
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CASE REPORT article
Front. Med., 16 October 2024
Sec. Infectious Diseases: Pathogenesis and Therapy
Volume 11 - 2024 | https://doi.org/10.3389/fmed.2024.1435239
This article is part of the Research TopicGastrointestinal Tract Infections: A Global PerspectiveView all 19 articles
Mucormycosis is an aggressive fungal disease. Gastrointestinal mucormycosis is rare, but its clinical symptoms lack specificity and mortality is high. Here, we report a case of intestinal mucormycosis caused by Lichtheimia ramosa in a 65-year-old woman with diabetes mellitus. The patient exhibited extensive mucosal tissue damage in the colon, with broad, undivided filamentous fungal hyphae present in the intestinal tissue. Therefore, the patient was suspected to have a filamentous fungal infection. Colonic tissue samples were obtained for fungal culture, and the fungus was identified as L. ramosa based on morphology and DNA sequencing. This case highlights the importance of pathologists and microbiologists in identifying pathogenic fungi and the significance of screening for mucormycosis in high-risk patient populations.
Mucormycosis is a life-threatening, invasive fungal infection caused by fungi from the subphylum Mucoromycotina, order Mucorales, which primarily affects immunocompromised patients (1). These pathogens can infect any organ system, including the head, central nervous system, neck, skin, nasal cavity, lungs, and gastrointestinal tract (GI) (2). The most common sites of invasion are the rhinocerebral and pulmonary regions, with GI infections being the rarest (3).
GI mucormycosis accounts for only 5–15% of all mucormycosis cases but has one of the highest mortality rates among its clinical presentations (4). Common risk factors for GI mucormycosis in adults include diabetes, stem cell or solid organ transplantation, corticosteroid therapy, malnutrition, intestinal digestive surgery, and hemodialysis/peritoneal dialysis (4, 5). This infection most frequently occurs in the stomach, followed by the colon, small intestine, and esophagus, which are less common (6). Lichtheimia ramosa, previously known as Absidia ramose and a member of the saprotrophic zygomycetous fungi, is an uncommon pathogen within the order Mucorales (7). Herein, we present a rare case of invasive intestinal mycosis caused by L. ramosa.
On March 7th, 2022, a 65-year-old woman was admitted to the hospital with nausea, vomiting, and diarrhea. Initial treatment included antibiotic therapy and fluid replenishment to maintain electrolyte balance. On March 9th, she was transferred to our hospital for further management due to lip edema, gingival congestion, and dyspnea. During this period, she experienced over ten episodes of watery diarrhea accompanied by periumbilical pain, although she did not have a fever. Her medical history included diabetes, hypertension, and cerebral infarction, with no reported infectious diseases or exposure to similar illnesses. Despite her diabetes, she was not on antidiabetic medications; however, she continued her antihypertensive treatment. Physical examination revealed a body temperature of 36.6°C, blood pressure of 153/94 mm Hg, lip swelling, gingival congestion, rough breathing sounds, and audible wheezing.
Subsequent laboratory investigations after admission (March 9th) revealed a leukocyte count of 11.96 × 109/L (normal: 3.5–9.5 × 109/L), a neutrophil count of 11.27 × 109/L (normal: 1.8–6.3 × 109/L), and a lymphocyte count of 0.41 × 109/L (normal: 1.1–3.2 × 109/L), with a lymphocyte percentage of 3.4% (normal: 20–50%). Procalcitonin level was measured at 0.18 ng/mL (normal: 0–0.5 ng/mL), interleukin-6 at 67.97 pg./mL (normal: 0–5.4 pg./mL), fasting blood glucose at 12.31 mmol/L (normal 3.9–6.1 mmol/L), alanine transaminase at 527 U/L (normal: 7–40 U/L), aspartate transaminase at 556.3 U/L (normal: 13–35 U/L), and creatinine at 378 μmol/L (normal: 41–81 μmmol/L). Microbiological tests for Clostridium difficile toxin, Salmonella and Shigella spp., Aspergillus antigen (GM test), and fungal 1,3-β-D-glucan (G test) were all negative. A lymphocyte subpopulation test conducted 7 days after admission revealed a CD3+ T-cell count of 572/μL (normal: 955–2,860/μL), a CD4+ T-cell count of 304/μL (normal: 550–1,440/μL), a CD8+ T-cell count of 176/μL (normal: 320–1,250/μL), and a natural killer cell count of 34/μL (normal: 150–1,100/μL). Abdominal computed tomography revealed multiple hypodense foci in the liver, localized wall thickening in the lower intestine, and enlarged mesenteric lymph nodes (Figure 1A).
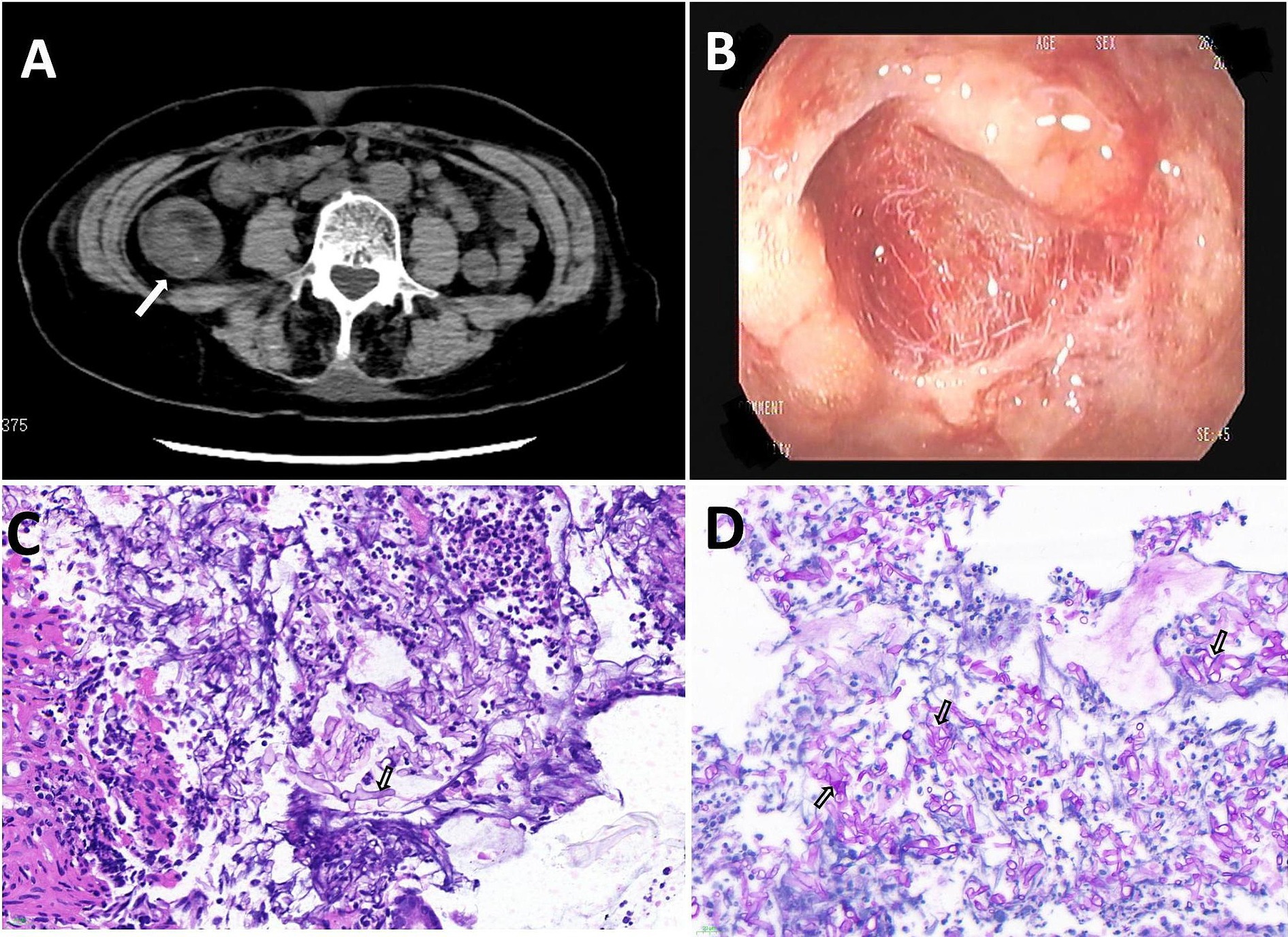
Figure 1. (A) Computed tomography (CT) revealed bowel wall thickening. (B) Gastroscopy revealed severe erosions, edema, and scattered superficial ulcers. (C,D) A broad, aseptate hyphae filamentous fungi was found in the intestinal tissue (Magnification: ×400). The tissue specimens were stained with Hematoxylin and Eosin staining (C), periodic acid Schiff (D).
The patient presented with bloody stools containing red mucosal tissue, indicative of intestinal mucosal damage. On March 14th, a colonoscopy revealed hyperemia, edema, erosion, and scattered superficial ulcers in the ileocecal region and throughout the colon, covered with a large amount of white material (Figure 1B) resistant to rinsing with dilute amphotericin B.
Microscopic examination of a colonic biopsy specimen showed severe chronic inflammation, acute active inflammation, cryptic inflammation, crypt abscesses, inflammatory exudation, and necrosis. Broad and undivided filamentous fungal hyphae were detected in the intestinal tissue using hematoxylin and eosin (HE) (Figure 1C) and Schiff periodate (PAS) staining (Figure 1D). Colon tissue samples cultured on Sabouraud agar medium exhibited colonies with grayish-white hyphae, a flat surface, and a velvety texture, rapidly spreading across the entire plate (Figure 2A). Further microscopic examination of lactophenol cotton blue stain revealed broad, aseptate branching hyphae. The sporangium stems, originating from creeping hyphae, were pear-shaped and had prominent conical columella (Figure 2B). DNA was extracted from the culture and subjected to sequencing with a fungal universal 18S rRNA primer pair (V4 regions: 3NDF, 5′-GGCAAGTCTGGTGCCAG-3′; and V4eukR2R, 3′-ACGGTATCTRATCRTCTTCG-5′). The colony was identified as L. ramosa on March 23th.

Figure 2. (A) White cottony growth of L. ramosa on the Sabouraud dextrose agar (SDA). (B) A lactophenol cotton blue (LCB) preparation showing pear-shaped sporangia and prominent conical columella.
After admission, the patient was treated with meropenem, cefoperazone/sulbactam, and hormone therapy. Based on intestinal histopathologic and tissue culture results, a mucorales infection was suspected, and the patient was diagnosed with intestinal-type mucormycosis on March 16th. Posaconazole was administered immediately for antifungal treatment. Despite prompt intervention, the disease progressed rapidly. Regrettably, the patient’s family decided to discontinue treatment on March 19th, 2022, due to the presence of multiple organ dysfunction syndrome. Subsequent follow-up indicated that the patient passed away post-discharge (Figure 3).
Gastrointestinal mucormycosis caused by L. ramosa is a rare opportunistic infection (8). The increasing use of glucocorticoids and immunosuppressants annually is also contributing to the rising incidence of mucormycosis. Diabetes has been a leading factor in mucormycosis and one of the important causes of fungal infections in humans (9). In China, poorly controlled diabetes has been identified as the most important risk factor for mucormycosis (10), similar to that in India, South America, the Middle East, and Africa. Mucormycosis commonly affects diabetic patients with uncontrolled blood sugar levels, predominantly causing rhino-orbito-cerebral infections due to Rhizopus species. However, gastrointestinal manifestations are rare, and infections caused by L. ramosa at the intestinal site are even more uncommon. Here, we present a fatal case of invasive intestinal infection with L. ramosa in a patient with poorly controlled diabetes mellitus.
The most frequently reported manifestations in patients of GI mucormycosis were abdominal pain, distention and fever, often depend on the area affected of the gastrointestinal tract. With intestinal mucormycosis, almost all patients have been reported to experience abdominal pain and fever (7, 11–13). Our patient has abdominal pain and diarrhea, but no fever. There are patients who were admitted for other reasons, such as a patient who was admitted due to shock, high fever, hypoxic respiratory failure and generalized symptoms of shaking and then found to have mucormycosis in the intestines (14). L. ramose is an opportunistic fungal pathogen that may result in a rare but serious mucormycosis infection. Mucormycosis caused by L. ramose infection was mainly found in the lungs, rhino-orbito-cerebral and skin (8, 15–17), and the intestinal mucormycosis caused by L. ramose is rare. There was only one report of a 53-year-old female patient with intestinal mycosis due to perforation of the transverse colon following antibacterial chemotherapy after esophageal cancer surgery, resulting in co-infection with L. ramose and Aspergillus calidoustus (7). The exact mechanism by which L. ramosa invades the intestine remains unclear, and may be related to the ingestion of spore-infected food.
GI mucormycosis is a highly invasive, virulent fungal infection. Early endoscopic biopsy is key to diagnosis. At present, the gold standard for the diagnosis of GI mucormycosis is tissue histopathology, which can diagnose 70% of cases. Culture and mucorales polymerase chain reaction (PCR) of tissue adds to the diagnostic yield (18). Semi-nested PCR for 18S rRNA gene and quantitative real-time PCR assays targeting the 28S rRNA gene are also molecular methods for early diagnosis of Mucorales (6). Mucormycosis lacks the cell wall components that can be detected on serum tests such as the 1,3-beta-D-glucan (BG) assay, which led to a negative test for the BG test. Therefore, clinicians should exercise caution and not exclude Mucorales infections solely based on G and GM test results. Instead, microbiological and histopathological examinations should be actively conducted in high-risk patients. Staining techniques such as PAS or silver hexamide staining of the tissue are recommended to enhance visualization of the fungal composition. In our patient, filamentous fungi were clearly detected by HE and PAS staining of intestinal mucosal tissue. Many patients are ineligible for surgery or endoscopic biopsy because of their poor physical condition, with most cases identified during autopsy. Even with the fungal culture of the intestinal tissue, there’s still no fungal growth in culture in more than 50% of cases (3, 19). Gastrointestinal tissue is not sterile tissue, and cultures will not be able to differentiate between harmless contamination and invasive infection. Therefore, the proportion of patients with confirmed premortal diagnoses of Mucorales spp. by tissue culture is quite small. In our case, the fungus was successfully cultured from the colon biopsy tissue and identified as L. ramosa via DNA sequencing using a fungal universal 18S rRNA primer pair.
The prognosis after the onset of gastrointestinal tract infection with Mucorales is poor, with a high mortality rate (20). Early surgical intervention is crucial in treating mucormycosis, including local debridement and the removal of infected tissues or organs when conditions permit. Additionally, timely and effective systemic antifungal therapy for mucormycosis is necessary after a definitive diagnosis. According to the literature, the empirical antifungal treatments for L. ramosa are amphotericin B and posaconazole. Amphotericin B is the most active drug against Lichtheimia spp. (21). In a case report, intestinal mycosis caused by co-infection with L. ramosa and Aspergillus calidoustus was successfully treated with liposomal amphotericin B after an emergency subtotal colectomy (7). However, amphotericin B has adverse effects, specifically severe nephrotoxicity. Furthermore, although amphotericin B liposomes can reduce nephrotoxicity, they should be used with caution in patients with renal impairment. Posaconazole is a recommended therapy that can serve as a complement or substitute for amphotericin B therapy (16). However, the efficacy of posaconazole remains controversial (22). In this study, the intestinal lesions of the patient were extensive and her physical condition was poor; therefore, surgery could not be performed. Due to compromised kidney function, the patient was treated with posaconazole instead of amphotericin B. Unfortunately, the patient discontinued treatment 10 days after admission and subsequently passed away at home. Hence, timely diagnosis and treatment are required to increase the chances of returning to health.
Intestinal mycosis can be difficult to diagnose and manage, owing to the various forms of the disease and the need to obtain and analyze tissue specimens at an early stage at an appropriate facility. The dramatic increase in Mucorales fungal infections demands more rapid and accurate diagnostic methods to identify such pathogens. We should actively control the basic diseases of susceptible people, and patients with high-risk factors should avoid contact with Mucorales-contaminated items and take anti-mold measures. Clinicians should remain highly suspicious of emerging fungal infections in high-risk patients, such as those with diabetes.
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
The studies involving humans were approved by the Ethics Committee of Taian City Central Hospital (Approval ID: 2023-05-02). The studies were conducted in accordance with the local legislation and institutional requirements. The intestinal mucosal tissue of the patient served as the sample tissue in this study. This tissue was subsequently cultured with microorganisms, and the resultant fungi were identified. Written informed consent for participation was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and institutional requirements. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
QL: Writing – original draft, Visualization, Formal analysis, Data curation. PC: Writing – original draft, Supervision, Investigation, Formal analysis. LX: Writing – review & editing, Validation, Investigation, Formal analysis. JZ: Writing – original draft, Validation, Methodology, Investigation. MJ: Writing – review & editing, Supervision, Project administration, Methodology, Investigation.
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
We thank Editage (www.editage.cn) for its linguistic assistance during the preparation of this manuscript.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Reid, G, Lynch, JP III, Fishbein, MC, Clark, NM, Lynch, JP 3rd, Michael, CF, et al. Mucormycosis. Semin Respir Crit Care Med. (2020) 41:099–114. doi: 10.1055/s-0039-3401992
2. Donnelly, JP, Chen, SC, Kauffman, CA, Steinbach, WJ, Baddley, JW, Verweij, PE, et al. Revision and update of the consensus definitions of invasive fungal disease from the European Organization for Research and Treatment of Cancer and the mycoses study group education and research consortium. Clin Infect Dis. (2020) 71:1367–76. doi: 10.1093/cid/ciz1008
3. Boris, Z, Tyson, A, and Christopher, F. Invasive gastrointestinal Mucormycosis. ACG Case Rep J. (2023) 10:e01161. doi: 10.14309/crj.0000000000001161
4. Addasi, Y, Nguyen, AH, Sabri, A, Ahmad, F, Rangray, R, and Velagapudi, M. Gastrointestinal Mucormycosis: a clinical review. Gastroenterology Res. (2023) 16:249–53. doi: 10.14740/gr1662
5. Kaur, H, Ghosh, A, Rudramurthy, S, and Chakrabarti, A. Gastrointestinal mucormycosis in apparently immunocompetent hosts-a review. Mycoses. (2018) 61:898–908. doi: 10.1111/myc.12798
6. Didehdar, M, chegini, Z, Moradabadi, A, Anoushirvani, AA, Tabaeian, SP, Yousefimashouf, M, et al. Gastrointestinal mucormycosis: a periodic systematic review of case reports from 2015 to 2021. Microb Pathog. (2022) 163:105388. doi: 10.1016/j.micpath.2022.105388
7. Kaneko, Y, Oinuma, KI, Terachi, T, Arimura, Y, Niki, M, Yamada, K, et al. Successful treatment of intestinal mycosis caused by a simultaneous infection with Lichtheimia ramosa and aspergillus calidoustus. Intern Med. (2018) 57:2421–4. doi: 10.2169/internalmedicine.0254-17
8. Geng, C, Lv, X, Li, J, Jiang, Q, Yang, R, and Zhan, P. Chronic subcutaneous infection due to Lichtheimia ramosa. J Eur Acad Dermatol Venereol. (2019) 33:e26–9. doi: 10.1111/jdv.15137
9. Dwivedi, S, Choudhary, P, Gupta, A, and Singh, S. The cross-talk between mucormycosis, steroids and diabetes mellitus amidst the global contagion of COVID-19. Crit Rev Microbiol. (2023) 49:318–33. doi: 10.1080/1040841x.2022.2052795
10. Lin-Wei, W, Pei-Qiu, Z, Xiao-Qing, C, and Jin, Y. Mucormycosis in mainland China: a systematic review of case reports. Mycopathologia. (2021) 187:1–14. doi: 10.1007/s11046-021-00607-4
11. Vikum, D, Nordøy, I, Torp Andersen, C, Fevang, B, Line, PD, Kolrud, FK, et al. A young, immunocompetent woman with small bowel and hepatic Mucormycosis successfully treated with aggressive surgical Debridements and antifungal therapy. Case Rep Infect Dis. (2017) 2017:1–4. doi: 10.1155/2017/4173246
12. Abdi Bati, W, Poornachandra, K, and Biniyam Alemayehu, A. Invasive intestinal mucormycosis in a 40-year old immunocompetent patient – a rarely reported clinical phenomenon: a case report. BMC Gastroenterol. (2020) 20:61. doi: 10.1186/s12876-020-01202-5
13. Antony, SJ, Parikh, MS, Ramirez, R, Applebaum, B, Friedman, G, and do, J. Gastrointestinal Mucormycosis resulting in a catastrophic outcome in an immunocompetent patient. Infect Dis Rep. (2015) 7:6031. doi: 10.4081/idr.2015.6031
14. Chintav, S, Stewart, Z, Jason, M, and Andrew, E. Colonic mucormycosis in an immunocompetent patient with endocarditis. IDCases. (2020) 20:e00773. doi: 10.1016/j.idcr.2020.e00773
15. Aboutalebian, S, Erami, M, Momen-Heravi, M, Charsizadeh, A, Hezaveh, SJH, Matini, AH, et al. A case of COVID-19-associated mucormycosis due to Lichtheimia ramosa. J Clin Lab Anal. (2023) 37:e24895. doi: 10.1002/jcla.24895
16. He, GQ, Xiao, L, Pan, Z, Wu, JR, Liang, DN, Guo, X, et al. Case report: a rare case of pulmonary mucormycosis caused by Lichtheimia ramosa in pediatric acute lymphoblastic leukemia and review of Lichtheimia infections in leukemia. Front Oncol. (2022) 12:949910. doi: 10.3389/fonc.2022.949910
17. Zhang, Q, Liu, H, Qiu, S, Wang, W, Yang, L, Chen, H, et al. A rare case of pulmonary coinfection by Lichtheimia ramosa and Aspergillus fumigatus in a patient with delayed graft function after renal transplantation. Transplant Proc. (2019) 51:551–5. doi: 10.1016/j.transproceed.2018.12.006
18. Xiao, L, Yinggai, S, and Ruoyu, L. The use of combined PCR, fluorescence in situ hybridisation and immunohistochemical staining to diagnose mucormycosis from formalin-fixed paraffin-embedded tissues. Mycoses. (2021) 64:1460–70. doi: 10.1111/myc.13382
19. Baxi, SN, Gohil, MR, Navadiya, AJ, Bapodra, MK, and Patel, HR. Comparative evaluation of histopathological analysis, KOH wet mount and fungal culture to diagnose fungal infections in post-COVID patients. Indian J Pathol Microbiol. (2023) 66:540–4. doi: 10.4103/ijpm.ijpm_663_21
20. Guinea, J, Escribano, P, Vena, A, Muñoz, P, Martínez-Jiménez, MC, Padilla, B, et al. Increasing incidence of mucormycosis in a large Spanish hospital from 2007 to 2015: epidemiology and microbiological characterization of the isolates. PLoS One. (2017) 12:e0179136. doi: 10.1371/journal.pone.0179136
21. Alastruey-Izquierdo, A, Cuesta, I, Walther, G, Cuenca-Estrella, M, and Rodriguez-Tudela, J. Antifungal susceptibility profile of human-pathogenic species of Lichtheimia. Antimicrob Agents Chemother. (2010) 54:3058–60. doi: 10.1128/aac.01270-09
Keywords: fungal infection, diabetes, mucormycosis, intestinal infection, Lichtheimia ramosa
Citation: Liu Q, Chen P, Xin L, Zhang J and Jiang M (2024) A rare intestinal mucormycosis caused by Lichtheimia ramosa in a patient with diabetes: a case report. Front. Med. 11:1435239. doi: 10.3389/fmed.2024.1435239
Received: 20 May 2024; Accepted: 27 September 2024;
Published: 16 October 2024.
Edited by:
Ponsiano Ocama, Makerere University, UgandaReviewed by:
Eman A. Gouda M. Youssef, Lundquist Institute for Biomedical Innovation, United StatesCopyright © 2024 Liu, Chen, Xin, Zhang and Jiang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Meijie Jiang, dGFpYW5tZWlqakAxNjMuY29t
†These authors share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.