
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Med. , 07 November 2024
Sec. Nuclear Medicine
Volume 11 - 2024 | https://doi.org/10.3389/fmed.2024.1464779
This article is part of the Research Topic Advances in PET-CT Imaging View all 14 articles
Purpose: [18F]AlF-NOTA-FAPI-04 demonstrates significant physiological uptake in the gallbladder and biliary tract system, representing a limitation of this positron emission tomography (PET) tracer. The aim of this study was to evaluate the impact of milk consumed prior to a PET/CT scan on [18F]AlF-NOTA-FAPI-04 uptake in normal abdominal organs.
Materials and methods: A total of 86 patients who underwent [18F]AlF-NOTA-FAPI-04 PET/CT imaging took part in this single-center retrospective clinical study at the Hunan Cancer Hospital between December 2020 and August 2021. Patients were divided into two groups according to their pre-PET scan diet: treated group, who consumed 250 mL of milk 10 ± 5 min after the tracer injection, while the control group was permitted no food intake subsequent to the radiotracer administration. The mean standardized uptake value (SUVmean) of gallbladder, liver, small intestine and pancreas were measured in 18F-FAPI and 18F-FDG PET/CT.
Results: There was a statistically significant difference in the 18F-FAPI uptake in the gallbladder between the treated group and the control group (p < 0.001). The average SUVmean in the treated group was 2.19 ± 2.01, which was significantly lower than the average SUVmean of 10.04 ± 9.66 in the control group. In the subgroup analysis of patients who underwent paired [18F]FDG and [18F]FAPI PET/CT scans, the 18F-FAPI uptake of liver and small intestine was significantly lower than the 18F-FDG uptake in both the treated group and the control group (p < 0.001).
Conclusion: This study suggests that milk consumption decreases physiological 18F-FAPI uptake in the gallbladder, potentially enhancing the diagnostic accuracy for gallbladder cancer.
Fibroblast activation protein (FAP), highly expressed in cancer-associated fibroblasts, is a type II transmembrane glycoprotein enzyme with peptidase activity (1–4). FAP inhibitors (FAPIs) labeled with radioactive tracers (68Ga, 18F, or 177Lu) are currently utilized in clinical practice for diagnosis and treatment in a wide range of malignant tumors and their associated metastases, demonstrating significant superiority over 18F-fluoro-2-deoxy-D-glucose (18F-FDG) in certain contexts. FAPI PET/CT has considerable promise for precise cancer assessment (5–9).
Among the extensively studied and reported PET molecular imaging probes, 68Ga-FAPI-04 demonstrates a remarkably high tumor-to-background ratio across more than 30 different types of cancer (10–13). However, the application of 68Ga-FAPI-04 is limited due to its relatively short half-life (68 min), low overall activity production (only sufficient for 2–3 patients in one batch), and sub-optimal spatial resolution. Due to its longer half-life of 110 min compared to [68Ga], [18F] facilitates large-scale production and long-distance transportation, making it the most commonly used radioisotope in clinical practice (14, 15). Several 18F-labeled FAPIs have been developed for either preclinical or clinical evaluation (16–21). [18F]AlF-NOTA-FAPI-04 is one of the 18F-labeled FAPIs that has demonstrated superior tumor imaging capabilities in several clinical evaluations, exhibiting improved physical properties, high yields, and favorable imaging characteristics. [18F]AIF-NOTA-FAPI-04 has the potential to serve as an ideal radiopharmaceutical for PET imaging (18, 22, 23). However, there are abundant differences in biodistribution between 18F-FAPI and 18F-FDG. Although 18F-FAPI uptake was lower than 18F-FDG in most normal tissues, the SUVmean of the gallbladder and pancreas was notably higher in 18F-FAPI compared to 18F-FDG (24). Previous studies have reported that 18F-FAPI demonstrates significant physiological uptake in the gallbladder and biliary tract system, which hampers the detection of their associated malignancies (20, 24). Oral intake of milk after 18F-FAPI administration may increase the hepatobiliary clearance rate of 18F-FAPI. Full-fat milk can induce the secretion of cholecystokinin(CCK) from the cells of the small intestine mucosa, with effects similar to those observed after direct administration of cholecystokinin, potentially stimulating gallbladder contraction and accelerating the transit of the tracer from the liver to the gastrointestinal tract (25). This approach, which involves the consumption of items such as full-fat milk or milkshakes, is commonly employed in nuclear medicine for myocardial perfusion imaging (26, 27).
The aim of this study was to assess the impact of fat intake on normal abdominal organs uptake of [18F] AlF-NOTA-FAPI-04 and to conduct a comparison on the physiological abdominal organ uptake of [18F] AlF-NOTA-FAPI-04 and 18F-FDG.
A total of 86 patients who underwent whole-body/abdominal [18F]AlF-NOTA-FAPI-04 PET/CT imaging at the Hunan Cancer Hospital between December 2020 and August 2021 were included in our study. Informed consent was obtained from each participant prior to 18F-FAPI PET/CT imaging. Patients were divided into two groups according to their diet before the PET scan: treated group, comprised of patients who consumed 250 mL milk 10 ± 5 min after the tracer injection. The volume of the milk was 250 mL, and contained 284 kJ/100 mL, fat content per 100 milliliters was 4.0 g. Control group, permitted no food intake subsequent to the radiotracer administration, which was the standard patient preparation. 64 patients underwent paired 18F-FDG and [18F]AlF-NOTA-FAPI-04 PET/CT scans.
This was a single-center retrospective study conducted at Hunan Cancer Hospital. This research complied with the Declaration of Helsinki’s recommendations for biomedical research involving human subjects and received approval from the Medical Ethics Committee of Hunan Cancer Hospital. Prior to the scan, patients in the treated group did give verbal informed consent to consume milk. The primary endpoint of this study was the physiological 18F-FAPI and 18F-FDG uptake in the gallbladder, liver, small intestine and pancreas, measured as mean standardized uptake value (SUVmean).
The F-18 radionuclide was synthesized in situ by subjecting O-18-H2O to a 9.8 MeV proton bombardment using a GE MINItrace cyclotron (GE HealthCare, Milwaukee, WI, USA). The FAPI-04 precursor was procured from PET Science and Technology CO., LTD (Beijing, China). [18F]AlF-NOTA-FAPI-04 was labeled using the procedure detailed by Jiang et al. (18). The manufacturing of 18F-FDG followed the standard procedure, utilizing the coincidence 18F-FDG synthesis module (AIO; TRSIS, China). Both [18F] AlF-NOTA-FAPI-04 and 18F-FDG exhibited a radiochemical purity exceeding 95%. The final product was sterile and met all the requirements stipulated by our institution before to use.
Patients must strictly fast for 4 h before imaging. The administered intravenous dose of both 18F-FAPI and 18F-FDG was 3.7 MBq (0.1 mCi)/kg. Fifteen minutes before the 18F-FDG injection, height, weight, and fasting blood glucose levels should be measured, with the blood glucose level required to be below 7.0 mmol/L; otherwise, an appropriate amount of insulin should be administered subcutaneously to ensure compliance with the standard. An hour following intravenous delivery, all patients underwent a PET/CT scan on a digital detector scanner (Discovery MI, GE, Healthcare, Milwaukee, WI, USA). The computed tomography (CT) scan covered the area from the whole skull to the upper thighs, using a tube voltage of 110 kV, a tube current of 120 mA, and a slice thickness of 3.75 mm. After the CT scan, a PET scan was done right away in 3D acquisition mode, taking 2 min for each position and 5 to 6-bed positions. Ordered subset expectation maximization (OSEM) was used to construct 18F-FDG and 18F-FAPI PET/CT images on an Advantage Workstation (AW 4.7, GE HealthCare, Milwaukee, WI, USA). After attenuation correction using the CT data, the reconstructed images were co-registered for analysis. The paired 18F-FDG and [18F]AlF-NOTA-FAPI-04 PET/CT scans were performed within 14 days.
All images were independently reviewed by two board-certified nuclear medicine physicians with expertise in interpreting PET/CT examinations. Any discrepancies in the image interpretations were resolved through consensus discussion. The intensity of physiological 18F-FAPI and 18F-FDG uptake in organs was quantified as the mean standardized uptake value (SUVmean). Areas of interest were drawn from tissues on the gallbladder, liver (right lobe), proximal jejunum and pancreas (tail/corpus). The volumes of interest (VOIs) were drawn in three consecutive slices on the PET images focused on the maximum voxel value for the mentioned organs, and the mean values of the SUV in the VOIs were recorded. To minimize a partial volume effect, VOIs were always positioned inside the bounds of the activity distribution. VOIs were delineated at 1 cm for minor tissues and at 2 cm for major organs such as the liver. Additionally, VOIs included intestinal walls and possible luminal content but not extraintestinal content. SUVmean were automatically extracted from the defined VOIs using the AW Workstation.
SPSS (version 25.0; SPSS Inc., Chicago, IL, USA) was employed for the analysis. Continuous variables were expressed as mean ± standard deviation (SD) when the data were normally distributed, otherwise, the median and interquartile range were reported. Categorical variables were represented as percentages (%). Fisher’s exact test or chi-square test was used to compare unordered categorical variables represented as numbers and percentages. Semiquantitative parameters measured using the 18F-FAPI and 18F-FDG were analyzed using the Mann–Whitney U test, with statistical significance defined by a probability (p) value ≤0.05.
Our cohort initially enrolled 105 consecutive patients, however, after excluding 15 patients with cholecystectomy and 4 patients with poor image quality, a total of 86 patients were ultimately included for the evaluation of the effect of pre-scan dietary preparations on the physiologic 18F-FAPI and 18F-FDG uptake of the gallbladder. The characteristics of the patients are summarized in Table 1. The treated group comprised 67 patients who drank milk subsequent to the radiotracer administration, while the Control group included 19 patients who underwent no food intake after the tracer injection. No statistically significant differences were observed between the two groups of patients in terms of age, gender, weight, body mass index, injection dose, and history of gastrectomy. All patients tolerated this test well, with no drug-related pharmacologic effects or physiologic reactions. No patient noticed any symptoms or experienced any adverse reactions during the injection process until the end of the examination.
The physiological 18F-FAPI uptake in various organs for the two groups are presented in Table 2. Quantitative analysis revealed moderate-to-low uptake in the average SUVmean in the liver, small intestine and pancreas. No significant differences were observed in the physiologic 18F-FAPI uptake in these organs between treated group and control group. There was a statistically significant difference in the 18F-FAPI uptake in the gallbladder between the treated group and the control group (p < 0.001). The average SUVmean in the treated group was 2.19 ± 2.01, which was significantly lower than the average SUVmean of 10.04 ± 9.66 in the control group. Figure 1 illustrates the distribution of physiological tracer uptake in the gallbladder, liver, small intestine and pancreas between the treated group and control group.
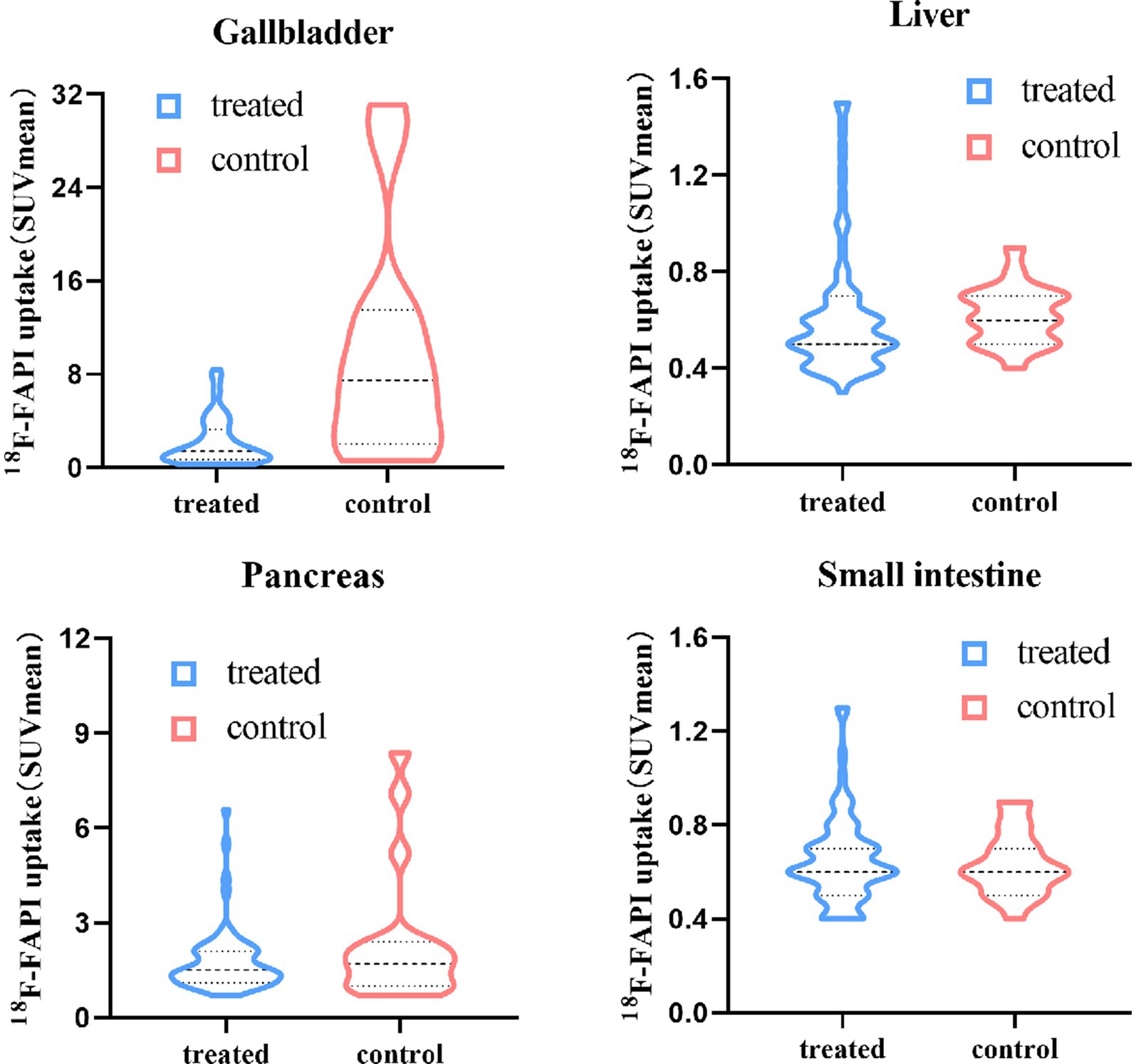
Figure 1. Physiological 18F-FAPI uptake in gallbladder, liver, small intestine and pancreas for different food intake protocols.
Subgroup analysis was performed on patients (n = 64) who underwent both 18F-FAPI and 18F-FDG PET/CT scan, In the treated group and control group, the 18F -FAPI uptake of the liver (p ≤ 0.001) and small intestine (p ≤ 0.001) were significantly lower compared to 18F-FDG uptake. However, in the treated group and control group, the 18F-FAPI uptake of the gallbladder (p ≤ 0.002) and pancreas (p ≤ 0.005) were significantly higher compared to 18F-FDG uptake (Table 3).
In a subgroup analysis of gastrectomy patients, there was significant difference in physiologic gallbladder uptake between two groups(p = 0.04), the average SUVmean in the treated group was 2.56 ± 2.12, which was significantly lower than the average SUVmean of 14.62 ± 14.71 in the control group. Apart from this, there were no difference in the physiologic 18F-FAPI uptake of liver, small intestine and pancreas between treated and control group after gastric resection (Table 4). Figure 2 illustrates the clear visual difference in the physiological 18F-FAPI uptake of gallbladder between the treated group and control group after gastric resection or without gastric resection.
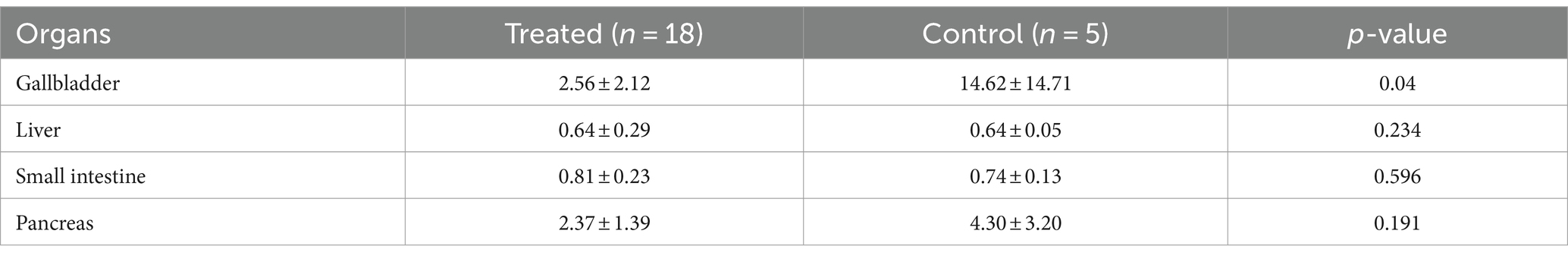
Table 4. Physiological 18F-FAPI uptake (SUVmean) in treated group and control group after gastric resection.
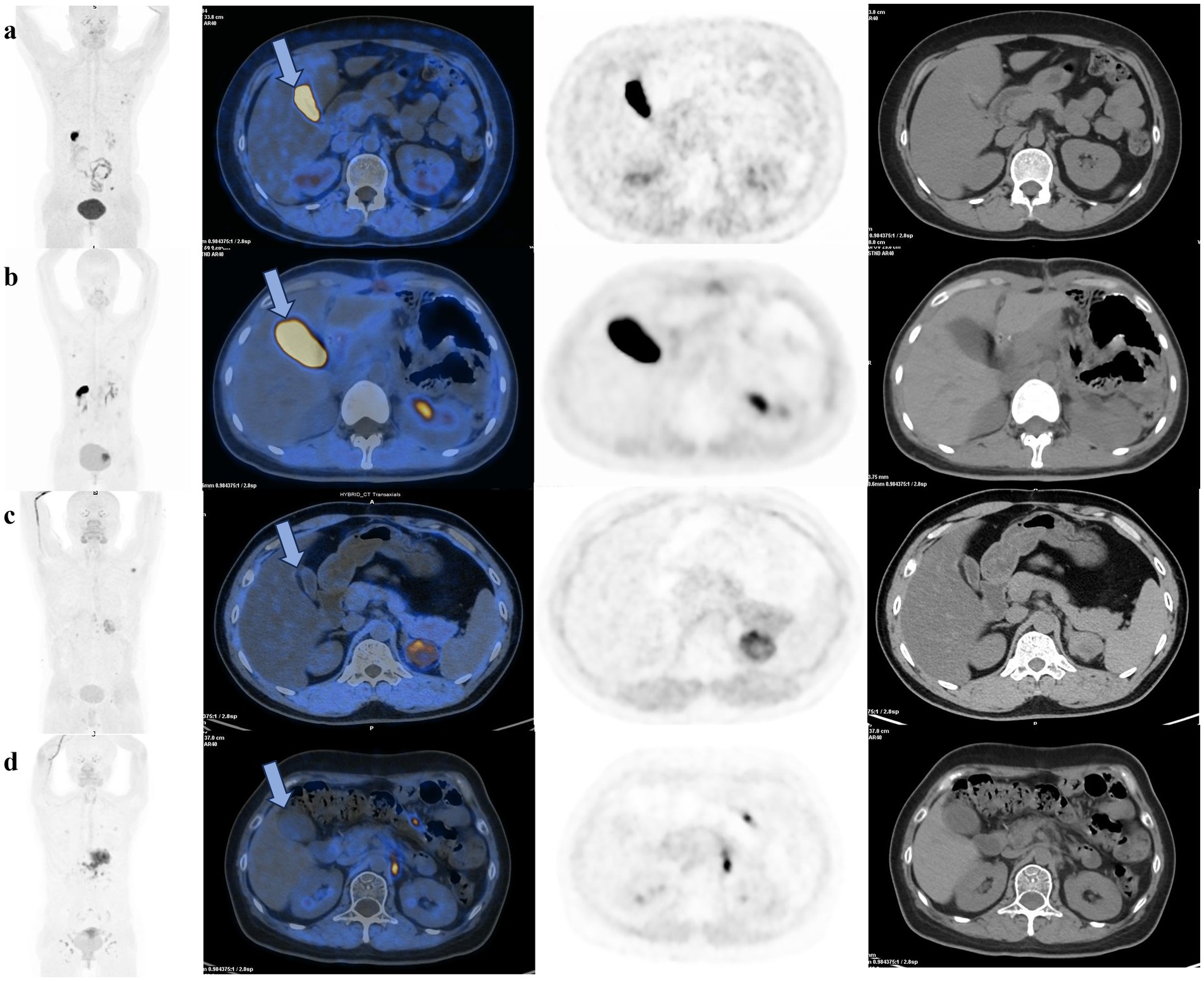
Figure 2. 18F-FAPI PET/CT scans of cancer patients demonstrate that pre-scan milk has a significant effect on reducing physiological uptake in the gallbladder after gastric resection or without gastric resection. (a) A patient without gastric resection in the control group showed significantly 18F-FAPI uptake in the gallbladder (blue arrow). (b) A patient after gastric resection in control group, with increased physiological uptake in the gallbladder (blue arrow). (c) A patient without gastric resection in the treated group, without visible gallbladder uptake (blue arrow). (d) A patient after gastric resection in the treated group, without visible gallbladder uptake (blue arrow) due to gallbladder emptying.
Radiolabelled FAPI has been reported to achieve better results in a variety of tumor imaging and is considered a suitable alternative to 18F-FDG (28, 29). The application of 68Ga-FAPI-04 is restricted due to its relatively short half-life, low overall activity production, and sub-optimal spatial resolution. On the other hand, 18F-labeled FAPIs have shown to possess superior tumor imaging abilities in various clinical evaluations, which exhibit improved physical properties, high yields, and favorable imaging characteristics (14, 15). Nevertheless, previous research demonstrated that 18F-FAPI has a generally high physiologic uptake in the normal gallbladder, reducing the diagnostic accuracy of primary and metastatic gallbladder lesions (20, 22, 24). We assessed the impact of pre-PET/CT ingestion of milk on the biodistribution of 18F-FAPI within normal abdominal organs in a tumor patient cohort.
In this study, physiological uptake of 18F-FAPI in the gallbladder was significantly lower in the treated group patients compared to the control group, which facilitates the visualization of gallbladder tumors. The principle of decreased gallbladder uptake of 18F-FAPI is based on the physiological metabolic characteristics of 18F-FAPI. Previous experiments on animals have indicated that 18F-FAPI is mainly excreted through the urinary and biliary systems (16). FAPI is a lipophilic tracer that can be excreted into the intestine by binding to bile acids in the biliary system (16, 18, 30). According to Heraghty’s study (31), a fatty meal can increase the hepatobiliary clearance of contrast agent, which is consistent with our findings. It is noteworthy that among the control group, two patients with the highest gallbladder uptake had undergone gastric cancer surgery years ago. Postoperative metabolic changes may alter bile acid production and lead to the formation of biliary sludge, increasing the incidence of gallbladder pathology and thus affecting gallbladder uptake (32).
Furthermore, we compared the results of 64 patients who underwent both 18F-FDG and 18F-FAPI PET/CT scans. We observed the physiologic 18F-FAPI uptake by the liver and small intestine in the treated group and control group was lower than 18F-FDG, and the 18F-FDG uptake of gallbladder and pancreas was significantly lower than the 18F-FAPI uptake in the treated group and control group, which is aligned with the previous studies (20, 22, 24, 33). Our study demonstrated that 18F-FAPI PET/CT can effectively reduce the physiological uptake of liver and small intestine, improving the 18F-FAPI visualization, thereby improving the lesion detection rate. A higher background 18F-FAPI uptake in gallbladder and pancreas might unbeneficial in detecting tumors and metastatic lesions in the abdominal cavity. However, one study has indicated that FAPI-PET is a reliable diagnostic method for pancreatic cancers (34), which suggests that a minor difference of SUVmean between 18F-FAPI and 18F-FDG cannot affect the accuracy of diagnosis in this type of cancer. We hypothesized that the slight increase in gallbladder uptake of treated group on 18F-FAPI PET/CT did not affect the detection of gallbladder lesions.
In the subgroup analysis of gastrectomy patient, the treated group and control groups did not exhibit substantial changes in liver, small intestines and pancreas uptake. However, the physiologic 18F-FAPI uptake of gallbladder in the treated group was significantly lower than the control group. There is a lack of definitive studies on physiologic 18F-FAPI uptake in gastrectomy patient. The physiological mechanisms related to the effect of fat intake on gallbladder contraction after gastrectomy remain unclear. Inoue. K highlighted that the release of CCK serves as the chief mechanism through which the ingestion of a fatty meal causes contraction of the gallbladder even after gastrectomy as well as before gastrectomy (35). Watanapa. P suggested that hyper-cholecystokininaemia persists for up to 15 months and may even increase with time after gastrectomy (36). However, some studies showed delayed emptying of the gallbladder after a gastric resection or vagotomy. The contraction of the gallbladder is caused by the stimulation of the vagal nerve and impaired gallbladder motor function could result from vagal denervation (37). Our study shows that milk consumption similarly promotes gallbladder emptying and decreases physiological gallbladder 18F-FAPI uptake in gastrectomy patients, which supports a major role for CCK in gallbladder contraction after gastrectomy.
Our study has several limitations: firstly, the sample size was small and the number of patients in the two groups were unbalanced. Statistical analysis may lack generalizability, and the conclusion need to be verified in larger studies. Secondly, SUVbw (normalized by Body Weight) is occasionally overestimated, particularly in obese individuals, which can lead to systematic bias for serial scans of patients with multiple follow-ups throughout the course of treatment. Our study would benefit from SUV measures normalized by lean body mass (38). Thirdly, the 18F-FAPI uptake of gallbladder was higher than the 18F-FDG uptake in the treated group. Consideration of 18F-FAPI or 18F-FDG for visualization is crucial in the comprehensive assessment of gallbladder cancer patients. Despites these limitations, it is believed that this study has undoubtedly enhanced our understanding of the influence of fat intake on [18F] AlF-NOTAFAPI-04 uptake in the normal abdominal organs.
In this retrospective study, we showed that the 18F-FAPI uptake of gallbladder in treated group was significantly lower than control group, which suggested that consumption of 250 mL of milk after the tracer injection potentially stimulate gallbladder contraction. Integration of pre-scan milk into routine 18F-FAPI PET/CT may enhance identification of gallbladder lesions, and may improve the diagnosis of gallbladder cancer in the future. On the other hand, the 18F-FAPI uptake of liver and small intestine was significantly lower than the 18F-FDG uptake, and the 18F-FDG uptake of gallbladder and pancreas was significantly lower than the 18F-FAPI uptake in both the treated group and the control group. 18F-FDG and 18F-FAPI serve as complementary tracers, thus dual tracer imaging holds significant clinical value.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving humans were approved by Medical Ethics Committee of Hunan Cancer Hospital. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and institutional requirements. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
JD: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Resources, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. WZ: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Resources, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. HL: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Resources, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. CJ: Supervision, Validation, Visualization, Writing – review & editing. HY: Funding acquisition, Supervision, Validation, Visualization, Writing – review & editing.
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This work was supported by Health Research Project of Hunan Provincial Health Commission (grant number W20243245), and Hunan Provincial Natural Science Foundation of China (grant number 2024JJ9250).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2024.1464779/full#supplementary-material
SUPPLEMENTARY FIGURE S1 | Schematic diagram of the volume of interest (VOI) delineation. The volumes of interest (VOIs) were drawn in three consecutive slices on the PET images focused on the maximum voxel value for the mentioned organs, and the mean values of the SUV in the VOIs were recorded. VOIs were delineated at 1 cm for minor tissues and at 2 cm for major organs such as the liver. The VOI of gallbladder and small intestine are located within the lumen, excluding the wall.
1. Brennen, WN, Isaacs, JT, and Denmeade, SR. Rationale behind targeting fibroblast activation protein-expressing carcinoma-associated fibroblasts as a novel chemotherapeutic strategy. Mol Cancer Ther. (2012) 11:257–66. doi: 10.1158/1535-7163.Mct-11-0340
2. Liu, R, Li, H, Liu, L, Yu, J, and Ren, X. Fibroblast activation protein: a potential therapeutic target in cancer. Cancer Biol Ther. (2012) 13:123–9. doi: 10.4161/cbt.13.3.18696
3. Christiansen, VJ, Jackson, KW, Lee, KN, Downs, TD, and McKee, PA. Targeting inhibition of fibroblast activation protein-α and prolyl oligopeptidase activities on cells common to metastatic tumor microenvironments. Neoplasia. (2013) 15:348–58. doi: 10.1593/neo.121850
4. Hamson, EJ, Keane, FM, Tholen, S, Schilling, O, and Gorrell, MD. Understanding fibroblast activation protein (FAP): substrates, activities, expression and targeting for cancer therapy. Proteomics Clin Appl. (2014) 8:454–63. doi: 10.1002/prca.201300095
5. Lindner, T, Loktev, A, Altmann, A, Giesel, F, Kratochwil, C, Debus, J, et al. Development of Quinoline-based Theranostic ligands for the targeting of fibroblast activation protein. J Nucl Med. (2018) 59:1415–22. doi: 10.2967/jnumed.118.210443
6. Giesel, FL, Kratochwil, C, Lindner, T, Marschalek, MM, Loktev, A, Lehnert, W, et al. (68)Ga-FAPI PET/CT: biodistribution and preliminary dosimetry estimate of 2 DOTA-containing FAP-targeting agents in patients with various cancers. J Nucl Med. (2019) 60:386–92. doi: 10.2967/jnumed.118.215913
7. Kratochwil, C, Flechsig, P, Lindner, T, Abderrahim, L, Altmann, A, Mier, W, et al. (68)Ga-FAPI PET/CT: tracer uptake in 28 different kinds of Cancer. J Nucl Med. (2019) 60:801–5. doi: 10.2967/jnumed.119.227967
8. Lindner, T, Altmann, A, Krämer, S, Kleist, C, Loktev, A, Kratochwil, C, et al. Design and development of (99m)Tc-labeled FAPI tracers for SPECT imaging and (188)re therapy. J Nucl Med. (2020) 61:1507–13. doi: 10.2967/jnumed.119.239731
9. Watabe, T, Liu, Y, Kaneda-Nakashima, K, Shirakami, Y, Lindner, T, Ooe, K, et al. Theranostics targeting fibroblast activation protein in the tumor stroma: (64)cu- and (225)ac-labeled FAPI-04 in pancreatic Cancer xenograft mouse models. J Nucl Med. (2020) 61:563–9. doi: 10.2967/jnumed.119.233122
10. Guo, W, Pang, Y, Yao, L, Zhao, L, Fan, C, Ke, J, et al. Imaging fibroblast activation protein in liver cancer: a single-center post hoc retrospective analysis to compare [(68)Ga]Ga-FAPI-04 PET/CT versus MRI and [(18)F]-FDG PET/CT. Eur J Nucl Med Mol Imaging. (2021) 48:1604–17. doi: 10.1007/s00259-020-05095-0
11. Pang, Y, Zhao, L, Luo, Z, Hao, B, Wu, H, Lin, Q, et al. Comparison of (68)Ga-FAPI and (18)F-FDG uptake in gastric, duodenal, and colorectal cancers. Radiology. (2021) 298:393–402. doi: 10.1148/radiol.2020203275
12. Qin, C, Liu, F, Huang, J, Ruan, W, Liu, Q, Gai, Y, et al. A head-to-head comparison of (68)Ga-DOTA-FAPI-04 and (18)F-FDG PET/MR in patients with nasopharyngeal carcinoma: a prospective study. Eur J Nucl Med Mol Imaging. (2021) 48:3228–37. doi: 10.1007/s00259-021-05255-w
13. Zhang, Z, Jia, G, Pan, G, Cao, K, Yang, Q, Meng, H, et al. Comparison of the diagnostic efficacy of (68) Ga-FAPI-04 PET/MR and (18)F-FDG PET/CT in patients with pancreatic cancer. Eur J Nucl Med Mol Imaging. (2022) 49:2877–88. doi: 10.1007/s00259-022-05729-5
14. Fowler, JS, and Ido, T. Initial and subsequent approach for the synthesis of 18FDG. Semin Nucl Med. (2002) 32:6–12. doi: 10.1053/snuc.2002.29270
15. Sahnoun, S, Conen, P, and Mottaghy, FM. The battle on time, money and precision: Da[(18)F] id vs. [(68)Ga]liath. Eur J Nucl Med Mol Imaging. (2020) 47:2944–6. doi: 10.1007/s00259-020-04961-1
16. Toms, J, Kogler, J, Maschauer, S, Daniel, C, Schmidkonz, C, Kuwert, T, et al. Targeting fibroblast activation protein: Radiosynthesis and preclinical evaluation of an (18)F-labeled FAP inhibitor. J Nucl Med. (2020) 61:1806–13. doi: 10.2967/jnumed.120.242958
17. Giesel, FL, Adeberg, S, Syed, M, Lindner, T, Jiménez-Franco, LD, Mavriopoulou, E, et al. FAPI-74 PET/CT using either (18)F-AlF or cold-kit (68)Ga labeling: biodistribution, radiation dosimetry, and tumor delineation in lung Cancer patients. J Nucl Med. (2021) 62:201–7. doi: 10.2967/jnumed.120.245084
18. Jiang, X, Wang, X, Shen, T, Yao, Y, Chen, M, Li, Z, et al. FAPI-04 PET/CT using [(18)F]AlF labeling strategy: automatic synthesis, quality control, and in vivo assessment in patient. Front Oncol. (2021) 11:649148. doi: 10.3389/fonc.2021.649148
19. Lindner, T, Altmann, A, Giesel, F, Kratochwil, C, Kleist, C, Krämer, S, et al. (18)F-labeled tracers targeting fibroblast activation protein. EJNMMI Radiopharm Chem. (2021) 6:26. doi: 10.1186/s41181-021-00144-x
20. Wang, S, Zhou, X, Xu, X, Ding, J, Liu, S, Hou, X, et al. Clinical translational evaluation of Al(18)F-NOTA-FAPI for fibroblast activation protein-targeted tumour imaging. Eur J Nucl Med Mol Imaging. (2021) 48:4259–71. doi: 10.1007/s00259-021-05470-5
21. Hu, K, Li, J, Wang, L, Huang, Y, Li, L, Ye, S, et al. Preclinical evaluation and pilot clinical study of [(18)F]AlF-labeled FAPI-tracer for PET imaging of cancer associated fibroblasts. Acta Pharm Sin B. (2022) 12:867–75. doi: 10.1016/j.apsb.2021.09.032
22. Wei, Y, Zheng, J, Ma, L, Liu, X, Xu, S, Wang, S, et al. [(18)F]AlF-NOTA-FAPI-04: FAP-targeting specificity, biodistribution, and PET/CT imaging of various cancers. Eur J Nucl Med Mol Imaging. (2022) 49:2761–73. doi: 10.1007/s00259-022-05758-0
23. Li, X, Lu, N, Lin, L, Chen, Y, Yang, S, Wang, H, et al. (18)F-FAPI-04 outperforms (18)F-FDG PET/CT in clinical assessments of patients with pancreatic adenocarcinoma. J Nucl Med. (2024) 65:206–12. doi: 10.2967/jnumed.123.266283
24. Mu, X, Huang, X, Li, M, Sun, W, and Fu, W. Comparison of physiological uptake of normal tissues in patients with cancer using 18F-FAPI-04 and 18F-FAPI-42 PET/CT. Front Nucl Med. (2022) 2:2. doi: 10.3389/fnume.2022.927843
25. Inoue, Y, Komatsu, Y, Yoshikawa, K, Akahane, M, Isayama, H, Ohtomo, K, et al. Biliary motor function in gallstone patients evaluated by fatty-meal MR cholangiography. J Magn Reson Imaging. (2003) 18:196–203. doi: 10.1002/jmri.10340
26. Purbhoo, K, and Vangu, MD. Efficacy of full-fat milk and diluted lemon juice in reducing infra-cardiac activity of (99m)Tc sestamibi during myocardial perfusion imaging. Cardiovasc J Afr. (2015) 26:171–6. doi: 10.5830/cvja-2015-033
27. Hofman, M, McKay, J, and Nandurkar, D. Efficacy of milk versus water to reduce interfering infra-cardiac activity in 99mTc-sestamibi myocardial perfusion scintigraphy. Nucl Med Commun. (2006) 27:837–42. doi: 10.1097/01.mnm.0000237989.60196.71
28. Dong, Y, Zhou, H, Alhaskawi, A, Wang, Z, Lai, J, Yao, C, et al. The superiority of fibroblast activation protein inhibitor (FAPI) PET/CT versus FDG PET/CT in the diagnosis of various malignancies. Cancers (Basel).. (2023) 15:1193. doi: 10.3390/cancers15041193
29. Li, C, Tian, Y, Chen, J, Jiang, Y, Xue, Z, Xing, D, et al. Usefulness of [(68)Ga]FAPI-04 and [(18)F]FDG PET/CT for the detection of primary tumour and metastatic lesions in gastrointestinal carcinoma: a comparative study. Eur Radiol. (2023) 33:2779–91. doi: 10.1007/s00330-022-09251-y
30. Kou, Y, Jiang, X, Yao, Y, Shen, J, Jiang, X, Chen, S, et al. Physiological tracer distribution and benign lesion incidental uptake of Al18F-NOTA-FAPI-04 on PET/CT imaging. Nucl Med Commun. (2022) 43:847–54. doi: 10.1097/mnm.0000000000001563
31. Heraghty, N, and Peters, AM. Hepatic bile acid transport increases in the postprandial state: a functional (11)C-CSar PET/CT study in healthy humans. JHEP Rep. (2021) 3:100357. doi: 10.1016/j.jhepr.2021.100357
32. Alsallamin, I, Chakhachiro, D, Bawwab, A, Nassar, M, and Alsallamin, A. Prevalence of symptomatic gallbladder disease after bariatric surgery: a literature review. Cureus. (2023) 15:e37777. doi: 10.7759/cureus.37777
33. Giesel, FL, Kratochwil, C, Schlittenhardt, J, Dendl, K, Eiber, M, Staudinger, F, et al. Head-to-head intra-individual comparison of biodistribution and tumor uptake of (68)Ga-FAPI and (18)F-FDG PET/CT in cancer patients. Eur J Nucl Med Mol Imaging. (2021) 48:4377–85. doi: 10.1007/s00259-021-05307-1
34. Chen, H, Pang, Y, Wu, J, Zhao, L, Hao, B, Wu, J, et al. Comparison of [(68)Ga]Ga-DOTA-FAPI-04 and [(18)F] FDG PET/CT for the diagnosis of primary and metastatic lesions in patients with various types of cancer. Eur J Nucl Med Mol Imaging. (2020) 47:1820–32. doi: 10.1007/s00259-020-04769-z
35. Inoue, K, Fuchigami, A, Hosotani, R, Kogire, M, Huang, YS, Miyashita, T, et al. Release of cholecystokinin and gallbladder contraction before and after gastrectomy. Ann Surg. (1987) 205:27–32.
36. Watanapa, P, Flaks, B, Oztas, H, Deprez, PH, Calam, J, and Williamson, RC. Enhancing effect of partial gastrectomy on pancreatic carcinogenesis. Br J Cancer. (1992) 65:383–7. doi: 10.1038/bjc.1992.77
37. Hahm, J, Park, J, Cho, Y, Eun, C, Lee, Y, Choi, H, et al. Changes in gallbladder motility in gastrectomized patients. Korean J Intern Med. (2000) 15:19–24. doi: 10.3904/kjim.2000.15.1.19
Keywords: milk, [18F]AlF-NOTA-FAPI-04, positron emission tomography, gallbladder, SUVmean
Citation: Dai J, Zhou W, Liu H, Jiang C and Ye H (2024) Impact of fat intake on [18F]AlF-NOTA-FAPI-04 uptake in normal abdominal organs. Front. Med. 11:1464779. doi: 10.3389/fmed.2024.1464779
Received: 15 July 2024; Accepted: 28 October 2024;
Published: 07 November 2024.
Edited by:
Francesco Dondi, Università degli Studi di Brescia, ItalyReviewed by:
Shun Huang, Southern Medical University, ChinaCopyright © 2024 Dai, Zhou, Liu, Jiang and Ye. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hui Ye, eXV4aW43NTgzMUAxNjMuY29t
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.