- 1Division of Antimicrobial Stewardship, New York-Presbyterian Queens, Flushing, NY, United States
- 2Columbia University School of Nursing, New York, NY, United States
- 3Paratek Pharmaceuticals, Inc., King of Prussia, PA, United States
Introduction: The 2019 American Thoracic Society/Infectious Disease Society of America guidelines recommend respiratory fluoroquinolones to treat community-acquired bacterial pneumonia (CABP) in adults with comorbidities. Fluoroquinolones are effective against both typical and atypical pathogens. However, fluoroquinolone treatment has a risk of adverse effects, and the Food and Drug Administration has issued black box safety warnings for their use. Inpatient use of fluoroquinolones has reduced as a result; however, most antibiotic courses are completed as outpatients and discharge prescriptions account for the majority of fluoroquinolone use. As such, a new treatment option is needed to replace fluoroquinolones. Omadacycline is an aminomethylcycline antibiotic with a broad spectrum of activity and is available as a once-daily intravenous or bioequivalent oral formulation.
Methods: This study assessed the safety and clinical efficacy of omadacycline compared with moxifloxacin for the treatment of adult CABP patients with Pneumonia Severity Index (PSI) risk class II/III and ≥1 comorbidity through a post-hoc analysis of the phase 3 OPTIC study (NCT02531438).
Results: In total, 239 omadacycline- and 222 moxifloxacin-treated patients were assessed. The median age was similar between groups (omadacycline: 57 years; moxifloxacin: 58 years), with 26.0% and 26.6%, respectively, ≥65 years of age. Early clinical response was 91.6% for patients with ≥1 comorbidity treated with omadacycline and 91.4% for those treated with moxifloxacin. Post-treatment evaluation results for overall response were 89.1% in the omadacycline group and 87.4% in the moxifloxacin group.
Conclusion: Safety warnings have reduced inpatient use of fluoroquinolones; however, outpatient and discharge prescriptions account for the majority of fluoroquinolone use. Outpatients with comorbidities need an efficacious alternative to fluoroquinolones. Omadacycline maintains the similar efficacy and benefits of fluoroquinolones as a once-daily, monotherapy, bioequivalent oral option with potent in vitro activity against the most common CABP pathogens, including S. pneumoniae and atypical pathogens, but offers a materially different safety profile consistent with its tetracycline heritage. In conclusion, both omadacycline and moxifloxacin exhibited similar efficacy in patients with PSI risk class II/III and comorbidities. Omadacycline fulfills an unmet need as an oral monotherapy treatment option for adult patients with CABP, which will further reduce the use of fluoroquinolones.
Clinical trial registration: https://www.clinicaltrials.gov/study/NCT02531438, identifer: NCT02531438; https://www.clinicaltrialsregister.eu/ctr-search/search?query=2013-004071-13, identifier: EudraCT #2013-004071-13.
1. Introduction
For the treatment of community-acquired bacterial pneumonia (CABP) in adults with comorbidities, the 2019 American Thoracic Society (ATS)/Infectious Diseases Society of America (IDSA) guidelines recommend either a combination therapy of amoxicillin/clavulanate or cephalosporin plus macrolide or doxycycline or monotherapy with a respiratory fluroquinolone (1). Fluoroquinolones are recommended as monotherapy for treating CABP as they are effective against the most common bacterial pathogens, both typical and atypical (1). However, fluoroquinolone treatment has a risk of adverse effects (AE). The most commonly identified AE is tendinopathy, with other reported AE domains including muscular, cognitive, psychiatric, and gastroenterological AEs (2). These AEs may become severe and require the discontinuation of fluoroquinolone treatment. Streptococcus pneumoniae is the most prevalent cause of CABP (3) and has become increasingly resistant to oral antibiotics, such as macrolides, penicillin, cephalosporins, and doxycycline (4–6). The Centers for Disease Control and Prevention (CDC) have classified drug-resistant Streptococcus pneumoniae as a serious public threat (7). In the United States, pneumonia and influenza are the ninth leading cause of death (8). Globally, lower respiratory infections, such as pneumonia, are the leading cause of death from communicable diseases (9). Alternative treatments are required to address the high unmet need, particularly concerning oral once-daily, fluoroquinolone-sparing, monotherapy antibiotic options.
Omadacycline is an aminomethylcycline antibiotic with in vitro activity against a broad spectrum of pathogens, including drug-resistant Streptococcus pneumoniae, β-lactamase-positive Haemophilus influenzae, and atypical pathogens such as Legionella pneumophila (10). Owing to modifications to the core tetracycline structure, omadacycline maintains antibacterial activity against pathogens expressing bacterial ribosomal protection proteins and tetracycline-specific efflux pumps, which are the two most common tetracycline resistance mechanisms (11). Omadacycline is available as a once-daily intravenous (IV) or bioequivalent oral formulation (12). The phase 3 OPTIC (Omadacycline for Pneumonia Treatment in the Community; NCT02531438) study demonstrated similar clinical efficacy between omadacycline and moxifloxacin, a respiratory fluoroquinolone, in adult patients with CABP (13). OPTIC enrolled patients with various degrees of disease severity and comorbidities. This allowed further analysis of outcomes for a subset of patients for whom outpatient treatment could be considered when applying the preferential, validated prediction rule for prognosis; the Pneumonia Severity Index (PSI); and who would require combination therapy with amoxicillin/clavulanate or cephalosporin AND macrolide or doxycycline OR monotherapy with respiratory fluoroquinolone if treated according to the ATS/IDSA community-acquired pneumonia (CAP) treatment guidelines (1). The ATS/IDSA guidelines continue to recommend respiratory fluoroquinolones for the management of CAP, despite black box safety warnings. This is due to their established efficacy in CAP, low resistance rates, broad coverage of pathogens associated with CAP, including typical and atypical pathogens, high bioavailability, and the convenience of monotherapy (1). Omadacycline shares these beneficial attributes with fluoroquinolones; however, the tetracycline class, including omadacycline, has a materially different safety profile, as evidenced by the FDA warnings listed in the prescribing information (14). The objective of this study was to determine the safety and clinical efficacy of omadacycline compared with moxifloxacin for the treatment of adult patients with CABP and comorbidities who were eligible for treatment as outpatients in the phase 3 OPTIC study.
2. Methods
In the OPTIC study (NCT02531438), patients were eligible for inclusion if they had at least three of the following four CABP symptoms: cough, dyspnea, pleuritic chest pain, or purulent sputum; two or more abnormal vital signs, with at least one clinical sign or laboratory finding associated with CABP, radiologically confirmed pneumonia, and be characterized as Pneumonia Severity Index (PSI) risk class II, III, or IV (13). This post-hoc analysis was performed for all patients with PSI risk classes II or III and at least one comorbidity (asthma/chronic obstructive pulmonary disease [COPD], diabetes mellitus, heart disease, liver disease, and renal disease), and outcomes were further described for each comorbidity. Patient disposition and randomization are shown in Figure 1. Adult patients aged 18 years or older were randomly assigned 1:1 to receive 7 to 14 days of either omadacycline (two doses of 100 mg every 12 h administered IV, then every 24 h, with the option to transition to 300 mg every 24 h, taken orally after at least 3 days) or moxifloxacin (400 mg every 24 h administered IV, with the option to transition to 400 mg every 24 h, taken orally after at least 3 days).
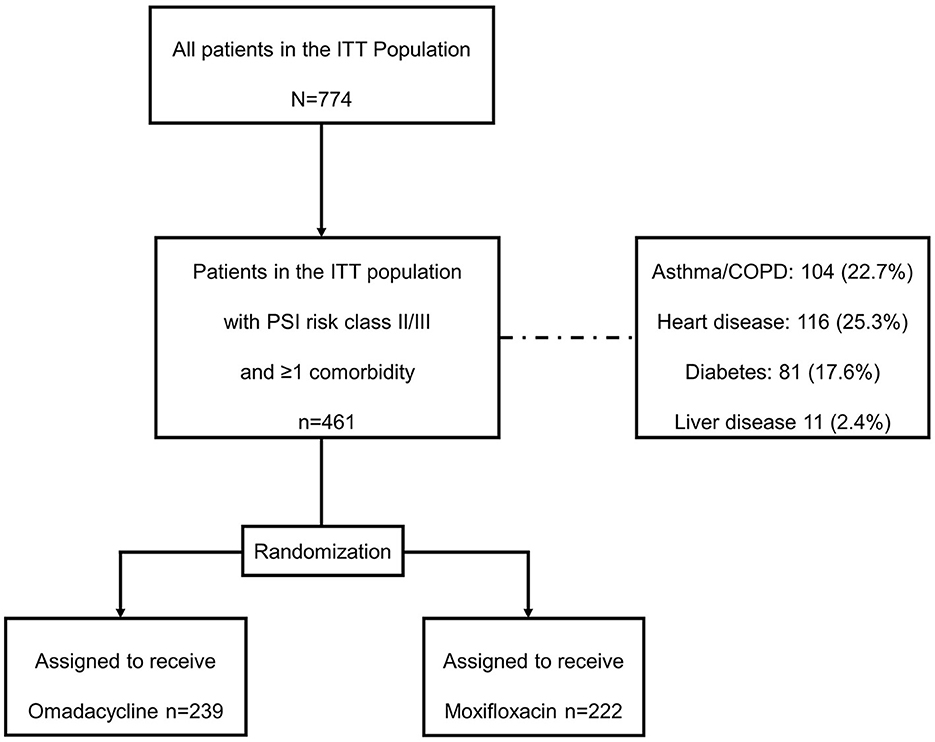
Figure 1. Patient disposition. COPD, chronic obstructive pulmonary disease; ITT, intention-to-treat; PSI, Pneumonia Severity Index.
The primary efficacy outcome was an early clinical response (ECR), defined in the OPTIC study as clinical success, assessed at 72–120 h after the first dose of a trial drug in the intention-to-treat (ITT) population (13). Clinical success was defined as survival with improvement of at least one level compared with baseline (e.g., from moderate to mild) in at least two CABP symptoms with no worsening in other CABP symptoms. A key secondary endpoint was the investigator-assessed clinical response at the post-treatment evaluation (PTE) performed 5–10 days after the last dose of the trial drug, which was defined as survival with resolution or improvement in the signs and symptoms of infection to the extent that further antibacterial therapy was not necessary (13).
3. Results
3.1. Patient demographics
Baseline demographics and clinical characteristics for the 239 omadacycline- and 222 moxifloxacin-treated patients who were included in this analysis are shown in Table 1. Demographics between both groups were generally similar, with a median age of 57 years for the omadacycline group and 58 years for the moxifloxacin group, with 26.0% and 26.6%, respectively, 65 years of age or older. The mean body weight was similar between the two groups (79 kg in the omadacycline group and 80 kg in the moxifloxacin group), and each group had an even split of patients in the three BMI categories of under 25 kg/m2, 25–30 kg/m2 and >30 kg/m2, respectively. The majority of patients had normal renal function (74% of the omadacycline group and 77% of the moxifloxacin group, with 11% and 9% with moderate impairment and 16% and 15% with mild impairment). Approximately half of each group had a medical history of hypertension, and around one-quarter had asthma, COPD, or heart disease. One-fifth of patients had diabetes; liver disease was observed in 2% of the omadacycline group and 3% of the moxifloxacin group.
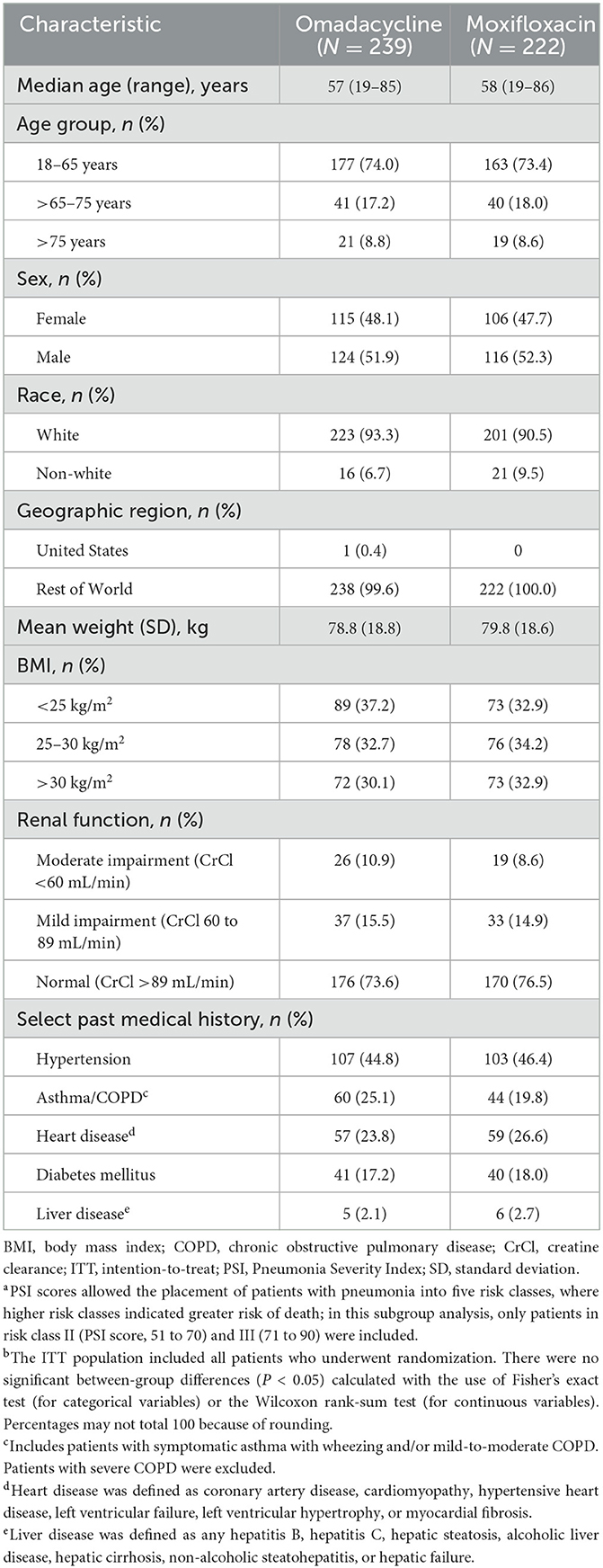
Table 1. Baseline demographics and clinical characteristics in patients with a Pneumonia —Severity Indexa risk class of II or III and ≥1 comorbidity, ITT populationb.
3.2. Early clinical response
Overall, ECR was 91.6% for patients with at least one comorbidity treated with omadacycline and 91.4% for moxifloxacin-treated patients (Table 2). Similarly, clinical response rates observed in patients with at least two comorbidities remained high, at 94.6% for omadacycline and 94.5% for moxifloxacin.
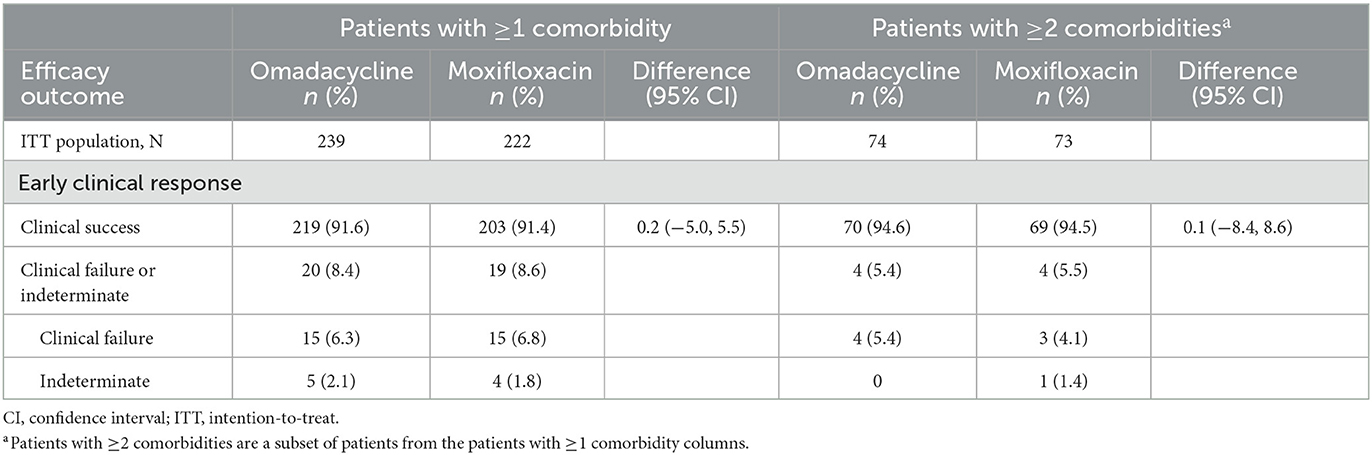
Table 2. Clinical response at early clinical response and post-treatment evaluation in Pneumonia Severity Index risk class II/III patients by number of comorbidities, ITT population.
3.3. Post-treatment evaluation
PTE results remained consistent for overall response in both treatment groups (omadacycline: 89.1%; moxifloxacin: 87.4%; Figure 2) and across individual comorbidity subgroups (omadacycline: 84.9% [any renal impairment] to 93.0% [chronic heart disease]; moxifloxacin: 88.6% [asthma/COPD] to 94.9% [chronic heart disease]). There were no significant differences in the PTE efficacy of either omadacycline or moxifloxacin.
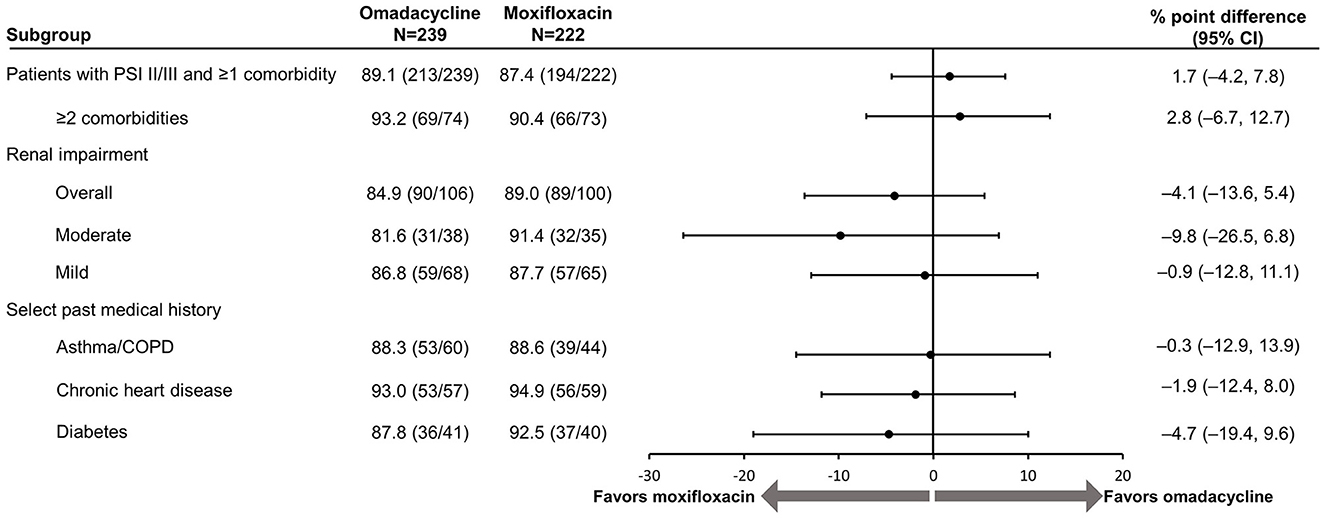
Figure 2. Efficacy of omadacycline and moxifloxacin by comorbidity at post-treatment evaluation, ITT population. CI, confidence interval; COPD, chronic obstructive pulmonary disease; ITT, intention-to-treat; PSI, Pneumonia Severity Index. Renal impairment was defined as creatinine clearance ≤89 mL/min (mild impairment: >60–89 mL/min, moderate impairment: >30–60 mL/min). The 95% CI is based on the Miettinen and Nurminen method without stratification (8). Scores on the PSI are used to place patients with pneumonia into risk classes that range from I to V, with higher risk classes indicating a greater risk of death; in this analysis, only patients in risk classes II (PSI score, 51–70) and III (71–90) were included.
3.4. Safety
Table 3 shows that treatment-emergent adverse event rates were similar across treatment groups (omadacycline: 38.8%; moxifloxacin: 44.6%), with the most reported adverse events being alanine aminotransferase increase (3.8% vs. 4.1%) and gamma-glutamyltransferase increase (3.8% vs. 2.3%) for omadacycline-treated patients and diarrhea (1.3% vs. 6.3%) for moxifloxacin-treated patients. Discontinuation due to an adverse event was infrequent in both treatment groups (omadacycline: 4.2%; moxifloxacin: 5.9%).
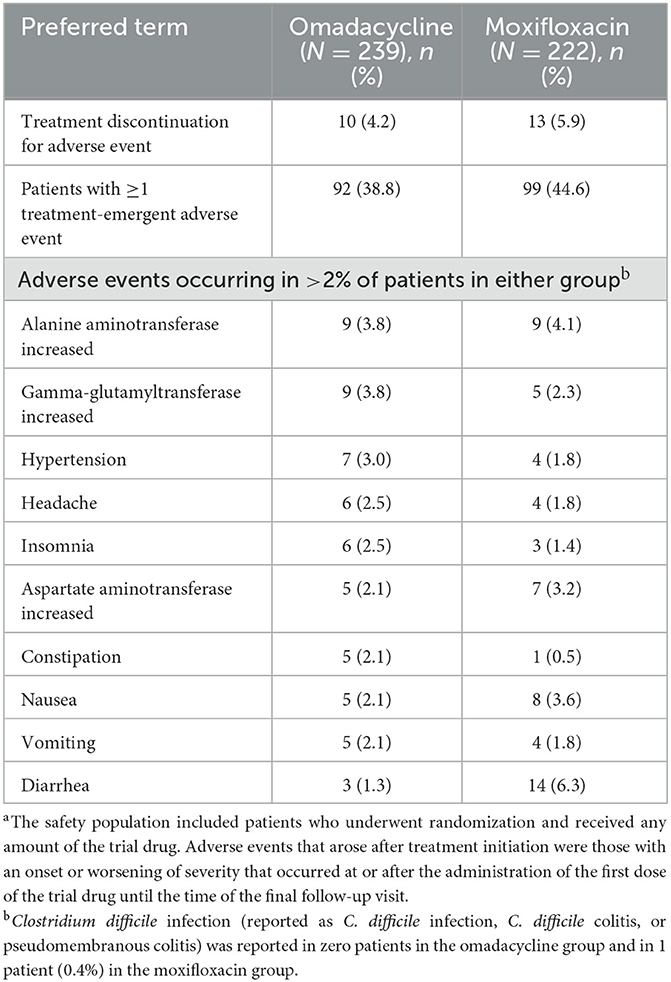
Table 3. Treatment-emergent adverse events in patients with Pneumonia Severity Index risk class II/III and ≥1 comorbidity, safety populationa.
4. Discussion
This study assessed the safety and clinical efficacy of omadacycline compared with the respiratory fluoroquinolone moxifloxacin for the treatment of adult CABP patients with PSI risk class II/III and at least one comorbidity through a post-hoc analysis of the phase 3 OPTIC study. Given their underlying comorbidities, these patients could have been considered eligible for outpatient treatment according to the 2019 ATS/IDSA CAP treatment guidelines (1) and warranted expanded treatment with combination therapy or a respiratory fluoroquinolone monotherapy.
In the OPTIC study, omadacycline was non-inferior to moxifloxacin for the treatment of adult patients with CABP (13). This analysis further supports the results of the OPTIC study, as omadacycline demonstrated similar clinical efficacy to a respiratory fluoroquinolone for the treatment of CABP in adult patients with comorbidities.
Safety concerns have been associated with the fluoroquinolone class since 2008, when the US Food and Drug Administration (FDA) added the first black box warning (15). To date, there are four unique black box warnings and recommendations to limit the use of fluoroquinolones. These include limitations of use for the treatment of acute bacterial sinusitis, acute exacerbations of chronic bronchitis, and uncomplicated urinary tract infections (15). The risks generally outweigh the benefits, as potential serious AEs may be considered worse than the conditions the fluoroquinolones are treating; it is necessary to consider alternative treatment options (15). In response to the FDA warnings, hospitals and antimicrobial stewardship teams have significantly reduced the hospital prescribing of fluoroquinolones; however, most of the antibiotic course is often completed as an outpatient (16), and discharge prescriptions remain unchanged, accounting for the majority of fluoroquinolone use (17).
Fluoroquinolones remain one of the most commonly prescribed antibiotic classes (18–20), likely due to all the reasons cited in the ATS/IDSA guidelines (1), in addition to a lack of awareness of additional options such as omadacycline. The 2019 ATS/IDSA CAP treatment guidelines recommend that patients with any number of comorbidities receive a broader-spectrum treatment due to their increased risk for poor outcomes and risk factors for drug resistance (1). ATS/IDSA guidelines recommend combination therapy to provide the greatest coverage as it is difficult to differentiate between pneumonia caused by typical or atypical pathogens, and patients with comorbidities are at higher risk of severe infection by these pathogens (1). Infection with atypical pathogens, such as Legionella spp., may lead to severe pneumonia, and fluoroquinolones can provide broad coverage when used as monotherapy (21). Respiratory fluoroquinolones maintain many advantages over combination therapy; all are once-daily monotherapy, bioequivalent oral therapy with potent in vitro activity against the most common cause of bacterial pneumonia, S. pneumoniae, and, in addition, have good penetration into the respiratory tissues and fluids (1, 22). All of these attributes, along with CAP being one of the most common indications for antibiotics, result in the unchanged and high utilization of fluoroquinolones for outpatients (17). Despite this, older patients with comorbidities are the group at greatest risk of fluoroquinolone-related adverse events (15).
Outpatients with comorbidities need an efficacious alternative to fluoroquinolones, given the warnings and precautions associated with the antibiotic class. Omadacycline, a derivative of the tetracycline class, has a materially different safety profile from fluoroquinolones, as evident in the warnings and precautions section of the prescribing information (10, 23, 24). While the TEAEs were similar across treatment groups, the study was not powered to detect a difference, and such a study would require a significant increase in enrollment. Given this limitation, post-marketing safety reporting is required by drug manufacturers. In addition, healthcare providers and the public can report via MedWatch, the FDA's medical safety reporting program, which also publishes safety alerts, including those issued for respiratory fluoroquinolones (25). As such, given that omadacycline maintains similar efficacy (13) and the benefits of respiratory fluoroquinolones without the associated black-boxed warnings, it is an important treatment option for patients with CABP, particularly those with comorbidities.
Limitations of this analysis include all limitations inherent to a post-hoc study design, such as a restriction of statistical power due to the smaller patient groups following the stratification of the larger cohort into the subgroups assessed. In addition, the patients included in this analysis were considered eligible for outpatient treatment based on objective scoring systems only and did not consider the provider's clinical judgment. Finally, patients may have comorbidities that were excluded from the OPTIC study, specifically other forms of chronic heart, lung, liver, or renal diseases other than those specified herein, and malignancy, alcoholism, and asplenia, which were not captured as comorbidities in OPTIC.
In summary, both omadacycline and moxifloxacin exhibited similar efficacy in patients with PSI risk class II/III and comorbidities. Omadacycline fulfills an unmet need as an oral monotherapy treatment option for adult patients with CABP, which will further reduce the use of fluoroquinolones.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving human participants were reviewed and approved by the relevant Institutional Review Board or Ethics Committee at each of the 86 participating sites in the OPTIC study. The patients/participants provided their written informed consent to participate in this study.
Author contributions
All authors contributed to analyzing and interpreting the data, drafting the manuscript, and approving the final manuscript.
Funding
This study received funding from Paratek Pharmaceuticals, Inc. The funder had the following involvement with the study: study design, collection, analysis, and interpretation of data; writing, reviewing, and editing this article; and the decision to submit it for publication. Medical writing assistance was provided by Meridian HealthComms Ltd, Plumley, UK, funded by Paratek Pharmaceuticals, Inc according to Good Publication Practice (GPP4).
Acknowledgments
The authors thank all participating patients and their families, all study co-investigators, and the research coordinators.
Conflict of interest
NW is a consultant for Paratek Pharmaceuticals, Inc. GDR reports travel support to present a poster version of this manuscript. SC is a consultant for Paratek Pharmaceuticals, Inc. MA-G and KW are employees and shareholders of Paratek Pharmaceuticals, Inc.
The remaining author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Olson G, Davis AM. Diagnosis and treatment of adults with community-acquired pneumonia. An official clinical practice guideline of the American thoracic society and infectious diseases society of America. Am J Respir Crit Care Med Oct 1. (2019) 200:e45–67. doi: 10.1164/rccm.201908-1581ST
2. Golomb BA, Koslik HJ, Redd AJ. Fluoroquinolone-induced serious, persistent, multisymptom adverse effects. BMJ Case Rep. 2015:bcr2015209821. doi: 10.1136/bcr-2015-209821
3. Feldman C, Anderson R. The role of streptococcus pneumoniae in community-acquired pneumonia. Semin Respir Crit Care Med Aug. (2020) 41:455–69. doi: 10.1055/s-0040-1702193
4. Aliberti S, Cook GS, Babu BL, Reyes LF, Rodriguez AH, Sanz F, et al. International prevalence and risk factors evaluation for drug-resistant Streptococcus pneumoniae pneumonia. J Infect. (2019) 79:300–11. doi: 10.1016/j.jinf.2019.07.004
5. Campbell GDJ, Silberman R. Drug-resistant Streptococcus pneumoniae. Clin Infect Dis. (1998) 26:1188–95. doi: 10.1086/520286
6. Jenkins TC, Sakai J, Knepper BC, Swartwood CJ, Haukoos JS, Long JA, et al. Risk factors for drug-resistant Streptococcus pneumoniae and antibiotic prescribing practices in outpatient community-acquired pneumonia. Acad Emerg Med Jun. (2012) 19:703–6. doi: 10.1111/j.1553-2712.2012.01365.x
7. Centers fDCaP. Antibiotic Resistance Threats in the United States 2019. (2019). Available online at: https://www.cdc.gov/drugresistance/pdf/threats-report/2019-ar-threats-report-508.pdf (accessed 19 December, 2022).
9. World HO. The Top 10 Causes of Death. (2023). Available online at: https://www.who.int/news-room/fact-sheets/detail/the-top-10-causes-of-death (accessed February, 2023).
10. Karlowsky JA, Steenbergen J, Zhanel GG. Microbiology and preclinical review of omadacycline. Clin Infect Dis. (2019) 69:S6–S15. doi: 10.1093/cid/ciz395
11. Draper MP, Weir S, Macone A, Donatelli J, Trieber CA, Tanaka SK, et al. Mechanism of action of the novel aminomethylcycline antibiotic omadacycline. Antimicrob Agents Chemother. (2014) 58:1279–83. doi: 10.1128/AAC.01066-13
12. Burgos RM, Rodvold KA. Omadacycline: a novel aminomethylcycline. Infect Drug Resist. (2019) 12:1895–915. doi: 10.2147/IDR.S171352
13. Stets R, Popescu M, Gonong JR, Mitha I, Nseir W, Madej A, et al. Omadacycline for community-acquired bacterial pneumonia. N Engl J Med Feb 7. (2019) 380:517–27. doi: 10.1056/NEJMoa1800201
14. Sakoulas G, Nowak M, Geriak M. Omadacycline in treating community-based infections: a review and expert perspective. Expert Rev Anti Infect Ther Mar. (2023) 21:255–65. doi: 10.1080/14787210.2023.2174100
15. Food aDA. FDA Updates Warnings for Fluoroquinolone Antibiotics on Risks of Mental Health and Low Blood Sugar Adverse Reactions. (2018). Available online at: https://www.fda.gov/news-events/press-announcements/fda-updates-warnings-fluoroquinolone-antibiotics-risks-mental-health-and-low-blood-sugar-adverse (accessed December 20, 2022)
16. Yogo N, Haas MK, Knepper BC, Burman WJ, Mehler PS, Jenkins TC. Antibiotic prescribing at the transition from hospitalization to discharge: a target for antibiotic stewardship. Infect Control Hosp Epidemiol Apr. (2015) 36:474–8. doi: 10.1017/ice.2014.85
17. Vaughn VM, Seelye SM, Wang XQ, Wiitala WL, Rubin MA, Prescott HC. Inpatient and discharge fluoroquinolone prescribing in veterans affairs hospitals between 2014 and 2017. Open Forum Infect Dis. (2020) 7:ofaa149. doi: 10.1093/ofid/ofaa149
18. Sankar A, Swanson KM, Zhou J, Jena AB, Ross JS, Shah ND, et al. Association of fluoroquinolone prescribing rates with black box warnings from the US food and drug administration. JAMA Netw Open Dec 1. (2021) 4:e2136662. doi: 10.1001/jamanetworkopen.2021.36662
19. Hicks LA, Bartoces MG, Roberts RM, Suda KJ, Hunkler RJ, Jr THT, et al. US outpatient antibiotic prescribing variation according to geography, patient population, and provider specialty in 2011. Clin Infect Dis. (2015) 60:1308–16. doi: 10.1093/cid/civ076
20. Baggs J, Fridkin SK, Pollack LA, Srinivasan A, Jernigan JA. Estimating national trends in inpatient antibiotic use among US hospitals from 2006 to 2012. JAMA Intern Med Nov 1. (2016) 176:1639–48. doi: 10.1001/jamainternmed.2016.5651
21. Garin N, Marti C, Skali LA, Prendki V. Atypical pathogens in adult community-acquired pneumonia and implications for empiric antibiotic treatment: a narrative review. Microorganisms. (2022) 10:12 doi: 10.3390/microorganisms10122326
22. Balfour JA, Lamb HM. Moxifloxacin: a review of its clinical potential in the management of community-acquired respiratory tract infections. Drugs Jan. (2000) 59:115–39. doi: 10.2165/00003495-200059010-00010
23. Macone AB, Caruso BK, Leahy RG, Donatelli J, Weir S, Draper MP, et al. In vitro and in vivo antibacterial activities of omadacycline, a novel aminomethylcycline. Antimicrob Agents Chemother. (2014) 58:1127–35. doi: 10.1128/AAC.01242-13
24. Pfaller MA, Huband MD, Shortridge D, Flamm RK. Surveillance of omadacycline activity tested against clinical isolates from the USA: report from the SENTRY antimicrobial Surveillance Program, 2019. J Glob Antimicrob Resist Dec. (2021) 27:337–51. doi: 10.1016/j.jgar.2021.09.011
Keywords: community-acquired bacterial pneumonia, omadacycline, fluoroquinolones, antibiotic resistance, oral antibiotics
Citation: Rodriguez GD, Warren N, Yashayev R, Chitra S, Amodio-Groton M and Wright K (2023) Omadacycline in the treatment of community-acquired bacterial pneumonia in patients with comorbidities: a post-hoc analysis of the phase 3 OPTIC trial. Front. Med. 10:1225710. doi: 10.3389/fmed.2023.1225710
Received: 19 May 2023; Accepted: 11 July 2023;
Published: 28 July 2023.
Edited by:
Werner Bernd Spur, Rowan University School of Osteopathic Medicine, United StatesReviewed by:
Kingsley Yin, Rowan University School of Osteopathic Medicine, United StatesMatthew Geriak, Sharp Memorial Hospital, United States
Copyright © 2023 Rodriguez, Warren, Yashayev, Chitra, Amodio-Groton and Wright. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Kelly Wright, S2VsbHkud3JpZ2h0QHBhcmF0ZWtwaGFybWEuY29t
 George D. Rodriguez1,2
George D. Rodriguez1,2 Roman Yashayev
Roman Yashayev Kelly Wright
Kelly Wright