
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Mar. Sci. , 11 December 2024
Sec. Marine Ecosystem Ecology
Volume 11 - 2024 | https://doi.org/10.3389/fmars.2024.1510854
This article is part of the Research Topic The Threat of Invasive Alien Species and the Challenge of Climate Change View all articles
Introduction: Biomass allocation between aboveground and belowground pools in salt marshes has distinct effects on salt marsh stability, and is influenced by climate warming and reproductive investment. However, the lack of studies on the effect of latitudinal variations in reproductive investments and biomass allocation in salt marshes makes it difficult to explore mechanisms of marsh plant growth to climate warming across geographical scales. The rapid invasion of the salt marsh grass Spartina alterniflora into lower latitude marshes around the world provides an opportunity to investigate biomass allocation and reproductive investment across latitudes, helping to understand how salt marshes respond to climate warming.
Methods: Therefore, we investigated aboveground biomass (AGB), belowground biomass (BGB), total biomass, sexual reproduction traits (inflorescence biomass, flowering culm), asexual reproduction traits (shoot number, rhizome biomass), among S. alterniflora at 19 sites in 10 geographic locations over a latitudinal gradient of ~2000 km from Dongying (37.82°N, high latitude) to Danzhou (19.73°N, low latitude) in China.
Results: The AGB, BGB, and total biomass displayed hump shaped relationships with latitude, but the BGB: AGB ratio decreased with increasing latitude (i.e. increased linearly with temperature). Interestingly, we found that the BGB: AGB ratio negatively correlated with sexual reproductive investment, but positively correlated with asexual reproductive investment.
Discussion: While conceptual and numerical models of salt marsh stability and carbon accumulation often infer responses based on aboveground biomass, our study suggests that salt marsh responses to climate warming based on aboveground biomass and static allocations may bias estimates of future salt marsh production driven by climate warming.
Biomass allocation is an essential plant functional trait that reflects plant adaptive strategies (Poorter et al., 2012). Moreover, biomass allocation affects ecosystem functions based on aboveground biomass (AGB) and belowground biomass (BGB) allocation, such as primary production (Kirwan et al., 2009), storm surge abatement (Gedan et al., 2011), and carbon storage (Ma et al., 2021). Temperature and precipitation are the main factors affecting biomass allocation over wide latitudinal gradients, the effects and relative importance are different between terrestrial ecosystems (Liu et al., 2021; Ma et al., 2021; Qi et al., 2019; Wang et al., 2016). Resource allocation to reproduction is also influenced by temperature (Yue et al., 2020), precipitation (Wang et al., 2018), water depth (Li et al., 2019), and salinity (Xue et al., 2018). Furthermore, in suboptimal growth conditions, plants can suppress reproductive allocation but conserve vegetative allocation to ensure survivorship (Wenk and Falster, 2015). The trade-off between growth and reproduction suggests that reproductive investment affects the plant’s biomass allocation (Lowry et al., 2019). There are few studies on the latitudinal pattern of aboveground and belowground biomass allocation in salt marshes (but see Crosby et al., 2017). Empirical evidence strongly supports the effects of climate warming and reproductive investment on biomass allocation separately (Ma et al., 2021; Wenk and Falster, 2015), but the combined response of allocation to both warming and reproductivity remains unknown.
In salt marshes, the aboveground organs are the main contributors to primary production, efficient in reducing wave height (Gedan et al., 2011), and helping to increase sediment deposition (Jiang et al., 2024; Mudd et al., 2010). Meanwhile, the belowground organs are the main location of carbon storage, which can increase subsurface marsh volume, enhance soil shear strength, and reduce erosion (Coleman and Kirwan, 2019; Kirwan and Megonigal, 2013; Silliman et al., 2019). While increasing temperatures can yield both positive and negative effects on salt marshes, their impact varies depending on the geographic location (Wernberg et al., 2024). In colder latitudes, warming tends to stimulate the growth of marsh plants, whereas in the tropical range limits of salt marshes, it tends to suppress or have minimal impact on their growth (Coldren et al., 2019; Smith et al., 2022). A manipulative warming experiment suggests that elevated temperatures have different effects between above and belowground organs, with optimum warming shown to be helpful for belowground biomass accumulation (Noyce et al., 2019). Latitudinal gradients of biomass allocation can help to explore the mechanisms of marsh plant growth and the potential response of salt marshes to climate warming at the geographical scale (Crosby et al., 2017). However, previous studies have always focused on the latitudinal pattern of AGB (Kirwan et al., 2009; Liu et al., 2016; Xu et al., 2020), with few studies examining changes in BGB with latitude. The timing of flowering and seeds set also varies with latitude (Crosby et al., 2015; Liu et al., 2016), which should influence biomass allocation (Chen et al., 2019; Sun et al., 2001). Therefore, the combined effects of climate warming and reproductive investment may affect above- and belowground biomass allocation along latitudinal gradients, potentially altering the stability and function of salt marshes.
Spartina alterniflora is a widely distributed salt marsh plant, native to the United States (27°N ~ 45°N) (Kirwan et al., 2009; Strong and Ayres, 2013). Previous studies in the U.S. found that S. alterniflora AGB decreased with increasing latitude, while BGB increased with increasing latitude, and that the BGB: AGB ratio increased with latitude in its native range (Crosby et al., 2017; Gross et al., 1991; Kirwan et al., 2009). S. alterniflora has been invading salt marshes for more than 40 years in China, and now occurs over ~ 20° of latitude, from 19°N ~ 40°N (An et al., 2007; Liu et al., 2016, 2018). A variety of plant functional traits show different latitudinal patterns between its native and invasive range, including reproduction traits (e.g., flowering culm, seeds set, flowering time), growth traits (e.g., size, density) and AGB (Liu et al., 2016, 2020a). These works suggest that the contrasting adaptation strategies may occurs between native and invasive ranges (Liu et al., 2020b).
Previous studies compared the trait differences between invasive and native ranges of S. alterniflora (Liu et al., 2016, 2020b). Yet most studies on biomass allocation have been conducted at local scales and focused on the response to some abiotic factors in the native (Biçe et al., 2023; Darby and Turner, 2008; Snedden et al., 2015) and invasive ranges (Tang et al., 2022; Zhao et al., 2010). The broader scale pattern of this latitudinal variation in biomass allocation of invasive S. alterniflora and its relationship with reproductive investment remains unknown. Furthermore, S. alterniflora in China extends to lower latitudes (to ~ 19°N) than studies in the US (to ~ 27°N, native range), providing a unique opportunity to reveal the more complicated latitudinal biomass allocation patterns than have been documented in the native range. In this study, we address two questions: 1) does the biomass allocation of S. alterniflora vary across latitudes within its invasive range in China? 2) do environmental factors and reproductive investment influence biomass allocation?
To investigate the latitudinal variations in biomass allocation and reproduction investment, we conducted field survey across latitude. The study locations encompassed a stretch of the Pacific coast in China, covering a distance exceeding 2000 km. Ten specific locations were chosen to represent the majority of S. alterniflora distribution in China (Figure 1). These 10 study locations ranged from Danzhou, Hainan (19.73°N), in the south to Dongying, Shandong (37.82°N), in the north (Table 1). Since the introduced in 1979, rapid spread of S. alterniflora happened in China coastal, the invasion history of S. alterniflora in each location was range from 1985 to 2000 (An et al., 2007), except Hannan in 2015 (Zhang et al., 2017). We conducted field surveys from August to October 2021, which represented the end of the growing season at all locations (Chen et al., 2021). At this time, the peak of flowering had passed and plants were beginning to senesce.
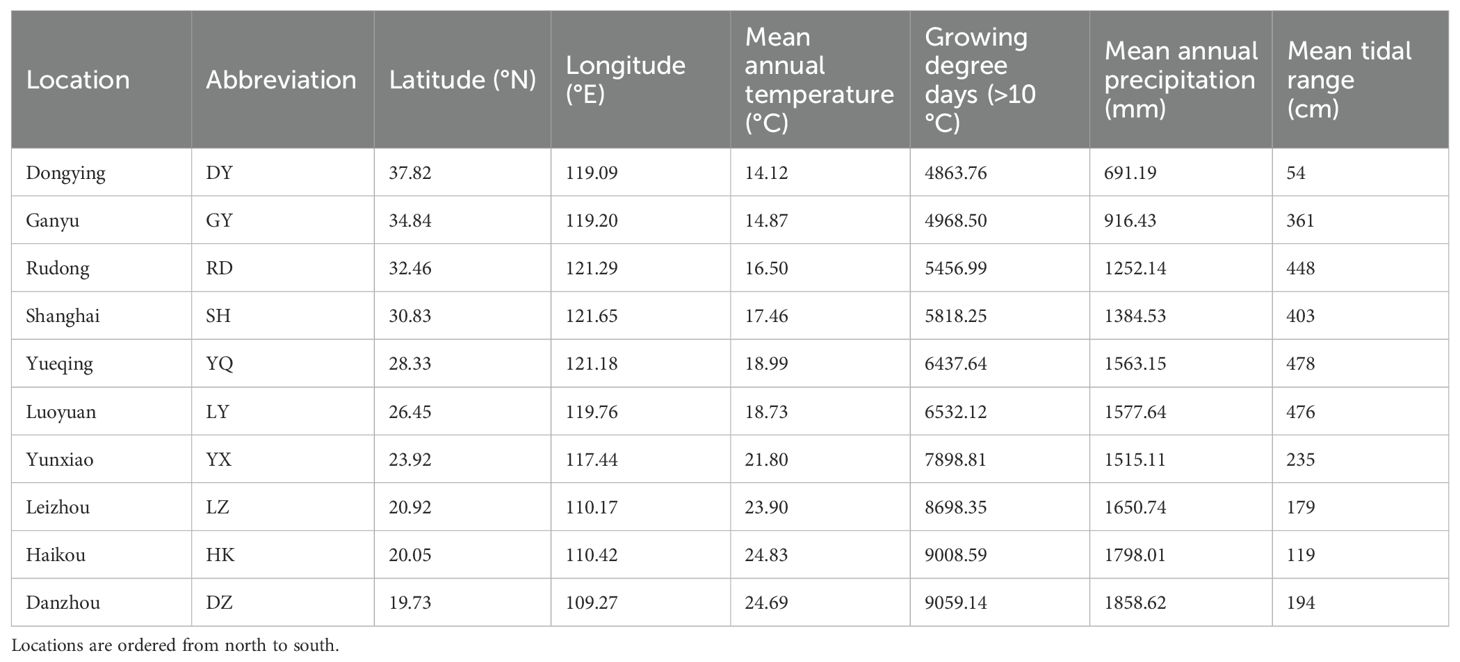
Table 1. Location name, abbreviation (used in figures), latitude and longitude, mean annual temperature, growing degree day, mean annual precipitation and mean tidal range of ten survey locations on the coast of China.
At each location, we worked at two sites, 2-3 km apart (except Danzhou). At each site, we sampled three to five 0.5*0.5 m quadrats, with at least 30 m spacing between quadrats. To standardized elevation effects across all sites, we defined the best-growing as the tallest plants at each site. And study showed that plant height of S. alterniflora have a humped relationships with elevation (Li et al., 2018), tallest plants usually growing at mid/low marsh. All quadrats were sampled in the mid/low marsh, where S. alterniflora had the tallest plant and grows best (Liu et al., 2020b). In each quadrat, all plants (including adult plants and seedlings) were counted and used as an asexual reproduction trait (Xue et al., 2018). Then flowering culms were counted and used to calculate the ratio of flowering culms to adult plants, which served as a sexual reproduction trait (Liu et al., 2016). All the aboveground parts were harvested at ground level and immediately weighed in the field. A single representative shoot, which was tallest with complete inflorescence (Liu et al., 2022b), from each quadrat was taken to the laboratory, dried at 60 °C to constant mass, and weighed. The dry mass: fresh mass ratio of these shoots in each site (total 3-5 shoots) was used to calculate aboveground dry mass for each quadrat, served as aboveground biomass (AGB). Furthermore, the inflorescence biomass was determined for each representative shoot, served as another reproduction trait (Sun et al., 2001). A cylindrical soil core (15 cm diameter and 20 cm height) containing roots was removed and washed from each quadrat, most of the belowground organs was in the upper 20 cm soil profile (Darby and Turner, 2008). Belowground organs were divided into roots and rhizomes, and were taken to the laboratory dried at 60 °C for 72 h to determine the root biomass and rhizome biomass. All biomass data, including aboveground biomass, root biomass, and rhizome biomass were calculated proportionally to biomass per square meter. The belowground biomass (BGB) is equal to the sum of root biomass and rhizome biomass. The total biomass is equal to the sum of AGB and BGB. The ratio of rhizome biomass to belowground biomass (referred to as Rhizome: BGB ratio) serves as an indicator of asexual reproduction trait (Kaldy and Dunton, 2000), and the ratio of belowground biomass to aboveground biomass (referred to as the BGB: AGB ratio) serves as an indicator of biomass allocation strategy.
To investigate the relationship between S. alterniflora biomass allocation and local environmental factors, we collected three surface soil samples (under 5-10 cm from soil surface) near the quadrats at each site. Soil water content (WC) and soil porewater salinity (PS) were measured using the soil rehydration method (Pennings and Richards, 1998). We also collocated surface soil with a 100 cm3 ring knife at the same quadrats to measure soil bulk density (BD) by the bulk density ring method (Yang et al., 2018). The annual mean tidal range (MTR), which served as a local environmental factor, was obtained from the stations closest to each local in the National Marine Data and Information Service (http://www.nmdis.gov.cn).
To analyze the relationship between S. alterniflora biomass allocation and climate environmental factors, we collected daily meteorological data, including daily mean temperature and daily rainfall, from the city closest to each site during 2012 to 2021 (Nearly 10 years) in Climate Information for the China Meteorological Data Sharing Service System (https://data.cma.cn/). Mean annual temperature (MAT), annual number of growing days (> 10 °C) and mean annual precipitation (MAP) were calculated to represent the climate differences at each site. Growing degree days (GDD) measure the number of degrees that daily temperatures exceed a threshold temperature (10 °C) necessary for significant plant growth and therefore reflect both the temperature and duration of the growing season (Kirwan et al., 2009).
For statistical analysis purposes, we assumed that S. alterniflora in each quadrat was derived from an independent patch, so that each quadrat was a replicate. Thus, we have 5-10 replicates at each location. Regression analyses, including linear regression and polynomial regression, were used to identify the latitudinal trend of total biomass, AGB, BGB, BGB: AGB ratio, inflorescence biomass, flowering culm, shoot number and Rhizome: BGB ratio, furthermore used to identify the relationships between BGB: AGB ratio and reproduction traits, which include inflorescence biomass, flowering culm, shoot number and Rhizome: BGB ratio.
We used structural equation modeling (SEM) to explore the pathways of how environmental variables affected the BGB: AGB ratio. The SEM was based on the following hypotheses: 1) environmental factors can affect BGB: AGB ratio directly or indirectly by regulating reproductive investment (e.g., sexual reproduction and asexual reproduction) that drive biomass allocation. 2) environmental factors should divide in two parts, climate environmental factors (named climate environment) and non-climate environmental factors (named local environment), which have different effects on BGB: AGB ratio and reproductive investment. Owing to the large number of explanatory variables and strong correlations among variables (Supplementary Tables S1, S2), we developed multivariate functional indices through principal component analysis (PCA) before SEM. The first principal component (PC1) from the PCA conducted for the MAT, MAP and GDD were introduced in the SEMs to represent the climate environment. The first principal component (PC1) from the PCA conducted for the MTR, WC, PS and BD were introduced in the SEMs to represent the local environment. The first principal component (PC1) from the PCA conducted for the flowering culm and inflorescence biomass were introduced in the SEMs to represent the sexual investment. The first principal component (PC1) from the PCA conducted for the ratio of Rhizome: BGB ratio and shoot number were introduced in the SEMs to represent the asexual investment. We used Fisher’s C test, the Akaike information criteria (AIC) value, and P value to evaluate the goodness of these models. All statistical analyses were performed on raw data from each quadrat, and were conducted using R version 4.1.2 with the ‘vegan’ package for RDA analysis (Dixon, 2003), and the ‘piecewiseSEM’ package for SEM analyses (Lefcheck, 2016).
Total biomass (R2 = 0.40, P < 0.001), aboveground biomass (AGB) (R2 = 0.56, P < 0.001), and belowground biomass (BGB) (R2 = 0.08, P = 0.024) all demonstrated a humped relationship with latitude (Figures 2A–C). Interestingly, the maximum values for total, aboveground, and belowground biomasses were found at similar latitudes. Specifically, the maximum AGB was 3854.95 ± 315.55 g/m², observed in YQ (28.33°N); the maximum BGB was 1658.38 ± 91.96 g/m², found in SH (30.83°N); and the maximum total biomass was 5492.23 ± 206.51 g/m², also located in SH (30.83°N). Furthermore, the ratio of BGB to AGB showed a linear decrease with increasing latitude (R2 = 0.39, P < 0.001) (Figure 3). The maximum value of the BGB: AGB ratio was 0.94 ± 0.07, found in LZ (20.92°N), while the minimum BGB: AGB ratio was 0.16 ± 0.03, observed in RD (32.46°N).
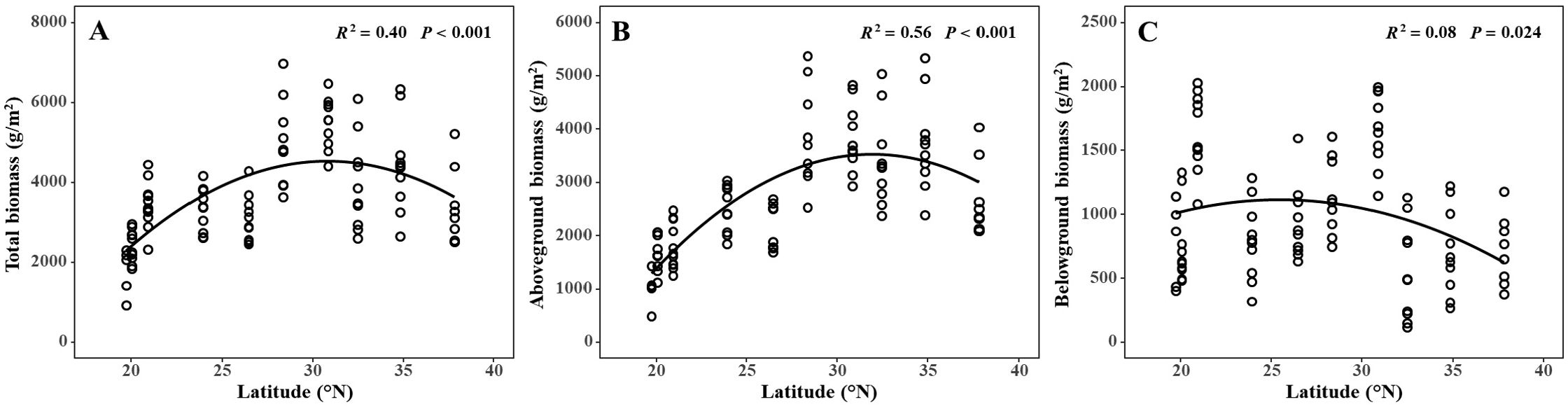
Figure 2. The relationships between (A) total biomass, (B) aboveground biomass and (C) belowground biomass and latitude.
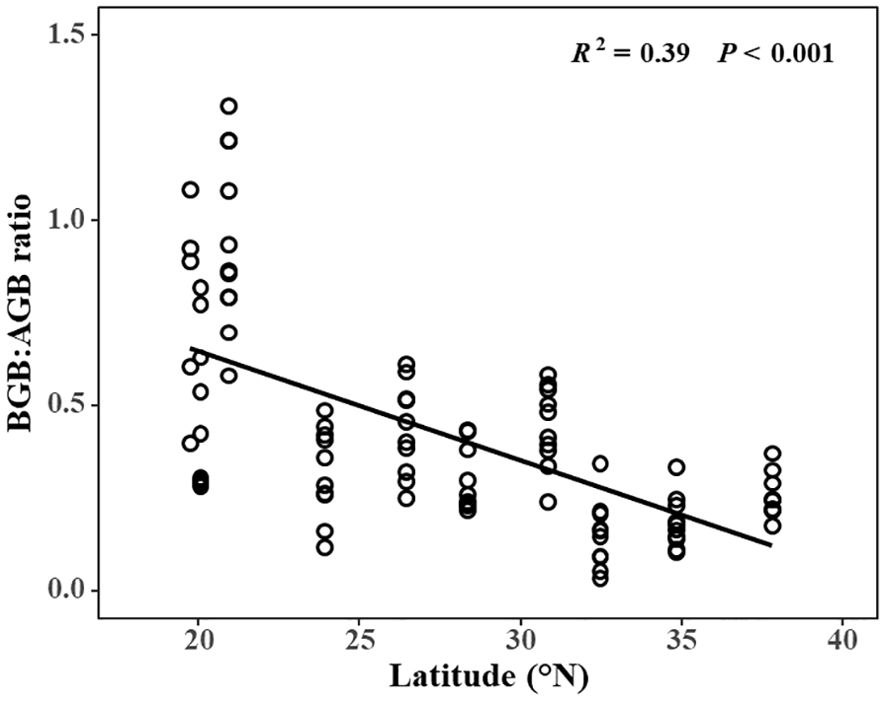
Figure 3. Relationship between the ratio of belowground biomass to aboveground biomass and latitude.
Sexual reproduction traits, specifically inflorescence biomass and flowering culm, showed a linear increase with latitude (Figures 4A, C. R2 = 0.41, P < 0.001; R2 = 0.37, P < 0.001). The inflorescence biomass varied from 0.16 ± 0.02 g/plant to 2.63 ± 0.29 g/plant, while the proportion of flowering culm to total number of adult plants ranged from 29.34 ± 3.63% to 79.14 ± 3.33%. In contrast, asexual reproduction traits, namely shoot number and Rhizome: BGB ratio, decreased linearly with increasing latitude (Figures 4B, D. R2 = 0.39, P < 0.001, R2 = 0.23, P < 0.001). The shoot number ranged from 126.00 ± 7.28 ind./m² to 547.27 ± 45.42 ind./m². Rhizome: BGB ratio varied from 34.76 ± 2.65% to 73.26 ± 3.89%. These findings highlight the distinct responses of sexual and asexual reproduction traits at latitudinal gradients.
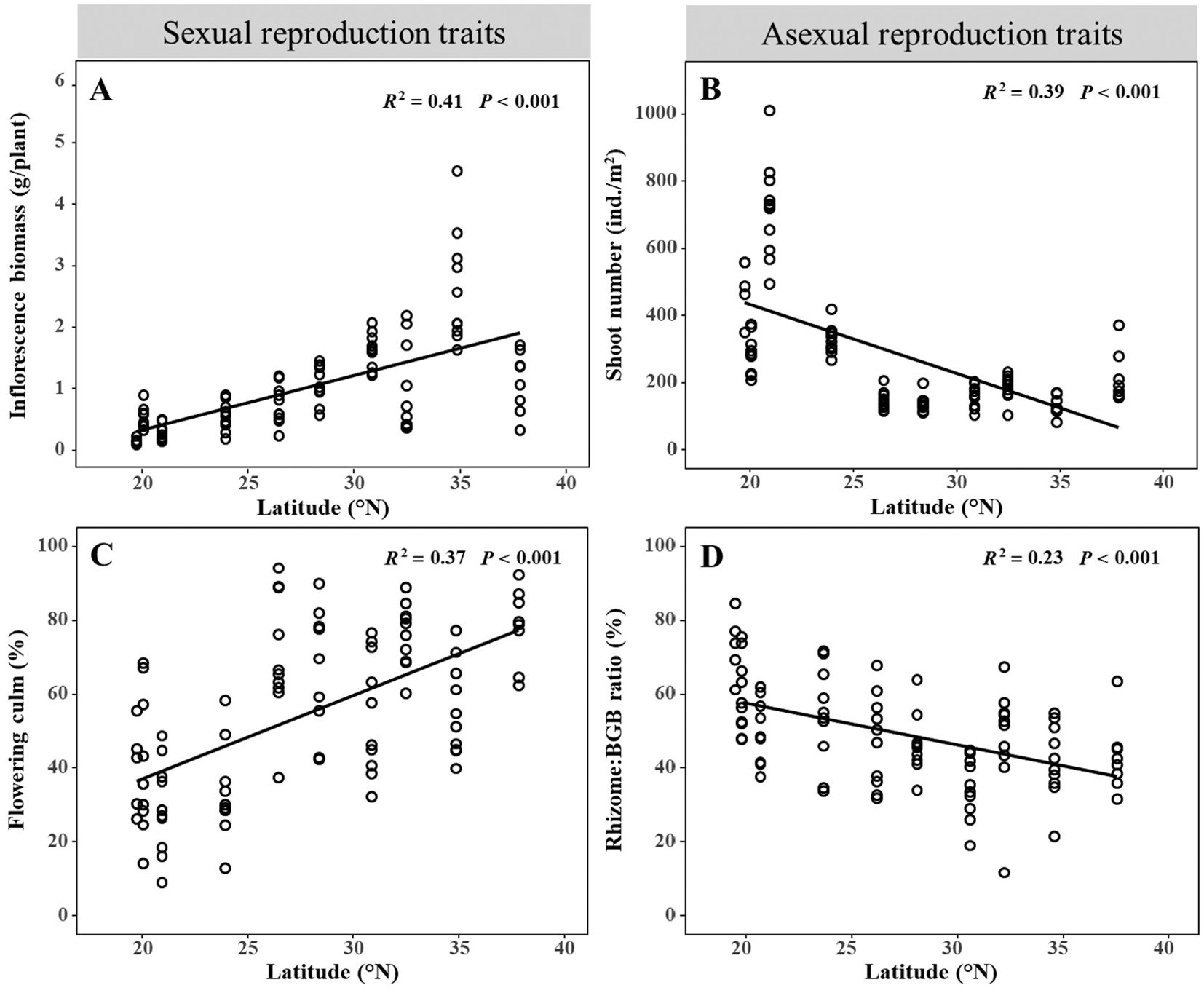
Figure 4. Relationships between sexual reproduction traits and asexual reproduction traits and latitude. Sexual reproduction traits include (A) inflorescence biomass and (C) flowering culm, asexual reproduction traits include (B) shoot number and (D) Rhizome: BGB ratio.
The BGB: AGB ratio demonstrated contrasting relationships with sexual and asexual reproduction traits, as clearly depicted in the provided scatter plots (Figure 5). For sexual reproduction traits (Figures 5A, C), the BGB: AGB ratio showed a linear decrease with an increase in inflorescence biomass (R2 = 0.21, P < 0.001) and flowering culm (R2 = 0.18, P < 0.001). On the other hand, for asexual reproduction traits (Figures 5B, D), the BGB: AGB ratio increased linearly with an increase in shoot number (R2 = 0.48, P < 0.001) and Rhizome: BGB ratio (R2 = 0.09, P = 0.003).
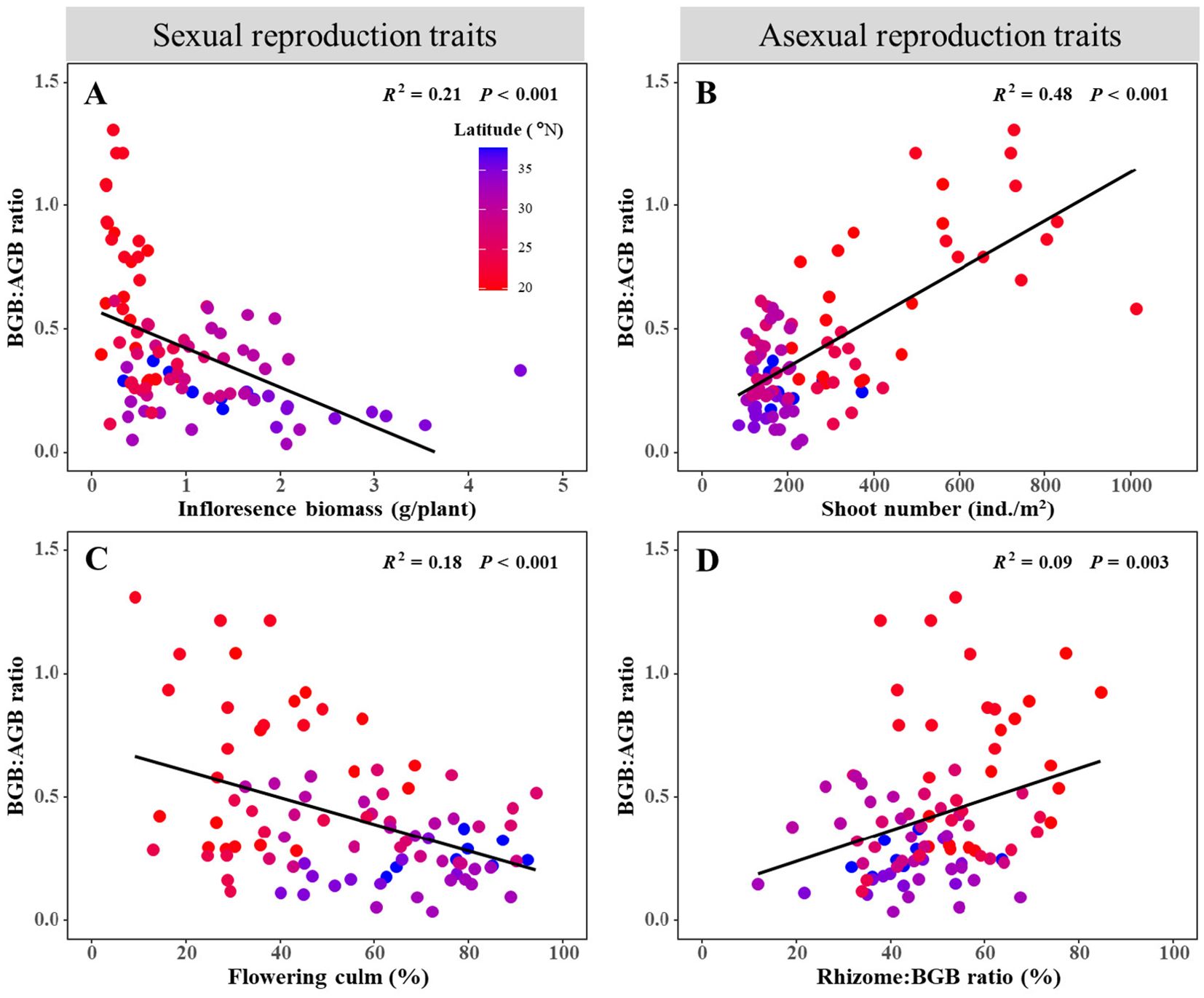
Figure 5. Relationships between sexual reproduction traits, asexual reproduction traits, and the ratio of belowground biomass to aboveground biomass. Sexual reproduction traits include (A) inflorescence biomass and (C) flowering culm, Asexual reproduction traits include (B) shoot number and (D) Rhizome: BGB ratio. The dots of different colors represent the latitude of the quadrat.
SEMs showed that the BGB: AGB ratio of S. alterniflora increased with climate environment and decreased with local environment (Figure 6). As indicated by the standardized total effects, climate environment (MAT, MAP, GDD) was more important than local environment (MTR, WC, PS, BD) in explaining the BGB: AGB ratio of S. alterniflora. SEMs revealed that the direct effects of climate and local environment on BGB: AGB ratio, but the indirect effects of climate and local environment on BGB: AGB ratio were found only in asexual investment, and the asexual investment increased with climate environment and decreased with local environment. The results of SEMs showed that both climate and local environment could directly affect BGB: AGB ratio. And then, climate and local environments could indirectly affect BGB: AGB ratio by changing the asexual reproduction.
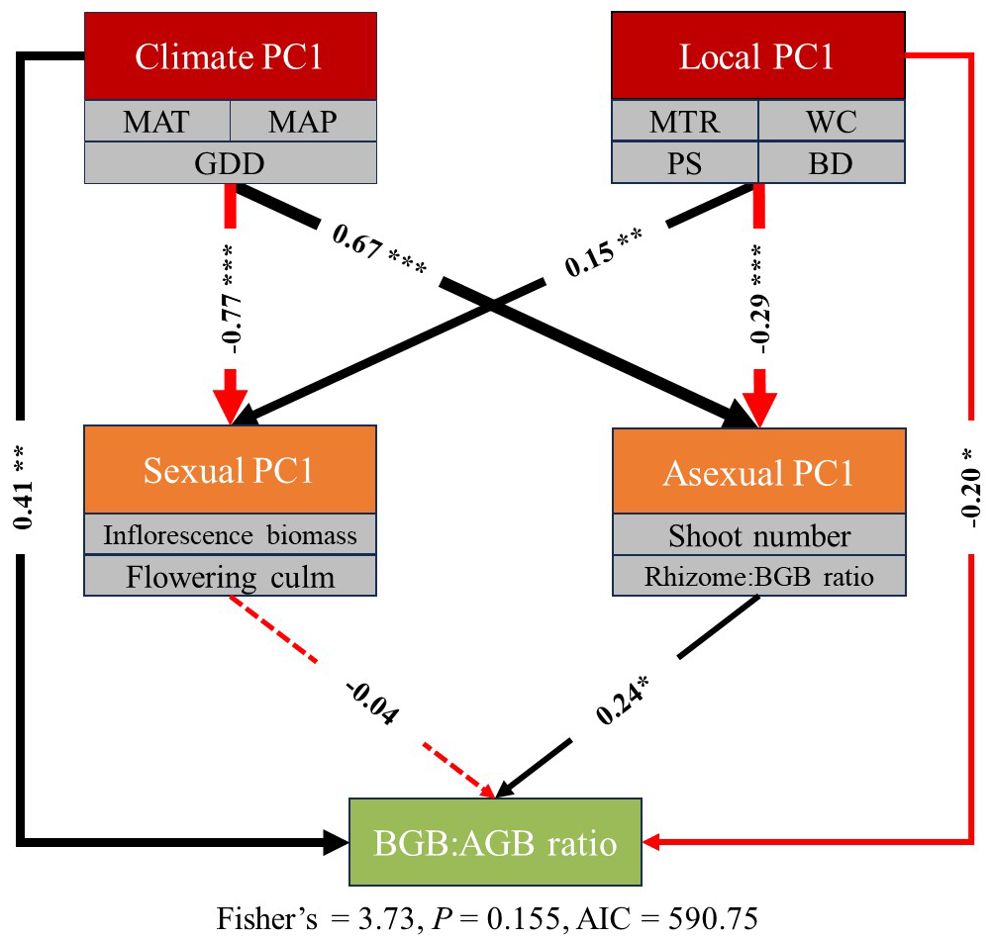
Figure 6. Structural equation models illustrate the plausible effects of environmental factors and reproductive investments on the biomass allocation of Spartina alterniflora along latitude gradients. Black and red arrows represent significant positive and negative pathways, respectively, and dashed arrows indicate nonsignificant pathways. Values next to the arrows represent standardized effect sizes with statistical significance (* P<0.05; ** P<0.01; *** P<0.001), arrow width is proportional to the strength of the relationship. MAT = mean annual temperature, MAP = mean annual precipitation, GDD = growing degree day, MTR = mean tidal range, WC = soil water content, PS = soil porewater salinity, BD = soil bulk density.
Our research discovered a linear decrease in BGB: AGB ratio from low to high latitude, indicating that S. alterniflora allocates more biomass belowground in southern marshes than in northern marshes in China. This variation in belowground biomass allocation suggests that ecosystem functions may differ between high and low latitudes. Previous studies focused on the effect of environmental conditions (e.g., temperature) on BGB: AGB ratio in the native range (Crosby et al., 2017), but our study found that reproductive investment also had a major effect on the biomass allocation of S. alterniflora across latitude in China. This finding underscores the importance of considering latitudinal trends in reproductive investment when predicting saltmarsh productivity and its impact on ecosystem function in the future. This new perspective could lead to more accurate predictions and better management strategies for these critical ecosystems.
Niche theory posits that plants perform optimally in middle latitudes, with performance decreasing below or above these middle latitudes (Cody, 1991). Our findings corroborate this theory, as we observed that AGB, BGB, and total biomass were highest in the mid-latitudes. The latitudinal trend in AGB is consistent with previous studies on AGB of S. alterniflora in China (Liu et al., 2016; Xu et al., 2020). However, the latitudinal trends in AGB and BGB are inconsistent with earlier studies in S. alterniflora in its native range, where AGB decreased linearly from low to high latitude (Kirwan et al., 2009; Liu et al., 2020a). Due to the methodological challenges inherent in observing and quantifying BGB, there is a limited body of research investigating the latitudinal patterns of BGB in S. alterniflora in China. However, the available studies indicate a linear increase in BGB with latitude in its native range (Crosby et al., 2017; Gross et al., 1991). Overall, the difference in latitudinal trends between invasive and native ranges of BGB may depend on the distribution and the differences in environmental factors along latitudes (Liu et al., 2020a). Research conducted across a broader latitudinal gradient suggests that the decline in productivity of S. alterniflora at low and high latitude regions may translate to marshes more susceptible to the effects of sea level rise.
In this study, AGB and BGB were associated with most of environmental factors (Supplementary Figure S1, Supplementary Table S3), but the mean annual temperature, which decreases linearly with latitude, is the most important environment factor. The results suggest an optimal temperature for S. alterniflora growth, which is consistent with the law of limiting factors, that plants often perform better in favorable environmental conditions (Singh and Lal, 1935), and that deviations from these optimal conditions plant performance reduce plant size and biomass (Liu et al., 2016; Liu and Pennings, 2019). The result of AGB was consistent with previous studies (Liu et al., 2016, 2020a), as both high and low temperatures inhibit plant growth and physiological metabolism (Hatfield and Prueger, 2015). The effect of temperature on BGB was also well studied in a manipulative warming experiment, where a moderate amount of warming maximized root growth (Noyce et al., 2019). The warming experiment and our latitudinal investment suggest that global warming may have different impacts on salt marsh productivity across different latitudes. At lower latitudes, high temperatures exceed the optimal temperature of S. alterniflora, so that climate warming will reduce the growth of S. alterniflora. This reduction in biomass may result in lower latitude salt marshes being more vulnerable to sea level rise.
High BGB: AGB ratios at high latitudes in terrestrial ecosystems have been attributed to cold winter temperatures (Vogel et al., 2008), though complex vegetation types and various controlling factors obscure a consistent pattern in BGB: AGB ratio with latitude (Jin et al., 2022; Qi et al., 2019; Tedla et al., 2019). In contrast, we found that BGB: AGB ratios were highest in low latitudes, and decreased linearly with increasing latitude, indicating that S. alterniflora allocated relatively more biomass to belowground in southern than northern marshes in China. The latitudinal trend in BGB: AGB ratio is also inconsistent with an earlier study in S. alterniflora in its native range, in which BGB: AGB ratio increased linearly from low to high latitude (Crosby et al., 2017). This may be attributed to variances in the lowest latitudes between the two studies: 19.73°N in our study and 32.55°N in Crosby’s study. Additionally, there appears to be a linearly increasing trend from RD (32.46°N) to DY (37.82°N). Discrepancies in temperature, precipitation and other environment factors between the native and invasive ranges could also contribute to differential responses (Poorter et al., 2012). Biomass allocation in plants confirms to optimal partitioning theory (Mccarthy and Enquist, 2007) and is influenced by a variety of environmental factors, such that BGB: AGB ratio typically decreases with temperature, and exhibits a humped relationship with precipitation (Qi et al., 2019). Our study found BGB: AGB ratio decreased linearly from low to high latitude. The decreasing temperature with latitude may be the most important factor in our study (Supplementary Figure S3). The results are consistent with a previous study, where four seagrass species showed a higher BGB: AGB ratio with temperature increases, because higher temperatures increase leaf respiration but decrease leaf photosynthesis, made them reallocate more AGB to BGB (George et al., 2018). Furthermore, early research showed first flowering day earlier at low latitude with higher temperature (Chen et al., 2021), earlier flowering had positive effect on BGB: AGB ratio (Liu et al., 2021), because most of photosynthetic products will transfer to belowground after flowering (Crosby et al., 2015). However, a warming experiment in a C4 Spartina patens marsh suggests that temperature had a non-linear effect on BGB and the BGB: AGB ratio, where BGB: AGB ratios were maximized for temperatures only slightly higher than ambient (Noyce et al., 2019). The effect of temperature on biomass allocation is also affected by other environmental factors (Ma et al., 2021). Our study found that both climate and local environments affect the BGB: AGB ratio of S. alterniflora. The salinity decreasing along the latitudinal gradient also affected the BGB: AGB ratio (Supplementary Figure S1), consistent with a previous study (Zhou et al., 2021) that illustrated the increase in soil salinity inhibits the absorption of nitrogen by plant roots, more biomass allocates to belowground is needed to absorption of nitrogen (Tang et al., 2022). Even though the results corroborate the optimal partitioning theory. But the effect of different invasion history on latitudinal biomass allocation is not clearly. Future studies need to test the effects of different invasion history on biomass allocation of S. alterniflora in different latitudes, which can help to better understand the underly mechanisms of latitudinal biomass allocation.
Our results may indicate that, in the future, although global warming will reduce the productivity of S. alterniflora, rising temperatures may encourage the plant to allocate more biomass into the belowground part, which could partly mitigate the decline in ground elevation and the erosion from sea level rising. And studies showed that S. alterniflora growth better than native species under flooding and salinity stress (Xue et al., 2018; Xu et al., 2020), suggest that S. alterniflora was more tolerant than natives in future scenarios of increased sea-level and saltwater intrusion (Borges et al., 2021), the maintaining of S. alterniflora may help for costal erosion control under future climate change (Strong and Ayres, 2013). However, S. alterniflora had negative impact on the native coastal ecosystems (An et al., 2007). Many native species, including salt marshes and mangroves, endangered birds and benthonic animal are threatened by S. alterniflora invasion (Ma et al., 2003; Zhang et al., 2012). Furthermore, the widely spread of S. alterniflora in China also had damage in maricultural activities, soil formation, and accumulation of nutrients (Gao et al., 2016), harm of S. alterniflora may out weights its benefits.
Previous work in a salt marsh warming experiment suggests that biomass allocation of plants responds to temperature warming through internal changes in nitrogen supply and demand (Noyce et al., 2019; Bruns et al., 2024). This work finds that root:shoot ratios are maximized at intermediate temperatures because plants must allocate biomass to roots to satisfy high nitrogen demand even as nitrogen supplied by mineralization is relatively low (Noyce et al., 2019; Bruns et al., 2024). Our finding that BGB: AGB ratios increase linearly with temperature differs from the findings of an optimum (non-linear) response observed in the warming experiment, and suggests additional mechanisms may be at play. For example, the latitudinal gradient in aboveground biomass (Figure 2) suggests that N demand should be lowest at low latitudes (i.e. low AGB), yet has the highest BGB: AGB ratio.
Previous studies indicate that reproductive investment also affects BGB: AGB ratio, such that increased sexual reproduction will reduce the BGB: AGB ratio (Sun et al., 2001; Skarpaas et al., 2016). In this study, the latitudinal patterns of sexual reproduction traits and asexual reproduction were contrasted in S. alterniflora. The latitudinal trend in sexual reproduction traits was also found in previous studies (Chen et al., 2021; Liu et al., 2016), which was embodied in the advance of flowering phenology (Crosby et al., 2015; Liu et al., 2022a) and the increase in seed set with increases in latitude (Liu et al., 2016). These increases may be due to shorter growth cycles and higher synchronization of flowering phenology at higher latitudes (Qiu et al., 2018). The latitudinal trends of asexual reproduction traits show that plants use the same resource pool for reproduction, and an increase in the investment in sexual reproduction inevitably reduces the investment in asexual reproduction (Thompson and Eckert, 2004). Furthermore, sexual reproduction cost more than asexual reproduction, consumes a lot of energy in individual growth to support flowering, cause less investment in asexual reproduction (Skarpaas et al., 2016).
Plants that favor sexual reproduction usually exhibit increased plant size and larger above-ground biomass (Liu and Pennings, 2019). Sexual reproduction requires substantial energy and this inhibits plant energy reserves in the belowground part of the plant (Liu et al., 2022a). In this study, the increasing sexual reproduction investment led to a decrease in the BGB: AGB ratio. On the other hand, asexual reproduction components, such as rhizomes, are an important part of belowground biomass, so that belowground biomass allocation decreases with the decrease in asexual reproduction (Xie et al., 2016). Furthermore, in the asexual reproduction stage, plants allocate more resources to the asexual reproductive organs which leads to a decrease in total biomass (Chen et al., 2019), resulting in a high BGB: AGB ratio at low latitudes. Overall, the lower input of sexual reproduction but the higher input of asexual reproduction resulted in a higher BGB: AGB ratio in low-latitude populations. Although the reasons for the difference in the reproductive investment of S. alterniflora along the latitude gradient remain to be studied, the higher investment in sexual reproduction in high latitude and the higher investment in asexual reproduction in low latitude contribute to a better understanding of the latitudinal biomass allocation pattern, indicating that the latitudinal trends of reproductive investment should be considered in the calculation of saltmarsh productivity on ecosystem function in the future.
S. alterniflora produces the most biomass at mid-latitudes, which is consistent with niche theory and the observed latitudinal trend in biomass indicates the presence of an optimal temperature range for salt marshes. Therefore, a warming-induced productivity decline will be found at low-latitude marshes with the future climate warming. But the BGB: AGB ratio decreased with increasing latitude (i.e. warming temperatures), so that an increase in the allocation to asexual reproduction could help to maintain belowground biomass with the future climate warming in low latitudes. In salt marsh, allocation to belowground organs regulate soil elevation gain, thereby influencing the future stability of salt marshes, and thus to prevent erosion from sea level rise.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
YC: Data curation, Formal analysis, Investigation, Methodology, Visualization, Writing – original draft, Writing – review & editing. FW: Investigation, Writing – review & editing. YW: Investigation, Writing – review & editing. YG: Investigation, Writing – original draft. MK: Conceptualization, Writing – review & editing. WL: Conceptualization, Formal analysis, Funding acquisition, Investigation, Supervision, Validation, Writing – review & editing. YZ: Conceptualization, Funding acquisition, Investigation, Supervision, Writing – review & editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. Funding for this project was provided by the National Key R and D Program of China (2022YFC3105401), and the National Natural Science Foundation of China (32025026, 32222054).
We thank H. Lu, D. Peng, X. Chen, J. Wang provided feedback throughout this investigated. We thank H. Zhou, W. Wu, X. Chen, Q. Wang conducted field measurements and sample collections.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declare that no Generative AI was used in the creation of this manuscript.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmars.2024.1510854/full#supplementary-material
An S. Q., Gu B. H., Zhou C. F., Wang Z. S., Deng Z. F., Zhi Y. B., et al. (2007). Spartina invasion in China: Implications for invasive species management and future research. Weed Res. 47, 183–191. doi: 10.1111/j.1365-3180.2007.00559.x
Biçe K., Schalles J., Sheldon J. E., Alber M., Meile C. (2023). Temporal patterns and causal drivers of aboveground plant biomass in a coastal wetland: Insights from time-series analyses. Front. Mar. Sci. 10. doi: 10.3389/fmars.2023.1130958
Borges F. O., Santos C. P., Paula J. R., Mateos-Naranjo E., Redondo-Gomez S., Adams J. B., et al. (2021). Invasion and extirpation potential of native and invasive Spartina species under climate change. Front. Mar. Sci. 8. doi: 10.3389/fmars.2021.696333
Bruns N. E., Noyce G. L., Megonigal J. P., Kirwan M. L. (2024). A test of functional balance theory for wetland biomass allocation in a global change experiment. Geophys. Res. Lett. 51, e2024GL110902. doi: 10.1029/2024GL110902
Chen X., Cheng X., Zhu H., Bañuelos G., Shutes B., Wu H. (2019). Influence of salt stress on propagation, growth and nutrient uptake of typical aquatic plant species. Nord. J. Bot. 37 (12), e02411. doi: 10.1111/njb.02411
Chen X., Liu W., Pennings S. C., Zhang Y. (2021). Plasticity and selection drive hump-shaped latitudinal patterns of flowering phenology in an invasive intertidal plant. Ecology 102 (5), e03311. doi: 10.1002/ecy.3311
Coldren G. A., Langley J. A., Feller I. C., Chapman S. K. (2019). Warming accelerates mangrove expansion and surface elevation gain in a subtropical wetland. J. Ecol. 107, 79–90. doi: 10.1111/1365-2745.13049
Coleman D. J., Kirwan M. L. (2019). The effect of a small vegetation dieback event on salt marsh sediment transport. Earth Surf. Proc. Land. 44, 944–952. doi: 10.1002/esp.4547
Crosby S. C., Angermeyer A., Adler J. M., Bertness M. D., Deegan L. A., Sibinga N., et al. (2017). Spartina alterniflora biomass allocation and temperature: implications for salt marsh persistence with sea-level rise. Estuar. Coast. 40, 213–223. doi: 10.1007/s12237-016-0142-9
Crosby S. C., Ivens-Duran M., Bertness M. D., Davey E., Deegan L. A., Leslie H. M. (2015). Flowering and biomass allocation in U.S. Atlantic coast. Spartina alterniflora. Am. J. Bot. 102, 669–676. doi: 10.3732/ajb.1400534
Darby F. A., Turner R. E. (2008). Below-and aboveground Spartina alterniflora production in a Louisiana salt marsh. Estuar. Coast. 31, 223–231. doi: 10.1007/s12237-007-9014-7
Dixon P. (2003). VEGAN, a package of R functions for community ecology. J. Veg. Sci. 14, 927–930. doi: 10.1111/j.1654-1103.2003.tb02228.x
Gao J., Feng Z., Chen L., Wang Y., Bai F., Li J. (2016). The effect of biomass variations of Spartina alterniflora on the organic carbon content and composition of a salt marsh in northern Jiangsu Province. China. Ecol. Eng. 95, 160–170. doi: 10.1016/j.ecoleng.2016.06.088
Gedan K. B., Kirwan M. L., Wolanski E., Barbier E. B., Silliman B. R. (2011). The present and future role of coastal wetland vegetation in protecting shorelines: answering recent challenges to the paradigm. Climatic Change 106, 7–29. doi: 10.1007/s10584-010-0003-7
George R., Gullström M., Mangora M. M., Mtolera M. S. P., Björk M. (2018). High midday temperature stress has stronger effects on biomass than on photosynthesis: A mesocosm experiment on four tropical seagrass species. Ecol. Evo. 8, 4508–4517. doi: 10.1002/ece3.3952
Gross M. F., Hardisky M. A., Wolf P. L., Klemas V. (1991). Relationship between aboveground and belowground biomass of. Spartina alterniflora (Smooth Cordgrass). Estuaries 14, 180. doi: 10.2307/1351692
Hatfield J. L., Prueger J. H. (2015). Temperature extremes: Effect on plant growth and development. Weather Clim. Extreme. 10, 4–10. doi: 10.1016/j.wace.2015.08.001
Jiang Y., Yang D., Huang J., Wen Y., Tang H., Xu J., et al. (2024). Invasive Spartina alterniflora alters sediment organic carbon mineralization dynamics in a coastal wetland of Southeastern China. Front. Mar. Sci. 11. doi: 10.3389/fmars.2024.1400381
Jin Y., Liu C., Qian S. S., Luo Y., Zhou R., Tang J., et al. (2022). Large-scale patterns of understory biomass and its allocation across China’s forests. Sci. Total Environ. 804, 150169. doi: 10.1016/j.scitotenv.2021.150169
Kaldy J., Dunton K. (2000). Above- and below-ground production, biomass and reproductive ecology of Thalassia testudinum (turtle grass) in a subtropical coastal lagoon. Mar. Ecol. Prog. Ser. 193, 271–283. doi: 10.3354/meps193271
Kirwan M. L., Guntenspergen G. R., Morris J. T. (2009). Latitudinal trends in Spartina alterniflora productivity and the response of coastal marshes to global change. Glob. Change Biol. 15, 1982–1989. doi: 10.1111/j.1365-2486.2008.01834.x
Kirwan M. L., Megonigal J. P. (2013). Tidal wetland stability in the face of human impacts and sea-level rise. Nature 504, 53–60. doi: 10.1038/nature12856
Lefcheck J. S. (2016). PIECEWISESEM: Piecewise structural equation modelling in R for ecology, evolution, and systematics. Method. Ecol. Evol. 7, 573–579. doi: 10.1111/2041-210X.12512
Li L., Barrett S. C. H., Song Z., Chen J. (2019). Sex-specific plasticity of reproductive allocation in response to water depth in a clonal, dioecious macrophyte. Am. J. Bota. 106, 42–50. doi: 10.1002/ajb2.1218
Li R., Yu Q., Wang Y., Wang Z. B., Gao S., Flemming B. (2018). The relationship between inundation duration and Spartina alterniflora growth along the Jiangsu coast, China. Estuar. Coast. Shelf S. 213, 305–313. doi: 10.1016/j.ecss.2018.08.027
Liu W., Chen X., Strong D. R., Pennings S. C., Kirwan M. L., Chen X., et al. (2020a). Climate and geographic adaptation drive latitudinal clines in biomass of a widespread saltmarsh plant in its native and introduced ranges. Limnol. Oceanogr. 65, 1399–1409. doi: 10.1002/lno.11395
Liu W., Chen X., Wang J., Zhang Y. (2022a). Does the effect of flowering time on biomass allocation across latitudes differ between invasive and native salt marsh grass Spartina alterniflora? Ecol. Evol. 12, e8681. doi: 10.1002/ece3.8681
Liu M., Mao D., Wang Z., Li L., Man W., Jia M., et al. (2018). Rapid invasion of spartina alterniflora in the coastal zone of mainland China: new observations from Landsat OLI images. Remote Sens-Basel 10, 1933. doi: 10.3390/rs10121933
Liu W., Maung-Douglass K., Strong D. R., Pennings S. C., Zhang Y. (2016). Geographical variation in vegetative growth and sexual reproduction of the invasive Spartina alterniflora in China. J. Ecol. 104, 173–181. doi: 10.1111/1365-2745.12487
Liu W., Pennings S. C. (2019). Self-thinning and size-dependent flowering of the grass Spartina alterniflora across space and time. Funct. Ecol. 33, 1830–1841. doi: 10.1111/1365-2435.13384
Liu W., Wang W., Zhang Y. (2022b). Differences in leaf traits of Spartina alterniflora between native and invaded habitats: Implication for evolution of alien species competitive ability increase. Ecol. Indic. 138, 108799. doi: 10.1016/j.ecolind.2022.108799
Liu Y., Xu M., Li G., Wang M., Li Z., De Boeck H. J. (2021). Changes of aboveground and belowground biomass allocation in four dominant grassland species across a precipitation gradient. Front. Plant Sci. 12. doi: 10.3389/fpls.2021.650802
Liu W., Zhang Y., Chen X., Maung-Douglass K., Strong D. R., Pennings S. C. (2020b). Contrasting plant adaptation strategies to latitude in the native and invasive range of Spartina alterniflora. New Phytol. 226, 623–634. doi: 10.1111/nph.16371
Lowry D. B., Popovic D., Brennan D. J., Holeski L. M. (2019). Mechanisms of a locally adaptive shift in allocation among growth, reproduction, and herbivore resistance in Mimulus guttatus. Evolution 73, 1168–1181. doi: 10.1111/evo.13699
Ma Z., Li B., Jing K., Zhao B., Tang S., Chen J. (2003). Effects of tidewater on the feeding ecology of hooded crane (Grus monacha) and conservation of their wintering habitats at Chongming Dongtan, China. Ecol. Res. 18, 321–329. doi: 10.1046/j.1440-1703.2003.00557.x
Ma H., Mo L., Crowther T. W., Maynard D. S., Van Den Hoogen J., Stocker B. D., et al. (2021). The global distribution and environmental drivers of aboveground versus belowground plant biomass. Nat. Ecol. Evol. 5, 1110–1122. doi: 10.1038/s41559-021-01485-1
Mccarthy M. C., Enquist B. J. (2007). Consistency between an allometric approach and optimal partitioning theory in global patterns of plant biomass allocation. Funct. Ecol. 21, 713–720. doi: 10.1111/j.1365-2435.2007.01276.x
Mudd S. M., D'Alpaos A., Morris J. T. (2010). How does vegetation affect sedimentation on tidal marshes? Investigating particle capture and hydrodynamic controls on biologically mediated sedimentation. J. Geophys. Res.-Earth Surf. 115, F03029. doi: 10.1029/2009JF001566
Noyce G. L., Kirwan M. L., Rich R. L., Megonigal J. P. (2019). Asynchronous nitrogen supply and demand produce nonlinear plant allocation responses to warming and elevated CO2. Proc. Natl. Acad. Sci. 116, 21623–21628. doi: 10.1073/pnas.1904990116
Pennings S. C., Richards C. L. (1998). Effects of wrack burial in salt-stressed habitats: Batis maritima in a southwest Atlantic salt marsh. Ecography 21, 630–638. doi: 10.1111/j.1600-0587.1998.tb00556.x
Poorter H., Niklas K. J., Reich P. B., Oleksyn J., Poot P., Mommer L. (2012). Biomass allocation to leaves, stems and roots: Meta-analyses of interspecific variation and environmental control. New Phytol. 193, 30–50. doi: 10.1111/j.1469-8137.2011.03952.x
Qi Y., Wei W., Chen C., Chen L. (2019). Plant root-shoot biomass allocation over diverse biomes: A global synthesis. Glob. Ecol. Conserv. 18, e00606. doi: 10.1016/j.gecco.2019.e00606
Qiu S., Xu X., Liu S., Liu W., Liu J., Nie M., et al. (2018). Latitudinal pattern of flowering synchrony in an invasive wind-pollinated plant. Proc. R. Soc B-Biol. Sci. 285, 20181072. doi: 10.1098/rspb.2018.1072
Silliman B. R., He Q., Angelini C., Smith C. S., Kirwan M. L., Daleo P., et al. (2019). Field experiments and meta-analysis reveal wetland vegetation as a crucial element in the coastal protection paradigm. Curr. Biol. 29, 1800–1806. doi: 10.1016/j.cub.2019.05.017
Singh B. N., Lal K. N. (1935). Limitations of Blackman’s law of limiting factors and harder’s concept of relative minimum as applied to photosynthesis. Plant Physiol. 10, 245–268. doi: 10.1104/pp.10.2.245
Skarpaas O., Meineri E., Bargmann T., Pötsch C., Töpper J., Vandvik V. (2016). Biomass partitioning in grassland plants along independent gradients in temperature and precipitation. Perspect. Plant Ecol. Evol. Syst. 19, 1–11. doi: 10.1016/j.ppees.2016.01.006
Smith A. J., Noyce G. L., Megonigal J. P., Guntenspergen G. R., Kirwan M. L. (2022). Temperature optimum for marsh resilience and carbon accumulation revealed in a whole-ecosystem warming experiment. Glob. Change Biol. 28, 3236–3245. doi: 10.1111/gcb.16149
Snedden G. A., Cretini K., Patton B. (2015). Inundation and salinity impacts to above- and belowground productivity in Spartina patens and Spartina alterniflora in the Mississippi River deltaic plain: Implications for using river diversions as restoration tools. Ecol. Eng. 81, 133–139. doi: 10.1016/j.ecoleng.2015.04.035
Strong D. R., Ayres D. R. (2013). Ecological and evolutionary misadventures of Spartina. Annu. Rev. Ecol. Evol. S 44, 389–410. doi: 10.1146/annurev-ecolsys-110512-135803
Sun S., Gao X., Cai Y. (2001). Variations in sexual and asexual reproduction of Scirpus mariqueter along an elevational gradient. Ecol. Res. 16, 263–274. doi: 10.1046/j.1440-1703.2001.00395.x
Tang L., Zhou Q. S., Gao Y., Li P. (2022). Biomass allocation in response to salinity and competition in native and invasive species. Ecosphere 13, e3900. doi: 10.1002/ecs2.3900
Tedla B., Dang Q.-L., Inoue S. (2019). White birch has limited phenotypic plasticity to take advantage of increased photoperiods at higher latitudes north of the seed origin. For. Ecol. Manage. 451, 117565. doi: 10.1016/j.foreco.2019.117565
Thompson F. L., Eckert C. G. (2004). Trade-offs between sexual and clonal reproduction in an aquatic plant: Experimental manipulations vs. Phenotypic correlations. J. Evol. Biol. 17, 581–592. doi: 10.1111/j.1420-9101.2004.00701.x
Vogel J. G., Bond-Lamberty B. P., Schuur E. A. G., Gower S. T., Mack M. C., O’Connell K. E. B., et al. (2008). Carbon allocation in boreal black spruce forests across regions varying in soil temperature and precipitation. Glob. Change Biol. 14, 1503–1516. doi: 10.1111/j.1365-2486.2008.01600.x
Wang P., Heijmans M. M. P. D., Mommer L., Van Ruijven J., Maximov T. C., Berendse F. (2016). Belowground plant biomass allocation in tundra ecosystems and its relationship with temperature. Environ. Res. Lett. 11, 55003. doi: 10.1088/1748-9326/11/5/055003
Wang Z., Xie L., Prather C. M., Guo H., Han G., Ma C. (2018). What drives the shift between sexual and clonal reproduction of Caragana stenophylla along a climatic aridity gradient? BMC Plant Biol. 18, 91. doi: 10.1186/s12870-018-1313-6
Wenk E. H., Falster D. S. (2015). Quantifying and understanding reproductive allocation schedules in plants. Ecol. Evol. 5, 5521–5538. doi: 10.1002/ece3.1802
Wernberg T., Thomsen M. S., Baum J. K., Bishop M. J., Bruno J. F., Coleman M. A., et al. (2024). Impacts of climate change on marine foundation species. Annu. Rev. Mar. Sci. 16, 247–282. doi: 10.1146/annurev-marine-042023-093037
Xie X.-F., Hu Y.-K., Pan X., Liu F.-H., Song Y.-B., Dong M. (2016). Biomass allocation of stoloniferous and rhizomatous plant in response to resource availability: A phylogenetic meta-Analysis. Front. Plant Sci. 7. doi: 10.3389/fpls.2016.00603
Xu X., Liu H., Liu Y., Zhou C., Pan L., Fang C., et al. (2020). Human eutrophication drives biogeographic salt marsh productivity patterns in China. Ecol. Appl. 30, e02045. doi: 10.1002/eap.2045
Xue L., Li X., Zhang Q., Yan Z., Ding W., Huang X., et al. (2018). Elevated salinity and inundation will facilitate the spread of invasive Spartina alterniflora in the Yangtze River Estuary, China. J. Exp. Mar. Biol. Ecol. 506, 144–154. doi: 10.1016/j.jembe.2018.06.008
Yang Y., Dou Y., An S., Zhu Z. (2018). Abiotic and biotic factors modulate plant biomass and root/shoot (R/S) ratios in grassland on the Loess Plateau, China. Sci. Total Environ. 636, 621–631. doi: 10.1016/j.scitotenv.2018.04.260
Yue S., Zhang X., Xu S., Zhang Y., Zhao P., Wang X., et al. (2020). Reproductive strategies of the seagrass Zostera japonica under different geographic conditions in northern China. Front. Mar. Sci. 7. doi: 10.3389/fmars.2020.574790
Zhang D., Hu Y., Liu M., Chang Y., Yan X., Bu R., et al. (2017). Introduction and spread of an exotic plant, Spartina alterniflora, along coastal marshes of China. Wetlands 37, 1181–1193. doi: 10.1007/s13157-017-0950-0
Zhang Y., Huang G., Wang W., Chen L., Lin G. (2012). Interactions between mangroves and exotic Spartina in an anthropogenically disturbed estuary in southern China. Ecology 93, 588–597. doi: 10.1890/11-1302.1
Zhao Y. J., Qing H., Zhao C. J., Zhou C. F., Zhang W. G., Xiao Y., et al. (2010). Phenotypic plasticity of Spartina alterniflora and Phragmites australis in response to nitrogen addition and intraspecific competition. Hydrobiologia 637, 143–155. doi: 10.1007/s10750-009-9992-5
Keywords: invasive plants, latitude, biomass allocation, trade-off, saltmarsh, global warming
Citation: Chen Y, Wu F, Wang Y, Guo Y, Kirwan ML, Liu W and Zhang Y (2024) Latitudinal trends in the biomass allocation of invasive Spartina alterniflora: implications for salt marsh adaptation to climate warming. Front. Mar. Sci. 11:1510854. doi: 10.3389/fmars.2024.1510854
Received: 14 October 2024; Accepted: 25 November 2024;
Published: 11 December 2024.
Edited by:
Francesco Tiralongo, University of Catania, ItalyCopyright © 2024 Chen, Wu, Wang, Guo, Kirwan, Liu and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wenwen Liu, bHd3QHhtdS5lZHUuY24=; Yihui Zhang, enloQHhtdS5lZHUuY24=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.