tomentum, a cutis composed of thin-walled, dense hyphae, hyphae 6–18 μm wide, cylindrical, not or slightly constricted at the septa, hyaline.
Specimens examined: China: Jilin Province, Jilin City, Zuojia Town, 7 September 2016, Jize Xu, HMJU00124.
Habitat and distribution: Solitary, on grass of forest. Known from China and Mexico.
Notes: M. cinereifolia is in clade B and belonging to subgenus Melanoleuca. Differing from interpretations of Sánchez-García et al. (2013), the material from Jilin produces a solid stipe which is pruinose or finely floccose at base.
Melanoleuca griseobrunnea Antonín, Ďuriška & Tomšovský, Plant Systematics and Evolution 303: 1195. 2017 (Figures 7C, 9V–Y, 10I,J, 11I–L).
Pileus 2.0–3.5 cm diameter, applanate with or without a shallow central depression and with small central umbo, dark gray-brown, sometimes paler toward margin, surface smooth, glabrous to finely tomentose, not viscid when moist, slightly pruinose at margin, margin slightly incurved. Lamellae adnexed to slightly decurrent with tooth, pale dirty cream, 16–19 lamellae/cm in the edge of the pileus, with intercalated lamellulae. Stipe 2.5–4.5 × 0.3–0.5 cm, gray brown, apex slightly paler, cylindrical, slightly clavate at base, pruinose or finely floccose at apex. Context 0.7–0.9 mm thick at pileus center, whitish to grayish brownish, smell fungoid, taste mild with unpleasant aftertaste. Spore print whitish.
Basidiospores (5.7) 6.3–7.6 × (3.9) 4.1–5.4 μm, Q = 1.40–1.51, (n = 30), elliptical, ovoid, with verruculose ornamentation, amyloid. Basidia 18–42 × 6.0–12 μm, clavate, subcylindrical or subfusoid, with four sterigmata. Cheilocystidia 25–46 × 6.0–10.5 μm, urticiform of both the exscissa- and brevipes-type. Pleurocystidia similar to cheilocystidia. Trama regular, inamyloid. Pileipellis a cutis constituted of subradially arranged, thin-walled hyphae, hyphae 5–13 μm wide, cylindrical, hyaline.
Specimens examined: China: Ningxia Hui Autonomous Region, Yinchuan City, Suyukou National Forest Park, 13 August 2018, Jize Xu, HMJU00134.
Habitat and distribution: Scattered on sandy soil. Known from China and Korea.
Notes: M. griseobrunnea is placed in clade A and belonging to subgenus Urticocystis. The species is related to M. porphyropoda, but M. griseobrunnea is distinguished by having urticiform cystidia (Yu et al., 2014). Differing from holotype (Antonín et al., 2017), the edges of lamellae for Chinese collections are jagged, and the stipes are slightly whitish pruinose.
Melanoleuca microcephala (P. Karst.) Singer, Cavanillesia 7: 123. 1935 (Figures 7I, 8D–F, 9D–F, 10G,H).
Pileus 1.8–2.5 cm diameter, plane or slightly depressed at disc, round or nearly circular, pale brown, sometimes lighter toward margin, surface dry, smooth, margin pale, incurved, slightly cracked. Lamellae adnate to slightly decurrent, white, 20–25 lamellae/cm in the edge of the pileus, with intercalated lamellulae. Stipe 5.3–6.3 × 0.3–0.5 cm, white at apex, became darker toward the base, light gray brown at base, cylindrical, slightly expanded at base. Context 0.5–0.7 mm thick at pileus center, milky white, odor none, taste mild. Spore print white.
Basidiospores (6.8) 7.2–8.3 × (4.8) 5.0–5.9 μm, Q = 1.37–1.41, (n = 30), elliptical, smooth or with verruculose ornamentation, amyloid. Basidia 35–40 × 6.8–8.0 μm, clavate, with four sterigmata. Cheilocystidia and pleurocystidia absent. Trama regular, inamyloid. Pileipellis a cutis consisted of intertwined, thin-walled, dense hyphae, hyphae 6–18 μm wide, cylindrical, not or slightly constricted at the septa, hyaline.
Specimens examined: China: Gansu Province, Zhangye City, Minle Town, 9 August 2018, Jize Xu, HMJU00133; Tianshui City, 8 August 2018 Jize Xu, HMJU00132, HMJU00138.
Habitat and distribution: Scattered on grass. Known from China and Italy.
Notes: M. microcephala is included in clade A and belonging to subgenus Urticocystis. Differing from interpretations of Fontenla et al. (2013), the stipe of the material from Gansu is slightly longer, and the color of stipe is darker.
Melanoleuca nivea Métrod ex Boekhout, Persoonia 13: 417. 1988 (Figures 7E,F, 9N–Q, 10K,L, 11E–H).
Pileus 3.0–5.0 cm diameter, convex to plane, mostly with low broad umbo, white to pale gray-brown, sometimes with some ochraceous spots, surface smooth, glabrous, slightly viscid when moist, margin somewhat inflexed and slightly exceeding. Lamellae emarginate to adnate, ventricose, thin, up to 8 mm wide, whitish or pale cream, 13–15 lamellae/cm in the edge of the pileus, with intercalated lamellulae, entire to minutely flocculose edge. Stipe 3–5.5 × 0.4–0.8 cm, whitish to pale grayish beige, finally becoming grayish and brownish toward base, cylindrical, somewhat broadening toward base, longitudinally striate, whitish pruinose, glabrous in lower parts when matured. Context 0.4 mm thick at pileus center, whitish, brown to orange-brown, smell faint, somewhat rancid, taste weak, unpleasant. Spore print yellowish white.
Basidiospores 6.5–8.3 (9.1) × 4.1–5.0 μm, Q = 1.52–1.98, (n = 30), elongate, moderately densely ornamented with rather coarse amyloid warts. Basidia 23–33 × 7–9 pm, clavate, with four sterigmata. Cheilocystidia (35) 40–65 × 9–15 (20) μm, fusiform, partly tending to lageniform, mostly with the apex acute and encrusted by crystals. Pleurocystidia similar to cheilocystidia. Trama regular, inamyloid. Pileipellis an ixocutis composed of radially arranged, thin-walled hyphae, hyphae 5–8 μm wide, cylindrical, hyaline.
Specimens examined: China: Liaoning Province, Huludao City, Bailang Mountain, 28 July 2016, Jize Xu, HMJU00148.
Habitat and distribution: Single on the ground of forest. Known from China and Netherlands.
Notes: M. nivea is placed in clade B and belonging to subgenus Melanoleuca. Differing from holotype (Boekhout, 1988), the Chinese collections produce a white to pale gray-brown pileus and darker colored stipe.
Melanoleuca pseudopaedida Bon, Docums Mycol. 20: 58. 1990 (Figures 7D, 8A–C, 9A–C, 10M–O).
Pileus 2.0–3.6 cm diameter, plane to depressed at disc, brown to fuliginous, becoming pale, some with white spots, surface smooth, glabrous, slightly viscid when moist, margin slightly incurved, slightly cracked. Lamellae adnate to emarginate, pale gray-brown, 16–18 lamellae/cm in the edge of the pileus, with intercalated lamellulae, edges entire, whitish pruinose. Stipe 2.5–4.5 × 0.3–0.5 cm, pale at apex, brown at center, cylindrical, longitudinally striate, hollow when matured. Context 0.5–0.7 mm thick at pileus center, dirty beige, odor none, taste absent. Spore print white.
Basidiospores (6.7) 7.0–8.4 (8.7) × 5.0–6.0 μm, Q = (1.26) 1.30–1.58, (n = 30), elliptical to subovoid, densely ornamented with amyloid warts. Basidia 24–30 × 6.8–8.0 μm, clavate, with two or four sterigmata. Cheilocystidia and pleurocystidia absent. Trama regular, inamyloid. Pileipellis tomentum, a cutis composed of thin-walled hyphae, hyphae 6–14 μm wide, cylindrical, not or slightly constricted at the septa, hyaline.
Specimens examined: China: Qinghai Province, Haixi State, Wulan Town, 7 August 2018, Jize Xu, HMJU00155.
Habitat and distribution: Solitary, on grass. Known from China and France.
Notes: M. pseudopaedida is included in clade A and belonging to subgenus Urticocystis. Differing from holotype (Bon, 1990), the lamellae of the Chinese collections are whitish pruinose and the color is paler.
For the ITS-nrLSU ML analysis, 110 sequences relating to 29 species were added. The ML tree represented as Figure 12 shows detailed results with high bootstrapping values. Phylogenetic analysis produced two Clades: A and B. Clade A was formed in I node, with the bootstrapping value of 81%, while Clade B was formed in II node, having a bootstrapping value of 100%. Melanoleuca galerina and M. subgrammopodia were independently separated in III node, with a bootstrapping value of 87%. Both M. galerina and M. subgrammopodia were included in Clade A.
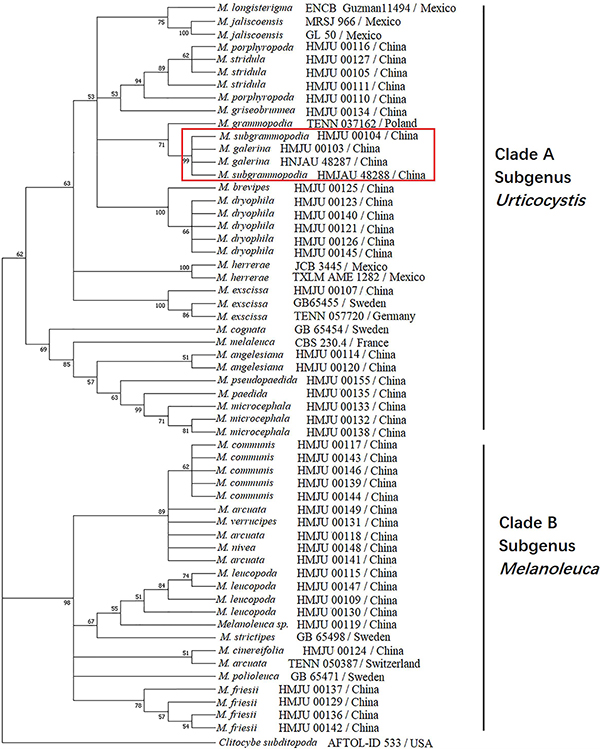
Figure 12. Maximum likelihood tree based on analyses of the ITS and nrLSU sequence data with Clitocybe subditopoda as the outgroup.
A combination of ITS and nrLSU sequences were used to estimate the divergence time of Melanoleuca. The MCC tree represented in Figure 13 shows two diverged clades in Melanoleuca since 33.31 million years ago. Clade A contains 18 independent species: M. griseobrunnea, M. pseudopaedida, M. microcephala, M. galerina, M. subgrammopodia, M. angelesiana, M. porphyropoda X. D. Yu, M. stridula (Fr.) Singer, M. dryophila Murrill, M. brevipes (Bull.) Pat., M. exscissa (Fr.) Singer, M. paedida (Fr.) Kühner & Maire, M. grammopodia (Bull.) Murrill, M. herrerae Sánchez-García & J. Cifuentes, M. longisterigma Sánchez-García & J. Cifuentes, M. jaliscoensis Sánchez-García, J. Cifuentes & Guzm.-Dáv., M. cognata (Fr.) Konrad & Maubl. and M. melaleuca (Pers.) Murrill. Clade B contains 9 species: M. cinereifolia, M. communis, M. nivea, M. leucopoda X. D. Yu, M. strictipes (P. Karst.) Jul. Schäff., M. polioleuca (Fr.) Kühner & Maire, M. friesii (Bres.) Bon, M. arcuata (Bull.) Singer and M. verrucipes (Fr.) Singer. Furthermore, M. galerina and M. subgrammopodia diverged 11.48 million years ago.
In this study, two new species from northeastern China were described in detail. Based on Boekhout (1988) M. galerina belongs to the subgenus Melanoleuca and M. subgrammopodia belongs to the section Grammpodiae in the subgenus Urticocystis. However, using phylogenetic analyses, the species in our investigations are divided into two Clades. The species in clade A are with urticiform cystidia or without cystidia, and in clade B have macrocystidia. This result was corroborated by Vizzini et al. (2011) whereby the authors divided the genus Melanoleuca into two subgenera: the subgenus Urticocystis mainly included the species with urticiform cystidia or without cystidia, while the subgenus Melanoleuca mainly included the species with macrocystidiate. Therefore, in this study, our phylogenetic analyses of Melanoleuca species using combined ITS and nrLSU sequences suggests that M. galerina and M. subgrammopodia are new species belonging to the subgenus Urticocystis.
In our investigations, the results of divergence time were in line with phylogenetic analysis, and supports that M. galerina and M. subgrammopodia are new species. In addition, results also indicate that divergence time of a lineage could be used as a criterion for ranking taxa (Zhao et al., 2016; Chen et al., 2017). But, the selection of proper calibration points provided by fossils is an important aspect. Moreover, reliable calibration points can provide evidence for divergence time (Feng, 2012).
The datasets generated can be found in NCBI, accession numbers can be found in Table 1.
JX wrote the manuscript. JX, XY, ML, and CZ carried out the experiments. JX, CZ, and JH collected the specimens. LG and YL designed the experiments. OM had a contribution in revising the manuscript and taking SEM photographs.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Antonín, V., Ďuriška, O., Gafforov, Y., Jančovičová, S., Para, R., and Tomšovský, M. (2017). Molecular phylogenetics and taxonomy in Melanoleuca exscissa group, (Tricholomataceae, Basidiomycota) and the description of M. griseobrunnea sp. nov. Plant Syst. Evol. 303, 1181–1198. doi: 10.1007/s00606-017-1430-y
Bau, T., and Li, Y. (1999). Species diversity of macrofungi studies in Daqinggou nature reserve. J. Jilin Agric. Univ. 3, 36–45.
Berbee, M. L., and Taylor, J. W. (2010). Dating the molecular clock in fungi – how close are we? Fungal Biol. Rev. 24, 1–16. doi: 10.1016/j.fbr.2010.03.001
Boekhout, T. (1988). Notulae ad floram agaricinam neerlandicam, XVI-New taxa, new combinations in Melanoleuca Pat. and notes on rare species in the Netherlands. Persoonia 13, 397–431.
Chen, J., Callac, P., Parra, L. A., Karunarathna, S. C., He, M. Q., Moinard, M., et al. (2017). Study in Agaricus subgenus minores and allied clades reveals a new American subgenus and contrasting phylogenetic patterns in Europe and Greater Mekong Subregion. Persoonia 38, 170–196. doi: 10.3767/003158517X695521
Chen, Y. Q. (2007). Studies on the Macrofungal Diversity in Laoyeling Mountain of Jilin. Master’s thesis, Jilin Agricultural University, Jilin.
Du, M. H., Gao, L. X., Li, J. Z., Chai, C. Y., Jia, S., and Wang, S. M. (2016). Preliminary investigation of diversity of macrofungi in Henan Funiu mountain nature reserve. Edible and Medicinal Mushrooms 24, 306–311.
Feng, B. (2012). Molecular Phylogeny and Biogeography of Boletus L. s.s. and Hydnum L. dissertation, Kunming Institute of Botany, Kunming.
Floudas, D., Binder, M., Riley, R., Barry, K., Blanchette, R. A., Henrissat, B., et al. (2012). The Paleozoic origin of enzymatic lignin decomposition reconstructed from 31 fungal genomes. Science 336, 1715–1719. doi: 10.1126/science.1221748
Fontenla, R., Lavorato, C., and Para, R. (2013). Observations on the genus Melanoleuca. On some taxa erected by Karsten. Micol. Veg. Mediterr. 28, 117–126.
Grgurinovic, C. A. (1985). Studies on J. B. Cleland’s fungal herbarium-1: Melanoleuca (Agaricales). Mycotaxon 23, 223–232.
Guindon, S., Delsuc, F., Dufayard, J. F., and Gascuel, O. (2009). Estimating maximum likelihood phylogenies with PhyML. Methods Mol. Biol. 537, 113–137. doi: 10.1007/978-1-59745-251-9_6
He, L. P., Ma, C. S., Zhang, S. Y., Wang, J. H., Gu, Z. D., and Jin, Q. Y. (2014). Macrofungi resources in Chagangliang Provincial Nature Reserve of Gansu. J. Gansu For. Sci. Technol. 4, 46–53.
Hibbett, D. S. (2014). Major Events in the Evolution of the Fungi. Princeton, NJ: Princeton University Press.
Hibbett, D. S., Grimaldi, D., and Donoghue, M. J. (1997). Fossil mushrooms from miocene and cretaceous ambers and the evolution of homobasidiomycetes. Am. J. Bot. 84:981. doi: 10.2307/2446289
Hyde, K. D., Udayanga, D., Manamgoda, D. S., Tedersoo, L., Larsson, E., Abarenkov, K., et al. (2013). Incorporating molecular data in fungal systematics: a guide for aspiring researchers. Curr. Res. Environ. Appl. Mycol. 3:132. doi: 10.5943/cream/3/1/1
Kalmer, A., Acar, I., and Tekpinar, A. D. (2018). Phylogeny of some Melanoleuca species (Fungi: Basidiomycota) in Turkey and identification of Melanoleuca angelesiana A.H. Sm. as a first record. Kastamonu Univ. J. For. Fac. 18, 314–326. doi: 10.17475/kastorman.499076
Kirk, P. M., Cannon, P. F., Minter, D. W., and Stalpers, J. A. (2008). Ainsworth & Bisby’s Dictionary of the Fungi, 10th Edn. Wallingford: CAB International.
Kornerup, A., and Wanscher, J. H. (1978). Methuen Handbook of Colour, 3rd Edn. London: Eyre Methuen.
Lin, C. Y., Wei, J. D., Cheng, H. J., Ye, J., and Yeh, K. Y. (2017). Embedded-based graphics processing unit cluster platform for multiple sequence alignments. Evol. Bioinform. Online 13, 1–10. doi: 10.1177/1176934317724764
Nylander, J. A. A. (2004). MrModeltest 2.2 Program Distributed by the Author. Uppsala: Uppsala University.
Posada, D., and Crandall, K. A. (1998). Modeltest: testing the model of DNA substitution. Bioinformatics 4, 817–818. doi: 10.1093/bioinformatics/14.9.817
Rehner, S. A., and Samuels, G. J. (1994). Taxonomy and phylogeny of Gliocladiu analysed from nuclear large subunit ribosomal DNA sequences. Mycol. Res. 98, 625–634. doi: 10.1016/S0953-7562(09)80409-7
Sánchez-García, M., Cifuentes-Blanco, J., and Matheny, P. B. (2013). Taxonomic revision of the genus Melanoleuca in Mexico and description of new species. Rev. Mex. Biodivers. 84, S111–S127.
Singer, R. (1986). The Agaricales in Modern Taxonomy, 4th Edn. Koenigstein: Koeltz Scientific Books.
Smith, A. H. (1944). New North American Agarics. Mycological 36, 242–262. doi: 10.1080/00275514.1944.12017545
Smith, S. Y., Currah, R. S., and Stockey, R. A. (2004). Cretaceous and eocene poroid hymenophores from Vancouver Island, British Columbia. Mycologia 96, 180–186. doi: 10.1080/15572536.2005.1183301
Stamatakis, A. (2006). Raxml-vi-hpc: maximum likelihood-based phylogenetic analyses with thousands of taxa and mixed models. Bioinformatics 22, 2688–2690. doi: 10.1093/bioinformatics/btl446
Sun, L. H., Song, G., Wang, L. Y., and Yun, X. F. (2012). Ecological diversity of macro fungi in Helan Mountain. J. Anhui Agric. Sci. 40, 2219–2222.
Talavera, G., and Castresana, J. (2007). Improvement of phylogenies after removing divergent and ambiguously aligned blocks from protein sequence alignments. Syst. Biol. 56, 564–577. doi: 10.1080/10635150701472164
Tian, H. M., Liu, T. Z., Tian, Y. C., Ren, X. Q., Qiu, Y. L., and Li, X. D. (2018). List of macrofungi in the xilamulun river basin I. J. Chifeng Univ. 34, 26–29.
Vilgalys, R., and Hester, M. (1990). Rapid genetic identification and mapping of enzymatically amplified ribosomal dna from several Cryptococcus species. J. Bacteriol. 172, 4238–4246. doi: 10.1128/jb.172.8.4238-4246
Vizzini, A., Para, R., Fontenla, R., Ghignone, S., and Ercole, E. (2011). A preliminary ITS phylogeny of Melanoleuca (Agaricales) with special reference to European taxa. Mycotaxon 118, 361–381. doi: 10.5248/118.361
Vladimír, A., Imre, R., and Lajos, B. (2014). Melanoleuca juliannae (Basidiomycota, Tricholomataceae), a new species from subgen. Urticocystis. Phytotaxa 170, 013–023. doi: 10.11646/phytotaxa.170.1.2
Wang, J. R. (2013). Biodiversity of Macrofungi in Shandong Province. dissertation, Jilin Agricultural University, Jilin.
Wei, J., Fan, Y. J., and Yan, W. (2015). A preliminary report on macrofungi from Wulashan area of Yin mountain. Guangdong Agric. Sci. 9, 149–153.
White, T. J., Bruns, T., Lee, S., and Taylor, J. (1990). 38 – Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. PCR Protoc. Guide Methods Appl. 38, 315–322. doi: 10.1016/B978-0-12-372180-8.50042-1
Wilgenbusch, J. C., and Swofford, D. (2003). Inferring evolutionary trees with PAUP∗. Curr. Protoc. Bioinformatics 00, 6.4.1–6.4.28. doi: 10.1002/0471250953.bi0604s00
Xia, X. (2013) DAMBE5: a comprehensive software package for data analysis in molecular biology and evolution. Mol. Biol. Evol. 30, 1720–1728. doi: 10.1093/molbev/mst064
Yu, X. D., Lv, S. X., Ma, D., Li, F. F., Lin, Y., and Zhang, L. (2014). Two new species of Melanoleuca (Agaricales, Basidiomycota) from northeastern China, supported by morphological and molecular data. Mycoscience 55, 456–461. doi: 10.1016/j.myc.2014.01.007
Zhang, W. M., Li, T. H., and Song, B. (2001). General situation of macrofungi in Guangdong Province. Ecol. Sci. 4, 48–58.
Zhao, R. L., Guo, J. L., Sánchez-Ramírez, S., Stata, M., Yang, Z. L., Gang, W., et al. (2017). A six-gene phylogenetic overview of Basidiomycota and allied phyla with estimated divergence times of higher taxa and a phyloproteomics perspective. Fungal Divers. 84, 43–74. doi: 10.1007/s13225-017-0381-5
Zhao, R. L., Karunarathna, S., and Raspé, O. (2011). Major clades in tropical Agaricus. Fungal Divers. 51, 279–296. doi: 10.1007/s13225-011-0136-7
Zhao, R. L., Zhou, J. L., Chen, J., Margaritescu, S., Sánchez-Ramírez, S., Hyde, K. D., et al. (2016). Towards standardizing taxonomic ranks using divergence times – a case study for reconstruction of the Agaricus taxonomic system. Fungal Divers. 78, 239–292. doi: 10.1007/s13225-016-0357-x
Keywords: taxonomy, phylogeny, divergence time, morphological characteristics, infrageneric classifications
Citation: Xu J, Yu X, Lu M, Hu J, Moodley O, Zhang C, Gong L and Li Y (2019) Phylogenetic Analyses of Some Melanoleuca Species (Agaricales, Tricholomataceae) in Northern China, With Descriptions of Two New Species and the Identification of Seven Species as a First Record. Front. Microbiol. 10:2167. doi: 10.3389/fmicb.2019.02167
Received: 31 March 2019; Accepted: 04 September 2019;
Published: 26 September 2019.
Edited by:
Rui-Lin Zhao, Institute of Microbiology (CAS), ChinaReviewed by:
Taihui Li, Guangdong Academy of Sciences, ChinaCopyright © 2019 Xu, Yu, Lu, Hu, Moodley, Zhang, Gong and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jize Xu, eHVqaXplQDE2My5jb20=; Chunlan Zhang, NDU4NTYwOTY5QHFxLmNvbQ==; Lei Gong, ZnVuZ3VzX2xhYkAxMjYuY29t; Yu Li, eHVqejgwMkBuZW51LmVkdS5jbg==
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.