- 1Chinese Academy of Sciences (CAS) Key Laboratory of Marine Ecology and Environmental Sciences, Institute of Oceanology, Chinese Academy of Sciences, Qingdao, China
- 2Laboratory for Marine Ecology and Environmental Science, Qingdao National Laboratory for Marine Science and Technology, Qingdao, China
- 3Centre for Ocean Mega-Science, Chinese Academy of Sciences, Qingdao, China
- 4School of Marine and Atmospheric Sciences, Stony Brook University, Stony Brook, NY, United States
Harmful algal blooms (HABs) of the ichthytoxic dinoflagellate Margalefidinium polykrikoides have caused mass mortality of marine life around the world. While its toxic effects can impact fish, bivalves, coral, zooplankton, and even other phytoplankton, the toxin(s) and allelochemical(s) eliciting these impacts have yet to be definitely identified, leaving open the question as to whether its toxicity and allelopathic effects are caused by the same chemical agents. In this study, we investigated the ability of 10 strains of M. polykrikoides with different geographic origins and ribotypes to cause mortality in two strains of the dinoflagellate, Akashiwo sanguinea (allelopathy), and the sheepshead minnow, Cyprinodon variegatus (toxicity). Results showed that the potency of allelopathy against both strains of A. sanguinea and toxicity to the fish were significantly correlated across strains of M. polykrikoides (p < 0.001 for all). These results strongly support the notion that the major allelochemicals and toxins of M. polykrikoides are identical chemicals, an ecological strategy that may be more energetically efficient than the separate synthesis of toxins and allelochemicals as has been reported in other HABs. Our results also highlight the vital significance of the definitive identification of allelochemicals and toxins of M. polykrikoides and of the quantitative characterization of these compounds in the field where HABs of M. polykrikoides occur during blooms.
Introduction
Margalefidinium (= Cochlodinium) polykrikoides is a harmful algal bloom (HAB) species that has been responsible for mass mortalities of aquatic organisms worldwide (Dorantes-Aranda et al., 2009b; Jiang et al., 2009; Jose Dorantes-Aranda et al., 2010; Kudela and Gobler, 2012; Cui et al., 2020; Basti et al., 2021). As an ichthyotoxic and HAB-causing species, M. polykrikoides not only has caused fish kills across North America and Asia, but also has been known to cause rapid mortality in bivalves, zooplankton, and corals (Kim et al., 1999; Dorantes-Aranda et al., 2009b; Jiang et al., 2009; Tang and Gobler, 2009b; Tang and Gobler, 2009a; Bauman et al., 2010; Tang and Gobler, 2010; Griffith et al., 2019). Beyond being toxic to marine animals, M. polykrikoides is also strongly allelopathic to other phytoplankton (Kim et al., 1999; Dorantes-Aranda et al., 2009b; Tang and Gobler, 2009b; Tang and Gobler, 2009a; Tang and Gobler, 2010). Still, the traits and interactions of toxicity and allelopathy and how they may facilitate M. polykrikoides blooms remain unclear.
Allelochemicals are chemical agents secreted by photosynthetic organisms that affect (mainly negatively) the growth, health, behavior, or population biology of organisms, mainly plants (Whittaker and Feeny, 1971). Smayda (1997) hypothesized that harmful algae form blooms by four major pathways, with two of them pertinent to allelochemically enhanced interspecific competition. Oppositely, the target species of toxicity are animals (Rice, 1979; Smayda, 1997; Legrand et al., 2003; Granéli and Hansen, 2006).
Many harmful algae can be both allelopathic to other algae and toxic to marine animals. In several cases, allelochemicals and toxins seem to be different compounds as have been demonstrated for the karlotoxin-producer Karlodinium veneficum (Yang et al., 2019), the paralytic shellfish toxin-producer Alexandrium spp. (Tillmann and John, 2002; Tillmann et al., 2007; Tillmann et al., 2008), and the brevetoxin producer Karenia brevis (Kubanek et al., 2005). For some algae, however, their toxicity to animals and allelopathic effects on phytoplankton appear to be caused by common mechanisms, for instance, for Prymnesium parvum (Singh et al., 2001; Granéli and Hansen, 2006).
The identity and mechanism of toxins and allelochemicals of M. polykrikoides have been the subject of debate. The toxins of M. polykrikoides cause damage to different cell types, including hemolysis in fish erythrocytes (Kim et al., 1999; Dorantes-Aranda et al., 2009a; Kim and Oda, 2010). The conceivable ichthyotoxic substances produced by M. polykrikoides have been hypothesized to include reactive oxygen species (ROS) (Tang and Gobler, 2009a), sterols, fatty acids (Giner et al., 2016), and mucopolysaccharides (Kim et al., 2002; Kim and Oda, 2010). The short-term nature of M. polykrikoides toxicity (minutes in the absence of live cells) and the ability of anti-oxidation compounds to mitigate its toxicity have suggested that ROS are a likely source of this HABs toxicity (Tang and Gobler, 2009b; Jiang et al., 2009). Further studies have also suggested that the existence of a synergistic action of ROS and polyunsaturated fatty acids, docosahexaenoic and eicosapentanoic, that are produced by M. polykrikoides may contribute to lipid peroxidation (Dorantes-Aranda et al., 2009a; Dorantes-Aranda et al., 2009b), which is associated with an increase in the solute permeability in the membrane cells, causing swelling and lysis of the vacuoles of the membrane liposomes (Girotti, 1990) and severe damage in fish gill liposomes (Kim et al., 1999). In addition, polysaccharides can exert a damaging effect on branchial cells (Dorantes-Aranda et al., 2009a; Dorantes-Aranda et al., 2009b; Kim and Oda, 2010), which may also contribute to the ichthyotoxic effects of M. polykrikoides. As for the allelochemicals of M. polykrikoides, they have been reported to affect the growth and survival of many planktonic species (Gobler et al., 2008; Mulholland et al., 2009; Tang and Gobler, 2009b; Tang and Gobler, 2009a; Richlen et al., 2010; Koch et al., 2014; Pérez-Morales et al., 2017; Hattenrath-Lehmann et al., 2019). However, the exact allelopathic mechanisms remain controversial and largely unknown. Prior investigations of M. polykrikoides (Kim et al., 1999; Tang and Gobler, 2010) and other allelopathy-causing species (Oda et al., 1992; Marshall et al., 2005; van Rijssel et al., 2008) have suggested that various compounds including ROS, PUFA (polyunsaturated fatty acid), and unidentified toxic metabolites may act as allelochemicals.
The goal of this study was to compare the toxic effects of M. polykrikoides on fish to its allelopathic effects on other phytoplankton in terms of characterizing the chemical nature of the toxin(s) and allelochemical(s) produced by the species. Given that prior research has established significant variation in the strength of allelopathy and toxicity among clonal isolates of M. polykrikoides (Tang and Gobler, 2010; Wang et al., 2020), we compared the ability of 10 clones isolated from the Atlantic and Pacific Oceans and representing two major ribotypes. Results demonstrated a high degree of similarity between allelochemical potency and ichthyotoxicity across the clones studied.
Materials and methods
Cultures and culturing conditions
Ten strains of M. polykrikoides were isolated from coastal areas of the United States, Mexico, and Japan (Table 1). The identity of all strains was confirmed with large subunit (LSU) rDNA sequencing. Two strains of Akashiwo sanguinea (ASNP6 and AS2) isolated from the Northport Bay, New York, USA in 19 August 2011 (Tang and Gobler, 2015) were used as the target species of allelopathic tests. All cultures were maintained in exponential phase growth in sterile GSe medium with a salinity of 31−32 made with an autoclave and 0.2 µm filtered seawater, and maintained at 21°C in an incubator with a 12-h light:12-h dark cycle providing ~100 μmol quanta m– 2 s– 1. Cultures were grown with a mixture of penicillin and streptomycin (2% v/v dilution of stock with 200 U·ml−1 penicillin and 0.2 mg·ml−1 streptomycin) to discourage the growth of bacteria.
Testing the allelopathy of M. polykrikoides
Cultures of CPSB-1B, CPSB-1G, CP1, CPNB-3, CPNB-6, CPSB-2A, CPGSB-1, and ASNP-6 were maintained in exponential growth phase in 500-ml conical flasks in GSe medium with 2% antibiotics mixture. For experiments, 10 ml of M. polykrikoides and 1 ml of ASNP-6 (both at ~103 cells ml−1) were added to one well of a six-well culture plate. One milliliter of ASNP-6 was added into 10 ml of GSe medium as control. Each treatment and control were established in triplicate. After 48 h, all samples (11 ml) were preserved with 2% Lugol’s solution (final) and quantified under a light microscope. Cultures of CPSB-2A, CP1, CPSB-1B, and CPSB-1G were also co-cultured with A. sanguinea (ASNP-6) for 72 h to test the allelopathic effects using the same methods described above for the 48-h assay, the only difference being that six replicates were set in each group of the latter experiment (n = 6).
Other strains of M. polykrikoides, CPINS129, CPCB10, CPPV1, and CP1, were tested with A. sanguinea strain AS2 as target species. All strains of M. polykrikoides were diluted to different cell densities (650−4,000 cells ml−1 for CP1, 2,300−4,300 cells ml−1 for CPINS129, 500−1,700 cells ml−1 for CPCB10, and 5,300−9,900 cells ml−1 for CPPV1) and then co-cultured with AS2 in a six-well culture plate with 9 ml of M. polykrikoides and 1 ml of A. sanguinea culture in one well, respectively. One milliliter of AS2 with the same cell density as treatments was added to 9 ml of GSe medium as control. All the treatments and controls were in triplicate. Samples were preserved after 24 h with Lugol’s solution (2% final) for subsequent cell density quantification.
To quantify and compare the allelopathic effects of M. polykrikoides among strains with different cell densities, a parameter, “Relative Mortality compared with control of A. sanguinea”, was defined as: Relative Mortality (AS) = [(Mean cell density of mono-cultured A. sanguinea − mean cell density of A. sanguinea co-cultured with M. polykrikoides)/mean cell density of mono-cultured A. sanguinea] × 100%, but is called “Mortality (AS)” below for simplicity.
Testing the toxicity of M. polykrikoides
We used 14-day-old (0.4–0.5 cm in length) Cyprinodon variegatus (sheepshead minnows) for toxicity experiments. All 10 strains of M. polykrikoides (CP1, CPINS129, CPCB10, CPPV1, CPSB-1B, CPSB-1G, CPSB-2A, CPNB-3, CPNB-6, and CPGSB-1) were maintained in exponential growth phase and diluted to several cell densities (350−4,700 cells ml−1 for CP1, 1,200−4,800 cells ml−1 for CPINS129, 300−700 cells ml−1 for CPCB10, 1,500−3,000 cells ml−1 for CPPV1, 1,200−4,000 cells ml−1 for CPSB-1B, 1,800 cells ml−1 for CPSB-1G, 2,700−5,500 cells ml−1 for CPSB-2A, 450−1,700 cells ml−1 for CPNB-3, 1,400 cells ml−1 for CPNB-6, and 1,400 cells ml−1 for CPGSB-1) before the experiment. Fish bioassays were performed in six-well plates with 10 ml of culture and one fish in each well, and one fish was added to 10 ml of GSe medium as control. All the treatments and controls were in replicates of six (n = 6) and were maintained at room temperature without aeration. The survival of fishes was recorded at 24 h. Some treatments (CP1, CPSB-1B, CPSB-1G, CPSB-2A, CPNB-3, CPNB-6, and CPGSB-1) lasted for 6 days to compare the mean death time of fishes in different cultures. A probit regression analysis of cell densities of CP1, CPINS129, and CPSB-2A, and mortality of sheepshead minnows was used to determine the median lethal dose (LD50) and compare the toxicity of different strains of M. polykrikoides when the cell densities were unequal across experiments.
Statistics
One-way ANOVAs and multiple comparison tests were used to compare the cell densities of M. polykrikoides, mortalities of A. sanguinea compared with control, and death time of sheepshead minnows among treatments using SPSS. For the toxicity experiments, G-tests were performed to assess the significance of toxic effects (Woolf, 1957). Spearman correlation coefficients were calculated to examine the correlation between the rank of allelopathy and toxicity of different M. polykrikoides. In all cases, the significance level was set at p < 0.05.
Results
Comparing allelopathic intensity of different strains of M. polykrikoides
During 48-h experiments with A. sanguinea strain ASNP-6, cell densities of seven strains of M. polykrikoides were similar with the exception of CPSB-1B (H), which was higher than CPSB-1G (H) (Figure 1, Table S1-2, p = 0.001), but this made no difference in Mortality (AS) (Table S1-1, p = 0.55), indicating that CPSB-1G was allelopathically stronger than CPSB-1B. CPSB-1B was more allelopathic than CPNB-6 given that CPSB-1B (L) had higher Mortality (AS) (Figure 1, Table S1-1, p = 0.001) but had equal cell density (Table S1-2, p = 0.08). CPNB-6 had a lower cell density than CP1 (Figure 1, Table S1-2, p = 0.01) but induced equal Mortality (AS) as CP1 (Figure 1, Table S1-1, p = 0.68), indicating that CPNB-6 was more allelopathic than CP1. As for CPNB-6 and CPGSB-1, the two strains exhibited no difference in cell density (Table S1-2, p = 0.69) but the former strain was more allelopathic (Figure 1, Table S1-1, p = 0.01). Similarly, CP1 and CPSB-2A exhibited no difference in cell density (Figure 1, Table S1-2, p = 0.66), but the former strain induced higher Mortality (AS) (Figure 1, Table S1-1, p = 0.01). Moreover, CPNB-3 (H), CPSB-2A, and CPGSB-1 led to roughly equal inhibition to ASNP-6 (Table S1, p > 0.05 for each pairwise comparison of the three strains) but decreased in order in cell density (Figure 1, Table S1, p < 0.05 in each pair of the three strains), indicating that the allelopathic strength was CPGSB-1 > CPSB-2A > CPNB-3. Although we cannot discern the difference in allelopathic strength of CP1 and CPGSB-1, the two strains were weaker than CPNB-6 but stronger than CPSB-2A, meaning the overall rank order of strength was CPSB-1G > CPSB-1B > CPNB-6 > CP1 ≈ CPGSB-1 > CPSB-2A > CPNB-3.
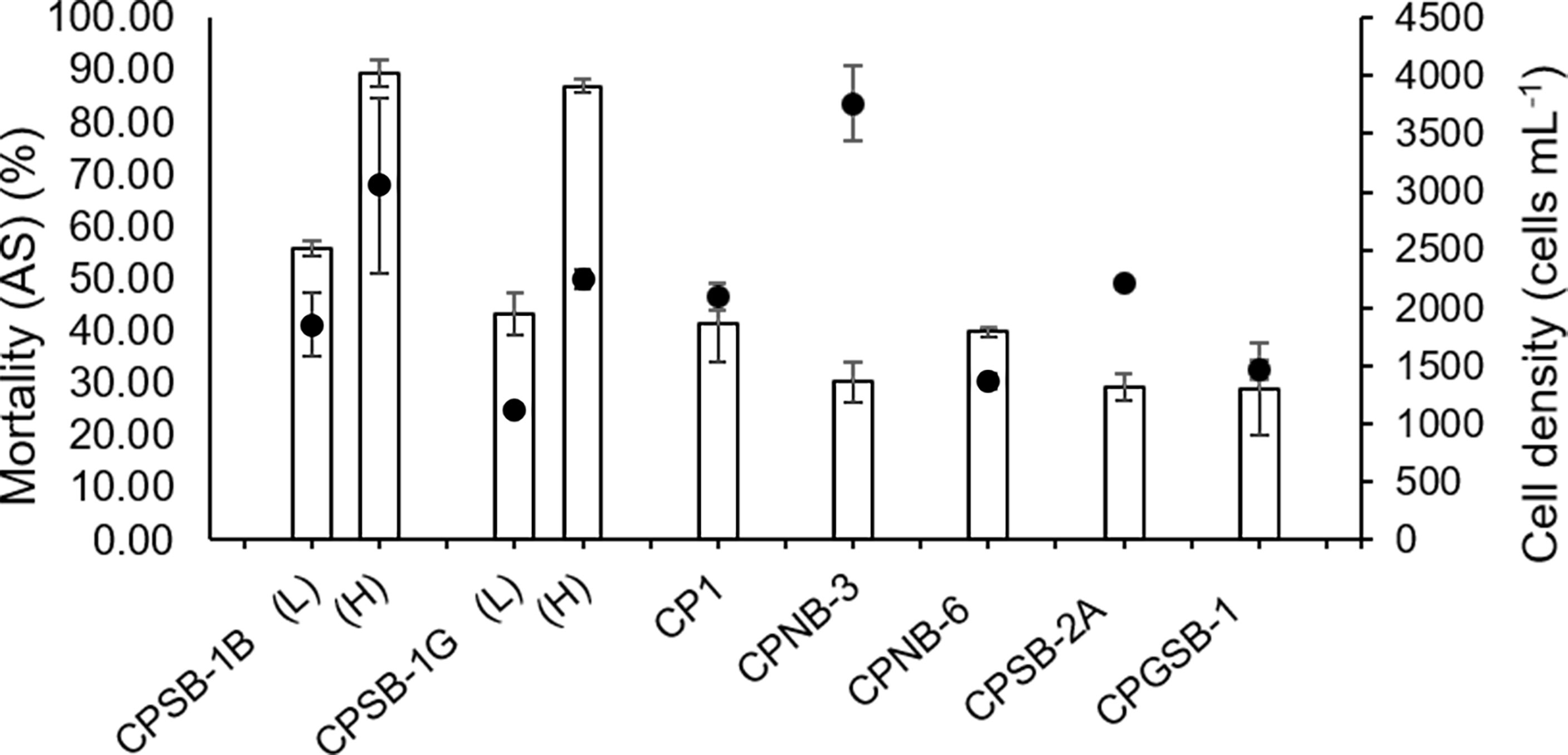
Figure 1 Allelopathic effects of M. polykrikoides on ASNP-6 in 48 h expressed as Mortality compared with control of A. sanguinea [Mortality (AS)]. The dots represent the cell density of different strains of M. polykrikoides. Each data point is the mean of triplicates (n = 3). Error bars indicate ±1 SD of n = 3.
In the experiment of ASNP-6 co-cultured with CPSB-2A, CP1, CPSB-1G, and CPSB-1B for 72 h, CPSB-2A had the highest cell density and the lowest Mortality (AS) (Figure 2, p < 0.05, one-way ANOVA) and thus was the least allelopathic strain. CP1 had a higher cell density than CPSB-1G (Figure 2, p = 0.001) but showed no difference in inducing Mortality (AS) (Figure 2, p = 0.13). Additionally, CP1 showed no difference in cell density with CPSB-1B (Figure 2, p = 0.09) but induced lower Mortality (AS) (Figure 2, p = 0.001). Thus, CP1 ranked after CPSB-1B and CPSB-1G in allelopathic intensity. Concluding from the above results, the allelopathic intensity in decreasing order was CPSB-1G > CPSB-1B > CPNB-6 > CP1 ≈ CPGSB-1 > CPSB-2A > CPNB-3.
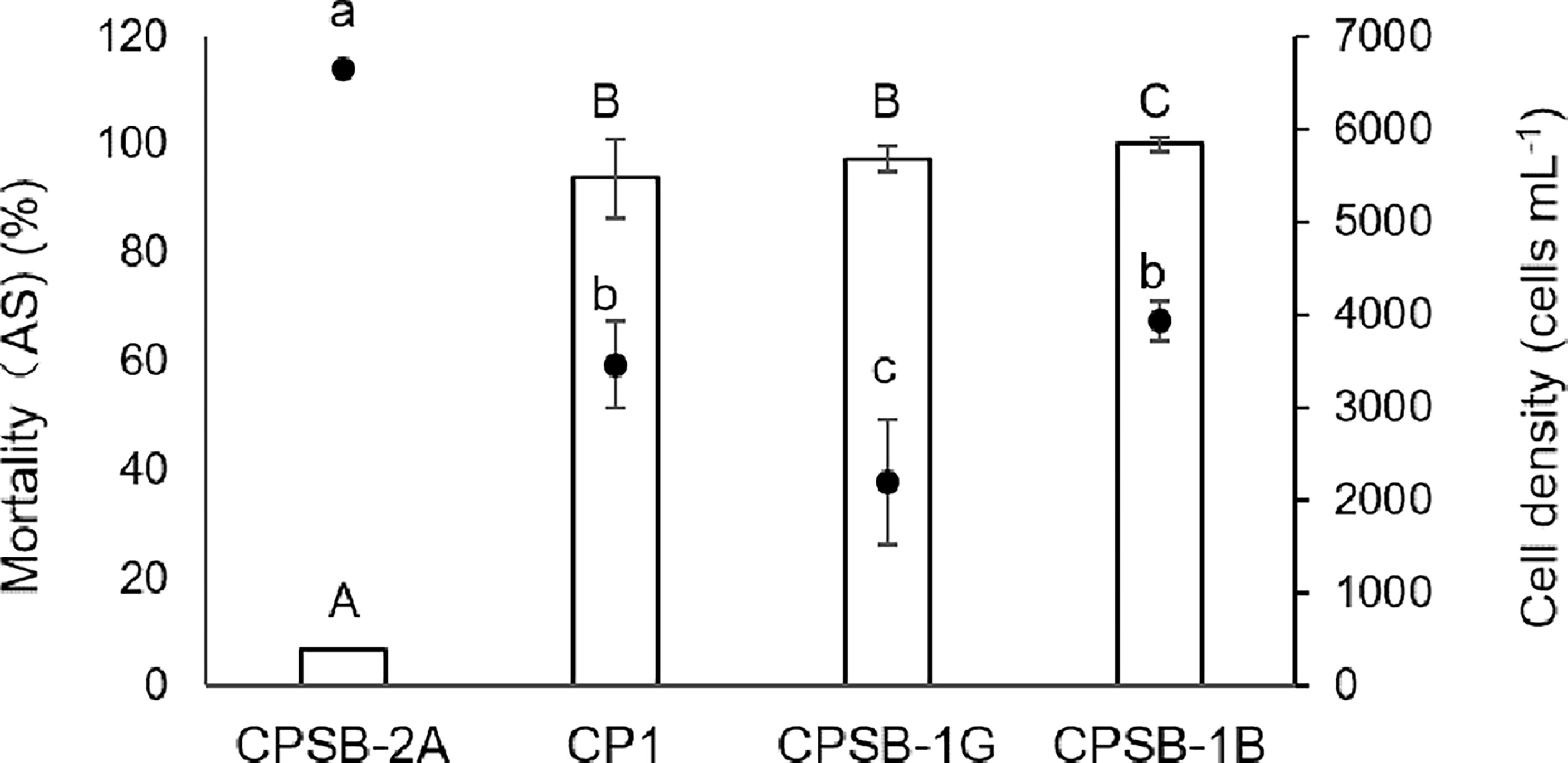
Figure 2 Allelopathic effects of M. polykrikoides on ASNP-6 in 72 h expressed as Mortality compared with control of A. sanguinea [Mortality (AS)]. The dots represent the cell density of different strains of M. polykrikoides. Each data point is the mean of sextuplicate (n = 6). Error bars indicate ±1 SD of n = 6. Different letters in uppercase indicate significant differences (p < 0.05) among mortalities, and different letters in lowercase indicate significant differences (p < 0.05) among cell densities.
In the allelopathic experiment using AS2 as the target species, four M. polykrikoides clones, namely, CP1, CPINS129, CPCB10, and CPPV1, were tested (Figure 3). As shown in Mortality (AS), CPCB10 at 500 cells ml−1 was more potent than CP1 at 650 cells ml−1 (Figure 3, Table S2-1, p = 0.001) despite similar cell densities (Table S2-2, p = 0.05). Compared with CPINS129 at a cell density of 2,300 cells ml−1, CP1 of 1,900 cells ml−1 had a lower cell density (Figure 3, Table S2-2, p = 0.001) but had a higher Mortality (AS) (Figure 3, Table S2-1, p = 0.00). In addition, CCPV1 of 8,300 cells ml−1 and CPINS129 of 4,300 cells ml−1 led to similar Mortality (AS) (Figure 3, Table S2-1, p = 0.09); however, the former had a higher cell density (Figure 3, Table S2-2, p = 0.00). As a result, the allelopathic intensity in decreasing order of the four strains was CPCB10 > CP1 > CPINS129 > CPPV1.
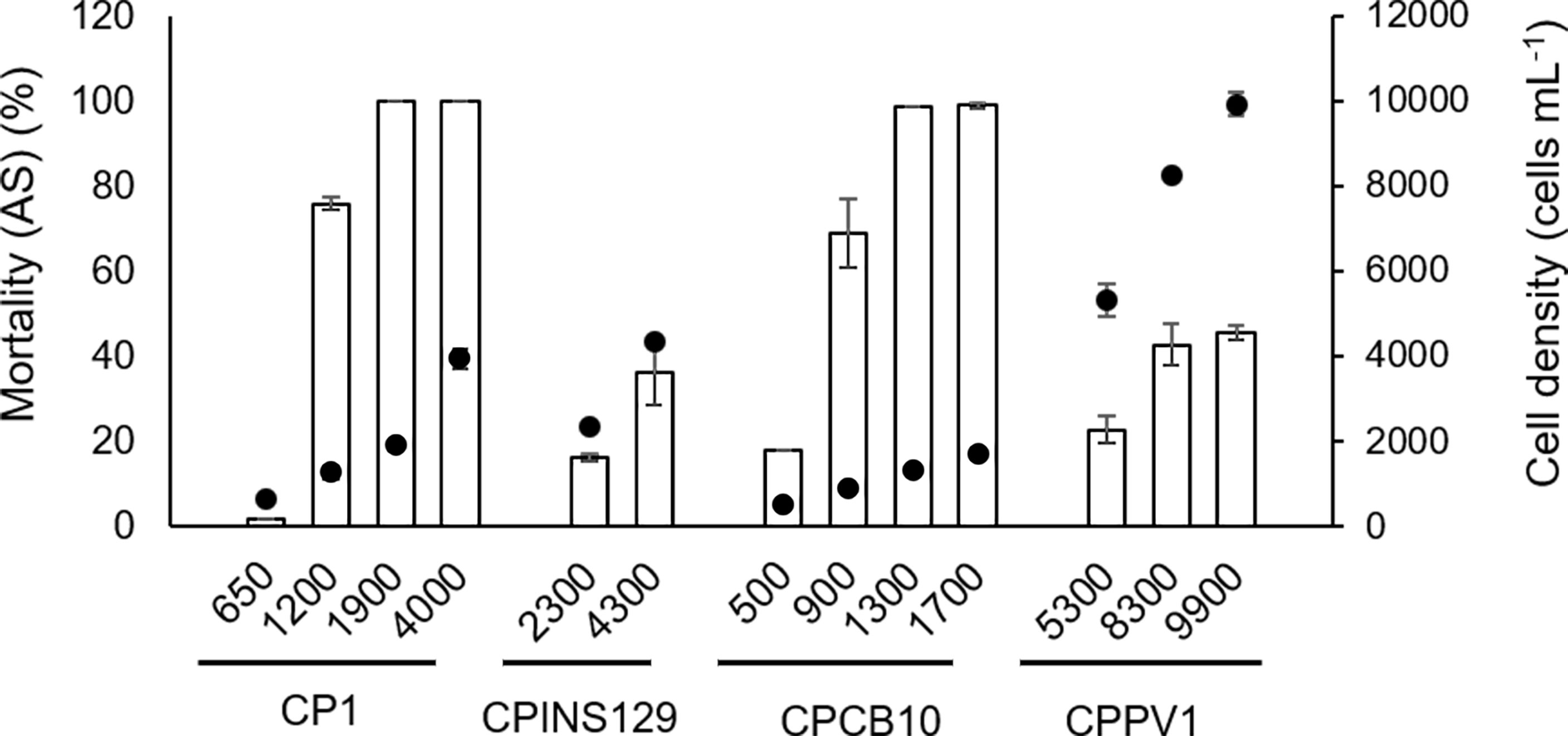
Figure 3 Allelopathic effects of M. polykrikoides on AS2 in 24 h expressed as Mortality compared with control of A. sanguinea [Mortality (AS)]. The dots represent the cell density of different strains of M. polykrikoides. Each data point is the mean of triplicates (n = 3). Error bars indicate ±1 SD of n = 3.
Comparing ichthyotoxicity among M. polykrikoides strains
Ten strains of M. polykrikoides at different cell densities were co-cultured with 14-day-old sheepshead minnows to test their toxicity (Figure 4). Four groups of M. polykrikoides (CPCB10 in 700 cells ml−1, CPSB-1B in 1,200 cells ml−1, CPNB-6 in 1,400 cells ml−1, and CPSB-1G in 1,800 cells ml−1) all caused 100% mortality of sheepshead minnows within 24 h (Figure 4, Table S3, p < 0.05 for pairwise comparisons), and the mean death time of sheepshead minnows was 1.9 h, 4.5 h, 5.4 h, and 13.1 h, respectively, suggesting that their toxic intensity decreased in the order CPCB10 > CPSB-1B > CPNB-6 > CPSB-1G. According to the results of regression analysis, CP1 caused 80% mortality of sheepshead minnows at a cell density of 1,874 cells ml−1 (Figure S1), which was lower than 100% mortality of sheepshead minnows co-cultured with 1,800 cells ml−1 of CPSB-1G (Figure 4). CP1 caused 60% mortality of sheepshead minnows at a cell density of 1,400 cells ml−1 (Figure S1), which was higher than the mortality of sheepshead minnows caused by CPGSB-1 (50%) at the same cell density (Figure 4). The LD50 dose of CPINS129 and CPSB-2A was 6,611 cells ml−1 and 5,170 cells ml−1, respectively (Figure S1), indicating that CPSB-2A was more ichthyotoxic than CPINS129. Moreover, CPPV1 and CPNB-3 caused the lowest mortality of sheepshead minnows among the 10 strains (Figure 4). Conclusively, the decreasing order of toxic intensity of the 10 strains was CPCB10 > CPSB-1B > CPNB-6 > CPSB-1G > CP1> CPGSB-1 > CPSB-2A> CPINS129 > CPPV1 ≈ CPNB-3.
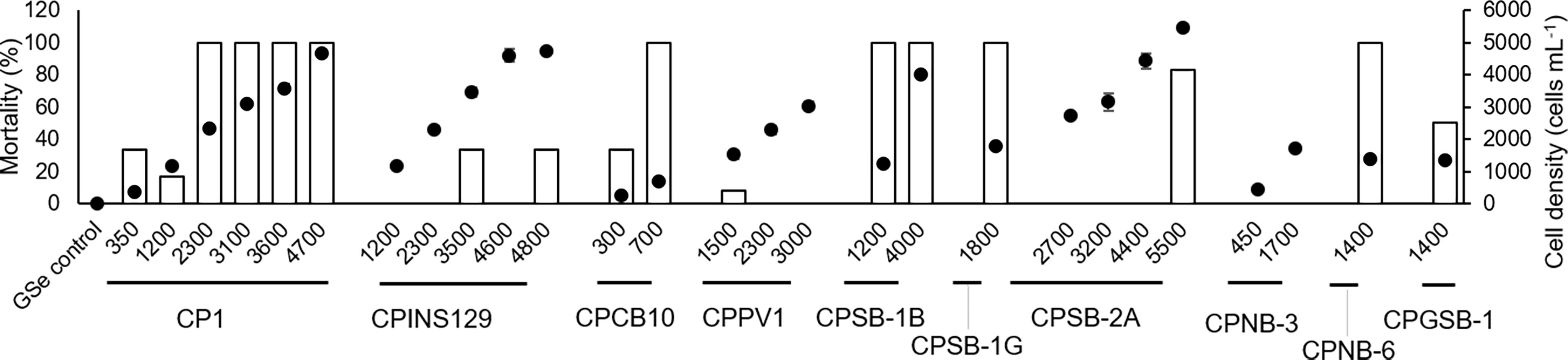
Figure 4 Toxic effects of M. polykrikoides on sheepshead minnows Cyprinodon variegatus in 24 h expressed as mortality (%) of sheepshead minnows (bars). The corresponding cell density of different strains of M. polykrikoides was also shown as dots for comparison. Error bars indicate ±1 SD of n = 6.
The death time of sheepshead minnows was also recorded in some groups (Figure 5). In groups of CPSB-2A (5,500 cells ml−1), CP1 (3,100 cells ml−1), CPSB-1G (1,800 cells ml−1), CPNB-6 (1,400 cells ml−1), and CPSB-1B (1,200 cells ml−1), the death time of sheepshead minnows was less than 20 h and did not differ among the strains (Figure 5, Table S4-2, p > 0.05 for pairwise comparisons), and cell densities of these groups decreased in order [Figure 5, Table S4-1, p < 0.05 for pairwise comparisons excluding groups CPNB-6 in 1,400 cells ml−1 vs. CPSB-1B in 1,200 cells ml−1 (p = 0.13)], indicating that the order of ichthyotoxicity of the five strains was CPSB-1B, CPNB-6 > CPSB-1G > CP1 > CPSB-2A. The death time of sheepshead minnows in groups of CPSB-2A and CPGSB-1 showed no significant difference (Figure 5, Table S4-2, p = 0.23), but CPGSB-1 had a lower cell density (Figure 5, Table S4-1, p = 0.001), indicating that the toxicity of CPGSB-1 was greater than CPSB-2A. The death time of sheepshead minnows in groups of CPNB-3 was significantly longer than in other groups as most fishes survived the duration of the experiment (Table S4-2, p = 0.001), indicating that the toxicity of CPNB-3 was the weakest among the seven strains.
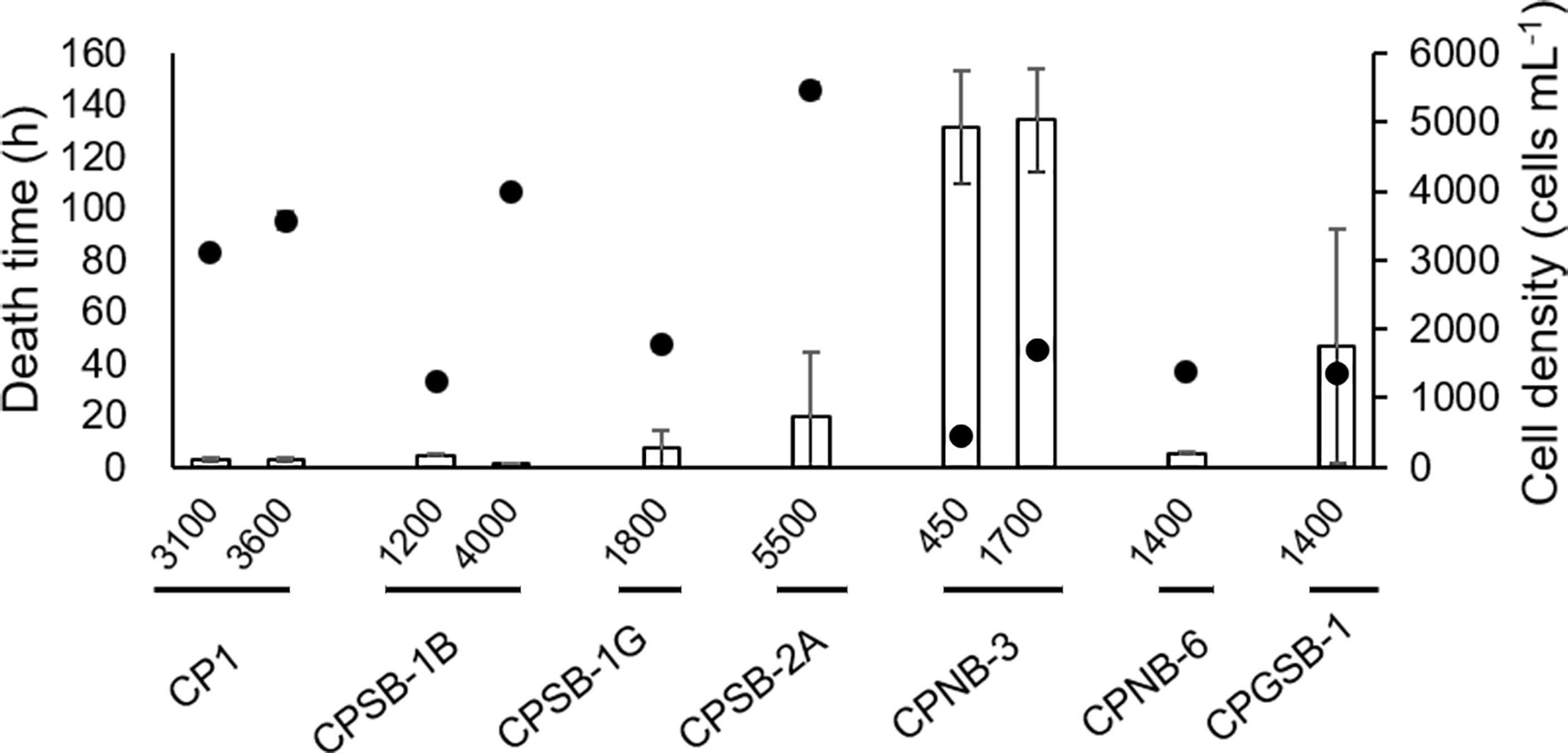
Figure 5 Toxic effects of M. polykrikoides on sheepshead minnows Cyprinodon variegatus expressed as death time. The dots represent the cell density of different strains of M. polykrikoides. Error bars indicate ±1 SD of n = 6.
Correlation of allelopathy and toxicity of M. polykrikoides
The allelopathic and toxic strengths of each strain were ranked according to the orders of allelopathy, which were CPSB-1G > CPSB-1B > CPNB-6 > CP1 ≈ CPGSB-1 > CPSB-2A > CPNB-3, and CPCB10 > CP1 > CPINS129 > CPPV1, and the order of toxicity, which was CPCB10 > CPSB-1B > CPNB-6 > CPSB-1G > CP1> CPGSB-1 > CPSB-2A > CPINS129 > CPPV1 ≈ CPNB-3 (Table S5), upon which the allelopathy and ichthyotoxicity of the strains were compared and found to be linearly correlated (Figure 6). Spearman’s rank correlation analysis showed that the allelopathic effects and ichthyotoxicity were significantly positively correlated (Spearman’s correlation coefficient = 0.88, p = 0.01, n = 7 for toxicity to sheepshead minnows and allelopathy to ASNP-6; Spearman’s correlation coefficient = 1.0, p < 0.0001, n = 4 for toxicity to sheepshead minnows and allelopathy to AS2; Figure 6), illustrating consistency between the allelopathy and ichthyotoxicity of the M. polykrikoides strains.
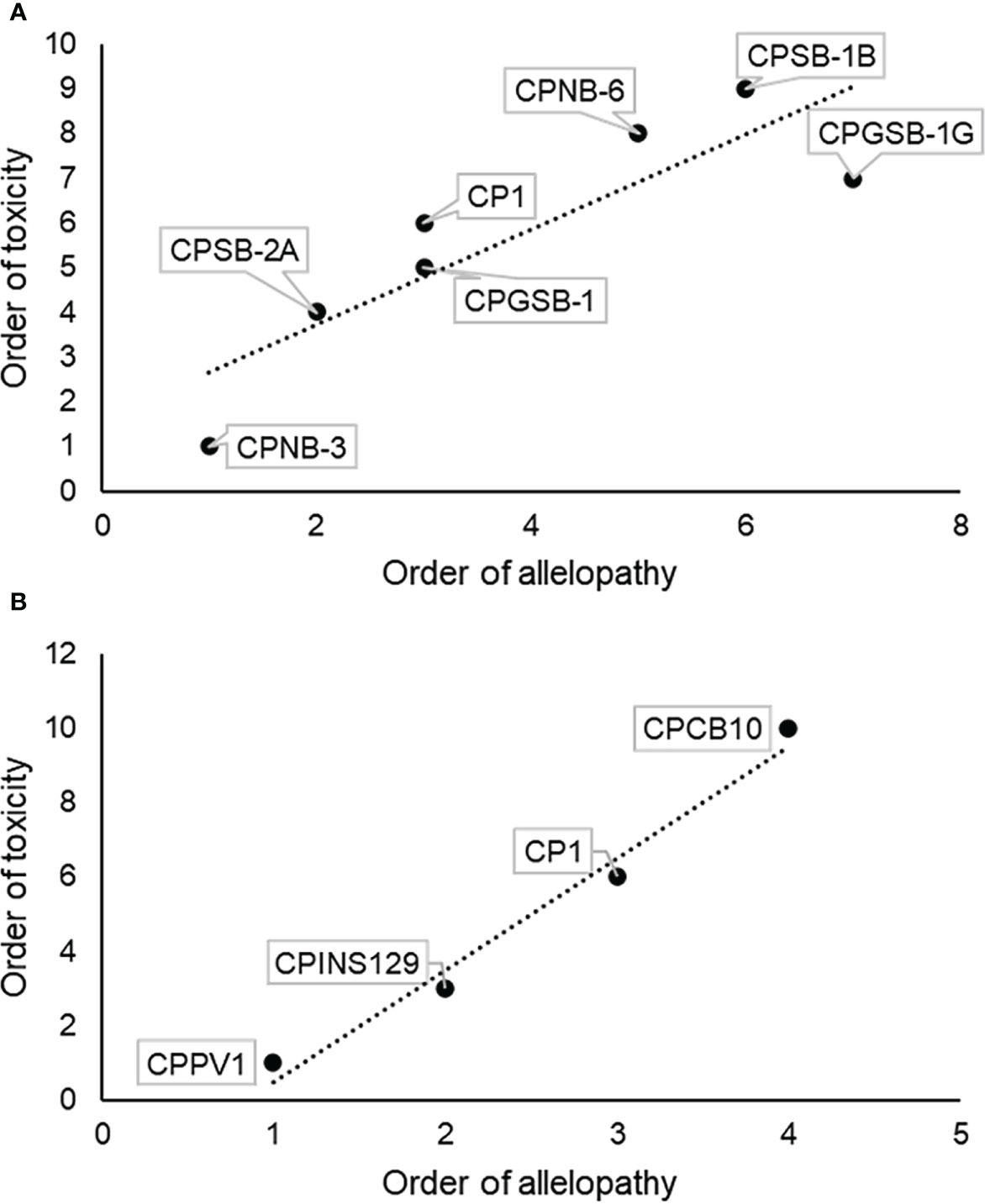
Figure 6 Correlation analysis of allelopathic intensity and toxic intensity of different strains of M. polykrikoides. (A) Order of allelopathic effects of M. polykrikoides on ASNP-6 and toxic effects of M. polykrikoides on sheepshead minnows. (B) Order of allelopathic effects of M. polykrikoides on AS2 and toxic effects of M. polykrikoides on sheepshead minnows.
Discussion
The consistency between allelopathy and toxicity among strains of M. polykrikoides
Margalefidinium polykrikoides strains exhibit variations in both toxicity and allelopathy (Tang and Gobler, 2010; Wang et al., 2020). By phylogenetic analysis of LSU rDNA of M. polykrikoides strains, at least four ribotypes have been identified globally (Iwataki et al., 2008; Reñé et al., 2013). Prior research has shown that different ribotypes of M. polykrikoides differ in toxicity (Wang et al., 2020), making this trait useful for providing perspective to investigate the relationships and chemical nature of allelopathy and toxicity of M. polykrikoides.
Here, 10 strains were studied that varied in different geographic origins, ribotypes, and isolation seasons (Table 1) but were cultured under uniform conditions and maintained in exponential growth. While differences in growth rates and maximum cell concentrations sometimes caused differences in experimental cell densities used, a gradient in cell densities was typically made for comparable treatment densities across strains. Fortunately, we obtained enough data to compare the allelopathy and toxicity between two strains, which yielded similar results and a similar order of strain potency.
Even though the intensity of allelopathy and toxicity of M. polykrikoides varied in different strains, especially strains of different ribotypes (Wang et al., 2020), the potencies in toxicity and allelopathy for the different strains of M. polykrikoides were consistent, as strains displaying potent toxicity also exhibited strong allelopathy. This consistency provides potent evidence for the hypothesis that chemical agents that are responsible for inhibitory and lethal effects on phytoplankton and marine animals, i.e., allelochemicals and toxins, were the same compounds.
Possible ecological implications of the consistency between allelopathy and toxicity
Plant secondary metabolism is a term for pathways and small-molecule products of metabolism (i.e., secondary metabolites) that are non-essential for the survival of the organism (Kossel, 1891). However, a wide variety and high diversity of secondary metabolites produced by plants are an important part of plant defense system against pathogenic attacks and environmental stresses, including toxins and allelochemicals (Yang et al., 2018). Producing secondary metabolites is thought to have a minor energetic cost (Waterman, 1992). Many harmful algae (e.g., Alexandrium spp., K. brevis, and K. veneficum) produce toxins and allelochemicals that are different compounds (Tillmann and John, 2002; Kubanek et al., 2005; Tillmann et al., 2007; Tillmann et al., 2008; Yang et al., 2019). In contrast, the findings of this study suggest that M. polykrikoides produces a singular class of compounds that inhibit competitors and potential predators (e.g., zooplankton and planktivorous fish), representing a potential energetic cost-saving for this HAB-causing species.
Expectations in identifying toxins and allelochemicals of M. polykrikoides
While prior studies have identified several kinds of compounds that may be the toxins made by M. polykrikoides, more evidence is needed to verify the actual toxicity of these substances. It has been suggested that the toxins of M. polykrikoides may be ROS (Kim et al., 1999; Tang and Gobler, 2009a). ROS-scavenging enzymes (peroxidase and catalase) have been shown to mitigate the toxicity and allelopathy of M. polykrikoides and multiple attributes of the toxicity are consistent with ROS being the toxic principle. For example, the rapidly diminished toxicity (in minutes) observed in M. polykrikoides cells that were freshly killed (Tang and Gobler, 2009a) was consistent with the short half-life of ROS compounds. Furthermore, M. polykrikoides exhibited the highest toxicity during the exponential growth phase of cultures, which aligns with reports of ROS production by actively growing, rather than stationary phase, cells of the species (Tang and Gobler, 2009a). While one study found that the O2- and H2O2 in a toxic strain of M. polykrikoides were at trace levels (Kim and Oda, 2010), other ROS compounds were not measured in that study. Mucopolysaccharides produced by M. polykrikoides may be attributed to the smothering of fishes (Kim and Oda, 2010), but no study has affirmed this finding and there were no visual signs of polysaccharides on fish during our study. Giner et al. (2016) extracted lipids of M. polykrikoides cells and analyzed the compositions of fatty acid and sterol in crude lipids, which consisted of a high proportion of PUFAs (47% of total fatty acids), dinosterol (40% of total sterols), and dihydrodinosterol (32% of total sterols). The identified fatty acids and sterols may contribute to long-term deleterious effects on invertebrates but were unlikely to be effective substances responsible for the acute toxicity to fish (Giner et al., 2016). In addition, according to the definition of allelopathy, allelochemicals sensu stricto refer to the substances that are excreted from the producing cells. Thus, the crude lipids extracted with organic solvents from cells may include many more substances than extracellular secretions. While some fatty acids with hemolytic property have also been identified in M. polykrikoides (Dorantes-Aranda et al., 2009a), bioassays of these substances have not been implemented and thus their toxic effects remain unknown. In addition, it has been proved that direct physical contact between test animals and algal cells is not necessary for M. polykrikoides to cause mortality, which means the toxins of M. polykrikoides could be easily released to the extracellular milieu (Tang and Gobler, 2009a).
Our finding strongly suggests that the allelochemicals and toxins of M. polykrikoides are the same chemical agents, which could be a cost-saving or energy-saving and thus ecologically advantageous strategy, especially so if the toxin(s) and allelochemical(s) are synthesized via a simple pathway. In this regard, the multiple chemical agents proposed to be responsible for the toxicity and allelopathy of the species as reviewed above (e.g., ROS, mucopolysaccharides, fatty acids, and sterols) are certainly not to be all true. It is, therefore, important to fully identify the toxins and allelochemicals of M. polykrikoides for the sake of both understanding the bloom ecology and mitigating the harmful effects of the species in the field.
Conclusion
We confirmed that the ichthyotoxicity and allelopathy of M. polykrikoides are strain specific and vary with different geographic origins and ribotypes. We further found that the order of ichthyotoxicity and allelopathy from strong to weak of the 10 strains of M. polykrikoides was positively correlated. These results strongly suggest that major allelochemicals and toxins of M. polykrikoides are identical chemicals, which could be an energy-saving and thus ecologically advantageous strategy.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
Author contributions
HY analyzed the data, searched the literature, and wrote the manuscript. CG supervised the research, edited the manuscript, and acquired the funding. YT designed and performed the experiments, edited the manuscript, and acquired the funding. All authors read and approved the final version of the manuscript.
Funding
This work was financially supported by the National Natural Science Foundation of China (No. 41976134), the Science and Technology Basic Resources Investigation Program of China (2018FY100204), and the Chicago Community Trust.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmars.2022.941205/full#supplementary-material
Supplementary Figure 1 | Regression curves about cell density of M. polykrikoides strains (CP1, CPINS129 and CPSB-2A) and mortality of sheepshead minnows by probit regression analysis.
Supplementary Table 1 | Multiple comparison test of allelopathic effects of M. polykrikoides on ASNP-6 in 48 h.
Supplementary Table 2 | Multiple comparison test of allelopathic effects of M. polykrikoides on AS2 in 24 h.
Supplementary Table 3 | Multiple comparison test of cell densities of M. polykrikoides in toxic experiment co-cultured with sheepshead minnows in 24 h.
Supplementary Table 4 | Multiple comparison test of 6-day toxic effects of M. polykrikoides on sheepshead minnows.
Supplementary Table 5 | Ranks of allelopathic and toxic intensities of different M. polykrikoides strains.
References
Basti L., Go J., Okano S., Higuchi K., Nagai S., Nagai K. (2021). Sublethal and antioxidant effects of six ichthyotoxic algae on early-life stages of the Japanese pearl oyster. Harmful Algae 103, 102013. doi: 10.1016/j.hal.2021.102013
Bauman A. G., Burt J. A., Feary D. A., Marquis E., Usseglio P. (2010). Tropical harmful algal blooms: An emerging threat to coral reef communities? Mar. Pollut. Bull. 60 (11), 2117–2122. doi: 10.1016/j.marpolbul.2010.08.015
Cui Y., Chun S.-J., Baek S.-S., Baek S. H., Kim P.-J., Son M., et al. (2020). Unique microbial module regulates the harmful algal bloom (Cochlodinium polykrikoides) and shifts the microbial community along the southern coast of Korea. Sci. Total Environ. 721, 137725. doi: 10.1016/j.scitotenv.2020.137725
Dorantes-Aranda J. J., Garcia-de la Parra L. M., Alonso-Rodríguez R., Morquecho L. (2009a). Hemolytic activity and fatty acids composition in the ichthyotoxic dinoflagellate Cochlodinium polykrikoides isolated from bahía de la paz, gulf of California. Mar. Pollut. Bull. 58 (9), 1401–1405. doi: 10.1016/j.marpolbul.2009.06.007
Dorantes-Aranda J. J., Garcia-de la Parra L. M., Alonso-Rodríguez R., Morquecho L., Voltolina D. (2009b). Toxic effect of the harmful dinoflagellate Cochlodinium polykrikoides on the spotted rose snapper Lutjanus guttatus. environ. Toxicol 25 (4), 319–326. doi: 10.1002/TOX.20507
Giner J.-L., Ceballos H., Tang Y.-Z., Gobler C. J. (2016). Sterols and fatty acids of the harmful dinoflagellate Cochlodinium polykrikoides. Chem. Biodiversity 13 (2), 249–252. doi: 10.1002/cbdv.201500215
Girotti A. W. (1990). Photodynamic lipid peroxidation in biological systems. Photochem. Photobiol. 51 (4), 497–509. doi: 10.1111/J.1751-1097.1990.TB01744.X
Gobler C. J., Berry D. L., Anderson O. R., Burson A., Koch F., Rodgers B. S., et al. (2008). Characterization, dynamics, and ecological impacts of harmful Cochlodinium polykrikoides blooms on eastern long island, NY, USA. Harmful Algae 7 (3), 293–307. doi: 10.1016/j.hal.2007.12.006
Granéli E., Hansen P. J. (2006). “Allelopathy in harmful algae: A mechanism to compete for resources?,” in Ecology of harmful algae. Eds. Granéli E., Turner J. T. (Berlin, Heidelberg: Springer Publishers), 189–201.
Griffith A. W., Shumway S. E., Gobler C. J. (2019). Differential mortality of north Atlantic bivalve molluscs during harmful algal blooms caused by the dinoflagellate, Cochlodinium (a.k.a. Margalefidinium) polykrikoides. Estuaries Coasts 42 (1), 190–203. doi: 10.1007/s12237-018-0445-0
Hattenrath-Lehmann T. K., Jankowiak J., Koch F., Gobler C. J. (2019). Prokaryotic and eukaryotic microbiomes associated with blooms of the ichthyotoxic dinoflagellate Cochlodinium (Margalefidinium) polykrikoides in new York, USA, estuaries. PloS One 14 (11), e0223067. doi: 10.1371/journal.pone.0223067
Iwataki M., Kawami H., Mizushima K., Mikulski C. M., Doucette G. J., Relox J. R., et al. (2008). Phylogenetic relationships in the harmful dinoflagellate Cochlodinium polykrikoides (Gymnodiniales, dinophyceae) inferred from LSU rDNA sequences. Harmful Algae 7 (3), 271–277. doi: 10.1016/j.hal.2007.12.003
Jiang X., Tang Y., Lonsdale D. J., Gobler C. J. (2009). Deleterious consequences of a red tide dinoflagellate Cochlodinium polykrikoides for the calanoid copepod Acartia tonsa. mar. Ecol. Prog. Ser. 390, 105–116. doi: 10.3354/meps08159
Jose Dorantes-Aranda J., Maria Garcia-de la Parra L., Alonso-Rodriguez R., Morquecho L., Voltolina D. (2010). Toxic effect of the harmful dinoflagellate Cochlodinium polykrikoides on the spotted rose snapper Lutjanus guttatus. environ. Toxicol 25 (4), 319–326. doi: 10.1002/tox.20507
Kim C. S., Lee S. G., Lee C. K., Kim H. G., Jung J. (1999). Reactive oxygen species as causative agents in the ichthyotoxicity of the red tide dinoflagellate Cochlodinium polykrikoides. J. Plankton Res. 21 (11), 2105–2115. doi: 10.1093/plankt/21.11.2105
Kim D., Oda T. (2010). “Possible factors responsible for the fish-killing mechanisms of the red tide phytoplankton, Chattonella marina and Cochlodinium polykrikoides,” in Coastal Environmental and Ecosystem Issues of the East China Sea, eds Ishimatsu A., Lie H.-J.. (Tokyo: TERRAPUB and Nagasaki University), 245–268.
Kim D., Oda T., Muramatsu T., Kim D., Matsuyama Y., Honjo T. (2002). Possible factors responsible for the toxicity of Cochlodinium polykrikoides, a red tide phytoplankton. Comp. Biochem. Physiol. C-Toxicol. Pharmacol. 132 (4), 415–423. doi: 10.1016/S1532-0456(02)00093-5
Koch F., Burson A., Tang Y. Z., Collier J. L., Fisher N. S., Sañudo-Wilhelmy S., et al. (2014). Alteration of plankton communities and biogeochemical cycles by harmful Cochlodinium polykrikoides (Dinophyceae) blooms. Harmful Algae 33, 41–54. doi: 10.1016/j.hal.2014.01.003
Kubanek J., Hicks M. K., Naar J., Villareal T. A. (2005). Does the red tide dinoflagellate Karenia brevis use allelopathy to outcompete other phytoplankton? Limnol. Oceanogr. 50 (3), 883–895. doi: 10.4319/lo.2005.50.3.0883
Kudela R. M., Gobler C. J. (2012). Harmful dinoflagellate blooms caused by cochlodinium sp.: Global expansion and ecological strategies facilitating bloom formation. Harmful Algae 14, 71–86. doi: 10.1016/j.hal.2011.10.015
Legrand C., Rengefors K., Fistarol G. O., Granéli E. (2003). Allelopathy in phytoplankton - biochemical, ecological and evolutionary aspects. Phycologia 42 (4), 406–419. doi: 10.2216/i0031-8884-42-4-406.1
Marshall J.-A., Ross T., Pyecroft S., Hallegraeff G. (2005). Superoxide production by marine microalgae: II. towards understanding ecological consequences and possible functions. Mar. Biol. 147 (2), 541–549. doi: 10.1007/S00227-005-1597-6
Mulholland M. R., Morse R. E., Boneillo G. E., Bernhardt P. W., Filippino K. C., Procise L. A., et al. (2009). Understanding causes and impacts of the dinoflagellate, Cochlodinium polykrikoides, blooms in the Chesapeake bay. Estuaries Coasts 32 (4), 734–747. doi: 10.1007/s12237-009-9169-5
Oda T., Akaike T., Sato K., Ishimatsu A., Takeshita S., Muramatsu T., et al. (1992). Hydroxyl radical generation by red tide algae. Arch. Biochem. Biophys. 294 (1), 38–43. doi: 10.1016/0003-9861(92)90133-H
Pérez-Morales A., Band-Schmidt C. J., Martínez-Díaz S. F. (2017). Mortality on zoea stage of the pacific white shrimp Litopenaeus vannamei caused by Cochlodinium polykrikoides (Dinophyceae) and chattonella spp. (Raphidophyceae). Mar. Biol. 164 (3), 57. doi: 10.1007/s00227-017-3083-3
Reñé A., Garcés E., Camp J. (2013). Phylogenetic relationships of Cochlodinium polykrikoides margalef (Gymnodiniales, dinophyceae) from the Mediterranean Sea and the implications of its global biogeography. Harmful Algae 25, 39–46. doi: 10.1016/j.hal.2013.02.004
Richlen M. L., Morton S. L., Jamali E. A., Rajan A., Anderson D. M. (2010). The catastrophic 2008-2009 red tide in the Arabian gulf region, with observations on the identification and phylogeny of the fish-killing dinoflagellate Cochlodinium polykrikoides. Harmful Algae 9 (2), 163–172. doi: 10.1016/j.hal.2009.08.013
Singh D. P., Tyagi M., Kumar A., Thakur J., Kumar A. (2001). Antialgal activity of a hepatotoxin-producing cyanobacterium, Microcystis aeruginosa. World J. Microbiol. Biotechnol. 17 (1), 15–22. doi: 10.1023/A:1016622414140
Smayda T. J. (1997). Harmful algal blooms: Their ecophysiology and general relevance to phytoplankton blooms in the sea. Limnol. Oceanogr. 42 (5), 1137–1153. doi: 10.4319/lo.1997.42.5_part_2.1137
Tang Y. Z., Gobler C. J. (2009a). Characterization of the toxicity of Cochlodinium polykrikoides isolates from northeast US estuaries to finfish and shellfish. Harmful Algae 8 (3), 454–462. doi: 10.1016/j.hal.2008.10.001
Tang Y. Z., Gobler C. J. (2009b). Cochlodinium polykrikoides blooms and clonal isolates from the northwest Atlantic coast cause rapid mortality in larvae of multiple bivalve species. Mar. Biol. 156 (12), 2601–2611. doi: 10.1007/s00227-009-1285-z
Tang Y. Z., Gobler C. J. (2010). Allelopathic effects of Cochlodinium polykrikoides isolates and blooms from the estuaries of long island, new York, on co-occurring phytoplankton. Mar. Ecol. Prog. Ser. 406, 19–31. doi: 10.3354/meps08537
Tang Y. Z., Gobler C. J. (2015). Sexual resting cyst production by the dinoflagellate Akashiwo sanguinea: A potential mechanism contributing to the ubiquitous distribution of a harmful alga. J. Phycol. 51 (2), 298–309. doi: 10.1111/jpy.12274
Tillmann U., Alpermann T., John U., Cembella A. (2008). Allelochemical interactions and short-term effects of the dinoflagellate Alexandrium on selected photoautotrophic and heterotrophic protists. Harmful Algae 7 (1), 52–64. doi: 10.1016/j.hal.2007.05.009
Tillmann U., John U. (2002). Toxic effects of alexandrium spp. on heterotrophic dinoflagellates: an allelochemical defence mechanism independent of PSP-toxin content. Mar. Ecol. Prog. Ser. 230, 47–58. doi: 10.3354/meps230047
Tillmann U., John U., Cembella A. (2007). On the allelochemical potency of the marine dinoflagellate Alexandrium ostenfeldii against heterotrophic and autotrophic protists. J. Plankton Res. 29 (6), 527–543. doi: 10.1093/plankt/fbm034
van Rijssel M., de Boer M. K., Tyl M. R., Gieskes W. W. C. (2008). Evidence for inhibition of bacterial luminescence by allelochemicals from Fibrocapsa japonica (Raphidophyceae), and the role of light and microalgal growth rate. Hydrobiologia 596 (1), 289–299. doi: 10.1007/s10750-007-9104-3
Wang H., Hu Z., Shang L., Leaw C. P., Lim P. T., Tang Y. Z. (2020). Toxicity comparison among four strains of Margalefidinium polykrikoides from China, Malaysia, and USA (belonging to two ribotypes) and possible implications. J. Exp. Mar. Biol. Ecol. 524, 151293. doi: 10.1016/j.jembe.2019.151293
Waterman P. G. (1992). Roles for secondary metabolites in plants. Ciba Found. Symp. 171, 255–269; discussion 269–275.
Whittaker R. H., Feeny P. P. (1971). Allelochemics: Chemical interactions between species. Science 171 (3973), 757–770. doi: 10.1126/SCIENCE.171.3973.757
Woolf B. (1957). The log likelihood ratio test (the G-test) - methods and tables for tests of heterogeneity in contingency tables. Ann. Hum. Genet. 21 (4), 397–409. doi: 10.1111/j.1469-1809.1972.tb00293.x
Yang H., Hu Z., Xu N., Tang Y. Z. (2019). A comparative study on the allelopathy and toxicity of four strains of Karlodinium veneficum with different culturing histories. J. Plankton Res. 41 (1), 17–29. doi: 10.1093/plankt/fby047
Keywords: Margalefidinium polykrikoides, ichthyotoxicity, allelopathy, strain variation, toxins, allelochemicals
Citation: Yang H, Gobler CJ and Tang YZ (2022) Consistency between the ichthyotoxicity and allelopathy among strains and ribotypes of Margalefidinium polykrikoides suggests that its toxins are allelochemicals. Front. Mar. Sci. 9:941205. doi: 10.3389/fmars.2022.941205
Received: 11 May 2022; Accepted: 01 August 2022;
Published: 19 August 2022.
Edited by:
Aifeng Li, Ocean University of China, ChinaReviewed by:
Leila Basti, Tokyo University of Marine Science and Technology, JapanYixiao Xu, Nanning Normal University, China
Copyright © 2022 Yang, Gobler and Tang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Christopher J. Gobler, Y2hyaXN0b3BoZXIuZ29ibGVyQHN0b255YnJvb2suZWR1; Ying Zhong Tang, eWluZ3pob25nLnRhbmdAcWRpby5hYy5jbg==
 Huijiao Yang
Huijiao Yang Christopher J. Gobler
Christopher J. Gobler Ying Zhong Tang
Ying Zhong Tang