- 1Department of Chemical Pharmaceutical and Agricultural Sciences, University of Ferrara, Ferrara, Italy
- 2Department of Biology and Biotechnology, Faculty of Science, The Hashemite University, Zarqa, Jordan
- 3Department of Environmental Sciences Informatics and Statistics, Ca’ Foscari University of Venice, Venice, Italy
Seagrasses play a vital role in marine ecosystems worldwide. However, until recently these ecosystems were experiencing declines due to various global and local threats. In response to this issue, initiatives have been launched to combat seagrass loss by addressing local and regional major stressors and actively engaging in restoration efforts by transplantation. Although seagrass restoration has progressed significantly with the development of numerous transplant techniques, these are not always crowned with success. This is often due to the fact that the environmental parameters of water, sediment and biota of the recipient sites are not carefully considered in their suitability for transplantation. In this study, the multi-year experiences and data from numerous environmental surveys in transitional water systems have been condensed to define limit values for the ideal growth conditions and the extreme values in which the survival of four aquatic angiosperm species is possible: Cymodocea nodosa, Zostera marina, Zostera noltei and Ruppia cirrhosa. Approaches to transplants, seasonality and critical issues have been explored. The identified limits and parameters of water, sediment and biota will help to define the suitability of a recipient site for the rooting of seagrasses, increasing the chances of success for transplant operations.
1 Introduction
Seagrass meadows, or better aquatic angiosperm meadows (to consider also the species of the genus Ruppia which, not being exclusively marine, are not universally accepted as seagrasses but are classified as freshwater plants - Short, 2003) are often considered the stamp of approval for the environmental quality of coastal and transitional water systems (TWS) and are the landmark of nearly pristine conditions (Sfriso et al., 2021). However, these aquatic angiosperms are also vulnerable to increasing human pressures in coastal areas such as: nutrient loading, siltation, mechanical disturbance, direct boat propeller damage, dredging and reclamation of marine areas, pollution, aquaculture, introduction of new competitors such as exotic marine organisms (Duarte et al., 2004), boat traffic, destructive fishing practices and harbor activities (Nordlund and Gullström, 2013). All these represent important forcing factors inducing severe decline in seagrass coverage. A comprehensive analysis of 215 studies (Waycott et al., 2009) revealed that seagrasses have been receding rapidly, with an average annual loss of 110 km² since 1980. Since the initial documentation of seagrass areas in 1879, approximately 29% of the known extent vanished with a significant escalation, increasing from a median of 0.9% per year before 1940 to 7% per year since 1990 (Waycott et al., 2009). In a more recent work by Dunic et al. (2021), an update of the situation confirms how losses still outweigh gains of seagrass meadows but it outlines how improvements and even reversals in the global trend have been recorded locally especially in the Temperate North Atlantic East (De los Santos et al., 2019; Zoffoli et al., 2021).
Seagrass loss has a significant impact on coastal biodiversity, leading to alterations in food webs and depletion of harvestable resources (Sfriso et al., 2021). The degradation of seagrass meadows leads to changes in vegetation composition and structure, primarily characterized by the replacement of seagrasses with problematic macroalgae such as: Gracilariaceae, Solieriaceae, Cladophoraceae and Ulvaceae (Morand and Briand, 1996). Especially the latter in eutrophic conditions present an unstable fast pulsing growth that can compromise ecosystem stability and trigger hypo-anoxic crises in early summer due to biomass collapse (Sfriso and Sfriso, 2017). In more extreme conditions, blooms of phytoplankton and cyanobacteria may occur (Sorokin and Zakuskina, 2010; Munari and Mistri, 2012). The presence of high and persistent biomasses of these macroalgae leads to a gradual disappearance of aquatic angiosperms and sensitive macroalgal species, resulting in a cascading loss of biodiversity and ecosystem services (Short and Wyllie-Echeverria, 1996; Orth et al., 2006; Burkholder et al., 2007; Boudouresque et al., 2009; UNEP, 2020; Rodil et al., 2022).
The global loss of seagrass meadows and the parallel growing awareness of their environmental value are leading to additional attention for projects aimed at their restoration and/or rehabilitation (Airoldi and Beck, 2007; Nellemann et al., 2009; Barbier et al., 2011; Unsworth et al., 2015; Nordlund et al., 2016; Sfriso et al., 2019a; Unsworth et al., 2019; Da Ros et al., 2020; UNEP, 2020; Calvo et al., 2021; Pansini et al., 2022).
Human-mediated restoration/rehabilitation is achieved in different ways, starting from assessing the reasons for seagrass loss and consequently trying to eliminate the drivers of pressure inducing the decline with different managing options (e.g., establishment of marine protected areas, imposing boating access-restrictions, using wastewater treatment systems to reduce eutrophication and digging canals to favor water circulation in choked areas with high water residence time) and both supporting and speeding up the natural recovery of seagrass meadows (e.g., substrate stabilization activities, tidal exchange restoration if altered, management of freshwater inflow). Therefore, human-mediated seagrass restoration/rehabilitation is recommended only in those cases where the seagrass ecosystem has been damaged to such an extent that the self-recovery is no longer possible. The evaluation of possible bottlenecks in the recruitment of new natural seagrass meadows (e.g., physical barriers preventing the connectivity between meadows, limited sexual reproduction in the dominant seagrass species and lacking or scarce seed production and dispersion) and activities to overcome these recruitment bottlenecks should be addressed first (Airoldi and Beck, 2007; Duarte et al., 2020; UNEP-Nairobi Convention, 2020). If all these human-mediated activities oriented to help the natural restoration of seagrass meadows would be unsuccessful or, anyway, not so effective, the active seagrass restoration by transplantation should be considered.
In other cases, human-mediated seagrass restoration can be employed as a form of compensatory mitigation by creating new seagrass meadows in areas that appear to be suitable for their growth in order to balance their destruction and recession from other areas due to anthropogenic activities (e.g., port expansion, land reclamation – UNEP-Nairobi Convention, 2020). Moreover, human-mediated creation of new seagrass meadows has been suggested to mitigate the effects of climate change, by acting as “blue carbon farming” sites aiming to reduce ocean acidification (Nellemann et al., 2009; Duarte et al., 2020).
The TWS coastal areas present very high temporal fluctuations and spatial variability of environmental conditions, therefore the identification of a transplantation area can be cryptic to assess, such as the ideal seasons and methods to implement the seagrass restoration.
The literature on the topic of seagrass transplantation is extremely broad and varied, as numerous environmental restoration project results have been published; however, in these papers, the limits between success (hereinafter referred to as “a long-lasting and persistent establishment of seagrass meadows, even when limited in size”) and failure of a seagrass restoration activity are not always clear (Zedler, 2007; Tan et al., 2020). The presence of this gray area especially for TWS seagrasses, where failures are only occasionally reported, does not allow to define clear limits of the environmental conditions that may be critical for rooting of the transplanted species.
The conditions that facilitate the seagrass rooting, as previously stated by Tan et al. (2020), are generally well documented and among these there are: light and water transparency (Duarte, 1991; Greve and Binzer, 2004), limited nutrients availability (Touchette and Burkholder, 2000; Sfriso et al., 2005a; Sfriso et al., 2017a), water hydrodynamics (Fonseca and Kenworthy, 1987; Schanz and Asmus, 2003), and sediment type (Erftemeijer and Middelburg, 1993; Van Katwijk and Wijgergangs, 2004). However, so far only general trends usually have been indicated and few studies provided mean values (Garmendia et al., 2010; Garmendia, 2021; Garmendia et al., 2023), ranges and limits of physico-chemical parameters to define a recipient site suitability for transplant. In Italy for marine seagrass species, such as Posidonia oceanica (L.) Delile, some efforts have been carried out to define the most influential aspects for monitoring and evaluating the success of restoration programmes (see Life SEPOSSO Project, LIFE16 GIE/IT/000761; Curiel et al., 2021; Mancini et al., 2022; Pansini et al., 2022) and actions to reduce meadow degradation (see Life SEAFOREST Project, LIFE16 CCM/IT/000121). However, a study carried out in the lagoon of Venice in the framework of the EU Life SERESTO seagrass transplantation Project (LIFE12 NAT/IT/000331) highlighted the challenge related to the identification of fixed ranges of physico-chemical values decisive for a long-lasting settlement of TWS seagrass meadows (Sfriso et al., 2021). Therefore, in order to facilitate the success of future transplantation activities in TWS, in this study we aim to: 1) define reliable ranges and limits of the main physico-chemical parameters of water and sediment for which it is possible to find and/or successfully transplant seagrasses; 2) identify seasons, techniques and species most suitable for a successful colonization of different areas; 3) fill in knowledge gaps on transplantation of the less common species R. cirrhosa.
In this study we decided to consider four main species of aquatic angiosperms present in Mediterranean TWS: Cymodocea nodosa (Ucria) Ascherson, Zostera marina Linnaeus, Zostera noltei Hornemann and Ruppia cirrhosa (Petagna) Grande (Duarte et al., 2004).
2 Materials and methods
2.1 Study areas
Samples were collected by our marine ecology research team in the framework of many projects carried out in the Venice Lagoon in this last decade (Life12 NAT/IT/000331-SeResto; Venice2021; Mo.Ve.Co. III 2018; LIFE19 NAT/IT/000264-TRANSFER) both with temporal (monthly sampling per one year in single stations) and spatial frequency (late spring-early summer sampling stations spread in the whole lagoon). Sampling stations and study areas are recorded in Figure 1.
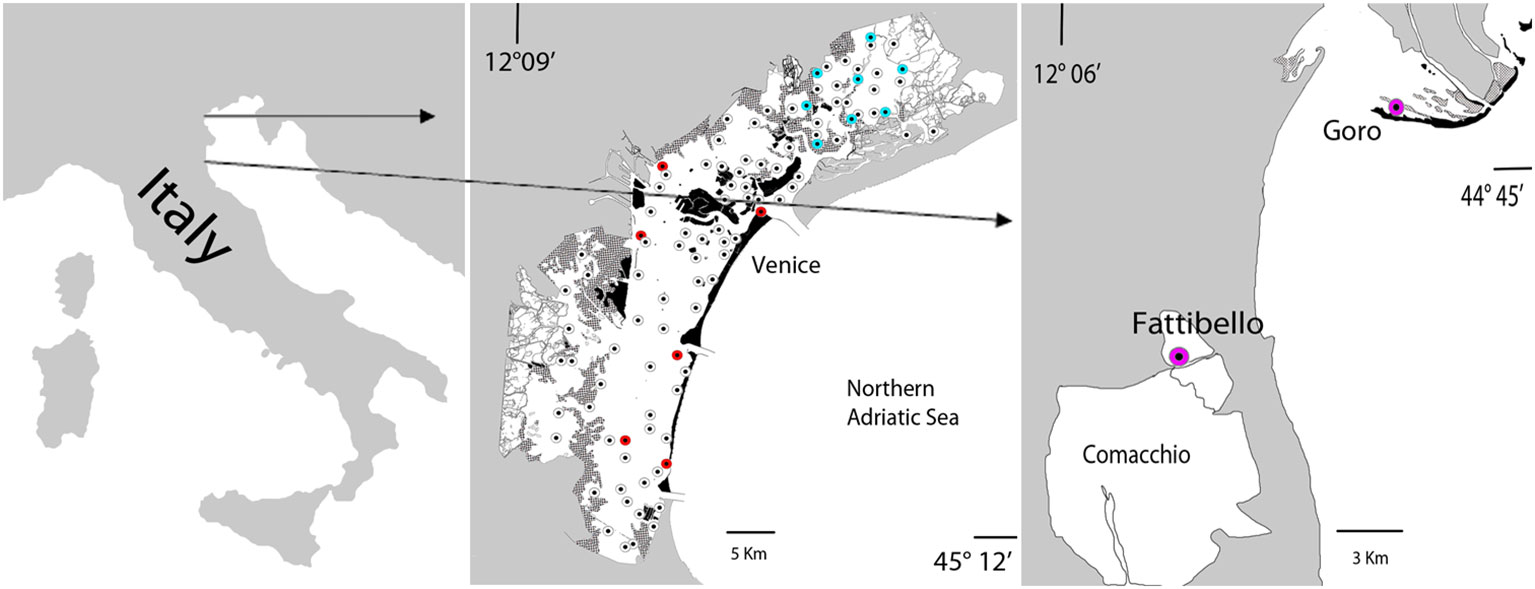
Figure 1 Map of the sampling stations in the Venice Lagoon, Goro Lagoon, Fattibello pond (Italy). White dots (Mo.Ve.Co.III), red dots (Venice2021), blue dots (Life12 NAT/IT/000331-SeResto); purple dots (LIFE19 NAT/IT/000264-TRANSFER).
The Venice Lagoon, situated in the northern Adriatic Sea, is the largest coastal lagoon in the Mediterranean Sea. It spans an area of 549 km2 and has an average depth of approx. 1.2 meters. The lagoon is connected to the Adriatic Sea through three inlets: Lido, Malamocco, and Chioggia, in that order from North to South. Tidal influence is significant in the lagoon, with approximately 60% of the water volume exchanged with the sea every 12 hours (Masiol et al., 2014). The primary sources of freshwater inflow into the lagoon are the Dese and Silone rivers, which contribute 44% of the total freshwater, while the Marzenego river provides additional smaller inputs. The central basin, which is the most anthropized, has experienced significant impacts from industrial waste, sewage, and anthropogenic activities in the past. However, in recent years, there has been a reduction in these impacts and the lagoon has shown signs of environmental recovery. The southern basin has lower nutrient levels and is densely populated by seagrasses (mainly C. nodosa and Z. marina), while the northern basin has limited water turnover and is mainly colonized by Z. noltei and R. cirrhosa, with a spot presence of Z. marina and C. nodosa along the edges of the canals with higher water exchange. In the past, many of these species disappeared due to excessive nutrients, clam fishing, and a bloom of picocyanobacteria (Sorokin et al., 2004). However, efforts have been made to reintroduce aquatic angiosperms into the affected areas, resulting in a significant recolonization and recovery (Sfriso et al., 2022).
In the Life SeResto project (a seagrass restoration project funded by the European Union to favor the recolonization by aquatic angiosperm in the northern basin of the Venice Lagoon in an area of interest of approx. 36.6 km2), water and sediment parameters and macrophytes collected from April 2014 to June 2017 (29 surveys) were analyzed in 8 stations representative of the overall environmental conditions of the area. The surveys were conducted monthly for one year at the project’s start and end, with quarterly surveys during the intermediate period. The number of samples implemented for our investigation was 232 (8 stations per 29 surveys) for water column and macrophytes analysis and 64 (8 stations per 2 samples per year per 4 years) for sediments evaluation.
During the Mo.Ve.Co. III (2018) project, water column and surface sediment parameters and macrophytes (aquatic angiosperms and macroalgae) were sampled in late spring-early summer in 87 stations spread in the whole lagoon to determine the ecological status according to the European Water Framework Directive (WFD 2000/60/EC).
Additional information was acquired in the “Venice 2021 mission” of CORILA (Consortium for the Coordination of the Research related to the Venice Lagoon System), a survey carried out in order to provide integrated knowledge for the management of the Venice Lagoon ecosystem upon the activation of the MOSE gate system (tidal regulating barriers with mobile gates). Samples were collected monthly for one year, before and after the operation of the “MOSE” system, in proximity of the three water inlets of the lagoon and in three stations placed inside the lagoon. On the whole, the total number of samples was 144 (6 stations per 24 months).
We integrated the data collected from surveys carried out monthly over one year in two additional stations (24 samples), one monitored in the lagoon of Goro and the other at Fattibello (Comacchio Valleys) in the framework of the project LIFE19 NAT/IT/000264-TRANSFER, a seagrass restoration project funded by the European Union to favor the seagrass recolonization in the lagoons of the Po Delta (Italy), in the lagoons of Amvrakikos (Greece) and in Mar Menor (Spain). The lagoon of Goro is a small shallow-water embayment, placed in the southernmost basin of the Po River Delta system, covering an area of 26 km2, whereas Fattibello is a small, choked pond of 6 km2, located in the northern side of the Comacchio Ponds, weakly affected by the flow of the tides.
2.2 Environmental parameters
The main physico-chemical parameters of the water column [temperature; pH (pHw); Eh (Ehw); salinity; dissolved oxygen (DO); dissolved inorganic nitrogen (DIN), as sum of nitrite, nitrates and ammonium; reactive phosphorus (RP); reactive silicates (RSi); total suspended solids (TSS); total chlorophyll-a (Chl-a tot), as sum of chlorophyll-a and pheophytin-a; water transparency by Secchi disk] and sediment [pH (pHs); Eh (Ehs); total nitrogen (Ntot); organic carbon (Corg); organic phosphorus (Porg); the fine fraction <63 µm (Fines); moisture; density] were analyzed following the procedures described in Sfriso et al. (2019b) and Sfriso et al. (2020a) and Strickland and Parsons (1984). Settled particulate matter (SPM) was collected monthly for one year in the stations by sedimentation traps according to the procedure described in Sfriso et al. (2005b).
2.3 Macrophyte variables
In all the aforementioned stations, together with the main environmental physico-chemical parameters, the presence/absence and cover of aquatic angiosperms, the biomass and cover of macroalgae, the number of total macrophyte taxa, macrophyte sensitive taxa (Sens.), the number of crustose calcareous algae (CCA) and the abundance of Chlorophyta and Rhodophyta were also monitored (Sfriso et al., 2014; Sfriso et al., 2017a; Sfriso et al., 2020b). The ecological status and the list of sensitive species of each station was determined by the application of the Macrophyte Quality Index (MaQI, Sfriso et al., 2014).
2.4 Data summary and statistical analysis
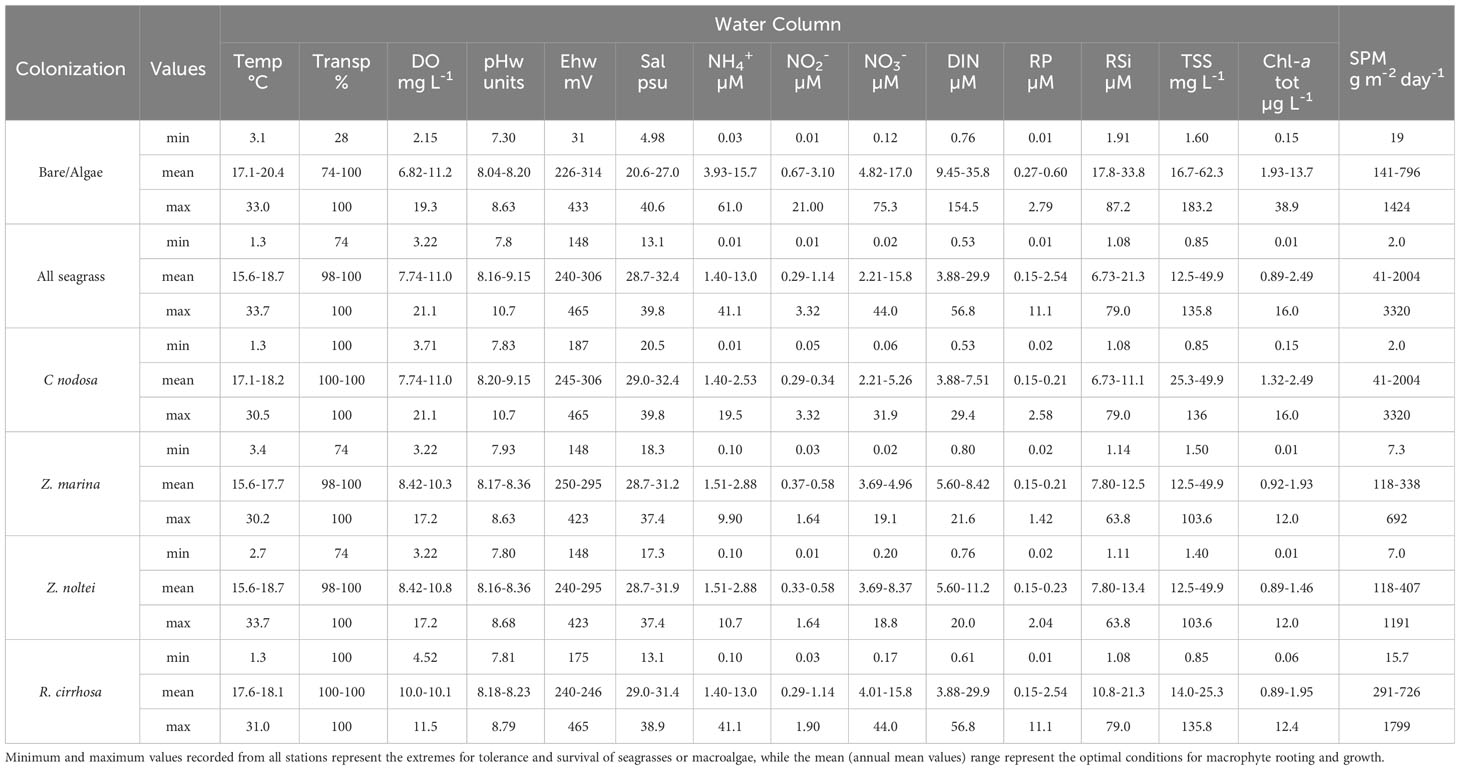
The data collected in all the surveys were used to calculate the annual mean, minimum and maximum values of the physico-chemical parameters of sediment and water for which seagrass presence was recorded in the sampling site or in which successful colonization was achieved. The minimum and maximum values describe the extreme tolerance limits found in the present study in the Northern Adriatic Sea, the mean instead was expressed as a range (summarizing the annual -12 months- data ranging from different stations) and describe the ideal conditions for seagrass growth.
All the analyses were performed with the software Statistica 7.1 (Statsoft). The spring dataset was processed by Spearman’s non-parametric analysis and principal component analysis after data normalization (PCA; including 103 stations sampled in June, 16 water parameters, 12 sediment parameters, 12 macrophyte variables) from: Mo.Ve.Co. III, 2018; SeResto, 2014-2017; CORILA mission, 2020-2021; Fattibello, Goro, 2022-2023; Cagnoni (1997).
3 Results
3.1 Relevant parameters/variables
The non-parametric Spearman’s coefficients between the physico-chemical parameters and macrophyte variables are shown in Table 1, highlighting the most relevant ones from the highest number of significant values. In this hierarchy, water transparency, Chl-a tot and Porg in the sediment gather in the top three most significant values, followed by Fines (sediment fraction < 63 µm), nitrates (NO3-), Ptot and Pinorg in surface sediments. Therefore, together with sediment moisture, Ehs and water temperature, we obtain the 10 parameters that most heavily influenced macrophytes presence, abundance, and type.
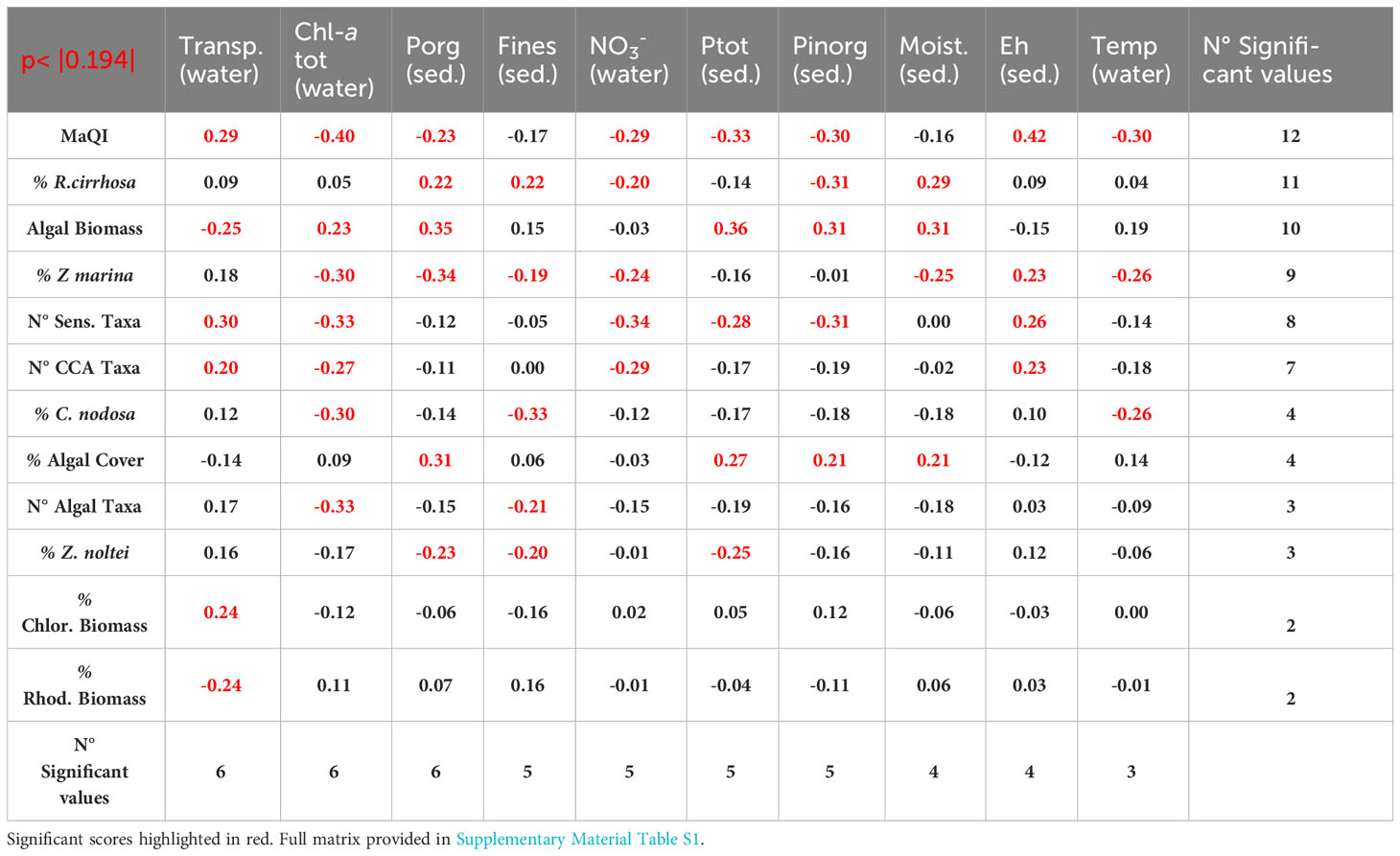
Table 1 Spearman’s non-parametric correlation matrix of the ten most significant parameters of sediment and water column with macrophyte variables.
Overall, water transparency had a positive effect on CCA, sensitive macroalgal species, Chlorophyta, and aquatic angiosperm growth, conversely high Chl-a values (i.e. high phytoplankton densities) had a depressing effect especially on seagrass, CCA, sensitive macroalgal species.
Similarly, high values of Porg in the sediment were positively linked to the presence of algal biomass, algal cover and R. cirrhosa cover and inversely to seagrasses. Among these first parameters, R. cirrhosa stands out from other aquatic angiosperms, not only because it was not correlated, and therefore not affected, by water transparency and phytoplankton (Chl-a), but also since it was positively correlated with Porg, together with algal biomass and cover and with other sedimentary parameters like Corg, Ntot and Ctot, associated with sediments of eutrophic areas. In the same way, high values of Fines had a negative effect on all aquatic angiosperms, except on R. cirrhosa, which was also positively correlated with high levels of sediment moisture. The associations between environmental parameters and macrophyte variables are better visualized by the PCA analysis. The plotting of the first two components, explaining a cumulative variance of 26.9%, is reported in Figure 2. On the right side of the graph, the parameters that negatively influenced on the angiosperm presence and growth can be found: i.e. in the sediments, high amounts of Fines, high values of moisture, Corg, Porg and Ntot and, in the water column, high Chl-a (phytoplankton) concentrations, high water temperature, high RSi, RP and TSS (turbidity) values. Conversely, on the left side of the graph, the values associated with the presence of Z. marina, Z. noltei, and C. nodosa are present: high salinity, high water transparency and pHs, well oxidized waters and sediments (Ehw/Ehs), high sediment density. These parameters were also associated with the presence of a higher number of macroalgal taxa, especially sensitive and calcified species and higher ecological scores, according to the MaQI ecological quality index. R. cirrhosa falls in the middle of the graph, showing to be less sensitive to the environmental trophic level and the ecological status compared to the other angiosperm species.
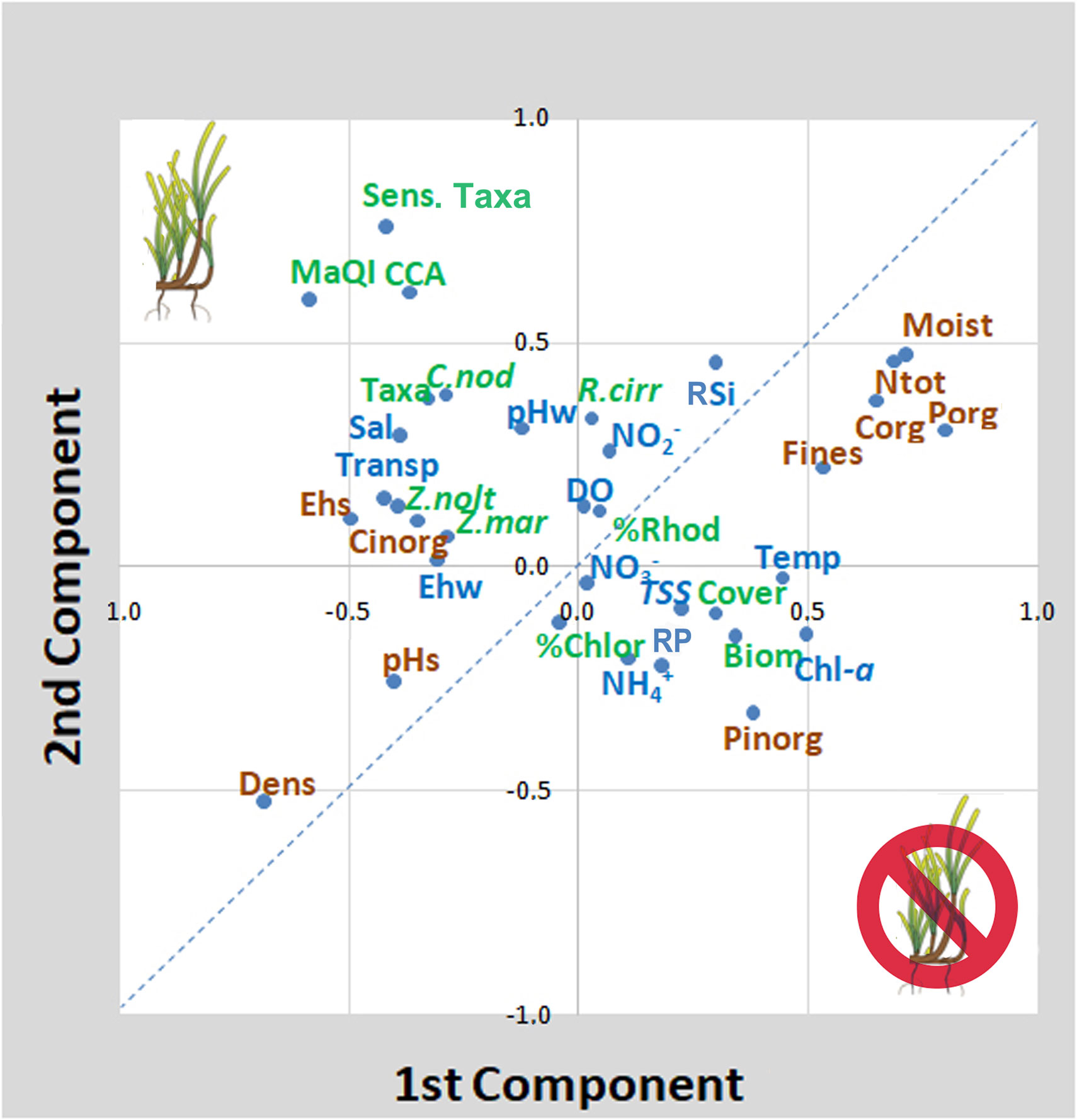
Figure 2 Principal Component Analysis of spring variables. The first two components explain a cumulative variance of 26.9%. Brown writings (sediment parameters); blue writings (water parameters); green writings (macrophyte biota parameters). The dotted line discriminates parameters/variables associated with high quality from those associated with poor quality. Biom. (biomass); CCA (crustose coralline algae); Chl-a (total chlorophyll-a); Chlor. (Chlorophyta); C. nod (C. nodosa); Cover (coverage); Corg (organic carbon); Dens. (density); DO(dissolved oxygen); Ehw/Ehs (Eh of the water column/sediment); MaQI (macrophyte quality index); Moist (moisture); NH4+ (ammonium); NO2- (nitrite); NO3-(nitrate); Ntot (total nitrogen); pHw/pHs (pH of water/sediment); Porg/Pinorg (organic/inorganic phosphorus); R. cirr (R. cirrhosa); Rhod. (Rhodophyta), RP (reactive phosphorus); RSi (reactive silicates); Sens. Taxa (macroalgae sensitive species); Taxa (total number of macroalgae species); Temp. (Temperature of water); Transp. (Transparency); TSS (total suspended solids); Z. mar (Z. marina); Z. nolt (Z. noltei).
3.2 The limits for success: water column
In this part of data elaboration, we tried to define extreme limits and ideal environmental conditions out of which the survival of aquatic angiosperms is disadvantaged and will lead to their disappearance in the short-medium term or, conversely, will allow a new plant introduction and successful growth. Additionally, these values can help to predict a shift in ecologic succession between different species with changing environmental conditions and the suitability of each species to different environmental parameters.
In Tables 2 and 3, the ranges of the annual means, recorded in several stations on monthly basis (the ideal condition range), and the minimum/maximum values, recorded in all the stations sampled on temporal and spatial basis where macrophytes were present (limits of tolerance and survival), are reported. In this way, the mean and the extreme values of each physico-chemical parameter of water and sediment or macrophyte variable, which favor or hamper the angiosperm presence and rule their transplant success, can be determined.
As recoded in Table 1 water transparency and Chl-a are prominent factors in aquatic angiosperm rooting, survival and growth. All the plants thrived in waters with a mean water transparency allowing a bottom visibility higher than 98%, i.e. affected only by occasional turbidity events; conversely, macroalgae lived also in turbid waters (especially red algae, such as Gracilariaceae and Solieriaceae) and tolerated very low minimum values (less than 30% of bottom visibility). Likewise, Chl-a, that traces the presence of phytoplankton, hampered aquatic angiosperm optimal growth at mean concentrations higher than 2.5 µg L-1, while macroalgae well tolerated mean concentrations up to 13.7 µg L-1. The TSS ideal average range for Z. noltei, Z. marina and C. nodosa was between 12.5 and 49.9 mg L-1, with higher extreme values for macroalgae (183 mg L-1). SPM, instead, describes a higher tolerance of seagrasses, especially C. nodosa for areas characterized by higher hydrodynamics and sedimentation rates associated with marine-like areas. Macroalgae showed the widest tolerance limit range for salinity and were present between 5.0 and 40.6 psu. Among aquatic angiosperms, R. cirrhosa survived at the lowest values (13.1 psu), but overall aquatic angiosperms thrived between 28.7 and 32.4 psu, while macroalgae preferred lower salinities living in the optimal range 20.6-27.0 psu.
Macroalgae showed the widest tolerance range for DIN, as for salinity. Indeed, the maximum recorded DIN value was 154 µM. The ideal range in terms of DIN values, in which macroalgae and phytoplankton thrived competing with aquatic angiosperms, started from mean values of 9.45 µM, reaching even 35.8 µM. The seagrasses Z. marina and Z. noltei lived within maximum DIN concentrations of 21.6 µM. The species R. cirrhosa, instead, was more tolerant proliferating well up to 56.8 µM. The situation was similar for RP; also in this case, the ideal growing ranges for R. cirrhosa showed higher values at the upper limit (RP: 11.1 µM) in comparison with the other aquatic angiosperms (maximum tolerated RP was 2.58 µM), even in comparison with the maximum scores from areas colonized by macroalgae (RP: 2.79 µM). Eventually, about the pH of the water column (pHw) no important differences within the limits of ideal conditions were noticed, although some differences were recorded in the extreme minimum values. Macroalgae were found also in the presence of the lowest pH scores (pHw: 7.30), while the minimum pHw values found for aquatic angiosperms were higher than 7.80. CCA species live as epiphytes on aquatic angiosperms and large macroalgae in our samples and were represented by 5 species (Pneophyllum fragile Kützing, Hydroliton boreale (Foslie) Y. M. Chamberlain, H. cruciatum (Bressan) Y. M. Chamberlain, H. farinosum (J. V. Lamouroux) D. Penrose et Y. M. Chamberlain, Melobesia membranacea (Esper) J. V. Lamouroux), showing a monthly mean number of 0.40-2.41 species on the leaves of aquatic angiosperms, on annual basis. Conversely, the mean number of CCA on macroalgae was in the range 0-0.6 species. The situation was quite similar for the number of sensitive species and the total macroalgal biodiversity, which was the highest in the presence of aquatic angiosperms. In this context, the mean macroalgal biomass well tolerated by aquatic angiosperms was in the range between 32 and 792 g fw m-2. However, during these surveys, among the aquatic angiosperm prairies, macroalgal biomasses up to 7500 g fw m-2 were also recorded.
3.3 The limits for success: sediment
The nature of the sedimentary substrate is often crucial for effective colonization by aquatic angiosperms and sediment physico-chemical characteristics and trophy play a key role in angiosperm rooting. High levels of Ntot in surface sediments were better tolerated by R. cirrhosa and macroalgae, while seagrasses had an upper tolerance limit of 4.66 mg g-1 and an ideal condition range within 1.77 mg g-1. Similarly, Corg was poorly tolerated by all aquatic angiosperms at values higher than 59.2 mg g-1 and presented ideal conditions below 23.5 mg g-1. Porg displayed higher values in the upper optimal ranges for R. cirrhosa (199 µg g-1), whereas in the presence of macroalgae reached 239 µg g-1. Fines, Ehs, moisture and density of the sediments were also important in determining the establishment of plants. The optimal percent of Fines for R. cirrhosa was in the range of muddy sediment, i.e. 74.6-91.1%; conversely, a wider range was tolerated by macroalgae (34.4-94.3%) and seagrasses (2.55-95.0%), which displayed the widest optimal ranges at values between 1.27 and 98.7% thriving also in sandy sediments (especially C. nodosa). Similarly, aquatic angiosperms thrived at higher sediment density (0.68-1.34 g dw cm-3). The Ehs displayed the widest range for aquatic angiosperms, especially in C. nodosa (growing both in choked and marine-like areas) ranging for optimal values in the range -190 - +124. R. cirrhosa was present in areas characterized by the lowest Ehs scores (range: -250 - +73).
4 Discussion
4.1 Transplantation approaches and critical issues
The survival of aquatic angiosperms is not always supported by environmental conditions and their transplant is not ubiquitously guaranteed in any type of TWS, regardless of the type of technique implemented to perform the transplant. A critical analysis performed by Curiel et al. (2021) summarized in few focal points the common mistakes that can compromise the success of a seagrass transplantation project. The highlighted points deal with: seabed alteration interventions that fail to alleviate habitat stress (e.g. excavation of canals, creation of salt marshes); adverse effects of climate change, such as high summer temperatures, prolonged low tide and emersion periods; macroalgal blooms leading to anoxic crises; execution of transplants on inconsistent sediments or in shallow bathymetry; poor alignment of the intervention’s seasonality with species characteristics; incorrect levelling of transplanted sods concerning the surrounding substrate; mistakes in selecting suitable species for transplantation. The main problems are therefore linked to the choice of unsuitable transplant sites (due to inappropriate bathymetry, water trophy, recipient substrate); to the transplant of the wrong species, not only in the wrong places but also in the wrong seasons, and to potential critical conditions triggered by extreme climatic events or disrupting species. To the critical points listed above by Curiel et al. (2021), we should add those raised by Cronau et al. (2023), such as the occurrence of extreme events (e.g. storms, siltation, salinity fluctuations, or drought) – fairly typical in some coastal ecosystems – which can readily compromise restoration efforts. Additionally, disruptions in trophic interactions could theoretically impede the progress of recovery attempts. For instance, the absence of apex predators (often linked to fishing activities) could result in an overabundance of meso-predators, which, in turn, might excessively exploit invertebrate grazers essential for managing the growth of epiphytic algae on seagrass leaves. Therefore, if direct anthropogenic impacts (e.g. pollution discharge, sediment dredging, unauthorized fishing) or unpredictable extreme climatic events (e.g. heat waves) are excluded for the choice of the intervention site, it is a preliminary need to monitor the main physico-chemical conditions of water column and surface sediments from the candidate recipient sites, which are the first drivers for recruitment, thriving or survival of new aquatic angiosperms.
Provided that sites suitable for aquatic angiosperm transplantation are identified on the basis of the limits described in Table 2 and Table 3, numerous and different approaches to the transplantation have been so far implemented in different projects in function of the species and donor/recipient substrates (Ganassin and Gibbs, 2008; Tan et al., 2020). In Figure 3 and Table S2, some aquatic angiosperm restoration trials published to date for C. nodosa, Z. marina, Z. noltei and R. cirrhosa are summarized, with a focus on species, locations, transplant approaches, planting periods, monitoring strategies and outcomes. Globally, these trials can be divided firstly into two main groups: mechanical transplants and manual transplants. Both methods require knowledge of plant species growth habits, seasonality, life histories and nearby available seagrass stocks to be used as donor meadows (either cultivated or wild, with the wild meadows usually being the most used). Different parts of the donor meadow can be used for the transplant activity (e.g. entire shoots, just the rhizomes, fragments, seeds or seedlings) and each of these parts can be prepared with different methods to be used as planting units. Different planting arrangements were also tested, but these were reported to have no effect on plant survival (Suykerbuyk et al., 2012). The planting units in transplant projects can be sediment-free (as anchored or unanchored sprigs, shoots or rhizomes), sediment-intact (as sods, cores or plugs, characterized by sediment plus intact rhizome/root systems) or seeds/seedlings (Ganassin and Gibbs, 2008; Matheson et al., 2017). Generally, sediment-intact transplanting units are preferred since the root and rhizome system is relatively undamaged and they also provide a reservoir of the original rooting medium (Fonseca et al., 1998; Matheson et al., 2017). However, single shoot/rhizome transplants ensure an increased dispersion of implants, elevating the likelihood of successful outcomes (Sfriso et al., 2019a; Zhang et al., 2021). Furthermore, in favorable conditions (Sfriso et al., 2021) the transplants of individual rhizomes resulted in clusters displaying a higher average growth rate compared to sods (rhizomes: 0.19 cm day-1, sods: 0.14 cm day-1). The greater abundance of single transplants can foster the rapid formation of expansive angiosperm meadows within a single year, with a survival of single shoots/rhizomes reported after one year ranging from 39% (Sfriso, 2018) to 95% (Davis and Short, 1997).
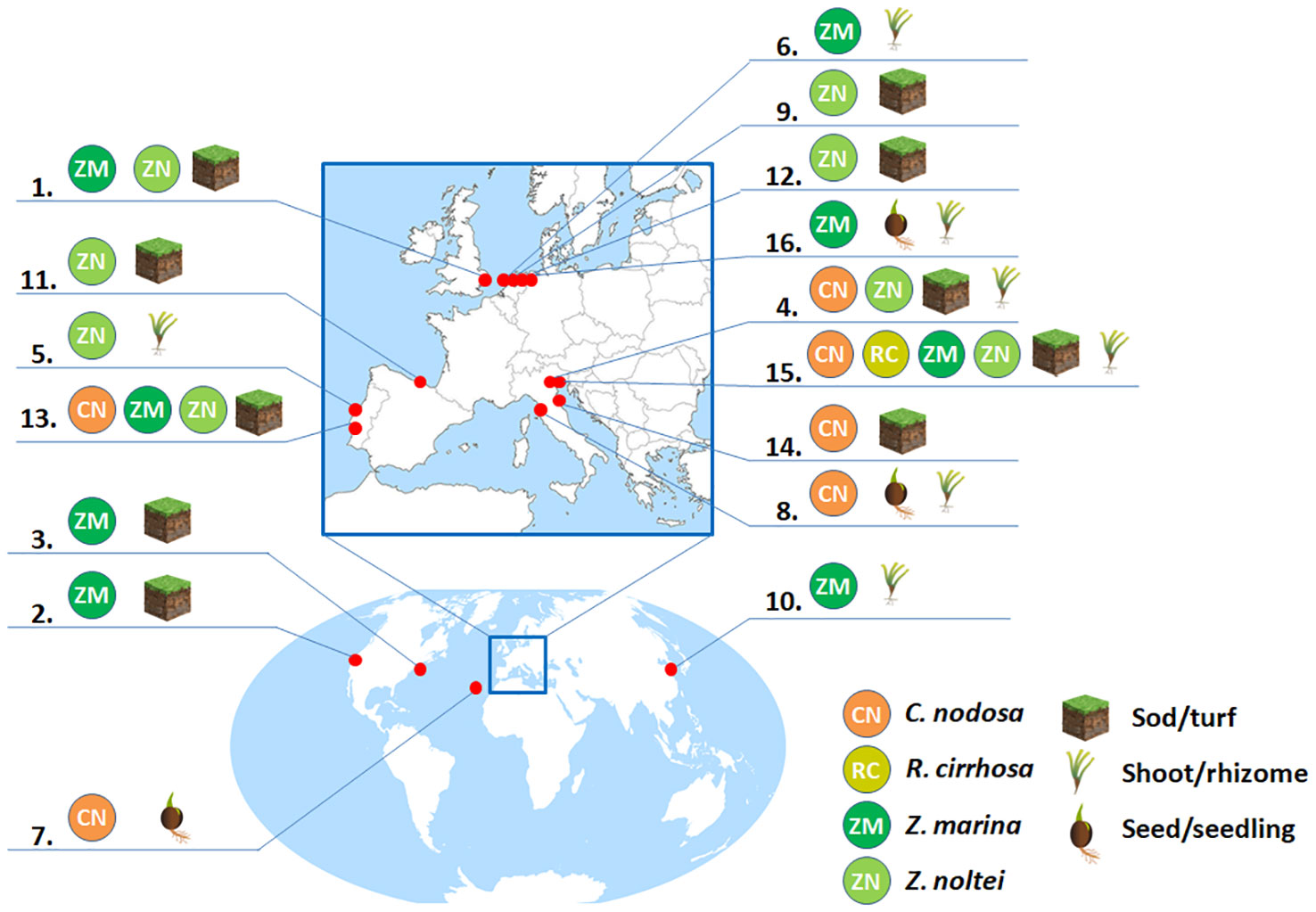
Figure 3 Seagrass restoration trials reported for C. nodosa, R. cirrhosa, Z. marina and Z. noltei. Additional information provided in supplementary material Table S1. 1. Ranwell et al. (1974), 2. Zimmerman et al. (1995), 3. Davis and Short (1997), 4. Curiel et al. (2003), 5. Martins et al. (2005), 6. Bos and Van Katwijk (2007), 7. Zarranz and Gonza (2010), 8. Balestri and Lardicci (2012), 9. Suykerbuyk et al. (2012), 10. Li and Lee (2010)/ Li et al. (2014), 11. Valle et al. (2015), 12. Suykerbuyk et al. (2016), 13. Paulo et al. (2019), 14. Da Ros et al. (2020), 15. Sfriso et al. (2021), 16. Cronau et al. (2023).
In environments characterized by high hydrodynamics, like open coastal areas or where severe storms rage, larger planting units have generally more chances of survival than smaller ones or single shoots (Suykerbuyk et al., 2016; Paulo et al., 2019), because they offer greater anchorage and less rhizome disturbance (Paling et al., 2001). Conversely, sediment-free transplants are more favored in habitats characterized by fine sands, moderate water movement and good light availability (Orth et al., 1999; Paling et al., 2007). In order to protect the transplants and mark the transplanted areas, sheltering or sediment stabilizing devices (e.g. meshes, fences, screens of wooden fascines, artificial seagrass mats) and poles or buoys can also be used. However, these protective and marking structures must be arranged with attention, as they can limit the water circulation by creating choked areas in which algal blooms can flourish, especially in sites with low hydrodynamics. Additionally, marking poles or buoys often act as a collection point for fast growing pleustophytic algae, such as the Ulvaceae, which remain trapped on signalling poles and wooden fascines accumulating and suffocating the nearby transplanted seagrasses (a problem recorded in the framework of the seagrass restoration project Life SERESTO - Sfriso et al., 2017b). Many authors also raised critical issues on the presence of antagonistic species that can compromise the success of transplantation activities and to which attention should be paid. These are well known fast-growing macroalgae, both epiphytic and pleustophytic. In our case however macroalgae were often present in association with aquatic angiosperms, especially represented by the Chlorophycea Chaetomorpha linum (O.F. Müller) Kützing. In the past we found that this species could reach biomasses also higher than 20 kg fw m-2, without affecting significantly plant survival as it would happen in the presence of high biomasses of fast growing r-strategy thionitrophilous species, such as Ulvaceae, Gracilariaceae, Solieriaceae and other Cladophoraceae. As appears from all the statistical analyses, C. linum is a sensitive species and apparently does not suffocate and kill the underlying aquatic angiosperms as it is associated with environments characterized by good-high ecological conditions.
Sediment bioturbation by benthic animal species were also reported to produce substantial losses of transplants, especially in early life stages like seeds and seedlings. Among these, the lugworm Arenicola marina Linnaeus (Hughes et al., 2000; Suykerbuyk et al., 2012; Cronau et al., 2023), the ragworms Nereis diversicolor Müller (Hughes et al., 2000) and Platynereis dumerilii Audouin et Milne Edwards (Cronau et al., 2023) and the green crab Carcinus maenas Linnaeus (1758) were reported (Davis and Short, 1997; Cronau et al., 2023).
4.2 What species, where and when
Given that the local availability of the seagrass species within acceptable and practicable limits of explantation and transport is a fundamental prerequisite, it is noted that all the articles investigated in Figure 3 and Table S2 on transplant trials of the last decades focused on transplant techniques, seasonality and the status of the prairies of the donor and recipient sites, with serious shortcomings in consideration of the water and sediment parameters/variables of the recipient sites, which are instead fundamental in predicting a successful rooting of aquatic angiosperms. Frequently, the criteria for choosing the sites are not clearly identified or the selected areas were close to already present prairies which probably presented similar and suitable conditions such as to guarantee the success of transplant operations. The authors focused on the texture of the sediments reporting how: Z. marina thrives in silty sediments; Z. noltei is prevalent in inner areas characterized by predominantly silty sediments, shallow waters and muddy expanses; C. nodosa establishes itself in regions characterized by elevated salinity and coarse sediments (Curiel et al., 2021) and R. cirrhosa thrives in very fine sediments (Munari et al., 2023). However, our results show that the texture of the sediments is not the only parameter to take into consideration, given the wide tolerance ranges for Fines of aquatic angiosperms, and that organic carbon, organic phosphorus and total nitrogen appear to be parameters of the sediment most significant in site selection. Similarly, seasonality is an important parameter in ensuring a successful colonization of the species, but up to date most of the transplant trials (Table S2) took place in late spring/early summer without taking into account the life cycle of the species. In this regard, it has been reported that is advisable to refrain from transplanting Z. marina in summer. Conversely, autumn is advisable as a transplant season for Z. marina being less stressful for the transplants and producing higher shoot expansion (Li and Lee, 2010; Li et al., 2014). This fact was confirmed by Sfriso et al. (2021), who reported autumn as the best season for Z. marina transplants, late spring and autumn for Z. noltei, late spring and early summer for C. nodosa and summer-autumn for R. cirrhosa.
The literature on R. cirrhosa transplants is quite poor. This species is different from the other investigated here; in fact, it is associated with conditions of environmental quality on average lower, living in choked and eutrophic areas in very fine sediments. These conditions are well tolerated as it is also highlighted by the limits proposed in Table 2 and Table 3. Besides, R. cirrhosa was reported to be associated with poor levels of environmental quality determined by means of the M-AMBI benthic quality index in the Comacchio Ponds – Italy (Munari et al., 2023). This species manifested an unstable presence in TWS, quickly forming meadows in just one year, then disappearing and reappearing after few years (Sfriso et al., 2021). These premises seem to suggest that R. cirrhosa could be a poor choice in a transplant strategy; however, the wide tolerance range of this species can make it an exceptional pioneer species in choked areas that are strongly affected by freshwater influence or are characterized by a high trophic status, where the survival of other aquatic angiosperms is disadvantaged. R. cirrhosa meadows displayed a positive influence on macrofaunal abundance and diversity (Boström et al., 2011; Munari et al., 2023) and, moreover, an area colonized by this species is generally one step higher on an ecological quality scale than beds of nuisance macroalgae, avoiding summer biomass collapse. Therefore, R. cirrhosa can represent a pioneer species in an ecological restoration project by initiating a process of ecological succession and sediment stabilization, which can subsequently favor the colonization by other seagrass species, such as Z. noltei or Z. marina.
4.3 Using the limits
The limits for plant rooting, survival and growth proposed here are not meant to be considered as insurmountable barriers, also considering the geographical delimitation of the investigated sites that are Mediterranean microtidal lagoons. These limits represent a guideline for determining the suitability of a site for transplantation of different species and to evaluate the chances of success, which are lower the farther away are the parameters/variables from the average/optimum reported values. Our proposal on their use in a preliminary site evaluation is as follows. A spatial and temporal punctiform sampling on the candidate recipient site allows to understand whether we are within the minimum and maximum survival limits for the various parameters/variables. However, a single sampling is too little to provide a reliable average of the environmental parameters/variables and to identify whether we are within the optimum range. For this reason, a minimum average value of 2 surveys (spring and autumn) at least is proposed, but possibly better one per season, to mediate the environmental conditions during the year. The most relevant parameters to consider in this evaluation, and that should be prioritized, are: water transparency, total Chl-a and nitrates or DIN, for the water column; Porg, Pinorg and Fines percent, for the sediments, and the presence/absence of sensitive macroalgae, especially CCA species. These calcareous species are particularly sensitive to environmental changes, more than aquatic angiosperms. In fact, they respond to environmental changes in a few months and are easily monitored because they grow as epiphytes on all large macroalgae, so their presence is easily monitored, even in the absence of angiosperms and in the presence of a few thalli of other species. Their presence/absence alone is the best index for evaluating an environment in good or bad environmental conditions (Sfriso et al., 2020b).
In this study, the previous experiences and available data on the Adriatic microtidal lagoons have been elaborated for the definition of physico-chemical parameters, macroalgal variables and limit values, optimal for rooting and growth, as well as survival of the aquatic angiosperms that should be monitored on a potential recipient site before carrying out the transplant activity in plant restoration. The parameters/variables defined here represent a guideline to select suitable sites for angiosperm transplants and for the identification of critical conditions that may compromise or optimize the success of transplant actions.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Author contributions
AAS: Conceptualization, Visualization, Writing – original draft, Funding acquisition. KS: Writing – review & editing, Writing – original draft. MM: Funding acquisition, Supervision, Writing – review & editing. CM: Supervision, Writing – review & editing. AJ: Writing – review & editing. AB: Investigation, Writing – review & editing. YT: Investigation, Writing – review & editing. AS: Data curation, Formal analysis, Methodology, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. AAS realized this publication during a research grant co-funded by the European Social Fund (ESF)—Italian National Operational Programme (NOP) on Research and Innovation 2014–2020 (article 24, clause 3, letter a) of Italian Law n. 240 of 30 December 2010 and of Italian ministerial decree n. 1062 of 10 August 2021, grant number 2021-PON-DM-1062-AS-RIC.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmars.2023.1299428/full#supplementary-material
References
Airoldi L., Beck M. W. (2007). Loss, status and trends for coastal marine habitats of Europe. Oceanography Mar. Biol: Annu. Rev. 45, 345–405. doi: 10.1201/9781420050943.ch7
Balestri E., Lardicci C. (2012). Nursery-propagated plants from seed: A novel tool to improve the effectiveness and sustainability of seagrass restoration. J. Appl. Ecol. 49 (6), 1426–1435. doi: 10.1111/j.1365-2664.2012.02197.x
Barbier E. B., Hacker S. D., Kennedy C., Koch E. W., Stier A. C., Silliman B. R. (2011). The value of estuarine and coastal ecosystem services. Ecol. Monogr. 81 (2), 169–193. doi: 10.1890/10-1510.1
Bos A. R., Van Katwijk M. M. (2007). Planting density, hydrodynamic exposure and mussel beds affect survival of transplanted intertidal eelgrass. Mar. Ecol. Prog. Ser. 336, 121–129. doi: 10.3354/meps336121
Boström C., Pittman S. J., Simenstad C., Kneib R. T. (2011). Seascape ecology of coastal biogenic habitats. Mar. Ecol. Prog. Ser. 427, 191–218. doi: 10.3354/meps09051
Boudouresque C. F., Bernard G., Pergent G., Shili A., Verlaque M. (2009). Regression of Mediterranean seagrasses caused by natural processes and anthropogenic disturbances and stress: A critical review. Botanica Marina 52 (5), 395–418. doi: 10.1515/BOT.2009.057
Burkholder J. M., Tomasko D. A., Touchette B. W. (2007). Seagrasses and eutrophication. J. Exp. Mar. Biol. Ecol. 350 (1), 46–72. doi: 10.1016/j.jembe.2007.06.024
Cagnoni S. (1997). Dynamics of phytocoenosis development in Ruppia cirrhosa (Petagna) Grande and Zostera noltii Hornem. in a salt marsh clear in relation to their microenvironments. [master's thesis] (Venice (IT: Ca' Foscari University of Venice).
Calvo S., Calco R., Luzzu F., Raimondi V., Assenzo M., Cassetti F. P., et al. (2021). Performance assessment of Posidonia oceanica (L.) Delile restoration experiment on dead matte twelve years after planting—structural and functional meadow features. Water 13, 724. doi: 10.3390/w13050724
Cronau R. J. T., de Fouw J., van Katwijk M. M., Bouma T. J., Heusinkveld J. H. T., Hoeijmakers D., et al. (2023). Seed- versus transplant-based eelgrass (Zostera marina L.) restoration success in a temperate marine lake. Restor. Ecol. 31 (1), e13786. doi: 10.1111/rec.13786
Curiel D., Kraljević-Pavelić S., Kovačev A., Miotti C., Rismondo A. (2021). Marine seagrasses transplantation in confined and coastal adriatic environments: methods and results. Water 13 (16), 2289. doi: 10.3390/w13162289
Curiel D., Scarton F., Rismondo A., Marzocchi M. (2003). Transplanting seagrasses in the Venice Lagoon: Results and perspectives. Proceedings of the Sixth International Conference on the Mediterranean Coastal Environment MEDCOAST03. 2, 853–864.
Da Ros Z., Corinaldesi C., Anno A. D., Gambi C., Torsani F., Danovaro R. (2020). Restoration of Cymodocea nodosa seagrass meadows: Efficiency and ecological implications. Restor. Ecol. 29 (2), e13313. doi: 10.1111/rec.13313
Davis R. C., Short F. T. (1997). Restoring eelgrass, Zostera marina L., habitat using a new transplanting technique: The horizontal rhizome method. Aquat. Bot. 59 (1), 1–15. doi: 10.1016/S0304-3770(97)00034-X
De los Santos C. B., Krause-Jensen D., Alcoverro T., Marbà N., Duarte C. M., van Katwijk M. M., et al. (2019). Recent trend reversal for declining European seagrass meadows. Nat. Commun. 10, 3356. doi: 10.1038/s41467-019-11340-4
Duarte C. M. (1991). Seagrass depth limits. Aquat. Bot. 40 (4), 363–377. doi: 10.1016/0304-3770(91)90081-F
Duarte C. M., Agusti S., Barbier E., Britten G. L., Castilla J. C., Gattuso J. P., et al. (2020). Rebuilding marine life. Nature 580 (7801), 39–51. doi: 10.1038/s41586-020-2146-7
Duarte C. M., Marbà N., Santos R. (2004). ““What may cause loss of seagrasses?”,” in European seagrasses: An introduction to monitoring and management. Eds. Borum J., Duarte C. M., Krause-Jensen D., Greve T. M. (The M&MS project - Monitoring and Managing of European Seagrasses), 24–32.
Dunic J. C., Brown C. J., Connolly R. M., Turschwell M. P., Côté I. M. (2021). Long- term declines and recovery of meadow area across the world’s seagrass bioregions. Glob Change Biol. 27, 4096–4109. doi: 10.1111/gcb.15684
Erftemeijer P., Middelburg J. (1993). Sediment-nutrient interactions in tropical seagrass beds: A comparison between a terrigenous and a carbonate sedimentary environment in South Sulawesi (Indonesia). Mar. Ecol. Prog. Ser. 95, 187–198. doi: 10.3354/meps095187
Fonseca M. S., Kenworthy W. J. (1987). Effects of current on photosynthesis and distribution of seagrasses. Aquat. Bot. 27 (1), 59–78. doi: 10.1016/0304-3770(87)90086-6
Fonseca M. S., Kenworthy W. J., Thayer G. W. (1998). Guidelines for the conservation and restoration of seagrasses in the United States and adjacent waters (Vol. 12). NOAA Coast. Ocean Program Decision Anal. Ser. 12, 1–222.
Ganassin C., Gibbs P. J. (2008). A review of seagrass planting as a means of habitat compensation following loss of seagrass meadow—Fisheries Final Report Series. Aust. Government NSW Department Primary Industries. 96, 1–35.
Garmendia J. M. (2021). “Restauración de praderas marinas: estuarios vascos.”, in Praderas marinas, tesoros de biodiversidad Publicación científico-técnica. Eds. Vázquez V. V., Gómez P. S., Redondo G. V. (Oleiros, Spain: CEIDA – Centro de Extensión Universitaria e Divulgación Ambiental de Galicia. Oleiros), 57–74.
Garmendia J. M., Rodríguez J. G., Borja Á., Franco J. (2010). Clasificación de los estuarios del País Vasco como zonas potenciales para la restauración de praderas intermareales de Zostera noltii. Rev. Investigación Marina 17 (4), 40–61.
Garmendia J. M., Rodríguez J. G., Borja A., Pouso S., Del Campo A., Galparsoro I., et al. (2023). Restoring seagrass meadows in Basque estuaries: nature-based solution for successful management. Nature-Based Solutions 4, 100084. doi: 10.1016/j.nbsj.2023.100084
Greve T. M., Binzer T. (2004). “Which factors regulate seagrass growth and distribution?,” in European seagrasses: An introduction to monitoring and management. Eds. Borum J., Duarte C. M., Krause-Jensen D., Greve T. M. (The M&MS project - Monitoring and Managing of European Seagrasses), 19–23.
Hughes R. G., Lloyd D., Ball L., Emson D. (2000). The effects of the polychaete Nereis diversicolor on the distribution and transplanting success of Zostera noltii. Helgoland Mar. Res. 54 (2), 129–136. doi: 10.1007/s101520050011
Li W. T., Kim Y. K., Park J. I., Zhang X., Du G. Y., Lee K. S. (2014). Comparison of seasonal growth responses of Zostera marina transplants to determine the optimal transplant season for habitat restoration. Ecol. Eng. 71, 56–65. doi: 10.1016/j.ecoleng.2014.07.020
Li W. T., Lee K. S. (2010). Adaptation success of Zostera marina to a new transplant environment. Algae 25 (1), 27–35. doi: 10.4490/algae.2010.25.1.027
Mancini G., Ventura D., Casoli E., Belluscio A., Ardizzone G. D. (2022). Transplantation on a Posidonia oceanica meadow to facilitate its recovery after the Concordia shipwrecking. Mar. pollut. Bull. 179, 113683. doi: 10.1016/j.marpolbul.2022.113683
Martins I., Neto J. M., Fontes M. G., Marques J. C., Pardal M. A. (2005). Seasonal variation in short-term survival of Zostera noltii transplants in a declining meadow in Portugal. Aquat. Bot. 82 (2), 132–142. doi: 10.1016/j.aquabot.2005.03.006
Masiol M., Facca C., Visin F., Sfriso A., Pavoni B. (2014). Interannual heavy element and nutrient concentration trends in the top sediments of Venice Lagoon (Italy). Mar. pollut. Bull. 89 (1), 49–58. doi: 10.1016/j.marpolbul.2014.10.036
Matheson F. E., Reed J., Santos V. M. D., Mackay G., Cummings V. J. (2017). Seagrass rehabilitation: Successful transplants and evaluation of methods at different spatial scales. New Z. J. Mar. Freshw. Res. 51 (1), 96–109. doi: 10.1080/00288330.2016.1265993
Morand P., Briand X. (1996). Excessive Growth of Macroalgae: A symptom of environmental disturbance. Botanica Marina 39, 491–516. doi: 10.1515/botm.1996.39.1-6.491
Munari C., Casoni E., Cozzula C., Pasculli A., Pezzi M., Sciuto K., et al. (2023). The ecological role of Ruppia cirrhosa (Petagna) Grande in a choked lagoon. Water 15 (12), 2162. doi: 10.3390/w15122162
Munari C., Mistri M. (2012). Ecological status assessment and response of benthic communities to environmental variability: The Valli di Comacchio (Italy) as a study case. Mar. Environ. Res. 81, 53–61. doi: 10.1016/j.marenvres.2012.08.008
Nellemann C., Corcoran E., Duarte C. M., Valdés L., DeYoung C., Fonseca L., et al. (2009). Blue Carbon (A Rapid Response Assessment. United Nations Environment Programme, GRID-Arendal). Available at: www.grida.no.
Nordlund L. M., Gullström M. (2013). Biodiversity loss in seagrass meadows due to local invertebrate fisheries and harbour activities. Estuarine Coast. Shelf Sci. 135, 231–240. doi: 10.1016/j.ecss.2013.10.019
Nordlund L. M., Koch E. W., Barbier E. B., Creed J. C. (2016). Seagrass ecosystem services and their variability across genera and geographical regions. PloS One 11 (10), 1–23. doi: 10.1371/journal.pone.0163091
Orth R. J., Carruthers T. J. B., Dennison W. C., Duarte C. M., Fourqurean J. W., Heck K. L., et al. (2006). A global crisis for seagrass ecosystems. BioScience 56 (12), 987–996. doi: 10.1641/0006-3568(2006)56[987:AGCFSE]2.0.CO;2
Orth R. J., Harwell M. C., Fishman J. R. (1999). A rapid and simple method for transplanting eelgrass using single, unanchored shoots. Aquat. Bot. 64 (1), 77–85. doi: 10.1016/S0304-3770(99)00007-8
Paling E. I., Van Keulen M., Tunbridge D. J. (2007). Seagrass Transplanting in Cockburn Sound, Western Australia: A Comparison of Manual Transplantation Methodology Using Posidonia sinuosa Cambridge et Kuo. Restor. Ecol. 15 (2), 240–249. doi: 10.1111/j.1526-100X.2007.00207.x
Paling E. I., van Keulen M., Wheeler K., Phillips J., Dyhrberg R. (2001). Mechanical seagrass transplantation in Western Australia. Ecol. Eng. 16 (3), 331–339. doi: 10.1016/S0925-8574(00)00119-1
Pansini A., Bosch-Belmar M., Berlino M., Sarà G., Ceccherelli G. (2022). Collating evidence on the restoration efforts of the seagrass Posidonia oceanica: current knowledge and gaps. Sci. Total Environ. 851, 158320. doi: 10.1016/j.scitotenv.2022.158320
Paulo D., Cunha A. H., Boavida J., Serrão E. A., Gonçalves E. J., Fonseca M. (2019). Open coast seagrass restoration. Can we do it? Large scale seagrass transplants. Front. Mar. Sci. 6. doi: 10.3389/fmars.2019.00052
Ranwell D. S., Wyer D. W., Boorman L. A., Pizzey J. M., Waters R. J. (1974). Zostera transplants in norfolk and suffolk, Great Britain. Aquaculture 4, 185–198. doi: 10.1016/0044-8486(74)90033-7
Rodil F., Lohrer A. M., Thrush S. F., Norkko A. (2022). Positive contribution of macrofaunal biodiversity to secondary production and seagrass carbon metabolism. Ecology 103, e3648. doi: 10.1002/ecy.3648
Schanz A., Asmus H. (2003). Impact of hydrodynamics on development and morphology of intertidal seagrasses in the Wadden Sea. Mar. Ecol. Prog. Ser. 261, 123–134. doi: 10.3354/meps261123
Sfriso A. (2018). LIFE12 NAT/IT/000331—La salvaguardia dell’habitat lagunare di Venezia tramite il ripopolamento di fanerogame marine. - “Habitat 1150* (Coastal lagoon) recovery by SEagrass RESTOration. A new strategic approach to meet HD & WFD objectives”. (Habitat Directive Water Framework Directive).
Sfriso A., Bonometto A., Boscolo R., Bruno L., Buosi A., Facca C., et al. (2017b) Trapianto delle piante acquatiche per il ripristino dell’habitat «lagune costere» — Linee guida dall’esperienza del progetto life natura SERESTO. Available at: http://www.lifeseresto.eu/lifeseresto/wp-content/uploads/2018/05/guida-2017.pdf.
Sfriso A., Buosi A., Facca C., Sfriso A. A. (2017a). Role of environmental factors in affecting macrophyte dominance in transitional environments: The Italian Lagoons as a study case. Mar. Ecol. 38 (2), e12414. doi: 10.1111/maec.12414
Sfriso A., Buosi A., Facca C., Sfriso A. A., Tomio Y., Juhmani A. S., et al. (2021). Environmental restoration by aquatic angiosperm transplants in transitional water systems: The Venice Lagoon as a case study. Sci. Total Environ. 795, 148859. doi: 10.1016/j.scitotenv.2021.148859
Sfriso A., Buosi A., Mistri M., Munari C., Franzoi P., Sfriso A. A. (2019b). Long-term changes of the trophic status in transitional ecosystems of the northern Adriatic Sea, key parameters and future expectations: The lagoon of Venice as a study case. Nat. Conserv. 34, 193–215. doi: 10.3897/natureconservation.34.30473
Sfriso A., Buosi A., Sciuto K., Wolf M. A., Tomio Y., Juhmani A., et al. (2022). Effect of ecological recovery on macrophyte dominance and production in the venice lagoon. Front. Mar. Sci. 9. doi: 10.3389/fmars.2022.882463
Sfriso A., Buosi A., Tomio Y., Juhmani A. S., Chiesa S., Greco M., et al. (2020a). Sediment carbon variations in the venice lagoon and other transitional water systems of the Northern Adriatic Sea. Water 12 (12), 3430. doi: 10.3390/w12123430
Sfriso A., Buosi A., Tomio Y., Juhmani A., Facca C., Sfriso A. A., et al. (2019a). Aquatic angiosperm transplantation: A tool for environmental management and restoring in transitional water systems. Water 11 (10), 2135. doi: 10.3390/w11102135
Sfriso A., Buosi A., Wolf M. A., Sciuto K., Molinaroli E., Moro I., et al. (2020b). Microcalcareous seaweeds as sentinels of trophic changes and CO2 trapping in transitional water systems. Ecol. Indic. 118, 106692. doi: 10.1016/j.ecolind.2020.106692
Sfriso A., Facca C., Bonometto A., Boscolo R. (2014). Compliance of the macrophyte quality index (MaQI) with the WFD, (2000/60/EC) and ecological status assessment in transitional areas: The Venice lagoon as study case. Ecol. Indic. 46, 536–547. doi: 10.1016/j.ecolind.2014.07.012
Sfriso A., Facca C., Ceoldo S. (2005a). Recording the occurrence of trophic level changes in the lagoon of Venice over the ‘90s. Environ. Int. 31 (7), 993–1001. doi: 10.1016/j.envint.2005.05.009
Sfriso A., Facca C., Marcomini A. (2005b). Sedimentation rates and erosion processes in the lagoon of Venice. Environ. Int. 31, 983–992. doi: 10.1016/j.envint.2005.05.008
Sfriso A. A., Sfriso A. (2017). In situ biomass production of Gracilariaceae and Ulva rigida: the Venice Lagoon as a study case. Botanica Marina 60 (3), 271–283. doi: 10.1515/bot-2016-0061
Short F. T., Wyllie-Echeverria S. (1996). Natural and human-induced disturbance of seagrasses. Environ. Conserv. 23 (1), 17–27. doi: 10.1017/S0376892900038212
Sorokin P. Y., Sorokin Y. I., Boscolo R., Giovanardi O. (2004). Bloom of picocyanobacteria in the venice lagoon during summer–autumn 2001: ecological sequences. Hydrobiologia 523 (1), 71–85. doi: 10.1023/B:HYDR.0000033096.14267.43
Sorokin Y. I., Zakuskina O. Y. (2010). Features of the Comacchio ecosystem transformed during persistent bloom of picocyanobacteria. J. Oceanography 66 (3), 373–387. doi: 10.1007/s10872-010-0033-9
Strickland J. D. H., Parsons T. R. (1984). A Practical Handbook of Seawater Analyses (2nd Ed.) (Ottawa, Canada: Bulletin of Fishery Research Board of Canada).
Suykerbuyk W., Bouma T. J., van der Heide T., Faust C., Govers L. L., Giesen W. B. J. T., et al. (2012). Suppressing antagonistic bioengineering feedbacks doubles restoration success. Ecol. Appl. 22 (4), 1224–1231. doi: 10.1890/11-1625.1
Suykerbuyk W., Govers L. L., Bouma T. J., Giesen W. B. J. T., de Jong D. J., van de Voort R., et al. (2016). Unpredictability in seagrass restoration: Analysing the role of positive feedback and environmental stress on Zostera noltii transplants. J. Appl. Ecol. 53 (3), 774–784. doi: 10.1111/1365-2664.12614
Tan Y. M., Dalby O., Kendrick G. A., Statton J., Sinclair E. A., Fraser M. W., et al. (2020). Seagrass restoration is possible: insights and lessons from Australia and New Zealand. Front. Mar. Sci. 7. doi: 10.3389/fmars.2020.00617
Touchette B. W., Burkholder J. M. (2000). Review of nitrogen and phosphorus metabolism in seagrasses. J. Exp. Mar. Biol. Ecol. 250 (1–2), 133–167. doi: 10.1016/S0022-0981(00)00195-7
UNEP (2020). Out of the blue: The value of seagrasses to the environment and to people (Nairobi: UNEP).
UNEP-Nairobi Convention/WIOMSA (2020). Guidelines for Seagrass Ecosystem Restoration in the Western Indian Ocean Region Vol. 63 (Nairobi: UNEP). Available at: www.nairobiconvention.org/www.wiomsa.org.
Unsworth R. K. F., Collier C. J., Waycott M., Mckenzie L. J., Cullen-unsworth L. C. (2015). A framework for the resilience of seagrass ecosystems. Mar. pollut. Bull. 100 (1), 34–46. doi: 10.1016/j.marpolbul.2015.08.016
Unsworth R. K. F., Mckenzie L. J., Collier C. J., Jarvis J. C., Jones B. L., Nordlund L. M. (2019). Global challenges for seagrass conservation. Ambio 48, 801–815. doi: 10.1007/s13280-018-1115-y
Valle M., Garmendia J. M., Chust G., Franco J., Borja Á. (2015). Increasing the chance of a successful restoration of Zostera noltii meadows. Aquat. Bot. 127, 12–19. doi: 10.1016/j.aquabot.2015.07.002
Van Katwijk M. M., Wijgergangs L. J. M. (2004). Effects of locally varying exposure, sediment type and low-tide water cover on Zostera marina recruitment from seed. Aquat. Bot. 80 (1), 1–12. doi: 10.1016/j.aquabot.2004.04.003
Waycott M., Duarte C. M., Carruthers T. J. B., Orth R. J., Dennison W. C., Olyarnik S., et al. (2009). Accelerating loss of seagrasses across the globe threatens coastal ecosystems. PNAS 106 (30), 12377–12381. doi: 10.1073/pnas.0905620106
Zarranz M. E., Gonza N. (2010). Restoration of Cymodocea nodosa seagrass meadows through seed propagation: germination in vitro, seedling culture and field transplant. Botanica Marina 53 (2), 173–181. doi: 10.1515/BOT.2010.019
Zedler B. J. (2007). An unclear, subjective descriptor of restoration outcomes. Ecol. Restor. 25 (3), 162–168. doi: 10.3368/er.25.3.162
Zhang Y. S., Gittman R. K., Donaher S. E., Trackenberg S. N., van der Heide T., Silliman B. R. (2021). Inclusion of intra- and interspecific facilitation expands the theoretical framework for seagrass restoration. Front. Mar. Sci. 8. doi: 10.3389/fmars.2021.645673
Zimmerman R. C., Reguzzoni J. L., Alberte R. S. (1995). Eelgrass (Zostera marina L.) transplants in San Francisco Bay: Role of light availability on metabolism, growth and survival. Aquat. Bot. 51 (1), 67–86. doi: 10.1016/0304-3770(95)00472-C
Keywords: seagrasses, restoration, transplants, parameters, success, coastal areas
Citation: Sfriso AA, Sciuto K, Mistri M, Munari C, Juhmani A-S, Buosi A, Tomio Y and Sfriso A (2023) Where, when, how and what seagrass to transplant for long lasting results in transitional water systems: the cases of Cymodocea nodosa, Zostera marina, Zostera noltei and Ruppia cirrhosa. Front. Mar. Sci. 10:1299428. doi: 10.3389/fmars.2023.1299428
Received: 22 September 2023; Accepted: 27 November 2023;
Published: 22 December 2023.
Edited by:
Ibon Galparsoro, Technological Center Expert in Marine and Food Innovation (AZTI), SpainReviewed by:
Daniele Ventura, Sapienza University of Rome, ItalyJoxe Mikel Garmendia, Technology Center Expert in Marine and Food Innovation (AZTI), Spain
Copyright © 2023 Sfriso, Sciuto, Mistri, Munari, Juhmani, Buosi, Tomio and Sfriso. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Andrea Augusto Sfriso, c2ZybmRyQHVuaWZlLml0
 Andrea Augusto Sfriso
Andrea Augusto Sfriso Katia Sciuto
Katia Sciuto Michele Mistri
Michele Mistri Cristina Munari
Cristina Munari Abdul-Salam Juhmani
Abdul-Salam Juhmani Alessandro Buosi
Alessandro Buosi Yari Tomio
Yari Tomio Adriano Sfriso
Adriano Sfriso