- 1Department of Pulmonary and Critical Care Medicine, Center of Respiratory Medicine, National Center for Respiratory Medicine, National Clinical Research Center for Respiratory Diseases, China-Japan Friendship Hospital, Beijing, China
- 2Department of Respiratory and Critical Care Medicine, Shengjing Hospital of China Medical University, Shenyang, Liaoning, China
- 3Center for Diagnosis and Management of Pulmonary Vascular Diseases, Department of Cardiology, Fuwai Hospital, Chinese Academy of Medical Sciences (CAMS) and Perceiving, Beijing, China
Background: Sarcopenia often occurs as a comorbidity in many diseases which ultimately affects patient prognosis. However, it has received little attention in patients with idiopathic pulmonary fibrosis (IPF). This systematic review and meta-analysis aimed at determining the prevalence and risk factors of sarcopenia in patients with IPF.
Methods: Embase, MEDLINE, Web of Science, and Cochrane databases were searched using relevant MeSH terms until December 31, 2022. The Newcastle-Ottawa Scale (NOS) was used for quality assessment and data analysis were performed using Stata MP 17.0 (Texas, USA). A random effects model was adopted to account for differences between articles, and the I2 statistic was used to describe statistical heterogeneities. Overall pooled estimates obtained from a random effects model were estimated using the metan command. Forest plots were generated to graphically represent the data of the meta-analysis. Meta-regression analysis was used for count or continuous variables. Egger test was used to evaluate publication bias and, if publication bias was observed, the trim and fill method was used.
Main results: The search results showed 154 studies, and five studies (three cross-section and two cohort studies) with 477 participants were finally included. No significant heterogeneity was observed among studies included in the meta-analysis (I2 = 16.00%) and our study's publication bias is low (Egger test, p = 0.266). The prevalence of sarcopenia in patients with IPF was 26% (95% CI, 0.22–0.31). The risk factors for sarcopenia in patients with IPF were age (p = 0.0131), BMI (p = 0.001), FVC% (p < 0.001), FEV1% (p = 0.006), DLco% (p ≤ 0.001), and GAP score (p = 0.003).
Conclusions: The pooled prevalence of sarcopenia in patients with IPF was 26%. The risk factors for sarcopenia in IPF patients were age, BMI, FVC%, FEV1%, DLco%, and GAP score. It is important to identify these risk factors as early as possible to improve the life quality of patients with IPF.
1. Introduction
Sarcopenia is a disease characterized by progressive and systematic loss of skeletal muscle mass and a decline in muscle quality and function (1). It is recognized as a complication in chronic diseases such as cancer, chronic obstructive pulmonary disease and idiopathic pulmonary fibrosis (IPF) (2, 3). These diseases combined with sarcopenia carries a risk of adverse outcomes, such as physical disability, poor quality of life and death (4). IPF is a chronic, progressive respiratory disease which has been reported to co-occur with sarcopenia (5–9). Previous studies have not given enough attention to sarcopenia in patients with IPF, partly due to the median survival time of patients with IPF only being 2–4 years (10) but with the development of antifibrotic therapy, some patients with IPF may live longer (11, 12). Considering the adverse outcomes that sarcopenia can cause in patients with IPF on life time and life quality, it is necessary to increase the awareness of sarcopenia as a comorbidity.
The aim of this review and meta-analysis was to determine the prevalence of sarcopenia in patients with IPF, and to analyze and explore the risk factors of sarcopenia in patients with IPF.
2. Methods
2.1. Search strategy
This systematic review and meta-analysis have been conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) Guidelines 2020 (13).
An electronic database search was conducted until December 31, 2022 in four electronic databases (PubMed, Embase, Cochrane Library, and Web of Science), mainly using the following subject heading and free-text terms: “Idiopathic Pulmonary Fibroses,” “Pulmonary Fibroses, Idiopathic,” “Idiopathic Fibrosing Alveolitis, Chronic Form,” “Fibrosing Alveolitis, Cryptogenic,” “Fibrocystic Pulmonary Dysplasia,” “Dysplasia, Fibrocystic Pulmonary,” “Fibrocystic Pulmonary Dysplasias,” “Pulmonary Dysplasia, Fibrocystic,” “Cryptogenic Fibrosing Alveolitis,” “Cryptogenic Fibrosing Alveolitides,” “Fibrosing Alveolitides, Cryptogenic,” “Pulmonary Fibrosis, Idiopathic,” “Familial Idiopathic Pulmonary Fibrosis,” “Idiopathic Pulmonary Fibrosis, Familial,” “Usual Interstitial Pneumonia,” “Usual Interstitial Pneumonia,” “Usual Interstitial Pneumonias,” “Interstitial Pneumonitis, Usual,” “Pneumonitides, Usual Interstitial,” “Pneumonitis, Usual Interstitial,” “Usual Interstitial Pneumonitides,” “Usual Interstitial Pneumonitis,” “Sarcopenia,” “Sarcopenias,” “Muscle atrophy,” “Sarcopenic,” “Muscle attenuation,” “Muscle loss,” “Muscle depletion”. Only studies in English were included, and searches were limited to human studies. The detailed search strategies are shown in Supplementary material.
2.2. Selection criteria
The inclusion criteria were as follows: (1) studies reporting the association between sarcopenia and IPF; (2) studies using valid and objective methods to diagnose IPF rather than self-reporting; (3) observational studies (cross-sectional studies and cohorts) or clinical trials (randomized and non-randomized); (4) the prevalence of sarcopenia was reported as a percentage or calculated from the results. The exclusion criteria were as follows: (1) the study type was a review, case report, conference abstract, comment, or editorial; (2) sarcopenia is not clearly defined; and (3) the data were obviously incorrect or incomplete.
2.3. Data extraction
The full texts of relevant studies were reviewed independently by two authors (JL and YL) after titles and abstract screening. Reference lists of relevant articles were manually searched. A cross-check of the following data was performed independently by two authors (JL and YL): first author, publication year, country, study design, sample size, age, sex, BMI, smoking history, diagnostic criteria for sarcopenia, prevalence of sarcopenia, and clinical impact of sarcopenia: spirometry parameters, dyspnea symptoms, exercise capacity, and mortality risk. Any disagreements were the subject of discussion between the two authors or resolution with the help of a third author (XZ).
2.4. Quality assessment
The Newcastle-Ottawa Scale (NOS) was used for cross-section and cohort studies (14). An NOS score of 0–3, 4–6, or 7–9 describes a study as low quality, moderate quality, or high quality, respectively. An independent quality assessment procedure was conducted by two authors (JL and YL), and any disagreements were resolved by a third author (XZ).
2.5. Statistical analysis
Effective sizes were extracted and reported where associations were made between sarcopenia and IPF. Articles included in this meta-analysis need to be reported the prevalence of sarcopenia in patients with IPF, or if the article provided sufficient information to calculate the prevalence of sarcopenia in patients with IPF. Data analyses were performed using Stata MP 17.0 (Texas, USA). A random effects model was used to account for differences between articles and the I2 statistic was used to describe statistical heterogeneities (15). The meta-analysis was graphically represented by forest plots. A meta-regression analyses was used for variables such as sample size, mean age, percentage of males, mean body mass index (BMI), ever smoke, smoking history (packet per year), the percentage value of forced vital capacity (FVC%), the percentage value of first second forced expiratory volume (FEV1%), forced expiratory volume in 1 s/forced volume vital capacity ratio (FEV1/FVC), the percentage value of diffusing capacity for carbon monoxide (DLco%), gender, age, the Gender-Age-Physiology (GAP) score, and the distance of 6-min walk test (6MWD). Moreover, we used meta-regression to explore whether there are differences in comorbidities, such as diabetes, hypertension, malignancy, and cardiac disease. Egger test was used to evaluate publication bias. The trim and fill method was used if publication bias was observed (16).
3. Results
3.1. Search result
Figure 1 shows a flow chart of the preferred reporting items for systematic reviews and meta-analysis of selected articles. The four databases yielded 154 potential articles. After removing duplicate articles, 122 progressed to title and abstract screening. Of these, 106 articles were excluded, resulting in 16 articles for full-text screening. Ultimately, five publications with 477 participants were included. The overall quality of the included studies was “high” and “moderate” and the prevalence of sarcopenia was reported in all five studies.
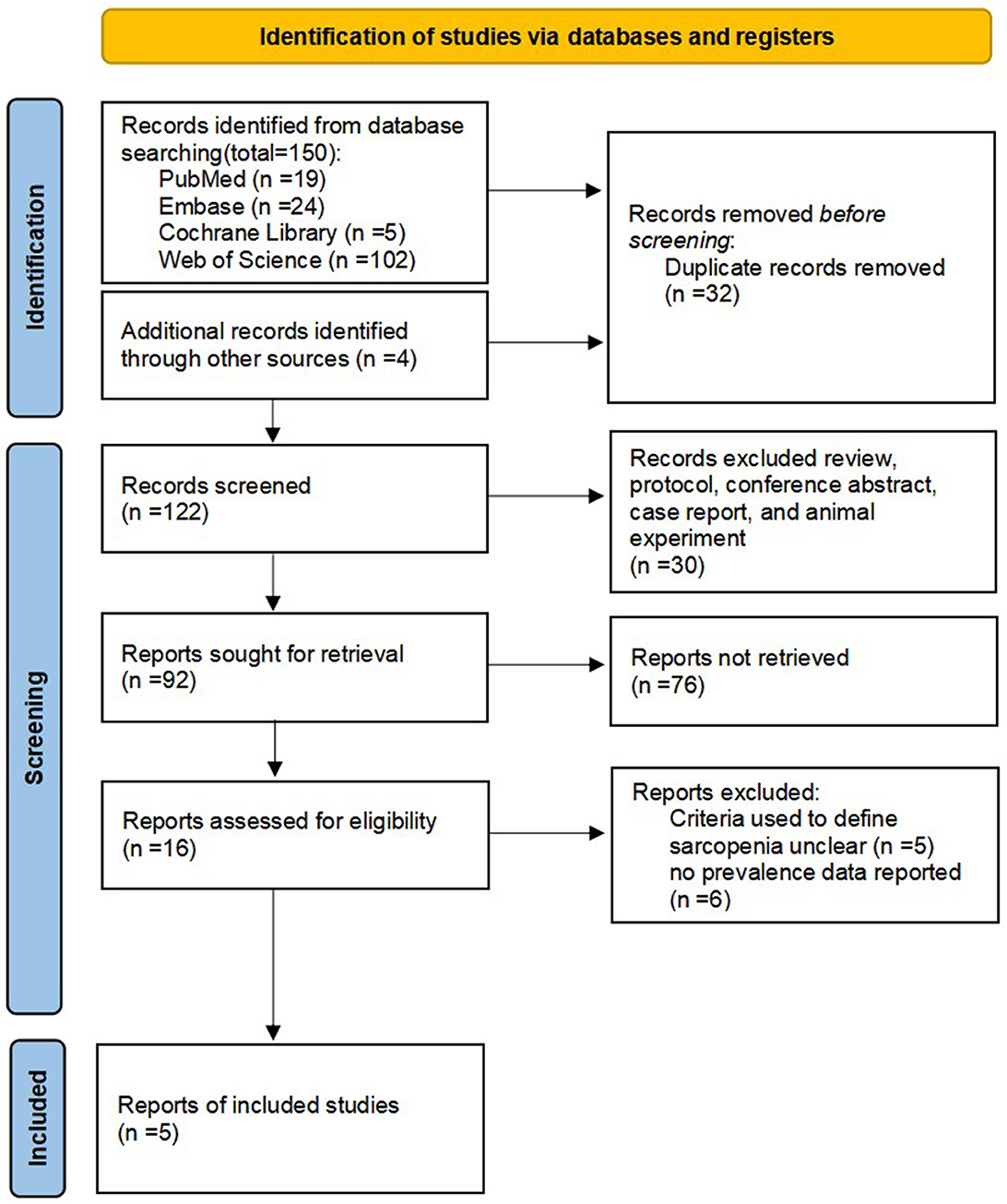
Figure 1. Preferred reporting items for systematic reviews and meta-analyses flow diagram of article selection.
Table 1 gives details on the included studies and their sample size, which ranged from 41 to 180. The mean or median age of the study participants ranged from 69.1 to 74.6 years old. There are two cohort and three cross-section publications included in our study. The diagnostic criteria used in the studies were as follows: the lowest quartile of the muscle index at T4 (T4MI), and the lowest quartile of cross-sectional area of the pectoralis muscles (PMcas), the European Working Group on Sarcopenia in Older People 2019 (EWGSOP2 2019) (17), and the Asian Working Group for Sarcopenia 2019 (AWGS 2019) (18). Most of the studies were conducted in Asia (four studies). A summary of the diagnostic criteria used to assess sarcopenia in these included studies is presented in Table 2.
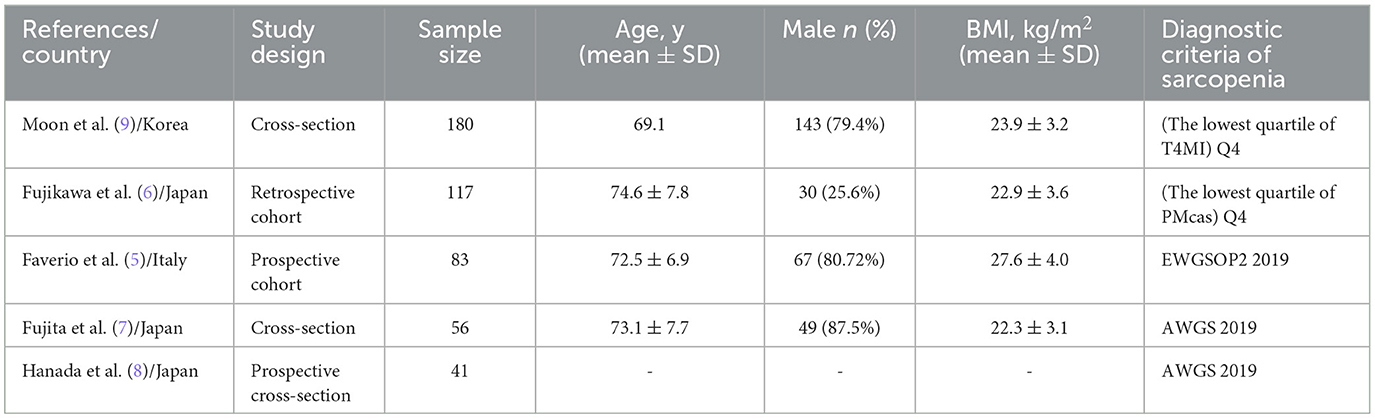
Table 1. Characteristic of the included studies regarding the prevalence of sarcopenia in subjects with idiopathic pulmonary fibrosis.
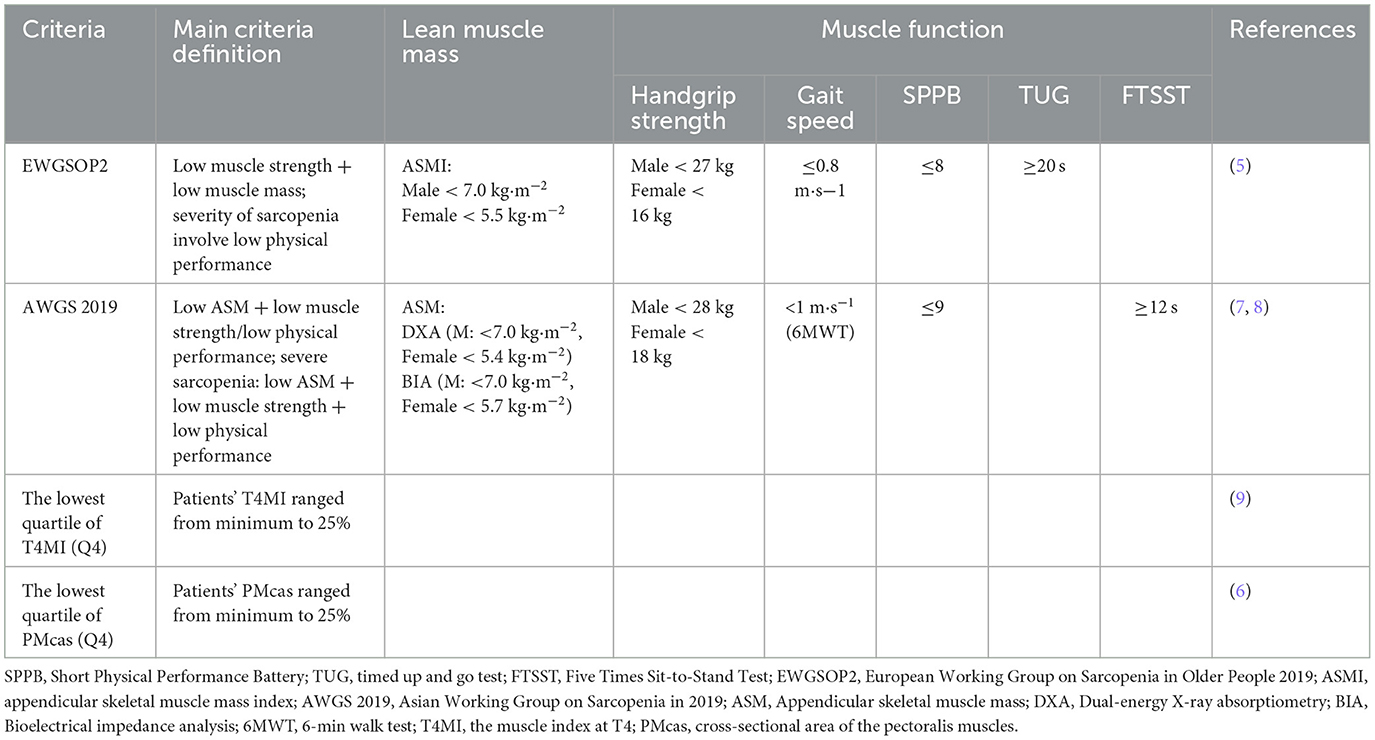
Table 2. Criteria and cut-off points to diagnose sarcopenia in individuals with IPF in the different studies.
3.2. Meta-analysis
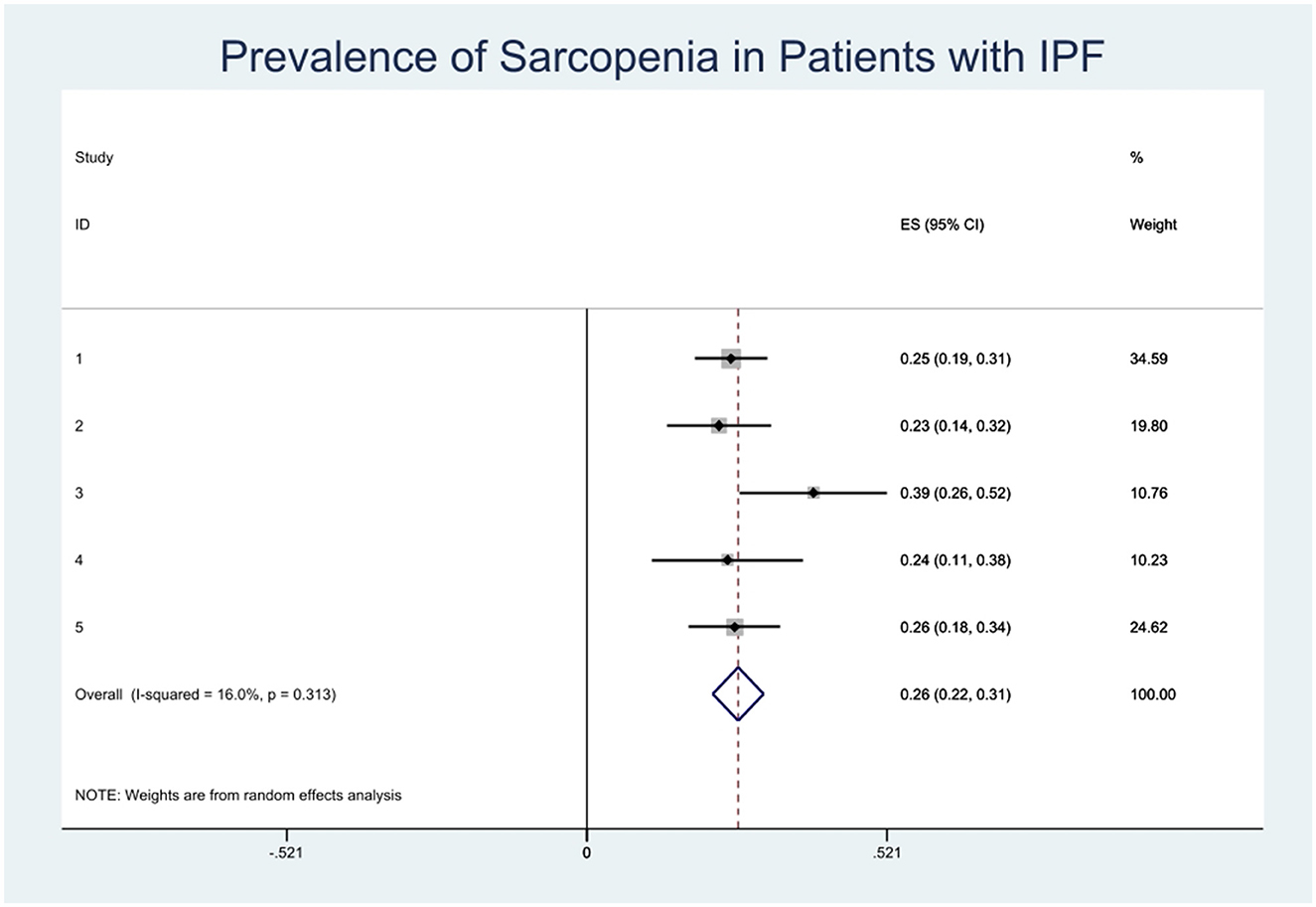
The combined prevalence of sarcopenia in patients with IPF was 26% (95% CI, 0.22–0.31). No significant heterogeneity was observed among the studies included in this meta-analysis (I2 = 16.0%) (Figure 2). We carried out sensitivity analysis on included articles and the group mean of four articles fell within the 95% confidence interval of the population, indicating that only one of the five groups of data included had a different impact on the overall results (Figure 3).
In the meta-regression analysis, age, BMI, FVC%, FEV1%, DLco%, and GAP score were associated with sarcopenia prevalence among patients with IPF (p < 0.05). Demographically, patients with sarcopenia have a higher mean age (p = 0.0131) and lower BMI (p = 0.001); In terms of pulmonary function test results, patients with sarcopenia had lower FVC% (p < 0.001), FEV1% (p = 0.006), and DLco% (p < 0.001). Moreover, patients with sarcopenia were more likely to have a higher GAP score (p = 0.003). However, no significant differences were observed between these two groups (IPF patients with sarcopenia, and IPF patients without sarcopenia) with regards to gender (p = 0.630), ever smoke (p = 0.063), smoking history (pack/year) (p = 0.109), FEV1/FVC (p = 0.069), or 6MWD (p = 0.189). After analyzing the available data, we did not find which comorbidities were more common in IPF patients with sarcopenia, no significant difference was found in terms of diabetes (p = 0.995), hypertension (p = 0.44), malignancy (p = 0.554), and cardiovascular disease (p = 0.554).
The results of Egger test showed that the publication bias is low (p = 0.2660).
4. Discussion
This meta-analysis demonstrated that the overall prevalence of sarcopenia in patients with IPF is 26%, and the all-inclusive prevalence of sarcopenia in patients with IPF ranged from 23 to 39%. The highest was observed in a study by Fujita et al. (7) from Japan. The group mean was outside the 95% confidence interval of the population in sensitivity analysis. It is known that the prevalence of sarcopenia increases with age, but in the Fujita et al. study (7) the mean age was older than others, so differences in the included patients' characteristics led to the high prevalence.
We found certain deficiencies in the included studies. First, they used different diagnostic criteria in identifying sarcopenia which could affect the prevalence. A meta-analysis also explored definition can affect the prevalence of sarcopenia (19). Second, we could not acquire the proportion of each age group of these included studies, the prevalence of IPF and sarcopenia all increases with age (20, 21), retired people used to be older and are less physically active. Third, people who were in serious condition and who cannot take pulmonary function or muscle mass tests were not represented in these studies. What's more, prevalence also depends on the setting, and it is more often seen in patients in hospital, post-acute care settings or care homes than in the community (22). These differences can lead to an underestimation in the prevalence of sarcopenia in patients with IPF.
In our study, age, BMI, FVC%, FEV1%, DLco%, and GAP score were considered as risk factors of sarcopenia in patients with IPF. We found that older patients with IPF were more likely to have sarcopenia, consistent with a previous study (23), which also suggested that the prevalence of sarcopenia is as high as 15% in patients over the age of 65 and 50% in people over the age of 80. Thus, when faced with aged patients with IPF, sarcopenia should be considered. In terms of lung function, patients with sarcopenia have worse FVC, FEV1, and DLco. Decreased lung function and symptoms of dyspnea can lead to a reduction in physical activity, which may lead to atrophic muscular disorder (24). In turn, the decrease in muscle mass and function can also lead to poor lung function and dyspnea, creating a vicious cycle. In our study, patients in the sarcopenia group had a higher GAP score, which is the same in studies by Faverio et al. (5) and Fujita et al. (7). As GAP score is the most commonly accepted prognostic scoring system, IPF patients with sarcopenia have a higher GAP score and a poorer prognosis (25).
There were no significant differences in gender, ever smoke, smoking history (pack/year), FEV1/FVC, and 6MWD. A previous study (9) divided and analyzed male and female patients separately as male and female patients exhibited significant differences in muscle mass, but we could not do this as the information was insufficient and some criterion for the diagnoses of sarcopenia were different between males and females. A cohort study (26) has showed that IPF is a male dominant disease, and males may develop IPF earlier because they are more exposed to fibrotic triggers. In our included studies, males also account for the majority of patients, but there is no significant difference in the prevalence of sarcopenia in male and female IPF patients. Further studies are needed to access the statistical data and understand the mechanisms that may account for the sex differences (27). The 6MWD is commonly used as a test to objectively assess the functional exercise capacity of patients with moderate to severe lung disease (28). In patients with IPF, decreased pulmonary diffusion function has a strong impact on exercise tolerance, while the impact of reduced muscle mass and function on exercise tolerance is negligible.
Chronic diseases were recognized as risk factors of sarcopenia. However, we did not found significant difference in terms of diabetes, hypertension, malignancy, and cardiac disease between these two groups (IPF patients with sarcopenia and IPF patients without sarcopenia). This may be due to the different types of diseases targeted by each study, and there is still not enough relevant data.
As for anti-fibrosis drugs (such as nintedanib and pirfenidone) on sarcopenia, statistical analysis could not be made in the enrolled studies, because the definitions of treatment were not clear enough. The use of nintedanib or pirfenidone can lead to anorexia and gastrointestinal disturbance (29), which may effect anti-fibrosis therapy and nutritional health in patients with IPF. A recent study by West et al. declared that adverse events after inhaled pirfenidone were all mild or moderate (30), which indicated that inhaled pirfenidone may lessen the adverse impact of anti-fibrotic therapy.
In sarcopenia, nutrient supplementation have been shown to be effective interventions. Nutritional supplementation have been shown to benefit muscle strength and exercise tolerance in patients with chronic obstructive pulmonary disease (31). An RCT trial has demonstrated that a vitamin D and leucine-enriched whey protein oral nutritional supplement can improve muscle mass and lower-extremity function among sarcopenic older adults (32). Another study showed that astaxanthin improves muscle strength in healthy elderly in addition to the elevation in endurance and walking distance (33). It seems that additional nutritional supplements are also beneficial for IPF patients to prevent sarcopenia. Further studies are needed to explore the types, dosages, and safety of nutrient supplements.
In our study, we found no significant difference in the prevalence of sarcopenia in patients with IPF among the included studies and the publication bias was low which indicate the included studies have good homogeneity. Considering the physical condition of patients who can participant in these clinical trials were fair (mean FVC% 71.34–86%, mean DLco% 46.8–71%, mean 6MWD 352.77 ± 97.65 m), it is easy to under-estimate the prevalence of sarcopenia in patients with IPF for enrollment studies. For this reason, we should pay more attention to these risk factors in patients with IPF to identify sarcopenia as early as possible.
5. Limitations
Our study has some limitations. Firstly, the sample size was small and all the information was collected in hospital settings. Secondly, there is no unified approach to diagnose sarcopenia among different races and populations. Thirdly, the current evidence was insufficient to infer prognosis.
Given these limitations, these results should be interpreted with caution. When assessing the risk of sarcopenia in patients with IPF, differences between study settings (i.e., community residents vs. patients in hospitals or nursing care facilities) should be considered. Larger multiethnic, prospective cohort studies should be designed to evaluate sarcopenia prevalence in patients with IPF.
6. Conclusion
This study pooled prevalence of sarcopenia in patients with IPF is 26% and the risk factors for sarcopenia in patients with IPF were age, BMI, FVC%, FEV1%, DLco%, and GAP score. Further researches are needed to determine the relationship between sarcopenia and IPF, and to ensure the development of personalized assessment and treatment strategies.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
GH conceived this manuscript and was responsible for conceptualization and study design. JL, YL, and XZ conducted the database search, study evaluation, and data extraction. JL and YL conducted the statistical analysis. JL, YL, MD, RT, QZ, YB, JM, and ZW participated in the manuscript writing and revising. All authors interpreted the data analyses, read, and approved the final manuscript.
Funding
This study was supported by National High Level Hospital Clinical Research Funding (2022-NHLHCRF-LX-01), the Elite Medical Professionals Project of China-Japan Friendship Hospital (No. ZRJY2021-BJ08), and the Non-profit Central Research Institute Fund of Chinese Academy of Medical Sciences (No. 2020-PT320-001). Funding information for this article has been deposited with the Crossref Funder Registry.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2023.1187760/full#supplementary-material
References
1. Nishikawa H, Asai A, Fukunishi S, Nishiguchi S, Higuchi K. Metabolic syndrome and sarcopenia. Nutrients. (2021) 13:519. doi: 10.3390/nu13103519
2. Cruz-Jentoft AJ, Sayer AA. Sarcopenia. Lancet. (2019) 393:2636–46. doi: 10.1016/S0140-6736(19)31138-9
3. Hou G, Kang J. More attention to comprehensive assessment and individualized therapy of chronic obstructive pulmonary disease. J Transl Int Med. (2015) 3:39–42. doi: 10.1515/jtim-2015-0001
4. Cruz-Jentoft AJ, Bahat G, Bauer J, Boirie Y, Bruyère O, Cederholm T, et al. Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing. (2019) 48:16–31. doi: 10.1093/ageing/afy169
5. Faverio P, Fumagalli A, Conti S, Madotto F, Bini F, Harari S, et al. Sarcopenia in idiopathic pulmonary fibrosis: a prospective study exploring prevalence, associated factors and diagnostic approach. Respir Res. (2022) 23:228. doi: 10.1186/s12931-022-02159-7
6. Fujikawa T, Kondo S, Saito T, Inoue T, Otake K, Misu S, et al. Impact of sarcopenia defined by carina-level skeletal muscle mass on the long-term prognosis of patients with idiopathic pulmonary fibrosis. Respir Med Res. (2022) 82:100965. doi: 10.1016/j.resmer.2022.100965
7. Fujita K, Ohkubo H, Nakano A, Mori Y, Fukumitsu K, Fukuda S, et al. Frequency and impact on clinical outcomes of sarcopenia in patients with idiopathic pulmonary fibrosis. Chron Respir Dis. (2022) 19:14799731221117298. doi: 10.1177/14799731221117298
8. Hanada M, Sakamoto N, Ishimoto H, Kido T, Miyamura T, Oikawa M, et al. A comparative study of the sarcopenia screening in older patients with interstitial lung disease. BMC Pulm Med. (2022) 22:45. doi: 10.1186/s12890-022-01840-3
9. Moon SW, Choi JS, Lee SH, Jung KS, Jung JY, Kang YA, et al. Thoracic skeletal muscle quantification: low muscle mass is related with worse prognosis in idiopathic pulmonary fibrosis patients. Respir Res. (2019) 20:35. doi: 10.1186/s12931-019-1001-6
10. American Thoracic Society. Idiopathic pulmonary fibrosis: diagnosis and treatment. International consensus statement. American Thoracic Society (ATS), and the European Respiratory Society (ERS). Am J Respir Crit Care Med. (2000) 161:646–64. doi: 10.1164/ajrccm.161.2.ats3-00
11. Naoi H, Suzuki Y, Mori K, Aono Y, Kono M, Hasegawa H, et al. Impact of antifibrotic therapy on lung cancer development in idiopathic pulmonary fibrosis. Thorax. (2022) 77:727–30. doi: 10.1136/thoraxjnl-2021-218281
12. Karampitsakos T, Diep PP, Loth DW, Nadeem I, Khurtsidze E, Wijsenbeek MS, et al. ERS International Congress 2022: highlights from the Interstitial Lung Diseases Assembly. ERJ Open Res. (2023) 9:2022. doi: 10.1183/23120541.00584-2022
13. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. (2021) 372:n71. doi: 10.1136/bmj.n71
14. Ma LL, Wang YY, Yang ZH, Huang D, Weng H, Zeng XT. Methodological quality (risk of bias) assessment tools for primary and secondary medical studies: what are they and which is better? Mil Med Res. (2020) 7:7. doi: 10.1186/s40779-020-00238-8
15. Cumpston M, Li T, Page MJ, Chandler J, Welch VA, Higgins JP, et al. Updated guidance for trusted systematic reviews: a new edition of the Cochrane Handbook for Systematic Reviews of Interventions. Cochrane Database Syst Rev. (2019) 10:Ed000142. doi: 10.1002/14651858.ED000142
16. Duval S, Tweedie R. Trim and fill: a simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics. (2000) 56:455–63. doi: 10.1111/j.0006-341X.2000.00455.x
17. Cruz-Jentoft AJ, Bahat G, Bauer J, Boirie Y, Bruyère O, Cederholm T, et al. Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing. (2019) 48:601. doi: 10.1093/ageing/afz046
18. Chen LK, Woo J, Assantachai P, Auyeung TW, Chou MY, Iijima K, et al. Asian working group for sarcopenia: 2019 consensus update on sarcopenia diagnosis and treatment. J Am Med Dir Assoc. (2020) 21:300–7.e2. doi: 10.1016/j.jamda.2019.12.012
19. Tseng CW, Wang KL, Fu PK, Huang CY, Hsieh TY, Hsieh CW, et al. GAP score and CA-153 associated with one-year mortality in anti-MDA-5 antibody-positive patients: a real-world experience. J Clin Med. (2021) 10:241. doi: 10.3390/jcm10225241
20. Kitamura A, Seino S, Abe T, Nofuji Y, Yokoyama Y, Amano H, et al. Sarcopenia: prevalence, associated factors, and the risk of mortality and disability in Japanese older adults. J Cachexia Sarcopenia Muscle. (2021) 12:30–8. doi: 10.1002/jcsm.12651
21. Martinez FJ, Collard HR, Pardo A, Raghu G, Richeldi L, Selman M, et al. Idiopathic pulmonary fibrosis. Nat Rev Dis Primers. (2017) 3:17074. doi: 10.1038/nrdp.2017.74
22. Kim M, Won CW. Prevalence of sarcopenia in community-dwelling older adults using the definition of the European Working Group on Sarcopenia in Older People 2: findings from the Korean Frailty and Aging Cohort Study. Age Ageing. (2019) 48:910–6. doi: 10.1093/ageing/afz091
23. Cohen S, Nathan JA, Goldberg AL. Muscle wasting in disease: molecular mechanisms and promising therapies. Nat Rev Drug Discov. (2015) 14:58–74. doi: 10.1038/nrd4467
24. Wall BT, Dirks ML, van Loon LJ. Skeletal muscle atrophy during short-term disuse: implications for age-related sarcopenia. Ageing Res Rev. (2013) 12:898–906. doi: 10.1016/j.arr.2013.07.003
25. Lee SH, Kim SY, Kim DS, Kim YW, Chung MP, Uh ST, et al. Predicting survival of patients with idiopathic pulmonary fibrosis using GAP score: a nationwide cohort study. Respir Res. (2016) 17:131. doi: 10.1186/s12931-016-0454-0
26. Sesé L, Nunes H, Cottin V, Israel-Biet D, Crestani B, Guillot-Dudoret S, et al. Gender differences in idiopathic pulmonary fibrosis: are men and women equal? Front Med. (2021) 8:713698. doi: 10.3389/fmed.2021.713698
27. Awano N, Inomata M, Kuse N, Tone M, Yoshimura H, Jo T, et al. Quantitative computed tomography measures of skeletal muscle mass in patients with idiopathic pulmonary fibrosis according to a multidisciplinary discussion diagnosis: a retrospective nationwide study in Japan. Respir Investig. (2020) 58:91–101. doi: 10.1016/j.resinv.2019.11.002
28. Agarwala P, Salzman SH. Six-minute walk test: clinical role, technique, coding, and reimbursement. Chest. (2020) 157:603–11. doi: 10.1016/j.chest.2019.10.014
29. Faverio P, Bocchino M, Caminati A, Fumagalli A, Gasbarra M, Iovino P, et al. Nutrition in patients with idiopathic pulmonary fibrosis: critical issues analysis and future research directions. Nutrients. (2020) 12:131. doi: 10.3390/nu12041131
30. West A, Chaudhuri N, Barczyk A, Wilsher ML, Hopkins P, Glaspole I, et al. Inhaled pirfenidone solution (AP01) for IPF: a randomised, open-label, dose-response trial. Thorax. (2023). doi: 10.1136/thorax-2022-219391
31. Rawal G, Yadav S. Nutrition in chronic obstructive pulmonary disease: a review. J Transl Int Med. (2015) 3:151–4. doi: 10.1515/jtim-2015-0021
32. Bauer JM, Verlaan S, Bautmans I, Brandt K, Donini LM, Maggio M, et al. Effects of a vitamin D and leucine-enriched whey protein nutritional supplement on measures of sarcopenia in older adults, the PROVIDE study: a randomized, double-blind, placebo-controlled trial. J Am Med Dir Assoc. (2015) 16:740–7. doi: 10.1016/j.jamda.2015.05.021
Keywords: IPF, sarcopenia, risk factors, review, meta-analysis
Citation: Li J, Lu Y, Deng M, Tong R, Zhang Q, Bian Y, Miao J, Wang Z, Zhou X and Hou G (2023) Prevalence and risk factors of sarcopenia in idiopathic pulmonary fibrosis: a systematic review and meta-analysis. Front. Med. 10:1187760. doi: 10.3389/fmed.2023.1187760
Received: 16 March 2023; Accepted: 16 May 2023;
Published: 08 June 2023.
Edited by:
Paschalis Steiropoulos, Democritus University of Thrace, GreeceReviewed by:
Fotios Drakopanagiotakis, Democritus University of Thrace, GreeceTheodoros Karampitsakos, University of South Florida, United States
Copyright © 2023 Li, Lu, Deng, Tong, Zhang, Bian, Miao, Wang, Zhou and Hou. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Gang Hou, aG91Z2FuZ2NtdUAxNjMuY29t
†These authors have contributed equally to this work
 Jiaye Li
Jiaye Li Ye Lu
Ye Lu Mingming Deng
Mingming Deng Run Tong
Run Tong Qin Zhang
Qin Zhang Yiding Bian
Yiding Bian Jinrui Miao1
Jinrui Miao1 Xiaoming Zhou
Xiaoming Zhou Gang Hou
Gang Hou