
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Mar. Sci. , 23 January 2025
Sec. Marine Biology
Volume 11 - 2024 | https://doi.org/10.3389/fmars.2024.1528582
This article is part of the Research Topic Current Research on Fish Otoliths and their Applications View all 10 articles
 Miao Xiang1,2†
Miao Xiang1,2† Haoran Liu1,3,4†
Haoran Liu1,3,4† Bo Li5
Bo Li5 Hongxi Guo5
Hongxi Guo5 Chuankun Zhu6
Chuankun Zhu6 Man Wang1,3
Man Wang1,3 Jie Wang1,3
Jie Wang1,3 Songguang Xie1,7
Songguang Xie1,7 Lei Zhang1,3*
Lei Zhang1,3*In this study, we assessed the growth patterns and population characteristics of the Odontobutis potamophila in Nansi Lake to inform evidence-based management recommendations for the sustainable development of fisheries in the region. A collection of O. potamophila was taken from Nansi Lake between August 2017 and July 2018 (except February) to estimate the age structure, growth pattern, and mortality of the population. Edge-type analysis of sagittal otoliths indicated that a single annulus was formed from March to May. The results revealed that the age structure of O. potamophila consisted of four age groups (0-3 ages), with 0-1 year-old fish comprising about 85.11% of the harvest. An isometric growth pattern was observed, with the Logistic growth function providing the best fit for combined sexes. (n=591, R2 = 0.622). Total mortality (Z), natural mortality (M), and fishing mortality (F) were computed as 1.69, 0.53, and 1.16/year, respectively. Meanwhile, the exploitation rate (E) was 0.69/year, in accordance with the rule of thumb (E>0.5, the fish stock was undergoing overfishing), which indicated the stock has been overfished slightly. These results showed that brief generation-time, dominance of juvenile fish and relatively high natural mortality of the O. potamophila provide essential information on the native fish species in Nansi Lake, which may be essential for conservation strategies and artificial propagation. At the same time, these results provide critical data for sustainable fisheries management in Nansi Lake and similar ecosystems.
Determination of age is of fundamental importance in the fields of fish biology and population management studies (de Santana et al., 2020; Nazir and Khan, 2020). The age data thus obtained can be used to calculate growth rates, mortality and productivity of fish populations (Campana, 2001; Ma et al., 2011). Annuli are usually formed in hard structures, e.g., scales, otoliths, fin rays and vertebrae, reflecting seasonal growth patterns, particularly in temperate waters (Nazir and Khan, 2020; Phelps et al., 2011; Ma et al., 2011). In the context of age identification for this species, although scales are a conventionally employed method, they are less precise in distinguishing older individuals. Otoliths, or ear bones, have been identified as a potential alternative, with studies indicating the presence of clear annual rings. The development of a method for age identification through the comparison of different materials is a future research direction. Field surveys have revealed that Nansi Lake has been significantly impacted by anthropogenic activities, leading to miniaturization of fish. Individuals of O. potamophila in the river are typically small in size, with large specimens being rare. Among the hard structures used to determine ages, otoliths are recommended in most of the investigated species and researches with most distinguishable annuli (Campana and Thorrold, 2001; Ferri and Brzica, 2022). In order to use any hard structure to determine age, it is critical to identify the annuli in the structure and validate its annual periodicity before any applications (Nazir and Khan, 2020).
The dark sleeper, Odontobutis potamophila (Günther, 1861), is a valuable aquaculture species due to its high-quality meat and market demand. It is commonly found in floodplain lakes across eastern China (Zhao et al., 2017; Wang et al., 2017). It is usually a by-catch species of shrimp trap and occurs in the harvest mainly during the spawning period from March to June. The O. potamophila needs a specific nest to lay eggs during the spawning period, with males guarding the nest and the fertilized eggs until the eggs hatched (Hao, 1960). Populations of this species have declined sharply in the last several decades in most of the lakes due to multiple factors, specifically overfishing and habitat degradation (Tang et al., 2015; Zhang et al., 2018).
However, the paucity of basic biological information regarding this species is a matter of concern. A significant body of research has recently been dedicated to O. potamophila, with studies focusing on reproduction, husbandry, and larval rearing (Liu et al., 2008). Phylogenetic analysis of the mitochondrial 12S rRNA sequence was conducted to ascertain the taxonomic composition of Chinese odontobutis. The analysis revealed the presence of four species: O. potamophila, Odontobutis sinensis, Odontobutis haifengensis and Odontobutis yaluensis. To date, no research has been conducted on the age determination of this species, which consequently hinders further biological study.
Previously, the maximum age of this species in Taihu Lake and Chaohu Lake was determined through the analysis of otolith size, with a maximum age of four years old confirmed (Li et al., 2007; Sun et al., 1996). Meanwhile, the Odontobutis sinensis in Honghu Lake was confirmed to be in the age range of 1-3 years old by using the scale (Xiang et al., 2021). Nevertheless, the maximum age measurements for this species in Nansi Lake have yet to be documented. The experimental results demonstrated that the age inferred based on otolith mass was largely consistent with the actual age, confirming that otolith mass is an accurate and rapid method of age identification (Han et al., 2022). The significant positive correlation between otolith weight and fish age suggests that otoliths can be used as a valid indicator for estimating fish age, especially in age groups where there is no overlap between otolith weight and body length. This method can provide a high level of accuracy. Nevertheless, it is important to note that the accuracy of this method may be compromised in circumstances where there is overlap between the parameters under consideration. Consequently, further validation and refinement of the analysis is required to ensure the optimal performance of this technique (Britton and Blackburn, 2014; Khan et al., 2018).
In this study, Nansi Lake is a unique study site because it is a key node of the South-to-North Water Diversion East Route Project, which has important ecological protection and water resource allocation roles. Therefore, we firstly analyzed the sectioned sagittal otoliths to distinguish any potential marks of annuli in Nansi Lake,. We then validated the annual periodicity of the marks, and used the validated annuli to determine age structure and to calculate growth patterns mortality of the population. The findings of this study, which concern the age identification of the O. potamophila and population parameters, may shed light on the growth patterns and population dynamics of O. potamophila. They may also provide critical data for assessing the reproductive potential and structural stability of the population. Furthermore, they may be essential for developing rational fishing strategies and for assessing the health of fishery resources.
Nansi Lake (34°27′-35°20′N; 116°34′-117°21′E) (Figure 1) belongs to the Huai River watershed, on the border between Shandong Province and Jiangsu Province, China. It is the largest freshwater lake in northern China, with a total surface area of 1266 km2 and an average water depth of 1.40 m (Wang et al., 2019; Qin et al., 2020). This lake is a part of the East Route of the South-to-North Water Transfer Project, serving as both the route and a storage water. A major part of the lake is included in a natural reserve. As a result, water quality, habitat and fish resource are well protected in this lake, and O. potamophila maintains a considerable population abundance, and frequently occurred in the harvest of fishermen. Sampling was conducted monthly from August 2017 through July 2018 (except for February 2018). No data from February were available due to the failure to launch nets for collection because of ice closure. Furthermore, the marked differences in the seasonal distribution of fish communities, the very low temperatures recorded in February and the low activity of fish during the winter months meant that only a very small number of samples could be collected. Benthic fyke nets are defined as nets that are typically 15 meters in length and 0.6 meters in width, with a mesh size of 4 mm. Each net contains approximately 20 trap boxes, each equipped with three entrances that permit entry for shrimp and fish, but prevent exiting. O. potamophila is a bycatch species in benthic fyke nets, predominantly encountered in nearshore areas with water depths of approximately 1 meter during harvesting operations (Qu et al., 2020; Guo et al., 2013). Therefore we used benthic fyke nets. The nets were 15-m long and 0.6-m wide with a 4-mm mesh size (Qin et al., 2020). The specimens were kept on ice immediately after sampling and brought into the laboratory for further analysis.
The fish was measured for standard length (SL, to 0.1mm) and weighed for body weight (BW, to 0.1g). It was then dissected to open its abdomen and determined of sex by visually checking the gonads. Fish can only be determined of sex when developed to late-stage II, but not earlier. Then both right and left sagittal otoliths were extracted and usually only one of them was used for analysis. Preliminary observations on whole sagittal otoliths polished slices (Cross-section grinding) under a dissecting microscope with transmitted light revealed identifiable opaque/translucent opaque. Using a dissecting microscope, sagittal otoliths were analyzed under transmitted light to identify opaque, which appeared as alternating opaque and tran. The otoliths were polished to get the transverse section (Ding et al., 2016). Periodic opaque/tran were visible in the otoliths when examined with transmitted light. Each otolith was identified to one of two marginal types, opaque or translucent. Monthly percentages of otoliths with opaque and translucent edges were calculated to validate the annual periodicity of the opaque to determine the months of transitions from opaque growth to translucent growth (Panfili and Morales-Nin, 2002; Soeth et al., 2019).
Age was determined by identifying and counting the inner margin of opaque that are assumed to form annually. Age was determined by the first author alone. Three independent readings were made with a minimum period of 2 weeks between each reading. We referred to He et al. (2008) to define age class and to calculate precise age of each fish. Each fish was assigned to an age class. When a fish was caught in the calendar year of its birth, it was assigned to age 0; if it was caught in the year following the calendar year of its birth, it was assigned to age 1, etc (Devries and Frie, 1996). To construct the growth function, each fish was also assigned a more precise age by fixing its birth date as 1 May, the middle of the main spawning season (Hao, 1960), and considering the date of capture (Stewart and Hughes, 2007; He et al., 2008). For a fish caught during April through June with a translucent margin zone outside of the last annulus, its age was calculated as , where A is the age in years, N is the number of annuli counted, and is the number of days from its nominated birth date to capture. Otherwise, age was calculated as (He et al., 2008).
The SL-at-age data were described by the following three growth models: 1) the von Bertalanffy growth function (VBGF) , where is the standard length at age t, is the asymptotic length, K is the growth coefficient and t0 is the hypothetical age at zero-length; 2) the logistic growth function (LGF) and 3) the Gompertz growth function (GGF) . Parameter estimates were obtained for the three models using nonlinear regression. Akaike Information Criterion (AICc) was used to assess the best-fit model based on goodness of fit and parsimony for males, females, and all individuals pooled (Akaike, 1983). The model with the smallest AICc value was selected as the best among the candidates. These three models are frequently employed in the fitting of growth equations, and it is important to note that there is no species that is exclusively associated with a single equation. The age-growth data were used to fit all three models simultaneously, and by using the Akaike information criterion and the comparison of the R2 of the equations, it was determined that the most suitable growth equation type for O. potamophila in this study was Logistic model. Of course, the most common one must be VB model, and the results showed that the R2 of VB model and Logistic model were approximately equal, but the AICc of Logistic was smaller and the estimated limiting body length is more in line with the current reality. The AICc difference (Δi) was computed for candidate models to evaluate relative model support. Models with Δi <2 have substantial support, while there is considerably less support for models with 4 <Δi <7, and models with Δi >10 have essentially no support and might be omitted from further consideration (Anderson & Burnham, 2002). The three growth functions for males and females were compared using the analysis of residual sums of squares (RSS) method (Chen et al., 1992). And the mean size of males and females was also compared. The growth performance index (GPI) (phi-prime) was calculated using the following formula (Pauly and Munro, 1984): = log10K + 2log10 .
The length-weight relationship (LWR) was described for each sex by , where a is the intercept of the regression or shape coefficient and b is the allometric or slope parameter (Ricker, 1975). Analysis of covariance (ANCOVA) was used to compare (log-transformed) LWR relationships for males and females (length and weight as dependent and independent variables, respectively, and sex as a covariate). The difference between b and isometric growth value (b = 3) was tested by t-test (Froese, 2006).
To obtain the total mortality (Z), the catch curve analysis was applied (Ricker, 1975). The natural mortality (M) were obtained from Pauly (1980) empirical equation: log10M = -0.0066 – 0.279log10 + 0.6543log10K + 0.4634log10T, where T is the average annual water temperature (in the present study, T = 17.31°C from (Qu et al., 2020). Fishing mortality (F) was estimated from the equation F = Z – M (Gulland, 1965), and exploitation rate (E) was estimated using the equation of (Elliott, 1983): E = F/Z. We used nonparametric statistics to analyze when data was non-normality and non-homogeneous. Statistical tests were conducted using SPSS 19.0 software and R 3.5.3 (R Project for Statistical Computing, http://www.r-project.org/).
A total of 591 individuals were analyzed of otoliths, of which 294 males (SL: 54.18-145.58 mm; BW: 3.75-88.52g) and 297 females (SL: 55.97-135.96 mm; BW: 4.34-85.32g) could be identified (Table 1). The mean SL was not statistically different between males (92.21 ± 17.73mm) and females (91.03 ± 14.9 mm; p=0.926>0.05).
There were inner margin of the translucent zone in the plane of the whole sagittal otoliths (Figure 2). The edge type was translucent during the months August-November. In December, the otolith margin of some fish was opaque, and then this proportion increased to its maximum in March of the following year, and afterwards decreased during March and May. In June, 100% of the otolith edges were translucent. Thus, the shift in growth pattern from translucent to opaque occurred in March and May (Figure 3). Additionally, estimated age ranged from 0 to 3 years, i.e. 4 age classes. Individuals of age 0 and 1 accounted for 85.11% of the harvest (Figure 4).
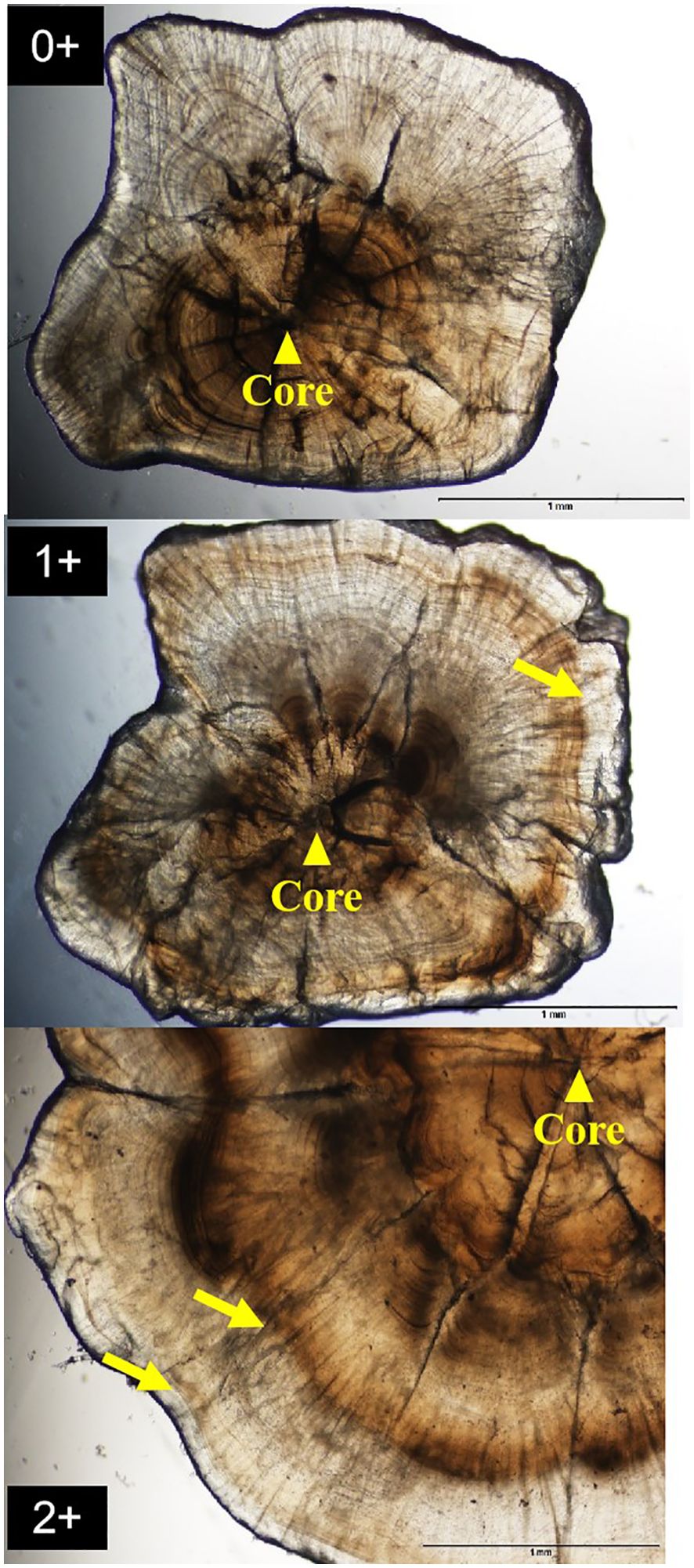
Figure 2. Photomicrograph from the sagittal otolith of a 0-3 years Odontobutis potamophila. Yellow arrow shows otolith core; black dot shows the position of opaque bands (scale bar = 1mm).
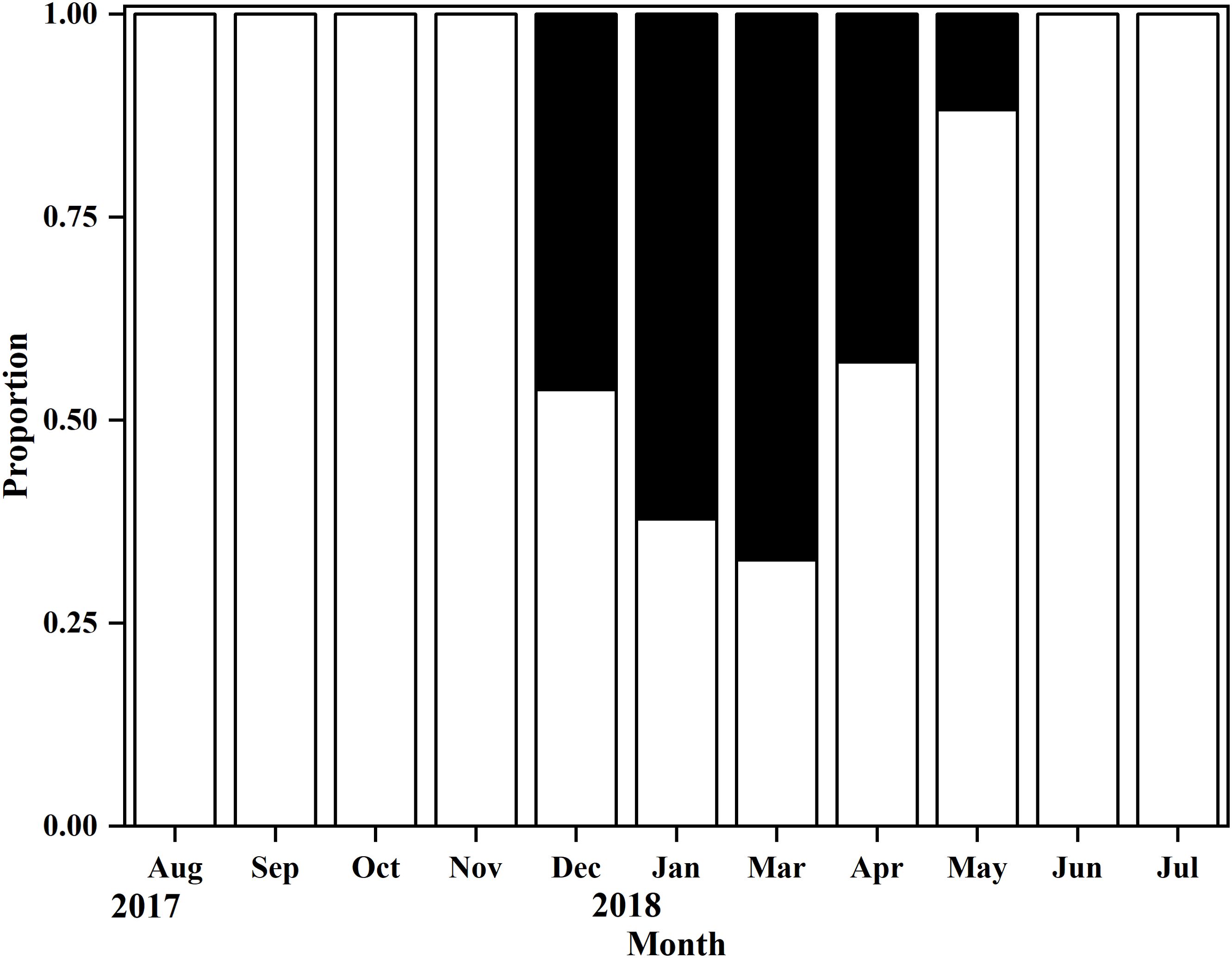
Figure 3. Monthly edge-type analysis expressed as percentages of opaque (black bars) or translucent (blank bars) edges for fish collected from Nansi Lake during August 2017 to through July 2018.
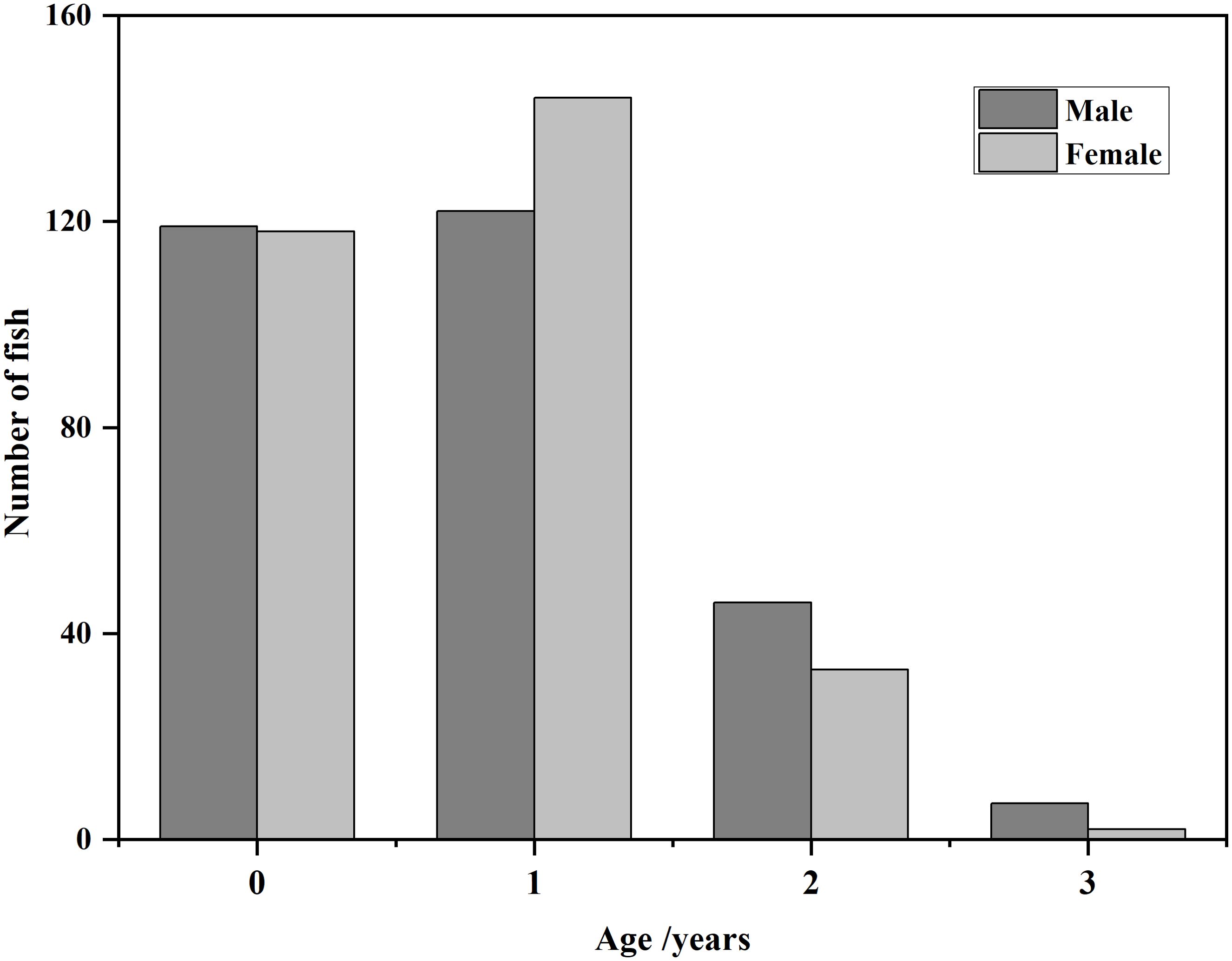
Figure 4. Age compositions of Odontobutis potamophila collected from the Nansi Lake during August 2017 through July 2018.
The RSS showed no significant difference in SL-at-age relationships between males and females (F < F0.05, 3, 2= 19.1643, p > 0.05, Table 2). We therefore combined data from both sexes to fit growth function. The LGF had the lowest AICc and Δi (Table 3), thus, was the best function fitting the SL-at-age relationship (n=591, R2 = 0.622; Figure 5). The GPI () of the population was 4.14.
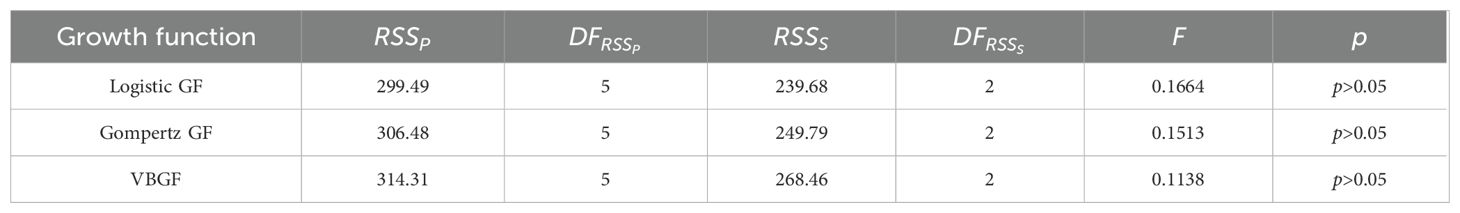
Table 2. Full spelt comparison of growth functions between males and females of Odontobutis potamophila collected from Nansi Lake during August 2017 through July 2018.
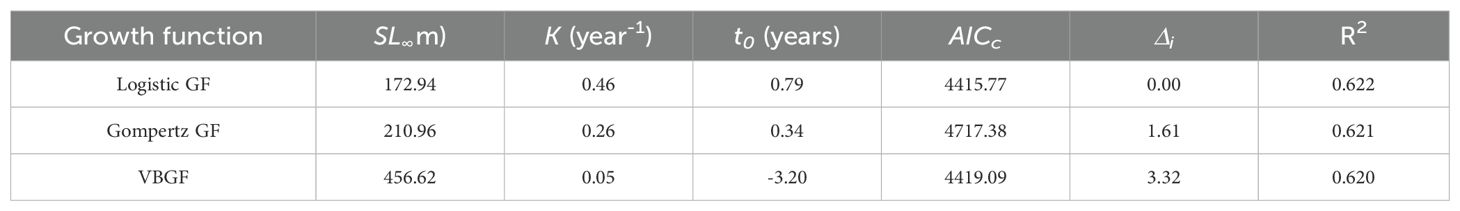
Table 3. Comparison of growth functions and model fit parameters for male and female populations of Odontobutis potamophila.
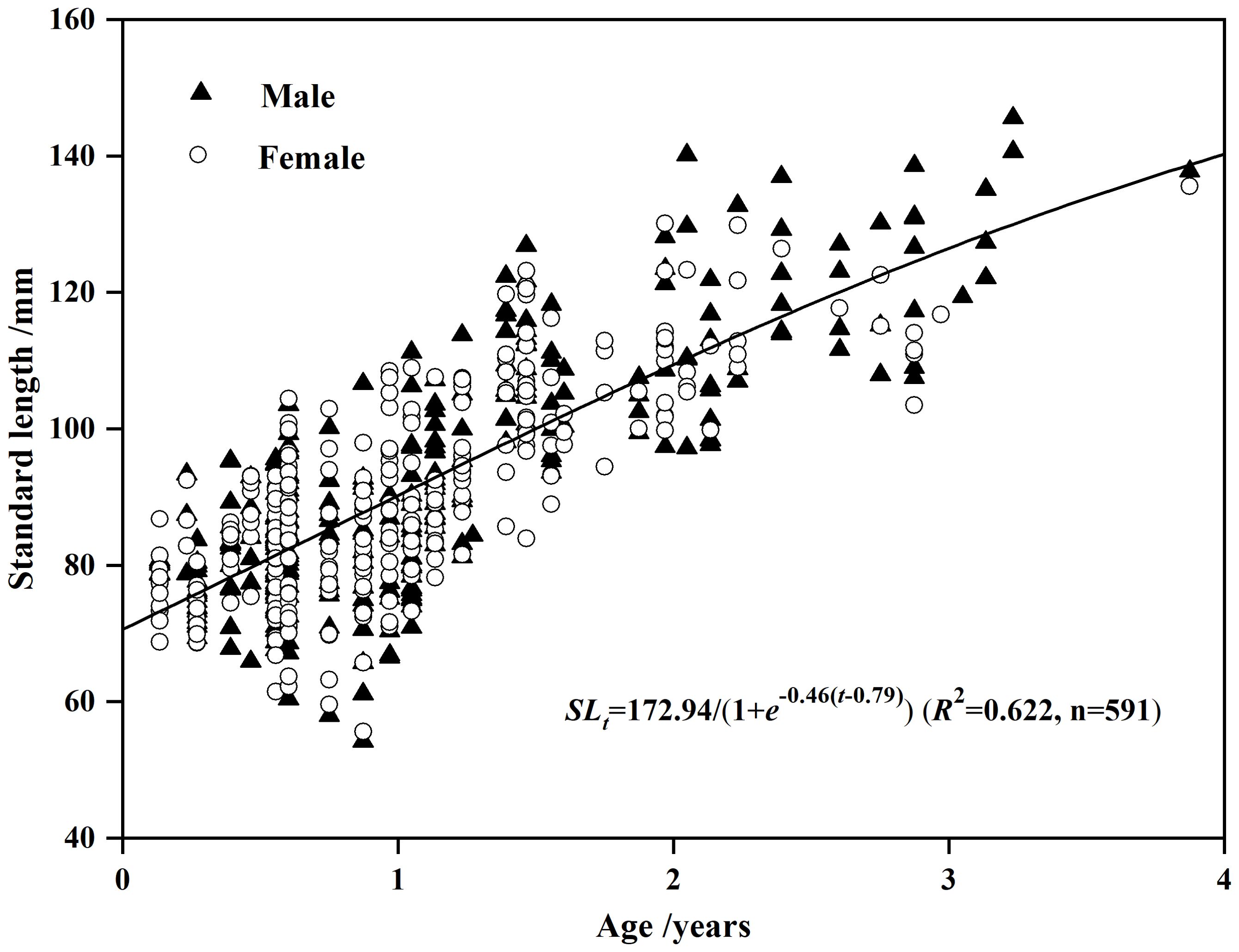
Figure 5. Logistic growth curve for Odontobutis potamophila collected from Nansi Lake during August 2017 through July 2018. Open circles, females; black triangles, males. The growth function is , R2 = 0.622 (n = 591). The growth curve demonstrates the pooled data for male and female dark sleepers collected in Nansi Lake.
The slope (b-values) of the SL-to-BW regressions for both males and females did not differ from the value of 3 (Male: t = 1.13<t0.05, p>0.05; Female: t = 1.10<t0.05, p>0.05). The LWRs were also not significantly different between males and females (ANCOVA, F = 3.382, p = 0.066). The LWR for pooled sexes was (n = 591, R2 = 0.949) (Figure 6).
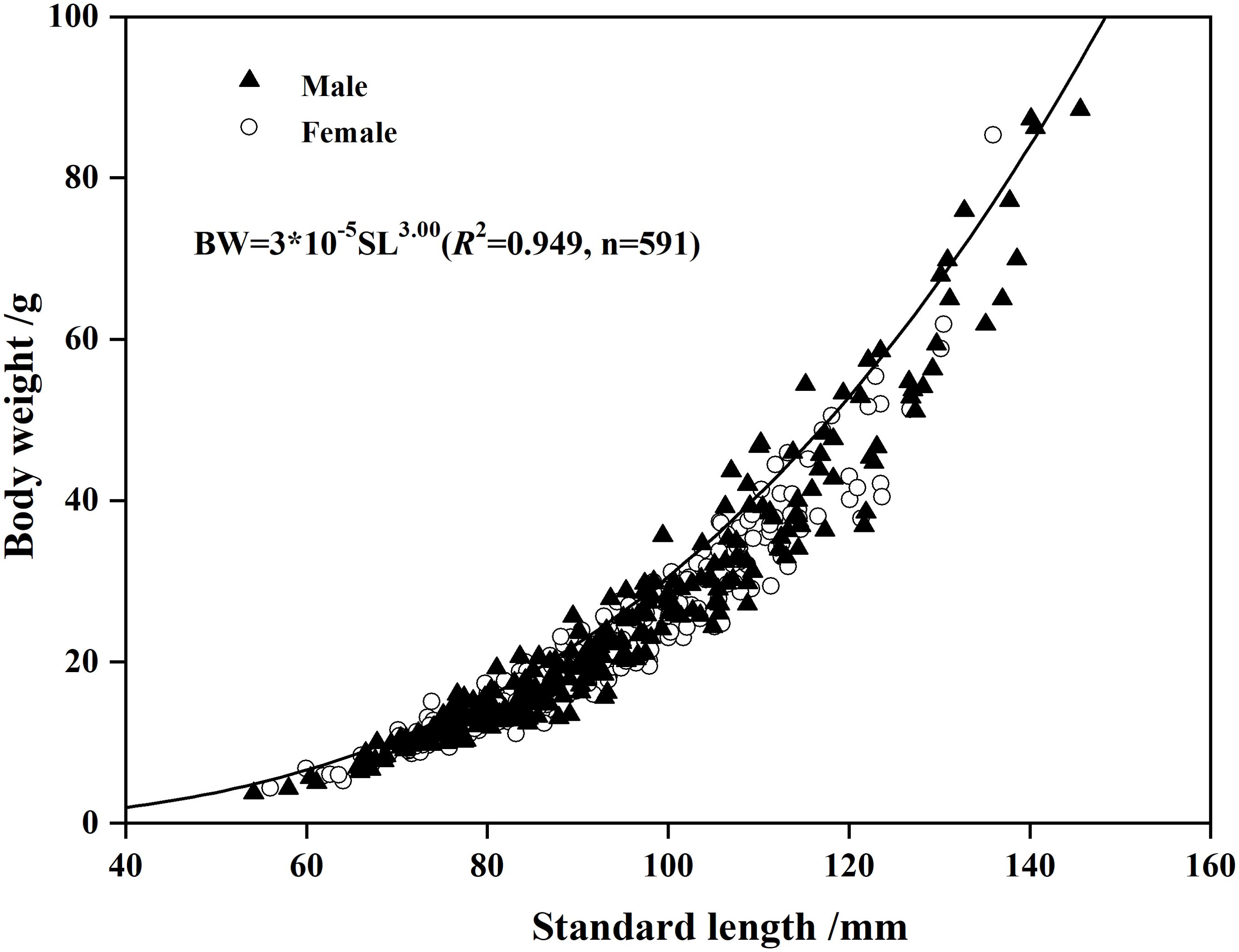
Figure 6. Relationship of standard length (SL) to body weight (BW) for Odontobutis potamophila collected from Nansi Lake during August 2017 through July 2018.The function is (n = 591, R2 = 0.949).
The total mortality rates (Z) obtained via the age structure catch curve was 1.69/year (n=591, R2 = 0.97; Figure 7). The natural mortality (M) estimated was 0.53/year, the fishing mortality rate (F) was 1.16/year, and the exploitation rate (E) was 0.69/year.
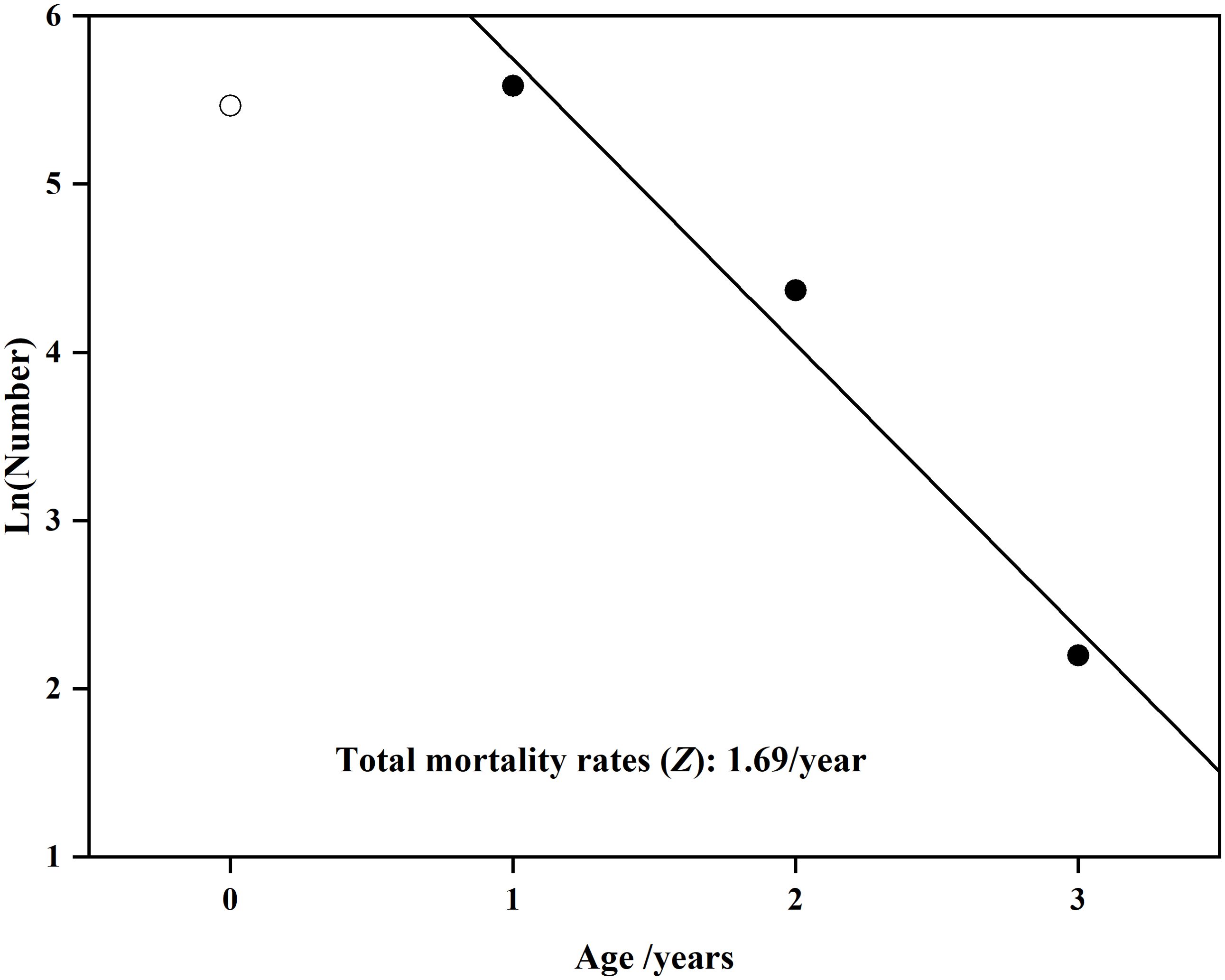
Figure 7. Linear regression fitted to the descending limb of age structure catch curve. Slope of the regression provided an estimate of the total mortality rates (Z) for Odontobutis potamophila collected from Nansi Lake.
This is the first study to provide age-verified information on the sagittal otoliths, growth, and mortality of O. potamophila from Nansi Lake. Annuli in otoliths of fish are typically identified by successive opaque/translucent growth zones (Yoneda et al., 2007; Uehara et al., 2020), and such growth zones were also observed in our study. The otolith edge-type analyses revealed an annual pattern of opaque zone deposition. The opaque zone deposition in otoliths occurred from December 2017 to May 2018 appears to be associated with increased water temperatures in the study area. The translucent zone was laid down when water temperatures increased, whereas the decrease in water temperature was accompanied by the laying down of the opaque zone. This temperature-dependent seasonal growth pattern in otoliths has been observed in many fish species, including Coilia mystus (He et al., 2008), Pelates octolineatus (Veale et al., 2015), Siniperca kneri (Zhao et al., 2018), and Pseudorhombus jenynsii (Coulson et al., 2020). The results of the present study show that the transition from the opaque zone to the translucent zone occurs in March and May (Figure 3), at the during the spawning season, similar to that previously reported for this species in Taihu Lake (Sun and Guo, 1996).
Information about the age structure and growth parameters of the fish population would be used for fish stock assessment (Hollyman, 2017).These parameters can be utilized to modify fishing strategies, thereby promoting ecosystem restoration and regeneration. This can be achieved by reducing fishing effort, implementing selective fishing, establishing marine protected areas and rebuilding depleted stocks, among other measures. The aforementioned measures are intended to reduce pressures on marine ecosystems, conserve biodiversity and achieve the long-term sustainability of fisheries resources. Otolith analysis demonstrated that the maximum age of this species caught in Nansi Lake was 3 years in both sexes (Figure 4), indicating that this species is short-lived. The maximum age that we estimated is likely to be close to the maximum age (4-year in Taihu Lake and Chaohu Lake respectively) for this species. Individuals of age-0 and -1 accounted for 85.11% of the harvest, suggesting that younger fish dominated Nansi Lake population (Figure 4). Given the short lifespan of the O. potamophila, which is similar to that of Neogobius melanostomus, a highly successful invasive species in North America and Europe (Kornis et al., 2017). it is intriguing to consider the potential parallels in sexual maturation and reproduction. However, given the absence of direct examination of maturity-at-age and measurement of gonad development in the O. potamophila, it remains unfeasible to make conclusive inferences regarding its reproductive capacity. Consequently, future research should concentrate on these aspects to facilitate a more comprehensive understanding of the reproductive patterns of the O. potamophila. This would facilitate a more informed comparison with N. melanostomus and could provide valuable insights into the ecological and evolutionary strategies of the O. potamophila (Cerwenka et al., 2023). This study shows that small dataset variations significantly affect the selection of the best-fit growth model. The ARSS showed no significant difference in the SL-at-age relationship between males and females (Table 2). Data from both sexes were then combined to fit growth functions and subsequently, the Logistic GF was concluded to be the best model to describe the growth pattern of Nansi Lake population based on the AICc and Δi selection. Smaller values of AICc and ΔI are more appropriate, and larger R2 (Huang et al., 2022). This study is also the first to estimate growth parameters for this species worldwide. The Logistic GF parameters obtained from the pooled dataset (=172.94 mm, K=0.46 year-1, t0 = 0.79 year, =4.14) were much larger than the values reported by Zhu et al. (1999) of the same genus O. potamophila in Bao’an Lake, China (Female: =152.90 mm, K=0.41 year-1, t0 = 0.16 year, =1.41; Male: =167.10 mm, K=0.37 year-1, t0 = 0.11 year, =1.36).
Sexual growth dimorphism was not evident in length-weight relationships (Afrooz et al., 2014). There were no statistical differences in the LWRs between sexes and demonstrated isometric growth patterns. The b-values exhibits differences in waters from different regions (Sun and Guo, 1996; Liu, 2001; Tang et al., 2015), while the observed differences in this value may be attributed to differences in habitat, sample size used for disease, and genetic diversity (Wootton, 1998; Dong et al., 2019) (Table 4).
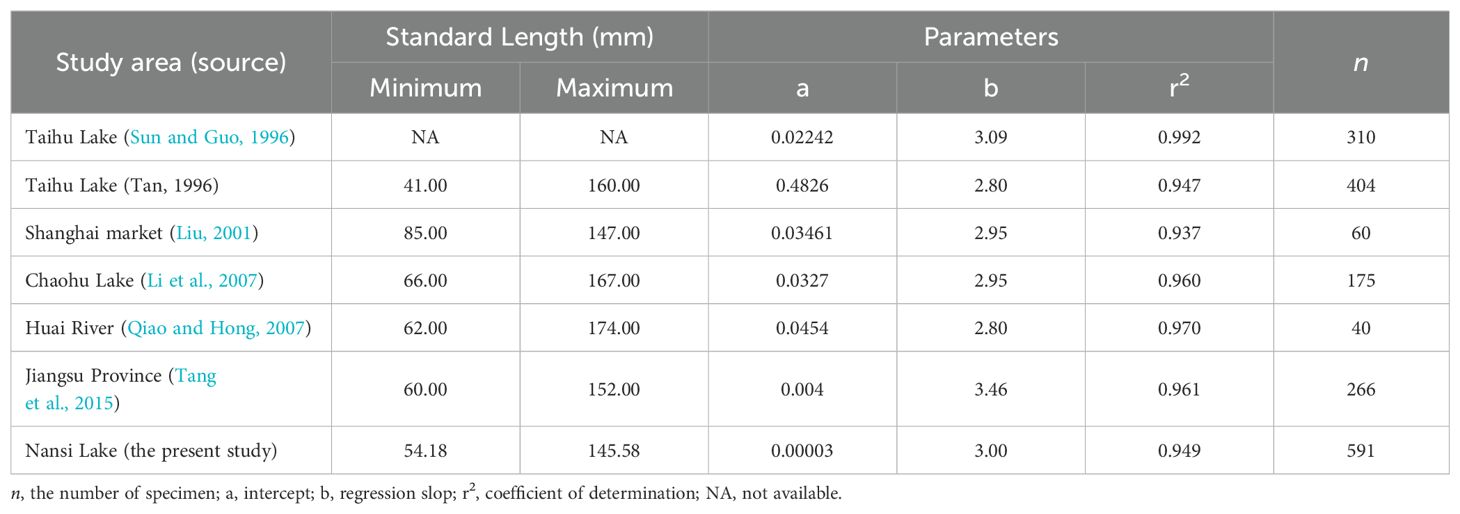
Table 4. Standard length-body weight relationships of Odontobutis potamophila of different populations from literatures.
Although our results showed no statistical difference in the mean SL between sexes, males tended to be larger than females by mRNA-seq and miRNA-seq analysis (Zhao et al., 2017; Zhang et al., 2018). This indicated some trade-off between growth and reproduction. Because male displays parental care behavior which consumes a lot of energy during the spawning period (Hao, 1960). Prior to sexual maturity, males invest more energy into somatic growth while females put energy into gonads, which induces males tended to be larger. Additionally, large individuals gain an advantage during courtship and obtain more chances to mate (Ahti et al., 2020). The observed lack of difference in size between males and females may be due to the fact that the samples were collected randomly and did not differentiate between males and females at the same time of the year. In addition, the samples were grown in the same environment, which could have affected the observations.
In this study, the total mortality (Z), natural mortality (M), fishing mortality (F) and exploitation rate (E) were evaluated for the first time in the Nansi Lakes, which is essential for the implementation of sustainable fisheries management strategies (Ogle, 2016). It was determined that the Z value of 1.69 per year was less than the value for small but high-mortality species, which may be attributed to varying fishing effort and the lake’s heterogeneous habitat (Dong et al., 2019). The M/K ratio is a pivotal metric in evaluating and overseeing the well-being of fishery resources. It plays a pivotal role in formulating fisheries management policies that are grounded in scientific principles. The M/K ratio of 1.15 was within the 1.12-2 range. The M/K ratio of 1.15 fell within the 1.12-2.0 range, indicating that the M value was reasonably well estimated (Pauly, 1980). The M value of 0.53/year was higher than the 0.2-0.3/year value, suggesting that species in the Nansi Lakes have a relatively short lifespan and relatively high natural mortality rates among species (Pauly, 1980). The fishing mortality rate (F) was found to be 1.17 per year, a figure that is considerably lower than those previously reported. This discrepancy may be attributed to the utilization of disparate fishing gears (Ye et al., 2014; Dong et al., 2019). Based on the estimates of F and Z, the exploitation rate E for the Nansi lakes was 0.69/year. Either VBGF or logistic growth function, they are used to estimate and K. In accordance with the rule of thumb (E > 0.5 indicates that the stock is experiencing overexploitation), the study found that the findings suggest that the stock is slightly overexploited (Clovis and Simon, 2024), emphasizing the need for stricter fisheries management policies. For example, implementing seasonal restrictions or establishing catch limits may help protect younger age classes. This study represents the inaugural examination of specific pairs of O. potamophila growth and mortality rates, which may be considered in future research, commencing with other lakes (e.g., Taihu Lake) for specific causes.
In conclusion, the results of this study indicate that opaque areas in O. potamophila otoliths form annually and that the opaque areas that form are a consequence of O. potamophila ageing. Meanwhile, our results regarding the age structure, growth, and mortality provided fundamental information for stock assessment and sustainable management strategies for this important commercial fishery resource. The relatively small maximum size, low maximum age, predominance of younger fish and relatively high natural mortality determined an opportunistic life-history trait, This opportunistic life history may lead O. potamophila to grow rapidly in the early stages of their life cycle in order to reach reproductive conditions as soon as possible, and to reproduce multiple times during their life cycle in order to increase their reproductive opportunities. Based on our conclusions that the life span of O. potamophila is concentrated in the 0-1 year age range, it is possible that O. potamophila reach sexual maturity at a relatively young age in order to start reproducing as early as possible and produce a large number of surviving offspring, suggesting a relative resilient to exploitation (Hitt et al., 2020). Furthermore, the opportunistic and equilibrium traits of O. potamophila may enhance its resilience to exploitation and adaptability to changing environments. These findings are crucial for formulating conservation policies and sustainable fishery practices, as in the case of the invasive goby Tridentiger bifasciatus in Nansi Lake (Mashiko, 1976; Qin et al., 2020). Consequently, future studies on the reproductive biology, parental care behavior and feeding habits of this species will contribute to management of fishery resources and assessing reproductive biology or parental care behaviors.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
The animal study was approved by The Research Committee of the Institute of Hydrobiology, Chinese Academy of Sciences. The study was conducted in accordance with the local legislation and institutional requirements.
MX: Conceptualization, Formal analysis, Writing – original draft. HL: Data curation, Writing – review & editing. BL: Visualization, Writing – original draft. HG: Investigation, Writing – original draft. CZ: Methodology, Writing – original draft. MW: Software, Writing – review & editing. JW: Supervision, Writing – review & editing. SX: Funding acquisition, Project administration, Writing – review & editing. LZ: Funding acquisition, Resources, Visualization, Writing – review & editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. The Major Science and Technology Project of Hubei Province (grant number 2022BBA009); This study was supported by the Science and Technology Project of Jiangsu Province (grant number BE2022421); Huai’an Major Science and Technology Project (grant number HAN202213); Huai’an Natural Science Research Program (grant number HAB202377); Jiangsu Collaborative Innovation Center of Regional Modern Agriculture & Environmental Protection (grant number HSXT30322);Central Public-interest Scientific Institution Basal Research Fund (No.YFI202415).
We would like to express my gratitude to all those who took part in the field sampling.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declare that no Generative AI was used in the creation of this manuscript.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmars.2024.1528582/full#supplementary-material
Afrooz E., Gholamreza E., Seyed A. R. H. (2014). Length-weight relationship and Fulton’s condition factor of Carasobarbus luteus (Heckel 1843) in Hoor Al-azim wetland. J. Ecol. Natural Environ. 6, 238–243. doi: 10.5897/jene2014.0449
Ahti P. A., Kuparinen A., Uusi-Heikkilä S. (2020). Size does matter—the eco-evolutionary effects of changing body size in fish. Environ. Rev. 28, 311–324. doi: 10.1139/er-2019-0076
Anderson D. R., Burnham K. P. (2002). Avoiding pitfalls when using information-theoretic methods. J. wildlife Manage., 912–918. doi: 10.2307/3803155
Britton J. R., Blackburn R. (2014). Application and utility of using otolith weights in the ageing of three flatfish species. Fisheries Res. 154, 147–151. doi: 10.1016/j.fishres.2014.02.005
Campana S. E. (2001). Accuracy, precision and quality control in age determination, including a review of the use and abuse of age validation methods. J. Fish Biol. 59, 197–242. doi: 10.1111/j.1095-8649.2001.tb00127.x
Campana S. E., Thorrold S. R. (2001). Otoliths, increments, and elements: keys to a comprehensive understanding of fish populations? Can. J. Fisheries Aquat. Sci. 58, 30–38. doi: 10.1139/f00-177
Cerwenka A. F., Brandner J., Dashinov D., Geist J. (2023). Small but Mighty: The Round Goby (Neogobius melanostomus) as a Model Species of Biological Invasions. Diversity 15, 528. doi: 10.3390/d15040528
Chen Y., Jackson D., Harvey H. (1992). A comparison of von Bertalanffy and polynomial functions in modeling fish growth data. Can. J. Fisheries Aquat. Sci. 49, 1228–1235. doi: 10.1139/f92-138
Clovis H. I. A., Simon A. M. (2024). Understanding overfishing: A literature review. Asian J. Fisheries Aquat. Res. 26, 61–71. doi: 10.9734/ajfar/2024/v26i1727
Coulson P. G., Hodgkinson D. J., Beckley L. E. (2020). Age validation and growth of the small-tooth flounder Pseudorhombus jenynsii from estuaries and coastal waters in south-western Australia. Ichthyological Res. 68, 249–262. doi: 10.1007/s10228-020-00784-0
de Santana H. S., Dei Tos C., Minte-Vera C. V. (2020). A review on the age and growth studies of freshwater fish in South America. Fisheries Res. 222, 105410. doi: 10.1016/j.fishres.2019.105410
Devries D. R., Frie R. V. (1996). “Determination of age and growth,” in Fisheries techniques, 2nd ed. Eds. Murphy B. R., Willis D. W. (Bethesda, MD: American Fisheries Society), 732.
Ding C. Z., Jiang X. M., Chen L. Q., Juan T., Chen Z. M. (2016). Growth variation of Schizothorax dulongensis Huang 1985 along altitudinal gradients: Implications for the Tibetan Plateau fishes under climate change. J. Appl. Ichthyology 32, 729–733. doi: 10.1111/jai.13102
Dong X. H., Xiang T., Ju T., Li R. J., Ye S. W., Lek S., et al. (2019). Age, growth, mortality and recruitment of thin sharpbelly Toxabramis swinhonis Günther 1873 in three shallow lakes along the middle and lower reaches of the Yangtze River basin, China. PeerJ 7, e6772. doi: 10.7717/peerj.6772
Elliott J. (1983). Fish stock assessment. A manual basic Methods by J. A. Gulland. Biometrics 40, 869–870. doi: 10.2307/2530936
Ferri J., Brzica A. (2022). Age, growth, and utility of otolith morphometrics as predictors of age in the european barracuda, sphyraena sphyraena (Sphyraenidae): preliminary data. Fishes 7, 68. doi: 10.3390/fishes7020068
Froese R. (2006). Cube law, condition factor and weight-length relationships: history, meta-analysis and recommendations. J. Appl. Ichthyology 22, 241–253. doi: 10.1111/j.1439-0426.2006.00805.x
Gulland J. (1965). “Estimation of mortality rates,” in Annex to Artic Fisheries Working Group Reports (International Council for the Exploration of the Sea (ICES, Copenhagen). doi: 10.1002/sim.4780040210
Guo C. B., Ye S. W., Lek S., Liu J. S., Zhang T. L., Yuan J., et al. (2013). The need for improved fishery management in a shallow macrophytic lake in the Yangtze River Basin: Evidence from the food web structure and ecosystem analysis. Ecol. Model. 26, 137–147. doi: 10.1016/j.ecolmodel.2013.07.013
Han M. X., Qin J. W., Duan Y. J., Luo X. N., Jiang Y. S. (2022). Relationships between otolith quality and age in the Odontobutis yaluens. J. Liaodong Univ. 29, 6–11. doi: 10.14168/j.issn.1673-4939.2022.01.02
Hao T. H. (1960). An ecological study of a fresh-water goby Odontobutis obscura (Temm. et schl.) in Liangtze Lake of Hupei. Acta Hydrobiologica Sin. 2, 145–158.
He W. P., Li Z. J., Liu J. S., Li Y. X., Murphy B. R., Xie S. G. (2008). Validation of a method of estimating age, modelling growth, and describing the age composition of Coilia mystus from the Yangtze Estuary, China. Ices J. Mar. Sci. 65, 1655–1661. doi: 10.1093/icesjms/fsn143
Hitt N. P., Rogers K. M., Kelly Z. A., Henesy J., Mullican J. E. (2020). Fish life history trends indicate increasing flow stochasticity in an unregulated river. Ecosphere 11, e03026. doi: 10.1002/ecs2.3026
Huang S.-C., Chang S.-K., Lai C.-C., Yuan T.-L., Weng J.-S., He J.-S. (2022). Length-Weight Relationships, Growth Models of Two Croakers (Pennahiamacrocephalus and Atrobucca nibe) off Taiwan and Growth Performance Indices of Related Species. Fishes 7, 281. doi: 10.3390/fishes7050281
Khan M. A., Nazir A., Banday U. Z. (2018). Utility of otolith weight to estimate age of Labeo bata (Actinopterygii: Cypriniformes: Cyprinidae) inhabiting the Ganga River. Acta Ichthyologica Piscatoria 48, 257–260. doi: 10.3750/aiep/02342
Kornis M. S., Weidel B. C., Vander Zanden M. J. (2017). Divergent life histories of invasive round gobies (Neogobius melanostomus) in Lake Michigan and its tributaries. Ecol. Freshw. Fish 26, 563–574. doi: 10.1111/eff.12300
Li Y. H., Wan Q., Zou C. W., Gao L. (2007). Determination of the biology of the Odontobutis obscurus in Chaohu Lake. J. Aquaculture 06, 6–9.
Liu L. P. (2001). Primary study on reproductive biology of Odontobutis potamophila. Inland Fisheries 11, 4–5.
Liu M., Hu X., Han Q., Luo Y. (2008). Variation of the proteinic enzymes activities during the embryonic and larval development of Odontobutis potamophila. Freshwater Fish 38, 39–41.
Ma B., Xie C., Huo B., Yang X., Li P. (2011). Age validation, and comparison of otolith, vertebra and opercular bone for estimating age of Schizothorax o’connori in the Yarlung Tsangpo River, Tibet. Environ. Biol. Fishes 90, 159–169. doi: 10.1007/s10641-010-9727-5
Mashiko K. (1976). Reproductive behavior of an eleotrid goby Odontobutis obscurus in aquaria. Japanese J. ichthyology 23, 69–78. doi: 10.11369/jji1950.23.69
Nazir A., Khan M. A. (2020). Stock-specific assessment of precise age and growth in the long-whiskered catfish Sperata aor from the Ganges River. Mar. Freshw. Res. 71, 1693–1701. doi: 10.1071/MF19315
Panfili J., Morales-Nin B. (2002). “Semi-direct validation,” in Manual of Fish Sclerochronology. Eds. Panfili J., Pontual H., Troadec H., Wright P. J. (Ifremer-IRD, Brest), pp 129–pp 134.
Pauly D. (1980). On the interrelationships between natural mortality, growth parameters, and mean environmental temperature in 175 fish stocks. ICES J. Mar. Sci. 39, 175–192. doi: 10.1093/icesjms/39.2.175
Pauly D., Munro J. (1984). Once more on the comparison of growth in fish and invertebrates. Fishbyte Newslett. Network Trop. Fisheries Scientists 2, 21.
Phelps Q. E., Edwards K. R., Willis D. W. (2011). Precision of five structures for estimating age of common carp. North Am. J. Fisheries Manage. 27, 103–105. doi: 10.1577/M06-045.1
Qiao D. L., Hong L. (2007). Morphological biology and fecundity of Odontobutis obscura in Huai River water system. Chin. J. Ecol. 26, 228–232.
Qin J., Xiang M., Jia M. X., Cheng F., Zhang L., Schmidt B. V., et al. (2020). Combined opportunistic and equilibrium life-history traits facilitate successful invasions of the Shimofuri goby (Tridentiger bifasciatus). Aquat. Invasions 15, 514–528. doi: 10.3391/ai.2020.15.3.10
Qu X., Chen Y., Liu H., Xia W., Lu Y., Gang D., et al. (2020). A holistic assessment of water quality condition and spatiotemporal patterns in impounded lakes along the eastern route of China’s South-to-North water diversion project. Water Res. 185, 116275. doi: 10.1016/j.watres.2020.116275
Ricker W. E. (1975). Computation and interpretation of biological statistics of fish populations. Bull. Fisheries Res. Board Canada 191, 401.
Soeth M., Fávaro L. F., Spach H. L., Daros F. A., Woltrich A. E., Correia A. T. (2019). Age, growth, and reproductive biology of the Atlantic spadefish Chaetodipterus faber in southern Brazil. Ichthyological Res. 66, 140–154. doi: 10.1007/s10228-018-0663-2
Stewart J., Hughes J. M. (2007). Age validation and growth of three commercially important hemiramphids in south-eastern Australia. J. Fish Biol. 70, 65–82. doi: 10.9734/ajfar/2024/v26i1727
Sun G. Y., Guo X. Y. (1996). Studies on the biology of Odontobutis potamophila in Taihu Lake. J. fisheries China 20, 194–202.
Sun G. Y., Guo X. Y., Guo X. Y. (1996). Biological studies on the sandtongue snakehead in the Taihu River. J. Aquat. Sci. 03), 2–11.
Tan B. p.. (1996). A preliminary study on the growth and feeding habits of the sand pond snakehead in Taihu Lake. Journal of Hubei Agricultural University 01, 31–37. doi: 10.1111/jai.12492
Tang S. K., Zhang T. Q., Wang M. H., Zhou G., Zhong L. Q., Li D. M., et al. (2015). Length-weight relationships of seven freshwater fishes from the JiangSu province, China. J. Appl. Ichthyology 31, 231–232. doi: 10.1111/jai.12492
Uehara M., Ebisawa A., Ohta I. (2020). Comparative age-specific demography of four commercially important deep-water snappers: implication for fishery management of a long-lived lutjanid. J. Fish Biol. 97, 121–136. doi: 10.1111/jfb.14332
Veale L., Coulson P., Hall N., Hesp A., Potter I. C. (2015). Age and size compositions, habitats, growth and reproductive characteristics of a terapontid (Pelates octolineatus) in coastal waters. Mar. Freshw. Res. 66, 535–548. doi: 10.1071/mf14079
Wang P. P., Ren M., Chen S. Q., Yin S. W., Zhao C., Zhang. H. Y., et al. (2017). Characterization and development of 56 EST-SSR markers derived from the transcriptome of Odontobutis potamophila. Genet. Mol. Res. 16, 4238. doi: 10.4238/gmr16029129
Wang F., Zhang S. L., Hou H. P., Yang Y. Y., Gong Y. L. (2019). Assessing the changes of ecosystem services in the Nansi Lake wetland, China. Water 11, 788. doi: 10.3390/w11040788
Wootton R. (1998). Ecology of teleost fishes. 2nd ed. (Boston: Kluwer Academic Publishers). doi: 10.1016/0044-8486(91)90031-2
Xiang M., Rypel A. L., Cheng F., Qin J., Zhang L., Chen Y., et al. (2021). Shift towards opportunistic life-History of sleeper in response to multi-Decadal overfishing. Water 13, 2582. doi: 10.3390/w13182582
Ye S., Moreau J., Zeng W., Zhang T., Liu J., Li Z. (2014). Growth and mortality of two small fishes, Toxabramis swinhonis Günther 1873 and Hyporhamphus intermedius (Cantor 1842), in a Yangtze shallow lake (China) assessed by length frequency analysis. J. Appl. Ichthyology 30, 479–484. doi: 10.1111/jai.12405
Yoneda M., Kurita Y., Kitagawa D., Ito M., Tomiyama T., Goto T., et al. (2007). Age validation and growth variability of Japanese flounder Paralichthys olivaceus off the Pacific coast of northern Japan. Fisheries Sci. 73, 585–592. doi: 10.1111/j.1444-2906.2007.01371.x
Zhang H., Zhao C., Yin S., Li Z., Cao Q., Li X., et al. (2018). Characterization and identification of single nucleotide polymorphism within the IGF-1R gene associated with growth traits of Odontobutis potamophila, J. World Aquacult. Soc 49, 366–379. doi: 10.1111/jwas.12504
Zhao S. S., Cheng F., Hou G., Hu Z. Y., Xie S. G. (2018). Opportunistic-tended life history traits of Siniperca kneri in the Three Gorges Reservoir, China: potential responses to impoundment. J. Oceanology Limnology 37, 694–705. doi: 10.1007/s00343-019-7364-1
Zhao C., Zhang G., Yin S., Li Z., Wang Q., Chen S., et al. (2017). Integrated analysis of mRNA-seq and miRNA-seq reveals the potential roles of sex-biased miRNA-mRNA pairs in gonad tissue of dark sleeper (Odontobutis potamophila). BMC Genomics 18, 613. doi: 10.1186/s12864-017-3995-9
Keywords: age validation, logistic growth function, Odontobutis potamophila, otolith, overfishing indicators
Citation: Xiang M, Liu H, Li B, Guo H, Zhu C, Wang M, Wang J, Xie S and Zhang L (2025) Otolith annulus validation and population dynamics of dark sleeper Odontobutis potamophila: insights for sustainable fisheries management. Front. Mar. Sci. 11:1528582. doi: 10.3389/fmars.2024.1528582
Received: 15 November 2024; Accepted: 27 December 2024;
Published: 23 January 2025.
Edited by:
Josipa Ferri, University of Split, CroatiaReviewed by:
Aafaq Nazir, National Taiwan University, TaiwanCopyright © 2025 Xiang, Liu, Li, Guo, Zhu, Wang, Wang, Xie and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lei Zhang, emhhbmdsZWlAaWhiLmFjLmNu
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.