- 1Department of Medicine, University of California, Irvine, Irvine, CA, United States
- 2Department of Surgery, University of Washington, Seattle, WA, United States
- 3No Resistance Consulting, Birmingham, AL, United States
- 4Division of Pulmonary and Critical Care Medicine, Department of Medicine, Washington University School of Medicine, St. Louis, MO, United States
- 5College of Pharmacy, University of Rhode Island, Kingston, RI, United States
- 6Department of Emergency Medicine, Valleywise Health, Arizona State University, Phoenix, AZ, United States
- 7Infectious Disease Clinical Outcomes Research Unit, Division of Infectious Disease, Lundquist Research Institute at Harbor-UCLA, Torrance, CA, United States
- 8GST Micro LLC, North, VA, United States
- 9American Family Care, Birmingham, AL, United States
Antibiotic-resistant pathogens cause over 35,000 preventable deaths in the United States every year, and multiple strategies could decrease morbidity and mortality. As antibiotic stewardship requirements are being deployed for the outpatient setting, community providers are facing systematic challenges in implementing stewardship programs. Given that the vast majority of antibiotics are prescribed in the outpatient setting, there are endless opportunities to make a smart and informed choice when prescribing and to move the needle on antibiotic stewardship. Antibiotic stewardship in the community, or “smart prescribing” as we suggest, should factor in antibiotic efficacy, safety, local resistance rates, and overall cost, in addition to patient-specific factors and disease presentation, to arrive at an appropriate therapy. Here, we discuss some of the challenges, such as patient/parent pressure to prescribe, lack of data or resources for implementation, and a disconnect between guidelines and real-world practice, among others. We have assembled an easy-to-use best practice guide for providers in the outpatient setting who lack the time or resources to develop a plan or consult lengthy guidelines. We provide specific suggestions for antibiotic prescribing that align real-world clinical practice with best practices for antibiotic stewardship for two of the most common bacterial infections seen in the outpatient setting: community-acquired pneumonia and skin and soft-tissue infection. In addition, we discuss many ways that community providers, payors, and regulatory bodies can make antibiotic stewardship easier to implement and more streamlined in the outpatient setting.
Introduction
Every year in the United States (US), antibiotic-resistant pathogens are implicated in at least 35,000 deaths and over 2.8 million infections (1). Fundamentals of antibiotic stewardship dictate that clinicians can reduce the impact of antibiotic resistance by carefully prescribing antibiotics only when needed, with the right drug, dosage, and duration (2). While hospital-based stewardship programs have demonstrated remarkable value and healthcare benefit, the expansion of stewardship to the outpatient setting—including primary care clinics, urgent care (UC) settings, and skilled nursing facilities—may be less successful unless consideration is given to the unique nature of outpatient healthcare. This article describes the scope of the problem with outpatient stewardship in the US and systematic challenges limiting implementation, offering some pragmatic solutions to facilitate implementation.
What’s the problem?
In the US in 2019, 250 million oral antibiotic prescriptions were written in the outpatient setting—roughly the equivalent of eight antibiotic prescriptions for every 10 people (Figure 1A) (3–5). One-third (∼47 million) of these outpatient antibiotic prescriptions are considered unnecessary (6). This is largely attributable to antibiotics prescribed for viral infections (e.g., viral upper respiratory infections, pharyngitis, and middle ear infections), as well as non-bacterial conditions such as allergy/asthma and bronchitis (7, 8).
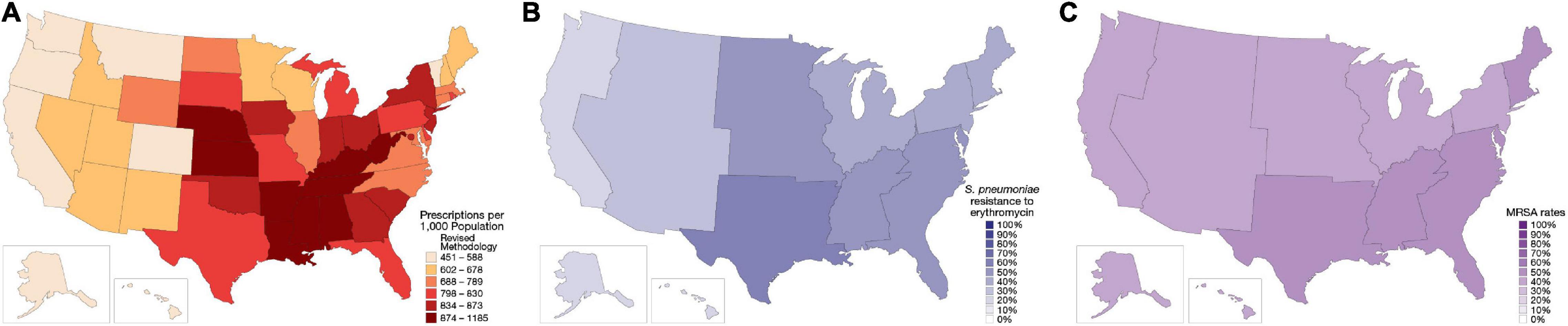
Figure 1. Regional distribution of antibiotic prescribing patterns and antibiotic resistance within the United States (US). (A) Outpatient antibiotic prescription rates from the Centers for Disease Control and Prevention, 2018 (3). (B) Erythromycin-resistant Streptococcus pneumoniae phenotype rates, 2019 (98). (C) Methicillin-resistant Staphylococcus aureus (MRSA) rates, as a percentage of all S. aureus isolates, 1997–2017 (51). Resistance rates were derived from isolates collected at US hospitals in the SENTRY surveillance program.
Antibiotics for common acute infections are often prescribed for 10 or more days of therapy, which is longer than needed (9–11). At 129 Veteran’s Affairs medical centers, 40% of antibiotic prescriptions for pneumonia were for 8 days or longer (11). In a single-center study, 42% of uncomplicated skin infections treated in the ambulatory setting were prescribed antibiotic therapy for ≥ 10 days (9). Excessive antibiotic duration is associated with a higher risk of Clostridioides difficile–associated diarrhea and drug toxicity (12–14).
Depending on the infection type, some 25–50% of antibiotic prescriptions for bacterial infections do not align with current guidelines (6, 9, 15, 16) or may fail to adequately consider local resistance patterns. The current guidelines from the Infectious Disease Society of America and American Thoracic Society indicate that macrolide monotherapy is a first-line treatment option for the typical patient with community-acquired pneumonia (CAP; those with no comorbidities or risk factors for methicillin-resistant Staphylococcus aureus [MRSA] or Pseudomonas aeruginosa), but only if local Streptococcus pneumoniae resistance rates are < 25% (17). S. pneumoniae is resistant to macrolides in around 40–50% of isolates in the US, and most US regions exhibit resistance rates > 25% (Figure 1B) (18–21). Despite relatively clear guidance from the CAP guidelines and established patterns of antimicrobial resistance, azithromycin, a macrolide, remains the most commonly prescribed agent in the US, accounting for about 30–40% of outpatient CAP prescriptions written (22).
While some local public health agencies and health systems provide clinicians with local resistance information, these data are becoming more challenging to obtain (23). Furthermore, even if an antibiogram (i.e., antibiotic susceptibility test report) is available, primary care providers may benefit from expert interpretation of the data, including the data source and how they affect the risk/benefit decision for therapy. Antibiotic resistance profiles can differ substantially between isolates collected in the outpatient setting versus inpatient setting and, therefore, antibiograms produced by hospitals should be interpreted carefully when applied to outpatients.
Why don’t we just have antibiotic stewardship in all outpatient settings?
Primary care physicians, advanced practice providers, and dentists account for the majority of outpatient antibiotic prescriptions written (24). Prescribers come from diverse specialties, geographic locations, and practice types (e.g., private vs. health system affiliates) (25). Implementation of effective antibiotic stewardship must be customized to each specific care setting and requires some expertise to establish. Moreover, for any substantial change in outpatient antibiotic use to be successfully implemented, outpatient clinicians need the resources and time to address inappropriate antibiotic prescribing.
Though antibiotic stewardship was originally introduced in inpatient care, regulatory bodies, and public health agencies are now implementing antimicrobial stewardship requirements in outpatient settings (26). The Centers for Disease Control and Prevention (CDC) adapted their inpatient stewardship recommendations to the outpatient setting, noting that clinicians should demonstrate a commitment to optimizing antibiotic prescribing and patient safety, take at least one action for policy or practice to improve antibiotic prescribing, track prescribing practices and provide regular feedback to clinicians, and provide educational resources and expertise on optimizing antibiotic prescribing (26). The Los Angeles County Department of Public Health has incorporated many of the CDC’s Core Elements of antibiotic stewardship into their Targeting Appropriate Prescribing in Outpatient Settings (TAP Out) program, which reduced inappropriate prescribing and provided well-received peer comparison reports on prescribing habits (27).
Recently, the Joint Commission, which is the largest healthcare accrediting body in the US, has been applying new antibiotic prescribing standards to accredited ambulatory healthcare (i.e., outpatient clinics, UC, or worksite clinics; Supplementary Table 1) (28, 29). One barrier to implementing antibiotic stewardship in outpatient settings is the lack of accountability for outpatient antibiotic stewardship through traditional regulatory bodies, i.e., the Centers for Medicare and Medicaid Services. Alternatively, payors may be able to play an important role in outpatient stewardship.
Several antibiotic stewardship programs have been developed specifically for implementation in skilled nursing facilities. For instance, the Agency for Health Care Quality created a four-part approach that includes methods to monitor and maintain a stewardship program (30). However, data from this program do not seem to have been published to date. Concurrently, one large health insurance organization has created its own antibiotic stewardship program, but again the effects are not publicly known (31). Full compliance at the participating sites may be difficult due to staffing shortages and lack of systems or protocols. While skilled nursing facilities have successfully implemented infection control measures (32), there is a need for more education and administrative oversight to fully implement the intended nature of antibiotic stewardship (33).
According to a 2018 Pew Trust report, almost 46% of antibiotic prescriptions written in the UC setting were unnecessary (24). These were mainly for respiratory tract infections. However, despite recent efforts by the Academy of Urgent Care Medicine, which developed an antibiotic stewardship education program, very few sites have completed the training to gain accreditation in antibiotic stewardship.
“It’s not me”
Prescribers don’t think they’re part of the antibiotic prescribing problem. Almost all surveyed physicians say that, in general, there is a problem with antibiotic resistance and inappropriate prescribing in the US (34, 35). However, only about 50% of these surveyed physicians see the problem as occurring in their specific practice. This disconnect continues to fuel the problem, and we all need to accept responsibility and survey our prescribing habits.
“I don’t have the data, and I don’t have the support to implement”
The average healthcare provider seeing patients in the community is not supported by health system-based education, interventions, and staff to guide appropriate prescribing practices. Therefore, the provider is left to navigate this complex field independently, sourcing guidelines and continuing education materials, and implementing stewardship practices. The prime example of this is the UC provider who usually works in isolation without regular peer-to-peer interaction, which is a crucial component of a successful antibiotic stewardship program.
Guideline disconnect
National health agencies (the CDC) and professional organizations (Infectious Diseases Society of America) have published a variety of resources for clinicians on antibiotic prescribing, for particular infections and for more appropriate use of antibiotics in general (17, 36–42). However, the complexity of the documents, the length of time between document updates, and the inclusion of some content that doesn’t reflect real-world practice leads many community providers to turn instead to alternative resources, including decision support information sites such as UpToDate and Epocrates, or rely on their medical training (8, 43). Some of the guidelines lack specific recommendations on duration of therapy, therapy choice, or how to interpret local resistance patterns.
Pressure to prescribe
Patients (and parents of young patients) often expect and may even pressure a provider for an antibiotic prescription when it is not indicated. About 84% of providers surveyed said they feel at least moderate pressure from patients for an antibiotic prescription (34). Patients’ and parents’ expectations for an antibiotic prescription can increase antibiotic prescribing (44). However, some of the perceived pressure from the perspective of the provider may not be the intention of the patient/parent, who instead is looking for reassurance and a better explanation of the management plan (2). For the independent practitioner in the outpatient setting, leaving the patient’s expectations unfulfilled risks having a “dissatisfied customer.” Some providers practice defensive prescribing of antibiotics, out of concern for missing bacterial infections and the possible medicolegal ramifications (45).
Smart prescribing for outpatients
Here, we want to address smart prescribing for two of the most common bacterial infections seen in the outpatient setting, CAP and skin and soft-tissue infection (SSTI). For CAP and SSTI, several organizations have released updated clinical practice guidelines within the last 7 years (17, 38, 42). Despite the advances in therapeutic options, many prescribers in the outpatient setting are unaware of these updates or have not received continuing education about updates from previous guidelines.
Community-acquired pneumonia
S. pneumoniae is the most commonly isolated bacterial pathogen in patients with pneumonia without underlying chronic lung disease; other causative pathogens include Haemophilus influenzae, Mycoplasma pneumoniae, S. aureus, and Legionella pneumophila (21, 46). A bacterial pathogen is isolated in about 25–50% of CAP cases, with many patients having no pathogen detected, and viral pathogens occurring in some cases (46, 47).
In the community and UC/emergency department settings, the most commonly prescribed antibiotics for CAP are azithromycin and fluoroquinolones, accounting for 50–66% of all prescriptions for CAP treatment (7, 11, 22). While many providers prescribe an antibiotic empirically for CAP, local data on pathogens and susceptibility (if available) could better inform the treatment approach. From a robust collection of isolates from North America, the susceptibility rates of S. pneumoniae to levofloxacin were high (97–99%) and remained stable from 2010 to 2014. There was a decrease in susceptibility rates over this period for other common antibiotics, such as amoxicillin, erythromycin, tetracycline (which can be used as a surrogate for doxycycline susceptibility), and trimethoprim–sulfamethoxazole (also known as co-trimoxazole; Figure 2), which may have the potential to render these agents less appropriate for empiric treatment of CAP (20). More recent studies show that resistance rates of S. pneumoniae to macrolides (e.g., azithromycin) are approximately 40–50% in the US (18, 19, 21). Inappropriate use of antibiotics can lead to selection of resistant mutants either within a class or, less commonly, with other agents, known as co-resistance. Thus, such collateral damage has to be considered. Based on national rates of antimicrobial resistance to S. pneumoniae, azithromycin monotherapy for CAP is not recommended. Lack of specificity in our national guidelines leaves most providers guessing at best available therapy rather than following expert guidance.
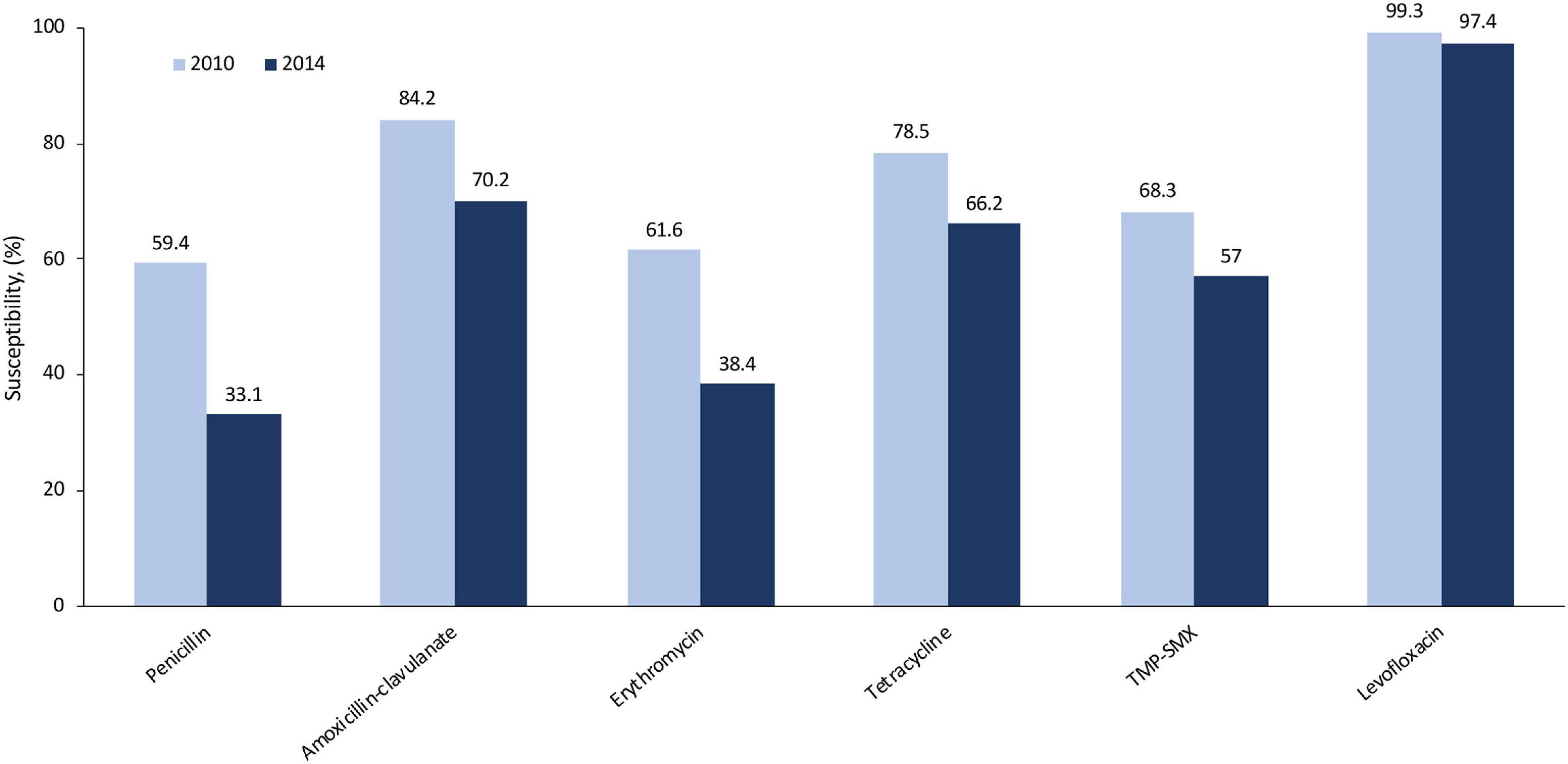
Figure 2. Susceptibility rates of Streptococcus pneumoniae to common antibiotics in North America (2010, 2014) using CLSI breakpoints (20). Amoxicillin–clavulanate rates were determined using non-meningitis breakpoints. CLSI, Clinical and Laboratory Standards Institute; TMP/SMX, trimethoprim–sulfamethoxazole.
Skin infection
S. aureus is the most commonly isolated pathogen from SSTIs, with Group A streptococci and P. aeruginosa also found to a lesser extent (48, 49). About half of all S. aureus isolates from SSTI cases in the US are MRSA strains (Figure 1C) (49, 50). Gram-negative pathogens, when they occur in SSTI, are more likely to be associated with surgical-site infections of the abdominal wall, or infections in the anal and perineal region (49).
Global susceptibility of S. aureus isolates between 1997 and 2016 showed susceptibility of methicillin-susceptible S. aureus (MSSA) isolates to many older agents was > 95%, except for penicillin and erythromycin (Figure 3) (51). The susceptibility of MRSA to these older antibiotics was generally lower than methicillin-susceptible S. aureus. However, the susceptibility rates did increase over the last two decades, possibly as a result of the spread of MRSA clones that are more susceptible to these agents. Many of the more recently approved antibiotics demonstrated susceptibility rates of > 99% against MRSA, except for levofloxacin (23% susceptible, from 72,000 isolates), delafloxacin (74% susceptible, from > 10,000 isolates), and ceftaroline (92% susceptible, from > 40,000 isolates).
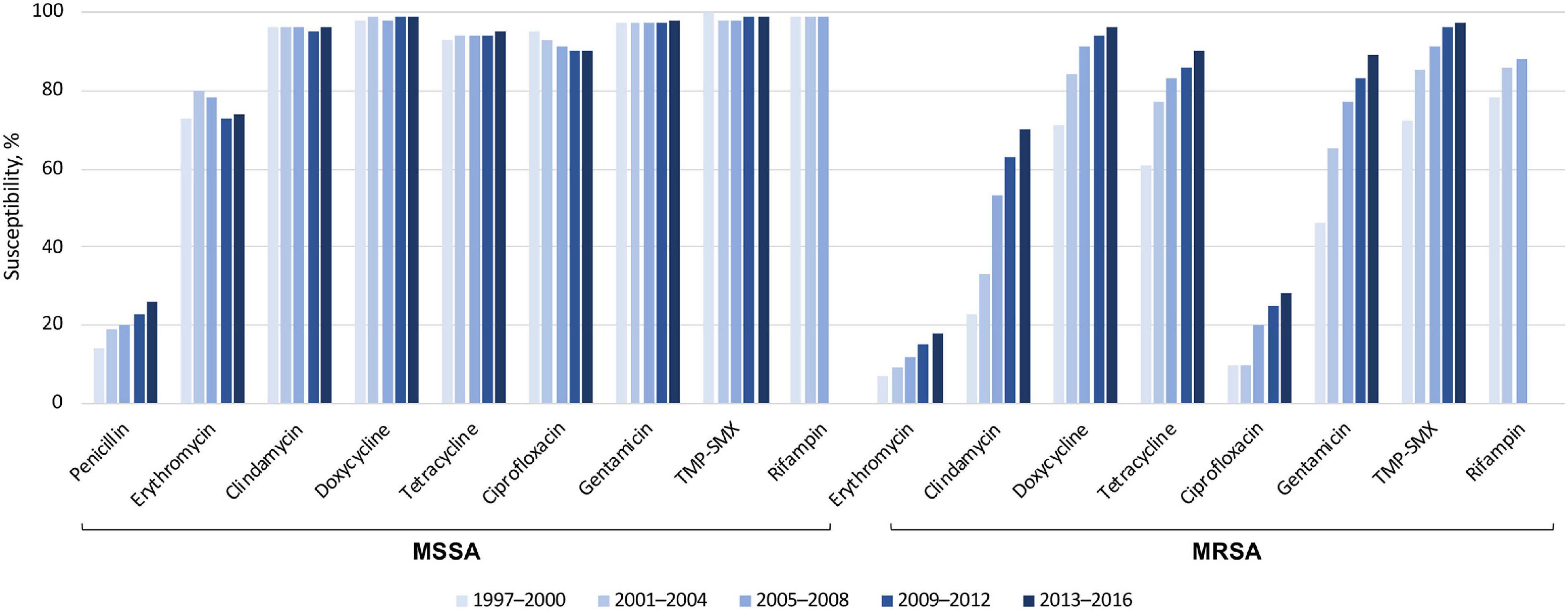
Figure 3. Susceptibility of > 191,000 S. aureus isolates to older antibiotics, from a global surveillance program (51). MRSA, methicillin-resistant Staphylococcus aureus; MSSA, methicillin-susceptible S. aureus; TMP-SMX, trimethoprim–sulfamethoxazole.
Uncomplicated (superficial), purulent SSTIs can often be treated by incision and drainage alone, while non-purulent SSTIs require antibiotics (38, 39, 52, 53). Antibiotic therapy when added to incision and drainage for abscesses can lead to a moderate improvement in efficacy (in one randomized study: 82–83% clinical success, depending on which antibiotic regimen was selected, vs. 68.9% in the incision and drainage only group), though this improvement may be limited to patients who have a positive culture for S. aureus (53). Antibiotic therapy for SSTI often consists of cephalexin and/or trimethoprim–sulfamethoxazole, though different agents may be used by provider choice and for certain types of infections (9, 54, 55). If MRSA is known or suspected to be present in the lesion, guideline-recommended treatments include vancomycin, linezolid, clindamycin, daptomycin, ceftaroline, doxycycline, minocycline, and trimethoprim–sulfamethoxazole; of note, cephalexin is not a recommended agent for treating known or suspected MRSA infections (38, 40, 42). SSTIs are often treated in the UC/emergency department setting, where providers may justifiably err on the side of treating with antibiotics because of the episodic nature of patient care and the lack of follow-up. This episodic nature of care occurs for many patients in the US with various conditions, including other infectious diseases, and may not readily be addressable without a systemic change in the availability and interconnectedness of electronic medical records (EMRs) or in the architecture of healthcare delivery and reimbursement.
Smart prescribing in the outpatient setting
Given the number of infections that occur annually for CAP and SSTI in the US, there are millions of chances to make a smart and informed choice when prescribing antibiotics. Antibiotic stewardship in the community, or “smart prescribing” as we suggest, should factor in antibiotic efficacy, safety, local resistance rates, and overall cost, in addition to patient-specific factors and disease presentation, to arrive at an appropriate therapy.
Almost half of surveyed providers said they would need “a lot of help” to implement antibiotic stewardship practices (34). We recognize the magnitude of the challenge and have assembled this easy-to-use best practice guide for providers in the outpatient setting who lack the time or resources to develop a plan or consult lengthy guidelines.
Measure existing prescribing habits
From EMR prescribing data, providers can identify one or two issues within their practice to address (e.g., inappropriate prescribing for a particular diagnosis code; peer benchmarking for antibiotic duration and dosing), and determine what action to take (56–58). Providers can then monitor the issue(s) periodically (e.g., monthly) to see if the data are improving (59). To obtain an approximate idea of how many patients fail initial treatment, providers can examine antibiotic refills, antibiotic switches, emergency department visits, and hospitalizations within 30 days of the initial prescription, though these data may be limited by the interconnectedness of EMRs or the patient obtaining all of their care within one health system. In the absence of an EMR, providers could review a patient’s recent medical history to determine previous treatments, and treatment failures on an individual basis. Other data and aggregate analyses require electronic systems and knowledge to interpret the results.
Choose an appropriate drug, dose, and duration
Recommendations are provided for the most common pathogens and patient populations in CAP (Table 1) and SSTI (Table 2). These recommendations are for the “standard” patient with one of these bacterial infections; a good rule of thumb is that for ∼80% of cases, your treatment should fall along these lines.
Community prescribing tends to follow standard dosing of antibiotics, but providers should be aware of the potential need for dose adjustments, for example related to body size or comorbid conditions (e.g., renal or hepatic impairment). For some patients, providers will need to take a different approach to antibiotic treatment based on certain patient or infection factors (Tables 1, 2). In all cases, providers should use their best judgment, tailor their treatment choice to each patient (their medical history, presentation, comorbid conditions, risk factors, and lifestyle), and use a shorter course whenever possible. Additionally, providers should watch out for certain safety issues that would suggest choosing an alternate antibiotic (Supplementary Table 2).
Delayed prescribing (“watchful waiting”) may assist in avoiding inappropriate prescribing related to patient pressure to prescribe, and thus reduce antibiotic resistance, by advising patients to return if symptoms do not improve within a few days or worsen (2, 60). It can also be a useful tool to allay a patient’s concerns that they present with at the initial visit. For UC settings, providers can offer the patient an antibiotic prescription with specific directions to fill it only if their symptoms haven’t improved in a few days, or write a future date on the prescription to be filled under the same circumstances. In cases where clinicians are uncertain of infections, a delayed prescription may be an appropriate safety net.
Case management
Ideally, a nurse or case manager should follow up with the patient at Day 2–3 after beginning antibiotic treatment to see if there are signs of an early response to treatment or any worsening symptoms. However, additional staffing may be needed to achieve this, which might be difficult to implement in certain practices. Alternatively, groups of providers can hold regular debriefing sessions to discuss cases and note any patterns of disease presentation or treatment failure. For patients with a skin infection, the provider can draw a circle around the initial extent of the infection and instruct the patient to call or send a photo of the lesion size at Day 1–2. This protocol also aids a second provider who sees the patient to assess the treatment response.
Making smart prescribing easier
Simplify guidance documents
Providers need guidance from experts that is easy to find and use, and reflects the real-world scenarios that they are faced with every day (43).
Know your local resistance patterns
Ask your local health department or community hospital for information (61). If those resources can’t routinely provide this information, reach out for help to a local infectious disease specialist, who can be found at the hospital or through a local chapter of the Infectious Diseases Society of America, or to the laboratory where you send your routine culture data. Laboratories that are accredited by the College of American Pathologists are required to publish an annual antibiogram.
Rapid assessments
Rapid diagnostic tests are available for various viral and bacterial pathogens for respiratory, gastrointestinal, sexually transmitted, and central nervous system infections, most of which provide results within 15–45 min (62). Using highly sensitive molecular diagnostic tests can significantly reduce unnecessary testing and treatment, including inappropriate antibiotic prescribing, though the results vary by pathogen and disease state. However, rapid diagnostic tests are often reimbursed in a flat fee payment per patient for outpatient providers. As such, the significantly higher costs of molecular and polymerase chain reaction testing must be absorbed by the provider. Unfortunately, this is not an economically feasible option. Diagnostic stewardship is likely a route to ensuring tests are undertaken in the appropriate patient and that the rapid accurate results assist with case management. Payers should be made aware of this situation and that the use of “expensive” tests upfront can reduce costs in the longer term.
Additional challenges to practical implementation of rapid assessments are sensitivity of the test and time and staff required to train and perform quality control of the test. Providers should also be aware that bacterial colonization (rather than infection) can return a positive result based on highly sensitive molecular diagnostic tests, which would not routinely warrant antibiotic treatment.
Patient/parent education
Suppose providers feel that the patient or parent is expecting an antibiotic prescription. In that case, the provider can explain why an antibiotic isn’t needed and give other actionable treatment advice so the patient/parent feels that they walked away from the visit with useful information (Supplementary Table 3) (2, 63). Even simple interventions, such as clinicians posting an informational letter in examination rooms with a signed commitment to use antibiotics appropriately, can reduce inappropriate prescribing by 20% (Supplementary Figure 1 shows an example) (64). The CDC has many handouts, posters, and web images, in English and Spanish, from the “Be Antibiotics Aware” campaign that can be shared with patients and caregivers (65). One effective example is the “Viruses or Bacteria: What’s got you sick?” poster, which shows a checklist of common conditions and whether an antibiotic is indicated or not (Supplementary Figure 2). When appropriate, hand your patient one of the CDC’s “prescription” sheets for symptom relief of common cold/viral illness (66). Providers can also obtain training that’s been specifically designed around improving their communication skills regarding antibiotic prescribing (67).
If a patient needs an antibiotic, encourage them to adhere to dosing instructions, and explain why this is important. In some situations, it may be worth explaining why you are prescribing a specific antibiotic (e.g., a narrower-spectrum vs. a broader-spectrum one) for the patient’s infection. In all cases, let the patient know about the likely disease course with treatment, potential side effects, and when to follow up.
Market the practice as accredited for antibiotic stewardship
The College of Urgent Care Medicine offers an Antibiotic Stewardship Commendation to practices that provide evidence of their compliance with the CDC’s Core Elements (68). Practices that receive this accreditation can advertise their achievement in their clinic and online.
Automated systems
EMR systems can be useful tools toward better antibiotic prescribing practices. In addition to making data collection easier (what was prescribed for a particular diagnosis), the EMR system can include prompts for particular interventions, automatically populated fields that comply with current guidelines, and step-through decision making (69). Unfortunately, the financial and logistical hurdles to implement these features in an EMR may be too high for smaller practices to overcome.
Provider behavioral change
In addition to the concern for missing an infection and the possible consequences (e.g., patient morbidity/mortality and litigation), diagnostic uncertainty drives a substantial amount of unnecessary antibiotic prescribing (70). There is an inherent contradiction between avoiding the downstream consequences of failed therapy and limiting inappropriate prescribing of antibiotics. While some internal factors that motivate providers’ prescribing habits would be difficult to change without a larger overhaul of the US healthcare system and law reform, some efforts can affect behavioral change in inappropriate antibiotic prescribing. Programs aimed at re-educating healthcare providers on appropriate antibiotic prescribing, providing individualized feedback, and peer comparisons can significantly reduce inappropriate prescribing (57, 71). For example, a recent study in a rural community setting included physician education through presentations on antibiotic stewardship and appropriate, guideline-concordant prescribing; feedback emails on guideline-discordant prescribing for a particular indication; and recommendations on how physicians could improve their prescribing. Additionally, patient education materials were distributed to clinics, from the CDC’s “Be Antibiotics Aware” campaign (71). This resulted in an absolute decrease of ∼15% in inappropriate prescribing during the 6-month influenza season. A randomized controlled trial of three different types of antibiotic prescribing interventions in primary care (N = 248 clinicians) found that the most significant reductions in inappropriate prescribing occurred after the providers (1) had to include written justification in the patient’s EMR for why the prescription was necessary, becoming a permanent part of the record; and (2) received regularly updated rankings of their prescribing rate compared with that of the top-performing peers (58, 72).
Risk stratification in community-acquired pneumonia
Common laboratory tests, such as complete blood counts and basic metabolic profiles, can be used to generate a risk score for adults that is highly predictive of 30-day all-cause death (73). Disease-specific risk scores, such as the Pneumonia Severity Index or the Confusion, Urea nitrogen, Respiratory rate, and Blood pressure (CURB) score (or alternatively a CRB65 score), can identify adult patients considered low risk who may be suitable candidates for outpatient therapy, and patients at high risk of death who require inpatient treatment and follow-up (74).
Controversies
Costs are part of the bigger picture of antibiotic treatment. In the outpatient setting, typically the only cost limit to the antibiotic is whether the patient’s health insurance will cover the prescription and if the patient can afford the co-pay. In the bigger picture of healthcare and societal costs of infections, while a patient may initially have an inexpensive treatment with an oral generic antibiotic for their particular infection, if the patient experiences treatment failure (potentially due to inappropriate drug, dose, or duration), then the overall cost of treating that infection escalates significantly (75). Cost savings unquestionably come into play when deciding between intravenous and oral drugs, thereby decreasing or eliminating inpatient or outpatient parenteral antibiotic therapy costs (76, 77).
While there have been several new antibiotics developed in the last decade, their use is often limited by institutional policies that they should be “saved” for special/last-resort use (78, 79). In practice, this can have the unintended consequence that non-ideal antibiotics are prescribed instead, potentially adding fuel to the fire of antibiotic resistance. Though there is a push by regulatory bodies to develop new antibiotics to combat antibiotic resistance threats, antibiotic stewardship practices may actually be having a negative effect on the research and development pipeline (1, 78, 80). So, we are left to wonder, what is an appropriate place in infection management for newer agents that have less acquired resistance or were designed to overcome common resistance mechanisms (79, 81)?
Conclusion
Regardless of the treatment setting where it is implemented, antibiotic stewardship is an evolving field (82, 83). Community prescribers can help move the needle on antibiotic stewardship by keeping in mind the “4 Ds”: prescribe an antibiotic for a bacterial infectious Disease, with the appropriate Drug, Dose, and Duration. To truly make headway with smart prescribing in the outpatient setting, more help from public health agencies, regulatory bodies, and payors is needed to provide education, practical support for implementation, and financial incentives for smart prescribing, as well as guidance from a multidisciplinary group on a pragmatic approach to appropriate antibiotic use in the community.
Author contributions
All authors contributed to data interpretation and reviewing/editing the manuscript and approved the final version to be published and were accountable for the work.
Funding
An unrestricted educational grant supported this manuscript and a 2-day roundtable meeting that preceded it, from Paratek Pharmaceuticals, Inc. (King of Prussia, PA) to GST Micro LLC (North, VA). The grantor had no role in preparing the manuscript or the decision to submit the manuscript for publication.
Acknowledgments
Agnella Izzo Matic, Ph.D., CMPP of AIM Biomedical, LLC (Fairfield, CT) provided medical writing and editorial support, which was funded by GST Micro LLC.
Conflict of interest
AA served as primary or co-investigator of clinical trials sponsored by, NeuroRx Pharma, Pulmotect, Blade Therapeutics, Novartis, Takeda, Humanigen, Eli Lilly, PTC Therapeutics, Octapharma, Fulcrum Therapeutics, and Alexion; and as a speaker and/or consultant for BMS, Pfizer, BI, Portola, Sunovion, Mylan, Salix, Alexion, AstraZeneca, Novartis, Nabriva, Paratek, Bayer, Tetraphase, Achaogen, La Jolla, Ferring, Seres, Millennium, PeraHealth, HeartRite, AseptiScope, and Sprightly. ED served as a consultant for Botanix Pharmaceuticals. BK received research funding from Savara Pharmaceuticals; served on advisory boards for GST Micro and Shionogi Pharmaceuticals; acted as a consultant for Atheneum; and a speaker for Boehringer Ingelheim and La Jolla. KL served as an advisor on grants sponsored by Merck, Pfizer, and as a consultant for Paratek Pharmaceuticals and Ferring Pharmaceuticals. FL served on the speakers’ bureau for AbbVie. GT served as an advisor on grants sponsored by Ferring Pharmaceuticals and Spero Pharmaceuticals, as a consultant for Taro Pharmaceuticals and Provepharm, and participated in a DSMB for Vail Scientific, and was an employee of GST Micro LLC. SV was employed as Vice President by American Family Care. GH was employed by company No Resistance Consulting.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2022.901980/full#supplementary-material
Abbreviations
CAP, community-acquired pneumonia; CDC, Centers for Disease Control and Prevention; EMR, electronic medical record; MRSA, methicillin-resistant Staphylococcus aureus; SSTI, skin and soft-tissue infection; UC, urgent care; US, United States.
References
1. Centers for Disease Control and Prevention. Antibiotic Resistance Threats in the United States, 2019. US Department of Health and Human Services. (2019). Available online at: https://www.cdc.gov/drugresistance/pdf/threats-report/2019-ar-threats-report-508.pdf (accessed September 14, 2021).
2. Fleming-Dutra KE, Mangione-Smith R, Hicks LA. How to prescribe fewer unnecessary antibiotics: talking points that work. Am Fam Physician. (2016) 94:200–2.
3. Centers for Disease Control and Prevention. Outpatient Antibiotic Prescriptions — United States, 2019. (2019). Available online at: https://www.cdc.gov/antibiotic-use/pdfs/Annual-Report-2019-H.pdf (accessed September 14, 2021).
4. Blue Cross Blue Shield. Antibiotic Prescription Fill Rates Declining in the U.S. (2017). Available online at: https://www.bcbs.com/the-health-of-america/reports/antibiotic-prescription-rates-declining-in-the-US (accessed September 14, 2021).
5. Fischer MA, Mahesri M, Lii J, Linder JA. Non-visit-based and non-infection-related antibiotic use in the US: a cohort study of privately insured patients during 2016-2018. Open Forum Infect Dis. (2021) 8:ofab412. doi: 10.1093/ofid/ofab412
6. Pew Charitable Trusts. Antibiotic Use in Outpatient Settings. (2016). Available online at: https://www.pewtrusts.org/-/media/assets/2016/05/antibioticuseinoutpatientsettings.pdf (accessed December 14, 2021).
7. Donnelly JP, Baddley JW, Wang HE. Antibiotic utilization for acute respiratory tract infections in U.S. emergency departments. Antimicrob Agents Chemother. (2014) 58:1451–7. doi: 10.1128/AAC.02039-13
8. Johnson MC, Hulgan T, Cooke RG, Kleinpell R, Roumie C, Callaway-Lane C, et al. Operationalising outpatient antimicrobial stewardship to reduce system-wide antibiotics for acute bronchitis. BMJ Open Qual. (2021) 10:e001275. doi: 10.1136/bmjoq-2020-001275
9. Hurley HJ, Knepper BC, Price CS, Mehler PS, Burman WJ, Jenkins TC. Avoidable antibiotic exposure for uncomplicated skin and soft tissue infections in the ambulatory care setting. Am J Med. (2013) 126:1099–106. doi: 10.1016/j.amjmed.2013.08.016
10. Lee RA, Centor RM, Humphrey LL, Jokela JA, Andrews R, Qaseem A. Appropriate use of short-course antibiotics in common infections: best practice advice from the American College of Physicians. Ann Intern Med. (2021) 174:822–7. doi: 10.7326/M20-7355
11. Lowery JLI, Alexander B, Nair R, Heintz BH, Livorsi DJ. Evaluation of antibiotic prescribing in emergency departments and urgent care centers across the veterans’ health administration. Infect Control Hosp Epidemiol. (2021) 42:694–701. doi: 10.1017/ice.2020.1289
12. Mulligan P, Shah N, Acree M, Grant J, Ravichandran U, Ismail N. Adherence to antibiotic stewardship program associated with shorter course of treatment and fewer adverse events. Infect Control Hosp Epidemiol. (2021) 1(Suppl. 1):S30–1.
13. Brown KA, Khanafer N, Daneman N, Fisman DN. Meta-analysis of antibiotics and the risk of community-associated Clostridium difficile infection. Antimicrob Agents Chemother. (2013) 57:2326–32. doi: 10.1128/AAC.02176-12
14. Slimings C, Riley TV. Antibiotics and healthcare facility-associated Clostridioides difficile infection: systematic review and meta-analysis 2020 update. J Antimicrob Chemother. (2021) 76:1676–88. doi: 10.1093/jac/dkab091
15. Shivley NR, Buehrle DJ, Clancy CJ, Decker BK. Prevalence of inappropriate antibiotic prescribing in primary care clinics within a veterans affairs health care system. Antimicrob Agents Chemother. (2018) 62:e00337–18. doi: 10.1128/AAC.00337-18
16. Jenkins TC, Knepper BC, Moore SJ, O’Leary ST, Caldwell B, Saveli CC, et al. Antibiotic prescribing practices in a multicenter cohort of patients hospitalized for acute bacterial skin and skin structure infection. Infect Control Hosp Epidemiol. (2014) 35:1241–50. doi: 10.1086/678056
17. Metlay JP, Waterer GW, Long AC, Anzueto A, Brozek J, Crothers K, et al. Diagnosis and treatment of adults with community-acquired pneumonia: an official clinical practice guideline of the American thoracic society and infectious diseases society of America. Am J Respir Crit Care Med. (2019) 200:e45–67. doi: 10.1164/rccm.201908-1581ST
18. Gupta V, Yu KC, Schranz J, Gelone SP. A multicenter evaluation of the US prevalence and regional variation in macrolide-resistant S. pneumoniae in ambulatory and hospitalized adult patients in the US. Open Forum Infect Dis. (2021) 8:ofab063. doi: 10.1093/ofid/ofab063
19. Keedy K, Li J, Nenninger A, Sheets A, Fernandes P, Tillotson G. Antibiotic susceptibility of Streptococcus pneumoniae in the US in 2014. In: Poster at the MAD-ID Annual Meeting, Poster 2016. Orlando, FL (2014).
20. Flamm RK, Rhomberg PR, Huband MD, Farrell DJ. Activity of omadacycline tested against Streptococcus pneumoniae from a global surveillance program. In: Poster at the Interscience Conference of Antimicrobial Agents and Chemotherapy Meeting, Poster C-554. San Diego, CA (2014). doi: 10.1016/j.diagmicrobio.2017.10.010
21. Torres A, Cilloniz C, Niederman MS, Menéndez R, Chalmers JD, Wunderink RG, et al. Pneumonia. Nat Rev Dis Prim. (2021) 7:25. doi: 10.1038/s41572-021-00259-0
22. Tillotson G, Lodise T, Classi P, Mildvan D, MCKinnell JA. Antibiotic treatment failure and associated outcomes among adult patients with community-acquired pneumonia in the outpatient setting: a real-world US insurance claims database study. Open Forum Infect Dis. (2020) 26:ofaa065. doi: 10.1093/ofid/ofaa065
23. McKinnell JA, Epson E, Horwich-Scholefield S, Humphries R, Hindler J, Miller L, et al. The microbiology laboratory is a valuable, but largely underutilized partner in antimicrobial stewardship and antimicrobial resistance monitoring. Open Forum Infect Dis. (2016) 1:S201. doi: 10.1093/ofid/ofw172.673
24. Pew Charitable Trusts. Outpatient Antibiotic Prescribing Varied Across the United States in 2018: Fact Sheet. (2020). Available online at: https://www.pewtrusts.org/en/research-and-analysis/fact-sheets/2020/10/outpatient-antibiotic-prescribing-varied-across-the-united-states-in-2018 (accessed September 14, 2021).
25. Public Health England. English Surveillance Programme for Antimicrobial Utilisation and Resistance (ESPAUR): Report 2019 to 2020, London, UK. (2020). Available online at: https://www.gov.uk/government/publications/english-surveillance-programme-antimicrobial-utilisation-and-resistance-espaur-report (accessed September 14, 2021).
26. Sanchez GV, Fleming-Dutra KE, Roberts RM, Hicks LA. Core elements of outpatient antibiotic stewardship. MMWR Morb Mort Wkly Rep. (2016) 65:1–12. doi: 10.15585/mmwr.rr6506a1
27. Acute Communicable Disease Control. Targeting Appropriate Prescribing in Outpatient Settings (TAP OUT). Los Angeles County Department of Public Health. (2021). Available online at: http://publichealth.lacounty.gov/Acd/TAPOUT.htm (accessed December 14, 2021).
28. Baker DW, Hyun D, Neuhauser MM, Bhatt J, Srinivasan A. Leading practices in antimicrobial stewardship: conference summary. Jt Comm J Qual Patient Saf. (2019) 45:517–23. doi: 10.1016/j.jcjq.2019.04.006
29. The Joint Commission. R3 Report, Issue 23: Antimicrobial Stewardship in Ambulatory Health Care. (2019). Available online at: https://www.jointcommission.org/standards/r3-report/r3-report-issue-23-antimicrobial-stewardship-in-ambulatory-health-care/ (accessed December 14, 2021).
30. Agency for Healthcare Research and Quality. Toolkit 2: Monitor and Sustain Stewardship. (2016). Available online at: https://www.ahrq.gov/nhguide/toolkits/implement-monitor-sustain-program/toolkit2-monitor-sustain-program.html (accessed November 23, 2021).
31. PharMerica. Antibiotic Stewardship Program. (2021). Available online at: https://pharmerica.com/who-we-are/clinical-programs/antibiotic-stewardship/ (accessed November 23, 2021).
32. Gouin KA, Cool AJ, Stone ND, Hicks L, Slifka KMJ, Almendinger K, et al. Review of nursing home antibiotic stewardship citation deficiencies to identify opportunities to improve antibiotic stewardship implementation, 2018-2019. Open Forum Infect Dis. (2021) 8:S55–6. doi: 10.1093/ofid/ofab466.088
33. McKinnell JA. Understanding Antimicrobial Stewardship (ASP) for Nursing Homes in California. (2019). Available online at: http://publichealth.lacounty.gov/acd/docs/SNFSymposium2019/AntimicrobialStewardshipNursingHomesCA.pdf (accessed September 14, 2021).
34. Pew Charitable Trusts. Survey of Doctors Reveals Challenges, Strategies for Reducing Inappropriate Antibiotic Use. (2020). Available online at: https://www.pewtrusts.org/en/research-and-analysis/articles/2020/08/06/survey-of-doctors-reveals-challenges-strategies-for-reducing-inappropriate-antibiotic-use (accessed September 14, 2021).
35. Pew Charitable Trusts. National Survey Reveals Barriers to Outpatient Antibiotic Stewardship Efforts. (2020). Available online at: https://www.pewtrusts.org/en/research-and-analysis/issue-briefs/2020/08/national-survey-reveals-barriers-to-outpatient-antibiotic-stewardship-efforts (accessed September 14, 2021).
36. Barlam TF, Cosgrove SE, Abbo LM, MacDougall C, Schuetz AN, Septimus EJ, et al. Implementing an antibiotic stewardship program: guidelines by the infectious diseases society of America and the society for healthcare epidemiology of America. Clin Infect Dis. (2016) 62:e51–77. doi: 10.1093/cid/ciw118
37. Centers for Disease Control and Prevention. Core Elements of Hospital Antibiotic Stewardship Programs, US Department of Health and Human Services. (2019). Available online at: https://www.cdc.gov/antibiotic-use/core-elements/hospital.html (accessed December 14, 2021).
38. Duane TM, Huston JM, Collom M, Beyer A, Parli S, Buckman S, et al. Surgical infection society 2020 updated guidelines on the management of complicated skin and soft tissue infections. Surg Infect. (2021) 22:383–99. doi: 10.1089/sur.2020.436
39. Lipsky BA, Dryden M, Gottrup F, Nathwani D, Seaton RA, Stryja J. Antimicrobial stewardship in wound care: a position paper from the British society for antimicrobial chemotherapy and European wound management association. J Antimicrob Chemother. (2016) 71:3026–35. doi: 10.1093/jac/dkw287
40. Pollack CVJ, Amin A, Ford WTJ, Finley R, Kaye KS, Nguyen HH, et al. Acute bacterial skin and skin structure infections (ABSSSI): practice guidelines for management and care transitions in the emergency department and hospital. J Emerg Med. (2015) 48:508–19. doi: 10.1016/j.jemermed.2014.12.001
41. Ramirez JA, Musher DM, Evans SE, Cruz CD, Crothers KA, Hage CA, et al. Treatment of community-acquired pneumonia in immunocompromised adults: a consensus statement regarding initial strategies. Chest. (2020) 158:1896–911. doi: 10.1016/j.chest.2020.05.598
42. Stevens DL, Bisno AL, Chambers HF, Dellinger EP, Goldstein EJC, Gorbach SL, et al. Practice guidelines for the diagnosis and management of skin and soft tissue infections: 2014 update by the infectious diseases society of America. Clin Infect Dis. (2014) 59:e10–52. doi: 10.1093/cid/ciu444
43. Spellberg B, Wright WF, Shaneyfelt T, Centor RM. The future of medical guidelines: standardizing clinical care with the humility of uncertainty. Ann Intern Med. (2021) 174:1740–2. doi: 10.7326/M21-3034
44. Sirota M, Round T, Samaranayaka S, Kostopoulou O. Expectations for antibiotics increase their prescribing: causal evidence about localized impact. Health Psychol. (2017) 36:402–9. doi: 10.1037/hea0000456
45. Tebano G, Dyar OJ, Beovic B, Béraud G, Thilly N, Pulcini C. Defensive medicine among antibiotic stewards: the international ESCMID AntibioLegalMap survey. J Antimicrob Chemother. (2018) 73:1989–96. doi: 10.1093/jac/dky098
46. Musher DM, Abers MS, Bartlett JG. Evolving understanding of the causes of pneumonia in adults, with special attention to the role of pneumococcus. Clin Infect Dis. (2017) 65:1736–44. doi: 10.1093/cid/cix549
47. Jain S, Self WH, Wunderink RG, Fakhran S, Balk R, Bramley AM, et al. Community-acquired pneumonia requiring hospitalization among U.S. adults. N Engl J Med. (2015) 373:415–27. doi: 10.1056/NEJMoa1500245
48. Cammarata S, Tillotson GS, Murray J, Kurtz S, Gupta V. Demographics of culture positive patients in the admission period with skin and skin structure infection in the US: a multicenter evaluation of pathogen distribution. In: Poster at the 28th European Congress of Clinical Microbiology and Infectious Diseases, Poster E0285. Madrid (2018).
49. Kaye KS, Petty LA, Shorr AF, Zilberberg MD. Current epidemiology, etiology, and burden of acute skin infections in the United States. Clin Infect Dis. (2019) 68:S193–9. doi: 10.1093/cid/ciz002
50. Esposito S, Noviello S, Leone S. Epidemiology and microbiology of skin and soft tissue infections. Curr Opin Infect Dis. (2016) 29:109–15. doi: 10.1097/QCO.0000000000000239
51. Diekema DJ, Pfaller MA, Shortridge D, Zervos M, Jones RN. Twenty-year trends in antimicrobial susceptibilities among Staphylococcus aureus from the SENTRY antimicrobial surveillance program. Open Forum Infect Dis. (2019) 6:S47–53. doi: 10.1093/ofid/ofy270
52. Amin A, Cerceo EA, Deitelzweig SB, Pile JC, Rosenberg DJ, Sherman BM. Hospitalist perspective on the treatment of skin and soft tissue infections. Mayo Clin Proc. (2014) 89:1436–51. doi: 10.1016/j.mayocp.2014.04.018
53. Daum RS, Miller LG, Immergluck L, Fritz S, Creech CB, Young D, et al. A placebo-controlled trial of antibiotics for smaller skin abscesses. N Engl J Med. (2017) 376:2545–55. doi: 10.1056/NEJMoa1607033
54. Kamath RS, Sudhakar D, Gardner JG, Hemmige V, Safar H, Musher DM. Guidelines vs actual management of skin and soft tissue infections in the emergency department. Open Forum Infect Dis. (2018) 5:ofx188. doi: 10.1093/ofid/ofx188
55. Brindle R, Williams M, Barton E, Featherstone P. Assessment of antibiotic treatment of cellulitis and erysipelas: a systematic review and meta-analysis. JAMA Dermatol. (2019) 155:1033–40. doi: 10.1001/jamadermatol.2019.0884
56. Leung V, Langford BJ, Ha R, Schwartz KL. Metrics for evaluating antibiotic use and prescribing in outpatient settings. JAC Antimicrob Resist. (2021) 3:dlab098. doi: 10.1093/jacamr/dlab098
57. Yadav K, Meeker D, Mistry RD, Doctor JN, Fleming-Dutra KE, Fleischman RJ, et al. A multifaceted intervention improves prescribing for acute respiratory infection for adults and children in emergency department and urgent care settings. Acad Emerg Med. (2019) 26:719–31. doi: 10.1111/acem.13690
58. Gong CL, Hay JW, Meeker D, Doctor JN. Prescriber preferences for behavioural economics interventions to improve treatment of acute respiratory infections: a discrete choice experiment. BMJ Open. (2016) 6:e012739. doi: 10.1136/bmjopen-2016-012739
59. Brotherton AL. Metrics of antimicrobial stewardship programs. Med Clin N Am. (2018) 102:965–76. doi: 10.1016/j.mcna.2018.05.008
60. Dobson EL, Klepser ME, Pogue JM, Labreche MJ, Adams AJ, Gauthier TP, et al. Outpatient antibiotic stewardship: interventions and opportunities. J Am Pharm Assoc. (2017) 57:464–73. doi: 10.1016/j.japh.2017.03.014
61. Humphries R, Mendez J, Miller LG, Miner A, Fernandes P, Richter S, et al. The regional antibiogram is an important public health tool to improve empiric antibiotic selection, Stenotrophomonas maltophilia as a case example. Open Forum Infect Dis. (2017) 4:S258. doi: 10.1093/ofid/ofx163.563
62. Bouzid D, Zanella M-C, Kerneis S, Visseaux B, May L, Schrenzel J, et al. Rapid diagnostic tests for infectious diseases in the emergency department. Clin Microbiol Infect. (2021) 27:182–91. doi: 10.1016/j.cmi.2020.02.024
63. Stivers T, Timmermans S. Arriving at no: patient pressure to prescribe antibiotics and physicians’ responses. Soc Sci Med. (2021) 290:114007. doi: 10.1016/j.socscimed.2021.114007
64. Meeker D, Knight TK, Friedberg MW, Linder JA, Goldstein NJ, Fox CR, et al. Nudging guideline-concordant antibiotic prescribing: a randomized clinical trial. JAMA Intern Med. (2014) 174:425–31. doi: 10.1001/jamainternmed.2013.14191
65. Centers for Disease Control and Prevention. Antibiotics Prescribing and Use: Patient Education and Promotional Resources. (2021). Available online at: https://www.cdc.gov/antibiotic-use/materials-references/index.html (accessed September 14, 2021).
66. Centers for Disease Control and Prevention. Educational Resources for Healthcare Professionals. (2021). Available online at: https://www.cdc.gov/antibiotic-use/training/materials.html (accessed October 13, 2021).
67. University of Washington, interactive Medical Training Resources [iMTR]. Dialogue Around Respiratory Illness Treatment. (2022). Available online at: https://www.uwimtr.org/dart/ (accessed May 11, 2022).
68. The College of Urgent Care Medicine. Antibiotic Stewardship Commendation. (2021). Available online at: https://www.ucaoa.org/Quality-Programs/Commendations/Antibiotic-Stewardship-Commendation (accessed September 14, 2021).
69. Kuper KM, Nagel JL, Kile JW, May LS, Lee FM. The role of electronic health record and “add-on” clinical decision support systems to enhance antimicrobial stewardship programs. Infect Control Hosp Epidemiol. (2019) 40:501–11. doi: 10.1017/ice.2019.51
70. Tarrant C, Krockow EM. Antibiotic overuse: managing uncertainty and mitigating against overtreatment. BMJ Qual Saf. (2022) 31:163–7. doi: 10.1136/bmjqs-2021-013615
71. Cummings PL, Alajajian R, May LS, Grant R, Greer H, Sontz J, et al. Utilizing behavioral science to improve antibiotic prescribing in rural urgent care settings. Open Forum Infect Dis. (2020) 7:ofaa174. doi: 10.1093/ofid/ofaa174
72. Meeker D, Linder JA, Fox CR, Friedberg MW, Persell SD, Goldstein NJ, et al. Effect of behavioral interventions on inappropriate antibiotic prescribing among primary care practices: a randomized clinical trial. JAMA. (2016) 315:562–70. doi: 10.1001/jama.2016.0275
73. Horne BD, May HT, Muhlestein JB, Ronnow BS, Lappé DL, Renlund DG, et al. Exceptional mortality prediction by risk scores from common laboratory tests. Am J Med. (2009) 122:550–8. doi: 10.1016/j.amjmed.2008.10.043
74. Aujesky D, Auble TE, Yealy DM, Stone RA, Obrosky DS, Meehan TP, et al. Prospective comparison of three validated prediction rules for prognosis in community-acquired pneumonia. Am J Med. (2005) 118:384–92. doi: 10.1016/j.amjmed.2005.01.00
75. Cunha CB. The pharmacoeconomic aspects of antibiotic stewardship programs. Med Clin North Am. (2018) 102:937–46. doi: 10.1016/j.mcna.2018.05.010
76. Ektare V, Khachatryan A, Xue M, Dunne M, Johnson K, Stephens J. Assessing the economic value of avoiding hospital admissions by shifting the management of gram+ acute bacterial skin and skin-structure infections to an outpatient care setting. J Med Econ. (2015) 18:1092–101. doi: 10.3111/13696998.2015.1078339
77. Gray A, Dryden M, Charos A. Antibiotic management and early discharge from hospital: an economic analysis. J Antimicrob Chemother. (2012) 67:2297–302. doi: 10.1093/jac/dks194
78. Vickers RJ, Bassetti M, Clancy CJ, Garey KW, Greenberg DE, Nguyen M-H, et al. Combating resistance while maintaining innovation: the future of antimicrobial stewardship. Future Microbiol. (2019) 14:1331–41. doi: 10.2217/fmb-2019-0227
79. Miller LG. Another new antibiotic for skin infections and why infectious disease specialists are hypocrites. Clin Infect Dis. (2019) 68:1223–4. doi: 10.1093/cid/ciy720
80. Beyer P, Paulin S. The antibacterial research and development pipeline needs urgent solutions. ACS Infect Dis. (2020) 6:1289–91. doi: 10.1021/acsinfecdis.0c00044
81. Polk C, Sampson MM, Roshdy D, Davidson LE. Skin and soft tissue infections in patients with diabetes mellitus. Infect Dis Clin North Am. (2021) 35:183–97. doi: 10.1016/j.idc.2020.10.007
82. Morris AM, Calderwood MS, Fridkin SK, Livorsi DJ, McGregor JC, Mody L, et al. Research needs in antibiotic stewardship. Infect Control Hosp Epidemiol. (2019) 40:1334–43. doi: 10.1017/ice.2019.276
83. Barlam TF. The state of antibiotic stewardship programs in 2021: the perspective of an experienced steward. Antimicrob Steward Health Epidemiol. (2021) 1:e20.
84. Singh N, Rogers P, Atwood CW, Wagener MM, Yu VL. Short-course empiric antibiotic therapy for patients with pulmonary infiltrates in the intensive care unit: a proposed solution for indiscriminate antibiotic prescription. Am J Respir Crit Care Med. (2000) 162:505–11. doi: 10.1164/ajrccm.162.2.9909095
85. Dunbar LM, Khashab MM, Kahn JB, Zadeikis N, Xiang JX, Tennenberg AM. Efficacy of 750-mg, 5-day levofloxacin in the treatment of community-acquired pneumonia caused by atypical pathogens. Curr Med Res Opin. (2004) 20:555–63. doi: 10.1185/030079904125003304
86. Dunbar LM, Wunderink RG, Habib MP, Smith LG, Tennenberg AM, Khashab MM, et al. High-dose, short-course levofloxacin for community-acquired pneumonia: a new treatment paradigm. Clin Infect Dis. (2003) 37:752–60. doi: 10.1086/377539
87. Greenberg D, Givon-Lavi N, Sadaka Y, Ben-Shimol S, Bar-Ziv J, Dagan R. Short-course antibiotic treatment for community-acquired alveolar pneumonia in ambulatory children: a double-blind, randomized, placebo-controlled trial. Pediatr Infect Dis J. (2014) 33:136–42. doi: 10.1097/INF.0000000000000023
88. Uranga A, España PP, Bilbao A, Quintana JM, Arriaga I, Intxausti M, et al. Duration of antibiotic treatment in community-acquired pneumonia: a multicenter randomized clinical trial. JAMA Intern Med. (2016) 176:1257–65. doi: 10.1001/jamainternmed.2016.3633
89. Asche C, McAdam-Marx C, Seal B, Crookston B, Mullins CD. Treatment costs associated with community-acquired pneumonia by community level of antimicrobial resistance. J Antimicrob Chemother. (2008) 61:1162–8. doi: 10.1093/jac/dkn073
90. Webb BJ, Dascomb K, Stenehjem E, Vikram HR, Agrwal N, Sakata K, et al. Derivation and multicenter validation of the drug resistance in pneumonia clinical prediction score. Antimicrob Agents Chemother. (2016) 60:2652–63. doi: 10.1128/AAC.03071-15
91. Hepburn MJ, Dooley DP, Skidmore PJ, Wllis MW, Starnes WF, Hasewinkle WC. Comparison of short-course (5 days) and standard (10 days) treatment for uncomplicated cellulitis. Arch Intern Med. (2004) 164:1669–74. doi: 10.1001/archinte.164.15.1669
92. Prokocimer P, De Anda C, Fang E, Mehra P, Das A. Tedizolid phosphate vs linezolid for treatment of acute bacterial skin and skin structure infections: the ESTABLISH-1 randomized trial. JAMA. (2013) 309:559–69. doi: 10.1001/jama.2013.241
93. Moran GJ, Fang E, Corey GR, Das AF, De Anda C, Prokocimer P. Tedizolid for 6 days versus linezolid for 10 days for acute bacterial skin and skin-structure infections (ESTABLISH-2): a randomised, double-blind, phase 3, non-inferiority trial. Lancet Infect Dis. (2014) 14:696–705. doi: 10.1016/S1473-3099(14)70737-6
94. Corey GR, Good S, Jiang H, Moeck G, Wikler M, Green S, et al. Single-dose oritavancin versus 7–10 days of vancomycin in the treatment of gram-positive acute bacterial skin and skin structure infections: the SOLO II noninferiority study. Clin Infect Dis. (2015) 60:254–62. doi: 10.1093/cid/ciu778
95. British Lymphology Society, The Lymphoedema Support Network. Consensus Document on the Management of Cellulitis in Lymphoedema. (2016). Available online at: https://www.lymphoedema.org/wp-content/uploads/2020/01/cellulitis_consensus.pdf (accessed September 14, 2021).
96. Jenkins TC, Knepper BC, Moore SJ, Saveli CC, Pawlowski SW, Perlman DM, et al. Microbiology and initial antibiotic therapy for injection drug users and non-injection drug users with cutaneous abscesses in the era of community-associated methicillin-resistant Staphylococcus aureus. Acad Emerg Med. (2015) 22:993–7. doi: 10.1111/acem.12727
97. Jackson KA, Bohm MK, Brooks JT, Asher A, Nadle J, Bamberg WM, et al. Invasive methicillin-resistant Staphylococcus aureus infections among persons who inject drugs – six sites, 2005-2006. MMWR Morb Mortal Wkly Rep. (2018) 67:625–8. doi: 10.15585/mmwr.mm6722a2
98. Shortridge D, Streit JM, Huband MD, Flamm RK. Delafloxacin activity against drug-resistant Streptococcus pneumoniae, Haemophilus influenzae, Haemophilus parainfluenzae, and Moraxella catarrhalis from US medical centers (2014-2018). Open Forum Infect Dis. (2019) 6(Suppl. 2):S577–8. doi: 10.1093/ofid/ofz360.1446
Keywords: antibiotic stewardship, antimicrobial stewardship, therapeutic antibacterial agents, microbial drug resistance, pneumonia, infectious skin diseases, overprescribing, inappropriate prescribing
Citation: Amin AN, Dellinger EP, Harnett G, Kraft BD, LaPlante KL, LoVecchio F, McKinnell JA, Tillotson G and Valentine S (2022) It’s about the patients: Practical antibiotic stewardship in outpatient settings in the United States. Front. Med. 9:901980. doi: 10.3389/fmed.2022.901980
Received: 22 March 2022; Accepted: 27 June 2022;
Published: 27 July 2022.
Edited by:
Yi-Wei Tang, Cepheid, United StatesReviewed by:
Oana Sandulescu, Carol Davila University of Medicine and Pharmacy, RomaniaJohn D. Walley, University of Leeds, United Kingdom
Copyright © 2022 Amin, Dellinger, Harnett, Kraft, LaPlante, LoVecchio, McKinnell, Tillotson and Valentine. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Alpesh N. Amin, anamin@hs.uci.edu
 Alpesh N. Amin
Alpesh N. Amin
