
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
MINI REVIEW article
Front. Med., 29 July 2022
Sec. Pulmonary Medicine
Volume 9 - 2022 | https://doi.org/10.3389/fmed.2022.954990
This article is part of the Research TopicRoad Trip from Mild to Severe Asthmatic Inflammation: The Traffic Lights of Biomarkers in Asthma ManagementView all 9 articles
PAR2, a receptor activated by serine proteases, has primarily pro-inflammatory roles in the airways and may play a role in asthma pathogenesis. PAR2 exerts its effects in the lungs through activation of a variety of airway cells, but also activation of circulating immune cells. There is evidence that PAR2 expression increases in asthma and other inflammatory diseases, although the regulation of PAR2 expression is not fully understood. Here we review the available literature on the potential role of PAR2 in asthma pathogenesis and propose a model of PAR2-mediated development of allergic sensitization. We also propose, based on our previous work, that PAR2 expression on peripheral blood monocyte subsets has the potential to serve as a biomarker of asthma severity and/or control.
Protease-Activated Receptors (PAR) are a family of G- protein coupled receptors with 4 members, PAR1–4. PARs are activated by serine proteases through a unique mechanism; the extracellular N terminal of the receptor is cleaved by serine proteases and the new N terminal, the tethered ligand (TL), folds and activates the receptor (1). A variety of serine proteases produced by inflammatory and other cells or from microorganisms can activate PAR receptors (2–10). Synthetic ligands that mimic TL sequences, called PAR activating peptides (PAR-AP), can activate PAR1, PAR2, and PAR4 without the requirement for proteolysis. Many of the studies we will review below use PAR-AP to study PAR-mediated effects, since unlike natural proteases they do not induce PAR-independent effects. Among PARs, PAR2 has a wide expression pattern (11) and has been linked to inflammation in the skin (12), gastrointestinal tract (13) and lungs (14), as well as in inflammatory pain (15).
Asthma is a complex inflammatory disease of the airways and one of the most common chronic diseases worldwide (16). Based on the intensity of therapy required to maintain disease control, asthma can be classified as mild, moderate and severe (17). Severe asthma represents less than 10% of patients with asthma but is responsible for a large share of asthma associated morbidity and health care costs (18). Identification of patients with severe asthma to allow timely institution of appropriate therapy is an important clinical problem. Asthma presents with multiple phenotypes and endotypes (19). Identification of endotypes of asthma is the result of our increased understanding of the pathophysiology of the disease including the role that various immune pathways play in disease development and progression. Allergic asthma is the most common form of asthma, but allergic asthma is also a heterogeneous condition that could be associated with different endotypes (20). Serine proteases present in the airways have been associated with the pathogenesis of allergic asthma through their ability to activate PAR2.
The first evidence suggesting a role for PAR2 in asthma came from studies showing pro-inflammatory effects of PAR2-mediated airway epithelial cell activation (21, 22). More direct evidence came in 2002 when Schmidlin et al. showed that PAR2 knockout (KO) mice were protected from the development of eosinophilic airway inflammation and airway hyperresponsiveness (AHR) in response to ovalbumin (23). The latter observation has since been reproduced in murine models that utilize ovalbumin, but also various biologically relevant allergens (24–27).
The airway epithelium, the first organ encountered by inhaled particles, pollutants and allergens, is viewed as an important immune organ aimed to protect the organism from environmental insults (28, 29), but is also involved in the pathogenesis of respiratory inflammatory diseases, including asthma (30, 31). PAR2-mediated activation of airway epithelial cells has been reported to release a number of factors that play important roles in asthma pathogenesis (Figure 1); these factors include remodeling proteases such as matrix metalloproteases (21), the neutrophil chemotactic factor IL-8 (22, 32–34), IL-6 (22, 35, 36), GM-CSF that affects multiple innate and adaptive immune cells (36, 37), the Th2 polarizing mediators TSLP (38, 39) and IL-25 (40) and various chemokines such as eotaxin (37, 41) and CCL-2 (42). These observations suggest that PAR2-mediated activation of the airway epithelium may release inflammatory mediators that polarize the immune response toward the Th2 phenotype and attract innate and adaptive immune cells to the airways (43).
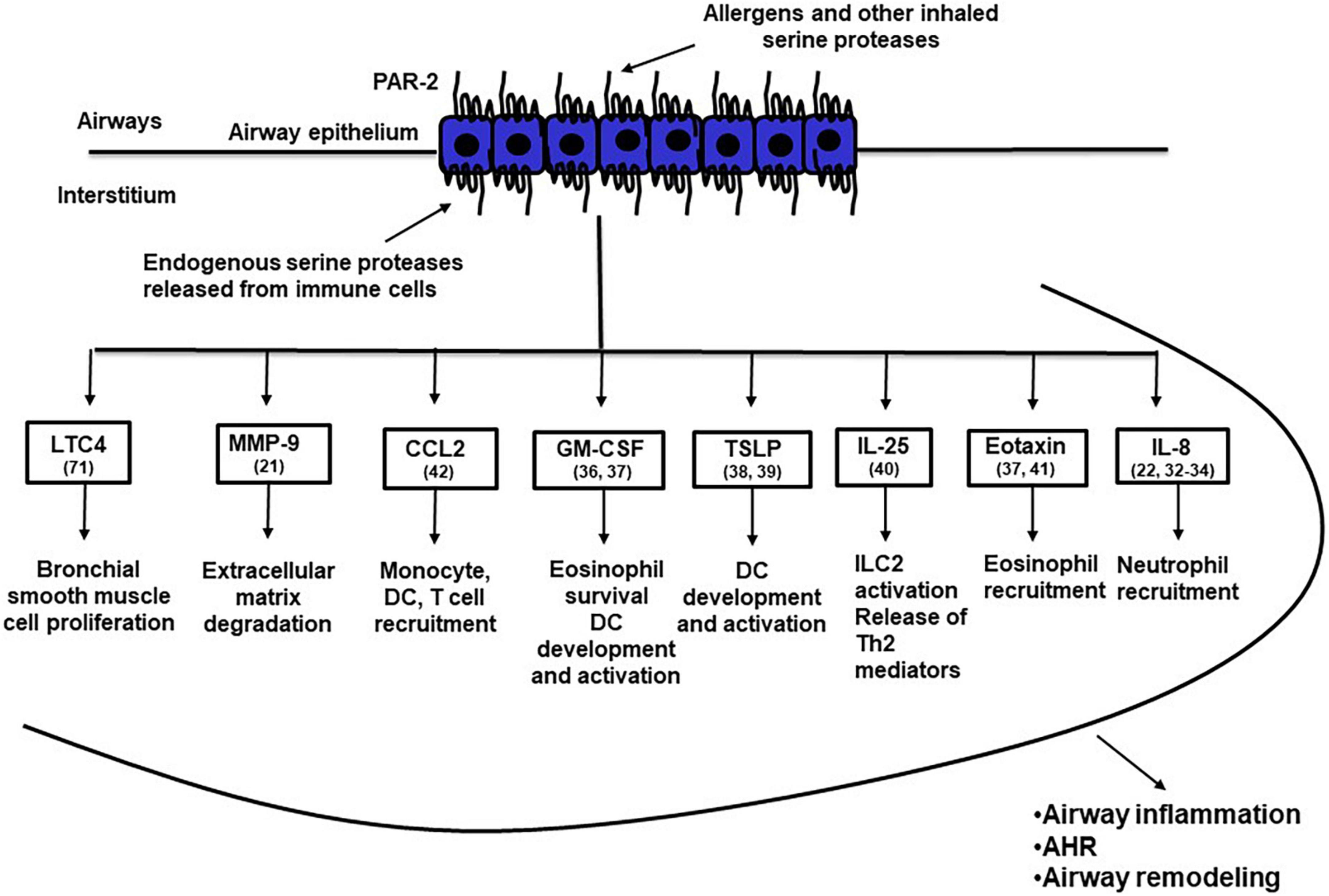
Figure 1. PAR-2 activated airway epithelial cells release a variety of inflammatory mediators that may induce airway inflammation, airway hyperresponsiveness and airway remodeling. The numbers in brackets after the name of specific inflammatory mediators indicate the reference of the main text where that mediator is discussed. AHR, airway hyperresponsiveness; CCL2, CC motif chemokine ligand 2; DC, dendritic cell; GM-CSF, granulocyte-macrophage colony-stimulating factor; ILC2, type 2 innate lymphoid cells; IL-25, interleukin 25; IL-8, interleukin 8; LTC4, leukotriene C4; MMP-9, matrix metalloproteinase 9; TSLP, thymic stromal lymphopoietin.
Development of allergic airway inflammation in humans and animal models can be divided into two steps; initial encounter with the antigen locally or systemically, leads to allergic sensitization with development of antigen-specific Th2 cells and production of IgE, while a subsequent exposure of a sensitized individual to the same antigen leads to the development of eosinophilic airway inflammation and AHR. PAR2 activation may participate in the development of allergic sensitization by inducing a deviation of the nasal/airway mucosa immune response against a foreign innocuous antigen from the default pathway of tolerance to allergic sensitization and production of antigen-specific IgE (44). This PAR2 effect exhibits striking similarities to the effects of TLR4 activation in the airways (45). We propose that in the airways PAR2 recognizes both internal and external “danger” signals, namely serine proteases released from inflammatory and other cells or inhaled through the air, respectively. PAR2 activation under these circumstances leads to activation of the innate and adaptive immune system through soluble mediators from the airway epithelium and to allergic sensitization (Figure 2). In addition to TNF (44), many other factors may also mediate, at least in part, the deviation of the immune system toward a Th2 phenotype and allergic sensitization following PAR2 activation, some of them factors released by the airway epithelium (Figure 1). In addition to the indirect effects of PAR2 activation on the adaptive immune system shown in Figure 2, endogenous and/or exogenous serine proteases may directly activate adaptive immune cells, since they also express PAR2 (46). Finally, in sensitized individuals, PAR2 activation during repeat exposures to sensitizing allergens results in release of inflammatory mediators important for the development of allergic airway inflammation, AHR and airway remodeling (14, 47, 48).
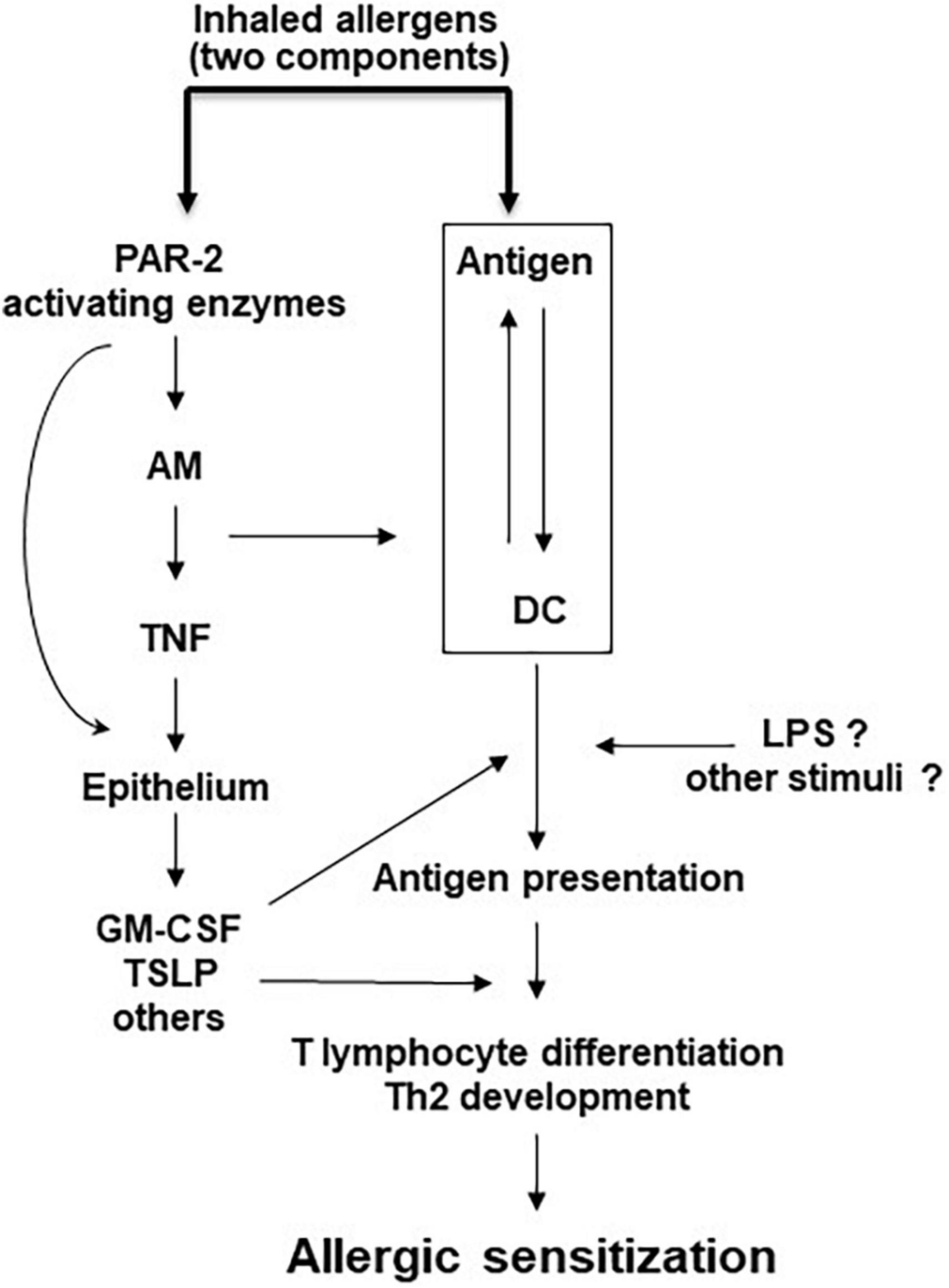
Figure 2. Model of PAR-2-mediated allergic sensitization. Allergic sensitization requires the recognition of an antigen by DC and also PAR-2 activation by an allergen with serine protease activity, or by an independent serine protease. Other stimuli may facilitate this process. AM, alveolar macrophages; DC, dendritic cells; GM-CSF, granulocyte macrophage colony stimulating factor; LPS, lipopolysaccharide; TNF, tumor necrosis factor; TSLP, thymic stromal lymphopoietin.
However, the role of PAR2 in the airways may be more complex that discussed so far. PAR2 activation causes relaxation of trachea preparations and protects from bronchoconstriction in vivo through the release of PGE2 from airway epithelial cells (49). Similarly, in a rabbit model of pollen allergy, PAR2 activation in the airways just prior to allergen challenge decreased allergen-mediated bronchoconstriction, eosinophil infiltration and AHR (50). These observations indicate that PAR2 may also have a protective effect against the development of signs and symptoms associated with asthma. The reasons for the potential dual effect of PAR2 activation have not been identified. We also do not know whether these “protective” effects of PAR2 activation can acutely or chronically antagonize the better studied pro-inflammatory effects. Finally, we don’t know if these protective effects would also be evident in human airways.
The vast majority of the in vivo data on the role of PAR2 in allergic inflammation come from animal studies. There is limited information on the pro-inflammatory potential of PAR2 in humans in vivo. PAR2-AP have been shown to induce inflammation when applied to humans intradermally (51), but these peptides have not been administered to humans through any other routes.
PAR2 may also affect allergic airway inflammation through its expression on a variety of immune cells. Both monocytes and macrophages express PAR2 (52) and its expression is altered in airway inflammatory conditions (53). PAR2 activation leads to cytokine production from monocytes and macrophages (48, 54, 55), affects macrophage differentiation (56, 57) and has antiviral effects. In vitro differentiated DC do not express PAR2 (52), but PAR2 is needed for their normal maturation (58). PAR2 contributes to DC antigen uptake and facilitates the presence of mature DC in draining lymph nodes in vivo, but in these case it is not clear if the effects are direct (44, 59). Direct PAR2 activation on naive T cells by proteases may induce IL-4 release and lead to allergic inflammation (46). PAR2 activation induces various inflammatory, but also antiviral pathways in neutrophils (60–63). Finally, eosinophils may also express PAR2, but the role of PAR2 in eosinophil functions is controversial (64–66).
Airway smooth muscle cells also play an important role in asthma pathogenesis (67). In addition, asthma, especially severe disease, is characterized by airway remodeling that includes airway smooth muscle hyperplasia and hypertrophy and fibrosis (30, 68). Two studies using house dust mite (HDM), showed that allergen proteases also induce proliferation of asthmatic bronchial smooth muscle cells through PAR2-dependent mechanisms (69, 70). These observations suggest that PAR2-mediated smooth muscle activation, either directly or indirectly through LTC4 released from epithelial cells (71), may contribute to the smooth muscle hypertrophy and/or hyperplasia seen in patients with asthma.
There are in vivo observations that PAR2 activation is involved in fibrosis, but these come primarily from fibroproliferative lung diseases such as idiopathic pulmonary fibrosis. A murine study showed that PAR2 contributes to the development of pulmonary fibrosis, while targeting PAR2 affords protection from bleomycin-induced fibrosis (72). Another study showed that mast cell tryptase induces lung fibroblast proliferation via PAR2-activation (73), suggesting that activated mast cells may induce fibrotic changes in asthma through PAR2 activation.
If PAR2 is important for development of allergic sensitization and inflammation, then interfering with its expression or activation may be a viable approach for prevention and/or treatment of allergic diseases. However, triggers relevant to asthma may upregulate PAR2 expression in the airways, which in turn may exacerbate allergic airway inflammation. PAR2 expression is increased on the airway epithelium of asthmatic individuals (74) and on the nasal mucosa epithelium of patients with allergic rhinitis (75, 76). Various inflammatory mediators upregulate PAR2 expression on endothelial cells (77, 78), mast cells (79, 80) and other cells (81–83), and the same may be true for airway epithelial cells. Also, cockroach (34), HDM (84), and mold (33) allergen extracts upregulate PAR2 expression on airway epithelial cells, possibly through proteases contained within the extracts. In addition, inflammatory stress, which is present in asthmatic airways, may regulate PAR2 expression in the lung through hypoxia, as has been shown to do in endothelial cells (85).
Bronchial smooth muscle cells from asthmatic individuals maintain higher PAR2 mRNA and protein expression than cells from normal individuals after ex vivo culture (86), suggesting the possibility that epigenetic changes due to the chronic inflammation in the airways may affect PAR2 expression. We recently showed that insulin regulates PAR2 expression in primary human airway epithelial cells through the FOXO1 transcription factor (87), which may indicate that insulin resistance, often associated with asthma (88, 89), may be associated with alterations of PAR2 expression. Finally, genetic factors regulating PAR2 expression cannot be excluded as PAR2 SNPs have been shown to increase mRNA stability and increase expression of PAR2 in PBMCs (90) and synovial tissue (91).
Prevention of PAR2 activation by allergens or endogenous proteases may also have therapeutic benefits in asthma. Unfortunately, development of small molecule inhibitors has been problematic, and only recently such PAR2 inhibitors are being described (92). A monoclonal humanized antibody has also been described, but has not been tested whether it is functional in vivo (93). Another antibody (MEDI 0618) is undergoing phase I evaluation and results may be available soon.1
Personalized medicine offers promise for improved diagnosis and treatment for inflammatory diseases including lung diseases (94, 95), but in asthma the lack of easily obtainable biomarkers to identify specific phenotypes and/or endotypes (96), limits the applicability of this approach. Many biomarkers have been tested and they all have their advantages and disadvantages (97). Identification of patients with severe asthma is an important clinical question, since these individuals require more intense treatment and close follow up to avoid asthma morbidity. Biomarkers that could identify those individuals and predict their response to therapy are in great demand (98).
Over the last few years our laboratory has been studying the utility of PAR2 expression as a biomarkers of asthma severity and control. Cells obtained through induced sputum would be ideal for these studies, but they are not easily accessible, except in specialized centers. Therefore, we have focused on peripheral blood cells. We validated that a subset of peripheral blood monocytes express surface PAR2, as has been shown before (48), but our more interesting observation was that cell surface PAR2 expression on peripheral blood intermediate monocytes (IMMo) correlated with disease severity (99). In particular, patients with severe asthma had higher% of IMMo expressing PAR2 and higher total number of PAR2-expressing IMMo in their peripheral blood compared to subjects with mild/moderate disease. Other cells, including eosinophils, neutrophils, and CD4+ lymphocytes, showed low PAR2 expression and no differences in expression between the two populations with different asthma severity, as there was also no difference between the two groups in PAR2 expression in classical monocytes. Our data showed that PAR2 expression on IMMo was an excellent marker to discriminate between subjects with severe and those with mild/moderate asthma. PAR2 expression on monocytes of patients with rheumatoid arthritis (100), granulomatosis with polyangiitis (101) and primary antiphospholipid syndrome (102) also correlates with disease activity. However, asthma is the first inflammatory condition where changes in PAR2 expression in a specific monocyte subgroup are associated with disease severity.
In addition, the% of PAR2-expressing IMMo in peripheral blood correlated with the dose of inhaled steroids prescribed to these subjects and was higher in subjects that had experienced at least one exacerbation over the last year. Unfortunately, we did not have detailed information on the proximity, total number and severity of exacerbations to understand whether subjects with a recent exacerbation were those with an exacerbation prone phenotype, a phenotype that has been shown to have prognostic significance for severe asthma.
It is also interesting that in the population with a recent asthma exacerbation PAR2 mRNA expression also correlated with the numbers of Th2 cells in the peripheral blood, indicating that PAR2 expression may also be associated with T2 inflammation, although the mechanisms leading to this association are not clear. It is interesting that PAR2-mediated activation of macrophages induces IL-4 secretion (103), which might contribute to T2 environment in peripheral tissues and may even support the development of allergic sensitization, an effect that follows PAR2 activation in murine studies (26, 44). It is also possible that the same triggers that lead to T2 disease or factors present in subjects with T2 diseases, are those that upregulate PAR2 on monocytes. To this effect we have evidence that LPS, which through TLR4 activation can facilitate allergic sensitization (45) or CCR5 that is increased in the airways of subjects with asthma (104, 105), can both upregulate PAR2 on human IMMo in vitro (106).
From our data comparing PAR2 expression in IMMo between subjects with severe and mild/moderate asthma, it is not clear whether PAR2 upregulation on the surface of IMMo depends on asthma severity or the presence of uncontrolled inflammation that can be present in severe disease. Two studies shed some light to this question. In a recent study we showed that PAR2 expression on the surface of IMMo is increased during an asthma exacerbation (107). In this study we showed that PAR2 expression on peripheral blood IMMo is higher in subjects presenting to the Emergency Department (ED) with an exacerbations compared to subjects with stable disease. PAR2 expression comes down to levels present in subjects with stable disease 2 weeks after the ED presentation and after the exacerbation has been treated. It is possible that increased inflammation in the days leading to an exacerbation is the reason for increased PAR2 expression. Increased systemic inflammation may lead to increased PAR2 expression on IMMo, as suggested by our results using a human allergy challenge model. In that case, inhalation allergen challenge induced an early (6 h) increase in PAR2 expression on peripheral blood IMMo that was sustained at 24 h (107). It would be interesting to know whether the same changes in PAR2 expression are also seen in inflammatory cells in the airways. Studies are underway in our laboratory to understand whether PAR2 expression increases in induced sputum and/or BAL cells after an allergen challenge and also in subjects with uncontrolled versus controlled asthma.
One of the requirements for PAR2 expression on peripheral blood IMMo to be used as a biomarker is that this value is stable and reproducible during the course of stable disease. Our data however, show that this may not be the case (108). We recruited 20 stable asthmatics and repeated the evaluation of PAR2 expression on peripheral blood IMMo every 3 months for a year. We found that even though the% of IMMo expressing PAR2 was stable in the whole population, there were differences in expression for specific individuals that could not be explained with the available clinical data. In this study we had no subjects that experienced an asthma exacerbation. It is possible that changes preceding exacerbations will be greater that the fluctuation of values during a stable course of the disease and therefore, this value may be useful as predictive biomarker for asthma exacerbations.
Being able to evaluate the activation state of the receptor, instead of its presence on the cell surface, may be a more accurate approach to evaluate the activity of this inflammatory pathway and may also function as a biomarker that could be used in asthma. A recent study showed that the small peptide liberated from the receptor when it is cleaved by activating serine proteases can be detected in human serum and its levels increase in patients with rheumatoid arthritis and responds to treatment of the disease with specific biologics (109). It will be interesting to test whether the levels of this peptide also change in asthma and whether it may be used as another biomarker for asthma severity and/or control.
Asthma is an inflammatory disease of the airways. Even though we can treat successfully the disease in the vast majority of subjects with mild or moderate asthma, we still are not able to fully address the needs of patients with severe disease. In addition, we know that exacerbations, especially severe exacerbations requiring urgent care, can happen at any point even in patients with mild disease and reliable biomarkers to predict such events are missing.
Our current knowledge on the potential role of PAR2 in allergic asthma, indicates that markers of activation of PAR2-related pathways may be candidates for biomarkers. Our current observations may allow the development of new hypotheses regarding potential biomarkers of asthma severity or impending exacerbations, that could be tested in future studies.
VG, NS, and HV were responsible for study design and authored the final draft of review article. All authors read and approved the final review article.
This research was supported by the Canadian Institute of Health Research (CIHR) and the Lung Association of Alberta and NWT.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
AM, Alveolar macrophage; AP, Activating peptide; AHR, Airway hyper responsiveness; BAL, Bronchial alveolar lavage; CCR5, C-C chemokine receptor type 5; DC, Dendritic cell; ED, Emergency department; FOXO1, Forkhead Box O1; GM-CSF, Granulocyte macrophage colony stimulating factor; HDM, House dust mite; IL, Interleukin; IgE, Immunoglobulin E; IMMo, Intermediate monocytes; LPS, Lipopolysaccharides; LTC4, Leukotriene C4; PAR, Protease activated receptor; SNP, Single nucleotide polymorphism; TL, Tethered ligand; TLR4, Toll like receptor 4; TNF, Tumor necrosis factor; TSLP, Thymic stromal lymphopoietin.
1. Heuberger DM, Schuepbach RA. Protease-activated receptors (PARs): mechanisms of action and potential therapeutic modulators in PAR-driven inflammatory diseases. Thromb J. (2019) 17:4. doi: 10.1186/s12959-019-0194-8
2. Nystedt S, Emilsson K, Wahlestedt C, Sundelin J. Molecular cloning of a potential proteinase activated receptor. Proc Natl Acad Sci USA. (1994) 91:9208–12. doi: 10.1073/pnas.91.20.9208
3. Bohm SK, Khitin LM, Grady EF, Aponte G, Payan DG, Bunnett NW. Mechanisms of desensitization and resensitization of proteinase-activated receptor-2. J Biol Chem. (1996) 271:22003–16. doi: 10.1074/jbc.271.36.22003
4. Cottrell GS, Amadesi S, Grady EF, Bunnett NW. Trypsin IV, a novel agonist of protease-activated receptors 2 and 4. J Biol Chem. (2004) 279:13532–9. doi: 10.1074/jbc.M312090200
5. Corvera CU, Dery O, McConalogue K, Bohm SK, Khitin LM, Caughey GH, et al. Mast cell tryptase regulates rat colonic myocytes through proteinase-activated receptor 2. J Clin Invest. (1997) 100:1383–93. doi: 10.1172/JCI119658
6. Camerer E, Huang W, Coughlin SR. Tissue factor- and factor X-dependent activation of protease-activated receptor 2 by factor VIIa. Proc Natl Acad Sci USA. (2000) 97:5255–60. doi: 10.1073/pnas.97.10.5255
7. Smith R, Jenkins A, Lourbakos A, Thompson P, Ramakrishnan V, Tomlinson J, et al. Evidence for the activation of PAR-2 by the sperm protease, acrosin: expression of the receptor on oocytes. FEBS Lett. (2000) 484:285–90. doi: 10.1016/S0014-5793(00)02146-3
8. Dulon S, Leduc D, Cottrell GS, D’Alayer J, Hansen KK, Bunnett NW, et al. Pseudomonas aeruginosa elastase disables proteinase-activated receptor 2 in respiratory epithelial cells. Am J Respir Cell Mol Biol. (2005) 32:411–9. doi: 10.1165/rcmb.2004-0274OC
9. Oikonomopoulou K, Hansen KK, Saifeddine M, Tea I, Blaber M, Blaber SI, et al. Proteinase-activated receptors, targets for kallikrein signaling. J Biol Chem. (2006) 281:32095–112. doi: 10.1074/jbc.M513138200
10. Ramsay AJ, Dong Y, Hunt ML, Linn M, Samaratunga H, Clements JA, et al. Kallikrein-related peptidase 4 (KLK4) initiates intracellular signaling via protease-activated receptors (PARs). KLK4 and PAR-2 are co-expressed during prostate cancer progression. J Biol Chem. (2008) 283:12293–304. doi: 10.1074/jbc.M709493200
11. Rothmeier AS, Ruf W. Protease-activated receptor 2 signaling in inflammation. Semin Immunopathol. (2012) 34:133–49. doi: 10.1007/s00281-011-0289-1
12. Moormann C, Artuc M, Pohl E, Varga G, Buddenkotte J, Vergnolle N, et al. Functional characterization and expression analysis of the proteinase-activated receptor-2 in human cutaneous mast cells. J Invest Dermatol. (2006) 126:746–55. doi: 10.1038/sj.jid.5700169
13. Lohman RJ, Cotterell AJ, Suen J, Liu L, Do AT, Vesey DA, et al. Antagonism of protease-activated receptor 2 protects against experimental colitis. J Pharmacol Exp Ther. (2012) 340:256–65. doi: 10.1124/jpet.111.187062
14. Asaduzzaman M, Nadeem A, Arizmendi N, Davidson C, Nichols HL, Abel M, et al. Functional inhibition of PAR2 alleviates allergen-induced airway hyperresponsiveness and inflammation. Clin Exp Allergy. (2015) 45:1844–55. doi: 10.1111/cea.12628
15. Zhao P, Lieu T, Barlow N, Metcalf M, Veldhuis NA, Jensen DD, et al. Cathepsin S causes inflammatory pain via biased agonism of PAR2 and TRPV4. J Biol Chem. (2014) 289:27215–34. doi: 10.1074/jbc.M114.599712
16. Dharmage SC, Perret JL, Custovic A. Epidemiology of asthma in children and adults. Front Pediatr. (2019) 7:246. doi: 10.3389/fped.2019.00246
17. Holguin F, Cardet JC, Chung KF, Diver S, Ferreira DS, Fitzpatrick A, et al. Management of severe asthma: a European Respiratory Society/American Thoracic Society guideline. Eur Respir J. (2020) 55:1900588. doi: 10.1183/13993003.00588-2019
18. Wenzel SE. Severe adult asthmas: integrating clinical features, biology, and therapeutics to improve outcomes. Am J Respir Crit Care Med. (2021) 203:809–21. doi: 10.1164/rccm.202009-3631CI
19. Fajt ML, Wenzel SE. Asthma phenotypes and the use of biologic medications in asthma and allergic disease: the next steps toward personalized care. J Allergy Clin Immunol. (2015) 135:299–310. doi: 10.1016/j.jaci.2014.12.1871
20. Chupp GL, Kaur R, Mainardi A. New therapies for emerging endotypes of asthma. Annu Rev Med. (2020) 71:289–302. doi: 10.1146/annurev-med-041818-020630
21. Vliagoftis H, Schwingshackl A, Milne CD, Duszyk M, Hollenberg MD, Wallace JL, et al. Proteinase-activated receptor-2-mediated matrix metalloproteinase-9 release from airway epithelial cells. J Allergy Clin Immunol. (2000) 106:537–45. doi: 10.1067/mai.2000.109058
22. Asokananthan N, Graham PT, Fink J, Knight DA, Bakker AJ, McWilliam AS, et al. Activation of protease-activated receptor (PAR)-1, PAR-2, and PAR-4 stimulates IL-6, IL-8, and prostaglandin E2 release from human respiratory epithelial cells. J Immunol. (2002) 168:3577–85. doi: 10.4049/jimmunol.168.7.3577
23. Schmidlin F, Amadesi S, Dabbagh K, Lewis DE, Knott P, Bunnett NW, et al. Protease-activated receptor 2 mediates eosinophil infiltration and hyperreactivity in allergic inflammation of the airway. J Immunol. (2002) 169:5315–21. doi: 10.4049/jimmunol.169.9.5315
24. Page K, Ledford JR, Zhou P, Dienger K, Wills-Karp M. Mucosal sensitization to German cockroach involves protease-activated receptor-2. Respir Res. (2010) 11:62. doi: 10.1186/1465-9921-11-62
25. Arizmendi NG, Abel M, Mihara K, Davidson C, Polley D, Nadeem A, et al. Mucosal allergic sensitization to cockroach allergens is dependent on proteinase activity and proteinase-activated receptor-2 activation. J Immunol. (2011) 186:3164–72. doi: 10.4049/jimmunol.0903812
26. Davidson CE, Asaduzzaman M, Arizmendi NG, Polley D, Wu Y, Gordon JR, et al. Proteinase-activated receptor-2 activation participates in allergic sensitization to house dust mite allergens in a murine model. Clin Exp Allergy. (2013) 43:1274–85. doi: 10.1111/cea.12185
27. Takizawa T, Tamiya M, Hara T, Matsumoto J, Saito N, Kanke T, et al. Abrogation of bronchial eosinophilic inflammation and attenuated eotaxin content in protease-activated receptor 2-deficient mice. J Pharmacol Sci. (2005) 98:99–102. doi: 10.1254/jphs.SCZ050138
28. Tam A, Wadsworth S, Dorscheid D, Man SF, Sin DD. The airway epithelium: more than just a structural barrier. Therapeut Adv Respirat Dis. (2011) 5:255–73. doi: 10.1177/1753465810396539
29. Hallstrand TS, Hackett TL, Altemeier WA, Matute-Bello G, Hansbro PM, Knight DA. Airway epithelial regulation of pulmonary immune homeostasis and inflammation. Clin Immunol. (2014) 151:1–15. doi: 10.1016/j.clim.2013.12.003
30. Hough KP, Curtiss ML, Blain TJ, Liu RM, Trevor J, Deshane JS, et al. Airway remodeling in asthma. Front Med. (2020) 7:191. doi: 10.3389/fmed.2020.00191
31. Hellings PW, Steelant B. Epithelial barriers in allergy and asthma. J Allergy Clin Immunol. (2020) 145:1499–509. doi: 10.1016/j.jaci.2020.04.010
32. Kauffman HF, Tamm M, Timmerman JA, Borger P. House dust mite major allergens Der p 1 and Der p 5 activate human airway-derived epithelial cells by protease-dependent and protease-independent mechanisms. Clin Mol Allergy. (2006) 4:5. doi: 10.1186/1476-7961-4-5
33. Chiu LL, Perng DW, Yu CH, Su SN, Chow LP. Mold allergen, pen C 13, induces IL-8 expression in human airway epithelial cells by activating protease-activated receptor 1 and 2. J Immunol. (2007) 178:5237–44. doi: 10.4049/jimmunol.178.8.5237
34. Lee MF, Wang NM, Liu SW, Lin SJ, Chen YH. Induction of interleukin 8 by American cockroach allergens from human airway epithelial cells via extracellular signal regulatory kinase and jun N-terminal kinase but not p38 mitogen-activated protein kinase. Ann Allergy Asthma Immunol. (2010) 105:234–40. doi: 10.1016/j.anai.2010.07.008
35. Asokananthan N, Graham PT, Stewart DJ, Bakker AJ, Eidne KA, Thompson PJ, et al. House dust mite allergens induce proinflammatory cytokines from respiratory epithelial cells: the cysteine protease allergen, Der p 1, activates protease-activated receptor (PAR)-2 and inactivates PAR-1. J Immunol. (2002) 169:4572–8. doi: 10.4049/jimmunol.169.8.4572
36. Kale SL, Arora N. Per a 10 activates human derived epithelial cell line in a protease dependent manner via PAR-2. Immunobiology. (2015) 220:525–32. doi: 10.1016/j.imbio.2014.10.018
37. Vliagoftis H, Befus AD, Hollenberg MD, Moqbel R. Airway epithelial cells release eosinophil survival-promoting factors (GM-CSF) after stimulation of proteinase-activated receptor 2. J Allergy Clin Immunol. (2001) 107:679–85. doi: 10.1067/mai.2001.114245
38. Jia X, Zhang H, Cao X, Yin Y, Zhang B. Activation of TRPV1 mediates thymic stromal lymphopoietin release via the Ca2+/NFAT pathway in airway epithelial cells. FEBS Lett. (2014) 588:3047–54. doi: 10.1016/j.febslet.2014.06.018
39. Kouzaki H, O’Grady SM, Lawrence CB, Kita H. Proteases induce production of thymic stromal lymphopoietin by airway epithelial cells through protease-activated receptor-2. J Immunol. (2009) 183:1427–34. doi: 10.4049/jimmunol.0900904
40. Kouzaki H, Tojima I, Kita H, Shimizu T. Transcription of interleukin-25 and extracellular release of the protein is regulated by allergen proteases in airway epithelial cells. Am J Respir Cell Mol Biol. (2013) 49:741–50. doi: 10.1165/rcmb.2012-0304OC
41. Sun G, Stacey MA, Schmidt M, Mori L, Mattoli S. Interaction of mite allergens Der p3 and Der p9 with protease-activated receptor-2 expressed by lung epithelial cells. J Immunol. (2001) 167:1014–21. doi: 10.4049/jimmunol.167.2.1014
42. Wang H, Yi T, Zheng Y, He S. Induction of monocyte chemoattractant protein-1 release from A549 cells by agonists of protease-activated receptor-1 and -2. Eur J Cell Biol. (2007) 86:233–42. doi: 10.1016/j.ejcb.2006.12.003
43. Gandhi VD, Vliagoftis H. Airway epithelium interactions with aeroallergens: role of secreted cytokines and chemokines in innate immunity. Front Immunol. (2015) 6:147. doi: 10.3389/fimmu.2015.00147
44. Ebeling C, Lam T, Gordon JR, Hollenberg MD, Vliagoftis H. Proteinase-activated receptor-2 promotes allergic sensitization to an inhaled antigen through a TNF-mediated pathway. J Immunol. (2007) 179:2910–7. doi: 10.4049/jimmunol.179.5.2910
45. Eisenbarth SC, Piggott DA, Huleatt JW, Visintin I, Herrick CA, Bottomly K. Lipopolysaccharide-enhanced, toll-like receptor 4-dependent T helper cell type 2 responses to inhaled antigen. J Exp Med. (2002) 196:1645–51. doi: 10.1084/jem.20021340
46. Liang G, Barker T, Xie Z, Charles N, Rivera J, Druey KM. Naive T cells sense the cysteine protease allergen papain through protease-activated receptor 2 and propel TH2 immunity. J Allergy Clin Immunol. (2012) 129:1377–86e13. doi: 10.1016/j.jaci.2012.02.035
47. Asaduzzaman M, Davidson C, Nahirney D, Fiteih Y, Puttagunta L, Vliagoftis H. Proteinase-activated receptor-2 blockade inhibits changes seen in a chronic murine asthma model. Allergy. (2018) 73:416–20. doi: 10.1111/all.13313
48. Johansson U, Lawson C, Dabare M, Syndercombe-Court D, Newland AC, Howells GL, et al. Human peripheral blood monocytes express protease receptor-2 and respond to receptor activation by production of IL-6, IL-8, and IL-1{beta}. J Leukoc Biol. (2005) 78:967–75. doi: 10.1189/jlb.0704422
49. Cocks TM, Fong B, Chow JM, Anderson GP, Frauman AG, Goldie RG, et al. A protective role for protease-activated receptors in the airways. Nature. (1999) 398:156–60. doi: 10.1038/18223
50. D’Agostino B, Roviezzo F, De Palma R, Terracciano S, De Nardo M, Gallelli L, et al. Activation of protease-activated receptor-2 reduces airways inflammation in experimental allergic asthma. Clin Exp Allergy. (2007) 37:1436–43. doi: 10.1111/j.1365-2222.2007.02793.x
51. Seeliger S, Derian CK, Vergnolle N, Bunnett NW, Nawroth R, Schmelz M, et al. Proinflammatory role of proteinase-activated receptor-2 in humans and mice during cutaneous inflammation in vivo. FASEB J. (2003) 17:1871–85. doi: 10.1096/fj.02-1112com
52. Colognato R, Slupsky JR, Jendrach M, Burysek L, Syrovets T, Simmet T. Differential expression and regulation of protease-activated receptors in human peripheral monocytes and monocyte-derived antigen-presenting cells. Blood. (2003) 102:2645–52. doi: 10.1182/blood-2002-08-2497
53. Roche N, Stirling RG, Lim S, Oliver BG, Oates T, Jazrawi E, et al. Effect of acute and chronic inflammatory stimuli on expression of protease-activated receptors 1 and 2 in alveolar macrophages. J Allergy Clin Immunol. (2003) 111:367–73. doi: 10.1067/mai.2003.6
54. Day SB, Zhou P, Ledford JR, Page K. German cockroach frass proteases modulate the innate immune response via activation of protease-activated receptor-2. J Innate Immun. (2010) 2:495–504. doi: 10.1159/000317195
55. Yamaguchi R, Yamamoto T, Sakamoto A, Ishimaru Y, Narahara S, Sugiuchi H, et al. Mechanism of interleukin-13 production by granulocyte-macrophage colony-stimulating factor-dependent macrophages via protease-activated receptor-2. Blood Cells Mol Dis. (2015) 55:21–6. doi: 10.1016/j.bcmd.2015.03.006
56. Nhu QM, Shirey KA, Pennini ME, Stiltz J, Vogel SN. Proteinase-activated receptor 2 activation promotes an anti-inflammatory and alternatively activated phenotype in LPS-stimulated murine macrophages. Innate Immun. (2012) 18:193–203. doi: 10.1177/1753425910395044
57. Steven R, Crilly A, Lockhart JC, Ferrell WR, McInnes IB. Proteinase-activated receptor-2 modulates human macrophage differentiation and effector function. Innate Immun. (2013) 19:663–72. doi: 10.1177/1753425913479984
58. Fields RC, Schoenecker JG, Hart JP, Hoffman MR, Pizzo SV, Lawson JH. Protease-activated receptor-2 signaling triggers dendritic cell development. Am J Pathol. (2003) 162:1817–22. doi: 10.1016/S0002-9440(10)64316-7
59. Ramelli G, Fuertes S, Narayan S, Busso N, Acha-Orbea H, So A. Protease-activated receptor 2 signalling promotes dendritic cell antigen transport and T-cell activation in vivo. Immunology. (2010) 129:20–7. doi: 10.1111/j.1365-2567.2009.03144.x
60. Bryzek D, Ciaston I, Dobosz E, Gasiorek A, Makarska A, Sarna M, et al. Triggering NETosis via protease-activated receptor (PAR)-2 signaling as a mechanism of hijacking neutrophils function for pathogen benefits. PLoS Pathog. (2019) 15:e1007773. doi: 10.1371/journal.ppat.1007773
61. Bock A, Tucker N, Kelher MR, Khan SY, Gonzalez E, Wohlauer M, et al. Alpha-enolase causes proinflammatory activation of pulmonary microvascular endothelial cells and primes neutrophils through plasmin activation of protease-activated receptor 2. Shock. (2015) 44:137–42. doi: 10.1097/SHK.0000000000000394
62. Bakele M, Lotz-Havla AS, Jakowetz A, Carevic M, Marcos V, Muntau AC, et al. An interactive network of elastase, secretases, and PAR-2 protein regulates CXCR1 receptor surface expression on neutrophils. J Biol Chem. (2014) 289:20516–25. doi: 10.1074/jbc.M114.575803
63. Feld M, Shpacovitch V, Ehrhardt C, Fastrich M, Goerge T, Ludwig S, et al. Proteinase-activated receptor-2 agonist activates anti-influenza mechanisms and modulates IFNgamma-induced antiviral pathways in human neutrophils. Biomed Res Int. (2013) 2013:879080. doi: 10.1155/2013/879080
64. Miike S, McWilliam AS, Kita H. Trypsin induces activation and inflammatory mediator release from human eosinophils through protease-activated receptor-2. J Immunol. (2001) 167:6615–22. doi: 10.4049/jimmunol.167.11.6615
65. Bolton SJ, McNulty CA, Thomas RJ, Hewitt CR, Wardlaw AJ. Expression of and functional responses to protease-activated receptors on human eosinophils. J Leukoc Biol. (2003) 74:60–8. doi: 10.1189/jlb.0702351
66. Vliagoftis H, Lacy P, Luy B, Adamko D, Hollenberg M, Befus D, et al. Mast cell tryptase activates peripheral blood eosinophils to release granule-associated enzymes. Int Arch Allergy Immunol. (2004) 135:196–204. doi: 10.1159/000081304
67. Camoretti-Mercado B, Lockey RF. Airway smooth muscle pathophysiology in asthma. J Allergy Clin Immunol. (2021) 147:1983–95. doi: 10.1016/j.jaci.2021.03.035
68. Kaczmarek KA, Clifford RL, Knox AJ. Epigenetic changes in airway smooth muscle as a driver of airway inflammation and remodeling in asthma. Chest. (2019) 155:816–24. doi: 10.1016/j.chest.2018.10.038
69. Miglino N, Roth M, Tamm M, Borger P. House dust mite extract downregulates C/EBPalpha in asthmatic bronchial smooth muscle cells. Eur Respir J. (2011) 38:50–8. doi: 10.1183/09031936.00068010
70. Trian T, Allard B, Dupin I, Carvalho G, Ousova O, Maurat E, et al. House dust mites induce proliferation of severe asthmatic smooth muscle cells via an epithelium-dependent pathway. Am J Respir Crit Care Med. (2015) 191:538–46. doi: 10.1164/rccm.201409-1582OC
71. Perng DW, Wu YC, Chang KT, Wu MT, Chiou YC, Su KC, et al. Leukotriene C4 induces TGF-beta1 production in airway epithelium via p38 kinase pathway. Am J Respir Cell Mol Biol. (2006) 34:101–7. doi: 10.1165/rcmb.2005-0068OC
72. Lin C, von der Thusen J, Daalhuisen J, ten Brink M, Crestani B, van der Poll T, et al. Pharmacological targeting of protease-activated receptor 2 affords protection from bleomycin-induced pulmonary fibrosis. Mol Med. (2015) 21:576–83. doi: 10.2119/molmed.2015.00094
73. Akers IA, Parsons M, Hill MR, Hollenberg MD, Sanjar S, Laurent GJ, et al. Mast cell tryptase stimulates human lung fibroblast proliferation via protease-activated receptor-2. Am J Physiol Lung Cell Mol Physiol. (2000) 278:L193–201. doi: 10.1152/ajplung.2000.278.1.L193
74. Knight DA, Lim S, Scaffidi AK, Roche N, Chung KF, Stewart GA, et al. Protease-activated receptors in human airways: upregulation of PAR-2 in respiratory epithelium from patients with asthma. J Allergy Clin Immunol. (2001) 108:797–803. doi: 10.1067/mai.2001.119025
75. Dinh QT, Cryer A, Trevisani M, Dinh S, Wu S, Cifuentes LB, et al. Gene and protein expression of protease-activated receptor 2 in structural and inflammatory cells in the nasal mucosa in seasonal allergic rhinitis. Clin Exp Allergy. (2006) 36:1039–48. doi: 10.1111/j.1365-2222.2006.02537.x
76. Lee HM, Kim HY, Kang HJ, Woo JS, Chae SW, Lee SH, et al. Up-regulation of protease-activated receptor 2 in allergic rhinitis. Ann Otol Rhinol Laryngol. (2007) 116:554–8. doi: 10.1177/000348940711600712
77. Nystedt S, Ramakrishnan V, Sundelin J. The proteinase-activated receptor 2 is induced by inflammatory mediators in human endothelial cells. Comparison with the thrombin receptor. J Biol Chem. (1996) 271:14910–5. doi: 10.1074/jbc.271.25.14910
78. Ritchie E, Saka M, Mackenzie C, Drummond R, Wheeler-Jones C, Kanke T, et al. Cytokine upregulation of proteinase-activated-receptors 2 and 4 expression mediated by p38 MAP kinase and inhibitory kappa B kinase beta in human endothelial cells. Br J Pharmacol. (2007) 150:1044–54. doi: 10.1038/sj.bjp.0707150
79. Grab DJ, Garcia-Garcia JC, Nikolskaia OV, Kim YV, Brown A, Pardo CA, et al. Protease activated receptor signaling is required for African trypanosome traversal of human brain microvascular endothelial cells. PLoS Negl Trop Dis. (2009) 3:e479. doi: 10.1371/journal.pntd.0000479
80. Zhang H, Lin L, Yang H, Zhang Z, Yang X, Zhang L, et al. Induction of IL-13 production and upregulation of gene expression of protease activated receptors in P815 cells by IL-6. Cytokine. (2010) 50:138–45. doi: 10.1016/j.cyto.2010.02.006
81. Xiang Y, Masuko-Hongo K, Sekine T, Nakamura H, Yudoh K, Nishioka K, et al. Expression of proteinase-activated receptors (PAR)-2 in articular chondrocytes is modulated by IL-1beta, TNF-alpha and TGF-beta. Osteoarthritis Cartilage. (2006) 14:1163–73. doi: 10.1016/j.joca.2006.04.015
82. Gruber BL, Marchese MJ, Santiago-Schwarz F, Martin CA, Zhang J, Kew RR. Protease-activated receptor-2 (PAR-2) expression in human fibroblasts is regulated by growth factors and extracellular matrix. J Invest Dermatol. (2004) 123:832–9. doi: 10.1111/j.0022-202X.2004.23445.x
83. Sokolova E, Aleshin S, Reiser G. Expression of protease-activated receptor (PAR)-2, but not other PARs, is regulated by inflammatory cytokines in rat astrocytes. Neurochem Int. (2012) 60:276–85. doi: 10.1016/j.neuint.2011.12.016
84. Zheng J, Liu W, Fan Y, Ye X, Xia W, Wang H, et al. Suppression of connexin 26 is related to protease-activated receptor 2-mediated pathway in patients with allergic rhinitis. Am J Rhinol Allergy. (2012) 26:e5–9. doi: 10.2500/ajra.2012.26.3740
85. Svensson KJ, Kucharzewska P, Christianson HC, Skold S, Lofstedt T, Johansson MC, et al. Hypoxia triggers a proangiogenic pathway involving cancer cell microvesicles and PAR-2-mediated heparin-binding EGF signaling in endothelial cells. Proc Natl Acad Sci USA. (2011) 108:13147–52. doi: 10.1073/pnas.1104261108
86. Allard B, Bara I, Gilbert G, Carvalho G, Trian T, Ozier A, et al. Protease activated receptor-2 expression and function in asthmatic bronchial smooth muscle. PLoS One. (2014) 9:e86945. doi: 10.1371/journal.pone.0086945
87. Gandhi VD, Shrestha Palikhe N, Hamza SM, Dyck JRB, Buteau J, Vliagoftis H. Insulin decreases expression of the proinflammatory receptor proteinase-activated receptor-2 on human airway epithelial cells. J Allergy Clin Immunol. (2018) 142:1003–6e8. doi: 10.1016/j.jaci.2018.04.040
88. Karlina R, Flexeder C, Musiol S, Bhattacharyya M, Schneider E, Altun I, et al. Differential effects of lung inflammation on insulin resistance in humans and mice. Allergy (2022). [Online ahead of print]. doi: 10.1111/all.15226
89. Baffi CW, Wood L, Winnica D, Strollo PJ Jr., Gladwin MT, Que LG, et al. Metabolic syndrome and the lung. Chest. (2016) 149:1525–34. doi: 10.1016/j.chest.2015.12.034
90. Lee JH, Kim KW, Gee HY, Lee J, Lee KH, Park HS, et al. A synonymous variation in protease-activated receptor-2 is associated with atopy in Korean children. J Allergy Clin Immunol. (2011) 128:1326–34e3. doi: 10.1016/j.jaci.2011.06.036
91. Han SL, Zhang YJ, Zhou M, Luan C, Wang P, Zhai L. Association of PAR-2 gene polymorphisms with the inflammatory response and susceptibility to knee osteoarthritis in the Chinese han population. Genet Test Mol Biomarkers. (2019) 23:84–90. doi: 10.1089/gtmb.2018.0219
92. McIntosh KA, Cunningham MR, Bushell T, Plevin R. The development of proteinase-activated receptor-2 modulators and the challenges involved. Biochem Soc Trans. (2020) 48:2525–37. doi: 10.1042/BST20200191
93. Giblin P, Boxhammer R, Desai S, Kroe-Barrett R, Hansen G, Ksiazek J, et al. Fully human antibodies against the Protease-Activated Receptor-2 (PAR-2) with anti-inflammatory activity. Hum Antibodies. (2011) 20:83–94. doi: 10.3233/HAB-2011-0243
94. Godman B, Finlayson AE, Cheema PK, Zebedin-Brandl E, Gutierrez-Ibarluzea I, Jones J, et al. Personalizing health care: feasibility and future implications. BMC Med. (2013) 11:179. doi: 10.1186/1741-7015-11-179
95. Subramanian M, Wojtusciszyn A, Favre L, Boughorbel S, Shan J, Letaief KB, et al. Precision medicine in the era of artificial intelligence: implications in chronic disease management. J Transl Med. (2020) 18:472. doi: 10.1186/s12967-020-02658-5
96. Popovic-Grle S, Stajduhar A, Lampalo M, Rnjak D. Biomarkers in different asthma phenotypes. Genes. (2021) 12:801. doi: 10.3390/genes12060801
97. Ray A, Camiolo M, Fitzpatrick A, Gauthier M, Wenzel SE. Are we meeting the promise of endotypes and precision medicine in asthma? Physiol Rev. (2020) 100:983–1017. doi: 10.1152/physrev.00023.2019
98. Fowler SJ, Sterk PJ. Breath biomarkers in asthma: we’re getting answers, but what are the important questions? Eur Respir J. (2019) 54:1901411. doi: 10.1183/13993003.01411-2019
99. Shrestha Palikhe N, Nahirney D, Laratta C, Gandhi VD, Vethanayagam D, Bhutani M, et al. Increased protease-activated receptor-2 (PAR-2) expression on CD14++CD16+ peripheral blood monocytes of patients with severe asthma. PLoS One. (2015) 10:e0144500. doi: 10.1371/journal.pone.0144500
100. Crilly A, Burns E, Nickdel MB, Lockhart JC, Perry ME, Ferrell PW, et al. PAR(2) expression in peripheral blood monocytes of patients with rheumatoid arthritis. Ann Rheum Dis. (2012) 71:1049–54. doi: 10.1136/annrheumdis-2011-200703
101. Jiang B, Grage-Griebenow E, Csernok E, Butherus K, Ehlers S, Gross WL, et al. The role of proteinase 3 (PR3) and the protease-activated receptor-2 (PAR-2) pathway in dendritic cell (DC) maturation of human-DC-like monocytes and murine DC. Clin Exp Rheumatol. (2010) 28:56–61.
102. Lopez-Pedrera C, Aguirre MA, Buendia P, Barbarroja N, Ruiz-Limon P, Collantes-Estevez E, et al. Differential expression of protease-activated receptors in monocytes from patients with primary antiphospholipid syndrome. Arthritis Rheum. (2010) 62:869–77. doi: 10.1002/art.27299
103. Garcia-Gonzalez G, Sanchez-Gonzalez A, Hernandez-Bello R, Gonzalez GM, Franco-Molina MA, Coronado-Cerda EE, et al. Triggering of protease-activated receptors (PARs) induces alternative M2 macrophage polarization with impaired plasticity. Mol Immunol. (2019) 114:278–88. doi: 10.1016/j.molimm.2019.08.004
104. Berkman N, Krishnan VL, Gilbey T, Newton R, O’Connor B, Barnes PJ, et al. Expression of RANTES mRNA and protein in airways of patients with mild asthma. Am J Respir Crit Care Med. (1996) 154:1804–11. doi: 10.1164/ajrccm.154.6.8970374
105. Rojas-Ramos E, Avalos AF, Perez-Fernandez L, Cuevas-Schacht F, Valencia-Maqueda E, Teran LM. Role of the chemokines RANTES, monocyte chemotactic proteins-3 and -4, and eotaxins-1 and -2 in childhood asthma. Eur Respir J. (2003) 22:310–6. doi: 10.1183/09031936.03.00084802
106. Shrestha Palikhe NWY, Gandhi VD, Cameron L, Vliagoftis H editors. Lipopolysaccaride (LPS) induction of Proteinase-Activated Receptor-2 (PAR-2) expression on CD14++CD16+ peripheral blood monocytes. Annual EAACI meeting 2018, May 26-30, Munich, Germany. Munich: EAACI (2018).
107. Palikhe NS, Gandhi VD, Wu Y, Sinnatamby T, Rowe BH, Mayers I, et al. Peripheral blood intermediate monocyte protease-activated receptor-2 expression increases during asthma exacerbations and after inhalation allergen challenge. Ann Allergy Asthma Immunol. (2021) 127:249–56e2. doi: 10.1016/j.anai.2021.04.016
108. Shrestha Palikhe N, Bosonea AM, Laratta C, Gandhi VD, Nahirney D, Hillaby A, et al. Stability of peripheral blood immune markers in patients with asthma. Allergy Asthma Clin Immunol. (2019) 15:30. doi: 10.1186/s13223-019-0343-4
Keywords: PAR-2, asthma, biomarker, severity, allergic disease
Citation: Gandhi VD, Shrestha Palikhe N and Vliagoftis H (2022) Protease-activated receptor-2: Role in asthma pathogenesis and utility as a biomarker of disease severity. Front. Med. 9:954990. doi: 10.3389/fmed.2022.954990
Received: 27 May 2022; Accepted: 15 July 2022;
Published: 29 July 2022.
Edited by:
A. A. Roger Thompson, The University of Sheffield, United KingdomReviewed by:
Beatrice Ludovica Ritondo, University of Rome Tor Vergata, ItalyCopyright © 2022 Gandhi, Shrestha Palikhe and Vliagoftis. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Harissios Vliagoftis, aGFyaUB1YWxiZXJ0YS5jYQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.