
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Med., 21 June 2022
Sec. Hepatology
Volume 9 - 2022 | https://doi.org/10.3389/fmed.2022.837898
This article is part of the Research TopicHepatitis B virus related chronic liver diseaseView all 5 articles
Background: Some people infected with the hepatitis B virus (HBV) with a normal level of alanine aminotransferase (ALT) are at risk of disease progression. We evaluated the value of platelet-to-portal vein width ratio (PPR) and platelet-to-spleen thickness ratio (PSR) to predict progressive liver fibrosis among patients with HBV infection with HBV e antigen (HBeAg)-negativity and a normal ALT level.
Methods: HBV surface antigen (HBsAg)-positive and HBeAg-negative individuals with a normal ALT level were enrolled. The inflammation grade (G) and fibrosis stage(S) were analyzed according to pathological features. Then, two groups (<S2 vs. ≥S2) among people with a normal ALT level were divided based on the pathological diagnosis, and the clinical characteristics were summarized.
Results: Seventy-three individuals among 142 patients with HBsAg-positivity and HBeAg-negativity had a normal ALT level. Also, 83.56% (61/73) individuals showed progressive liver fibrosis (≥S2). The ALT level and aspartate aminotransferase (AST) between the two groups differed (21.01 ± 7.40 vs. 25.37 ± 7.90 U/L, p = 0.08; 29.49 ± 13.56 vs. 30.16 ± 21.88 U/L, p = 0.92, respectively). Portal-vein width, serum levels of albumin and globulin, AST-to-Platelet Ratio Index (APRI), and Fibrosis 4 (FIB-4) score were not significantly different between the two groups (p > 0.05). The platelet count, PPR, and PSR were significantly different between the two groups [(145.92 ± 14.55) ×109/L vs. (126.38 ± 23.85) ×109/L, p = 0.008; 10.80 ± 1.30 vs. 9.01 ± 1.97, p = 0.004; 4.21 ± 0.65 vs. 3.33 ± 0.89, p = 0.02, respectively]. The PPR and PSR decreased gradually upon fibrosis aggravation (p < 0.05). Based on the cut off value of the PPR (9.07) and PSR (3.54), their sensitivity and specificity was 0.917 and 0.525, and 0.833 and 0.541, respectively.
Conclusion: The PPR and PSR can be employed to assess earlier fibrosis progression among patients with HBV infection with HBeAg-negativity and a normal ALT level.
The natural history of hepatitis B virus (HBV) infection can be divided into four stages: immune tolerance, immune clearance, inactive carrier status, and reactivity (1). “Immune tolerance” refers to the non-responsive state after the immune system of the body meets a specific antigen (2). Previously, it was believed that most patients with chronic HBV infection were in the “immune tolerance period” (ITP) (3). Patients in the ITP have slight inflammatory necrosis and/or liver fibrosis, and a poor response to antiviral therapy. Simultaneously, spontaneous HBV e antigen (HBeAg) serological conversion and sustained remission are possible during the ITP. Otherwise, long-term antiviral therapy in the ITP can lead to drug resistance and other adverse events, and even increase the economic burden of patients. Therefore, most patients with immune tolerance of chronic HBV infection do not need antiviral therapy (4, 5).
There is a lack of sensitive and specific markers to measure the immune tolerance of patients after HBV infection. However, in the definition of the ITP of HBV infection by various hepatology societies, a continuously normal level of alanine aminotransferase (ALT) is regarded as a basic characteristic (6–8). However, there is also evidence that significant fibrosis occurs in a large proportion of HBV-infected patients with a normal ALT level who are considered ITP (9). Hepatocellular carcinoma has been found in some patients in the ITP (10, 11). Liver histopathology of HBV-infected people with a normal ALT level indicates that about one-third to one-half of cases are not in the ITP (12–14). Therefore, clinicians must judge if patients of chronic HBV infection with a normal ALT level are in the ITP.
Liver biopsy is the “gold standard” for determining if the liver has inflammation and fibrosis. However, ascertaining if a patient has liver inflammation by liver biopsy is impractical because it is invasive. Accurate assessment of the degree of liver fibrosis in patients with a normal ALT level in a non-invasive manner is crucial to ascertain if patients are immune-tolerant.
We investigated the basic clinical and laboratory characteristics of progressive liver fibrosis among patients with HBV infection who were HBeAg-negative and had a normal ALT level.
The study protocol was approved by the Clinical Research Ethics Committee of Fuling Center Hospital of Chongqing City (Chongqing, China). Written informed consent was obtained from patients (or their legal surrogates) before data collection.
The inclusion criteria were: (i) serum hepatitis B surface antigen (HBsAg)-positivity for ≥6 months; (ii) serum HBeAg-negativity; (iii) never treated with antiviral agents.
The exclusion criteria were: (i) infection with hepatitis A, C, D, E or other viruses; (ii) history of alcohol and/or drug intake that caused liver damage; (iii) non-alcoholic fatty liver disease; (iv) magligant liver tumor and definite cirrhosis; (v) history of hereditary metabolic liver disease or autoimmune liver disease; (vi) history of hypertension, diabetes mellitus, coronary heart disease, or metabolic syndrome; (vii) history of traumatic fractures.
A total of 165 patients who underwent liver biopsy for assessment of disease progression from July 2019 to June 2021 at Fuling Center Hospital of Chongqing City were evaluated, retrospectively (Figure 1). Twenty-three patients were excluded. Hence, 142 patients with HBsAg-positivity and HBeAg-negativity were enrolled. Of these, 73 patients had a normal ALT level (defined as ALT <40U/L). All patients underwent ultrasound-guided percutaneous liver biopsy followed by calculation of the METAVIR score to assess the inflammation grade (G) and fibrosis stage (S). Then, patients were divided into two groups: progressive liver fibrosis (S ≥ 2) and non-progressive fibrosis (S <2).
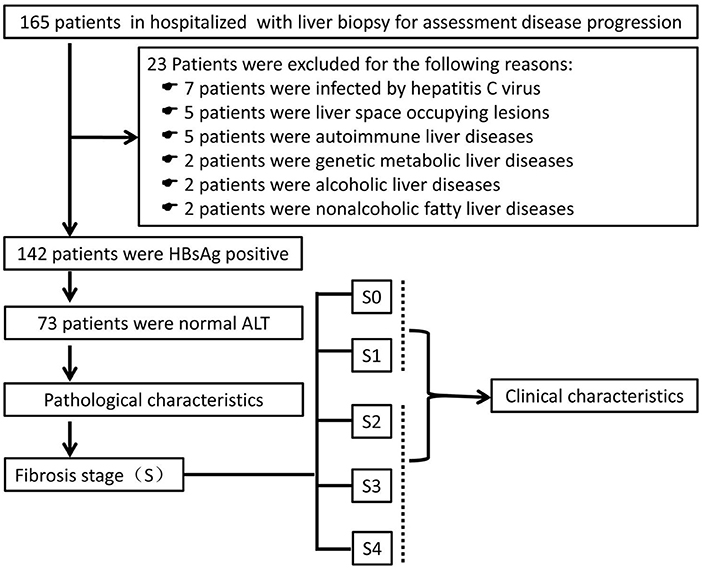
Figure 1. Screening, enrollment and grouping of the study. ALT, alanine aminotransferase; HBsAg, hepatitis B surface antigen.
The clinical and laboratory data of all patients were collected upon hospital admission. These data comprised: age; platelet count; serum levels of ALT, aspartate aminotransferase (AST), albumin, globulin, and albumin/globulin (A/G) ratio; portal vein width (PVW); spleen thickness.
Serum levels of HBeAg or HBsAg were quantified using a standardized electro-chemiluminescent immunoassay (Architect™ HBeAg; Abbott, Chicago, IL, USA). HBV DNA was measured using COBAS TaqMan v2.0 (limit of detection = 20 IU/mL; Roche, Basel, Switzerland). Laboratory tests were done upon admission to Fuling Center Hospital of Chongqing City.
Ultrasound-guided percutaneous liver puncture was carried out. Then, hematoxylin and eosin-stained liver biopsies were tested by two specialist pathologists in a blinded manner. G ≥ 2 was considered “moderate/severe necroinflammation”, and S ≥ 2 was regarded as “liver fibrosis progression state” according to the METAVIR scoring system (15–17).
SPSS 22.0 (IBM, Armonk, NY, USA) was used for statistical analyses. Continuous variables (presented as frequencies and percentages or the mean ± SD) were compared using the Student's t-test or non-parametric Mann–Whitney U-test. Categorical data were analyzed with the chi-squared test and Fisher's exact test. Receiver-operating characteristic (ROC) curves were generated and the area under the ROC curve (AUC) was calculated and compared. Binary logistic regression was employed to consider the cutoff value. Optimal cutoff values were selected to maximize specificity and sensitivity. P < 0.05 was considered significant.
Twenty three patients of the 165 patients were excluded: infection with the hepatitis C virus, 7 patients; space-occupying lesion in the liver, 5 patients; autoimmune liver disease, 5 patients; genetic metabolic liver disease, 2 patients; alcoholic liver disease, 2 patients; non-alcoholic fatty liver disease, 2 patients. Thus, 142 patients were enrolled in this study, and 73 individuals had a normal ALT level (Figure 1).
Of 142 patients, the number with an inflammation grade of 0, 1, 2, 3, and 4 was 0, 16, 100, 23, and 3, respectively (Table 1). For patients with inflammation grade 0, 1, 2, 3, and 4, the percentage with a fibrosis stage ≥2 was 0, 18.75, 93, 100, and 100%, respectively. Overall, 83.80% (119/142) of individuals had progressive liver fibrosis (S ≥2).
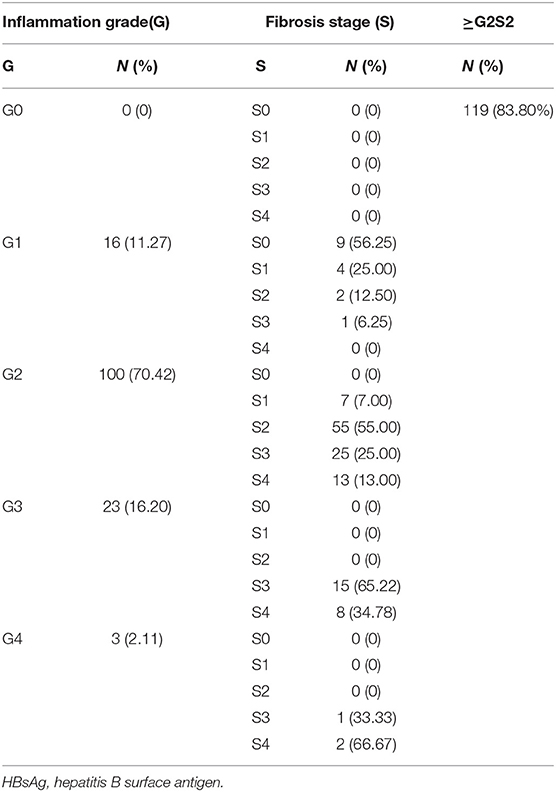
Table 1. Inflammation grade and fibrosis stage of 142 patients with HBsAg-positivity and HBeAg-negativity.
For the 73 patients with a normal ALT level, the number with an inflammation grade of 0, 1, 2, 3, and 4 was 0, 10, 56, 7, and 0, respectively. For patients with inflammation grade 0, 1, 2, 3, and 4, the percentage with a fibrosis stage ≥2 was 0, 10.00, 94.64, 100, and 0%, respectively. Overall, 83.56% (61/73) patients had progressive liver fibrosis (S ≥ 2) (Table 2).
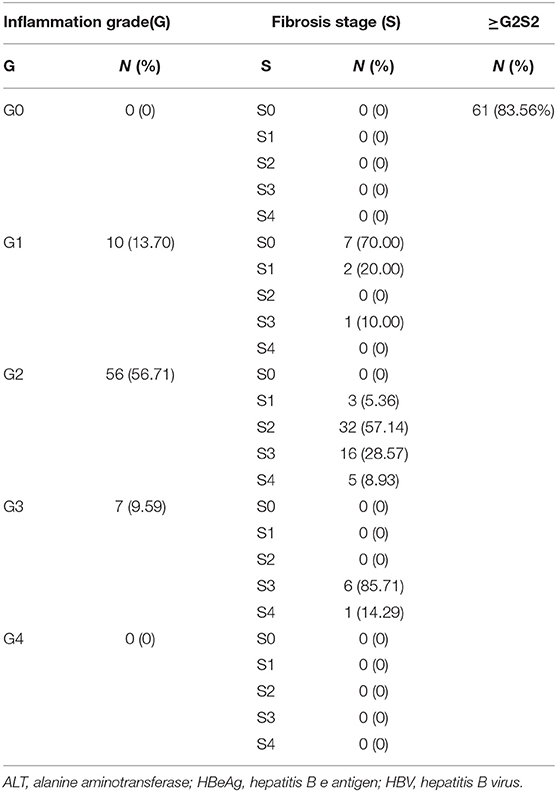
Table 2. Inflammation grade and fibrosis stageamong73 HBV-infected patients with HBeAg-negativity and a normal ALT level.
Levels of ALT and AST were not significantly different between the group with fibrosis stage <2 and those with fibrosis stage ≥2 (21.01 ± 7.40 vs. 25.37 ± 7.90 U/L, p = 0.08; 29.49 ± 13.56 vs. 30.16 ± 21.88 U/L, p = 0.92, respectively). PVW, albumin level, globulin level, A/G ratio, APRI, and FIB-4 score were not significantly different between the group with fibrosis stage <2 and the group with fibrosis stage ≥2 (p > 0.05). The platelet count, platelet-to-portal vein width ratio (PPR), and platelet-to-splenomegaly ratio (PSR) were significantly different between the group with fibrosis stage <2 and the group with fibrosis stage ≥2 [(145.92 ± 14.55) ×109/L vs. (126.38 ± 23.85) ×109/L, p = 0.008; 10.80 ± 1.30 vs. 9.01 ± 1.97, p = 0.004; 4.21 ± 0.65 vs. 3.33 ± 0.89, p = 0.02, respectively] (Table 3).
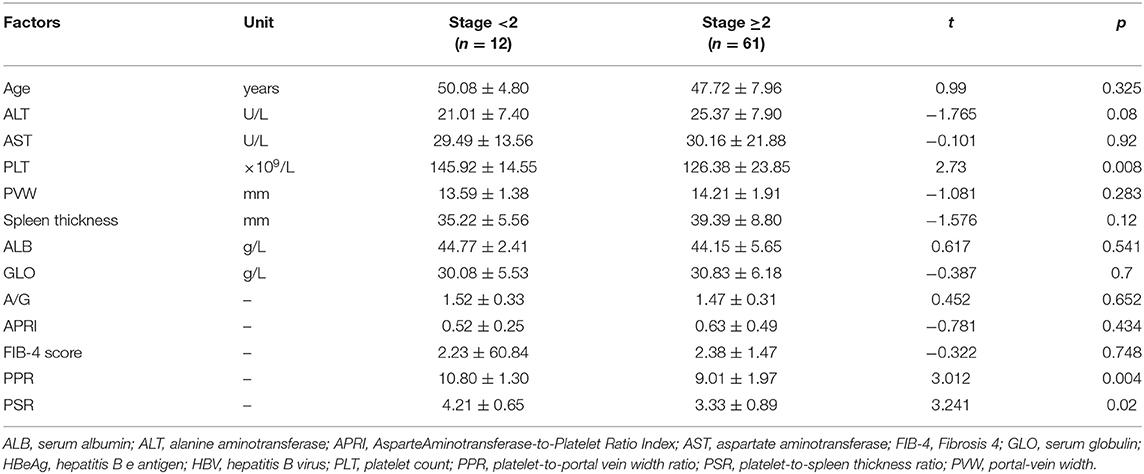
Table 3. Characteristics of liver-fibrosis progression among HBV-infected patients with HBeAg-negativity and a normal ALT level.
The PPR and PSR decreased gradually with aggravation of liver fibrosis (p < 0.05) (Table 4). Binary logistic regression analysis revealed the PPR and PSR to be independent risk factors for advanced fibrosis among people with HBV infection and HBeAg-negativity and a normal ALT level. Analysis of ROC curves was applied to evaluate the performance of the PPR and PSR for prediction of progressive liver fibrosis among people with HBV infection with HBeAg-negativity and a normal ALT level.
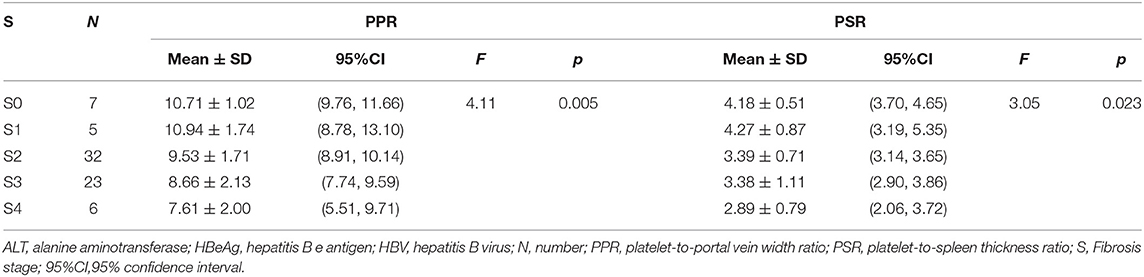
Table 4. PPR and PSR in advanced liver fibrosis among HBV-infected patients with HBeAg-negativity and a normal ALT level.
The AUC of the PPR and PSR was 0.751 and 0.786, respectively (Figure 2). The optimal cut off value of the PPR and PSR was 9.07 and 3.54, respectively. The corresponding sensitivity and specificity of the PPR were 0.917 and 0.525, and those of the PSR were 0.833 and 0.541, respectively (Table 5).
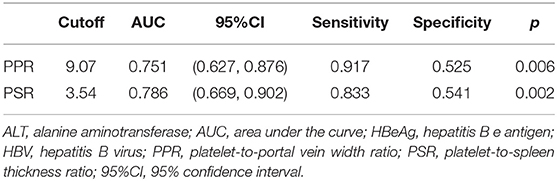
Table 5. Efficacy of the PPR and PSR for evaluating hepatic-fibrosis progression among HBV-infected patients with HBeAg-negativity and a normal ALT level.
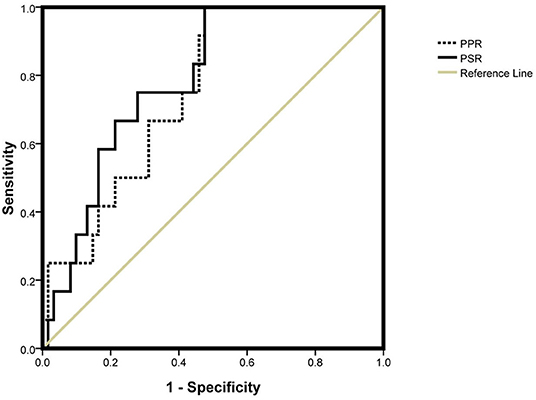
Figure 2. AUC of the PPR and PSR. PPR, platelet-to-portal vein width ratio; PSR, platelet-to-spleen thickness ratio.
Approximately 257 million people are infected by the HBV worldwide (18). Recent studies have shown that ~19% of people infected with the HBV require antiviral treatment (19). For chronic HBV infection, antiviral therapy can prevent the progression of fibrosis, cirrhosis, and cancer of the liver, and reduce the mortality associated with liver disease. Hence, antiviral therapy is crucial (20–22).
The serum level of ALT reflects the host immunity to viral challenge, and is one of the most sensitive biomarkers for liver inflammation. Therefore, an increase in the serum level of ALT is a good indication of hepatic necroinflammation, and is one of the crucial indexed to evaluate disease status for CHB patients (23), and is one of the parameter for determining initiation of antiviral therapy (8, 24, 25). However, in real-world clinical practice, the ALT level is not completely consistent with the degree of liver inflammation and progressive fibrosis. Inflammation and fibrosis as judged by histology has been documented in people with a HBV infection and a normal level of ALT (6, 26, 27).
We found that 83.80% (119/142) of individuals with an HBV infection had progressive fibrosis. Simultaneously, 83.56% (61/73) of patients with a normal ALT level had progressive fibrosis (fibrosis stage ≥2). ALT is a marker of liver dysfunction. In China, about 62.4% of HBV-infected patients have a normal ALT level, and these patients are often mistaken for having immune tolerance. Changes in the ALT level are not always consistent with the degree of inflammation of liver tissue. Hence, errors may arise in judging if patients need antiviral therapy based on the ALT level. Therefore, a normal ALT level cannot be used to ascertain if patients need treatment.
Liver biopsy is the gold standard to assess fibrosis/cirrhosis in HBV patients objectively (7). Antiviral therapy should be applied to HBV patients with a Knodell Histology Activity Index ≥4 or moderate/severe necroinflammation and/or fibrosis with a normal ALT level (20, 21). We observed that, in HBV-infected patients with a normal ALT level, the progression of inflammation and fibrosis could not be determined by hepatic biochemical indices (ALT, AST, albumin, globulin, A/G ratio), imaging (PVW, spleen thickness), or other characteristics (e.g., age) alone. These data suggest that we should reconsider the guiding role of the ALT level for starting antiviral therapy in HBV patients. We also under took evaluation by non-invasive methods, such as the APRI and FIB-4 Score. These are recommended by the World Health Organization as non-invasive evaluations of inflammation and fibrosis of the liver. However, these two indices are not applicable to HBV-infected patients with a normal ALT level.
We noted a significant difference in the platelet count between patients with fibrosis stage <2 and patients with fibrosis stage ≥2. Hence, fibrosis progression in HBV-infected patients with a normal ALT level was inversely proportional to the number of platelets. Thus, although the ALT was normal in HBV-infected patients, inflammation and progressive fibrosis may be present in the liver tissue. Patients with progressive liver fibrosis require antiviral therapy regardless of ALT status (8, 20, 28). Early antiviral therapy in these patients could also achieve a greater immune response (29). Our results suggest that HBV-infected patients with a normal ALT level may also have significant hepatic pathological changes and a high risk of liver cancer or death.
Thrombocytopenia has been observed in 76–85% of patients with progressive liver disease. Thrombocytopenia is secondary to hypersplenism, possible immune-mediated mechanisms, direct viral suppression of platelet production, and reduced production of thrombopoietin (30, 31). Thrombocytopenia in individuals with chronic liver disease is associated with portal venous pressure and hypersplenism. The platelet number is influenced by many factors, whereas the portal-vein width and spleen thickness are mainly associated with portal hypertension during liver fibrosis/cirrhosis. We combined the platelet count with the portal-vein width and spleen thickness into the PPR and PSR. The PPR and PSR could be used to predict progressive fibrosis among HBV-infected patients with HBeAg-negativity and a normal ALT level. We calculated the sensitivity of the PPR and PSR to be 0.917 and 0.833, respectively, where as the specificity of the PPR and PSR was only 0.525 and 0.541, respectively. Such low specificity may be related to the many factors influencing thrombocytopenia, as well as our small study cohort.
Liver biopsy is invasive, so timely and accurate assessment of the need for early liver biopsy is extremely important to detect progressive liver fibrosis. We demonstrated that patients with progressive fibrosis had a lower PPR and PSR. Further subgroup analysis showed that the PPR and PSR tended to decline gradually with fibrosis progression. Therefore, the PPR and PSR could reflect the stages of progressive fibrosis among HBV-infected patients with HBeAg-negativity and a normal ALT level. Using the PPR and PSR might provide better predictive accuracy than using the ALT level alone, especially for determining who needs a liver biopsy. In other words, the clinical guiding significance of PPR and PSR lies in that for HBV infected patients with a normal ALT and HBeAg negative, the PPR and PSR of patients should be closely observed. If the PPR of these patients is lower than 9.07 and PSR is lower than 3.54, liver biopsy should be mobilized as far as possible to obtain evidence of liver fibrosis progression in HBV infected patients. In contrast, if the appeal criteria are not met, follow-up may necessary and liver biopsy may not be required for the time being. It has certain guiding significance for saving medical resources and avoiding expanding indications of liver biopsy.
Our study had three main limitations. First, the study cohort was small, which hampered the robustness of statistical analyses. The small sample size was because liver biopsy is invasive and many patients refuse it. Second, our cohort may be not representative of all HBV-infected patients. Third, we excluded HBeAg-positive patients. Greater efforts should be made to explore more non-invasive methods to enable accurate judgment of disease progression for patients with a normal ALT level.
Our study revealed the value of using the PPR and PSR for predicting progressive fibrosis among patients with HBV infection with HBeAg-negativity and a normal ALT level. The PPR and PSR could help to screen patients who need a liver biopsy for the time being.
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
The study protocol was approved by the Clinical Research Ethics Committee of Fuling Center Hospital of Chongqing City (Chongqing, China). Written informed consent to participate in this study was obtained from patients (or their legal surrogates) before data collection.
LL and YS collected the data. MF carried out data analyses. JX contributed to the conception and design of the study. WY drafted and revised the manuscript. All authors approved the final version of the manuscript.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
We would like to thank the Department of Hepatology & Translation Medicine, Fuling Center Hospital of Chongqing City, for their support in helping to complete this study.
1. Yim HJ, Lok AS. Natural history of chronic hepatitis B virus infection: what we knew in 1981 and what we know in 2005. Hepatology. (2006) 43:20956. doi: 10.1002/hep.20956
2. Dudley FJ, Fox RA, Sherlock S. Cellular immunity and hepatitis-associated, Australia antigen liver disease. Lancet. (1972) 1:723–6. doi: 10.1016/S0140-6736(72)90234-6
3. Razavi-Shearer D, Gamkrelidze I, Nguyen MH, Chen DS, Van Damme P, Abbas Z, et al. Global prevalence, treatment, and prevention of hepatitis B virus infection in 2016: a modelling study. Lancet Gastroenterol Hepatol. (2018) 3:383–403. doi: 10.1016/S2468-1253(18)30056-6
4. Wong GLH, Chan HLY, Yu Z, Chan HY, Tse CH, Wong VWS. Liver fibrosis progression is uncommon in patients with inactive chronic hepatitis B: A prospective cohort study with paired transient elastography examination. J Gastroenterol Hepatol. (2013) 28:1842–8. doi: 10.1111/jgh.12327
5. Gish RG, Given BD, Lai CL, Locarnini SA, Lau JY, Lewis DL, et al. Chronic hepatitis B: Virology, natural history, current management and a glimpse at future opportunities. Antiviral Res. (2015) 121:47–58. doi: 10.1016/j.antiviral.2015.06.008
6. Feld JJ, Ayers M, El-Ashry D, Mazzulli T, Tellier R, Heathcote EJ. Hepatitis B virus DNA prediction rules for hepatitis B e antigen-negative chronic hepatitis B. Hepatology. (2007) 46:1057–70. doi: 10.1002/hep.21811
7. Wai CT, Cheng CL, Wee A, Dan YY, Chan E, Chua W, et al. Non-invasive models for predicting histology in patients with chronic hepatitis B. Liver Int. (2006) 26:666–72. doi: 10.1111/j.1478-3231.2006.01287.x
8. Sarin SK, Kumar M, Lau GK, Abbas Z, Chan HL, Chen CJ, et al. Asian-Pacific clinical practice guidelines on the management of hepatitis B: a 2015 update. Hepatol Int. (2016) 10:1–98. doi: 10.1007/s12072-015-9675-4
9. Liu N, Yang N, Ma W, Yang S, Hu C, Li J, et al. Efficacy of antiviral treatment in liver biopsy-proven immune-tolerant chronic hepatitis b patients: a retrospective cohort study. Front Med. (2021) 8:363. doi: 10.3389/fmed.2021.655530
10. Mason WS, Gill US, Litwin S, Zhou Y, Peri S, Pop O, et al. HBV DNA integration and clonal hepatocyte expansion in chronic hepatitis B patients considered immune tolerant. Gastroenterology. (2016) 151:986–98. doi: 10.1053/j.gastro.2016.07.012
11. Protzer U. Knolle P. “To Be or Not to Be”: immune tolerance in chronic hepatitis B. Gastroenterology. (2016) 151:805–6. doi: 10.1053/j.gastro.2016.09.038
12. Kumar M, Sarin SK, Hissar S, Pande C, Sakhuja P, Sharma BC, et al. Virologic and histologic features of chronic hepatitis B virus-infected asymptomatic patients with persistently normal ALT. Gastroenterology. (2008) 134:1376–84. doi: 10.1053/j.gastro.2008.02.075
13. Nguyen MH, Garcia RT, Trinh HN, Lam KD, Weiss G, Nguyen HA, et al. Histological disease in Asian-Americans with chronic hepatitis B, high hepatitis B virus DNA, and normal alanine aminotransferase levels. Am J Gastroenterol. (2009) 104:2206–13. doi: 10.1038/ajg.2009.248
14. Liao B, Wang Z, Lin S, Ying X, Yi J, Min X, et al. Significant fibrosis is not rare in chinese chronic hepatitis B patients with persistent normal ALT. PLoS ONE. (2013) 8:e78672. doi: 10.1371/journal.pone.0078672
15. Knodell RG, Ishak KG, Black WC, Chen TS, Craig R, Kaplowitz N, et al. Formulation and application of a numerical scoring system for assessing histological activity in asymptomatic chronic active hepatitis. Hepatology. (1981) 1:431–5. doi: 10.1002/hep.1840010511
16. Bedossa P, Poynard T. An algorithm for the grading of activity in chronic hepatitis C. The METAVIR Cooperative Study Group. Hepatology. (1996) 24:289–93. doi: 10.1002/hep.510240201
17. Bravo AA, Sheth SG, Chopra S. Liver biopsy. N Engl J Med. (2001) 344:495–500. doi: 10.1056/NEJM200102153440706
18. Smith S, Harmanci H, Hutin Y, Hess S, Bulterys M, Peck R, et al. Global progress on the elimination of viral hepatitis as a major public health threat: an analysis of WHO Member State responses 2017. JHEP Rep. (2019) 1:81–9. doi: 10.1016/j.jhepr.2019.04.002
19. Tan M, Bhadoria AS, Cui F, Tan A, Van Holten J, Easterbrook P, et al. Estimating the proportion of people with chronic hepatitis B virus infection eligible for hepatitis B antiviral treatment worldwide: a systematic review and meta-analysis. Lancet Gastroenterol Hepatol. (2021) 6:106–19. doi: 10.1016/S2468-1253(20)30307-1
20. Terrault NA, Lok ASF, McMahon BJ, Chang KM, Hwang JP, Jonas MM, et al. Update on prevention, diagnosis, and treatment of chronic hepatitis B: AASLD 2018 hepatitis B guidance. Hepatology. (2018) 67:1560–99. doi: 10.1002/hep.29800
21. European Association For The Study Of The Liver. EASL 2017 Clinical Practice Guidelines on the management of hepatitis B virus infection. J Hepatol. (2017) 67:370–98. doi: 10.1016/j.jhep.2017.03.021
22. Liu J, Liang W, Jing W, Liu M. Countdown to 2030: eliminating hepatitis B disease, China. Bull World Health Organiz. (2019) 97:230. doi: 10.2471/BLT.18.219469
23. Du Y, Du B, Fang X, Shu M, Cai W. ALT flare predicts hepatocellular carcinoma among antiviral treated patients with chronic hepatitis b: a cross-country cohort study. Front Oncol. (2021) 10:3125. doi: 10.3389/fonc.2020.615203
24. Tong MJ, Pan CQ, Hann HW, Kowdley KV, Han SH, Min AD, et al. The management of chronic hepatitis B in Asian Americans. Dig Dis Sci. (2011) 56:3143–62. doi: 10.1007/s10620-011-1841-5
25. Han SH, Durazo FA, Saab S, Tong MJ. A proposed, evidence-based approach to the treatment of chronic Hepatitis B. J Clin Gastroenterol. (2011) 45:259–66. doi: 10.1097/MCG.0b013e3181f312f5
26. Tai DI, Lin SM, Sheen IS, Chu CM, Lin DY, Liaw YF. Long-term outcome of hepatitis B e antigen-negative hepatitis B surface antigen carriers in relation to changes of alanine aminotransferase levels over time. Hepatology. (2009) 49:1859–67. doi: 10.1002/hep.22878
27. Tsang PS, Trinh H, Garcia RT, Phan JT, Ha NB, Nguyen H, et al. Significant prevalence of histologic disease in patients with chronic hepatitis B and mildly elevated serum alanine aminotransferase levels. Clin Gastroenterol Hepatol. (2008) 6:569–74. doi: 10.1016/j.cgh.2008.02.037
28. Terrault NA, Bzowej NH, Chang KM, Hwang JP, Jonas MM, Murad MH, et al. guidelines for treatment of chronic hepatitis B. Hepatology. (2016) 63:261–83. doi: 10.1002/hep.28156
29. Zhao Q, Liu K, Zhu X, Yan L, Ding Y, Xu Y, et al. Anti-viral effect in chronic hepatitis B patients with normal or mildly elevated alanine aminotransferase. Antiviral Res. (2020) 184:13. doi: 10.1016/j.antiviral.2020.104953
30. Qamar AA, Grace ND, Groszmann RJ, Garcia-Tsao G, Bosch J, Burroughs AK, et al. Incidence, prevalence, and clinical significance of abnormal hematologic indices in compensated cirrhosis. Clin Gastroenterol Hepatol. (2009) 7:689–95. doi: 10.1016/j.cgh.2009.02.021
Keywords: fibrosis stage, hepatitis B virus, normal ALT, platelet-to-portal vein width ratio, platelet-to-spleen thickness ratio
Citation: Feng M, Lei L, Xu J, Shi Y and Yang W (2022) Platelet-to-Portal Vein Width Ratio and Platelet-to-Spleen Thickness Ratio Can Be Used to Predict Progressive Liver Fibrosis Among Patients With HBV Infection With HBeAg-Negativity and a Normal ALT Level. Front. Med. 9:837898. doi: 10.3389/fmed.2022.837898
Received: 17 December 2021; Accepted: 25 May 2022;
Published: 21 June 2022.
Edited by:
Rinaldo Pellicano, Molinette Hospital, ItalyReviewed by:
Hirsh Trivedi, Beth Israel Deaconess Medical Center and Harvard Medical School, United StatesCopyright © 2022 Feng, Lei, Xu, Shi and Yang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jian Xu, MTEwNjEzNDUxNEBxcS5jb20=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.