- 1Fourth Affiliated Hospital, Zhejiang University School of Medicine, Yiwu, China
- 2Department of Obstetrics and Gynecology, The Second Affiliated Hospital of Zhejiang Chinese Medical University, Hangzhou, China
- 3Zhejiang University School of Medicine, Hangzhou, China
- 4Women's Hospital, Zhejiang University School of Medicine, Hangzhou, China
- 5Department of Gynecology, First Affiliated Hospital, Heilongjiang University of Chinese Medicine, Harbin, China
- 6Heilongjiang Province Hospital, Harbin, China
Objective: To evaluate the effect of hyperinsulinemia (HI) and insulin resistance (IR) on endocrine, metabolic, and reproductive outcomes in women without polycystic ovary syndrome (PCOS) undergoing assisted reproduction.
Materials and Methods: The study included 1,104 non-PCOS women undergoing in vitro fertilization/intracytoplasmic sperm injection-fresh embryo transfer. HI was evaluated by serum fasting insulin (FIN), and IR was evaluated by homeostatic model assessment of insulin resistance index (HOMA-IR). In addition, biometric, sex hormone, and metabolic parameters were measured. Independent t-test, linear, and logistic regression examined associations between HI, IR, and endocrine, metabolic, ovarian stimulation characteristics, and reproductive outcomes.
Results: Women with HI and IR had lower levels of progesterone, luteinizing hormone, follicle-stimulating hormone, estradiol, high-density lipoproteins, and increased levels of triglycerides low-density lipoproteins. For ovarian stimulation characteristics, those with HI and IR had a longer duration of stimulation, a higher total gonadotropin dose, and a lower peak estradiol level. Linear regression confirmed these associations. For reproductive outcomes, HI and IR were not associated with clinical pregnancy, live birth, and miscarriage.
Conclusions: HI and IR did not impair reproductive outcomes in non-PCOS women undergoing assisted reproduction.
Introduction
Infertility and assisted reproduction technology (ART) are associated with considerable emotional and financial burdens. However, it is usually difficult to predict the results of assisted reproduction because it is influenced by multiple factors such as the social, psychological, and physical status of the patient (1–3).
Hyperinsulinemia (HI) and insulin resistance (IR) status have been well-documented to be associated with an increased risk of type 2 diabetes, hypertension, cardiovascular disease, and non-alcoholic fatty liver disease (4–6). Insulin has also been suggested to play an important role in female reproductive health and follicle development. Periovulatory insulin signaling is essential for ovulation, cell differentiation of the granulosa, and female fertility (7). An in vitro study found that IR could decrease steroidogenesis and increase apoptosis of human granulosa cells (8). Preimplantation exposure to high concentrations of insulin-like growth factor I results in lower implantation rates in mice (9). Elevated insulin levels could compromise mice decidualization in early-stage pregnancy (10). Previous studies suggested that IR was an independent risk factor for spontaneous abortion and recurrent pregnancy loss (11, 12).
IR and HI are found more frequently in women with polycystic ovary syndrome (PCOS) (13, 14). HI and IR can lead to worse endocrine, metabolic, and fertility outcomes in women with PCOS who underwent ovulation induction (15). Preconception impaired glucose tolerance was independently associated with adverse pregnancy outcomes in women with PCOS who underwent fresh or frozen embryo transfer (16). A systematic review found that IR is a risk factor for spontaneous abortion in women with PCOS who underwent assisted reproductive treatment (17).
Despite all of the above, the evidence for the effect of HI and IR on women without PCOS is limited. Therefore, we designed this study to investigate associations between HI, IR, and endocrine, metabolic, and reproductive outcomes in a cohort of women who did not have PCOS.
Materials and Methods
Study Design
This was a retrospective cohort study of subjects from January 2018 to October 2020 in a center for reproductive medicine of Women's Hospital, Zhejiang University School of Medicine in China. The study was approved by the ethics committee of the hospital (IRB-20200235-R) and was conducted in accordance with the Declaration of Helsinki. Because this was a retrospective study and only de-identified data were analyzed, the ethics committee waived the standard requirement of informed consent from the women in the study.
Study Population
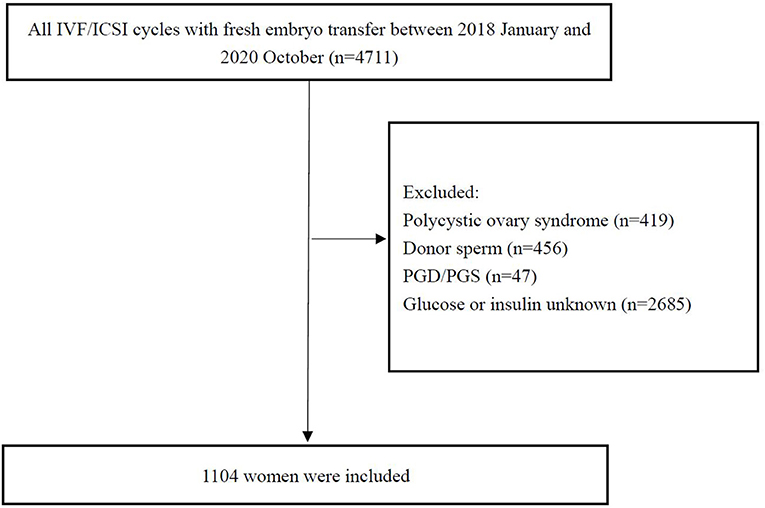
Patients undergoing the first in vitro fertilization (IVF)/intracytoplasmic sperm injection (ICSI) cycle with fresh embryo transfer from January 2018 to October 2020 were recruited in the study. Briefly, the inclusion criteria were the following: (1) women 18–45 years old; (2) fresh embryo transfer and IVF/ICSI; and (3) fasting insulin (FIN) and glucose (FG) evaluated before treatment. The exclusion criteria were (1) women with the diagnosis of PCOS: PCOS was diagnosed by Rotterdam criteria [two out of three of oligo-amenorrhea, biochemical or clinical hyperandrogenism, and polycystic ovaries on transvaginal ultrasound (18)]; (2) the use of an egg or sperm donor; (3) preimplantation genetic diagnosis or screening; (4) lack of FIN and glucose evaluation. Data from 4,711 women were initially screened, of which 1,104 were included in the final analysis. A flow chart of the study procedure is shown in Figure 1.
At the initial evaluation, age, height, weight, systolic blood pressure (SBP), and diastolic blood pressure (DBP) were measured, and body mass index (BMI) was calculated. Reproductive data included infertility diagnosis, primary infertility, and duration of infertility. Blood samples were collected after an overnight fast on Day 2–4 of the menstrual cycle prior to the beginning of pituitary down-regulation. Anti-Mullerian hormone (AMH), basal follicle-stimulating hormone (FSH), basal luteinizing hormone (LH), basal estradiol (E2), basal progesterone (P), basal testosterone (T), basal prolactin (PRL), total cholesterol (TC), low-density lipoprotein (LDL), high-density lipoprotein (HDL), triglycerides (TG), FG, and FIN were measured. Insulin resistance assessment of the homeostasis model (HOMA-IR) was calculated using the formula [HOMA-IR = (FIN × FG)/22.5]. HI was defined as the upper 10 percentile of FIN in this current cohort. IR was defined as HOMA-IR >2.69 according to a previous study (19).
Laboratory Assessment
FSH, LH, E2, T, and PRL were measured by chemiluminescence using a Roche E801 analyzer (Roche, Switzerland) according to the manufacturer's protocol. TC, HDL, LDL, and TG were measured using a Beckman AU5800 chemistry analyzer (Beckman Coulter, United States) according to the manufacturer's protocol. FG was measured using a Beckman AU5800 chemistry analyzer (Beckman Coulter, United States). The intra-assay laboratory coefficients of variation for FG ranged from 0.97 to 1.03%, and the between-assay coefficients of variation ranged from 1.40 to 2.96%. Finally, FIN was measured using an Abbott i2000SR immunoassay analyzer (Abbott, United States). The intra-assay laboratory coefficients of variation for FIN ranged from 1.75 to 1.80%, and the between-assay coefficients of variation ranged from 1.14 to 1.45%.
Intervention
In the short-acting gonadotrophin-releasing hormone agonist protocol (GnRH-a) (Triptorelin, Ferring AG, Germany), GnRH-a was administered daily in the mid-luteal phase until trigger. After 14 days, serum LH, FSH, and E2 were measured, and recombinant FSH (Gonal-F, Merck Serono, Switzerland) was administered daily when FSH and LH were < 5 IU/L and E2 was < 50 pg/mL. In the gonadotrophin-releasing hormone antagonist (GnRH-A) (Cetrorelix, Merck Serono, France) protocol, recombinant FSH was administered on Day 2 or 3 of the menstrual cycle until the trigger, and GnRH-A was used daily when the leading follicles reached a mean diameter of 14 mm until the trigger. In both protocols, when three follicles reached a mean diameter of 17 mm, or two follicles reached a mean diameter of 18 mm, recombinant hCG (Ovidrel, Serono, Italy) was administered subcutaneously. Oocyte retrieval was performed 36 h after recombinant hCG injection. The retrieved oocytes were inseminated by conventional IVF, ICSI, or IVF/ICSI (50% IVF and 50% ICSI). The embryos were cultured in G1-plus medium (Vitrolife, Switzerland) at 37°C in an incubator with 6% carbon dioxide. Up to 2 embryos were transferred at the cleavage stage on Day 3 after fertilization.
Outcome Measures
The outcomes of ovarian stimulation were the duration of ovarian stimulation, the total dose of gonadotropin, the peak E2, the endometrial thickness, the number of retrieved oocytes, the number of two-pronuclear embryos (2PN), and the number of embryo transfers. Reproductive outcomes were live birth, clinical pregnancy, and miscarriage. Live birth was defined as the delivery of a viable infant after 28 weeks of gestation. Clinical pregnancy was confirmed by visualization of at least one gestational sac on ultrasound. Miscarriage was defined as a pregnancy loss before 28 weeks of gestation (20).
Statistical Analysis
SPSS statistics 24.0 (IBM) was used to analyze the data. Means and standard deviations, frequencies, and percentages derived from demographic and reproductive characteristics data were calculated. The independent t-test was used to compare the means between groups. Linear regression was used to evaluate associations between FIN, HOMA-IR, and outcomes of endocrine, metabolic, and ovarian stimulation before and after adjustment for age and BMI. Effects were described as coefficients β with a 95% confidence interval (CI). Logistic regression analyses were used to identify potential effects of FIN and HOMA-IR on clinical pregnancy, live birth, and miscarriage before and after adjusting for age and BMI. The effects were described as odds ratios (OR) with 95% CI. All reported p-values are two-sided, and p < 0.05 was considered statistically significant.
Results
Baseline Characteristics
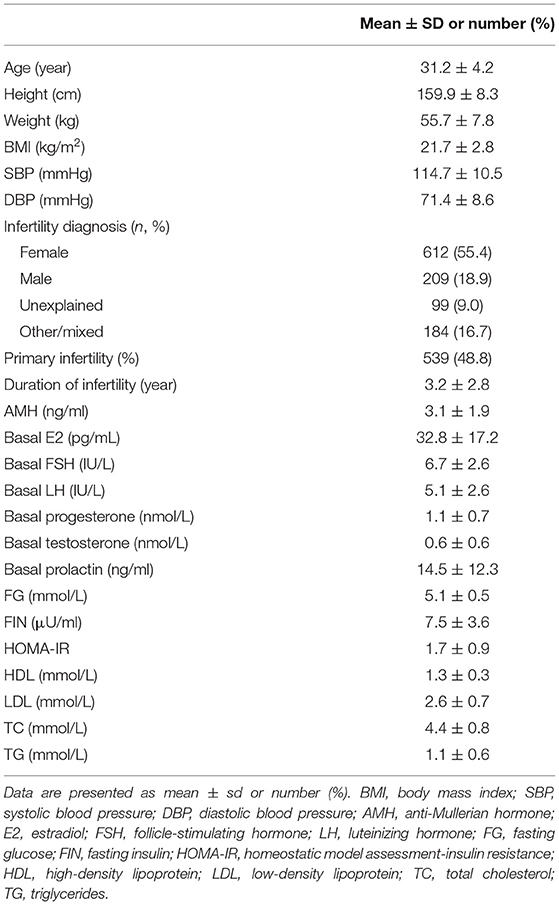
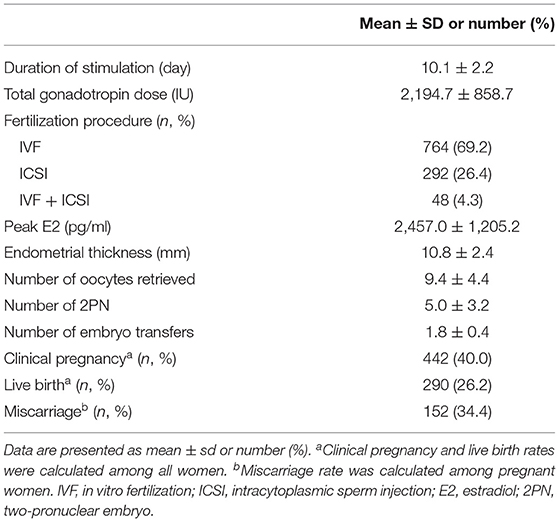
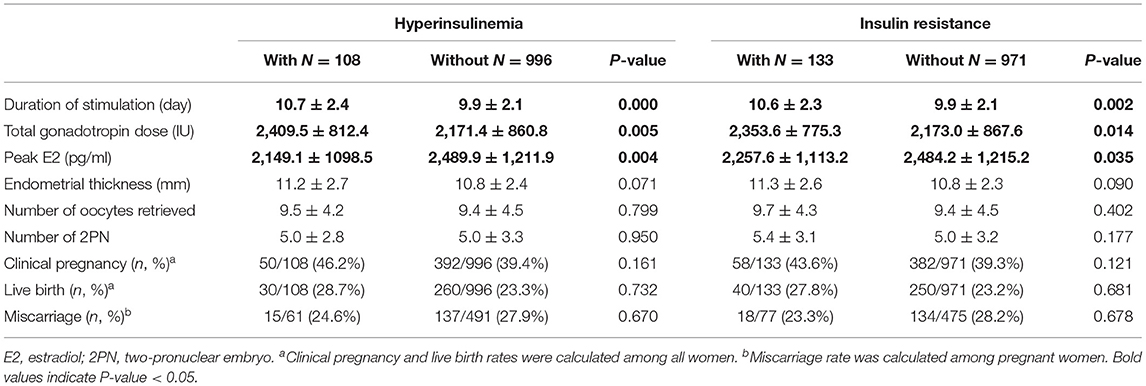
The baseline characteristics of the 1,104 women included in the study are shown in Table 1. The mean age was 31.2 ± 4.2 years, and the mean BMI was 21.7 ± 2.8. Infertility diagnosis, duration of infertility, primary infertility, AMH, basal E2, LH, FSH, P, T, PRL, TC, LDL, HDL, TG, glucose, FIN, and HOMA-IR were also reported in Table 1. The mean FIN and HOMA-IR were 7.5 ± 3.6 μU/ml and 1.7 ± 0.9. HI and IR were identified in 108 (9.8%) and 133 (12.0%) women. In addition, the duration of ovarian stimulation, the total dose of gonadotropin, the peak level of E2, the thickness of the endometrial, the type of fertilization, the number of retrieved oocytes, the number of 2PN, and the number of embryo transfers were listed in Table 2. A total of 442 women (40.0%) achieved clinical pregnancy, 290 (26.2%) had live births, and 152 (34.4%) had miscarriages.
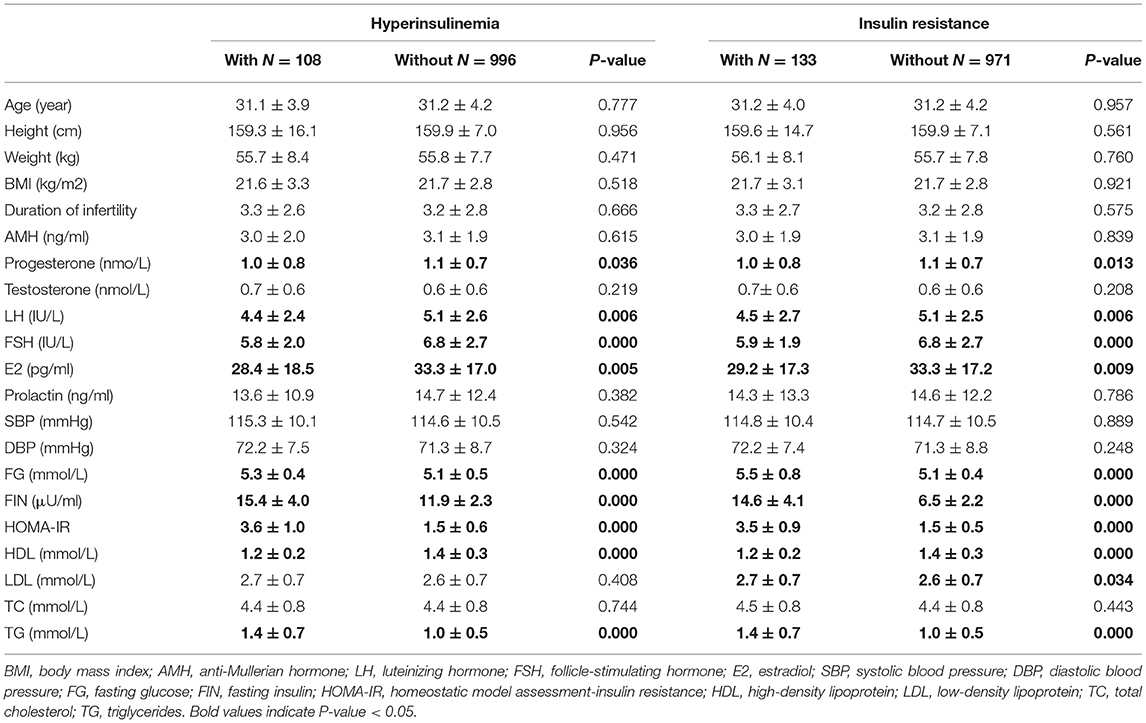
Baseline clinical and biochemical characteristics based on the presence or absence of HI and IR are presented in Table 3. Women with HI had significantly lower P, LH, FSH, and E2 levels than women without HI. Similarly, IR was associated with lower levels of P, LH, FSH, and E2. AMH and PRL were not associated with HI and IR (Table 3).
The results also demonstrated that women with HI had significantly higher levels of FG and TG and lower HDL levels. Similarly, women with IR had significantly higher levels of FG, LDL, and TG and lower HDL levels. Blood pressure and TC did not have an association with HI and IR (Table 3).
Characteristics of Ovarian Stimulation and Reproductive Outcomes
The results in Table 4 demonstrated that women with HI had a significantly longer duration of stimulation and higher total gonadotropin dose but a lower peak level of E2. Similarly, women with IR had a significantly longer duration of stimulation and higher total gonadotropin dose but a lower peak E2. Endometrial thickness, number of retrieved oocytes, number of 2PN, clinical pregnancy, miscarriage, and live birth rates were not statistically different between women with and without HI and IR.
Linear Regression
FIN and HOMA-IR were viewed as continuous variables in linear regression to further explore the association with HI and IR. FIN was positively associated with T, FG, and TG levels, while negatively associated with P, LH, FSH, E2, and HDL levels. HOMA-IR was positively associated with T, FG, LDL, and TG levels, while negatively associated with P, LH, FSH, E2, and HDL levels. After adjusting for age and BMI, these associations still existed (Supplementary Table 1). For ovarian stimulation and reproductive outcomes, FIN and HOMA-IR were both positively associated with stimulation duration and total gonadotropin dose and negatively associated with peak E2. These associations were still significant after adjustment for age and BMI (Supplementary Table 2).
Logistic Regression
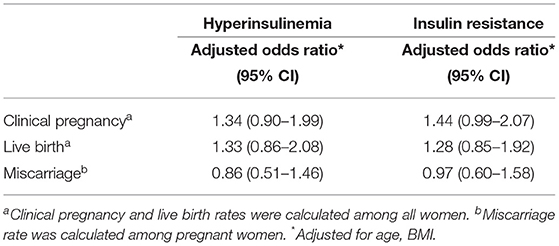
Logistic regression showed that HI and IR were not associated with clinical pregnancy, live birth, and miscarriage (Table 5). Furthermore, when viewed as a continuous variable, FIN and HOMA-IR did not have associations with clinical pregnancy, live birth, and miscarriage (Supplementary Table 3).
Discussion
In the current retrospective cohort study of women without PCOS who underwent assisted reproduction, HI and IR were associated with worse sex hormone and metabolic parameters. In addition, HI and IR were also associated with poorer cycle stimulation characteristics, including higher gonadotropin requirements, longer duration of gonadotropin stimulation, and lower peak E2 level. However, HI and IR did not affect reproductive outcomes including pregnancy, live birth, and miscarriage.
As expected, HI and IR were associated with metabolic hormones and sex hormones, which is similar in women with PCOS (15). HI and IR are characterized by abnormal secretion of sex hormones and insulin response. IR and HI can promote the liver secretion of very-low-density lipoproteins and activate liver endothelial lipase that promote HDL decomposition and therefore decrease HDL (21). Insulin inhibits the activation of TGlipase (a key rate-limiting enzyme for lipid mobilization), resulting in increased lipid mobilization and TG synthesis (22). HI and IR are also associated with the function of the hypothalamic-pituitary-ovary axis. Mice with conditional knockout of the insulin receptor in neurons show impaired maturation of the ovarian follicle due to hypothalamic dysregulation of LH (23). We did not find an association between HI, IR, and testosterone, but previous studies suggested that insulin is associated with concentrations of sex hormone-binding globulins, which might affect bioavailability of androgens (24).
In PCOS, enlarged ovarian volume and excess follicles were associated with IR (25). In women without PCOS, the total follicle count was significantly higher in those with a HOMA-IR >2.5, and HOMA-IR was positively correlated with the total follicle count in this group of women (26). IR was a predictor of ovarian hyperstimulation syndrome in women with non-PCOS (27). Our results showed that HI and IR were associated with a longer duration of ovarian stimulation, a higher total gonadotropin dose, and a lower peak E2 level, but not with the number of retrieved oocytes. Together, these findings suggested that HI and IR might inhibit folliculogenesis by suppressing FSH and increasing resistance to gonadotropin in follicle development (28). Although we did not find an association between HI, IR, and endometrial thickness, increasing the dose of gonadotropin and a longer duration of stimulation might indicate impaired endometrial receptivity.
HI and IR lead to poor reproductive outcomes in women with PCOS undergoing ovulation induction and assisted reproduction (15, 17). This could partly explain why drugs for controlling blood glucose could possibly improve reproductive outcomes in women with PCOS (29, 30). However, the current study found that HI and IR were not associated with clinical pregnancy rate, live birth rate, and miscarriage rate in women without PCOS. Although some studies reported that metabolic and ovarian stimulation outcomes might negatively affect reproductive outcomes (31–38), the current study did not recommend treating HI and IR for non-PCOS women undergoing assisted reproduction. However, the women in our study were relatively young, lean, and had a lower prevalence of HI and IR. Therefore, these associations still need research in different populations with a higher BMI.
Some previous studies have investigated clinical predictors of the starting dose of recombinant FSH in the assisted reproduction procedure. For example, a study found that obese women require significantly higher amounts of gonadotropins to achieve similar success rates in IVF compared to normal weight women (39). Another study used age and ovarian reserve markers to optimize the initial dose of recombinant FSH, leading to a more customized initial dose and improved cost-effectiveness. But for women with high AMH, it does not appear adequate (40). Our results indicated that IR and HI might help construct a better algorithm to decide the starting dose of controlled ovarian stimulation.
HI and IR are routinely monitored in clinical settings to assess diabetes and cardiovascular diseases. Considering the key role of IR in women with PCOS, insulin sensitizers, including metformin and inositol isoforms, can benefit women with PCOS due to their safety profile and effectiveness (41–43). With the modern obesity epidemic [44], the prevalence of HI and IR is believed to be increasing, even in women without PCOS. The current study suggests that there may be benefits in evaluating HI and IR status for young, lean women without PCOS before assisted reproduction treatment. Future clinical studies should focus on whether pretreatment improving HI and IR status is beneficial for women without PCOS.
The main strength of the current study was the large sample size. The observed associations remained significant after adjustment for age and BMI. But it also had several limitations. One is the design of the retrospective study. Although the large sample size, the adjustments made, and the multivariate regression analysis conducted, the presence of confounding bias cannot be completely excluded. Many women were excluded from the whole cohort due to the lack of glucose and insulin measurements, which could decrease the generalizability of our conclusion. Additionally, our population studied was relatively young and lean; more research is needed in specific subpopulations of patients with more frequent HI and IR, such as more obese women. Finally, as only a single measure of preconception serum insulin and glucose levels was available, it was impossible to investigate associations between HI and IR in sequential cycles and the cumulative live birth rate.
The results of the current study suggested that HI and IR impaired endocrine, metabolic, and ovarian stimulation outcomes but not reproductive outcomes in non-PCOS women undergoing assisted reproduction. However, more prospective studies are needed to confirm these associations.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
Ethics Statement
The studies involving human participants were reviewed and approved by Ethics Committee of the Women's Hospital, School of Medicine, Zhejiang University. The ethics committee waived the requirement of written informed consent for participation.
Author Contributions
JX designed the study and critically revised the manuscript. W-YC and XL performed data analysis and drafted the manuscript. W-YC, XL, JS, CD, and WW collected data. DJ and JZ revised the manuscript. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2021.736320/full#supplementary-material
References
1. Wu AK, Elliott P, Katz PP, Smith JF. Time costs of fertility care: the hidden hardship of building a family. Fertil Steril. (2013) 99:2025–30. doi: 10.1016/j.fertnstert.2013.01.145
2. Rooney KL, Domar AD. The relationship between stress and infertility. Dialogues Clin Neurosci. (2018) 20:41–7. doi: 10.31887/DCNS.2018.20.1/klrooney
3. Kushnir VA, Barad DH, Albertini DF, Darmon SK, Gleicher N. Systematic review of worldwide trends in assisted reproductive technology 2004-2013. Reprod Biol Endocrinol. (2017) 15:6. doi: 10.1186/s12958-016-0225-2
4. Després JP, Lamarche B, Mauriège P, Cantin B, Dagenais GR, Moorjani S, et al. Hyperinsulinemia as an independent risk factor for ischemic heart disease. N Engl J Med. (1996) 334:952–7. doi: 10.1056/NEJM199604113341504
5. Zavaroni I, Bonini L, Gasparini P, Barilli AL, Zuccarelli A, Dall'Aglio E, et al. Hyperinsulinemia in a normal population as a predictor of non-insulin-dependent diabetes mellitus, hypertension, and coronary heart disease: the barilla factory revisited. Metabolism. (1999) 48:989–94. doi: 10.1016/S0026-0495(99)90195-6
6. Facchini FS, Hua N, Abbasi F, Reaven GM. Insulin resistance as a predictor of age-related diseases. J Clin Endocrinol Metabo. (2001) 86:3574–8. doi: 10.1210/jcem.86.8.7763
7. Sekulovski N, Whorton AE, Shi M, Hayashi K, MacLean JA. Periovulatory insulin signaling is essential for ovulation, granulosa cell differentiation, and female fertility. FASEB J. (2020) 34:2376–91. doi: 10.1096/fj.201901791R
8. Belani M, Shah P, Banker M, Gupta S. Dual effect of insulin resistance and cadmium on human granulosa cells - In vitro study. Toxicol Appl Pharmacol. (2016) 313:119–30. doi: 10.1016/j.taap.2016.10.019
9. Pinto AB, Schlein AL, Moley KH. Preimplantation exposure to high insulin-like growth factor I concentrations results in increased resorption rates in vivo. Human Rep. (2002) 17:457–62. doi: 10.1093/humrep/17.2.457
10. Zhang C, Yang C, Li N, Liu X, He J, Chen X, et al. Elevated insulin levels compromise endometrial decidualization in mice with decrease in uterine apoptosis in early-stage pregnancy. Arch Toxicol. (2019) 93:3601–15. doi: 10.1007/s00204-019-02601-8
11. Tian L, Shen H, Lu Q, Norman RJ, Wang J. Insulin resistance increases the risk of spontaneous abortion after assisted reproduction technology treatment. J Clin Endocrinol Metab. (2007) 92:1430–3. doi: 10.1210/jc.2006-1123
12. Craig LB, Ke RW, Kutteh WH. Increased prevalence of insulin resistance in women with a history of recurrent pregnancy loss. Fertil Steril. (2002) 78:487–90. doi: 10.1016/S0015-0282(02)03247-8
13. Escobar-Morreale HF. Polycystic ovary syndrome: definition, aetiology, diagnosis and treatment. Nat Rev Endocrinol. (2018) 14:270–84. doi: 10.1038/nrendo.2018.24
14. DeUgarte CM, Bartolucci AA, Azziz R. Prevalence of insulin resistance in the polycystic ovary syndrome using the homeostasis model assessment. Fertil Steril. (2005) 83:1454–60. doi: 10.1016/j.fertnstert.2004.11.070
15. Zhang D, Yang X, Li J, Yu J, Wu X. Effect of hyperinsulinaemia and insulin resistance on endocrine, metabolic and fertility outcomes in women with polycystic ovary syndrome undergoing ovulation induction. Clin Endocrinol. (2019) 91:440–8. doi: 10.1111/cen.14050
16. Wei D, Zhang B, Shi Y, Zhang L, Zhao S, Du Y, et al. Effect of preconception impaired glucose tolerance on pregnancy outcomes in women with polycystic ovary syndrome. J Clin Endocrinol Metab. (2017) 102:3822–9. doi: 10.1210/jc.2017-01294
17. Sun Y-F, Zhang J, Xu Y-M, Cao Z-Y, Wang Y-Z, Hao G-M, et al. High BMI and insulin resistance are risk factors for spontaneous abortion in patients with polycystic ovary syndrome undergoing assisted reproductive treatment: a systematic review and meta-analysis. Front Endocrinol. (2020) 11:592495. doi: 10.3389/fendo.2020.592495
18. Rotterdam ESHRE/ASRM-Sponsored PCOS consensus workshop group. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome (PCOS). Hum Reprod. (2004) 19:41–7. doi: 10.1093/humrep/deh098
19. Li R, Zhang Q, Yang D, Li S, Lu S, Wu X, et al. Prevalence of polycystic ovary syndrome in women in China: a large community-based study. Hum Reprod. (2013) 28:2562–9. doi: 10.1093/humrep/det262
20. Shi Y, Sun Y, Hao C, Zhang H, Wei D, Zhang Y, et al. Transfer of fresh versus frozen embryos in ovulatory women. N Engl J Med. (2018) 378:126–36. doi: 10.1056/NEJMoa1705334
21. Nikkilä EA, Kuusi T, Harno K, Tikkanen M, Taskinen MR, editors. Lipoprotein Lipase Hepatic Endothelial Lipase are Key Enzymes in the Metabolism of Plasma High Density Lipoproteins. New York, NY: Springer US (1980).
22. Sozen I, Arici A. Hyperinsulinism and its interaction with hyperandrogenism in polycystic ovary syndrome. Obstet Gynecol Surv. (2000) 55:321–8. doi: 10.1097/00006254-200005000-00026
23. Brüning JC, Gautam D, Burks DJ, Gillette J, Schubert M, Orban PC, et al. Role of brain insulin receptor in control of body weight and reproduction. Science. (2000) 289:2122–5. doi: 10.1126/science.289.5487.2122
24. Wallace IR, McKinley MC, Bell PM, Hunter SJ. Sex hormone binding globulin and insulin resistance. Clin Endocrinol. (2013) 78:321–9. doi: 10.1111/cen.12086
25. Hong S-H, Sung Y-A, Hong YS, Jeong K, Chung H, Lee H. Polycystic ovary morphology is associated with insulin resistance in women with polycystic ovary syndrome. Clin Endocrinol. (2017) 87:375–80. doi: 10.1111/cen.13380
26. Dickerson EH, Cho LW, Maguiness SD, Killick SL, Robinson J, Atkin SL. Insulin resistance and free androgen index correlate with the outcome of controlled ovarian hyperstimulation in non-PCOS women undergoing IVF. Hum Reprod. (2010) 25:504–9. doi: 10.1093/humrep/dep393
27. Nikbakht R, Zargar M, Moramezi F, Ziafat M, Tabesh H, Sattari AR, et al. Insulin resistance and free androgen as predictors for ovarian hyperstimulation syndrome in non-PCOS women. Horm Metab Res. (2020) 52:104–8. doi: 10.1055/a-1079-5342
28. Franks S, Stark J, Hardy K. Follicle dynamics and anovulation in polycystic ovary syndrome. Hum Reprod Update. (2008) 14:367–78. doi: 10.1093/humupd/dmn015
29. Salamun V, Jensterle M, Janez A, Vrtacnik Bokal E. Liraglutide increases IVF pregnancy rates in obese PCOS women with poor response to first-line reproductive treatments: a pilot randomized study. Eur J Endocrinol. (2018) 179:1–11. doi: 10.1530/EJE-18-0175
30. Morley LC, Tang T, Yasmin E, Norman RJ, Balen AH. Insulin-sensitising drugs (metformin, rosiglitazone, pioglitazone, D-chiro-inositol) for women with polycystic ovary syndrome, oligo amenorrhoea and subfertility. Cochrane Database Syst Rev. (2017) 11:CD003053. doi: 10.1002/14651858.CD003053.pub6
31. Bacchetti T, Morresi C, Vignini A, Tiano L, Orlando P, Montik N, et al. HDL functionality in follicular fluid in normal-weight and obese women undergoing assisted reproductive treatment. J Assist Reprod Genet. (2019) 36:1657–64. doi: 10.1007/s10815-019-01523-9
32. Browne RW, Bloom MS, Shelly WB, Ocque AJ, Huddleston HG, Fujimoto VY. Follicular fluid high density lipoprotein-associated micronutrient levels are associated with embryo fragmentation during IVF. J Assist Reprod Genet. (2009) 26:557–60. doi: 10.1007/s10815-009-9367-x
33. Kim K, Bloom MS, Browne RW, Bell EM, Yucel RM, Fujimoto VY. Associations between follicular fluid high density lipoprotein particle components and embryo quality among in vitro fertilization patients. J Assist Reprod Genet. (2017) 34:1–10. doi: 10.1007/s10815-016-0826-x
34. Nagy RA, van Montfoort APA, Groen H, Homminga I, Andrei D, Mistry RH, et al. Anti-oxidative function of follicular fluid HDL and outcomes of modified natural cycle-IVF. Sci Rep. (2019) 9:12817. doi: 10.1038/s41598-019-49091-3
35. Cai W-Y, Luo X, Chen E, Lv H, Fu K, Wu X-K, et al. Serum lipid levels and treatment outcomes in women undergoing assisted reproduction: a retrospective cohort study. Front Endocrinol. (2021) 12:633766. doi: 10.3389/fendo.2021.633766
36. Munch EM, Sparks AE, Zimmerman MB, Van Voorhis BJ, Duran EH. High FSH dosing is associated with reduced live birth rate in fresh but not subsequent frozen embryo transfers. Hum Reprod. (2017) 32:1402–9. doi: 10.1093/humrep/dex094
37. Joo BS, Park SH, An BM, Kim KS, Moon SE, Moon HS. Serum estradiol levels during controlled ovarian hyperstimulation influence the pregnancy outcome of in vitro fertilization in a concentration-dependent manner. Fertil Steril. (2010) 93:442–6. doi: 10.1016/j.fertnstert.2009.02.066
38. Zhang W, Tian Y, Xie D, Miao Y, Liu J, Wang X. The impact of peak estradiol during controlled ovarian stimulation on the cumulative live birth rate of IVF/ICSI in non-PCOS patients. J Assist Reprod Genet. (2019) 36:2333–44. doi: 10.1007/s10815-019-01568-w
39. Burnik Papler T, Vrtačnik Bokal E, Prosenc Zmrzljak U, Stimpfel M, Laganà AS, Ghezzi F, et al. PGR and PTX3 gene expression in cumulus cells from obese and normal weighting women after administration of long-acting recombinant follicle-stimulating hormone for controlled ovarian stimulation. Arch Gynecol Obstet. (2019) 299:863–71. doi: 10.1007/s00404-018-5031-y
40. Di Paola R, Garzon S, Giuliani S, Laganà AS, Noventa M, Parissone F, et al. Are we choosing the correct FSH starting dose during controlled ovarian stimulation for intrauterine insemination cycles? Potential application of a nomogram based on woman's age and markers of ovarian reserve. Arch Gynecol Obstet. (2018) 298:1029–35. doi: 10.1007/s00404-018-4906-2
41. Sharpe A, Morley LC, Tang T, Norman RJ, Balen AH. Metformin for ovulation induction (excluding gonadotrophins) in women with polycystic ovary syndrome. Cochrane Database Syst Rev. (2019) 12:CD013505. doi: 10.1002/14651858.CD013505
42. Paul C, Laganà AS, Maniglio P, Triolo O, Brady DM. Inositol's and other nutraceuticals' synergistic actions counteract insulin resistance in polycystic ovarian syndrome and metabolic syndrome: state-of-the-art and future perspectives. Gynecol Endocrinol. (2016) 32:431–8. doi: 10.3109/09513590.2016.1144741
Keywords: assisted reproduction, insulin resistance, hyperinsulinemia, metabolic, ovarian stimulation
Citation: Cai W-Y, Luo X, Song J, Ji D, Zhu J, Duan C, Wu W, Wu X-K and Xu J (2022) Effect of Hyperinsulinemia and Insulin Resistance on Endocrine, Metabolic, and Reproductive Outcomes in Non-PCOS Women Undergoing Assisted Reproduction: A Retrospective Cohort Study. Front. Med. 8:736320. doi: 10.3389/fmed.2021.736320
Received: 05 July 2021; Accepted: 29 November 2021;
Published: 07 January 2022.
Edited by:
Erol Tavmergen, Ege University, TurkeyReviewed by:
Antonio Simone Laganà, University of Insubria, ItalySvend Lindenberg, Copenhagen Fertility Center, Denmark
Nazan Yurtcu, Sivas Cumhuriyet University Faculty of Medicine, Turkey
Copyright © 2022 Cai, Luo, Song, Ji, Zhu, Duan, Wu, Wu and Xu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jian Xu, eHVqQHpqdS5lZHUuY24=
†These authors share first authorship
 Wang-Yu Cai
Wang-Yu Cai Xi Luo
Xi Luo Jianyuan Song
Jianyuan Song Danpin Ji2
Danpin Ji2 Wei Wu
Wei Wu Jian Xu
Jian Xu