- 1Department of Spine Surgery, Weihai Municipal Hospital, Cheeloo College of Medicine, Shandong University, Weihai, China
- 2Department of Medical Imaging, Weihai Wendeng District People’s Hospital, Weihai, China
- 3Department of Traumatic Orthopaedics, Shandong Provincial Hospital Affiliated to Shandong First Medical University, Jinan, China
Background: The association between cholesterol and triglycerides with the lumbar bone mineral density (BMD) was widely investigated, but the results remained conflicting. This study aimed to investigate the relationship between total cholesterol, triglycerides, and total lumbar BMD in adults.
Materials and methods: This cross-sectional study included 1,985 individuals aged 50 years and over. The data on total cholesterol, triglycerides, total lumbar BMD, and other covariates were obtained from the National Health and Nutritional (NHANES) between 2017 and March 2020 pre-pandemic. Multivariate logistic regression models were utilized to investigate the association between cholesterol, triglycerides, and total lumbar BMD. Smooth curve fittings and generalized additive models were also used to analyze the potential non-linearity.
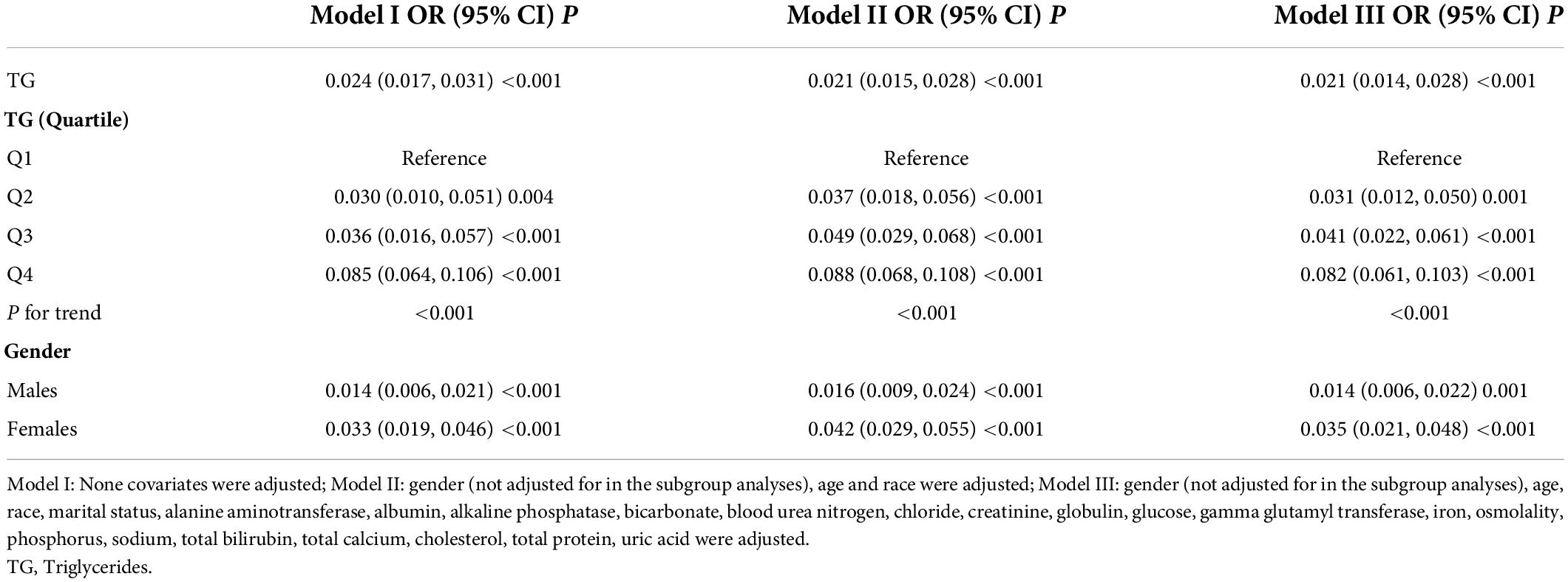
Results: A total of 901 men and 1,084 women with a mean age of 63.02 ± 8.72 years (age 50–80 years) were included in this study. In multivariate regression analysis, the association between cholesterol and total lumbar BMD was negative (β = −0.026, 95% CI: −0.033, −0.020). This relationship still existed after adjusted for gender and race (β = −0.018, 95% CI: −0.025, −0.012) and fully adjusted for all covariates (β = −0.022, 95% CI: −0.029, −0.015). The association between triglycerides and total lumbar BMD was positive (β = 0.024, 95% CI: 0.017, 0.031). This relationship still existed after adjusted for gender and race (β = 0.021, 95% CI: 0.015, 0.028) and fully adjusted for all covariates (β = 0.021, 95% CI: 0.014, 0.028). In threshold effect analysis, the relationship between triglycerides and total lumbar BMD was an inverted U-shaped curve with the inflection point at 2.597 mmol/L.
Conclusion: High levels of total cholesterol and relatively low levels of triglycerides are significantly associated with the total lumbar BMD in adults aged 50 years and over.
Introduction
Osteoporosis is one of the most prevalent public health threats in elderly populations, characterized by microarchitectural deterioration, reduced lumbar bone mineral density (BMD), and skeletal fragility. The diagnosis of osteoporosis is based on the BMD measured by dual-energy X-ray absorptiometry (DXA). Fracture is the most serious clinical consequence of osteoporosis. It is estimated that 1.5 million fractures were caused by osteoporosis per year in the United States (1). Osteoporosis-related fractures can result in poor quality of life, high healthcare costs, and an increased risk of death. Low BMD is one of the important risk factors for fractures (2).
Atherosclerosis is another severe hazard to health and quality of life. The metabolic disorder of cholesterol and triglycerides is the main cause of atherosclerosis. Atherosclerosis and osteoporosis often coincide with each other in elderly people (3), yielding the metabolism of cholesterol and triglycerides associated with BMD. To assess the potential role of cholesterol and triglycerides in osteoporosis, different studies have been investigated but the results remained conflicting. Therefore, the objective of the current study was to evaluate the relationship between total cholesterol, triglycerides, and total lumbar BMD in adults aged over 50 years using a nationally representative sample from the NHANES 2017–2020.
Materials and methods
Study population
Data were obtained from the NHANES between 2017 and March 2020 pre-pandemic for this study. NHANES databases are cross-sectional surveys conducted by the National Center for Health Statistics, providing multitudinous health and nutrition data of the general United States population.
The data of NHANES are publicly available on the internet. Full detailed information about NHANES can be found on the website.1 The conduct of NHANES was approved by the NCHS Ethics Review Board, and the signed written informed consents were obtained from all included participants (4).
A total of 15,560 individuals aged 50 years and over were enrolled for this study from the NHANES between 2017 and March 2020 pre-pandemic. After exclusion of 13,575 subjects with missing total lumbar BMD (n = 13439) or total cholesterol, triglycerides (n = 136) data, and 1,985 subjects remained for the final analysis (Figure 1).
Study variables
The exposure variables were total cholesterol and triglycerides. Total cholesterol and triglycerides were conducted with blood serum at Advanced Research and Diagnostic Laboratory, using Roche Cobas 6000 (c501 module).
The outcome variable was total lumbar BMD. The measurement of total lumbar BMD was provided by DAX scans, administered by trained and certified radiology technologists. The spine scans were acquired on Hologic Discovery model A densitometers (Hologic, Inc., Bedford, MA, United States) in 2017–2018 and on Hologic Horizon model A densitometers (Hologic, Inc., Bedford, MA, United States) in 2019–2020. A cross-calibration study was conducted to assure the accuracy of the NHANES longitudinal assessment.
Multivariate models contain variables that might confound the links between total cholesterol, triglycerides, and total lumbar BMD. The data on gender, age, race, and marital status were obtained from questionnaires. The data on alanine aminotransferase, albumin, alkaline phosphatase, bicarbonate, blood urea nitrogen, chloride, creatinine, globulin, glucose, gamma-glutamyl transferase, iron, osmolality, phosphorus, sodium, total bilirubin, total calcium, total protein, and uric acid were obtained from standard biochemistry profile analysis with a Roche Cobas 6000. Detailed information about these covariates can be obtained from the NHANES website.
Statistical analyses
The study participants were stratified into quartiles according to total cholesterol and triglycerides levels. Weighted multivariable linear regression models were applied to analyze the association between total cholesterol, triglycerides, and total lumbar BMD. Weighted smooth curve fittings and generalized additive models were used to analyze the potential non-linearity. Two-piecewise linear regression models were used to calculate the threshold effects if non-linearity associations existed. Three models were built: Model I, an unadjusted model; Model II, minimally adjusted for gender, age, and race; Model III, fully adjusted for all covariates (listed in Tables 1, 2). All analyses were performed using the Empower-Stats software (version 2.0. X&Y Solutions, Boston, MA, United States) and statistical software R (version 3.4.3). The p < 0.05 was considered statistically significant.
Results
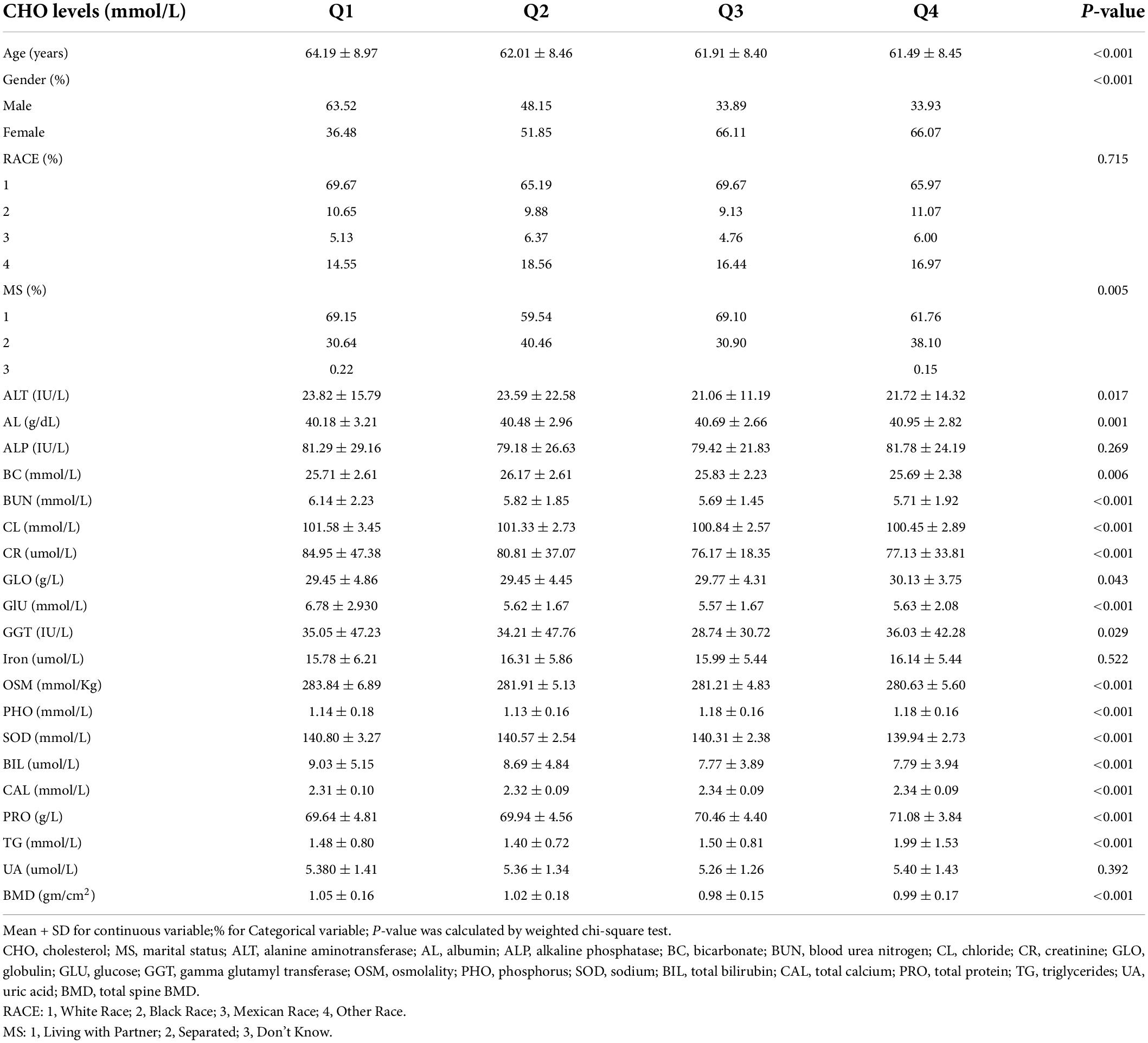
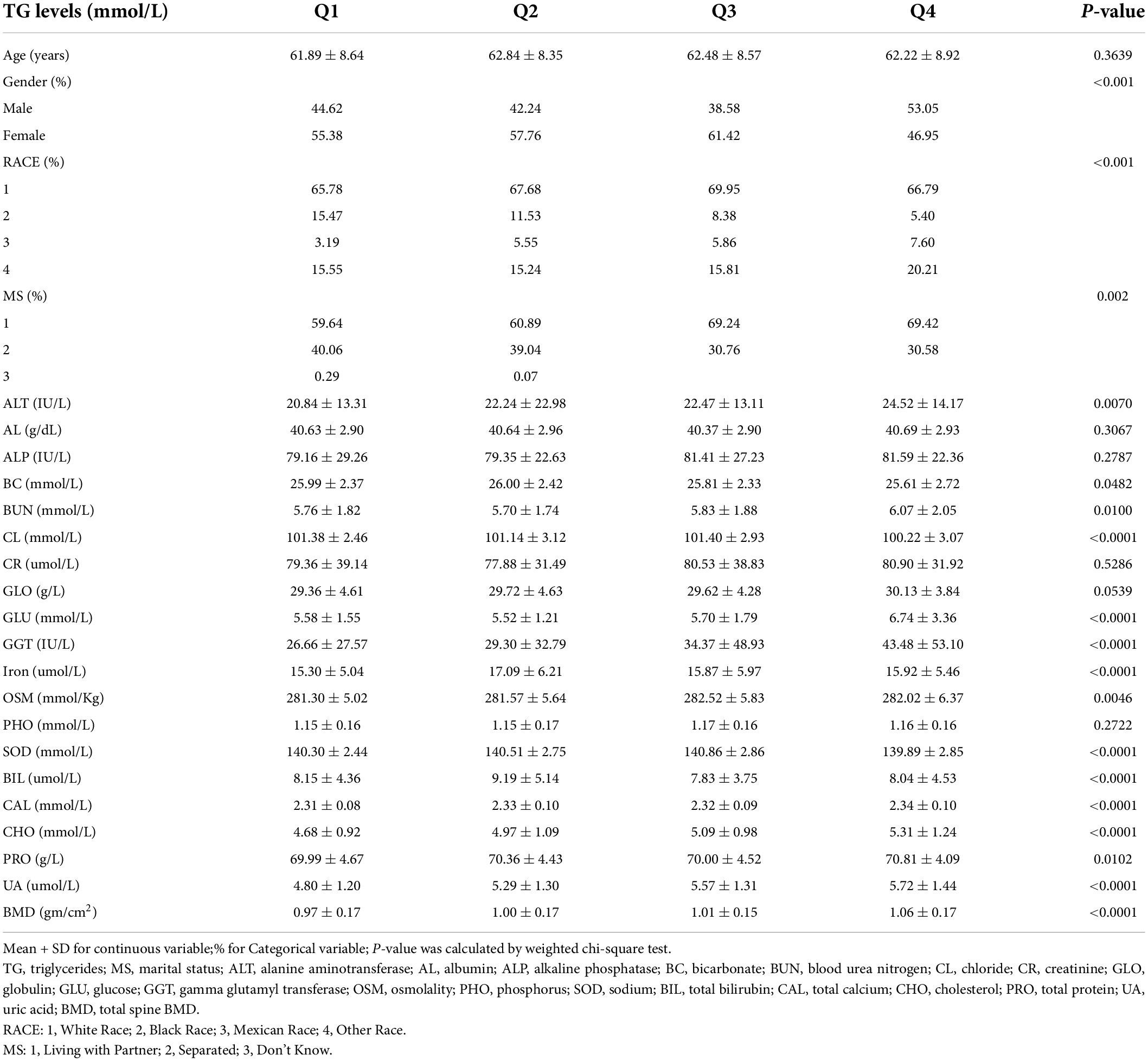
There are 901 men and 1,084 women included in this study. Age averaged 63.02 ± 8.72 years with a range of 50–80 years. Baseline characteristics of all subjects, classified by quartiles of total cholesterol and triglycerides levels, are, respectively, presented in Tables 1, 2. As shown in Table 1, subjects with lower total cholesterol levels were older and the lowest lumbar BMD was in the Q3 group. In Table 2, subjects with lower triglycerides levels had lower BMD and the lowest lumbar BMD was in the Q1 group.
The association between total cholesterol and total lumbar BMD was negative in all three regression models (Table 3): model 1 (β = −0.026, 95% CI: −0.033, −0.020); model II (β = −0.018, 95% CI: −0.025, −0.012); model III (β = −0.022, 95% CI: −0.029, −0.015). Stratified by quartile level of total cholesterol, the trend test remained significant in Q3 and Q4 subgroups (p < 0.001). In the subgroup analysis stratified by gender, this negative association remained in the women group: model 1 (β = −0.027, 95% CI: −0.036, −0.017); model II (β = −0.028, 95% CI: −0.037, −0.019); model III (β = −0.028, 95% CI: −0.036, −0.019). In the men group, this association was no longer significant in models I and II but became negative after controlling in the fully adjusted model III (β = −0.011, 95% CI: −0.022, −0.001).
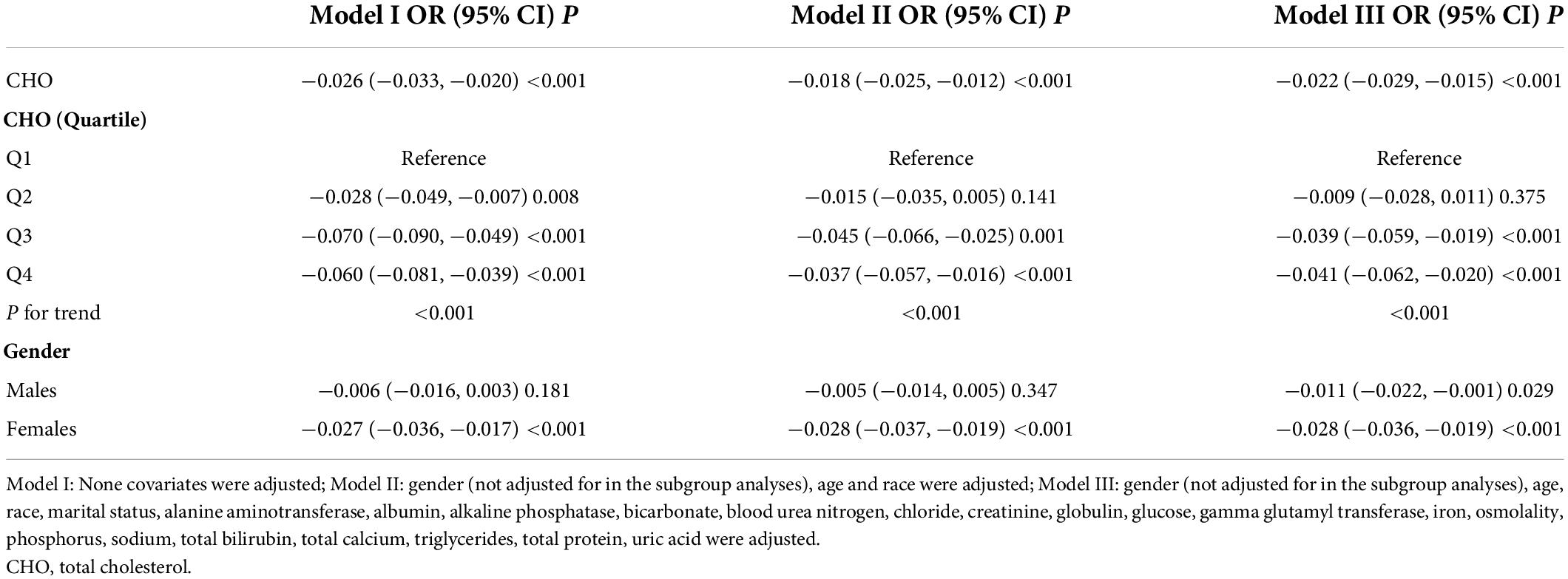
Table 3. Associations between total cholesterol (mmol/L) and total spine bone mineral density (BMD) (gm/cm2).
The association between triglycerides and total lumbar BMD was positive in all three regression models (Table 4): model 1 (β = 0.024, 95% CI: 0.017, 0.031); model II (β = 0.021, 95% CI: 0.015, 0.028); and model III (β = 0.021, 95% CI: 0.014, 0.028). Moreover, the trend remained significant among the different triglycerides level quartile groups (p for trend <0.001). In the subgroup analysis stratified by gender, we also observed a positive association between triglycerides and total lumbar BMD after controlling for potential confounding factors, with all p-values < 0.001.
The non-linear relationship between total cholesterol, triglycerides, and total lumbar BMD is shown in Figures 2, 3, respectively. Using a two-piecewise linear regression model, the point of inflection in the U-shaped association was at a level of 6.077 mol/L for total cholesterol and 2.597 mol/L for triglycerides. On both sides of the inflection point, there were higher cholesterol levels and lower lumbar BMD (Table 5). To the left of the inflection point, there was a higher triglycerides level and lumbar BMD. To the right of the inflection point, there was a higher triglycerides level and lower lumbar BMD, but the difference was not statistically significant (Table 6).
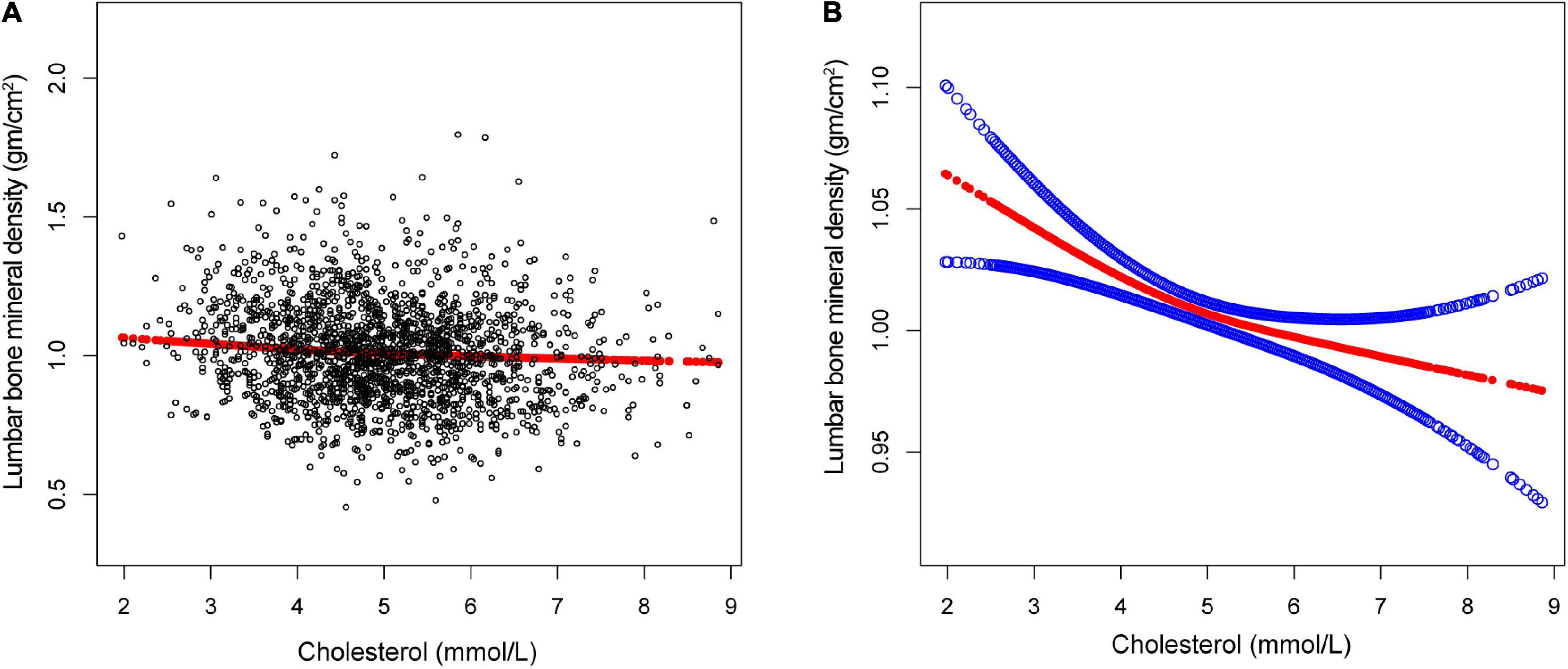
Figure 2. The association between total cholesterol and lumbar bone mineral density (BMD). (A) Each black point represents a single participant cholesterol sample. (B) Solid red line represents the smooth curve fit between variables. Blue bands represent the 95% of confidence interval from the fit. Gender, age, race, marital status, alanine aminotransferase, albumin, alkaline phosphatase, bicarbonate, blood urea nitrogen, chloride, creatinine, globulin, glucose, gamma glutamyl transferase, iron, osmolality, phosphorus, sodium, total bilirubin, total calcium, triglycerides, total protein, and uric acid were adjusted.
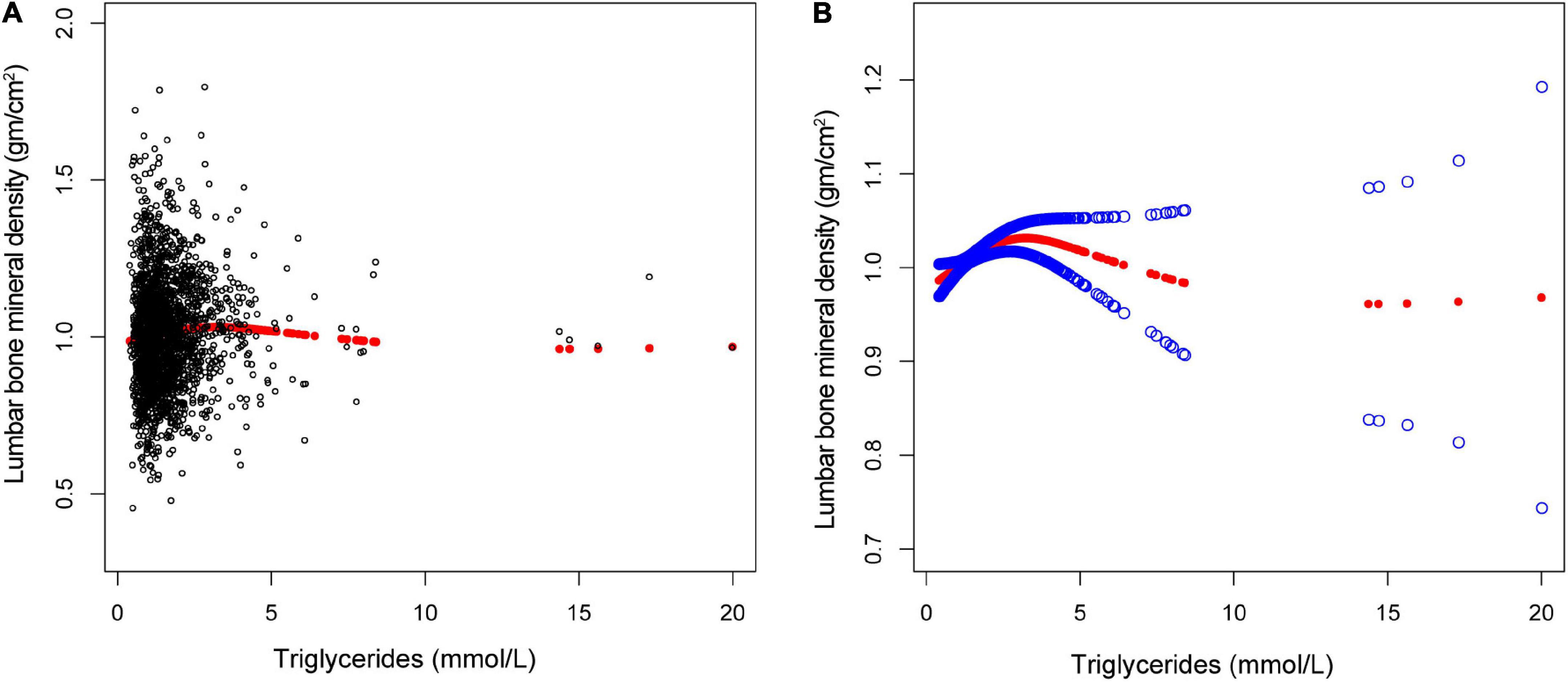
Figure 3. The association between triglycerides and lumbar bone mineral density (BMD). (A) Each black point represents a single participant triglycerides sample. (B) Solid red line represents the smooth curve fit between variables. Blue bands represent the 95% of confidence interval from the fit. Gender, age, race, marital status, alanine aminotransferase, albumin, alkaline phosphatase, bicarbonate, blood urea nitrogen, chloride, creatinine, globulin, glucose, gamma glutamyl transferase, iron, osmolality, phosphorus, sodium, total bilirubin, total calcium, cholesterol, total protein, and uric acid were adjusted.
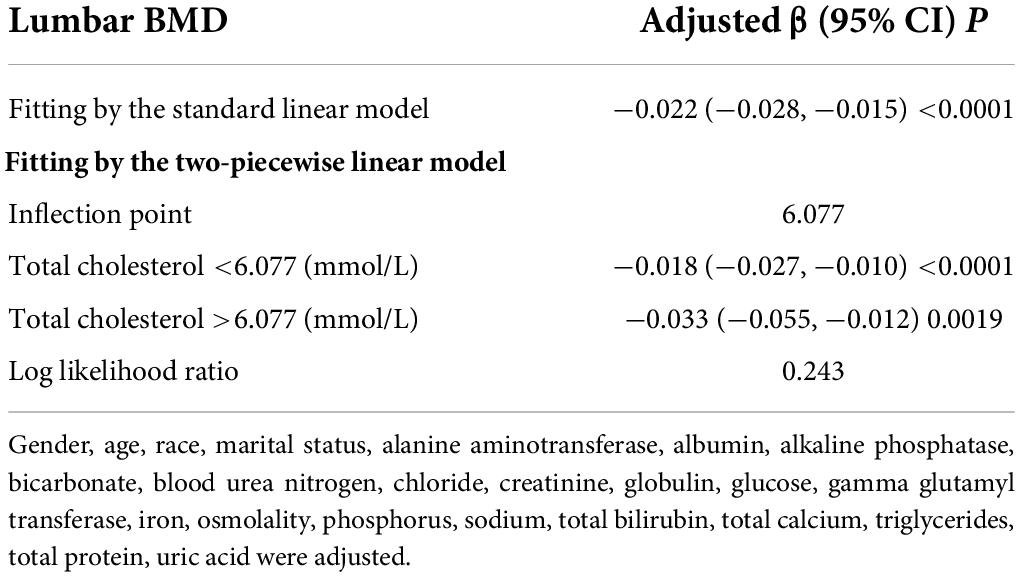
Table 5. Threshold effect analysis of total cholesterol on lumbar BMD using the two-piecewise linear regression model.
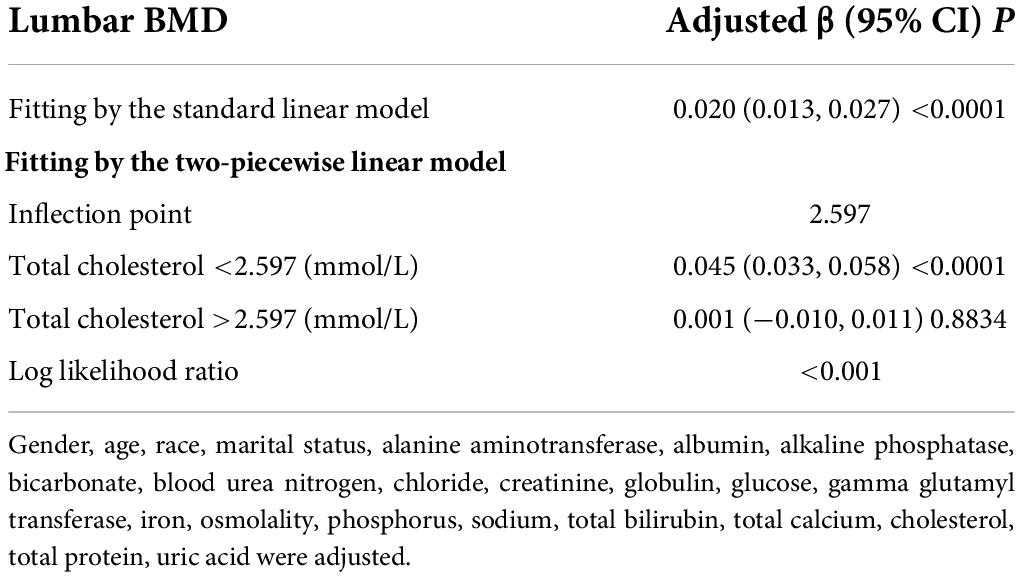
Table 6. Threshold effect analysis of triglycerides on lumbar BMD using the two-piecewise linear regression model.
Discussion
In this current study, we used the lasted representative data of NHANES (2017–2020) to evaluate the associations between total cholesterol, triglycerides, and total lumbar BMD in aged 50 years and over. The results revealed a negative association between total cholesterol and total lumbar BMD and a positive association between triglycerides and total lumbar BMD. Specifically, we identified a U-shaped association between cholesterol and total lumbar BMD, with an inverted U-shaped association between triglycerides and total lumbar BMD.
Abnormal cholesterol metabolism has been associated with several metabolic diseases. Numerous studies investigated the association between cholesterol and BMD in the general population, but conclusions were inconsistent. In most studies, a higher level of cholesterol was found to be associated with an increased risk of osteoporosis or a lower BMD (5–8). A non-association between cholesterol and BMD was also reported (9–11). Our findings that higher total cholesterol levels are associated with lower spine BMD do support the most previous research studies. However, in the subgroup analysis stratified by quartile level of total cholesterol, the negative association between cholesterol and lumbar BMD remained only in Q3 and Q4 subgroups. In the subgroup analysis stratified by gender, this negative association remained in the women group. These results suggest that different subgroups of the same variable may also affect this association. The mechanism of negative associations between cholesterol and osteoporosis is still unclear. The oxidized lipid could be one of the reasons to cause osteoporosis, by inhibiting osteoblast differentiation and reducing bone mineralization (12, 13). Other potential mechanisms may involve bone metabolic abnormalities due to inflammatory regulators and flow-limiting atherosclerotic hypoperfusion (14, 15).
Hypertriglyceridemia is one of the most common lipid abnormalities. Many diseases caused by hypertriglyceridemia have been identified. The links between triglyceride and BMD were widely studied in the general population with different conclusions. Multiple evidence supported that increased triglyceride level was significantly associated with a decreased BMD or osteoporosis (16). Consistent with the above, a positive association between them was also observed (17). In order to clarify the relationship between triglyceride and total spine BMD, our present study showed a positive association between them. In the subgroup analysis stratified by quartile level of triglycerides or by gender, this positive correlation maintains consistency. Our findings also suggested that there is an inverted U-shaped relationship between triglyceride and BMD by further threshold effect analysis. When a triglyceride is less than 2.597 (mmol/L), the relationship between triglyceride levels and lumbar BMD is positively correlated. When a triglyceride is greater than 2.597 (mmol/L), it seemed that an increased triglyceride level is associated with a decreased BMD, but the difference was not statistically significant. This may imply that triglyceride has a dose-dependent effect on total lumbar BMD. A normal or moderately high level of triglyceride plays a beneficial role on bone, while a very high level of triglyceride may be unrelated to bone health. Similar to our finding, the dose–response relationship of triglyceride to BMD also has been reported in previous studies (18).
Our study has several strengths compared to the previous studies. First, we used the lasted data from NHANES between 2017 and March 2020 pre-pandemic in aged 50 years and over. Second, our results demonstrated a positive association between triglyceride and total lumbar BMD with a point of inflection at 2.597 mmol/L. The inverted U-shaped association between triglycerides and total lumbar BMD was not revealed in the previous studies. The relationships between cholesterol, triglycerides, and BMD have been conflicting. The possible reasons for these differences may be associated with heterogeneity in sample size, participant type, and covariate correction. There are several limitations to this study. First, the NHANES database is a cross-sectional survey, thus temporality and causation cannot be inferred from this study. More fundamental mechanistic research and large sample prospective studies are needed to understand the particular mechanism of them. Second, data collection for the NHANES 2019–2020 cycle was not completed due to the coronavirus disease 2019 pandemic. Last, other possible covariates, such as therapeutic intervention, autoimmune, and other conditions, did not take into account. Different covariates may influence the links between cholesterol, triglycerides, and lumbar BMD. High-quality studies on the relationship between cholesterol, triglycerides, and total lumbar BMD are required to confirm or oppose our findings.
Conclusion
In people aged 50 years and over, our research discovered a negative association between total cholesterol and total lumbar BMD and a positive association between triglycerides and total lumbar BMD. There was an inverted U-shaped association between triglycerides and total lumbar BMD with a point of inflection at 2.597 mmol/L.
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/Supplementary material.
Ethics statement
Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
PW performed and interpreted statistics and also drafted the initial manuscript. CC and CS were responsible for data collection and statistical analysis. JJ and YW supported the manuscript writing. WM contributed to the study design for the whole research and revised the manuscript. All authors read and approved the final manuscript.
Funding
This study was supported by the Weihai Municipal Hospital incubation project (FH2021JY06), Shandong Provincial Natural Science Foundation (ZR202102270170), and Shandong Medical and Health Science Technology Development Program (2019WS233).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2022.923730/full#supplementary-material
Footnotes
References
1. Black DM, Rosen CJ. Clinical practice. Postmenopausal osteoporosis. N Engl J Med. (2016) 374:254–62. doi: 10.1056/NEJMcp1513724
2. Compston JE, McClung MR, Leslie WD. Osteoporosis. Lancet. (2019) 393:364–76. doi: 10.1016/S0140-6736(18)32112-3
3. Mishra BH, Mishra PP, Mononen N, Hilvo M, Sievanen H, Juonala M, et al. Uncovering the shared lipidomic markers of subclinical osteoporosis-atherosclerosis comorbidity: The young finns study. Bone. (2021) 151:116030. doi: 10.1016/j.bone.2021.116030
4. Zipf G, Chiappa M, Porter KS, Ostchega Y, Lewis BG, Dostal J National health and nutrition examination survey: Plan and operations, 1999–2010. Vital Health Stat Ser 1. (2013) 56:1–37.
5. Yang XL, Cui ZZ, Zhang H, Wei XT, Feng GJ, Liu L, et al. Causal link between lipid profile and bone mineral density: A mendelian randomization study. Bone. (2019) 127:37–43. doi: 10.1016/j.bone.2019.05.037
6. Kan B, Zhao Q, Wang L, Xue S, Cai H, Yang S. Association between lipid biomarkers and osteoporosis: A cross-sectional study. BMC Musculoskeletal Disord. (2021) 22:759. doi: 10.1186/s12891-021-04643-5
7. Zhang Q, Zhou J, Wang Q, Lu C, Xu Y, Cao H, et al. Association between bone mineral density and lipid profile in Chinese women. Clin Interventions Aging. (2020) 15:1649–64. doi: 10.2147/CIA.S266722
8. Yang Y, Liu G, Zhang Y, Xu G, Yi X, Liang J, et al. Association between bone mineral density, bone turnover markers, and serum cholesterol levels in type 2 diabetes. Front Endocrinol. (2018) 9:646. doi: 10.3389/fendo.2018.00646
9. Zolfaroli I, Ortiz E, Garcia-Perez MA, Hidalgo-Mora JJ, Tarin JJ, Cano A. Positive association of high-density lipoprotein cholesterol with lumbar and femoral neck bone mineral density in postmenopausal women. Maturitas. (2021) 147:41–6. doi: 10.1016/j.maturitas.2021.03.001
10. Alay I, Kaya C, Cengiz H, Yildiz S, Ekin M, Yasar L. The relation of body mass index, menopausal symptoms, and lipid profile with bone mineral density in postmenopausal women. Taiwanese J Obstetrics Gynecol. (2020) 59:61–6. doi: 10.1016/j.tjog.2019.11.009
11. Niu P, Li H, Liu D, Zhang YF, Liu Y, Liang C. Association between hdl-c and bone mineral density: An cross-sectional analysis. Int J Gen Med. (2021) 14:8863–72. doi: 10.2147/IJGM.S334972
12. Parhami F, Morrow AD, Balucan J, Leitinger N, Watson AD, Tintut Y, et al. Lipid oxidation products have opposite effects on calcifying vascular cell and bone cell differentiation. A possible explanation for the paradox of arterial calcification in osteoporotic patients. Arteriosclerosis Thrombosis Vasc Biol. (1997) 17:680–7. doi: 10.1161/01.atv.17.4.680
13. Parhami F, Garfinkel A, Demer LL. Role of lipids in osteoporosis. Arteriosclerosis Thrombosis Vasc Biol. (2000) 20:2346–8. doi: 10.1161/01.ATV.20.11.2346
14. Demer LL, Tintut Y. Mechanisms linking osteoporosis with cardiovascular calcification. Curr Osteoporosis Rep. (2009) 7:42–6. doi: 10.1007/s11914-009-0008-1
15. Lello S, Capozzi A, Scambia G. Osteoporosis and cardiovascular disease: An update. Gynecol Endocrinol Off J Int Soc Gynecol Endocrinol. (2015) 31:590–4. doi: 10.3109/09513590.2015.1041908
16. Xu D, di Wang K, Yang J. Triglyceride can predict the discordance between QCT and DXA screening for BMD in old female patients. Dis Markers. (2020) 27:8898888. doi: 10.1155/2020/8898888
17. Saoji R, Das RS, Desai M, Pasi A, Sachdeva G, Das TK, et al. Association of high-density lipoprotein, triglycerides, and homocysteine with bone mineral density in young indian tribal women. Arch Osteoporosis. (2018) 13:108. doi: 10.1007/s11657-018-0525-6
Keywords: total cholesterol, triglycerides, lumbar bone mineral density, osteoporosis, NHANES
Citation: Wang P, Chen C, Song C, Jia J, Wang Y and Mu W (2022) High cholesterol and low triglycerides are associated with total lumbar bone mineral density among adults aged 50 years and over: The NHANES 2017–2020. Front. Med. 9:923730. doi: 10.3389/fmed.2022.923730
Received: 11 May 2022; Accepted: 18 July 2022;
Published: 08 August 2022.
Edited by:
Giovanni Maga, Institute of Molecular Genetics Luigi Luca Cavalli-Sforza, ItalyReviewed by:
Rajeev Aurora, Saint Louis University, United StatesMara Carsote, Carol Davila University of Medicine and Pharmacy, Romania
Copyright © 2022 Wang, Chen, Song, Jia, Wang and Mu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Weidong Mu, sdphweidongmu@126.com
 Peng Wang1
Peng Wang1