- 1Department of Hepatology Division 2, Beijing Ditan Hospital, Capital Medical University, Beijing, China
- 2Department of Gynecology and Obstetrics, Beijing Ditan Hospital, Capital Medical University, Beijing, China
- 3Department of Hepatology Division 2, Peking University Ditan Teaching Hospital, Beijing, China
Objective: To explore any correlation between serum urate (SU) level or insulin resistance (IR) and metabolic dysfunction associated steatotic liver disease (MASLD) in patients with metabolic syndrome (MS).
Methods: Data from all MASLD patients, diagnosed by liver biopsy, were enrolled and divided into MASLD alone group and MASLD with MS group. They were subdivided into hyperuricemia group and normal SU group to find correlation between SU/IR and MASLD in patients with MS and independent risk factors for MASLD.
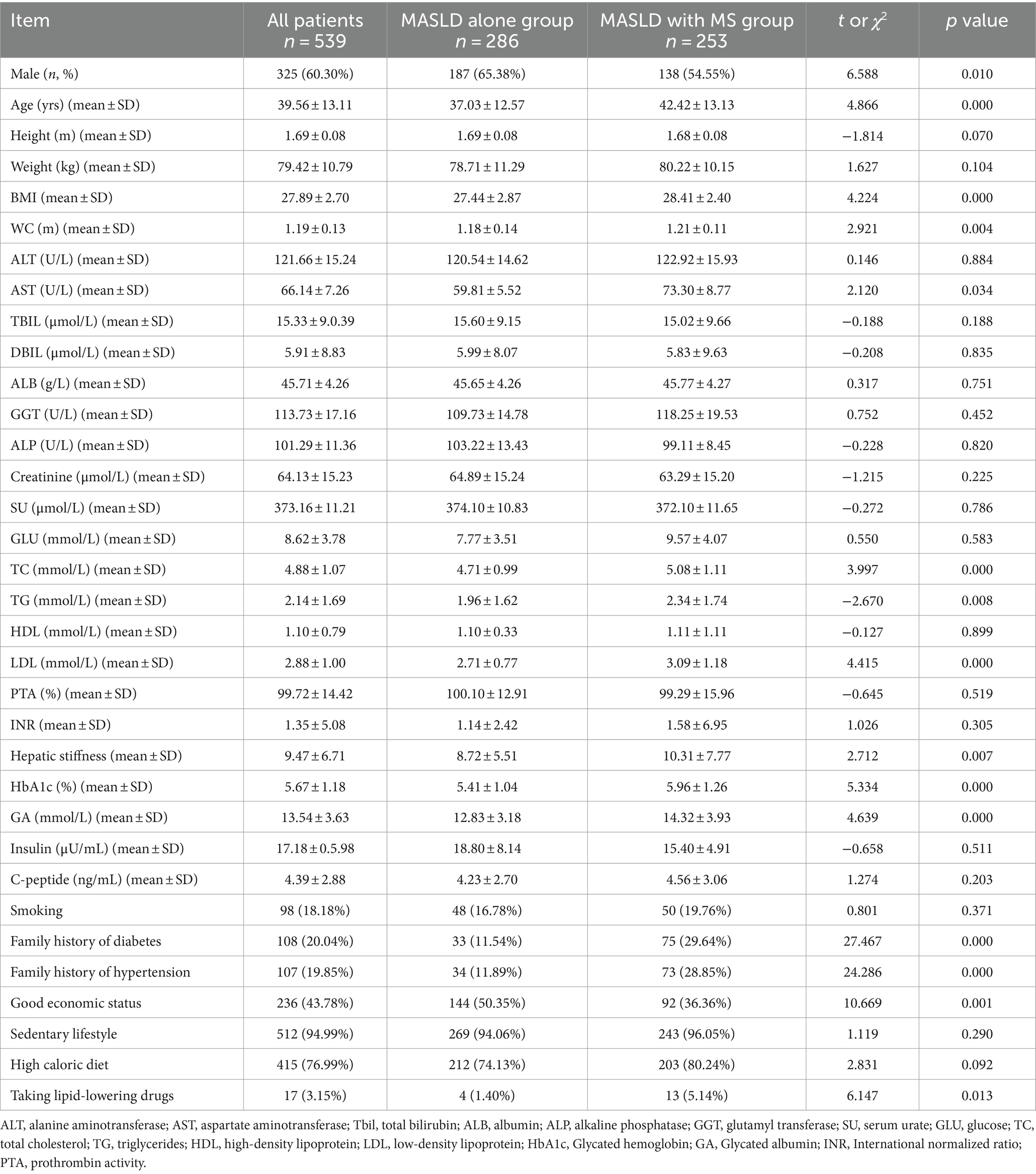
Results: Data from 539 MASLD patients were analyzed. Body mass index (BMI) (p = 0.000), waist circumference (WC) (p = 0.004), and low-density lipoprotein (LDL) (p = 0.000) were dramatically higher in MASLD with MS group than those with MASLD alone; MASLD with MS patients had significantly more family history of diabetes (p = 0.000) and hypertension (p = 0.000) than patients with MASLD alone. Height (p = 0.000), weight (p = 0.000), BMI (p = 0.000) and WC (p = 0.001), and LDL (p = 0.007) were dramatically higher in hyperuricemia patients than those with normal SU. SU was inversely associated with age (p = 0.000) and high-density lipoprotein (HDL) (p = 0.003), and positively correlated with weight (p = 0.000), BMI (p = 0.000) and WC (p = 0.000), TG (p = 0.000), and LDL (p = 0.000). Logistic Regression analysis showed that age (p = 0.031), TG (p = 0.002), LDL (p = 0.010), HbA1c (p = 0.026), and family history of hypertension (p = 0.000) may be independent risk factors for MASLD in patient with MS.
Conclusion: Insulin resistance (IR) in MASLD patients with MS, but not higher SU levels, has closer correlation with the occurrence of MASLD in patients with family history of hypertension and diabetes having higher BMI, LDL, HbA1c.
Introduction
The metabolic dysfunction associated steatotic liver disease (MASLD), a major health burden, the most common cause of chronic liver disease all over the world, has lately risen with the estimated worldwide prevalence of about 38% (1) of the general population (2–4). MASLD is a disease caused by the interaction of multiple factors such as genetics, diet, and lifestyle (5). It is characterized by excessive accumulation of triglyceride within the cytoplasm of hepatocytes (exceeding 5% of liver weight) due to both increased inflow of free fatty acids and de novo hepatic lipogenesis and insufficient mitochondrial capacity for beta oxidation in individuals without alcohol consumption (6). Its clinical manifestations include metabolic dysfunction associated hepatic steatosis (MAHS; simple benign condition of MAFL), metabolic dysfunction associated steatohepatitis (MASH; inflammatory subtype of MAFL with lobular inflammation and apoptosis), that can progress to liver cirrhosis, liver failure and liver cancer if left untreated (7, 8). Patients with MASLD often have systemic hypertension, dyslipidaemia, insulin resistance/diabetes which are prognostic variables common to the metabolic syndrome (MS) (9). MASLD is the hepatic manifestation of the MS which is often associated with abnormal liver enzyme levels such as elevated levels of alanine aminotransferase (ALT) and aspartate aminotransferase (AST) (10, 11). Though growing evidence suggest bidirectional relationship between MASLD and MS because MASLD can predispose to MS, but hepatic steatosis reportedly can occur independently of insulin resistance (12–15). MASLD can occur in individuals who are not obese (16, 17). In studies of hepatic steatosis, mice over-expressing DGAT2, an enzyme that catalyzes the final step of hepatic triglyceride biosynthesis, was demonstrated to develop hepatic steatosis with normal plasma glucose and insulin levels and normal insulin tolerance (12). The primary therapy for most patients with MASLD is weight loss. However, pioglitazone (an anti-diabetic medication) (18, 19), and Glucagon-like peptide-1 (GLP-1) receptor agonists (20, 21) have shown promising hepatic outcomes in patients with MASLD. For MASLD diagnosis, liver biopsy is the gold standard to diagnose any form of hepatic inflammation, fibrosis or injury associated with it. Nevertheless, owing to its invasive nature, it has not been routinely used to diagnose various liver diseases.
MASLD was demonstrated as a manifestation of metabolic syndrome (MS) (22, 23) which has high incidence rate in the adult population leading to the increase in the cost of the global public health. MS is a non-communicable complex pathophysiologic state of a group of interrelated diseases characterized by at least any three or more of the following conditions: abdominal obesity (greater waist circumference), high fasting blood glucose, insulin resistance, high triglycerides, lower high-density lipoprotein cholesterol (HDL), higher low-density lipoprotein cholesterol (LDL), high blood pressure and hypertension (24). MS has become the major health hazard of modern world caused primarily by increased consumption of high calorie and low-fiber fast food, sedentary lifestyle of reduced physical activity including genetic/epigenetic makeup of individual that can lead to cardiovascular and cerebrovascular diseases (25). The liver plays a central role in metabolic syndrome due to its role in glucose and triglyceride overproduction because two key components of MS, glucose and triglycerides, are overproduced by the fatty liver.
Serum urate (SU) is the end-product of purine (endogenous and exogenous) metabolism in humans and the great apes because of loss-of- function mutations during primate evolution in the gene of uricase enzyme, that oxidizes uric acid to more soluble allantoin (26, 27). Due to uricase inactivation the SU level is 7 to 8-fold higher in humans (≈240–360 μM) compared to other mammals (≈30–50 μM in mice) (27). Thus, higher SU levels may have selective advantage in the evolution of hominids, may be related to memory with less SU level linked to neurodegenerative disorders, including Alzheimer’s Disease (28). Approximately two-third of the SU is produced endogenously, and the remaining comes from dietary purines (29). The normal SU level in humans is the result of net balance between biosynthesis of SU primarily in liver, reabsorption of urate in renal proximal tubules and secretion in the renal tubule and intestine (30). Most SU is filtered freely in the kidneys, with roughly 90% of the urate from glomerular filtrate is reabsorbed via urate transporters in the proximal tubule (31). About 70% of the total uric acid from our body passes through the kidneys and the rest via intestinal and biliary secretion (29). Ultimately, after urate reabsorption, only 3–10% of the filtered urate is excreted in the urine (32). Abnormalities in SU metabolism and its decreased excretion by the kidneys are one of the major causes of hyperuricemia and gout development (33). Dysregulation of xanthine oxidoreductase (XOD), the enzyme that catalyzes the endogenous production of SU primarily in liver and urate transporters (34) that reabsorb urate in renal proximal tubule and secrete urate in renal tubule and intestine, and their genetic variabilities are the major causes for the development of hyperuricemia. SU is elevated in metabolic syndrome (MS) and diabetes (35) as a consequence of insulin resistance and the effects of insulin to reabsorb more urate resulting in reduced urinary urate excretion (36). It is debatable that elevated SU levels can lead to insulin resistance. In fact, there is a positive relationship between serum insulin and elevated SU levels, in healthy volunteers and people with diabetes (36). Insulin resistance also leads to impaired SU excretion at a low urinary pH, contributing to the formation of urate stones (37). These genetic data are consistent with a causal role of insulin to control SU levels (36). Insulin resistance is considered the major mechanism in the development and progression of MASLD/MASH as a result of impaired insulin signaling that leads to increase intracellular fatty acid-derived metabolites such as diacylglycerol, fatty acyl CoA or ceramides (6). It is debatable whether elevated level of SU is the causative factor of MASLD, because allopurinol (an inhibitor of XOD) treatment to reduce serum urate level was shown to significantly increase the triglyceride values (38). However, febuxostat (another inhibitor of XOD) treatment, was shown to suppress the development of nonalcoholic steatohepatitis in a rodent model (39).
Previous studies on MASLD, MS, and blood uric acid levels have rarely been based on liver biopsy for diagnosis of MASLD patients. In this study, MASLD patients were diagnosed using liver biopsy to find any correlation between MASLD and insulin resistance or SU level using prognostic variables from MASLD patients without or with MS.
Materials and methods
Subjects
Patients admitted to Beijing Ditan Hospital from October 2008 to December 2018 underwent liver biopsy, for the diagnosis of MASLD. Inclusion criteria: MASLD diagnosis of all patients by liver biopsy. Exclusion criteria: (1) Liver diseases caused by alcoholic hepatitis, autoimmune hepatitis etc.; (2) liver diseases caused by viral infections such as EB virus (Epstein–barr Virus, EBV), CMV (Cytomegalovirus), HIV (Human Immunodeficiency Virus); (3) mental diseases; (4) liver tumors. This study was approved by the Ethics Committee of Beijing Ditan Hospital and the ethics ID was Jing Di Lun Ke Zi 2018 No. 052-01.
Diagnostic criteria for liver tissue biopsy and histopathology
A 16G liver puncture needle was used under ultrasound guidance for liver tissue puncture, and the length of the tissue specimen was required to be at least 1.0 cm (1.5–2.5 cm). Liver biopsy specimens were consecutively sliced and subjected to routine H–E, reticular fibrosis, and/or Masson staining. The Scheuer scoring system was used to evaluate the staging of liver fibrosis (S0–S4) and inflammation grading (G0–G4), with S3–S4 defined as advanced liver fibrosis. According to the Brunt grading system, fat degeneration was evaluated and divided into four levels: F0 (<5%), F1 (5–33%), F2 (33–66%), and F3 (≥66%). All pathological sections were independently observed and evaluated by two experienced pathologists. In case of any disagreement, a third pathologist was there for arbitration.
Clinical index detection
Liver function (Wako Pure Chemical Industries, Ltd., JAPAN) and kidney function (Sekisui Medical CALCo, Ltd., JAPAN) were detected using Hitachi fully automated biochemical analyzer (Hitachi Ltd). International standardized ratio (Beckman coulter, America) was measured. In this study, the upper bound of alanine transaminase (ALT) Aspartate transaminase (AST) detection value is 40 U/L, the upper normal value of total bilirubin (TBIL) is 18.8 μmol/L, the upper bound of the Gamma-glutamyl transferase (GGT) detection value is 60 U/L, the upper limit of the Alkaline phosphatase (ALP) detection value is 125 U/L, and the lower bound of the normal albumin detection value is 40 g/L.
Statistical analyses
All data were subjected to statistical analyses using Statistical Package for the Social Sciences (SPSS 26.0 software; Chicago, IL, USA), GraphPad Prism 6 and WPS Office version 5.5.1 (7991) software. Before performing the analysis, the Kolmogorov–Smirnov method was used to analyze all data for the normality test. The count data were shown using a descriptive analysis and a percentage, and the comparison of data between two groups was performed by the Fisher’s exact test or chi-square test. The statistical description of normally distributed data were expressed by the mean ± standard deviation (Mean ± SD), and the comparison of data between two groups was performed by two independent samples t-test; non-normally distributed data were described using the median (Q1, Q3), and comparison between groups were performed by the nonparametric M-U test. Univariate and multivariate Logistic regression were used to analyze risk factors for MASLD with MS. All statistical tests were used two-sided, statistically significant if p < 0.05.
Results
Basic clinical characteristics of patients with MASLD
In this retrospective study, we collected data of MASLD patients from the outpatient department of Beijing Ditan Hospital, Capital Medical University. Table 1 showed a total of 539 MASLD patients (325 males and 214 females, aged 39.56 ± 13.11 years) diagnosed by liver biopsy. Patients were grouped according to whether they had MS. Patients with MASLD alone and patients with MASLD combined with MS were 53.06% (286) and 46.94% (253), respectively. MASLD patients with MS were significantly older than patients with MASLD alone (p < 0.001), as is shown in Table 1. Height and weight were not significantly different between two groups, but BMI (p < 0.001) and waist circumference (p = 0.004) were greater in MASLD with MS than those with MASLD alone. There were no significant differences in ALT (Alanine Transaminase), TBIL (Total Bilirubin), and DBIL (Direct/conjugated Bilirubin) levels between two groups, but the AST (aspartate aminotransferase) levels in MASLD patients with MS (p = 0.031) were higher (73.30 ± 87.65 vs. 59.81 ± 55.28) than those in patients with MASLD alone. There were no significant differences in blood glucose, SU, and creatinine levels, and triglyceride and HDL between two groups, while the levels of total cholesterol (p < 0.001) and LDL (p < 0.001) were higher (cholesterol: 5.08 ± 1.11 vs. 4.71 ± 0.99; LDL: 3.09 ± 1.18 vs. 2.71 ± 0.77) in MASLD patients with MS than patients with MASLD alone. Hepatic stiffness (p = 0.007) was greater in patients with MASLD combined with MS (10.31 ± 7.77 vs. 8.72 ± 5.51) than that in patients with MASLD alone. Glycated hemoglobin (HbA1c) (5.96 ± 1.26 vs. 5.41 ± 1.04, p = 0.000) and glycated albumin (14.32 ± 3.93 vs. 12.83 ± 3.18, p < 0.001) were higher in MASLD patients with MS than those in patients with MASLD alone. However, there were no significant differences in insulin and C peptide levels between two groups which could be due to their low half lives in serum (approximately 30 min for C-peptide and 5–6 min for insulin). More MASLD patients with MS had a family history of diabetes (p < 0.001) and hypertension (28.85% vs. 11.89%, p < 0.001) than patients with MASLD alone. Economic status of MASLD patients with MS (36.36% vs. 50.35%, p < 0.001) was worse than that patients with MASLD alone. The percentage of patients taking lipid-lowering drugs in MASLD with MS group was significantly higher (5.14% vs. 1.40%) than the patients in NAFLD alone group (p = 0.013) (Table 1).
Comparison of MS components between hyperuricemia and normal SU patients
In this study, data from 539 MASLD patients, confirmed by liver biopsy, were collected, in which blood urate data from two patients were missing. Therefore, data from 537 patients were included for the comparison of MS components between hyperuricemia subgroup and the normal SU subgroup. In this group of MASLD patients, the proportion of male patients was higher (60.34% male and 39.66% female) than that of female patients, and there was significant statistical difference in the proportion of male patients between two groups (hyperuricemia vs. normal SU group) (p = 0.001). Average age of hyperuricemia patients was significantly lower than those with normal serum urate level, as is shown in Table 2. Height (p = 0.000), weight (p < 0.001), BMI (p < 0.001) and waist circumference (p = 0.001) were significantly higher in hyperuricemia patients than those with normal serum urate. There was no significant difference in transaminase level between two groups. However, the albumin (ALB) level (p < 0.001), glycated albumin level (a marker of glycemic control) (p = 0.044) and creatinine level (p < 0.001) in hyperuricemia patients were significantly higher than that of normal SU patients. Although there was no significant difference in triglyceride (TG) level between two groups, total cholesterol (TC) (p = 0.044) and low-density lipoprotein (LDL) levels (p = 0.007) were significantly higher in hyperuricemia group than those in normal blood urate group. Interestingly, in Table 2 of our study, MASLD patients with hyperuricemia had lower HDL level (p = 0.045) with higher platelet to HDL ratio (PHR) (p = 0.033), than that of MASLD patients with normal serum urate level. The glycated albumin (GA) of hyperuricemia patients was markedly lower than that of normal blood urate patients (p = 0.044); the percentage of hyperuricemia patients with family history of hypertension (p = 0.001) and high-calorie diet (p < 0.001) was markedly lower than that of normal blood urate patients in Table 2. In Table 2 of this study, MASLD patients with hyperuricemia have lower HDL level (1.04 ± 0.24 mmol/L) with the chance of higher PHR, than that of MASLD patients with normal serum urate level.
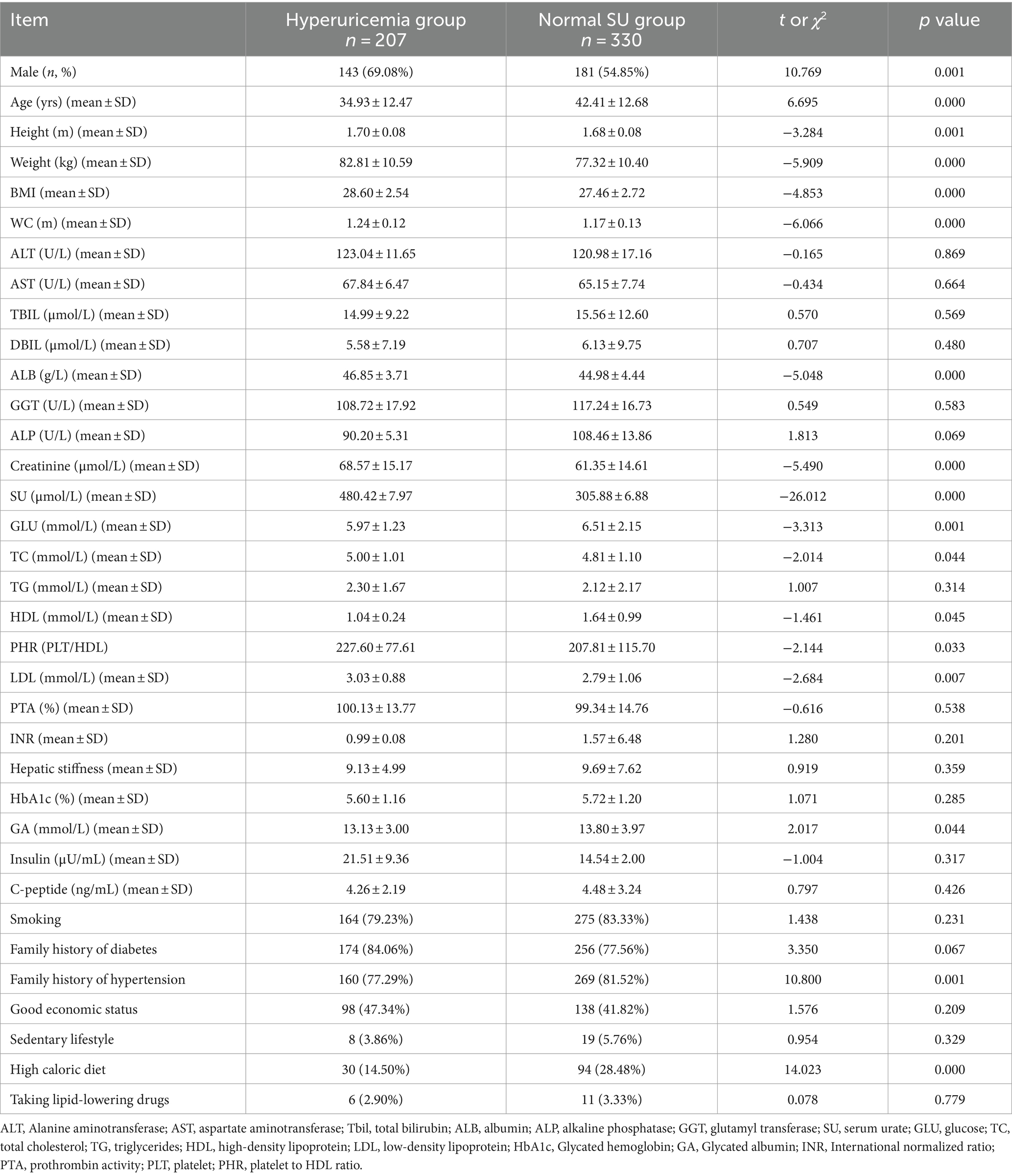
Table 2. Comparison of MS components between MASLD patients with hyperuricemia and MASLD patients with normal SU levels.
Correlation analysis of SU levels and MS components in MASLD patients
The relevance analysis of serum urate (SU) levels and MS components in MASLD patients found that SU levels were positively correlated with height (p < 0.001), weight (p < 0.001), BMI (p < 0.001) and waist circumference (p < 0.001), ALT (p = 0.005), and ALB (p < 0.001) in Figure 1, while SU levels were negatively correlated with age (p < 0.001) in Figure 1. SU levels were negatively correlated with blood glucose (p < 0.001), and HDL (p = 0.003), HbA1C (p = 0.004) and glycated albumin (p = 0.018) in Figure 2, while SU levels were positively correlated with LDL (p < 0.001), creatinine (p < 0.001), TC (p = 0.006), TG (p < 0.001), and insulin levels (p = 0.010) in Figure 2 suggesting higher SU level as a result of insulin resistance/hyperinsulinemia might lead to MASLD in patients with MS.
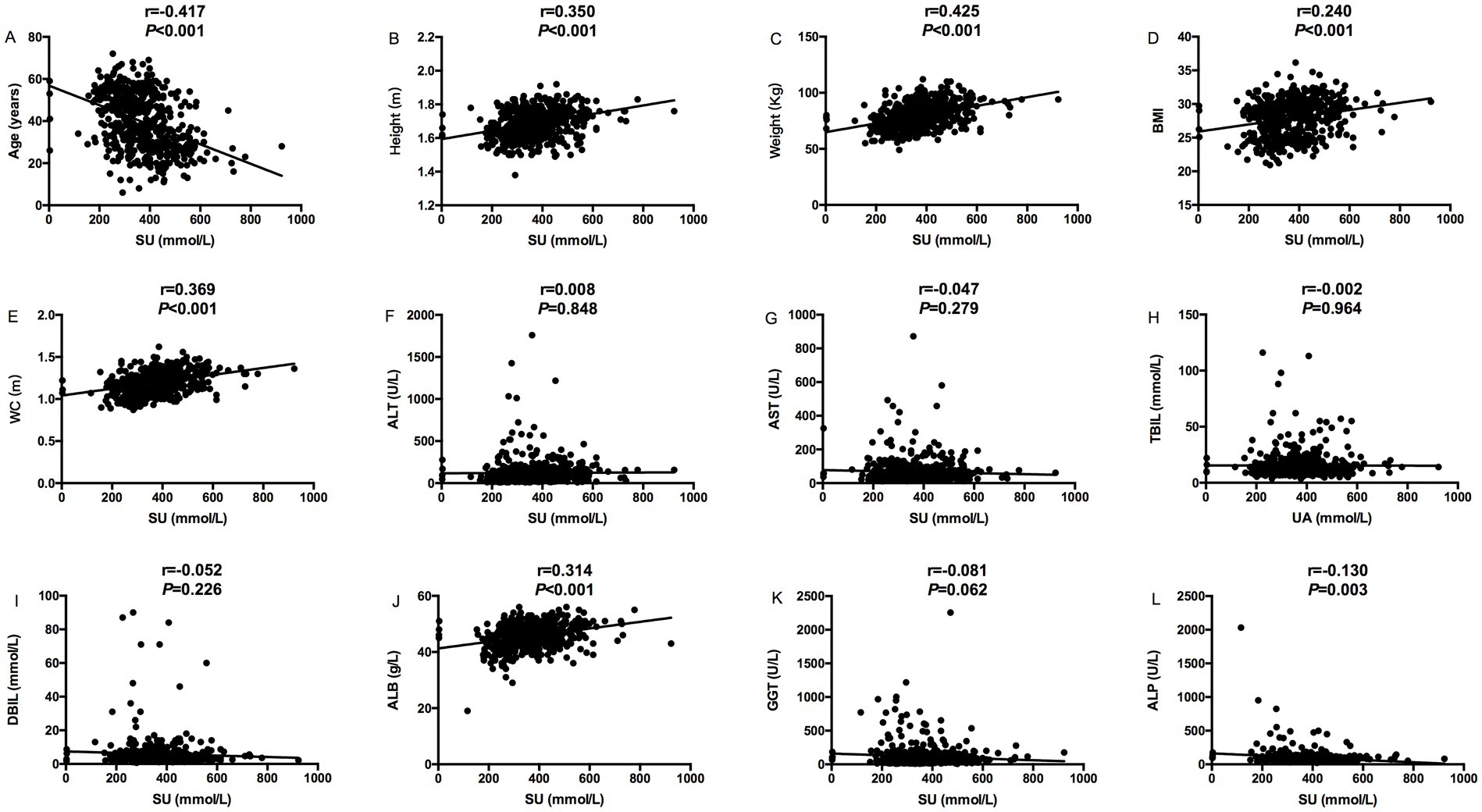
Figure 1. Correlation analysis between serum urate levels and MS components (Age, Height, Weight, BMI, WC, ALT, AST, TBIL, DBIL, ALB, and GGT); (A) The correlation between serum urate levels and age; (B) The correlation between serum urate levels and height; (C) The correlation between serum urate levels and weight; (D) The correlation between serum urate levels and BMI; (E) The correlation between serum urate levels and WC; (F) The correlation between serum urate levels and ALT; (G) The correlation between serum urate levels and AST; (H) The correlation between serum urate levels and TBIL; (I) The correlation between serum urate levels and DBIL; (J) The correlation between serum urate levels and ALB; (K) The correlation between serum urate levels and GGT; (L) The correlation between serum urate levels and ALP.
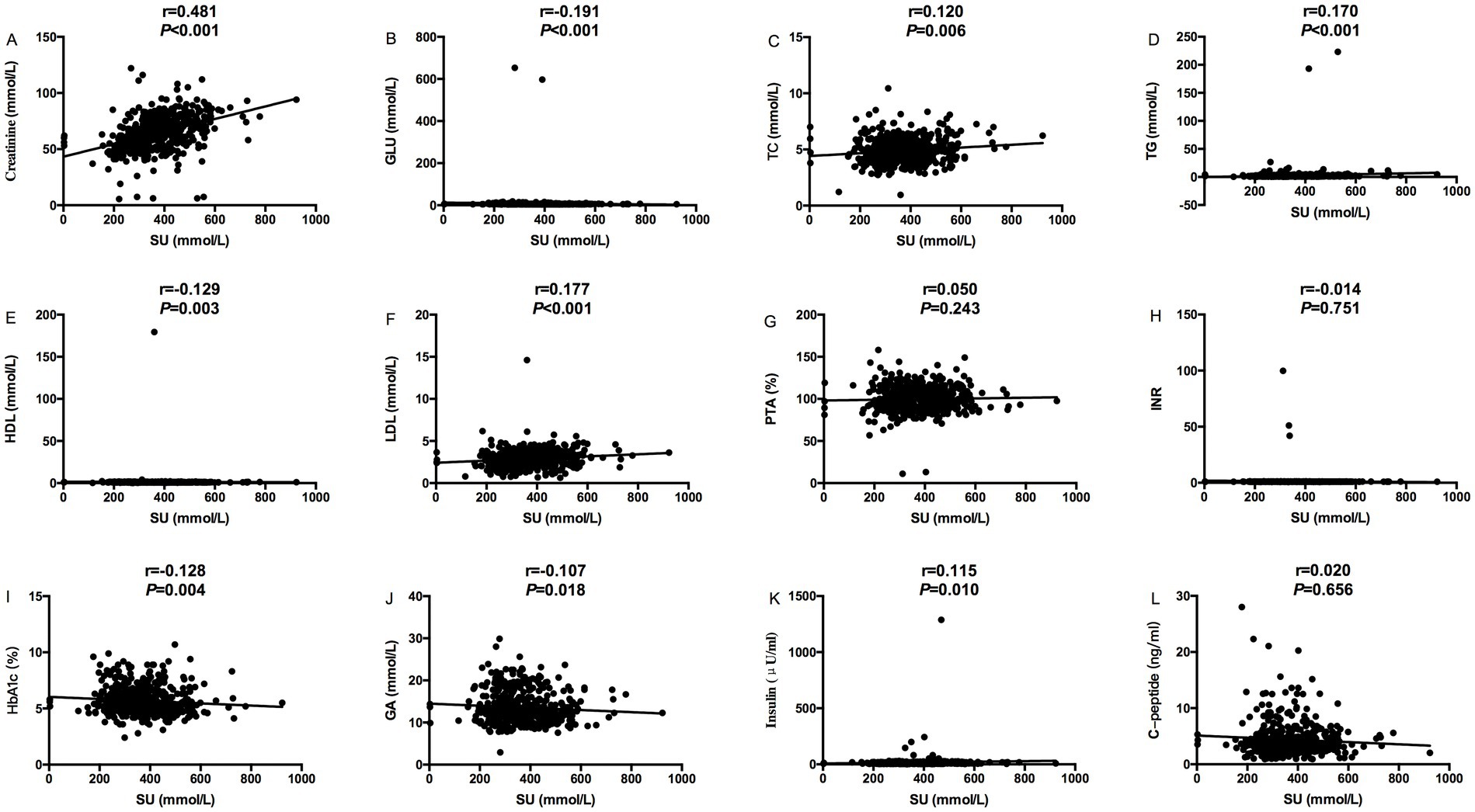
Figure 2. Relevance analysis between serum urate levels and MS components (Creatinine, GLu, blood lipids, PTA, INR, HbA1c, GA, C-peptide and Insulin); (A) The correlation between serum urate levels and Creatinine; (B) The correlation between serum urate levels and GLU; (C) The correlation between serum urate levels and TC; (D) The correlation between serum urate levels and TG; (E) The correlation between serum urate levels and HDL; (F) The correlation between serum urate levels and LDL; (G) The correlation between serum urate levels and PTA; (H) The correlation between serum urate levels and INR; (I) The correlation between serum urate levels and HbA1c; (J) The correlation between serum urate levels and GA; (K) The correlation between serum urate levels and Insulin; (L) The correlation between serum urate levels and C-peptide.
Risk factor analysis for MASLD with MS
Univariate and multivariate Logistic regression analysis showed risk factors for MASLD with MS. The results showed that age (p = 0.031), triglyceride (p = 0.002), LDL (p = 0.010), glycated hemoglobin (p = 0.026), and family history of hypertension (p < 0.001) were independent risk factors for MASLD patients combined with MS in Table 3.
Discussion
MASLD has become a chronic liver disease affecting the health of people (with a prevalence of about 38%) all over the world, and is considered to be the liver manifestation of MS, which is closely related to obesity and the risk of liver complications related to diabetes, hypertension and non-alcoholic steatohepatitis (40, 41). Understanding the pathogenesis and risk factors of MASLD is very important because the number one cause of mortality of MASLD patients is cardiovascular disease (CVD) rather than liver disease (1). MASLD patients typically have one or more MS components, such as hypertension, dyslipidemia, abnormal blood sugar, or insulin resistance (9). As mentioned above, MASLD is correlated to MS, whereas, diabetes, obesity and dyslipidemia are considered as important risk factors of MASLD (42) that could be linked through changes in metabolism. Although liver biopsy is the gold standard to diagnose MASLD, it is an invasive procedure. However, in previous studies, the diagnostic methods for MASLD were mostly based on the combination of medical history, imaging, and biochemical indicators, etc. This retrospective study is based on data collected from MASLD patients who were properly diagnosed through liver biopsy. MASLD occurs when excess fat is accumulated in hepatocyte in the absence of any significant alcohol consumption due to insufficient mitochondrial capacity for beta oxidation. Currently, there is no proper approved pharmaceutical treatment modality for MASLD except recommendation for altering a patient’s predisposing factors, like low-calorie diet and increased physical activity. Many clinical trials are currently under development to find a new promising pharmacological agents for the treatment of MASLD. In a phase 2 trial, for patients with MASH and moderate or severe fibrosis, treatment with tirzepatide for 52 weeks is more effective in relieving MASH without worsening fibrosis (43). Tao et al. 45 have suggested that FOT1 is a promising new treatment option for all stages and future clinical trials of MAFLD (44). Treatment with denifanstat significantly improves the disease activity, MASH resolution, and fibrosis, which supports the entry of denifanstat into Phase 3 development (45). The anti-diabetic medication like pioglitazone (18, 19), and Glucagon-like peptide-1 (GLP-1) receptor agonists (20, 21) have hinted promising results in patients with MASLD. Modest wine (but not beer or liquor) consumption was also suggested for decreased prevalence of suspected MASLD (46) that could be associated with the protective effect of grape-sourced resveratrol. To confirm the therapeutic efficacy of resveratrol for MASLD large-scale randomized controlled trials is necessary.
MS is a group of diseases with multiple components related to each other. It is characterized by three or more of the following conditions: overweight, high waist circumference or obesity, high triglycerides, low high-density lipoprotein cholesterol, abnormal blood glucose, insulin resistance or diabetes, and elevated blood pressure. Our study showed that in MASLD patients, the proportion of male patients was higher than that of female patients. Riazi et al. (47) and Ballestri et al. (48) suggested that the incidence of MASLD in males was higher than that in females, which was consistent with our finding. Our study also showed that age, BMI, and waist circumference in MASLD patients combined with MS were significantly higher than those in patients with MASLD alone. This may be because overweight or obese patients with MASLD are more likely to accompany with MS (49, 50). MS patients may have a history of dyslipidemia, diabetes and hypertension. Our results showed that the levels of cholesterol, low-density lipoprotein, glycated hemoglobin and glycated albumin (51), and family history of diabetes and hypertension in MASLD patients with MS were significantly higher than those in patients with MASLD alone. So MASLD patients with dyslipidemia, diabetes and hypertension were more likely to have MS. In addition, our study showed that the liver stiffness of MASLD patients combined with MS was higher than that of patients with MASLD alone, which may be related to abnormal metabolic factors such as blood glucose, dyslipidemia, and obesity, et al., accelerating the progression of MASLD to liver fibrosis and cirrhosis. Due to abnormal blood lipids in MS patients, the probability of using lipid-lowering drugs may be higher than that of patients with MASLD alone. Logistic regression analysis in this study showed that age, triglycerides, low-density lipoprotein, glycated hemoglobin, and family history of hypertension are independent risk factors for MASLD patients combined with MS (Table 3).
Insulin resistance (IR) and fat accumulation in the liver are strongly related (52). Multiple studies have shown that insulin resistance/hyperinsulinemia can lead to hyperglycemia, hypertension, dyslipidemia, hyperuricemia, elevated inflammatory markers, and endothelial dysfunction (53). Progression of insulin resistance can lead to metabolic syndrome (MS), metabolic dysfunction associated steatotic liver disease (MASLD), and type 2 diabetes. In this study, among MASLD patients diagnosed through liver biopsy, the weight, BMI, and waist circumference of hyperuricemia patients were significantly higher than patients with normal SU level, suggesting apparent role of higher SU levels (54, 55). However, the difference in serum C-peptide level between MASLD with hyperuricemia group and MASLD with normal SU group is insignificant (4.26 ± 2.19 ng/mL, 4.48 ± 3.24 ng/mL in Table 2) but much higher than normal level (0.9–1.8 ng/mL) suggesting hyperinsulinemia/insulin resistance but not SU level is likely to be correlated with MASLD. Thus, progression of IR most likely leads to MS and MASLD.
In summary, this retrospective study was intended to find the correlation between SU levels or IR and MASLD in patients (diagnosed with liver biopsy) with MS, as well as the independent risk factors for MASLD in patients with MS. After thorough analyzes, it is concluded that IR/hyperinsulinemia but not SU level has closer correlation with MASLD in patients with MS than patients with MASLD alone. Older age, overweight or obesity, higher HbA1C and glycated albumin levels, higher LDL levels and hyperuricemia caused by IR most likely lead to MASLD in older patients with family history of diabetes and hypertension.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding authors.
Ethics statement
The studies involving humans were approved by Ethics Committee of Beijing Ditan Hospital. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
WC: Conceptualization, Data curation, Methodology, Project administration, Software, Writing – original draft, Formal analysis, Investigation, Resources, Supervision, Validation, Writing – review & editing. TJ: Data curation, Investigation, Methodology, Software, Writing – original draft. WD: Data curation, Formal analysis, Investigation, Software, Writing – original draft. SWa: Data curation, Investigation, Methodology, Software, Writing – original draft. XL: Data curation, Formal analysis, Investigation, Methodology, Writing – original draft. ZZ: Formal analysis, Investigation, Methodology, Software, Writing – original draft. LZ: Investigation, Software, Writing – review & editing. YL: Investigation, Methodology, Software, Writing – review & editing. MC: Data curation, Investigation, Writing – review & editing. RL: Data curation, Formal analysis, Investigation, Writing – review & editing. SWu: Data curation, Investigation, Software, Writing – review & editing. GS: Formal analysis, Investigation, Software, Writing – review & editing. YG: Data curation, Investigation, Software, Writing – review & editing. HH: Formal analysis, Investigation, Software, Writing – review & editing. XC: Data curation, Investigation, Writing – review & editing. LH: Data curation, Investigation, Software, Writing – review & editing. MX: Data curation, Investigation, Writing – review & editing. WY: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Software, Writing – review & editing. YX: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Software, Writing – original draft, Writing – review & editing. ML: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This study was funded by the National Key Research and Development Program (2022YFC2603500 and 2022YFC2603505); Beijing Municipal Health Commission High-Level Public Health Technical Personnel Construction Project (Discipline leader-03-26); the Capital Health Research and Development of Special Public Health Project (2022-1-2172); the Digestive Medical Coordinated Development Center of Beijing Hospitals Authority (XXZ0302) and Beijing Hospitals Authority Clinical Medicine Development of Special Funding Support (XMLX 202127).
Acknowledgments
We are grateful to all the staff and patients involved in this research.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Minetti, ET, Hamburg, NM, and Matsui, R. Drivers of cardiovascular disease in metabolic dysfunction-associated steatotic liver disease: the threats of oxidative stress. Front Cardiovasc Med. (2024) 11:1469492. doi: 10.3389/fcvm.2024.1469492
2. Hou, XH, Zhu, YX, Lu, HJ, Chen, HF, Li, Q, Jiang, S, et al. Non-alcoholic fatty liver disease’s prevalence and impact on alanine aminotransferase associated with metabolic syndrome in the Chinese. J Gastroenterol Hepatol. (2011) 26:722–30. doi: 10.1111/j.1440-1746.2010.06509.x
3. Younossi, ZM, Koenig, AB, Abdelatif, D, Fazel, Y, Henry, L, and Wymer, M. Global epidemiology of nonalcoholic fatty liver disease-metaanalytic assessment of prevalence, incidence, and outcomes. Hepatology. (2016) 64:73–84. doi: 10.1002/hep.28431
4. Argo, CK, and Caldwell, SH. Epidemiology and natural history of nonalcoholic steatohepatitis. Clin Liver Dis. (2009) 13:511–31. doi: 10.1016/j.cld.2009.07.005
5. Nseir, W, Hellou, E, and Assy, N. The role of dietary and lifestyle changes in non-alcoholic fatty liver disease. World J Gastroenterol. (2014) 20:9338–44. doi: 10.3748/wjg.v20.i28.9338
6. Engin, A . Non-alcoholic fatty liver disease. Adv Exp Med Biol. (2017) 960:443–67. doi: 10.1007/978-3-319-48382-5_19
7. Kanwal, F, Kramer, JR, Mapakshi, S, Natarajan, Y, Chayanupatkul, M, Richardson, PA, et al. Risk of hepatocellular carcinoma in patients with non-alcoholic fatty liver disease. Gastroenterology. (2018) 155:1828–1837.e2. e2. doi: 10.1053/j.gastro.2018.08.024
8. Kodama, K, Kawaguchi, T, Hyogo, H, Nakajima, T, Ono, M, Seike, M, et al. Clinical characteristics of hepatocellular carcinoma in patients with non-alcoholic fatty liver disease. J Gastrointest Liver Dis. (2019) 34:1626–32. doi: 10.1111/jgh.14608
9. Dixon, JB, Bhathal, PS, and O'Brien, PE. Nonalcoholic fatty liver disease: predictors of nonalcoholic steatohepatitis and liver fibrosis in the severely obese. Gastroenterology. (2001) 121:91–100. doi: 10.1053/gast.2001.25540
10. Clark, JM, Brancati, FL, and Diehl, AM. The prevalence and etiology of elevated aminotransferase levels in the United States. Am J Gastroenterol. (2003) 98:960–7. doi: 10.1111/j.1572-0241.2003.07486.x
11. Edmison, J, and McCullough, AJ. Pathogenesis of non-alcoholicsteatohepatitis: human data. Clin Liver Dis. (2007) 11:75–104. doi: 10.1016/j.cld.2007.02.011
12. Monetti, M, Levin, MC, Watt, MJ, Sajan, MP, Marmor, S, Hubbard, BK, et al. Dissociation of hepatic steatosis and insulin resistance in mice overexpressing DGAT in the liver. Cell Metab. (2007) 6:69–78. doi: 10.1016/j.cmet.2007.05.005
13. Kantartzis, K, Machicao, F, Machann, J, Schick, F, Fritsche, A, Häring, HU, et al. The DGAT2 gene is a candidate for the dissociation between fatty liver and insulin resistance in humans. Clin Sci (Lond). (2009) 116:531–7. doi: 10.1042/CS20080306
14. Niebergall, LJ, Jacobs, RL, Chaba, T, and Vance, DE. Phosphatidylcholine protects against steatosis in mice but not non-alcoholic steatohepatitis. Biochim Biophys Acta. (2011) 1811:1177–85. doi: 10.1016/j.bbalip.2011.06.021
15. Yki-Järvinen, H . Non-alcoholic fatty liver disease as a cause and a consequence of metabolic syndrome. Lancet Diabetes Endocrinol. (2014) 2:901–10. doi: 10.1016/S2213-8587(14)70032-4
16. Kim, HJ, Kim, HJ, Lee, KE, Kim, DJ, Kim, SK, Ahn, CW, et al. Metabolic significance of nonalcoholic fatty liver disease in nonobese, nondiabetic adults. Arch Intern Med. (2004) 164:2169–75. doi: 10.1001/archinte.164.19.2169
17. Sinn, DH, Gwak, GY, Park, HN, Kim, JE, Min, YW, Kim, KM, et al. Ultrasonographically detected non-alcoholic fatty liver disease is an independent predictor for identifying patients with insulin resistance in non-obese, non-diabetic middle-aged Asian adults. Am J Gastroenterol. (2012) 107:561–7. doi: 10.1038/ajg.2011.400
18. Colca, JR, and Scherer, PE. The metabolic syndrome, thiazolidinediones, and implications for intersection of chronic and inflammatory disease. Mol Metab. (2022) 55:101409. doi: 10.1016/j.molmet.2021.101409
19. Takahashi, H, Kessoku, T, Kawanaka, M, Nonaka, M, Hyogo, H, Fujii, H, et al. Ipragliflozin improves the hepatic outcomes of patients with diabetes with NAFLD. Hepatol Commun. (2022) 6:120–32. doi: 10.1002/hep4.1696
20. Ghosal, S, Datta, D, and Sinha, B. A meta-analysis of the effects of glucagon-like-peptide 1 receptor agonist (GLP1-RA) in nonalcoholic fatty liver disease (NAFLD) with type 2 diabetes (T2D). Sci Rep. (2021) 11:22063. doi: 10.1038/s41598-021-01663-y
21. Rezaei, S, Tabrizi, R, Nowrouzi-Sohrabi, P, Jalali, M, Atkin, SL, Al-Rasadi, K, et al. GLP-1 receptor agonist effects on lipid and liver profiles in patients with nonalcoholic fatty liver disease: systematic review and meta-analysis. Can J Gastroenterol Hepatol. (2021) 2021:8936865–11. doi: 10.1155/2021/8936865
22. Marchesini, G, Bugianesi, E, Forlani, G, Cerrelli, F, Lenzi, M, Manini, R, et al. Non alcoholic fatty liver disease, steatohepatitis, and metabolic syndrome. Hepatology. (2003) 37:917–23. doi: 10.1053/jhep.2003.50161
23. Del Ben, M, Polimeni, L, Brancorsini, M, Di Costanzo, A, D'Erasmo, L, Baratta, F, et al. Non alcoholic fatty liver disease, metabolic syndrome, and protein 3 gene variants containing patatin like phospholipase domains. Eur J Med. (2014) 25:566–570. doi: 10.1016/j.ejim.2014.05.012
24. Wainwright, P, and Byrne, CD. Bidirectional relationships and disconnects between NAFLD and features of the metabolic syndrome. Int J Mol Sci. (2016) 17:367. doi: 10.3390/ijms17030367
25. Saklayen, MG . The global epidemic of the metabolic syndrome. Curr Hypertens Rep. (2018) 20:12. doi: 10.1007/s11906-018-0812-z
26. Wu, XW, Muzny, DM, Lee, CC, and Caskey, CT. Two independent mutational events in the loss of urate oxidase during hominoid evolution. J Mol Evol. (1992) 34:78–84. doi: 10.1007/BF00163854
27. So, A, and Thorens, B. Uric acid transport and disease. J Clin Invest. (2010) 120:1791–9. doi: 10.1172/JCI42344
28. Mandal, AK, and Mount, DB. Interaction between ITM2B and GLUT9 links urate transport to neurodegenerative disorders. Front Physiol. (2019) 10:1323. doi: 10.3389/fphys.2019.01323
29. Schlesinger, N . Dietary factors and hyperuricaemia. Curr Pharm Des. (2005) 11:4133–8. doi: 10.2174/138161205774913273
30. Mandal, AK, and Mount, DB. The molecular physiology of uric acid homeostasis. Annu Rev Physiol. (2015) 77:323–45. doi: 10.1146/annurev-physiol-021113-170343
31. Bobulescu, IA, and Moe, OW. Renal transport of uric acid: evolving concepts and uncertainties. Adv Chronic Kidney Dis. (2012) 19:358–71. doi: 10.1053/j.ackd.2012.07.009
32. Taniguchi, A, and Kamatani, N. Control of renal uric acid excretion and gout. Curr Opin Rheumatol. (2008) 20:192–7. doi: 10.1097/BOR.0b013e3282f33f87
33. Liu, R, Han, C, Wu, D, Xia, X, Gu, J, Guan, H, et al. Prevalence of hyperuricemia and gout in mainland China from 2000 to 2014: a systematic review and meta-analysis. Biomed Res Int. (2015) 2015:762820. doi: 10.1155/2015/762820
34. Mandal, AK, Mercado, A, Foster, A, Zandi-Nejad, K, and Mount, DB. Uricosuric targets of tranilast. Pharmacol Res Perspect. (2017) 5:e00291. doi: 10.1002/prp2.291
35. Copur, S, Demiray, A, and Kanbay, M. Uric acid in metabolic syndrome: does uric acid have a definitive role? Eur J Intern Med. (2022) 103:4–12. doi: 10.1016/j.ejim.2022.04.022
36. Mandal, AK, Leask, MP, Estiverne, C, Choi, HK, Merriman, TR, and Mount, DB. Genetic and physiological effects of insulin on human urate homeostasis. Front Physiol. (2021) 12:713710. doi: 10.3389/fphys.2021.713710
37. Spatola, L, Angelini, C, Badalamenti, S, Maringhini, S, and Gambaro, G. Kidney stones diseases and glycaemic statuses: focus on the latest clinical evidences. Urolithiasis. (2017) 45:457–60. doi: 10.1007/s00240-016-0956-8
38. Ziga-Smajic, N, Skrbo, S, Muratovic, S, Pehlivanovic, B, Lagumdzija, D, and Omerovic, N. Comparison of the effects of allopurinol and febuxostat on the values of triglycerides in hyperuricemic patients. Med Arch. (2020) 74:172–6. doi: 10.5455/medarh.2020.74.172-176
39. Nakatsu, Y, Seno, Y, Kushiyama, A, Sakoda, H, Fujishiro, M, Katasako, A, et al. The xanthine oxidase inhibitor febuxostat suppresses development of nonalcoholic steatohepatitis in a rodent model. Am J Physiol Gastrointest Liver Physiol. (2015) 309:G42–51. doi: 10.1152/ajpgi.00443.2014
40. Baratta, F, Pastori, D, Angelico, F, Balla, A, Paganini, AM, Cocomello, N, et al. Nonalcoholic fatty liver disease and fibrosis associated with increased risk of cardiovascular events in a prospective study. Clin Gastroenterol Hepatol. (2020) 18:2324–2331.e4. doi: 10.1016/j.cgh.2019.12.026
41. Mantovani, A, Petracca, G, Beatrice, G, Csermely, A, Lonardo, A, Schattenberg, JM, et al. Non-alcoholic fatty liver disease and risk of incident chronic kidney disease: an updated meta-analysis. Gut. (2022) 71:156–62. doi: 10.1136/gutjnl-2020-323082
42. EASL . EASD&EASO clinical practice guidelines for the management of nonalcoholic fatty liver disease. J Hepatol. (2016) 64:1388–402. doi: 10.1016/j.jhep.2015.11.004
43. Loomba, R, Hartman, ML, Lawitz, EJ, Vuppalanchi, R, Boursier, J, Bugianesi, E, et al. Tirzepatide for metabolic dysfunction-associated steatohepatitis with liver fibrosis. N Engl J Med. (2024) 391:299–310. doi: 10.1056/NEJMoa2401943
44. Tao, L, Yang, X, Ge, C, Zhang, P, He, W, Xu, X, et al. Integrative clinical and preclinical studies identify FerroTerminator1 as a potent therapeutic drug for MASH. Cell Metab. (2024) 36:2190–2206.e5. doi: 10.1016/j.cmet.2024.07.013
45. Loomba, R, Bedossa, P, Grimmer, K, Kemble, G, Bruno Martins, E, McCulloch, W, et al. Denifanstat for the treatment of metabolic dysfunction-associated steatohepatitis: a multicentre, double-blind, randomised, placebo-controlled, phase 2b trial. Lancet. Gastroenterol Hepatol. (2024) 10:S2468-1253(24)00246-00242. doi: 10.1016/S2468-1253(24)00246-2
46. Dunn, W, Xu, R, and Schwimmer, JB. Modest wine drinking and decreased prevalence of suspected nonalcoholic fatty liver disease. Hepatology. (2008) 47:1947–54. doi: 10.1002/hep.22292
47. Riazi, K, Azhari, H, Charette, JH, Underwood, FE, King, JA, Afshar, EE, et al. The prevalence and incidence of NAFLD worldwide: a systematic review and meta-analysis. Lancet Gastroenterol Hepatol. (2022) 7:851–61. doi: 10.1016/S2468-1253(22)00165-0
48. Ballestri, S, Nascimbeni, F, Baldelli, E, Marrazzo, A, Romagnoli, D, and Lonardo, A. NAFLD as a sexual dimorphic disease: role of gender and reproductive status in the development and progression of nonalcoholic fatty liver disease and inherent cardiovascular risk. Adv Ther. (2017) 34:1291–326. doi: 10.1007/s12325-017-0556-1
49. Di Bonito, P, Di Sessa, A, Licenziati, MR, Corica, D, Wasniewska, M, Umano, GR, et al. Is metabolic syndrome useful for identifying youths with obesity at risk for NAFLD? Children (Basel). (2023) 10:233. doi: 10.3390/children10020233
50. Green, M, Arora, K, and Prakash, S. Microbial medicine: prebiotic and probiotic functional foods to target obesity and metabolic syndrome. Int J Mol Sci. (2020) 21:2890. doi: 10.3390/ijms21082890
51. D'Alessandro, A, Mirasole, C, and Zolla, L. Haemoglobin glycation (Hb1Ac) increases during red blood cell storage: a MALDI-TOF mass-spectrometry-based investigation. Vox Sang. (2013) 105:177–80. doi: 10.1111/vox.12029
52. Chen, Z, Yu, R, Xiong, Y, Du, F, and Zhu, S. Erratum in: A vicious circle between insulin resistance and inflammation in nonalcoholic fatty liver disease. Lipids Health Dis. (2018) 17:33. doi: 10.1186/s12944-018-0678-8
53. Reungjui, S, Roncal, CA, Mu, W, Srinivas, TR, Sirivongs, D, Johnson, RJ, et al. Thiazide diuretics exacerbate fructose-induced metabolic syndrome. J Am Soc Nephrol. (2007) 18:2724–31. doi: 10.1681/ASN.2007040416
54. Feng, Y, Fu, M, Guan, X, Wang, C, Yuan, F, Bai, Y, et al. Uric acid mediated the association between BMI and postmenopausal breast Cancer incidence: a bidirectional Mendelian randomization analysis and prospective cohort study. Front Endocrinol (Lausanne). (2021) 12:742411. doi: 10.3389/fendo.2021.742411
Keywords: metabolic dysfunction associated steatotic liver disease, hyperuricemia, metabolic syndrome, liver biopsy, low-density lipoprotein, hyperinsulinemia
Citation: Cao W, Jiang T, Deng W, Wang S, Li X, Zhang Z, Zhang L, Lu Y, Chang M, Liu R, Wu S, Shen G, Gao Y, Hao H, Chen X, Hu L, Xu M, Yi W, Xie Y and Li M (2024) Insulin resistance has closer correlation with the occurrence of metabolic dysfunction associated steatotic liver disease diagnosed by liver biopsy. Front. Med. 11:1384927. doi: 10.3389/fmed.2024.1384927
Edited by:
Sheetalnath Rooge, University of Kansas Medical Center, United StatesReviewed by:
Asim Kumar Mandal, Brigham and Women’s Hospital and Harvard Medical School, United StatesDhananajay Kumar, Louisiana State University Health Shreveport, United States
Copyright © 2024 Cao, Jiang, Deng, Wang, Li, Zhang, Zhang, Lu, Chang, Liu, Wu, Shen, Gao, Hao, Chen, Hu, Xu, Yi, Xie and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Minghui Li, d3VobTIwMDBAc2luYS5jb20=; Yao Xie, eGlleWFvMDAxMjAxODRAc2luYS5jb20=; Wei Yi, eWl3ZWkxMjE1QDE2My5jb20=
†These authors have contributed equally to this work
 Weihua Cao
Weihua Cao Tingting Jiang
Tingting Jiang Wen Deng1†
Wen Deng1† Lu Zhang
Lu Zhang Ruyu Liu
Ruyu Liu Shuling Wu
Shuling Wu Mengjiao Xu
Mengjiao Xu Wei Yi
Wei Yi Yao Xie
Yao Xie Minghui Li
Minghui Li