
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
MINI REVIEW article
Front. Med. , 31 January 2024
Sec. Gastroenterology
Volume 11 - 2024 | https://doi.org/10.3389/fmed.2024.1327864
 Elisabetta Dell’Unto1,2
Elisabetta Dell’Unto1,2 Gianluca Esposito1,2
Gianluca Esposito1,2 Maria Rinzivillo2
Maria Rinzivillo2 Matteo Marasco1,2,3
Matteo Marasco1,2,3 Bruno Annibale1,2
Bruno Annibale1,2 Francesco Panzuto1,2*
Francesco Panzuto1,2*Gastric neuroendocrine neoplasms (g-NENs) are rare tumors arising from the gastric enterochromaffin-like cells. Recent data suggests an increased detection rate, attributed to more frequent esophagogastroduodenoscopies. While type 3 g-NENs were historically deemed aggressive, emerging research indicates potential for conservative management, especially endoscopic resection, in well-differentiated, small tumors. European guidelines now advocate for endoscopic intervention in selected cases, but North American guidelines remain more conservative. Key factors influencing outcomes are tumor size, grading, and depth of gastric wall infiltration. Endoscopic resection has shown promise for tumors confined to submucosal layers without lymphovascular invasion. Given the complexities, a multidisciplinary team approach is essential for management decisions. Current insights are largely based on retrospective studies, underscoring the need for prospective research to optimize endoscopic approaches.
Gastric neuroendocrine neoplasms (g-NENs) are rare tumors that grow from the gastric enterochromaffin-like cells (ECL cells), with a yearly incidence and prevalence of 0.4 and 3 per 100,000 people, respectively (1). The frequency of these neoplasms has increased in recent years, most likely due to the growing use of routine esophagogastroduodenoscopies (EGD) and the resulting increase in incidental findings (1, 2). As with other NENs, g-NENs can be classified as well-differentiated G1 tumors with a Ki-67 of 3%, well-differentiated G2 tumors with a Ki-67 of 3–20%, and well-differentiated G3 tumors with a Ki-67 of >20%. Gastric neuroendocrine carcinomas (g-NECs) are poorly differentiated tumors with a Ki-67 value greater than 20% (3). Furthermore, g-NENs can be divided into three subgroups based on the presence of an underlying gastric pathology and the presence or absence of hypergastrinemia/ECL hyperplasia: type 1 g-NENs, which account for 75–80% of all g-NENs and are associated with chronic atrophic gastritis (CAG). They are commonly low-grade (G1), well-differentiated, small and multiple lesions, with a risk of metastasis <5% and long-term survival of almost 100%; type 2 g-NEN, which rise in the context of multiple endocrine neoplasia type 1 syndrome, and have an intermediate risk of metastasis ranging from 10 to 30%; sporadic type 3 g-NENs, which rise in the absence of a background pathology, are usually single lesions with larger size (>1 cm) and, compared to other g-NENs, have a greater potential to generate metastasis (up to 50%), resulting in a worse long-term survival (5-year survival rate 70%) (4). Type 3 g-NENs, similar to types 1 and 2, belong to the ECL-cell neuroendocrine tumor family. They predominantly occur in males and are characterized by the absence of hypergastrinemia, and normal oxyntic mucosa surrounding the tumor, which lacks hyperplastic or dysplastic ECL-cell proliferation. The proliferation rates of type 3 g-NENs vary, encompassing low-grade (G1), intermediate-grade (G2), to high-grade (G3) classifications (5). Because the prognosis of these lesions is strictly dependent on tumor type, adequate bioptic sampling of the stomach is required to correctly define the assessment of g-NENs and to properly establish the diagnostic-therapeutic path (4, 6). Traditionally, type 3 g-NENs have been considered aggressive tumors, nearly similar to gastric adenocarcinoma, and thus treated with a radical approach (radical surgery with lymphadenectomy). Nonetheless, in recent years, smaller and more indolent type 3 g-NENs have been incidentally discovered, leading to more conservative care in a subset of patients (4). Because of the increased use of EGD in recent decades, type 3 g-NENs are more usually discovered early, with a reduced size and stage (7). As a result, the scientific community has been debating whether a more conservative method (endoscopic resection) could be feasible in certain patients (8). Different guidelines from Europe and US offer varying recommendations, reflecting the heterogeneity in clinical understanding and approaches to these tumors. This divergence in guidelines underscores the need for harmonized consensus and further research to establish clear management pathways for type 3 g-NENs.
This review aims to provide insights into the management of type 3 g-NENs, emphasizing the potential significance of endoscopic treatment in their care. Specifically, we will focus on the discrepancies among the various guidelines available for managing these patients. Given the need to regard poorly differentiated NEC as distinct, more aggressive disorders in which endoscopic therapy plays no role, this review concentrates on well-differentiated NETs (thus, the term NET will be used throughout the manuscript). We included data identified by searching the MEDLINE database with no date restriction using the following string of search (“gastric neuroendocrine neoplasms” OR “type 3 gastric neuroendocrine tumors” OR “type 3 gastric carcinoids”) AND (“endoscopy” OR “endoscopic treatment” OR “endoscopic resection” OR “endoscopic management” OR “endoscopic mucosal resection” OR “endoscopic submucosal dissection”). We included only articles deemed relevant to the objectives of this review and written in English. Guidelines from the leading gastroenterology/endoscopy and neuroendocrine tumor scientific societies were consulted.
International guidelines provide heterogeneous recommendations for the management of type 3 gNETs (Table 1). Endoscopic resection of tumors measuring <10 mm and of low grade (G1) is feasible, as per the recent European Neuroendocrine Tumor Society (ENETS) guidance paper, provided metastases are ruled out and the depth of gastric wall invasion is evaluated using endoscopic ultrasonography (4). Also, the European Society of Gastrointestinal Endoscopy (ESGE) suggests that endoscopic resection may be a viable option for type 3 gNETs that are less than 20 mm in diameter, show exclusive submucosal invasion, and have a negative gallium-68 DOTATOC scan beforehand (9). The American Society for Gastrointestinal Endoscopy (ASGE) states that, due to the high likelihood of lymph node metastases, type 3 gNETs should undergo surgical treatment. However, endoscopic excision might be considered for small, well-differentiated tumors (less than 10 mm) (10). Nonetheless, the North American Neuroendocrine Tumors Society (NANETS) guidelines issued in 2010 firmly maintain the indication for extensive surgery for type 3 gNETs (11). NCCN guidelines recommend surgical approaches, such as partial or total gastrectomy (with lymphadenectomy), as the “preferred” method for type 3 gNETs, considering endoscopic resection as an option when EUS or other imaging have ruled out regional lymphadenopathy without specifying indications regarding tumor size (12). Both the Nordic guidelines and the UK guidelines suggest to treat type 3 gNETs in the same way as adenocarcinomas, by performing surgical resection plus lymph node dissection (13, 14). The shift in recommendations toward a more conservative approach in the more recent guidelines (Table 1; Figure 1) is due to recent reports that, although based on small retrospective patient series, have highlighted the potential for safe and effective endoscopic therapy in these patients. The more conservative approach recommended by the ESGE (9) and ENETS (4) guidelines may be due to these guidelines being more recent than others. This underscores the importance of updating guidelines promptly as new scientific evidence becomes available, especially in type 3 gNETs where the scientific evidence is scant.
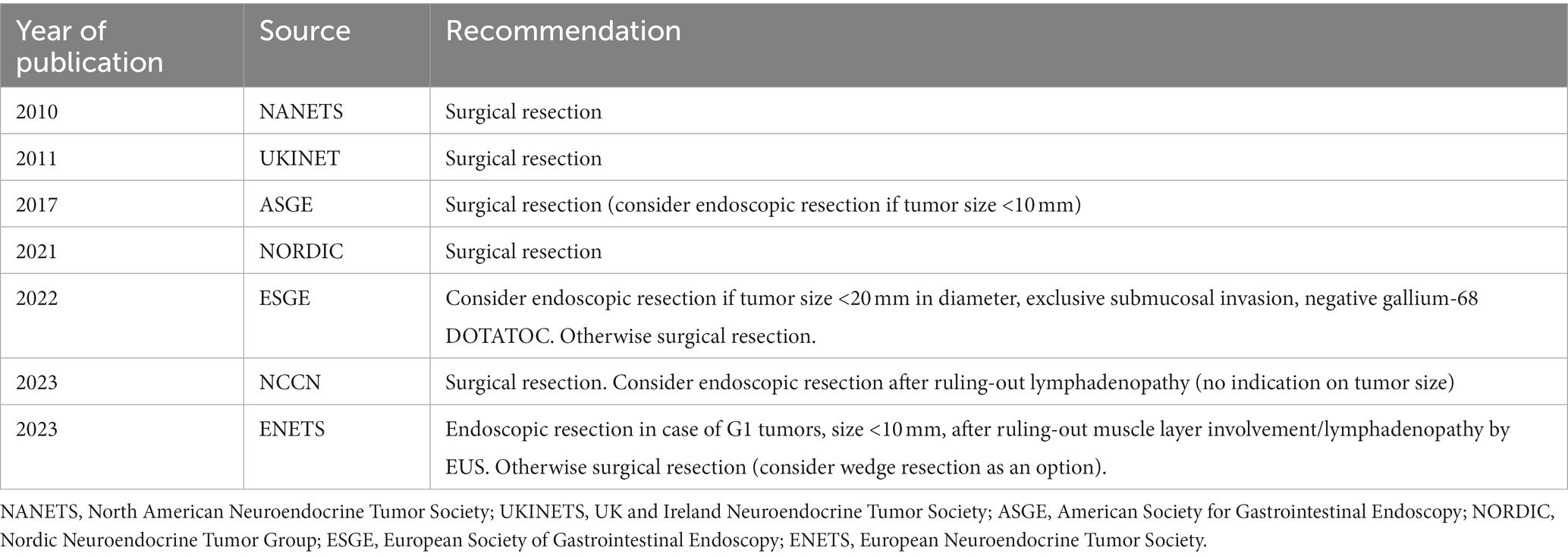
Table 1. Recommendations for treating type 3 gastric NETs according to the different international guidelines.
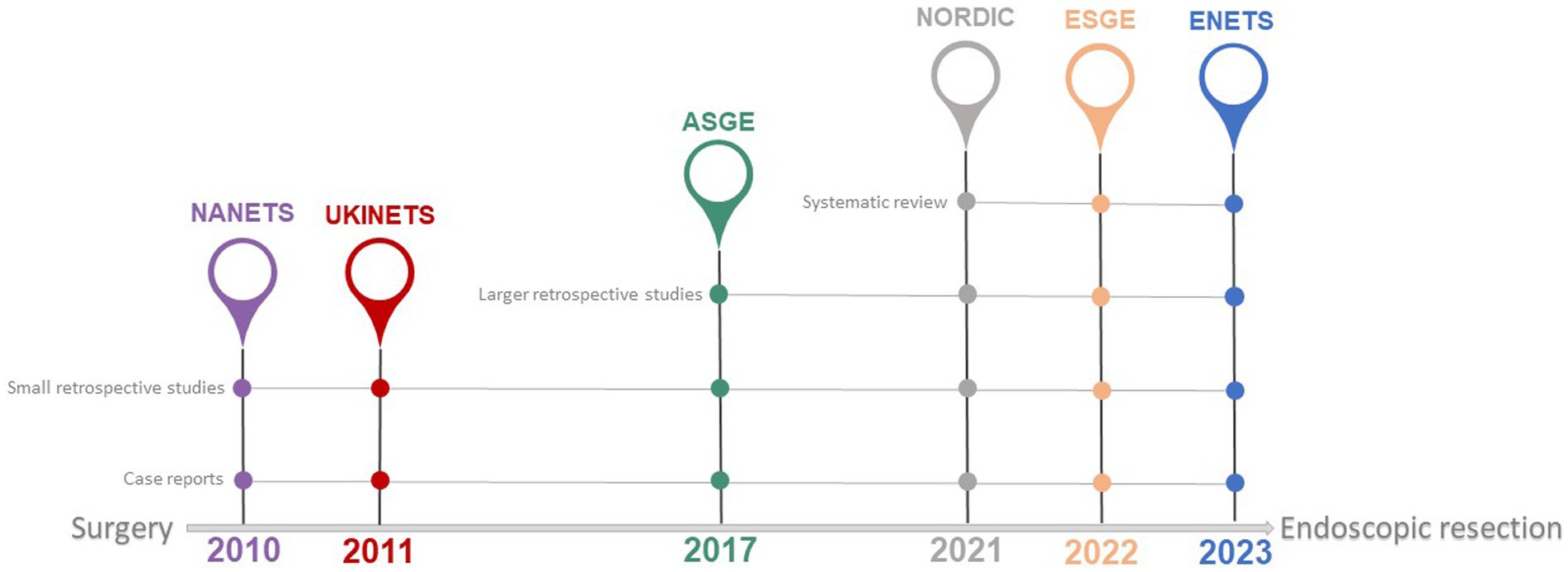
Figure 1. Available international guidelines on management of type 3 gNETs. Year of publication by leading scientific societies involved in the management of type 3 gNETs. A shift toward a more conservative approach is observed from older to newer guidelines (see also Table 1). The continuous grey line indicates the nature of the literature supporting the recommendations in the referenced guidelines. NANETS, North American Neuroendocrine Tumor Society; UKINETS, UK and Ireland Neuroendocrine Tumor Society; ASGE, American Society for Gastrointestinal Endoscopy; NORDIC, Nordic Neuroendocrine Tumor Group; ESGE, European Society of Gastrointestinal Endoscopy; ENETS, European Neuroendocrine Tumor Society.
Even a few new publications can alter the existing recommendations. Excharcou et al. analyzed 229 individuals with type 3 gNETs in a recent systematic review that comprised 10 nonrandomized retrospective investigations on this subset of tumors. Overall, 51.5% (n = 118) of them were well-differentiated G1-G2 NETs, and 121 patients with small and confined lesions received endoscopic excision with Endoscopic Mucosal Resection (EMR) or Endoscopic Submucosal Dissection (ESD) (15). Only one patient, 68 months after the endoscopic resection of a 16 mm G1 NET, had a nodal recurrence during follow-up, implying that selected cases have a better prognosis than previously thought (15). Hirasawa and colleagues investigated similar findings, reporting data on 144 patients with well-differentiated G1-G2 type 3 gNET, 63 of whom had endoscopic resection (48–76.2% - without any other subsequent therapy) (16). Only one patient experienced disease progression during follow-up after receiving conservative endoscopic resection alone, and the 5-year overall survival in the endoscopic group was 100%, with a 5-year recurrence-free survival of 97.6%. It should be highlighted that all type 3 gNETs undergoing endoscopic resection were restricted to the mucosa and submucosa, with a median tumor size of 7 mm, indicating that the best prognostic results are reserved for carefully selected patients (16).
Since an endoscopic strategy to the care of type 3 gNETs had been proposed, several authors pondered what the optimal resection technique was to obtain a complete excision of these lesions. Excharcou et al. reported a rate of complete endoscopic resection (R0) for type 3 gNETs using EMR or ESD of 72–80%, with the highest values obtained using the ESD technique (8); whereas Min et al. obtained a rate of R0 resection in 86.4% of the cases, using primarily ESD but also EMR/modified-EMR (15). However, the optimum endoscopic resection approach in this situation is still debated, despite the fact that ESD appears to be the most successful (17).
The identification of prognostic factors capable of predicting disease progression and assisting clinicians in deciding which patient is eligible for endoscopic therapy in those with type 3 gNET is particularly challenging, given the lack of supporting scientific evidence.
Consistent with findings from Excharcou (15) and Hirasawa (16), numerous retrospective studies (8, 18–21) observed positive outcomes following endoscopic resection of well-differentiated, small, low-grade type 3 gNETs limited to the submucosal layers without lymphovascular invasion. Conversely, some studies reported poorer outcomes for type 3 gNETs, often involving cases with larger, higher-grade lesions (22–24). As a result, it is critical to stratify the population based on the presence of unfavorable risk factors, which should guide therapeutic decisions. The most important features that seem to affect the clinical outcomes of patients with a diagnosis of type 3 gNETs, according to a recent systematic review of the literature, are size, grading, and depth of gastric wall infiltration (25). In Hirasawa’s paper, larger G2 lesions with deeper invasion of the gastric wall had a statistically significant higher risk of lymph nodal involvement, with a consequent worse prognosis. The importance of tumor size is highlighted further by the fact that in some cases, extremely small (5 mm) type 3 gNETs were accidentally excised utilizing biopsies and did not recur during follow-up (16). In terms of grading, the Ki67 index is one of the most important risk factors for GEP-NENs in general (26), but its relevance in gNETs is yet unknown (21). In any case, it is prudent to exercise caution before planning an endoscopic resection for a gNET with a high Ki67, given the predictable risk of a more unfavorable biological behavior. Moreover, in this patient setting, there is no evidence to suggest that endoscopic resection is safe. Unsurprisingly, deeper infiltration of the stomach wall with involvement of the muscolaris propria (and beyond) is a risk factor for lymph nodal or distant metastases (18, 24, 27). Endoscopic ultrasound (EUS) is becoming increasingly important in the diagnostic path in this context, to better determine the depth of tumor invasion and lymph node status. Although the role of EUS is well-established in evaluating NETs originating from other parts of the digestive system, such as pancreatic primaries, its role in gastric-originating forms has not been thoroughly studied. The paucity of data on this subject is a major challenge when addressing the scientific literature related to the efficacy of endoscopic treatment of gNETs. Nevertheless, EUS is recommended in patients with type 3 gNETs by the major recent international guidelines, primarily for planning the appropriate mode of endoscopic resection and for conducting a local disease staging to exclude the presence of loco-regional lymph nodes (28).
Despite being thought of aggressive tumors, type 3 gNETs are increasingly being recognized as more indolent lesions, treatable with conservative endoscopic treatment in selected patients with small, low-grade (G1) lesions. In the absence of standardized selection criteria, such as specific tumor size or Ki67 cut-off levels, it is imperative to evaluate each case individually within a multidisciplinary team discussion (29). Due to the disease’s rarity, there are little data in the literature, primarily from non-randomized retrospective studies with small and widely heterogeneous populations. The guidelines from various scientific societies do not entirely agree on when to use endoscopic resection for type 3 gNETs (Table 1). Specifically, they do not provide clear risk factors to assist clinicians in patient selection. However, there is a trend toward a more conservative endoscopic approach for small tumors (with <1 cm suggested as the cut-off limit, although not standardized), provided that accurate disease staging, including the use of EUS, has been conducted to rule out deep gastric wall invasion and/or lymph node involvement.
There is a pressing need for prospective data to determine the optimal therapeutic algorithm for type 3 gNETs, particularly for small tumors discovered incidentally. As awareness in this specific patient setting increases, there is a growing consensus that traditional surgical approaches may be supplanted by more conservative endoscopic management. Prospective clinical trials are crucial to identify the most effective endoscopic procedure for achieving complete curative resection. Although recent major guidelines uniformly advocate for EUS in disease staging—to both rule out lymph node metastases and assess involvement of the gastric deep layers—no studies have assessed the accuracy of EUS for this particular patient population. Scientific societies focused on NET management should initiate multicenter studies to address these gaps, with the goal of formulating therapeutic recommendations rooted in a robust evidence-based approach.
ED: Conceptualization, Writing – original draft. GE: Writing – review & editing. MR: Writing – review & editing. MM: Writing – review & editing. BA: Supervision, Writing – review & editing. FP: Conceptualization, Funding acquisition, Supervision, Writing – original draft.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This research was supported by grant Ateneo Sapienza RM12218161A53B1D.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Dasari, A, Shen, C, Halperin, D, Zhao, B, Zhou, S, Xu, Y, et al. Trends in the incidence, prevalence, and survival outcomes in patients with neuroendocrine tumors in the United States. JAMA Oncol. (2017) 3:1335–42. doi: 10.1001/jamaoncol.2017.0589
2. Rossi, RE, and Massironi, S. The increasing incidence of neuroendocrine neoplasms worldwide: current knowledge and open issues. J Clin Med. (2022) 11:3794. doi: 10.3390/jcm11133794
3. Rindi, G, Mete, O, Uccella, S, Basturk, O, La Rosa, S, Brosens, LAA, et al. Overview of the 2022 WHO classification of neuroendocrine neoplasms. Endocr Pathol. (2022) 33:115–54. doi: 10.1007/s12022-022-09708-2
4. Panzuto, F, Ramage, J, Pritchard, DM, Van Velthuysen, MF, Schrader, J, Begum, N, et al. Guidance paper for gastroduodenal neuroendocrine Tumours (NETs) G1–G3. J Neuroendocrinol. (2023) 35:e13306. doi: 10.1111/jne.13306
5. La Rosa, S, and Vanoli, A. Gastric neuroendocrine neoplasms and related precursor lesions. J Clin Pathol. (2014) 67:938–48. doi: 10.1136/jclinpath-2014-202515
6. Esposito, G, Angeletti, S, Cazzato, M, Galli, G, Conti, L, Di Giulio, E, et al. Narrow band imaging characteristics of gastric polypoid lesions: a single-center prospective pilot study. Eur J Gastroenterol Hepatol. (2020) 32:701–5. doi: 10.1097/meg.0000000000001697
7. Lahner, E, Pilozzi, E, Esposito, G, Galli, G, and Annibale, B. Gastric carcinoid in the absence of atrophic body gastritis and with low Ki67 index: a clinical challenge. Scand J Gastroenterol. (2014) 49:506–10. doi: 10.3109/00365521.2013.878381
8. Exarchou, K, Kamieniarz, L, Tsoli, M, Victor, A, Oleinikov, K, Khan, MS, et al. Is local excision sufficient in selected grade 1 or 2 type III gastric neuroendocrine neoplasms? Endocrine. (2021) 74:421–9. doi: 10.1007/s12020-021-02775-1
9. Deprez, PH, Moons, LMG, O’Toole, D, Gincul, R, Seicean, A, Pimentel-Nunes, P, et al. Endoscopic Management of Subepithelial Lesions Including Neuroendocrine Neoplasms: European Society of Gastrointestinal Endoscopy (ESGE) guideline. Endoscopy. (2022) 54:412–29. doi: 10.1055/a-1751-5742
10. Faulx, AL, Kothari, S, Acosta, RD, Agrawal, D, Bruining, DH, Chandrasekhara, V, et al. The role of endoscopy in subepithelial lesions of the GI tract. Gastrointest Endosc. (2017) 85:1117–32. doi: 10.1016/j.gie.2017.02.022
11. Kulke, MH, Anthony, LB, Bushnell, DL, de Herder, WW, Goldsmith, SJ, Klimstra, DS, et al. NANETS treatment guidelines. Pancreas. (2010) 39:735–52. doi: 10.1097/mpa.0b013e3181ebb168
12. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®). Neuroendocrine and adrenal tumors version 1. (2023) Available at: https://www.nccn.org/professionals/physician_gls/pdf/neuroendocrine.pdf
13. Janson, ET, Knigge, U, Dam, G, Federspiel, B, Grønbaek, H, Stålberg, P, et al. Nordic guidelines 2021 for diagnosis and treatment of Gastroenteropancreatic neuroendocrine neoplasms. Acta Oncol. (2021) 60:931–41. doi: 10.1080/0284186x.2021.1921262
14. Ramage, JK, Ahmed, A, Ardill, J, Bax, N, Breen, DJ, Caplin, ME, et al. Guidelines for the Management of Gastroenteropancreatic Neuroendocrine (including carcinoid) Tumours (NETs). Gut. (2011) 61:6–32. doi: 10.1136/gutjnl-2011-300831
15. Exarchou, K, Howes, N, and Pritchard, DM. Systematic review: Management of Localised low-grade Upper Gastrointestinal Neuroendocrine Tumours. Aliment Pharmacol Ther. (2020) 51:1247–67. doi: 10.1111/apt.15765
16. Hirasawa, T, Yamamoto, N, and Sano, T. Is endoscopic resection appropriate for type 3 gastric neuroendocrine tumors? Retrospective Multicenter Study. Digest Endos. (2020) 33:408–17. doi: 10.1111/den.13778
17. Esposito, G, Dell’Unto, E, Ligato, I, Marasco, M, and Panzuto, F. The meaning of R1 resection after endoscopic removal of gastric, duodenal and rectal neuroendocrine tumors. Expert Rev Gastroenterol Hepatol. (2023) 17:785–93. doi: 10.1080/17474124.2023.2242261
18. Min, B-H, Hong, M, Lee, JH, Rhee, P-L, Sohn, TS, Kim, S, et al. Clinicopathological features and outcome of type 3 gastric neuroendocrine Tumours. Br J Surg. (2018) 105:1480–6. doi: 10.1002/bjs.10901
19. Kwon, YH. Long-term follow up of endoscopic resection for type 3 gastric NET. World J Gastroenterol. (2013) 19:8703–8. doi: 10.3748/wjg.v19.i46.8703
20. Hanna, A, Kim-Kiselak, C, Tang, R, Metz, DC, Yang, Z, DeMatteo, R, et al. Gastric neuroendocrine tumors: reappraisal of type in predicting outcome. Ann Surg Oncol. (2021) 28:8838–46. doi: 10.1245/s10434-021-10293-7
21. Panzuto, F, Campana, D, Massironi, S, Faggiano, A, Rinzivillo, M, Lamberti, G, et al. Tumour type and size are prognostic factors in gastric neuroendocrine neoplasia: a multicentre retrospective study. Dig Liver Dis. (2019) 51:1456–60. doi: 10.1016/j.dld.2019.04.016
22. Li, Y-L, Qiu, X-D, Chen, J, Zhang, Y, Li, J, Xu, J-M, et al. Clinicopathological characteristics and prognosis of 77 cases with type 3 gastric neuroendocrine Tumours. World J Gastrointestinal Oncol. (2020) 12:1416–27. doi: 10.4251/wjgo.v12.i12.1416
23. Jiao, X, Wang, Z, Peng, X, Zhang, L, and Zhou, L. Effects of tumor types on treatment strategy formulation and prognostic evaluation of gastric neuroendocrine tumors. Future Oncol. (2020) 16:2197–207. doi: 10.2217/fon-2020-0150
24. Endo, S, Dousei, T, Yoshikawa, Y, Hatanaka, N, Taniyama, K, Yamauchi, A, et al. Gastric neuroendocrine tumors in our institutions according to the WHO 2010 classification. Int Surg. (2013) 97:335–9. doi: 10.9738/cc134.1
25. Laffi, A, Lania, AGA, Ragni, A, Di Vito, V, Liccardi, A, Rubino, M, et al. Gastric neuroendocrine tumors (g-NETs): a systematic review of the management and outcomes of type 3 g-NETs. Cancers. (2023) 15:2202. doi: 10.3390/cancers15082202
26. Panzuto, F, Merola, E, Pavel, ME, Rinke, A, Kump, P, Partelli, S, et al. Stage IV gastro-Entero-pancreatic neuroendocrine neoplasms: a risk score to predict clinical outcome. Oncologist. (2017) 22:409–15. doi: 10.1634/theoncologist.2016-0351
27. Vanoli, A, La Rosa, S, Miceli, E, Klersy, C, Maragliano, R, Capuano, F, et al. Prognostic evaluations tailored to specific gastric neuroendocrine neoplasms: analysis of 200 cases with extended follow-up. Neuroendocrinology. (2018) 107:114–26. doi: 10.1159/000489902
28. Zilli, A, Arcidiacono, PG, Conte, D, and Massironi, S. Clinical impact of endoscopic ultrasonography on the Management of Neuroendocrine Tumors: lights and shadows. Dig Liver Dis. (2018) 50:6–14. doi: 10.1016/j.dld.2017.10.007
Keywords: gastric carcinoids, neuroendocrine tumors, guidelines, endoscopic resection, management
Citation: Dell’Unto E, Esposito G, Rinzivillo M, Marasco M, Annibale B and Panzuto F (2024) Type 3 gastric neuroendocrine neoplasms: the rising promise of conservative endoscopic management. Front. Med. 11:1327864. doi: 10.3389/fmed.2024.1327864
Received: 25 October 2023; Accepted: 15 January 2024;
Published: 31 January 2024.
Edited by:
Mohamed Rahouma, NewYork-Presbyterian, United StatesReviewed by:
Zhaohai Yang, University of Pennsylvania, United StatesCopyright © 2024 Dell’Unto, Esposito, Rinzivillo, Marasco, Annibale and Panzuto. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Francesco Panzuto, Francesco.panzuto@uniroma1.it
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.