
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Med. , 15 December 2021
Sec. Precision Medicine
Volume 8 - 2021 | https://doi.org/10.3389/fmed.2021.776876
This article is part of the Research Topic Women in Science - Precision Medicine 2021 View all 9 articles
Our previous study manifested that lanthanum chloride (LaCl3) can enhance the anticancer ability of cisplatin (DDP) in ovarian cancer cells. Here, ovarian cancer cells SKOV3 and SKOV3/DDP were subjected to DDP and LaCl3. Cell viability, apoptosis, DNA repair, and PI3K/Akt pathway were detected. LaCl3 induced more cell death and apoptosis caused by DDP in two cell lines, accompanied by upregulation of Bax and Cleaved caspase 3 proteins, and downregulation of Bcl-2 protein. LaCl3 also could decrease RAD51 protein by inactivation of the PI3K/Akt pathway. These data indicated that LaCl3 could be a potential drug to modulate DDP resistance by inactivating of PI3K/Akt pathway and attenuating DNA repair in ovarian cancer.
Ovarian cancer is the most lethal gynecologic cancer. The first-line treatment for ovarian cancer is cisplatin (DDP)-based chemotherapy after cytoreductive surgery. However, the DDP resistance of ovarian cancer during the treatment is an important reason for the treatment failure. The 5-years survival rate is <40% (1, 2).
Cisplatin (DDP) often attacks DNA to cause DNA damage and lead to cell apoptosis; therefore, an enhanced DNA repair plays a key role in DDP resistance (3, 4). Survival pathways were necessary for cell survival and involved in chemoresistance. The PI3K/Akt survival pathway was a way to play an important role in cell survival and DDP resistance in ovarian cancer. Akt can activate the cyclin D1, NF-kB, mTOR, RAD51 or inhibit the caspase 9, p21, p27 to inhibit cell apoptosis (5–7). Lots of research target the DNA repair and PI3K/Akt pathway to explore new drugs to reverse the DDP resistance and improve the prognosis of ovarian cancer (8, 9). However, there are still no effective therapies due to the side effect. Therefore, it is important to explore novel drugs to conquer the resistance of DDP.
Lanthanum chloride (LaCl3) is a complex of rare earth elements. Recent studies have reported that LaCl3 can inhibit the proliferation and migration of cancer cells and can be an effective drug to kill cancer cells (10–12). Our previous study indicated that LaCl3 could augment the anticancer ability of DDP (13, 14). However, the mechanisms of LaCl3 acts on ovarian cancer remain unclear.
Research showed that LaCl3 can downregulate the PI3K/Akt signaling pathway to cause cytotoxicity; and LaCl3 can inactivate the Akt signaling pathway to induce autophagy (15, 16). Considering the important role of the PI3K/ Akt pathway in DDP resistance. We suggest that LaCl3 may reverse DDP resistance via PI3K/Akt pathway. Therefore, in this study, the relationship of LaCl3 and PI3K/Akt was explored using DDP sensitive and DDP resistance ovarian cancer cells. Preliminary data showed that LaCl3 could inactivate the PI3K/Akt pathway to inhibit DNA repair, eventually enhancing the antitumor ability of DDP in ovarian cancer.
Human ovarian cancer cell lines SKOV3 and SKOV3/DDP (identified by STR; Cell bank, Type Culture Collect., Chin. Sci., Shanghai, China) were cultured in RPIM-1640 (Gibco, Beijing, China) supplemented with 10% fetal bovine serum (Biological Industries, Israel), at 37°C and 5% CO2. SKOV3/DDP was a resistance subline of SKOV3 that grew in 0.75 μg/ml of DDP (Yunnan Phytopharm., Kunming, China). Cells were cultured with a DDP-free medium for 5 days before experiments to avoid interferences caused by residual DDP (17, 18).
Cells were seeded in a 96-well plate (5,000 cells per well) and exposed to DDP (0, 1, 2, 4, 8, 16, 32, and 64 μmol/L) or exposed to LaCl3 (0, 0.5, 1, 1.5, 2, 2.5, and 3 μmol/L) for 48 h. Cell viability was determined with a CCK-8 assay (MedChemExpress, United States). The half-maximal inhibition concentration (IC50) of DDP was calculated by the probit regression. The IC50 of DDP and maximum unharmful concentration of LaCl3 were used in the following experiments.
Subsequently, cells were subjected to DDP (IC50) combined with LaCl3 (1.5 μmol/L) for 24, 48, and 72 h, and cell viability was determined.
Proteins were extracted after cells were exposed to DDP (IC50) and/or LaCl3 (1.5 μmol/L) for 48 h using RIPA buffer (Beyotime, Chongqing, China) supplemented with phenylmethanesulfonyl fluoride. Proteins were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred to a polyvinylidene fluoride membrane (Merck Millipore, Billerica, MA). Primary antibodies as follow: anti-PI3K/p-PI3K (catalog numbers: bs-0128R, bs-332R) (Bioss Biotech., Beijing, China), anti-Akt/p-Akt (catalog numbers: 4691T, 4060T) (Cell Signaling Technology, USA), anti-Bcl2 (catalog numbers: 4223S) (Cell Signaling Technology), anti-Bax (catalog numbers: 5023S) (Bioss Biotech., Beijing, China), anti-Cleaved caspase 3 (catalog numbers: 9661T) (Cell Signaling Technology, USA), anti-RAD51 (catalog numbers: ab133534) (Abcam, UK), and anti-β-actin (catalog numbers: 66009-1-lg) (Proteintech, Wuhan, China). The secondary antibody was a goat anti-rabbit IgG antibody (catalog numbers: 7076S) (Cell Signal. Technol.). β-actin was the reference.
Apoptosis cells were detected using an Annexin V assay (Keygen Biotech., Nanjing, China) after Cells were treated with DDP (IC50) and/or LaCl3 (1.5 μmol/L) for 48 h.
Cells were exposed to SC79 (an activator of Akt: 4 μg/mL;) (MedChemExpress) co-culture with DDP (IC50) and/or LaCl3 (1.5 μmol/L) for 48 h. Then CCK8 was used to detect the survival cells, and western blot was used to detect the RAD51 protein.
Data were processed with the software SPSS 26 (IBM, Armonk, United States). ANOVA and t-test were performed. The difference was significant if p < 0.05.
The IC50 values of DDP were 5 and 15 μmol/L for SKOV3 and SKOV3/DDP cells, respectively, confirming the resistance phenotype of SKOV3/DDP (Figure 1A). The percentages of survival cells were more than 90% in SKOV3 and SKOV3/DDP cells after exposure to LaCl3 (0.5, 1, and 1.5 μmol/L). However, the percentage of survival cells was <90% following exposure to higher concentrations of LaCl3 (≥ 2 μmol/L), and the percentage of dead cells in SKOV3/DDP cells was higher than in SKOV3 (p =0.032, p = 0.001) (Figure 1B). Therefore, 1.5 μmol/L of LaCl3 was used in the subsequent experiments.
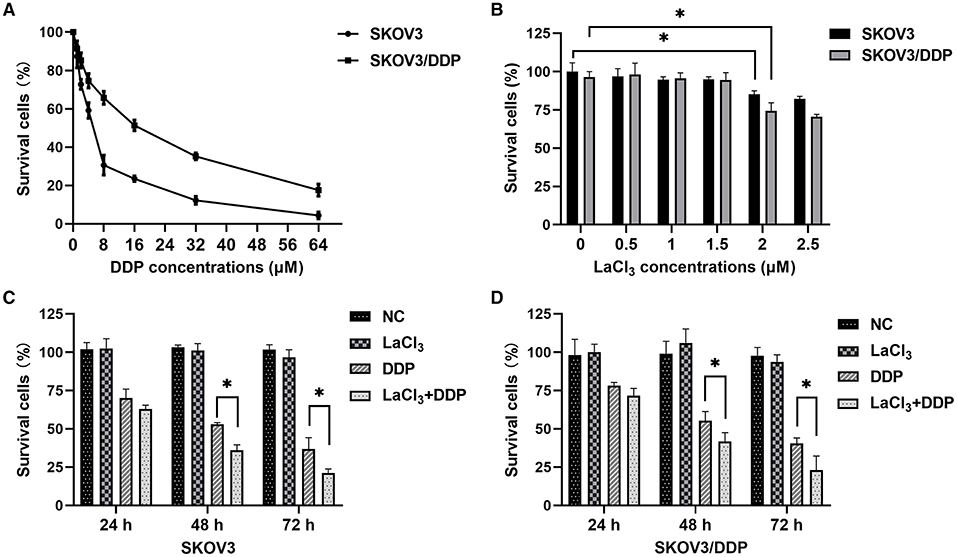
Figure 1. LaCl3 promoted cell death caused by DDP in ovarian cancer cells (n = 3). Percentages of survival cells after DDP treatment; higher IC50 values were noted and confirmed the resistance phenotype of SKOV3/DDP (A). Percentages of survival cells after LaCl3 treatment; the percentages more than 90% after exposure to LaCl3 (0.5, 1, and 1.5 μmol/L), and <90% following exposure to higher concentrations of LaCl3 (≥2 μmol/L) (B). LaCl3 (1.5 μmol/L) decreased the survival percentage in SKOV3 cells following DDP (5.0 μmol/L) treatment (C). LaCl3 (1.5 μmol/L) decreased the survival percentage in SKOV3/DDP cells following DDP (15 μmol/L) treatment (D). *p < 0.05.
The percentage of the dead cells was increased in SKOV3 cells following exposure to DDP combined with LaCl3 compared with DDP alone (p = 0.001–0.025) (Figure 1C). In SKOV3/DDP cells, we observed the same results (p = 0.013–0.026) (Figure 1D). These results indicated that LaCl3 enhanced the cytotoxicity of DDP.
DDP caused SKOV3 and SKOV3/DDP cell apoptosis, and the combination of LaCl3 and DDP led to a higher percentage of apoptotic SKOV3 and SKOV3/DDP cells (p = 0.005, p = 0.001) (Figure 2).
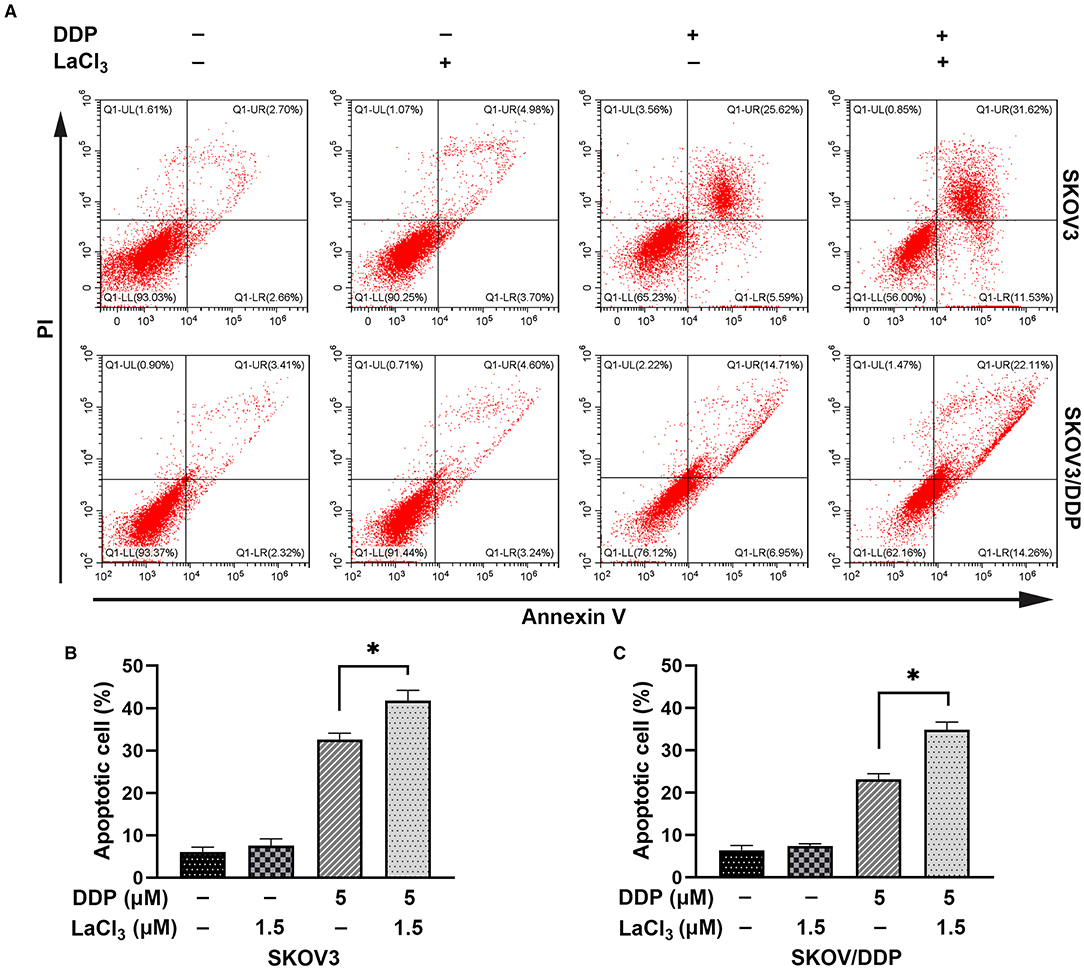
Figure 2. LaCl3 promoted cell apoptosis caused by DDP in ovarian cancer cells (n = 3). Apoptosis was detected by the annexin V assay; the apoptotic percentage in SKOV3 cells was increased after DDP combined with LaCl3 (A,B). The apoptotic percentage in SKOV3/DDP cells was increased after DDP combined with LaCl3 (A,C). *p < 0.05.
Subsequently, apoptosis-related proteins Bax, Bcl-2, and Cleaved-caspase 3 were determined. In SKOV3 and SKOV3/DDP cells, the levels of Bax and Cleaved-caspase 3 were increased after treatment with LaCl3 and DDP (p = 0.045, p = 0.046, p = 0.001, and p = 0.002) (Figure 3); the Bcl-2 was decreased in SKOV3 and SKOV3/DDP cells after treatment with LaCl3 and DDP (p = 0.043, p = 0.017) (Figure 3). These data indicated that LaCl3 promoted cell apoptosis due to DDP both in SKOV3 and SKOV3/DDP cells.
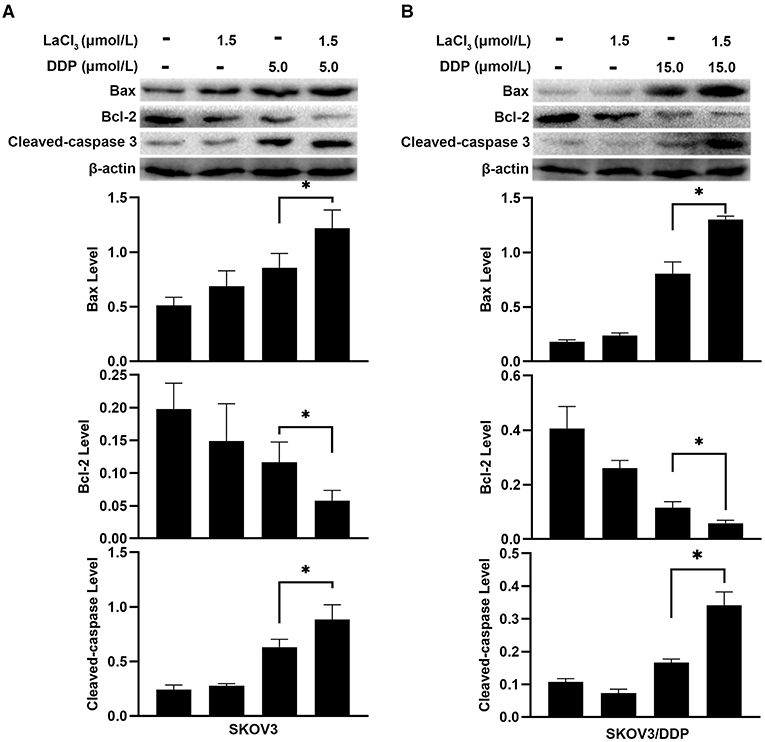
Figure 3. LaCl3 regulated apoptosis-related proteins with DDP in ovarian cancer cells (n = 3). DDP induced the expression of Bax and Cleaved-caspase 3, LaCl3 further increased them; the Bcl-2 was decreased following LaCl3 and DDP exposure in SKOV3 cells (A) and in SKOV3/DDP cells (B). *p < 0.05.
DNA repair was assayed by detecting RAD51 since RAD51 is a key molecule for homologous recombination (HR) (19). Recent studies indicated that the PI3K/Akt can induce the expression of RAD51 (7, 20). Western blot was used to detect the expression of RAD51 and PI3K/Akt. DDP increased the RAD51 level, which means DDP not only induced DNA damage but also initiated DNA repair. However, LaCl3 decreased the level of RAD51 in both cell lines (p = 0.002, p = 0.004) (Figures 4A,B). DDP induced the phosphorylation of PI3K/Akt, the levels of p-PI3K and p-Akt were decreased in two cell lines following LaCl3 exposure (p = 0.04, p = 0.042, p = 0.011, p = 0.001) (Figures 4C,D).
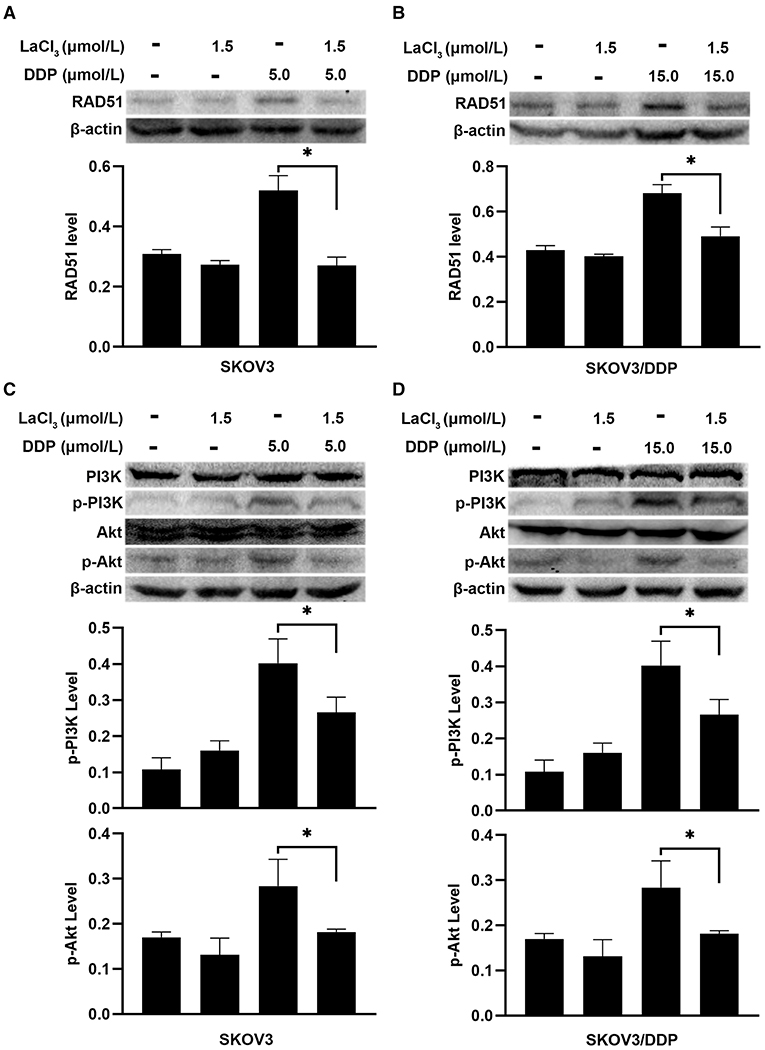
Figure 4. LaCl3 inhibited DNA repair by inactivation of PI3K/Akt pathway in ovarian cancer cells (n = 3). DDP induced the expression of RAD51, LaCl3 inhibited its expression in SKOV3 cells (A) and SKOV3/DDP cells (B). The level of p-PI3K and p-Akt was increased after DDP exposure, and such an inductive effect was inhibited in SKOV3 cells (C) and SKOV3/DDP cells (D) following LaCl3 exposure. *p < 0.05.
To further demonstrate the action of PI3K/Akt on RAD51, the SC79 (an activator of Akt) was added in SKOV3 and SKOV3/DDP cells. The level of RAD51 was upregulated in both cells after exposure to SC79 (p = 0.045, p = 0.011) (Figures 5A,B). The percentages of survival cells were increased after exposure to SC79 in SKOV3 and SKOV3/DDP cells (p = 0.034, p = 0.035) (Figures 5C,D). The data indicated that LaCl3 could inhibit DNA repair via PI3K/Akt pathway and promote the action of DDP.
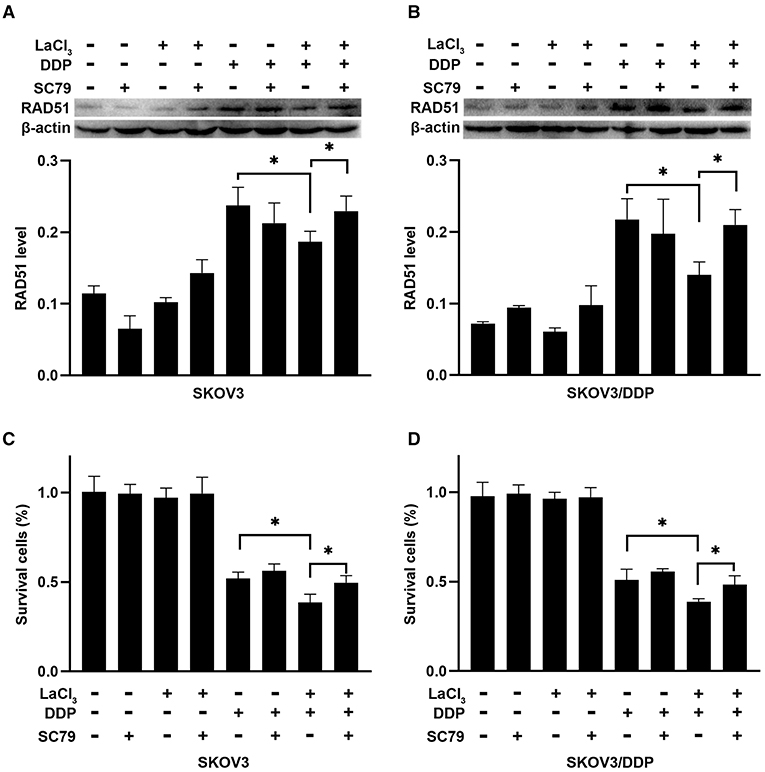
Figure 5. SC79 attenuated the effect of LaCl3 combined with DDP in ovarian cancer cells (n = 3). The RAD51 was upregulated in SKOV3 cells (A) and SKOV3/DDP cells (B) following SC79 (4 μg/mL) exposure. Percentages of survival cells were increased in SKOV3 cells (C) and SKOV3/DDP cells (D) following SC79 treatment. *p < 0.05.
DDP resistance is a key obstacle for the treatment of ovarian cancer. Hence, it is of particular importance to explore new drugs to reverse DDP resistance. Here, we demonstrated that LaCl3 (≥2 μmol/L) caused cell death in SKOV3 and SKOV3/DDP cells. Interestingly, the percentage of dead cells in SKOV3/DDP cells was higher than in SKOV3 (p = 0.032, p = 0.001), and this needs further study to validate that the LaCl3 was more lethal on DDP resistant cells in the future. However, the lower concentration of LaCl3 (1.5 μmol/L) that was unharmful to ovarian cancer cells could increase cell death due to DDP. This was consistent with our previous study that LaCl3 can enhance the cytotoxicity of DDP in ovarian cancer cells (13).
Cytotoxicity of DDP was regulated by apoptosis (21, 22). Hence, enhancing cell apoptosis was the main target for drugs to reverse DDP resistance (23). The combination of DDP and LaCl3 led to the highest apoptotic percentage in SKOV3 and SKOV3/DDP cells. Then, the Bax and Cleaved caspase 3 were most expressed in both cells following DDP and LaCl3 exposure, and Bcl-2 was the least. These were consistent with the previous study that LaCl3 can regulate the protein expressions of Akt, Bcl-2, Bcl-xl, Bax, Bad, caspase-3, and caspase-9 to promote cell apoptosis (24); and indicated that LaCl3 promote cell apoptosis caused by DDP to conquer DDP resistance.
The main target of DDP is DNA, DDP attacks DNA to cause a break, and unrepair damage leads to apoptosis. RAD51 is the key protein of HR for repairing DNA damage (25, 26). In this study, the level of RAD51 was upregulated following DDP exposure, which meant DDP inducing DSB, then starting DNA repair. This possibly explains the reason that the cytotoxicity of DDP was decreased during treatment and eventually led to DDP resistance. However, the RAD51 level was decreased after LaCl3 exposure. These were consistent with the results of the percentages of dead and apoptotic cells following DDP and LaCl3 exposure and consistent with previous studies that conquer DDP resistance by inhibiting DNA repair (27). Our results primarily indicated that LaCl3 can enhance apoptosis by inhibiting DNA repair.
The PI3K/Akt pathway is an important survival pathway that is involved in DDP resistance in ovarian cancer (28). Here, the phosphorylation of PI3K and Akt was activated by DDP, but the inductive effect of DDP was attenuated after LaCl3 exposure. These results were consistent with previous studies that LaCl3 can inhibit the PI3K/Akt pathway to cause cytotoxicity (15, 16). Activation of Akt can increase the expression of RAD51, while inactivation of Akt downregulates the level of RAD51 to enhance cell apoptosis caused by DNA-damaging drugs (29). Hence, the SC79 (an activator of Akt) was added to neutralize the inhibiting effect of LaCl3, the level of RAD51 was increased that caused by DDP combined with LaCl3 exposure, while the percentages of dead cells were deceased. The data demonstrated that LaCl3 inactivated PI3K/Akt pathway to downregulate the expression of RAD51.
In conclusion, the LaCl3 could attenuate the DDP resistance of ovarian cancer cells via inhibiting PI3K/Akt pathway, downregulated RAD51 to inhibit DNA repair, and eventually promoted cell apoptosis due to DDP. Thus, LaCl3 can be a potential drug for the treatment of ovarian cancer and DDP resistance.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
SF performed the experiments. SF and PZ drafted the manuscript. XC and FL checked the manuscript. FW designed the study and checked the manuscript. All authors have given approval to the final version of the manuscript.
This work was supported by the Natural Science Foundation of Science and Technology Department of Jiangxi Province (20192BAB205076) and Natural Science Foundation of Jiangxi Province (20202BABL206101).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2021.776876/full#supplementary-material
1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. (2019) 69:7–34. doi: 10.3322/caac.21551
2. Christie EL, Bowtell DDL. Acquired chemotherapy resistance in ovarian cancer. Ann Oncol. (2017) 28:viii13–viii15. doi: 10.1093/annonc/mdx446
3. Damia G, Broggini M. Platinum resistance in ovarian cancer: Role of DNA Repair. Cancers (Basel). (2019) 11:119. doi: 10.3390/cancers11010119
4. Helleday T. Homologous recombination in cancer development, treatment and development of drug resistance. Carcinogenesis. (2010) 31:955–60. doi: 10.1093/carcin/bgq064
5. Luo J, Yao JF, Deng XF, Zheng XD, Jia M, Wang YQ, et al. 14, 15-EET induces breast cancer cell EMT and cisplatin resistance by up-regulating integrin αvβ3 and activating FAK/PI3K/AKT signaling. J Exp Clin Cancer Res. (2018) 37:23. doi: 10.1186/s13046-018-0694-6
6. Brasseur K, Gévry N, Asselin E. Chemoresistance and targeted therapies in ovarian and endometrial cancers. Oncotarget. (2017) 8:4008–42. doi: 10.18632/oncotarget.14021
7. Ko JC, Chen JC, Wang TJ, Zheng HY, Chen WC, Chang PY, et al. Astaxanthin down-regulates Rad51 expression via inactivation of AKT kinase to enhance mitomycin C-induced cytotoxicity in human non-small cell lung cancer cells. Biochem Pharmacol. (2016) 105:91–100. doi: 10.1016/j.bcp.2016.02.016
8. Wang B, Hou D, Liu Q, Wu T, Guo H, Zhang X, et al. Artesunate sensitizes ovarian cancer cells to cisplatin by downregulating RAD51. Cancer Biol Ther. (2015) 16:1548–56. doi: 10.1080/15384047.2015.1071738
9. Cao WQ, Zhai XQ, Ma JW, Wu T, Guo H, Zhang X, et al. Natural borneol sensitizes human glioma cells to cisplatin-induced apoptosis by triggering ROS-mediated oxidative damage and regulation of MAPKs and PI3K/AKT pathway. Pharm Biol. (2020) 58:72–9. doi: 10.1080/13880209.2019.1703756
10. Zhang Z, Wang J, Li J, Xu S. Telomerase-mediated apoptosis of chicken lymphoblastoid tumor cell line by lanthanum chloride. Biol Trace Elem Res. (2011) 144:657–67. doi: 10.1007/s12011-011-9027-8
11. Yu L, Xiong J, Guo L, Miao L, Liu S, Guo F. The effects of lanthanum chloride on proliferation and apoptosis of cervical cancer cells: involvement of let-7a and miR-34a microRNAs. Biometals. (2015) 28:879–90. doi: 10.1007/s10534-015-9872-6
12. Dai Y, Li J, Li J, Yu L, Dai G, Hu A, et al. Effects of rare earth compounds on growth and apoptosis of leukemic cell lines. In Vitro Cell Dev Biol Anim. (2002) 38:373–5. doi: 10.1290/1071-2690(2002)038<0373:EORECO>2.0.CO;2
13. Wang F, Zhu Y, Fang S, Li S, Liu S. Lanthanum chloride enhances cisplatin-induced apoptosis in ovarian cancer cells. Cell Mol Biol (Noisy-le-grand). (2016) 62:1–5.
14. Wang F, Zhu Y, Fang S, Li S, Liu S. Effect of lanthanum chloride on tumor growth and apoptosis in human ovarian cancer cells and xenograft animal models. Exp Ther Med. (2018) 16:1143–8. doi: 10.3892/etm.2018.6299
15. Gao X, Yang J, Li Y, Yu M, Liu S, Han Y, et al. Lanthanum chloride induces autophagy in rat hippocampus through ROS-mediated JNK and AKT/mTOR signaling pathways. Metallomics. (2019) 11:439–53. doi: 10.1039/C8MT00295A
16. Zheng L, Zhang J, Yu S, Ding Z, Song H, Wang Y, et al. Lanthanum chloride causes neurotoxicity in rats by upregulating miR-124 expression and targeting PIK3CA to regulate the PI3K/Akt signaling pathway. Biomed Res Int. (2020) 2020:5205142. doi: 10.1155/2020/5205142
17. Fang S, Luo Y, Zhang Y, Wang H, Liu Q, Li X, et al. NTNG1 Modulates cisplatin resistance in epithelial ovarian cancer cells via the GAS6/AXL/Akt Pathway. Front Cell Dev Biol. (2021) 9:652325. doi: 10.3389/fcell.2021.652325
18. Liu Q, Zhong X, Zhang Y, Li X, Qian G, Yu T. Ultrasound enhances ZD2767P-carboxypeptidase G2 against chemoresistant ovarian cancer cells by altering the intracellular pharmacokinetics of ZD2767D. Mol Pharm. (2020) 17:1922–32. doi: 10.1021/acs.molpharmaceut.0c00008
19. Laurini E, Marson D, Fermeglia A, Aulic S, Fermeglia M, Pricl S. Role of Rad51 and DNA repair in cancer: A molecular perspective. Pharmacol Ther. (2020) 208:107492. doi: 10.1016/j.pharmthera.2020.107492
20. Philip CA, Laskov I, Beauchamp MC, Marque M, Amin O, Bitharas J, et al. Inhibition of PI3K-AKT-mTOR pathway sensitizes endometrial cancer cell lines to PARP inhibitors. BMC Cancer. (2017) 17:638. doi: 10.1186/s12885-017-3639-0
21. Kong F, Liu X, Zhou Y, Hou X, He J, Li Q, et al. Downregulation of METTL14 increases apoptosis and autophagy induced by cisplatin in pancreatic cancer cells. Int J Biochem Cell Biol. (2020) 122:105731. doi: 10.1016/j.biocel.2020.105731
22. Ghosh S. Cisplatin: The first metal based anticancer drug. Bioorg Chem. (2019) 88:102925. doi: 10.1016/j.bioorg.2019.102925
23. Qian G, Dai L, Yu T. Thioridazine sensitizes cisplatin against chemoresistant human lung and ovary cancer cells. DNA Cell Biol. (2019) 38:718–24. doi: 10.1089/dna.2019.4715
24. Wang J, Wu T, Ma L, Guo Y, Huang Y, Zheng L. Action of akt pathway on la-induced hippocampal neuron apoptosis of rats in the growth stage. Neurotox Res. (2020) 38:434–46. doi: 10.1007/s12640-020-00206-z
25. He L, Luo L, Zhu H, et al. FEN1 promotes tumor progression and confers cisplatin resistance in non-small-cell lung cancer. Mol Oncol. (2017) 11:640–54. doi: 10.1002/1878-0261.12058
26. Zhang C, Chen L, Peng D, et al. METTL3 and N6-methyladenosine promote homologous recombination-mediated repair of DSBs by modulating DNA-RNA hybrid accumulation. Mol Cell. (2020) 79:425–442.e7. doi: 10.1016/j.molcel.2020.06.017
27. Xu L, Sun Z, Wei X, et al. The inhibition of MARK2 suppresses cisplatin resistance of osteosarcoma stem cells by regulating DNA damage and repair. J Bone Oncol. (2020) 23:100290. doi: 10.1016/j.jbo.2020.100290
28. Ma H, Cao W, Ding M. MicroRNA-31 weakens cisplatin resistance of medulloblastoma cells via NF-κB and PI3K/AKT pathways. Biofactors. (2020) 46:831–8. doi: 10.1002/biof.1616
Keywords: lanthanum chloride, cisplatin resistance, ovarian cancer, PI3K, Akt
Citation: Fang S, Zhang P, Chen X, Liu F and Wang F (2021) Lanthanum Chloride Sensitizes Cisplatin Resistance of Ovarian Cancer Cells via PI3K/Akt Pathway. Front. Med. 8:776876. doi: 10.3389/fmed.2021.776876
Received: 14 September 2021; Accepted: 15 November 2021;
Published: 15 December 2021.
Edited by:
Alice Chen, National Cancer Institute, United StatesReviewed by:
Zhi Shi, Jinan University, ChinaCopyright © 2021 Fang, Zhang, Chen, Liu and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Fen Wang, MjcyNTI0Mzg3QHFxLmNvbQ==
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.