
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Mar. Sci., 12 April 2021
Sec. Marine Evolutionary Biology, Biogeography and Species Diversity
Volume 8 - 2021 | https://doi.org/10.3389/fmars.2021.641236
This article is part of the Research TopicBiodiversity and Distribution of Benthic Invertebrates - From Taxonomy to Ecological Patterns and Global ProcessesView all 16 articles
Oceanic islands are known by their unique evolutionary histories and high endemicity caused by isolation. This is the first survey on the biodiversity of Peracarida from Trindade Island, a volcanic island located about 1,160 km off the Brazilian coast, with the first reports of Tanaidacea from the island and the description of Synapseudes isis sp. nov. and three new species of Amphipoda (Ampithoe thaix sp. nov., Elasmopus gabrieli sp. nov., and Eusiroides lucai sp. nov.). The results of the phylogenetic analysis of Synapseudes based on morphological characters and its biogeography through the Bayesian Binary MCMC analysis (BBM) suggested an Indo-Pacific origin for the genus. Finally, the biodiversity of Trindade Island is compared to that of the Abrolhos Archipelago, the closest islands from the coast on the continental shelf, suggesting a high endemicity of Peracarida, corresponding to 44% of Amphipoda and 50% of Tanaidacea species in the island of Trindade.
Oceanic islands are of great interest because of their isolation, high endemicity, and evolutionary history (Pinheiro et al., 2017). The Trindade and Martin Vaz Archipelago, located southeastern off Brazil, is a unique place with high endemism rate compared to other Atlantic Ocean localities and the seamounts that connect Trindade and Martin Vaz to the South American continent, known as Vitória-Trindade Seamount Chain (VTC). These areas are considered a biodiversity hotspot for fishes and invertebrates (Pinheiro et al., 2015), with the number of new species described for VTC, and especially to the Trindade Island (TR), still rapidly increasing (Lima et al., 2019; Cunha et al., 2020; Pachelle and Tavares, 2020; Simone and Cavallari, 2020).
Among the environments found in TR, the rocky shores comprise a large amount of the coastal area, with tidepools being found all over the island (Macieira et al., 2015), providing a stressful and changing habitat based on tide dynamics coupled with environmental characteristics and ecological processes that drive the community (Gibson, 1986). Such intertidal environment can harbor macrophytal beds that allow the increase of biodiversity by providing substrata to epiphytic algae and bryozoans and food resources and shelter to several vertebrates and invertebrates (Duffy and Hay, 1991; Christie et al., 2009). In the island of Trindade, 36 species of macroalgae were previously reported, including nine species of brown algae (Pereira-Filho et al., 2011).
These marine macrophytes form habitat patches to the epifaunal assemblages dominated by crustaceans of the order Peracarida (Tanaka and Leite, 2003), such as amphipods, isopods, and tanaidaceans, that have direct development and consequently low dispersion, favoring the establishment of endemic species (Hurtado et al., 2016; Pinheiro et al., 2017). However, data on Peracarida biodiversity from TR remain scarce, with only two amphipod species reported from the archipelago so far and one endemic to TR (Oliveira, 1951; Barnard, 1965; Andrade and Senna, 2017). Records of isopods from TR are still restricted to terrestrial fauna and ectoparasites (Barth, 1958; Moreira, 1977; Souza et al., 2013), while tanaidaceans are unknown.
Although benthic Peracarida members are considered to present high levels of endemicity, they show good ability to disperse long distances, especially on macroalgae, which provide both high food value and floating potential, once organisms with direct development may reproduce and their offspring recruit within the parental raft (Thiel, 2003). Therefore, according to Błażewicz-Paszkowycz et al. (2012), transport in floating algae (rafting) and vicariance are likely to create new habitats and to provide dispersion as well as to isolate populations, allowing them to diversify and speciate. However, the lack of studies on the events that have driven the current distribution of Peracarida associated with macroalgae hinders a better understanding of the evolutionary biogeographic processes involved.
Therefore, we aim to report on the Peracarida fauna associated with the brown algae of the genus Dictyota sp. from TR, with the first report of tanaidaceans and the description of one new species of Synapseudes (Tanaidacea: Apseudomorpha) and three new species of Amphipoda. We also comment on the phylogenetic position of the new species Synapseudes isis sp. nov. based on morphological characters and perform an event-based biogeographic analysis of the genus to further understand the processes underlying the faunal diversity associated with macroalgae worldwide. Finally, we provide a discussion on the biogeographical patterns inferred for Peracarida comparing the taxa from TR with those from the Abrolhos Archipelago, the closest islands from the coast on the continental shelf.
Specimens were collected from the brown algae of the genus Dictyota sp. under permission SISBIO 60924-3. Sampling was carried out on Trindade Island in September 2018 and April 2019 at Piscinas do Parcel (20°31′10.812″S, 20°31′10.812″W), Ilha do Sul (20°31′27.5″S, 29°19′25.2″W), and Piscina da Praia do Lixo (20°31′27.624″S, 29°19′22.188″W) (Figure 1).

Figure 1. Location of Trindade Island and Abrolhos Archipelago and sampling localities of Dictyota sp. at Trindade Island. (A) Ilha do Sul. (B) Piscina da Praia do Lixo. (C,D) Piscina do Parcel.
The island of Trindade is located at the eastern limit of the VTC (20°30′S, 29°20′W) together with Martin Vaz, in southeastern Brazil, the easternmost group of islands off Brazil, about 1,160 km from the coast (Figure 1). Trindade and Martin Vaz hold the most isolated intertidal habitats within the Brazilian Province and are surrounded by calcareous algal reefs throughout the littoral zone (Gasparini and Floeter, 2001).
Algal fronds were collected underwater by free diving, from 1 to 10 m, stored in fabric bags (0.2 mm mesh size) within seawater and washed off for fauna separation. The associated fauna was fixed and preserved in ethanol 90% for later identification. Appendages and mouthparts of dissected specimens were mounted on glass slides and sealed with glycerol. Drawings were made with a camera lucida at a Zeiss Axioscope stereomicroscope and digitally drawn with Inkscape. Types are deposited at the Museu de Zoologia da Universidade Estadual de Campinas (ZUEC).
We inferred the phylogenetic position of Synapseudes isis sp. nov. by analyzing published characters from the revision of Synapseudinae Guţu (1972). A character matrix was developed with 26 terminal taxa and 22 morphological characters, including 13 characters from the cephalothorax (including antennae, mouthparts, and cheliped), three from the pereon (including pereopods and gills), and six from the pleon (Supplementary Materials 1, 2). Characters were combined into multistate groupings to avoid overly dependent characters, resulting in nine binary characters, 11 multistate characters, and two continuous characters. Polarization of the characters was conducted through outgroup comparison. Metapseudes wilsoni Błażewicz-Paszkowycz and Bamber, 2007, and Ronabus idios (Gardiner, 1973) were chosen as out-groups based on Heard et al. (2018).
Data matrix of discrete characters was constructed using MorphoBank 3.0 (O’Leary and Kaufman, 2012), and continuous characters were subsequently added at the data file. We adopted the parsimony criterion and performed phylogenetic reconstructions in TNT version 1.1 (Goloboff et al., 2008). A heuristic analysis was conducted using the traditional search algorithm, with 8,000 replications and 500 trees held per replicate. Polymorphic characters were considered unordered. The branch-swapping algorithm used was “tree bisection and reconnection” (TBR). Branch support was calculated using the relative Bremer support (subtrees up to 10 extra steps; relative fit difference of 0.9; Bremer, 1994) implemented in TNT. Character polarization was conducted a posteriori according to Nixon and Carpenter (1993), and character optimization was made with Winclada (Nixon, 2002). For character discussion, the abbreviation used [SX(Y)] means the “state Y of character X” (Iwasa-Arai and Serejo, 2018; Iwasa-Arai et al., 2019). Synapomorphic characters whose secondary homology (sensu De Pinna, 1991) was rejected are herein referred to as “homoplastic synapomorphy” synapomorphies (e.g., Wheeler et al., 1993; Gomes-da-Silva and Souza-Chies, 2017; Iwasa-Arai and Serejo, 2018).
The abbreviations used are as follows: A, antenna; Che, cheliped; CI, consistency index; Ep, pleonal epimera; f, female; Gn, gnathopod; Hd, head; l, left; L, length; LL, lower lip; m, male; Md, mandible; Mx, maxilla; Mxp, Maxilliped; P, pereopod; Per, pereonite; Pl, pleon; r, right; RI, retention index; S, state; T, telson; and U, uropod; UL, upper lip.
The distribution range of Synapseudes was divided into 12 areas based on the presence of one or more endemic species according to the marine realms proposed by Costello et al. (2017). These areas are Mediterranean (A), North Pacific (B), Mid-Tropical North Pacific (C), Caribbean and Gulf of Mexico (D), Gulf of California (E), Indo-Pacific and Indian Ocean (F), Coral Sea (G), Offshore Western Pacific (H), Offshore South Atlantic (I), and Rio de la Plata (J).
To examine the possible ancestral ranges of Synapseudes, we used the Bayesian Binary MCMC analysis (BBM) implemented in the program Reconstruct Ancestral State in Phylogenies (RASP; Yu et al., 2015) by using the morphological phylogeny described above (see section “Materials and Methods, Phylogenetic Analysis”) as input topology. BBM was primarily designed for reconstructing the ancestral state of given nodes by calculating the probabilities of ancestral ranges using the probabilities of each area unit generated by MrBayes (Ronquist and Huelsenbeck, 2003), and it was chosen because of the ability to deal with phylogenetic uncertainty. The MCMC chains of BBM analysis were run simultaneously for two million generations, with a sampling frequency of every 100 generations and a 10% burn-in. The maximum number of areas for this analysis was kept at four.
Eleven species of Peracarida are reported herein associated with Dictyota sp. from TR. Since records of peracarid crustaceans for this location are scarce, all observations but one are new records for the island. Of the two amphipod species previously reported, Elasmopus besnardi Oliveira, 1951, is currently considered a synonym of Elasmopus brasiliensis (Dana, 1853; Horton et al., 2020), and Cymadusa trindadensis Andrade and Senna, 2017, endemic to TR, was the only previously reported species (Figure 2). Herein, we give the first records of Tanaidacea for TR, comprising two species, one new species of Apseudomorpha and one new record of Tanaidomorpha.
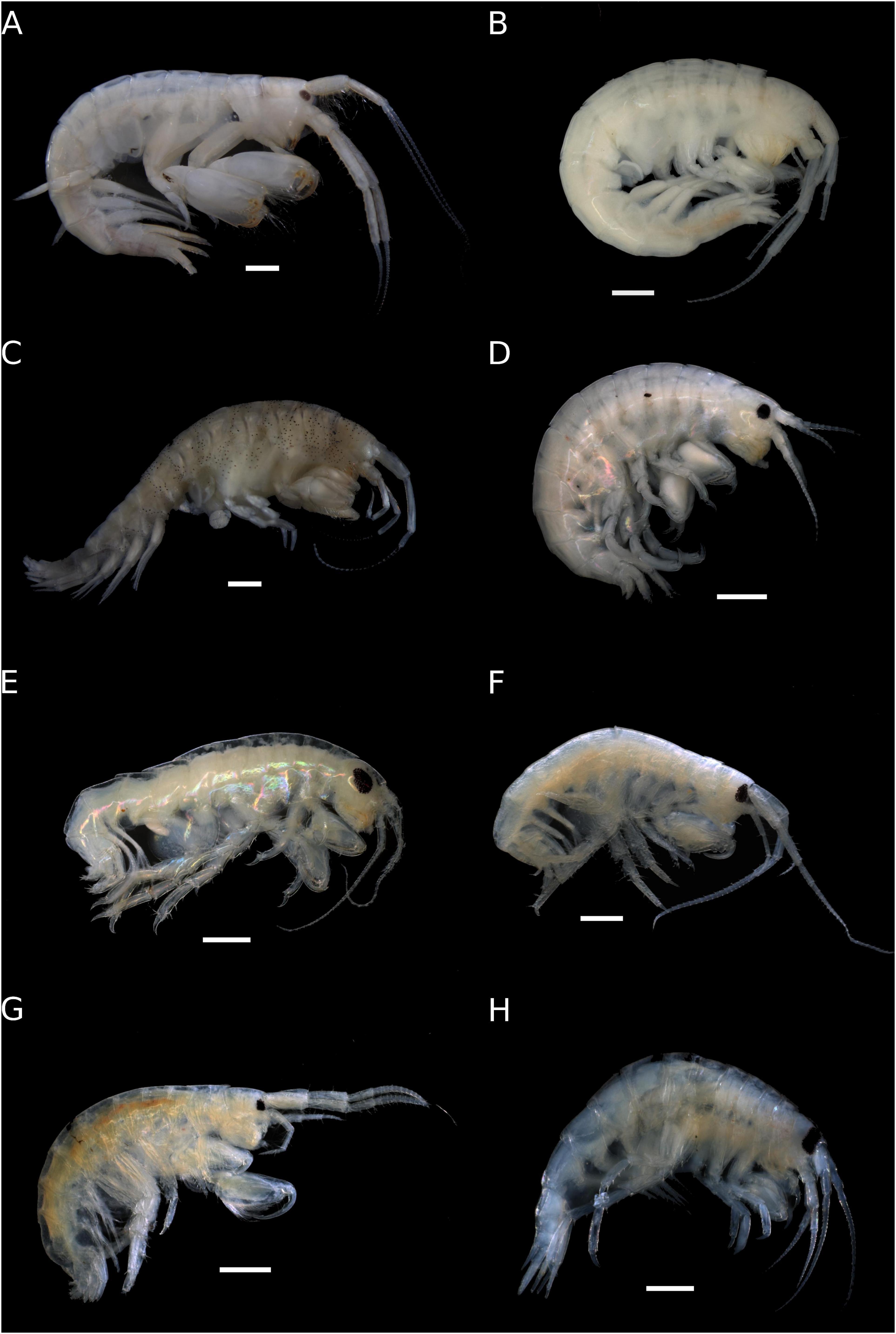
Figure 2. Amphipoda from Trindade Island. (A) Ampithoe marcuzzii male (ZUEC-CRU-4354). (B) Ampithoe marcuzzii female (ZUEC-CRU-4355). (C) Cymadusa trindadensis (ZUEC-CRU-4359). (D) Hyale niger (ZUEC-CRU-4360). (E) Protohyale macrodactyla (ZUEC-CRU-4362). (F) Elasmopus gabrieli sp. nov. (ZUEC-CRU-5364) (G) Elasmopus viracochai (ZUEC-CRU-4367). (H) Eusiroides lucai sp. nov. (ZUEC-CRU-4368). Scale bar: 1 mm.
Order Amphipoda Latreille, 1816
Suborder Senticaudata Lowry and Myers, 2013
Family Ampithoidae Boeck, 1871
Genus Ampithoe Leach, 1814
Ampithoe marcuzzii Ruffo, 1954
Ampithoe marcuzzii Ruffo, 1954: 120, figs I–II.—Barnard, 1958: 25.—Ortíz et al., 2007: 484.—Siqueira, 2012: 28, Anexo 1B.—Martín et al., 2013: 1705.—Paz-Ríos et al., 2013b: 9, fig. 9.—Campos et al., 2020: 2, figs. 1–3.
Ampithoe cf. marcuzzii—LeCroy, 2002: 245, fig. 262.
Material examined: Three individuals (not sexed), Piscinas do Parcel (20°31′10.812″S, 29°19′25.8″W), from Dictyota sp., September 2018, T. P. Macedo col. (ZUEC-CRU-4354); three individuals (not sexed), Ilha do Sul (20°31′27.5″S, 29°19′25.2″W), from Dictyota sp., April 2019, I. Batistela col. (ZUEC-CRU-4354).
Remarks: Ampithoe marcuzzii was described from Los Roques, Venezuela, and its current distribution ranges the western Atlantic Ocean, from Florida to state of São Paulo (SP), southeastern Brazil (Serejo and Siqueira, 2018; Campos et al., 2020). Specimens from TR are larger (up to 14 mm) than in the original description by Ruffo (1954), as observed by Siqueira (2012) in SP.
Ampithoe suapensis Correia et al., 2016
Ampithoe suapensis Correia et al., 2016: 196, figs 1–4.
Material examined: Five individuals (not sexed), Piscinas do Parcel (20°31′10.812″S, 29°19′25.8″W), from Dictyota sp., September 2018, T. P. Macedo col. (ZUEC-CRU-4356).
Remarks: Ampithoe suapensis was previously known only from its type locality in Suape Beach, state of Pernambuco, Brazil. The TR specimens agree well with the original description of A. suapensis.
Ampithoe thaix Siqueira and Iwasa-Arai sp. nov.
http://zoobank.org/F4432581-ECA0-49EE-90C0-3DE39566F7E1
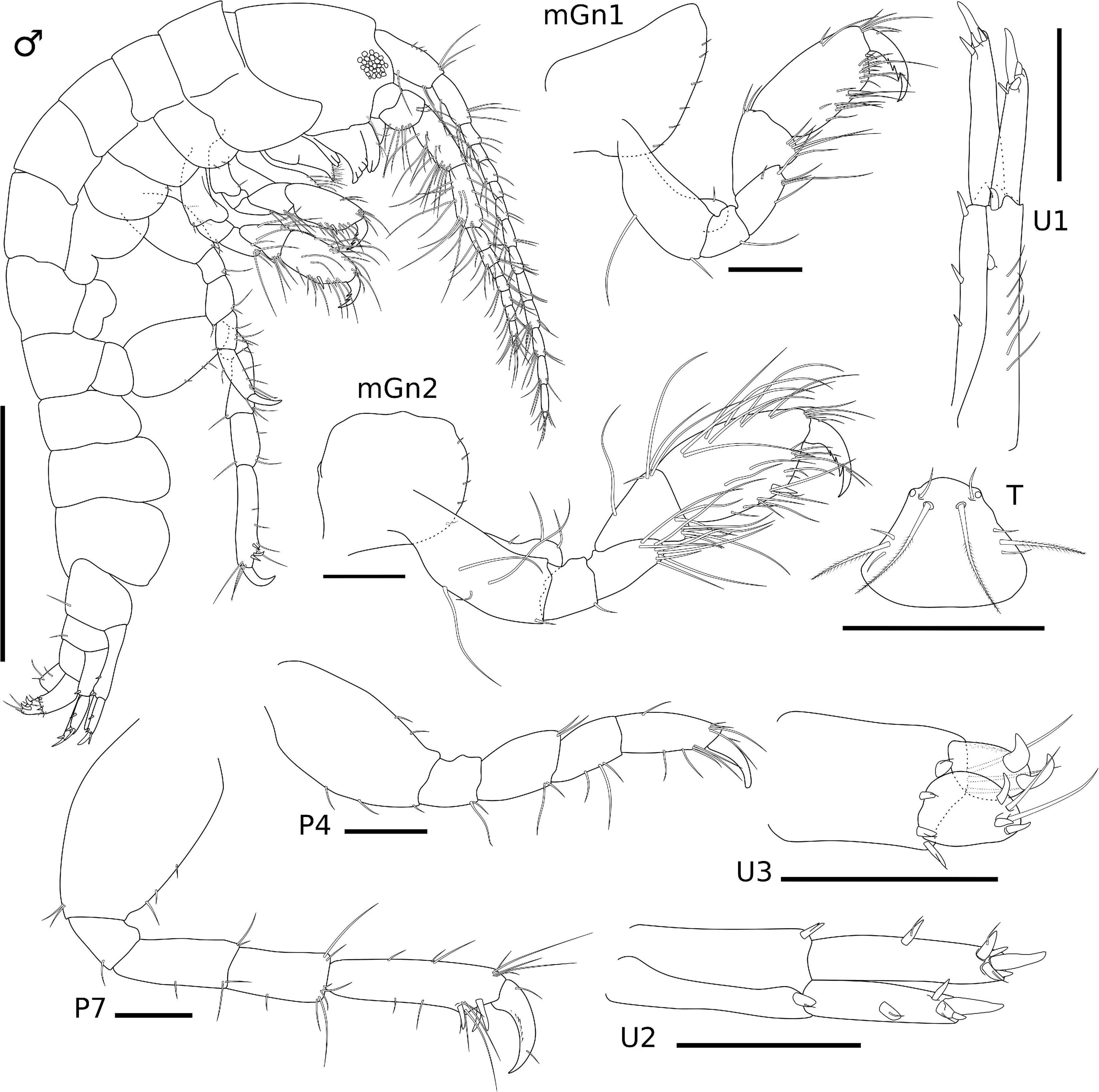
Figure 3. Ampithoe thaix Siqueira and Iwasa-Arai sp. nov. Male (ZUEC-CRU- 4357). Scale bar: 1 mm. Gnathopods, pereopods, uropods, and telson. Scale bar: 100 μm.
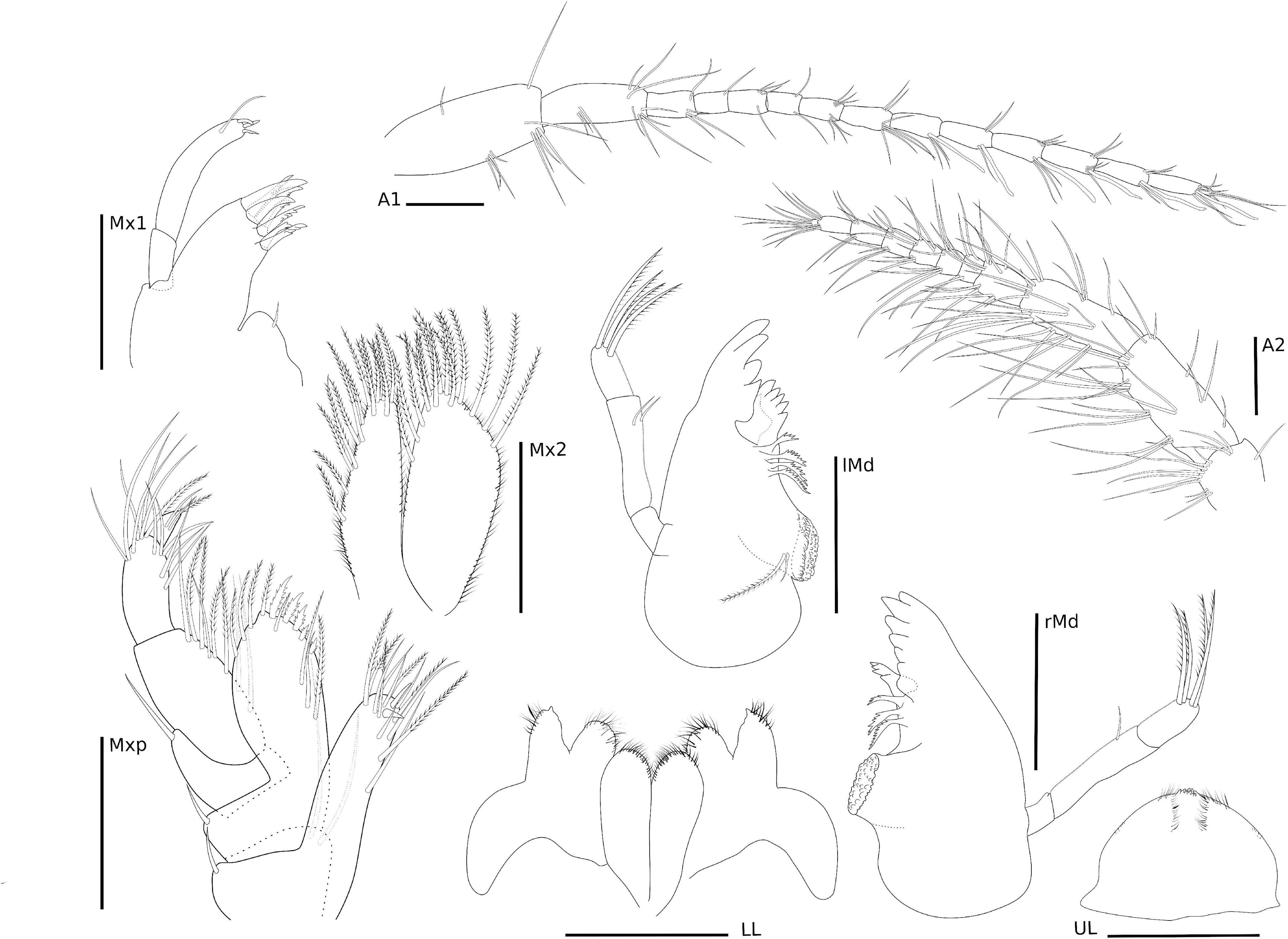
Figure 4. Ampithoe thaix Siqueira and Iwasa-Arai sp. nov. Antennae and mouth parts. Scale bar: 100 μm.
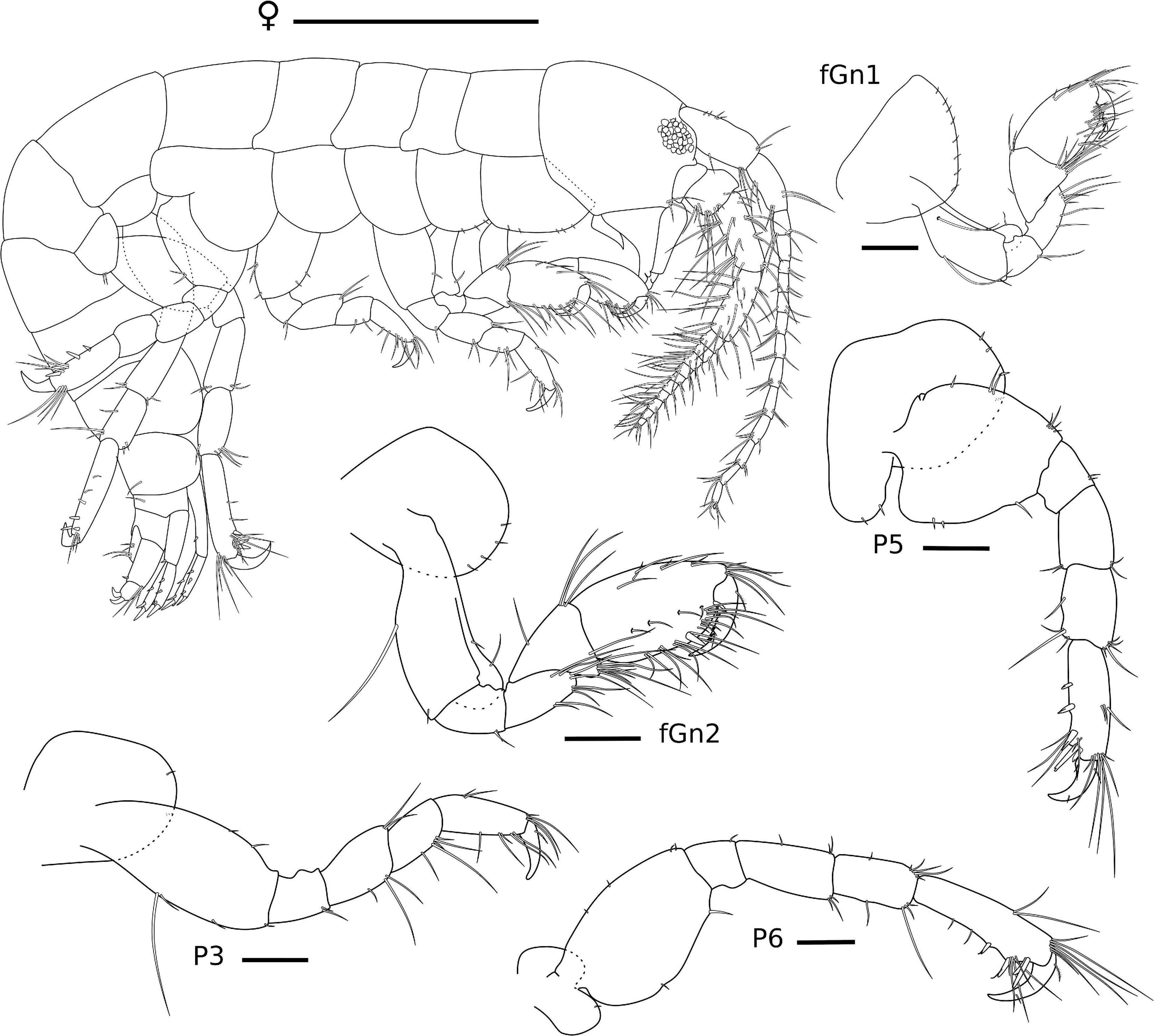
Figure 5. Ampithoe thaix Siqueira and Iwasa-Arai sp. nov. Female (ZUEC-CRU-4358). Scale bar: 1 mm. Gnathopods and pereopods. Scale bar: 100 μm.
Material examined: Holotype: male, Piscinas do Parcel (20°31′10.812″S, 29°19′25.8″W), from Dictyota sp., September 2018, T. P. Macedo col. (ZUEC-CRU-4357).
Paratype: one female, same as holotype. (ZUEC-CRU-4358).
Diagnosis: A2 heavily setose. Md palp article 2 2 × longer than article 3, with one or two setae on the margin. Mx1, inner plate with one seta. Gn1 slightly shorter than Gn2, Gn1 coxa produced anteriorly; propodus subrectangular and setose, palm transverse. Gn2, coxa oval; carpus with long setae on the anterior margin, propodus subrectangular, palm transverse with one robust seta. P5–7 propodus with 3–5 robust setae posteriorly. U1 with round spur apical margin of the peduncle, inner and outer rami lacking anterior robust setae. T subtriangular.
Etymology: This species is named after the marine biologist Thais Peixoto Macedo.
Description: Male (ZUEC-CRU-4357). Body (Figure 3). Length 4.7 mm.
Eyes rounded and well developed. A1 moderately setose; flagellum 13-articulate. A2 heavily setose; flagellum 8-articulate. UL rounded. LL notched, inner and outer lobes setose apically. rMd, incisor and lacinia mobilis seven- and four-toothed, respectively, accessory setal row with three serrated setae; palp 3-articulate, article 1 3.2 × shorter than article 2; article 2 2 × longer than article 3; article 3 1.5 × longer than article 1; article 3 with four long plumose setae apically. lMd, molar with one long plumose seta and one short seta; incisor and lacinia mobilis four- and five-toothed, respectively, accessory setal row with four serrated setae; palp 3-articulate, article 3 with four long plumose setae apically, similar to right palp. Mx1, inner plate small and rounded, inner margin with one single short seta; outer plate with seven stout serrated setae apically; palp 2-articulate with three stout setae and one long seta. Mx2, inner plate as long as outer plate, with marginal row of short setae, seven long plumose setae on the inner margin and six long plumose apical setae; outer plate, row of short setae on the inner and outer margins, long plumose setae on the apical and inner margin. Mxp, inner plate about 3 × longer than wide, inner margin and apex with long setae and plumose long setae, apical margin with two robust setae; outer plate, 1.8 × longer than wide, inner margin with three long plumose setae and five serrate robust setae, outer and apical margins with row of long plumose setae; palp 4-articulate, article 1, subtriangular, outer margin with one long setae, article 2 subrectangular, inner margin with row of long plumose setae, article 3 subrectangular inner margin with six long setae, outer margin with five long setae, article 4 with two long setae and one plumose seta (Figure 4).
Gn1, slightly smaller than Gn2; coxa subtriangular, enlarged anteriorly, ventral margin bearing row of short setae; basis 2.3 × longer than wide, anterodistal margin with one short seta, posterior margin with two setae; ischium subrectangular, 1.1 × longer than wide, posterior margin with one long seta; merus 1.5 × longer than wide, posterior margin with five long setae; carpus 1.1 × longer than wide, anterodistal margin with two setae, posterior margin setose; propodus subrectangular, 1.8 × longer than wide, anterior margin with two setae, anterodistal angle with four setae, posterior margin setose, posterodistal margin with one robust seta, palm transverse; dactylus 1.5 × longer than palm, serrated, with two setae. Gn2, coxa oval, ventral margin bearing row of short setae; basis 1.8 × longer than wide, anterior margin with three setae, posterior margin with three setae, inner face with two long setae; ischium small and subquadrate, as long as wide, posterior margin with one short seta; merus, 1.8 × longer than wide, posterior margin setose; carpus, 1.3 × longer than wide, anterior margin with one short and four long setae, posterior margin setose; propodus subrectangular, 1.8 × longer than wide, anterior and posterior margins setose; palm transverse, with one robust seta delimiting palm; dactylus 1.5 × longer than palm, with two setae (Figure 3).
P3 not recovered (described from female). P4, coxa not recovered, basis subrectangular, 1.8 × longer than wide, anterior margin with two setae, posterior margin with two setae; ischium small and subrectangular, 1.2 × longer than wide, posterior margin with two long setae; merus subrectangular, 1.4 × longer than wide, anterior margin with two setae, posterior margin with two setae; carpus subrectangular, 1.5 × longer than wide, posterior margin with three setae; propodus subrectangular, 2.5 × longer than wide, anterior margin with five setae, posterior margin with five setae; dactylus slightly curved, with one short seta. P5 not recovered (described from female). P6 not recovered (described from female). P7, coxa not recovered, basis 1.5 × longer than wide, anterior margin with three setae, posterior margin with two setae; ischium small and subrectangular, 1.2 × longer than wide, posterior margin with one seta; merus subrectangular, 2.2 × longer than wide, anterior and posterior margins with setae; carpus subrectangular, 2.1 × longer than wide, anterior margin with two setae, posterior margin with six setae; propodus elongate, 3.8 × longer than wide, anterior margin with eight long setae, posterior margin with three robust setae and six setae; dactylus slightly curved, with two short setae (Figure 3).
U1, peduncle 3.5 × longer than wide, with distoventral rounded spur, inner margin with three robust setae, outer margin with two robust setae and a row of long setae; inner ramus 1.2 × longer than outer ramus, apical margin with four robust setae; outer ramus, apical margin with four robust setae. U2, peduncle 2.1 × longer than wide, inner and outer margins with one robust setae each; inner ramus 1.2 × longer than outer ramus, inner margin with one robust seta, apical margin with five robust setae; outer ramus, outer margin with one robust seta, apical margin with four robust setae. U3, peduncle slightly elongate, about 1.6 × longer than wide, with three long setae and four robust setae; inner ramus slightly longer than outer ramus, apical margin with two long setae and three robust setae; outer ramus, apical margin with two curved robust setae. T subtriangular, lateral margins with one long plumose and two short setae, distolateral angles with one cusp and one short seta each (Figure 4).
Female (ZUEC-CRU-4758). Body (Figure 5). Length 4.8 mm.
Gn1, slightly smaller than Gn2; coxa subtriangular, enlarged anteriorly; basis 2.6 × longer than wide, anterior and posterior margins with one long and one short setae each; ischium small and subrectangular, 1.3 × longer than wide, posterodistal margin with two setae; merus 1.8 × longer than wide, posterior margin setose; carpus 1.8 × longer than wide, anterior margin with three setae, posterior margin setose; propodus subrectangular, 1.6 × longer than wide, anterodistal and posterior margin setose, palm slightly convex, lacking robust seta; dactylus 3 × longer than palm, serrated, with one plumose seta. Gn2, coxa oval, ventral margin with row of short setae; basis 2.4 × longer than wide, anterior margin with two short setae, posterior margin with one short seta; ischium small and subrectangular, 1.4 × longer than wide; merus 1.7 × longer than wide, posterodistal margin setose; carpus triangular, 1.3 × wider than long, anterior margin with few setae, posterior margin setose; propodus 1.7 × longer than wide, anterior and posterior margins setose; palm slightly convex, with one robust seta delimiting palm; dactylus 2.2 × longer than palm, with one plumose seta (Figure 5).
P3, coxa oval, with one short seta; basis 2.2 × longer than wide, anterior margin with two setae, posterior margin with one long and three short setae; ischium subrectangular, 1.2 × longer than wide, posterior margin with two setae; merus subrectangular, 1.3 × longer than wide, anterior margin with two setae, posterior margin with three setae; carpus subrectangular, 1.8 × longer than wide, posterior margin with five setae; propodus subrectangular, 1.5 × longer than wide, anterior margin with six setae, posterior margin with five setae; dactylus slightly curved, with one short seta. P5, coxa, ventral margin rounded with three short setae, lobe with two short setae; basis 1.2 × longer than wide, anterior margin with seven setae, posterior margin with three short setae; ischium subrectangular, 1.3 × wider than long, anterior margin with two short setae; merus subrectangular, 1.7 × longer than wide, anterior and posterior margins with two setae each; carpus subrectangular, 1.6 × longer than wide, anterior margin with a tuft of apical setae, posterior margin with four setae; propodus elongate, 3 × longer than wide, anterior margin with three setae and a tuft of apical setae, posterior margin with five robust and five simple setae; dactylus slightly curved, with two short setae. P6, coxa, ventral margin rounded and naked, lobe with one short setae; basis 1.5 × longer than wide, anterior margin with four short setae, posterior margin with two short setae; ischium subrectangular, as wide as long, anterior margin with one short seta; merus subrectangular, 2 × longer than wide, anterior and posterior margins with three setae each; carpus subrectangular, 1.8 × longer than wide, anterior margin with a tuft of apical setae, posterior margin with four setae; propodus elongate, 3.4 × longer than wide, anterior margin with two long setae and a tuft of apical setae, posterior margin with four robust and seven setae; dactylus slightly curved, with two short setae (Figure 5).
Type locality: Trindade Island, Espírito Santo state, Brazil.
Distribution: Currently known only from the type locality.
Remarks: The genus Ampithoe is the most speciose among Ampithoidae (Sotka et al., 2017), with 78 species described worldwide (Horton et al., 2020). Six species of Ampithoe were previously reported from the Brazilian coast: Ampithoe divisura Shoemaker, 1938, Ampithoe marcuzzii Ruffo, 1954, Ampithoe ramondi Audouin, 1826, Ampithoe robustimana Andrade and Senna, 2017, Ampithoe seticoxae Serejo and Licínio, 2002, and Ampithoe suapensis Correia et al., 2016 (Serejo and Siqueira, 2018). Among these species, Ampithoe thaix sp. nov. is morphologically closer to A. divisura and A. suapensis by presenting a round projection on the distal margin of the uropod 1 peduncle. Although the validity of A. divisura was previously questioned (LeCroy et al., 2009), Ampithoe thaix sp. nov. can be easily differentiated from the A. divisura specimens from the Brazilian coast identified by Serejo and Licínio (2002) by lacking a digitiform process on gnathopod 2 palm and an anterodistal propodus projection, as well as by presenting robust setae on propodus of pereopods 5–7 (vs. striated in A. divisura). Ampithoe thaix sp. nov. can also be differentiated from A. suapensis based on characters from mandible, gnathopods, uropods, and telson. The mandible palp of Ampithoe thaix sp. nov. is large and robust, with four plumose setae on article 3, while A. suapensis presents a short and slender mandible palp with three setae on the apex. The propodus of gnathopod in A. thaix sp. nov. is subrectangular and setose on both dorsal and ventral margins, and the palm is defined by a robust seta, while the propodus of gnathopod 1 is oval and setose only on the palmar margin, which is not defined by a robust seta. The uropods of A. thaix sp. nov. have asymmetric rami without setae on the inner margins, different from A. suapensis, where asymmetry is only observed on uropod 2, and both uropods 1 and 2 of A. suapensis have setae on the inner margins of the rami. Finally, A. thaix sp. nov. and A. suapensis can be distinguished by the telson shape, which is subtriangular in A. thaix sp. nov. and trapezoidal in A. suapensis. Ampithoe thaix sp. nov. is the third Ampithoe species reported from Brazilian oceanic islands.
Genus Cymadusa Savigny, 1816
Cymadusa trindadensis Andrade and Senna, 2017
Cymadusa trindadensis Andrade and Senna, 2017: 378, figs 12–15.
Material examined: Four females, Piscinas do Parcel (20°31′10.812″S, 29°19′25.8″W), from Dictyota sp., September 2018, T. P. Macedo col. (ZUEC-CRU-4359).
Remarks: Cymadusa trindadensis is so far endemic to TR. Type specimens were collected over 40 years ago and lacked complete antenna. Specimens found herein differ from the original description in the number of articles in the antenna 1 flagellum, which is bi-articulate in C. trindadensis types and uni-articulate in the examined specimens. The number of robust and short setae on uropods 1 and 3 also varies, with the present specimens presenting uropod 1 with marginal robust setae on both rami, while in types, it is present only on the inner margin of the outer ramus, and uropod 3 bearing four robust setae and nine long setae on the apical margin of the inner ramus, whereas types have only four setae, as well as one short robust seta on the distolateral margin, which is not present in the original description. The outer margin has four marginal setae instead of one, as described by Andrade and Senna (2017).
Family Hyalidae BulyĊeva, 1957
Genus Hyale Rathke, 1836
Hyale niger (Haswell, 1879)
Allorchestes niger Haswell, 1879: 319.—1885: 95, pl. 11 figs 1–3.
Hyale nigra.—Stebbing, 1906: 571.—Schellenberg, 1928: 659, fig. 204.—Ledoyer, 1972: 273.—1979: 137, fig. 89.—1986: 1002, fig. 397.—J. L. Barnard, 1974: 66.—Serejo, 1999: 600, figs 5–7.—Leite, 2011: 176, fig. 3.14B.—Leite et al., 2011: 328.
Hyale niger.—Lowry and Stoddart, 2003: 129.
Material examined: Sixteen individuals (not sexed), Piscinas do Parcel (20°31′10.812″S, 29°19′25.8″W), from Dictyota sp., September 2018, T. P. Macedo col. (ZUEC-CRU-4360). One individual (not sexed), Ilha do Sul (20°31′27.5″S, 29°19′25.2″W), from Dictyota sp., April 2019, I. Batistela col. (ZUEC-CRU-4361).
Remarks: Hyale niger is a species with disjunct distribution, described for southeastern Australia and later found in Brazil and Madagascar (Serejo, 1999). Future work encompassing its broad distribution, detailed morphology, and molecular data may elucidate the species status. The specimens from TR agree well with the specimens found in the southeastern Brazilian continental coast, where the species is usually observed especially in Sargassum spp. from São Paulo state (Leite, 2011; Leite et al., 2011).
Protohyale Bousfield and Hendrycks, 2002
Protohyale macrodactyla Stebbing, 1899
Hyale macrodactylus Stebbing, 1899: 404, pl. 31d.
Hyale macrodactyla.—Stebbing, 1906: 565, fig. 96.—Oliveira, 1953: 339. Ledoyer, 1972: 273, pl. 77A.—1986: 1001, fig. 396.—Serejo, 1999: 592, figs 1, 2.—Leite, 2011.—Leite et al., 2011.
Protohyale (Protohyale) macrodactyla.—Bousfield and Hendrycks, 2002: 79.
Protohyale macrodactyla.—LeCroy et al., 2009: 959.—Paz-Ríos et al., 2013a: 4, fig. 3.
Material examined: Seven individuals (not sexed), Piscinas do Parcel (20°31′10.812″S, 29°19′25.8″W), from Dictyota sp., September 2018, T. P. Macedo col. (ZUEC-CRU-4362); 18 individuals (not sexed), Ilha do Sul (20°31′27.5″S, 29°19′25.2″W), from Dictyota sp., April 2019, I. Batistela col. (ZUEC-CRU-4363).
Remarks: Protohyale macrodactyla is a common species found in the Atlantic Ocean. The species was originally described from the Caribbean and later found along the Brazilian coast (Stebbing, 1899; Serejo, 1999; Leite, 2011; Leite et al., 2011). The TR material agrees well with the specimens found on the Brazilian continental coast.
Family Maeridae Krapp-Schickel, 2008
Genus Elasmopus Costa, 1853
Elasmopus gabrieli Siqueira and Iwasa-Arai sp. nov.
http://zoobank.org/9AA8C7EB-56BD-4071-82E3-49045225BF82
(Figures 2F, 6–8)
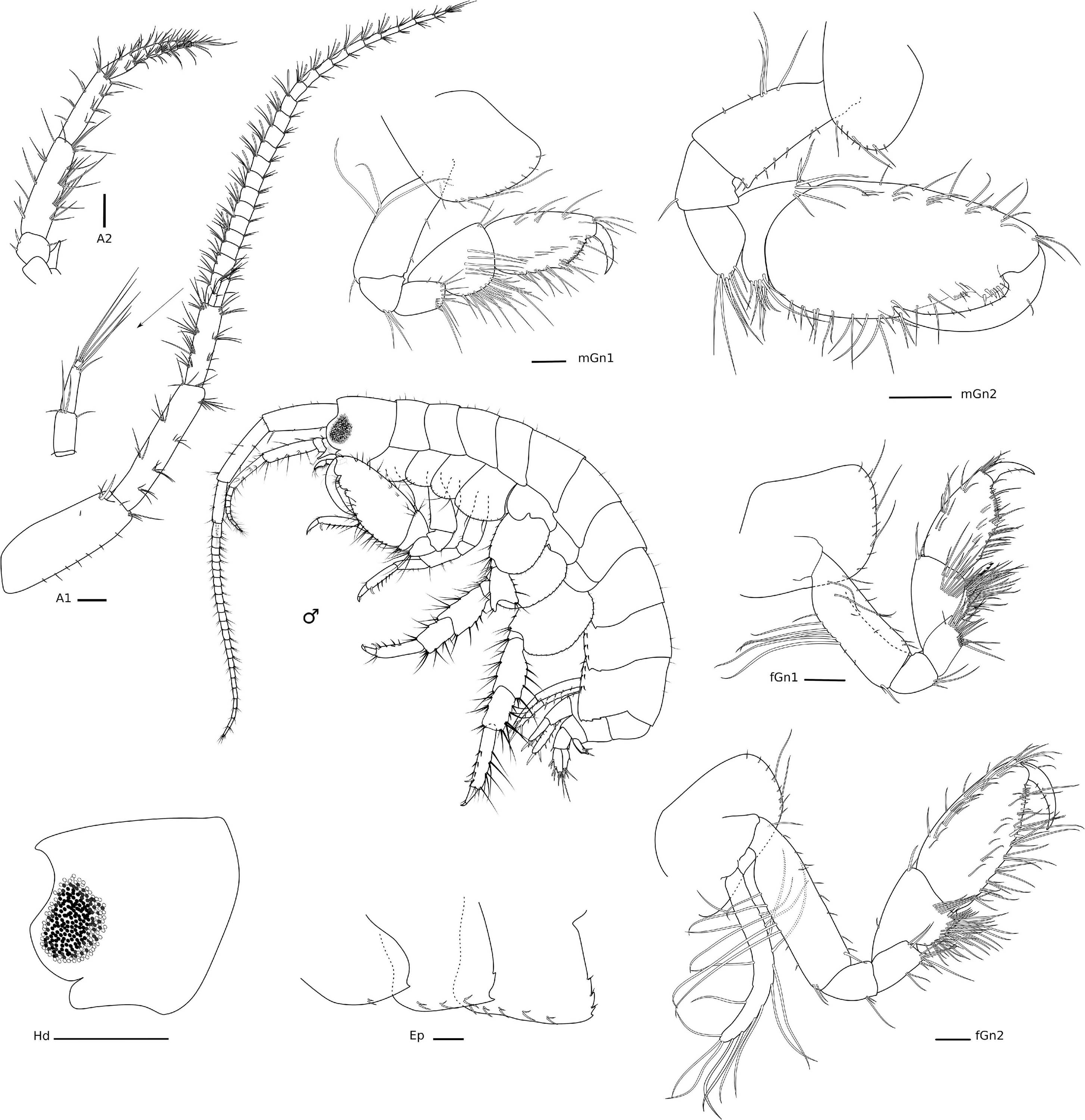
Figure 6. Elasmopus gabrieli Siqueira and Iwasa-Arai sp. nov. Male (ZUEC-CRU-5364). Head and gnathopods. Scale bar: 0.5 mm. Antennae and epimeral plates. Scale bar: 100 μm.
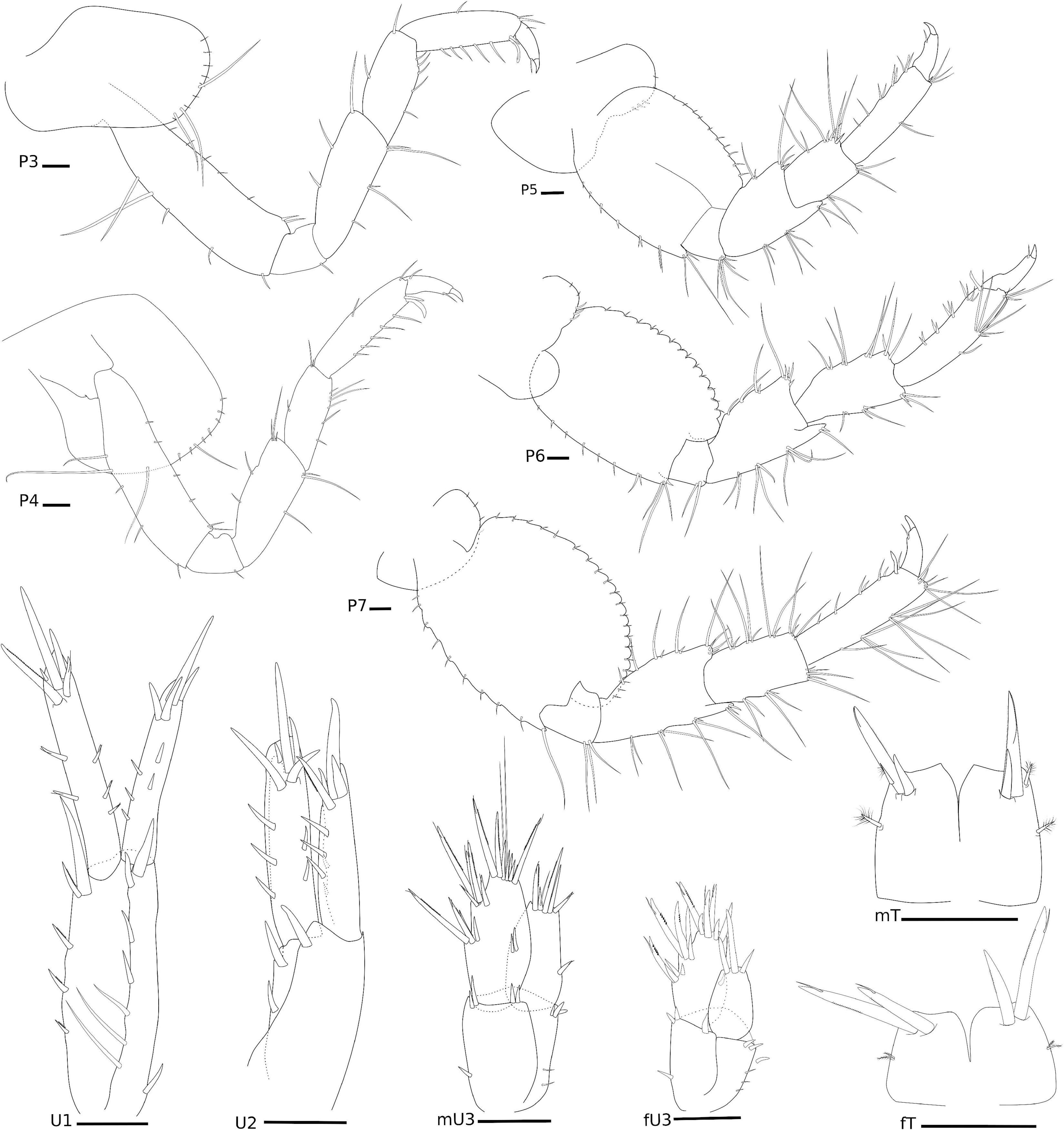
Figure 8. Elasmopus gabrieli Siqueira and Iwasa-Arai sp. nov. Pereopods, uropods, and telson. Scale bar: 100 μm.
Material examined: Holotype: male, Piscinas do Parcel (20°31′10.812″S, 29°19′25.8″W), from Dictyota sp., September 2018, T. P. Macedo col. (ZUEC-CRU-5364).
Paratypes: one male, two females, two juveniles, same as holotype (ZUEC-CRU-5365). Two females, one juvenile, same as holotype (ZUEC-CRU-5366).
Diagnosis: A1 1/2 of body length, with accessory flagellum 3-articulate. A2 short, 1/3 the length of A1. Md palp article 3 falcate, with plumose setae along the margin, margin concave with three long setae on the apex. Mxp palp with 4-articulate with terminal unguis. Gn1 propodus palm serrated. Gn2 propodus oval, with triangular process delimiting palm, with one subquadrate and one subrectangular processes close to the dactylus articulation. Pereopods 3–7 propodus with row of robust setae on the posterior margin and one locking seta distally. Pereopods 5–7 basis slightly serrated and convex. Epimeral plate 3 with the posterior margin slightly serrated with four notches. U3 with several long plumose robust setae. T cleft (approximately 1/2 on its length), lobes apex slightly concave with one long robust plumose seta and one short robust seta.
Etymology: This species is named after the son of SGLS, Gabriel Siqueira.
Description: Male (ZUEC-CRU-5364). Body (Figure 6). Length 8.5 mm.
Head with eyes rounded and well developed (Figure 6), lateral cephalic lobes rounded, with anteroventral notch. A1 longer than A2, about 0.5 × body length, peduncular article 1 with one robust seta and three setae on the distolateral margin and row of short setae laterally; peduncular articles 2 and 3 with tufts of setae on the lateral and distal margins; accessory flagellum 3-articulate, shorter than the first proximal article of the flagellum, with three long setae on the apex; flagellum 23-articulate and 1.1 × longer than peduncular articles 1–3 combined, with aesthetascs present from articles 3–19. A2 stout, 3 × shorter than A1; peduncular articles setose; flagellum 8-articulate, 2.3 × shorter than peduncular articles 3–5 combined, proximal article longest, setae present in all articles. UL rounded, setose apically. LL notched, inner and outer lobes setose apically, outer lobe with one small projection on the inner margin. rMd, molar with one long plumose seta; incisor with three teeth and medial part of the plate without evident teeth, lacinia mobilis seven-toothed; accessory setal row with three serrated setae. lMd, molar with one long plumose seta; incisor and lacinia mobilis two- and five-toothed, respectively; accessory setal row with four serrated setae; palp 3-articulate, article 1 short, 1.5 × longer than wide, article 2 elongate, 2 × longer than wide, article 3 falcate, similar in size to article 2, with three long setae apically and a row of plumose setae on the inner margin, with 21 setae in total, 18 of them plumose and three simple setae. Mx1, inner plate small and subtriangular, inner margin with row of thin setae, apex with two long plumose setae; outer plate with seven stout serrated setae apically; palp 2-articulate, article 2 with long setae apically and on the inner margin. Mx2, inner plate as long as the outer plate, with marginal row of short setae on the inner margin, three long plumose setae on the inner margin and several long apical setae; outer plate, row of long setae apically, with two long plumose setae on the inner margin. Mxp, inner plate about 2.4 × longer than wide, inner margin and apex with plumose long setae; outer plate, 1.8 × longer than wide, inner margin with four long plumose setae and seven serrated robust setae; palp 4-articulate, article 1, subtriangular, naked, article 2 subrectangular, inner margin with row of long setae, article 3 subrectangular inner margin with long setae, outer margin with five long setae, thin setae apically, article 4 with three setae, unguis with one short seta, falcate (Figure 7).
Gn1 subchelate, smaller than Gn2; coxa subtriangular, enlarged anteriorly, ventral margin bearing row of short and long setae; basis 2.5 × longer than wide, anterior margin with two short setae, posterior margin with two long setae, posterodistal margin with one seta; ischium subtriangular, as long as wide, posterior margin with a tuft of setae; merus subrectangular, about 1.5 × longer than wide, distal margin setose; carpus 1.6 × longer than wide, anterodistal margin with two setae, posterior margin heavily setose; propodus subrectangular, 1.8 × longer than wide, anterior margin with three pairs of short setae and one long seta each, anterodistal angle with a tuft of setae, posterior margin setose with a row of setae, palm transverse, serrated, with long and short setae along its margin and delimited by a robust seta; dactylus falcate, with two short setae. Gn2 subchelate, coxa oval, ventral margin bearing row of short setae and three long setae; basis 2.1 × longer than wide, anterior margin with row of setae, posterior margin with three long and two short setae; ischium small and subquadrate, 1.2 × longer than wide, posterodistal margin with one seta; merus subrectangular, 1.6 × longer than wide, posterodistal margin setose; carpus subtriangular, 1.3 × wider than long, anterodistal margin with one robust and five long setae, posterior margin setose with a row of plumose setae and one long seta distally; propodus elongate, 2.1 × longer than wide, anterior margin with row of short setae, anterodistal angle with a tuft of setae, posterior margin setose, with one triangular process delimiting palm and one distal subquadrate and one proximal subrectangular processes with four robust setae distally, palm slightly concave; dactylus falcate, fitting palm, posterior margin with tiny setae, with one single short seta on the anterior margin, and apically blunt (Figure 6).
P3, coxa oval, 1.8 × longer than wide, anterior margin two long setae; basis subrectangular, 3.5 × longer than wide, anterodistal margin with two short setae, posterior margin with three short setae; ischium small and subrectangular, as long as wide, posterior margin with one short seta; merus subrectangular, 2.6 × longer than wide, anterior margin with three setae, posterior margin with five setae; carpus subrectangular, 2.1 × longer than wide, anterior margin with two setae, posterior margin with five setae; propodus subrectangular, 3.4 × longer than wide, anterior margin with one seta, posterior margin with five and one posterodistal locking setae; dactylus stout, with one distal short seta. P4, coxa oval, ventrally produced, 1.3 × longer than wide, anterior margin with row of short setae and one long seta, ventral margin with long seta; basis subrectangular, 3.5 × longer than wide, anterior margin with sparse short setae, anterodistal margin with two short setae, posterior margin with four setae; ischium small and subrectangular, 1.1 × longer than wide, with one short setae posteriorly; merus subrectangular, 2.4 × longer than wide, anterior margin with five setae, posterior margin with three setae; carpus subrectangular, 2.5 × longer than wide, anterior margin with three setae, posterior margin with six setae; propodus subrectangular, 3.7 × longer than wide, anterior margin with two setae, posterior margin with five and one robust setae, and one posterodistal locking seta; dactylus stout, with two short setae. P5, coxa bilobed, posterior lobe narrower, with three short robust setae; basis oval, 1.3 × longer than wide, anterior margin with a row of short setae, posterior margin convex and slightly serrated; ischium subquadrate, as wide as long, posterodistal margin with tuft of setae; merus subrectangular, 1.3 × longer than wide, anterior margin with five setae and one short robust seta, posterior margin with two tufts of setae, posterodistal margin produced with three setae; carpus subrectangular, 1.5 × longer than wide, anterior margin with long setae, anterodistal margin with one short robust seta, posterior margin with seven setae; propodus subrectangular, 3.5 × longer than wide, anterior margin with two setae, anterodistal margin with three setae and one short robust seta, posterior margin with two single and three paired setae; dactylus stout, with one short seta. P6, coxa bilobed, posterior lobe narrower, with three short robust setae and two short setae; basis oval, 1.3 × longer than wide, anterior and posterior margins with a row of short setae, posterior margin convex and casteloserrated; ischium small and subquadrate, 1.1 × wider than long, anterodistal margin with two setae; merus subtriangular, 1.6 × longer than wide, anterior margin with tufts of setae, anterodistal margin produced, with two setae, posterior margin with two long and two short setae, posterodistal margin with four short setae; carpus subtriangular, 1.9 × longer than wide, anterior and posterior margins with tufts of setae; propodus subrectangular, 4.2 × longer than wide, anterior margin with four setae, posterior margin with four paired robust setae, and one posterodistal locking seta; dactylus stout, with one short seta. P7, coxa naked; basis oval, 1.1 × longer than wide, anterior and posterior margins with a row of short setae, posterior margin convex and slightly casteloserrated; ischium small and subrectangular, 1.1 × longer than wide, posterodistal margin with three long setae; merus subrectangular, 1.7 × longer than wide, anterior margin with pairs of setae, anterodistal margin with tuft of setae, posterior margin with three pairs of setae, posterodistal margin produced; carpus subrectangular, 1.8 × longer than wide, anterior margin with three tufts of three setae each, anterodistal margin with four setae, posterior margin with 10 setae; propodus subrectangular, 3.5 × longer than wide, anterior margin with tufts of long setae, posterior margin with seven setae and one locking setae distally; dactylus stout, with one seta (Figure 8).
Ep, epimeron 1 with one oblique ledge on surface, with two setae on the ventral margin, with one small posterior projection; epimeron 2 flattened ventrally, with three pairs of setae on the ventral margin, posterior margin with two small projections; epimeron 3 largest, slightly serrated, flattened ventrally, with five pairs of setae on the ventral margin, posterior margin with four projections, three of them with one seta (Figure 6).
U1, peduncle 2.7 × longer than wide, inner margin with four short pinnate robust setae and one long robust seta apically, outer margin with four robust setae and three long setae, one robust seta posteriorly; inner ramus 1.4 × longer than outer ramus, inner margin with two robust setae, outer margin with three robust setae, apical margin with five robust setae; outer ramus, inner margin with three robust seta, outer margin with two robust setae, apical margin with four robust setae. U2, peduncle 2 × longer than wide, inner margin with two robust setae, outer margin with one robust seta; inner ramus 1.3 × longer than outer ramus, inner margin with three robust setae, outer margin with two robust setae, apical margin with five robust setae; outer ramus, inner margin with two robust setae, outer margin with three robust setae, apical margin with four robust setae, one of them pinnate. U3, peduncle subrectangular, about 1.5 × longer than wide, inner margin with one robust seta, outer margin with two setae, distal margin with seven robust setae; outer ramus longer than inner ramus, outer margin with nine robust setae, four of them pinnated, inner margin with two robust setae, apical margin with two setae and eight robust setae, three of them pinnated; inner ramus, outer margin with one robust seta, apical margin with five robust setae, two of them pinnated. T cleft (about 1/2 length), lateral margins with one pappose seta, posterolateral margins with two robust setae (one long and plumose, one short) and one pappose seta (Figure 8).
Female (ZUEC-CRU-4365). Length 7.9 mm.
Gn1 similar to male, subchelate, smaller than Gn2. Gn2 subchelate, coxa oval, ventral margin bearing row of short setae and two long setae; basis 3.1 × longer than wide, anterior margin with row of setae, posterior margin with two medium and two short setae; ischium small and subquadrate, 1.4 × longer than wide, posterodistal margin with one long and two short setae; merus subrectangular, 1.4 × longer than wide, posterodistal margin setose; carpus subtriangular, 1.3 × wider than long, anterodistal margin with two setae, posterior margin heavily setose, with a row of plumose and a row of simple long setae; propodus elongate, 2.2 × longer than wide, anterior margin with row of short seta, anterodistal angle with a tuft of setae, posterior margin setose, lacking processes, palm slightly concave, delimited by two robust setae; dactylus falcate, length similar to palm, with four short setae and apically blunt (Figure 6).
U3, peduncle subrectangular, about 1.5 × longer than wide, inner margin with one robust seta, outer margin with three setae, distal margin with six robust setae; outer ramus longer than inner ramus, inner margin with eight robust setae, three of them pinnated, outer margin with one robust seta, apical margin with six robust setae, one of them pinnated; inner ramus, margins smooth, apical margin with three robust setae. T cleft (about ½ length), lateral margins with two pappose setae, posterolateral margins with two robust setae (Figure 8).
Type locality: Trindade Island, Espírito Santo, Brazil.
Distribution: Currently known only from the type locality.
Remarks: Elasmopus besnardi was firstly described from TR based on a female specimen, and it was reported as nomen dubium by Ruffo (1959). It is currently considered a synonym of E. brasiliensis (Dana, 1853; Horton et al., 2020), with types of the E. besnardi being lost (Souza-Filho and Serejo, 2012) and the species never reported after its description. Elasmopus gabrieli sp. nov. female differs from the former E. besnardi in several characters, including accessory flagellum 3-articulate, shorter than first proximal article of flagellum (vs. accessory flagellum 2-articulate, larger than first proximal article of flagellum), articles 2 and 3 of mandibular palp similar in length (vs. article 3 larger than 2), palm of gnathopods delimited by robust setae and length dactylus similar to palm (vs. not delimited palm and length dactylus shorter to palm); inner ramus of uropod 3 without robust setae on the inner margins (vs. inner margins with robust setae) and telson cleft about ½ length (vs. telson totally cleft). Alves et al. (2016) observed that the species of Elasmopus have some morphological patterns and therefore suggested the species separation into four groups, in which E. gabrieli sp. nov. can be placed in group 3, together with E. longipropodus Senna and Souza-Filho, 2011, E. rapax Costa, 1853, E. thalyae Gouillieux and Sorbe, 2015, E. thomasi Ortiz and Lalana, 1994, and E. yupanquii Alves et al., 2016, by having three palmar processes on gnathopod 1 of the male. Within the group, E. gabrieli sp. nov. is more related to E. longipropodus from Brazil and E. thalyae from France by sharing the following characters: antenna 1 accessory flagellum with three articles, pereopod 5 basis with convex posterior margin, epimeral plate 3 serrated, and uropod 3 inner ramus slightly shorter than the outer ramus. Elasmopus gabrieli sp. nov. differs from E. thalyae by having gnathopod 1 palm serrated (vs. smooth), three processes on gnathopod 2 palm, one proximal triangular, and one subquadrate and one subrectangular processes distally (vs. triangular, triangular and subquadrate, respectively), epimeron 1–3 with short setae on the ventral margin (vs. long and short setae), telson cleft 0.5 × length (vs. 0.84 × length), lobes apical margin slightly concave with undeveloped cusps (vs. lobes apical margins concave with well-developed cusps). Elasmopus gabrieli sp. nov. also differs from E. longipropodus by having an accessory flagellum shorter than the first proximal article of the flagellum of antenna 1 (vs. larger than the first proximal article of the flagellum), mandible palp articles 2 and 3 with the same length (vs. article 2 shorter than article 3), article 3 falcate with plumose setae along its concave margin and with three long setae on the apex (vs. four apical setae), gnathopod 2 propodus 2 × longer than wide (vs. 2.5 × longer than wide), palm defined by a triangular process, palm margin with a subquadrate and a subrectangular process distally (vs. a subacute process and a palmar corner defined by a strong acute triagular process), length dactylus similar to palm (vs. length dactylus shorter to palm), pereopods 3–7 propodus with one locking seta distally (without locking seta), uropod 3 with two setae on the inner margin of the outer ramus (vs. one seta), and telson with undeveloped cusps, with two robust seta, one long and plumose, and one short in each lobe (vs. with apicolateral cusps well developed, with three robust setae).
Krapp-Schikel and Ruffo (1990) grouped the species Elasmopus canarius Krapp-Schikel and Ruffo, 1990, E. pectenicrus (Bate, 1862), E. serricatus J. L. Barnard, 1969, E. spinibasus Sivaprakasam, 1970, E. crenulatus Berents, 1983, E. laufolii Myers, 1986, based on the presence of a very dense fringe of long setae on the posterior margin of gnathopod 2 of the male and by a casteloserrate posterior margin of the basis of pereopods 6 and 7. The herein described species, E. gabrieli sp. nov., shares the casteloserrate pattern on the basis of pereopods 6 and 7; however, it lacks a very dense fringe of long setae on gnathopod 2 of the male. Most of these species have an intertropical distribution in the Indo-Pacific region, and future cladistic biogeographic analysis may elucidate the evolution of these characters across the ocean basins.
Elasmopus viracochai Alves et al., 2016
Elasmopus viracochai Alves et al., 2016: 21, figs 17–22.
Material examined: One male, Ilha do Sul (20°31′27.5″S, 29°19′25.2″W), from Dictyota sp., April 2019, I. Batistela col. (ZUEC-CRU-4367).
Remarks: Elasmopus viracochai was so far known only from its type locality, in Ceará state, northeastern Brazil. Only one male specimen was found in TR samples, and it agrees well with the original description.
Family Pontogeneiidae Stebbing, 1906
Genus Eusiroides Stebbing, 1888
Eusiroides lucai Siqueira and Iwasa-Arai sp. nov.
http://zoobank.org/B1EE8F0A-A0DB-43DB-846B-66122E836110
(Figures 2H, 9, 10)
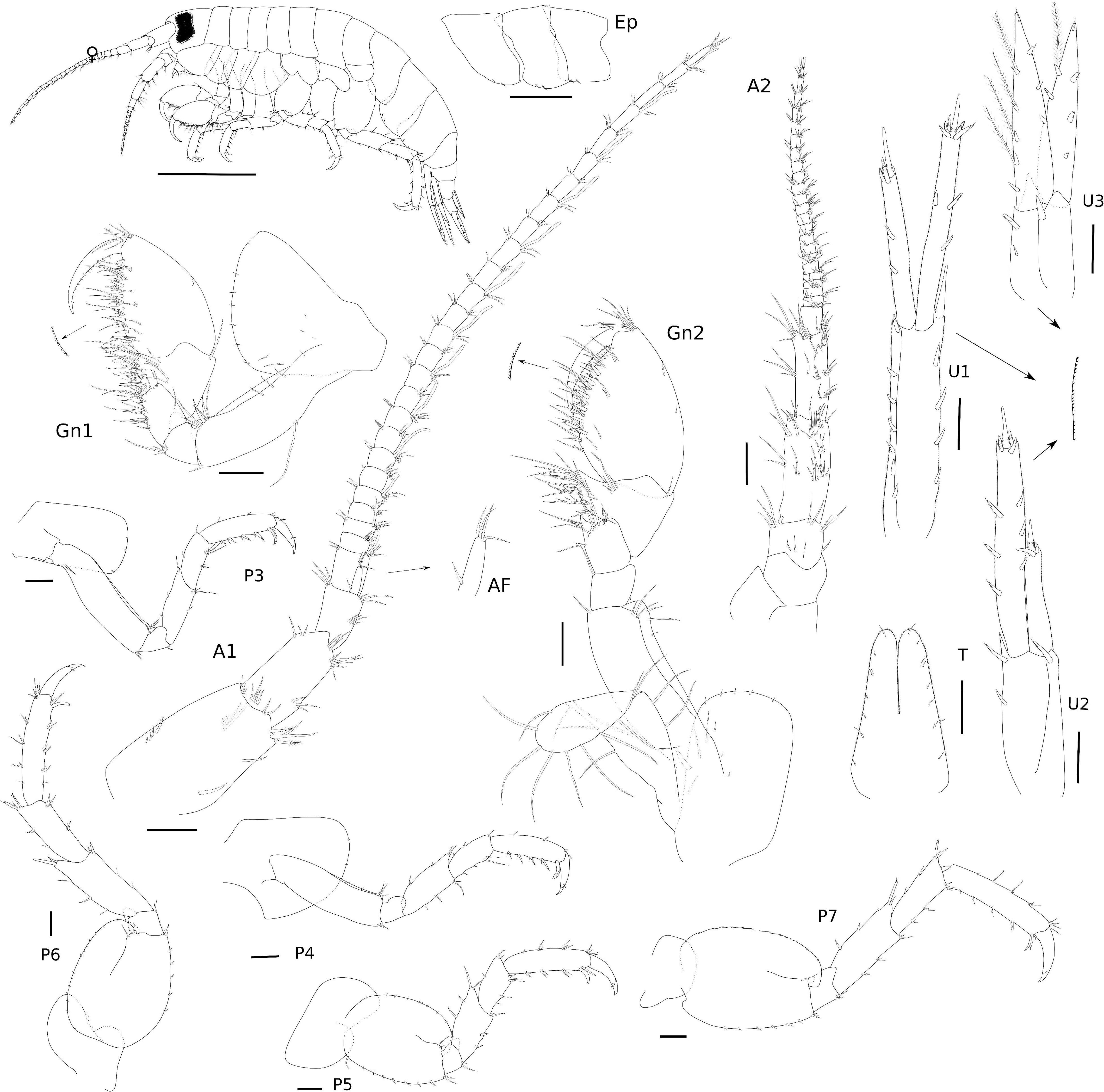
Figure 9. Eusiroides lucai Siqueira and Iwasa-Arai sp. nov. Female (ZUEC-CRU-4368). Scale bar: 1 mm. Antennae, gnathopods, and epimeral plates. Scale bar: 100 μm.
Material examined: Holotype: female, Piscinas do Parcel (20°31′10.812″S, 29°19′25.8″W), from Dictyota sp., September 2018, T. P. Macedo col. (ZUEC-CRU-4368).
Diagnosis: Antennae with stout peduncular articles, with calceoli on flagellum; accessory flagellum of A1 uni-articulate, shorter than first proximal article of flagellum. rMd molar without seta, incisor with two teeth, one wide and the other weakly developed, lacinia mobilis serrated. lMd incisor with three teeth, lacinia mobilis six-toothed; palp article 3 with three long plumose setae apically. Mx1 outer plate with 10 stout serrated setae apically, palp 2-articulate. Mx2, inner plate longer than outer plate. Mxp palp with five articles, article 5 falcate. Gnathopods similar to each other, dactylus falcate, with proximal plumose seta. P3–7 propodus with row of robust setae on the posterior margin and one locking seta distally. Pleonal epimera each with two marginal setae ventrally; epimera 2 and 3 with oblique edge on surface; epimeron 3 with one process posteriorly. U1 peduncle with row of robust setae. U2 shorter than U1, inner ramus 2 × shorter than the outer ramus. U3 inner ramus with a row of plumose setae. T deeply cleft (approximately 1/2 on its length).
Etymology: This species is named after the son of SGLS, Luca Siqueira.
Description: Female (ZUEC-CRU-4368). Body (Figure 9). Length 8.3 mm.
Head with eyes reniform and well developed. A1 2.1 × shorter than body length; peduncular article 1 with two plumose setae distolateral margin and one plumose seta laterally, with a few setae on the lateral and distal margins; peduncular articles 2 and 3 with setae on the lateral and distal margins; accessory flagellum uni-articulate, shorter than the first proximal article of the flagellum, with four setae on the apex; flagellum 26-articulate and 2.1 × longer than peduncular articles 1–3 combined, with calceoli present from articles 2 to 10, and aesthetascs present from articles 2 to 25 discontinuously. A2 stout, 1.7 × shorter than A1; peduncular articles setose; flagellum 23-articulate, slightly longer than peduncular articles 3–5 combined, proximal article longest, with calceoli present from articles 1 to 20. UL rounded, setose in medial region. LL notched, inner lobe setose on the outer margin; outer lobes setose apically and on the inner margin. rMd, molar without setae; incisor with two teeth, one wide and the other weakly developed, lacinia mobilis serrated; accessory setal row with three serrated setae; palp 3-articulate, article 3 with three long plumose setae apically. lMd, molar with two short setae; incisor and lacinia mobilis three- and six-toothed, respectively, accessory setal row with three serrated setae and one seta; palp 3-articulate, article 3 with three long plumose setae apically. Mx1, inner plate small and rounded, inner margin with two short setae; outer plate with 10 stout serrated setae apically; palp 2-articulate with seven long setae. Mx2, inner plate longer than outer plate; inner plate, marginal row of short setae, robust setae on the inner margin and short setae apically; outer plate, long plumose setae on the apical and inner margin. Mxp, inner plate small, 1.3 × shorter than the outer plate, inner plate, inner margin and apex with four plumose long setae, apical margin with five robust setae; outer plate, 3.2 × longer than wide, inner margin and apex with row of long plumose setae; palp 5-articulate, article 1, subtriangular, apex with two long setae, article 2 subrectangular, inner margin with row of long setae, article 3 subrectangular inner margin with long setae, outer margin with six serrated long setae, three long plumose setae and short seta, article 4 with three short setae; article 5 naked, falcate (Figure 10).
Gn1, as long as Gn2; coxa subtriangular, enlarged anteriorly, ventral margin bearing row of short setae; basis 3 × longer than wide, anterior margin with three setae, posterior margin with one long and one short setae, anterodistal margin with a tuft of setae and two robust setae, posterodistal margin with one seta; ischium subquadrate, as long as wide, posterior margin with a tuft of setae; merus subrectangular, about 1.2 × longer than wide, posterior margin heavily setose; carpus 1.1 × wider than long, anterodistal margin with one long seta, posterior margin heavily setose; propodus oval, 1.4 × longer than wide, anterior margin with one short seta, anterodistal angle with a tuft of setae, posterior margin setose with a row of robust setae, palm slightly convex, serrated; dactylus falcate, with a proximal plumose setae and a row of short setae. Gn2, coxa subrectangular, ventral margin bearing row of short setae; basis 3 × longer than wide, anterodistal margin with three setae, posterior margin with one seta; ischium small and subquadrate, as long as wide, posterodistal margin with one long seta; merus, 1.4 × longer than wide, with a setose posterodistal projection, distal margin with a row of setae; carpus, 1.2 × wider than long, anterodistal margin with one short seta, posterior margin setose; propodus oval, 1.6 × longer than wide, anterior margin with one short seta, anterodistal angle with a tuft of setae, posterior margin setose with a row of robust setae, palm slightly convex, serrated; dactylus falcate, with a proximal plumose setae and a row of short setae (Figure 9).
P3, coxa subrectangular, 1.7 × longer than wide, anterior margin with a row of short setae; basis subrectangular, 3.6 × longer than wide, with proximal long seta and anterodistal margin with three setae; ischium small and subrectangular, 1.5 × longer than wide, posterior margin with one short seta; merus subrectangular, 2.5 × longer than wide, anterior margin with three setae, posterior margin with three setae; carpus subrectangular, 2.2 × longer than wide, anterior margin with two setae, posterior margin with five setae; propodus subrectangular, 3.8 × longer than wide, anterior margin with four setae, posterior margin with four simple and four robust setae, and one posterodistal locking seta; dactylus falcate, bi-articulated, with one plumose seta and two short setae. P4, coxa acutely produced backward posteriorly, 1.1 × longer than wide, anterior margin with two short setae; basis subrectangular, 3.6 × longer than wide, anterodistal margin with three setae and posterodistal margin with two setae; ischium small and subrectangular, 1.1 × longer than wide, naked; merus subrectangular, 2.3 × longer than wide, anterior margin with four setae, posterior margin with four setae; carpus subrectangular, 2.1 × longer than wide, anterior margin with three setae, posterior margin with four setae; propodus subrectangular, 3.7 × longer than wide, anterior margin with four setae, posterior margin with four slender and four robust setae, and one posterodistal locking seta; dactylus falcate, bi-articulated, with one plumose seta and one short setae. P5, coxa bilobed, posterior lobe narrower and more ventrally produced than the anterior lobe, naked; basis oval, 1.3 × longer than wide, anterior and posterior margins with a row of short setae; ischium small and subrectangular, 1.3 × wider than long, posterodistal margin with two setae; merus subrectangular, 1.5 × longer than wide, anterior margin with four setae, posterior margin with two robust setae and posterodistal margin with two setae; carpus subrectangular, 1.6 × longer than wide, anterior margin with three setae, posterior margin with five setae; propodus subrectangular, 4 × longer than wide, anterior margin with tufts of setae, posterior margin with three paired setae, and one posterodistal locking seta; dactylus falcate, bi-articulate, with two short setae (Figure 9). P6, coxa bilobed, posterior lobe narrower and more ventrally produced than the anterior lobe, naked; basis oval, 1.2 × longer than wide, anterior and posterior margins with a row of short setae; ischium small and subrectangular, 1.1 × wider than long, posterodistal margin with two setae; merus subrectangular, 2.4 × longer than wide, anterior margin with three setae, anterodistal margin with two robust setae, posterior margin with two setae and posterodistal margin with two robust setae; carpus subrectangular, 2.5 × longer than wide, anterior margin with two setae and three robust setae, posterior margin with seven setae; propodus subrectangular, 4.7 × longer than wide, anterior margin with tufts of setae, posterior margin with three paired robust setae, and one posterodistal locking seta; dactylus falcate, bi-articulate, with one short seta and one plumose seta. P7, coxa oval, naked; basis oval, 1.3 × longer than wide, anterior and posterior margins with a row of short setae; ischium small and subrectangular, 1.1 × wider than long, posterodistal margin with two setae; merus subrectangular, 2.3 × longer than wide, anterior margin with three setae, anterodistal margin with two robust setae, posterior margin with three pairs of setae; carpus subrectangular, 2.5 × longer than wide, anterior margin with three setae and three robust setae, posterior margin with six slender setae and one robust seta; propodus subrectangular, 4.7 × longer than wide, anterior margin with tufts of setae, posterior margin with paired robust setae; dactylus falcate (Figure 9).
Ep, epimeron 1 with one oblique ledge on the surface, with two setae on the ventral margin anteriorly; epimeron 2 with two setae on the ventral margin anteriorly; epimeron 3 largest, flattened ventrally, with two setae on the anterior half of the ventral margin, posterior margin with one tooth (Figure 9).
U1, margins with tiny setae, peduncle 4.7 × longer than wide, inner margin with six robust setae, outer margin with four robust setae, margins with tiny setae; inner ramus 1.2 × longer than the outer ramus, inner margin with two robust setae, apical margin with three robust setae; outer ramus, inner margin with one robust seta, outer margin with two robust setae, apical margin with six robust setae. U2, peduncle 2.3 × longer than wide, inner margin with two robust setae, outer margin with one robust seta; inner ramus 2 × shorter than the outer ramus, inner and outer margins with three robust setae each, apical margin with four robust setae; outer ramus, apical margin with three robust setae, margins with tiny setae. U3, lanceolate and foliaceous, peduncle subrectangular, about 1.7 × longer than wide, inner margin with two robust setae, outer margin with one robust seta; inner ramus as long as outer ramus, inner margin with five robust setae and four long plumose setae, outer margin with two robust setae, apex with one seta; outer ramus, inner margin with three robust setae and one plumose seta, outer margin with three robust setae, apex with one seta, margins with tiny setae. T cleft (about 1/2 length), lateral margins with row of setae, margins with tiny setae, lobes with round apex (Figure 9).
Type locality: Trindade Island, Espírito Santo state, Brazil.
Distribution: Currently known only from the type locality.
Remarks: The genus Eusiroides currently has 16 described species (Horton et al., 2020), which are very similar according to the morphology of antennae, eyes, gnathopods, and uropod 3 (Barnard and Karaman, 1991). Species of Eusiroides can be distinguished from each other by the presence or absence of teeth and serrated posterior margin of epimeron 3 and by telson shape (cleft, moderate or deeply cleft) (Barnard, 1932; Ledoyer, 1982; Thomas, 1993). Based on these characters, Eusiroides lucai sp. nov. is more similar to E. georgiana K. H. Barnard, 1932, by having a tooth on the posterior margin of epimeron 3; however, E. lucai sp. nov. can be distinguished from its congeneric by presenting gnathopods 1 and 2 palm serrated, pereopods 3–4 with one distal locking seta on propodus, uropods 1–3 with tiny setae on the margins, inner ramus of uropod 2 2 × shorter than the outer ramus (vs. 1.5), telson ½ cleft (vs. 2/3), lateral margin of lobes with row of setae and apex round with one seta (vs. one seta on the lateral margins and acute apex). Although the species was described based on a single specimen, its isolated geographical distribution and its conspicuous morphological characters suggest E. lucai sp. nov. to be a new species, and further samplings on TR are likely to report more individuals. This is the third record of Pontogeniidae for Brazil, where Eusiroides sp. was reported from Pernambuco state (Santos and Soares, 1999), and Tethygeneia longleyi (Shoemaker, 1933) was previously reported from São Paulo and Paraná states (southeastern Brazil).
Order Tanaidacea Dana, 1849
Suborder Apseudomorpha Sieg, 1980
Superfamily Apseudoidea Leach, 1814
Family Metapseudidae Lang, 1970
Subfamily Synapseudinae Guţu, 1972
Genus Synapseudes Miller, 1940
Synapseudes isis Segadilha, Siqueira and Iwasa-Arai sp. nov.
http://zoobank.org/733CEA04-27C6-42D5-AE0F-3D048DF66F6E
(Figures 11, 12)
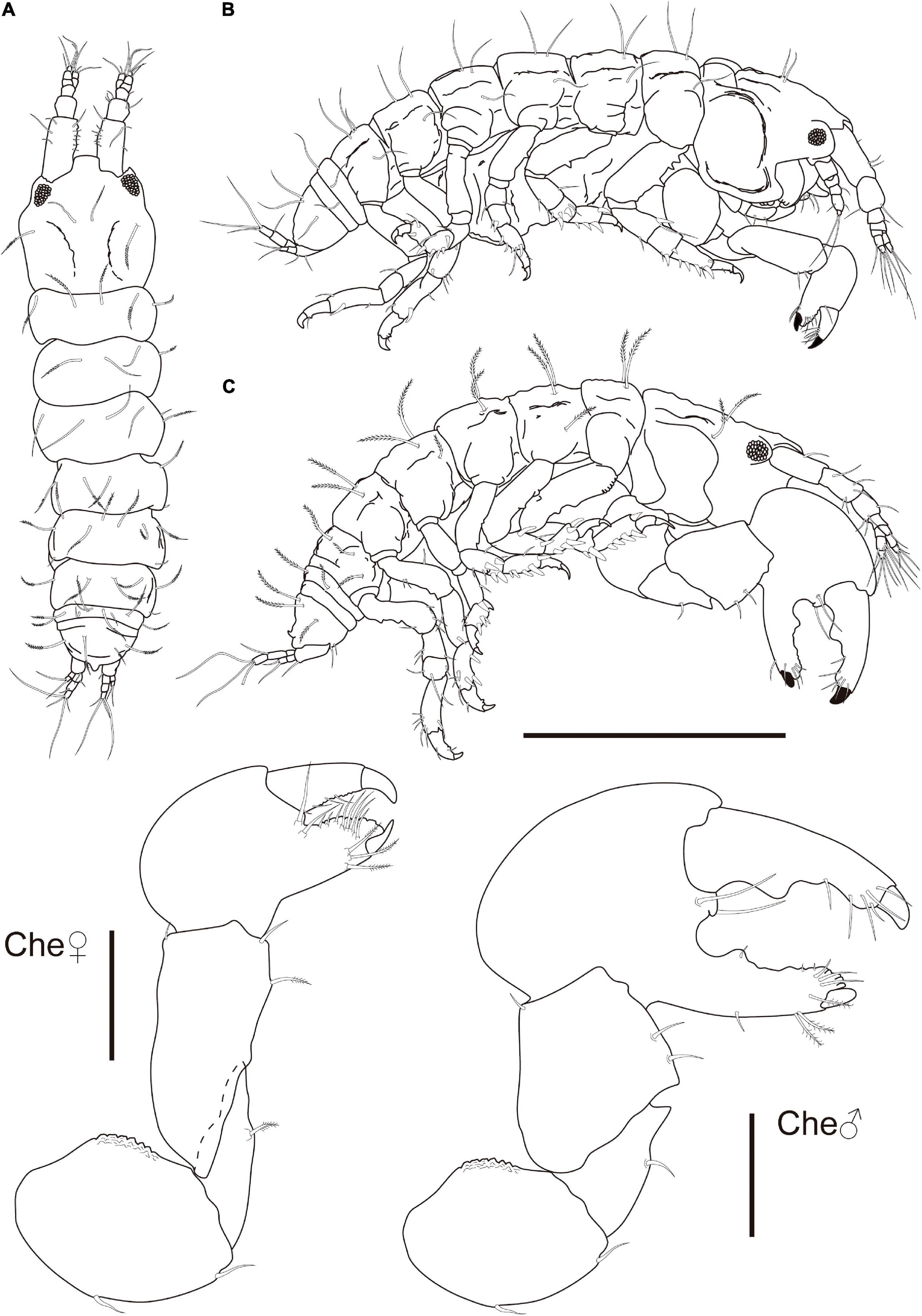
Figure 11. Synapseudes isis Segadilha, Siqueira, and Iwasa-Arai sp. nov. Female (ZUEC-CRU-4369). (A) Ventral view. (B) Lateral view. (C) Male (ZUEC-CRU-4370) lateral view. Scale bar: 0.5 mm.
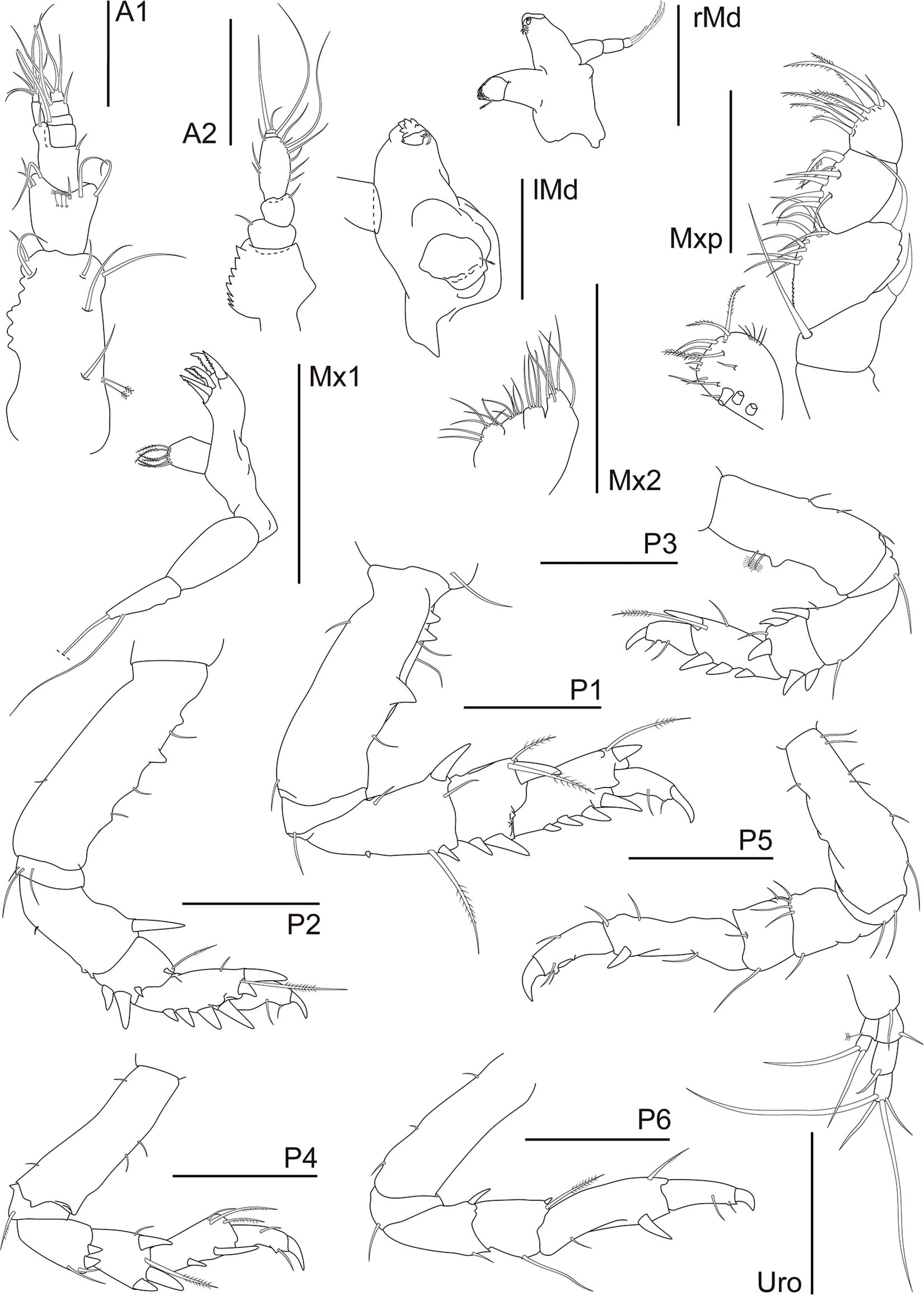
Figure 12. Synapseudes isis Segadilha, Siqueira, and Iwasa-Arai sp. nov. Mouthparts, pereopods, and uropod. Scale bar: 100 μm.
Material examined: Holotype: female, Ilha do Sul (20°31′27.5″S, 29°19′25.2″W), from Dictyota sp., April 2019, I. Batistela col. (ZUEC-CRU-4369).
Paratypes: one male, same as holotype (ZUEC-CRU-4370).
Diagnosis: Female. Body 4 × longer than wide. Cephalothorax shorter than Per 1–3 lengths combined. Rostrum appearing undifferentiated, truncated. Pleon with three segments appearing functionally fused; but with two pleonite remnants delineated by sutures, at least dorsally. Antennule article 2 without tubercles or spiniform apophysis. Antenna article 1 with inner margin strongly serrated; flagellum bi-articulate terminating in one long and one short setae. Mxp palp article 1 with distolateral seta strongly developed; palp article 2 distolateral stout seta 1.5 × longer than that on palp article 1. Cheliped merus, carpus, and propodus with plumose setae ventrally; fixed finger with row of 10 setae on the incisive margin. Pereopod 1 propodus with four stout spiniform setae on the ventral margin. P1–3 basis with three, two, and one spiniform apophyses on the ventral margin, respectively. Uropod endopod composed of three articles and exopod of two articles.
Male. Cheliped basis dorsal margin with strong crenulation; merus ventral margin with medial seta and large pointed apophyses distally; carpus more trapezoidal shaped than in female, ventral margin with two setae and small acute subdistal apophyses; propodus with two setae near articulation of dactylus; fixed finger with one rounded tubercle, three submarginal and 3 min marginal setae on the outer incisive margin. Dactylus with two rounded tubercles and six setae on the ventral margin.
Etymology: The specific epithet is a noun in apposition after the oceanographer Isis Batistela.
Description: Female (ZUEC-CRU-4369). Body (Figure 11). Length 1.1 mm, almost 4 × longer than wide.
Cephalothorax 27% of TBL, shorter than Per 1–3 lengths combined, longer than wide; rostrum truncated with broad base, smooth; eyelobes well defined, pigment and ommatidia present. Carapace with finely plumose setae on the lateral margin and near each ocular lobe (Figure 11).
A1 shorter than cephalothorax. Peduncle with four articles. Article 1 robust, 2.2 × longer than wide; inner margin with row of seven irregularly shaped blunt teeth or tubercles and two setae distally; outer margin with two medial penicillate and one and three subdistal setae. Article 2 as long as wide; with six setae distally. Article 3 slightly longer than wide; with distal seta. Article 4 0.7 × longer than wide; with distal seta. Outer flagellum with three articles. Article 1 wider than long, with distal aesthetasc. Article 2 0.8 × longer than wide; with aesthetasc and seta distally. Article 3 short, with three terminal setae. Inner flagellum with two articles. Article 1 twice longer than wide, naked. Article 2 minute, distally with six setae. A2 peduncle with four articles: Article 1 robust with bulge, with serrated inner margin. Article 2 inner margin with seta; squama absent. Article 3 about as long as article 2; outer margin with seta. Article 4 1.8 × longer than wide, inner margin with two medial and one distal setae; with seven setae along the outer margin. Flagellum with two articles. Article 1 with very long seta and article 2 with two terminal setae of different widths. rMd incisor smooth; setiferous lobe with reduced bifurcate lacinia mobilis and three serrated setae, pars molaris with one acute and two blunt lobes and plumose seta distally. Palp with three articles; article 1 longest, 1.3 × longer than article 2, naked; article 2 1.5 × longer than wide, naked; article 3 with three serrated setae. lMd serrate pars incisiva, lacinia mobilis smooth; setiferous lobe with three serrated setae, pars molaris robust, blunt, with plumose seta distally; mandibular palp broken during dissection. Mx1 outer endite with apparently six terminal strong serrated spines; inner endite bearing six finely plumose setae. Palp bi-articulate with two long subdistal setae. Mx2 with long seta on the outer margin. Movable endite outer lobe with distal margin bearing four long setae; inner lobe of movable endite with four setae. Fixed endite outer lobe with six setae; inner fixed endite with seven setae. Mxp basis naked, narrow. Palp article 1 trapezoidal, with long seta (reaching article 3) on the inner distal margin and robust spine on the outer distal margin; article 2 longest, inner margin finely serrated proximally, with 10 setae, outer margin with two small apophyses and distal long seta; article 3 with six setae along inner margin and one plumose seta distally; article 4 with six plumose inner setae and three subdistal setae. Endite inner margin with three subcylindrical tubercles proximally and five subdistal setae; five setae apically; outer margin with five thin setae (Figure 12).
Che basis, 1.2 × longer than wide; ventral margin with medial and distal setae; dorsal margin with strong crenulation. Merus triangular; ventral margin with plumose seta subdistally. Carpus 2.7 × longer than wide, longer than basis, widest distally; ventral margin with medial plumose and distal setae; dorsal margin with minute distodorsal seta. Propodus 1.4 × longer than wide; with two setae near the articulation of the dactylus; fixed finger with three ventral plumose setae, with five submarginal and three marginal setae on the outer incisive margin. Dactylus and unguis slightly longer than fixed finger; with four setae on the ventral margin (Figure 11).
Pereon about 60% of TBL, all pereonites wider than long, all with two finely plumose setae on the subdistal dorsal margin and one seta on each anterolateral margin; Per 1–3 wider than others; Per 2–3 longer than Per 1; Per 6 shortest. P1 coxa lacking apophysis, with seta. Basis 2.9 × longer than wide; ventral margin with subproximal and distal setae; three setae and two smaller and one larger triangular apophyses along the dorsal margin. Ischium wider than long, with ventral seta. Merus 1.9 × longer than wide, widest distally; ventral margin with small medial spine and subdistal long plumose seta and spine; dorsal margin with proximal seta and distal seta and large spine. Carpus about as long as wide, widest distally, ventral margin with two subdistal spines; distodorsal margin with two plumose setae (one longer than the other) and one spine. Propodus twice longer than wide, with four spines along the ventral margin; dorsal margin with subdistal long plumose seta and spine. Dactylus together with unguis shorter than the propodus, dactylus longer than the unguis; dactylus with 2 minute ventral setae and small subdistal denticle. Unguis curved. P2 coxa lacking apophysis, naked. Basis 3.1 × longer than wide; ventral margin with minute medial seta; three setae and one rounded and two triangular apophyses along the dorsal margin. Ischium wider than long, with three ventral setae. Merus 1.9 × longer than wide, widest distally; ventral margin with minute medial seta; distodorsal margin with large spine. Carpus 0.8 × longer than wide; ventral margin with subdistal spines and two distal apophyses; distodorsal margin with plumose seta and small spine. Propodus 1.8 × longer than wide; with four spines along the ventral margin; dorsal margin with medial seta, distal long plumose seta, spine, and triangular apophyses. Dactylus together with unguis shorter than the propodus, dactylus longer than the unguis; dactylus with 2 minute ventral setae and small subdistal denticle. Unguis curved. P3 basis 2.7 × longer than wide; ventral margin with two medial and one distal setae; dorsal margin with two penicillate setae and rounded apophysis. Ischium with ventral seta. Merus 1.9 × longer than wide, widest distally; ventral margin subdistal seta; distodorsal margin with seta and spine. Carpus 0.8 × longer than wide; ventral margin with two subdistal spines; distodorsal margin with one plumose seta and two spines. Propodus twice longer than wide; with three spines along the ventral margin; dorsal margin with medial seta, distal long plumose seta and spine. Dactylus together with unguis shorter than the propodus, dactylus longer than the unguis; dactylus with 2 minute ventral setae and small subdistal denticle. Unguis curved. P4 basis 3.4 × longer than wide; ventral margin with proximal and distal setae; dorsal margin with two setae. Ischium wider than long, with ventral plumose seta. Merus 1.5 × longer than wide; widest distally; distoventral margin with two setae and two spines. Carpus 1.4 × longer than wide; ventral margin with spine; distodorsal margin with two setae (one longer than other) and one spine. Propodus 2.7 × longer than wide; ventral margin with three spines; distodorsal margin with two plumose and one simple setae. Dactylus curved, together with unguis shorter than the propodus, dactylus longer than the unguis, with two (one medial and one subdistal) ventral setae and small subdistal denticle. Unguis curved. P5 basis 3 × longer than wide; with six setae along the ventral margin; dorsal margin subproximal seta. Ischium with two ventral setae. Merus about as long as wide; distoventral margin with seta; distodorsal margin with four setae. Carpus 0.8 × longer than wide; ventral margin with seta; distodorsal margin with seta. Propodus 3.7 × longer than wide; ventral margin with medial seta and subdistal spine; dorsal margin with subdistal and distal setae. Dactylus together with unguis shorter than the propodus, dactylus longer than the unguis, with one middorsal and two subdistal ventral setae and small subdistal denticle ventrally. Unguis curved. P6 basis 3.3 × longer than wide; with three setae along the dorsal margin. Ischium with ventral setae. Merus 1.5 × longer than wide; ventral margin with subdistal seta; distodorsal margin with spine. Carpus 1.2 × longer than wide; ventral margin with subdistal seta; distodorsal margin with seta and spine. Propodus 3.7 × longer than wide; ventral margin with subproximal seta and subdistal spine; dorsal margin with subdistal seta. Dactylus together with unguis shorter than the propodus, dactylus longer than the unguis; dactylus with one subdistal ventral seta. Unguis curved (Figure 12).
Pleon about 13% of TBL, shorter than Per 1–2 lengths combined, slightly longer than wide; last three pleonites fused to pleotelson, all indications of pleonites similar in length, without pleopods; pleonite 1 with three pairs of finely plumose dorsal setae and one seta on each lateral margin; pleonite 3 with two finely plumose setae dorsally. Pleotelson length 0.2 × that of all pleonites combined, apex large and rounded tip. Pleopods absent.
U biramous. Basal article longer than wide; inner margin with distal seta. Exopod with two articles, shorter than endopod articles 1–2 combined; article 1 with penicillate seta; article 2 with two distal setae. Endopod with three articles; article 1 with distal seta; article 2 longest, with distal seta; article 3 with two long and two short terminal setae (Figure 12).
Male (ZUEC-CRU-4370). Body (Figure 11). Length 1.1 mm.
Che, chela symmetrical. Similar but more robust and distinctly larger than those of the female. Basis ventral margin with medial and distal setae; dorsal margin with strong crenulation. Merus triangular, ventral margin with medial seta and large pointed apophyses distally. Carpus broad and short, more trapezoidal shaped than in female, ventral margin with two setae and small acute subdistal apophyses. Propodus with two setae near articulation of dactylus; fixed finger with one medial and three subdistal plumose setae on the ventral margin; with one rounded tubercle, three submarginal and 3 minute marginal setae on the outer incisive margin. Dactylus and unguis slightly longer than the fixed finger; with two rounded tubercles and six setae on the ventral margin.
Type locality: Trindade Island, Espírito Santo state, Brazil.
Distribution: Currently known only from the type locality.
Remarks: Synapseudes isis sp. nov., Synapseudes menziesi Bǎcescu, 1976, S. pinosensis (Guţu and Ortiz, 2009) and S. rectifrons Guţu, 1996, are distinguished from the other 23 members of the genus as follows: bi-articulate antennal flagellum terminating in a long seta, remnants of two pleonites present, and uropod endopod composed of three articles. The new species from Brazil can be separated from S. pinosensis by having the ventral margin of pereopod 1 propodus with four stout spiniform setae (three spiniform setae in S. pinosensis) and antennule article 2 without distal spiniform apophysis (peduncular article 2 inner distally with a dentiform expansion in S. pinosensis). Synapseudes isis sp. nov. is different from S. menziesi by rostrum appearing undifferentiated, truncated (rostrum with irregularly bilobed anterior margin in S. menziesi) and Maxilliped palp article 2 distolateral stout seta 1.5 × longer than that on palp article 1 (as long as in S. menziesi).
Synapseudes isis sp. nov. closely resembles the other Brazilian species S. rectifrons but can be distinguished from it by antennule article 2 without tubercles or spines (with a spine on the inner margin and a small tubercle on the outer margin in S. rectifrons), antenna article 1 with inner margin strongly serrated (smooth in S. rectifrons) and maxilliped palp article 1 with distolateral seta strongly developed (thin and weakly developed in S. rectifrons). Also, S. isis sp. nov. appears unique by having cheliped merus, carpus, and propodus with plumose setae ventrally, cheliped fixed finger with row of 10 setae on the incisive margin, and pereopod 1–3 with three, two, and one spiniform apophyses on the ventral margin of the basis, respectively.
Suborder Tanaidomorpha Sieg, 1980
Family Leptocheliidae Lang, 1973
Genus Chondrochelia Guţu, 2016
Chondrochelia dubia (Krøyer, 1842)
Material examined: Nine females, Piscinas do Parcel (20°31′10.812″S, 29°19′25.8″W), from Dictyota sp., September 2018, T. P. Macedo col. (ZUEC-CRU-4371); three females, Ilha do Sul (20°31′27.5″S, 29°19′25.2″W), from Dictyota sp., April 2019, I. Batistela col. (ZUEC-CRU-4372).
Remarks: Chondrochelia is a shallow water genus with dimorphic males and females, and C. dubia was firstly described as Tanais dubius from Brazil, and currently shows a worldwide distribution. Differences in morphological characters among Chondrochelia species are subtle, and further molecular studies may elucidate species delimitation within the genus. The new record agrees with the overall diagnosis of C. dubia and extends its distribution to the island of Trindade.
Heuristic searches resulted in four most parsimonious trees (L = 65 steps; CI = 50 and RI = 61), which were combined into a strict consensus tree, and this topology was used for the following data interpretation (Figure 13). Clade letters are represented below branches for the clades discussed ahead.
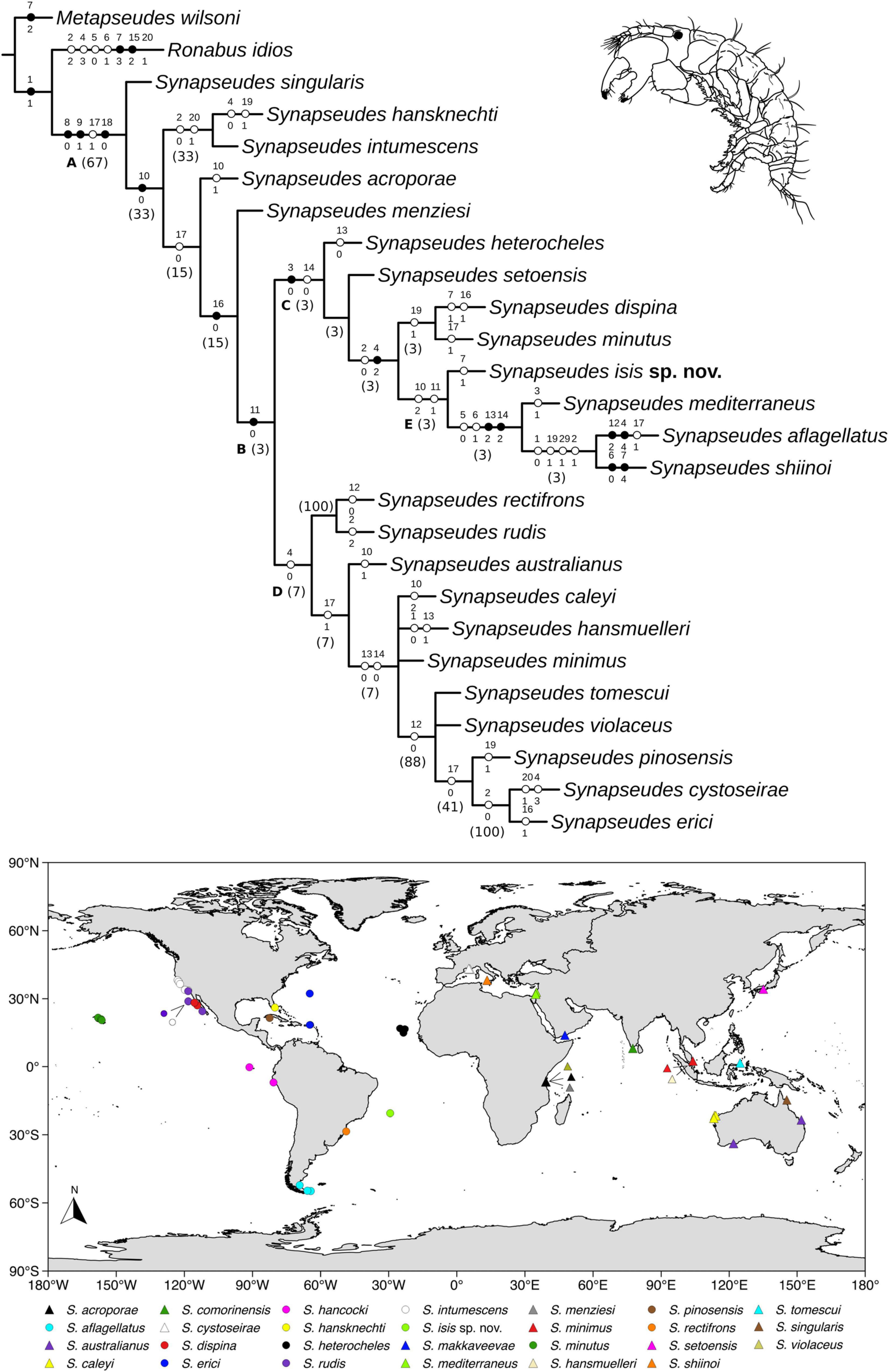
Figure 13. Details of the Synapseudes Miller, 1940 consensus cladogram. Letters below branches represent clades mentioned in the discussion, numbers in parenthesis correspond to Bremmer relative support. Black circles represent non-homoplastic synapomorphies; white circles represent homoplastic synapomorphies. Numbers above branches represent the characters, and states are represented below. Map shows the current distribution of Synapseudes.
Clade A encompasses the genus Synapseudes, supported by four synapomorphies, including three non-homoplastic synapomorphies: presence of three terminal setae on mandibular palp [S6(0)], presence of setae on palp article 2 of Md [S7(1)], and two coupling hooks (retinacula) on endite of Mxp [S19(0)]; and one homoplastic synapomorphy: four articles on the uropodal endopod [S16(1)].
Clade B includes two clades of Synapseudes (clades C and D), and it is supported by two pleonites [S17(0)]. Clade C is composed of S. heterocheles (Vanhöffen, 1914), S. setoensis Shiino, 1951, S. dispina Menzies, 1953, S. minutus Miller, 1940, S. isis sp. nov., S. mediterraneus Bǎcescu, 1977, S. aflagellatus Sieg, 1986, and S. shiinoi Riggio, 1973, grouped by one non-homoplastic synapomorphy, the presence of three spiniform setae on the ventral margin of the propodus of pereopod 2 [S10(0)], and one homoplastic synapomorphy, one article on antennal flagellum [S12(0)]. Clade D comprises S. rectifrons, S. rudis Menzies, 1953, S. australianus Bǎcescu, 1981, S. caleyi Heard et al., 2018, S. hansmuelleri Guţu, 2006, S. minimus Guţu, 2006, S. tomescui Guţu, 2006, S. violaceus Bǎcescu, 1976, S. pinosensis, S. cystoseirae Amar and Cazaubon, 1978, and S. erici Błażewicz-Paszkowycz et al., 2011. This clade is clustered by the absence of distal spiniform apophyses on antennular peduncle article 2 [S13(0)].
Finally, clade E comprises S. isis sp. nov. as the sister clade of S. mediterraneus, S. aflagellatus, and S. shiinoi, supported by the homoplastic synapomorphy of three pleonites [S17(1)] and symmetrical chelipeds in male [S18(2)].
The results of the BBM analysis indicated that dispersal played a key role in shaping the current distribution patterns of Synapseudes (Figure 14), totaling 15 dispersals between areas within the genus. According to the analysis, the neighbor realms (G) Coral Sea and (F) Indo-Pacific and Indian Ocean are the two most likely ancestral areas of Synapseudes (node 46), with 50.50 and 22.48% marginal probability, respectively. The analysis suggests an early dispersal to the North Pacific (B) and the Caribbean and Gulf of Mexico (D), followed by sympatric speciation events along the Indo-Pacific and Indian Oceans.
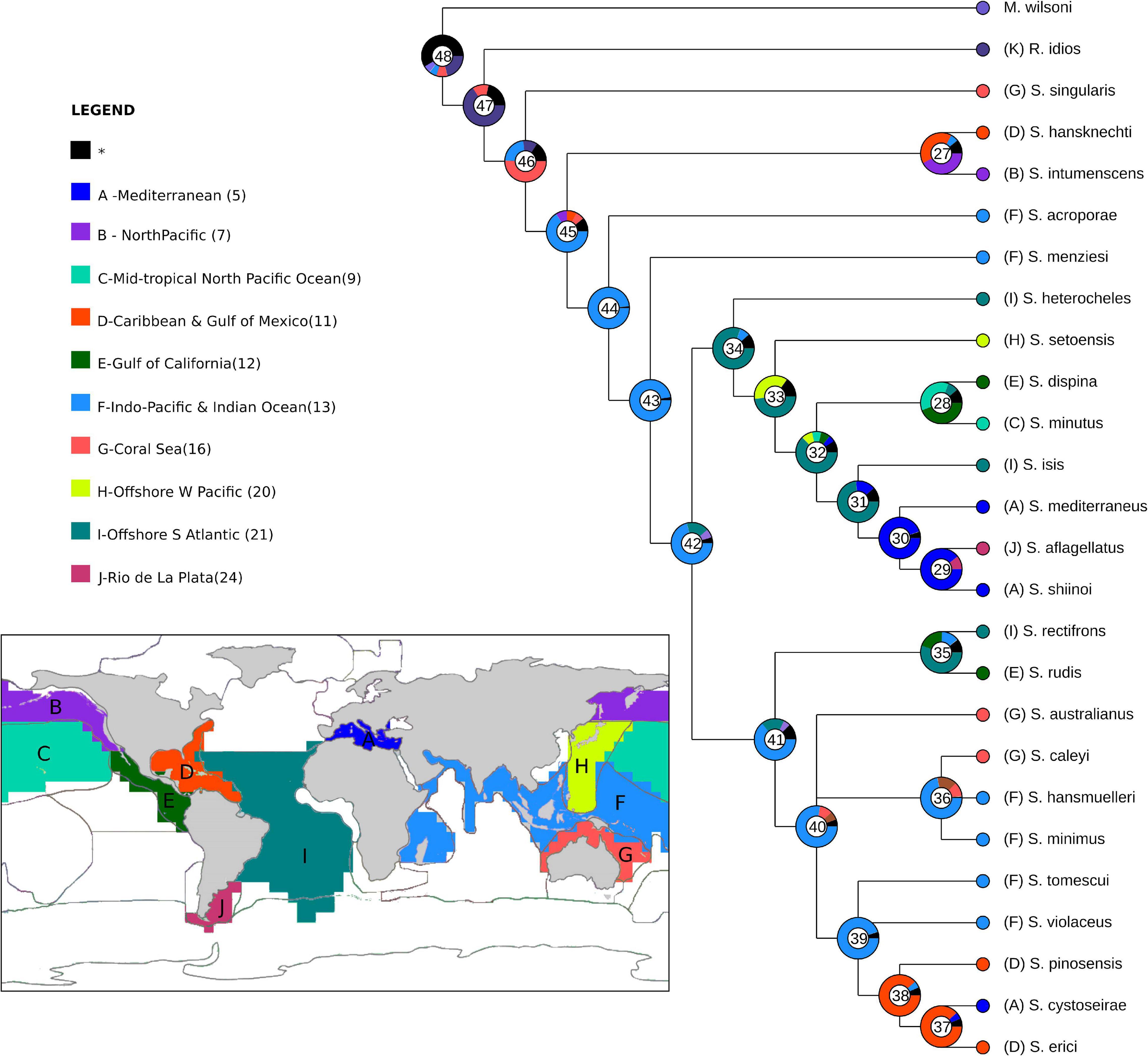
Figure 14. Bayesian Binary MCMC (BBM) analysis of Synapseudes Miller, 1940. Map shows realms proposed by Costello et al. (2017), and letters in map correspond to realms with Synapseudes Miller, 1940 distribution records. Numbers in legend correspond to realms numbers by Costello et al. (2017). Colors on terminals correspond to current distribution and colors on nodes represent potential distribution of ancestors.
A transoceanic dispersion is proposed for ancestors on node 42, with a single dispersion from the Pacific to the Atlantic Ocean before the separation into two main Synapseudes clades. The first main clade (clade C; Figure 13) shares a strict evolutionary relationship with the Atlantic followed by a late dispersal to the Mediterranean (A). On the other hand, the second main clade (clade D; Figure 13) mostly remains in the Indo-Pacific and Coral Sea realms, with a second dispersal to the Caribbean and Gulf of Mexico.
Concerning the new species from TR, Synapseudes isis sp. nov., its ancestor’s area of distribution (node 31) suggests the last Atlantic speciation before the dispersal to the Mediterranean, with 73.26 and 15.25% marginal probabilities, respectively.
According to the classical Theory of Island Biogeography (IBT) proposed by MacArthur and Wilson (1967), species richness is positively correlated with island area and negatively correlated with isolation. Additionally, Rosenzweig (1995) also commented on the importance of island age for predicting species richness. The General Dynamic Theory of Oceanic Island Biogeography (GDM; Whittaker et al., 2008) also takes into account island age as an important factor for predicting island species richness on islands, together with island area and isolation, but relies on not only the island birth but also the death processes that lead to erosion and consequent habitat loss (Hachich et al., 2019). Although these factors are known to influence species richness, species richness is highly determined by the taxon studied, as observed for reef fishes, seaweed, and marine gastropods in shallow waters (Hachich et al., 2015). Therefore, the results presented herein aim to contribute to the knowledge of Peracarida biogeography in shallow water habitats from oceanic islands.
The fauna of Peracarida from TR indeed agrees with the isolation of the island, in which only 11 species were sampled associated with Dictyota sp., whereas 30 species were found in the same algae at the Abrolhos Bank (Cunha et al., 2013), a 46,000 km2 projection of the Brazilian continental shelf (Dutra et al., 2005), including two orders (Cumacea and Isopoda) not observed in TR (Figures 1, 15). The Abrolhos Bank harbors the largest and most diverse reef complex of the South Atlantic (Leão, 1982; Dutra et al., 2005) presenting high levels of coral endemism (Leão and Dominguez, 2000); however, benthic invertebrates such as crustaceans and polychaetes showed low levels of endemicity for the area (Paiva, 2005; Young and Serejo, 2005; Cunha et al., 2013).
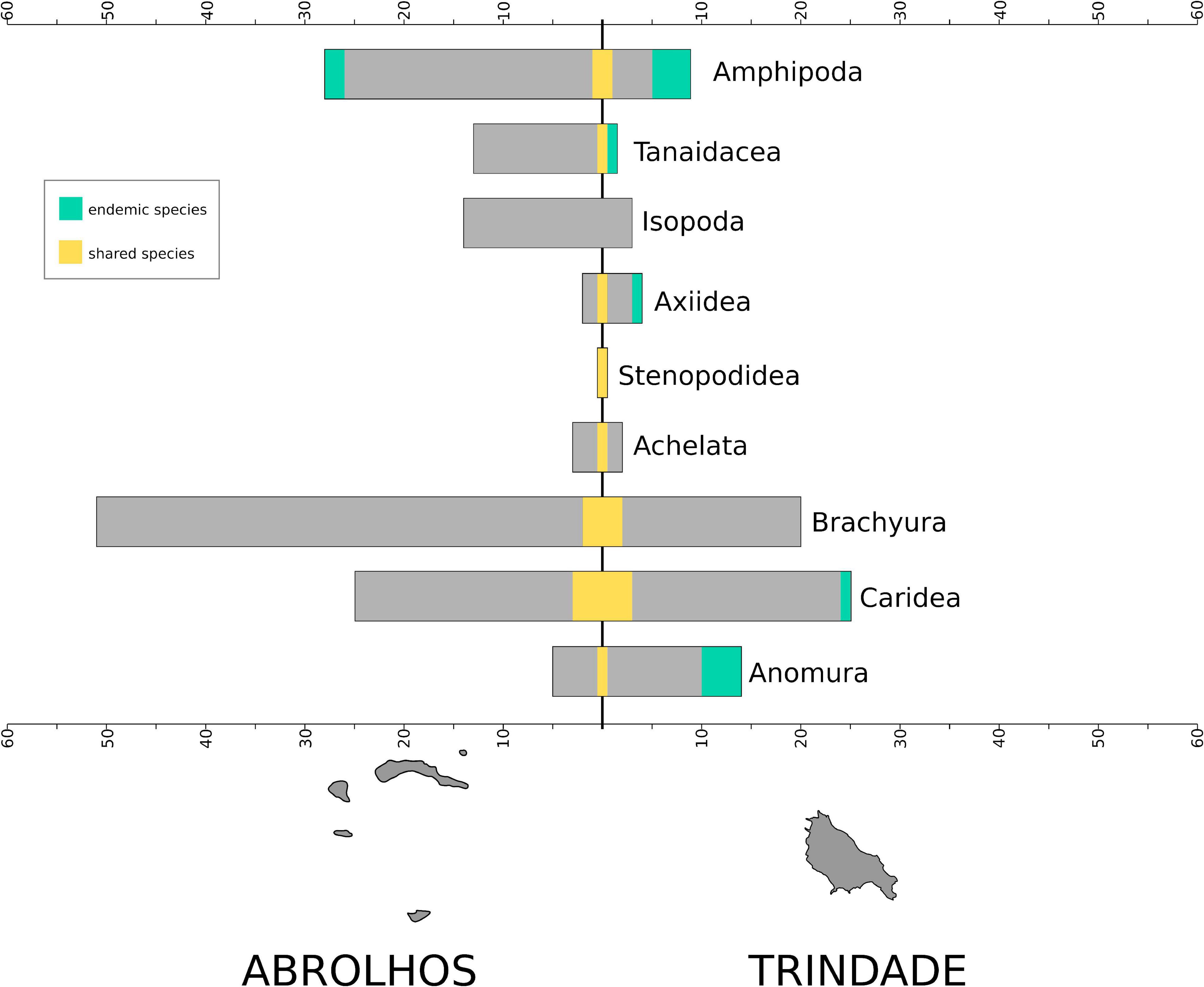
Figure 15. Crustacea species richness reported from Abrolhos Archipelago (left) and Trindade Island (right). Gray bars correspond to species not shared by both islands, yellow bars correspond to shared species, and seafoam green bars correspond to endemic species. References: Amphipoda: Cunha et al. (2013), Andrade and Senna (2017), Serejo and Siqueira (2018); Tanaidacea: Brum (1973); Guţu (1996), Cunha et al. (2013); Isopoda: Barth (1958), Moreira (1977), Cunha et al. (2013), Souza et al. (2013), Campos-Filho et al. (2018); Axiidea: Pachelle and Tavares (2020); Stenopodidea: Soledade et al. (2015), Tavares et al. (2017); Achelata: Faria-Júnior et al. (2014), Tavares et al. (2017); Brachyura: Almeida and Coelho (2008), Tavares et al. (2017); Caridea: Soledade et al. (2017), Tavares et al. (2017), Lima et al. (2019).
Regarding its endemicity, Peracarida is one of the most endemic taxa among the marine invertebrate fauna (Costello et al., 2017; Arfianti and Costello, 2020), and TR shows a high endemicity rate of 45%, higher than that observed for other Crustacea taxa previously reported for the island, such as Anomura, Axiidea, and Caridea, with 28, 25, and 4%, respectively (Anker et al., 2016; Tavares et al., 2017; Lima et al., 2019; Pachelle and Tavares, 2020). Other Decapoda taxa such as Achelata, Brachyura, and Stenopodidea lacked any species endemic to TR (Tavares et al., 2017). Nonetheless, we believe that the actual number of Peracarida species from TR is still underestimated, once only Peracarida members associated with Dictyota up to 10 m deep were sampled herein. Therefore, considering the broad range of Peracarida lifestyles, from planktonic to benthic and parasitic, further samplings are recommended to better assess TR biodiversity.
The cladistic analysis of Synapseudes supported the monophyly of the genus and agreed with the erection of the genus Ronabus by Heard et al. (2018). Ronabus is separated from Synapseudes based on the number of antennal flagella [S2(2)], the number of antennal terminal setae [S4(3)], the number of articles in the inner and outer antennular flagellum [S5(0) and S6(1)], the number of terminal setae on the mandibular palp [S7(3)], number of articles on the uropodal exopod [S15(2)], and telson with bulgae [S20(1)]. The analysis also showed two main clades within Synapseudes (clades C and D), which are discussed according to its distribution (Figure 13).
The BBM analysis of the genus Synapseudes is the first event-based biogeographic attempt to understand the dynamics of fauna associated with macroalgae over long periods of time. Even though it is well known that macroalgae are the best drifter substrata particularly for Peracarida because of lack of larval phase (Thiel, 2003), providing shelter and nutrition even in harsh environments, knowledge on the evolutionary processes that have driven the current distribution is scarce.
We recovered the Indo-Pacific + Coral Sea realms as the ancestral distribution area of Synapseudes (Figure 14), supporting the proposal of an Indo-Pacific “center of origin,” where species originated in the biodiversity center colonize marginal areas and speciate (Bowen et al., 2013). Six species presented the current distribution within the realm of Indo-Pacific and Indian Ocean (realm F), followed by three species distributed in the Mediterranean (realm A), the Caribbean and Gulf of Mexico (realm D), and Offshore South Atlantic (realm I). Therefore, the BBM analysis suggests an Indo-Pacific + Coral Sea origin followed by multiple dispersals to other ocean realms. From the Indo-Pacific + Coral Sea origin, the first dispersal of Synapseudes ancestors was to the North Pacific (realm B) and to the Caribbean and Gulf of Mexico (realm D), while consecutive sympatric speciations continued to raise species number in the Indo-Pacific (Figure 14).
The separation of Synapseudes into two main clades corresponds to the first dispersal to the Offshore South Atlantic (realm I), where most of the ancestors speciated, suggesting a niche suitability for the genus. A possible return to the Pacific Ocean is suggested on node 33, where three dispersals to new realms within the Pacific are observed (nodes 28, 32, and 33), while the ancestors that remained in the Atlantic later dispersed to the neighboring realms of the Mediterranean (realm A) and Rio de La Plata (realm J). The second main clade remained in the Indo-Pacific region, with a second dispersal to the Atlantic, whereas successive sympatric speciations in the Indo-Pacific and Coral Sea continued to happen. Lastly, another dispersal to the Caribbean and Gulf of Mexico (realm D) followed by sympatric speciation were observed for Synapseudes (Figure 14).
This first attempt to understand the evolution of Peracarida associated with macroalgae suggests multiple dispersions from an Indo-Pacific center of origin, and better understanding of the paleocurrent dynamics, other Peracarida taxa phylogenetics, and molecular data will certainly help unpuzzling the historical biogeography of fauna–macroalgae association.
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/Supplementary Material.
TI-A and SS: conceptualization. TI-A, SS, and JS: methodology and formal analysis. TI-A: writing–original draft. TI-A, SS, JS, and FL: writing–review and editing. FL: funding acquisition and supervision.
This study was funded by the Fundação de Amparo à Pesquisa do Estado de São Paulo-FAPESP (grant numbers 2018/00488–7 and 2018/10313–0).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
We are thankful to Projeto Ecológico de Longa Duração– Ilhas Oceânicas Brasileiras (PELD-ILOC), Thais Peixoto Macedo and Isis Batistela (Universidade Federal de Santa Catarina) for collecting the Dictyota material from the Trindade Island. We thank Francisco Andrade Montenegro for helping with Synapseudes drawings. We are also grateful to Profs. Silvio Nihei, Fabio Laurindo, and Andre Carvalho (Universidade de São Paulo) for reviewing the manuscript and for the helpful comments and suggestions. Finally, we thank FAPESP for TI-A’s scholarship (2018/00488-7) and TI-A, SS and FL grant (2018/10313-0).
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmars.2021.641236/full#supplementary-material
Supplementary Material 1 | Morphological characters used on Synapseudes phylogenetic analysis.
Supplementary Material 2 | Taxa and characters matrix.
Almeida, A. O., and Coelho, P. A. (2008). Estuarine and marine brachyuran crabs (Crustacea: Decapoda) from Bahia, Brazil: checklist and zoogeographical considerations. Lat. Am. J. Aquat. Res. 36, 183–222. doi: 10.3856/vol36-issue2-fulltext-4
Alves, J., Johnsson, R., and Senna, A. R. (2016). On the genus Elasmopus Costa, 1853 from the Northeastern coast of Brazil with five new species and new records. Zootaxa 4184, 1–40. doi: 10.11646/zootaxa.4184.1.1
Amar, R., and Cazaubon, A. (1978). Une nouvelle espèce de Synapseudes Crustacea, Tanaidacea des côtes méditerrannéennes. Téthys 8, 327–333.
Andrade, L. F., and Senna, A. R. (2017). Four new species of Cymadusa Savigny, 1816 (Amphipoda: Ampithoidae) and new records of C. filosa Savigny, 1816 from Brazilian coast. Zootaxa 4226, 359–389. doi: 10.11646/zootaxa.4226.3.3
Anker, A., Tavares, M., and Mendonça, J. B. (2016). Alpheid shrimps (Decapoda: Caridea) of the Trindade & Martin Vaz Archipelago, off Brazil, with new records, description of a new species of Synalpheus and remarks on zoogeographical patterns in the oceanic islands of the tropical southern Atlantic. Zootaxa 4138, 1–58. doi: 10.11646/zootaxa.4138.1.1
Arfianti, T., and Costello, M. J. (2020). Global biogeography of marine amphipod crustaceans: latitude, regionalization, and beta diversity. Mar. Ecol. Prog. Ser. 638, 83–94. doi: 10.3354/meps13272
Audouin, V. (1826). Explication sommaire des planches de crustacesde l’Egypte et de la Syrie, publiees par Jules-Cesar Savigny, membre de l’Institut; offrant un expose des caracteres naturels des genres, avec la distinction des especes. Description de l’Egypte, Histoire Naturelle 1, 77–98.
Bǎcescu, M. (1976). Representatives of the family Synapseudidae (Crustacea, Tanaidacea) from the Tanzanian coral reefs: one new genus (Curtipleon) and three new species of Synapseudes. Trav. du Muséum Natl. d’Histoire Nat. ‘Grigore Antipa’ 17, 51–63.
Bǎcescu, M. (1977). Heterotanais longidactylus n. sp. and Synapseudes mediterraneus n. sp., Tanaidacea new for the eastern Mediterranean fauna. Rev. Roum. Biol. Série Biol. Anim. 22, 119–125.
Bǎcescu, M. (1981). Contribution to the knowledge of the Monokonophora (Crustacea, Tanaidacea) of the eastern Australia coral reefs. Rev. Roum. Biol. Série Biol. Anim. 26, 111–120.
Barnard, J. L. (1958). A remarkable new genus of corophiid amphipod from coastal marine bottoms of southern California. Bull. South Calif. Acad. Sci. 57, 85–90.
Barnard, J. L. (1965). Marine Amphipoda of the family Ampithoidae from southern California. Proc. U. S. Natl. Mus. 118, 1–46. doi: 10.5479/si.00963801.118-3522.1
Barnard, J. L. (1969). Gammaridean Amphipoda of the rocky intertidal of California: Monterey Bay to La Jolla. Bul. U. S. Natl. Mus. 258, 1–230. doi: 10.5479/si.03629236.258.1
Barnard, J. L. (1974). Gammaridean Amphipoda of Australia, part II. Smithson. Contr. Zool. 139, 1–148. doi: 10.5479/si.00810282.139
Barnard, J. L., and Karaman, G. S. (1991). The families and genera of marine Amphipoda (except marine Gammaroids). Rec. Aust. Mus. Suppl. 13, 1–866. doi: 10.3853/j.0812-7387.13.1991.91
Barth, R. (1958). Observações biológicas e meteorológicas feitas na Ilha de Trindade. Mem. Inst. Oswaldo Cruz 56, 261–279. doi: 10.1590/s0074-02761958000100013
Bate, C. S. (1862). Catalogue of the specimens of amphipodous Crustacea in the collection of the British Museum. Br. Mus. 1–399.
Berents, P. B. (1983). The Melitidae of Lizard Island and adjacent reefs, The Great Barrier Reef, Australia (Crustacea: Amphipoda). Rec. Aust. Mus. 35, 101–143. doi: 10.3853/j.0067-1975.35.1983.313
Błażewicz-Paszkowycz, M., Bamber, R., and Anderson, G. (2012). Diversity of Tanaidacea (Crustacea: Peracarida) in the world’s oceans–how far have we come? PLoS One 7:e33068. doi: 10.1371/journal.pone.0033068
Błażewicz-Paszkowycz, M., and Bamber, R. N. (2007). New apseudomorph tanaidaceans (Crustacea: Peracarida: Tanaidacea) from eastern Australia: apseudidae, whiteleggiidae, metapseudidae and pagurapseudidae. Mem. Mus. Vic. 64, 107–148. doi: 10.24199/j.mmv.2007.64.11
Błażewicz-Paszkowycz, M., Heard, R. W., and Bamber, R. N. (2011). A new species of shallow-water metapseudid (Crustacea: Malacostraca: Peracarida: Tanaidacea) from Bermuda (Caribbean). Bull. Peabody Mus. Nat. Hist. 52, 127–134. doi: 10.3374/014.052.0103
Boeck, A. (1871). Crustacea Amphipoda borealia et arctica. Forhandlinger i Videnskabs- Selskabet i Christiania 1870, 83–280.
Bousfield, E. L., and Hendrycks, E. A. (2002). The Talitroidean amphipod family Hyalidae revised with emphasis on the North Pacific fauna: systematic and distributional ecology. Amphipacifica 3, 17–134.
Bowen, B. W., Rocha, L. A., Toonen, R. J., and Karl, S. A. (2013). The origins of tropical marine biodiversity. Trends Ecol. Evol. 28, 359–366. doi: 10.1016/j.tree.2013.01.018
Bremer, K. (1994). Branch support and tree stability. Cladistics 10, 295–304. doi: 10.1111/j.1096-0031.1994.tb00179.x
Brum, I. N. (1973) Contribuição ao conhecimento da fauna do Arquipélago de Abrolhos, Bahia, Brasil, N°4. Crustacea–Tanaidacea. Boletim do Museu de História Natural da Universidade Federal de Minas Gerais. Zoologia 18, 1–14.
BulyĊeva, A. N. (1957) Beach-fleas of the seas of the USSR and adjacent waters (Amphipoda — Talitroidea). Akademiia Nauk SSSR, Opredeliteli po Faune SSSR 65, 1–185.
Campos, J. M. P., Correia, F. R., and Rosa Filho, J. S. (2020). Primeiro registro de Ampithoe marcuzzii Ruffo, 1954 (Crustacea: Amphitoidae) para o Nordeste do Brasil. Pesquisa e Ensino em Ciências Exatas e da Natureza 4, 01–07.
Campos-Filho, I. S., Cardoso, G. M., and Aguiar, J. O. (2018). Catalogue of terrestrial isopods (Crustacea, Isopoda, Oniscidea) from Brazil: an update with some considerations. Nauplius 26:e2018038. doi: 10.1590/2358-2936e2018038
Christie, H., Norderhaug, K. M., and Fredriksen, S. (2009). Macrophytes as habitat for fauna. Mar. Ecol. Prog. Ser. 396, 221–233. doi: 10.3354/meps08351
Correia, F. R., Guedes-Silva, E., and Souza-Filho, J. F. (2016). A new species of Ampithoe Leach, 1814 (Senticaudata, Ampithoidae) from Brazilian coast. Zootaxa 4136, 195–200. doi: 10.11646/zootaxa.4136.1.12
Costa, A. (1853). Descrizione di tre nuovi Crostacei del Mediterraneo discoperti dal Rev. G.F. Hope. Fauna del Regno di Napoli 83, 1–10.
Costello, M. J., Tsai, P., Wong, P. S., Cheung, A. K. L., Basher, Z., and Chaudhary, C. (2017). Marine biogeographic realms and species endemicity. Nat. Commun. 8:1057. doi: 10.1038/s41467-017-01121-2
Cunha, R., Tavares, M., and Mendonça, J. B. J. (2020). Asteroidea (Echinodermata) from shallow-waters of the remote oceanic archipelago Trindade and Martin Vaz, southeastern Atlantic, with taxonomic and zoogeographical notes. Zootaxa 4742:zootaxa.4742.1.2. doi: 10.11646/zootaxa.4742.1.2
Cunha, T. J., Güth, A. Z., Bromberg, S., and Sumida, P. Y. G. (2013). Macrofauna associated with the brown algae Dictyota spp. (Phaeophyceae, Dictyotaceae) in the Sebastião Gomes Reef and Abrolhos Archipelago, Bahia, Brazil. Cont. Shelf Res. 70, 140–149. doi: 10.1016/j.csr.2013.09.001
Dana, J. D. (1849). Conspectus crustaceorum, conspectus of the Crustacea of the U.S. exploring expedition. Am. J. Sci. Arts 8, 424–428.
Dana, J. D. (1853). Crustacea. Part II. United States Exploring Expedition During the Years 1838, 1839, 1840, 1841, 1842; Under the Command of Charles Wilkes 14: 689–1618, 96 pls. doi: 10.5962/bhl.title.23237
De Pinna, M. C. C. (1991). Concepts and tests of homology in the cladistic paradigm. Cladistics 7, 367–394. doi: 10.1111/j.1096-0031.1991.tb00045.x
Duffy, J. E., and Hay, M. E. (1991). Food and shelter as determinants of food choice by an herbivorous marine amphipod. Ecology 72, 1286–1298. doi: 10.2307/1941102
Dutra, G., Allen, G., Werner, T., and McKenna, S. (2005). A Rapid Marine Biodiversity Assessment of the Abrolhos Bank, Bahia, Brazil. The RAP Bulletin of Biological Assessment, 38th Edn. Washington, DC: Conservation International.
Faria-Júnior, E., de Carvalho Gaeta, J., and Freire, A. S. (2014). Una actualización de las especies de langosta (Panulirus White, 1847) en el Parque Nacional de Abrolhos, Norte de Brazil/An update on the lobster species (Panulirus White, 1847) from the Abrolhos Marine National Park, Northern Brazil. Rev. Investig. Marinas 33, 37–42.
Gardiner, L. F. (1973). New species of the genera Synapseudes and Cyclopoapseudes with notes on morphological variation, postmarsupial development, and phylogenetic relationships within the family Metapseudidae (Crustacea: Tanaidacea). Zool. J. Linn. Soc. 53, 25–58. doi: 10.1111/j.1096-3642.1973.tb01410.x
Gasparini, J. L., and Floeter, S. R. (2001). The shore fishes of Trindade Island, western south Atlantic. J. Nat. Hist. 35, 1639–1656. doi: 10.1080/002229301317092379
Gibson, R. N. (1986). “Intertidal teleosts: life in a fluctuating environment,” in The Behaviour of Teleost Fishes, ed. T. J. Pitcher (Boston, MA: Springer), 388–408. doi: 10.1007/978-1-4684-8261-4_15
Goloboff, P. A., Farris, J. S., and Nixon, K. C. (2008). TNT, a free program for phylogenetic analysis. Cladistics 24, 774–786. doi: 10.1111/j.1096-0031.2008.00217.x
Gomes-da-Silva, J., and Souza-Chies, T. T. (2017). What actually is Vriesea? A total evidence approach in a polyphyletic genus of Tillandsioideae (Bromeliaceae, Poales). Cladistics 34, 181–199. doi: 10.1111/cla.12200
Gouillieux, B., and Sorbe, J. C. (2015). Elasmopus thalyae sp. nov.(Crustacea: Amphipoda: Maeridae), a new benthic species from soft and hard bottoms of Arcachon Bay (SE Bay of Biscay). Zootaxa 3905, 107–118. doi: 10.11646/zootaxa.3905.1.6
Guţu, M. (1972). Phylogenetic and systematic considerations upon the Monokonophora (Crustacea – Tanaidacea) with the suggestions of a new family and several new subfamilies. Revue Roumaine de Biologie, S rie de Zoologie 17, 297–305.
Guţu, M. (1996). Tanaidaceans (Crustacea, Peracarida) from Brazil, with description of new taxa and systematical remarks on some families. Trav. du Muséum Natl. d’Histoire Nat. ‘Grigore Antipa’ 36, 23–133.
Guţu, M. (2006). New Apseudomorph Taxa (Crustacea, Tanaidacea) of the World Ocean. Bucharest: Curtea Veche.
Guţu, M. (2016). Systematic Novelties of the Enigmatic Universe of the Leptocheliids (Crustacea: Tanaidacea). Bucharest: ePublishers.
Guţu, M., and Ortiz, M. (2009). A new genus and two new species of the Metapseudids from the Southern waters of Cuba (Crustacea: Tanaidacea: Apseudomorpha). Trav. du Muséum Natl. d’Histoire Nat. ‘Grigore Antipa’ 52, 87–99.
Hachich, N. F., Bonsall, M. B., Arraut, E. M., Barneche, D. R., Lewinsohn, T. M., and Floeter, S. R. (2015). Island biogeography: patterns of marine shallow-water organisms in the Atlantic Ocean. J. Biogeogr. 45, 1871–1882. doi: 10.1111/jbi.12560
Hachich, N. F., Ferrari, D. S., Quimbayo, J. P., Pinheiro, H. T., and Floeter, S. R. (2019). “Island biogeography of marine shallow-water organisms,” in Encyclopedia of the World’s Biomes, eds M. Goldstein and D. DellaSala (Amsterdam: Elsevier), 61–75. doi: 10.1016/B978-0-12-409548-9.11947-5
Heard, R. W., Stępień, A., Drumm, D. T., Błażewicz, M., and Anderson, G. (2018). Systematic and taxonomic observations on the subfamily Synapseudinae Guţu, 1972 and related metapseudid taxa (Crustacea: Tanaidacea: Apseudomorpha), with the erection of a new genus and descriptions of three new species. Zootaxa 4370, 301–344. doi: 10.11646/zootaxa.4370.4.1
Horton, T., Lowry, J., De Broyer, C., Bellan-Santini, D., Coleman, C. O., Corbari, L., et al. (2020). World Amphipoda Database. Elasmopus besnardi Oliveira, 1951. Accessed through: World Register of Marine Species. Avaliable at: http://www.marinespecies.org/aphia.php?p=taxdetails&id=531472 (accessed December 3, 2020).
Hurtado, L. A., Mateos, M., Mattos, G., Liu, S., Haye, P. A., and Paiva, P. C. (2016). Multiple transisthmian divergences, extensive cryptic diversity, occasional long-distance dispersal, and biogeographic patterns in a marine coastal isopod with an amphi-American distribution. Ecol. Evol. 6, 7794–7808. doi: 10.1002/ece3.2397
Iwasa-Arai, T., and Serejo, C. S. (2018). Phylogenetic analysis of the family Cyamidae (Crustacea: Amphipoda): a review based on morphological characters. Zool. J. Linn. Soc., zlx 101, 1–29.
Iwasa-Arai, T., Siqueira, S. G. L., Machado, G. B. D. O., and Leite, F. P. P. (2019). Phylogenetic reconstruction of the genus Pseudaeginella (Amphipoda: Caprellidae), with the description of a new species from Brazil. System. Biodivers. 17, 179–189. doi: 10.1080/14772000.2019.1572668
Krapp-Schikel, G., and Ruffo, S. (1990). Marine amphipods of the Canary Islands with description of a new species of Elasmopus. Misc. Zool. 14, 53–58.
Krapp-Schickel, T. (2008). What has happened with the Maera-clade (Crustacea, Amphipoda) during the last decades? Bolletino del Museo di Storia Naturale di Verona 32, 3–32.
Lang, K. (1970). Taxonomische und phylogenetische Untersuchungen über die Tanaidaceen. 4. Aufteilung der Apseudiden in vier Familien nebst Aufstellung von zwei Gattungen und einer Art der neuen Familie Leiopidae. Ark. för Zool. 22, 595–626.
Lang, K. (1973). Taxonomische und phylogenetische Untersuchungen über die Tanaidaceen (Crustacea). 8. Die Gattungen Leptochelia Dana, Paratanais Dana, Heterotanais G.O. Sars und Nototanais Richardson. Dazu einige Bemerkungen über die Monokonophora und ein Nachtrag. Zool. Scr. 2, 197–229. doi: 10.1111/j.1463-6409.1974.tb00752.x
Latreille, P. A. (1816). Nouveau Dictionnaire d’histoire naturelle, appliquée aux arts, à l’Agriculture, à l’Economic rurale et domestique, à la Médecine, etc. Par une Société de Naturalistes et d’Agriculteurs. Nouvelle Édition. Paris. 1, 467–469.
Leach, W. E. (1814). “Crustaceology,” in The Edinburgh Encyclopedia, ed. D. Brewster (Edinburgh: William Blackwood), 383–437.
Leão, Z. M. A. N. (1982). Morphology, Geology and Developmental History of the Southermost Coral Reefs of Western Atlantic. Abrolhos Bank, Brazil. Doctoral Thesis, University of Miami, Coral Gables, FL.
Leão, Z. M. A. N., and Dominguez, J. M. L. (2000). “Tropical coast of Brazil,” in Seas at the Millennium: An Environmental Evaluation, Vol. 1, ed. C. R. C. Sheppard (Pergamon: Europe, the Americas and West Africa), 719–729.
LeCroy, S. E., Gasca, R., Winfield, I., Ortiz, M., and Escobar-Briones, E. (2009). “Amphipoda (Crustacea) of the Gulf of Mexico,” in Gulf of Mexico–Origins, Waters, and Biota, Vol. 1, eds D. L. Felder and D. K. Camp (College Station, TX: Texas A&M University Press), 941–972.
LeCroy, S. E. (2002). “An illustrated identification guide to the nearshore marine and estuarine Gammaridean Amphipoda of Florida,” in Families Ampeliscidae, Amphilochidae, Ampithoidae, Aoride, Argissidae, and Haustoriidae, Vol. 2, (Tallahassee, FL: Florida Department of Environmental Protection), 213.
Ledoyer, M. (1972). Presence de Perioculodes aequimanus (Kossmann) dans les eaux mediterranennes (region de Marseille) et comparaison avec P. longimanus (Bate et Westwood) (Crustacea, Amphipoda). Bull. Mus. Natl. d’histoire Nat. Zool. 491, 775–781.
Ledoyer, M. (1982). Crustacés amphipodes gammariens. Familles des Acanthonotozomatidae à Gammaridae. Faune de Madagascar 59, 1–598.
Leite, F. P. P. (2011). “Amphipoda,” in Biodiversidade e Ecossistemas Bentônicos Marinhos do Litoral Norte de São Paulo Susteste do Brasil, eds A. C. Z. Amaral and S. A. H. Nallin (Campinas: Unicamp/IB), 327–333.
Leite, F. P. P., Siqueira, S. G. L., Oliveira, D. A., Hoff, C., Requel, A. C., Brumatt, P. N., et al. (2011). “Peracáridos dos substratos biológicos de costões rochosos,” in Biodiversidade e Ecossistemas Bentônicos Marinhos do Litoral Norte de São Paulo Susteste do Brasil, eds A. C. Z. Amaral and S. A. H. Nallin (Campinas: Unicamp/IB), 327–333.
Lima, D., Tavares, M., and Mendonça, J. B. (2019). Paguroids (Decapoda: Anomura: Diogenidae and Paguridae) of the remote oceanic Archipelago Trindade and Martin Vaz, off southeast Brazil, with new records, description of three new species and zoogeographical notes. Zootaxa 4694, 1–63. doi: 10.11646/zootaxa.4694.1.1
Lowry, J. K., and Myers, A. A. (2013). A phylogeny and classification of the Senticaudata subord. nov. (Crustacea: Amphipoda). Zootaxa 3610, 1–80. doi: 10.11646/zootaxa.3610.1.1
Lowry, J. K., and Stoddart, H. E. (2003). “Crustacea: malacostraca: peracarida: Amphipoda, cumacea, mysidacea,” in Zoological Catalogue of Australia, Vol. 19.2B, eds P. L. Beesley and W. W. K. Houston (Melbourne: CSIRO Publishing), 1–479.
MacArthur, R. H., and Wilson, E. O. (1967). The theory of Island Biogeography. Princeton: Princeton University Press.
Macieira, R. M., Simon, T., Pimentel, C. R., and Joyeux, J.-C. (2015). Isolation and speciation of tidepool fishes as a consequence of Quaternary sea-level fluctuations. Environ. Biol. Fishes 98, 385–393. doi: 10.1007/s10641-014-0269-0
Martín, A., Díaz, Y., Miloslavich, P., Escobar-Briones, E., Guerra-García, J. M., Ortiz, M., et al. (2013). Regional diversity of Amphipoda in the Caribbean Sea. Rev. Biol. Trop. 61, 1681–1720. doi: 10.15517/rbt.v61i4.12816
Menzies, R. J. (1953). No. 9- The apseudid Chelifera of the eastern tropical and north temperate Pacific Ocean. Bull. Mus. Comp. Zool. Harvard Coll. 107, 443–496.
Miller, M. A. (1940). The Isopod Crustacea of the Hawaiian Islands (Chelifera and Valvifera). Bernice Pauahi Bish. Mus. Occas. Pap. 15, 295–319.
Moreira, P. S. (1977). Occurrence and ecological notes on Rocinela signata (Isopoda, Flabellifera) off Brazil. Bolm. Inst. Oceanogr. 26, 293–301. doi: 10.1590/s0373-55241977000200007
Myers, A. A. (1986). Amphipoda from the South Pacific: Niue Island. J. Nat. Hist. 20, 1381–1392. doi: 10.1080/00222938600770921
Nixon, K. C. (2002). Winclada (Beta) (Version 1.00.08). Avaliable at: http://cladistics.com/about_winc.html. (accessed 26 July 2020).
O’Leary, M. A., and Kaufman, S. G. (2012). MorphoBank 3.0: Web Application for Morphological Phylogenetics and Taxonomy. Avaliable at: http://www.morphobank.org (accessed July 7, 2020).
Oliveira, L. P. H. (1951). The genus Elasmopus on the coast of Brazil with description of Elasmopus besnardi n. sp., and E. fusimanus n. sp. (Crustacea, Amphipoda). Bolm. Inst. Paul. Oceanogr. 2, 1–17. doi: 10.1590/s0100-42391951000200001
Oliveira, L. P. H. (1953). Crustacea Amphipoda do Rio de Janeiro. Mem. Inst. Oswaldo Cruz 51, 289–376. doi: 10.1590/s0074-02761953000100009
Ortiz, M., and Lalana, R. (1994). Two new species of the genus Elasmopus (Amphipoda, Gammaridea), from the Cuban marine waters. Trav. du Muséum Natl. d’Histoire Nat. ‘Grigore Antipa’ 34, 293–302.
Ortíz, M., Martín, A., and Díaz, Y. J. (2007). Lista y referencias de los crustáceos anfípodos (Amphipoda: Gammaridea) del Atlántico occidental tropical. Rev. Biol. Trop. 55, 479–498. doi: 10.15517/rbt.v55i2.6026
Pachelle, P. P. G., and Tavares, M. (2020). Axiidean ghost shrimps (Decapoda: Axiidae, Callianassidae, Callichiridae, Micheleidae) of the Trindade and Martin Vaz Archipelago, Vitória-Trindade Seamounts Chain and Abrolhos, off southeastern Brazil. Zootaxa 4758, 103–126. doi: 10.11646/zootaxa.4758.1.4
Paiva, P. C. (2005). “Soft-bottom polychaetes of the Abrolhos Bank,” in A Rapid Marine Biodiversity Assessment of the Abrolhos Bank, Bahia, Brazil. The RAP Bulletin of Biological Assessment, eds G. Dutra, G. Allen, T. Werner, and S. McKenna (Washington, DC: Conservation International), 87–90.
Paz-Ríos, C. E., Simões, N., and Ardisson, P.-L. (2013a). Intertidal and shallow water amphipods (Amphipoda : Gammaridea and Corophiidea) from Isla Pérez, Alacranes Reef, southern Gulf of Mexico. Nauplius 21, 179–194. doi: 10.1590/S0104-64972013000200005
Paz-Ríos, C. E., Simões, N., and Ardisson, P.-L. (2013b). Records and observations of amphipods (Amphipoda : Gammaridea and Corophiidea) from fouling assemblages in the Alacranes Reef, southern Gulf of Mexico. Mar. Biodiv. Rec. 6, 1–16. doi: 10.1007/978-3-319-23534-9_1
Pereira-Filho, G. H., Amado-Filho, G. M., Guimarães, S. M., Moura, R. L., Sumida, P. Y., Abrantes, D. P., et al. (2011). Reef fish and benthic assemblages of the Trindade and Martin Vaz island group, southwestern Atlantic.Braz. J. Oceanogr. 59, 201–212. doi: 10.1590/s1679-87592011000300001
Pinheiro, H. T., Bernardi, G., Simon, T., Joyeux, J. C., Macieira, R. M., and Gasparini, J. L. (2017). Island biogeography of marine organisms. Nature 549, 82–85. doi: 10.1038/nature23680
Pinheiro, H. T., Mazzei, E., Moura, R. L., Amado-Filho, G. M., Carvalho-Filho, A., Braga, A. C., et al. (2015). Fish biodiversity of the Vitória-Trindade Seamount Chain, southwestern Atlantic: an updated database. PLoS One 10:e0118180. doi: 10.1371/journal.pone.0118180
Riggio, S. (1973). Segnalazione del genere Synapseudes Miller, 1940 (Crustacea Peracarida Anisopoda) nel Mediterraneo con la Descrizione preliiminare di Synapseudes shiinoi n. sp. Mem. Biol. Mar. Oceanogr. Mess. N.S. 3, 11–19.
Ronquist, F., and Huelsenbeck, J. P. (2003). MrBayes3: bayesian phylogenetic inference undermixed models. Bioinformatics 19, 1572–1574. doi: 10.1093/bioinformatics/btg180
Rosenzweig, M. L. (1995). Species Diversity in Space and Time. Cambridge: Cambridge University Press.
Ruffo, S. (1954). Studi sui crostacei anfipodi. XL. Nuovi anfipodi raccolti nel Venezuelan dal Prof. G. Marcuzzi. Mem. Mus. Civ. Storia Nat. Ver. ona 4, 117–125.
Ruffo, S. (1959). Contributions to the knowledge of the Red Sea No. 13. Contributo alia conoscenze degli anfipodi del Mar Rosso (1) (Materiali raccolti a Ghardaqa e nel Golfo di Aqaba). Bull. Sea Fish. Res. Stat. Haifa 20, 1–26. doi: 10.1080/11250003809436977
Santos, J. P., and Soares, C. M. A. (1999). Crustacea Amphipoda Gammaridea da Praia de Piedade – Jaboatão dos Guararapes – Pernambuco – Brasil. Trab. Oceanog. Univ. Fed. 27, 61–72. doi: 10.5914/tropocean.v27i2.2818
Savigny, J. C. (1816). Observations generales sur la bouche des arachnides, des crustaces et des entomostraces, Memories sur les Animaux sans Vertebres. Second Memorie 1, 39–117.
Senna, A. R., and Souza-Filho, J. F. (2011). A new species of the Elasmopus rapax complex (Crustacea: Amphipoda: Maeridae) from Brazilian waters. Cah. Biol. Mar. 52:57.
Serejo, C. S. (1999). “Taxonomy and distribution of the family Hyalidae (Amphipoda, Talitroidea) on the Brazilian coast,” in Crustaceans and the Biodiversity Crisis. Proceedings of the Fourth International Crustacea Congress. Crustacean Issues, eds F. R. Schram and J. C. V. Klein (Leiden: Brill), 591–616.
Serejo, C. S., and Licínio, M. V. S. (2002). The genus Ampithoe (Crustacea, Amphipoda, Ampithoidae) from the Brazilian coast. Arq. Mus. Nac. 60, 41–60.
Serejo, C. S., and Siqueira, S. G. L. (2018). Catalogue of the Order Amphipoda from Brazil (Crustacea, Peracarida): suborders amphilochidea, senticaudata and order ingolfiellida. Zootaxa 4431, 1–139. doi: 10.11646/zootaxa.4431.1.1
Shiino, S. M. (1951). On two new species of the family Apseudidae found at Seto. Rep. Facul. Fish. Prefect. Univ. Mie 1, 11–25.
Shoemaker, C. R. (1933). Two New Genera and Six New Species of Amphipoda From Tortugas. Papers of the Tortugas Laboratory. Washington, DC: Carnegie Institute of Washington, 245–256.
Shoemaker, C. R. (1938). Three new species of the amphipod genus Ampithoe from the west coast of America. J. Wash. Acad. Sci. 28, 15–25.
Sieg, J. (1986). “Crustacea Tanaidacea of the antarctic and the subantarctic. 1. On material collected at tierra del Fuego, Isla de los Estados, and the west coast of the Antarctic Peninsula,” in Biology of the Antarctic Seas 18, Vol. 45, ed. L. S. Korniker (Washington, D.C: American Geophysical Union). the Antarctic Research Series.
Simone, L. R. L., and Cavallari, D. C. (2020). A new species of Macrocypraea (Gastropoda, Cypraeidae) from Trindade Island, Brazil, including phenotypic differentiation from remaining congeneric species. PLoS One 15:e0225963. doi: 10.1371/journal.pone.0225963
Siqueira, S. G. L. (2012). Alga parda Sargassum Furcatum e Anfípodes Ampitoídeos Associados como Potenciais Bioindicadores de Poluição por Hidrocarbonetos de Petróleo. Ph. D. Thesis, Universidade Estadual de São Paulo, São Paulo, 126.
Sivaprakasam, T. E. (1970). A new species and a new record of Amphipoda (Crustacea) from the Gulf of Mannar. J. Mar. Biol. Assoc. India 10, 274–282.
Soledade, G. O., Fonseca, M. S., and Almeida, A. O. (2015). Shallow-water stenopodidean and caridean shrimps from Abrolhos Archipelago, Brazil: new records and updated checklist. Zootaxa 3905, 52–68.
Soledade, G. O., Santos, G. G., Pinheiro, U., and Almeida, A. O. (2017). New records of association between caridean shrimps (Decapoda) and sponges (Porifera) in Abrolhos Archipelago, northeastern Brazil. Nauplius 25. doi: 10.1590/2358-2936e2017027
Sotka, E. E., Bell, T., Hughes, L. E., Lowry, J. K., and Poore, A. G. (2017). A molecular phylogeny of marine amphipods in the herbivorous family Ampithoidae. Zool. Scr. 46, 85–95. doi: 10.1111/zsc.12190
Souza, L. L., Azevedo, H. J. C. C., Vargas, A. B., Senna, A. R., and Souza, L. A. (2013). First record of Porcellionides pruinosus (Brandt, 1833)(Oniscidea: Porcellionidae) from Trindade Island, off Espírito Santo state coast, Brazil. Bol. Mus. Biol. Mello Leitão 32, 71–78.
Souza-Filho, J. F., and Serejo, C. S. (2012). Redescription and designation of a neotype for Eudevenopus capuciatus (Oliveira, 1955) (Crustacea: Amphipoda: Platyschnopidae) from Brazilian waters, with comments on its cuticular ultrastructures. Cah. Biol. Mar. 53, 469–484.
Stebbing, T. R. R. (1888). Report on the Amphipoda collected by H.M.S. Challenger during the years 1873–76. Report on the Scientific Results of the Voyage of H.M.S. Challenger during the Years 1873–1876. Zool. 29, 1–1737.
Stebbing, T. R. R. (1899). Amphipoda from the copenhagen museum and other sources. Part II. Trans. Linn. Soc. Lond. Zool. 8, 395–432. doi: 10.1111/j.1096-3642.1899.tb00202.x
Stebbing, T. R. R. (1906). Amphipoda I. Gammaridea. Das Tierreich 21, 1–806. doi: 10.11646/zootaxa.646.1.1
Tanaka, M. O., and Leite, F. P. P. (2003). Spatial scaling in the distribution of macrofauna associated with Sargassum stenophyllum (Mertens) Martius: analyses of faunal groups, gammarid life habits, and assemblage structure. J. Exp. Mar. Biol. Ecol. 293, 1–22. doi: 10.1016/s0022-0981(03)00233-8
Tavares, M., Carvalho, L., and Mendonça, J. B. (2017). Towards a review of the decapod Crustacea from the remote oceanic Archipelago of Trindade and Martin Vaz, South Atlantic Ocean: new records and notes on ecology and zoogeography. Pap. Avulsos Zool. Mus. Zool. Univ. São Paulo 57, 157–176. doi: 10.11606/0031-1049.2017.57.14
Thiel, M. (2003). “Rafting of benthic macrofauna: important factors determining the temporal succession of the assemblage on detached macroalgae,” in Migrations and Dispersal of Marine Organisms, eds M. B. Jones, A. Ingólfsson, E. Ólafsson, G. V. Helgason, K. Gunnarsson, and J. Svavarsson (Dordrecht: Springer), 49–57. doi: 10.1023/b:hydr.0000008486.37391.60
Thomas, J. D. (1993). Identification manual for marine Amphipoda (Gammaridea): I. Common coral Reef and Rocky Bottom Amphipods of South Florida. Tallahassee, FL: Florida Department of Environmental Protection, 1–83. Final Report, Contract No. SP290.
Vanhöffen, E. (1914). Die Isopoden der deutschen Südpolar-Expedition 1901-1903. Deutsche Südpolar-Expedition, Zoologie 15, 447–598.
Wheeler, W. C., Schuh, R. T., and Bang, R. (1993). Cladistic relationships among higher groups of Heteroptera: congruence between morphological and molecular data sets. Insect Syst. Evol. 24, 121–137. doi: 10.1163/187631293x00235
Whittaker, R. J., Triantis, K. A., and Ladle, R. J. (2008). A general dynamic theory of oceanic island biogeography. J. Biogeogr. 35, 977–994. doi: 10.1111/j.1365-2699.2008.01892.x
Young, P. S., and Serejo, C. S. (2005). “Crustacea of the Abrolhos Region, Brazil,” in A Rapid Marine Biodiversity Assessment of the Abrolhos Bank, Bahia, Brazil, Vol. 38, eds G. F. Dutra, G. R. Allen, T. Werner, and S. A. McKenna (Washington, DC: Conservation International), 91–95.
Keywords: dispersal, event-based biogeography, cladistics, morphology, taxonomy
Citation: Iwasa-Arai T, Siqueira SGL, Segadilha JL and Leite FPP (2021) The Unique Amphipoda and Tanaidacea (Crustacea: Peracarida) Associated With the Brown Algae Dictyota sp. From the Oceanic Trindade Island, Southwestern Atlantic, With Biogeographic and Phylogenetic Insights. Front. Mar. Sci. 8:641236. doi: 10.3389/fmars.2021.641236
Received: 13 December 2020; Accepted: 09 March 2021;
Published: 12 April 2021.
Edited by:
Jose Manuel Guerra-García, Seville University, SpainReviewed by:
Jesser F. Souza-Filho, Federal University of Pernambuco, BrazilCopyright © 2021 Iwasa-Arai, Siqueira, Segadilha and Leite. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Tammy Iwasa-Arai, YXJhaXRhbW15QGdtYWlsLmNvbQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.