- Emergency and Critical Care Center, Department of Emergency Medicine, Zhejiang Provincial People’s Hospital, People’s Hospital of Hangzhou Medical College, Hangzhou, Zhejiang, China
Traditional disease prediction models and scoring systems for acute pancreatitis (AP) are often inadequate in providing concise, reliable, and effective predictions regarding disease progression and prognosis. As a novel interdisciplinary field within artificial intelligence (AI), machine learning (ML) is increasingly being applied to various aspects of AP, including severity assessment, complications, recurrence rates, organ dysfunction, and the timing of surgical intervention. This review focuses on recent advancements in the application of ML models in the context of AP.
1 Introduction
Acute pancreatitis (AP) is an inflammatory disorder affecting the parenchyma and peripancreatic tissue, characterized by severe abdominal pain, elevated pancreatic enzymes, and pancreatitis-related changes on abdominal imaging. The incidence of AP has shown a rising trend globally, with an average occurrence rate of 34 cases per 100,000 individuals. Approximately 20% of patients progress to either moderately severe acute pancreatitis (MSAP, accompanied by transient [≤48 h] organ dysfunction and/or local complications such as necrosis of pancreatic or peripancreatic tissue) or severe acute pancreatitis (SAP, accompanied by persistent [>48 h] organ failure), the mortality rate can reach as high as 20–40% (1).
Machine learning (ML) is a category of artificial intelligence tools in which virtual agents learn an optimized set of rules through trial and error—a policy that maximizes expected returns (2). ML has many ideal characteristics that can help with medical decision-making, and these algorithms are able to infer the best decision from suboptimal training sets. ML has been successfully applied to medical problems in the past, such as diabetes and sepsis (3, 4).
Machine learning has demonstrated significant potential in the field of medicine, particularly in disease diagnosis and prognosis. Over the past decade, the utilization of ML algorithms based on databases for acute pancreatitis has become increasingly prevalent. Numerous studies have employed ML algorithms to forecast AP mortality rates (5), severity (6–8), complications (9), recurrence rates (10), as well as surgical or intervention strategies (7), with ML exhibiting robust reliability in these domains.
In recent years, ML algorithms and the prediction models based on them have generated significant interest among researchers. A growing body of evidence indicates that ML plays a crucial role in predicting acute pancreatitis diagnosis and prognosis. This review aims to offer an overview of the specific applications of ML in AP, with the expectation that artificial intelligence can furnish more evidence-based support for clinical practice in the future.
2 The role of ML in predicting AP mortality
According to the 2012 revision of Atlanta classification (RAC) (11), SAP accompanied by persistent organ failure carries a high mortality rate, ranging from 20 to 40% (1). When complicated by late-stage infections, the mortality rate becomes exceedingly high. Traditional scoring systems for predicting mortality are complex and limited. A systematic review revealed that the positive predictive values of the APACHE II score (AcutePhysiology and Chronic Health Evaluation score, widely used in the classification of critically ill patients and prognosis prediction, which can make a quantitative evaluation of the patient’s condition, a higher score indicates a more serious condition, a poorer prognosis, and a higher rate of mortality), Ranson score (one of the earliest scoring systems for predicting the severity of AP and is primarily used to predict the severity of biliary pancreatitis), and Glasgow criteria (emphasis on objective laboratory indicators, including 8 indicators, assessed in 48h of admission to the hospital) were only 69, 63, and 66% respectively (12). Although APACHE II provides the best predictive value for mortality, there is currently no single scoring system that can reliably predict the mortality rate of acute pancreatitis. Therefore, in recent years, numerous early prediction models based on ML algorithms have been developed. These models offer valuable insights for early intervention and potentially reducing the mortality rate of SAP.
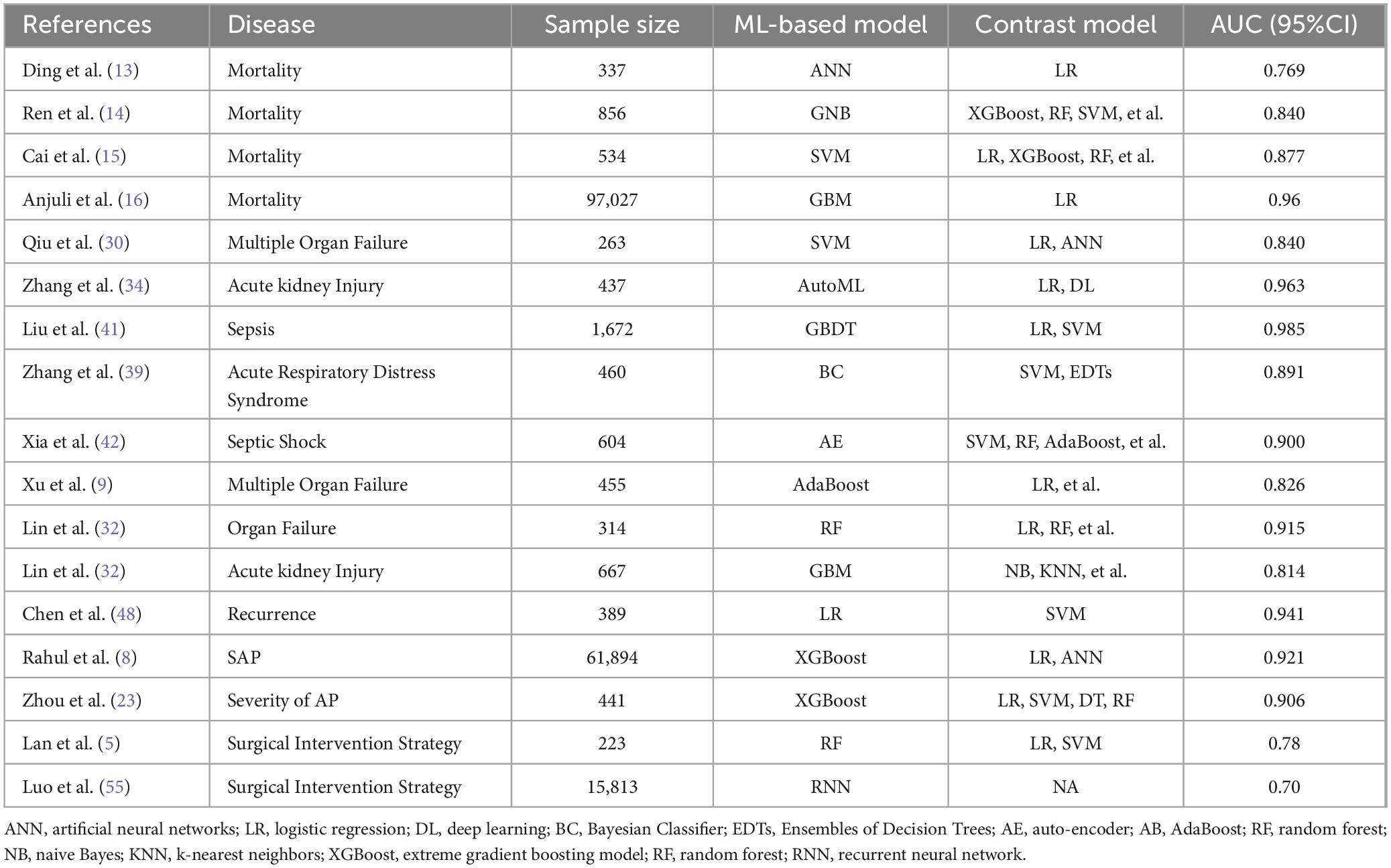
Ding et al. initially developed an artificial neural network (ANN) model using the MIMIC-III database, achieving an area under the receiver operating characteristic curve (AUC) of 0.769, which outperformed logistic regression with an AUC of 0.607, Ranson score with 0.652, and SOFA score with 0.401 in predicting in-hospital mortality rate for AP patients (13). The ANN model demonstrated superior overall performance and early-stage risk stratification capability for high-risk AP patients. Building on this, Ren et al. identified 856 AP admitted to the intensive care unit (ICU) from the MIMIC-IV database and developed 9 ML models. Among these, they selected the Gaussian naive Bayes (GNB) model, which demonstrated an AUC, accuracy, sensitivity, and specificity of 0.840, 0.787, 0.839, and 0.792 respectively—making it the most effective among all models tested (14). The GNB model’s ability to identify high mortality risk in AP patients admitted to the ICU was further validated using an external database. Similarly, ML models, especially support vector machine (SVM) models, play a crucial role in predicting 28-day all-cause mortality in patients with SAP and analyzing their risk factors (15). The superior attributes of these models compared to traditional scoring systems enhance their effectiveness in early identification of SAP patients and reducing their mortality risk.
However, when it comes to specific causal diagnosis of AP for predicting mortality rates, the predictive capability of gradient boosting machine (GBM) machine learning models appears to be insignificant. Luthra collected 97,027 patients with biliary pancreatitis from the Nationwide Readmission Database over a 4-year period and compared the differences in predicting AP patient mortality between the GBM machine learning model and multivariate logistic regression analysis, finding that the GBM machine learning model had a higher positive predictive value (47.3% vs 35.9%) and lower sensitivity (40.1% vs 46.7%) (16). Therefore, he believes that in a large national database, traditional analysis and GBM machine learning model are comparable and not inferior, and the application of machine learning in managing database-based models for predicting hospital mortality due to common disease states is limited. It is worth noting that after statistical analysis, he found that the inpatient mortality rate of biliary pancreatitis was 0.97%, and hospital stay, age, SAP, patient income quartile, and sepsis were determined as the main predictors of mortality in biliary pancreatitis after it was determined.
3 The role of ML in predicting AP severity
A recent study in Japan showed that the mortality rate of SAP is about 16.7% (17), and early identification and personalized precision treatment can reduce the mortality rate of SAP. Previous studies have shown that precision treatment within 48 h of admission can significantly reduce mortality from SAP (18).
Due to the severity of SAP, high mortality rate, and association with organ failure, early identification and intervention of SAP patients are crucial. However, traditional scoring systems often require more than 24 h to perform and have limited accuracy. To address this, Luo et al. constructed and compared the predictive performance of five different ML models in training and validation cohorts, concluding that the random forest (RF) model performed the best and could be used to guide treatment and improve clinical outcomes (19). The AUC, accuracy, sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) of the RF model were 0.961, 86.0, 90.0, 81.5, 84.4, and 88.0% in the training cohort, and 0.969, 90.1, 88.6, 91.5, 91.2, and 89.0% in the validation cohort, which were significantly higher than those of other scoring systems (20, 21), the RF model has a higher accuracy in predicting SAP in the early stages of AP (22). Similarly, after developing and comparing different ML prediction models in terms of their effectiveness in predicting the severity of AP, Rahul et al. concluded that the extreme gradient boosting (XGBoost) model showed the best performance in predicting SAP, which can accurately predict SAP at an early stage and provide assistance to clinicians in identifying and intervening in SAP earlier (8, 23, 24). XGBoost is a machine learning technique that integrates regression tree gradient lifting methods and has gained widespread recognition in the machine learning literature (25–27), data mining challenges, and disease outcome prediction. Given its ability to predict SAP by combining imaging findings and clinical indicators, as well as its capacity to effectively handle missing values commonly encountered in clinical settings (8), early classification and identification of AP can provide valuable guidance for improved integration of medical resources.
4 The role of ML in predicting AP complications
4.1 Organ failure (OF)
Approximately 20% of patients develop organ failure in AP (28), and the presence of persistent organ dysfunction is a key factor in distinguishing between MAP, MSAP and SAP. Once OF occurs, the mortality rate can be as high as 30% (29), while also increasing the risk of infected pancreatic necrosis. Therefore, early identification of AP complicated by OF has a crucial impact on the emergency management of AP patients and plays a vital role in improving survival rates.
Four studies designed ML models to predict OF (9, 30–32). Qiu established models based on SVM, logistic regression (LR), and ANN to predict multiple organ failure (MOF) (30). The area under receiver operating characteristic curve (AUROC) values of these three models were not significantly different at 0.840, 0.832, and 0.834 respectively. Additionally, there was no significant difference in the AUROC compared to the traditional APACHE II score with an AUC value of 0.814 where P > 0.05. He believes that the three ML models can all be effective prognostic tools for predicting MOF in MSAP and SAP, and recommends using ANN, which only requires hematocrit, kinetic-time, IL-6, and creatinine as four common parameters. A multicenter cohort study employed complete blood count, serum biochemical markers, and coagulation indicators to develop 6 ML-based algorithm models for predicting MOF (9). Among these, the Adaptive Boosting algorithm (AdaBoost) exhibited superior predictive performance with an AUC of 0.826, sensitivity of 0.805, and specificity of 0.733. IL-6, creatinine, and kinetic time in coagulation indicators were identified as the three most significant independent variables, and monitoring these features can aid in preventing AP-related MOF. Numerous studies have indicated that the conventional use of ANN models is superior to APACHE II scores and LR models in predicting disease severity, MOF, and mortality, and the ANN models can accurately classify 96.2% of patients (31, 33). Lin et al. collected data from 314 Hyperlipidemic acute pancreatitis (HLAP) patients and established LR, NB (Naive Bayes), KNN (K-Nearest Neighbors), DT (Decision Tree) and RF models (32). The AUC values were 0.838, 0.824, 0.853, 0.897, and 0.915 respectively, all significantly higher than those of traditional prediction scoring systems. Among them, the RF model exhibited the highest predictive AUC for OF in HLAP patients with a sensitivity of 0.828 and accuracy of 0.814 among the 5 models tested. They concluded that the RF model outperforms other models as well as clinical scoring systems in predicting the occurrence of OF in HLAP patients and is beneficial for early intervention in high-risk HLAP patients for OF prevention.
AP-related OF mainly involves respiratory, circulatory, and renal failure. Some retrospective clinical analyses have confirmed the role of ML in acute kidney injury (AKI) associated with AP (34–36). Zhang et al. developed an automated machine learning (AutoML) algorithm prediction model that intelligently selects from a range of algorithms and hyperparameters to tailor models for specific datasets (34), enabling early prediction of AKI in AP patients. It demonstrates superior performance compared to traditional LR, requiring less time and achieving higher accuracy, thus significantly improving work efficiency. This warrants its clinical application and promotion. Lin et al. extracted data from the MIMIC-IV database to build a predictive ML model for SAP-AKI using 1,235 cases of SAP patients (35). The models included GBM, GLM, KNN, NB, ANN, RF, and SVM with AUC values of 0.814, 0.812, 0.671, 0.812, 0.688, 0.809 and 0.810 respectively. This highlights the significant role of GBM in predicting SAP-AKI and can assist clinical practitioners in identifying high-risk patients and intervening promptly to reduce mortality rates in intensive care units. It is also worth noting that systemic inflammatory response is inherently associated with the process of AKI and may be caused by local inflammation within renal tissues (37).
Acute respiratory distress syndrome (ARDS) is a common complication of AP, with approximately 30% of SAP patients developing ARDS (38), resulting in a mortality rate of up to 37% (28). Two retrospective analysis studies exploring ML models for AP-associated ARDS have yielded positive results (39, 40), successfully establishing predictive models based on ML. Compared with other models, the Bayesian Classifier (BC) model achieving the highest AUC at 0.891 and demonstrating the best predictive performance (39). The Ensemble Decision Trees (EDT) showed good predictive capabilities, with the highest accuracy (0.891) and precision (0.800). It is noteworthy that lower PaO2 and Ca2+ levels upon admission, as well as elevated CRP, Procalcitonin, Lactic Acid, Neutrophil-Lymphocyte Ratio, White Blood Cell Count, and Amylase levels are significantly associated with an increased risk of developing ARDS in AP patients; among these features, PaO2 is identified as the most important predictor.
4.2 Sepsis
ML techniques also demonstrate significant advantages in predicting and evaluating septic shock. In a large retrospective cohort study (41), 1,672 AP from the MIMIC III and MIMIC IV databases were selected to construct six ML models, including SVM, KNN, Multilayer Perceptron (MLP), LR, Gradient Boosting Decision Tree (GBDT), and AdaBoost. The GBDT model demonstrated superior performance in predicting sepsis among AP patients with an AUC of 0.985 on the test set, outperforming LR, Systemic Inflammatory Response Syndrome (SIRS) score, Bedside Index for Severity in Acute Pancreatitis (BISAP) score, Sequential Organ Failure Assessment (SOFA) score, quick SOFA (qSOFA), and APACHE II scores in sepsis prediction. Similarly, another retrospective study data established multiple ML models for early prediction of septic shock in AP with sepsis (42), with the final auto-encoder (AE) model achieving the highest AUC on the validation set (AUC 0.900, accuracy 0.868), while the AUC on the test set was 0.879 and the accuracy was 0.790. The AE model performed better than traditional scoring systems in predicting septic shock in AP with sepsis within 28 days after admission.
5 The role of ML in predicting AP recurrence rate
Recurrent acute pancreatitis (RAP) is defined as a history of at least two episodes of AP with no evidence of pancreatic tissue or functional abnormalities during the remission period. It represents a distinct subtype of pancreatitis, and statistics indicate that 17–22% of diagnosed AP patients will experience recurrence (43). RAP serves as a significant risk factor for the development of chronic pancreatitis (CP), with up to 36% of RAP patients ultimately progressing to CP (44). CP is often accompanied by comorbidities such as diabetes, malnutrition, steatorrhea, and weight loss. Long-term follow-up studies have revealed that 1.3% of CP patients may progress to pancreatic cancer over an 8-year period (45), significantly impacting their quality of life and prognosis. Therefore, early identification and timely intervention for individuals at risk for developing RAP following an episode of acute pancreatitis may mitigate the incidence rates of both RAP and pancreatic cancer while enhancing long-term quality of life.
Radiomics is an emerging field that optimizes existing imaging resources to extract high-throughput quantitative features from medical images (46, 47). These features are further analyzed using predefined algorithms to develop models for clinical decision-making. Currently, radiomics has been widely applied in the precise analysis of tumors and their metastases (46).
ML models based on radiomics research for predicting RAP are currently underutilized in clinical practice. Two retrospective analysis studies have confirmed the role of SVM models in predicting and distinguishing RAP (10, 48). The SVM model demonstrates a significantly higher AUC than traditional clinical models (0.941 vs. 0.712, p = 0.000), with similar conclusions observed in the validation dataset (0.929 vs. 0.671, p = 0.000) (48). The SVM model constructed using radiomic features can effectively differentiate between patients with functional abdominal pain, RAP, and CP, achieving an overall average accuracy of 82.1%. For patients diagnosed with RAP solely based on symptoms of abdominal pain and laboratory values or those for whom imaging studies during AP episodes are unavailable, radiomics may serve as a valuable diagnostic adjunct (10).
6 The role of ML in predicting AP surgical intervention strategy
Infected pancreatic necrosis (IPN) is the most severe local complication in the late stage of AP. Once IPN occurs, it indicates SAP, with a mortality rate as high as 30% (49). Treatment often involves a series of surgical debridement procedures known as “Step-up” strategies, including percutaneous catheter drainage (PCD), endoscopic transgastric necrosectomy, video-assisted minimally invasive surgery, and open surgery (50). There has been significant debate regarding the timing of surgical intervention for IPN. Research suggests that early surgery results in a mortality rate exceeding 50% (51), while delaying surgery until 4 weeks after the onset of IPN can reduce both complications and mortality rates (52). With the advancement of modern minimally invasive techniques, early endoscopic drainage during the course of AP has also proven to be safe and effective (53, 54). Early, timely, and accurate prediction of IPN occurrence and determination of the optimal timing for surgical intervention are crucial factors guiding subsequent treatment decisions.
Lan et al. included 223 patients with IPN who underwent surgical treatment for AP (5). They classified IPN patients based on whether the surgery was performed within 4 weeks using LR, SVM, and RF models. The RF model demonstrated a higher classification accuracy (0.80) compared to SVM (0.78) and LR (0.71). Additionally, they identified IL-6, infectious necrosis, fever, and CRP as key factors in determining the timing of surgical intervention for IPN patients. The ML model can effectively predict the optimal timing for surgical intervention in IPN, providing valuable guidance for clinicians in developing personalized surgical strategies for IPN patients.
Another large-scale retrospective clinical study involving 15,813 patients with AP has developed a novel ML model based on recurrent neural network (RNN) to predict the timing of surgical intervention for IPN (55). This model, known as Phased Long Short-Term Memory (Phased-LSTM), achieved an AUC greater than 0.70 and demonstrated stronger interpretability, making it suitable for predicting the optimal timing for surgery. The developed model visualizes specific surgical timings and changes in laboratory indicators from onset to discharge for AP patients, enabling comprehensive monitoring of patients with necrotizing pancreatitis throughout their hospitalization. Due to the ability of LSTM to forget and update long-term states, its performance surpassed that of SVM and RF, highlighting the advantages of time series models in handling temporal data.
7 Discussion
With the improvement in living standards, the incidence of AP has been increasing annually in recent years, with a rise of approximately 2–5% per year (56). Concurrently, the proportion of SAP is also rising. SAP is closely associated with multiple organ failure and has a high mortality and recurrence rate. Once the condition progresses to IPN and OF, the mortality rate can reach up to 30% (1). Therefore, early prediction of the severity of AP, the occurrence of complications, and the timing of intervention is crucial for clinical decision-making and timely intervention. However, traditional clinical prediction models, which are often based on multivariable analysis, are challenging to construct within 24h widespread clinical application. Consequently, it is imperative to develop a simple, effective, and clinically implementable model for early prediction of AP progression.
Artificial Intelligence (AI) encompasses a range of subfields within computer science. In recent years, advancements in algorithms such as ML, statistical learning, deep learning, and cognitive computing have played a pivotal role in the diagnosis and treatment of diseases such as sepsis and cancer (4, 57). ML, a subset of AI, is a burgeoning interdisciplinary field that integrates statistics, computer science, and other areas. It is not only used for text mining and classification in computer science, but is also increasingly applied in clinical practice. Various ML algorithm models for disease prediction and diagnosis have been developed based on AI technologies and are now widely accepted in the medical field. Recently, ML has begun to be applied to areas such as the severity of AP, complications, recurrence rates, organ dysfunction, and the timing of surgical intervention. This review focuses on recent advancements in the application of ML models in the context of AP (refer to Table 1).
We have observed that the majority of current ML models do not account for several important factors, including the etiology of AP and the stratification of severity. Additionally, most of the data utilized are retrospective, although these models have been validated on test and validation sets, their reliability still requires confirmation through clinical practice. Many studies are single-center with small sample sizes and lack external validation. Most research focuses on binary classification of AP into SAP and non-SAP. To date, there have been no ML models that provide accurate prognostication based on the 2012 Atlanta classification, which includes SAP, MSAP, and mild acute pancreatitis (MAP). Notably, most ML models remain limited to predicting traditional severity and complications, with a significant gap in predictive models for recurrence rates, optimal timing for surgery, pancreatic necrosis accumulation, and local complications such as infectious pancreatic necrosis. Future research should address these areas.
8 Conclusion
In conclusion, ML has proven to be an excellent predictor of mortality, severity, complications, recurrence, organ dysfunction, and timing of surgical intervention in acute pancreatitis, and is superior to traditional scoring systems such as the APACHE II score, the BISAP score, the SOFA score, and other traditional systems. However, much more prospective clinical studies are needed to validate this idea.
Author contributions
ZT: Conceptualization, Data curation, Writing – original draft, Writing – review and editing. GL: Investigation, Writing – review and editing. YZ: Formal analysis, Project administration, Writing – review and editing. QL: Software, Validation, Writing – review and editing. WC: Resources, Writing – review and editing. JT: Methodology, Supervision, Writing – review and editing. SJ: Supervision, Writing – review and editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This study was supported by the Health Science and Technology Plan of Zhejiang Province, China (2023KY051) and the Clinical Research Project of Zhejiang Provincial Administration of Traditional Chinese Medicine, China (2023ZL253).
Acknowledgments
We acknowledge all the doctors and nurses in the Emergency Department of Zhejiang Provincial hospital. And we thank all the participators in this review.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Boxhoorn L, Voermans RP, Bouwense SA, Bruno MJ, Verdonk RC, Boermeester MA, et al. Acute pancreatitis. Lancet. (2020) 39610252:726–34.
2. Bennett CC, Hauser K. Artificial intelligence framework for simulating clinical decision-making: a Markov decision process approach. Artif Intell Med. (2013) 571:9–19. doi: 10.1016/j.artmed.2012.12.003
3. Gulshan V, Peng L, Coram M, Stumpe MC, Wu D, Narayanaswamy A, et al. Development and validation of a deep learning algorithm for detection of diabetic retinopathy in retinal fundus photographs. JAMA. (2016) 31622:2402–10.
4. Komorowski M, Celi LA, Badawi O, Gordon AC, Faisal AA. The artificial intelligence clinician learns optimal treatment strategies for sepsis in intensive care. Nat Med. (2018) 2411:1716–20.
5. Lan L, Guo Q, Zhang Z, Zhao W, Yang X, Lu H, et al. Classification of infected necrotizing pancreatitis for surgery within or beyond 4 weeks using machine learning. Front Bioeng Biotechnol. (2020) 8:541.
6. Kiss S, Pinter J, Molontay R, Nagy M, Farkas N, Sipos Z, et al. Hungarian pancreatic study G: early prediction of acute necrotizing pancreatitis by artificial intelligence: a prospective cohort-analysis of 2387 cases. Sci Rep. (2022) 121:7827. doi: 10.1038/s41598-022-11517-w
7. Shi N, Lan L, Luo J, Zhu P, Ward TRW, Szatmary P, et al. Predicting the need for therapeutic intervention and mortality in acute pancreatitis: a two-center international study using machine learning. J Pers Med. (2022) 12:616. doi: 10.3390/jpm12040616
8. Thapa R, Iqbal Z, Garikipati A, Siefkas A, Hoffman J, Mao Q, et al. Early prediction of severe acute pancreatitis using machine learning. Pancreatology. (2022) 221:43–50.
9. Xu F, Chen X, Li C, Liu J, Qiu Q, He M, et al. Prediction of multiple organ failure complicated by moderately severe or severe acute pancreatitis based on machine learning: a multicenter cohort study. Mediators Inflamm. (2021) 2021:5525118.
10. Mashayekhi R, Parekh VS, Faghih M, Singh VK, Jacobs MA, Zaheer A. Radiomic features of the pancreas on CT imaging accurately differentiate functional abdominal pain, recurrent acute pancreatitis, and chronic pancreatitis. Eur J Radiol. (2020) 123:108778. doi: 10.1016/j.ejrad.2019.108778
11. Banks PA, Bollen TL, Dervenis C, Gooszen HG, Johnson CD, Sarr MG, et al. Acute pancreatitis classification working G: classification of acute pancreatitis–2012: revision of the Atlanta classification and definitions by international consensus. Gut. (2013) 621:102–11. doi: 10.1136/gutjnl-2012-302779
12. Gravante G, Garcea G, Ong SL, Metcalfe MS, Berry DP, Lloyd DM, et al. Prediction of mortality in acute pancreatitis: a systematic review of the published evidence. Pancreatology. (2009) 95:601–14.
13. Ding N, Guo C, Li C, Zhou Y, Chai X. An artificial neural networks model for early predicting in-hospital mortality in acute pancreatitis in MIMIC-III. Biomed Res Int. (2021) 2021:6638919. doi: 10.1155/2021/6638919
14. Ren W, Zou K, Huang S, Xu H, Zhang W, Shi X, et al. Prediction of in-hospital mortality of intensive care unit patients with acute pancreatitis based on an explainable machine learning algorithm. J Clin Gastroenterol. (2024) 586:619–26. doi: 10.1097/MCG.0000000000001910
15. Cai W, Wu X, Chen Y, Chen J, Lin X. Risk factors and prediction of 28-day-all cause mortality among critically ill patients with acute pancreatitis using machine learning techniques: a retrospective analysis of multi-institutions. J Inflamm Res. (2024) 17:4611–23. doi: 10.2147/JIR.S463701
16. Luthra AK, Porter K, Hinton A, Chao WL, Papachristou GI, Conwell DL, et al. A comparison of machine learning methods and conventional logistic regression for the prediction of in-hospital mortality in acute biliary pancreatitis. Pancreas. (2022) 5110:1292–9. doi: 10.1097/MPA.0000000000002208
17. Yasuda H, Horibe M, Sanui M, Sasaki M, Suzuki N, Sawano H, et al. Etiology and mortality in severe acute pancreatitis: a multicenter study in Japan. Pancreatology. (2020) 203:307–17.
18. Petrov MS, Pylypchuk RD, Uchugina AF. A systematic review on the timing of artificial nutrition in acute pancreatitis. Br J Nutr. (2009) 1016:787–93. doi: 10.1017/S0007114508123443
19. Luo Z, Shi J, Fang Y, Pei S, Lu Y, Zhang R, et al. Development and evaluation of machine learning models and nomogram for the prediction of severe acute pancreatitis. J Gastroenterol Hepatol. (2023) 383:468–75. doi: 10.1111/jgh.16125
20. Valverde-Lopez F, Matas-Cobos AM, Alegria-Motte C, Jimenez-Rosales R, Ubeda-Munoz M, Redondo-Cerezo E. BISAP, RANSON, lactate and others biomarkers in prediction of severe acute pancreatitis in a European cohort. J Gastroenterol Hepatol. (2017) 329:1649–56. doi: 10.1111/jgh.13763
21. Pando E, Alberti P, Mata R, Gomez MJ, Vidal L, Cirera A, et al. Early changes in blood urea Nitrogen (BUN) can predict mortality in acute pancreatitis: comparative study between BISAP score, APACHE-II, and other laboratory markers-A prospective observational study. Can J Gastroenterol Hepatol. (2021) 2021:6643595. doi: 10.1155/2021/6643595
22. Yuan L, Ji M, Wang S, Wen X, Huang P, Shen L, et al. Machine learning model identifies aggressive acute pancreatitis within 48 h of admission: a large retrospective study. BMC Med Inform Decis Mak. (2022) 221:312. doi: 10.1186/s12911-022-02066-3
23. Zhou Y, Han F, Shi XL, Zhang JX, Li GY, Yuan CC, et al. Prediction of the severity of acute pancreatitis using machine learning models. Postgrad Med. (2022) 1347:703–10.
24. Zhao Y, Wei J, Xiao B, Wang L, Jiang X, Zhu Y, et al. Early prediction of acute pancreatitis severity based on changes in pancreatic and peripancreatic computed tomography radiomics nomogram. Quant Imaging Med Surg. (2023) 133:1927–36. doi: 10.21037/qims-22-821
25. Li Q, Yang H, Wang P, Liu X, Lv K, Ye M. XGBoost-based and tumor-immune characterized gene signature for the prediction of metastatic status in breast cancer. J Transl Med. (2022) 201:177. doi: 10.1186/s12967-022-03369-9
26. Lu D, Peng J, Wang Z, Sun Y, Zhai J, Wang Z, et al. Dielectric property measurements for the rapid differentiation of thoracic lymph nodes using XGBoost in patients with non-small cell lung cancer: a self-control clinical trial. Transl Lung Cancer Res. (2022) 113:342–56. doi: 10.21037/tlcr-22-92
27. Hou N, Li M, He L, Xie B, Wang L, Zhang R, et al. Predicting 30-days mortality for MIMIC-III patients with sepsis-3: a machine learning approach using XGboost. J Transl Med. (2020) 181:462. doi: 10.1186/s12967-020-02620-5
28. Schepers NJ, Bakker OJ, Besselink MG, Ahmed Ali U, Bollen TL, Gooszen HG, et al. Dutch pancreatitis study G: impact of characteristics of organ failure and infected necrosis on mortality in necrotising pancreatitis. Gut. (2019) 686:1044–51. doi: 10.1136/gutjnl-2017-314657
29. Petrov MS, Shanbhag S, Chakraborty M, Phillips AR, Windsor JA. Organ failure and infection of pancreatic necrosis as determinants of mortality in patients with acute pancreatitis. Gastroenterology. (2010) 1393:813–20.
30. Qiu Q, Nian YJ, Guo Y, Tang L, Lu N, Wen LZ, et al. Development and validation of three machine-learning models for predicting multiple organ failure in moderately severe and severe acute pancreatitis. BMC Gastroenterol. (2019) 191:118.
31. Hong WD, Chen XR, Jin SQ, Huang QK, Zhu QH, Pan JY. Use of an artificial neural network to predict persistent organ failure in patients with acute pancreatitis. Clinics (Sao Paulo). (2013) 681:27–31.
32. Lin W, Huang Y, Zhu J, Sun H, Su N, Pan J, et al. Machine learning improves early prediction of organ failure in hyperlipidemia acute pancreatitis using clinical and abdominal CT features. Pancreatology. (2024) 243:350–6. doi: 10.1016/j.pan.2024.02.003
33. Mofidi R, Duff MD, Madhavan KK, Garden OJ, Parks RW. Identification of severe acute pancreatitis using an artificial neural network. Surgery. (2007) 1411:59–66.
34. Zhang R, Yin M, Jiang A, Zhang S, Xu X, Liu L. Automated machine learning for early prediction of acute kidney injury in acute pancreatitis. BMC Med Inform Decis Mak. (2024) 241:16.
35. Lin S, Lu W, Wang T, Wang Y, Leng X, Chi L, et al. Predictive model of acute kidney injury in critically ill patients with acute pancreatitis: a machine learning approach using the MIMIC-IV database. Ren Fail. (2024) 461:2303395. doi: 10.1080/0886022X.2024.2303395
36. Yang D, Zhao L, Kang J, Wen C, Li Y, Ren Y, et al. Development and validation of a predictive model for acute kidney injury in patients with moderately severe and severe acute pancreatitis. Clin Exp Nephrol. (2022) 268:770–87.
37. Yilmaz H, Cakmak M, Inan O, Darcin T, Akcay A. Can neutrophil-lymphocyte ratio be independent risk factor for predicting acute kidney injury in patients with severe sepsis? Ren Fail. (2015) 372:225–9.
38. Fei Y, Gao K, Li WQ. Prediction and evaluation of the severity of acute respiratory distress syndrome following severe acute pancreatitis using an artificial neural network algorithm model. HPB (Oxford). (2019) 217:891–7.
39. Zhang M, Pang M. Early prediction of acute respiratory distress syndrome complicated by acute pancreatitis based on four machine learning models. Clinics (Sao Paulo). (2023) 78:100215. doi: 10.1016/j.clinsp.2023.100215
40. Yang D, Kang J, Li Y, Wen C, Yang S, Ren Y, et al. Development of a predictive nomogram for acute respiratory distress syndrome in patients with acute pancreatitis complicated with acute kidney injury. Ren Fail. (2023) 452:2251591. doi: 10.1080/0886022X.2023.2251591
41. Liu F, Yao J, Liu C, Shou S. Construction and validation of machine learning models for sepsis prediction in patients with acute pancreatitis. BMC Surg. (2023) 231:267.
42. Xia Y, Long H, Lai Q, Zhou Y. Machine learning predictive model for septic shock in acute pancreatitis with sepsis. J Inflamm Res. (2024) 17:1443–52.
43. Sankaran SJ, Xiao AY, Wu LM, Windsor JA, Forsmark CE, Petrov MS. Frequency of progression from acute to chronic pancreatitis and risk factors: a meta-analysis. Gastroenterology. (2015) 1496:1490–1500.e1491.
44. Ahmed Ali U, Issa Y, Hagenaars JC, Bakker OJ, Van Goor H, Nieuwenhuijs VB, et al. Dutch pancreatitis study G: risk of recurrent pancreatitis and progression to chronic pancreatitis after a first episode of acute pancreatitis. Clin Gastroenterol Hepatol. (2016) 145:738–46. doi: 10.1186/s13054-016-1208-6
45. Hao L, Zeng XP, Xin L, Wang D, Pan J, Bi YW, et al. Incidence of and risk factors for pancreatic cancer in chronic pancreatitis: a cohort of 1656 patients. Dig Liver Dis. (2017) 4911:1249–56. doi: 10.1016/j.dld.2017.07.001
46. Gillies RJ, Kinahan PE, Hricak H. Radiomics: images are more than pictures. They are data. Radiology. (2016) 2782:563–77.
47. Lambin P, Rios-Velazquez E, Leijenaar R, Carvalho S, Van Stiphout RG, Granton P, et al. Radiomics: extracting more information from medical images using advanced feature analysis. Eur J Cancer. (2012) 484:441–6.
48. Chen Y, Chen TW, Wu CQ, Lin Q, Hu R, Xie CL, et al. Radiomics model of contrast-enhanced computed tomography for predicting the recurrence of acute pancreatitis. Eur Radiol. (2019) 298:4408–17. doi: 10.1007/s00330-018-5824-1
49. De-Madaria E, Buxbaum JL. Advances in the management of acute pancreatitis. Nat Rev Gastroenterol Hepatol. (2023) 2011:691–2.
50. Hollemans RA, Bakker OJ, Boermeester MA, Bollen TL, Bosscha K, Bruno MJ, et al. Dutch pancreatitis study G: superiority of step-up approach vs open necrosectomy in long-term follow-up of patients with necrotizing pancreatitis. Gastroenterology. (2019) 1564:1016–26. doi: 10.1053/j.gastro.2018.10.045
51. Mowery NT, Bruns BR, Macnew HG, Agarwal S, Enniss TM, Khan M, et al. Surgical management of pancreatic necrosis: a practice management guideline from the Eastern Association for the Surgery of Trauma. J Trauma Acute Care Surg. (2017) 832:316–27. doi: 10.1097/TA.0000000000001510
52. Leppaniemi A, Tolonen M, Tarasconi A, Segovia-Lohse H, Gamberini E, Kirkpatrick AW, et al. 2019 WSES guidelines for the management of severe acute pancreatitis. World J Emerg Surg. (2019) 14:27.
53. Van Grinsven J, Van Santvoort HC, Boermeester MA, Dejong CH, Van Eijck CH, Fockens P, et al. Dutch pancreatitis study G: timing of catheter drainage in infected necrotizing pancreatitis. Nat Rev Gastroenterol Hepatol. (2016) 135:306–12.
54. Huang D, Li Q, Lu Z, Jiang K, Wu J, Gao W, et al. From “step-up” to “step-jump”: a leap-forward intervention for infected necrotizing pancreatitis. Chin Med J (Engl). (2021) 1353:285–7. doi: 10.1097/CM9.0000000000001877
55. Luo J, Lan L, Peng L, Li M, Lu H, Yang D, et al. Predicting timing of surgical intervention using recurrent neural network for necrotizing pancreatitis. IEEE Access. (2020) 8:207905-207913.
56. Tenner S, Vege SS, Sheth SG, Sauer B, Yang A, Conwell DL, et al. American college of gastroenterology guidelines: management of acute pancreatitis. Am J Gastroenterol. (2024) 1193:419–37.
Keywords: artificial intelligence, machine-learning model, acute pancreatitis, severity, complications, recurrence, mortality
Citation: Tan Z, Li G, Zheng Y, Li Q, Cai W, Tu J and Jin S (2025) Advances in the clinical application of machine learning in acute pancreatitis: a review. Front. Med. 11:1487271. doi: 10.3389/fmed.2024.1487271
Received: 27 August 2024; Accepted: 16 December 2024;
Published: 07 January 2025.
Edited by:
Rahul Kashyap, WellSpan Health, United StatesReviewed by:
Priyal Mehta, Saint Vincent Hospital, United StatesMuhammad Daniyal Waheed, Maroof International Hospital, Pakistan
Copyright © 2025 Tan, Li, Zheng, Li, Cai, Tu and Jin. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Senjun Jin, amluc2pfMjAwOEAxNjMuY29t
†ORCID: Zhaowang Tan, orcid.org/0000-0002-1845-6765
 Zhaowang Tan†
Zhaowang Tan† Qian Li
Qian Li Senjun Jin
Senjun Jin