- 1Department of Life Sciences, MARE - Marine and Environmental Sciences Centre/ARNET - Aquatic Research Network, University of Coimbra, Coimbra, Portugal
- 2Institute of Marine Sciences, University of California Santa Cruz, Santa Cruz, CA, United States
- 3People and Nature, Environmental Defense Fund, Monterey, CA, United States
- 4School of Ocean and Earth Science, University of Southampton, Southampton, United Kingdom
- 5Centre d’Etudes Biologiques de Chizé UMR7372 Centre national de la recherche scientifique (CNRS) La Rochelle Université, Villiers-en-Bois, France
- 6Division of Ecology and Evolution, Research School of Biology, The Australian National University, Canberra, ACT, Australia
- 7The University of Western Australia (UWA) Oceans Institute and the School of Biological Sciences, The University of Western Australia, Perth, W A, Australia
Editorial on the Research Topic
Tracking marine megafauna for conservation and marine spatial planning
1. Introduction
Marine megafauna are an important component of marine ecosystems providing a range of cultural, regulating and provisioning ecosystem services to humans (Dunn et al., 2019; Hammerschlag et al., 2019). They transport energy, nutrients, and other materials vertically and horizontally through the oceans (Roman et al., 2014; Kiszka et al., 2015; Estes et al., 2016; Hammerschlag et al., 2019), and through their large size and often high mobility, influence other species through consumption and risk avoidance behaviour. Marine megafauna also include important focal species (Zacharias and Roff, 2001) in marine conservation and management, given their role as sentinel species or ecological indicators (Hazen et al., 2019).
Despite their important role in ecosystems, the widespread and increasing threats faced by many marine megafauna taxa has led to a poor conservation status of many species (e.g., Rees et al., 2016; Dias et al., 2019; Nelms et al., 2021; Jorgensen et al., 2022). Understanding and mitigating the threats faced by marine megafauna is challenging (Lascelles et al., 2014; Reisinger et al., 2022) because both the threats and the marine environment are highly dynamic in space and time, and animals’ occurrences vary with shifts in environmental and oceanographic conditions at different scales. Additionally, these animals are often highly mobile, making it difficult to pinpoint the occurrences of different individuals in specific locations.
There are several definitions for the term ‘marine megafauna’. For example, Estes et al. (2016) define marine megafauna as species with maximum reported mass >45 kg, including 338 extant species of cetaceans, pinnipeds, sirenians, the sea otter (Enhyrda lutris), the polar bear (Ursus maritimus), the emperor penguin (Aptenodytes forsteri), marine reptiles, bony and cartilaginous fishes, cephalopods, and the giant clam (Tridacna gigas). Here, we take the view of Authier et al. (2017), who do not impose a strict body mass threshold, but consider marine megafauna to be a coherent group based on their ecological similarities (at or near the top of food webs, with no or few predators) and that share conservation issues.
This Research Topic covers a broad taxonomic representation, spanning seabirds, cetaceans, sea turtles, pinnipeds, elasmobranchs, teleosts, a sirenian, the polar bear, and a large crustacean, the red king crab (Paralithodes camtschaticus). Articles submitted address how biologging is being used to understand the movement behaviour and distribution of marine megafauna, and how this information can play a key role to prioritise conservation goals. The resulting 34 articles illustrate how biologging is informing conservation of marine megafauna, and in light of these studies, we discuss challenges, methodological implications and future directions for biologging in conservation.
2. Patterns and processes
A massive increase in the use of biologging, including miniaturised animal-attached tags for logging or relaying data about animal movements, behaviour, physiology, or environment (Rutz and Hays, 2009; Williams et al., 2020), has been instrumental in providing this otherwise hard to come by information (Hussey et al., 2015). Due to technological limitations (e.g., tag weight), funding or other resource limitations, remoteness, difficulty of capture, and handling or tagging, the movements and distributions of many species, populations or life stages remain poorly understood (Pereira et al., 2022). Many studies in this Research Topic provide baseline information important for conservation (e.g., Laidre et al., Schorr et al., Figure 1). For example, Setyawan et al. used a combination of methods to document the movements of juvenile reef manta rays (Mobula alfredi) in Indonesia and found that individuals are largely resident within the Wayag lagoon, emphasising the need for area-based protection. Biologging studies also enable an understanding of the patterns and processes driving range shifts (Cloyed et al.). For instance, Aune et al. tagged invasive red king crabs to better understand their habitat requirements and environmental conditions facilitating their invasion into new fjords in Norway.
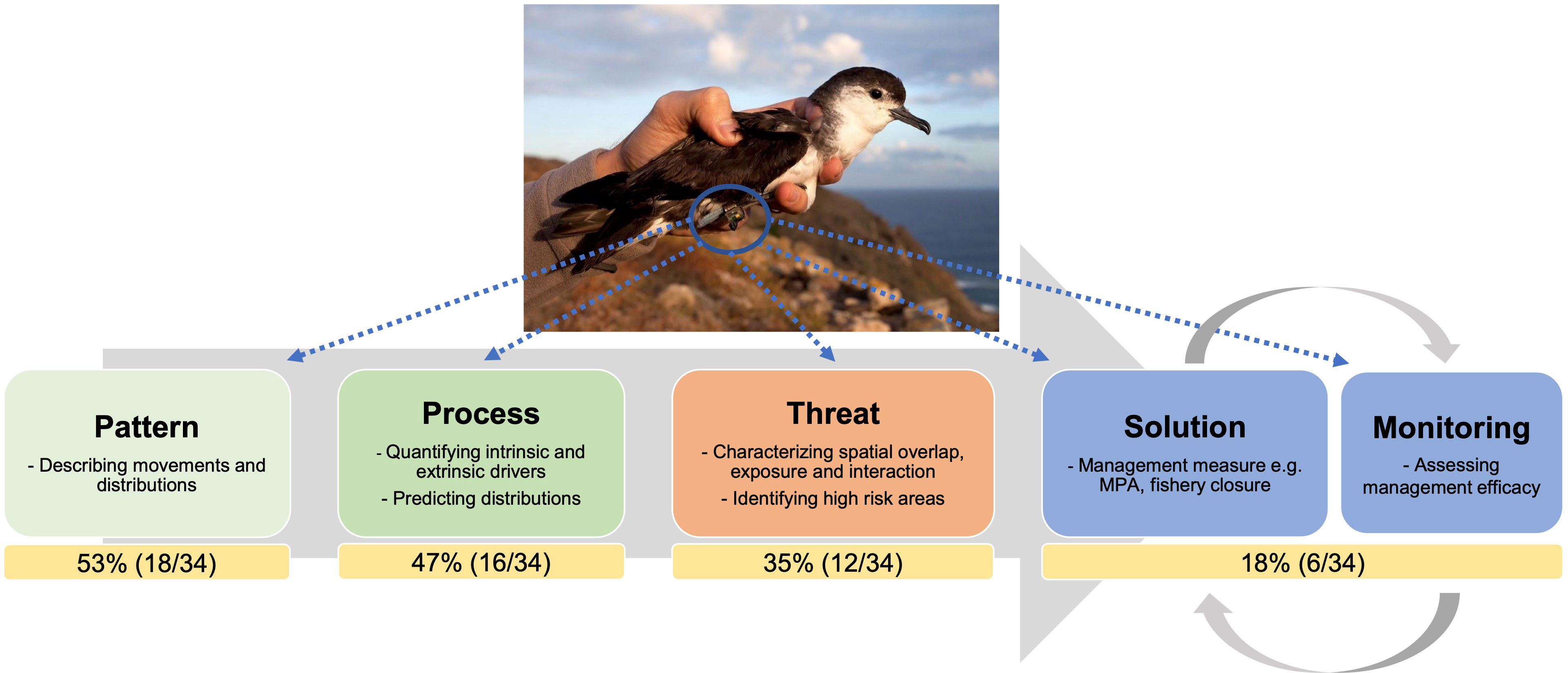
Figure 1 Biologging data provide a range of useful information to address management questions, including essential ecological data that allow assessing species distributions, movement patterns, and the processes that drive their behaviour, as well as identifying overlap or interactions with threats. The percentage and number of studies in each category is shown by a yellow box. Studies can contribute to one or more categories, so the percentages do not sum to 100. The majority (53%) of studies in this Research Topic provide essential ecological information (e.g., pattern and process), whereas much fewer (18%) provide a direct policy recommendation or assess the efficacy of conservation measures. Photo credit: Macaronesian shearwater (Puffinus baroli) with a geolocator by Luís Ferreira (www.luis-ferreira.com).
Biologging also provides the opportunity to explore habitat requirements and quantify the environmental drivers of species distribution. Studies in this Research Topic demonstrate that bottom depth (Dehnhard et al.), topography (Wyles et al.), marine productivity (Thiebot et al.) and sea ice cover (Harcourt et al., Fortune et al.), among other factors, influence the movement and foraging decisions of marine megafauna (Figure 1). However, their importance differs between populations or species, highlighting the need for species-and population-specific studies (Carter et al., O’Hanlon et al.). Moreover, some studies have failed to find effects of environmental features on foraging decisions, likely because wide-ranging species (such as seabirds) may have weak or broad preferences for available habitats (Halpin et al., Morten et al.). Biologging studies also enable an understanding of individual-level patterns and processes driving movement or foraging strategies. For example, foraging strategies of individuals can vary according to sub-colony (Morinay et al.) and be related to colony size (Rebstock et al.) and individual-level specialisation independent of these factors (Descamps et al.). In most habitat modelling studies, environmental variables are commonly assumed to be surrogates of the distribution and abundance of lower trophic level prey, but very few incorporate data on prey abundance (Chambault et al.). Proud et al. showed that incorporating 3-dimensional prey distributions from active acoustics can improve predictions of the foraging distributions of king penguins (Aptenodytes patagonicus).
3. Overlap and interaction with anthropogenic stressors
By pinpointing where, why and how marine megafauna overlap, interact and respond to anthropogenic activities, biologging can highlight areas for protection and contribute to a more sustainable exploitation of marine resources (Hays et al., 2019; Grémillet et al., 2022). In this Research Topic, we focus on the ways in which biologging has extended our ability to measure the exposure of marine megafauna to anthropogenic threats (Figure 1) such as fisheries, shipping, and offshore platforms and seismic surveys.
3.1. Fisheries
Fisheries have impacted marine megafauna worldwide, either directly as target catch, bycatch (Lewison et al., 2004), or through indirect interactions such as competition for food resources (Grémillet et al., 2018). Biologging has provided crucial information on the extent to which species overlap with and are exposed to fishing activity (e.g. Clay et al., 2019; Queiroz et al., 2019). For example, by comparing the distributions of satellite-tagged white sharks (Carcharodon carcharias) and fishing effort, Kock et al. showed that sharks overlapped with longline and gillnet fisheries within 25% of South Africa’s Exclusive Economic Zone (EEZ). High-precision tracking of individual animals and fishing vessels can also reveal the extent to which marine taxa interact with fishing vessels (Weimerskirch et al., 2020, Morten et al., Reisinger et al.).
3.2. Shipping
Shipping is increasing globally, presenting a growing threat to marine megafauna (Pirotta et al., 2019) and biologging may provide valuable information regarding where and when marine species are exposed to vessel strikes, which are often undetected and unreported (Womersley et al., 2022). Pasanisi et al. mapped for the first time the oceanic areas of high exposure between loggerhead turtles (Caretta caretta) and shipping traffic in the western Mediterranean, while Thiebot et al. showed that key habitats of four Arctic seabirds are not fully covered by a designated shipping avoidance area, recommending a northward extension that better affords protection. By incorporating high-resolution data on vertical movements, both Fonseca et al. and Oliveira et al. show that fin (Balaenoptera physalus) and sperm whales (Physeter macrocephalus) may be more vulnerable to collision on the sea surface at night, or when conducting fast dive ascents, respectively.
3.3. Offshore platforms and seismic surveys
Increasing global energy demands have led to the expansion of both petrochemical and renewable platforms, which may pose significant threats to marine biodiversity (Venegas-Li et al., 2019; Harwood and King, 2022). Yet, few studies have attempted to quantify the extent to which animals interact with these structures. Collins et al. showed that foraging Leach’s Storm-Petrels (Hydrobates leucorhous) flew within the surroundings of an oil platform in 17.5% of the trips, though birds rarely approached them at night. Rebstock et al. also showed that Magellanic penguins (Spheniscus magellanicus) from multiple colonies off the east coast of Argentina extensively overlapped with a large area permitted for hydrocarbon exploration and where seismic surveys are regularly carried out.
4. Establishment and monitoring of area-based conservation measures
There is an increasing global push towards setting aside large swathes of the ocean as marine protected areas (MPA), however, their efficacy in protecting marine megafauna habitats remains unclear, particularly for species with vast home ranges. Biologging data play an increasingly important role in MPA design, implementation and management (Hays et al., 2019) and multispecies studies have shed light into the degree to which wide-ranging species are protected by MPAs throughout key life-history stages (Handley et al., 2020; Hindell et al., 2020; Baylis et al., 2021).
Several studies showed that MPAs in their current form allow some protection (Harcourt et al., Kock et al., Patrício et al.); for instance, the Ross Sea MPA off Antarctica encompassed all Weddell seal (Leptonychotes weddellii) habitat given their largely coastal distribution (Harcourt et al.), while green turtles (Chelonia mydas) in West Africa spent 78% of their time within five sites in a regional MPA network, which are mostly no-take zones (Patrício et al.). In contrast, other studies found that current MPA boundaries present gaps in protection (Carter et al., Conners et al.). In the United Kingdom, the distribution of grey seals (Halichoerus grypus) does not match the distribution of Special Areas for Conservation (SACs), which were legally designated for their protection (Carter et al.) Conners et al. compiled biologging data from 36 species to compare space use in relation to the size and location of global MPAs, and found them too small to encompass the complete home ranges of most species (MPAs covered <5% of core areas). It is suggested that MPAs focus on targeting seasonal aggregations and critical life history stages, and be enacted alongside other management measures such as bycatch mitigation.
5. Megafauna as ecosystem indicators
While this Research Topic has largely focused on how biologging can inform the conservation of studied species, it is widely recognised that marine megafauna can act as sentinels indicating the state of ecosystems, habitats or other species (e.g., prey distributions) that are more challenging to monitor directly (Hazen et al., 2019; Jelicich et al., 2022). Two complementary studies present the use of seabirds as indicators of humpback whale (Megaptera novaeangliae) abundance in US waters; Cimino et al. tracked western gulls (Larus occidentalis) in the California Current over a 7-year period and found that gulls often feed in association with whales, while Silva et al. found tagged great shearwaters (Ardenna gravis) in the Stellwagen Bank National Marine Sanctuary overlapped with whale distributions. Both studies suggest that seabirds could be used as indicators of whales and their prey (e.g., krill), given the relative ease and low cost of tagging seabirds relative to other species.
6. Methodological implications and the future of biologging for conservation
While biologging has provided a wealth of knowledge on the distribution and behaviour of megafauna, it should be viewed as a complement to other methods, providing information where traditional tools cannot. In this Research Topic, we also included several studies that examine species distributions through alternative means. For example, passive acoustic monitoring of humpback whales, which have characteristic calls, established their occurrence between the coast of South Africa and the Antarctic shelf across a multi-year period (Shabangu and Kowarski). Bottom-mounted acoustic hydrophones revealed social interactions of North Pacific minke whales (Balaenoptera acutorostrata) (Martin et al.). Active acoustic tracking combines concepts of acoustic monitoring (networks of receivers) and biologging (animal-mounted acoustic devices emitting data) to assess key aspects of the movement ecology of marine megafauna (Alexandri and Diamant). Besides acoustic technologies, the combination of animal-mounted biologging data with - non exhaustively - data from autonomous underwater vehicles, buoys, or ship-based observations (Cimino et al., Silva et al.) could be used to improve the conservation of marine megafauna, especially those species which inhabit cryptic ecosystems, such as underneath the sea ice.
Studies based on a single colony or year often may not be representative of the space use of a population or species across time (e.g., Dehnhard et al., Morinay et al.). This highlights the need for large datasets to improve our ability to provide useful information to policy makers and conservation bodies. A recent example is MegaMove (www.megamove.org), a global consortium endorsed by the UN Ocean Decade (https://www.oceandecade.org/), of hundreds of researchers from around the world focused on advancing conservation of marine megafauna through strategic mitigation of threats guided by an innovative global science effort. Refinements of habitat modelling approaches to better predict the foraging distributions of animals from untracked populations (Ronconi et al.) is another way to address fundamental questions at large scale. Improving the performance of species distribution models (Goetz et al.) is, however, of the utmost importance, as low performance models make real-time management challenging (Halpin et al.).
There is no doubt that technological advances will continue to bring progress to the biologging field and enhance our knowledge of the ecology of marine megafauna. In parallel with the improvement in the miniaturisation of devices, their autonomy and hydrodynamic design1, the use of new sensors and biologging approaches will help tackle conservation issues. This is already illustrated by seabird-borne devices that monitor the Automated Identification Systems (AIS) of fishing vessels (Weimerskirch et al., 2020). Animal-borne oceanographic devices may include new biochemical and physical sensors, increasing our ability to monitor the oceans and understand the interactions between marine megafauna and their environment.
The increasing availability of biologging data with new levels of complexity also brings a new challenge associated with the efficient and effective analyses of those datasets. Analyses of large and complex datasets require substantial computational capacity and a high degree of analytical proficiency (Joo et al., 2020; Grémillet et al., 2022). Multidisciplinary collaborations will be key to develop new ways to analyse data and investigate the new complexities that arise from merging multiple datasets. Making the resources available online in the form of open data and codes, which has facilitated the reproducibility of results among the scientific community (Sequeira et al., 2019; Williams et al., 2020) has been encouraged. Yet, practical examples where these studies have led to clearly identifiable real-world changes in conservation or marine spatial planning efforts are scarce. This is demonstrated in this Research Topic whereby the majority (n = 18, 53%) of studies were largely focused on describing spatial and behavioural patterns of marine megafauna populations, with few (n = 6, 18%) directly assessing the efficacy of management measures (Figure 1). Future studies should therefore be designed to address specific conservation goals and promote early engagement among scientists and stakeholders in the decision-making processes, in order to maximise the use of ecological information into effective conservation measures (Hays et al., 2019).
Author contributions
All authors listed have made a substantial, direct, and intellectual contribution to the work and approved it for publication.
Acknowledgments
We thank all authors, reviewers, and editors that were involved in this Research Topic and greatly contributed to the quality and success of the work presented here. We also thank our colleagues Vitor Paiva and Jaime Ramos for proofreading a final version of the manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Footnotes
- ^ Note the recent emergence of non-rigid biologgers, like the pliable “Marine Skin” logger, represents a huge improvement in respecting the hydrodynamics of marine organisms (Shaikh et al., 2019).
References
Authier M., Spitz J., Blanck A., Ridoux V. (2017). Conservation science for marine megafauna in Europe: Historical perspectives and future directions. Deep Sea Res. Part II: Topical Stud. Oceanography 141, 1–7. doi: 10.1016/j.dsr2.2017.05.002
Baylis A. M. M., Lecea A. M., Tierney M., Orben R. A., Ratcliffe N., Wakefield E., et al. (2021). Overlap between marine predators and proposed marine managed areas on the Patagonian shelf. Ecol. Appl. 31, e02426. doi: 10.1002/eap.2426
Clay T. A., Small C., Tuck G. N., Pardo D., Carneiro A. P. B., Wood A. G., et al. (2019). A comprehensive large-scale assessment of fisheries bycatch risk to threatened seabird populations. J. Appl. Ecol. 56, 1882–1893. doi: 10.1111/1365-2664.13407
Dias M. P., Martin R., Pearmain E. J., Burfield I. J., Small C., Phillips R. A., et al. (2019). Threats to seabirds: A global assessment. Biol. Conserv. 237, 525–537. doi: 10.1016/j.biocon.2019.06.033
Dunn D. C., Harrison A.-L., Curtice C., DeLand S., Donnelly B., Fujioka E., et al. (2019). The importance of migratory connectivity for global ocean policy. Proc. R. Soc. B: Biol. Sci. 286, 20191472. doi: 10.1098/rspb.2019.1472
Estes J. A., Heithaus M., McCauley D. J., Rasher D. B., Worm B. (2016). Megafaunal impacts on structure and function of ocean ecosystems. Annu. Rev. Environ. Resour. 41, 83–116. doi: 10.1146/annurev-environ-110615-085622
Grémillet D., Chevallier D., Guinet C. (2022). Big data approaches to the spatial ecology and conservation of marine megafauna. ICES J. Mar. Sci. 79, 975–986. doi: 10.1093/icesjms/fsac059
Grémillet D., Ponchon A., Paleczny M., Palomares M.-L. D., Karpouzi V., Pauly D. (2018). Persisting worldwide seabird-fishery competition despite seabird community decline. Curr. Biol. 28, 4009–4013.e2. doi: 10.1016/j.cub.2018.10.051
Hammerschlag N., Schmitz O. J., Flecker A. S., Lafferty K. D., Sih A., Atwood T. B., et al. (2019). Ecosystem function and services of aquatic predators in the anthropocene. Trends Ecol. Evol. 34, 369–383. doi: 10.1016/j.tree.2019.01.005
Handley J. M., Pearmain E. J., Oppel S., Carneiro A. P. B., Hazin C., Phillips R. A., et al. (2020). Evaluating the effectiveness of a large multi-use MPA in protecting key biodiversity areas for marine predators. Diversity Distributions 26, 715–729. doi: 10.1111/ddi.13041
Harwood A. J. P., King S. (2022). “Seabirds and marine renewable energy sources,” in Seabird biodiversity and human activities. Eds. Ramos J. A., Pereira L. (Boca Raton, Florida: CRC Press, Taylor&Francis), 106–125. doi: 10.1201/9781003047520-9/seabirds-marine-renewable-energy-sources-andrew-harwood-sue-king
Hays G. C., Bailey H., Bograd S. J., Bowen W. D., Campagna C., Carmichael R. H., et al. (2019). Translating marine animal tracking data into conservation policy and management. Trends Ecol. Evol. 34, 459–473. doi: 10.1016/j.tree.2019.01.009
Hazen E. L., Abrahms B., Brodie S., Carroll G., Jacox M. G., Savoca M. S., et al. (2019). Marine top predators as climate and ecosystem sentinels. Front. Ecol. Environ. 17, 565–574. doi: 10.1002/fee.2125
Hindell M. A., Reisinger R. R., Ropert-Coudert Y., Hückstädt L. A., Trathan P. N., Bornemann H., et al. (2020). Tracking of marine predators to protect southern ocean ecosystems. Nature 580, 87–92. doi: 10.1038/s41586-020-2126-y
Hussey N. E., Kessel S. T., Aarestrup K., Cooke S. J., Cowley P. D., Fisk A. T., et al. (2015). Aquatic animal telemetry: A panoramic window into the underwater world. Science 348. doi: 10.1126/science.1255642
Jelicich R. M., Berón P., Copello S., Dellabianca N. A., García G., Labrada-Martagón V., et al. (2022). “Marine megafauna Sea turtles, seabirds and marine mammals,” in Marine biology. Eds. Pan J., Pratolongo P. (Boca Raton, Florida: CRC Press, Taylor&Francis), 297–324. doi: 10.1201/9780429399244-14/marine-megafauna-sea-turtles-seabirds-marine-mammals-rocío-mariano-jelicich-paula-berón-sofia-copello-natalia-dellabianca-germán-garcía-vanessa-labrada-martagón-natalia-paso-viola-jesica-paz-luciana-riccialdelli-analía-san-martin-juan-pablo-seco-pon-mónica-torres-marco-favero
Joo R., Boone M. E., Clay T. A., Patrick S. C., Clusella-Trullas S., Basille M. (2020). Navigating through the r packages for movement. J. Anim. Ecol. 89, 248–267. doi: 10.1111/1365-2656.13116
Jorgensen S., Micheli F., White T., Van Houtan K., Alfaro-Shigueto J., Andrzejaczek S., et al. (2022). Emergent research and priorities for shark and ray conservation. Endangered Species Res. 47, 171–203. doi: 10.3354/esr01169
Kiszka J., Heithaus M., Wirsing A. (2015). Behavioural drivers of the ecological roles and importance of marine mammals. Mar. Ecol. Prog. Ser. 523, 267–281. doi: 10.3354/meps11180
Lascelles B., Notarbartolo Di Sciara G., Agardy T., Cuttelod A., Eckert S., Glowka L., et al. (2014). Migratory marine species: their status, threats and conservation management needs. Aquat. Conservation: Mar. Freshw. Ecosyst. 24, 111–127. doi: 10.1002/aqc.2512
Lewison R. L., Crowder L. B., Read A. J., Freeman S. A. (2004). Understanding impacts of fisheries bycatch on marine megafauna. Trends Ecol. Evol. 19, 598–604. doi: 10.1016/j.tree.2004.09.004
Nelms S., Alfaro-Shigueto J., Arnould J., Avila I., Bengtson Nash S., Campbell E., et al. (2021). Marine mammal conservation: over the horizon. Endangered Species Res. 44, 291–325. doi: 10.3354/esr01115
Pereira J., Paiva V., Krüger L., Votier S. C. (2022). “Tracking seabirds for conservation and marine spatial planning,” in Seabird biodiversity and human activities. Eds. Ramos J. A., Pereira L. (Boca Raton, Florida: CRC Press, Taylor&Francis), 58–74. doi: 10.1201/9781003047520-5/tracking-seabirds-conservation-marine-spatial-planning-jorge-pereira-vitor-paiva-lucas-krüger-stephen-votier
Pirotta V., Grech A., Jonsen I. D., Laurance W. F., Harcourt R. G. (2019). Consequences of global shipping traffic for marine giants. Front. Ecol. Environ. 17, 39–47. doi: 10.1002/fee.1987
Queiroz N., Humphries N. E., Couto A., Vedor M., da Costa I., Sequeira A. M. M., et al. (2019). Global spatial risk assessment of sharks under the footprint of fisheries. Nature 572, 461–466. doi: 10.1038/s41586-019-1444-4
Rees A., Alfaro-Shigueto J., Barata P., Bjorndal K., Bolten A., Bourjea J., et al. (2016). Are we working towards global research priorities for management and conservation of sea turtles? Endangered Species Res. 31, 337–382. doi: 10.3354/esr00801
Reisinger R. R., Johnson C., Friedlaender A. S. (2022). “Marine mammal movement ecology in a conservation and management context,” in Marine mammals: the evolving human factor ethology and behavioral ecology of marine mammals. Eds. Sciara G., Würsig B. (Springer International Publishing), 149–192. doi: 10.1007/978-3-030-98100-6_5
Roman J., Estes J. A., Morissette L., Smith C., Costa D., McCarthy J., et al. (2014). Whales as marine ecosystem engineers. Front. Ecol. Environ. 12, 377–385. doi: 10.1890/130220
Rutz C., Hays G. C. (2009). New frontiers in biologging science. Biol. Lett. 5, 289–292. doi: 10.1098/rsbl.2009.0089
Sequeira A. M. M., Heupel M. R., Lea M. A., Eguíluz V. M., Duarte C. M., Meekan M. G., et al. (2019). The importance of sample size in marine megafauna tagging studies. Ecol. Appl. 29, e01947. doi: 10.1002/eap.1947
Shaikh S. F., Mazo-Mantilla H. F., Qaiser N., Khan S. M., Nassar J. M., Geraldi N. R., et al. (2019). Noninvasive featherlight wearable compliant “Marine skin”: Standalone multisensory system for deep-Sea environmental monitoring. Small 15, 1804385. doi: 10.1002/smll.201804385
Venegas-Li R., Levin N., Morales-Barquero L., Kaschner K., Garilao C., Kark S. (2019). Global assessment of marine biodiversity potentially threatened by offshore hydrocarbon activities. Global Change Biol. 25, 2009–2020. doi: 10.1111/gcb.14616
Weimerskirch H., Collet J., Corbeau A., Pajot A., Hoarau F., Marteau C., et al. (2020). Ocean sentinel albatrosses locate illegal vessels and provide the first estimate of the extent of nondeclared fishing. Proc. Natl. Acad. Sci. 117, 3006–3014. doi: 10.1073/pnas.1915499117
Williams H. J., Taylor L. A., Benhamou S., Bijleveld A. I., Clay T. A., Grissac S., et al. (2020). Optimizing the use of biologgers for movement ecology research. J. Anim. Ecol. 89, 186–206. doi: 10.1111/1365-2656.13094
Womersley F. C., Humphries N. E., Queiroz N., Vedor M., da Costa I., Furtado M., et al. (2022). Global collision-risk hotspots of marine traffic and the world’s largest fish, the whale shark. Proc. Natl. Acad. Sci. 119, 2117440119. doi: 10.1073/pnas.2117440119
Keywords: biologging, climate change, fisheries, offshore energy production, marine predators, marine protected areas, movement
Citation: Pereira JM, Clay TA, Reisinger RR, Ropert-Coudert Y and Sequeira AMM (2023) Editorial: Tracking marine megafauna for conservation and marine spatial planning. Front. Mar. Sci. 9:1119428. doi: 10.3389/fmars.2022.1119428
Received: 08 December 2022; Accepted: 16 December 2022;
Published: 04 January 2023.
Edited and Reviewed by:
Laura Airoldi, University of Padova Chioggia Hydrobiological Station, ItalyCopyright © 2023 Pereira, Clay, Reisinger, Ropert-Coudert and Sequeira. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jorge M. Pereira, jorge.pereira@uc.pt
 Jorge M. Pereira
Jorge M. Pereira