- 1Division of Infectious Diseases, Wayne State University, Detroit, MI, United States
- 2Department of Medicine, Loyola University Chicago Stritch School of Medicine, Maywood, IL, United States
- 3GST Micro LLC, Henrico, VA, United States
Clostridioides difficile infection poses significant clinical challenges due to its recurrent nature. Current antibiotic management does not address the underlying issue, that of a disturbed gastrointestinal microbiome, called dysbiosis. This provides a supportive environment for the germination of C. difficile spores which lead to infection and toxin production as well as an array of other health conditions. The use of microbiome restoration therapies such as live biotherapeutics can reverse dysbiosis and lead to good clinical outcomes. Several such therapies are under clinical investigation.
1. Introduction
Clostridioides difficile infection (CDI) is an urgent threat, both for the patient and healthcare professionals. CDI is one of the most common healthcare-associated infections and aggressive action is required to combat this threat (1). There are an estimated 467,400 cases of healthcare- and community-associated CDI cases annually in the United States and a cumulative incidence of 8 per 100,000 person-years in the European Union (2, 3). The estimated direct medical cost of CDI in the US is $5.4 billion (2014 dollars) (4).
Patients with CDI often present with watery diarrhea and abdominal pain, but symptoms can also include fever, hypotension, or ileus in more severe cases (5); complications can include sepsis or colectomy/ileostomy (6–11). Testing for CDI is recommended for patients who have unexplained, new onset diarrhea (at least 3 unformed stools over ≥24 h) using a nucleic acid amplification test alone or as part of an algorithm that includes glutamate dehydrogenase or stool toxin test (12). The current recommended treatment regimen for an initial episode of CDI is fidaxomicin (200 mg BID q10d) or vancomycin (125 mg, QID q10d) as an acceptable alternative (13).
Unfortunately, in approximately 25% of cases, CDI recurs within 1–2 months of the initial infection (6, 7, 14, 15). Recurrence is often associated with more severe disease, increased costs, and hypervirulent strains of C. difficile (16–19). After a first recurrence, patients are substantially more likely to have a subsequent recurrence, with approximately 50–60% of these patients experiencing multiply recurrent CDI (6, 7, 20, 21).
2. Gut dysbiosis and Clostridioides difficile infection
Initial episodes of CDI are almost always precipitated by antibiotic use, so much so that it has the strongest association of any identified risk factor for CDI (6, 7, 22, 23). Other common risk factors for CDI include older age, use of gastric acid suppressants, comorbid conditions such as kidney disease and cardiovascular disease, and recent healthcare exposure (24–27).
Clostridioides difficile is found in the gut of some healthy individuals and is kept in check, residing in a dormant spore state, by a healthy gut microbiota (28). Underlying the pathophysiology of CDI is disruption of the gut microbiota, or gut dysbiosis. Dysbiosis has been defined as “any change to the composition of resident commensal communities relative to [those] found in healthy individuals” (29). This can include a loss of beneficial microbes, reduced diversity of gut species, or expansion of a pathogenic species (29). In patients with CDI, the gut microbiota exhibits a loss of diversity, which can worsen with recurrent CDI (30). With gut dysbiosis, C. difficile spores can germinate and produce exotoxins, disrupting the intestinal mucosa and causing CDI-associated diarrhea (31, 32).
The inciting dysbiosis for CDI can arise for several reasons. Antibiotics that are considered significant disruptors of the gut microbiota also have the strongest association with developing CDI (33–37). Older age brings changes in the gut microbiota, which could be influenced by a change in diet, lifestyle, or immune senescence (30, 38, 39). Patients taking chronic gastric acid suppressants, who are often older, show significant increases in gut Enterococcus, Streptococcus, and Staphylococcus species (40).
Clostridioides difficile spores require a germinant to transform from the spore state to the growing, vegetative cell, in the form of specific bile acids. Primary bile acids are synthesized by hepatocytes and transformed into secondary bile acids by certain members of the healthy gut microbiota (28, 41). Bile acids derived from cholic acid promote the germination of C. difficile spores, while bile acids derived from chenodeoxycholic acid (CDCA) inhibit germination (41). In addition, vegetative cell growth of C. difficile is inhibited by CDCA. In animal studies and in humans, hosts with higher levels of secondary bile acids were more resistant to developing CDI, whereas hosts with higher levels of primary bile acids were more susceptible (41).
Perhaps counterintuitively, CDI is treated with antibiotics. While antibiotics may eliminate the initial infection, they alter the composition of the gut microbiota, including widespread reduction in diversity by commonly-used vancomycin (29, 30). With the continued burden of recurrent CDI, that does not appear to be lessening with increased infection control measures or changes in antimicrobial prescribing, a non-antibiotic approach may offer an alternative means of addressing the disease (2).
3. Restoring the gut microbiota in Clostridioides difficile infection
Given the underlying state of gut dysbiosis that fosters CDI, an ideal goal for patients with CDI is eubiosis, or restoring the gut microbiota to a healthy state (28, 29). Microbiota-based therapies have been investigated by Western medicine as a treatment for gut dysbiosis since the 1950s (42). Since then, their use has increased steadily, in parallel with our understanding of gut microbiota disruption as an underlying cause of CDI as well as many other gastrointestinal disorders.
Fecal microbiota transplantation (FMT) is the delivery of intestinal microbiota from a healthy donor to a recipient to mitigate disease by modifying the structure and/or function of the gut microbiota (43, 44). FMT is currently recommended in the CDI treatment guidelines as an option at the second or subsequent recurrence (12, 45). In addition to CDI treatment, including FMT, changes to underlying risk factors should be considered for their effect on the gut microbiota, such as discontinuing gastric acid suppressants or altering systemic antimicrobial therapy for a non-CDI infection.
The goal of FMT is to restore the gut microbiota to a healthy state and replace dysbiotic microbes with taxa/species that are associated with healthy host microbiota (46, 47). The expectation is that reintroduced healthy species will engraft and out-compete C. difficile, thus eliminating dysbiosis and providing colonization resistance (48). FMT can return metabolite levels and profiles, including bile acids and short-chain fatty acids, to a healthy state, presumably as a result of enzymatic activity provided by normal host microbiota (48).
Reduced presence of Bacteroides spp. appears to be associated with negative consequences for GI disorders, including CDI (49). Bacteria in the phyla Bacteroidetes are abundant in healthy gut microbiota and likely play a key role in bacterial metabolism and the gut environment (28). The presence of Bacteroides spp. and their surface proteins and metabolites may activate the host immune system to limit entry and proliferation of potential pathogens or exert an antibacterial effect (50, 51).
The initial literature regarding FMT for CDI was primarily case reports and retrospective cohort studies as the therapy was being investigated (52–54). While these studies often showed positive patient outcomes, namely prevention of CDI recurrence for several months after treatment, by nature of their study design the resulting data were prone to selection bias. More recently, prospective and randomized controlled trials of FMT have been completed, generally demonstrating FMT as a safe and effective therapy for CDI with treatment success rates of ∼75% (55–57). A recent prospective, real-world observational study of medically complex patients receiving FMT for CDI reported 78% (4,195/5,344) of patients exhibited clinical cure, with 3.6% of patients experiencing a serious adverse event (58). FMT has also been shown to decrease mortality in patients with refractory severe or fulminant CDI (59).
Performing FMT can be operationally challenging, including costs and logistical concerns around screening donors and processing stool (58, 60). Additionally, there is no standard protocol for FMT composition, route of delivery, number of infusions, or dosage, variables that could all affect treatment outcomes (61).
4. Approaches to restoring the gut microbiota
Live biotherapeutic products (LBPs) have been developed as an extension of the initial FMT studies, in part as a way to standardize products and outcomes being measured. LBPs contain live microbes that are able to prevent, treat or cure a disease (62). Several LBPs have been or are currently being studied for CDI. The goal of treatment with LBPs for CDI is similar to FMT, namely to restore the gut microbiota to a healthier state (63).
LBPs that are currently in late-stage development differ in their approach toward product composition and delivery. SER-109 (Seres Pharmaceuticals, Lexington MA) is an oral capsule (4 capsules once daily q3d) containing spores of ∼50 specific species of only Firmicutes that are isolated from healthy donors (64). SER-109 was designed on the premise that Firmicutes can compete metabolically with C. difficile for essential nutrients and bile acids (63). While a phase 2 study of SER-109 did not show a significant difference versus placebo in patients with multiply recurrent CDI, in those patients who did show SER-109 engraftment by microbiome analysis, there was also a significant increase in secondary bile acids (65). From a phase 3 study of patients who had 3 or more episodes of CDI, the treatment success after SER-109 was 88% (recurrence rate of 12%) (66).
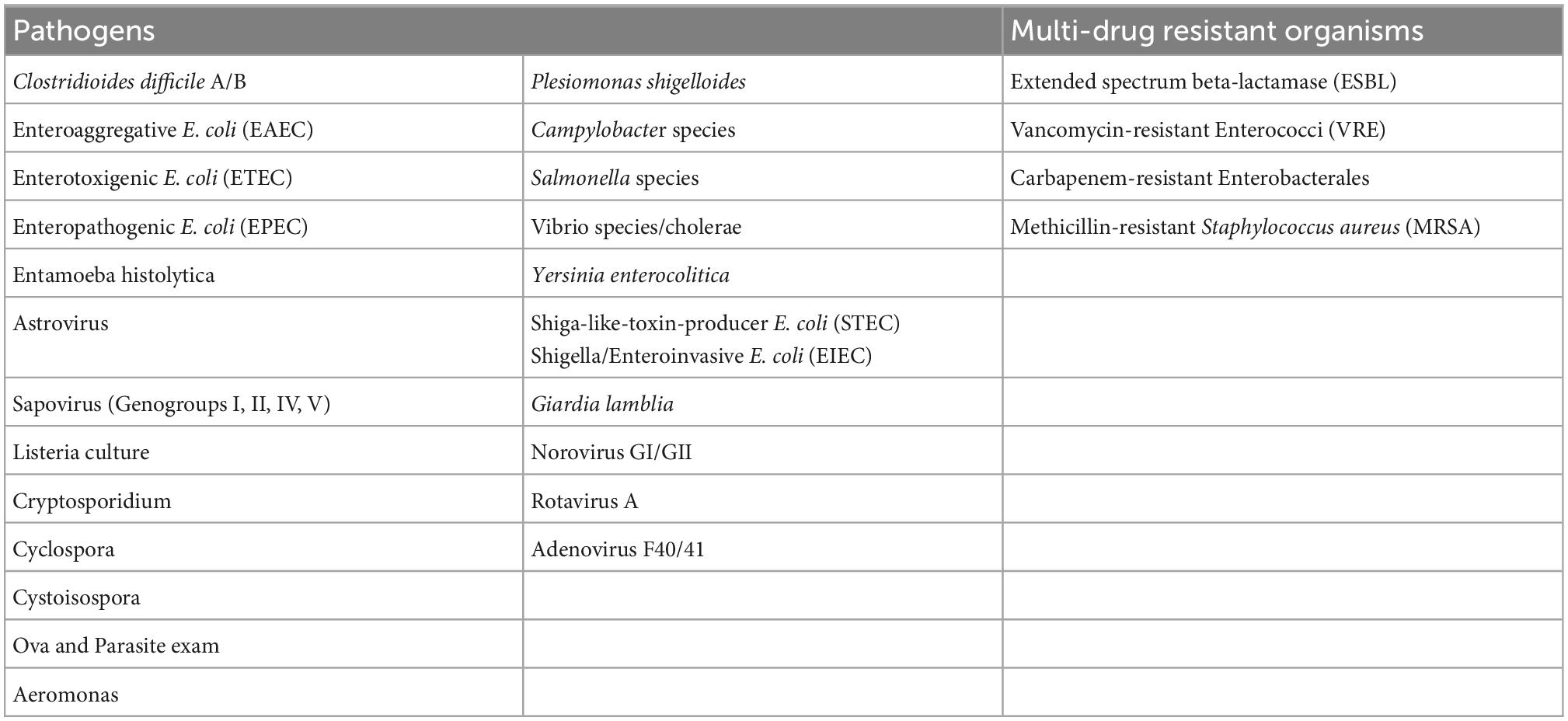
RBX2660 (Ferring Pharmaceuticals, Parsippany NJ) is a biologically-sourced, broad consortium microbiota-based live biotherapeutic product (LBP) that is processed from the stool of healthy donors, standardized and administered rectally (67). The product was approved on November 30, 2022 by the FDA as Rebyota as a live biotherapeutic for the treatment of recurrent C. difficile infection (REBYOTA | FDA) RBX2660 is screened for 29 different species of pathogens as shown in Table 1. Results from a phase 3 trial of RBX2660, analyzed with a Bayesian hierarchical model formally incorporating data from a phase 2b trial, showed a treatment success rate of 70.6% (68). Long-term data (up to 24 months) after treatment with RBX2660 in a phase 2 trial showed durable treatment success, with more than 90% of treatment responders remaining CDI-free at 6, 12, and 24 months (69, 70). Microbiota analyses from this phase 2 trial also showed a highly dysbiotic composition before treatment, which converged toward the RBX2660 composition within 7 days after treatment (69, 71). Taxa that were restored to predominance after RBX2660 included Bacteroidia and Clostridia while gammaproteobacteria and bacilli, the deleterious organisms, were reduced. Administration of RBX2660 delivery is via enema, without the need for bowel preparation or colonoscopy and can be used in patients who are not able to take an oral product.
CP101 (Finch Therapeutics, Somerville, MA, USA) is an oral capsule (10 capsules taken once) delivering a full-spectrum microbiota product that showed 75% efficacy in preventing CDI recurrence in a phase 2 trial (72). A phase 3 trial of CP101 is currently recruiting patients (NCT05153499). Several other microbiota-based products in earlier stages of development have been or are currently being investigated for CDI (63).
The negative physical effects of gut dysbiosis are clear, but emerging evidence also points to psychological effects as well. Psychological consequences of CDI are reported by ∼70% of people who have active or previous infection (73). From an analysis of Medicare Fee-for-service beneficiaries, within a 12-month period after an initial CDI episode, approximately 15–20% of the cohort had newly diagnosed psychiatric conditions (anxiety, depression, delirium) (7). After receiving a microbiota-based LBP for CDI treatment in a phase 2 trial setting, participants exhibited statistically significant and clinically meaningful improvements in the mental component score of the SF-36 assessment of quality of life (QoL) (74). From a phase 3 randomized, controlled trial, using a the CDiff32, a CDI-specific measure of QoL, patients receiving an LBP reported significant improvements in mental health-related QoL as early as week 1, which continued throughout the 8-week blinded study period (75). While definitive mechanisms linking changes in the gut microbiota to mental state have not been determined, it is clear that there is a link (76).
5. Discussion
A healthy gut microbiota is associated with many aspects of health and resistance to CDI as well as other diseases. Restoring healthy gut microbial communities can help break the vicious cycle of recurrence in CDI patients. The outcomes of treatment with live biotherapeutic products have been measured in terms of short- and long-term clinical observations and microbiome changes, which modify the metabolic processes in the gut and elicit positive changes in mental aspects associated with CDI. The availability of regulated standardized products will be welcome additions to the armamentarium against C. difficile infections.
Author contributions
TC and GT conceived the idea for the work, wrote the original draft, revised and edited the manuscript. GH wrote the original draft and revised and edited the manuscript. All authors agreed to be accountable for the content of the work and approved the final version for publication.
Funding
The authors report no funding for this report. Publication fees were funded by Ferring Pharmaceuticals Inc. (Parsippany, NJ, USA).
Acknowledgments
Medical writing and editorial support were provided by Agnella Izzo Matic, Ph.D., CMPP (AIM Biomedical, LLC) and funded by Ferring Pharmaceuticals Inc.
Conflict of interest
TC is a speaker for Abbvie Inc., Cepheid, Ferring Pharmaceuticals Inc., and Pfizer Inc.; and a consultant for Cepheid, Ferring Pharmaceuticals Inc., Pfizer Inc., and Shionogi Inc. GT is a consultant to Ferring Pharmaceuticals Inc., Spero Therapeutics, and a speaker for Hikma Pharmaceuticals and was employed by GST Micro LLC. GH is a consultant to Ferring Pharmaceuticals Inc., and BioK+.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Centers for Disease Control and Prevention. Antibiotic Resistance Threats in the United States, 2019. Atlanta, GA: US Department of Health and Human Services (2019).
2. Guh A, Mu Y, Winston L, Johnston H, Olson D, Farley M, et al. Trends in U.S. burden of Clostridioides difficile infection and outcomes. N Engl J Med. (2020) 382:1320–30. doi: 10.1056/NEJMoa1910215
3. Balsells E, Shi T, Leese C, Lyell I, Burrows J, Wiuff C, et al. Global burden of Clostridium difficile infections: a systematic review and meta-analysis. J Glob Health. (2019) 9:010407. doi: 10.7189/jogh.09.010407
4. Desai K, Gupta S, Dubberke E, Prabhu V, Browne C, Mast T. Epidemiological and economic burden of Clostridium difficile in the United States: estimates from a modeling approach. BMC Infect Dis. (2016) 16:303. doi: 10.1186/s12879-016-1610-3
5. Gerding D, File TMJ, McDonald L. Diagnosis and treatment of Clostridium difficile infection (CDI). Infect Dis Clin Pr (Baltim Md). (2016) 24:3–10. doi: 10.1097/IPC.0000000000000350
6. Feuerstadt P, Boules M, Stong L, Dahdal D, Sacks N, Lang K, et al. Clinical complications in patients with primary and recurrent Clostridioides difficile infection: a real-world data analysis. SAGE Open Med. (2021) 9:1–8. doi: 10.1177/2050312120986733
7. Feuerstadt P, Nelson W, Teigland C, Dahdal D. Clinical burden of recurrent Clostridioides difficile infection in the medicare population: a real-world claims analysis. Antimicrob Steward Heal Epidemiol. (2022) 2:e60. doi: 10.1017/ash.2022.2
8. Falcone M, Russo A, Iraci F, Carfagna P, Goldoni P, Vullo V, et al. Risk factors and outcomes for bloodstream infections secondary to Clostridium difficile infection. Antimicrob Agents Chemother. (2016) 60:252–7. doi: 10.1128/AAC.01927-15
9. Ianiro G, Murri R, Sciumè G, Impagnatiello M, Masucci L, Ford A, et al. Incidence of bloodstream infections, length of hospital stay and survival in patients with recurrent Clostridioides difficile infection treated with fecal microbiota transplantation or antibiotics: a prospective cohort study. Ann Intern Med. (2019) 171:695–702. doi: 10.7326/M18-3635
10. Kasper A, Nyazee H, Yokoe D, Mayer J, Mangino J, Khan Y, et al. A multicenter study of Clostridium difficile infection-related colectomy, 2000–2006. Infect Control Hosp Epidemiol. (2012) 33:470–6. doi: 10.1086/665318
11. Rodrigues R, Barber G, Ananthakrishnan AN. A comprehensive study of costs associated with recurrent Clostridium difficile infection. Infect Control Hosp Epidemiol. (2017) 38:196–202. doi: 10.1017/ice.2016.246
12. McDonald L, Gerding D, Johnson S, Bakken J, Carroll K, Coffin S, et al. Clinical practice guidelines for Clostridium difficile infection in adults and children: 2017 update by the infectious diseases society of America (IDSA) and society for healthcare epidemiology of America (SHEA). Clin Infect Dis. (2018) 66:e1–48. doi: 10.1093/cid/cix1085
13. Johnson S, Lavergne V, Skinner A, Gonzales-Luna A, Garey K, Kelly C, et al. Clinical practice guideline by the infectious diseases society of America (IDSA) and society for healthcare epidemiology of America (SHEA): 2021 focused update guidelines on management of Clostridioides difficile infection in adults. Clin Infect Dis. (2021) 73:e1029–44. doi: 10.1093/cid/ciab549
14. Leffler D, Lamont J. Clostridium difficile infection. N Engl J Med. (2015) 372:1539–48. doi: 10.1056/NEJMra1403772
15. Lessa F, Mu Y, Bamberg W, Beldavs Z, Dumyati G, Dunn J, et al. Burden of Clostridium difficile infection in the United States. N Engl J Med. (2015) 372:825–34. doi: 10.1056/NEJMoa1408913
16. Song J, Kim Y. Recurrent Clostridium difficile infection: risk factors, treatment, and prevention. Gut Liver. (2019) 13:16–24. doi: 10.5009/gnl18071
17. Shields K, Araujo-Castillo RV, Theethira T, Alonso C, Kelly C. Recurrent Clostridium difficile infection: from colonization to cure. Anaerobe. (2015) 34:59–73. doi: 10.1016/j.anaerobe.2015.04.012
18. Appaneal H, Caffrey A, Beganovic M, Avramovic S, LaPlante K. Predictors of Clostridioides difficile recurrence across a national cohort of veterans in outpatient, acute, and long-term care settings. Am J Heal Pharm. (2019) 76:581–90. doi: 10.1093/ajhp/zxz032
19. Nour Abou Chakra C, Pepin J. Risk factors for recurrence, complications and mortality in Clostridium difficile infection: a systematic review. PLoS One. (2014) 9:e98400. doi: 10.1371/journal.pone.0098400
20. Ma G, Brensinger C, Wu Q, Lewis J. Increasing incidence of multiply recurrent Clostridium difficile infection in the United States: a cohort study. Ann Intern Med. (2017) 167:152–8. doi: 10.7326/M16-2733
21. Sheitoyan-Pesant C, Abou Chakra C, Pépin J, Marcil-Héguy A, Nault V, Valiquette L. Clinical and healthcare burden of multiple recurrences of Clostridium difficile infection. Clin Infect Dis. (2016) 62:574–80. doi: 10.1093/cid/civ958
22. Davies K, Lawrence J, Berry C, Davis G, Yu H, Cai B, et al. Risk factors for primary Clostridium difficile infection; results from the observational study of risk factors for Clostridium difficile infection in hospitalized patients with infective diarrhea (ORCHID). Front Public Heal. (2020) 8:293. doi: 10.3389/fpubh.2020.00293
23. Eze P, Balsells E, Kyaw M, Nair H. Risk factors for Clostridium difficile infections – an overview of the evidence base and challenges in data synthesis. J Glob Heal. (2017) 7:010417. doi: 10.7189/jogh.07.010417
24. Collins C, Ayturk M, Flahive J, Emhoff T Jr., Frederick AJ, Santry H. Epidemiology and outcomes of community acquired Clostridium difficile infections in medicare beneficiaries. J Am Coll Surg. (2014) 218:1141–7.e1. doi: 10.1016/j.jamcollsurg.2014.01.053
25. Isidro J, Mendes A, Serrano M, Henriques A, Oleastro M. Overview of Clostridium difficile Infection: Life Cycle, Epidemiology, Antimicrobial Resistance and Treatment. Clostridium Difficile – A Comprehensive Overview. (2017). doi: 10.5772/intechopen.69053
26. Smits W, Lyras D, Lacy D, Wilcox M, Kuijper E. Clostridium difficile infection. Nat Rev Dis Prim. (2016) 2:16020. doi: 10.1038/nrdp.2016.20
27. Khanna S, Gupta A, Baddour L, Pardi D. Epidemiology, outcomes, and predictors of mortality in hospitalized adults with Clostridium difficile infection. Intern Emerg Med. (2016) 11:657–65. doi: 10.1007/s11739-015-1366-6
28. Theriot C, Young V. Interactions between the gastrointestinal microbiome and Clostridium difficile. Annu Rev Microbiol. (2015) 69:445–61. doi: 10.1146/annurev-micro-091014-104115
29. Petersen C, Round J. Defining dysbiosis and its influence on host immunity and disease. Cell Microbiol. (2014) 16:1024–33. doi: 10.1111/cmi.12308
30. Shin J, Warren C. Collateral damage during antibiotic treatment of C. difficile infection in the aged host: insights into why recurrent disease happens. Gut Microbe. (2017) 8:504–10. doi: 10.1080/19490976.2017.1323616
31. Fernández-García L, Blasco L, López M, Tomás M. Clostridium difficile Infection: Pathogenesis, Diagnosis and Treatment. Clostridium Difficile – A Comprehensive Overview. (2017). doi: 10.5772/67754
32. Abt M, McKenney P, Pamer E. Clostridium difficile colitis: pathogenesis and host defence. Nat Rev Microbiol. (2016) 14:609–20. doi: 10.1038/nrmicro.2016.108
33. Brown K, Langford B, Schwartz K, Diong C, Garber G, Daneman N. Antibiotic prescribing choices and their comparative C. difficile infection risks: a longitudinal case-cohort study. Clin Infect Dis. (2021) 72:836–44. doi: 10.1093/cid/ciaa124
34. Brown K, Khanafer N, Daneman N, Fisman D. Meta-analysis of antibiotics and the risk of community-associated Clostridium difficile infection. Antimicrob Agents Chemother. (2013) 57:2326–32. doi: 10.1128/AAC.02176-12
35. Tabak Y, Srinivasan A, Yu K, Kurtz S, Gupta V, Gelone S, et al. Hospital-level high-risk antibiotic use in relation to hospital-associated Clostridioides difficile infections: retrospective analysis of 2016–2017 data from US hospitals. Infect Control Hosp Epidemiol. (2019) 40:1229–35. doi: 10.1017/ice.2019.236
36. Pepin J, Saheb N, Coulombe M, Alary M, Corriveau M, Authier S, et al. Emergence of fluoroquinolones as the predominant risk factor for Clostridium difficile-associated diarrhea: a cohort study during an epidemic in Quebec. Clin Infect Dis. (2005) 41:1254–60. doi: 10.1086/496986
37. Bhalodi A, van Engelen T, Virk H, Wiersinga W. Impact of antimicrobial therapy on the gut microbiome. J Antimicrob Chemotheri. (2019) 74:i6–15. doi: 10.1093/jac/dky530
38. Nagpal R, Mainali R, Ahmadi S, Wang S, Singh R, Kavanagh K, et al. Gut microbiome and aging: physiological and mechanistic insights. Nutr Heal Aging. (2018) 4:267–85. doi: 10.3233/NHA-170030
39. Donskey C. Clostridium difficile in older adults. Infect Dis Clin N Am. (2017) 31:743–56. doi: 10.1016/j.idc.2017.07.003
40. Imhann F, Bonder M, Vila A, Fu J, Mujagic Z, Vork L, et al. Proton pump inhibitors affect the gut microbiome. Gut. (2016) 65:740–8. doi: 10.1136/gutjnl-2015-310376
41. Aguirre A, Sorg J. Gut associated metabolites and their roles in Clostridioides difficile pathogenesis. Gut Microbe. (2022) 14:2094672. doi: 10.1080/19490976.2022.2094672
42. Eiseman B, Silen W, Bascom G, Kauvar A. Fecal enema as an adjunct in the treatment of pseudomembranous enterocolitis. Surgery. (1958) 44:854–9.
43. U Food and Drug Administration. Enforcement Policy Regarding Investigational New Drug Requirements for Use of Fecal Microbiota for Transplantation to Treat Clostridium difficile Infection Not Responsive to Standard Therapies. (2020). Available online at: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/enforcement-policy-regarding-investigational-new-drug-requirements-use-fecal-microbiota (accessed December 21, 2022).
44. Kelly C, Kim A. The AGA’s fecal microbiota transplantation national registry: an important step toward understanding risks and benefits of microbiota therapeutics. Gastroenterology. (2017) 152:681–4. doi: 10.1053/j.gastro.2017.01.028
45. van Prehn J, Reigadas E, Vogelzang E, Bouza E, Hristea A, Guery B, et al. European society of clinical microbiology and infectious diseases: 2021 update on the treatment guidance document for Clostridioides difficile infection in adults. Clin Microbiol Infect. (2021) 27:S1–21. doi: 10.1016/j.cmi.2021.09.038
46. Kaakoush N. Fecal transplants as a microbiome-based therapeutic. Curr Opin Microbiol. (2020) 56:1–8. doi: 10.1016/j.mib.2020.05.008
47. Khoruts A, Staley C, Sadowsky M. Faecal microbiota transplantation for Clostridioides difficile: mechanisms and pharmacology. Nat Rev Gastroenterol Hepatol. (2021) 18:67–80. doi: 10.1038/s41575-020-0350-4
48. Segal J, Mullish B, Quraishi M, Iqbal T, Marchesi J, Sokol H. Mechanisms underpinning the efficacy of faecal microbiota transplantation in treating gastrointestinal disease. Ther Adv Gastroenterol. (2020) 13:1–14. doi: 10.1177/1756284820946904
49. Shin A, Preidis G, Shulman R, Kashyap P. The gut microbiome in adult and pediatric functional gastrointestinal disorders. Clin Gastroenterol Hepatol. (2019) 17:256–74. doi: 10.1016/j.cgh.2018.08.054
50. Wexler H. Bacteroides: the good, the bad, and the nitty-gritty. Clin Microbiol Rev. (2007) 20:593–621. doi: 10.1128/CMR.00008-07
51. Yekani M, Baghi H, Naghili B, Vahed S, Sóki J, Memar M. To resist and persist: important factors in the pathogenesis of Bacteroides fragilis. Microb Pathog. (2020) 149:104506. doi: 10.1016/j.micpath.2020.104506
52. Perler B, Chen B, Phelps E, Allegretti J, Fischer M, Ganapini V, et al. Long-term efficacy and safety of fecal microbiota transplantation for treatment of recurrent Clostridioides difficile infection. J Clin Gastroenterol. (2020) 54:701–6. doi: 10.1097/MCG.0000000000001281
53. Bafeta A, Yavchitz A, Riveros C, Batista R, Ravaud P. Methods and reporting studies assessing fecal microbiota transplantation a systematic review. Ann Intern Med. (2017) 167:34–9. doi: 10.7326/M16-2810
54. Drekonja D, Reich J, Gezahegn S, Greer N, Shaukat A, MacDonald R, et al. Fecal microbiota transplantation for Clostridium difficile infection a systematic review. Ann Intern Med. (2015) 162:630–8. doi: 10.7326/M14-2693
55. Tariq R, Pardi D, Bartlett M, Khanna S. Low cure rates in controlled trials of fecal microbiota transplantation for recurrent Clostridium difficile infection: a systematic review and meta-analysis. Clin Infect Dis. (2019) 68:1351–8. doi: 10.1093/cid/ciy721
56. Saha S, Mara K, Pardi D, Khanna S. Long-term safety of fecal microbiota transplantation for recurrent Clostridioides difficile infection. Gastroenterology. (2021) 160:1961–9. doi: 10.1053/j.gastro.2021.01.010
57. Quraishi M, Widlak M, Bhala N, Moore D, Price M, Sharma N, et al. Systematic review with meta-analysis: the efficacy of faecal microbiota transplantation for the treatment of recurrent and refractory Clostridium difficile infection. Aliment Pharmacol Ther. (2017) 46:479–93. doi: 10.1111/apt.14201
58. Osman M, Budree S, Kelly C, Panchal P, Allegretti J, Kassam Z, et al. Effectiveness and safety of fecal microbiota transplantation for Clostridioides difficile infection: results from a 5,344 patient cohort study. Gastroenterology. (2022) 163:319–22. doi: 10.1053/j.gastro.2022.03.051
59. Cheng Y, Phelps E, Nemes S, Rogers N, Sagi S, Bohm M, et al. Fecal microbiota transplant decreases mortality in patients with refractory severe or fulminant Clostridioides difficile infection. Clin Gastroenterol Hepatol. (2020) 18:2234.e–43.e. doi: 10.1016/j.cgh.2019.12.029
60. Gundacker N, Morrow C, Rodriguez M. Letter: a simple out-patient faecal microbiota transplant technique. Aliment Pharmacol Ther. (2016) 44:101. doi: 10.1111/apt.13648
61. Ianiro G, Maida M, Burisch J, Simonelli C, Hold G, Ventimiglia M, et al. Efficacy of different faecal microbiota transplantation protocols for Clostridium difficile infection: a systematic review and meta-analysis. United Eur Gastroenterol J. (2018) 6:1232–44. doi: 10.1177/2050640618780762
62. United States Food and Drug Administration. Early Clinical Trials with live Biotherapeutic Products: Chemistry, Manufacturing, and Control Information: Guidance for Industry. Silver Spring, MD: United States Food and Drug Administration (2016).
63. Gonzales-Luna A, Carlson T. Follow your gut: microbiome-based approaches in the developmental pipeline for the prevention and adjunctive treatment of Clostridioides difficile infection (CDI). Curr Infect Dis Rep. (2020) 22:22. doi: 10.1007/s11908-020-00729-8
64. Khanna S, Pardi D, Kelly C, Kraft C, Dhere T, Henn M, et al. A novel microbiome therapeutic increases gut microbial diversity and prevents recurrent Clostridium difficile infection. J Infect Dis. (2016) 214:173–81. doi: 10.1093/infdis/jiv766
65. McGovern B, Ford C, Henn M, Pardi D, Khanna S, Hohmann E, et al. SER-109, an investigational microbiome drug to reduce recurrence after Clostridioides difficile infection: lessons learned from a phase 2 trial. Clin Infect Dis. (2021) 72:2132–40. doi: 10.1093/cid/ciaa387
66. Feuerstadt P, Louie T, Lashner B, Wang E, Diao L, Bryant J, et al. SER-109, an oral microbiome therapy for recurrent Clostridioides difficile infection. N Engl J Med. (2022) 386:220–9. doi: 10.1056/NEJMoa2106516
67. Orenstein R, Dubberke E, Hardi R, Ray A, Mullane K, Pardi D, et al. Safety and durability of RBX2660 (Microbiota Suspension) for recurrent Clostridium difficile infection: results of the PUNCH CD study. Clin Infect Dis. (2016) 62:596–602. doi: 10.1093/cid/civ938
68. Khanna S, Assi M, Lee C, Yoho D, Louie T, Knapple W, et al. Efficacy and safety of RBX2660 in PUNCH CD3, a phase III, randomized, double-blind, placebo-controlled trial with a bayesian primary analysis for the prevention of recurrent Clostridioides difficile infection. Drugs. (2022) 82:1527–38. doi: 10.1007/s40265-022-01797-x
69. Orenstein R, Dubberke E, Khanna S, Lee C, Yoho D, Johnson S, et al. Durable reduction of Clostridioides difficile infection recurrence and microbiome restoration after treatment with RBX2660: results from an open-label phase 2 clinical trial. BMC Infect Dis. (2022) 22:245. doi: 10.1186/s12879-022-07256-y
70. Orenstein R. The role of microbiome-based therapeutics in Clostridioides difficile infection: durable, long-term results of RBX2660. Infect Dis Ther. (2022). doi: 10.1007/s40121-022-00714-9 [Online ahead of print].
71. Kwak S, Choi J, Hink T, Reske K, Blount K, Jones C, et al. Impact of investigational microbiota therapeutic RBX2660 on the gut microbiome and resistome revealed by a placebo-controlled clinical trial. Microbiome. (2020) 8:125. doi: 10.1186/s40168-020-00907-9
72. Khanna S, Kelly C, Louie T, Fisher M, Hota S, Misra B, et al. CP101, an investigational orally administered microbiome therapeutic, increases intestinal microbiome diversity and prevents recurrent C. difficile infection: results from a randomized, placebo-controlled trial. Am J Gastroenterol. (2021) 116:S57. doi: 10.14309/01.ajg.0000772996.83378.7c
73. Lurienne L, Bandinelli P, Galvain T, Coursel C, Oneto C, Feuerstadt P. Perception of quality of life in people experiencing or having experienced a Clostridioides difficile infection: a US population survey. J Patient Rep Outcomes. (2020) 4:14. doi: 10.1186/s41687-020-0179-1
74. Guthmueller B, Orenstein R, Dubberke E, Khanna S, Gerding D, Mische S, et al. Quality of life increased after treatment for participants in a phase 2b randomized, double-blinded, placebo-controlled clinical trial of RBX2660 for preventing recurrent Clostridium difficile infections. Am J Gastroenterol. (2016) 111:S1524. doi: 10.14309/00000434-201810001-02738
75. Feuerstadt P, Dubberke E, Guo A, Harvey A, Yang M, García-Horton V, et al. Significant improvement in health-related quality of life (HRQL) with RBX2660: results from a phase 3, randomized, placebo-controlled trial in recurrent Clostridioides difficile infection (PUNCH CD3). Open Forum Infect Dis. (2022) 9:ofac492.577. doi: 10.1093/ofid/ofac492.577
Keywords: microbiota, microbiome, fecal microbiota transplant, Clostridioides difficile infection, Clostridium difficile, recurrent CDI
Citation: Chopra T, Hecht G and Tillotson G (2023) Gut microbiota and microbiota-based therapies for Clostridioides difficile infection. Front. Med. 9:1093329. doi: 10.3389/fmed.2022.1093329
Received: 08 November 2022; Accepted: 15 December 2022;
Published: 09 January 2023.
Edited by:
Angel Lanas, University of Zaragoza, SpainReviewed by:
Thomas Louie, University of Calgary, CanadaCopyright © 2023 Chopra, Hecht and Tillotson. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Teena Chopra,  dGNob3ByYUBtZWQud2F5bmUuZWR1
dGNob3ByYUBtZWQud2F5bmUuZWR1
 Teena Chopra1*
Teena Chopra1* Gail Hecht
Gail Hecht Glenn Tillotson
Glenn Tillotson