
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
MINI REVIEW article
Front. Med. , 14 February 2024
Sec. Ophthalmology
Volume 11 - 2024 | https://doi.org/10.3389/fmed.2024.1307682
Dry eye is a widespread chronic inflammatory disease that causes fatigue, tingling, burning, and other symptoms. Dry eye is attributed to rheumatic diseases, diabetes, hormone disorders, and contact lenses, which activate inflammatory pathways: mitogen-activated protein kinases (MAPK) and nuclear factor-B (NF-κB), promote macrophage inflammatory cell and T cell activation, and inflammation factors. Clinicians use a combination of anti-inflammatory drugs to manage different symptoms of dry eye; some of these anti-inflammatory drugs are being developed. This review introduces the dry eye inflammation mechanisms and the involved inflammatory factors. We also elucidate the anti-inflammatory drug mechanism and the detection limits.
Dry eye is a prevalent ocular surface disease worldwide, accounting for 11.3% of people over 50, and adversely affects the visual quality, work, and life of patients (1). Dry eye is a multifactorial disease involving conjunctival goblet cells, lacrimal glands, and meibomian glands. These structures affect electrolytes, water, mucin, and lipids in the tear film, causing instability, hypertonicity, and inflammation (2). The tear film, divided into two layers, has the functions of lubrication, antibacterial, nutrition, and wound healing. The inner layer close to the cornea is the aqueous mucus layer, and the apical epithelial cells of conjunctiva and corneal surface secrete mucin for the aqueous mucus layer; the closer to the cornea, the higher the mucin amount (3). Under normal conditions, the blink reflex is performed 5–10 times/s, renewing tears, evenly distributing on the ocular surface, and promoting meibomian lipid release. The corneal nociceptive nerve innervates the blink reflex, originates from the ciliary nerve derived from the trigeminal node, and transmits through the cornea-scleral limbus to innervate the facial nucleus of orbicularis muscle and regulates the lacrimal gland or facial muscles. When the corneal sensitivity is reduced, resulting in less reflex tearing and fewer nerve impulses from the trigeminal nerve, the lacrimal gland secrete less (4). Dry eye is divided into anti-tear and evaporative types. Antitear dry eye is often associated with autoimmune diseases and is characterized by decreased lacrimal and other gland secretion. Evaporative dry eye and lack of meibomian gland lipid, blink rate reduces, main show is tear evaporation increased, but the amount of tear secretion is normal (5).
Inflammation is essential in dry eye pathogenesis; activating inflammatory pathways and releasing inflammatory factors lead to a vicious dry eye cycle (6). The dry eye clinical diagnosis must often understand the patient’s symptoms, environment, occupation, diet, contact lens usage, autoimmune disease, and surgery histories. Infrared imaging of the meibomian glands was given at times to patients to determine whether dry eyes were accompanied by meibomian gland dysfunction (7, 8). With the development of research on dry eye mechanism and diagnosis, the treatment approach should not be limited to artificial tears (ATs). Anti-inflammatory therapy seems to be a more suitable way to treat dry eyes. This review describes the dry eye inflammation mechanism, the associated inflammatory factors, and the anti-inflammatory treatment.
Inflammatory cells possess an immune role on the ocular surface, including neutrophils, macrophages, natural killer, dendritic, and T cells (9). Macrophages infiltrate the corneal epithelium of dry eye patients exhibiting higher levels than the control; therefore, macrophages may be related to inflammation grade and neovascularization (10). Natural killer cells induce epithelial cell death while secreting interferon-γ (IFN-γ), which triggers an inflammatory cascade (11). Activated Th0 migrates to dendritic cells, together with the corneal antigen between lymphatic endothelial and dendritic cells, interacting with each other (12). T cells are divided into CD4+/CD8+ T cells and CD4+ CD8+ double-positive T cells. CD4+ T cells include effector T cells: T helper 1 and 17 (Th1 and Th17) cells. CD8+ T lymphocytes, located in the secondary T cell zone of the thymus, are activated by antibodies on histocompatibility complex 1 (MHC-1) to form cytotoxic T lymphocytes with high CD57 content by antigen-presenting cells (APCs) (13, 14). Th17 cells are important in chronic diseases and disease relapse. Th-17 cell differentiation is initiated by the STAT3 signaling pathway activated by transforming growth factor-β (TGF-β) and interleukin (IL)-6 secreted by epithelial and dendritic cells. Activated Th17 secretes IL-17A, promotes nuclear factor-B (NF-κB) and mitogen-activated proteikinases (MAPK) signaling pathways to induce inflammation, stimulates metalloproteinase production, destroys the epithelial junction structure, and affects the corneal barrier function (15, 16); IL-12/23 can drive Th17 cells to affect ocular surface autoimmunity (17). Although IL-12/23 can stimulate the memory of Th17 cells, IL-7/15 can maintain this memory function for a long time and even lead to chronic inflammation (18). The memory effect of Th17 on dry eye could be alleviated by anti-IL-15 treatment (19). Th1 cells mediate cellular immunity; IL-4 promotes IL-12 secretion by APC cells in dry-eye patients, which induces Th1 cells to secrete IFN-γ and tumor necrosis factor-alpha (TNF-α) that enhance the endoplasmic reticulum stress, reduce Ca2+ concentration, and stimulate cholinergic agonist secretion to reduce mucin secretion by goblet cells. This inflammatory response promotes conjunctival goblet and lacrimal gland cell apoptosis (Figure 1) (20) and stimulates endothelial cells to secrete CXCL9/10/11 chemokines to aggregate inflammatory cells (21). Th2 cells mediate humoral immunity by secreting IL-4/5/13 due to the induction of WGATAR nucleotide consensus sequence (GATA-3) derived by IL-2/7 (22).
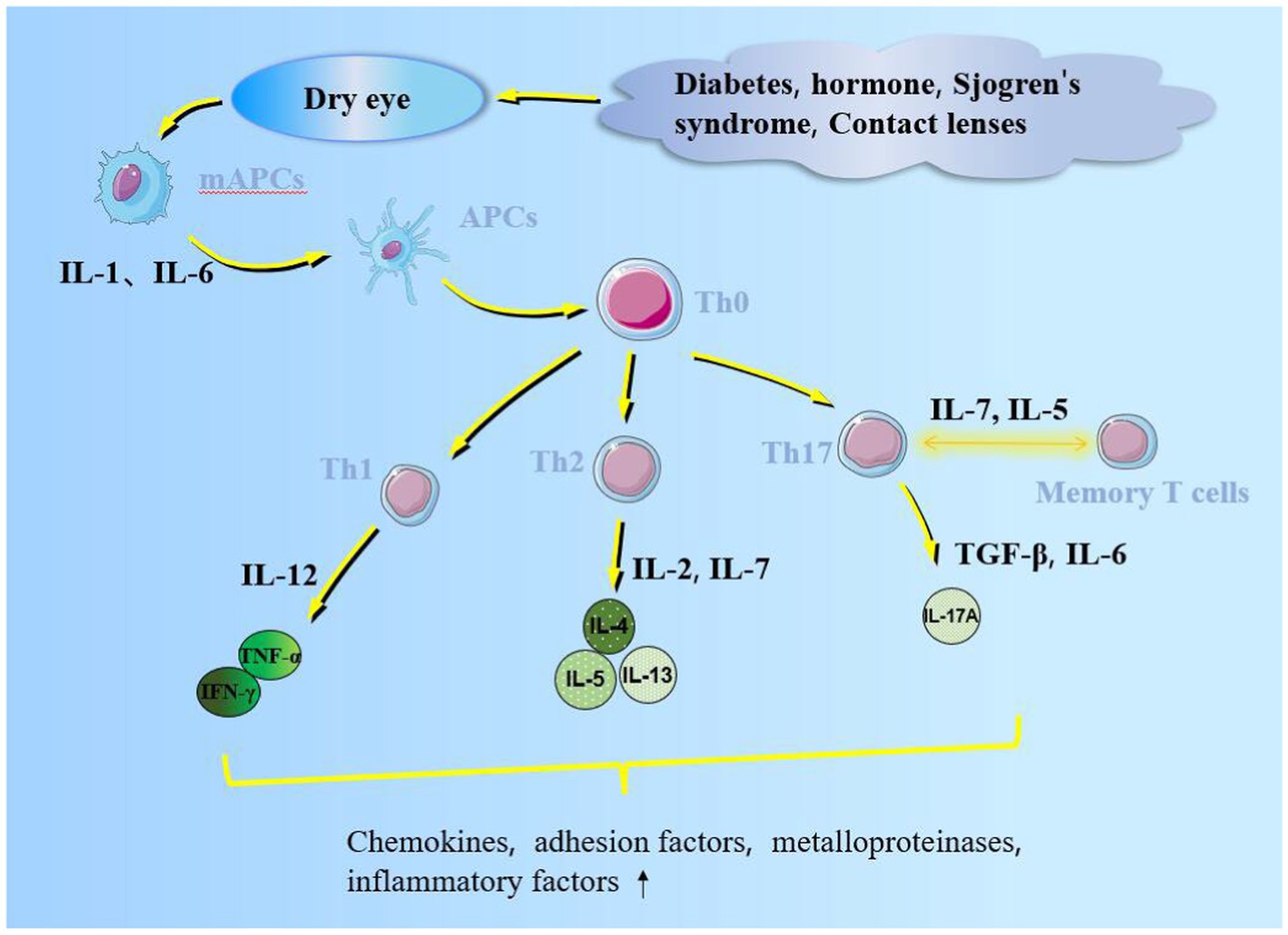
Figure 1. Inflammatory factors promote APC maturation. Mature APCs migrate through lymphatic vessels into regional lymph nodes with cytokines and chemokines. Additionally, it promotes naive T cells (Th 0) to form helper, memory, and regulatory T cells, which act on the ocular surface, secrete pro-inflammatory cytokines, and affect the corneal barrier function.
The predisposing factors of dry eye include an inflammatory environment, tear hypertonicity, microtrauma, microorganisms, infection, and other stress, which trigger related inflammatory pathways. The common pathways included MAPK, NF-κB, and TGF-β, including three subtypes: JNK, ERK, and p38. The p38 pathway regulates Th1/2 cytokine secretion (23), activates NF-κβ downstream of the MAPK pathway, and translocates NF-κB to the nucleus to transcript pro-inflammatory factors (24). The inflammatory factors, TNF, IL, and matrix metalloproteinases (MMPs) expressions can activate the NF-κB pathway to amplify inflammatory effects (25). NF-κB can promote epithelial cells to assemble inflammasomes, activate caspase-1 after receiving inflammatory signals, and promote inflammatory factors IL-1β/18 expression (Figure 2) (26). These inflammatory factors can cause damage-associated molecular patterns (DAMPs) and activate pattern recognition receptors (PRRS) such as toll-like receptor 4 (TLR4). TLR4 can directly activate caspase-8 to promote inflammasome assembly further (Figure 1) (27). These inflammatory factors promote a corneal proteolytic enzyme, such as corneal MMP-3/9, which can hydrolyze the connexin between corneal cells and destroy the dense barrier structure of the cornea. Increased inflammatory factors and MMPs exacerbated the ocular surface state of high permeability, aggravating the dry eye symptoms and contributing to the chronic inflammatory reaction (28).
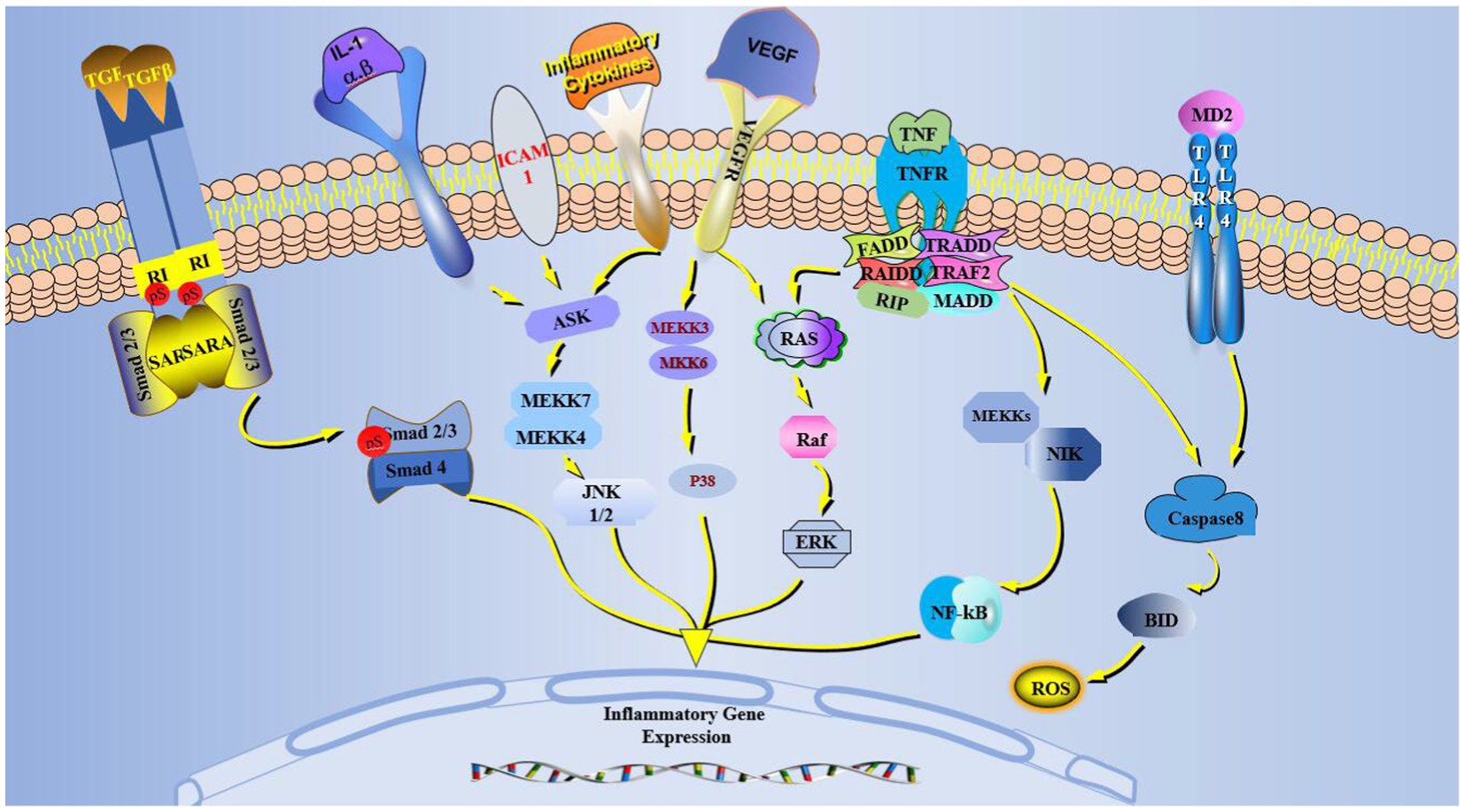
Figure 2. MAPK, VEGF, TGF-β, TNF, TLR-4 pathways in dry eye activated cells. Inflammation and VEGF activate MAPK, which generates JNK, P38, and ERK through ASK and Ras. TNF activates Ras and induces NF-κB production. TLR-4 activation induces ROS and inflammatory body production in cells. The TGF-β pathway binds and phosphorylates Smad2/3/4. The newly generated JNK, P38, ERK, NF-κB, and Smad2/3/4 in the cytoplasm enter the nucleus and affect the inflammatory gene expression.
Blood vessels in normal cornea tend to be antiangiogenesis factor (Thrombospondin-1) (TSP-1); TSP-1 and endothelial cells of CD36 and CD47 inhibit angiogenesis (29). Vascular endothelium maintains the balance between internal and external blood vessels. Systemic diseases, such as Sjögren’s syndrome and diabetes mellitus, can cause systemic chronic inflammation, destroy the endothelium, and affect various organ structures. In endothelial dysfunction, after increased ROS, nitric oxide (NO) bioavailability is reduced, cell adhesion molecules are increased, and leukocyte infiltration is enhanced (30). Adhesion molecule intercellular adhesion molecule-1 (ICAM-1) in white blood cells, endothelial cells, and epithelial cell surface expression belongs to the immunoglobulin superfamily of albumin. The functionally associated antigen-1 (LFA-1) ligand rolls along the vessel wall and increases T cell adhesion to the endothelium in dry eye pathogenesis (31). The decrease of lacrimal gland secretion in diabetic dry eye patients is related to the lacrimal gland microvascular damage. In dry eye patients, the increased conjunctival blood vessel diameter increases the blood vessel density and blood flow rate, mainly related to cellular cytosine, NO, prostaglandins PGI2, PGE2, and histamine (32).
Lymphatic vessels grow parallel in the limbus to form a network that maintains homeostasis by draining interstitial fluid and metabolites. During inflammation, blood vessels leak, generating increased interstitial fluid. Accordingly, lymphatic dilation allows more fluid and inflammatory cells to enter the lymphatic vessels to alleviate inflammation and maintain homeostasis (33). IL-1 induces IL-17 to promote vascular endothelial growth factor-D (VEGF-D) and VEGFR-3 expression in lymphatic vessels and increase lymphatic endothelial cell proliferation under dry stress (12). The VEGF and VEGF receptor families regulate lymphangiogenesis, among which VEGF-A/B mainly promote angiogenesis, while VEGF-C/D mainly promote lymphangiogenesis (34). VEGF-A/C are generated by tissues and then flow into lymph nodes, initiating lymph node production (35). VEGF-C/D activate ERK and AKT by binding to VEGFR3 to induce lymphatic migration and germination, and these lymphatic vessels migrate to the center of the cornea, which has a larger diameter of lymphatic vessels that grow into the cornea than the lymphatic vessels of the limbus (36, 37). The corneal lymphatic diameter and area decreased in VEGF-C-blocked dry-eye mice (38). VEGF-A expressed by B cells interacts with VEGFR-2, activating lymphatic proliferation without affecting new lymphatic vessel germination, besides inducing CD11b+ macrophage recruitment in the cornea injury site and promoting VEGF-A/C/D (39).
Diabetes is an aggravating factor in dry eye patients. Due to neuropathy and metabolic disorders, diabetes may affect dry eye symptoms such as dryness, foreign body sensation, and burning sensation. The corneal epithelial layer of diabetic patients has high fragility, low thick bottom, and low cell density, weakening the barrier function (40). A high glucose environment promotes tissue protein glycosylation, AGE, and its receptor, which promotes the NF-kB signaling pathways activation and ROS (41). In the nucleus, NF-κB causes lacrimal gland cells to enter the polyol pathway. Glucose forms sorbitol under the action of aldose reductase; the accumulated sorbitol increases the lacrimal gland cell osmotic pressure, impairing the function of the lacrimal gland after cell edema (42). Oral aldose reductase inhibitors increase tear film evaporation time; therefore, insulin secretion affects meibomian gland function, lacrimal gland, and corneal epithelial proliferation (43). High glucose reduces the effect of trigeminal nerve nutrition, corneal nerve fiber density, length is reduced, patient blink rate drops, and it appears tear evaporation capacity increases. Panretinal laser therapy can affect corneal neuropathy and cause dry eye (44). In diabetic patients, meibomian gland duct obstruction, acinar atrophy, and surrounding inflammatory manifestations lead to meibomian gland dysfunction and dry eye (45). In the urea chain within induced diabetic rats, the lacrimal gland weight and volume are lower, while inflammation and related factors such as IL, TNF, and AGE were higher than in the control (46).
Sjögren’s syndrome is an autoimmune disease of the exocrine gland (such as tear ducts, salivary, sebaceous, and sweat glands). In women between 20–40 years old and with a high postmenopausal incidence, Sjögren’s autoantibody syndrome interferes with and damages a toadstool receptors, causing systemic symptoms (47). About 11% of dry eye patients can be diagnosed with Sjögren’s syndrome (48), which is characterized by swelling of lymphocytes in the lacrimal gland and conjunctiva, T lymphocyte attack with meibomian gland shedding, and mucin defense (49). In patients with Sjögren’s syndrome, antigen-antibody reaction may occur in the corneal stroma, inducing inflammation and causing collagen dissolution and corneal mechanobiology destruction (50). In patients with Sjögren’s syndrome complicated with dry eye, the positive rate of cornea staining is higher than in ordinary patients with dry eye.
The changes in corneal substromal nerve fibers in patients with Sjögren’s syndrome are debated; the chronic neuropathic pain in patients with dry eye in Sjögren’s syndrome may be related to decreased corneal substromal nerve. Li et al. (51) suggested that different disease courses or development stages may change corneal substromal nerve fibers differently. Ran et al. (52) suggested that corneal subbasal nerve fibers may be related to eye injury, tear production, and the response degree to such damaging stimuli.
Women are more likely to suffer from dry eye due to sex hormones, as androgens promote lipid production while inhibited by estrogens (53). Androgen has been shown to have a regulatory role in the whole body of sebaceous glands, including the eye meibomian gland. The meibomian gland, as the largest sebaceous gland in the human body, produces 5α-reductase (54) and contains androgen receptors in the nucleus, which can convert testosterone into the active form of dihydrotestosterone, promoting DNA and protein synthesis in the lacrimal gland and increasing lacrimal gland secretion (55). For males, approximately 50% of the androgen production comes from the gonads; for women, almost all androgen originates from the adrenal glands, synthesized from adrenal steroid precursor (56). The probability of dry eye increases with age, especially in postmenopausal women, when the activity and content of the steroid precursor dehydroepiandrosterone (DHEA) in the adrenal gland and steroidogenic enzymes in the eye tissue decrease. Serum testosterone in premenopausal women had a negative correlation stability, and postmenopausal positively correlated. Accordingly, tears were positively correlated in premenopausal women; the opposite was true for postmenopausal (57). The local application seems to be better for the androgen treatment of dry eye than general replacement therapy. Estrogen has a certain anti-inflammatory effect; it can promote TNF-α and IL-6 expression and lymphocyte immune response. The decrease in sex hormones may cause dry eye symptoms in menopausal women. Conversely, for pregnant women in the third trimester, dry eye symptoms are caused by high estrogen and progesterone levels (58). Estrogen therapy for dry eye is controversial; for menopausal women, randomized controlled trials have shown that ATs combined with estradiol eye drops are more effective than ATs alone (59). Coksuer et al. (60) showed that postmenopausal women continued to take drospirenone 2 mg + estradiol 1 mg for six months, BUT and Schimer trial results improved. Although hormone therapy has a certain therapeutic effect on dry eye, long-term application may increase dry eye symptoms.
After using contact lenses, a more common term is end-of-day (EOD) symptoms, which refers to the dryness and discomfort of patients after removing contact lenses at night. Over half of people who wear contact lenses for a long time can be diagnosed with dry eye (61). The water content of contact lenses is inversely proportional to the prevalence of dry eye (62). Moreover, hydrogel contact lens material was more likely to lead to dry eye than silicone hydrogel material (63); the tear film lipid becomes thinner, and the osmolarity increases, which increases tear evaporation (64). Contact lenses of about 2 mm cover the conjunctival area; in a normal blink at the same time, contact lenses will move along the conjunctiva. Therefore, occasional contact lens breakage, leaving debris between the contact lens and the cornea, and other small debris may cause corneal and conjunctival wear and induce inflammation. IL-6/17A, MMP-9, ICAM-1, and granulocyte-macrophage colony stimulating factor (GM-CSF) levels increased in patients wearing contact lenses (65). The meibomian glands in contact lens wearers gradually shorten from the distal end and are more severely damaged than in ordinary dry eye patients. Conjunctival goblet cell density decreased after 3 months of contact lens wear (66, 67). Mild dry eye in contact lens wearers causes ocular surface inflammation and activation of dendritic cells in the cornea. The increased number of activated dendritic cells can decrease corneal basal nerve fibers. However, when dendritic cells are reduced, the corneal subbasal nerve fibers may increase due to regeneration (68). Prolactin, lipocalin-1, lactoferrin, and lysozyme are decreased in tears of dry eye patients. Compared with normal dry eye patients, prolactin content increased in those wearing contact lenses while the proline content decreased (69, 70).
Clinically, some patients with moderately severe dry eye drops use ring spore in ring spore essence, an isolate from the fungus immune modulators. The drug is positively charged due to its low water solubility and interacts with the negatively charged mucin in the tear film to improve its bioavailability as an oil-water emulsion (71). Cyclosporine is mainly metabolized in the liver of mammals and is not metabolized in the eye, where it mainly accumulates on the ocular surface and corneal epithelium. Continuous use of cyclosporine for 12 months hardly causes damage to the corneal endothelium (72). T cells can take up cyclosporine, which induces inflammation after being dropped on the ocular surface. By binding to cyclophilin in T cells, cyclosporine inhibits calcineurin, an enzyme required for T cell proliferation. However, due to the action of cyclosporine, T-cell proliferation is limited, and inflammatory factor formation is reduced (73).
Presently, 0.05%–0.1% cyclosporine eye drops are commonly used in the market. In dry eye patients without systemic diseases, 0.05% cyclosporine is recommended for 3 months. In patients with dry eye associated with systemic disease, 0.05% ring spore treatment is recommended for 6 months (74). In dry eye patients, 0.05% cyclosporine was used to take a conjunctival epithelial biopsy, revealing that conjunctival goblet cells and soluble mucin increased (75). In Mexican patients, 0.1% cyclosporine A was more effective in improving dry eyes, red eyes, and eye fatigue. The tear side effect is weak. Some studies have compared the effects of 0.05%, 0.1%, 0.2%, and 0.4% cyclosporine, indicating that 0.1% cyclosporine improved dry eye indexes (76). However, some dry eye patients may experience conjunctival congestion and tingling when using cyclosporine. Therefore, combining cyclosporine and other anti-inflammatory drugs in eye drops can reduce the drug-caused discomfort (77).
Cortical sterols and glucocorticoid receptor inhibition of inflammation factors promote lymphocyte apoptosis. The common corticosteroids in clinical practice include prednisone, dexamethasone, and fluorometholone. The stromal layer is hydrophilic because the corneal epithelium and endothelium are hydrophobic. Ethyl acetate, alcohols, and salts are commonly used as carriers of corticosteroids: acetic ester and alcohol derivatives to improve the fat-soluble carrier, phosphate, and hydrochloride to improve the water-soluble carrier (78). Methyl prednisone can reduce dry eye on MAPK pathway activation in mice, reducing inflammatory cytokine transcription in corneal epithelium to relieve dry eye inflammation (79). MMP-9, TNF-α, and IL-1β levels in tears of patients with dry eye were decreased after topical dexamethasone treatment. They also demonstrated that topical dexamethasone alleviated the hypertonic state of tears by inhibiting NF-κB activation through caspase-1 (80). Due to the inhibitory effect of dexamethasone on IL-1β, subsequent MMP-1 promoter activation is reduced, thereby inhibiting ERK and JNK phosphorylation (81). Dexamethasone controls MMP-8, which promotes corneal neutrophil infiltration and increases MMP-1/9/13 expression while impairing corneal healing in its absence. TIMPs are a two-way adjustment factor: TIMP-2 binds to MMP-2/14 to promote wound healing, and the opposite was observed for TIMP-1.
In conclusion, dexamethasone can treat the early inflammation of dry eye by reducing MMPs and TIMP-1, increasing MMP-8 transcription, and reducing neutrophil infiltration and inflammatory factor production (82). Long-term use of corticosteroids has side effects, such as increased intraocular pressure, infection aggravation, and posterior capsule opacity. Therefore, short-term initiation with soft steroids such as fluorometholone and loteprednol may be considered (83, 84).
Nonsteroidal antiinflammatory drugs (NSAIDs) are a safer alternative to corticosteroids for ocular inflammation control. Diclofenac sodium, bromfenac, and pranoprofen are common eye drops. NSAIDs inhibit cyclo-plus oxidase synthesis. COX, divided into COX-1/2, promotes arachidonic acid production of thromboxane, which induces inflammation (85). COX-2 was not present in normal cells but was sensitive to moderate to severe inflammation and chemical burns (86). NSAIDs and ATs were more effective in treating dry eyes than controls using ATs alone (87). Many think non-selective NSAID and COX-2 inhibition of COX-1 are simultaneously, but COX-1 inhibition affects the prostaglandin generated to promote corneal epithelium healing. Nimesulide, a selective non-steroidal anti-inflammatory drug, can preferentially inhibit COX-2, which is suitable for experimental animal dry eye rabbit models. In the dry eye rat model, tear osmolality increases, and extracellular ions are transferred into the cells to maintain stability, accelerating corneal cell apoptosis. During this period, the nuclear factor of T cell 5 (NFAT5) is activated, which regulates organic permeate formation and slows the increased intracellular ions (88). Topical administration of diclofenac sodium can stimulate NFAT5 production and reduce corneal epithelial damage without affecting tear volume (89). While NSAID drugs can alleviate the symptoms of dry eye patients, there is literature pointing out that NSAIDs can interfere with corneal defects in patients with corneal epithelium healing. NSAIDs can reduce the corneal sensitivity of ordinary individuals, and patients with dry eye, especially patients with Sjögren’s syndrome, should be carefully used. Severe cases may be accompanied by the possibility of corneal perforation (90).
Currently, the antibiotics used in dry eye treatment include ofloxacin, Azithromycin, Doxycycline and minocycline tetracycline class. Local use of ofloxacin eye drops, although stability can be improved less in dry eye treatment (91). For dry eye caused by contact lens wear, 1.0% azithromycin eye solution can prolong the comfortable time of contact lens wearing and improve the dry eye degree (92). For dry eye with meibomian gland dysfunction, azithromycin can inhibit bacterial lipase formation, reduce meibomian gland lipid degradation by lipase, and promote the lipid content in tear film (93).
Azithromycin can stimulate meibomian gland cell differentiation, increase intracellular cholesterol, cholesterol lipid, phospholipid, and lysosome accumulation, promote new protein secretion, and improve dry eye (94, 95). As a broad spectrum of large ring lactone class antibiotic, azithromycin has a better permeability; it can block NF-κB activation, inhibiting inflammatory cytokines IL-6/8 (96). For dry eye patients with acne rosacea, tetracycline can reduce inflammatory factors IL-1α and TNF-α (97). Doxycycline inhibits MMP-9 formation by inhibiting TGF-β and activating the Smad and MAPK signaling pathways in corneal epithelial cells (98). Doxycycline can reduce the increased MMP-1/3/9/13 and inhibit JNK activation in human corneal epithelial cells under a hypertonic environment (99). Azithromycin and doxycycline can restore the carotenoid level in the tear film of patients with dry eyes. Therefore, the patients do not have dry eye symptoms and signs. Moreover, azithromycin seems more effective than doxycycline in improving meibomian gland secretion (100).
Autologous blood serum contains epithelial growth factor (EGF), TGF-β, vitamin A, lysozyme, and fibronectin, which can improve epithelial cell density and promote the migration and repair of corneal epithelial cells (101, 102). For moderate dry eye, 20% autologous serum has a similar effect to 50% autologous serum; consequently, the recommended concentration is 20%, while for severe dry eye, 50% autologous serum is recommended (103). However, the treatment time is long, and it must be used for 3 months after the end of treatment, and the effect may be weakened with the extension of treatment time (104). Additionally, autologous serum should be sterile when prepared, used, and stored. Once contamination occurs, microorganisms will proliferate in the serum, likely aggravating the disease (105).
Hormone therapy for dry eye in postmenopausal women has been a research focus. The main mechanism of hormone therapy for dry eye is to reduce lacrimal gland inflammation, increase IgA levels, and promote meibomian gland secretion (106). Estrogen alone is more effective than estrogen combined with progesterone in improving tear and ocular surface symptoms in postmenopausal dry-eye women (107); especially for patients under the age of 50 menopause, the improvement effect is more significant (108). However, hormone therapy may increase intraocular pressure and even aggravate dry eye; this adverse risk may increase with age (109). IL-1 receptor antagonist (IL-1Ra, Anakinra) can effectively bind to IL-1R1, reduce inflammatory body production by macrophages, block the subsequent inflammatory response, and relieve pain in patients with dry eyes (110, 111). Meibomian gland massage, hot compress, and intense pulsed light (IPL) therapy are common for patients with meibomian gland dysfunction. Meibomian gland massage and hot compress can mainly open the obstructed meibomian gland mouth and remove the secretion accumulated in the meibomian gland (112). However, this moment of dredging on improving the meibomian gland dysfunction in patients with dry eye effect is short (113).
IPL therapy uses about 500–1,200 nm wavelength light in the eye and skin; it raises the skin temperature, induces superficial vascular coagulation, and while promoting meibomian gland secretion, it also attenuates the activity of inflammatory factors in the capillaries (114). IPL can target the pigment oxidase enzymes in cells that initiate the photobiomodulatory cycle, and the energy generated is used for cell repair (115). IPL can prolong the appearance of broken, repair rough corneal epithelium, and protect against mites (116). However, because the IPL energy strengthens when used, it may cause burns, the risk of bubbles, stabbing pain, and skin pigmentation (117). Mesenchymal stem cells (MSCs) can be transformed into various cells while regulating immune responses, repairing tissues, reducing CD4+ T cell infiltration, and reducing inflammation (118). Dry eye in the rat using bromine deoxyuridine labeled MSCs eye drops a week later, the conjunctival goblet cell increased, and tear volume and stability were improved (119).
The lymphocyte infiltration degree in the lacrimal gland of Sjögren syndrome dry eye mice decreased, and aquaporin 5 expression increased after intraperitoneal injection of bone marrow-derived MSCs (120). In addition to drug treatment of dry eye, dietary supplement research is widespread. Schirmer score and Schirmer score of dry eye patients who took royal jelly for 8 weeks were significantly improved. This may affect the potential mechanism of mitochondrial function in the lacrimal gland; AMPK phosphorylation improves the level of lachrymal energy to repair the tear (121, 122). Manuka honey, compared with normal honey, has higher polyphenol compounds and a large amount of methyl glyoxal, which makes it have better anti-inflammatory and antioxidant capacity (123), as it can improve ocular surface index, meibomian gland function and MMP-9 level (124).
Continuous supplementation of omega-3 fatty acids for 3 months reduced tear osmolality and increased tear film stability while reducing IL-17A levels (125). The ratio of ω-3 to ω-6 fatty acids determines whether inflammation occurs. Omega-6 fatty acids generated class arachidic acid has a pro-inflammatory role; omega-3 fatty acids were mostly eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA). EPA inhibits prostaglandin, leukotriene, and thrombinin production. DHA can be shortened and re-saturated to produce EPA and anti-inflammatory prostaglandins (126). In addition, added n-3 polyunsaturated fatty acids (n-3PUFA) and alpha lipoic acid (ALP) can increase tear secretion (127). Vitamin D can inhibit dendritic cell maturation, reduce inflammation, improve corneal endothelium, tear film index hyperosmolar state, and repair barrier function (128).
ATs are the most basic and conventional treatment for dry eyes. The different classifications of eye drugs can be divided into alternative and ocular surface wetting agents, more tears regulators three categories (129). Common moisturizer ingredients are carboxymethyl cellulose, carbomer, hyaluronic acid, betaine, sorbitol, glycerin, and salt ions. The structure of moisturizers is similar to mucin secreted by goblet cells, which can replenish water and improve tear film stability (19, 130). The common type of multi-action tear substitute is hypotonic ATs, which can treat dry eye by reducing the hyperosmolar condition. The hydroxyl and carboxyl groups of the drug can interact with the hydrogen bonds in the tear film (131). However, visual quality improves in some patients and deteriorates in others after using ATs (132, 133).
A dry eye with neuropathic pain can be relieved by nerve stimulation or gabapentin. Brinton et al. (134) implanted a nerve stimulator into the nasal submucosa of experimental rabbits. They gave a certain stimulation intensity and frequency every day, revealing that the volume of tears, the concentration of tear protein, and the content of lipids in the tears of rabbits increased, and the osmolality decreased. In 1927, the nasolacrimal reflex (NLR) was proposed; when the nasal mucosa is stimulated, it starts from the ophthalmic branch of trigeminal nerve, passes through the afferent nerve to anterior ethmoidal nerve, reaches the superior salivary nucleus (SSN) of midbrain and pons, and then passes through the parasympathetic branch of efferent nerve to meet the lacrimal nerve, innervating the lacrimal gland and accessory lacrimal gland, and controls tear production (135, 136).
Additionally, the electrical stimulation of tear ducts and lacrimal nerve, sieve before nerve, trigeminal nerve, and the cornea can increase the number of tears (137, 138). Gabapentin can bind to the α2δ subunit of presynaptic N-type voltage calcium channel, reduce excitatory neurotransmitter release, increase γ-aminobutyric acid (GABA) content, and relieve pain (139, 140). Gabapentin, for neurological encephalitis, diabetic neuropathy, and nerve after herpes zoster pain medication, showed the side effects of central nervous system suppression, such as dizziness, headache, and drowsiness (141). Vitamin A can enhance tear film stability and stimulate production (142). Ren et al. (143) demonstrated that oral vitamin B1 and mecobalamin in dry eye patients improved corneal nerve length, width, and reflectivity to accelerate epithelial cell repair. Additionally, maintaining eye hygiene and removing mites have certain benefits for dry eye treatment; using tea tree oil to remove mites can improve dry eye symptoms (144).
Dry eye is a common disease that is usually managed using ATs. The main way to detect dry eye inflammation is to measure the cytokines in tears, such as anti-inflammatory cytokines IL-1b, IL-8, IL-17A, IFN-g, TNF-a or anti-inflammatory factor IL-10. The commonly used tear collection methods include Schirmer test paper collection and glass microcapillary collection. The difference is that the latter is suitable for the detection of a wider range of proteins in tears, while the former is more suitable for the detection of inflammatory factors (145, 146). With the wide application of new examination techniques in clinical practice, the number of inflammatory cells and dendritic cells in the corneal stroma of dry eye patients has increased by confocal microscopy. Currently widely used is the HRT3 confocal microscope produced by Heidelberg, Germany, which has a magnification of 800 times. The lateral resolution was 1–2 μm and the axial resolution was 5–10 μm. The field of view is smaller because of the larger magnification. For examination, topical anesthesia was performed in advance and the microscope objective was covered with a polymethyl methacrylate cap. The instrument probe was placed in contact with the corneal surface, and the images were collected by manually adjusting the controller until the corneal substromal nerve fiber layer could be clearly seen (147, 148).
With primary diseases such as Sjögren’s syndrome, diabetes patients should be actively treating the primary disease. A dry eye with inflammation produces various inflammatory factors (Table 1). Therefore, anti-inflammatory treatment combined with ATs seems more effective in improving dry eye mechanisms. Non-steroidal anti-inflammatory drugs can be used in patients with long-term contact lens wearing because of papillae and follicles in the palpebral conjunctiva. 0.1% cyclosporine is not recommended for moderate dry eyes because it irritates the eye, and 0.05% cyclosporine can be added first. Using corticosteroids can be anti-inflammatory and improve dry eye for postoperative dry eye. For dry eyes associated with meibomian gland dysfunction, and with the meibomian gland massage and hot compress, strong pulse therapy appears to relieve the discomfort of the patient instantly and reduce the tarsal cyst and stye.
In treating meibomian glands, steroid hormones such as dexamethasone, Pyridostigmine and antibiotic azithromycin can achieve twice the effect with half the effort. Compared with other anti-inflammatory drugs, the autologous serum has more immunoreactive ingredients and seems better for use in concurrent keratitis under conditions of protection from microbial infection. Although dry eye is more common in elderly female patients, it is necessary to monitor sex hormone levels while regularly reviewing dry eye. Therefore, it was suitable for endocrine disorder patients. Diet therapy is closer to life, and it is appropriate to eat some royal jelly and manuka honey, which contain omega-3 fatty acids and vitamins A/B1/D, for some patients are more likely to accept (Table 2).
Various anti-inflammatory drugs correspond to dry eyes with different symptoms and signs. As for the dosage and time of medication, rheumatic diseases should be recommended if the patient’s tear score is deficient at the first visit. In terms of medication, ATs were given. A conventional dose of anti-inflammatory drugs was given according to the signs, and the patient was advised to review within a certain period. According to the review of dry eye score, intraocular pressure is considered, and an anti-inflammatory drug is used. Anti-inflammatory drugs should not be given without a time limit and should be discontinued during ongoing patient review. The information should include the patient’s age, gender, lifestyle, job characteristics, diet, drug reaction conditions, and acceptance. In clinical practice, patients with dry eye who often visit again feel that their ocular discomfort has not been significantly relieved. Therefore, we should ask about the patient’s medical history in detail, such as using regular medication and the work and living environment status. After careful physical examination, the regimen should be re-determined, and a combination of antibiotics may be considered.
There are many anti-inflammatory studies on dry eye treatment, but a few studies on the course and dosage. This may be related to the fact that dry eye is a complex multifactorial disease. Conventional anti-inflammatory drugs, such as non-steroidal drugs and corticosteroids, are routinely used in clinical practice. Drugs that inhibit inflammatory pathways and alleviate inflammatory factors are not widely used due to the lack of extensive clinical trials. In conclusion, dry eye treatment should be multiple and comprehensive, particularly combined anti-inflammatory drugs and ATs. For the future anti-inflammatory treatment of dry eye, we continue to work hard to clarify the dosage and the development of new drugs (149).
LC: Writing – original draft. CW: Writing – review & editing. HZ: Funding acquisition, Methodology, Project administration, Resources, Writing – review & editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by Provincial Bureau Project of Jilin Province (2020SCZ48); Science and Technology Department of Jilin Province Research Fund (20200201537JC).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Farrand, K , Fridman, M , Stillman, I , and Schaumberg, D . Prevalence of diagnosed dry eye disease in the United States among adults aged 18 years and older. Am J Ophthalmol. (2017) 182:90–8. doi: 10.1016/j.ajo.2017.06.033
2. Lam, H , Bleiden, L , de Paiva, CS , Farley, W , Stern, ME , and Pflugfelder, SC . Tear cytokine profiles in dysfunctional tear syndrome. Am J Ophthalmol. (2009) 147:198–205 e1. doi: 10.1016/j.ajo.2008.08.032
3. Pflugfelder, S , and Stern, M . Biological functions of tear film. Exp Eye Res. (2020) 197:108115. doi: 10.1016/j.exer.2020.108115
4. Cozzini, T , Piona, C , Marchini, G , Merz, T , Brighenti, T , Bonetto, J, et al. In vivo confocal microscopy study of corneal nerve alterations in children and youths with type 1 diabetes. Pediatr Diabetes. (2021) 22:780–6. doi: 10.1111/pedi.13219
5. Mittrucker, HW , Visekruna, A , and Huber, M . Heterogeneity in the differentiation and function of CD8+ T cells. Arch Immunol Ther Exp. (2014) 62:449–58. doi: 10.1007/s00005-014-0293-y
6. Blanco-Vázquez, M , Vázquez, A , Fernández, I , Novo-Diez, A , Martínez-Plaza, E , García-Vázquez, C, et al. Inflammation-related molecules in tears of patients with chronic ocular pain and dry eye disease. Exp Eye Res. (2022) 219:109057. doi: 10.1016/j.exer.2022.109057
7. O'Neil, E , Henderson, M , Massaro-Giordano, M , and Bunya, V . Advances in dry eye disease treatment. Curr Opin Ophthalmol. (2019) 30:166–78. doi: 10.1097/ICU.0000000000000569
8. Thia, ZZ , Ho, YT , Shih, KC , and Tong, L . New developments in the management of persistent corneal epithelial defects. Surv Ophthalmol. (2023) 68:1093–114. doi: 10.1016/j.survophthal.2023.06.001
9. Strauss-Albee, D , Horowitz, A , Parham, P , and Blish, C . Coordinated regulation of NK receptor expression in the maturing human immune system. J Immunol. (2014) 193:4871–9. doi: 10.4049/jimmunol.1401821
10. Chen, Y , Chauhan, S , Lee, H , Saban, D , and Dana, R . Chronic dry eye disease is principally mediated by effector memory Th17 cells. Mucosal Immunol. (2014) 7:38–45. doi: 10.1038/mi.2013.20
11. Smith, V , Rishmawi, H , Hussein, H , and Easty, D . Tear film MMP accumulation and corneal disease. Br J Ophthalmol. (2001) 85:147–53. doi: 10.1136/bjo.85.2.147
12. Chauhan, S , Jin, Y , Goyal, S , Lee, H , Fuchsluger, T , Lee, H, et al. A novel pro-lymphangiogenic function for Th17/IL-17. Blood. (2011) 118:4630–4. doi: 10.1182/blood-2011-01-332049
13. Bron, A , de Paiva, C , Chauhan, S , Bonini, S , Gabison, E , Jain, S, et al. TFOS DEWS II pathophysiology report. Ocul Surf. (2017) 15:438–510. doi: 10.1016/j.jtos.2017.05.011
14. Tsubota, K , Yokoi, N , Shimazaki, J , Watanabe, H , Dogru, M , Yamada, M, et al. New perspectives on dry eye definition and diagnosis: a consensus report by the Asia dry eye society. Ocul Surf. (2017) 15:65–76. doi: 10.1016/j.jtos.2016.09.003
15. Belmonte, C , Nichols, J , Cox, S , Brock, J , Begley, C , Bereiter, D, et al. TFOS DEWS II pain and sensation report. Ocul Surf. (2017) 15:404–37. doi: 10.1016/j.jtos.2017.05.002
16. Marfurt, C , Anokwute, M , Fetcko, K , Mahony-Perez, E , Farooq, H , Ross, E, et al. Comparative anatomy of the mammalian corneal subbasal nerve plexus. Invest Ophthalmol Vis Sci. (2019) 60:4972–84. doi: 10.1167/iovs.19-28519
17. Oba, S , Araki-Sasaki, K , Chihara, T , Kojima, T , Murat, D , and Takahashi, K . Aberrant corneal homeostasis in neurosurgery-induced neurotrophic keratopathy. J Clin Med. (2022) 11:3804. doi: 10.3390/jcm11133804
18. Chesney, J , Bacher, M , Bender, A , and Bucala, R . The peripheral blood fibrocyte is a potent antigen-presenting cell capable of priming naive T cells in situ. Proc Natl Acad Sci U S A. (1997) 94:6307–12. doi: 10.1073/pnas.94.12.6307
19. Labbé, A , Alalwani, H , Van Went, C , Brasnu, E , Georgescu, D , and Baudouin, C . The relationship between subbasal nerve morphology and corneal sensation in ocular surface disease. Invest Ophthalmol Vis Sci. (2012) 53:4926–31. doi: 10.1167/iovs.11-8708
20. Pelegrino, F , Volpe, E , Gandhi, N , Li, D , Pflugfelder, S , and de Paiva, C . Deletion of interferon-γ delays onset and severity of dacryoadenitis in CD25KO mice. Arthritis Res Ther. (2012) 14:R234. doi: 10.1186/ar4077
21. Antonelli, A , Ferrari, S , Giuggioli, D , Ferrannini, E , Ferri, C , and Fallahi, P . Chemokine (C-X-C motif) ligand (CXCL)10 in autoimmune diseases. Autoimmun Rev. (2014) 13:272–80. doi: 10.1016/j.autrev.2013.10.010
22. Durali, D , de Goër de Herve, MG , Giron-Michel, J , Azzarone, B , Delfraissy, JF̧ , and Taoufik, Y . In human B cells, IL-12 triggers a cascade of molecular events similar to Th1 commitment. Blood. (2003) 102:4084–9. doi: 10.1182/blood-2003-02-0518
23. Di Mitri, D , Sambucci, M , Loiarro, M , De Bardi, M , Volpe, E , Cencioni, MT, et al. The p38 mitogen-activated protein kinase cascade modulates T helper type 17 differentiation and functionality in multiple sclerosis. Immunology. (2015) 146:251–63. doi: 10.1111/imm.12497
24. Zhou, HY , Shin, EM , Guo, LY , Youn, UJ , Bae, K , Kang, SS, et al. Anti-inflammatory activity of 4-methoxyhonokiol is a function of the inhibition of iNOS and COX-2 expression in RAW 264.7 macrophages via NF-κB, JNK and p38 MAPK inactivation. Eur J Pharmacol. (2008) 586:340–9. doi: 10.1016/j.ejphar.2008.02.044
25. Ouyang, W , Wu, Y , Lin, X , Wang, S , Yang, Y , Tang, L, et al. Role of CD4+ T helper cells in the development of BAC-induced dry eye syndrome in mice. Invest Ophthalmol Vis Sci. (2021) 62:25. doi: 10.1167/iovs.62.1.25
26. Chi, W , Hua, X , Chen, X , Bian, F , Yuan, X , Zhang, L, et al. Mitochondrial DNA oxidation induces imbalanced activity of NLRP3/NLRP6 inflammasomes by activation of caspase-8 and BRCC36 in dry eye. J Autoimmun. (2017) 80:65–76. doi: 10.1016/j.jaut.2017.02.006
27. Lopes, A , Bekker, C , Hillen, M , Blokland, S , Hinrichs, A , Pandit, A, et al. The transcriptomic profile of monocytes from patients with Sjögren’s syndrome is associated with inflammatory parameters and is mimicked by circulating mediators. Front Immunol. (2021) 12:701656. doi: 10.3389/fimmu.2021.701656
28. Luo, L , Li, D-Q , Doshi, A , Farley, W , Corrales, RM , and Pflugfelder, SC . Experimental dry eye stimulates production of inflammatory cytokines and MMP-9 and activates MAPK signaling pathways on the ocular surface. Invest Ophthalmol Vis Sci. (2004) 45:4293. doi: 10.1167/iovs.03-1145
29. Ellenberg, D , Azar, D , Hallak, J , Tobaigy, F , Han, K , Jain, S, et al. Novel aspects of corneal angiogenic and lymphangiogenic privilege. Prog Retin Eye Res. (2010) 29:208–48. doi: 10.1016/j.preteyeres.2010.01.002
30. Hu, R , Siu, C , Lau, E , Wang, W , Lau, C , and Tse, H . Impaired nitrate-mediated dilatation could reflect nitrate tolerance in patients with coronary artery disease. Int J Cardiol. (2007) 120:351–6. doi: 10.1016/j.ijcard.2006.10.011
31. Johnson, L , Clasper, S , Holt, A , Lalor, P , Baban, D , and Jackson, D . An inflammation-induced mechanism for leukocyte transmigration across lymphatic vessel endothelium. J Exp Med. (2006) 203:2763–77. doi: 10.1084/jem.20051759
32. Chen, W , Deng, Y , Jiang, H , Wang, J , Zhong, J , Li, S, et al. Microvascular abnormalities in dry eye patients. Microvasc Res. (2018) 118:155–61. doi: 10.1016/j.mvr.2018.03.015
33. Brunner, G , Roux, M , Falk, T , Bresch, M , Böhm, V , Blödorn-Schlicht, N, et al. The peripheral lymphatic system is impaired by the loss of neuronal control associated with chronic spinal cord injury. Am J Pathol. (2022) 192:1448–57. doi: 10.1016/j.ajpath.2022.06.012
34. Yang, J , Walia, A , Huang, Y , Han, K , Rosenblatt, M , Azar, D, et al. Understanding lymphangiogenesis in knockout models, the cornea, and ocular diseases for the development of therapeutic interventions. Surv Ophthalmol. (2016) 61:272–96. doi: 10.1016/j.survophthal.2015.12.004
35. Kataru, R , Jung, K , Jang, C , Yang, H , Schwendener, R , Baik, J, et al. Critical role of CD11b+ macrophages and VEGF in inflammatory lymphangiogenesis, antigen clearance, and inflammation resolution. Blood. (2009) 113:5650–9. doi: 10.1182/blood-2008-09-176776
36. Goyal, S , Chauhan, S , El Annan, J , Nallasamy, N , Zhang, Q , and Dana, R . Evidence of corneal lymphangiogenesis in dry eye disease: a potential link to adaptive immunity? Arch Ophthalmol. (2010) 128:819–24. doi: 10.1001/archophthalmol.2010.124
37. Clahsen, T , Büttner, C , Hatami, N , Reis, A , and Cursiefen, C . Role of endogenous regulators of hem- and lymphangiogenesis in corneal transplantation. J Clin Med. (2020) 9:479. doi: 10.3390/jcm9020479
38. Goyal, S , Chauhan, S , and Dana, R . Blockade of prolymphangiogenic vascular endothelial growth factor C in dry eye disease. Arch Ophthalmol. (2012) 130:84–9. doi: 10.1001/archophthalmol.2011.266
39. Patnam, M , Dommaraju, S , Masood, F , Herbst, P , Chang, J , Hu, W, et al. Lymphangiogenesis guidance mechanisms and therapeutic implications in pathological states of the cornea. Cells. (2023) 12:319. doi: 10.3390/cells12020319
40. Yusufoğlu, E , Güngör Kobat, S , and Keser, S . Evaluation of central corneal epithelial thickness with anterior segment OCT in patients with type 2 diabetes mellitus. Int Ophthalmol. (2023) 43:27–33. doi: 10.1007/s10792-022-02384-5
41. Madonna, R , Balistreri, C , Geng, Y , and De Caterina, R . Diabetic microangiopathy: pathogenetic insights and novel therapeutic approaches. Vasc Pharmacol. (2017) 90:1–7. doi: 10.1016/j.vph.2017.01.004
42. Zhang, X , Zhao, L , Deng, S , Sun, X , and Wang, N . Dry eye syndrome in patients with diabetes mellitus: prevalence, etiology, and clinical characteristics. J Ophthalmol. (2016) 2016:8201053. doi: 10.1155/2016/8201053
43. Fujishima, H , Shimazaki, J , Yagi, Y , and Tsubota, K . Improvement of corneal sensation and tear dynamics in diabetic patients by oral aldose reductase inhibitor, ONO-2235: a preliminary study. Cornea. (1996) 15:368–72. doi: 10.1097/00003226-199607000-00006
44. Dias, A , Batista, T , Roma, L , Módulo, C , Malki, L , Dias, L, et al. Insulin replacement restores the vesicular secretory apparatus in the diabetic rat lacrimal gland. Arq Bras Oftalmol. (2015) 78:158–63. doi: 10.5935/0004-2749.20150041
45. Riss, B , and Binder, S . Corneal sensitivity after photocoagulation for diabetic retinopathy. Albrecht Von Graefes Arch Klin Exp Ophthalmol. (1981) 217:143–7. doi: 10.1007/BF00418989
46. Yu, T , Shi, W , Song, A , Gao, Y , Dang, G , and Ding, G . Changes of meibomian glands in patients with type 2 diabetes mellitus. Int J Ophthalmol. (2016) 9:1740–4. doi: 10.18240/ijo.2016.12.06
47. Alves, M , Calegari, V , Cunha, D , Saad, M , Velloso, L , and Rocha, E . Increased expression of advanced glycation end-products and their receptor, and activation of nuclear factor kappa-B in lacrimal glands of diabetic rats. Diabetologia. (2005) 48:2675–81. doi: 10.1007/s00125-005-0010-9
48. Manfrè, V , Chatzis, L , Cafaro, G , Fonzetti, S , Calvacchi, S , Fulvio, G, et al. Sjögren’s syndrome: one year in review 2022. Clin Exp Rheumatol. (2022) 40:2211–24. doi: 10.55563/clinexprheumatol/43z8gu
49. Langguth, D , Morris, S , Clifford, L , Wilson, R , Neil, J , Hogan, P, et al. Specific testing for “isolated” anti-52 kDa SSA/Ro antibodies during standard anti-extractable nuclear antigen testing is of limited clinical value. J Clin Pathol. (2007) 60:670–3. doi: 10.1136/jcp.2006.040360
50. André, F , and Böckle, BC . Sjögren’s syndrome. JDDG. J Dtsch Dermatol Ges. (2022) 20:980–1002. doi: 10.1111/ddg.14823_g
51. Wang, H , Wang, P , Chen, T , Zou, J , Li, Y , Ran, X, et al. Analysis of clinical characteristics of immune-related dry eye. J Ophthalmol. (2017) 2017:8532397. doi: 10.1155/2017/8532397
52. Li, F , Zhang, Q , Ying, X , He, J , Jin, Y , Xu, H, et al. Corneal nerve structure in patients with primary Sjögren’s syndrome in China. BMC Ophthalmol. (2021) 21:211. doi: 10.1186/s12886-021-01967-7
53. Hao, R , Chou, Y , Ding, Y , Liu, Z , Wang, Y , Ren, X, et al. Corneal subbasal nerve analysis in patients with primary Sjögren’s syndrome: a novel objective grading method and clinical correlations. Int Ophthalmol. (2023) 43:779–93. doi: 10.1007/s10792-022-02478-0
54. Peck, T , Olsakovsky, L , and Aggarwal, S . Dry eye syndrome in menopause and Perimenopausal age group. J Midlife Health. (2017) 8:51–4. doi: 10.4103/jmh.JMH_41_17
55. Schirra, F , Suzuki, T , Dickinson, D , Townsend, D , Gipson, I , and Sullivan, D . Identification of steroidogenic enzyme mRNAs in the human lacrimal gland, meibomian gland, cornea, and conjunctiva. Cornea. (2006) 25:438–42. doi: 10.1097/01.ico.0000183664.80004.44
56. Song, X , Zhao, P , Wang, G , and Zhao, X . The effects of estrogen and androgen on tear secretion and matrix metalloproteinase-2 expression in lacrimal glands of ovariectomized rats. Invest Ophthalmol Vis Sci. (2014) 55:745–51. doi: 10.1167/iovs.12-10457
57. Puy, L , Turgeon, C , Gagné, D , Labrie, Y , Chen, C , Pelletier, G, et al. Localization and regulation of expression of the FAR-17A gene in the hamster flank organs. J Invest Dermatol. (1996) 107:44–50. doi: 10.1111/1523-1747.ep12297867
58. Mathers, W , Stovall, D , Lane, J , Zimmerman, M , and Johnson, S . Menopause and tear function: the influence of prolactin and sex hormones on human tear production. Cornea. (1998) 17:353–8. doi: 10.1097/00003226-199807000-00002
59. Huang, J , Lu, M , and Ding, C . Na+/K+-ATPase expression changes in the rabbit lacrimal glands during pregnancy. Curr Eye Res. (2013) 38:18–26. doi: 10.3109/02713683.2012.725797
60. Sator, M , Joura, E , Golaszewski, T , Gruber, D , Frigo, P , Metka, M, et al. Treatment of menopausal keratoconjunctivitis sicca with topical oestradiol. Br J Obstet Gynaecol. (1998) 105:100–2. doi: 10.1111/j.1471-0528.1998.tb09358.x
61. Coksuer, H , Ozcura, F , Oghan, F , Haliloglu, B , and Coksuer, C . Effects of estradiol-drospirenone on ocular and nasal functions in postmenopausal women. Climacteric. (2011) 14:482–7. doi: 10.3109/13697137.2010.539724
62. Nichols, J , Willcox, M , Bron, A , Belmonte, C , Ciolino, J , Craig, J, et al. The TFOS international workshop on contact lens discomfort: executive summary. Invest Ophthalmol Vis Sci. (2013) 54:TFOS7–TFOS13. doi: 10.1167/iovs.13-13212
63. Nichols, J , and Sinnott, L . Tear film, contact lens, and patient-related factors associated with contact lens-related dry eye. Invest Ophthalmol Vis Sci. (2006) 47:1319–28. doi: 10.1167/iovs.05-1392
64. Tyagi, G , Alonso-Caneiro, D , Collins, M , and Read, S . Tear film surface quality with rigid and soft contact lenses. Eye Contact Lens. (2012) 38:171–8. doi: 10.1097/ICL.0b013e31824da99c
65. Guillon, M , and Maissa, C . Contact lens wear affects tear film evaporation. Eye Contact Lens. (2008) 34:326–30. doi: 10.1097/ICL.0b013e31818c5d00
66. Lim, C , Stapleton, F , and Mehta, J . Review of contact lens-related complications. Eye Contact Lens. (2018) 44:S1–S10. doi: 10.1097/ICL.0000000000000481
67. Markoulli, M , Papas, E , Cole, N , and Holden, B . Effect of contact lens wear on the diurnal profile of matrix metalloproteinase 9 in tears. Optom Vis Sci. (2013) 90:419–29. doi: 10.1097/OPX.0b013e31828d7d3b
68. Arita, R , Itoh, K , Inoue, K , Kuchiba, A , Yamaguchi, T , and Amano, S . Contact lens wear is associated with decrease of meibomian glands. Ophthalmology. (2009) 116:379–84. doi: 10.1016/j.ophtha.2008.10.012
69. Sapkota, K , Franco, S , Sampaio, P , and Lira, M . Effect of three months of soft contact lens wear on conjunctival cytology. Clin Exp Optom. (2016) 99:336–41. doi: 10.1111/cxo.12373
70. Liu, Q , Xu, Z , Xu, Y , Zhang, J , Li, Y , Xia, J, et al. Changes in corneal dendritic cell and sub-basal nerve in long-term contact lens wearers with dry eye. Eye Contact Lens. (2020) 46:238–44. doi: 10.1097/ICL.0000000000000691
71. Zhou, L , Beuerman, R , Chan, C , Zhao, S , Li, X , Yang, H, et al. Identification of tear fluid biomarkers in dry eye syndrome using iTRAQ quantitative proteomics. J Proteome Res. (2009) 8:4889–905. doi: 10.1021/pr900686s
72. Lallemand, F , Schmitt, M , Bourges, JL , Gurny, R , Benita, S , and Garrigue, JS . Cyclosporine A delivery to the eye: a comprehensive review of academic and industrial efforts. Eur J Pharm Biopharm. (2017) 117:14–28. doi: 10.1016/j.ejpb.2017.03.006
73. Acheampong, A , Shackleton, M , Tang-Liu, D , Ding, S , Stern, M , and Decker, R . Distribution of cyclosporin A in ocular tissues after topical administration to albino rabbits and beagle dogs. Curr Eye Res. (1999) 18:91–103. doi: 10.1076/ceyr.18.2.91.5381
74. Liddicoat, AM , and Lavelle, EC . Modulation of innate immunity by cyclosporine A. Biochem Pharmacol. (2019) 163:472–80. doi: 10.1016/j.bcp.2019.03.022
75. Kujawa, A , and Rózycki, R . A 0.05% cyclosporine treatment of the advanced dry eye syndrome. Klin Ocz. (2005) 107:280–6.
76. Rhee, M , and Mah, F . Clinical utility of cyclosporine (CsA) ophthalmic emulsion 0.05% for symptomatic relief in people with chronic dry eye: a review of the literature. Clin Ophthalmol. (2017) 11:1157–66. doi: 10.2147/OPTH.S113437
77. Stevenson, D , Tauber, J , and Reis, B . Efficacy and safety of cyclosporin A ophthalmic emulsion in the treatment of moderate-to-severe dry eye disease: a dose-ranging, randomized trial. the cyclosporin A phase 2 study group. Ophthalmology. (2000) 107:967–74. doi: 10.1016/S0161-6420(00)00035-X
78. Sheppard, J , Scoper, S , and Samudre, S . Topical loteprednol pretreatment reduces cyclosporine stinging in chronic dry eye disease. J Ocul Pharmacol Ther. (2011) 27:23–7. doi: 10.1089/jop.2010.0085
79. Abadia, B , Calvo, P , Ferreras, A , Bartol, F , Verdes, G , and Pablo, L . Clinical applications of dexamethasone for aged eyes. Drugs Aging. (2016) 33:639–46. doi: 10.1007/s40266-016-0392-z
80. De Paiva, C , Corrales, R , Villarreal, A , Farley, W , Li, D , Stern, M, et al. Corticosteroid and doxycycline suppress MMP-9 and inflammatory cytokine expression, MAPK activation in the corneal epithelium in experimental dry eye. Exp Eye Res. (2006) 83:526–35. doi: 10.1016/j.exer.2006.02.004
81. Li, X , Jin, X , Wang, J , Li, X , and Zhang, H . Dexamethasone attenuates dry eye-induced pyroptosis by regulating the KCNQ1OT1/miR-214 cascade. Steroids. (2022) 186:109073. doi: 10.1016/j.steroids.2022.109073
82. Lu, Y , Fukuda, K , Liu, Y , Kumagai, N , and Nishida, T . Dexamethasone inhibition of IL-1-induced collagen degradation by corneal fibroblasts in three-dimensional culture. Invest Ophthalmol Vis Sci. (2004) 45:2998–3004. doi: 10.1167/iovs.04-0051
83. Bian, F , Pelegrino, F , Henriksson, J , Pflugfelder, S , Volpe, E , Li, D, et al. Differential effects of dexamethasone and doxycycline on inflammation and MMP production in murine alkali-burned corneas associated with dry eye. Ocul Surf. (2016) 14:242–54. doi: 10.1016/j.jtos.2015.11.006
84. Raizman, M . Corticosteroid therapy of eye disease. Arch Ophthalmol. (1996) 114:1000–1. doi: 10.1001/archopht.1996.01100140208016
85. Coursey, T , and de Paiva, C . Managing Sjögren’s syndrome and non-Sjögren syndrome dry eye with anti-inflammatory therapy. Clin Ophthalmol. (2014) 8:1447–58. doi: 10.2147/OPTH.S35685
86. Sancilio, L , Nolan, J , Wagner, L , and Ward, J . The analgesic and antiinflammatory activity and pharmacologic properties of bromfenac. Arzneimittelforschung. (1987) 37:513–9.
87. Aragona, P , and Di Pietro, R . Is it safe to use topical NSAIDs for corneal sensitivity in Sjögren’s syndrome patients? Expert Opin Drug Saf. (2007) 6:33–43. doi: 10.1517/14740338.6.1.33
88. Ji, Y , Seo, Y , Choi, W , Yeo, A , Noh, H , Kim, E, et al. Dry eye-induced CCR7+ CD11b+ cell lymph node homing is induced by COX-2 activities. Invest Ophthalmol Vis Sci. (2014) 55:6829–38. doi: 10.1167/iovs.14-14744
89. Garrett, Q , Khandekar, N , Shih, S , Flanagan, JL , Simmons, P , Vehige, J, et al. Betaine stabilizes cell volume and protects against apoptosis in human corneal epithelial cells under hyperosmotic stress. Exp Eye Res. (2013) 108:33–41. doi: 10.1016/j.exer.2012.12.001
90. Sawazaki, R , Ishihara, T , Usui, S , Hayashi, E , Tahara, K , Hoshino, T, et al. Diclofenac protects cultured human corneal epithelial cells against hyperosmolarity and ameliorates corneal surface damage in a rat model of dry eye. Invest Ophthalmol Vis Sci. (2014) 55:2547–56. doi: 10.1167/iovs.13-13850
91. Aragona, P , Stilo, A , Ferreri, F , and Mobrici, M . Effects of the topical treatment with NSAIDs on corneal sensitivity and ocular surface of Sjögren’s syndrome patients. Eye. (2005) 19:535–9. doi: 10.1038/sj.eye.6701537
92. McCabe, E , and Narayanan, S . Advancements in anti-inflammatory therapy for dry eye syndrome. Optometry. (2009) 80:555–66. doi: 10.1016/j.optm.2009.02.010
93. Nichols, J , Bickle, K , Zink, R , Schiewe, M , Haque, R , and Nichols, K . Safety and efficacy of topical azithromycin ophthalmic solution 1.0% in the treatment of contact lens-related dry eye. Eye Contact Lens. (2012) 38:73–9. doi: 10.1097/ICL.0b013e31823ff229
94. Qiao, J , and Yan, X . Emerging treatment options for meibomian gland dysfunction. Clin Ophthalmol. (2013) 7:1797–803. doi: 10.2147/OPTH.S33182
95. Liu, Y , Kam, W , Ding, J , and Sullivan, D . Can tetracycline antibiotics duplicate the ability of azithromycin to stimulate human meibomian gland epithelial cell differentiation? Cornea. (2015) 34:342–6. doi: 10.1097/ICO.0000000000000351
96. Liu, Y , Kam, W , Ding, J , and Sullivan, D . Effect of azithromycin on lipid accumulation in immortalized human meibomian gland epithelial cells. JAMA Ophthalmol. (2014) 132:226–8. doi: 10.1001/jamaophthalmol.2013.6030
97. Runda, N , Manna, S , Vanathi, M , Tandon, R , and Gupta, N . Tear film lipid layer thickness measurement from ocular surface analyzer as a marker to monitor treatment of meibomian gland dysfunction in a study comparing physiological detergent-free eyelid wipes with conventional therapy: a randomized trial. Indian J Ophthalmol. (2022) 70:1963–70. doi: 10.4103/ijo.IJO_2885_21
98. Solomon, A , Rosenblatt, M , Li, D , Monroy, D , Ji, Z , Lokeshwar, B, et al. Doxycycline inhibition of interleukin-1 in the corneal epithelium. Am J Ophthalmol. (2000) 130:688. doi: 10.1016/S0002-9394(00)00755-8
99. Kim, H , Luo, L , Pflugfelder, S , and Li, D . Doxycycline inhibits TGF-beta1-induced MMP-9 via Smad and MAPK pathways in human corneal epithelial cells. Invest Ophthalmol Vis Sci. (2005) 46:840–8. doi: 10.1167/iovs.04-0929
100. Li, DQ , Chen, Z , Song, XJ , Luo, L , and Pflugfelder, SC . Stimulation of matrix metalloproteinases by hyperosmolarity via a JNK pathway in human corneal epithelial cells. Invest Ophthalmol Vis Sci. (2004) 45:4302–11. doi: 10.1167/iovs.04-0299
101. Foulks, G , Borchman, D , Yappert, M , and Kakar, S . Topical azithromycin and oral doxycycline therapy of meibomian gland dysfunction: a comparative clinical and spectroscopic pilot study. Cornea. (2013) 32:44–53. doi: 10.1097/ICO.0b013e318254205f
102. Wilson, S , Chen, L , Mohan, R , Liang, Q , and Liu, J . Expression of HGF, KGF, EGF and receptor messenger RNAs following corneal epithelial wounding. Exp Eye Res. (1999) 68:377–97. doi: 10.1006/exer.1998.0603
103. Kokawa, N , Sotozono, C , Nishida, K , and Kinoshita, S . High total TGF-beta 2 levels in normal human tears. Curr Eye Res. (1996) 15:341–3. doi: 10.3109/02713689609007630
104. Ripa, M , Jabbehdari, S , Yazdanpanah, G , Lukacs, E , Karcher, B , Iqbal, O, et al. The role of multisystem disease in composition of autologous serum tears and ocular surface symptom improvement. Ocul Surf. (2020) 18:499–504. doi: 10.1016/j.jtos.2020.02.011
105. Kumari, N , Kusumesh, R , Kumari, R , Sinha, B , and Singh, V . Comparative evaluation of effectiveness of twenty versus fifty percent autologous serum eye drops in treatment of dry eye. Indian J Ophthalmol. (2023) 71:1603–7. doi: 10.4103/IJO.IJO_2684_22
106. Shtein, R , Shen, J , Kuo, A , Hammersmith, K , Li, J , and Weikert, M . Autologous serum-based eye drops for treatment of ocular surface disease: a report by the American Academy of Ophthalmology. Ophthalmology. (2020) 127:128–33. doi: 10.1016/j.ophtha.2019.08.018
107. Scott, G , Yiu, S , Wasilewski, D , Song, J , and Smith, R . Combined esterified estrogen and methyltestosterone treatment for dry eye syndrome in postmenopausal women. Am J Ophthalmol. (2005) 139:1109–10. doi: 10.1016/j.ajo.2004.11.042
108. Golebiowski, B , Badarudin, N , Eden, J , Gerrand, L , Robinson, J , Liu, J, et al. The effects of transdermal testosterone and oestrogen therapy on dry eye in postmenopausal women: a randomised, placebo-controlled, pilot study. Br J Ophthalmol. (2017) 101:926–32. doi: 10.1136/bjophthalmol-2016-309498
109. Feng, Y , Feng, G , Peng, S , and Li, H . The effects of hormone replacement therapy on dry eye syndromes evaluated by Schirmer test depend on patient age. Cont Lens Anterior Eye. (2016) 39:124–7. doi: 10.1016/j.clae.2015.09.002
110. Rocha, E , Mantelli, F , Nominato, L , and Bonini, S . Hormones and dry eye syndrome: an update on what we do and don’t know. Curr Opin Ophthalmol. (2013) 24:348–55. doi: 10.1097/ICU.0b013e32836227bf
111. Amparo, F , Dastjerdi, M , Okanobo, A , Ferrari, G , Smaga, L , Hamrah, P, et al. Topical interleukin 1 receptor antagonist for treatment of dry eye disease: a randomized clinical trial. JAMA Ophthalmol. (2013) 131:715–23. doi: 10.1001/jamaophthalmol.2013.195
112. Harrell, C , Markovic, B , Fellabaum, C , Arsenijevic, N , Djonov, V , and Volarevic, V . The role of interleukin 1 receptor antagonist in mesenchymal stem cell-based tissue repair and regeneration. Biofactors. (2020) 46:263–75. doi: 10.1002/biof.1587
113. Kovács, B , Láng, B , Takácsi-Nagy, A , Horváth, G , Czakó, C , Csorba, A, et al. Meibomian gland dysfunction and dry eye: diagnosis and treatment. Orv Hetil. (2021) 162:43–51. doi: 10.1556/650.2021.31958
114. Yan, S , and Wu, Y . Efficacy and safety of intense pulsed light therapy for dry eye caused by meibomian gland dysfunction: a randomised trial. Ann Palliat Med. (2021) 10:7857–65. doi: 10.21037/apm-21-1303
115. Mittal, R , Patel, S , and Galor, A . Alternative therapies for dry eye disease. Curr Opin Ophthalmol. (2021) 32:348–61. doi: 10.1097/ICU.0000000000000768
116. Toyos, R , Toyos, M , Willcox, J , Mulliniks, H , and Hoover, J . Evaluation of the safety and efficacy of intense pulsed light treatment with meibomian gland expression of the upper eyelids for dry eye disease. Photobiomodul Photomed Laser Surg. (2019) 37:527–31. doi: 10.1089/photob.2018.4599
117. Albietz, J , and Schmid, K . Intense pulsed light treatment and meibomian gland expression for moderate to advanced meibomian gland dysfunction. Clin Exp Optom. (2018) 101:23–33. doi: 10.1111/cxo.12541
118. Suwal, A , Hao, J , Zhou, D , Liu, X , Suwal, R , and Lu, C . Use of intense pulsed light to mitigate meibomian gland dysfunction for dry eye disease. Int J Med Sci. (2020) 17:1385–92. doi: 10.7150/ijms.44288
119. Beyazyıldız, E , Pınarlı, F , Beyazyıldız, O , Hekimoğlu, E , Acar, U , Demir, M, et al. Efficacy of topical mesenchymal stem cell therapy in the treatment of experimental dry eye syndrome model. Stem Cells Int. (2014) 2014:250230. doi: 10.1155/2014/250230
120. Lee, MJ , Ko, AY , Ko, JH , Lee, HJ , Kim, MK , Wee, WR, et al. Mesenchymal stem/stromal cells protect the ocular surface by suppressing inflammation in an experimental dry eye. Mol Ther. (2015) 23:139–46. doi: 10.1038/mt.2014.159
121. Aluri, HS , Samizadeh, M , Edman, MC , Hawley, DR , Armaos, HL , Janga, SR, et al. Delivery of bone marrow-derived mesenchymal stem cells improves tear production in a mouse model of Sjögren’s syndrome. Stem Cells Int. (2017) 2017:3134543. doi: 10.1155/2017/3134543
122. Inoue, S , Kawashima, M , Hisamura, R , Imada, T , Izuta, Y , Nakamura, S, et al. Clinical evaluation of a royal jelly supplementation for the restoration of dry eye: a prospective randomized double blind placebo controlled study and an experimental mouse model. PLoS One. (2017) 12:e0169069. doi: 10.1371/journal.pone.0169069
123. Imada, T , Nakamura, S , Kitamura, N , Shibuya, I , and Tsubota, K . Oral administration of royal jelly restores tear secretion capacity in rat blink-suppressed dry eye model by modulating lacrimal gland function. PLoS One. (2014) 9:e106338. doi: 10.1371/journal.pone.0106338
124. Zhang, Y , Si, J , Li, S , Zhang, G , Wang, S , Zheng, H, et al. Chemical analyses and antimicrobial activity of nine kinds of unifloral Chinese honeys compared to Manuka Honey (12+ and 20+). Molecules. (2021) 26:2778. doi: 10.3390/molecules26092778
125. Hu, J , Kong, L , Zhu, S , Ju, M , and Zhang, Q . Efficacy and safety of manuka honey for dry eye. Clin Exp Optom. (2023) 106:455–65. doi: 10.1080/08164622.2022.2106779
126. Deinema, L , Vingrys, A , Wong, C , Jackson, D , Chinnery, H , and Downie, L . A randomized, double-masked, placebo-controlled clinical trial of two forms of omega-3 supplements for treating dry eye disease. Ophthalmology. (2017) 124:43–52. doi: 10.1016/j.ophtha.2016.09.023
127. James, M , Gibson, R , and Cleland, L . Dietary polyunsaturated fatty acids and inflammatory mediator production. Am J Clin Nutr. (2000) 71:343S–8S. doi: 10.1093/ajcn/71.1.343S
128. Andrade, A , Salomon, T , Behling, C , Mahl, C , Hackenhaar, F , Putti, J, et al. Alpha-lipoic acid restores tear production in an animal model of dry eye. Exp Eye Res. (2014) 120:1–9. doi: 10.1016/j.exer.2013.12.014
129. Barragan, M , Good, M , and Kolls, J . Regulation of dendritic cell function by vitamin D. Nutrients. (2015) 7:8127–51. doi: 10.3390/nu7095383
130. Labetoulle, M , Benitez-Del-Castillo, J , Barabino, S , Herrero Vanrell, R , Daull, P , Garrigue, J, et al. Artificial tears: biological role of their ingredients in the management of dry eye disease. Int J Mol Sci. (2022) 23:2434. doi: 10.3390/ijms23052434
131. Eftimov, P , Yokoi, N , Melo, A , Daull, P , and Georgiev, G . Interactions of meibum and tears with mucomimetic polymers: a hint towards the interplay between the layers of the tear film. Int J Mol Sci. (2021) 22:2747. doi: 10.3390/ijms22052747
132. Zheng, X , Goto, T , and Ohashi, Y . Comparison of in vivo efficacy of different ocular lubricants in dry eye animal models. Invest Ophthalmol Vis Sci. (2014) 55:3454–60. doi: 10.1167/iovs.13-13730
133. Goto, E , Yagi, Y , Matsumoto, Y , and Tsubota, K . Impaired functional visual acuity of dry eye patients. Am J Ophthalmol. (2002) 133:181–6. doi: 10.1016/S0002-9394(01)01365-4
134. Montés-Micó, R , Cáliz, A , and Alió, J . Wavefront analysis of higher order aberrations in dry eye patients. J Refract Surg. (2004) 20:243–7. doi: 10.3928/1081-597X-20040501-08
135. Brinton, M , Kossler, A , Patel, Z , Loudin, J , Franke, M , Ta, C, et al. Enhanced tearing by electrical stimulation of the anterior ethmoid nerve. Invest Ophthalmol Vis Sci. (2017) 58:2341–8. doi: 10.1167/iovs.16-21362
136. Tauber, J , Karpecki, P , Latkany, R , Luchs, J , Martel, J , Sall, K, et al. Lifitegrast ophthalmic solution 5.0% versus placebo for treatment of dry eye disease: results of the randomized phase III OPUS-2 study. Ophthalmology. (2015) 122:2423–31. doi: 10.1016/j.ophtha.2015.08.001
137. Gupta, A , Heigle, T , and Pflugfelder, S . Nasolacrimal stimulation of aqueous tear production. Cornea. (1997) 16:645–8.
138. Brinton, M , Chung, J , Kossler, A , Kook, K , Loudin, J , Franke, M, et al. Electronic enhancement of tear secretion. J Neural Eng. (2016) 13:016006. doi: 10.1088/1741-2560/13/1/016006
139. Friedman, N , Butron, K , Robledo, N , Loudin, J , Baba, S , and Chayet, A . A nonrandomized, open-label study to evaluate the effect of nasal stimulation on tear production in subjects with dry eye disease. Clin Ophthalmol. (2016) 10:795–804. doi: 10.2147/OPTH.S101716
140. Attal, N , Cruccu, G , Haanpää, M , Hansson, P , Jensen, T , Nurmikko, T, et al. EFNS guidelines on pharmacological treatment of neuropathic pain. Eur J Neurol. (2006) 13:1153–69. doi: 10.1111/j.1468-1331.2006.01511.x
141. Ongun, N , and Ongun, G . Is gabapentin effective in dry eye disease and neuropathic ocular pain? Acta Neurol Belg. (2021) 121:397–401. doi: 10.1007/s13760-019-01156-w
142. Galor, A , Moein, H , Lee, C , Rodriguez, A , Felix, E , Sarantopoulos, K, et al. Neuropathic pain and dry eye. Ocul Surf. (2018) 16:31–44. doi: 10.1016/j.jtos.2017.10.001
143. Foulks, G . Pharmacological management of dry eye in the elderly patient. Drugs Aging. (2008) 25:105–18. doi: 10.2165/00002512-200825020-00003
144. Ren, X , Chou, Y , Wang, Y , Jing, D , Chen, Y , and Li, X . The utility of oral vitamin B1 and mecobalamin to improve corneal nerves in dry eye disease: an in vivo confocal microscopy study. Nutrients. (2022) 14:3750. doi: 10.3390/nu14183750
145. Zhao, CS , Chen, Y , Ying, G-S , and Asbell, PA . Association of tear cytokine ratios with symptoms and signs of dry eye disease: biomarker data from the dry eye assessment and management study. Curr Eye Res. (2023) 49:16–24. doi: 10.1080/02713683.2023.2262168
146. Kannan, R , Das, S , Shetty, R , Zhou, L , Ghosh, A , and Deshpande, V . Tear proteomics in dry eye disease. Indian J Ophthalmol. (2023) 71:1203–14. doi: 10.4103/IJO.IJO_2851_22
147. Sim, R , Yong, K , Liu, Y-C , and Tong, L . In vivo confocal microscopy in different types of dry eye and meibomian gland dysfunction. J Clin Med. (2022) 11:2349. doi: 10.3390/jcm11092349
148. Kheirkhah, A , Rahimi Darabad, R , Cruzat, A , Hajrasouliha, AR , Witkin, D , Wong, N, et al. Corneal epithelial immune dendritic cell alterations in subtypes of dry eye disease: a pilot in vivo confocal microscopic study. Invest Ophthalmol Vis Sci. (2015) 56:7179–85. doi: 10.1167/iovs.15-17433
Keywords: dry eye, inflammation, mechanism, therapy, review
Citation: Chu L, Wang C and Zhou H (2024) Inflammation mechanism and anti-inflammatory therapy of dry eye. Front. Med. 11:1307682. doi: 10.3389/fmed.2024.1307682
Received: 05 October 2023; Accepted: 14 January 2024;
Published: 14 February 2024.
Edited by:
Enzo Maria Vingolo, Sapienza University of Rome, ItalyReviewed by:
Hong Qi, Peking University Third Hospital, ChinaCopyright © 2024 Chu, Wang and Zhou. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hongyan Zhou, emhvdWhvbmd5QGpsdS5lZHUuY24=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.