- 1Department of Emergency, Peking University People's Hospital, Beijing, China
- 2Department of Infectious Diseases, Peking University People's Hospital, Beijing, China
- 3Department of Neurology, Peking University People's Hospital, Beijing, China
Campylobacter jejuni (C. jejuni), a Gram-negative bacterium, belongs to microaerobic bacteria. We reported a 21-year-old male patient diagnosed with hemophagocytic lymphohistiocytosis (HLH) due to C. jejuni infection, who presented with multiple clinical manifestations of peripheral nerve injury, such as ophthalmoplegia, facial paralysis, and urinary retention during the treatment. Electromyography showed neurogenic injury and the final diagnosis was Guillain-Barre Syndrome (GBS). After treatment of dexamethasone combined with immunoglobulin, the patient was discharged from the hospital with partial recovery of neurological symptoms.
Introduction
Campylobacter jejuni (C. jejuni), a Gram-negative bacterium, belongs to microaerobic bacteria. We reported a 21-year-old male patient diagnosed with hemophagocytic lymphohistiocytosis (HLH) due to C. jejuni infection, who presented with multiple clinical manifestations of peripheral nerve injury, such as ophthalmoplegia, facial paralysis, and urinary retention during the treatment. Electromyography showed neurogenic injury and the final diagnosis was Guillain-Barre Syndrome (GBS). After treatment of dexamethasone combined with immunoglobulin, the patient was discharged from the hospital with partial recovery of neurological symptoms.
Case Report
The patient, a 21-year-old Uygur male, had a history of fever and diarrhea for 8 days, with the highest body temperature of about 38.6°C, accompanied by chills, and sparse watery stool several times, without abdominal pain. Due to the intermittently increased temperature after taking non-steroidal anti-inflammatory drugs (NSAIDs), the patient was treated in the fever clinic but got a poor treatment effect, therefore he was admitted to the emergency department on October 10, 2021.
Physical examination on admission showed a body temperature of 38°C, pulse of 102 times/min, respiration of 23 times/min, and blood pressure of 125/72 mmHg, with clear consciousness, palpable spleen under the ribs. No other significant abnormalities were found in other physical examinations.
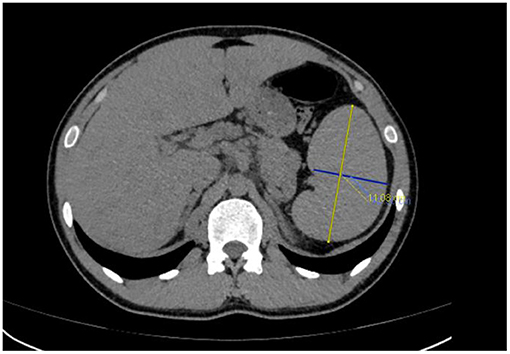
Emergency laboratory analysis in the emergency department indicated the following: white blood cell count decreased from normal range to 1.56 × 109/L (reference value, RV 3.5–9.5 × 109/L), hemoglobin was 166 g/L (RV 130–175 g/L), platelet decreased from normal range to 74 × 109/L (RV 125–350 × 109/L), aspartate aminotransferase was 150u/L (RV 15–40 u/L), lactate dehydrogenase was 1249 u/L (RV 109–245 u/L), triglyceride was 2.29 mmol/L (RV 0.45–1.7 mmol/L), sodium was 124.2 mmol/L (RV 137–147 mmol/L), fibrinogen decreased from normal range to 1.27 g/L (RV 2–4 g/L), sCD25 was 25,398 pg/mL (RV < 6,400pg/mL), ferritin was 3671u g/L (RV 30-400ng/mL), natural killer cell activity was 18.48% (RV ≥ 15.11%). G-test (serum (1,3)-β-D-glucan test) was positive, GM-test (galactomannan test) was negative, and blood culture, procalcitonin, c-reactive protein, erythrocyte sedimentation rate and T-SPOT.TB test were all negative. Mycoplasma pneumoniae antibody IgM (Passive Particle Agglutination, FUJIREBIO) was 1:80 positive (RV < 1:20), Legionella pneumophila antibody IgM (ELISA, Euroimmun) was 0.82 positive (RV < 0.8). The detection of Cytomegalovirus (CMV), Epstein Barr virus (EBV), Adenovirus and Coxsackievirus were all negative (real-time PCR). The oncological and immunological indexes were normal. Plain CT scan of the thoracoabdominal basin showed mild fatty liver and splenomegaly (Figure 1). There are many small lymph nodes in the hepatogastric space and retroperitoneal space.
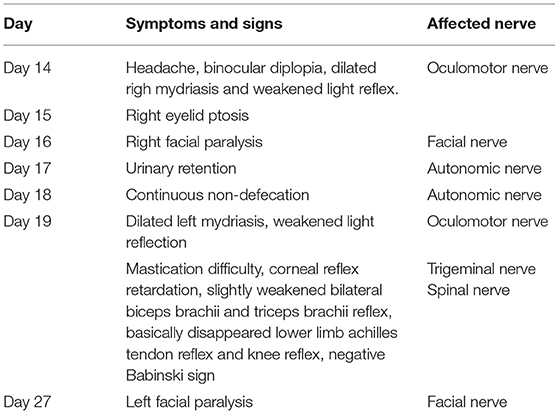
Referring to HLH-2004 diagnostic criteria, the patient met the following conditions: ① Temperature > 38.5°C for more than 7 days; ② Hemocytopenia (WBC <10 × 109/L, PLT <100 × 109/L, hemoglobin 166 g/L); ③Splenomegaly; ④Low fibrinogen (FIB <1.5 g/L) and high triglycerides (2.29 mmol/L), although not meeting the diagnostic criteria (3 mmol/L); ⑤Ferritin > 500u g/L;⑥sCD25 > 6,400 pg/mL. Considering the definitive diagnosis of HLH, the patient was treated with 20 g immunoglobulin QD (8 days) and 0.5 g imipenem cilastatin sodium Q8H, and his leukocytes, platelets, and fibrinogen gradually returned to normal levels. However, on day 14 after his onset, the patient developed a series of symptoms of peripheral nerve involvement, as shown in Table 1.
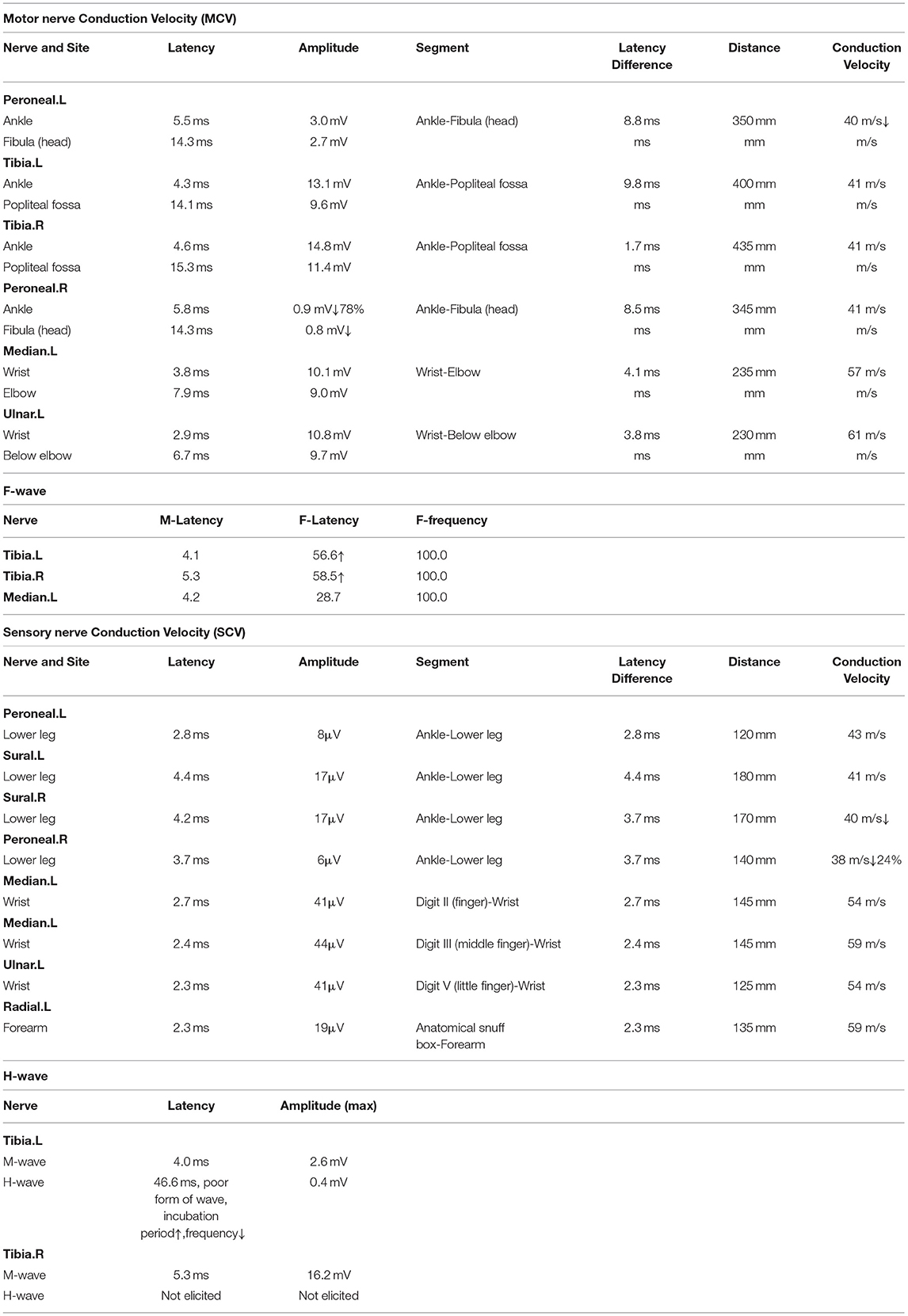
Lumbar puncture was performed on the 16th day. Cerebrospinal fluid showed a low pressure and turbid appearance, containing 150 white cells/μL (90% lymphocytes, 10% neutrophils), protein 1.09 g/L, glucose 2.37 mmol/L, and chlorine 100.6 mmol/L; Bacteria, fungi, cryptococcus, and Mycobacterium tuberculosis were all negative; Rubella virus, CMV and EBV IgM antibody and DNA were later reported as negative. CSF and serum albumin data: CSF and serum albumin was 3.27 and 37 g/L, respectively (October 19); while 1.09 and 32.6 g/L, respectively (October 22). The albumin quotient (albumin in CSF/albumin in serum) indicated that an increased permeability of the blood-brain. No abnormality was found in CSF next-generation sequencing technology (NGS) implemented by Biotechnology Co., Ltd. Briefly, they optimized the internal index adapter and real-time analysis pipeline, established the optimal threshold for pathogen identification, and performed rapid metagenomic-NGS analysis (1). Peripheral neuropathy, demyelination, paraneoplastic tumor, autoimmune brain-related antibodies, serum antibodies IgM and IgG of C. jejuni were positive by enzyme-linked immunosorbent assay (ELISA), performed by Dr. Hao from the first hospital of Peking University (2), The values of IgM and IgG were greater than the negative control values (the cut-off values were 0.08 and 0.16 respectively) even after 1:160 dilution. Given the clinical presentation and CSF findings, based on intravenous dexamethasone of 20mg QD, the treatment regimen was adjusted to meropenem 1000 mg Q8H combined with acyclovir 60 mg Q8H and Mecobalamin 500 ug QD. On the 18th day, the patient's body temperature dropped to normal. On the 19th day, the lumbar puncture was performed again, and the cerebrospinal fluid became clear. CSF laboratory examination showed white blood cell count of 91/μL (92% single nucleus, 8% multiple nuclei), protein level of 3.27 g/L, normal glucose and chlorine, as well as negative NGS. Electromyography (Table 2) and cranial MRI were generally normal.
Considering the peripheral nerve injury and cerebrospinal fluid protein cell separation, GBS secondary to C. jejuni infection was specifically classified as Miller Fisher syndrome (MFS) and Bickerstaff brainstem encephalitis. On the 23rd day of onset, the diameter of the right pupil gradually recovered to 4 mm the pupillary light reflex, and the closing function of the right upper eyelid gradually recovered. On the 26th day, dexamethasone was reduced to 10 mg QD. On the 27th day, the left pupil diameter recovered to about 4 mm, and the pupillary light reflex recovered. On the 28th day, the patient defecated autonomously. On the 41st day, spontaneous urination recovered, and the patient was hospitalized for 42 days. Before discharge, the patient had left bilateral facial paralysis and mild diplopia in both eyes. One month after discharge, the patient was followed up, leaving slight facial paralysis, and other symptoms returned to normal.
Discussion
C. jejuni, is a kind of Gram-negative bacteria, with which humans are easily infected. The incidence rate is high in summer and autumn, and the number of infected men is about 1.2–1.5 times that of women (3). C. jejuni, is the leading cause of bacterial diarrhea with an incubation period of about 2–7 days. The clinical manifestations are characterized by fever, abdominal pain, and red and white blood cells in feces. Parenteral infection is common in patients aged 40–70 or with hypo-immunity. Blood-borne infection can cause complications such as bacteremia and meningitis.
GBS is a group of acute autoimmune-mediated peripheral neuropathys, generally with a history of precursor infection in two-thirds of patients, and its common pathogens include C. jejuni, CMV, EBV, M. pneumonia, etc, of which C. jejuni is the most common (4). The patient was positive for M. pneumonia IgM antibody and weakly positive for L. pneumophila IgM antibody, we consider these to be false-positive results because the patient's body temperature dropped to normal without targeted treatment (5, 6). At the same time, GBS is also one of the serious complications caused by C. jejuni infection (7). Most of the clinical symptoms of GBS peak in about 2 weeks. The typical clinical manifestation is paresthesia of the distal limbs, accompanied by symmetrical weakness of the lower limbs, and then involving the muscles innervated by the upper limbs and cranial nerves. Autonomic dysfunction is also a common symptom, including abnormal pupil, intestinal, or bladder function. Protein-cell separation can be seen in cerebrospinal fluid. Nerve conduction velocity and electromyography contribute to GBS diagnosis and primary myelin injury determination. The infection rate of C. jejuni in GBS patients with diarrhea as a precursor is as high as 85%. In 50% of patients with C. jejuni enteritis, the bacteria cannot be isolated by fecal examination 2 weeks after diarrhea stops (7).
Acute inflammatory demyelinating polyneuropathy (AIDP) and acute motor axonal neuropathy (AMAN) are the two most common subtypes of GBS. The less common subtypes include acute motor-sensory axonal neuropathy (AMSAN) and Miller Fisher syndrome (MFS) (7). Asia has a high incidence rate of MFS, and the proportion of GBS patients is approximately 15–25%. The main clinical manifestations are ophthalmoplegia, ataxia, weakening, or disappearance of tendon reflex. The initial symptoms are diplopia and limb weakness. Neurogenic injury is common on EMG, with decreased nerve conduction velocity, and prolonged or absent F wave (8). The etiology and pathogenesis of MFS remain unclear. They are mainly related to the autoimmune response after infection. It is currently believed that antibodies generated after C. jejuni infection directly attack the presynaptic membrane and synaptic space (9, 10), resulting in impaired integrity of peripheral nerves and loss of nerve conduction-related functions. Recent studies have shown that anti-GQ1b, anti-GD1b, and anti-GT1a antibodies are related to the pathogenesis of MFS, among which anti-GQ1b is the most specific, mainly deposited in the paraganglionic area of oculomotor nerve, trochlear nerve, and abductor nerve, with the most abundant content in oculomotor nerve. After the occurence of clinical symptoms, the antibody titer increases rapidly and then decreases when the symptoms turn negative. Generally, the serum anti-GQ1b antibody turns negative one month after onset (11). Combined with the neurological manifestations, as well as the increased lymphocyte number and protein content in CSF, the diagnosis of the patient is closer to MFS and Bickerstaff brainstem encephalitis.The IgM and IgG antibodies of C. jejuni were positive in the blood samples while the ganglioside antibodies were negative in the cerebrospinal fluid. There are two possibilities to explain this false-negative phenomenon. Firstly, some studies have found that about 10–30% of MFS cases are negative in the routine detection of GQ1b antibodies. Due to the fact that GQ1 antibodies are calcium dependent, they may fail to bind the detection ligand in the absence of sufficient Ca2+. Secondly, rapid antibody depletion can also result in a negative test result (12, 13).
HLH, a reactive disease caused by infection, tumor, immune-related diseases, and other causes that activate the immune system, is mainly characterized by persistent fever, hepatosplenomegaly, pancytopenia, and hemophagocytosis in the bone marrow and lymphoid tissue (14, 15). HLH is divided into primary HLH and secondary HLH. Primary HLH is an autosomal or sex chromosome recessive disease, and secondary HLH is related to various underlying diseases.
Currently, there is no report of HLH caused by C. jejuni. A case report of HLH complicated with GBS caused by EB virus infection was found in the search database (16). C. jejuni has not been reported to simultaneously cause both HLH and GBS. After excluding other diagnostic possibilities, we considered that HLH was caused by C. jejuni infection, and then GBS appeared as a consequence of an autoimmune response.
GBS is generally considered to be elicited by diverse ganglioside antibodies or multiple antibody complexes generated by varying serotypes and genotypes of C. jejuni. It's been reported that the lipo-oligosaccharide (LOS) motifs of C. jejuni isolated from GBS patients resemble peripheral nerve gangliosides (GM1, GD1a, GQ1b, etc.). The key antigenic molecule is presumably the sialylated oligosaccharide motif on the LOS of C. jejuni, which contains the heptosyl residue peculiar to LOS and the oligosaccharide component simulating gangliosides. Molecular mimicry has significant effect on the production of the antibodies. A subtle degree of structural differences of key antigenic molecules in the pathogen may trigger a breakdown of tolerance and lead to autoimmunity (17). The combination of antigen and antibody, meanwhile, will elicit inflammatory cell infiltration and complement fixation, which would cause immune attacks on specific sites of peripheral and central nerves with similar antigenicity, thereby resulting in nerve injury. The key motif is also the ligand of Siglec-1 (CD169) needed for phagocytosis by antigen presenting cells (APC). Siglec-1 is a type1 transmembrane protein and belongs to the Sialic acid binding Ig-like lectin family. It plays a critical role in the maturation of colonic T cells and in the elicitation of autoantibodies (18). Furthermore, a recent study has found that GBS patients exhibit defective signaling of Siglec-10, an inhibitory receptor expressed by B cells, which may give rise to aberrant B cell responses against gangliosides or LOS. This indicates that there probably exists a regulatory mechanism that will restrict the risk of autoimmunity to self-glycolipids, and this mechanism may be undermined in patients with GBS (17).
In conclusion, given the irreversible damage of GBS, early identification and timely treatment of central nervous system diseases caused by GBS can prevent irreversible injury to the central nervous system, playing a vital role in the improvement of the long-term prognosis.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author/s.
Ethics Statement
Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author Contributions
All authors listed have made a substantial, direct, and intellectual contribution to the work and approved it for publication.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Zhang D, Zhang J, Du J, Zhou Y, Wu P, Liu Z, et al. Optimized Sequencing Adaptors Enable Rapid and Real-Time Metagenomic Identification of Pathogens During Runtime of Sequencing [Published Online Ahead of Print, 2022 Mar 15]. Clin Chem. (2022) 2022:Hvac024. doi: 10.1093/Clinchem/Hvac024
2. Hao HJ, Li HF, Yuan JM. Serum Antibody to Campylobacter Jejuni (CJ) in Guillain-Barre Syndrome (GBS) Patients and Its Significance. Chinese J Immunol. (2001) 17:608–10 [in Chinese].
3. Man SM. The Clinical Importance of Emerging Campylobacter Species. Nat Rev Gastroenterol Hepatol. (2011) 8:669–85. doi: 10.1038/Nrgastro.2011.191
4. Kaakoush NO, Castaño-Rodríguez N, Mitchell HM, Man SM. Global Epidemiology of Campylobacter Infection. Clin Microbiol Rev. (2015) 28:687–720. doi: 10.1128/CMR.00006-15
5. Como J, Moffa MA, Bhanot N, Min Z, Cole KS, Kuzyck J, et al. Potential False-Positive Urine Legionella Enzyme Immunoassay Test Results. Eur J Clin Microbiol Infect Dis. (2019) 38:1377–82. doi: 10.1007/S10096-019-03575-2
6. Herrera M, Aguilar YA, Rueda ZV, Muskus C. Vélez LA. Comparison of Serological Methods With PCR-Based Methods for the Diagnosis of Community-Acquired Pneumonia Caused by Atypical Bacteria. J Negat Results Biomed. (2016) 15:3. doi: 10.1186/S12952-016-0047-Y
7. Poropatich KO, Walker CL, Black RE. Quantifying the Association Between Campylobacter Infection and Guillain-Barré Syndrome: A Systematic Review. J Health Popul Nutr. (2010) 28:545–52. doi: 10.3329/Jhpn.V28i6.6602
8. Van Doorn PA, Ruts L, Jacobs BC. Clinical Features, Pathogenesis, and Treatment of Guillain-Barré Syndrome. Lancet Neurol. (2008) 7:939–50. doi: 10.1016/S1474-4422(08)70215-1
9. Chinese Chinese Society of Neurology, Chinese Guidelines for Diagnosis and Treatment of Guillain-Barré Syndrome 2019. Chinese J Neurol. (2019) 52:877–82 [in Chinese]. doi: 10.3760/cma.j.issn.1006
10. Hussain AM, Flint NJ, Livsey SA, Wong R, Spiers P, Bukhari SS. Bickerstaff's Brainstem Encephalitis Related to Campylobacter Jejuni Gastroenteritis. J Clin Pathol. (2007) 60:1161–2. doi: 10.1136/jcp.2006.046284
11. Fukami Y, Wong AH, Funakoshi K, Safri AY, Shahrizaila N, Yuki N. Anti-GQ1b Antibody Syndrome: Anti-Ganglioside Complex Reactivity Determines Clinical Spectrum. Eur J Neurol. (2016) 23:320–6. doi: 10.1111/ene.12769
12. Rocha Cabrero F, Morrison EH. Miller Fisher Syndrome. In: StatPearls. Treasure Island (FL): StatPearls Publishing (2021).
13. Uchibori A, Gyohda A. Chiba A. Ca(2+)-Dependent Anti-GQ1b Antibody in GQ1b-Seronegative Fisher Syndrome and Related Disorders. J Neuroimmunol. (2016) 298:172–7. doi: 10.1016/j.Jneuroim.2016.07.021
14. Horne A, Trottestam H, Aricò M, Egeler RM, Filipovich AH, Gadner H. Frequency and Spectrum of Central Nervous System Involvement in 193 Children With Haemophagocytic Lymphohistiocytosis. Br J Haematol. (2008) 140:327–35. doi: 10.1111/j.1365-2141.2007.06922.x
15. Al-Samkari H, Berliner N. Hemophagocytic Lymphohistiocytosis. Annu Rev Pathol. (2018) 13:27–49. doi: 10.1146/Annurev-Pathol-020117-043625
16. Kimoto K, Koga M, Odaka M, Hirata K, Takahashi M, Li J, et al. Relationship of Bacterial Strains to Clinical Syndromes of Campylobacter-Associated Neuropathies. Neurology. (2006) 67:1837–43. doi: 10.1212/01.wnl.0000244468.22377.6b
17. Laman JD, Huizinga R, Boons GJ, Jacobs BC. Guillain-Barré Syndrome: Expanding the Concept of Molecular Mimicry. Trends Immunol. (2022) 43:296–308. doi: 10.1016/j.It.2022.02.003
Keywords: Campylobacter jejuni, hemophagocytic lymphohistiocytosis, Guillain-Barre Syndrome, peripheral nerve injury, ophthalmoplegia, facial paralysis
Citation: Shi F-e, Chen M-f, Li Y-j, Dong G-y and Zhu J-h (2022) Campylobacter jejuni-Associated Hemophagocytic Lymphohistiocytosis and Guillain-Barre Syndrome: A Case Report. Front. Med. 9:895923. doi: 10.3389/fmed.2022.895923
Received: 14 March 2022; Accepted: 20 June 2022;
Published: 07 July 2022.
Edited by:
Linda Ann Spatz, City University of New York, United StatesReviewed by:
Andreas Erich Zautner, University Hospital Magdeburg, GermanyAnkit Malik, The University of Chicago, United States
Copyright © 2022 Shi, Chen, Li, Dong and Zhu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Gui-ying Dong, YW5hc3Rhc2lhZGd5QGZveG1haWwuY29t; Ji-hong Zhu, emh1amlob25nNjRAc2luYS5jb20=
 Fang-e Shi
Fang-e Shi Mei-fang Chen2
Mei-fang Chen2 Gui-ying Dong
Gui-ying Dong