
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CASE REPORT article
Front. Immunol. , 09 July 2024
Sec. Primary Immunodeficiencies
Volume 15 - 2024 | https://doi.org/10.3389/fimmu.2024.1414573
 Zhe Sun1,2,3,4†
Zhe Sun1,2,3,4† Ruoqu Wei1,2,3,4†
Ruoqu Wei1,2,3,4† Chaolan Pan1,2,3,4†
Chaolan Pan1,2,3,4† Cheng Ni1,2,3,4
Cheng Ni1,2,3,4 Xue Zhang1,2,3,4
Xue Zhang1,2,3,4 Wenbin Guan5
Wenbin Guan5 Ruhong Cheng1,2,3,4
Ruhong Cheng1,2,3,4 Yan Gu1,2,3,4
Yan Gu1,2,3,4 Hong Yu1,2,3,4
Hong Yu1,2,3,4 Kejun He6
Kejun He6 Zhen Zhang1,2,3,4*
Zhen Zhang1,2,3,4* Xia Yu1,2,3,4*
Xia Yu1,2,3,4* Zhirong Yao1,2,3,4*
Zhirong Yao1,2,3,4*Dedicator of cytokinesis 8 (DOCK8) deficiency represents a primary immunodeficiency with a wide range of clinical symptoms, including recurrent infections, atopy, and increased malignancy risk. This study presents a case of a 6-year-old girl with DOCK8 deficiency, characterized by severe, treatment-resistant herpetic infections who was successfully treated with siltuximab and glucocorticoids. The successful use of siltuximab in achieving remission highlights the pivotal role of interleukin-6 (IL-6) in DOCK8 deficiency pathogenesis and suggests that IL-6 modulation can be critical in managing DOCK8 deficiency-related viral infections, which may inform future therapeutic strategies for DOCK8 deficiency and similar immunodeficiencies.
Type 2 autosomal-recessive hyper-IgE syndrome (AR-HIES2) (OMIM 243700) is a combined immunodeficiency that exemplifies the broad clinical features of IEI (inborn errors of immunity) (1). Mutations in the gene encoding DOCK8 account for the majority of patients with AR-HIES2, referred to as DOCK8 deficiency, characterized by eosinophilia, elevated immunoglobulin E (IgE) levels, recurrent infections, atopic dermatitis (AD), allergies, autoimmunity and malignancy (2, 3). The DOCK8 protein plays an important role in cytoskeletal organization, affecting dendritic cell migration, the proliferation and apoptosis of T cells and B cells, and natural killer cell toxicity (4, 5). It causes susceptibility to cutaneous viral infections, especially with herpes simplex virus (HSV), human papillomavirus (HPV), molluscum contagiosum virus (MCV), and varicella-zoster virus (VZV) (1). These infections may be severe, persistent, and refractory to treatment, representing a major cause of morbidity and at times mortality in DOCK8 deficiency. Here, we describe a patient with DOCK8 deficiency and severe, progressive herpetic infections refractory to therapy with valacyclovir. Our patient got surprising relief after combination treatment of siltuximab and glucocorticoid in addition to antiviral treatment, suggesting a pivotal role of IL-6 in pathogenesis.
A 6-year-old Chinese girl, presenting with a two-year history of wart-like masses on her nose and lip, was referred to our dermatology clinic in May 2021. At age 4, a red papule appeared on her left side of nose, progressed in size and recurred shortly after they were excised three times with the pathological diagnosis of hemangioma in other hospitals. Gradually, asymptomatic granulomatous masses with purulent exudation in the right side of oral cavity developed in the recent 6 months (Figure 1A). The patient didn’t have any constitutional symptoms including fever, night sweats, fatigue and weight loss. She was born of a consanguineous marriage and exhibited a clinical history notable for recurrent respiratory tract infections, AD and foods allergies to milk, eggs, and seafood.
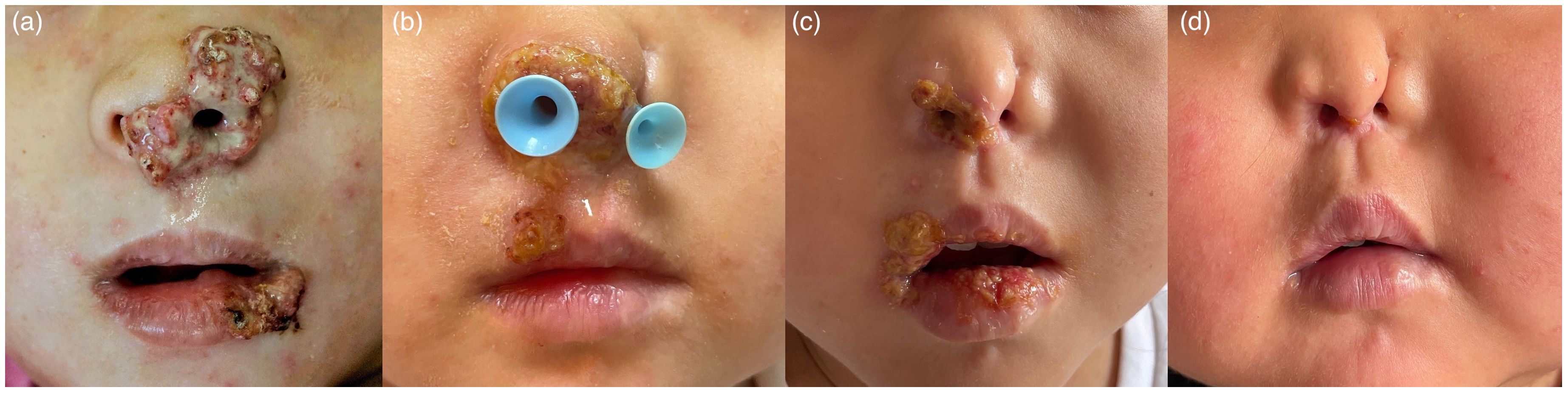
Figure 1 Representative pictures of the lesions in patient early in their infection. (A), after IFN-a therapy (B), after 4 doses of siltuximab (C), and 6 months after treatment (D), respective.
Physical examination revealed multiple enlarged lymph nodes in submental, submandibular and neck region. Laboratory results were presented in Table 1. Human immunodeficiency virus and HHV-8 serologies were negative. We took the nasal lesion for pathology and revealed an epithelioid hemangioma (Figures 2A, B). Biopsy from oral cavity showed superficial and deep perivascular inflammation with cavernous edema (Figures 2C, D). The pathology of enlarged right submandibular lymph node revealed reactive lymphoid hyperplasia with Castleman-like histology, including follicular hyperplasia, partly thickening of follicular mantle zone, and proliferation of plasma cells in the lymphoid interlobular area (Figures 2E, F). HSV-1 was confirmed by immunohistochemical analysis of the nasal lesion.
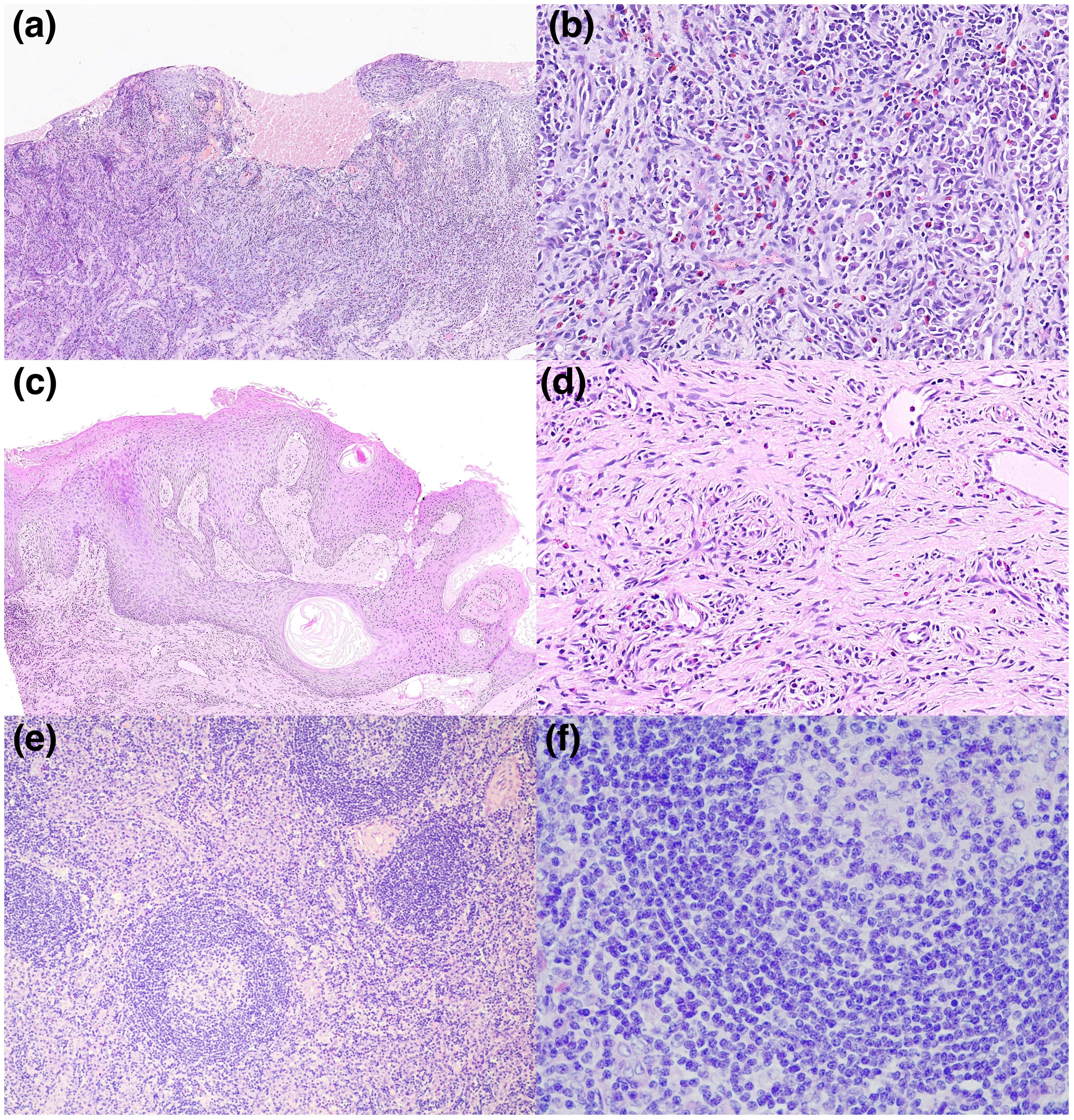
Figure 2 (A–F) Microscopies of skin biopsy specimens, hematoxylin and eosin (H&E). (A, B) Biopsy of nose displayed local ulceration of squamous epithelium with inflammatory exudation, granulation tissue hyperplasia and squamous pseudoepithelioma. A significant infiltration of neutrophils, eosinophils, and plasma cells is evident. Degenerative changes in the nuclei of the squamous epithelial cells, particularly at the site of the ulceration, aligns with the histopathological hallmarks consistent with HSV infection. (C, D) Biopsy of oral cavity showed superficial and deep perivascular inflammation with cavernous edema. (E, F) Biopsy of the enlarged right submandibular lymph node revealed follicular hyperplasia, partly thickening of follicular mantle zone, and proliferation of plasma cells in the lymphoid interlobular area, resembling Castleman’s disease. (A: H&E, x100; B: H&E, x400; C: H&E, x100; D: H&E, x400; E: H&E, x100; F: H&E, x400).
Approval was obtained from the ethics committee of Xinhua Hospital, Shanghai Jiaotong University School of Medicine, and all procedures were performed according to the principles of the Declaration of Helsinki. To identify whether the patient had primary immunodeficiency, whole-exome sequencing (WES) was performed after obtaining written informed consent. WES revealed a homozygous mutation in the DOCK8 gene, which was a ~58.6Kb genomic deletion in exons 1–2 inherited from her parents. We performed qRT-PCR for exon1, Intron1, exon2, Intron2 and exon3 (which was contained in this ~58.6Kb-size deletion) of DOCK8, and the proband and her parents showed the deletion (chr9:214506-273133) in exon 1 and exon 2 of DOCK8 (Figure 3). Combined with the clinical manifestations, laboratory results and WES findings, AR-HIES2 was established.
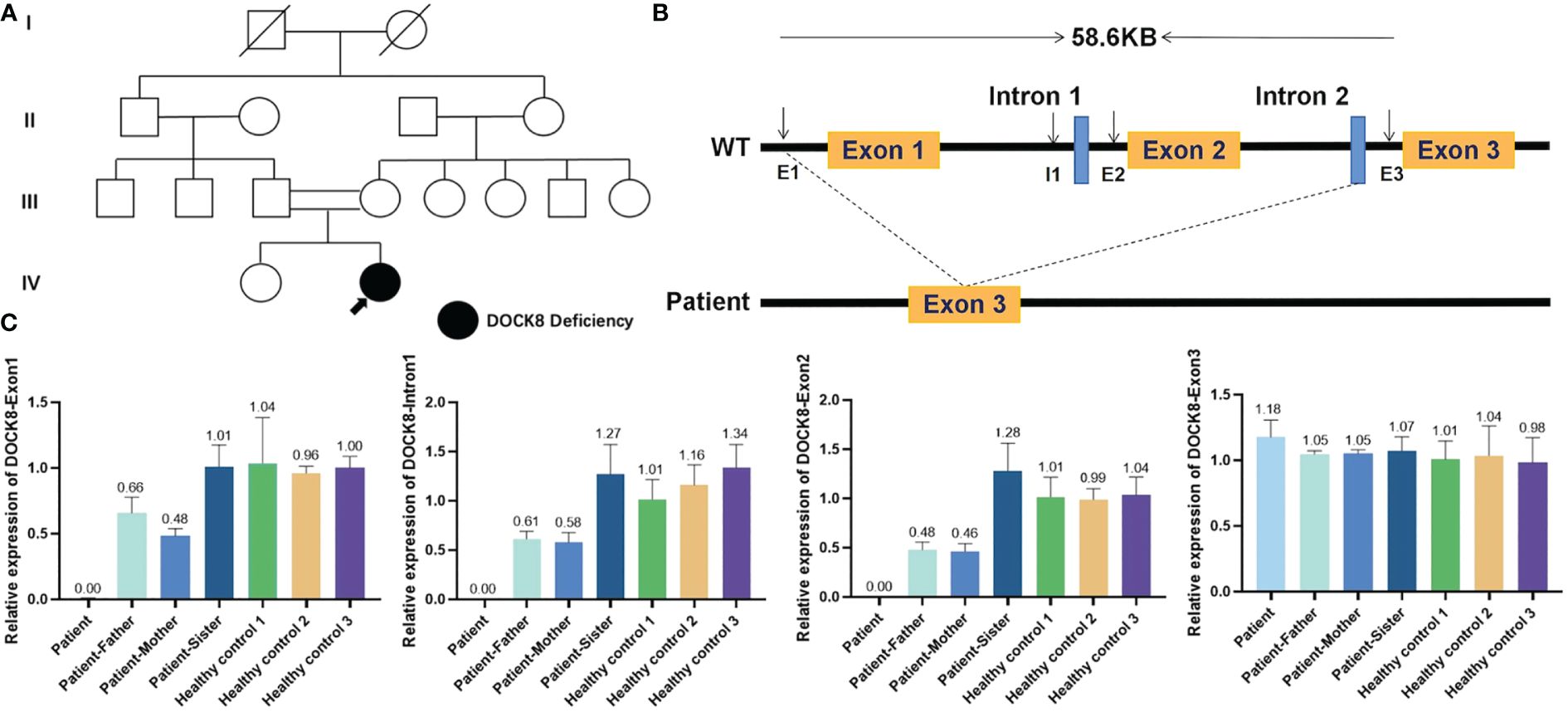
Figure 3 Deletion in dedicator of cytokinesis 8 (DOCK8). (A) Pedigree of a Chinese family with AR-HIES2. Filled symbol represents the patient. Open symbols represent unaffected individuals. The double lines represent a consanguineous union. Her parents and sister were all healthy. (B) Schematic representation of the wildtype DOCK8 gene, which comprises 48 exons. The patient has a homozygous mutation in the DOCK8 gene, which was a 58.6Kb genomic deletion in exons 1–2 inherited from her parents. (C) To verify whether the proband, her parents and sister had the deletion, we performed qRT-PCR for exon1, Intron1, exon2, Intron2 and exon3 (which was contained in this ~58.6Kb-size deletion) of DOCK8. RNA was isolated from the peripheral blood of the patient, her parents, her sister and three healthy controls by the RNAzol method. CT values were compared with that of ALB by the relative quantification system. The proband and her parents showed the deletion (chr9:214506-273133) in exon 1 and exon 2 of DOCK8.
The patient had experienced various treatments. Oral valaciclovir and intravenous immunoglobulin (1g/kg every month) were prescribed for 2 months, and the exudation of nose and oral cavity reduced while there was no visible shrinkage of granulomatous masses. As the granulomatous mass on the nose corroded the nasal cavity, we conducted surgical resection again but relapsed one month later (Figure 1B). Pegylated interferon-alpha 2a (IFNα-2a) through topical and subcutaneous injection were applied but had little effect. In light of persistent symptoms, the pathology of Castleman-like lymph node and high IL-6 levels, the patient was administered an IL-6 inhibitor, siltuximab (200mg every 3 weeks) and prednisone(1.5mg/kg/day) in addition to antiviral treatment. All other treatments mentioned above were discontinued due to their limited effects. Remarkably, this regimen induced a significant reduction in symptoms and lymph node size during the fourth therapy (Figure 1C). As we found CD3, CD4 and CD8 counts were low in our patient (Table 1), we administered trimethoprim-sulfamethoxazole for pneumocystis jirovecii prophylaxis, at a dosage of 0.24g twice daily, three times a week. The family refused further immunoglobulin replacement therapy due to the high cost and limited immediate effect. Prednisone was reduced by half and complete remission was achieved at the sixth therapy (Figure 1D). Except for IgE, eosinophils and IL-6 were within the normal range. We gave her thymalfasin and valaciclovir for immune enhancement. Prednisone was gradually tapered to a final dose of 4 mg every other day, starting with 8 mg daily for the first two months and 4 mg daily for the next two months, resulting in a sustained response throughout the six-month follow-up period. Although HSCT was suggested as a definitive treatment, it was not pursued due to economic factors.
DOCK8 deficiency is a rare IEIs seen predominantly in consanguineous populations, now recognized as a distinct form of combined immunodeficiency (2). It impairs cytotoxic CD8+ T-cell memory, NK T-cell populations and functionality, B-cell activation, antigen-specific response formation, and signaling via DOCK8 and MyD88-dependent toll-like receptors (5, 6). The DOCK8 gene has over 30 mutations identified across populations since 2009, with large deletions being most common, but also includes point mutations, splice site mutations, and small insertions/deletions, leading to little or no DOCK8 protein expression. There’s no significant difference in the phenotypic features of AR-HIES2 patients with various DOCK8 mutation genotypes (7). Individuals with DOCK8-deficiency have a high incidence of allergic diseases and eczema, hyper IgE, eosinophilia, and a profound type-2 CD4+ T helper cell (Th2) bias (8), leading to IL-6 overexpression (9). An increased susceptibility to mucocutaneous viral infections, particularly caused by HSV, HPV, MCV, and VZV, is a hallmark of DOCK8 deficiency, differentiating it from other hyper-IgE conditions (1). These DNA viruses establish latent infections after initial infections in the general population during childhood (2).
In the context of DOCK8 deficiency, HSV infections are notably severe, often becoming chronic and leading to resistance against systemic therapies like acyclovir or valacyclovir (10). HSCT is advocated for all affected individuals, ideally at an early stage of the disease (6). But this procedure is associated with a high risk of morbidity and mortality particularly with uncontrolled viral infection. Interferon therapy has been shown to be effective by inhibiting viral replication and activating effector lymphocytes, including NK and cytotoxic T cells (11, 12). However, our patient was unresponsive to antiviral and interferon therapies. Elevated IL-6 levels and a Castleman-like lymph node pathology suggested the use of siltuximab, which is anti-human IL-6 monoclonal antibody approved for idiopathic multicentric Castleman’s disease. The successful use of siltuximab in achieving remission demonstrates the immunomodulatory role of IL-6 in antiviral defense against HSV in the context of DOCK8 deficiency.
IL-6 plays diverse roles in plasma cell maturation, acute inflammatory mediator production, and vascular endothelial growth factor (VEGF) secretion (13, 14). HSV subverts the host’s immune system by encoding a homolog of human IL-6, referred to as viral IL-6 (vIL-6), in monocyte-derived mature dendritic cells (DCs) (15). High serum IL-6 levels enhance angiogenesis and increase vascular permeability by upregulating VEGF expression (16) and induces cytokine storms (13, 17). It might also contribute to B-cell proliferation and angiogenesis within lymph nodes via IL-6R-independent engagement of gp130. In rodent experiments, IL-6 transgenic mice displayed multiple lymph node swelling and follicular hyperplasia (18). In our patient, VEGF was strongly expressed in the plasma cells around the hyalinized vessels in the interfollicular region, and the pathology described as hemangioma verified our hypothesis that virally expressed VEGF may be responsible for proliferative granulomatous masses. Anti-IL-6 therapy may act by reducing cytokine storms driven by high serum IL-6 levels and inhibiting HSV replication. Clinical response to siltuximab observed in this individual also supports this contention. However, the multifaceted nature of IL-6’s actions highlights the need for a nuanced understanding, considering various regulatory factors. Further studies are needed to fully understand the specific mechanisms of IL-6 for advancing our knowledge and potential therapeutic interventions.
In summary, this report details a pediatric case of DOCK8 deficiency accompanied by severe, progressive herpetic infections non-responsive to standard antiviral and interferon therapies, yet responsive to siltuximab and glucocorticoid administration. Our findings suggest that IL-6 serves as a crucial modulator of immune response to HSV with implications for its regulatory capacity in inflammation, cellular, and humoral immunity, warranting further exploration into its specific mechanistic pathways. Anti-IL-6 therapy may be beneficial for DOCK8-deficient subjects who prove unresponsive to conventional antiviral chemotherapies, indicating a prospective avenue for clinical management of this immunodeficiency.
Our case describes a 6-year-old patient with DOCK8 deficiency and severe, progressive herpetic infection. Given the distinct clinical phenotype of DOCK8 deficiency, characterized by eczema, recurrent bacterial skin and lung infections, chronic viral skin infections, and severe allergies in combination with a cellular immunodeficiency and increased risk for malignancy, it would seem straightforward to diagnose affected patients early. The severe cutaneous phenotype in our patient responded to a combination of siltuximab and prednisone in addition to antiviral treatment. Our research findings indicate that IL-6 plays a crucial regulatory role in the immune response against HSV, demonstrating its role in inflammation regulation, cellular immunity, and humoral immunity. Anti-IL-6 therapy may be beneficial for DOCK8 deficient subjects who have been shown to be unresponsive to traditional antiviral chemotherapy. Our findings suggests that IL-6 emerges as a pivotal regulator in the immune response against HSV, demonstrating its roles in inflammation modulation, cellular immunity, and humoral immunity. Anti-IL-6 therapy may be beneficial in those DOCK8-deficient subjects who prove unresponsive to conventional antiviral chemotherapies.
The data presented in the study are deposited in the GeneBank repository, accession number BioSample: SAMN41533898 and SRA: SRR29181171.
The studies involving humans were approved by Ethics Committee of Xinhua Hospital, Shanghai Jiaotong University School of Medicine. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants’ legal guardians/next of kin. Written informed consent was obtained from the individual(s), and minor(s)’ legal guardian/next of kin, for the publication of any potentially identifiable images or data included in this article. Written informed consent was obtained from the participant/patient(s) for the publication of this case report.
ZS: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. RW: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. CP: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. CN: Writing – review & editing, Conceptualization, Data curation, Investigation, Project administration, Validation. XZ: Writing – review & editing, Data curation, Funding acquisition, Investigation, Methodology, Validation. WG: Writing – review & editing, Data curation, Formal analysis, Investigation, Methodology, Software. RC: Writing – review & editing, Conceptualization, Data curation, Investigation, Methodology, Software, Supervision. YG: Investigation, Methodology, Software, Writing – review & editing, Formal analysis. HY: Formal analysis, Investigation, Methodology, Software, Writing – review & editing. KH: Investigation, Methodology, Project administration, Supervision, Writing – review & editing. ZZ: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – review & editing. XY: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – review & editing. ZY: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – review & editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This publication presents independent research funded by the Medical-Engineering Cross Fund of Shanghai Jiao Tong University(YG2021QN57), National Nature Science Foundation of China (82203962). There was no commercial support for this study.
We thank the patients and their families for their ongoing participation in our treatment and follow-up. Their understanding was important in ensuring the success of the treatment. During the preparation of this work, the authors used ChatGPT4 for language editing and polishing. After using this tool, the authors reviewed and edited the content and take full responsibility for the content of the publication.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Biggs CM, Keles S, Chatila TA. DOCK8 deficiency: Insights into pathophysiology, clinical features and management. Clin Immunol (Orlando Fla.). (2017) 181:75–82. doi: 10.1016/j.clim.2017.06.003
2. Chu EY, Freeman AF, Jing H, Cowen EW, Davis J, Su HC, et al. Cutaneous manifestations of DOCK8 deficiency syndrome. Arch Dermatol. (2012) 148:79–84. doi: 10.1001/archdermatol.2011.262
3. Kasap N, Celik V, Isik S, Cennetoglu P, Kiykim A, Eltan SB, et al. A set of clinical and laboratory markers differentiates hyper-IgE syndrome from severe atopic dermatitis. Clin Immunol (Orlando Fla.). (2021) 223:108645. doi: 10.1016/j.clim.2020.108645
4. Su HC, Jing H, Angelus P, Freeman AF. Insights into immunity from clinical and basic science studies of DOCK8 immunodeficiency syndrome. Immunol Rev. (2019) 287:9–19. doi: 10.1111/imr.12723
5. Yang J, Liu Y. Autosomal recessive hyper-IgE syndrome caused by DOCK8 gene mutation with new clinical features: a case report. BMC Neurol. (2021) 21:288. doi: 10.1186/s12883-021-02324-3
6. Harada Y, Tanaka Y, Terasawa M, Pieczyk M, Habiro K, Katakai T, et al. DOCK8 is a Cdc42 activator critical for interstitial dendritic cell migration during immune responses. Blood. (2012) 119:4451–61. doi: 10.1182/blood-2012-01-407098
7. Xue L, Yang Y, Wang S. A novel large deletion of the DOCK8 gene in a Chinese family with autosomal-recessive hyper-IgE syndrome. J Eur Acad Dermatol Venereology: JEADV. (2015) 29:599–601. doi: 10.1111/jdv.12394
8. Janssen E, Wilkie H, Geha RS. Macabre TH2 skewing in DOCK8 deficiency. J Allergy Clin Immunol. (2021) 148:73–5. doi: 10.1016/j.jaci.2021.02.025
9. Smith KA, Maizels RM. IL-6 controls susceptibility to helminth infection by impeding Th2 responsiveness and altering the Treg phenotype in vivo. Eur J Immunol. (2014) 44:150–61. doi: 10.1002/eji.201343746
10. Aydin SE, Kilic SS, Aytekin C, Kumar A, Porras O, Kainulainen L, et al. DOCK8 deficiency: clinical and immunological phenotype and treatment options - a review of 136 patients. J Clin Immunol. (2015) 35:189–98. doi: 10.1007/s10875-014-0126-0
11. Keles S, Jabara HH, Reisli I, McDonald DR, Barlan I, Hanna-Wakim R, et al. Plasmacytoid dendritic cell depletion in DOCK8 deficiency: rescue of severe herpetic infections with IFN-α 2b therapy. J Allergy Clin Immunol. (2014) 133:1753–5.e3. doi: 10.1016/j.jaci.2014.03.032
12. Papan C, Hagl B, Heinz V, Albert MH, Ehrt O, Sawalle-Belohradsky J, et al. Beneficial IFN-α treatment of tumorous herpes simplex blepharoconjunctivitis in dedicator of cytokinesis 8 deficiency. J Allergy Clin Immunol. (2014) 133:1456–8. doi: 10.1016/j.jaci.2014.02.008
13. Kang S, Kishimoto T. Interplay between interleukin-6 signaling and the vascular endothelium in cytokine storms. Exp Mol Med. (2021) 53:1116–23. doi: 10.1038/s12276-021-00649-0
14. Aletaha D, Kerschbaumer A, Kastrati K, Dejaco C, Dougados M, McInnes IB, et al. Consensus statement on blocking interleukin-6 receptor and interleukin-6 in inflammatory conditions: an update. Ann rheumatic Dis. (2023) 82:773–87. doi: 10.1136/ard-2022-222784
15. Birzer A, Krawczyk A, Draßner C, Kuhnt C, Mühl-Zürber P, Heilingloh CS, et al. HSV-1 modulates IL-6 receptor expression on human dendritic cells. Front Immunol. (2020) 11:1970. doi: 10.3389/fimmu.2020.01970
16. Cohen T, Nahari D, Cerem LW, Neufeld G, Levi BZ. Interleukin 6 induces the expression of vascular endothelial growth factor. J Biol Chem. (1996) 271:736–41. doi: 10.1074/jbc.271.2.736
17. Santa Cruz A, Mendes-Frias A, Oliveira AI, Dias L, Matos AR, Carvalho A, et al. Interleukin-6 is a biomarker for the development of fatal severe acute respiratory syndrome coronavirus 2 pneumonia. Front Immunol. (2021) 12:613422. doi: 10.3389/fimmu.2021.613422
Keywords: DOCK8 deficiency, herpetic infections, IL-6, siltuximab, primary immunodeficiency
Citation: Sun Z, Wei R, Pan C, Ni C, Zhang X, Guan W, Cheng R, Gu Y, Yu H, He K, Zhang Z, Yu X and Yao Z (2024) Successfully treated with siltuximab and prednisone in a 7-year-old girl with DOCK8-deficiency presenting as recurrent wart-like lesions: a case report. Front. Immunol. 15:1414573. doi: 10.3389/fimmu.2024.1414573
Received: 18 April 2024; Accepted: 27 June 2024;
Published: 09 July 2024.
Edited by:
Ramsay Fuleihan, Columbia University, United StatesReviewed by:
Artem Kalinichenko, St. Anna Children’s Cancer Research Institute (CCRI), AustriaCopyright © 2024 Sun, Wei, Pan, Ni, Zhang, Guan, Cheng, Gu, Yu, He, Zhang, Yu and Yao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhirong Yao, eWFvemhpcm9uZ0B4aW5odWFtZWQuY29tLmNu; Xia Yu, eXV4aWFAeGluaHVhbWVkLmNvbS5jbg==; Zhen Zhang, emhhbmd6aGVuNDAyN0AxNjMuY29t
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.