- 1Department of Biomedical Sciences, Institute of Tropical Medicine (ITM), Antwerp, Belgium
- 2University of Antwerp, Antwerp, Belgium
- 3Research Foundation Flanders (FWO), Brussels, Belgium
- 4Unité Mixte de Recherche Processus Infectieux en Milieu Insulaire Tropical (UMR PIMIT), Université de La Réunion, CHU de La Réunion, Plateforme Technologique CYROI, Sainte-Clotilde, Réunion Island, France
- 5Plan National de Lutte contre le Paludisme, Moroni, Comoros
- 6Damien Foundation, Brussels, Belgium
- 7National Tuberculosis and Leprosy Control Program, Moroni, Comoros
- 8Université de La Réunion, Fédération de recherche Environnement, Biodiversité et Santé, Saint-Denis, Réunion Island, France
Introduction: Leprosy, one of the oldest known human diseases, continues to pose a global challenge for disease control due to an incomplete understanding of its transmission pathways. Ticks have been proposed as a potential contributor in leprosy transmission due to their importance as vectors for other infectious diseases.
Methods: In 2010, a sampling of ticks residing on cattle was conducted on the islands Grande Comore, Anjouan, and Mohéli which constitute the Union of the Comoros where leprosy remains endemic. To investigate the potential role of ticks as a vector in transmission of leprosy disease, molecular analyses were conducted.
Results: Out of the 526 ticks analysed, none were found to harbour Mycobacterium leprae DNA, as determined by a quantitative polymerase chain reaction (qPCR) assay targeting a family of dispersed repeats (RLEP) specific to M. leprae.
Discussion: Therefore, our results suggest that in the Union of the Comoros, ticks are an unlikely vector for M. leprae.
Introduction
Leprosy is a mutilating disease caused by the intracellular bacilli Mycobacteria leprae (M. leprae) and/or lepromatosis (1). Despite the World Health Organization (WHO) removing leprosy from its list of public health concerns in 2001, the lack of significant reduction in new cases and the detection of leprosy in children indicate that transmission of the disease is still ongoing (2). This is evident in regions where active measures are taken to identify cases, such as door-to-door screenings, which consistently uncover new leprosy patients. Additionally, the prevalence of severe disabilities at the time of diagnosis in many countries suggests delayed detection and diagnosis (3). As a result, it is becoming increasingly apparent that we only see the tip of the iceberg of the global leprosy burden.
The exact transmission route of leprosy has not been fully elucidated yet. Different sites have been identified as potential entry and exit for M. leprae bacilli to the human body, namely the nose, mouth and skin (4). The highest bacillary burden is found in the epidermis of leprosy patients (5). The most probable transmission route of leprosy is via the aerial route (6), caused by the prolonged close contact to leprosy patients. Especially multibacillary patients are considered to drive leprosy transmission, given the high bacterial load. However, the nine-banded armadillo (7, 8), red squirrels (9), and chimps (10) have been confirmed as animal reservoirs and zoonotic transmission of M. leprae has been confirmed by genotyping (8) infected armadillos and leprosy patients in the US. Thus, the question as to whether the transmission pathway is direct or (partially) vector-driven remains unresolved (4).
The vector competence of Amblyomma sculptum from the family of hard ticks (Ixodidae) for M. leprae was demonstrated by Ferreira et al. (11) by artificially feeding adult females with M. leprae Thai-53 infected rabbit blood. Transovarial transmission of M. leprae was confirmed by the M. leprae specific RLEP qPCR. These findings are supported by results of Tongluan et al. (12) who injected Amblyomma maculatum ticks at adult and nymph stage with an M. leprae Thai-53 suspension derived from infected nude mice footpads. They confirmed the presence of M. leprae DNA in F1 larvae and F1 nymphs via RLEP qPCR. Both studies were able to grow M. leprae in cell lines derived from ticks, viability was confirmed by examination of the normalized expression levels of the M. leprae esxA gene (12) or 16S rRNA RT-qPCR (11). Transmission of M. leprae to a vertebrate host followed by an infection was only shown for M. leprae cultivated in and isolated from tick-derived cell lines. When inoculated directly into the footpad, these bacilli are able to establish a prolonged infection in mice (11, 12). Blood-feeding tick larvae were able to transfer viable M. leprae to a rabbit model. However, rabbit skin was analysed already 5 days after inoculation, a time frame too short to confirm a stable infection of the vertebrate host (11). Even though these studies were mainly aiming towards being able to grow M. leprae bacilli in vitro in a cell line, the experimental data suggests that there is a possibility for a transmission route of leprosy via ticks after taking their blood meal on a person with leprosy.
The Union of the Comoros has the highest per capita incidence of leprosy in Africa [as high as seven cases per 10,000 individuals on Anjouan (13, 14)], making it the only country of the African continent that did not reach the elimination target of less than 1 patient/10,000 population postulated by the WHO (3, 15). Despite the persistent efforts of the National Tuberculosis and Leprosy Control Programme, including intensified screenings since 2008 and the administration of post-exposure prophylaxis within the framework of the PEOPLE and BE-PEOPLE studies (Clinicaltrials.gov: NCT03662022 and NCT05597280), leprosy, a poverty-related disease, remains endemic on the islands Anjouan and Mohéli. In contrast, the wealthiest of the three islands, Grande Comore, is not considered a leprosy endemic region. Leprosy has a long incubation period of several months to decades, with an average of 2–4 years (16), which implies ongoing transmission of the disease by the high proportion of affected children on Anjouan and Mohéli (2). The potential contribution of non-human animal and environmental reservoirs to the transmission of leprosy represents a knowledge gap towards interrupting leprosy transmission. Further, the role of ticks as biological or mechanical vector has not been confirmed by epidemiological studies yet. Therefore, this study sought to investigate the presence of M. leprae DNA in a tick collection obtained from the Union of the Comoros as a means of further elucidating the potential involvement of ticks as a vector in leprosy transmission.
Materials and methods
Samples
A total of 526 ticks from a previously described collection (17) from the Union of the Comoros were selected for screening for the presence of M. leprae DNA. Specimens were shipped and stored in molecular grade pure ethanol (Avantor, United States) at −20°C to the Institute of Tropical Medicine in Antwerp, Belgium. From the leprosy-endemic islands Anjouan (n = 134) and Mohéli (n = 129) 263 ticks were available. The prevalence of leprosy on Anjouan and Mohéli by the end of 2017 was 4.57/10,000 (18). A summary of leprosy prevalence per sampled district on Anjouan and Mohéli can be found in Supplementary Table S1. As a comparator, n = 263 ticks were selected from Grande Comore where leprosy is not endemic. All ticks were morphologically inspected and classified according to the guide by Walker et al. (19) before they were molecularly examined for the presence of M. leprae DNA.
DNA extraction
One half of each tick was used for DNA extraction. The ticks were ground with a mortar and pestle in 1 mL phosphate-buffered saline. To avoid DNA contamination mortars and pestles were autoclaved, treated with bleach, and rinsed prior to use and a new set was used for each sample. Subsequently, 200 μL of the resulting suspension were incubated with 200 μL in-house lysis buffer (Tris-HCl – pH 7.5, EDTA 0.5 M pH 8, 6 M GuHCl, Tween 20, Triton X-100, diatomaceous earth) and 20 μL proteinase K solution (Promega, United States) in a shaking incubator for 1 h at 60°C and 200 rpm. The lysed suspension was further extracted with the Maxwell® 16 FFPE PLUS Tissue LEV DNA purification Kit (Promega, United States), following the manufacturers’ protocol. To control for contamination throughout the extraction procedure, each run included a negative (molecular grade water) and a positive extraction control (suspension of mouse footpad infected with M. leprae Thai-53, BEI reference number: 19352).
qPCR assay
To quantify M. leprae DNA in the tick extracts, a qPCR assay targeting a family of dispersed repeats (RLEP) (20) was used as described previously (21) for 45 cycles (positivity cut-off <40 Cq), using the StepOnePlus™ qPCR cycler and StepOne software v2.3 (Applied Biosystems, United States), the primer and probe sequences and cycling conditions can be found in Supplementary Table S2. With this assay 36 out of 37 RLEP copies in the M. leprae genome are detected. Samples were tested in triplicate and considered positive when two of the three replicates were under the positivity cut-off. Non-template controls (molecular grade water) to control for contamination during the qPCR procedure and a gDNA (M. leprae NHDP, BEI reference number: 19350) standard curve for quantification with 1:10 dilutions from 3 × 105 to 3 × 101 RLEP copies were included in each run. An internal positive control (IPC, Eurogentec, Belgium) was spiked into each well to detect inhibition during the qPCR run.
Statistical analysis
To determine the significance of the difference between ticks selected from the leprosy endemic (Anjouan and Mohéli) and non-endemic (Grande Comore) islands, the one-proportion z-test was applied. The significance of the sample rate ratio of ticks investigated in this study compared to the complete tick collection by Yssouf et al. (17) was calculated with the Fisher’s exact test. All statistical analyses were performed with R, version 4.3.0 for macOS (The R foundation, Vienna, Austria), the alternative hypothesis, stating significant differences between variables, was accepted at a significance level of alpha = 0.05.
Results
Morphological classification of ticks
Of the 263 ticks from the endemic islands of Anjouan and Mohéli, 253 (96.2%) were identified as Rhipicephalus microplus and 10 (3.8%) as Amblyomma variegatum (Table 1). The sample rate ratio analysis of species classification showed that A. variegatum was slightly underrepresented in the subset examined in our study with a proportion of 3.8% compared to 9.8% in the complete original collection by Yssouf et al. (17) (Supplementary Table S3).
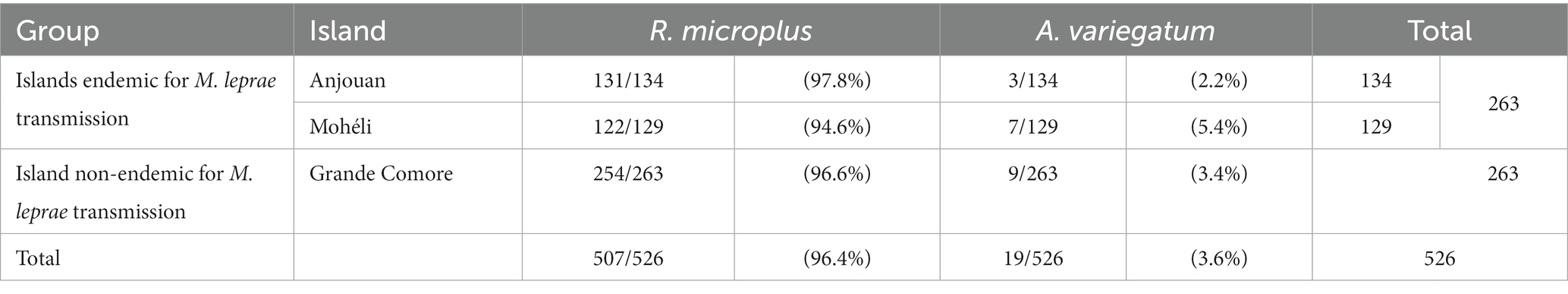
Table 1. Species distribution of ticks over the three islands of the Union of the Comoros classified according to Walker et al. (19).
In our study an additional classification of the ticks by developmental stage and sex was conducted. Most of the ticks from the endemic islands were adults (n = 184, 70.0%), followed by ticks in the nymph stage (n = 77, 29.3%). Only n = 2 larvae (0.8%) were available for analysis (Table 2). The majority of collected ticks was identified as female (n = 109, 81.3% from Anjouan; n = 102, 79.1% from Mohéli; n = 167, 63.5% from Grande Comore). For a small proportion of ticks (4.6%) the sex could not be identified in our study because the determining features in some nymphs and larvae were inconclusive (Table 2).
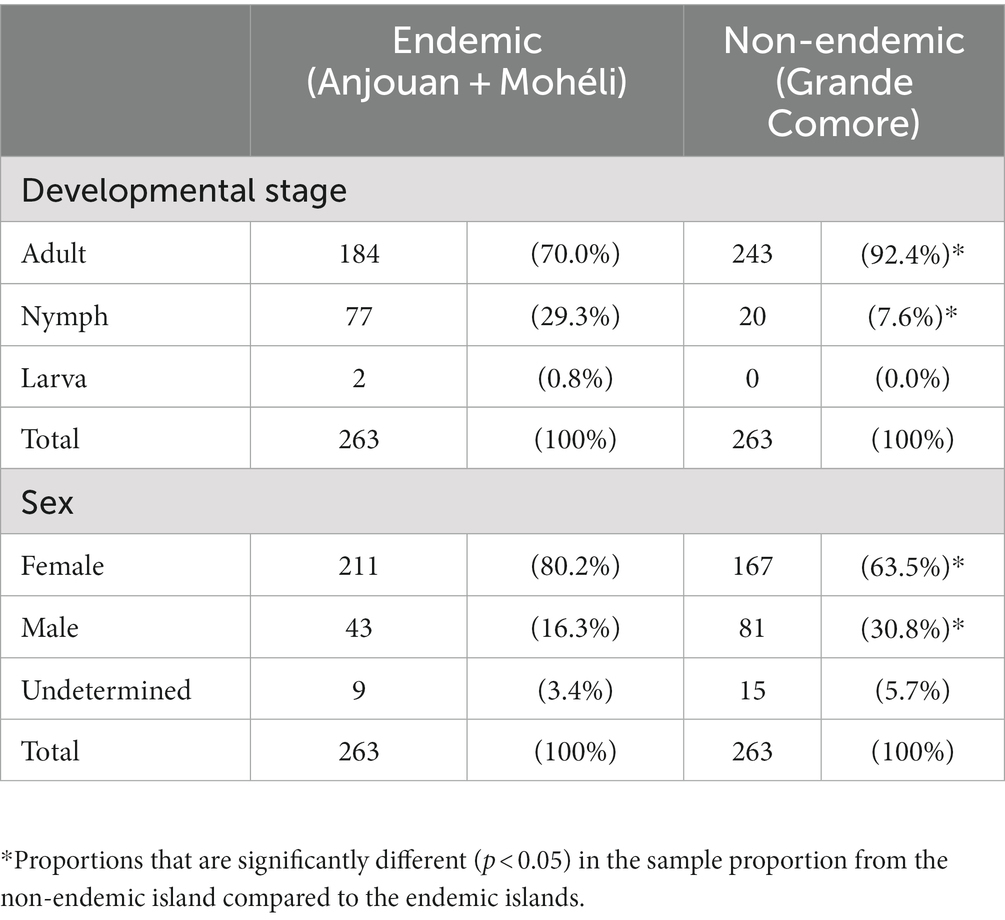
Table 2. Distribution of developmental stages and sex of ticks classified and investigated in this study.
Detection of M. leprae DNA by RLEP qPCR
None of the 526 tested DNA extracts from ticks resulted in a positive result in the RLEP qPCR. For none of the triplicates an amplification curve showed during the qPCR assay and therefore the positivity cut-off of 40 Cq was fulfilled. The limit of detection of the RLEP qPCR assay is as low as 30 RLEP copies per 2 μL added to each qPCR reaction, which correlates with approximately one M. leprae bacillus. All positive extraction controls resulted in a positive qPCR result. Negative extraction controls and non-template controls were negative on qPCR, indicating the absence of DNA contamination during the extractions and qPCR assays. IPC was spiked into the DNA extracts before qPCR quantification. Results were consistent within each qPCR run which confirms the absence of qPCR inhibition. A summary of the qPCR results of RLEP and IPC can be found in Supplemental File 1.
Discussion
This study is the first to use molecular tools to screen wild, animal-derived ticks from a leprosy endemic country for the presence of M. leprae. The absence of M. leprae DNA was confirmed in all tested specimens from the Comoros. Next to M. leprae, M. lepromatosis can also cause leprosy disease in humans (1). We have tested the leprosy patient cohort in the Comoros for the presence of M. lepromatosis DNA by qPCR assay, with results suggesting that M. leprae is the only causative agent for leprosy on the Comoros (manuscript in preparation). Therefore, in this study ticks were only screened for the presence of M. leprae DNA.
In the search for drivers for leprosy transmission, two previous studies (11, 12) identified ticks from the genus Amblyomma as potential competent vectors for M. leprae. More specifically, under experimental conditions the transovarial transmission and the survival of M. leprae in female ticks and tick-derived cells was confirmed. The majority of the wild tick collection analysed in our study were adult females, which are able to harbour and transmit M. leprae under experimental conditions. The small proportion of nymphs, which is the developmental stage most likely to parasitize humans and transmit other tick-borne diseases such as lyme disease (22) and ehrlichiosis (23), could explain our inability to detect M. leprae DNA in the tick collection that was studied.
Further, the tick collection consisted of a small ratio of Amblyomma ticks, the species with proven capacity to harbour M. leprae (11, 12), compared to R. microplus. Only 10 out of 263 (3.8%) ticks from the endemic islands Anjouan and Mohéli were A. variegatum while Yssouf et al. (17) classified 73 out of 742 (9.8%) ticks as A. variegatum. The reason for the different species distribution is that only a subset of the original collection was available for analyses at ITM, Antwerp. The selected number of ticks from Grande Comore, used as non-endemic controls, was matched to the species distribution found for the endemic islands in this study. Accordingly, the percentage of A. variegatum was smaller than the one found by Yssouf et al. on this island. However, both Rhipicephalus and Amblyomma ticks belong to the family of Ixodidae (or hard ticks). In their previous studies Tongluan et al. and Ferreira et al. were able to maintain M. leprae in Ixodes-derived cell lines which suggests a similar potential of all members of the Ixodidae family as a vector for M. leprae.
Even though the ticks analysed in our study were collected from cattle and goats and not from humans, feeding of cattle ticks on humans seems probable in situations where humans and livestock live closely together. For both R. microplus and A. variegatum which mainly feed on cattle and other large animals (24), such cross-over events have been reported (25–27). A recent publication by Faber et al. (28) is raising the hypothesis that a skin disease in water buffaloes described as lepra bubalorum could be caused by M. leprae and therefore act as animal reservoir. However, evidence for cases in Indonesia is only historical as there were no further reports for lepra bubalorum in cattle since 1961 (29) and there is no water buffalo population described in the Union of the Comoros (30).
Different other vectors have been suggested for the transmission of M. leprae, e.g., arthropods such as mosquitos (Aedes, Culex, Rhodnius) (31–33), flies (Musea, Calliphora and Stomoxys) (34), and sand flies (Phlebotomus, Sergentomyia). The latter are unlikely vectors as they cannot maintain viable M. leprae bacilli (35). Early studies on mosquitos confirmed the presence of acid-fast bacilli in the proboscis of mosquitos (A. aegypti and C. fatigans) after experimentally feeding on untreated leprosy patients (31, 32). However, viability determined by fluorescence microscopy reduced within seven days after feeding (32). Da Silva Neumann et al. have investigated R. prolixus, A. aegypti, and C. quinquefasciatus as possible vector, with the result that only R. prolixus has the ability to defecate infective M. leprae up to 20 days after infection with M. leprae Thai-53 infected rabbit blood (33). Additionally, amoeba have been found to have vector potential as they can phagocytose M. leprae. In vitro experiments showed that M. leprae can survive up to 72 h within the Acanthamoeba and up to 8 months in amoebal cysts while retaining infectivity for a nude mouse model (36, 37). However, for none of these vector candidates a clear correlation with leprosy infections in humans was identified.
Even though Ixodes ticks are potential competent vectors for M. leprae in vitro and pathogen transmission from livestock to humans via ticks is probable, all ticks from Anjouan, Mohéli, and Grande Comore that were investigated tested negative for M. leprae DNA. This finding lessens the chance that leprosy is a tick-borne zoonosis in the Union of the Comoros, rather than spread by human-to-human transmission.
Our results support the hypothesis that most leprosy infections are caused by human-to-human interactions rather than by a non-human animal or environmental reservoir of M. leprae and that close contact to a leprosy patient is the driving force of transmission. For the definitive exclusion of the role of ticks in the transmission of leprosy disease, a larger number of ticks also from other leprosy endemic regions should be analysed. The exploration of human-derived ticks and particularly ticks parasitising leprosy patients should be the focus of such studies. Further, qualitative case control studies investigating daily activities of leprosy patients and healthy controls will be useful for the generation of new hypotheses on the driving factors of leprosy transmission.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
Ethics statement
All ticks were manually sampled in the presence of cattle owners. No suffering was imposed to sampled animals and the disturbance associated with sampling was underneath the regulatory threshold requiring the submission of the protocol to an institutional ethics committee, as described on European Union Directive 2010/63/UE. For the study of ticks, ethical approval was not required in accordance with the national legislation and the institutional requirements.
Author contributions
LK, EC, BJ, and SB contributed to conception and design of the study. AY and PT were involved with the tick sampling and provided the tick collection. EC and MD-L were involved in the methodology, investigation, and validation. LK, EC, and SB performed the formal analysis and statistics. SB was the project administrator and together with BJ supervised the study. LK wrote the original manuscript draft. All authors contributed to the article and approved the submitted version.
Funding
This study was supported by a R2STOP research grant from effect: hope and The Mission To End Leprosy and EDCTP2 programme supported by the European Union (grant number RIA2017NIM-1847-PEOPLE). SB was supported by the Research Foundation Flanders (FWO), grant numbers 1189219N and 1189221N. LK was supported by the FWO, grant number 1SE7522N.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2023.1238914/full#supplementary-material
References
1. Deps, P, and Collin, SM. Mycobacterium lepromatosis as a second agent of Hansen’s disease. Front Microbiol. (2021) 12:1–7. doi: 10.3389/fmicb.2021.698588
2. Marijke Braet, S, Jouet, A, Aubry, A, Van Dyck-Lippens, M, Lenoir, E, Assoumani, Y, et al. Investigating drug resistance of Mycobacterium leprae in the Comoros: an observational deep-sequencing study. Lancet Microbe. (2022) 3:e693–700. doi: 10.1016/S2666-5247(22)00117-3
3. World Health Organization. Global leprosy (Hansen disease) update, 2021: moving towards interruption of transmission. Wkly Epidemiol Rec (2022); 36: 429–450. Available at: https://population.un.org/wpp/Download/Standard/Population/
4. Bratschi, MW, Steinmann, P, Wickenden, A, and Gillis, TP. Current knowledge on Mycobacterium leprae transmission: a systematic literature review. Lepr Rev. (2015) 86:142–55. doi: 10.47276/lr.86.2.142
5. Satapathy, J, Kar, B, and Job, C. Presence of Mycobacterium leprae in epidermal cells of lepromatous skin and its significance. Ind J Dermatol Venereol. (2005) 71:267–9. doi: 10.4103/0378-6323.16620
6. Araujo, S, Freitas, LO, Goulart, LR, and Goulart, IMB. Molecular evidence for the aerial route of infection of mycobacterium leprae and the role of asymptomatic carriers in the persistence of leprosy. Rev Infect Dis. (2016) 63:1412–20. doi: 10.1093/cid/ciw570
7. da Silva, MB, Portela, JM, Li, W, Jackson, M, Gonzalez-Juarrero, M, Hidalgo, AS, et al. Evidence of zoonotic leprosy in Pará, Brazilian Amazon, and risks associated with human contact or consumption of armadillos. G Pluschke, (Ed.) PLoS Negl Trop Dis. (2018); 12, doi: 10.1371/journal.pntd.0006532,:e0006532
8. Sharma, R, Singh, P, Loughry, WJ, Lockhart, JM, Inman, WB, Duthie, MS, et al. Zoonotic leprosy in the southeastern United States. Emerg Infect Dis. (2015) 21:2127–34. doi: 10.3201/eid2112.150501
9. Avanzi, C, Del-Pozo, J, Benjak, A, Stevenson, K, Simpson, VR, Busso, P, et al. Red squirrels in the British Isles are infected with leprosy bacilli. Science. (2016) 354:744–7. doi: 10.1126/science.aah3783
10. Hockings, KJ, Mubemba, B, Avanzi, C, Pleh, K, Düx, A, Bersacola, E, et al. Leprosy in wild chimpanzees. Verena J Schuenemann. (2021) 598:652–6. doi: 10.1038/s41586-021-03968-4
11. JdS, Ferreira, Souza, DA, Santos, JP, CCDU, Ribeiro, Baêta, BA, Teixeira, RC, et al. Ticks as potential vectors of Mycobacterium leprae: use of tick cell lines to culture the bacilli and generate transgenic strains. PJ Krause, (Ed.) PLoS Negl Trop Dis. (2018); 12, doi: 10.1371/journal.pntd.0007001,:e0007001
12. Tongluan, N, Shelton, LT, Collins, JH, Ingraffia, P, McCormick, G, Pena, M, et al. Mycobacterium leprae infection in ticks and tick-derived cells. Front Microbiol. (2021) 12:1–12. doi: 10.3389/fmicb.2021.761420
13. Ortuno-Gutierrez, N, Baco, A, Braet, S, Younoussa, A, Mzembaba, A, Salim, Z, et al. Clustering of leprosy beyond the household level in a highly endemic setting on the Comoros, an observational study. BMC Infect Dis. (2019) 19:501. doi: 10.1186/s12879-019-4116-y
14. Hasker, E, Baco, A, Younoussa, A, Mzembaba, A, Grillone, S, Demeulenaere, T, et al. Leprosy on Anjouan (Comoros): persistent hyper-endemicity despite decades of solid control efforts. Lepr Rev. (2017) 88:334–42. doi: 10.47276/lr.88.3.334
15. World Health Assembly 44. Forty-Fourth World Health Assembly, Geneva, 6–16, (1991): Resolutions and decisions, annexes
17. Yssouf, A, Lagadec, E, Bakari, A, Foray, C, Stachurski, F, Cardinale, E, et al. Colonization of Grande Comore Island by a lineage of Rhipicephalus appendiculatus ticks. Parasites Vectors. (2011) 4:1–8. doi: 10.1186/1756-3305-4-38
18. Programme National de Lutte contre la Tuberculose et la Lèpre (PNTL). Rapport Annuel 2019. Moroni, Union des Comores: Bulletins et mémoires de la Société médicale des hôpitaux de Paris (2019).
19. Walker, AR, Bouattour, A, Camicas, J-L, Estrada-Pena, A, Horak, IG, Latif, AA, et al. Ticks of domestic animals in Africa: a guide to identification of species. Edinburgh (Scotland). Biosci Rep. 63–161. (2003).
20. Woods, SA, and Cole, ST. A family of dispersed repeats in Mycobacterium leprae. Mol Microbiol. (1990) 4:1745–51. doi: 10.1111/j.1365-2958.1990.tb00552.x
21. Truman, RW, Andrews, PK, Robbins, NY, Adams, LB, Krahenbuhl, JL, and Gillis, TP. Enumeration of Mycobacterium leprae using real-time PCR. PLoS Negl Trop Dis. (2008) 2:e328. doi: 10.1371/journal.pntd.0000328
22. Matuschka, F-R, Fischer, P, Heiler, M, Blümcke, S, and Spielman, A. Stage-associated risk of transmission of the Lyme disease spirochete by europeanixodes ticks. Parasitol Res. (1992) 78:695–8. doi: 10.1007/BF00931523
23. Des, VF, and Fish, D. Transmission of the agent of human granulocytic Ehrlichiosis by host-seeking Ixodus scapularis (Acari: Ixodidae) in southern New York state. J Med Entomol. (1997) 34:379–82. doi: 10.1093/jmedent/34.4.379
24. Maina, AN, Jiang, J, Omulo, SA, Cutler, SJ, Ade, F, Ogola, E, et al. High prevalence of Rickettsia africae variants in Amblyomma variegatum ticks from domestic mammals in rural Western Kenya: implications for human health. Vector-Borne Zoonotic Dis. (2014) 14:693–702. doi: 10.1089/vbz.2014.1578
25. Kaur, N, Prasher, P, Kumar, K, and Dhingra, S. Rhipicephalus (Boophilus) microplus (arachnida: Ixodidae) larvae infestation of human eyelids. A rare case. Acarologia. (2019) 59:21–5. doi: 10.24349/acarologia/20194309
26. Ali, A, Khan, MA, Zahid, H, Yaseen, PM, Qayash Khan, M, Nawab, J, et al. Seasonal dynamics, record of ticks infesting humans, wild and domestic animals and molecular phylogeny of Rhipicephalus microplus in Khyber Pakhtunkhwa Pakistan. Front Physiol. (2019) 10:793. doi: 10.3389/fphys.2019.00793/full
27. Jensenius, M, Fournier, P-E, Vene, S, Hoel, T, Hasle, G, Henriksen, AZ, et al. African tick bite fever in travelers to rural sub-equatorial Africa. Clin Infect Dis. (2003) 36:1411–7. doi: 10.1086/375083
28. Faber, WR, Menke, H, Rutten, V, and Pieters, T. Lepra Bubalorum, a potential reservoir of Mycobacterium leprae. Front Microbiol. (2021) 12:1–6. doi: 10.3389/fmicb.2021.786921
29. Ploemacher, T, Faber, WR, Menke, H, Rutten, V, and Pieters, T. Reservoirs and transmission routes of leprosy; a systematic review. C Franco-Paredes, (Ed.) PLoS Negl Trop Dis. (2020); 14::e0008276. doi: 10.1371/journal.pntd.0008276
30. Minervino, AHH, Zava, M, Vecchio, D, and Borghese, A. Bubalus bubalis: a short story. Front Vet Sci. (2020) 7:7. doi: 10.3389/fvets.2020.570413/full
31. Narayanan, E, Sreevatsa, RAD, Kirchheimer, WF, and Bedi, BM. Persistence and distribution of Mycobacterium leprae in Aedes aegypti and Culex fatigans experimentally fed on leprosy patients. Lepr India. (1978) 50:26–37.
32. Banerjee, R, Banerjee, BD, Chaudhury, S, and Hati, AK. Transmission of viable Mycobacterium leprae by Aedes aegypti from lepromatous leprosy patients to the skin of mice through interrupted feeding. Lepr Rev. (1991) 62:21–6. doi: 10.5935/0305-7518.19910003
33. Da Silva, NA, De Almeida, DF, Da Silva, FJ, Fontes, ANB, Rosa, PS, Macedo, RE, et al. Experimental infection of Rhodnius prolixus (hemiptera, triatominae) with Mycobacterium leprae indicates potential for leprosy transmission. PLoS One. (2016) 11:1–14. doi: 10.1371/journal.pone.0156037
34. Geater, JG. The fly as potential vector in the transmission of leprosy. Lepr Rev. (1975) 46:279–86. doi: 10.5935/0305-7518.19750029
35. Sreevatsa, GBK, Ipe, IM, and Desikan, KV. Can sandflies be the vector for leprosy? Int J Lepr Other Mycobact Dis. (1992) 60:94–6.
36. Lahiri, R, and Krahenbuhl, JL. The role of free-living pathogenic amoeba in the transmission of leprosy: a proof of principle. Lepr Rev. (2008) 79:401–9. doi: 10.47276/lr.79.4.401
Keywords: leprosy, Mycobacterium leprae, ticks, transmission, vector, reservoir, cattle
Citation: Krausser L, Chauvaux E, Van Dyck-Lippens M, Yssouf A, Assoumani Y, Tortosa P, de Jong BC and Braet SM (2023) Ticks are unlikely to play a role in leprosy transmission in the Comoros (East Africa) as they do not harbour M. leprae DNA. Front. Med. 10:1238914. doi: 10.3389/fmed.2023.1238914
Edited by:
Sebastian Vernal, University of São Paulo, BrazilReviewed by:
Mallika Lavania, National Institute of Virology (ICMR), IndiaFilipe Rocha Lima, University of São Paulo, Brazil
Ana Maria Roselino, University of São Paulo, Brazil
Copyright © 2023 Krausser, Chauvaux, Van Dyck-Lippens, Yssouf, Assoumani, Tortosa, de Jong and Braet. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lena Krausser, bGVuYS5rcmF1c3NlckBleHQuaXRnLmJl
 Lena Krausser
Lena Krausser Elien Chauvaux
Elien Chauvaux Magalie Van Dyck-Lippens1
Magalie Van Dyck-Lippens1 Pablo Tortosa
Pablo Tortosa