- 1Laboratory of Enzyme Chemistry, G.B. Elyakov Pacific Institute of Bioorganic Chemistry Far Eastern Branch, Russian Academy of Sciences, Vladivostok, Russia
- 2Laboratory of Immunology, G.P. Somov Research Institute of Epidemiology and Microbiology, Vladivostok, Russia
Brown alga Fucus evanescens, widespread in the Far Eastern seas of Russia, is valuable source of sulfated polysaccharides—fucoidans with beneficial biological activities. The most homogenous fraction of fucoidan from F. evanescens was shown to be molecule containing linear main chain of alternating 2-sulfated 1,3- and 1,4-linked α-L-fucose residues. Few sulfate groups were found in position 4 of some 1,3-linked fucose residues. Acetyl groups occupied free C-3 of 1,4-linked residues and/or the C-4 of 1,3-linked fucose residues. Enzymatic hydrolysis, mild acid hydrolysis, and autohydrolysis of native fucoidan were used for elucidation of the fine structural characteristics of fucoidan from F. evanescens. The aim of this review to summarize published data on biological activities of fucoidan from F. evanescens: antiviral, anticoagulant, thrombolytic, hepatoprotective, immunomodulatory, anticancer, and their practical application.
Introduction
Brown algae contain several compounds with biological activities: polysaccharides, iodine organic products, mannitol, macro- and micro-elements, vitamins, unsaturated fatty acids, and other biogenic compounds. The aqueous ethanol extracts of the brown alga Fucus evanescens were enriched with tyrosine and phenylalanine (these amino acids are precursors of thyroxin biosynthesis in humans), proteins, phenol compounds, and chlorophyll (Imbs et al., 2009). In addition, F. evanescens is a promising source of fucoxanthin (Imbs et al., 2013). We present data about the structure, biological activities, and practical application of sulfated polysaccharide—fucoidan—from the F. evanescens.
We did not aim to compare the data about fucoidans from F. evanescens with fucoidans from other Fucus species, as there are published a number of reviews on various fucoidans. It is also difficult to compare our data with the data for fucoidans from other Fucus species, because the authors often use commercial products which are not structurally characterized completely. It is known that structural characteristics of fucoidans depend on the method of isolation and fractionation of the polysaccharides. The comparison with commercially available fucoidan from Fucus vesiculosus is not quite correct, because it contains a lot of impurities (Nishino et al., 1994).
We wanted that results about investigations of fucoidans from F. evanescens were accessible to other researchers, since most of the results were published in Russian journals.
Structure of Fucoidans from Fucus evanescens
The brown alga F. evanescens belongs to the family Fucaceae of the order Fucales. Fucoidans obtained from the alga of this family are sulfated fucans with a main chain of alternating residues of 1,3- and 1,4-linked α-L-fucose (Chevolot et al., 2001; Bilan et al., 2004, 2006).
Studies of the fucoidan content in F. evanescens, which depends on the location of algal harvest and on the conditions of the polysaccharide extraction procedure, were performed by our group (Table 1; Zvyagintseva et al., 2003). We found that this alga contained the highest fucoidan content compared to that of other brown algae harvested from the Sea of Okhotsk and Sea of Japan: Saccharina cichorioides, Saccharina japonica, and Saccharina gurjanovae.

Table 1. The fucoidan content in the brown alga Fucus evanescens inhabiting different regions of the Sea of Okhotsk (Zvyagintseva et al., 2003).
The structure of fucoidan from F. evanescens was studied by two research groups: Zvyagintseva et al. (Zvyagintseva et al., 2003; Kusaykin et al., 2006; Anastyuk et al., 2009, 2012; Silchenko et al., 2014) and Bilan et al. (2002). In this investigation (Bilan et al., 2002), crude fucoidan was obtained and separated into five fractions. The most homogenous fraction was chosen for structure elucidation. This fraction contained fucose, sulfate, and acetyl groups at a molar ratio of 1:1.23:0.36 and trace amounts of galactose and xylose. The desulfation and deacetylation of fucoidan were carried out to simplify the polysaccharide structure. The structures of the obtained modified fucoidans were studied by 1D (1H, 13C) and 2D (COSY, TOCSY, ROESY, NOESY, HSQC, and HMBC) NMR spectroscopy and methylation analysis. As a result, fucoidan was shown to be a regular molecule containing disaccharide repeating units, with a linear main chain of alternating 2-sulfated 1,3- and 1,4-linked α-L-fucose residues. A methylation analysis of desulfated fucoidan showed that 1,3- and 1,4-linked fucose residues had a ratio of 1.2:1. Few sulfate groups were found in position 4 of some 1,3-linked fucose residues. Acetyl groups occupied the free C-3 of 1,4-linked residues and/or the C-4 of 1,3-linked fucose residues (Figure 1A). It was shown that xylose residues were not linked to fucose and belonged to 1,4-β-D-xylan.
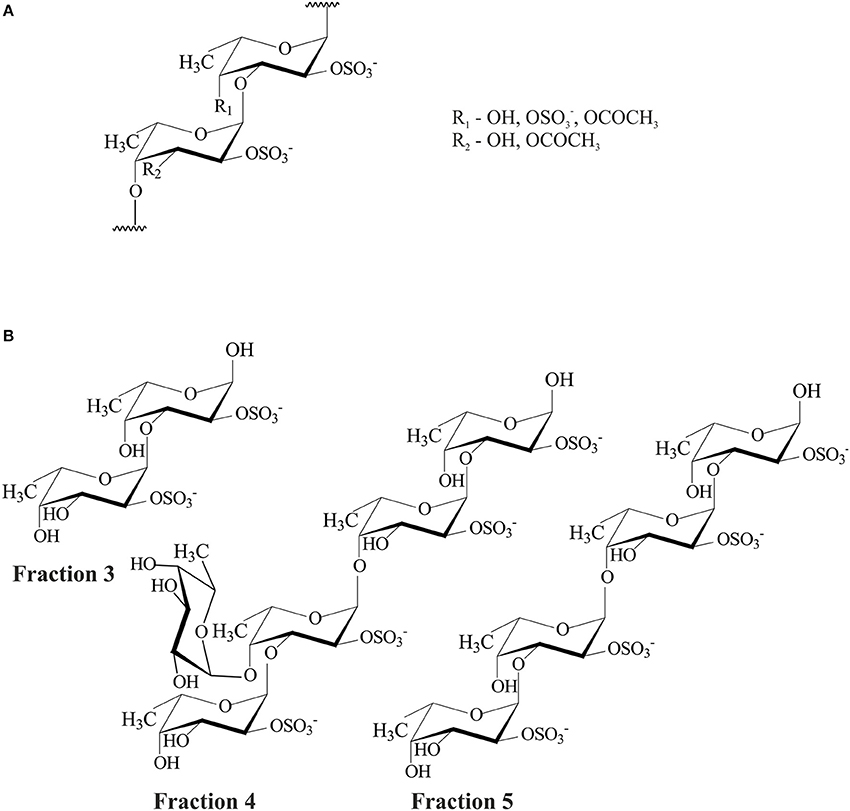
Figure 1. Structures of fucoidan from F. evanescens (A) and products of its enzymatic hydrolysis (B).
It is known that each alga can synthesize fucoidans of various structures. For example, 16 fractions of fucoidans with different contents of 1,3- and 1,4-α-L-fucose residues and degrees of sulfation and acetylation were obtained from commercially available fucoidans from F. vesiculosus, which is widely used for the study of biological activities of polysaccharides(Honya et al., 1999; Ale et al., 2011; Fitton, 2011; Fitton et al., 2015).
We developed a scheme to purify water-soluble polysaccharides from brown algae and to obtain the fraction of fucoidan from F. evanescens with other structural characteristics compared to those of the fucoidan mentioned above (Bilan et al., 2002). According to our data (Kusaykin et al., 2006), fucose and sulfate groups were found at a ratio of 1:0.43. This fraction also contained trace amounts of other monosaccharide residues. A methylation analysis of desulfated fucoidan showed that 1,3- and 1,4-linked fucose residues had a ratio of 3.5:1. Thus, this fraction was distinguished by higher contents of 1,3-linked fucose residues.
To elucidate the fine structural characteristics of fucoidan from F. evanescens, we used enzymatic hydrolysis, low-acid hydrolysis, and autohydrolysis of native fucoidan (Silchenko et al., 2014). Native fucoidan from F. evanescens was depolymerized using fucoidanase from the Vietnamese marine mollusk Lambis sp., which was specific for the α-1,4-O-glycosidic bonds. The products of enzymatic hydrolysis were separated into high- and low molecular weight (HMW and LMW) fractions. The LMW fraction (yield 73%) was fractionated by anion-exchange chromatography to obtain fractions 1, 2, 3, 4, and 5 with yields of 3.6, 4.0, 5.4, 13.7, and 15.8%, respectively. Structures of the more homogenous fractions 3, 4, and 5 were investigated by 1D (1H, 13C) and 2D (COSY, TOCSY, HSQC, and HMBC) NMR spectroscopy. Consequently, fucoidan from F. evanescens had a small amount (~2%) of single 1,4-linked fucose residues in the branches at C-4 of 1,3-linked fucose residues of the main chain. Fraction 3 mainly contained sulfated disaccharide, fraction 5 contained sulfated linear tetrasaccharide, and fraction 4 contained sulfated branched pentasaccharide; the other components of these fractions were present in trace amounts. These observations correlated with the data from the mass spectrometry analysis. Structures of fractions 3, 4, and 5 were characterized as α-L-Fucp-2-OSO-(1,3)-α-L-Fucp-2-OSO, α-L-Fucp-2-OSO-(1,3)-;α-L-Fucp-(1,4)-α-L-Fucp-2-OSO-(1,4)-α-L-Fucp-2-OSO-(1,3)-α-L-Fucp-2-OSO, and α-L-Fucp-2-OSO-(1,3)-α-L-Fucp-2-OSO-(1,4)-α-L-Fucp-2-OSO-(1,3)-α-L-Fucp-2-OSO, respectively (Figure 1B).
In addition, fucoidan was depolymerized under solvolytic conditions, and the LMW fraction was analyzed by MALDI-TOF MS and ESI MS/MS. It was shown that fuco-oligosaccharides (DP = 2–4) mainly had sulfation at C-2 and a prevalence of 1,3-linkages over the 1,4-linkages. Xylose and galactose residues were found to be associated with fucose residues in the followed fragments: Xyl-(1,4)-Fuc, Gal-(1,4)-Fuc, Gal-(1,4)-Gal-(1,4)-Fuc, and Gal-(1,4)-Gal. Fucose, galactose, and xylose residues were sulfated mainly at C-2 and less frequently at C-4. Residues of glucuronic acid were found in the composition of non-sulfated fucooligosaccharides: Fuc-(1,3)-GlcA, Fuc-(1,4)-Fuc-(1,3)-GlcA, and Fuc-(1,3)-Fuc-(1,4)-Fuc-(1,3)-GlcA (Anastyuk et al., 2009). In the next study, fucoidan was depolymerized by autohydrolysis, and the LMW fraction was analyzed by MALDI-TOF MS (Anastyuk et al., 2012). Mass spectrometry analysis showed the presence of galactose-containing fragments with the following structures: Gal-2-OSO3Na-(1,3)-Gal-2-OSO3Na, Gal-2,4-OSO3Na-(1,4)-Fuc, and Fuc-2-OSO3Na-(1,4)-Gal-2-OSO3Na.
Our results show that the intensity of the biosynthesis of fucoidan may significantly depend on the algae habitats. A structural investigation of fucoidan from F. evanescens with enzymatic, chemical, and physical methods allowed us to determine that the minor components, such as Xyl, Gal, and GlcA, are part of the fucoidan molecules from F. evanescens.
This part of the review was devoted to the structural investigation of fucoidans from F. evanescens and to the determination of the location of their minor structural elements. According to the literature, fucoidans are multifunctional polysaccharides (Ermakova et al., 2015). They possess a broad spectrum of biological activities: antiviral, anticoagulant, thrombolytic, hepatoprotective, immunomodulatory, and anticancer. Our aim was to summarize published data about the biological activities of fucoidans from F. evanescens and their practical application.
Biological Activity and Application
Antiviral Activity
Fucoidans have antiviral activity against several viruses: hepatitis C virus, influence virus, Newcastle disease virus, tick-bone encephalitis virus, Dengue virus, hemorrhagic fever with renal syndrome virus, and viruses of the Herpesviridae family (Fitton, 2011; Fitton et al., 2015).
It was found that fucoidan from F. evanescens has no cytotoxic effects on Jurkat and SC-1 cells within a concentration range of 0.001–100 μg/mL. The activity of fucoidan against lentiviral transduction of the Jurkat cells by pseudo-HIV-1 particles with HIV-1 gr120+gp41 or particles containing the G envelope protein from vesicular stomatitis virus (VSV) was studied. These viruses have different mechanisms of cell penetration. Fucoidan inhibits the lentiviral transduction of Jurkat cells by pseudo-HIV-1 particles with the HIV-1 gr120+gp41 envelope protein at 10 μg/mL. The removal of acetyl groups leads to a decrease in the molecular mass of polysaccharides (from 620 to 20 kDa) and their inhibitory activity (IC50 changed from 0.01 to 0.52 μg/mL). In addition, native and deacetylated fucoidans suppressed the infection of SC-1 cells by Mo-MuLV in the same manner (IC50 changed from 0.006 to 4.5 μg/mL). The fucoidan from F. evanescens did not provide a strong inhibition of antiviral activity against pseudo-HIV-1 particles with the envelope protein G of VSV (from 0.1 to 10 μg/mL). These data demonstrate the specific activity of fucoidan from F. evanescens against HIV-1 (Prokofjeva et al., 2013). Fucoidan from Fucus vesiculosus (0.1 μg/mL) possessed inhibitory action on HIV-1 reverse transcriptase (Queiroz et al., 2008).
Fucoidan from F. evanescens exhibited antiviral activity against Hantaan virus (Makarenkova et al., 2008). In vitro, fucoidan from F. evanescens (100–1000 μg/mL) showed virucidal and protective action on cells that were infected with tick-borne encephalitis (Makarenkova et al., 2012a).
Tobacco mosaic virus (TMV) can induce mosaic, mottled, necrotic, stunted, curled-leaf, and yellowing plant tissues. Fucoidan from the brown alga F. evanescens (1 mg/mL) inhibited more than 90% of the spread of an infection induced by TMV in tobacco leaves (Nicotiana tabacum L.) of two cultivars: Ksanti-nk and Samsun (Lapshina et al., 2006). However, the inhibitory effect of fucoidan decreased as the infection spread. Fucoidan directly affected the virus: agglutinated virions were found by electron microscopy in the mixture of TMV (2 μg/mL) with fucoidan (1 mg/mL). It was established that the effect of fucoidan on the plant occurred at the genetic level: the processing of tobacco leaves cv. Ksanti-nk with actinomycin D (10 μg/mL) for 24 h before inoculation with TMV infection almost completely inhibited the antiviral effect of fucoidan (Lapshina et al., 2007). The significance of this discovery is that it shows the biotechnological potential of fucoidan from F. evanescens.
Anticoagulant Activity
The anticoagulant activity of fucoidan from F. evanescens and another 10 brown algae from the Far Eastern part of Russia was determined by measuring the activated partial thromboplastin time (APTT), prothrombin time, and thrombin time. The mechanism of their anticoagulant effect was investigated on the activity of key enzymes of blood coagulation: thrombin and factor Xa. Regarding the most active fucoidan from Laminaria saccharina, the concentration required for the 50% inhibition of thrombin (IC50) was 8.3 μg/mL in the absence of AT III and 0.6 μg/mL in the presence of AT III. For the fucoidan from F. evanescens, IC50 was 9.4 μg/mL and 1.1 μg/mL. For comparison, effectiveness of binding (IC50) of fucoidan from F. vesiculosus to thrombin in the presence of ATIII was 2.1 μg/mL (Ustyuzhanina et al., 2013). All investigated fucoidans interact with AT III and promote thrombin activation, similar to heparin; however, in contrast to heparin, they may directly inhibit thrombin. Fucoidan from F. evanescens, similar to all of the examined fucoidans (except for fucoidan from Laminaria saccharina), weakly inhibited factor Xa in the presence of AT III, in contrast to heparin. None of studied fucoidans inhibited Xa in the absence of AT III. The authors concluded that the anticoagulant activity of the investigated fucoidans did not depend directly on contents of fucose and other neutral sugars or on the structure of the main chain (Ushakova et al., 2009).
Another group calculated the ratio of the inhibitory activity of fucoidan from F. evanescens aXa/aIIa as 1.3–2.3. The effect of fucoidan on the fibrinolysis system was observed by activating the endogenous fibrinolytic system of blood. It was found that the effect of this fucoidan as an anticoagulant in vitro and in vivo was comparable to the effect of heparin. It should be noted that the anticoagulant activity of fucoidan by its oral administration (5 and 10 mg/kg) in the studied doses is weak and recorded as immunotropic activity with parenteral and oral routes of administration (Kuznetsova et al., 2003a,b). The intravenous administration of fucoidan from F. evanescens to rats causes a dose-dependent increase in the plasma anticoagulant activity (Maistrovsky et al., 2010a).
Quantitative differences in the action of fucoidans may result from different sulfation degrees and the presence of various types of glycoside bonds in polysaccharide molecules (Drozd et al., 2006, 2011a,b; Kuznetsova et al., 2006). To investigate effect of fine elements of the structure of fucoidan from F. evanescens on anticoagulant activity, the preparations of fucoidan without protein and polyphenols, as well as deacetylated and depolymerized, were obtained. The deacetylation of fucoidan did not change ability of fucoidan to inhibit thrombin and Xa factor, while removing proteins and polyphenols decreased the polysaccharide activity. The depolymerization of fucoidan cause an increase in the ability to inhibit thrombin predominantly by heparin cofactor II. All of the investigated samples formed a complex with protamine sulfate (Lapikova et al., 2008).
For fractionated fucoidans from F. vesiculosus were showed that fucoidan with charge density of 0.5 sulfates per sugar unit and a size of 70 sugar units demonstrate desired procoagulant activities for improvement of haemostasis in factor VIII/factor IX-deficient plasma (Zhang et al., 2014).
The combination of the immunomodulating properties of the fucoidan from F. evanescens with their anticoagulant activity is promising for improving hemorheology and microcirculation and for reducing tendency to thrombosis during surgery and treatment (injury, combined lesions, intoxication, septicemia, and infectious diseases), as well as secondary immune deficiency.
Hepatoprotective Activity
Fucoidans showed a corrective effect on indicators of lymphocyte activation in patients with hepatitis C. Patients received the standart theraphy and the standard therapy with fucoidan from F. evanescens added (Filonova et al., 2010).
The hepatoprotective effect of fucoidan (oral administration) in experimental chronic toxic hepatitis in mice induced by carbon tetrachloride was registered with magnetic resonance and biochemical analysis. The oral administration of fucoidan led to the normalization of morphological structure and functional state of the liver. The obtained results demonstrate the hepatoprotective activity of fucoidan and open up new prospects for its clinical application in the treatment of hepatitis C.
Fucoidan has low toxicity and is soluble in water and acidic solutions. Currently, the fucoidan from F. evanescens has been standardized and produced in sufficient quantities for research, production of food supplements, and preclinical testing. This polysaccharide regulates the humoral and cellular factors of innate and acquired immunity (Kuznetsova et al., 2010).
The effect of fucoidan extracted from F. evanescens on endotoxin-induced damage in a mouse model of endotoxemia was studied. The results of this investigation demonstrate its preventive effect. The administration of fucoidan (parenteral or per os) increased the survival times of mice, led to the inhibition the increasing levels of proinflammatory cytokines (TNFα and IL-6), and attenuated hypercoagulation and microcirculatory disorders and secondary dystrophic-destructive changes in the liver, kidneys, lungs, and hearts of mice. In addition, fucoidan effectively regulated the immunity and hemostasis systems in experimental endotoxemia, attenuated the course of disseminated intravascular coagulation (DIC) syndrome, prevented endotoxin-induced damage in a mouse model of endotoxemia, and in the long term, has the potential for the development of a drug to reduce of the negative effects of endotoxin (Kuznetsova et al., 2014).
Effect on the Complement System
The increased or decreased activation of alternative pathway of complement (APC) is frustrating for the organism. Especially dangerous is its increased uncontrolled activation of autoimmune complexes, which are capable of causing damage to the organism's own cells. Therefore, it is important to search for new physiologically active substances with a corrective process to activate the complement system.
Fucoidans from F. evanescens, L. cichorioides, and L. japonica were used to study the effect of water-soluble polysaccharides from brown algae on APC. Highly sulfated fucoidan II from L. cichorioides showed the highest activity toward APC (IC50 = 0.7 mg/mL), for fucoidan II from F. evanescens IC50 = 9.2 mg/mL, and for fucoidan from L. japonica IC50 = 20 mg/mL. The authors used preparations of fucoidans with different sulfate group contents, and it was shown that this structural characteristic was not important for the activation of APC. The laminarans (1,3:1,6-β-D-glucans) from these algae were not active in this test. This finding supports the positive influence of fucose residues on the structure of polysaccharides for the activation of APC. This study demonstrates that fucoidan from F. evanescens is inhibitor of human complement activation in vitro (Zvyagintseva et al., 2000).
Fucoidan as Modificator of Enterosorbent
Fucoidan from F. evanescens was examined for the creation of a new immune enterosorbent (Konenkov et al., 2008). The surface of modified sorbent contains hydrophilic centers due to alumina, polar fragments of the polysaccharide, and hydrophobic regions of carbon sorbent. This composition allows the binding of highly toxic substances and provides a detoxifying effect of the sorbent. In addition, the porous structure of the sorbent and the nature of the chemical centers (weak acidic or basic centers) provide its prolonged escape and synergistic hepatoprotective effect.
Immunomodulatory hepatoprotective effects of the new immune enterosorbent and its beneficial effect on the mucous membrane of the gut were shown in vivo. It is possible to use the new sorbent for the treatment of patients with purulent septic diseases (Konenkov et al., 2008). The modified sorbent as the basis of medical dressings and medical creams and as part of complex medical devices can be used as a hemosorbent or enterosorbent or as a carrier for enzymes, cells and biologically active substances, promoting their prolonged action.
Anticancer Activity
The ability of fucoidan from F. evanescens to increase the apoptosis induced by etoposide (inhibitor of DNA topoisomerase II) was investigated. It has been shown that the incubation of MT-4 but not Namalwa cells in the presence of a highly purified preparation of fucoidan (500 μg/mL) increases the sensitivity of these cells to etoposide with the subsequent induction of caspase-3-independent pathways of apoptosis. The results suggested a new role for fucoidan: the combination of fucoidan with approved anticancer drugs for a synergistic effect (Philchenkov et al., 2006, 2007). Commercially available crude fucoidan from the brown seaweed F. vesiculosus (Sigma-Aldrich) became very attractive, in spite of the absence of sufficient data on its exact structure. A number of researchers used this product without any purification. Thus, it was demonstrated fucoidan from F. vesiculosus induced apoptosis of human lymphoma HS-Sultan cells (Aisa et al., 2005), myeloid leukemia U937 cells (Park et al., 2013), and HL-60, NB4, THP-1 cells (Jin et al., 2010) via the activation of caspase-9 and -3 accompanied by down-regulation of Bcl-2 and Bax expression, as well as changes in the phosphorylation of ERK, JNK, p38, and Akt kinases.
The antitumor and antimetastatic activities of fucoidan from F. evanescens were investigated in vivo using mice with transplanted Lewis lung adenocarcinoma (C57Bl/6 mice). Fucoidan (10 mg/kg) alone possess moderate antitumor and antimetastatic effects. In addition, this fucoidan potentiates the antimetastatic but not antitumor activities of cyclophosphamide (drug used for chemotherapy) and, at 25 mg/kg, increases the toxic effect of cyclophosphamide (Alekseyenko et al., 2007).
The fucoidan from F. vesiculosus (20 μg/mL) synergistically reduced cell growth in the EGFR/ERBB2-amplified cancer cell line (OE33) when it was combined with lapatinib, a targeted therapy that acts as a tyrosine kinase inhibitor in advanced HER2-positive breast cancer cells (Oh et al., 2014). The in vitro anticancer activity (soft agar model) of fucoidans derived from nine brown algae species was studied. The fucoidan from F. evanescens (200 μg/mL) was nontoxic to DLD-1 and HT-29 cells and inhibited their colony formation (DLD-1 at 50% and HT-29 at 30%; Vischuk et al., 2009). Several authors investigated the effect of fucoidans from F. vesiculosus on colorectal cancer growth. The fucodan was shown to suppress growth of human colon carcinoma HCT-15 cells on 62% at concentration 100 μg/ml (Hyun et al., 2009), and to induce substantial reductions in viable cell numbers of HT-29 and HCT116 at dose 20 μg/ml (Kim et al., 2010). On other hand, Han et al. examined the growth inhibiting effect of the same fucoidan on HT-29 cells. The cell growth was found to be significantly decreased following treatment with polysaccharide (200 μg/ml; Han et al., 2015). Because of the heterogeneity in structural characteristics within seaweed, differing extraction conditions used by researchers can give rise to the isolation of distinct fucan forms (Li et al., 2008). This fact can explain difference in doses of fucoidan using for the treatment of the same cell lines. This fucoidan (400 μg/mL) was also nontoxic to the melanoma cell lines SK-Mel-5 and SK-Mel-28 and inhibited the cell proliferation (48 h) of these cells in a dose-dependent manner. The fucoidan from F. evanescens (800 μg/mL) inhibited the colony formation of SK-MEL-5 (63%) and SK-MEL-28 (70%) cells, and the content of α-1,4-fucose residues in the fucoidan molecule is important for its inhibiting activity (Anastyuk et al., 2012).
The cancer-preventive efficacy of the fucoidan from F. evanescens in vitro and ex vivo was investigated. Fucoidan participates in the prevention of neoplastic cell transformation and in the progression of colon carcinomas through lymphokine-activated killer T-cell-originated protein kinase (TOPK; Vishchuk et al., 2016).
Immunomodulatory Activity
Fucoidan activates innate immune cells and can protect organisms against pathogenic microorganisms (Makarenkova et al., 2008). The mechanism of the stimulating effect of fucoidan from F. evanescens on immune cells was associated with an enhanced induction of cytokines by dendritic cells (DC; Makarenkova et al., 2010; Khilchenko et al., 2011) and with the maturation of the DC through the downregulation of scavenger receptor class-A type I and type II (SR-A; Jin et al., 2009; Zviagintseva et al., 2009).
Fucoidans enhance the induction of cytokines by dendritic cells (DC) in vitro (Makarenkova et al., 2010) and the maturation of the blood DC through the downregulation of scavenger receptor class-A type I and type II (SR-A; Jin et al., 2009). Fucoidan-induced maturation was eliminated by pretreatment with a TNF-α antibody. Specific inhibitors of p38 MAPK and glycogen synthase kinase 3 suppressed the TNF-a production and maturation of fucoidan-treated peripheral blood dendritic cells. Fucoidan from F. evanescens is effective inductor of the maturation of DC and of enhanced T cell stimulatory capacity (Jin et al., 2009; Zviagintseva et al., 2009). Our date have a good correlation with results obtained for fucoidan from F. vesiculosus (Kim and Joo, 2008).
Fucoidans from F. evanescens, Laminaria japonica, and Laminaria cichorioides possess immunotropic activity and can protect organisms against pathogenic microorganisms. These fucoidans are independent ligands for TLRs. The investigated fucoidans specifically interacted with TLR 2, TLR 4, and the heterodimer TLR 2/6. This interaction resulted in the activation of the transcription nuclear factor NF-κB, which is crucial for the formation of an immune response on the Th1 type (Makarenkova et al., 2012b,c). In addition, fucoidan from F. evanescens (from 10 to 100 μg/mL) can delay (but not suppress) in vitro cell death after UVB irradiation (human skin fibroblast HS68 and immortalized human keratinocyte HaCaT). An investigation of the mechanism of this activity indicated that fucoidans inhibit MMP-1 promoter activity and expression (Moon et al., 2008, 2009; Ku et al., 2010).
The influence of fucoidans from F. evanescens, L. cichorioides, and L. japonica (500 μg/mL) on the apoptosis of human peripheral blood lymphocytes was studied. The investigated fucoidans induced lymphocyte apoptosis through the mitochondrial pathway: they increased the proportion of cells with a low mitochondrial transmembrane potential and inhibited the expression of the Bcl-xL gene in blood lymphocytes (Gazha et al., 2015).
Antioxidant Activity
Fucoidans have been reported to show high antioxidant activity (Hu et al., 2010; Costa et al., 2011; Rodriguez-Jasso et al., 2014). The antioxidant properties of fucoidans are defined by their structural characteristics and are notably related to their molecular weight (Xue et al., 2001). The chemical composition of the sulfated polysaccharides that were extracted from F. evanescens is affected by the extraction method (Imbs et al., 2015a). The fractions of fucoidans from F. evanescens obtained by different extraction methods had different amount of polyphenols. The antioxidant activity of these fucoidans was strongly correlated with polyphenol content but not with sulfation degree or the uronic acid and fucose contents. It is likely that pure fucoidans do not possess antioxidant activity and that the activity observed is due to the presence of polyphenols.
Clinical Trials of Food Supplement Base of Fucoidan
At the G.B. Elyakov Pacific Institute of Bioorganic Chemistry of the Far Eastern Branch of the Russian Academy of Sciences, the fucoidan from F. evanescens was used to create the first Russian food supplement based on fucoidan: “Fucolam®.” “Fucolam®” possesses all of the properties that have been established for fucoidan from F. evanescens.
Clinical investigations using volunteers have shown that the application of “Fucolam®” for the treatment of patients with arteriosclerosis leads to the normalization of the cholesterol distribution among lipoprotein fractions and to a reduced atherogenic index (Maistrovsky et al., 2010b; Kryzhanovsky et al., 2014; Imbs et al., 2015b).
Prebiotics must have resistance to the absorption and activity of enzymes in the upper gastrointestinal tract and must provide species selectivity. The prebiotic potential of fucoidans was investigated in vitro and in vivo (Maistrovskiy et al., 2009). The results of this study indicate that fucoidan from F. evanescens do not digest in the upper gastrointestinal tract and stimulate the growth of bifidobacteria (3–5.8 times compared to that of the control) on a medium enriched with fucoidan (Kuznetsova et al., 2012). Thus, we created “Bifidomarin”—new symbiotic sour milk beverage with B. bifidum (Kuznetsova et al., 2012). “Bifidomarin” can be used in a variety of patients with gastrointestinal tract disorders. This sour milk drink normalizes microbiocenosis and restores both the immune and methanolic statuses (Zaporozhets et al., 2014).
The possible applications of fucoidan from F. evanescens are varied. Our investigations demonstrate the significant potential of “Fucolam®” and “Bifidomarin” as supplements to improve human health.
Author Contributions
RM, describing information about structural investigations of polysaccharides; NS, describing information about investigations of biological activity polysaccharides; TI, describing information about investigations of season variation of polysaccharides and content of polyphenols in brown algae; TNZ, describing information about investigations of biological activity of polysaccharides; OM, describing information about investigations of biological activity of polysaccharides; TSZ, describing information about investigations of biological activity of polysaccharides; NB, describing information about investigations of biological activity of polysaccharides; SE, describing common information about investigations of polysaccharides.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
This project was supported by grants of Russian Foundation for Basic Research (RFBR) No. 16-54-540004, 15-04-01004, and 15-I-5-011.
References
Aisa, Y., Miyakawa, Y., Nakazato, T., Shibata, H., Saito, K., Ikeda, Y., et al. (2005). Fucoidan induces apoptosis of human HS-sultan cells accompanied by activation of caspase-3 and down-regulation of ERK pathways. Am. J. Hematol. 78, 7–14. doi: 10.1002/ajh.20182
Ale, M. T., Maruyama, H., Tamauchi, H., Mikkelsen, J. D., and Meyer, A. S. (2011). Fucoidan from Sargassum sp. and Fucus vesiculosus reduces cell viability of lung carcinoma and melanoma cells in vitro and activates natural killer cells in mice in vivo. Int. J. Biol. Macromol. 49, 331–336. doi: 10.1016/j.ijbiomac.2011.05.009
Alekseyenko, T. V., Zhanayeva, S. Y., Venediktova, A. A., Zvyagintseva, T. N., Kuznetsova, T. A., Besednova, N. N., et al. (2007). Antitumor and antimetastatic activity of fucoidan, a sulfated polysaccharide isolated from the Okhotsk Sea Fucus evanescens brown alga. Bull. Exp. Biol. Med. 143, 730–732. doi: 10.1007/s10517-007-0226-4
Anastyuk, S. D., Shevchenko, N. M., Ermakova, S. P., Vishchuk, O. S., Nazarenko, E. L., Dmitrenok, P. S., et al. (2012). Anticancer activity in vitro of a fucoidan from the brown alga Fucus evanescens and its low-molecular fragments, structurally characterized by tandem mass-spectrometry. Carbohydr. Polym. 87, 186–194. doi: 10.1016/j.carbpol.2011.07.036
Anastyuk, S. D., Shevchenko, N. M., Nazarenko, E. L., Dmitrenok, P. S., and Zvyagintseva, T. N. (2009). Structural analysis of a fucoidan from the brown alga Fucus evanescens by MALDI-TOF and tandem ESI mass spectrometry. Carbohydr. Res. 344, 779–787. doi: 10.1016/j.carres.2009.01.023
Bilan, M. I., Grachev, A. A., Shashkov, A. S., Nifantiev, N. E., and Usov, A. I. (2006). Structure of a fucoidan from the brown seaweed Fucus serratus L. Carbohydr. Res. 341, 238–245. doi: 10.1016/j.carres.2005.11.009
Bilan, M. I., Grachev, A. A., Ustuzhanina, N. E., Shashkov, A. S., Nifantiev, N. E., and Usov, A. I. (2002). Structure of a fucoidan from the brown seaweed Fucus evanescens C.Ag. Carbohydr. Res. 337, 719–730. doi: 10.1016/S0008-6215(02)00053-8
Bilan, M. I., Grachev, A. A., Ustuzhanina, N. E., Shashkov, A. S., Nifantiev, N. E., and Usov, A. I. (2004). A highly regular fraction of a fucoidan from the brown seaweed Fucus distichus L. Carbohydr. Res. 339, 511–517. doi: 10.1016/j.carres.2003.10.028
Chevolot, L., Mulloy, B., Ratiskol, J., Foucault, A., and Colliec-Jouault, S. (2001). A disaccharide repeat unit is the major structure in fucoidans from two species of brown algae. Carbohydr. Res. 330, 529–535. doi: 10.1016/S0008-6215(00)00314-1
Costa, L. S., Fidelis, G. P., Telles, C. B., Dantas-Santos, N., Camara, R. B., Cordeiro, S. L., et al. (2011). Antioxidant and antiproliferative activities of heterofucans from the seaweed Sargassum filipendula. Mar. Drugs 9, 952–966. doi: 10.3390/md9060952
Drozd, N. N., Miftakhova, N. T., Savchik, E. Y., Kalinina, T. B., Makarov, V. A., Imbs, T. I., et al. (2011a). Antithrombotic and hemorrhagic activities of fucoidan isolated from Fucus evanescens brown algae. Eksp. Klin. Farmakol. 74, 26–30.
Drozd, N. N., Shevchenko, N. M., Ermakova, S. P., Lapikova, E. S., Makarov, V. A., and Zvyagintseva, T. N. (2011b). Influence of structural characteristics of fucoidans from brown seaweed on anticoagulant activity and mobility of their complexes with protamine sulfate in electric field. Pharm. Chem. J. 45, 45–50. doi: 10.1007/s11094-011-0560-8
Drozd, N. N., Tolstenkov, A. S., Makarov, V. A., Kuznetsova, T. A., Besednova, N. N., Shevchenko, N. M., et al. (2006). Pharmacodynamic parameters of anticoagulants based on sulfated polysaccharides from marine algae. Bull. Exp. Biol. Med. 142, 591–593. doi: 10.1007/s10517-006-0426-3
Ermakova, S., Kusaykin, M., Trincone, A., and Zvyagintseva, T. (2015). Are multifunctional marine polysaccharides a myth or reality? Front. Chem. 3:39. doi: 10.4081/ni.2016.6534
Filonova, N. V., Zaporozhets, T. S., Ermolitskaya, S. A., Lebedeva, L. V., Pozhidaeva, I. A., Zvyagintseva, T. N., et al. (2010). Functional activity of lymphocytes in peripheral blood of patients with chronic viral hepatitis C in case of comprehensive treatment with Fucus evanescens derived fucoidan. Pac. Med. J. 1, 55–58.
Fitton, J. H. (2011). Therapies from fucoidan; multifunctional marine polymers. Mar. Drugs 9, 1731–1760. doi: 10.3390/md9101731
Fitton, J. H., Stringer, D. N., and Karpiniec, S. S. (2015). Therapies from fucoidan: an update. Mar. Drugs 13, 5920–5946. doi: 10.3390/md13095920
Gazha, A. K., Zaporozhets, T. S., Kuznetsova, T. A., Zviagintseva, T. N., and Besednova, N. N. (2015). Effect of sulfated polysaccharides from brown algae on apoptosis of human peripheral blood lymphocytes. Bull. Exp. Biol. Med. 159, 617–619. doi: 10.1007/s10517-015-3028-0
Han, Y. S., Lee, J. H., and Lee, S. H. (2015). Fucoidan inhibits the migration and proliferation of HT-29 human colon cancer cells via the phosphoinositide-3 kinase/Akt/mechanistic target of rapamycin pathways. Mol. Med. Rep. 12, 3446–3452. doi: 10.3892/mmr.2015.3804
Honya, M., Mori, H., Anzai, M., Araki, Y., and Nishizawa, K. (1999). Monthly changes in the content of fucans, their constituent sugars and sulphate in cultured Laminaria japonica. Hydrobiologia 398–399, 411–416. doi: 10.1023/A:1017007623005
Hu, T., Liu, L., Chen, Y., Wu, J., and Wang, S. (2010). Antioxidant activity of sulfated polysaccharide fractions extracted from Undaria pinnatifida in vitro. Int. J. Biol. Macromol. 46, 193–198. doi: 10.1016/j.ijbiomac.2009.12.004
Hyun, J. H., Kim, S. C., Kang, J. I., Kim, M. K., Boo, H. J., Kwon, J. M., et al. (2009). Apoptosis inducing activity of fucoidan in HCT-15 colon carcinoma cells. Biol. Pharm. Bull. 32, 1760–1764. doi: 10.1248/bpb.32.1760
Imbs, T. I., Ermakova, S. P., Fedoreyev, S. A., Anastyuk, S. D., and Zvyagintseva, T. N. (2013). Isolation of fucoxanthin and highly unsaturated monogalactosyldiacylglycerol from brown alga Fucus evanescens C Agardh and in vitro investigation of their antitumor activity. Mar. Biotechnol. 15, 606–612. doi: 10.1007/s10126-013-9507-2
Imbs, T. I., Krasovskaya, N. P., Ermakova, S. P., Makarieva, T. N., Shevchenko, N. M., and Zvyagintseva, T. N. (2009). Comparative study of chemical composition and antitumor activity of aqueous-ethanol extracts of brown algae Laminaria cichorioides, Costaria costata, and Fucus evanescens. Russ. J. Mar. Biol. 35, 164–170. doi: 10.1134/S1063074009020084
Imbs, T. I., Skriptsova, A. V., and Zvyagintseva, T. N. (2015a). Antioxidant activity of fucose containing sulfated polysaccharides obtained from Fucus evanescens by different extraction methods. J. Appl. Psychol. 27, 545–553. doi: 10.1007/s10811-014-0293-7
Imbs, T. I., Zvyagintseva, T. N., and Ermakova, S. P. (2015b). ≪Fukolam≫ - the first food supplement based on fucoidan in Russia. Vestn. Far East Branch Russ. Acad. Sci. 6, 145–149.
Jin, J. O., Park, H. Y., Xu, Q., Park, J. I., Zvyagintseva, T. N., Stonik, V. A., et al. (2009). Ligand of scavenger receptor class A indirectly induces maturation of human blood dendritic cells via production of tumor necrosis factor-alpha. Blood 113, 5839–5847. doi: 10.1182/blood-2008-10-184796
Jin, J. O., Song, M. G., Kim, Y. N., Park, J. I., and Kwak, J. Y. (2010). The mechanism of fucoidan-induced apoptosis in leukemic cells: involvement of ERK1/2, JNK, glutathione, and nitric oxide. Mol. Carcinogen. 49, 771–782. doi: 10.1002/mc.20654
Khilchenko, S. R., Zaporozhets, T. S., Shevchenko, N. M., Zvyagintseva, T. N., Vogel, U., Seeberger, P., et al. (2011). Immunostimulatory activity of fucoidan from the brown alga Fucus evanescens: role of sulfates and acetates. J. Carbohyd. Chem. 30, 291–305. doi: 10.1080/07328303.2011.604456
Kim, E. J., Park, S. Y., Lee, J. Y., and Park, J. H. (2010). Fucoidan present in brown algae induces apoptosis of human colon cancer cells. BMC Gastroenterol. 10:96. doi: 10.1186/1471-230X-10-96
Kim, M. H., and Joo, H. G. (2008). Immunostimulatory effects of fucoidan on bone marrow-derived dendritic cells. Immunol. Lett. 115, 138–143. doi: 10.1016/j.imlet.2007.10.016
Konenkov, V. I., Liubarskii, M. S., Bgatova, N. P., Rachkovskaya, L. N., Borodin, I. I., Besednova, N. N., et al. (2008). Hepatoprotective properties comparison study of new composite sorbent. Vestn. Limfologii 1, 23–31.
Kryzhanovsky, S. P., Bogdanovich, L. Y., Kushnerova, N. F., and Shevchenko, N. M. (2014). Phospholipids and neutral lipids in dyslipidemic patients and correction of lipid metabolism polysaccharides sea kelp. Fundam. Res. 10, 1951–1958.
Ku, M. J., Jung, J. W., Lee, M. S., Cho, B. K., Lee, S. R., Lee, H. S., et al. (2010). Effect of Fucus evanescens fucoidan on expression of matrix metalloproteinase-1 promoter, mRNA, protein and signal pathway. J. Life Sci. 20, 1603–1610. doi: 10.5352/JLS.2010.20.11.1603
Kusaykin, M. I., Chizhov, A. O., Grachev, A. A., Alekseeva, S. A., Bakunina, I. Y., Nedashkovskaya, O. I., et al. (2006). A comparative study of specificity of fucoidanases from marine microorganisms and invertebrates. J. Appl. Psychol. 18, 369–373. doi: 10.1007/s10811-006-9042-x
Kuznetsova, T. A., Agafonova, I. G., Krokhmal, T. S., Zvyagintseva, T. N., and Filonova, N. V. (2010). Hepatoprotective properties of Fucus evanescens derived fucoidan. Pac. Med. J. 4, 32–35.
Kuznetsova, T. A., Besednova, N. N., Mamaev, A. N., Momot, A. P., Shevchenko, N. M., and Zvyagintseva, T. N. (2003a). Anticoagulant activity of fucoidan from brown algae Fucus evanescens of the Okhotsk Sea. Bull. Exp. Biol. Med. 136, 471–473. doi: 10.1023/B:BEBM.0000017096.72246.1f
Kuznetsova, T. A., Besednova, N. N., Somova, L. M., and Plekhova, N. G. (2014). Fucoidan extracted from Fucus evanescens prevents endotoxin-induced damage in a mouse model of endotoxemia. Mar. Drugs 12, 886–898. doi: 10.3390/md12020886
Kuznetsova, T. A., Besednova, N. N., Urvantseva, A. M., Bakunina, I. Y., Zvyagintseva, T. N., Drozd, N. N., et al. (2006). Comparative investigation of biological activity of fucoidans from brown seaweeds. Vestn. Far East Branch Russ. Acad. Sci. 6, 105–110.
Kuznetsova, T. A., Zaporozhets, T. S., Besednova, N. N., Shevchenko, N. M., Zvyagintseva, T. N., Mamaev, A. N., et al. (2003b). Immunostimulating and anticoagulating activity of fucoidan isolated from brown algae Fucus evanescens in Okhotsk Sea. Antibiot. Khimioter. 48, 11–13.
Kuznetsova, T. A., Zaporozhets, T. S., Makarenkova, I. D., Timchenko, N. F., Besednova, N. N., Zviagintseva, T. N., et al. (2012). Prebiotic potential of polysaccharides from the brown alga Fucus evanescens and perspectives for the clinical using. Pac. Med. J. 1, 37–40.
Lapikova, E. S., Drozd, N. N., Tolstenkov, A. S., Makarov, V. A., Zvyagintseva, T. N., Shevchenko, N. M., et al. (2008). Inhibition of thrombin and factor Xa by Fucus evanescens fucoidan and its modified analogs. Bull. Exp. Biol. Med. 146, 328–333.
Lapshina, L., Reunov, A., Nagorskaya, V., Zvyagintseva, T., and Shevchenko, N. (2006). Inhibitory effect of fucoidan from brown alga Fucus evanescens on the spread of infection induced by tobacco mosaic virus in tobacco leaves of two cultivars. Russ. J. Plant Physiol. 53, 246–251. doi: 10.1134/S1021443706020154
Lapshina, L., Reunov, A., Nagorskaya, V., Zvyagintseva, T., and Shevchenko, N. (2007). Effect of fucoidan from brown alga Fucus evanescens on a formation of TMV-specific inclusion in the cells of tobaco leaves. Russ. J. Plant Physiol. 54, 111–114. doi: 10.1134/S1021443707010165
Li, B., Lu, F., Wei, X., and Zhao, R. (2008). Fucoidan: structure and bioactivity. Molecules 13, 1671–1695. doi: 10.3390/molecules13081671
Maistrovskiy, K. V., Zaporozhets, T. S., Fedyanina, L. N., Kalenik, T. K., Motkina, E. V., and Imbs, T. I. (2009). Effects of immune response modulating agent fucoidan derived from Fucus evanescens brown algae on the parameters of antioxidative system, lipid and carbohydrate metabolism in mice. Pac. Med. J. 3, 97–99.
Maistrovsky, K. V., Rapovka, V. G., and Shevchenko, N. M. (2010a). Influence of fucoidan on procoagulant link of the hemostasis system patients with obliterating atherosclerosis of lower extremities vessels. Vestn. Ural Med. Acad. Sci. 2/1 29, 167–168.
Maistrovsky, K. V., Zaporozhets, T. S., Rapovka, V. G., Zvyagintseva, T. N., and Shevchenko, N. M. (2010b). Correcting lipid exchange in patients with obliterating atherosclerosis of lower limb vessels with Fucus evanescens-derived sulphated polysaccharide. Pac. Med. J. 4, 47–50.
Makarenkova, I. D., Akhmatova, N. K., Semenova, I. B., Besednova, N. N., Zviagintseva, T. N., and Shevchenko, N. M. (2010). Production of cytokines by murine bone marrow dendritic cells in vitro mediated by sulfated polysaccharides obtained from sea brown algae. J. Mikrobiol. Epidemiol. Immunobiol. 5, 34–39.
Makarenkova, I. D., Kompanets, G. G., Besednova, N. N., Slonova, R. A., Zvyagintseva, T. N., and Shevchenko, N. M. (2008). Inhibiting effects fucoidans on Hantaan virus adsorption on model of peritoneal macrophages in vitro. Vopr. Virusol. 53, 12–15.
Makarenkova, I. D., Leonova, G. N., Maystrovkaya, O. S., Zvyagintseva, T. N., Imbs, T. I., Ermakova, S. P., et al. (2012a). Antiviral effect of brown algae-derivate sulphated polysaccharides in case of experimental tick-borne encephalitis: tying structure and function. Pac. Med. J. 1, 44–46.
Makarenkova, I. D., Logunov, D. Y., Tukhvatulin, A. I., Semenova, I. B., Besednova, N. N., and Zvyagintseva, T. N. (2012c). Interactions between sulfated polsaccharides from sea brown algae and Toll-like receptors on HEK293 eukaryotic cells in vitro. Bull. Exp. Biol. Med. 154, 241–244.
Makarenkova, I. D., Logunov, D. Y., Tukhvatulin, A. I., Semenov, I. B., Zvyagintseva, T. N., Gorbach, V. I., et al. (2012b). Sulfated polysaccharides of brown seaweeds are ligands of Toll-like receptors. Biochem. (Moscow) Suppl. Ser. B Biomed. Chem. 6, 75–80. doi: 10.1134/S1990750812010118
Moon, H. J., Lee, S. H., Ku, M. J., Yu, B. C., Jeon, M. J., Jeong, S. H., et al. (2009). Fucoidan inhibits UVB-induced MMP-1 promoter expression and down regulation of type I procollagen synthesis in human skin fibroblasts. Eur. J. Dermatol. 19, 129–134. doi: 10.1684/ejd.2008.0611
Moon, H. J., Lee, S. R., Shim, S. N., Jeong, S. H., Stonik, V. A., Rasskazov, V. A., et al. (2008). Fucoidan inhibits UVB-induced MMP-1 expression in human skin fibroblasts. Biol. Pharm. Bull. 31, 284–289. doi: 10.1248/bpb.31.284
Nishino, T., Nishioka, C., Ura, H., and Nagumo, T. (1994). Isolation and partial characterization of a novel amino sugar-containing fucan sulfate from commercial Fucus vesiculosus fucoidan. Carbohyd. Res. 255, 213–224. doi: 10.1016/S0008-6215(00)90980-7
Oh, B., Kim, J., Lu, W., and Rosenthal, D. (2014). Anticancer effect of fucoidan in combination with tyrosine kinase inhibitor lapatinib. Evid. Based Complement. Altern. Med. 2014, 865375. doi: 10.1155/2014/865375
Park, H. S., Hwang, H. J., Kim, G. Y., Cha, H. J., Kim, W. J., Kim, N. D., et al. (2013). Induction of apoptosis by fucoidan in human leukemia U937 cells through activation of p38 MAPK and modulation of Bcl-2 family. Mar. Drugs 11, 2347–2364. doi: 10.3390/md11072347
Philchenkov, A. A., Zavelevich, M. P., Khranovskaya, N. N., Zaporozhets, T. S., Imbs, T. I., Zvyagintseva, T. N., et al. (2006). Fucoidan from Far-Eastern seaweeds modulate in vitro apoptosis in human MT-4 leukemic cells. Russ. J. Biother. 4, 30–37.
Philchenkov, A., Zavelevich, M., Imbs, T., Zvyagintseva, T., and Zaporozhets, T. (2007). Sensitization of human malignant lymphoid cells to etoposide by fucoidan, a brown seaweed polysaccharide. Exp. Oncol. 29, 181–185.
Prokofjeva, M. M., Imbs, T. I., Shevchenko, N. M., Spirin, P. V., Horn, S., Fehse, B., et al. (2013). Fucoidans as potential inhibitors of HIV-1. Mar. Drugs 11, 3000–3014. doi: 10.3390/md11083000
Queiroz, K. C., Medeiros, V. P., Queiroz, L. S., Abreu, L. R., Rocha, H. A., Ferreira, C. V., et al. (2008). Inhibition of reverse transcriptase activity of HIV by polysaccharides of brown algae. Biomed. Pharmacother. 62, 303–307. doi: 10.1016/j.biopha.2008.03.006
Rodriguez-Jasso, R. M., Mussatto, S. I., Pastrana, L., Aquilar, C. N., and Teixeira, J. A. (2014). Chemical composition and antioxidant activity of sulphated polysaccharides extracted from Fucus vesiculosus using different hydrothermal processes. Chem. Pap. 68, 203–209. doi: 10.2478/s11696-013-0430-9
Silchenko, A. S., Kusaykin, M. I., Zakharenko, A. M., Menshova, R. V., Khanh, H. H. N., Dmitrenok, P. S., et al. (2014). Endo-1,4-fucoidanase from Vietnamese marine mollusk Lambis sp. which producing sulphated fucooligosaccharides. J. Mol. Catal. B Enzym. 102, 154–160. doi: 10.1016/j.molcatb.2014.02.007
Ushakova, N. A., Morozevich, G. E., Ustiuzhanina, N. E., Bilan, M. I., Usov, A. I., Nifant'ev, N. E., et al. (2009). Anticoagulant activity of fucoidans from brown algae. Biochemistry 3, 77–83. doi: 10.1134/s1990750809010119
Ustyuzhanina, N. E., Ushakova, N. A., Zyuzina, K. A., Bilan, M. I., Elizarova, A. L., Somonova, O. V., et al. (2013). Influence of fucoidans on hemostatic system. Mar. Drugs 11, 2444–2458. doi: 10.3390/md11072444
Vischmuk, O. S., Ermakova, S. P., Pham, D. T., Shevchenko, N. M., Ly, B. M., and Zvyagintseva, T. N. (2009). Anticancer activity of brown algae-derived fucoidans. Pac. Med. J. 3, 86–89.
Vishchuk, O. S., Sun, H., Wang, Z., Ermakova, S. P., Xiao, J. X., Lu, T., et al. (2016). PDZ-binding kinase/T-LAK cell-originated protein kinase is a target of the fucoidan from brown alga Fucus evanescens in the prevention of EGF-induced neoplastic cell transformation and colon cancer growth. Oncotarget 7, 18763–18773. doi: 10.18632/oncotarget.7708
Xue, C. H., Fang, Y., Lin, H., Chen, L., Li, Z. J., Deng, D., et al. (2001). Chemical characters and antioxidative properties of sulfated polysaccharides from Laminaria japonica. J. Appl. Psychol. 13, 67–70. doi: 10.1023/A:1008103611522
Zaporozhets, T. S., Besednova, N. N., Kuznetsova, T. A., Zviagintseva, T. N., Makarenkova, I. D., Kryzhanovsky, S. P., et al. (2014). Prebiotic potential of polysaccharides and extracts of seaweeds. Russ. J. Mar. Biol. 40, 1–9. doi: 10.1134/S1063074014010106
Zhang, Z., Till, S., Jiang, C., Knappe, S., Reutterer, S., Scheiflinger, F., et al. (2014). Structure-activity relationship of the pro- and anticoagulant effects of Fucus vesiculosus fucoidan. Thromb. Haemost. 111, 429–437. doi: 10.1160/TH13-08-0635
Zviagintseva, T. N., Ermakova, S. P., Kusaikin, M. I., Shevchenko, N. M., Fedoreyev, S. A., and Kwak, J. Y. (2009). Agent Inducing the Dendritic Cell Maturation. Russian Patent No 2361598.
Zvyagintseva, T. N., Shevchenko, N. M., Nazarova, I. V., Scobun, A. S., Luk'yanov, P. A., and Elyakova, L. A. (2000). Inhibition of complement activation by water-soluble polysaccharides of some far-eastern brown seaweeds. Comp. Biochem. Physiol. Toxicol. Pharmacol. 126, 209–215. doi: 10.1016/s0742-8413(00)00114-6
Zvyagintseva, T. N., Shevchenko, N. M., Nazarova, I. V., Scobun, A. S., Luk'yanov, P. A., and Elyakova, L. A. (2003). Water-soluble polysaccharides of some far-eastern brown seaweeds. Distribution, structure, and their dependence on the developmental conditions. J. Exp. Mar. Biol. Ecol. 294, 1–13. doi: 10.1016/S0022-0981(03)00244-2
Keywords: brown seaweed, sulfated polysaccharide, application, food supplement, prebiotic
Citation: Menshova RV, Shevchenko NM, Imbs TI, Zvyagintseva TN, Malyarenko OS, Zaporoshets TS, Besednova NN and Ermakova SP (2016) Fucoidans from Brown Alga Fucus evanescens: Structure and Biological Activity. Front. Mar. Sci. 3:129. doi: 10.3389/fmars.2016.00129
Received: 17 March 2016; Accepted: 07 July 2016;
Published: 03 August 2016.
Edited by:
Donatella De Pascale, National Research Council, ItalyReviewed by:
Antje Labes, GEOMAR - Helmholtz Centre for Ocean Research Kiel, GermanyFernando Reyes, Fundación MEDINA, Spain
Copyright © 2016 Menshova, Shevchenko, Imbs, Zvyagintseva, Malyarenko, Zaporoshets, Besednova and Ermakova. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Svetlana P. Ermakova, c3ZldGxhbmFfZXJtYWtvdmFAaG90bWFpbC5jb20=
 Roza V. Menshova
Roza V. Menshova Natalia M. Shevchenko1
Natalia M. Shevchenko1 Tatiana I. Imbs
Tatiana I. Imbs Tatiana N. Zvyagintseva
Tatiana N. Zvyagintseva Tatyana S. Zaporoshets
Tatyana S. Zaporoshets Svetlana P. Ermakova
Svetlana P. Ermakova