- 1Department of Hematology/Oncology, University Children's Hospital Basel, Basel, Switzerland
- 2Intercontinental Cooperative Immune thrombocytopenia (ITP) Study Group, Basel, Switzerland
- 3Lymphoma Unit, Oncology Institute of Southern Switzerland, Bellinzona, Switzerland
- 4Centre de Référence des Cytopénies Auto-Immunes de l'Adulte, Service de Médecine Interne, CHU Henri Mondor, AP-HP, Université Paris-Est Créteil, Créteil, France
- 5Jane Anne Nohl Division of Hematology, Department of Medicine, Keck School of Medicine, University of Southern California, Los Angeles, CA, United States
- 6Medical Outpatient Clinic and Immunodeficiency Laboratory, Department of Biomedicine, University Hospital and University Basel, Basel, Switzerland
The pathogenesis of immune thrombocytopenia (ITP) is increasingly being elucidated, and its etiology is becoming more frequently identified, leading to a diagnostic shift from primary to secondary ITP. The overlap between autoimmunity, immunodeficiency, and cancer is evident, implying more interdisciplinarity in daily care. This mini-review is based on an expert meeting on ITP organized by the Intercontinental Cooperative ITP Study Group and presents the challenges of hematologists in understanding and investigating “out of the box” concepts associated with ITP.
Introduction
The triennial Intercontinental Cooperative ITP Study Group (ICIS) expert meeting on immune thrombocytopenia (ITP) was held for the sixth time in September 2019 in Locarno, Switzerland, where scientists and clinicians of pediatric and adult hematology, oncology, immunology, and genetics shared their ideas, experiences, and research findings to critically assess open questions and launch future joint projects (www.itpbasel.ch).
In the present mini-review, we discuss current approaches to understand ITP, following the paradigm “looking out of the box,” which aim to summarize some analogies and bridges between autoimmunity, infection, immunodeficiency, and cancer. It is without a doubt that the comprehension of pathophysiological overlap condition will help develop new targeted therapies.
The ITP Syndrome
ITP is an autoimmune bleeding disorder caused by various etiologies, which is characterized by increased platelet destruction and impaired production, resulting in a decreased platelet count. Primary ITP is idiopathic, whereas secondary ITP is linked to an underlying condition (1). The rate of secondary ITP in children has not been studied in detail but is assumed to be rare (2.4%) (2, 3). In adults, ~18–38% of ITP patients have an underlying disease, comorbid condition, and/or comedication, making the diagnosis of secondary ITP more probable (2, 4). Secondary ITP is known to be caused by systemic autoimmune disorders, primary or secondary immunodeficiency, infectious diseases, paraneoplastic syndromes (e.g., lymphomas and other malignancies), and drug-dependent antibodies (5).
Understanding, diagnosing, and treating patients with ITP require expertise in the rapidly growing field of autoimmune disorders. Indeed, there has been an increase in ITP knowledge in recent years (6), and the number of patients with primary ITP as an idiopathic hematological disorder is gradually decreasing. However, clinical research and patient care should continue to embrace both primary and secondary ITP because of differential diagnostic considerations and a holistic understanding of it.
Cines et al. (4) first used the term “ITP syndrome” in 2009 in their review paper. The “syndrome” is characterized by both the heterogeneity of pathogenesis and the variability in clinical courses, which is a hallmark of ITP, regardless of whether it is secondary or primary. In this cited review, Cines et al. presented the hypothesis that the level of the dysfunctional immune tolerance (central vs. peripheral) predicts the treatment response and clinical course. A mistake in tolerance induction during a normal germinal center response may explain the forms of disease that are cured by steroids or rituximab. In contrast, central tolerance defects may be less responsive to immunomodulation because a large proportion of the primary repertoire is autoreactive and may be reconstituted rapidly after therapy. This was also shown in an analysis of three ITP patients relapsing after splenectomy, as the expected reduction of the B-cell memory pool after removal of the spleen was absent, whereas a persisting intrinsic B-cell hyperfunction was measured (7).
In accordance to this assumption, infection-triggered ITP (e.g., childhood ITP) exhibits the best prognosis, whereas genetically determined immune deficiency-associated ITP exhibits a rather refractory course. However, additional factors such as environmental and genetic, age, concomitant disease, and medication may impact the clinical picture via variability in platelet turnover, propensity to bleed, risk of chronic disease, and response to ITP-directed therapy.
Autoimmunity Overlap Syndromes
ITP in patients with a systemic autoimmune disease, such as systemic lupus erythematosus or Sjögren syndrome, are evidently considered secondary ITP. Some other disease associations reveal a possible correlation or interaction, but this is yet to be clarified, for example, the one between autoimmune thyroid disorders and ITP. Hashimoto thyroiditis and Graves' disease are among the most common autoimmune disorders, and their association with ITP has extensively been discussed. The time intervals between the diagnosis of thyroid disease and that of ITP may range from months to years, still thyroid antibodies have been detected in 18–39% of ITP patients and overt hyperthyroidism in 7–8% (8–11). In children the prevalence of antithyroid antibodies was found to be significantly higher in chronic ITP (11.6%) than in the pediatric population (1.2%) (12). However, in children autoimmune thyroiditis does not seem to play a role as a prognostic factor of chronicity. Surprisingly, case reports have shown that ITP with hyperthyroidism leads to treatment-refractory thrombocytopenia (13, 14). Therefore, it remains unclear whether these underlying conditions or factors influence the phenotype and clinical course of ITP. Alternatively, these conditions can be postulated to reflect an underlying immune dysfunction, resulting in the loss of self-tolerance. When distinguishing secondary from primary ITP, there may be a thin line of separation.
Infection and Autoimmunity
Infectious diseases and vaccinations may trigger autoimmunity, which may be true for primary ITP of childhood and for ITP associated with infections caused by Helicobacter pylori, varicella zoster, human immunodeficiency virus (HIV), and hepatitis C virus as well as for the combined pediatric vaccine against measles, rubella and mumps (5, 6, 15). By contrast, vaccination and ITP in adults have not yet been proven to be correlated, and some studies have even suggested that influenza immunization is potentially protective against autoimmunity (16). The pathogenesis appears to be multifactorial in all cases. Molecular mimicry with the cross-reaction of antiviral antibodies and platelet surface glycoproteins is widely accepted. Other theories, including the production of platelets expressing viral antigens, binding of immune complexes, and formation of autoantibodies via epitope spread, have been discussed and studied. These phenomena can result in immune system dysregulation, leading to the clonal expansion of autoreactive B-/T-cell subsets or the disturbed balance between activating and inhibiting Fc-gamma receptors (5). However, virus-related mechanisms, including megakaryocyte infection; platelet surface alteration, accelerating their clearance; hypersplenism; hemophagocytosis; microangiopathic syndromes; and liver dysfunction, leading to reduced thrombopoietin production, may aggravate thrombocytopenia (5).
Immunodeficiency and Autoimmunity (Green Box)
Primary (genetically determined) and secondary (e.g., acquired by viral infections, drugs) dysfunction of the immune system have been linked to autoimmune disorders (Figure 1). Besides recurrent and opportunistic infections, the hallmarks of immunodeficiency are autoimmune cytopenia, such as ITP and autoimmune hemolytic anemia (17, 18). The clinician should therefore always consider the concomitant occurrence of autoimmune diseases and susceptibility to infections with caution. In the 40–50 s, the preventive and therapeutic administration of IgG concentrates (IVIG) resulted in a dramatic improvement of survival in numerous primary immunodeficiency diseases (PIDD) (19). In 1981, the immunomodulatory effect of IVIG in children with ITP (20) was observed to be the bridge to pathogenic and therapeutic challenges of the dysregulated immune system in autoimmune and immunodeficiency disorders. However, several disease associations are not well-understood, particularly the way of causality and recognition of the primary event.
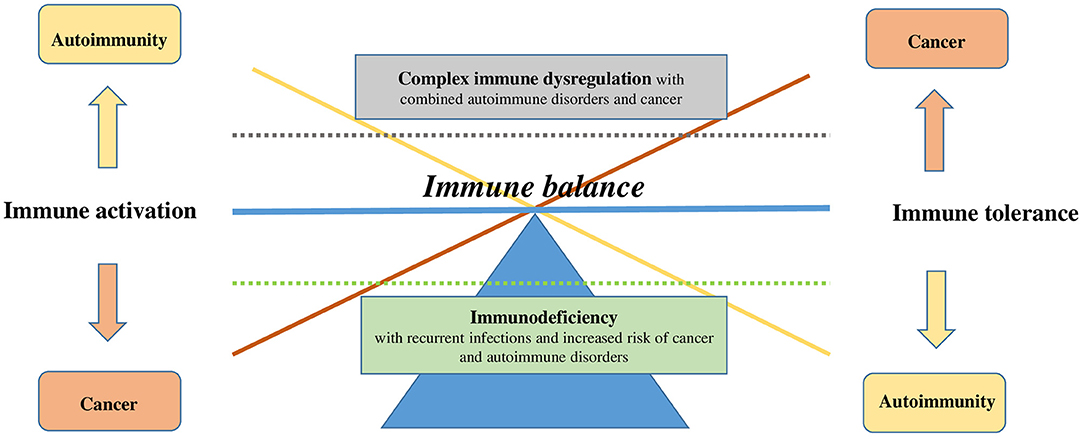
Figure 1. Dysregulation of immune balance. Immune balance can be disturbed on two levels: immune tolerance (on the right) or immune activation (on the left). Onset of a disease can be simplified by the shift in the balance on one side or the other (cancer in orange vs. autoimmunity in yellow) or on the vertical axis, represented by the dotted lines (immunodeficiency in green vs. complex immune dysregulation in gray).
Patients with primary and secondary immunodeficiency have also an increased risk of certain cancers (Figure 2). For instance, PIDD patients have a significantly increased risk for lymphoma (10-fold in men and 8-fold in women) (21). Subsequent to infection, malignancy is the most prevalent cause of mortality in both PIDD children and adults. The type of malignancy depends on the immunodeficiency type, patient age, probably the occurrence of certain viral (e.g., HHV8, EBV) or bacterial infections (e.g., Helicobacter pylori), and the treatment administered (e.g., stem cell transplantation), indicating that different pathogenic mechanisms are implicated (22, 23). However, the concept of aberrant immune surveillance in immunodeficiency resumes the clinical duality (24).
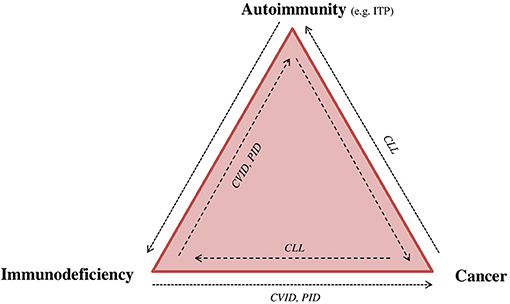
Figure 2. Immunodeficiency, autoimmunity, and cancer: “The immune trilogy.” Disturbance of the immune system can have multiple outcomes, such as cancer, autoimmunity or immune deficiency. The complexity and linkage between each of them can be demonstrated as “the immune trilogy.” Here are presented some examples of disease overlap. CLL, chronic lymphocytic leukemia; CVID, common variable immunodeficiency; ITP, immune thrombocytopenia; PID, primary immunodeficiency.
Cancer and Autoimmunity (Gray Box)
The first publication that reported the association between autoimmune disease and lymphoma was in 1966 (25). Subsequently, population studies have confirmed that certain autoimmune conditions, such as Sjögren syndrome, systemic lupus erythematosus, Hashimoto's disease, and rheumatoid arthritis, increase the risk of B-cell lymphoma (Figure 2) (26). Other reports have indicated a greater occurrence of T-cell lymphoma in celiac disease and ulcerative colitis patients (27). The frequency of lymphomas connected to an autoimmune disorder is estimated to be between 7% [e.g., Hodgkin's lymphoma (HL)] and 18% (e.g., marginal zone lymphoma) (28). Inversely, ITP occurs in the context of malignant lymphoproliferation (29), such as chronic lymphocytic leukemia (30), HL (31), and various types of non-Hodgkin's lymphomas (NHLs) (32, 33); it is rarely associated with T-cell NHL (29). The prevalence of ITP in HL has been estimated at 0.2–1% (31, 34), NHL at 0.76% (32), and chronic lymphocytic leukemia (CLL) at 1–5% (30). In a small case series, a high rate of lymphocytes with a CLL phenotype has been measured in autoimmune hemolytic anemia and ITP patients, assuming that some cases of ITP were probably initially misdiagnosed as primary forms (35). Cohort studies involving population-based nationwide databases showed an increased incidence for NHL and HL after the diagnosis of ITP, suggesting that ITP may also precede lymphoma onset (26, 36). However, the lymphoproliferative disease is generally considered to be the cause of thrombocytopenia, regardless of the diagnosis of cancer occurring months to years later. Several case reports have explained the causality of ITP and lymphoproliferation based on the assumption that treatments for lymphoma led to ITP resolution. In addition, tumor cells have been shown to produce antibodies against platelets or induce autoimmunity via antigen mimicry (37–39). In conclusion, whether ITP is a paraneoplastic phenomenon or an erroneous response of the immune system to the malignant cell remains unelucidated. Moreover, although it is unclear whether ITP may induce hematological malignancies, it could be possible, considering that the immunological process also occurs in the bone marrow, affecting stem cells.
The link between cancer and autoimmunity is the dysregulation of the immune system. T cells, B cells, and regulatory T and B cells as well as the innate cells (myeloid cells, dendritic cells) are involved in surveillance mechanisms of self and non-self (40–42). Many of the mechanisms that are considered essential in preventing autoimmunity suppress the immune response toward tumor cells (e.g., regulatory T cells) (40, 41, 43), placing cancer and autoimmunity in the opposite ends of the immune balance (Figure 1). Therefore, it appears quite remarkable that both conditions can co-occur, the reasons of which are probably multifactorial; further, the causality in one or another direction and the time scale are not always clear (44). The association of cancer and autoimmunity may also reveal an undiagnosed immunodeficiency syndrome.
For patients with autoimmune disorders, the risk of developing lymphoproliferative malignancies and solid tumors (45) is increased probably due to the combination of the underlying immune system dysregulation, immunosuppressive drug therapy, and chronic inflammation, thereby damaging tissue and further suppressing the immune system. Conversely, autoimmune disorders in cancer patients may appear as paraneoplastic phenomena or subsequent to immune therapies (e.g., checkpoint inhibitors) or as a second event based on the common genetic or environmental risk factors.
Although the pathogeneses of cancer and autoimmunity appear to be opposites, one common feature exists that supports the evolution of both diseases: chronic inflammation. Besides immune escape, cancer growth is well-known to be supported by a permissive tumor stroma that includes chronic inflammation and myeloid cells (46, 47). In autoimmune disorders, the dysregulation cascade becomes independent, facilitating the persistence and advancement of the disorder, regardless of the cause being removed, which can also be described as “chronic inflammation” and represents, in analogy to cancer, the microenvironment of autoimmune disorders (48).
Therapeutic Analogies and New Developments
Therapeutic modalities in cancer have been a focus of research for several years. Targeted drugs and immunotherapy are currently integrated in numerous multimodal treatment strategies, complementing the classical cytostatic or cytotoxic approach (chemotherapy and radiotherapy). Currently, immunotherapy, such as monoclonal antibodies and T-cell therapies, is dramatically expanding. Nevertheless, challenges in the field remain tremendous. Targeting a tumor-specific antigen does not automatically lead to an antitumoral immunological response. The immunological activity (Th1/Th2 balance) may even be different for different epitopes of the same antigen.
In autoimmunity, therapies have particularly been focused on immunosuppression and immunomodulation (5). However, a long-term sustained response is usually limited. Reconstitution of tolerance in ITP has mostly yielded disappointing results, with only 30% of patients showing a sustained response following dexamethasone pulse therapy or rituximab therapy (49, 50). Curing autoimmune disorders is probably best achieved with treatments at an early stage of autoimmunity and by using a multimodal approach like that with anti-cancer treatments. Autoimmunity may evolve and become resistant to treatments with time and insufficient treatments (autoimmune expansion), similar to cancer. Novel approaches for the management of ITP with combined therapies against T and B cells and thrombopoietin-receptor agonists early in the course of the disease appear to exhibit promising results (e.g., mycofenolate mofetil/dexamethasone, rituximab/dexamethasone/eltrombopag, and rituximab/belimumab; ongoing studies include NCT03156452, NCT02834286, NCT03154385, NCT02760251, and NCT04812483) (51–53).
Cutting-edge treatments based on the knowledge of cancer and autoimmunity is emerging. New therapies that activate or reproduce autoimmune disorder pathways have been developed to target cancer, and reciprocally, immunological escape mechanisms of cancer have been investigated to treat autoimmune disorders. Strategies in suppressing or activating Tregs (54–56), dendritic cells (57), and myeloid-derived suppressor cells (58) are currently being studied. These cells are part of the tumor environment and support cancer growth, promote angiogenesis, and suppress immunological responses via different pathways (e.g., immunosuppressive cytokine expression and checkpoint inhibitors) (46, 59, 60). The role of myeloid-derived suppressor cells in autoimmunity is now being studied in vitro, which may result in novel therapeutic strategies (61).
Conclusion
The pathogenesis of ITP is becoming increasingly understood, and secondary ITP is more likely identified. However, ITP remains to be a syndrome of complex immunological dysfunction. The clinical overlap between cancer, immunodeficiency, and autoimmune disorders exhibits an immunological activity that can simultaneously be overactive and suppressed or defective. Various endogenous (e.g., genetic predilection) and exogenous (e.g., infection) factors may trigger immune dysregulation with an intrinsic progression. Understanding analogies and bridges between cancer, immunodeficiency, and autoimmunity may help define driver immunological pathways, ultimately resulting in possible novel therapeutic strategies.
Author Contributions
AS wrote the manuscript. FC, BG, HL, and MR presented the topic at the ICIS meeting and reviewed the manuscript. TK and PI reviewed the manuscript and provided critical feedback. AS, PI, and TK conceived the original idea. All authors contributed to the article and approved the submitted version.
Conflict of Interest
AS: Amgen (research funds) and Novartis (advisory board). BG: served as an expert for Amgen, Novartis, Grifols, LFB, and Roche, and received grands for the research from Amgen. TK: Novartis (research funds), Amgen (research funds), and UCB biosciences (advisory board).
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Rodeghiero F, Stasi R, Gernsheimer T, Michel M, Provan D, Arnold DM, et al. Standardization of terminology, definitions and outcome criteria in immune thrombocytopenic purpura of adults and children: report from an international working group. Blood. (2009) 113:2386–93. doi: 10.1182/blood-2008-07-162503
2. Moulis G, Palmaro A, Montastruc JL, Godeau B, Lapeyre-Mestre M, Sailler L. Epidemiology of incident immune thrombocytopenia: a nationwide population-based study in France. Blood. (2014) 124:3308–15. doi: 10.1182/blood-2014-05-578336
3. Schifferli A, Heiri A, Imbach P, Holzhauer S, Seidel MG, Nugent D, et al. Misdiagnosed thrombocytopenia in children and adolescents: analysis of the Pediatric and Adult Registry on Chronic ITP. Blood Adv. (2021) 5:1617–26. doi: 10.1182/bloodadvances.2020003004
4. Cines DB, Bussel JB, Liebman HA, Luning Prak ET. The ITP syndrome. Blood. (2009) 113:6511–21. doi: 10.1182/blood-2009-01-129155
5. Cines DB, Liebman H, Stasi R. Pathobiology of secondary immune thrombocytopenia. Semin Hematol. (2009) 46(1 Suppl. 2):S2–14. doi: 10.1053/j.seminhematol.2008.12.005
6. Zufferey A, Kapur R, Semple JW. Pathogenesis and therapeutic mechanism in immune thrombocytopenia (ITP). J Clin Med. (2017) 6:16. doi: 10.3390/jcm6020016
7. Giordano P, Cascioli S, Lassandro G, Marcellini V, Cardinale F, Valente F, et al. B-cell hyperfunction in children with immune thrombocytopenic purpura persists after splenectomy. Pediatr Res. (2016) 79:262–70. doi: 10.1038/pr.2015.211
8. Cordiano I, Betterle C, Spadaccino CA, Soini B, Girolami A, Fabris F. Autoimmune thrombocytopenia (AITP) and thyroid autoimmune disease (TAD): overlapping syndromes? Clin Exp Immunol. (1998) 113:373–8. doi: 10.1046/j.1365-2249.1998.00677.x
9. Altintas A, Pasa S, Cil T, Bayan K, Gokalp D, Ayyildiz O. Thyroid and celiac diseases autoantibodies in patients with adult chronic idiopathic thrombocytopenic purpura. Platelets. (2008) 19:252–7. doi: 10.1080/09537100801894651
10. Cheung EM, Naik R, Keng M, Liebman HA. Thyroid disorder-related immune thrombocytopenia: clinical and laboratory characteristics. Blood. (2008) 112:4561. doi: 10.1182/blood.V112.11.4561.4561
11. Ioachimescu AG, Makdissi A, Lichtin A, Zimmerman RS. Thyroid disease in patients with idiopathic thrombocytopenia: a cohort study. Thyroid. (2007) 17:1137–42. doi: 10.1089/thy.2007.0066
12. Giordano P, Urbano F, Lassandro G, Bianchi FP, Tolva A, Saracco P, et al. Role of antithyroid autoimmunity as a predictive biomarker of chronic immune thrombocytopenia. Pediatr Blood Cancer. (2019) 66:e27452. doi: 10.1002/pbc.27452
13. Bowles KM, Turner GE, Wimperis JZ. Resolution of chronic severe refractory thrombocytopenia after treatment of hypothyroidism. J Clin Pathol. (2004) 57:995–6. doi: 10.1136/jcp.2004.016543
14. Gill H, Hwang YY, Tse E. Primary immune thrombocytopenia responding to antithyroid treatment in a patient with Graves' disease. Ann Hematol. (2011) 90:223–4. doi: 10.1007/s00277-010-0983-4
15. Liebman. Other thrombocytopenias. Semin Hematol. (2007) 44(4 Suppl. 5):S24–34. doi: 10.1053/j.seminhematol.2007.11.004
16. Grimaldi-Bensouda L, Michel M, Aubrun E, Leighton P, Viallard JF, Adoue D, et al. Immune thrombocytopenia study group. A case-control study to assess the risk of immune thrombocytopenia associated with vaccines. Blood. (2012) 120:4938–44. doi: 10.1182/blood-2012-05-431098
17. Fischer A, Provot J, Jais JP, Alcais A, Mahlaoui N, members of the CEREDIH French PID study group. Autoimmune and inflammatory manifestations occur frequently in patients with primary immunodeficiencies. J Allergy Clin Immunol. (2017) 140:1388–93.e8. doi: 10.1016/j.jaci.2016.12.978
18. Feuille EJ, Anooshiravani N, Sullivan KE, Fuleihan RL, Cunningham-Rundles C. Autoimmune cytopenias and associated conditions in CVID: a Report From the USIDNET Registry. J Clin Immunol. (2018) 38:28–34. doi: 10.1007/s10875-017-0456-9
19. Cohn EJ, Oncley JL, Strong LE, Hughes WL, Armstrong SH. chemical, clinical, and immunological studies on the products of human plasma fractionation. I. The characterization of the protein fractions of human plasma. J Clin Invest. (1944) 23:417–32. doi: 10.1172/JCI101508
20. Imbach P, Barandun S, d'Apuzzo V, Baumgartner C, Hirt A, Morell A, et al. High-dose intravenous gammaglobulin for idiopathic thrombocytopenic purpura in childhood. Lancet. (1981) 1:1228–31. doi: 10.1016/S0140-6736(81)92400-4
21. Mayor PC, Eng KH, Singel KL, Abrams SI, Odunsi K, Moysich KB, et al. Cancer in primary immunodeficiency diseases: cancer incidence in the United States Immune Deficiency Network Registry. J Allergy Clin Immunol. (2018) 141:1028–35. doi: 10.1016/j.jaci.2017.05.024
22. Haas OA. Primary immunodeficiency and cancer predisposition revisited: embedding two closely related concepts into an integrative conceptual framework. Front Immunol. (2019) 9:3136. doi: 10.3389/fimmu.2018.03136
23. Chua I, Quinti I, Grimbacher B. Lymphoma in common variable immunodeficiency: interplay between immune dysregulation, infection and genetics. Curr Opin Hematol. (2008) 15:368–74. doi: 10.1097/MOH.0b013e328302c7b6
24. Mortaz E, Tabarsi P, Mansouri D, Khosravi A, Garssen J, Velayati A, et al. Cancers related to immunodeficiencies: update and perspectives. Front Immunol. (2016) 7:365. doi: 10.3389/fimmu.2016.00365
25. Mellors RC. Autoimmune disease in NZB-Bl mice. II. Autoimmunity and malignant lymphoma. Blood. (1966) 27:435–48. doi: 10.1182/blood.V27.4.435.435
26. Landgren O, Engels EA, Pfeiffer RM, Gridley G, Mellemkjaer L, Olsen JH, et al. Autoimmunity and susceptibility to hodgkin lymphoma: a population-based case-control study in Scandinavia. JNCI. (2006) 98:1321–30. doi: 10.1093/jnci/djj361
27. Cellier C, Delabesse E, Helmer C, Patey N, Matuchansky C, Jabri B, et al. Refractory sprue, coeliac disease, and enteropathy-associated T-cell lymphoma. French Coeliac Disease Study Group. Lancet. (2000) 356:203–8. doi: 10.1016/S0140-6736(00)02481-8
28. Zintzaras E, Voulgarelis M, Moutsopoulos HM. The risk of lymphoma development in autoimmune diseases: a meta-analysis. Arch Intern Med. (2005) 165:2337–44. doi: 10.1001/archinte.165.20.2337
29. Ayesh Haj Yousef MH, Alawneh K, Zahran D, Aldaoud NH, Khader Y. Immune thrombocytopenia among patients with cancer and its response to treatment. Eur J Haematol. (2018) 101:185–90. doi: 10.1111/ejh.13091
30. Zent CS, Ding W, Schwager SM, Reinalda MS, Hoyer JD, Jelinek DF, et al. The prognostic significance of cytopenia in chronic lymphocytic leukemia/small lymphocytic lymphoma (CLL). Br J Haematol. (2008) 141:615–21. doi: 10.1111/j.1365-2141.2008.07086.x
31. Xiros N, Binder T, Anger B, Bohlke J. Heimpel H. Idiopathic thrombocytopenic purpura autoimmune hemolytic anemia in Hodgkin's disease. Eur J Haematol. (1988) 40:437–41. doi: 10.1111/j.1600-0609.1988.tb00853.x
32. Tan J, Wei J, Ni X, Zhou J. Immune thrombocytopenic purpura occurred prior to multiple extranodal diffuse large B cell lymphoma. Platelets. (2011) 22:81–3. doi: 10.3109/09537104.2010.517582
33. Hauswirth AW, Skrabs C, Schützinger C, Raderer M, Chott A, Valent P, et al. Autoimmune thrombocytopenia in non-Hodgkin's lymphomas. Haematologica. (2008) 93:447–50. doi: 10.3324/haematol.11934
34. Bradley SJ, Hudson GV, Linch DC. Idiopathic thrombocytopenic purpura in Hodgkin's disease: a report of eight cases. Clin Oncol (R Coll Radiol). (1993) 5:355–7. doi: 10.1016/S0936-6555(05)80085-8
35. Mittal S, Blaylock MG, Culligan DJ, Barker RN, Vickers MA. A high rate of CLL phenotype lymphocytes in autoimmune hemolytic anemia and immune thrombocytopenic purpura. Haematologica. (2008) 93:151–2. doi: 10.3324/haematol.11822
36. Fallah M., Liu X, Ji J, Försti A, Sundquist K, Hemminki K. Autoimmune diseases associated with non-Hodgkin lymphoma: a nationwide cohort study. Ann Oncol. (2014) 25:2025–30. doi: 10.1093/annonc/mdu365
37. Nobuoka A, Sakamaki S, Kogawa K, Fujikawa K, Takahashi M, Hirayama Y, et al. A case of malignant lymphoma producing autoantibody against platelet glycoprotein Ib. Int J Hematol. (1999) 70:200–6.
38. Vial G, Rivière E, Raymond A-A, James C, Di-Tommaso S, Dugot-Senant N, et al. Antigenic mimicry in paraneoplastic immune thrombocytopenia. Front Immunol. (2019) 10:523. doi: 10.3389/fimmu.2019.00523
39. Benvenuto M, Mattera R, Masuelli L, Tresoldi I, Giganti MG, Frajese GV, et al. The crossroads between cancer immunity and autoimmunity: antibodies to self antigens. Front Biosci (Landmark Ed). (2017) 22:1289–329. doi: 10.2741/4545
40. Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. (2011) 144:646–74. doi: 10.1016/j.cell.2011.02.013
41. Andersen MH. Cancer and autoimmunity. Semin Immunopathol. (2017) 39:241–3. doi: 10.1007/s00281-016-0617-6
42. Coussens LM, Werb Z. Inflammation and cancer. Nature. (2002) 420:860–7. doi: 10.1038/nature01322
43. Knochelmann HM, Dwyer CJ, Bailey SR, Amaya SM, Elston DM, Mazza-McCrann JM, et al. When worlds collide: Th17 and Treg cells in cancer and autoimmunity. Cell Mol Immunol. (2018) 15:458–69. doi: 10.1038/s41423-018-0004-4
44. Ehrenfeld M, Abu-Shakra M, Buskila D, Shoenfeld Y. The dual association between lymphoma and autoimmunity. Blood Cells Mol Dis. (2001) 27:750–6. doi: 10.1006/bcmd.2001.0442
45. Giat E, Ehrenfeld M, Shoenfeld Y. Cancer and autoimmune diseases. Autoimmun Rev. (2017) 16:1049–57. doi: 10.1016/j.autrev.2017.07.022
46. Umansky V, Blattner C, Fleming V, Hu X, Gebhardt C, Altevogt P, et al. Myeloid-derived suppressor cells and tumor escape from immune surveillance. Semin Immunopathol. (2017) 39, 295–305. doi: 10.1007/s00281-016-0597-6
47. Krejsgaard T, Lindahl LM, Mongan NP, Wasik MA, Litvinov IV, Iversen L, et al. Malignant inflammation in cutaneous T-cell lymphoma-a hostile takeover. Semin Immunopathol. (2017) 39, 269–82. doi: 10.1007/s00281-016-0594-9
48. Duan L, Rao X, Sigdel KR. Regulation of inflammation in autoimmune disease. J Immunol Res. (2019) 2019:7403796. doi: 10.1155/2019/7403796
49. Deshayes S, Khellaf M, Zarour A, Layese R, Fain O, Terriou L, et al. Long-term safety and efficacy of rituximab in 248 adults with immune thrombocytopenia: results at 5 years from the French prospective registry ITP-ritux. Am J Hematol. (2019) 94:1314–24. doi: 10.1002/ajh.25632
50. Naithani R, Mahapatra M, Kumar R, Mishra P, Saxena R. High dose dexamethasone therapy shows better responses in acute immune thrombocytopenia than in chronic immune thrombocytopenia. Platelets. (2010) 21:270–3. doi: 10.3109/09537101003637257
51. Gómez-Almaguer D, Colunga-Pedraza PR, Gómez-De León A, Gutiérrez-Aguirre CH, Cantú-Rodríguez OG, Jaime-Pérez JC. Eltrombopag, low-dose rituximab, and dexamethasone combination as frontline treatment of newly diagnosed immune thrombocytopenia. Br J Haematol. (2019) 184:288–90. doi: 10.1111/bjh.15070
52. Pell J, Greenwood R, Ingram J, Wale K, Thomas I, Kandiyali R, et al. Trial protocol: a multicentre randomised trial of first-line treatment pathways for newly diagnosed immune thrombocytopenia: standard steroid treatment versus combined steroid and mycophenolate. The FLIGHT trial. BMJ Open. (2018) 8:e024427. doi: 10.1136/bmjopen-2018-024427
53. Zhang M, Du X, Cheng Y, Cheng G. Safety and efficacy of eltrombopag plus pulsed dexamethasone as first-line therapy for immunethrombocytopenia. Br J Haematol. (2020) 189:369–78. doi: 10.1111/bjh.16327
54. Wang H, Franco F, Ho PC. Metabolic Regulation of Tregs in Cancer: opportunities for immunotherapy. Trends Cancer. (2017) 3:583–92. doi: 10.1016/j.trecan.2017.06.005
55. Hoeppli RE, Pesenacker AM. Targeting tregs in juvenile idiopathic arthritis and juvenile dermatomyositis-insights from other diseases. Front Immunol. (2019) 10:46. doi: 10.3389/fimmu.2019.00046
56. Zhang Q, Lu W, Liang CL, Chen Y, Liu H, Qiu F, Dai Z Chimeric antigen receptor (CAR) Treg: a promising approach to inducing immunological tolerance. Front Immunol. (2018) 9:2359. doi: 10.3389/fimmu.2018.02359
57. Coutant F, Miossec P. Altered dendritic cell functions in autoimmune diseases: distinct and overlapping profiles. Nat Rev Rheumatol. (2016) 12:703–15. doi: 10.1038/nrrheum.2016.147
58. Schupp J, Krebs FK, Zimmer N, Trzeciak E, Schuppan D, Tuettenberg A. Targeting myeloid cells in the tumor sustaining microenvironment. Cell Immunol. (2019) 343:103713. doi: 10.1016/j.cellimm.2017.10.013
59. Talmadge JE, Gabrilovich DI. History of myeloid-derived suppressor cells. Nat Rev Cancer. (2013) 13:739–52. doi: 10.1038/nrc3581
60. Sica A, Massarotti M. Myeloid suppressor cells in cancer and autoimmunity. J Autoimmun. (2017) 85:117–25. doi: 10.1016/j.jaut.2017.07.010
Keywords: immune thrombocytopenia, ITP syndrome, secondary ITP, autoimmunity, immunodeficiency, cancer, overlap syndromes
Citation: Schifferli A, Cavalli F, Godeau B, Liebman HA, Recher M, Imbach P and Kühne T (2021) Understanding Immune Thrombocytopenia: Looking Out of the Box. Front. Med. 8:613192. doi: 10.3389/fmed.2021.613192
Received: 01 October 2020; Accepted: 28 May 2021;
Published: 24 June 2021.
Edited by:
Giancarlo Castaman, University of Florence, ItalyReviewed by:
Efthymia Vlachaki, Aristotle University of Thessaloniki, GreecePaola Giordano, University of Bari Aldo Moro, Italy
Copyright © 2021 Schifferli, Cavalli, Godeau, Liebman, Recher, Imbach and Kühne. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Alexandra Schifferli, YWxleGFuZHJhLnNjaGlmZmVybGlAdWtiYi5jaA==
 Alexandra Schifferli
Alexandra Schifferli Franco Cavalli3
Franco Cavalli3 Howard A. Liebman
Howard A. Liebman Mike Recher
Mike Recher