- 1Department of Orthopedics, Taizhou Hospital of Zhejiang Province Affiliated to Wenzhou Medical University, Taizhou, China
- 2Department of Clinical Laboratory, Taizhou Hospital of Zhejiang Province Affiliated to Wenzhou Medical University, Taizhou, China
Objectives: To evaluate the impact of intra-articular injection with tranexamic acid (TXA) on total blood loss (TBL) and postoperative pain after arthroscopic rotator cuff repair (ARCR).
Methods: This study retrospectively included patients with full-thickness rotator cuff tears who underwent shoulder ARCR surgery in Taizhou hospital, China, between January 2018 and December 2020. Patients received 10 ml (100 mg/ml) of intra-articular TXA injection (TXA group) or 10 ml of normal saline (non-TXA group) after the incision was sutured. The primary variable was the type of drug injected into the shoulder joint at the end of the operation. The primary outcome were perioperative TBL and postoperative pain [measured by visual analog scale (VAS)]. The secondary outcomes were differences in red blood cell count, hemoglobin count, hematocrit, platelet count.
Results: A total of 162 patients were included, 83 patients in TXA group and 79 patients in non-TXA group. Notably, patients in TXA group were more likely to have lower TBL volume [261.21 (175.13–506.67) ml vs. 382.41 (236.11–593.31), P = 0.025], and postoperative VAS score ≤ 2 within 24 h (P = 0.031) compared with those in non-TXA group. In addition, the median hemoglobin count difference was significantly lower in TXA group than that of in non-TXA group (P = 0.045), while, the differences in median counts of red blood cell, hematocrit, and platelet between the two groups were comparable (all P > 0.05).
Conclusion: Intra-articular injection of TXA might reduce the TBL and degree of postoperative pain within 24 h after shoulder arthroscopy.
1. Introduction
Rotator cuff tears (RCTs) are a common shoulder disease that leads to the long-term pain and stiffness of the shoulder joint (1), often requiring rotator cuff repair surgery to restore function and relieve pain in the affected shoulder (2). As concepts and techniques improve, the number of arthroscopic rotator cuff repair (ARCR) interventions is increasing (3). Based on a previous research, it has been long believed that ARCR leads to only minimal blood loss (4). However, recent study by Liu et al. (5) reported that on the first postoperative day the estimated blood loss was approximately 300 ml, and the mean serum hemoglobin decreased by approximately 10 g/L. Thus, the bleeding from the surgical site can still affect the well-being of the patients and should be further discussed.
Tranexamic acid (TXA) is a synthetic analog of the amino acid lysine that competitively blocks the plasminogen lysine binding site, thereby inhibiting fibrinolysis (6). In recent years, TXA has been increasingly used in arthroscopic surgery to reduce intraoperative and postoperative bleeding. Previous studies have shown that intravenous administration of TXA before arthroscopic shoulder surgery could significantly reduce intraoperative bleeding (5), and intra-articular injection of TXA could reduce postoperative intra-articular bleeding on the first postoperative day in patients receiving arthroscopic anterior cruciate ligament construction surgery (7). To the best of our knowledge, no studies on the intra-articular injection of TXA in reducing intra-articular bleeding after ARCR have been published.
This study aimed to evaluate the impact of intra-articular injection with TXA on total blood loss (TBL) and postoperative pain after arthroscopic rotator cuff repair.
2. Methods
2.1. Study design and patients
This retrospective study included patients with RCTs who underwent shoulder arthroscopic surgery in Taizhou hospital, China, between January 2018 and December 2020. Non-probability sampling was used for this study. This retrospective study included all eligible patients with RCTs who underwent shoulder arthroscopic surgery in Taizhou hospital, China, between January 2018 and December 2020. Intra-articular TXA has been routinely administrated since March 2019 (TXA group), while patients before March 2019 were only administered 10 ml of saline (non-TXA group). Therefore, the patients treated between January 2018 and February 2019 were included in the non-TXA group, and those treated from March 2019 to December 2020 were included in the TXA group. Approval of Taizhou hospital ethics committee was obtained before the commencement of this study (K20220713). The informed consent was waived by the ethics committee due to the retrospective nature of the study.
Included patients underwent ARCR after being diagnosed with full-thickness RCTs of the postero-superior cuff by MR examination. Patients with biceps tendon and subscapularis injury were also included in this study. All patients received conservative treatment for more than 3 months without symptom relief. The exclusion criteria were as follows: (1) labral pathology requiring repair; (2) acute traumatic rotator cuff tears; (3) anticoagulation therapy before surgery; (4) abnormal coagulation profile (prothrombin time or activated partial thromboplastin time) before surgery; (5) renal or liver disorders; (6) irreparable massive rotator cuff tear.
To our knowledge, no studies have been published providing recommendations on the optimal intra-articular TXA dosage specifically for shoulder arthroscopic surgery. However, Chiang et al. (7) used the same arthroscopic technique to reconstruct the anterior cruciate ligament. In this study, a 10 ml (concentration:100 mg/ml) of intra-articular injection of TXA was found to reduce postoperative intra-articular bleeding during the first 24 h post-surgery. In addition, it also reduced the pain and the hemarthrosis severity in the early postoperative period. No systemic side effects or toxicity requiring aspiration was noted during the follow-up period. Therefore, we decided to apply the same dosage of TXA in our study.
2.2. Sample size
The sample size was calculated based on the TBL in a pilot study. The pilot TXA group consisted of 10 patients randomly selected from the rotator cuff tears patients treated with intra-articular injection of TXA during shoulder arthroscopic surgery. The pilot non-TXA group consisted of 10 patients randomly selected from the rotator cuff tears patients with intra-articular injection of normal saline. Mean and standard deviation (SD) were calculated for each pilot group. The calculated effect size was estimated using an α equal of 0.05 and a power (1-β) of 0.8. The final calculation effect size was 0.48. Based on this calculation, we determined that at least 70 patients were required for each group to achieve statistically significant results. The G*Power software version 3.1.9.7 was used to calculate the sample size (available at http://www.gpower.hhu.de; Heinrich Heine-University of Dusseldorf, Dusseldorf, Germany).
2.3. Surgery procedure
All patients underwent the shoulder arthroscopic surgery with the same surgical technique and all surgeries were performed by the same surgeons. After receiving general anesthesia combined with interscalene plexus block, all patients were placed in the lateral decubitus position. Normal saline containing epinephrine (1 mg/3 L) was used for intra-articular irrigation. A standardized arthroscopic approach for surgery was used with all patients.
First, an arthroscopic intra-articular examination was performed in all cases to probe whether the patients had other intra-articular lesions. We used 1 to 2 suture anchors (4.5 mm, TWINFIX Ultra PK Suture Anchor, Smith & Nephew) to repair the subscapularis if the subscapularis tendon was torn and recored the type of subscapularis tendon tears. Meanwhile, we performed a biceps tenotomy if the patients had a superior labrum anterior and posterior (SLAP) lesion or degenerative biceps tendon.
Then, via subacromial space arthroscopic vision, we performed acromiolpasty in cases with subacromial impingement syndorme and recored the type of postero-superior cuff tears. The double-row suture-bridge technique was used to repair the cuff tears (4.5 mm, Healix Healix Anchor System, Depuy and 4.5 mm, TWINFIX Ultra PK Suture Anchor, Smith & Nephew). Irreparable type C4 postero-superior cuff tears were eliminated from this study.
At the end of the operation, using a 14G puncture needle, 10 ml of TXA (100 mg/ml) or normal saline was injected into the shoulder joint through a posterior approach.
2.4. Postoperative care
Postoperatively, the operative limb was protected with an abduction brace for at least six weeks. During the duration of the hospital stay, all patients received postoperative intravenous injection of fumiprofen (50 mg, twice daily, Beijing Tide, China) for pain control. Passive movements, such as passive abduction and pendulum movement, began on the first day after surgery. All patients were discharged on the third day after surgery. In the third month after surgery, sports and task-specific exercises were permitted. Full return to sports and overhead activities was not permitted until the sixth month after surgery.
2.5. Data collection and definition
The following clinical parameters were collected: gender, age, height, weight, body mass index (BMI), affected side, systolic blood pressure (SBP), diastolic blood pressure (DBP), arterial pressure, inducement (non, fall down, sports, accident), underlying diseases (hypertension, diabetes), red blood cell count difference, hemoglobin count difference, hematocrit difference, platelet count difference, and intraoperative variables including type of postero-superior cuff tears and subscapularis tears, number of patients underwent acromiolpasty or long head of biceps tendon (LHBT) tenotomy, and duration of the surgery.
Classification of postero-superior cuff tears (C1, C2, C3, C4) and subscapularis tears (type 1, type 2, type 3, type 4, type 5) were performed according to the International Society of Arthroscopy, Knee Surgery and Orthopaedic Sports Medicine (ISAKOS) classification system (8).
2.6. Outcomes
The primary outcomes were TBL and postoperative pain. The TBL was calculated using the following equation (9): TBL = 70 ml × Weight × 2 × (preoperative hematocrit—postoperative hematocrit)/(preoperative hematocrit + postoperative hematocrit). The postoperative pain was measured by visual analog scale (VAS) score. The secondary outcomes were differences in red blood cell count, hemoglobin count, hematocrit, platelet count.
2.7. Statistical analysis
All statistical analysis were performed by SPSS software (version 20.0, license number: 4B6MINO86Z4LZV9AA7GHEC89P5TRNTOHAA3XKX5YW7GM2SWHCCTAFYBL3B3IKPMM7I9N3MSTBXOO8VPKXZHSEXGST8; IBM Corp., Armonk, NY).
2.7.1. Hypothese testing
We hypothesized that the intra-articular injection of TXA can reduce postoperative blood loss and pain. The blood loss was mainly evaluated by measuring the changes after surgery in TBL. The postoperative pain intensity was assessed using the VAS. The differences in the continuous variables between the 2 groups (TXA group and non-TXA group) were tested using the t-test for the normally distributed variables and the rank-sum test for the non-normally distributed variables. The null hypothesis tested is that the patients in TXA group and non-TXA group demonstrate the same postoperative blood loss and pain. The a level was set a priori at.05.
2.7.2. Qualitative data
The qualitative data, including gender, affected side, inducement, underlying diseases, and the supraspinatus and subscapularis tendon tear type, the number of patients who underwent acromioplasty and LHBY tenotomy were presented as percentages and compared using the chi-squared (χ2) test. A P-value below 0.05 was considered statistically significant.
2.7.3. Quantitative data
The quantitative data included age, height, weight, BMI, SBP, DBP, surgery duration, platelet count changes, and the VAS score. The normally distributed data were represented as mean ± standard deviation and compared using the t-test. Conversely, the non-normally distributed data were represented as median (quartile range) and compared using the rank-sum test. A P-value below 0.05 was considered statistically significant.
3. Results
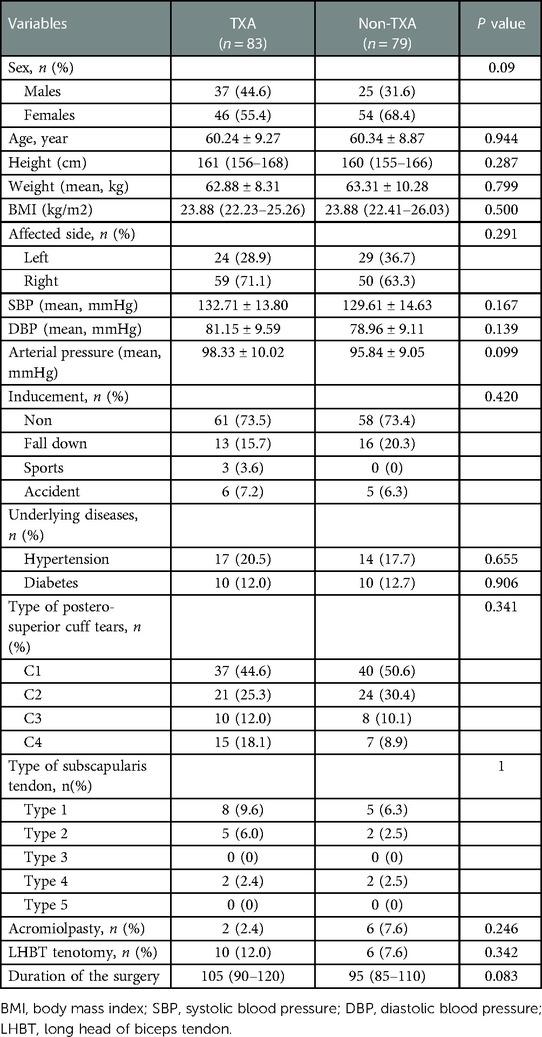
A total of 162 patients were included, with 83 patients in the TXA group and 79 patients in non-TXA group. Patients in TXA group were 60.24 ± 9.27 years old, with BMI of 23.88 (22.23–25.26) kg/m2, and patients in and non-TXA groups were 60.34 ± 8.87 years, with BMI of 23.88 (22.41–26.03) kg/m2. The basic characteristics were comparable between patients in TXA group and non-TXA group (All P > 0.05) (Table 1). Additionally, the intraoperative variables including the type of postero-superior cuff and scapular tendon tears, number of patients underwent acromiolpasty or LHBT tenotomy, and the duration of the surgery were not significantly different between the two groups (All P > 0.05) (Table 1).
Notably, patients in TXA group were more likely to have postoperative VAS pain score ≤ 2 within 24 h (P = 0.031), but not 2 h or 48 h after surgery (Table 2). The TBL volume in TXA group was significantly lower than non-TXA group [261.21 (175.13–506.67) ml vs. 382.41 (236.11–593.31), P = 0.025]. The median hemoglobin count difference before and after surgery was also significantly lower in TXA group than that of in non-TXA group (P = 0.045), while, the differences in median counts of red blood cell, hematocrit, and platelet between the two groups were comparable (all P > 0.05) (Table 2).
No thromboembolic adverse effects, wound complications, or infections were noted in either group at 3 months after surgery. At last follow-up visit, all patients were satisfied with the post-operative shoulder function via the effective rehabilitation. Regrettably, we do not have the detailed clinical outcome scores to correlate with the findings of our investigations.
4. Discussion
This study demonstrated that the TBL volume in patients who received intra-articular TXA injection during shoulder arthroscopy was significantly lower than in those who did not receive the injection. Patients in TXA group were also more likely to have postoperative VAS pain score ≤ 2 within 24 h than in the TXA group, indicating that TXA injection might reduce the TBL and degree of postoperative pain. The null hypothesis was rejected and the alternative hypothesis supported.
Controlling the intra-articular bleeding is important for recovery and prognosis after shoulder arthroscopy surgery. Previous studies have shown that TXA effectively decreased postoperative intra-articular bleeding in arthroscopic knee surgeries, such as partial meniscectomy (10) and anterior cruciate ligament reconstruction (11). However, prospective double-blind study by Li et al. (5) in 2020 reported that intravenous administration of TXA before shoulder arthroscopy significantly improved intra-operative bleeding, but not affected post-operative blood loss, red cell count, hemoglobin or hematocrit. In this study, intra-articular TXA injection during shoulder arthroscopy successfully prevented blood loss and hemoglobin loss—in line with the above knee surgery studies, but slightly different from Li study. To the best of our knowledge, there are no available clinical studies on the effect of intra-articular injection of TXA after ARCR, thus reported results are encouraging and worse to be further tested in the future prospective studies.
Hemarthrosis is considered to affect articular cartilage to a great degree, increasing the risk of postoperative infections, and leading to worse outcomes, especially in the early postoperative period (12, 13). Accordingly, reducing intra-articular bleeding is important to improve the joint mobility and decrease post-operative pain. Study by Nugent et al. (10) compared patients undergoing arthroscopic meniscectomy who received intravenous TXA and normal saline and concluded that TXA could improve early functional recovery and reduce postoperative hemarthrosis of the knee joint without increasing risk. In this study patients in TXA group were more likely to have postoperative VAS pain score ≤ 2 within 24 h after surgery, which was comparable with the results of TXA intravenous administration (6, 11) and intra-articular injection (7) in arthroscopic anterior cruciate ligament reconstruction, suggesting that the reduced amount of postoperative hemarthrosis could reduce the pain in the early postoperative period.
Currently, the drug delivery method for TXA in orthopedic surgical procedures remains under discussion. Although after intravenous administration TXA may efficiently penetrate large joints, it has a relatively short half-life (2 h) and might remain just above the effective plasma concentration for only 4 to 6 h (7). Several systematic meta-analyses have shown that topical (intra-articular) administration of TXA has comparable effectiveness to intravenous delivery in orthopedic surgical procedures (14–17). Moreover, some studies noted that intra-articular TXA administration may have the potential benefits of allowing higher local concentration while preventing the risk of adverse systemic effects (7, 18). Studies by Alshryda et al. (18) and Ahmed et al. (19) concluded that intra-articular TXA provides a greater benefit than intravenous in decreasing postoperative blood loss during arthroplasty. By binding to the lysine binding site of the plasminogen molecule, TXA could lessen the activation of plasminogen to plasmin, thus reducing the dissolution of the fibrin network and ultimately reducing the consumption of platelets needed for hemostasis (20). Therefore, based on the above and results of the present study, it could be suggested that local administration of TXA during shoulder arthroscopy exerts its effect in the early postoperative period without systemic risks.
Numerous studies evaluated the incidence of adverse events after the administration of TXA for arthroscopic surgery. A systematic review by Goldstein et al. found no incidence of deep vein thrombosis (DVT), infection, and arthrofibrosis events associated with the use of TXA perioperatively in arthroscopic surgery of anterior cruciate ligament reconstruction, meniscectomy, femoroacetabular impingement, and rotator curr repair (21, 22). In another systematic review by Sun et al., no wound infections or cardiac, thrombotic, or thromboembolic complications were recorded in patients treated with TXA for arthroscopic surgery (23). Similarly, in our study, no thromboembolic adverse effects, wound complications, or infections were noted in either group at 3 months follow-up. Although a longer follow-up period would have provided more information on the efficacy of TXA, many patients in our study did not attend follow-up appointments after 3 months. However, no thromboembolic adverse effects, wound complications, or infections were noted in the TXA and control groups. Based on these findings, we speculate that some patients may have missed the follow-up appointments because they did not experience any complications after surgery. Therefore based on our findings and those of previous studies, we concluded that intra-articular TXA is safe and effective at reducing postoperative bleeding and pain.
This study provided important clinical guidance of reducing postoperative intra-articular bleeding in patients who underwent ARCR surgery. Nevertheless, this study acknowledges the following limitations. Due to the retrospective nature, some selective bias might have been present. The optimal dosage, route, and timing of TXA application in ARCR surgery remains to be investigated. And, finally, longer follow-up is required to adequately detect adverse reactions to TXA. Further multicenter prospective studies with large sample size are needed to clarify these issues. Moreover, during the initial use of TXA, we only focused on the peri-operative total blood loss (TBL) and pain, as well as post-operative complications. We did not perform a detailed assessment of shoulder function during follow-up. Therefore, we were unable to evaluate the effect of intra-articular injection of TXA on the postoperative shoulder function of patients. This requires a more comprehensive and detailed test plan in the next prospective study.
In conclusion, this study suggested that intra-articular injection of TXA might reduce the TBL and degree of postoperative pain within 24 h after shoulder arthroscopy.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
Ethics statement
The studies involving human participants were reviewed and approved by Approval of Taizhou hospital ethics committee was obtained before the commencement of this study (K20220713). Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Author contributions
RZ and HJ: carried out the studies, participated in collecting data, and drafted the manuscript. LS: performed the statistical analysis and participated in its design. WX: participated in acquisition, analysis, or interpretation of data and draft the manuscript. All authors contributed to the article and approved the submitted version.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Novoa-Boldo A, Gulotta LV. Expectations following rotator cuff surgery. Curr Rev Musculoskelet Med. (2018) 11:162–6. doi: 10.1007/s12178-018-9470-7
2. Dougherty KA, Dilisio MF, Agrawal DK. Vitamin d and the immunomodulation of rotator cuff injury. J Inflamm Res. (2016) 9:123–31. doi: 10.2147/JIR.S106206
3. Huberty DP, Schoolfield JD, Brady PC, Vadala AP, Arrigoni P, Burkhart SS. Incidence and treatment of postoperative stiffness following arthroscopic rotator cuff repair. Arthroscopy. (2009) 25:880–90. doi: 10.1016/j.arthro.2009.01.018
4. Griffiths D, Jagiello J, Dheerendra S, Mcnamara C, Ahrens P. Blood loss in arthroscopic shoulder surgery: is it clinically important? Shoulder Elbow. (2013) 5:231–4. doi: 10.1111/sae.12032
5. Liu YF, Hong CK, Hsu KL, Kuan FC, Chen Y, Yeh ML, et al. Intravenous administration of tranexamic acid significantly improved clarity of the visual field in arthroscopic shoulder surgery. A prospective, double-blind, and randomized controlled trial. Arthroscopy. (2020) 36:640–7. doi: 10.1016/j.arthro.2019.10.020
6. Camarasa MA, Olle G, Serra-Prat M, Martin A, Sanchez M, Ricos P, et al. Efficacy of aminocaproic, tranexamic acids in the control of bleeding during total knee replacement: a randomized clinical trial. Br J Anaesth. (2006) 96:576–82. doi: 10.1093/bja/ael057
7. Chiang ER, Chen KH, Wang ST, Ma HL, Chang MC, Liu CL, et al. Intra-articular injection of tranexamic acid reduced postoperative hemarthrosis in arthroscopic anterior cruciate ligament reconstruction: a prospective randomized study. Arthroscopy. (2019) 35:2127–32. doi: 10.1016/j.arthro.2019.02.018
8. Calvo E, Rebollon C, Itoi E, Imhoff A, Savoie FH, Arce G. Reliable interobserver and intraobserver agreement of the international society of arthroscopy, knee surgery and orthopaedic sports medicine (isakos) classification system of rotator cuff tears. J Isakos. (2022) 7:56–61. doi: 10.1016/j.jisako.2021.12.004
9. Good L, Peterson E, Lisander B. Tranexamic acid decreases external blood loss but not hidden blood loss in total knee replacement. Br J Anaesth. (2003) 90:596–9. doi: 10.1093/bja/aeg111
10. Nugent M, May JH, Parker JD, Kieser DC, Douglas M, Pereira R, et al. Does tranexamic acid reduce knee swelling and improve early function following arthroscopic meniscectomy? A double-blind randomized controlled trial. Orthop J Sports Med. (2019) 7:1810913546. doi: 10.1177/2325967119866122
11. Felli L, Revello S, Burastero G, Gatto P, Carletti A, Formica M, et al. Single intravenous administration of tranexamic acid in anterior cruciate ligament reconstruction to reduce postoperative hemarthrosis and increase functional outcomes in the early phase of postoperative rehabilitation: a randomized controlled trial. Arthroscopy. (2019) 35:149–57. doi: 10.1016/j.arthro.2018.07.050
12. Salzler MJ, Lin A, Miller CD, Herold S, Irrgang JJ, Harner CD. Complications after arthroscopic knee surgery. Am J Sports Med. (2014) 42:292–6. doi: 10.1177/0363546513510677
13. Bahl V, Goyal A, Jain V, Joshi D, Chaudhary D. Effect of haemarthrosis on the rehabilitation of anterior cruciate ligament reconstruction–single bundle versus double bundle. J Orthop Surg Res. (2013) 8:5. doi: 10.1186/1749-799X-8-5
14. Chen TP, Chen YM, Jiao JB, Wang YF, Qian LG, Guo Z, et al. Comparison of the effectiveness and safety of topical versus intravenous tranexamic acid in primary total knee arthroplasty: a meta-analysis of randomized controlled trials. J Orthop Surg Res. (2017) 12:11. doi: 10.1186/s13018-017-0512-4
15. Sun X, Dong Q, Zhang YG. Intravenous versus topical tranexamic acid in primary total hip replacement: a systemic review and meta-analysis. Int J Surg. (2016) 32:10–8. doi: 10.1016/j.ijsu.2016.05.064
16. Zhang P, Liang Y, Chen P, Fang Y, He J, Wang J. Intravenous versus topical tranexamic acid in primary total hip replacement: a meta-analysis. Medicine. (2016) 95:e5573. doi: 10.1097/MD.0000000000005573
17. Wang H, Shen B, Zeng Y. Comparison of topical versus intravenous tranexamic acid in primary total knee arthroplasty: a meta-analysis of randomized controlled and prospective cohort trials. Knee. (2014) 21:987–93. doi: 10.1016/j.knee.2014.09.010
18. Alshryda S, Sukeik M, Sarda P, Blenkinsopp J, Haddad FS, Mason JM. A systematic review and meta-analysis of the topical administration of tranexamic acid in total hip and knee replacement. Bone Joint J. (2014) 96-B:1005–15. doi: 10.1302/0301-620X.96B8.33745
19. Ahmed S, Ahmed A, Ahmad S, Javed S, Aziz A. Blood loss after intraarticular and intravenous tranexamic acid in total knee arthroplasty. J Pak Med Assoc. (2018) 68:1434–7.30317337
20. Tengborn L, Blomback M, Berntorp E. Tranexamic acid–an old drug still going strong and making a revival. Thromb Res. (2015) 135:231–42. doi: 10.1016/j.thromres.2014.11.012
21. Goldstein K, Jones C, Kay J, Shin J, de Sa D. Tranexamic acid administration in arthroscopic surgery is a safe adjunct to decrease postoperative pain and swelling: a systematic review and meta-analysis. Arthroscopy. (2022) 38:1366–77. doi: 10.1016/j.arthro.2021.10.001
22. Belk JW, McCarty EC, Houck DA, Dragoo JL, Savoie FH, Thon SG. Tranexamic acid use in knee and shoulder arthroscopy leads to improved outcomes and fewer hemarthrosis-related complications: a systematic review of level i and ii studies. Arthroscopy. (2021) 37:1323–33. doi: 10.1016/j.arthro.2020.11.051
Keywords: rotator cuff tear, arthroscopic rotator cuff repair, tranexamic acid, blood loss, pain, retrospective study
Citation: Zhu R, Jiang H, Xu W, Shen L and Jin G (2023) Impact of intra-articular injection with tranexamic acid on total blood loss and postoperative pain after arthroscopic rotator cuff repair surgery. Front. Surg. 10:1052039. doi: 10.3389/fsurg.2023.1052039
Received: 23 September 2022; Accepted: 6 February 2023;
Published: 23 February 2023.
Edited by:
Jaimo Ahn, University of Michigan, United States© 2023 Zhu, Jiang, Xu, Shen and Jin. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Gang Jin amluZ0BlbnplbWVkLmNvbQ==
Specialty Section: This article was submitted to Orthopedic Surgery, a section of the journal Frontiers in Surgery
 Rangteng Zhu1
Rangteng Zhu1 Hantao Jiang
Hantao Jiang Gang Jin
Gang Jin