- 1Department of Anesthesiology, The First Affiliated Hospital of Shandong First Medical University, Jinan, China
- 2Huaiyin District Center for Disease Control and Prevent, Jinan, China
Study objective: To quantitatively assess and compare the efficacy and adverse effects of six different peripheral nerve block techniques after arthroscopic shoulder surgery (ASS).
Design: Bayesian network meta-analysis.
Methods: The PubMed, Embase, Web of Science, the Cochrane Central Register of Controlled Trials, China National Knowledge Infrastructure database, Chinese Scientific Journal database, Wan Fang databases were searched to retrieve randomized clinical trials comparing interscalene brachial plexus block, continuous interscalene brachial plexus block, supraclavicular brachial plexus block, suprascapular nerve block, combined suprascapular and axillary nerve block and local infiltration analgesia on postoperative pain, opioid consumption, and adverse effects (defined as Horner’s syndrome, dyspnea, hoarseness, vomiting, and nausea) after ASS under general anesthesia (GA). Two reviewers independently screened the literature, extracted data, and evaluated the risk of bias in the included studies.
Results: A total of 1,348 articles were retrieved initially and 36 randomized clinical trials involving 3,124 patients were included in the final analysis. The network meta-analysis showed that interscalene brachial plexus block was superior in reducing pain and opioid consumption compared to the five other interventions. However, adverse effects were reduced using suprascapular nerve block and combined suprascapular and axillary nerve block compared to interscalene brachial plexus block.
Conclusion: Interscalene brachial plexus block was superior in reducing pain and opioid consumption compared to other peripheral nerve blocks but had a higher frequency of adverse events.
Introduction
Arthroscopic shoulder surgery (ASS) is a commonly used procedure for shoulder surgery with minimal invasiveness, a wide field of vision, and rapid functional recovery (1, 2). Despite the popularity of the surgery, the severe postoperative pain becomes a complication after ASS (up to 45%) that prolongs the patient’s recovery period and seriously affect the quality of life (3). Thus, finding a safe and effective postoperative pain regimen is crucial.
Currently, general anesthesia (GA) is combined with a regional nerve block in ASS, which reduces postoperative requirements of analgesia (4). Interscalene brachial plexus block (ISB) is one of the most reliable and commonly performed regional techniques, which has been universally considered a standard technique in postoperative pain management for ASS (5, 6). However, it often associated with a risk of complications, including epidural or subarachnoid injection, Horner’s syndrome, dyspnea, hoarseness, intravascular injection, muscle or vascular injury, pneumothorax (7). Some peripheral nerve blocks involving ISB, continuous interscalene nerve block (CISB), supraclavicular nerve block (SCB), suprascapular nerve block (SSNB), suprascapular nerve block combined with axillary nerve block (SSAX) and local infiltration anesthesia (LIA) are also recommended to provide postoperative analgesia for ASS. The ranking of them in terms of efficacy and safety is still unknown, and an excellent method to investigate this is the network meta-analysis provided that certain assumptions are fulfilled.
Methods
This systematic review is reported according to the PRISMA declaration for Network Meta-analysis and the Cochrane Handbook for the Systematic Review of Interventions (8, 9). The study evaluated existing available data retrospectively, hence neither ethical approval nor patient consent is required.
Search strategy
A systematic literature search was designed and conducted separately by two authors to identify relevant randomized controlled trials (RCTs) on PubMed, Embase, Web of Science, the Cochrane Central Register of Controlled Trials, China National Knowledge Infrastructure database, Chinese Scientific Journal database, Wan Fang Database, from the date of database inception to 1st June 2022. There were no restrictions on publication year, region, or language. We used Medical Subject Headings (MeSH) Emtree terms, subject headings, and free-text terms in our search strategy, mainly include: “arthroscopic shoulder surgery” “arthroscopy,” “shoulder,” “nerve block,” “regional anesthesia,” “regional block,” “local block,” “interscalene nerve block,” “suprascapular nerve block,” “supraclavicular nerve block,” “suprascapular and axillary nerve blocks,” “pain,” and “analgesia.” We performed a further examination if the paper was presented in a non-English format due to certain restrictions in language.
Additionally, we conducted a battery of recursive searches and manual retrieval for major international conferences, which were presented only with an abstract that met our eligibility criteria. All above screening records will be managed using EndNote X9 (Thomson ISI Research Soft, Philadelphia, PA, USA). The established search strategies for each database were displayed in the “Search Strategies” supplement.
Eligibility criteria and exclusion criteria and data extraction
Inclusion criteria and exclusion criteria were determined as the priority according to PICO principle. Any study that compared the efficacy of anesthesia techniques as postoperative analgesia was thought suitable for our NMA. The inclusion and exclusion criteria were as follows. Participants: patients who underwent ASS under GA. Interventions: nerve block or regional anesthesia was administered in the operating room combined with GA. Comparators: interventions themselves or patients received GA alone. Outcomes: the primary outcome was pain scores (VAS or NRS) in the PACU or within 1 h, 2 or 4 h, 6 or 8 h, 24 h after surgery and opioids consumption in 24 h after surgery; the secondary outcomes were the incidence of adverse events. Study design: Only RCTs were included in this review. Exclusion criteria: contraindications to nerve block or local anesthetics, coagulopathy, neuropathy, and chronic opioid use.
Two authors (ZL and J-HW) independently identified the relevant articles. Both titles and abstracts were initially searched according to the established eligible criteria. Duplicate articles were also removed simultaneously. In addition, studies published only in abstract form without any available data were discarded. If there is disagreement, an independent reviewer (P-CS) will serve as the expert referee to ensure consensus was reached on all items. Studies were summarized into seven groups, CISB, ISB, SSNB, SCB, SSAX, LIA, control group (CG).
Outcome measures and quality assessment
Two authors extracted relevant data from the included articles independently as follows: first author(s), year of publication, patient characteristics, sample size, type of block used, pain scores, opioids consumption, incidence of complications (Horner syndrome, dyspnea, hoarseness, vomiting, and nausea). We extracted the mean and standard deviation (SD) of pain scores and opioids consumption as continuous outcomes. As for the dichotomous data, the incidence of side effects and complications were extracted from the articles.
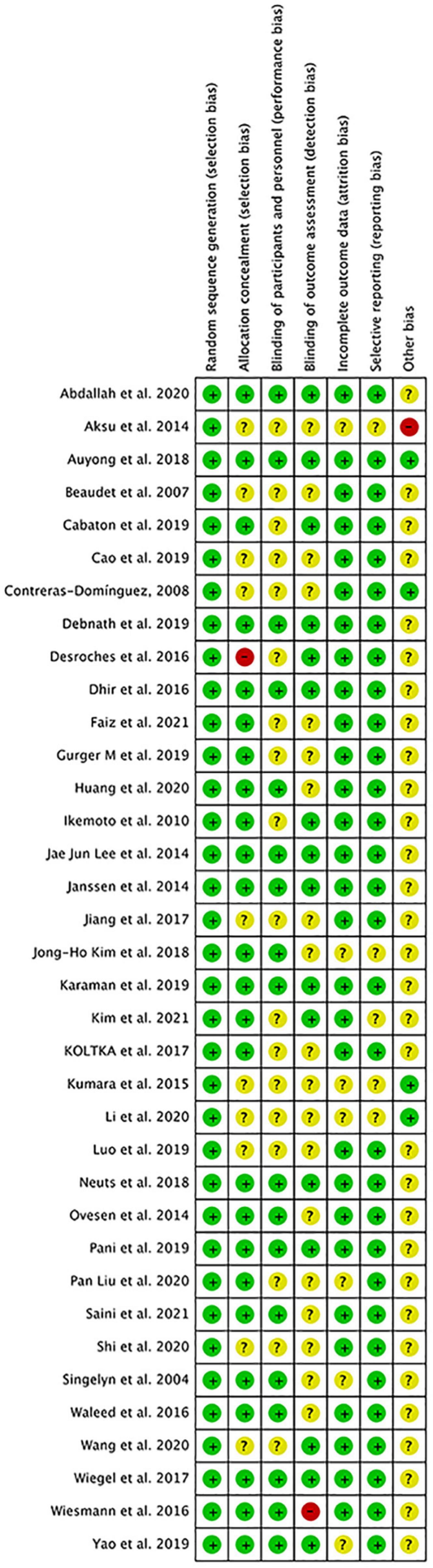
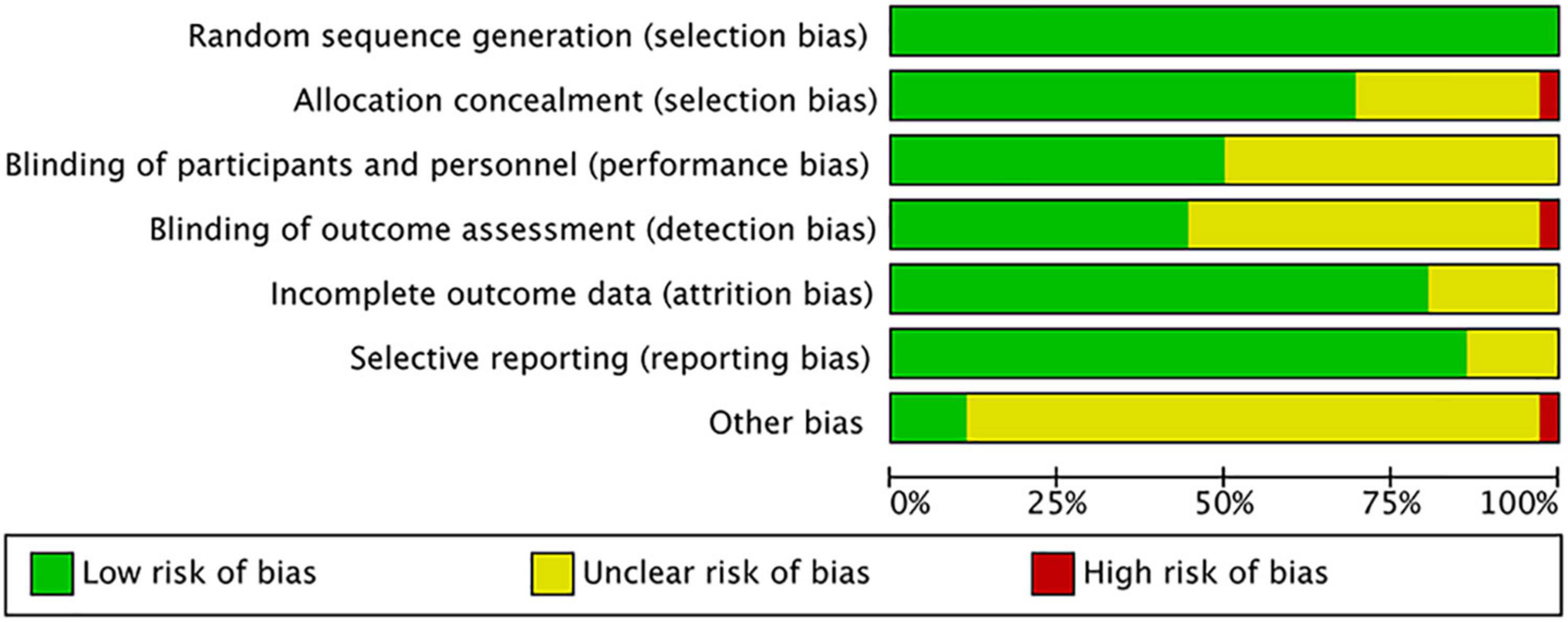
Two independent authors (ZL and J-HW) appraised and classified the risk of bias by using Cochrane’s risk of bias (ROB) tool. Seven assessment items were classified as low, high, or unclear rank, which included random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective outcome reporting, and “other issues” under the guidance of the guidelines of Cochran’s Handbook for Systematic Reviews of Interventions (8). The assessment of ROB was performed in Review Manager (Version 5.3). Additionally, the Grade approach was used to access the quality of evidence for each association (10).
Data analysis
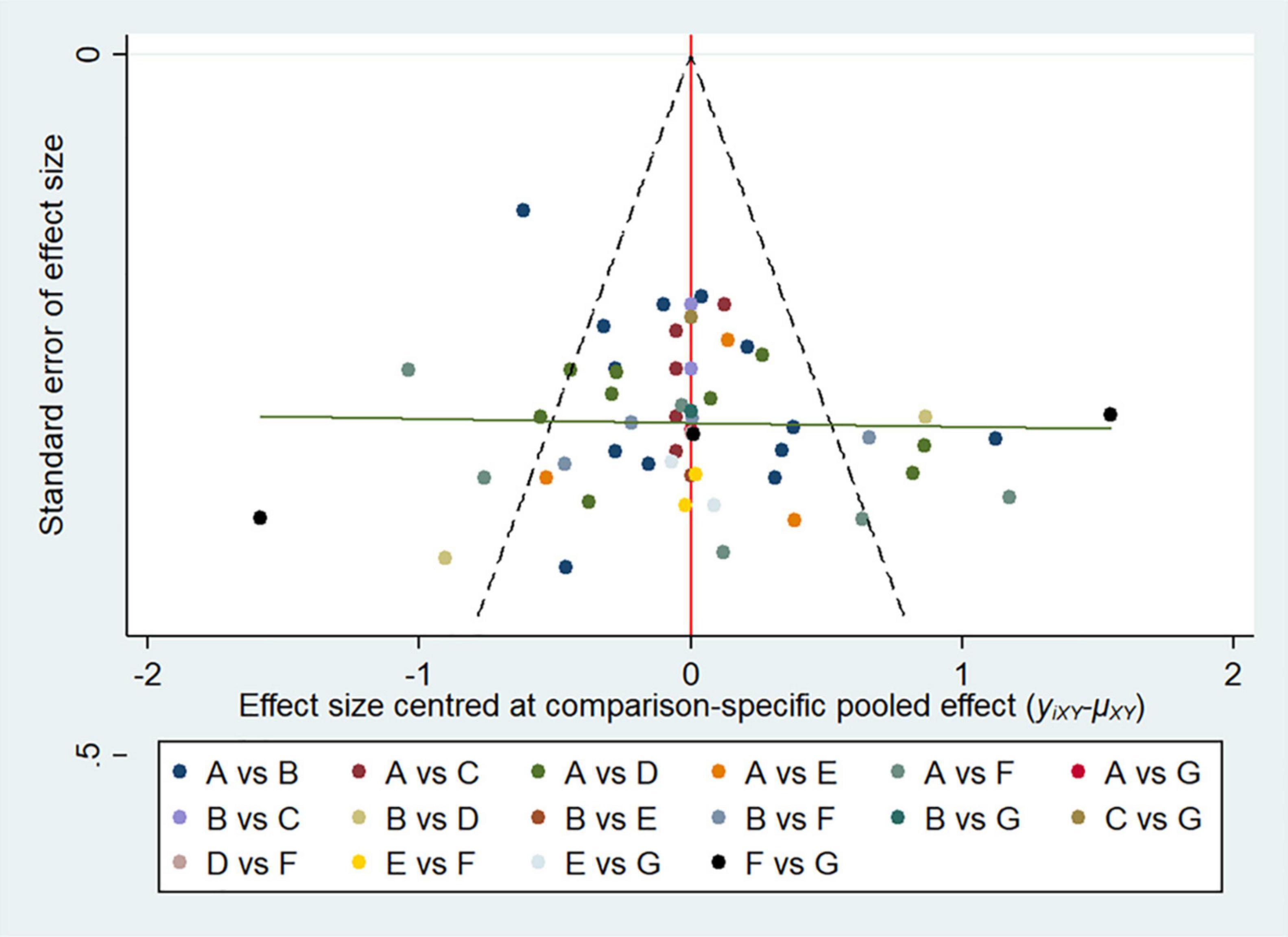
Firstly, a network plot was generated for all direct comparisons to simulate a fully connected network, and a comparison-adjusted network funnel plot for funnel plot asymmetry was applied to assess the publication bias. Both analyses were performed in STATA software, version 14.0 (Stata Corp., College Station, TX). Before performing data analyzing, we assessed the transitivity and consistency assumption carefully, which underlies NMA and concerns the validity of making indirect comparisons. The baseline characteristics of participants are described using summary characteristics for the following analysis (11–13). Based on the Bayesian network meta-analysis, a non-informative prior distribution was used to compare the six interventions (14). All the outcomes were analyzed using random-effects models via the Markov chain Monte Carlo (MCMC) method, which established three distinct chains with sufficient iteration (15–17). For continuous variable, we used the mean difference (MD) to pool the effect size, as well as their 95% confidence intervals. As for the incidence of side effects and complications, dichotomous data were summarized using the odds ratio (OR) and 95% confidence interval (CI) (18, 19). The surface under the cumulative ranking curve (SUCRA) was calculated to rank probability of each intervention (20). A higher SUCRA value represents the likelihood that the intervention is on the top rank or is highly effective; a SUCRA value of 0 indicates the lowest efficacy compared to other prevention (19). Convergence of iterations was assessed for each parameters using the Brooks-Gelman-Rubin method and visual analysis of trace plots. The network consistency was evaluated with the node-splitting approach, where P-values of less than 0.05 indicated the probability of inconsistency of the entire network frame. If necessary, another sensitivity analysis was conducted for studies (8, 16, 21, 22). The above of the Bayesian network analysis was performed using the OpenBUGS (ver. 3.2.3 rev 1012, Members of OpenBUGS Project Management Group) software.
Results
Baseline characteristics and quality of the included studies
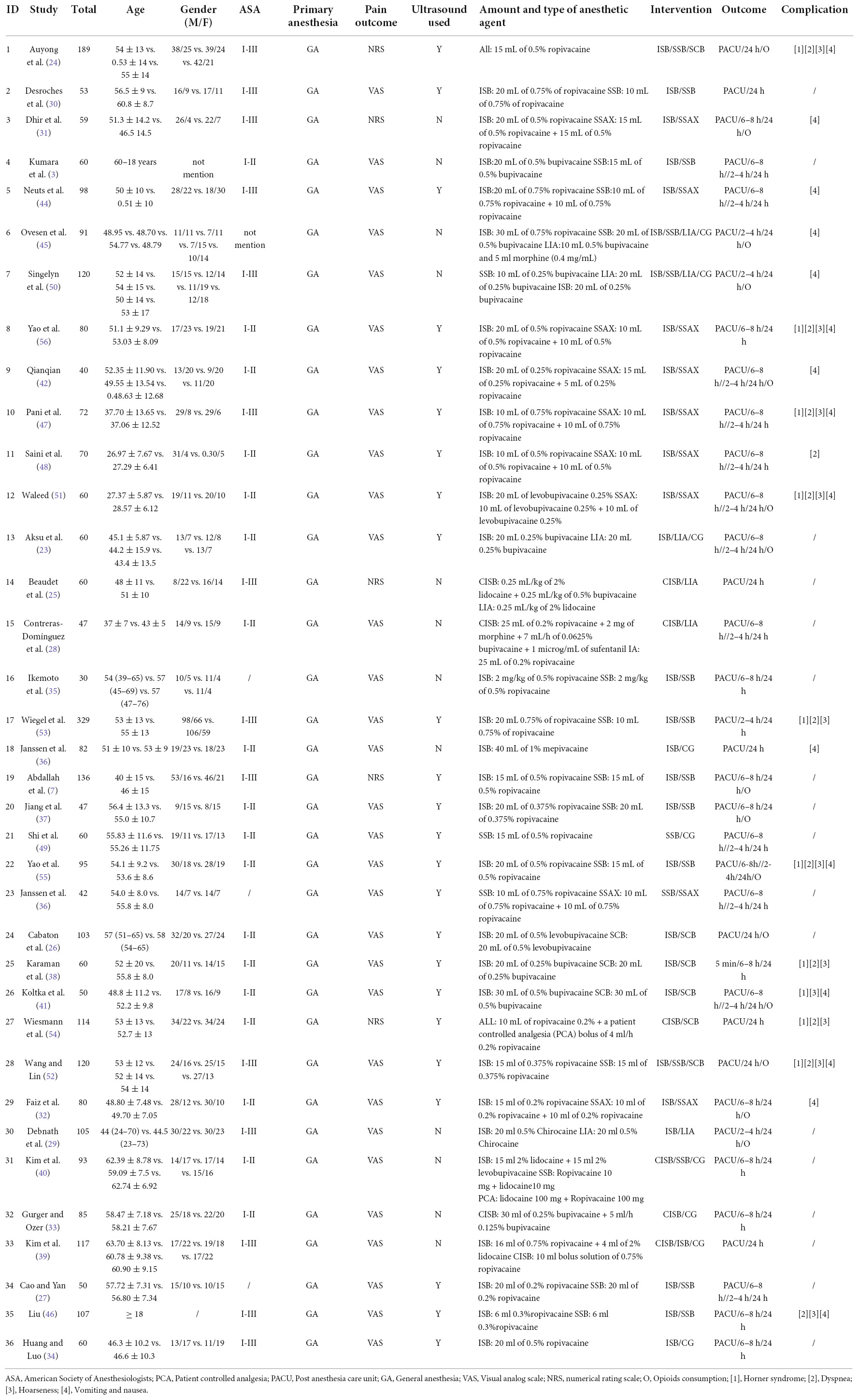
A total of 1,348 studies were identified initially by the electronic database searches and 45 discovered by manual searching as a supplement, and 935 articles were discarded due to duplication. After screening on the titles and abstracts, 241 articles were removed, and the 217 articles that met the criteria were remained to go through a further full-text examination. 181 articles were excluded for the following reasons: 104 did not represent a relevant data, 62 did not represent a relevant outcome, 15 were not randomized controlled trials. Finally, 36 RCTs were deemed eligible for the analysis with a unanimous agreement achieved between the review authors. The outline of literature search and selection procedures are shown in Figure 1. All searched reference lists were imported and managed in EndNote X9 software (Clarivate Analytics, London, United Kingdom). The basic characteristics of included studies were summarized in Table 1.
Thirty-six studies included in the review were published between 2004 and 2021, enrolling a total of 3,124 patients undergoing ASS for arthroscopic rotator cuff, subacromial decompression and other forms of shoulder surgery (3, 7, 23–56). The RCTs had a parallel (n = 4) or crossover (n = 32) design between six interventions. The sample size was largest for the ISB group (n = 1,174; 29 studies), followed by the SSNB group (n = 693; 17 studies), the CISB group (n = 415; 7 studies), SSAX group (n = 330; 10 studies), and control group (n = 289; 9 studies), the SCB group (n = 267; 6 studies), and LIA group (n = 149; 5 studies). A network plot was generated to visualize all direct comparisons (Figure 2).
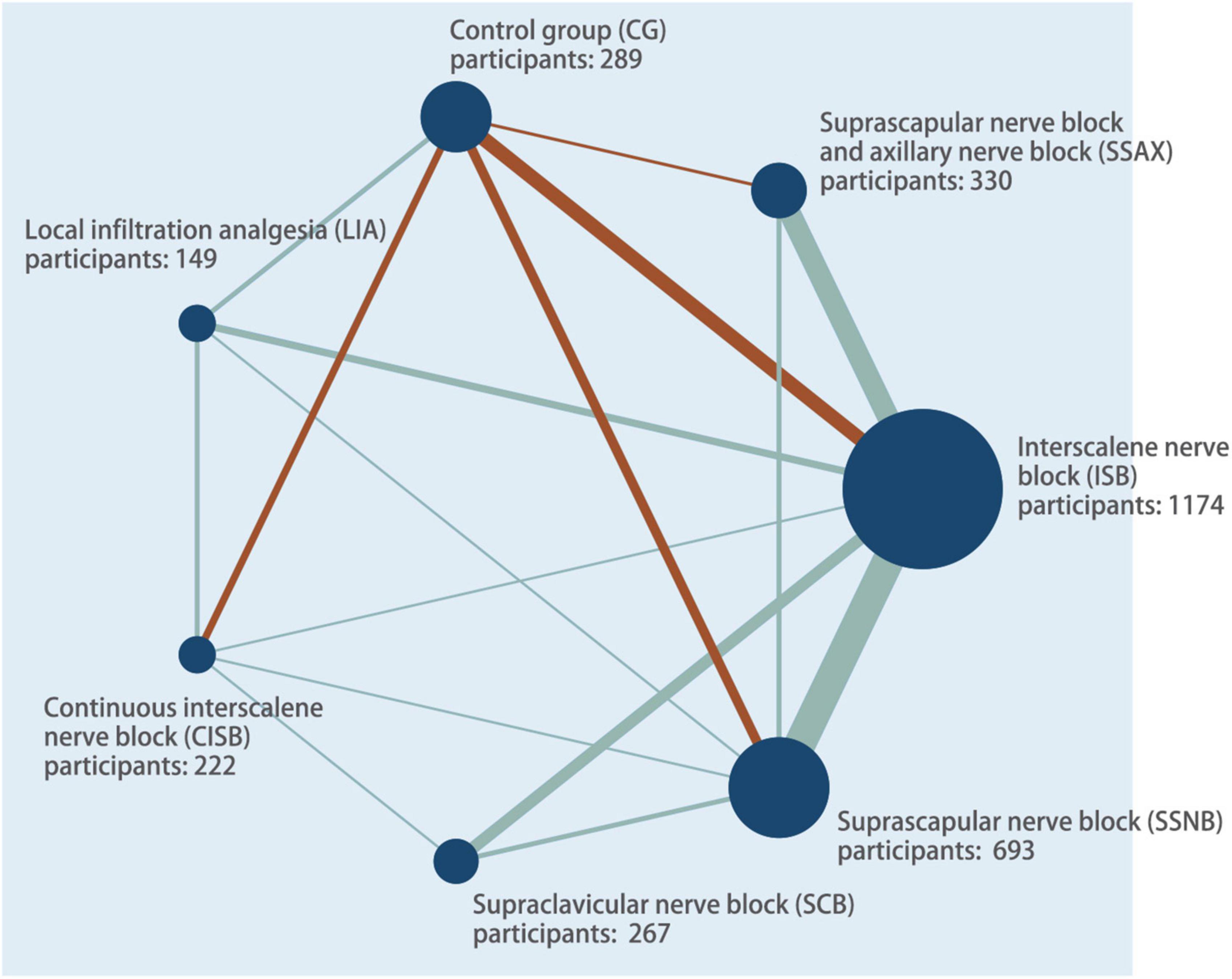
Figure 2. Network plot of all evidence of all the trails. The network plot of the intervention network shows the comparison of the sample size to provide anesthesia for patients undergoing arthroscopic shoulder surgery. Each node represented a different method of prevention with size of the node depending on the number of patients who received the intervention directly. The nodes were connected by lines indicating direct relationships between interventions, with the thickness of the line depending on the amount of direct evidence supporting the intervention.
The overall quality of included studies showed low variations. All the 36 included trials were randomly assigned and had a low risk of bias (ROB) in “Random sequence generation.” Five studies had a low ROB for the selective reporting item. Seven RCTs had a high or unclear ROB due to attrition. 25 used allocation concealment and 16 described the blinding of outcome assessment in detail. The assessment of quality of included studies were showed in Figures 3, 4. The funnel plot did not indicate publication bias due to its symmetrical distribution (Inverted funnel plot) (Figure 5).
Pain scores
Every study of postoperative pain scores has been associated with various nerve blocks or local analgesia. Thirty-one studies evaluated pain score by recording on a visual analog scale (VAS), a continuous scale based on a 0–10 cm (100 mm) in length. Five studies evaluated postoperative pain scores with a numerical rating scale (NRS) scoring, and the numbers (0–10) were administered in a numeric version of the VAS to evaluate pain intensity. The pain scores were evaluated at five time points (In the PACU or within 1 h after surgery, 2 or 4 h, 6 or 8 h, 24 h after surgery).
In the Post anesthesia care unit or within 1 h after surgery
A total of 36 studies reported pain scores in the PACU or within 1 h after surgery, including 7 groups (CG, ISB, CISB, SSNB, SCB, SSAX, LIA). CISB (MD = –3.14, 95% CI –4.47, –1.82), ISB (MD = –2.41, 95% CI –3.40, –1.41), SCB (MD = –2.34, 95% CI –3.79, –0.88), SSNB (MD = –1.66, 95% CI –2.73, –0.59), and SSAX (MD = –1.63, 95% CI –2.86, –0.39), provided significantly better analgesic effects compared to the CG group.
According to the SUCRA data (Supplementary Figure 1), CISB (SUCRA = 94.27%) and ISB (75.49%) had the highest efficacy, followed by SCB (69.36%), SSNB (39.64%), SSAX (38.79%), SSAX (31.18%), and control group (1.28%).
Within 2 or 4 h after surgery
Sixteen studies reported pain scores within 2 or 4 h after surgery and included 7 groups (CG, ISB, CISB, SSNB, SCB, SSAX, IA). ISB (MD = –2.02, 95% CI –3.49, –0.58) has significantly better outcomes than the CG group within 2 or 4 h after surgery.
According to the SUCRA data (Supplementary Figure 2), ISB (SUCRA = 85.56%) had the highest efficacy, followed by SCB (72.74%), SSNB (52.16%), CISB (48.53%), SSAX (48.23%), LIA (31.85%), and control group (10.92%).
Within 6 or 8 h after surgery
Twenty-three studies reported pain scores within 6 or 8 h after surgery and included 7 groups (Control group, ISB, CISB, SSNB, SCB, SSAX, LIA). ISB (MD = –1.69, 95% CI –2.54, –0.88), SCB (MD = –1.78, 95% CI –3.33, –0.24), SSNB (MD = –1.49, 95% CI –2.37, –0.63), CISB (MD = –1.39, 95% CI –2.50, –0.29) have significantly better outcomes than the CG group within 6 h or 8 h after surgery.
According to the SUCRA data (Supplementary Figure 3), ISB (SUCRA = 77.35%) had the highest efficacy, followed by SCB (75.37%), SSNB (62.93%), CISB (57.89%), LIA (47.02%), SSAX (27.53%), and control group (1.92%).
At 24 h after surgery
Thirty-six studies reported pain scores at 24 h after surgery and included 7 groups (Control group, ISB, CISB, SSNB, SCB, SSAX, LIA). SSNB (MD = –1.26, 95% CI –2.39, –0.10), SSAX (MD = –1.10, 95% CI –2.06, –0.11) have significantly better outcomes than the LIA group at 24 h after surgery.
The SUCRA data denoted that SSNB (SUCRA = 86.73%) and SSAX (SUCRA = 78.21%) had the highest efficacy, followed by ISB (SUCRA = 60.05%), CISB (SUCRA = 50.21%), SCB (SUCRA = 45.38%), LIA (SUCRA = 8.26%), and control group (21.16%) (Supplementary Figure 4).
Opioids consumption
Eighteen studies reported opioids consumption within 24 h after surgery and included 7 groups (Control group, ISB, CISB, SSNB, SCB, SSAX, LIA). ISB (MD = –12.9, 95% CI –17.15, –7.08), SCB (MD = –8.36, 95% CI –15.48, –1.33), SSNB (MD = –7.15, 95% CI –12.20, –2.15) have significantly better outcomes than the CG group within 6 h or 8 h after surgery (Supplementary Figure 5).
The SUCRA data showed that ISB (SUCRA = 97.23%) had the highest efficacy, followed by, SCB (SUCRA = 67.41%), SSNB (SUCRA = 57.91%), SSAX (SUCRA = 50.76%), CISB (SUCRA = 46.85%), LIA (SUCRA = 25.71%), and control group (21.16%).
Postoperative complications
Horner syndrome
Ten studies reported the incidence of Horner syndrome after surgery and included 5 groups (ISB, SSNB, SCB, CISB, SSAX). SSNB (OR = 0.15, 95% CI 0.01, 0.29), and SSAX (OR = 0.86, 95% CI 0.01, 0.67) significantly reduced the incidence of Horner syndrome compared to CISB group. SSNB (OR = 0.04, 95% CI 0.01, 0.13), SSAX (OR = 0.08, 95% CI 0.01, 0.32), and SCB (OR = 0.24, 95% CI 0.06, 0.58) significantly reduced the incidence of Horner syndrome compared to ISB group (Supplementary Figure 6).
Dyspnea
Twelve studies reported the incidence of dyspnea after surgery and included 5 groups (ISB, SSNB, SCB, CISB, SSAX). SSAX (OR = 0.12, 95% CI 0.02, 0.32) and SSNB (OR = 0.27, 95% CI 0.07, 0.62) significantly reduced the incidence of dyspnea syndrome compared to ISB group (Supplementary Figure 7).
Hoarseness
Eleven studies reported the incidence of hoarseness after surgery and included 5 groups (ISB, SSNB, SCB, CISB, SSAX). SSAX (OR = 0.29, 95% CI 0.03, 0.88) and SSNB (OR = 0.36, 95% CI 0.08, 0.84) significantly reduced the incidence of hoarseness compared to ISB group (Supplementary Figure 8).
Vomiting and nausea
Fourteen studies reported the incidence of vomiting after surgery and included 5 groups (ISB, SSNB, SCB, CISB, SSAX). SSNB (OR = 0.31, 95% CI 0.11, 0.71) and ISB (OR = 0.31, 95% CI 0.71, 0.84) significantly reduced the incidence of Horner syndrome compared to CISB group (Supplementary Figure 9).
Discussion
This NMA provides efficacy data on five variants of nerve blocks and intra-articular infiltration analgesia combined with GA, as well as the comparisons of some important complications. In the included study, all patients received nerve block before surgery. During the perioperative period, patients received GA with muscle relaxants, combined with multimodal analgesia. It is suggested that ISB are the most highly effective performed regional techniques for ASS in the early postoperative period (in the PACU or 1 h after surgery, 2 or 4 h, 6 or 8 h), while SSNB, SSAX provided provide better late postoperative shoulder analgesia (at 24 h after surgery). Moreover, SSNB, SSNB, SCB, may have a lower overall complication rate for Horner syndrome, dyspnea, hoarseness, vomiting and nausea than ISB and CISB.
ISB has been historically considered the gold standard in postoperative pain management for ASS, which was usually performed with an injection of local anesthetic at the nerve root level of the brachial plexus to block C5–7 between the anterior and middle scalene muscles (5, 57, 58). A systematic review by Warrender et al. recommend the use of ISBs as the most effective analgesic for outpatient undergoing ASS based on the evidence of 40 RCTs (4). Consistent with previous studies, our results also indicated that ISB significantly improved pain control in the early postoperative period compared with control group, particularly in the PACU or within 2 h or 4 h hours postoperatively. Following ISB, ipsilateral phrenic nerve block is a well-known complication, of which the rates of 16.6–38% have been reported in previous studies. The root cause is the interscalene insertion site is close to the phrenic nerve, and the unintended spread of local anesthesia could cause diaphragm paresis, thus reducing vital capacity and leading to dyspnea (59). Therefore, ISB would have been a relative contraindication in patients with serious pulmonary disease. Desai found that patients who received continuous interscalene infusion catheters (CISB) resulted in a clinically remarkable improvement during the first 24 postoperative hours compared with those who received a single shot ISB (5). It is indicated in our results CISB group provided a better analgesia than the ISB group in the early postoperative period.
Many studies suggested SSNB may be considered as an alternative when ISB is contraindicated to be used as an option for patients after ASS (60–62). A previous meta-analysis of 14 articles suggested that, SSNB showed inferior analgesic effect compared with ISB, particularly in the short-term period (in the PACU or within 1–2 h postoperatively) (2). At 24 h postoperative, there was no significant difference in analgesic effect between the SSNB and ISB groups. The results of this NMA are mostly consistent with previous systematic reviews. In the early postoperative time (in PACU or within 1 h), compared to the control group, the efficiency of the SSNB group was lower than that of the ISB group (ISB: MD = –2.41, 95% CI –3.40, –1.41; SSNB: MD = –1.66, 95% CI –2.73, –0.59). Additionally, compared to the ISB group, the SSNB group provided a lower analgesic effect than the ISB group (MD = –0.74, 95% CI –1.48, –0.01). At 24 h after surgery, the analgesic effect has no significant difference between two groups. The explanation for the imperfect early pain control of SSNB is that, the suprascapular nerve is considered to innervate about 70% shoulder joint, the other 30% is innervated by the lateral thoracic nerve and axillary nerve (2, 63). Therefore, we hypothesize that combined with axillary block, SSAX may provide improved postoperative pain control compared with SSNB alone. The results suggested that SSAX group significantly reduced pain scores compared with control group (in PACU or at 24 h) (64). However, there was no difference between the results of SSNB group and the SSAX group. Furthermore, in contrast to that of ISB, we find that the complication rates were significantly lower in the SSNB and SSAX groups.
Supraclavicular block (SCB) is also an alternative to ISB with a low incidence of side effects. Cornish found that although SCB were administered under the clavicle and above the first rib, the local anesthetics could spread cephalad between the anterior and middle scalene muscles (65). A meta-analysis by Guo et al. compared SCB with ISB in pain control after shoulder surgery, indicating that SCB provided similar analgesic efficacy compared to ISB with a low incidence of hoarseness and Horner syndrome (66), which is consistent with our results. Compared with control group, SCB group reduced significantly pain scores in PACU (MD = –2.34, 95% CI –3.79, –0.88).
Local infiltration analgesia (LIA) is a safe and valuable postoperative pain management technique for patients undergoing ASS, which was usually performed at the end of the shoulder surgery before wound closure. However, iatrogenic chondrolysis of the glenohumeral joint as a complication of local infiltration analgesia is a rare but recognized complication, especially in the case of high dose and long-term administration of bupivacaine (67). In our NMA, the results suggested that LIA play no significant role in reducing the pain score at all time periods.
Our study has several strengths. To our knowledge, this is the first network meta-analysis evaluating postoperative pain regimens after ASS. Additionally, high-quality meta-analysis could be performed owing to that only RCTs was eligible for the present analysis. The trials were generally at low risk of bias for most ROB domains. Furthermore, in order to guarantee an accurate and thorough evaluation of the total body of data, the GRADE approach was used to grade the quality of the studies. Our NMA provided comprehensive evidence-based clinical practice guidance regarding the perioperative pain regimens in patients undergoing ASS.
There are also potential limitations in this review. Due to the limitations of the literature, some new analgesic methods and rare complications of nerve block were not analyzed in this NMA. Moreover, different types, concentrations, volumes of local anesthesia were used in these trials, which may cause some deviations. Another limitation is related to the technology used. Some nerve blocks are performed under ultrasound guidance, while others are located only by nerve stimulation. Furthermore, there was heterogeneity between the included studies in terms of quality evaluation, outcome measures, and assessment time. Finally, the proficiency of the operators, postoperative analgesia used, and patient characteristics may affect the pooled results and occurrence of complications.
Conclusion
ISB was superior in reducing pain and opioid consumption compared to other peripheral nerve blocks but had a higher frequency of adverse events.
Data availability statement
The original contributions presented in this study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
ZL and J-hW helped substantial contributions to the conception or design of the work, the acquisition, analysis, interpretation of data for the work, drafting the manuscript, and revising it critically for important intellectual content. Y-bL and G-hW helped agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. P-cS helped final approval of the version to be published. All authors contributed to the article and approved the submitted version.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2022.1032253/full#supplementary-material
References
1. He Y, Liu J, Wang Z, Zhou P, Deng X, Yang L, et al. Analysis of the early clinical outcomes of arthroscopic debridement in the treatment of shoulder tuberculosis. J Orthop Surg Res. (2020) 15:550. doi: 10.1186/s13018-020-02086-7
2. Kay J, Memon M, Hu T, Simunovic N, Duong A, Paul J, et al. Suprascapular nerve blockade for postoperative pain control after arthroscopic shoulder surgery: a systematic review and meta-analysis. Orthop J Sports Med. (2018) 6:2325967118815859. doi: 10.1177/2325967118815859
3. Kumara AB, Gogia AR, Bajaj JK, Agarwal N. Clinical evaluation of post-operative analgesia comparing suprascapular nerve block and interscalene brachial plexus block in patients undergoing shoulder arthroscopic surgery. J Clin Orthop Trauma. (2016) 7:34–9. doi: 10.1016/j.jcot.2015.09.003
4. Warrender WJ, Syed UAM, Hammoud S, Emper W, Ciccotti MG, Abboud JA, et al. Pain management after outpatient shoulder arthroscopy: a systematic review of randomized controlled trials. Am J Sports Med. (2017) 45:1676–86. doi: 10.1177/0363546516667906
5. Desai N. Postoperative analgesia for shoulder surgery. Br J Hosp Med. (2017) 78:511–5. doi: 10.12968/hmed.2017.78.9.511
6. Dobie KH, Shi Y, Shotwell MS, Sandberg WS. New technique targeting the C5 nerve root proximal to the traditional interscalene sonoanatomical approach is analgesic for outpatient arthroscopic shoulder surgery. J Clin Anesth. (2016) 34:79–84. doi: 10.1016/j.jclinane.2016.03.064
7. Abdallah FW, Wijeysundera DN, Laupacis A, Brull R, Mocon A, Hussain N, et al. Subomohyoid anterior suprascapular block versus interscalene block for arthroscopic shoulder surgery: a multicenter randomized trial. Anesthesiology. (2020) 132:839–53. doi: 10.1097/aln.0000000000003132
8. Higgins JP, Jackson D, Barrett JK, Lu G, Ades AE, White IR. Consistency and inconsistency in network meta-analysis: concepts and models for multi-arm studies. Res Synth Methods. (2012) 3:98–110. doi: 10.1002/jrsm.1044
9. Hutton B, Salanti G, Caldwell DM, Chaimani A, Schmid CH, Cameron C, et al. The PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: checklist and explanations. Ann Intern Med. (2015) 162:777–84. doi: 10.7326/m14-2385
10. Brozek JL, Akl EA, Alonso-Coello P, Lang D, Jaeschke R, Williams JW, et al. Grading quality of evidence and strength of recommendations in clinical practice guidelines. Part 1 of 3. An overview of the GRADE approach and grading quality of evidence about interventions. Allergy. (2009) 64:669–77. doi: 10.1111/j.1398-9995.2009.01973.x
11. Caldwell DM, Ades AE, Higgins JP. Simultaneous comparison of multiple treatments: combining direct and indirect evidence. BMJ. (2005) 331:897–900. doi: 10.1136/bmj.331.7521.897
12. Jansen JP, Naci H. Is network meta-analysis as valid as standard pairwise meta-analysis? It all depends on the distribution of effect modifiers. BMC Med. (2013) 11:159. doi: 10.1186/1741-7015-11-159
13. Salanti G, Marinho V, Higgins JP. A case study of multiple-treatments meta-analysis demonstrates that covariates should be considered. J Clin Epidemiol. (2009) 62:857–64. doi: 10.1016/j.jclinepi.2008.10.001
14. Mengersen K, Stojanovski EJC. Bayesian methods in meta-analysis. In: S Chow editor. Encyclopedia of Biopharmaceutical Statistics. Oxfordshire: Taylor & Francis (2006). p. 116–21.
15. Mavridis D, Salanti G. A practical introduction to multivariate meta-analysis. Stat Methods Med Res. (2013) 22:133–58. doi: 10.1177/0962280211432219
16. van Valkenhoef G, Dias S, Ades AE, Welton NJ. Automated generation of node-splitting models for assessment of inconsistency in network meta-analysis. Res Synth Methods. (2016) 7:80–93. doi: 10.1002/jrsm.1167
17. Welton NJ. Evidence Synthesis for Decision Making in Healthcare. Hoboken, NJ: John Wiley & Sons (2012).
18. Mbuagbaw L, Rochwerg B, Jaeschke R, Heels-Andsell D, Alhazzani W, Thabane L, et al. Approaches to interpreting and choosing the best treatments in network meta-analyses. Syst Rev. (2017) 6:79. doi: 10.1186/s13643-017-0473-z
19. Salanti G, Ades AE, Ioannidis JP. Graphical methods and numerical summaries for presenting results from multiple-treatment meta-analysis: an overview and tutorial. J Clin Epidemiol. (2011) 64:163–71. doi: 10.1016/j.jclinepi.2010.03.016
20. Page MJ, Shamseer L, Altman DG, Tetzlaff J, Sampson M, Tricco AC, et al. Epidemiology and reporting characteristics of systematic reviews of biomedical research: a cross-sectional study. PLoS Med. (2016) 13:e1002028. doi: 10.1371/journal.pmed.1002028
21. Chaimani A, Higgins JP, Mavridis D, Spyridonos P, Salanti G. Graphical tools for network meta-analysis in STATA. PLoS One. (2013) 8:e76654. doi: 10.1371/journal.pone.0076654
22. Dias S, Welton NJ, Caldwell DM, Ades AE. Checking consistency in mixed treatment comparison meta-analysis. Stat Med. (2010) 29:932–44. doi: 10.1002/sim.3767
23. Aksu R, Biçer C, Ülgey A, Bayram A, Güneş I, Güney A, et al. Comparison of interscalene brachial plexus block and intra-articular local anesthetic administration on postoperative pain management in arthroscopic shoulder surgery. Braz J Anesthesiol. (2015) 65:222–9. doi: 10.1016/j.bjane.2014.06.005
24. Auyong DB, Hanson NA, Joseph RS, Schmidt BE, Slee AE, Yuan SC. Comparison of anterior suprascapular, supraclavicular, and interscalene nerve block approaches for major outpatient arthroscopic shoulder surgery: a randomized, double-blind, noninferiority trial. Anesthesiology. (2018) 129:47–57. doi: 10.1097/aln.0000000000002208
25. Beaudet V, Williams SR, Tétreault P, Perrault MA. Perioperative interscalene block versus intra-articular injection of local anesthetics for postoperative analgesia in shoulder surgery. Reg Anesth Pain Med. (2008) 33:134–8. doi: 10.1016/j.rapm.2007.10.005
26. Cabaton J, Nové-Josserand L, Mercadal L, Vaudelin T. Analgesic efficacy of ultrasound-guided interscalene block vs. supraclavicular block for ambulatory arthroscopic rotator cuff repair: a randomised noninferiority study. Eur J Anaesthesiol. (2019) 36:778–86. doi: 10.1097/eja.0000000000001065
27. Cao SWJ, Yan L. Effcacy of direct arthroscopy-guided suprascapular nerve block under shoulder arthroscopy in postoperative analgesia of rotator cuff repair. China J Endosc. (2019) 25:55–60. doi: 10.3969/j.issn.1007-1989.2019.11.008
28. Contreras-Domínguez V, Carbonell-Bellolio P, Sanzana ES, Ojeda-Greciet A, Orrego-Santos R. [Efficacy of a continuous interscalene block vs intra-articular analgesia for postoperative pain in arthroscopic acromioplasty]. Rev Esp Anestesiol Reanim. (2008) 55:475–80. doi: 10.1016/s0034-9356(08)70630-3
29. Debnath UK, Goel V, Saini S, Trehan N, Trehan R. A prospective study of two methods of analgesia in shoulder arthroscopic procedures as day case surgery. J Clin Orthop Trauma. (2020) 11:S368–71. doi: 10.1016/j.jcot.2019.06.008
30. Desroches A, Klouche S, Schlur C, Bauer T, Waitzenegger T, Hardy P. Suprascapular nerve block versus interscalene block as analgesia after arthroscopic rotator cuff repair: a randomized controlled noninferiority trial. Arthroscopy. (2016) 32:2203–9. doi: 10.1016/j.arthro.2016.03.013
31. Dhir S, Sondekoppam RV, Sharma R, Ganapathy S, Athwal GSA. Comparison of combined suprascapular and axillary nerve blocks to interscalene nerve block for analgesia in arthroscopic shoulder surgery: an equivalence study. Reg Anesth Pain Med. (2016) 41:564–71. doi: 10.1097/aap.0000000000000436
32. Faiz SHR, Mohseni M, Imani F, Attaee MK, Movassaghi S, Rahimzadeh P. Comparison of ultrasound-guided supra-scapular plus axillary nerve block with interscalene block for postoperative pain management in arthroscopic shoulder surgery; A double-blinded randomized open-label clinical trial. Anesth Pain Med. (2021) 11:e112540. doi: 10.5812/aapm.112540
33. Gurger M, Ozer AB. A comparison of continuous interscalene block versus general anesthesia alone on the functional outcomes of the patients undergoing arthroscopic rotator cuff repair. Eur J Orthop Surg Traumatol. (2019) 29:1659–66. doi: 10.1007/s00590-019-02482-8
34. Huang ZZW, Luo R. Impacts of ultrasound-guided brachial plexus block on fast track rehabil itation after shoulder arthroscopy. China Mod Doctor. (2020) 58:133–36.
35. Ikemoto RY, Murachovsky J, Prata Nascimento LG, Bueno RS, Oliveira Almeida LH, Strose E, et al. Prospective randomized study comparing two anesthetic methods for shoulder surgery. Rev Bras Ortop. (2010) 45:395–9. doi: 10.1016/s2255-4971(15)30386-4
36. Janssen H, Stosch R, Pöschl R, Büttner B, Bauer M, Hinz JM, et al. Blood pressure response to combined general anaesthesia/interscalene brachial plexus block for outpatient shoulder arthroscopy. BMC Anesthesiol. (2014) 14:50. doi: 10.1186/1471-2253-14-50
37. Jiang HWQ, Tang Y, Zheng M, Chen L. Application of ultrasound-guided anterior suprascapular nerve block in the analgesia of shoulder arthroscopic surgery. J Clin Anesthesiol. (2017) 12:1192–5.
38. Karaman T, Karaman S, Aşçı M, Tapar H, şahin A, Dogru S, et al. Comparison of ultrasound-guided supraclavicular and interscalene brachial plexus blocks in postoperative pain management after arthroscopic shoulder surgery. Pain Pract. (2019) 19:196–203. doi: 10.1111/papr.12733
39. Kim JH, Koh HJ, Kim DK, Lee HJ, Kwon KH, Lee KY, et al. Interscalene brachial plexus bolus block versus patient-controlled interscalene indwelling catheter analgesia for the first 48 hours after arthroscopic rotator cuff repair. J Shoulder Elbow Surg. (2018) 27:1243–50. doi: 10.1016/j.jse.2018.02.048
40. Kim JY, Kang MW, Lee HW, Noh KC. Suprascapular nerve block is an effective pain control method in patients undergoing arthroscopic rotator cuff repair: a randomized controlled trial. Orthop J Sports Med. (2021) 9:2325967120970906. doi: 10.1177/2325967120970906
41. Koltka AK, Büget M, Bingül ES, Erşen A, Küçükay S, Atalar AC, et al. Postoperative analgesia after arthroscopic shoulder surgery: a comparison between single-shot interscalene block and single-shot supraclavicular block. Agri. (2017) 29:127–31. doi: 10.5505/agri.2017.67984
42. Qianqian L. The Effect of Ultrasound-Guided Suprascapular Nerve Combined Axillary Nerve Block On Analgesia After Shoulder Arthroscopy. Ph.D. thesis. Shenyang: China Medical University (2019).
43. Lee JJ, Kim DY, Hwang JT, Lee SS, Hwang SM, Kim GH, et al. Effect of ultrasonographically guided axillary nerve block combined with suprascapular nerve block in arthroscopic rotator cuff repair: a randomized controlled trial. Arthroscopy. (2014) 30:906–14. doi: 10.1016/j.arthro.2014.03.014
44. Neuts A, Stessel B, Wouters PF, Dierickx C, Cools W, Ory JP, et al. Selective suprascapular and axillary nerve block versus interscalene plexus block for pain control after arthroscopic shoulder surgery: a noninferiority randomized parallel-controlled clinical trial. Reg Anesth Pain Med. (2018) 43:738–44. doi: 10.1097/aap.0000000000000777
45. Ovesen J, Falstie-Jensen T, Christensen CA. Comparison of Subacromial bursae block, suprascapular nerve block and interscalene brachial plexus block after arthroscopic shoulder surgery. Pain Stud Treat. (2014) 02:47845. doi: 10.4236/pst.2014.23017
46. Liu P. Effect of suprascapular nerve block on patients undergoing arthroscopic shoulder surgery. Trauma J. (2020) 25:589–90. doi: 10.3969/j.issn.1009-7147.2020.03.095
47. Pani N, Routray SS, Pani S, Mallik S, Pattnaik S, Pradhan A. Post-operative analgesia for shoulder arthroscopic surgeries: a comparison between inter-scalene block and shoulder block. Indian J Anaesth. (2019) 63:382–7. doi: 10.4103/ija.IJA_65_19
48. Saini S, Rao SM, Agrawal N, Gupta A. Comparison of analgesic efficacy of shoulder block versus interscalene block for postoperative analgesia in arthroscopic shoulder surgeries: a randomised trial. Indian J Anaesth. (2021) 65:451–7. doi: 10.4103/ija.IJA_110_21
49. Shi X, Wang Y, Chen L. The Application of Ultrasound-Guided Suprascapular Nerve Block Combined With General Anesthesia In Arthroscopic of Rotator Cuff Injury Repair. Chin J Med Front. (2020) 12:71–75. doi: 10.12037/YXQY.2020.07-13
50. Singelyn FJ, Lhotel L, Fabre B. Pain relief after arthroscopic shoulder surgery: a comparison of intraarticular analgesia, suprascapular nerve block, and interscalene brachial plexus block. Anesth Analg. (2004) 99:589–92. doi: 10.1213/01.Ane.0000125112.83117.49
51. Waleed A. Postoperative analgesia for arthroscopic shoulder surgery: comparison between ultrasound-guided interscalene block and combined suprascapular and axillary nerve blocks. Original Article. Ain Shams J Anaesthesiol. (2016) 9:536–41. doi: 10.4103/1687-7934.198260
52. Wang HDJ, Lin H. Comparison of three ultrasound-guided nerve block techniques in shoulder arthroscopic surgery. Traumatic J. (2020) 25:937–40. doi: 10.3969/j.issn.1009-7147.2020.05.056
53. Wiegel M, Moriggl B, Schwarzkopf P, Petroff D, Reske AW. Anterior suprascapular nerve block versus interscalene brachial plexus block for shoulder surgery in the outpatient setting: a randomized controlled patient- and assessor-blinded trial. Reg Anesth Pain Med. (2017) 42:310–8. doi: 10.1097/aap.0000000000000573
54. Wiesmann T, Feldmann C, Müller HH, Nentwig L, Beermann A, El-Zayat BF, et al. Phrenic palsy and analgesic quality of continuous supraclavicular vs. interscalene plexus blocks after shoulder surgery. Acta Anaesthesiol Scand. (2016) 60:1142–51. doi: 10.1111/aas.12732
55. Yao J, Huang H, Huang S, Zhang Z. Application effect of suprascapular nerve block in patients undergoing arthroscopic rotator cuff repair surgery. Tianjin Med J. (2019) 47:851–4.
56. Yi-ping L. The analgesia effect of ultrasound-guided suprascapular and axillary nerve blocks for shoulder rotator cuff repair under arthroscopy. J Gannan Med Univ. (2020) 40:786–89.
57. Yan S, Zhao Y, Zhang H. Efficacy and safety of interscalene block combined with general anesthesia for arthroscopic shoulder surgery: a meta-analysis. J Clin Anesth. (2018) 47:74–9. doi: 10.1016/j.jclinane.2018.03.008
58. Sánchez Novas D, Biscaiburo JP, Terrasa S, Vescovo A. Patient satisfaction and opioid requirements after ultrasound-guided interscalene block for arthroscopic shoulder surgery among operators with different levels of experience: a prospective observational study. J Clin Anesth. (2020) 62:109718. doi: 10.1016/j.jclinane.2020.109718
59. Oliver-Fornies P, Ortega Lahuerta JP, Gomez Gomez R, Gonzalo Pellicer I, Oliden Gutierrez L, Viñuales Cabeza J, et al. Diaphragmatic paralysis, respiratory function, and postoperative pain after interscalene brachial plexus block with a reduced dose of 10?ml levobupivacaine 0.25% versus a 20?ml dose in patients undergoing arthroscopic shoulder surgery: study protocol for the randomized controlled double-blind REDOLEV study. Trials. (2021) 22:287. doi: 10.1186/s13063-021-05216-6
60. Byeon GJ, Shin SW, Yoon JU, Kim EJ, Baek SH, Ri HS. Infusion methods for continuous interscalene brachial plexus block for postoperative pain control after arthroscopic rotator cuff repair. Korean J Pain. (2015) 28:210–6. doi: 10.3344/kjp.2015.28.3.210
61. Uquillas CA, Capogna BM, Rossy WH, Mahure SA, Rokito AS. Postoperative pain control after arthroscopic rotator cuff repair. J Shoulder Elbow Surg. (2016) 25:1204–13. doi: 10.1016/j.jse.2016.01.026
62. White L, Reardon D, Davis K, Velli G, Bright M. Anterior suprascapular nerve block versus interscalene brachial plexus block for arthroscopic shoulder surgery: a systematic review and meta-analysis of randomized controlled trials. J Anesth. (2022) 36:17–25. doi: 10.1007/s00540-021-03000-z
63. Aszmann OC, Dellon AL, Birely BT, McFarland EG. Innervation of the human shoulder joint and its implications for surgery. Clin Orthop Relat Res. (1996) 330:202–7. doi: 10.1097/00003086-199609000-00027
64. Zhao J, Xu N, Li J, Liang G, Zeng L, Luo M, et al. Efficacy and safety of suprascapular nerve block combined with axillary nerve block for arthroscopic shoulder surgery: a systematic review and meta-analysis of randomized controlled trials. Int J Surg. (2021) 94:106111. doi: 10.1016/j.ijsu.2021.106111
65. Cornish P. Supraclavicular block–new perspectives. Reg Anesth Pain Med. (2009) 34:607–8. doi: 10.1097/AAP.0b013e3181ada5af
66. Guo CW, Ma JX, Ma XL, Lu B, Wang Y, Tian AX, et al. Supraclavicular block versus interscalene brachial plexus block for shoulder surgery: a meta-analysis of clinical control trials. Int J Surg. (2017) 45:85–91. doi: 10.1016/j.ijsu.2017.07.098
Keywords: arthroscopic shoulder surgery, pain management, nerve block, complications, Bayesian network meta-analysis
Citation: Liu Z, Li Y-b, Wang J-h, Wu G-h and Shi P-c (2022) Efficacy and adverse effects of peripheral nerve blocks and local infiltration anesthesia after arthroscopic shoulder surgery: A Bayesian network meta-analysis. Front. Med. 9:1032253. doi: 10.3389/fmed.2022.1032253
Received: 21 September 2022; Accepted: 21 October 2022;
Published: 10 November 2022.
Edited by:
Shun Ming Chan, Tri-Service General Hospital, TaiwanReviewed by:
Kai Henrik Wiborg Lange, Nordsjællands Hospital, DenmarkAbhijit Nair, Ministry of Health, Oman
Dipasri Bhattacharya, Government of West Bengal, India
Copyright © 2022 Liu, Li, Wang, Wu and Shi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Peng-cai Shi, shipengcai1997@163.com
 Zheng Liu1
Zheng Liu1