- 1Department of Clinical Laboratory, Abrihajira Hospital, Amhara National Regional State, Abrihajira, Ethiopia
- 2Department of Medical Microbiology, School of Biomedical and Laboratory Sciences, College of Medicine and Health Sciences, University of Gondar, Gondar, Ethiopia
Introduction: Border areas are important sites for disseminating Mycobacterium tuberculosis among individuals living in such areas. This study examined patients with suspected pulmonary tuberculosis (PTB) visiting the Abrihajira and Metema hospitals in northwest Ethiopia to investigate the prevalence of rifampicin-resistant Mycobacterium tuberculosis (RR-MTB), multidrug-resistant Mycobacterium tuberculosis (MDR-MTB), and risk factors related to Mycobacterium tuberculosis infection.
Methods: A hospital-based cross-sectional study was conducted from February to August 2021 among 314 PTB presumptive patients. Xpert MTB/RIF and line probe assays (LPA) were used to process sputum samples. Data were imported into the Epi-Data 3.1 program and exported to Statistical Package for the Social Sciences (SPSS) version 20.0 (SPSS, Chicago, IL, United States) to conduct the analysis. A logistic regression analysis was used to investigate the relationship between the dependent and independent variables. A value of p of <0.05 denoted statistical significance.
Results: Of the total (314) PTB presumptive patients who participated in this study, 178 (56.69%) were men, and 165 (52.5%) were from 25 to 50 years of age with a median age of 35.00 (inter-quartile: 25–45 years). Among all patients, 12.7% had PTB by Gene Xpert and 7/314 (2.23%) were resistant to rifampicin. Among patients enrolled, 4/314 (1.27%) had MDR-MTB (resistant to RIF and INH) by LPA. Regarding the risk factors assessed, primary level of education, sputum production, night sweating, respiratory disorder, contact history of TB, history of MDR-MTB infection, history of alcohol use, and cigarette smoking showed statistical significance with the prevalence of PTB (p ≤ 0.05).
Discussion: This study observed a high prevalence of PTB, RR-MTB, and MDR-MTB compared with many other previous studies conducted in Ethiopia. Among the assessed risk factors that could be associated with the prevalence of PTB, eight were statistically significant. This prevalence, resistance, and statistically significant variables are the evidence to which more emphasis should be given to the country’s border areas.
Introduction
Pulmonary tuberculosis (PTB) is a disease owing to M. tuberculosis that affects predominantly the lungs (1). It is the world’s number one killer among infectious diseases and one of the top 10 causes of death (1, 2). Multidrug-resistant Mycobacterium tuberculosis (MDR-MTB) is the most important global public health issue, and a study depicted that biocide affects multiple sites in the bacterium and found that it can cause resistance in a non-specific way (3). In 2018, there were close to 10 million people with tuberculosis and 1.2 million tuberculosis-related deaths among human immunodeficiency virus (HIV)-negative people globally. Approximately 500,000 new cases of rifampicin-resistant (RR) tuberculosis also occurred in the same year, 70% of which were classified as multidrug-resistant tuberculosis (MDR-TB) (1, 4). The high prevalence of HIV, being in border regions, unstable residency, delayed early diagnosis, and inadequate early treatment initiation all contribute to the high burden of tuberculosis (TB) and MDR-TB in low-income nations (5). A disease management and control program must implement quick identification, early treatment initiation, ongoing surveillance, and regular monitoring of drug-resistant TB (6).
In December 2010, the World Health Organization (WHO) approved Xpert MTB/RIF (Cepheid, Sunnyvale, CA, USA). This automated molecular system detects both MTB and rifampicin-resistant (RR) tuberculosis concurrently to address the issue of early TB diagnosis in low-income countries (7). In over 90% of cases, RR-MTB is a surrogate marker for MDR-TB. It is primarily recommended for patients with TB/HIV co-infection, suspected MDR-TB, and pediatric patients, though it was indicated for all TB presumptive patients as of 2013 (8, 9). To diagnose TB and RR-MTB, Ethiopia introduced the Xpert MTB/RIF assay in 2014 (10). Ethiopia ranks 15th among high MDR-TB countries, with more than 5,800 projected MDR-TB cases yearly, making it one of the 30 countries with the highest TB, TB/HIV, and MDR-TB burdens (11).
Only a few Xpert MTB/RIF test investigations have been performed in Ethiopia. For example, Ataye (8.98%) (12), Eastern Amhara, Ethiopia (11%) (13), and Tigray (7.9%) reported a prevalence of 7.9–11% and 5.3–9% for MTB and RR-MTB, respectively. Only a few investigations on presumed PTB patients were undertaken in Ethiopia until 2021 in the nation’s border regions. These studies do not adequately reflect the PTB status of the entire country’s border region. Therefore, having representative data in the nation’s border region will close the knowledge gap and give policymakers and implementers up-to-date information.
Materials and methods
Study design, period, and area
A hospital-based cross-sectional study was conducted from February to August 2021 at Metema and Abrihajira hospitals. These hospitals are located in the West Gondar region of the Amhara National Regional State, 850 km from the capital of Ethiopia, Addis Ababa. These two hospitals offer a range of patient services, including outpatient, inpatient, gynecological, tuberculosis, and antiretroviral therapy (ART) clinics and various diagnostic and treatment services for other illnesses in the community. There are around 119 beds in the two hospitals: 85 beds at the Metema Hospital and 34 beds at the Abrihajira Hospital. It borders Eritrea to the north and Sudan to the west, and large-scale agricultural activities are being practiced in these areas of Ethiopia (14, 15).
Source and study population
The source population was all patients who were visiting Metema and Abrihajira hospitals. The study population was all the PTB presumptive patients seeking health services at the specified hospitals.
Sample size determination
The sample size was calculated using a single population proportion formula and a 5% margin of error at a 95% confidence interval (CI). The target populations for the study were all PTB presumptive patients at the Metema and Abrihajira hospitals, which served an estimated 160,000 people (N value) (13, 16). The prevalence (p) of PTB obtained from the previous study conducted at the University of Gondar Hospital, Northwest Ethiopia, was 24.6% (17).
n = p(1-p) = ( (0.246) (0.754) = 285
Where Z = 95% confidence interval (1.96);
p = prevalence rate from the previous study;
d = Margin of sampling error (5%);
q = 1-p;
n = number of sample units that will be studied.
With a 10% non-respondent rate in mind, 285 + 29 = 314 patients comprised the entire sample used in the current study. All of these study participants were included using a convenience sampling technique.
Socio-demographic data collection
Socio-demographic data were collected using a structured, closed-ended questionnaire-based face-to-face interview. A questionnaire covering the social-demographic, clinical, and PTB-related risk factors was formulated in English, then translated into the local (Amharic) language for data collection and retranslated into English for analysis and reporting.
Specimen collection, handling, and transportation
Based on the national TB diagnosis guideline (18), spot or morning sputum specimens were collected using two falcon tubes with 4–5 mL from each PTB presumptive patient. The first falcon tube sputum was stored at 2–8°C for a maximum of 1 week until testing at the Abrihajira and Metema primary hospitals, and the second one was stored at −20°C and transported to the University of Gondar Comprehensive Specialized Hospital by triple package systems at 2–4°C using an ice pack for solid culture (LJ) when the first specimen was positive for MTB/RIF by Gene Xpert MTB/RIF nested real-time polymerase chain reaction (PCR) at the Abrihajira and Metema hospitals and MDR-MTB testing by line probe assays (LPA) at the Amhara Public Health Institute.
The MTB/RIF Xpert Assay was conducted using the Gene Xpert machine model number 900-0513GxIvR2/Rev.C.2 (Cepheid, Inc.) to detect PTB and rifampicin (RIF) resistance, as described in previous studies (19, 20). Decontaminated samples were mixed with sodium hydroxide (NaOH) and isopropanol sample reagent buffer (1:3 ratio) and incubated at room temperature for 15 min. The GeneXpert MTB/RIF (Cepheid, Sunnyvale, Inc.) was loaded with 2 milliliters (mL) of the sample in the cartridge (GeneXpert MTB/RIF, Cepheid, Sunnyvale, CA). Results were generated after 2 h, indicating whether Mycobacterium tuberculosis was present and its RIF sensitivity.
Seven specimens positive for RR-MTB were inoculated onto Lowenstein–Jensen (LJ) slants, following previous methods (20, 21). They were incubated at 37°C for 4–6 weeks and colonies were checked twice weekly. LJ slant medium was considered positive when colonies appeared, confirmed by AFB smears.
After GeneXpert MTB/RIF (Cepheid, Sunnyvale, CA) examination, LPA was performed at the Amhara Public Health Institute Laboratory following the manufacturer’s protocol (Hain Life Science GmbH, Nehren, Germany) to detect RIF and Isoniazid (INH) resistance due to mutations in rpo, inhA, and katG genes. The hybridization procedure included denaturation, conjugation, and substrate application. As in a previous study, results were interpreted for Mycobacterium tuberculosis presence, resistance, sensitivity, or invalidity (18).
Operational definitions
Presumptive PTB patients: Patients were considered to have ‘presumptive TB’ if any of the following symptoms, regardless of duration, were present: cough, fever, night sweats, unintentional weight loss, chest pain, or loss of appetite.
Confirmed PTB: TB confirmed through bacteriological testing, with the detection of TB in a sputum sample using Xpert® MTB/RIF, was considered confirmed TB (22).
Quality control
The study used a pretested questionnaire in a comparable environment that was not part of the study. Close supervision and support during data collection and daily checks of completed questionnaires were made for correctness, completeness, and clarity of data. Internal and external quality checks were performed for the Gene Xpert assay AFB. Pre-analytical, analytical, and post-analytical quality control methods were used to ensure the reliability and validity of the study test procedures for sample collection, processing, and evaluation. A recently sub-cultured M. tuberculosis H37Rv strain was suspended for culture and LPA quality control. The biosafety cabinet is where all the culture’s handling and collection materials, including used pipettes and tubes, were kept. All materials were sealed before being placed in the autoclave, and gloves and other garbage were gathered in a plastic bag for the autoclave.
Data analysis
To conduct the analysis, the obtained data were input into the “Epi-Data 3.1” program and exported to Statistical Package for the Social Sciences (SPSS) version 20.0 (SPSS, Chicago, IL, USA). The data were compiled using descriptive statistics. The link between the dependent-and independent-variables (clinical features, socio-demographic characteristics, and predisposing factors) was investigated using bivariate and multivariate analysis. For the multivariable analysis, variables that demonstrated significance at p-values of 0.2 in the bivariate analysis were chosen. As measures of the strength of association, adjusted odds ratios (AOR) and their 95% confidence intervals (CIs) were used. Statistical significance was defined as a value of p ≤0.05.
Results
Socio-demographic data
The study included 314 PTB presumptive patients, whose median age was 35.0 (inter-quartile: 25–45 years), and those treated at the Metema and Abrihajira hospitals. Men comprised the majority of research participants (178; 56.69%), followed by married people (170; 54.1%), those who could not read or write (121; 38.5%), people who lived in cities (162; 51.6%), and people aged 25 to 50 years (165; 52.5%) (Table 1).
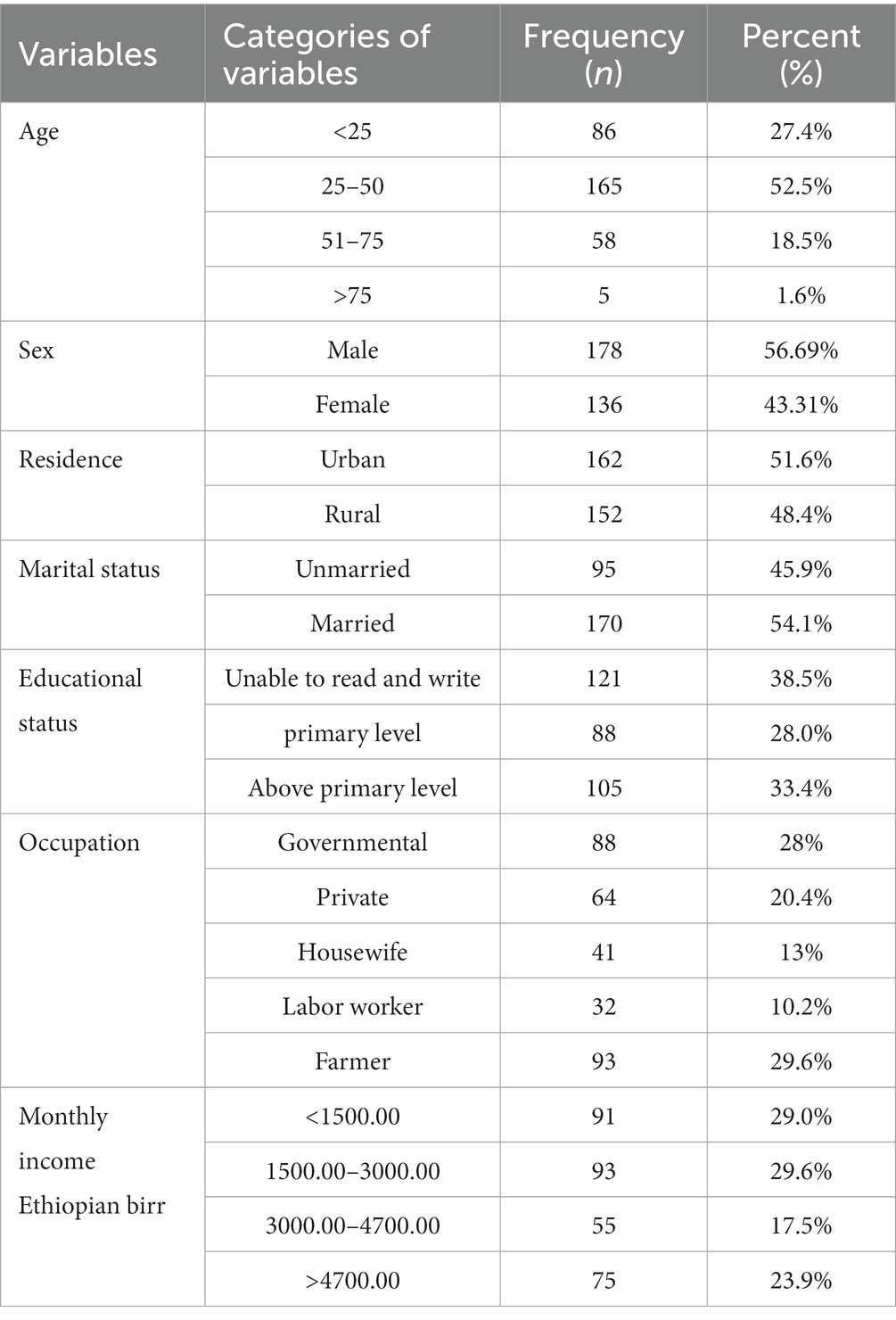
Table 1. Socio-demographic characteristics of PTB presumptive patients in northwestern Ethiopia, 2021 (N = 314).
Clinical presentation and behavioral data
All the study individuals included in this investigation displayed PTB clinical signs and symptoms, as illustrated in Table 2. Cases with loss of appetite (280, 89.2%), sputum production (276, 87.89%), weight loss (241, 76.75%), and night sweats (239, 76%) were the participants’ primary symptoms. Of all study participants, 94 (29.9%) had past contact with patients who were known to be infected with TB, 29 (9.2%) had previous contact with patients who were known to have MDR-TB, 62 (19.7%) had a history of alcohol use, 29 (6.9%) had a history of PTB, and 30 (9.6%) had a history of smoking cigarettes (Table 2).
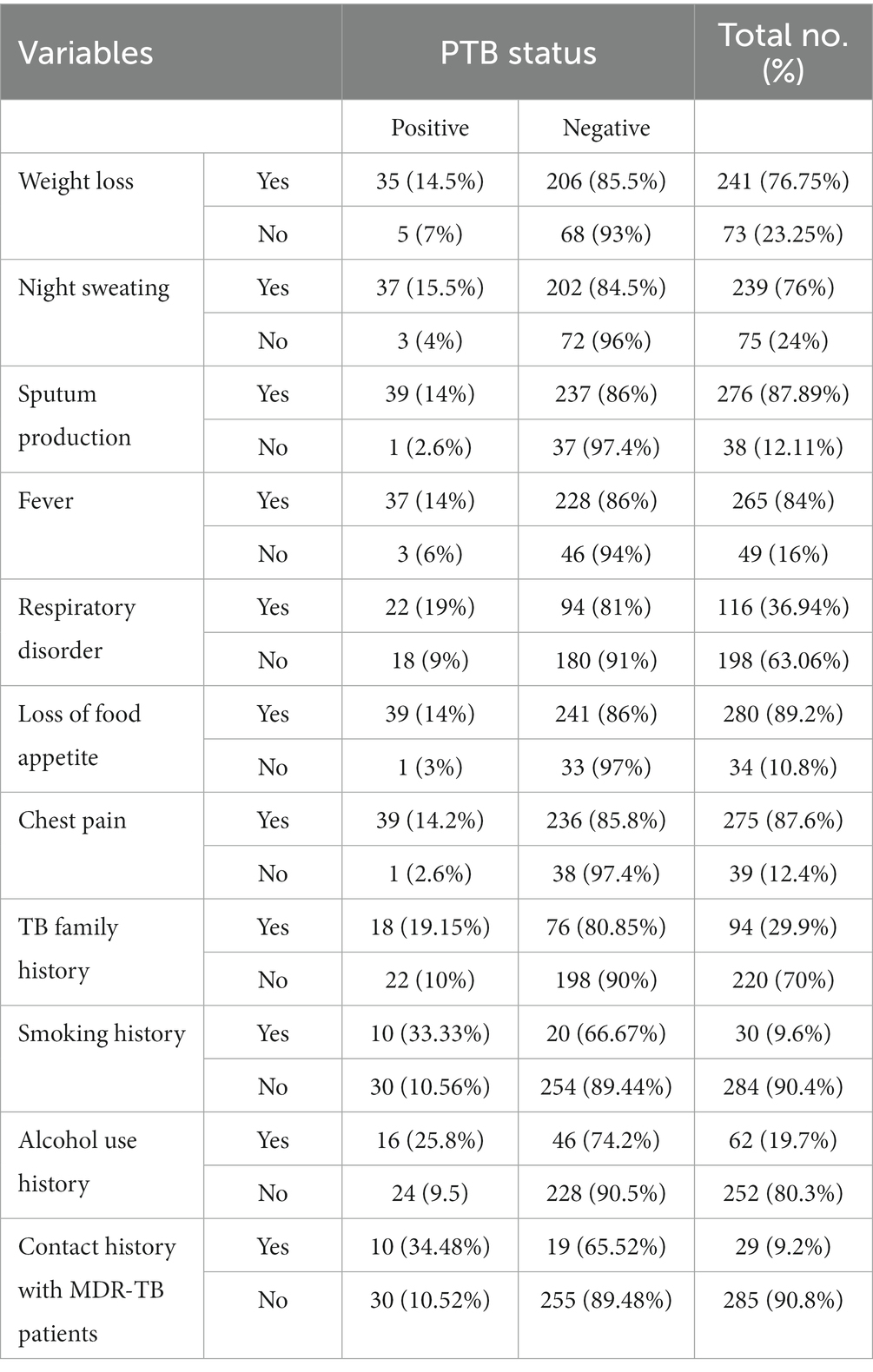
Table 2. PTB among PTB presumptive patients in relation to their clinical presentations in northwestern Ethiopia, 2021 (N = 314).
Prevalence of PTB, RR-MTB, and MDR-MTB
Forty (12.7%) of the study’s subjects were PTB-positive. The prevalence of RR-MTB was 2.2% across the entire study population (7/314) and was 17.5% (95% CI: 9.1–25.9) among individuals with PTB confirmation. MDR-MTB cases were 10% of the PTB confirmed cases and four (1.27%) of the study’s total participants. Study participants who were male, married, had only completed their primary education, were private workers, and had lower monthly incomes were shown to have higher PTB. Additionally, more men with RR-MTB and MDR-MTB were seen in the 25 to 50 age group among rural residents and unmarried research participants (Table 3).
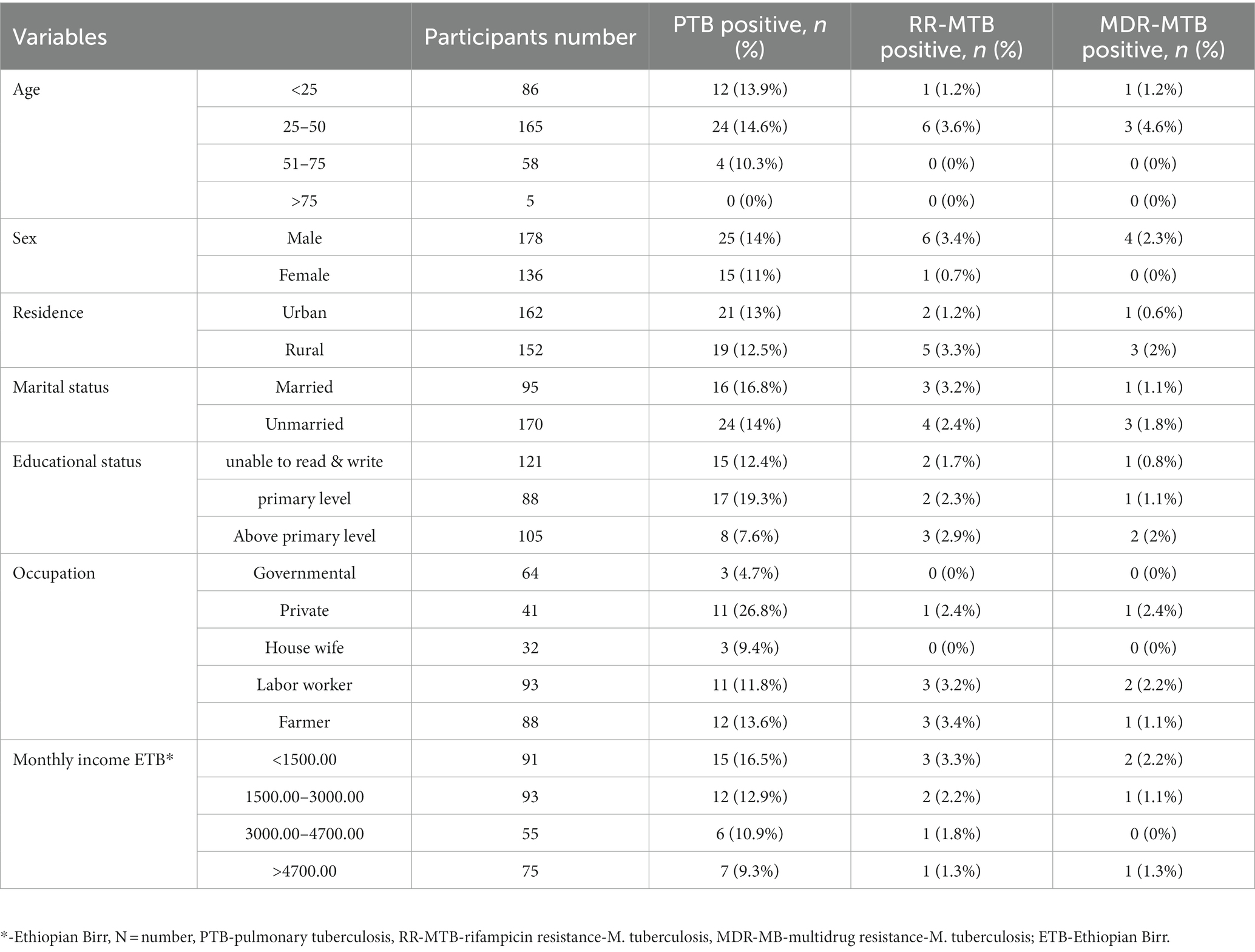
Table 3. PTB, RR-MTB, and MDR-MTB among PTB presumptive patients in northwestern Ethiopia, 2021 (N = 314).
Clinical risk factors associated with PTB
The majority of the subjects showed typical clinical signs and symptoms, which included nocturnal sweating (239, 76.1%), weight loss (241, 76.8%), coughing up sputum (274, 87.3%), and loss of appetite (280, 89.2%). The bi-variable analysis of patient characteristics revealed that patients’ lack of appetite, sputum production, night sweats, respiratory pain, fever, weight loss, and chest pain were significant clinical aspects. However, the multivariable model indicated that night sweating (AOR = 4.24 CI; 1.14–15.78), respiratory problems (AOR = 2.08 CI; 1.04–4.16), and sputum production (AOR =22.62 CI; 1.24–411.86) were significantly associated with PTB (Table 4).
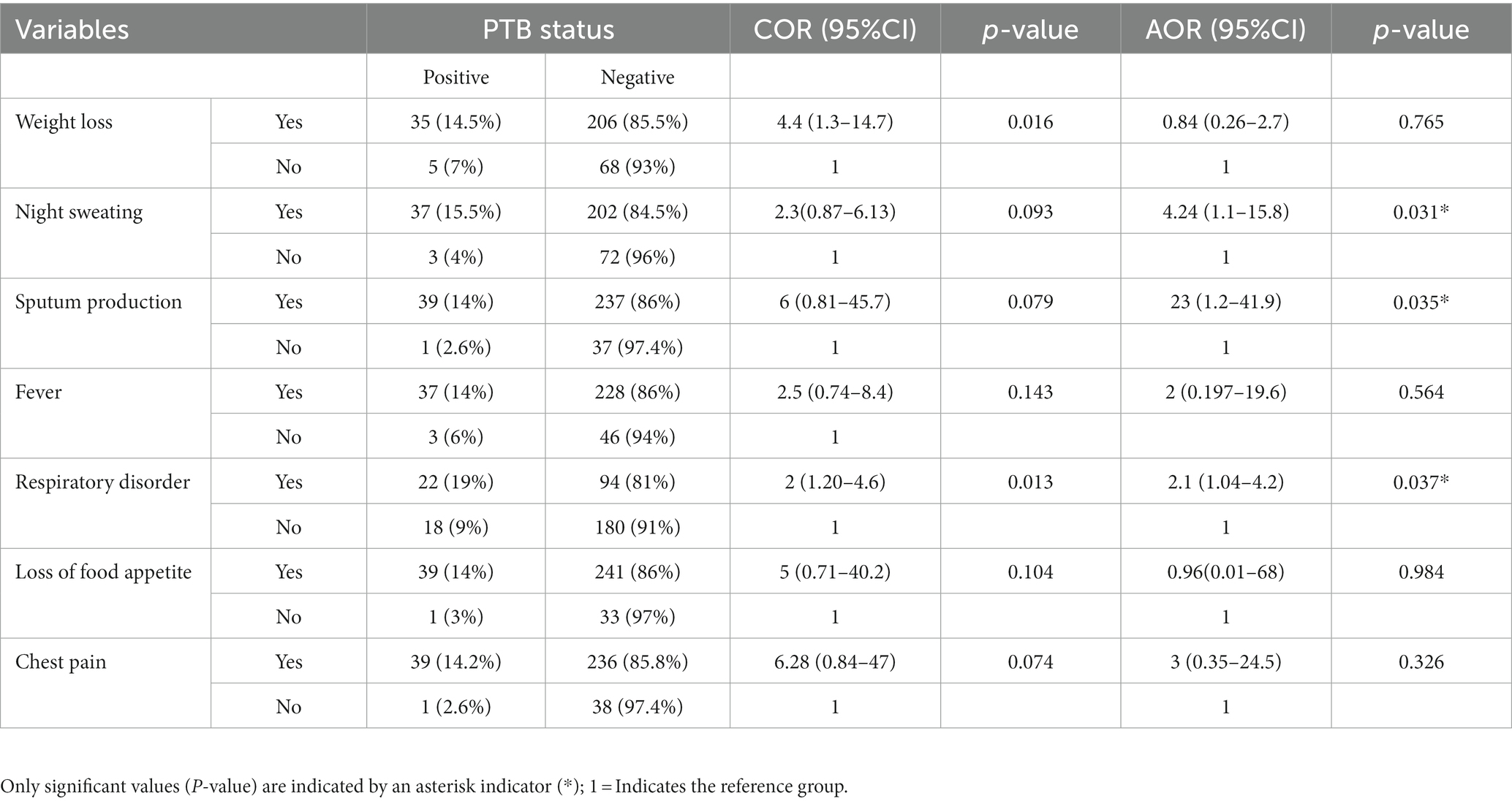
Table 4. Association of the clinical variables with PTB among PTB presumptive patients in northwestern Ethiopia, 2021 (N = 314).
Factors associated with PTB among PTB presumptive patients
Ten (34.48%) patients had a contact history with MDR-TB patients, 10 (33.33%) had a history of smoking cigarettes, and 18 (19.15%) of the study participants had a contact history with known PTB-infected patients. Patients with primary education (AOR = 3.76 CI; 1.3–10.5), contact history with confirmed PTB cases (AOR = 2.06 CI; 1.00–4.22), a history of alcohol use (AOR = 2.93 CI; 1.40–6.10), and a history of smoking cigarettes (AOR = 3.18 CI; 1.298–7.77) were more likely to contract the PTB infection than were patients without these risk factors (Table 5).
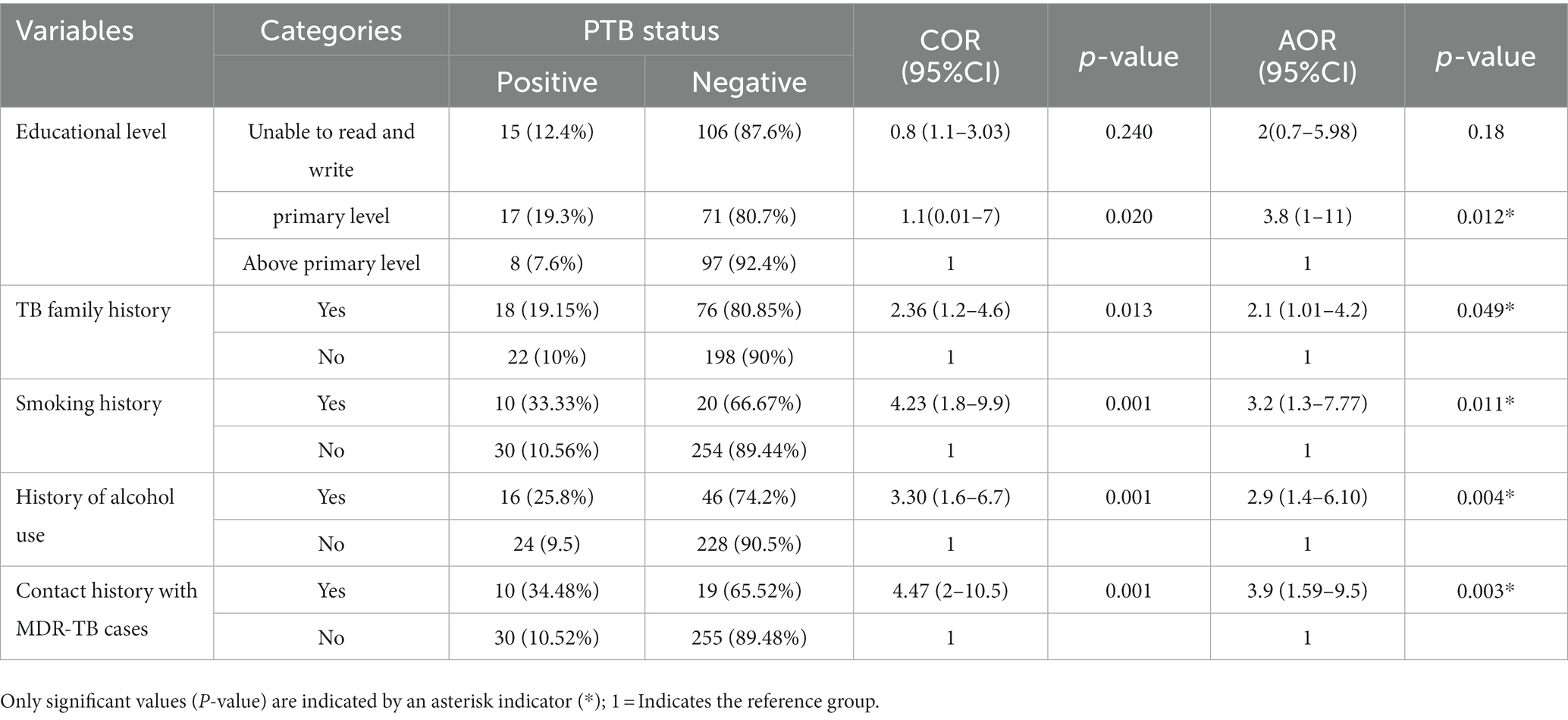
Table 5. Association of the demographic and behavioral characteristics with PTB among PTB presumptive patients in northwestern Ethiopia, 2021 (N = 314).
Discussion
The prevalence of pulmonary tuberculosis (PTB) in the current study was 12.7% (95% confidence interval: 9.2–16.6). This figure aligns with the findings from other studies conducted in similar settings, such as Addis Ababa Saint Paul’s Hospital, Ethiopia (10%) (23), Northeast Ethiopia (13.5%) (24), South Africa (11.79%) (25), and Nigeria (12.3%) (26). However, it is worth noting that our study reports a lower prevalence compared to research conducted in Debre Markos (19.8%) (27) and Gambella (20.0%) (28) in Ethiopia, as well as in China (32.12%) (29).
It is important to emphasize that the prevalence of PTB in our study is higher among PTB presumptive patients compared to some previous investigations within Ethiopia, such as Ataye, northeast Ethiopia (8.98%) (12), and Tigray, northwest Ethiopia (7.9%) (16). The variations in PTB prevalence observed across these studies may be attributed to differences in the characteristics of the study populations, geographical locations, and variations in laboratory techniques employed. Furthermore, it is noteworthy that the influx of daily laborers with low socioeconomic status from various regions of the country to the Metema and Abrihajira areas, where extensive agricultural activities take place, and the proximity of these study areas to the border with Sudan and Eritrea, might contribute to a lower level of awareness regarding the risk of PTB transmission from individuals with a history of TB (30, 31).
The risk of contracting pulmonary tuberculosis (PTB) is amplified among individuals of disadvantaged socioeconomic status, experiencing compromised immune function, engaging in smoking habits, and having proximity to actively ill PTB patients (12). The current study indicated that participants with a primary level of education faced approximately a 4-fold increased susceptibility to PTB compared to individuals with higher levels of educational attainment (10). Notably, individuals with lower educational attainment or limited literacy may exhibit deficiencies in fundamental health literacy, potentially hindering their understanding of PTB transmission and associated risk factors. Extensive global research corroborates these suppositions (32, 33).
According to this study, PTB presumptive patients with frequent interaction with confirmed PTB patients were 2.1 times more likely to have the illness than those without such contacts. Similarly, research in Ethiopia revealed a high correlation between PTB and previous interactions with PTB patients (32, 34). A significant risk factor for developing PTB was previous interaction with MDR-TB subjects. In comparison to individuals who did not have such a background, suspected PTB patients who had frequent interaction with MDR-TB patients were nearly four times more likely to develop the PTB. Another study in Ethiopia revealed a statistically significant correlation between PTB prevalence among PTB presumptive patients and interaction with MDR-TB patients (35). The current study identified cigarette smoking as a risk factor for PTB, and those who smoked had a 3-fold higher risk of developing the condition than non-smokers. In the current study, patients with a history of alcohol use were also nearly three times more likely than those who did not acquire PTB illness. Numerous studies have shown that drinking alcohol and smoking cigarettes directly harm blood vessels and biliary function, lowering immunity and increasing the risk of PTB (36–39).
In the present study, individuals manifesting symptoms of night sweats, respiratory distress, or sputum production exhibited a respective 4.2-fold, 2-fold, and 23-fold increased likelihood of having pulmonary tuberculosis (PTB) compared to their counterparts. This observation finds support in a prior study, which established a statistically significant association between PTB and the presence of sputum production and nocturnal sweating (32).
In contrast to the current study, research conducted at Addis Ababa and Ataye Primary Hospital in Ethiopia revealed no statistically significant correlations between PTB and the symptoms of night sweats, respiratory difficulties, or sputum production (10, 38). The disparities observed in these results may be attributed to variances in the study populations and geographical settings.
Gene Xpert assay-identified RR-MTB isolates are indicators of MDR-MTB. The presence of RR-MTB poses a significant global health (12). Rifampicin-resistant MTB is significantly prevalent in low-income countries, which may be explained by their socioeconomic and demographic makeup, including their high rates of malnutrition, overcrowding, inadequate medical treatment, and lack of social security (12). In the present study, the prevalence of rifampicin-resistant Mycobacterium tuberculosis (RR-MTB) was 7 out of 314 participants (2.2%) across the entire cohort. Among the subset of PTB-confirmed patients, this prevalence was notably higher at 7 out of 40 (17.5%). This finding closely aligns with prior research conducted on confirmed PTB patients in Gondar, Ethiopia, which reported a prevalence of 15.8% [17], as well as studies in Nigeria (13.9%) (40) and North India (10.5%) (41). However, it should be noted that our study observed a higher RR-MTB prevalence than previous investigations among confirmed PTB patients in Ataye (5.3%) (12), Afar (4.3%) (42), and Gambella (4.9%) (17), all within the Ethiopian context. This disparity can be elucidated by several factors within the study population, including a limited understanding of the mechanisms leading to drug resistance, restricted access to healthcare facilities, potential inaccuracies in patient diagnosis, treatment, and post-treatment monitoring, as well as suboptimal patient adherence. These factors may collectively account for the increased prevalence of rifampicin-resistant Mycobacterium tuberculosis (RR-MTB) observed in the present study [28]. However, the prevalence of RR-MTB is less common (33.3%) than that of the previous study conducted in Oromia, Ethiopia (43). We included suspicious individuals to identify PTB, whereas the study of the Oromia region included confirmed cases, which may have contributed to this variation. The discrepancies in prevalence across studies may also be explained by the diagnosis method, population geography, and study sets. The disparity in RR-MTB across the country may also be attributed to variations in patient selection and the study’s sample size. In the current investigation, the prevalence of MDR-MTB was 4/314 (1.27%) across all patients and 4/40 (10%) among PTB-confirmed individuals. This result is comparable to investigations done in Ethiopia among confirmed patients, where the rates were 7.8% (44) and 13.8% (45), respectively. The current study’s findings were less prevalent than those in Tigray, Ethiopia (16.7%) (45). We included presumptive cases, but other studies included recognized patients of RR-MTB to verify drug resistance profiles, which may have contributed to this variation. The results of the current study, however, were more significant than those of investigations conducted in South Gondar, Ethiopia (1.8%) (46), Central Ethiopia (1.5%) (47), and India (1.34%) (48). The disparities in access to medical services and health education and the presence of a considerable labor force and a transient resident population at the study site, as opposed to other regions, could plausibly account for the observed variation, as highlighted in this study.
Conclusion
After attempting to compare the results of the current study with numerous other prior studies conducted in Ethiopia, we found a greater prevalence of PTB, RR-MTB, and MDR-MTB. Five risk factors were statistically significant (p≤ 0.05) when considered as having a potential relationship with PTB prevalence. The high incidence, resistance, and statistically significant variables found in this study support the idea that national border regions should receive more attention. The findings of this study in the border region will fill knowledge gaps and give researchers, decision-makers, and implementers up-to-date information. The results should also disturb the scientific community because an additional study is required if only to lessen the total impact of tuberculosis in the region.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Ethics statement
Ethical approval (SBMLS/2724/2021) was obtained from the research and ethics review committee of the School of Biomedical Laboratory Sciences, College of Medicine and Health Sciences, University of Gondar. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants' legal guardians/next of kin.
Author contributions
MG: Conceptualization, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. YM: Software, Supervision, Validation, Visualization, Writing – review & editing. MJ: Software, Supervision, Validation, Visualization, Writing – review & editing. BW: Conceptualization, Data curation, Formal analysis, Writing – original draft.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Acknowledgments
The authors thank the Metema and Abrihajira primary hospitals, the Amhara Public Health Institute, the Amhara National Regional Health Bureau, and the University of Gondar Comprehensive Specialized Hospital for their general assistance. Additionally, a special thanks should go to the study participants.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Ahmed, A, Mekonnen, D, Shiferaw, AM, Belayneh, F, and Yenit, MK. Incidence and determinants of tuberculosis infection among adult patients with HIV attending HIV care in north-East Ethiopia: a retrospective cohort study. BMJ Open. (2018) 8:e016961. doi: 10.1136/bmjopen-2017-016961
2. Fahimzad, SA, Ghasem, M, Shiva, F, Ghadiri, K, Navidinia, M, and Karimi, A. Susceptibility pattern of Bacille Calmette-Guerin strains against pyrazinamide and other major anti-mycobacterial drugs. Arch Pediatr Infect Dis. (2015) 3:e17814. doi: 10.5812/pedinfect.17814
3. Mehdi, G, and Masoumeh, N. Overview perspective of bacterial strategies of resistance to biocides and antibiotics. Arch Clin Infect Dis. (2019) In Press:e65744. doi: 10.5812/archcid.65744
4. Annabel, B, Anna, D, and Hannah, M. Global tuberculosis report 2019. Geneva: World Health Organization. (2019) 15:7–9.
5. Abebe, G, Abdissa, K, Abdissa, A, Apers, L, Agonafr, M, de Jong, B, et al. Relatively low primary drug resistant tuberculosis in southwestern Ethiopia. BMC res. Notes. (2012) 5:1–6. doi: 10.1186/1756-0500-5-225
6. World Health Organization . Rapid сommunication: key changes to treatment of multidrug-and rifampicin-resistant tuberculosis (MDR/RR-TB). Geneva: World Health Organization (2018). 2 p.
7. Kelecha, WT, Teklegiorgis, SG-S, and Gemechu, MM. Rifampicin-resistance pattern of mycobacterium tuberculosis and associated risk factors among presumptive pulmonary and extra pulmonary tuberculosis patients at Madda Walabu university Goba referral hospital, Southeast Ethiopia. Research Square. (2021) [Preprint]. doi: 10.21203/rs.3.rs-652594/v1
8. World Health Organization . Companion handbook to the WHO guidelines for the programmatic management of drug-resistant tuberculosis. Geneva, Switzerland: WHO Press, World Health Organization (2014).
9. World Health Organization . Xpert MTB/RIF assay for the diagnosis TB meeting report (2016). Geneva, Switzerland: World Health Organization.
10. Balcha, TT, Sturegård, E, Winqvist, N, Skogmar, S, Reepalu, A, Jemal, ZH, et al. Intensified tuberculosis case-finding in HIV-positive adults managed at Ethiopian health centers: diagnostic yield of Xpert MTB/RIF compared with smear microscopy and liquid culture. PLoS One. (2014) 2-14:e85478. doi: 10.1371/journal.pone.0085478
11. World Health Organization . World health organization global tuberculosis report, 2017 (WHO/HTM/TB/2017.23). Geneva: World Health Organization (2017).
12. Gebretsadik, D, Ahmed, N, Kebede, E, Mohammed, M, and Belete, MA. Prevalence of tuberculosis by automated GeneXpert rifampicin assay and associated risk factors among presumptive pulmonary tuberculosis patients at Ataye District hospital, north East Ethiopia. Infect Drug Resist. (2020) 13:1507–16. doi: 10.2147/IDR.S248059
13. Wasihun, AG, Hailu, GG, and Dejene, TA. Prevalence of Mycobacterium tuberculosis (rifampicin-resistant MTB) and associated risk actors among pulmonary presumptive TB patients in eastern Amhara, Ethiopia: 2015–2019. Infect Dis Ther. (2021) 10:1299–308. doi: 10.1007/s40121-020-00368-5
14. Tarekegne, D, Jemal, M, Atanaw, T, Ebabu, A, Endris, M, Moges, F, et al. Prevalence of human immunodeficiency virus infection in a cohort of tuberculosis patients at Metema hospital, Northwest Ethiopia: a 3 years retrospective study. BMC Res Notes. (2016) 9:1–6. doi: 10.1186/s13104-016-2004-8
15. Melkamu, HT, Beyene, AM, and Zegeye, DT. Knowledge, attitude and practices of the resident community about visceral leishmaniasis in west Armachiho district, Northwest Ethiopia. Heliyon. (2020) 9:1–6. doi: 10.1016/j.heliyon.2019.e03152
16. Wasihun, AG, Dejene, TA, and Hailu, GG. Frequency of MTB and rifampicin resistance MTB using Xpert-MTB/RIF assay among adult presumptive tuberculosis patients in Tigray, northern Ethiopia: a cross sectional study. PLoS One. (2020) 15:e0240361. doi: 10.1371/journal.pone.0240361
17. Jaleta, KN, Gizachew, M, Gelaw, B, Tesfa, H, Getaneh, A, and Biadgo, B. Rifampicin-resistant Mycobacterium tuberculosis among tuberculosis-presumptive cases at University of Gondar Hospital, Northwest Ethiopia. Infect Drug Resist. (2017) 10:185–92. doi: 10.2147/IDR.S135935
18. World Health Organization . WHO consolidated guidelines on drug-resistant tuberculosis treatment. Geneva: World Health Organization (2019).
19. Allahyartorkaman, M, Mirsaeidi, M, Hamzehloo, G, Amini, SM, Zakiloo, M, and Nasiri, MI. Low diagnostic accuracy of Xpert MTB/RIF assay for extrapulmonary tuberculosis: a multicenter surveillance. Sci Rep. (2019) 9:1, 18515–18516. doi: 10.1038/s41598-019-55112-y
20. Aricha, SA, Kingwara, L, Mwirigi, NW, Chaba, L, Kiptai, T, Wahogo, J, et al. Comparison of GeneXpert and line probe assay for detection of mycobacterium tuberculosis and rifampicin-mono resistance at the National Tuberculosis Reference Laboratory. Kenya BMC Infect Dis. (2019) 19:852. doi: 10.1186/s12879-019-4470-9
21. Naveen, G, and Peerapur, BV. Comparison of of the Lowenstein-Jensen medium, the Middlebrook 7H10 medium and MB/BacT for the isolation of Mycobacterium Tuberculosis (MTB) from clinical specimens. J Clin Diagn Res. (2012) 6:1704–9. doi: 10.7860/JCDR/2012/4603.2635
22. World Health Organization . WHO operational handbook on tuberculosis. Module 1: Prevention - tuberculosis preventive treatment. Geneva: World Health Organization (2020).
23. Nugussie, DA, Mohammed, GA, and Tefera, AT. Prevalence of smear-positive tuberculosis among patients who visited Saint Paul’s specialized Hospital in Addis Ababa, Ethiopia. Biomed Res Int. (2017) 2017:1–5. doi: 10.1155/2017/6325484
24. Belay, M, Bjune, G, and Abebe, F. Prevalence of tuberculosis, HIV, and TB-HIV co-infection among pulmonary tuberculosis suspects in a predominantly pastoralist area, Northeast Ethiopia. Glob Health Action. (2015) 8:27949. doi: 10.3402/gha.v8.27949
25. Brunet, L, Pai, M, Davids, V, Ling, D, Paradis, G, Lenders, L, et al. High prevalence of smoking among patients with suspected tuberculosis in South Africa. Eur Respir J. (2011) 38:139–46. doi: 10.1183/09031936.00137710
26. Nwachukwu, N, Onyeagba, R, Nwaugo, V, Ugbogu, O, and Ulasi, A. Prevalence of pulmonary tuberculosis and its associated risk factors in Anambra state, Nigeria. Trends Food Sci Technol (2016). Available at: https://www.researchgate.net/publication/309202233_486_PREVALENCE_OF_PULMONARY_TUBERCULOSIS
27. Mulu, W, Abera, B, Yimer, M, Hailu, T, Ayele, H, and Abate, D. Rifampicin-resistance pattern of mycobacterium tuberculosis & associated factors among presumptive tuberculosis patients referred to Debre Markos referral hospital, Ethiopia: a cross-sectional study. BMC Res Notes. (2017) 10:1–8. doi: 10.1186/s13104-016-2328-4
28. Ejeta, E, Beyene, G, Bonsa, Z, and Abebe, G. Xpert MTB/RIF assay for the diagnosis of mycobacterium tuberculosis and rifampicin resistance in high human immunodeficiency virus setting in gambella regional state, Southwest Ethiopia. J Clin Tuberc Other Mycobact Dis. (2018) 12:14–20. doi: 10.1016/j.jctube.2018.06.002
29. Zhang, CY, Zhao, F, Xia, YY, Yu, YL, Shen, X, Lu, W, et al. Prevalence and risk factors of active pulmonary tuberculosis among elderly people in China: a population based cross-sectional study. Infect Dis Poverty. (2019) 8:7. doi: 10.1186/s40249-019-0515-y
30. Hayibor, KM, Bandoh, DA, Asante-Poku, A, and Kenu, E. Predictors of adverse TB treatment outcome among TB/HIV patients compared with non-HIV patients in the Greater Accra regional hospital from 2008 to 2016. Tuberc Res Treat. (2020) 2020:1–8. doi: 10.1155/2020/1097581
31. Dara, M, de Colombani, P, Petrova-Benedict, R, Centis, R, Zellweger, JP, Sandgren, A, et al. Minimum package for cross-border TB control and care in the WHO European region: a Wolfheze consensus statement. Eur Respir J. (2012) 40:1081–90. doi: 10.1183/09031936.00053012
32. Ayalew, ML, Yigzaw, WB, Tigabu, A, and Tarekegn, BG. Prevalence, associated risk factors and rifampicin resistance pattern of pulmonary tuberculosis among children at Debre Markos referral hospital, northwest. Ethiopia Infect Drug Resist. (2020) 13:3863–72. doi: 10.2147/IDR.S277222
33. Stevens, H, Ximenes, RA, Dantas, CM, and Rodrigues, LC. Risk factors for tuberculosis in older children and adolescents: a matched case–control study in Recife. Brazil Emerg Themes Epidemiol. (2014) 11:1–7. doi: 10.1186/s12982-014-0020-5
34. Gebrecherkos, T, Gelaw, B, and Tessema, B. Smear positive pulmonary tuberculosis & HIV co-infection in prison settings of North Gondar zone. Northwest Ethiopia BMC Public Health. (2016) 16:1–10. doi: 10.1186/s12889-016-3761-y
35. Hiruy, N, Melese, M, Habte, D, Jerene, D, Gashu, Z, Alem, G, et al. Comparison of the yield of tuberculosis among contacts of multidrug-resistant and drug-sensitive tuberculosis patients in Ethiopia using GeneXpert as a primary diagnostic test. Int J Infect Dis. (2018) 71:4–8. doi: 10.1016/j.ijid.2018.03.011
36. Mesfin, EA, Beyene, D, Tesfaye, A, Admasu, A, Addise, D, Amare, M, et al. Drug-resistance patterns of Mycobacterium tuberculosis strains and associated risk factors among multi drug-resistant tuberculosis suspected patients from Ethiopia. PLoS One. (2018) 13:e0197737. doi: 10.1371/journal.pone.0197737
37. Silva, DR, Muñoz-Torrico, M, Duarte, R, Galvão, T, Bonini, EH, Arbex, FF, et al. Risk factors for tuberculosis: diabetes, smoking, alcohol use, and the use of other drugs. J Bras Pneumol. (2018) 44:145–52. doi: 10.1590/s1806-37562017000000443
38. Zammarchi, L, Bartalesi, F, and Bartoloni, A. Tuberculosis in tropical areas and immigrants. Mediterr J Hematol Infect Dis. (2014) 6:e2014043. doi: 10.4084/MJHID.2014.043
39. Sinshaw, W, Kebede, A, Bitew, A, Tesfaye, E, Tadesse, M, Mehamed, Z, et al. Prevalence of tuberculosis, multidrug resistant tuberculosis and associated risk factors among smear negative presumptive pulmonary tuberculosis patients in Addis Ababa. Ethiopia BMC Infect Dis. (2019) 19:1–15. doi: 10.1186/s12879-019-4241-7
40. Nwadioha, SI, Nwokedi, EOP, Ezema, GC, Eronini, NC, Anikwe, A, AuduI, F, et al. Drug resistant M. tuberculosis in Benue, Nigeria. Microbiol Res J Int. (2014) 4:988–95. doi: 10.9734/BMRJ/2014/9084
41. Gupta, A, Mathuria, JP, Singh, SK, Gulati, AK, and Anupurba, S. Antitubercular drug resistance in four healthcare facilities in North India. J Health Popul Nutr. (2011) 29:583.
42. Gebrehiwet, GB, Kahsay, AG, Welekidan, LN, Hagos, AK, Abay, GK, and Hagos, DG. Rifampicin resistant tuberculosis in presumptive pulmonary tuberculosis cases in Dubti hospital, Afar. Ethiopia J Infect Dev Ctries. (2019) 13:21–7. doi: 10.3855/jidc.10462
43. Mulisa, G, Workneh, T, Hordofa, N, Suaudi, M, Abebe, G, and Jarso, G. Multidrug-resistant mycobacterium tuberculosis and associated risk factors in Oromia region of Ethiopia. Int J Infect Dis. (2015) 39:57–61. doi: 10.1016/j.ijid.2015.08.013
44. Asgedom, SW, Teweldemedhin, M, and Gebreyesus, H. Prevalence of multidrug-resistant tuberculosis and associated factors in Ethiopia: a systematic review. J Pathog. (2018) 2018:7104921. doi: 10.1155/2018/7104921
45. Welekidan, LN, Skjerve, E, Dejene, TA, Gebremichael, MW, Brynildsrud, O, Agdestein, A, et al. Characteristics of pulmonary multidrug-resistant tuberculosis patients in Tigray region, Ethiopia: a cross-sectional study. PLoS One. (2020) 15:e0236362. doi: 10.1371/journal.pone.0236362
46. Alelign, A, Zewude, A, Mohammed, T, Tolosa, S, Ameni, G, and Petros, B. Molecular detection of Mycobacterium tuberculosis sensitivity to rifampicin and isoniazid in South Gondar zone, Northwest Ethiopia. BMC Infect Dis. (2019) 19:343. doi: 10.1186/s12879-019-3978-3
47. Bedewi, Z, Mekonnen, Y, Worku, A, Medhin, G, Zewde, A, Yimer, G, et al. Mycobacterium tuberculosis in Central Ethiopia: drug sensitivity patterns and association with genotype. New Microbes New Infect. (2017) 17:69–74. doi: 10.1016/j.nmni.2017.02.003
Keywords: Abrihajira and Metema hospital, Xpert MTB/RIF assay, multidrug-resistant Mycobacterium tuberculosis, line probe assay, northwestern Ethiopia
Citation: Wubu B, Jemal M, Million Y and Gizachew M (2023) Pulmonary tuberculosis and multidrug-resistant Mycobacterium tuberculosis in northwestern Ethiopia: a hospital-based cross-sectional study among presumptive pulmonary tuberculosis patients. Front. Med. 10:1266780. doi: 10.3389/fmed.2023.1266780
Edited by:
Sam Donta, Falmouth Hospital, United StatesReviewed by:
Dongdong Li, Sichuan University, ChinaAyo Ajayi, Federal University Oye Ekiti, Nigeria
Muhammad Furqan Akhtar, Riphah International University (Lahore), Pakistan
Copyright © 2023 Wubu, Jemal, Million and Gizachew. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mucheye Gizachew, bXVjaGVnaXphQGdtYWlsLmNvbQ==
 Birhanu Wubu1
Birhanu Wubu1 Yihenew Million
Yihenew Million Mucheye Gizachew
Mucheye Gizachew