
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Med., 23 September 2022
Sec. Ophthalmology
Volume 9 - 2022 | https://doi.org/10.3389/fmed.2022.973025
 Jianbo Mao1,2
Jianbo Mao1,2 Nuo Chen2
Nuo Chen2 Shian Zhang1
Shian Zhang1 Yuyan Fang2
Yuyan Fang2 Zicheng Zheng3
Zicheng Zheng3 Sulan Wu2
Sulan Wu2 Xin Ye2
Xin Ye2 Yijing Chen2
Yijing Chen2 Yiqi Chen1,2
Yiqi Chen1,2 Lijun Shen1,2*
Lijun Shen1,2*Purpose: To investigate the associations between cytokine levels in the aqueous humor (AH) and hyperreflective foci (HF) on spectral-domain optical coherence tomography (SD-OCT) in neovascular age-related macular degeneration (nAMD) and polypoidal choroidal vasculopathy (PCV).
Methods: The prospective study included 63 eyes with nAMD, 44 with PCV, and 43 with cataracts (Controls). AH samples were obtained before anti-vascular endothelial growth factor (VEGF) therapy or cataract surgery. Cytokines interleukin 6 (IL-6), IL-8, IL-10, interferon-inducible protein 10 (IP-10), monocyte chemotactic protein 1 (MCP-1), and VEGF were measured by multiplex bead assay. Best-corrected visual acuity (BCVA), central macular thickness (CMT), and the number of HF were evaluated at baseline and 1 month after anti-VEGF treatment.
Results: No significances difference in IL-6 and IL-8 levels were noted among the three groups (P = 0.370 and P = 0.067). VEGF, IP-10, and IL-10 levels were significantly higher in nAMD and PCV groups than in Controls (all P < 0.05). In nAMD, HF was positively correlated with VEGF (rs = 0.300, P = 0.025) and in eyes with HF group, VEGF and IL-10 were significantly higher than those without HF (P = 0.008 and P = 0.022). In PCV, no correlation was observed between HF and cytokines (all P > 0.05). After anti-VEGF treatment, patients with HF in nAMD and PCV were predisposed to worse visual outcomes (P = 0.022 and P = 0.015) and a significantly greater reduction in CMT (P = 0.001 and P = 0.057). And nAMD patients with HF were more sensitive to anti-VEGF treatment than those without HF (P = 0.029).
Conclusions: In the nAMD group, HF was positively correlated with VEGF. Patients in nAMD with HF had elevated levels of VEGF and IL-10 and responded favorably to anti-VEGF. HF might serve as an inflammatory biomarker and a predictive factor for therapeutic efficacy in patients with nAMD.
Age-related macular degeneration (AMD) is a progressive chronic disease that causes visual impairment and severe vision loss (1). It is estimated that the global prevalence of AMD is 190 million persons by 2020, and will increase to 288 million persons in 2040 (2). AMD is mainly divided into dry (also known as non-vascular, non-exudative and atrophic) and wet (also known as neovascular and exudative) forms, with wet AMD causing the most severe vision loss (1, 2). Anti-VEGF therapy is now the most commonly used treatment for AMD with satisfactory outcomes. Polypoidal choroidal vasculopathy (PCV), a vascular disease of the choroid, is considered to be a common subtype of nAMD. However, recent studies in the fields of genetics, proteomics, and imaging have further clarified the distinction between nAMD and PCV (3). Previous research demonstrated that inflammation was likely to play a role in the pathogenesis of AMD and PCV (4, 5). Various local and systemic inflammatory molecules, including cytokines, have been proposed as biomarkers of AMD but at present, no specific and reliable marker has been found (6).
Hyperreflective foci (HF) was visualized as discrete, well-circumscribed lesions with greater reflectivity than the retinal pigment epithelium (RPE) band on spectral-domain optical coherence tomography (SD-OCT), which was considered a structural biomarker associated with disease progression, treatment response, and prognosis of several retinal diseases, including AMD (7), diabetic macular edema (8) and central serous chorioretinopathy (9). The origin of HF is not clear, but many studies supported the hypothesis that HF was as aggregates of activated microglial cells in the retina and possible in vivo biomarker of local inflammation (10, 11). Preliminary studies had shown that HF was correlated with complement factors upregulation in aqueous humor (AH) of patients with early stages of AMD (12). And the presence of HF might reflect a degree of local inflammation including complement activation in the eye (12). However, the association between HF and intraocular cytokines of patients with AMD and PCV was rarely reported. Better understanding of the relationship between HF and cytokines in AH of patients with AMD and PCV could provide new insights into the distinct pathophysiological processes between AMD and PCV. And this may provide individualized therapeutic strategies and prognosis of diseases.
In this study, we investigated the associations between AH concentrations of inflammatory cytokines and the number of HF on SD-OCT in patients with nAMD and PCV. Moreover, we evaluated the treatment responses to anti-VEGF injections in nAMD and PCV eyes with or without HF to explore the role of HF in treatment efficacy.
This prospective study was reviewed and performed at Eye Hospital of Wenzhou Medical University between May 2019 and January 2021, and the study protocol (No. 121-K107-01) was approved by the ethics committee of The Eye Hospital of Wenzhou Medical University. All of the procedures adhered to the tenets of the Declaration of Helsinki. Written informed consent was obtained from all subjects.
The subjects included 63 with nAMD (44 males, 19 females), 44 patients with PCV (28 males, 16 females), and 43 controls (14 males, 29 females) without any sight-threatening disease except for cataract who were scheduled for cataract surgery.
The inclusion criteria were (1) Patients with nAMD and PCV were diagnosed specifically with fluorescein angiography (FA) and indocyanine green angiography (ICGA), (2) nAMD and PCV patients were treatment-naive and scheduled to receive the anti-VEGF intravitreal injection, (3) central macular thickness (CMT) >250 μm, and (4) Patients aged over 18 years. The exclusion criteria were (1) Subjects who have undergone intraocular surgery or procedures within the last 6 months, (2) Patients who were diagnosed with systemic inflammatory medical conditions or malignancies, and (3) Patients suffering from other eye disorders such as diabetic retinopathy, retinal vascular occlusion or retinal detachment except for the included diseases in the study. The differential diagnosis of nAMD and PCV was based mainly on the ICGA findings. Eyes with nAMD showed classic choroidal neovascularization (CNV) or occult CNV with FFA, with no polypoidal lesions in ICGA. According to the origin and location of neovascular vessels, CNV is classified as type 1 CNV (beneath the RPE) and type 2 CNV (above the RPE) in the nAMD group (13). PCV presents as polypoid vasodilation with or without superficial choroidal vascular abnormalities.
Before anti-VEGF injection or cataract surgery, all patients underwent an extensive ophthalmologic examination that included best-corrected distance visual acuity (recorded as the logarithm of the minimum angle of resolution, logMAR), slit-lamp biomicroscopy, intraocular pressure measurement, and dilated funduscopic examination.
Patients initially diagnosed with nAMD or PCV who were in the acute phase received the anti-VEGF drugs ranibizumab (0.5 mg/0.05 ml, Lucentis; Novartis Pharma AG, Basel, Switzerland) or conbercept (0.5 mg/0.05 ml, KH902; Biotech Co., Ltd., Sichuan, China) therapy. Retreatment or change of treatment regimen was considered according to clinical response at a 1-month follow-up. AH samples were collected before cataract surgery in the control group or before intravitreal anti-VEGF drugs (ranibizumab or conbercept) injection into the eyes with nAMD or PCV. After topical anesthesia, ~0.05–0.1 ml of AH was withdrawn at the corneal limbus using a tuberculin syringe with a 30-gauge needle. The samples were immediately frozen and stored at −80°C until analysis of the cytokines. Luminex200 (BIO-RAD, Hercules, CA, USA) was used for the detection of VEGF, interleukin 6 (IL-6), IL-8, IL-10, interferon-inducible protein 10 (IP-10), and monocyte chemotactic protein 1 (MCP-1). The concentration of AH cytokine was calculated and presented as pg/ml from the standard curve of each specific cytokine provided by the kit (LXSAHM-06, RnD).
All patients with nAMD and PCV were imaged using Heidelberg Spectralis (Heidelberg Engineering, Heidelberg, Germany) before intravitreal anti-VEGF injections in the nAMD and PCV groups. A volume scan comprising 18 horizontal B-scans covering a 6 × 6 mm area of the macula region within the fovea centered was obtained using SD-OCT. Central macular thickness (CMT) was automatically calculated as the average retinal thickness within a circle having a 500-mm radius, centered on the fovea, based on the volume scan data containing the target circle area. The change in CMT (ΔCMT) at 1 month following anti-VEGF injection was calculated by subtracting the post-injection thickness from the baseline thickness. The CMTreduction_ratio was calculated as the ratio of ΔCMT to the remaining CMT, which itself was calculated as the baseline CMT minus 250 μm: (14, 15).
The CMTreduction_ratio responses to anti-VEGF therapy were classified as “sensitive” in which the CMTreduction_ratio was ≥30% or as “non-sensitive” in which the CMTreduction_ratio was < 30%.
nAMD or PCV eyes were divided into two groups based on the presence of HF, that is, HF positive (+) group and HF negative (–) group for analysis of the clinical characteristics. The presence of HF was defined as the presence of small focal hyperreflective material (as hyperreflective as RPE) observed in at least one available scan (16). The number of HF was manually counted within the 6-mm area centered on the fovea in the fovea-spanning horizontal raster scan (Figure 1). Two independent observers separately counted the number of HF, and the values averaged. The diameter of HF was limited to within a range of 20–50 μm to exclude counting small noise signals as HF and to prevent the inclusion of large confluent HF clumps, which are present as typical hard exudates in fundus photography.
All statistical analyses were performed using SPSS 26.0 for Windows (SPSS Inc., Chicago, IL, USA). Quantitative variables were tested for normal distribution by the Shapiro–Wilk test. Variables displaying skewed distribution were expressed as the median and interquartile range (IQR). Groups of discrete variables were compared by means of the Mann–Whitney U test or Kruskal–Wallis nonparametric analysis of variance. For the Kruskal–Wallis test, P-values were adjusted by the Bonferroni correction for multiple comparisons of continuous variables. Categorical variables were analyzed using the Chi-square test. Spearman rho tests were used for correlation analyses. A probability value (P-value) < 0.05 was considered statistically significant.
The average age of the patients with nAMD, PCV, and control was 70.0 (IQR, 61.0–82.0), 67.0 (IQR, 61.3–75.0), and 72.0 years (IQR, 64.0–76.0), respectively (Table 1). There was no statistical difference in mean age among the three groups (P = 0.200, Table 1). The male and female ratios were 44:19 and 28:16 in nAMD and PCV patients, respectively, and 14:29 in controls. Gender showed statistical difference among the three groups (P < 0.001). However, no significant differences were noted in six cytokines levels between male and female (all P > 0.05, Supplementary Table S1).
There were no significant differences in the levels of IL-6 and IL-8 among the three groups (P = 0.370 and P = 0.067, Table 1). The levels of VEGF (P = 0.002 and P = 0.025), IP-10 (P < 0.001 and P < 0.001), and IL-10 (P < 0.001 and P < 0.001) in nAMD and PCV groups were significantly higher than in controls. And MCP-1 levels were significantly elevated in the PCV group than in nAMD group (P = 0.021), but they did not differ from the control group (P = 0.102).
Based on baseline measurements of BCVA and SD-OCT parameters, there was no significant difference in BCVA among the three groups (P = 0.063, Table 1). CMT baseline value was significantly higher in the PCV group than in nAMD group (P = 0.002); however, the number of HF in patients with nAMD and PCV was not different (P = 0.677).
The AH levels of VEGF and IL-10 [median: 47.93 and 1.05 pg/ml, respectively, interquartile range (IQR), 32.13–64.47 and 0.82–1.22, respectively] were significantly higher in the nAMD patients with HF compared to those without HF (P = 0.008 and P = 0.022, respectively, Table 2). There were no significant differences in concentrations of AH cytokines IL-6, IP-10, MCP-1, or IL-8 between the nAMD patients with HF and without HF (all P > 0.05). Similarly, none of the cytokine levels investigated in the present study were different between the PCV patients with HF and without HF groups (all P > 0.05, Table 2).
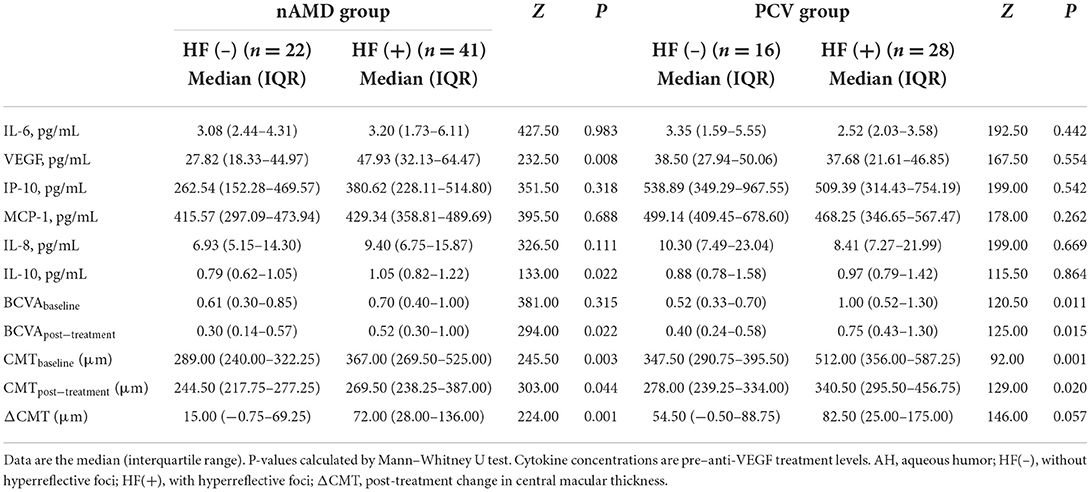
Table 2. AH concentrations (pg/mL) of cytokines, BCVA, and SD-OCT parameters in eyes with or without HF in the nAMD and PCV groups.
Baseline BCVA for nAMD patients without HF (median, 0.61; IQR, 0.30–0.85) was not significantly different from those with HF (median, 0.70; IQR, 0.40–1.00; P = 0.315, Table 2). However, for PCV patients, the baseline BCVA for those without HF, 0.52 (0.33–0.70), was better than for those with HF (P < 0.001). The post-treatment BCVA for nAMD and PCV patients with HF, (median, 0.52 and 0.75, respectively; IQR, 0.30–1.00 and 0.43–1.30, respectively), were worse than for those without HF (P = 0.022 and P = 0.015 respectively). The baseline CMT for nAMD and PCV patients with HF, (median, 367.00 and 512.00 μm; IQR, 269.50–525.00 and 356.00–587.25, respectively), was thicker than for those without HF (P = 0.003 and P = 0.001, respectively). Following anti-VEGF treatment, the decrease in CMT (ΔCMT) for patients with HF in the nAMD and PCV groups, 72.00 (28.00–136.00) and 82.50 μm (25.00–175.00) respectively, was greater than for those without HF (P = 0.001 and P = 0.057 respectively).
In the nAMD group, HF was correlated with the concentration of VEGF in AH (rs = 0.300, P = 0.025, Table 3) and with the CMTbaseline (rs = 0.476, P < 0.001). In the PCV group, HF was not correlated with any of the cytokine concentrations (all P > 0.05); however it was correlated with the BCVAbaseline (rs = 0.407, P = 0.007) and the CMTbaseline (rs = 0.531, P < 0.001). One month after anti-VEGF injection, HF were correlated with BCVApost − treatment (nAMD: rs = 0.298, P = 0.021; PCV: rs = 0.396, P = 0.009), CMTpost − treatment (nAMD: rs = 0.309, P = 0.016; PCV: rs = 0.411, P = 0.006), and the ΔCMT (nAMD: rs = 0.457, P < 0.001, PCV: rs = 0.307, P = 0.045).
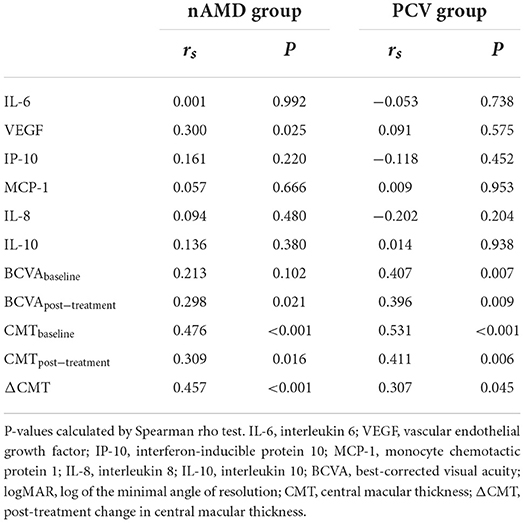
Table 3. Correlation between HF and AH concentrations (pg/mL) of cytokines and clinical characteristics in nAMD and PCV groups.
Different types of CNVs in nAMD had different expression patterns in the baseline AH cytokine levels and in the number of HF (Table 4). The AH levels of VEGF and IL-10 were significantly higher in type 2 CNV than those in type 1 CNV (P = 0.018 and P = 0.019, respectively). In contrast, none of the other cytokine levels, including IL-6, IP-10, MCP-1, or IL-8 were significantly different between nAMD types 1 and 2 CNVs. Moreover, the number of HF was significantly higher in type 2 CNV than that in type 1 CNV (P = 0.022).
Based on changes in the CMTreduction_ratio as a measure of anti-VEGF effectiveness, eyes in which the decrease was more than 30% were considered to be sensitive to anti-VEGF treatment. At 1 month after the treatment, 30 eyes (73.2%) of the nAMD with HF group were highly responsive (Figure 2). In contrast, only 10 eyes (45.5%) of the nAMD without HF group were as responsive. Thus, nAMD eyes with HF were more responsive to anti-VEGF treatment than those without HF (P = 0.029). For PCV, 18 eyes with HF (64.3%) and 10 eyes without HF (62.5%) had a sensitive response to anti-VEGF treatment. Thus, there was no difference in treatment responses to anti-VEGF between PCV eyes with HF and without HF (P = 0.906).
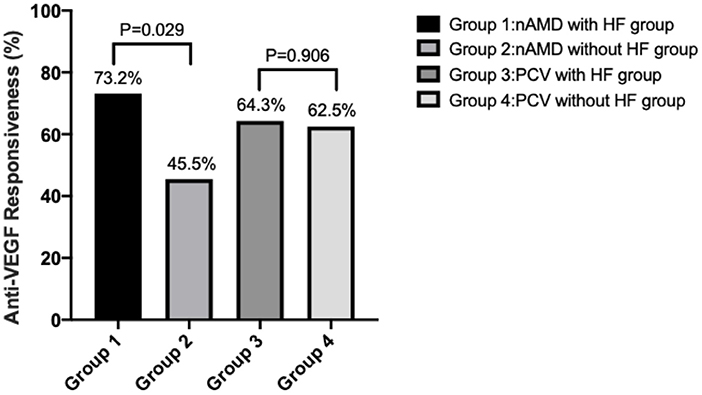
Figure 2. Sensitivity rate of anti-VEGF therapy in eyes with or without HF in the nAMD and PCV groups. Anti-VEGF responsiveness was calculated by CMTreduction_ratio.
The four most salient results emerging from this study were as follows: (1) AH levels of cytokines were differently expressed among three groups in which VEGF, IP-10, and IL-10 levels were higher in both nAMD and PCV groups compared with the control group. (2) In the nAMD group with HF, the AH levels of VEGF and IL-10 were significantly higher than those without HF. And the AH levels of VEGF were positively correlated with the number of HF. After anti-VEGF treatment, nAMD and PCV patients with HF were predisposed to worse visual outcomes and a significantly greater reduction in CMT than those without HF. (3) Compared to nAMD patients with type 1 CNV, those with Type 2 CNV had higher levels of AH VEGF and IL-10. (4) nAMD patients with HF were more sensitive to anti-VEGF treatment than those without HF.
Recent studies have shown that inflammation plays a critical role in the pathogenic progression of AMD (17, 18). Inflammation is a cascade of reactions triggered by the interaction of activated immune cells and secreted cytokines. VEGF is a cytokine of special interest because in current clinical practice intravitreal anti-VEGF injection has become the mainstay of treatment for patients with nAMD and PCV (19). However, there has been no consensus regarding the relative levels of VEGF in patients with nAMD and PCV. Some studies reported that increased VEGF levels in AH were present in both nAMD and PCV patients (20). However, others reached the opposite conclusion, indicating that there were no significant differences between nAMD, PCV, and controls (21, 22). Our data showed that AH VEGF concentrations were markedly increased in patients with nAMD and PCV compared with Controls. Joo et al. (21) and Agrawal et al. (22) reached a quite different conclusion from ours possibly due to the small sample sizes of their studies. Another plausible explanation was that compared with pancreatic diseases such as proliferative diabetic retinopathy, AMD and PCV had localized pathology and limited increase in VEGF production.
In our study, AH IP-10 concentrations were elevated in eyes with either nAMD or PCV. IP-10 was known for its vascular stabilization and anti-fibrotic activity. Previous in vitro studies have shown that IP-10 inhibits VEGF-mediated activation of m-calpain and interferes with newly formed blood vessels through the CXCR3 signaling pathway (23). Boulday et al. (24) reported that VEGF induced over-expression of IP-10 in endothelial cells in vitro and vivo. Considering all of this evidence, it seems that the upregulation of IP-10 was a compensatory response to excessively elevated VEGF. However, this compensatory upregulation of IP-10 was not sufficient to antagonize the angiogenic effect of VEGF in the pathogenesis of nAMD and PCV.
The third chemokine that was protruding elevated in the AH of both nAMD and PCV patients was IL-10. Previous studies have shown that IL-10 promotes angiogenesis by preventing macrophages from invading the choroid or by directly polarizing macrophages to a pro-angiogenic phenotype (25). Nakamura and colleagues found that elevated IL10 levels in the elderly activated STAT3 signaling, induced alternative macrophage activation and pathological angiogenesis (26). Thus, it was not surprising that increased levels of the inflammatory cytokine IL-10 were found in the AH of patients with nAMD and PCV.
MCP-1, as a mediator of inflammation and angiogenesis, was involved in various ocular diseases, such as branch retinal vein occlusion, proliferative diabetic retinopathy, and AMD (27–29). Histopathologic findings reported that arteriosclerotic-like hyalinization of choroidal vessels was characteristic of PCV (30). Several molecular and immunological studies showed that MCP-1 may be involved in the etiology, progression, and prognosis of atherosclerosis (31). This suggested that increased MCP-1 levels may play an essential role in the pathogenesis of PCV. Some investigators have reported that MCP-1 levels in eyes with AMD were significantly higher compared with controls (21); however, others suggested that MCP-1 levels in nAMD were not significantly different from controls (22, 32). In the present study, MCP-1 was elevated in the AH of PCV patients compared with the nAMD group, but not significantly different from the control group. It may be a potential cytokine explaining the difference between nAMD and PCV. Therefore, whether MCP-1 plays a pathogenic role in nAMD and PCV requires further investigation.
Numerous studies had shown that HF originated from activated microglial cells induced by inflammatory responses (16) and was associated with many retinal diseases, including AMD (7), diabetic macular edema (8), and central serous chorioretinopathy (9). We found the AH concentrations of VEGF and IL-10 were significantly elevated in the nAMD patients with HF compared to those without HF. Further, the AH levels of VEGF were positively correlated with the number of HF. Our results supported the hypothesis that eyes with larger amounts of HF at baseline may have a worse inflammatory reaction and more active choroidal neovascularization (33). And severe inflammatory responses are often accompanied by persistent changes in vascular permeability, so the increase in the amount of HF may reflect persistently high VEGF levels. After anti-VEGF treatment, patients with HF in either nAMD or PCV were predisposed to a worse visual outcome but had a significant reduction in CMT. A reasonable explanation for these findings is that anatomical restoration preceded functional improvement during 1 month after the anti-VEGF therapy. Segal et al. reported that the quantity of HF was associated with worst visual outcomes at baseline and at each follow-up visit (34). They hypothesized that eyes with larger amounts of HF at the initial examination may have more severe blood-retinal barrier damage, worse inflammatory reaction, and more active choroidal neovascularization leading to a poorer visual outcome (34).
With FFA and SD-OCT, the different types of nAMD CNVs can be differentiated from one another (35). Type 1 CNV is localized within the sub-RPE space, and type 2 CNV is present within the subretinal space. The VEGF and IL-10 concentrations were higher in the AMD type 2 CNV than in type 1 CNV. Because the AMD type 2 CNV existed above the RPE, cytokines secreted by them could likely spread easily within the vitreous and AH.
Based on the CMTreduction_ratio, nAMD patients with HF were more likely to be sensitive to anti-VEGF treatment compared to those without HF. This finding was consistent with Hsia, who found that the eyes with decreased HF after anti-VEGF treatment might have better visual acuity (36). Thus, it appears that HF might be a biomarker for predicting the effects of anti-VEGF treatment.
There are three limiting factors in our study. The first was the heterogeneity of the data (mixed treatment with ranibizumab and conbercept). Second, the manual counting of HF was time-consuming and lacks precision. Future automated software may be applied for more efficient and precise HF counting. Third, the AH samples maybe not be as valuable as vitreous fluid for detecting cytokine concentrations at the site of pathology in the retina and choroid. However, collecting vitreous samples is more invasive than collecting AH, and therefore not ethically justified for research on human subjects.
In summary, our study confirmed that SD-OCT structural parameters accompanied by differences in AH cytokine concentrations were involved in the pathogenesis of nAMD and PCV. nAMD patients with HF were more likely to respond favorably to anti-VEGF treatment than those without HF. HF could be used as an inflammatory biomarker and a predictive factor for the treatment efficacy in patients with nAMD.
Raw data supporting this study's findings will be made available from the authors upon reasonable request, without undue reservation.
All procedures involving human participants were in accordance with the ethical standards of the institutional and the National Research Committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. The study was approved by the Ethics Committee of The Eye Hospital of Wenzhou Medical University. All patients provided written informed consent for inclusion in the study.
JM, YC, and LS have contributed to conceptualizing, designing the study, and final revision of the manuscript. NC and SZ have contributed to performing the experiment, data analysis, and writing. YF, ZZ, and YC have involved in data collection. SW and XY have organized figures and reference revisions. All authors listed contributed to the manuscript with intellectual input and approved the final version for publication.
This work was financially supported by the Key Project of Zhejiang Medical Science and Technology Plan (WKJ-ZJ-2035) and Medical and Health Science Technology Project of Zhejiang Province (2022KY088).
The authors acknowledges NC, who has made genuine contributions to the manuscripts and SZ who endorses the data and conclusions is appreciated as well.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2022.973025/full#supplementary-material
1. Mitchell P, Liew G, Gopinath B, Wong TY. Age-related macular degeneration. Lancet. (2018) 392:1147–59. doi: 10.1016/S0140-6736(18)31550-2
2. Apte RS. Age-related macular degeneration. N Engl J Med. (2021) 385:539–47. doi: 10.1056/NEJMcp2102061
3. Yanagi Y, Foo VHX, Yoshida A. Asian age-related macular degeneration: from basic science research perspective. Eye. (2019) 33:34–49. doi: 10.1038/s41433-018-0225-x
4. Subhi Y, Krogh Nielsen M, Molbech CR, Oishi A, Singh A, Nissen MH, et al. Plasma markers of chronic low-grade inflammation in polypoidal choroidal vasculopathy and neovascular age-related macular degeneration. Acta Ophthalmol. (2019) 97:99–106. doi: 10.1111/aos.13886
5. Cheung CMG, Lai TYY, Ruamviboonsuk P, Chen S-J, Chen Y, Freund KB, et al. Polypoidal choroidal vasculopathy. Ophthalmology. (2018) 125:708–24. doi: 10.1016/j.ophtha.2017.11.019
6. Minaker SA, Mason RH, Lahaie Luna G, Bapat P, Muni RH. Changes in aqueous and vitreous inflammatory cytokine levels in neovascular age-related macular degeneration: a systematic review and meta-analysis. Acta Ophthalmol. (2021) 99:134–55. doi: 10.1111/aos.14537
7. Fragiotta S, Rossi T, Cutini A, Grenga PL, Vingolo EM. Predictive factors for development of neovascular age-related macular degeneration: a spectral-domain optical coherence tomography study. Retina. (2018) 38:245–52. doi: 10.1097/IAE.0000000000001540
8. Schreur V, Altay L, van Asten F, Groenewoud JMM, Fauser S, Klevering BJ, et al. Hyperreflective foci on optical coherence tomography associate with treatment outcome for anti-VEGF in patients with diabetic macular edema. PLoS ONE. (2018) 13:e0206482. doi: 10.1371/journal.pone.0206482
9. Borrelli E, Zuccaro B, Zucchiatti I, Parravano M, Querques L, Costanzo E, et al. Optical coherence tomography parameters as predictors of treatment response to eplerenone in central serous chorioretinopathy. JCM. (2019) 8:1271. doi: 10.3390/jcm8091271
10. Wu J, Zhang C, Yang Q, Xie H, Zhang J, Qiu Q, et al. Imaging hyperreflective foci as an inflammatory biomarker after anti-VEGF treatment in neovascular age-related macular degeneration patients with optical coherence tomography angiography. BioMed Res Int. (2021) 2021:1–7. doi: 10.1155/2021/6648191
11. Pilotto E, Torresin T, Bacelle ML, De Mojà G, Ferrara AM, Zovato S, et al. Hyper-reflective retinal foci as possible in vivo imaging biomarker of microglia activation in von Hippel-Lindau disease. PLoS ONE. (2022) 17:e0272318. doi: 10.1371/journal.pone.0272318
12. Sitnilska V, Enders P, Cursiefen C, Fauser S, Altay L. Association of imaging biomarkers and local activation of complement in aqueous humor of patients with early forms of age-related macular degeneration. Graefes Arch Clin Exp Ophthalmol. (2021) 259:623–32. doi: 10.1007/s00417-020-04910-6
13. Freund KB, Zweifel SA, Engelbert M. Do we need a new classification for choroidal neovascularization in age-related macular degeneration? Retina. (2010) 30:1333–49. doi: 10.1097/IAE.0b013e3181e7976b
14. Udaondo P, Hernández C, Briansó-Llort L, García-Delpech S, Simó-Servat O, Simó R. Usefulness of liquid biopsy biomarkers from aqueous humor in predicting anti-VEGF response in diabetic macular edema: results of a pilot study. JCM. (2019) 8:1841. doi: 10.3390/jcm8111841
15. Mao J, Zhang S, Zheng Z, Deng X, Liu C, Chen Y, Zhao S, Zhang Y, Shen L. Prediction of anti-VEGF efficacy in diabetic macular oedema using intraocular cytokines and macular optical coherence tomography. Acta Ophthalmol. (2022) 100:e891–8. doi: 10.1111/aos.15008
16. Coscas G, De Benedetto U, Coscas F, Li Calzi CI, Vismara S, Roudot-Thoraval F, et al. Hyperreflective dots: a new spectral-domain optical coherence tomography entity for follow-up and prognosis in exudative age-related macular degeneration. Ophthalmologica. (2013) 229:32–7. doi: 10.1159/000342159
17. Romero-Vazquez S, Llorens V, Soler-Boronat A, Figueras-Roca M, Adan A, Molins B. Interlink between inflammation and oxidative stress in age-related macular degeneration: role of complement factor H. Biomedicines. (2021) 9:763. doi: 10.3390/biomedicines9070763
18. Tan W, Zou J, Yoshida S, Jiang B, Zhou Y. The role of inflammation in age-related macular degeneration. Int J Biol Sci. (2020) 16:2989–3001. doi: 10.7150/ijbs.49890
19. Kim JH, Kim JW, Kim CG, Lee DW. Long-term switching between ranibizumab and aflibercept in neovascular age-related macular degeneration and polypoidal choroidal vasculopathy. Graefes Arch Clin Exp Ophthalmol. (2020) 258:1677–85. doi: 10.1007/s00417-020-04710-y
20. Zhou H, Zhao X, Yuan M, Chen Y. Comparison of cytokine levels in the aqueous humor of polypoidal choroidal vasculopathy and neovascular age-related macular degeneration patients. BMC Ophthalmol. (2020) 20:15. doi: 10.1186/s12886-019-1278-8
21. Joo J-H, Kim H, Shin J-H, Moon SW. Aqueous humor cytokine levels through microarray analysis and a sub-analysis based on optical coherence tomography in wet age-related macular degeneration patients. BMC Ophthalmol. (2021) 21:399. doi: 10.1186/s12886-021-02152-6
22. Agrawal R, Balne PK, Wei X, Bijin VA, Lee B, Ghosh A, et al. Cytokine profiling in patients with exudative age-related macular degeneration and polypoidal choroidal vasculopathy. Invest Ophthalmol Vis Sci. (2019) 60:376. doi: 10.1167/iovs.18-24387
23. Bodnar RJ, Yates CC, Wells A. IP-10 blocks vascular endothelial growth factor-induced endothelial cell motility and tube formation via inhibition of calpain. Circ Res. (2006) 98:617–25. doi: 10.1161/01.RES.0000209968.66606.10
24. Boulday G, Haskova Z, Reinders MEJ, Pal S, Briscoe DM. Vascular endothelial growth factor-induced signaling pathways in endothelial cells that mediate overexpression of the chemokine IFN-γ-inducible protein of 10 kDa in vitro and in vivo. J Immunol. (2006) 176:3098–107. doi: 10.4049/jimmunol.176.5.3098
25. Dace DS, Khan AA, Kelly J, Apte RS. Interleukin-10 promotes pathological angiogenesis by regulating macrophage response to hypoxia during development. PLoS ONE. (2008) 3:e3381. doi: 10.1371/journal.pone.0003381
26. Nakamura R, Sene A, Santeford A, Gdoura A, Kubota S, Zapata N, et al. IL10-driven STAT3 signalling in senescent macrophages promotes pathological eye angiogenesis. Nat Commun. (2015) 6:7847. doi: 10.1038/ncomms8847
27. Noma H, Yasuda K, Mimura T, Suganuma N, Shimura M. Retinal microcirculation and cytokines as predictors for recurrence of macular edema after intravitreal ranibizumab injection in branch retinal vein occlusion. JCM. (2020) 10:58. doi: 10.3390/jcm10010058
28. Song S, Yu X, Zhang P, Dai H. Increased levels of cytokines in the aqueous humor correlate with the severity of diabetic retinopathy. J Diabet Compl. (2020) 34:107641. doi: 10.1016/j.jdiacomp.2020.107641
29. Lechner J, Chen M, Hogg RE, Toth L, Silvestri G, Chakravarthy U, et al. Peripheral blood mononuclear cells from neovascular age-related macular degeneration patients produce higher levels of chemokines CCL2 (MCP-1) and CXCL8 (IL-8). J Neuroinflamm. (2017) 14:42. doi: 10.1186/s12974-017-0820-y
30. Nakashizuka H, Mitsumata M, Okisaka S, Shimada H, Kawamura A, Mori R, et al. Clinicopathologic findings in polypoidal choroidal vasculopathy. Invest Ophthalmol Vis Sci. (2008) 49:4729. doi: 10.1167/iovs.08-2134
31. Basurto L, Gregory MA, Hernández SB, Sánchez-Huerta L, Martínez AD, Manuel-Apolinar L, et al. Monocyte chemoattractant protein-1 (MCP-1) and fibroblast growth factor-21 (FGF-21) as biomarkers of subclinical atherosclerosis in women. Exp Gerontol. (2019) 124:110624. doi: 10.1016/j.exger.2019.05.013
32. Born LI, Wolfs RCW. Schreurs MWJ, Dik WA, Rothova A. Intraocular cytokine profile and autoimmune reactions in retinitis pigmentosa, age-related macular degeneration, glaucoma and cataract. Acta Ophthalmol. (2019) 97:185–92. doi: 10.1111/aos.13899
33. Parodi MB, Arrigo A, Romano F, Aragona E, Marchese A, Cicinelli MV, et al. Hyperreflective foci number correlates with choroidal neovascularization activity in angioid streaks. Invest Ophthalmol Vis Sci. (2018) 59:3314. doi: 10.1167/iovs.18-24291
34. Segal O, Barayev E, Nemet AY, Geffen N, Vainer I, Mimouni M. Prognostic value of hyperreflective foci in neovascular age-related macular degeneration treated with bevacizumab. Retina. (2016) 36:2175–82. doi: 10.1097/IAE.0000000000001033
35. Gualino V, Tadayoni R, Cohen SY, Erginay A, Fajnkuchen F, Haouchine B, et al. Optical coherence tomography, fluorescein angiography, and diagnosis of choroidal neovascularization in age-related macular degeneration. Retina. (2019) 39:1664–71. doi: 10.1097/IAE.0000000000002220
Keywords: aqueous humor, cytokine, hyperreflective foci, neovascular age-related macular degeneration, polypoidal choroidal vasculopathy, spectral-domain optical coherence tomography
Citation: Mao J, Chen N, Zhang S, Fang Y, Zheng Z, Wu S, Ye X, Chen Y, Chen Y and Shen L (2022) Association between inflammatory cytokines in the aqueous humor and hyperreflective foci on optical coherence tomography in patients with neovascular age-related macular degeneration and polypoidal choroidal vasculopathy. Front. Med. 9:973025. doi: 10.3389/fmed.2022.973025
Received: 19 June 2022; Accepted: 31 August 2022;
Published: 23 September 2022.
Edited by:
Alan G. Palestine, University of Colorado Anschutz Medical Campus, United StatesReviewed by:
Carlo Gesualdo, Università della Campania Luigi Vanvitelli, ItalyCopyright © 2022 Mao, Chen, Zhang, Fang, Zheng, Wu, Ye, Chen, Chen and Shen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lijun Shen, c2xqQG1haWwuZXllLmFjLmNu
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.