
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Med. , 26 September 2024
Sec. Intensive Care Medicine and Anesthesiology
Volume 11 - 2024 | https://doi.org/10.3389/fmed.2024.1481083
This article is part of the Research Topic Mechanisms and Interventions for Post-Operative Neurocognitive Disorder and Sleep Disruptions View all 7 articles
Background: Extubation failure (EF) is common in the intensive care unit (ICU) and is associated with poor prognosis, especially in high-risk patients. However, the efficacy of prophylactic noninvasive oxygen therapy (NIT), including noninvasive ventilation (NIV) and high-flow nasal cannula (HFNC), in reducing EF in high-risk patients remains controversial. Therefore, we aimed to evaluate the effect of post-extubation prophylactic NIT on EF in high-risk patients.
Methods: This was a retrospective observational study conducted in the ICU from March 2018 to December 2023. We included adult patients at high risk for reintubation who were mechanically ventilated for over 24 h and successfully passed the spontaneous breathing trial (SBT). Immediately after extubation, patients underwent NIT or conventional oxygenation therapy (COT). The primary outcome was the EF rate within 7 days after extubation.
Results: There were 440 patients in the NIT group and 274 in the COT group. After propensity-score matching, 227 subjects were enrolled in each group. NIT reduced the rate of EF (18.0% vs. 34.3%, p < 0.001) and reintubation (10.5% vs. 18.2% p = 0.003) compared with COT, which was confirmed in propensity-matched cohort (17.6% vs. 32.2%, p < 0.001; 11.5% vs. 19.8%, p = 0.014). Multivariate logistic regression analysis indicated that prophylactic NIT (p = 0.001) and higher ROX index (p = 0.022) were associated with reduced risk of EF. While higher fluid balance (p = 0.013), higher RSBI (p < 0.001), and the occurrence of delirium (p = 0.032) may be the risk factors for EF. Subgroup analysis showed that post-extubation NIT was more effective in elderly patients, and HFNC was non-inferior to NIV in reducing EF. While HFNC had a tendency to reduce the incidence of delirium.
Conclusion: Post-extubation prophylactic NIT is effective in reducing EF in high-risk patients, especially in the elderly patients. HFNC is an alternative treatment to NIV. Fluid balance, RSBI, ROX index, and delirium are associated with the occurrence of EF.
Extubation failure (EF) is common in the intensive care unit (ICU) and still occurs in 10–20% of mechanically ventilated patients who successfully complete the spontaneous breathing trial (SBT) (1). Among high-risk patients, that is, those older than 65 years or with any underlying chronic cardiac or respiratory disease, the EF rate is even as high as 48% (2). EF increases mortality by 25–50% and prolongs ICU stay and length of hospital stay (LOS) (3). Therefore, it is necessary to provide effective post-extubation respiratory support to prevent the occurrence of EF.
In addition to high-risk factors, delirium may also cause EF. Delirium is frequent in the ICU and may contribute to EF through altered consciousness, agitation and subsequent sedation, aspiration, and intolerance to noninvasive mechanical ventilation (NIV) (4). A reintubation rate of 22% has been reported among patients who developed delirium on the day of extubation (5). Identification of risk factors for EF is particularly important in predicting the occurrence of EF and reintubation.
NIV has been recommend for patients with hypercapnia. However, the effect of prophylactic use on reintubation and mortality in high-risk patients remains controversial (6, 7). In addition, NIV is susceptible to gastric distention, skin damage and claustrophobia, limiting its widespread use and reducing its efficacy in EF (8, 9). In contrast, high-flow nasal cannula (HFNC) improves patient comfort and tolerability (10). HFNC has also been reported to suppress delirium, which is a contributing factor to reintubation (11). In clinical practice, HFNC has emerged as a promising treatment strategy for patients with hypoxemic respiratory failure. In high-risk patients, HFNC was even comparable to NIV in preventing EF and reintubation (12). In recent years, an increasing number of studies referred to NIV and HFNC collectively as noninvasive oxygen therapy (NIT), and investigated its efficacy in ICU patients (13, 14).
A relevant randomized controlled trial (RCT) indicated that preventive use of NIT did not prevent reintubation compared with conventional oxygen therapy (COT) (15). However, the population was unselected and the efficacy of NIT in high-risk patients is unclear. Therefore, we conducted this retrospective observational cohort study to evaluate the efficacy of post-extubation prophylactic NIT to reduce EF in high-risk patients and to identify potential risk factors for EF.
This was a retrospective observational cohort study conducted in the ICU of the First Affiliated Hospital of Chongqing Medical University from March 2018 to December 2023. The study was approved by the institutional ethics committee of the First Affiliated Hospital of Chongqing Medical University and registered with the Chinese Clinical Trial Registry (ChiCTR2200061820). Informed consent was waived because of the retrospective observational nature of the study. All records and data were anonymized and de-identified prior to analysis.
We reviewed the records of all adult patients (≥ 18 years) admitted to the ICU and receiving MV for at least 24 h. In further screening, patients at high-risk of reintubation (7) who successfully passed the SBT and received post-extubation prophylactic NIV or HFNC and COT were included in the study. Patients were considered with high risk factors for reintubation if they fulfilled any of the following criteria as described in earlier studies: (1) age over 65 years; (2) had any underlying chronic cardiac or pulmonary disease. Underlying chronic cardiac diseases included left ventricular dysfunction, defined as left ventricular ejection fraction ≤ 45%; history of cardiogenic pulmonary edema; documented ischemic heart disease; or permanent atrial fibrillation. Underlying chronic pulmonary diseases included chronic obstructive pulmonary disease (COPD), obesity-hypoventilation syndrome, or restrictive pulmonary disease.
Exclusion criteria were as follows: (1) died before SBT or accidental extubation; (2) tracheotomy before weaning attempt; (3) intervention lasted less than 24 hours; (4) post-extubation surgery; (5) refusal of resuscitation and reintubation; (6) missing data.
Patients who received prophylactic NIV (BiPAP Vision, Philips Respironics, USA) immediately after extubation were classified as the NIT group. The course of NIV was at least 24 h, but could be interrupted by drinking, feeding, and clearing secretions. Depending on patient respiratory status, NIV could be continued until complete recovery. Positive end-expiratory pressure (PEEP) was set at 4–6 cmH2O, and pressure-support level was initially set at 8 cmH2O (titrated 1–2 cmH2O) to obtain a tidal volume of about 6–8 mL/kg. Fractional inspiratory oxygen ratio (FiO2) was adjusted to maintain peripheral capillary oxygen saturation (SpO2) above 92%.
Patients in the NIT group could also be treated with HFNC (Optiflow, Fisher and Paykel Healthcare, Canada) immediately after extubation for at least 24 h. Flow was initially set at 10 L/min and titrated upwards in 5 L/min steps until patients experienced discomfort. FiO2 was adjusted to maintain SpO2 above 92%. To provide sufficient humidification, the temperature of the heated humidifier was set to 37°C.
Patients in the control group received COT via face mask or nasal cannula. FiO2 was set to achieve SpO2 over 92%. And COT was administered according to patient needs.
The primary outcome was the rate of EF within 7 days following extubation. EF was defined as the need for reintubation or NIV rescue treatment (16). Secondary outcomes included reintubation within 7 days after extubation (2, 12), post-extubation respiratory failure (7), delirium on the day of extubation (17), in-hospital mortality, and post-extubation ICU stay and LOS. Patients were immediately reintubated if any of the following criteria was met: massive aspiration, uncontrollable agitation, sputum retention, hemodynamic deterioration unresponsive to vasoactive drugs, respiratory pauses with loss of consciousness or gasping for air, heart rate < 50 beats per min with loss of alertness, and cardiac or respiratory arrest. And respiratory failure was defined as the presence of any of the criteria below: respiratory rate > 35 breaths per minute, clinical signs suggesting respiratory distress, respiratory acidosis (pH < 7.35 and PaCO2 > 45 mmHg), hypoxemia (SpO2 ≤ 90% or PaO2:FiO2 ratio ≤ 120 mmHg at FIO2 > 0.4), decreased level of consciousness (GCS > 1 point decrease), and agitation. Delirium was defined as a disturbance of consciousness characterized by a sudden onset and a fluctuating course of attention accompanied by a change in perception or cognition. Delirium was routinely measured by ICU nurses using the Confusion Assessment Method for the ICU (CAM-ICU).
The following data were collected retrospectively from the medical records: age, gender, underlying diseases, main reason for intubation, acute physiology and chronic health evaluation II (APACHE II) score at ICU admission and at extubation, duration of MV before extubation, fluid balance and secretion volume 24h before extubation, use of vasopressors at extubation, hemoglobin, and Glasgow Coma Score (GCS). Vital signs and arterial blood gas parameters were collected before SBT and at extubation, including mean arterial pressure, heart rate, respiratory rate, tidal volume, SpO2, rapid shallow breathing index (RSBI), the ratio of SpO2/FiO2 to respiratory rate (ROX index), as well as pH, partial pressure of oxygen (PaO2), partial pressure of carbon dioxide (PaCO2), the ratio of PaO2 to FIO2 (oxygenation index).
Patients were divided into two subgroups based on age (> 65 years and ≤ 65 years) to demonstrate the impact of prophylactic NIT on EF, particularly in elderly high-risk patients. And another subgroup analysis was performed in the NIT group to determine whether HFNC was noninferior to NIV in reducing EF rate.
Due to the retrospective design of the study, propensity score matching (PSM) was performed to reduce the effects of selection bias and possible confounding factors between the two groups. The following variables were selected to generate the propensity score: age, gender, underling diseases, intubation period, APACHE II score at ICU admission and at extubation, fluid volume, secretion volume, hemoglobin, GCS, and physiological parameters before SBT and at extubation. After calculating the propensity scores, we matched patients with similar propensity scores in each group in a 1:1 ratio using the nearest neighbor method, with the caliper width set to 0.1.
Data were summarized as mean ± standard deviation (SD) or median (25th percentile, 75th percentile) depending on distribution. The Mann-Whitney U test was used for group comparisons of continuous variables when the data were abnormally distributed; otherwise, Student’s t-test was applied. Categorical variables were expressed as numbers (percentage) and compared using the chi-square test or Fisher’s exact test as appropriate. Univariate logistic regression analysis was used to identify independent factors related to EF within 7 days after extubation. Variables with p < 0.1 in the univariate analysis and other clinically significant variables were included in the conditional stepwise multivariable logistic regression. All statistical tests were 2-sided and p-values < 0.05 were considered statistically significant. Statistical analysis was performed using IBM SPSS Statistics 26.0 (SPSS Inc., Chicago, IL, USA).
Between March 2018 and December 2023, 3227 patients over 18 years old were admitted to the ICU receiving MV. Of these, 1746 patients were excluded due to MV duration less than 24 h. Among the remaining 1481 patients, 767 were excluded for the following reasons: death (n = 251) or tracheostomy (n = 61) before SBT attempt, accidental extubation (n = 75), without risk factors for reintubation (n = 225), receiving neither NIT nor COT intervention immediately after extubation (n = 17), duration of intervention less than 24 h (n = 48), refusal of resuscitation and reintubation (n = 33), post-extubation surgery (n = 21) and loss of information (n = 36). Overall, we analyzed data from 714 patients, including 440 patients in the NIT group and 274 patients in the COT group. The flow diagram is shown in Figure 1.
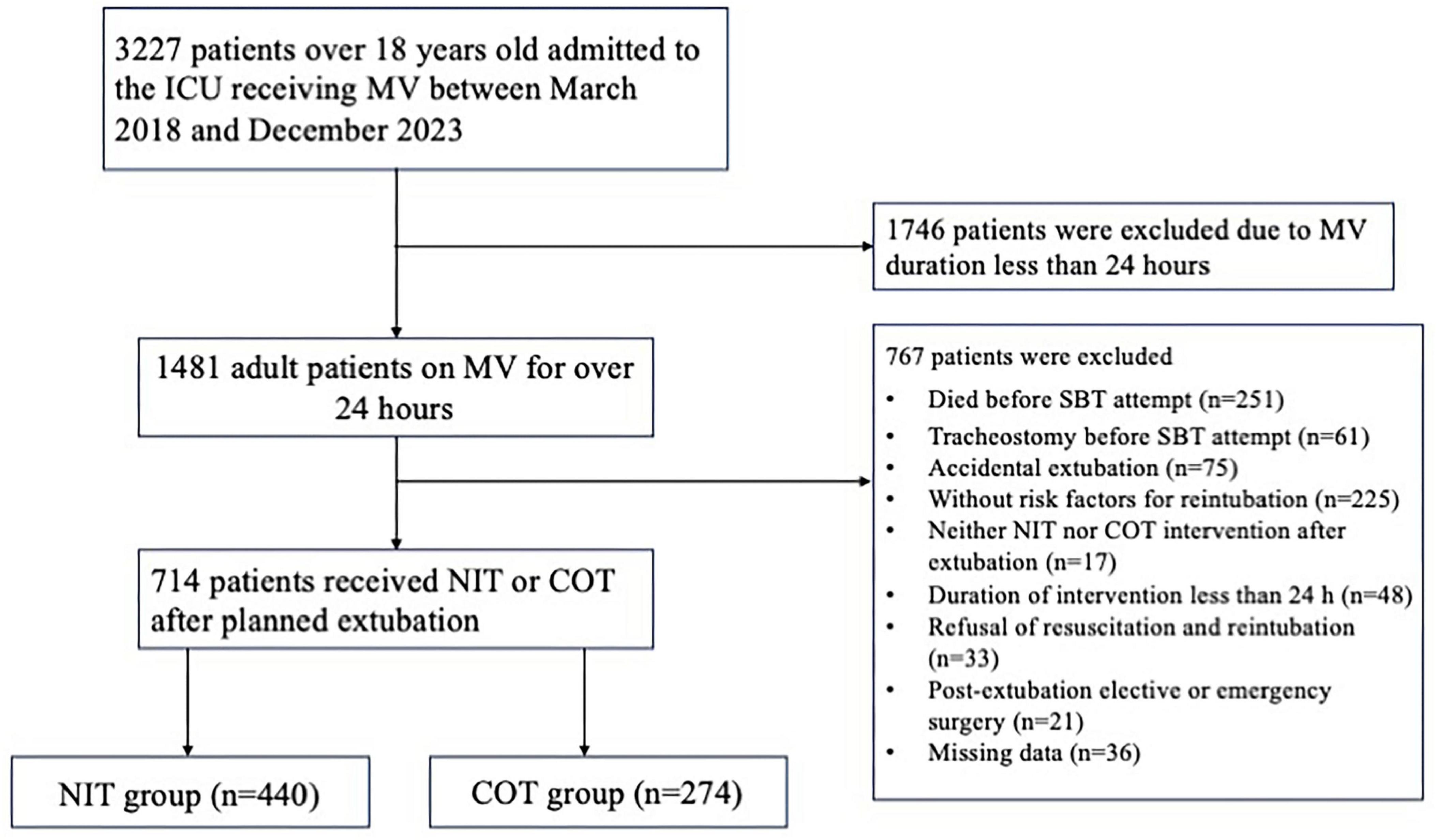
Figure 1. The flow diagram of study population. ICU, intensive care unit; MV, mechanical ventilation; SBT, spontaneous breathing trial; COT, conventional oxygen therapy; NIT, noninvasive oxygen therapy.
The baseline characteristics of both groups are presented in Table 1. There were more male patients in the NIT group than in the COT group (75.5% vs. 67.2%, p = 0.016). The NIT group had a higher proportion of patients with acute exacerbation of chronic obstructive pulmonary disease (AECOPD) (52.0% vs. 21.9%, p < 0.001), and a lower proportion of patients with pneumonia or acute respiratory distress syndrome (ARDS) than the COT group (42.5% vs. 57.3%, p < 0.001). Patients receiving NIT had a longer intubation period than those in the COT group [6 (4, 10) vs. 5.5 (3, 7) d, p = 0.001]. The amount of secretion 24 h before extubation was significantly higher in the NIT group than in the COT group [64 (38, 78) vs. 41 (21, 61) ml, p < 0.001]. The pre-SBT tidal volume was greater in the NIT group compared to the control group (474.0 ± 83.1 vs. 450.9 ± 78.3 ml, p < 0.001). Patients receiving prophylactic NIT had higher PaCO2 levels before SBT (48.7 ± 11.6 vs. 41.4 ± 9.4 mmHg, p < 0.001) and at extubation (47.1 ± 9.5 vs. 40.1 ± 5.9 mmHg, p < 0.001) than those in the COT group. After a 1:1 PSM, 227 matched subjects were included in each group. There were no significant differences in demographic and clinical characteristics between the two matched cohorts, except that patients in the NIT group had a higher secretion volume 24h before extubation (shown in Table 1).
The occurrence of EF in both groups is summarized in Table 2. Of the 440 patients treated with prophylactic NIT, 79 failed to extubate, with a lower incidence than the control group (18.0% vs. 34.3%, p < 0.001). In the propensity-matched cohort, the EF rate in the COT group was 32.2%, nearly 2 times that of the NIT group (p < 0.001).
In the overall cohort, NIT was associated with a lower reintubation rate (10.5% vs. 18.2%, p = 0.003) and in-hospital mortality (17.5% vs. 27.9%, p = 0.001) compared with COT. However, the incidence of respiratory failure and delirium were comparable between the two groups (39.1% vs. 43.6%, p = 0.227; 51.8% vs. 56.6%, p = 0.213). As shown in Table 2, NIT group had longer post-extubation ICU stay and post-extubation LOS than the control group [7(4, 11) vs. 5(3, 9) d, p < 0.001; 9 (6, 15) vs. 7 (4, 16) d, p = 0.004, respectively]. In the propensity-matched cohort, NIT reduced the incidence of reintubation compared with the COT (11.5% vs. 19.8%, p = 0.014). However, there were no significant differences in respiratory failure, delirium, in-hospital mortality, post-extubation ICU stay, and post-extubation LOS between the two matched cohorts.
Among the 714 patients in the study, 512 were over 65 years old (as shown in Table 3). In this subgroup, NIT reduced the rate of EF (16.3% vs. 38.0%, p < 0.001) and reintubation (12.5% vs. 23.0%, p = 0.002) compared with COT, as confirmed in the propensity-matched cohort (13.9% vs. 37.3%, p < 0.001; 8.9% vs. 24.7%, p < 0.001, respectively). In both cohorts, there were no differences in respiratory failure, delirium, post-extubation ICU stay or post-extubation LOS between the two groups. In the non-elderly high-risk subgroup (n = 202), NIT was not superior to COT in reducing EF, reintubation, respiratory failure, delirium, and in-hospital mortality, as demonstrated in the propensity-matched cohort. The LOS after extubation was 10.5d in the NIT group, 3.5d longer than in the COT group (p = 0.002). And in the propensity-matched cohort, NIT also prolonged post-extubation LOS compared with the control group [13 (9, 14) vs. 9 (3, 10)d, p = 0.017).
In the NIT group, 392 patients received prophylactic NIV after planned extubation and 48 patients received prophylactic HFNC. As illustrated in Table 4, HFNC was noninferior to NIV in reducing EF (p = 0.162), reintubation (p = 0.624), respiratory failure (p = 0.771), in-hospital mortality (p = 0.083), and shortening post-extubation ICU stay (p = 0.393) and post-extubation LOS (p = 0.754), which was also confirmed in the propensity-matched cohort. However, patients receiving HFNC had a lower incidence of delirium than those with NIV (35.4% vs. 59.2%, p = 0.002). After PSM, the rate of delirium was comparable between the two groups (46.7% vs. 62.4%, p = 0.100).
Univariate logistic regression analysis showed that there were significant differences in APACHE II score at extubation, fluid balance volume, secretion volume, intervention protocol, pre-SBT PaCO2, ROX index, RSBI, PEEP and delirium between the failed extubation group and the successful extubation group. After the above variables were inserted into the multivariable logistic regression analysis (as shown in Table 5), we found that prophylactic NIT was a protective factor for EF, both in the overall cohort (odds ratio [OR] = 0.20, 95% confidence interval [CI]: 0.06–0.73, p = 0.014) and in the propensity-matched cohort (OR = 0.06, 95% CI: 0.01–0.30, p = 0.001). Higher fluid balance 24h before extubation (OR = 1.02, 95% CI: 1.00–1.03, p = 0.002, for the overall cohort; OR = 1.01, 95% CI: 1.00–1.02, p = 0.013, for the propensity-matched cohort) and higher pre-extubation RSBI (OR = 1.19, 95% CI: 1.10–1.28, p < 0.001, for the overall cohort; OR = 1.17, 95%CI: 1.10–1.24, p < 0.001, for the propensity-matched cohort) were associated with an increased risk of EF. Delirium on the day of extubation appeared to be a risk factor for EF, both in the overall cohort (OR = 1.96, 95% CI: 1.27–2.54, p = 0.029) and in the propensity-matched cohort (OR = 1.78, 95% CI: 1.32–1.94, p = 0.032). Higher pre-SBT ROX index appeared to be a protective factor against EF (OR = 0.80, 95% CI: 0.64–1.00, p = 0.045, for the overall cohort; OR = 0.59, 95% CI: 0.37–0.93, p = 0.022, for the propensity-matched cohort).
In this cohort study, prophylactic NIT (including NIV and HFNC) was superior to COT in reducing the rate of EF within 7 days after extubation in patients at high-risk of reintubation, especially in those older than 65 years. HFNC was noninferior to NIV in high-risk patients. In addition, higher fluid balance 24 h before extubation, lower pre-SBT ROX index, higher pre-extubation RSBI, and delirium on the day of extubation increased the risk of EF.
NIT was associated with a lower incidence of EF and reintubation in high-risk patients. The high success rate may be due to the superiority of NIV and HFNC over COT. As we know, NIV administered following extubation opens the upper airway, prevents alveolar collapse, and improves oxygenation (18). In high-risk patients, inspiratory positive airway pressure (IPAP) can reduce respiratory workload and compensate for increased airway resistance (19). Expiratory positive airway pressure (EPAP) increases end-expiratory lung volume and decreases venous return, especially in patients with congestive heart failure (20). Compared with COT, HFNC provides more predictable FiO2 and preserves the mucosal function (21). In addition, HFNC generates a positive airway pressure (between 2 and 8 cmH2O at the pharyngeal level) similar to positive end-expiratory pressure (PEEP), which may benefit high-risk patients (22–24).
However, a recent RCT indicated that the application of NIT after extubation was not able to prevent reintubation compared with usual care, contrary to our results (15). The different findings may be related to the study population. In the study by Casey et al. (15), critically ill adult patients undergoing MV were included, whereas we only focused on mechanically ventilated patients with high-risk factors for reintubation. Moreover, in that study (15), patients in the usual-care group could also be treated with NIV or HFNC at the discretion of the attending physicians, which may reduce the occurrence of reintubation. Furthermore, in the study by Casey et al. (15), HFNC was predominantly used in the NIT group, which may influence the efficacy of NIT. Therefore, more studies are needed to investigate the effectiveness of NIT on reintubation and EF.
In the subgroup analysis, NIT immediately after extubation benefited elderly patients, which was consistent with a cohort study (25). However, we found no effect of NIT on EF and reintubation in non-elderly high-risk patients compared with COT. In fact, age is an important factor in reintubation (26). In addition to being older than 65 years, elderly patients are prone to be complicated with other risk factors, such as COPD and chronic heart failure. It has been suggested that patients with ≥ 4 risk factors may respond better to NIV (27), which may explain why NIT is more beneficial in older patients. In addition, the duration of prophylactic use of NIT varies by individual, which may affect the efficacy of NIT on EF and reintubation. Further studies are needed to determine the effect of the number of risk factors and duration of intervention on EF.
The respiratory support provided by HFNC is limited, as it may not provide stable positive pressure like NIV (27). However, subgroup analysis of the present study demonstrated that HFNC was noninferior to NIV in reducing EF, which was in accordance with the results of Hernández et al. (12). Numerous studies have confirmed that HFNC is significantly more comfortable and tolerable than NIV (12, 28, 29). In fact, the heating and humidification functions of HFNC allow gas delivery at an optimal humidity, effectively promoting secretion clearance while avoiding side effects such as mucosal dryness (30, 31). Interestingly, we found that HFNC tended to reduce the incidence of delirium, which was in agreement with the findings of Hernández et al. (12) and Stéphan et al. (28). Furthermore, HFNC has a CO2 flushing effect on the nasopharyngeal space, thereby decreasing anatomical dead space ventilation and CO2 rebreathing (32, 33).
In addition to prophylactic NIT, multivariate logistic regression analysis in our study showed that a higher ROX index before SBT was associated with a reduced risk of EF. The ROX index, defined as the ratio of SpO2/FiO2 to respiratory rate, is often used as a predictor of reintubation after HFNC failure, with moderate specificity (34, 35). An increasing number of articles have reported the role of ROX index in predicting NIV failure, but there is population heterogeneity, with different time periods for ROX index measurement (36, 37). A retrospective study showed that the ROX index at 6 h after ICU admission helped identify patients with ARDS at risk of NIT failure. Zablockis et al. (38) reported the role of ROX index within 24 h of admission in predicting NIV failure in patients with acute hypoxemic respiratory failure (38). To our knowledge, this study indicated for the first time that pre-SBT ROX index may be associated with the development of EF in high-risk patients. More prospective studies are needed in the future to verify the validity of the ROX index in predicting EF at different time points and to find the best threshold.
In the present study, higher fluid balance 24 h before extubation increased the risk of EF in high-risk patients, which was in agreement with previous studies (39, 40). Weaning-induced pulmonary edema is a common reason for EF (41). And cardiac dysfunction can occur during decannulation owing to increased preload and afterload of the right and left ventricles, triggering EF, especially in high-risk patients (42). Therefore, restricted fluid therapy may be one of the key measures for successful extubation.
Delirium is a common medical problem that is often characterized by transient fluctuations in attention, confusion, and disturbance of thought (43). Delirium has been reported to occur in 50 to 80% of mechanically ventilated patients (44). The incidence of delirium was as high as 54.8% in the high-risk patients included in this study. Older age, ventilator use, and benzodiazepine use increased the risk of delirium in ICU patients (45). This was confirmed in our subgroup analysis, which showed a higher incidence of delirium in patients older than 65 years than in those aged ≤ 65 years. In addition, delirium is a risk factor for EF and reintubation, which was consistent with our results. This may be related to the fact that delirium impedes pulmonary rehabilitation and out-of-bed activities. Not only that, but patients who develop delirium are often treated with benzodiazepines, and the cumulative effect of these sedative drugs can impair mental status after tracheal intubation removal, leading to EF and reintubation.
Although early weaning from MV after successful SBT improves prognosis, EF is inevitable and significantly increases the rate of reintubation. Therefore, it is important to choose an appropriate respiratory strategy to prevent EF, especially for high-risk patients. To prevent EF and increase the success rate of extubation, the modalities of COT, HFNC, and NIV are commonly used to support breathing. In clinical practice, NIV or HFNC could be used prophylactically after planned extubation to reduce the risk of EF in high-risk patients. And NIT is more effective in those older than 65 years. Reducing the incidence of EF and reintubation, and shortening the length of hospital stay are not only beneficial to patients and their families, but also avoid the waste of medical resources. In addition, combinational use of HFNC and NIV seems to be a promising method in post-extubated patients because the addition of HFNC to NIV could, at least theoretically, further improve gas exchange and decrease the work of breathing. In the future, larger sample size randomized controlled trials are needed to explore the effect of combination therapy on extubation failure and reintubation in high-risk patients.
There are several limitations in the study. First, this was a cohort study conducted in a single center, limiting the generalizability of the results. Evaluation methods and parameter settings in different hospitals may affect the effectiveness of NIT on EF. In the future, we will conduct a related multicenter randomized controlled study to further explore the effect of NIT in high-risk patients. Second, due to the nature of retrospective study, there may be potential biases such as selection bias, recalling bias, and confounding factors. These biases may affect the validity of the findings. To address selection bias, we established clear inclusion and exclusion criteria and used uniform and accepted diagnostic criteria. However, PSM was performed in the study to reduce the effect of selection bias and possible confounders between the two groups. The possibility of residual confounding may still exist after PSM. To further control for confounders, other statistical methods can be used, such as stratified analyses or multivariate adjustment analyses, which can help to identify and control for additional confounders. Considering the limited sample size, only two subgroup analyses were performed in this study. Third, although the assessment of SBT is standardized, clinical guidelines are updated over time and the attending physicians make the final decision. Fourth, the small sample size, particularly in the subgroup analysis involving the efficacy of NIV and HFNC, may weaken the strength of the evidence. However, HFNC was proven to be noninferior to NIV in reducing reintubation in relevant multicenter RCTs. Finally, respiratory mechanics parameters such as cough peak expiratory flow (CPEF), peak inspiratory pressure and peak expiratory pressure were missed due to retrospective data collection from medical records. These relevant parameters may be associated with EF. And CPEF is considered to be a useful tool for predicting extubation (46). A CPEF of < 60 L/min was associated with a significantly increased risk of EF (47). The advantage of CPEF is that it is simple, inexpensive, portable, easy to repeat, and has the potential to prevent reintubation. More respiratory mechanical parameters are needed to predict extubation success or failure in the future.
In conclusion, prophylactic use of NIT following planned extubation is effective in reducing the rate of EF in high-risk patients, especially in those over 65 age of years. HFNC is an alternative treatment to NIV in high-risk patients and increases patient comfort and tolerance. Furthermore, fluid balance, RSBI, ROX index and delirium may be good predictors of EF in high-risk patients.
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
The studies involving humans were approved by the institutional ethics committee of the First Affiliated Hospital of Chongqing Medical University. The studies were conducted in accordance with the local legislation and institutional requirements. The ethics committee/institutional review board waived the requirement of written informed consent for participation from the participants or the participants’ legal guardians/next of kin because Informed consent was waived because of the retrospective observational nature of the study.
XZ: Writing – original draft. LL: Writing – original draft. MM: Writing – review and editing. XL: Writing – review and editing.
The author(s) declare that no financial support was received for the research, authorship, and/or publication of the article.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Kaur R, Vines D, Patel A, Lugo-Robles R, Balk R. Early identification of extubation failure using integrated pulmonary index and high-risk factors. Respir Care. (2021) 66:1542–8. doi: 10.4187/respcare.08656
2. Ferrer M, Sellarés J, Valencia M, Carrillo A, Gonzalez G, Badia J, et al. Non-invasive ventilation after extubation in hypercapnic patients with chronic respiratory disorders: Randomised controlled trial. Lancet. (2009) 374:1082–8. doi: 10.1016/S0140-6736(09)61038-2
3. Thille A, Richard J, Brochard L. The decision to extubate in the intensive care unit. Am J Respir Crit Care Med. (2013) 187:1294–302. doi: 10.1164/rccm.201208-1523CI
4. Ely E, Shintani A, Truman B, Speroff T, Gordon S, Harrell F Jr., et al. Delirium as a predictor of mortality in mechanically ventilated patients in the intensive care unit. JAMA. (2004) 291:1753–62. doi: 10.1001/jama.291.14.1753
5. Thille A, Boissier F, Ben Ghezala H, Razazi K, Mekontso-Dessap A, Brun-Buisson C. Risk factors for and prediction by caregivers of extubation failure in ICU patients: A prospective study. Crit Care Med. (2015) 43:613–20. doi: 10.1097/CCM.0000000000000748
6. Vargas F, Clavel M, Sanchez-Verlan P, Garnier S, Boyer A, Bui H, et al. Intermittent noninvasive ventilation after extubation in patients with chronic respiratory disorders: A multicenter randomized controlled trial (VHYPER). Intensive Care Med. (2017) 43:1626–36. doi: 10.1007/s00134-017-4785-1
7. Thille A, Muller G, Gacouin A, Coudroy R, Decavèle M, Sonneville R, et al. Effect of postextubation high-flow nasal oxygen with noninvasive ventilation vs high-flow nasal oxygen alone on reintubation among patients at high risk of extubation failure: A randomized clinical trial. JAMA. (2019) 322:1465–75. doi: 10.1001/jama.2019.14901
8. Tan D, Walline J, Ling B, Xu Y, Sun J, Wang B, et al. High-flow nasal cannula oxygen therapy versus non-invasive ventilation for chronic obstructive pulmonary disease patients after extubation: A multicenter, randomized controlled trial. Crit Care. (2020) 24:489. doi: 10.1186/s13054-020-03214-9
9. Abrard S, Rineau E, Seegers V, Lebrec N, Sargentini C, Jeanneteau A, et al. Postoperative prophylactic intermittent noninvasive ventilation versus usual postoperative care for patients at high risk of pulmonary complications: A multicentre randomised trial. Br J Anaesth. (2023) 130:e160–8. doi: 10.1016/j.bja.2021.11.033
10. Maggiore S, Jaber S, Grieco D, Mancebo J, Zakynthinos S, Demoule A, et al. High-flow versus venturimask oxygen therapy to prevent reintubation in hypoxemic patients after extubation: A multicenter randomized clinical trial. Am J Respir Crit Care Med. (2022) 206:1452–62. doi: 10.1164/rccm.202201-0065OC
11. Wang Q, Peng Y, Xu S, Lin L, Chen L, Lin Y. The efficacy of high-flow nasal cannula (HFNC) versus non-invasive ventilation (NIV) in patients at high risk of extubation failure: A systematic review and meta-analysis. Eur J Med Res. (2023) 28:120. doi: 10.1186/s40001-023-01076-9
12. Hernández G, Vaquero C, Colinas L, Cuena R, González P, Canabal A, et al. Effect of postextubation high-flow nasal cannula vs noninvasive ventilation on reintubation and postextubation respiratory failure in high-risk patients: A randomized clinical trial. JAMA. (2016) 316:1565–74. doi: 10.1001/jama.2016.14194
13. Sryma P, Mittal S, Mohan A, Madan K, Tiwari P, Bhatnagar S, et al. Effect of proning in patients with COVID-19 acute hypoxemic respiratory failure receiving noninvasive oxygen therapy. Lung India. (2021) 38:S6–10. doi: 10.4103/lungindia.lungindia_794_20
14. Subramaniam A, Haji J, Kumar P, Ramanathan K, Rajamani A. Noninvasive oxygen strategies to manage confirmed Covid-19 patients in indian intensive care units: A survey. Indian J Crit Care Med. (2020) 24:926–31. doi: 10.5005/jp-journals-10071-23640
15. Casey J, Vaughan E, Lloyd B, Billas P, Jackson K, Hall E, et al. Protocolized postextubation respiratory support to prevent reintubation: A randomized clinical trial. Am J Respir Crit Care Med. (2021) 204:294–302. doi: 10.1164/rccm.202009-3561OC
16. Thille A, Boissier F, Ben-Ghezala H, Razazi K, Mekontso-Dessap A, Brun-Buisson C, et al. Easily identified at-risk patients for extubation failure may benefit from noninvasive ventilation: A prospective before-after study. Crit Care. (2016) 20:48. doi: 10.1186/s13054-016-1228-2
17. Michetti C, Griffen M, Teicher E, Rodriguez J, Seoudi H, Liu C, et al. FRIEND or FOE: A prospective evaluation of risk factors for reintubation in surgical and trauma patients. Am J Surg. (2018) 216:1056–62. doi: 10.1016/j.amjsurg.2018.07.004
18. Abrard S, Jean L, Rineau E, Dupré P, Léger M, Lasocki S. Safety of changes in the use of noninvasive ventilation and high flow oxygen therapy on reintubation in a surgical intensive care unit: A retrospective cohort study. PLoS One. (2021) 16:e0249035. doi: 10.1371/journal.pone.0249035
19. Thille A, Coudroy R, Nay M, Gacouin A, Decavèle M, Sonneville R, et al. beneficial effects of noninvasive ventilation after extubation in obese or overweight patients: A post hoc analysis of a randomized clinical trial. Am J Respir Crit Care Med. (2022) 205:440–9. doi: 10.1164/rccm.202112-2776LE
20. Thomrongpairoj P, Tongyoo S, Tragulmongkol W, Permpikul C. Factors predicting failure of noninvasive ventilation assist for preventing reintubation among medical critically ill patients. J Crit Care. (2017) 38:177–81. doi: 10.1016/j.jcrc.2016.11.038
21. Chanques G, Riboulet F, Molinari N, Carr J, Jung B, Prades A, et al. Comparison of three high flow oxygen therapy delivery devices: A clinical physiological cross-over study. Minerva Anestesiol. (2013) 79:1344–55. doi: 10.1186/ISRCTN15995925
22. Parke R, Bloch A, McGuinness S. Effect of very-high-flow nasal therapy on airway pressure and end-expiratory lung impedance in healthy volunteers. Respir Care. (2015) 60:1397–403. doi: 10.4187/respcare.04028
23. Parke R, McGuinness S. Pressures delivered by nasal high flow oxygen during all phases of the respiratory cycle. Respir Care. (2013) 58:1621–4. doi: 10.4187/respcare.02358
24. Natalini D, Grieco D, Santantonio M, Mincione L, Toni F, Anzellotti G, et al. Physiological effects of high-flow oxygen in tracheostomized patients. Ann Intensive Care. (2019) 9:114. doi: 10.1186/s13613-019-0591-y
25. Duan J, Bai L, Zhou L, Han X, Huang S. Decreasing re-intubation using prophylactic noninvasive ventilation in elderly patients: A propensity-matched study. J Crit Care. (2019) 50:77–81. doi: 10.1016/j.jcrc.2018.11.019
26. Valley T, Sjoding M, Ryan A, Iwashyna T, Cooke C. Association of intensive care unit admission with mortality among older patients with pneumonia. JAMA. (2015) 314:1272–9. doi: 10.1001/jama.2015.11068
27. Hernández G, Paredes I, Moran F, Buj M, Colinas L, Rodríguez M, et al. Effect of postextubation noninvasive ventilation with active humidification vs high-flow nasal cannula on reintubation in patients at very high risk for extubation failure: A randomized trial. Intensive Care Med. (2022) 48:1751–9. doi: 10.1007/s00134-022-06919-3
28. Stéphan F, Barrucand B, Petit P, Rézaiguia-Delclaux S, Médard A, Delannoy B, et al. High-flow nasal oxygen vs noninvasive positive airway pressure in hypoxemic patients after cardiothoracic surgery: A randomized clinical trial. JAMA. (2015) 313:2331–9. doi: 10.1001/jama.2015.5213
29. Maggiore S, Idone F, Vaschetto R, Festa R, Cataldo A, Antonicelli F, et al. Nasal high-flow versus Venturi mask oxygen therapy after extubation. Effects on oxygenation, comfort, and clinical outcome. Am J Respir Crit Care Med. (2014) 190:282–8. doi: 10.1164/rccm.201402-0364OC
30. Cortegiani A, Accurso G, Mercadante S, Giarratano A, Gregoretti C. High flow nasal therapy in perioperative medicine: From operating room to general ward. BMC Anesthesiol. (2018) 18:166. doi: 10.1186/s12871-018-0623-4
31. Frat J, Brugiere B, Ragot S, Chatellier D, Veinstein A, Goudet V, et al. Sequential application of oxygen therapy via high-flow nasal cannula and noninvasive ventilation in acute respiratory failure: An observational pilot study. Respir Care. (2015) 60:170–8. doi: 10.4187/respcare.03075
32. Nishimura M. High-flow nasal cannula oxygen therapy in adults: Physiological benefits, indication, clinical benefits, and adverse effects. Respir Care. (2016) 61:529–41. doi: 10.4187/respcare.04577
33. Liu Q, Shan M, Zhu H, Cao J, Chen R. Noninvasive ventilation with a helmet in patients with acute respiratory failure caused by chest trauma: A randomized controlled trial. Sci Rep. (2020) 10:21489. doi: 10.1038/s41598-020-78607-5
34. Fuentes Y, Carvajal K, Cardona S, Montaño G, Ibáñez-Prada, Bastidas A, et al. The respiratory rate-oxygenation index predicts failure of post-extubation high-flow nasal cannula therapy in intensive care unit patients: A retrospective cohort study. Rev Bras Ter Intensiva. (2022) 34:360–6. doi: 10.5935/0103-507X.20220477-en
35. Ferrer S, Sancho J, Bocigas I, Bures E, Mora H, Monclou E, et al. ROX index as predictor of high flow nasal cannula therapy success in acute respiratory failure due to SARS-CoV-2. Respir Med. (2021) 189:106638. doi: 10.1016/j.rmed.2021.106638
36. L S, Sehgal IS, Kajal K, Kataria S, Premkumar M, Singla K, et al. Factors associated with non-invasive oxygen therapy failure in COVID-19 pneumonia: A single center, retrospective study in a tertiary hospital in North India. Cureus. (2022) 14: e29721.
37. Innocenti F, Lazzari C, Paolucci E, De Paris A, Lagomarsini A, Guerra F, et al. Role of prognostic scores in predicting in-hospital mortality and failure of non-invasive ventilation in adults with COVID-19. Intern Emerg Med. (2022) 17:2367–77. doi: 10.1007/s11739-022-03058-x
38. Zablockis R, Šlekytė G, Mereškevičienė R, Kėvelaitienė K, Zablockienė B, Danila E. Predictors of noninvasive respiratory support failure in COVID-19 patients: A prospective observational study. Medicina (Kaunas). (2022) 58:769. doi: 10.3390/medicina58060769
39. Santos P, Ribas A, Quadros T, Blattner C, Boniatti M. Postextubation fluid balance is associated with extubation failure: A cohort study. Rev Bras Ter Intensiva. (2021) 33:422–7. doi: 10.5935/0103-507X.20210057
40. Maezawa S, Kudo D, Miyagawa N, Yamanouchi S, Kushimoto S. Association of body weight change and fluid balance with extubation failure in intensive care unit patients: A single-center observational study. J Intensive Care Med. (2021) 36:175–81. doi: 10.1177/0885066619887694
41. Frutos-Vivar F, Ferguson N, Esteban A, Epstein S, Arabi Y, Apezteguía C, et al. Risk factors for extubation failure in patients following a successful spontaneous breathing trial. Chest. (2006) 130:1664–71. doi: 10.1378/chest.130.6.1664
42. Upadya A, Tilluckdharry L, Muralidharan V, Amoateng-Adjepong Y, Manthous C. Fluid balance and weaning outcomes. Intensive Care Med. (2005) 31:1643–7. doi: 10.1007/s00134-005-2801-3
43. Kotfis K, Marra A, Ely EW. ICU delirium - a diagnostic and therapeutic challenge in the intensive care unit. Anaesthesiol Intensive Ther. (2018) 50:160–7. doi: 10.5603/AIT.a2018.0011
44. Ely E, Inouye S, Bernard G, Gordon S, Francis J, May L, et al. Delirium in mechanically ventilated patients: Validity and reliability of the confusion assessment method for the intensive care unit (CAM-ICU). JAMA. (2001) 286:2703–10. doi: 10.1001/jama.286.21.2703
45. Devlin J, Skrobik Y, Gélinas C, Needham D, Slooter A, Pandharipande P, et al. Clinical practice guidelines for the prevention and management of pain, agitation/sedation, delirium, immobility, and sleep disruption in adult patients in the ICU. Crit Care Med. (2018) 46:e825–73.
46. Abedini M, Froutan R, Bagheri Moghaddam A, Mazloum S. Comparison of “cough peak expiratory flow measurement” and “cough strength measurement using the white card test” in extubation success: A randomized controlled trial. J Res Med Sci. (2020) 25:52. doi: 10.4103/jrms.JRMS_939_19
Keywords: noninvasive oxygen therapy, extubation failure, high-risk patients, delirium, noninvasive ventilation, high-flow nasal cannula
Citation: Zheng X, Lu L, Ma M and Lei X (2024) Effect of prophylactic noninvasive oxygen therapy after planned extubation on extubation failure in high-risk patients: a retrospective propensity score-matched cohort study. Front. Med. 11:1481083. doi: 10.3389/fmed.2024.1481083
Received: 15 August 2024; Accepted: 11 September 2024;
Published: 26 September 2024.
Edited by:
Bin Yang, First Affiliated Hospital of Xiamen University, ChinaReviewed by:
Jie Tian, Sichuan University, ChinaCopyright © 2024 Zheng, Lu, Ma and Lei. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiaofeng Lei, MzQ5NTkyNjUwOUBxcS5jb20=
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.