
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Immunol. , 20 March 2024
Sec. Microbial Immunology
Volume 15 - 2024 | https://doi.org/10.3389/fimmu.2024.1370658
 Huifang Niu1
Huifang Niu1 Minfeng Zhou2
Minfeng Zhou2 Daniel Zogona1
Daniel Zogona1 Zheng Xing1
Zheng Xing1 Ting Wu1
Ting Wu1 Rui Chen2
Rui Chen2 Dandan Cui2*
Dandan Cui2* Fengxia Liang3*
Fengxia Liang3* Xiaoyun Xu1*
Xiaoyun Xu1*Metabolic diseases are comprehensive disease based on obesity. Numerous cumulative studies have shown a certain correlation between the fluctuating abundance of Akkermansia muciniphila and the occurrence of metabolic diseases. A. muciniphila, a potential probiotic candidate colonized in the human intestinal mucus layer, and its derivatives have various physiological functions, including treating metabolic disorders and maintaining human health. This review systematically explicates the abundance change rules of A. muciniphila in metabolic diseases. It also details the high efficacy and specific molecules mechanism of A. muciniphila and its derivatives in treating obesity, type 2 diabetes mellitus, cardiovascular disease, and non-alcoholic fatty liver disease.
The gut microbiota is recognized as one of the pivotal environmental factors in regulating host health. The compositional imbalance of intestinal microbiota is associated with the emergence of various diseases. Multiple studies have reported a significant correlation between metabolic diseases, including obesity (1), type 2 diabetes (T2DM) (2), cardiovascular disease (CVD) (3), and nonalcoholic fatty liver disease (NAFLD) (4) and a specific bacterium. In the process of observing different bacteria, Akkermansia muciniphila has been repeatedly mentioned as a potential candidate due to its unique function, high prevalence, and abundance in nearly all life stages. However, products containing A. muciniphila are unavailable in various countries. The exact mechanism underlying A. muciniphila, exerting a probiotic effect on the host, is not fully understood. In this manuscript, we reviewed the history of this microbe from its discovery to the first investigations associating A. muciniphila with metabolic diseases (Figure 1). We systematically elaborated on the significant role played by this bacterium in metabolic diseases and its mechanism of action through a review of current human and animal experiments.
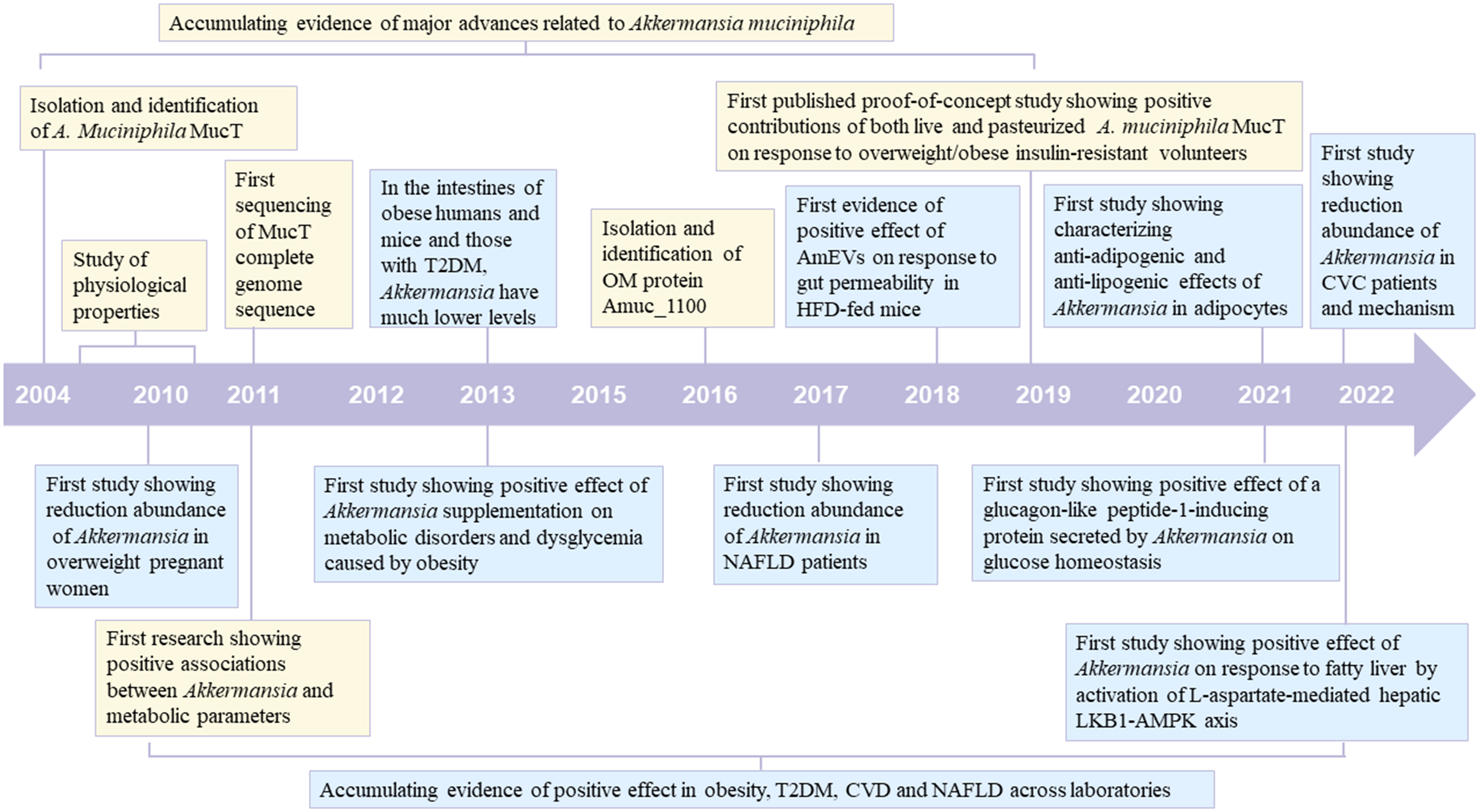
Figure 1 Timeline of major advances related to Akkermansia muciniphila and its positive effect on metabolic diseases (Obesity, T2DM, CVD and NAFLD). The blue box indicates major advances related to A. muciniphila. The yellow box indicates its positive effect on metabolic diseases T2DM, type 2 diabetes; CVD, cardiovascular disease; NAFLD, nonalcoholic fatty liver disease.
A. muciniphila, a number of the phylum Verrucomicrobia, is an oval-shaped, non-motile, strictly anaerobic Gram-negative strain. Since its isolation in 2004, the number of available studies about A. muciniphila has increased exponentially. A. muciniphila can be cultivated in the following media: brain heart soak, porcine gastric mucin medium, Columbia broth, trypsin soy, and synthetic medium (16 g/L soy protease, 4 g/L threonine, 25 mM glucose, and 25 mM N-acetylglucosamine). It has an optimal growth temperature of 37°C and an optimal pH of 6.5, and it must be cultured in either 100% N2 or 5% H2, 10% CO2, 85% N2 (5–8). A. muciniphila is highly abundant in the intestinal tract, accounting for approximately 1% to 4% of the total intestinal microbiota. A. muciniphila uses mucin as its sole carbon and nitrogen source, giving it a distinct survival advantage that is not strictly diet-dependent. In vitro, it preferentially utilizes monosaccharides, such as glucose and fructose, over N- acetylglucosamine and N-acetylgalactosamine to grow (9). The whole genome sequencing of MucT further confirmed that A. muciniphila primarily depends on a series of hydrolytic enzymes to degrade mucins, including proteases, sulfatases, β-N acetylhexosaminidase, glycosyl-hydrolases enzymes and sialidases.
A.muciniphila is highly prevalent and abundant in humans and animals. It primarily colonizes the outer mucus layer of the gastrointestinal tract, and its distribution in the intestinal tract was uneven, with the highest abundance in the cecum. A. muciniphila was also detected in rats, horses, rock thunderbirds, otters, dairy calves, guinea pigs, Burmese pythons, and zebrafish (10–16). In addition, A. muciniphila was found to be enriched in human breast milk, utilizing oligosaccharides in human breast milk as a carbon source (17), indicating that it has the potential for vertical transmission from mother to child. A large-scale population genomic analysis of the genus A. muciniphila using 88 isolated genomes and 2226 genomes showed that the human gut contains five candidate species of A. muciniphila (18). Their 16S rRNA gene sequences were surprisingly similar, but there were significant genome-wide differences. A. muciniphila strains from 22 Chinese intestines were isolated and identified by genomic fingerprinting (Enterobacterial Repetitive Intergenic Consensus Polymerase Chain Reaction), which unexpectedly indicated that they could be classified into 12 subclasses (19). Based on 39 isolates, an evolutionary tree was constructed using Single Nucleotide Polymorphism loci of core genes, and A. muciniphila was divided into three subgroups, AmI, AmII and AmIII, with significant variations in KEGG and GO functional annotations (20).
The functional activity of A. muciniphila as a potential candidate strain for alleviating metabolic diseases depends on its abundance level. Multiple factors affect the abundance of A. muciniphila in the host’s intestinal tract. Age is the first factor. Initially, vertical transmission of A. muciniphila to infants occurs by human breast milk, with one- month-old infants containing 2.05 to 4.36 log cells/g of feces. The number of A. muciniphila increases rapidly with age, nearly doubling in infants aged 6 to 12 months. By two years of age, a complete mucin-degrading microbiota is established, approaching the number found in adults. In addition, A. muciniphila concentrations are significantly lower in fecal samples of the elderly compared to adults (21). Diet is the second factor. Long-term consumption of high-sugar or high-fat diets alters the structure and composition of the intestinal microbiota and reduces the amount of A. muciniphila (22), whereas a restrictive diet has the opposite effect (23). It is the cause of the increased abundance of A. muciniphila in postoperative patients after gastric-bypass surgery (24). Similar to gastric bypass surgery, calorie restriction also positively influences the gut microbiota and the abundance of A. muciniphila. Recent studies indicate that intermittent fasting enhances the levels of Lactobacillus and Bifidobacterium in healthy mice while diminishing Helicobacter, Prevotella, and Bacteroides populations (25–27). Following 28 days of intermittent fasting treatment, db/db mice demonstrated a marked rise in Lactobacillus levels and the production of butyrate-producing Odoribacter in their intestines (28). Additionally, enrichment of A. muciniphila is frequently observed in individuals or animals undergoing calorie restriction (29–33). Changes in the gut microbiota due to calorie restriction ultimately result in increased levels of short-chain fatty acids (34). These fatty acids, upon binding to their respective G protein-coupled receptors, stimulate the production of peptide YY (PYY), thereby contributing to appetite suppression, decreased gastrointestinal motility, and reduced calorie intake. Supplementation with prebiotics, fructo- oligosaccharides, FODMAP (fermentable Oligo-, Di- and Mono-saccharides and Polyols-which includes fructose, lactose, oligosaccharides, polyols, and sugar alcohols (polyols, such as sorbitol, mannitol, xylitol and maltitol)), and dietary polyphenols may increase the abundance of A. muciniphila in healthy humans or animals (35). In addition, prebiotics can reverse the reduction of A. muciniphila abundance due to high-sugar or high-fat diets (36, 37). Studies have shown that the abundance of A. muciniphila in the feces of DIO mice decreased from 109/g to 107/g after being fed a high-fat diet (containing 60% fat) for 8 weeks; however, supplementation with low-fructo-oligosaccharides (0.3 g/day, high-fat diet) for 8 weeks restored its abundance to the original level (36). Additionally, in ob/ob mice, after 5 weeks of oral administration of low-fructo-oligosaccharides at a dose of 0.3 g/day, the abundance of A. muciniphila in the intestines increased by more than 80 times, along with a significant increase in the abundance of Bifidobacterium spp. and the E. rectale/C. coccoides group (38). Furthermore, a FODMAP diet has been found to alleviate Crohn’s Disease. A single-blind, randomized, crossover trial compared the effects of low (3.05%) and high intake of FODMAP (“typical Australian diet” containing 23.7% FODMAP) on Crohn’s Disease. The results showed that the low FODMAP diet reduced the total bacterial abundance, while A typical Australian diet increased the relative abundance of the butyrate-producing Clostridium cluster XIVa and the mucus-associated A. muciniphila, and reduced the torque of Fusobacterium (39). Moreover, dietary polyphenols play a role in regulating the gut microbiota. Compared with the control group, a high-fat diet containing 1% Concord grape polyphenols significantly increased the abundance of A. muciniphila in the intestines of C57BL/6J mice and reduced the ratio of Firmicutes to Bacteroidetes (40). Anthocyanin supplements significantly increased the β-diversity of the gut microbiota and the abundance of Bacteroidetes (41). Mustard polyphenols also reduced the ratio of Firmicutes to Bacteroidetes and increased the abundance of Lactobacillaceae, A. muciniphila, and Blautia (42). Orange polyphenols promoted the increase of Bifidobacterium and Lactobacillus (43). The third influencing factor is disease. As an important tissue in direct contacting with the external environment, disruption of the structure and abnormal composition of intestinal microorganisms can trigger disease, and in turn, disease may disrupt its healthy microecology. Most diseases, including metabolic disorders, neurodegenerative diseases, immune system disorders, cancer and intestinal inflammation, have a negative association with A. muciniphila (44). The final factor to consider is antibiotics. These drugs are used for preventing or treating bacterial infections and can be categorized based on their molecular structures into groups like β-lactams, penicillins, macrolides, tetracyclines, aminoglycosides, among others. Most antibiotics possess broad-spectrum activity. In a double-blind human trial, significant changes in the composition of intestinal microbiota were observed on days 7, 12, 17, and 27 following a 7-day eradication therapy comprising amoxicillin 500 mg qid, metronidazole 400 mg tid, and lansoprazole 30 mg bid. Comparing fecal microbiota abundance on day 1 and day 27 post-treatment revealed a decrease in Bacteroides genus and total lactobacilli, while the abundance of Bifidobacterium genus, Enterobacteriaceae, and Enterococcus/Streptococcus genus showed an increasing trend (45). The impact of antibiotics on gut microbiota is influenced by several factors, including spectrum of activity, pharmacokinetics, dosage, route of administration, and intestinal concentration (46). Additionally, a novel antibiotic, OPS-2071 (a quinolone class drug), demonstrates high antibacterial activity against other intestinal bacterial strains but notably lower activity against A. muciniphila. OPS-2071 significantly increases the colonization rate of A. muciniphila and the mucin content in the feces of healthy rats (47). Similar experimental results were observed in a human study using vancomycin treatment, where A. muciniphila significantly colonized the intestine after broad-spectrum antibiotic treatment (8), although the underlying mechanisms remain unclear. Additionally, geography, lifestyle, baseline levels, and the host genotype all impact its abundance.
The disruption of gut homeostasis in metabolic diseases is closely linked to intestinal immune function. Acting as a physiological barrier, the intestine prevents harmful substances from infiltrating the body. The intestinal barrier comprises biological, physical, chemical, and immune components (48). Among them, biological barrier refers to the fact that probiotic bacteria occupy intestinal space and compete for nutrients and survival resources, thereby limiting the growth and reproduction of pathogenic microorganisms (49). The composition and structure of the microbiota influence the host immune system by regulating the intestinal mucosal barrier, producing metabolic products, and modulating immune responses (50). Concurrently, the host immune system sustains intestinal microbial balance by recognizing and eliminating harmful microbes and controlling immune reactions (51). The intricate interplay between microbes and hosts is essential for preserving intestinal health, warding off infections, and modulating immune responses.
Metabolic disease is a comprehensive disease based on obesity. According to the International Diabetes Federation, the metabolic disease is diagnosed when central obesity is accompanied by any two of the following conditions: ① Triglyceride levels of 150 mg/dL (1.7 mmol/L) or more; ② High-density cholesterol decreasing to 40 mg/dL (1.3 mmol/L) in men and 50 mg/dL (1.29 mmol/L) in women; ③ Elevated blood pressure to >130 mm Hg systolic or >85 mm Hg diastolic, or a diagnosis of hypertension. ④ Fasting blood glucose exceeding 100 mg/dL (5.6 mmol/L) or diagnosed with T2DM. This criterion has been widely accepted and revised. Further analysis of the intestinal microbiota of patients with metabolic diseases (obesity, T2DM, CVD and NAFLD) indicated that the abundance of A. muciniphila microbiota was reduced, suggesting that there must be a direct or indirect association between the two. However, the exact mechanism underlying this association is not fully understood.
In recent years, people’s dietary needs have expanded beyond subsistence level. Obesity has become another significant consequence of the affluence of material possessions. It is now one of the leading causes of health risks and a risk factor for many diseases, including T2DM, NAFLD, and CVD (52, 53), making obesity one of the most important public health problems of the 21st century (54). The regulation of intestinal microbiota has garnered widespread interest as an effective strategy to prevent or treat obesity. A. muciniphila, the only Verrucomicrobia (phylum) genus that can be cultured in vitro, has been repeatedly mentioned in the context of obesity. The objective regulations that exist between the two has been gradually revealed. In 2010, it was observed for the first time that the abundance of A. muciniphila was lower in overweight pregnant women than in normal-weight pregnant women (55). Similar findings were observed in the stool samples of obese or overweight preschool children (56). Furthermore, a study analyzing fecal microbiota in 17 lean weight (BMI 19-24.99 kg/m2) and 15 obese women (BMI>30 kg/m2) using real-time fluorescence quantification PCR (qPCR) method revealed that A. muciniphila was more abundant in lean individuals than in obese ones (57). To further investigate the relationship between A. muciniphila and obesity, researchers successfully induced an obese mouse model with a high-fat diet and collected cecal feces. Analysis of feces by qPCR showed a 100-fold decrease in the population of A. muciniphila in the obese model group compared to lean littermates. The ob/ob mice are homozygous Lepob mutant mice with an invisible gene on chromosome 6 that causes obesity and advanced diabetes. Similarly, analysis of their intestinal feces showed a 3300-fold reduction in A. muciniphila population in ob/ob mice compared to lean littermates (36). The substantial changes in A. muciniphila genus abundance indicate a possible association between A. muciniphila and obesity. However, a few studies contradicted the above findings. For instance, A. muciniphila was more abundant in obese than in normal-weight children (58). To comprehensively elaborate the regularity between the two, substantial human and animal studies were conducted, which conclusively demonstrated that A. muciniphila abundance was reduced in obese individuals or animals (Table 1).
Based on the phenomenon of decreased A. muciniphila abundance due to obesity, researchers hypothesized that supplemental A. muciniphila could alleviate obesity or pathological features resulting from obesity. Amandine Everard et al. in 2013 investigated the beneficial effects of A. muciniphila on high-fat diet-induced obese mice (live and heat- killed at 121°C for 15 min). The results demonstrated that live A. muciniphila could alleviate fat-mass gain, insulin resistance, adipose tissue inflammation, and metabolic endotoxemia in obese mice, whereas heat-killed A. muciniphila had no such effects (36). In addition, numerous animal experiments have discovered that A. muciniphila supplementation also has physiological activities including reducing body weight, promoting metabolism, and enhancing intestinal barrier (Table 2), However, A. muciniphila exacerbated intestinal inflammation in salmonella-infected gnotobiotic mice, which is a negative effect (78). Studies have shown that A. muciniphila extracellular vesicles reduce intestinal permeability (72). A. muciniphila also exacerbates depletion of the mucus layer by consuming mucin, which in turn leads to thinning of the mucus layer and increased inflammation. The application of A. muciniphila in humans has been relatively slow due to the uncertainty of its functional activity and the lack of complete understanding of its mechanism. Hubert Plovier et al. first introduced pasteurized (70°C, 30 min) A. muciniphila and unexpectedly observed that pasteurization enhanced the functional activity of A. muciniphila, which reduced fat-gain, insulin resistance and dyslipidemia in obese mice (6). This surprising discovery expands the potential of A. muciniphila as a next-generation probiotic for food applications. In a subsequent randomized, double-blind, placebo-controlled exploratory study, overweight or obese volunteers were supplemented with 1010 live or pasteurized A. muciniphila daily for three months. This study provided groundbreaking evidence that A. muciniphila is safe and tolerable. In addition, pasteurized A. muciniphila still retains the capacity to lower total plasma cholesterol and reduce body weight, body fat and hip circumference in humans (73). Toxicological experiments have also confirmed the safety of pasteurized A. muciniphila as a food ingredient (79). Although current research results are promising, there are limitations in the study subjects. Therefore, further studies are required to expand the scope of subjects and demonstrate the relationship between A. muciniphila supplementation and improving obesity metabolic parameters.
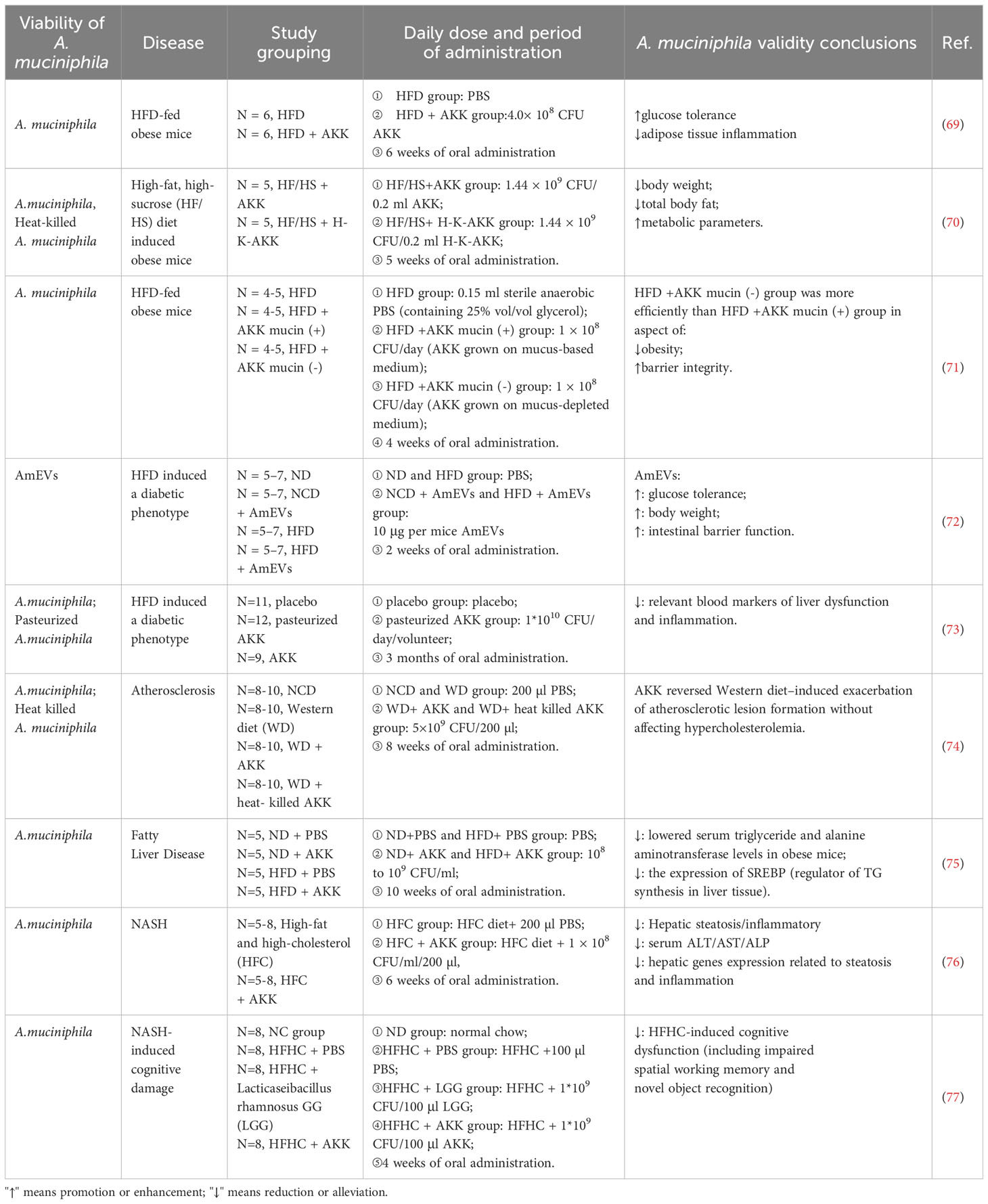
Table 2 Beneficial effects of A. muciniphila and its derivatives supplementation on metabolic diseases.
As the effects of A. muciniphila in alleviating obesity continue to be demonstrated, its underlying mechanisms are being explored. Obesity is a chronic metabolic disease caused by the excessive accumulation or abnormal distribution of organismal fat, mainly due to the imbalance between caloric intake and energy excretion of the body. When caloric intake exceeds energy excretion, excessive energy is stored as fat in adipocytes. Adipocytes are essential for fat synthesis and storage. Glycerol and fatty acids required for triglyceride synthesis are mainly provided by glucose metabolism. Glycerol is converted from dihydroxyacetone phosphate produced by glycolysis and fatty acids are synthesized from acetyl coenzyme A produced by the oxidative breakdown of glucose. Pasteurized A. muciniphila alleviates diet-induced obesity through increased energy expenditure and spontaneous physical activity. The energy expenditure is not associated with changes in thermogenic markers or white adipose tissue but with decreased expression of the lipid droplet-associated factor perilipin2 in brown and white fat. In addition, pasteurized A. muciniphila decreases carbohydrate absorption, increasing energy excretion in the feces (80). The treatment of 3T3-L1 cells with A. muciniphila cell lysate effectively diminished lipid accumulation and down-regulated mRNA expression of adipogenesis-associated proteins. A. muciniphila upregulates the expression of SERPINA3G in adipocytes and inhibits adipogenesis (81). Currently, the study of the molecular mechanism of A. muciniphila in alleviating obesity is still in the initial stage, additional studies are needed to further elucidate it.
Diabetes mellitus is a chronic metabolic disease caused by the deficiency in insulin secretion or the reduction in the body’s ability to utilize insulin. T2DM accounts for approximately 90% of diabetes mellitus cases worldwide. According to the 10th edition of the International Diabetes Federation, in 2021 there were 537 million adults (20-79 years old) with diabetes and more than 4 million deaths per year were caused by diabetes. Studies have demonstrated dramatic differences in the structure and composition of the gut microbiota in T2DM patients compared with healthy individuals, including A. muciniphila. In 2013, a study was conducted on normal, pre-diabetic and diabetic individuals using 16S rRNA sequencing of stool samples to quantify A. muciniphila. The results showed that A. muciniphila abundance was significantly lower in the pre-diabetic and diabetic groups compared to the normal group (82). Another study was conducted on the stool samples of patients with short-, medium- and long-term T2DM. The results indicated that the abundance of A. muciniphila in medium- and the long-term patient was significantly lower than that of short-term patients. The above studies strongly indicated a correlation between the pathological process and A. muciniphila abundance, and the longer the disease duration, the lower the A. muciniphila abundance. However, there are a few studies that contradict the above conclusion. Marion Régnier et al. induced a T2DM mouse model with a high-fat and high-sugar diet, collected feces, and analyzed them using 16S rRNA sequencing combined with qPCR. The results showed that A. muciniphila was more abundant in T2DM mice compared to normal mice (83). Another study showed that A. muciniphila was more abundant in T2DM patients than in healthy individuals (84). The inconsistency of the findings prompted a large number of studies to be conducted, which eventually concluded that there is a negative correlation between the two (Table 1). This negative correlation property suggests that A. muciniphila supplementation may reverse the underlying physiological indices of T2DM. To test this hypothesis, researchers induced T2DM mice with a high-fat diet and gavaged them 2 x 108 CFU/0.2 ml/day for four weeks. The results showed that A. muciniphila possessed the physiological activity to alleviate the elevated blood glucose induced by the high-fat diet (36). Meanwhile, Feifan Wu et al. reported that A.muciniphila could improve glucose tolerance, regulate intestinal microbiota destroyed by high-fat diets, promote acetate and propionate production, and enhance intestinal barrier function (22). Numerous subsequent studies confirmed these functional activities.
Obesity is a crucial factor in the pathogenesis of T2DM, as evidenced by studies conducted on animal models induced with high-fat diets combined with streptozotocin. The primary pathological mechanisms of T2DM have been identified as: ① Insufficient insulin secretion. Obesity often leads to dyslipidemia and chronically elevated levels of free fatty acids (FFAs). FFAs are essential to maintain the function of islet cell (85). However, a high level of FFA stimulation can lead to a decline in islet cell function. In one study, when isolated islets were exposed to high levels of FFA, insulin secretion significantly decreased over time and eventually ceased, leading to non-insulin secretion (86). High levels of FFA oxidation in islet cells reduced the expression of glucose transporter receptor 2, glucokinase and insulin genes, which directly affected insulin synthesis and secretion (87). As the functional activity of A. muciniphila in modifying diabetes is further investigated (Table 2), its intrinsic molecular mechanisms are being revealed. A. muciniphila has the function of reducing FFA levels by alleviating obesity. In addition, A. muciniphila improved the structure and composition of the intestinal microbiota and promoted the production of short-chain fatty acid (88). Acetic acid, propionic acid and butyric acid targeted G protein-coupled receptors 41 and 43 on the surface of intestinal L cells, which activated downstream molecular signaling and ultimately promoted the secretion of glucagon-like peptide-1(GLP-1) (89). Furthermore, A. muciniphila can secrete glucagon-like peptide-1-inducing protein P9. P9 bound to intercellular adhesion molecule 2 on the surface of L cells and activated downstream molecular pathways to stimulate the secretion of GLP-1 (90). GLP-1 is transported to the pancreas through blood vessels, and binds to its corresponding receptors to induce insulin secretion, ultimately achieving hypoglycemic effect. ② Insulin resistance. Obesity disrupts intestinal microbiota and elevated levels of lipopolysaccharides (LPS). High levels of LPS penetrate the intestinal barrier and activate the NF-κB/MAPKs signaling pathway, resulting in chronic low-grade inflammation (91). As chronic low-grade inflammation is generated, serine kinases (JNK, IKK) are activated and induce phosphorylation of insulin receptors, preventing insulin from binding properly to its receptors (92), leading to insulin resistance. LPS crossing the intestinal barrier is a central cause of chronic low-grade inflammation and is a key step in developing insulin resistance. The intestinal barrier function is maintained by two main factors: the thickness of the mucus layer and the degree of tight junctions between intestinal epithelial cells. The mucus primarily comprises water, inorganic salts and mucin, and mucin is its main functional component. The most abundant protein in the mucin complex is MUC2. The degree of tight junctions between intestinal epithelial cells is determined by the proliferation capacity of intestinal epithelial cells and the expression level of tight junction proteins. It has been reported that A. muciniphila upregulates MUC2, BIRC3 and TNFAIP3 (BIRC3 and TNFAIP3 are anti-apoptotic genes of intestinal epithelial cells involved in their proliferation process) via ADP-heptose-dependent activation of the ALPK1/TIFA pathway, which maintains intestinal barrier function and reduce its permeability (93). This subsequently inhibits chronic low-grade inflammation induced by LPS and insulin resistance. In addition, A. muciniphila promotes the expression of the tight junction proteins ZO-1, Occludin, and Claudin 3 (94). The outer membrane protein Amuc_1100 from A. muciniphila (95), and its extracellular vesicle (72) can enhance intestinal barrier function. In conclusion, A. muciniphila and its derivatives alleviate T2DM by promoting insulin secretion and reducing insulin resistance.
Metformin, as a first-line treatment for type 2 diabetes, has a good safety profile, but its exact mechanism of action is currently unclear. While traditionally thought to lower blood sugar levels by activating the hepatic AMPK pathway, thereby reducing hepatic glucose output (96–101), recent findings suggest metformin may also influence intestinal pathways (102, 103). Metformin can rapidly change the composition and structure of the intestinal microbial flora, mainly by reducing the diversity of the bacterial flora in mice and promoting the abundance of A. muciniphila and various short-chain fatty acid-producing microbial flora in the human intestine, including Butyrivibrio, Bifidobacterium bifidum, Megasphaera, and an operational taxonomic unit of Prevotella (104, 105). Metformin’s positive regulation of intestinal flora is beneficial to increase the production of short-chain fatty acids. Short-chain fatty acids can promote the proliferation of intestinal mucosal epithelial cells and enhance the expression of tight junction proteins, thereby improving intestinal barrier function (106). Strengthening the intestinal barrier effectively suppresses inflammation triggered by lipopolysaccharide penetration. Additionally, short-chain fatty acids bind to G-protein-coupled receptors, prompting intestinal L cells to release PYY and GLP-1. PYY curbs appetite and slows gastrointestinal motility, aiding in obesity management (107), while GLP-1 stimulates insulin release by binding to pancreatic islet B cell receptors, thereby regulating blood sugar levels (108). In summary, metformin’s modulation of intestinal flora is intricately linked to its anti-inflammatory, anti-obesity, and hypoglycemic effects.
During the modulation of gut microbiota, the impact of metformin on the levels of A. muciniphila is consistently highlighted. C57BL/6 mice were fed a high-fat diet (60% fat) for 28 weeks to induce metabolic disorders (obesity and T2D), and then treated with metformin at a dose of 300 mg/kg/day for 10 weeks to observe the effect of metformin on the intestinal microbiota. The findings indicated a notable increase in the abundance of A. muciniphila and Clostridium cocleatum in mice receiving metformin post high-fat diet (109). In an experiment using metformin to treat ulcerative colitis, the ability of metformin to increase the relative abundance of Lactobacillus and Akkermansia, reduce Clostridium erysipelvis at the genus level, and alter the composition of the intestinal microbiota was similarly demonstrated (110). Presently, there’s no evidence to suggest direct stimulation of A. muciniphila production by metformin; however, studies have noted a significant rise in goblet cell count in mouse intestines post-metformin treatment. This increase in mucin secretion by goblet cells directly correlates with the elevated abundance of A. muciniphila (69). In summary, metformin achieves the effect of alleviating T2DM by regulating the abundance of intes tinal flora, especially A. muciniphila.
CVD is the leading cause of mortality worldwide (111). According to the World Health Organization, approximately 17.9 million people died from CVD in 2019, accounting for 32% of all deaths worldwide. There are significant differences between the intestinal microbiota of CVD patients and healthy individuals. A survey of patients with major adverse cardiovascular and cerebrovascular events (MACCE) showed that Eubacterium eligens, A. muciniphila, Prevotella stercorea, and Eubacterium rectale were less abundant in MACCE patients than in the no-MACCE group (112) (Table 1). Notably the amount of A. muciniphila in the intestine of abdominal aortic aneurysms patients or mice was nearly depleted. This predicts that A. muciniphila may play a crucial role in their pathological process. A. muciniphila supplementation has been found to reverse Western diet-induced atherosclerosis, which is a major pathological process in CVD. In addition, A. muciniphila (2 × 108 CFU/180µl) improved cardiovascular disease in mouse models by altering the intestinal microbiota and immune system (113). A. muciniphila had also been demonstrated to improve cold-associated atrial fibrillation by inhibiting the formation trimethylamine (TMA)/trimethylamine N-oxide (TMAO) in a mouse model of cold-associated atrial fibrillation (114). Currently, the physiological activity of A. muciniphila for CVD has been validated to a certain extent. However, relevant studies and clinical data are still relatively scarce. Therefore, further studies need to be conducted.
Atherosclerosis, the leading cause of CVD (115), is manifested as atheromatous plaque material composed of lipid or fibrous substances in the entire aorta and arterial roots. The main mechanism of atherosclerosis: ① With the improvement of people’s living standard, high-fat diet gradually becomes people’s daily diet pattern. Long-term consumption of high-energy diets can induce obesity and cause abnormalities in the body’s lipid metabolism. As a result, high levels of oxidized low-density lipoprotein cholesterol in the plasma are not transferred out of the plasma in time to be deposited in the inner wall of the arterial vessels and trigger inflammation, resulting in the gradual formation of atheromatous deposits in the lumen of the arteries. Over time, this deposit eventually becomes fibrotic, resulting in narrowing of the arterial lumen, causing a loss of wall elasticity and plaque rupture. The ruptured plaques circulate with the blood, easily blocking the blood vessels and causing thrombosis, eventually leading to the occurrence of adverse CVD, including myocardial infarction and angina pectoris, which is fatal for human health and life (116). Oral administration of A. muciniphila can reverse weight gain and abnormal lipid metabolism caused by a high-fat diet and reduce plasma cholesterol levels. In addition, A. muciniphila can enhance intestinal barrier function, which effectively inhibits LPS-induced inflammation and indirectly prevents CVD. ② Choline, phosphatidylcholine, and L-carnitine are abundant in red meat, shellfish, eggs, and fish. These three substances can be metabolized by intestinal microbiota to TMA, a CVD biomarker. TMO absorbed by the organism is further converted to TMAO by hepatic flavin monooxygenase 3 (FMO3) in the liver. Pasteurized A. muciniphila can counteract the increase in FMO3 levels induced by high-fat diet, suggesting the possibility of A. muciniphila intervention in TMAO production. Furthermore, supplementing obese mice with A. muciniphila could promote the excretion of TMA and TMAO through urine, thereby reducing their levels in plasma. In conclusion, A. muciniphila can effectively inhibit the occurrence of CVD through different pathways.
NAFLD is a pathological syndrome characterized by excessive deposition of lipids in liver cells caused by factors other than alcohol and clear liver damage factors (primarily drugs, viral infections, and autoimmunity) (117). It has a high prevalence and incidence in many countries and can be divided into three categories based on its pathological process: simple fatty liver, non-alcoholic steatohepatitis (NASH), and liver cirrhosis. Intestinal microbiota has gained considerable attention as a potential therapeutic target for NAFLD. Studies showed that abundance of A. muciniphila was significantly reduced in gut of NASH and NAFLD patients (118) (Table 2). A study conducted on 46 NASH patients and 38 healthy controls also confirmed the above findings based on qPCR results of their stool samples (119). In addition, using saccharin/sucalose to induce the NAFLD mouse model resulted in a significant decrease in the abundance of A. muciniphila (120). However, while most studies suggest a negative relationship between the two, some findings contradict it. GV Moreira et al. induced a mouse model with NAFLD phenotype using a high-fat diet and observed an increase in the abundance of A. muciniphila in the intestinal tract of the model (4). The inconsistency of conclusions suggests the need for further exploration of the relationship between the two. The significant reduction in the abundance of A. muciniphila in NAFLD patients suggests its potential role in alleviating NAFLD. Yong Rao et al. used high cholesterol and high fat to induce mice and administered A. muciniphila at a dose of 1x108 CFU/mL/day for 6 weeks. The results showed that it effectively reversed NAFLD in the liver, including hepatic steatosis, inflammation, and liver injury (121). At the same time, A. muciniphila supplementation can significantly reduce liver fat and the risk of NAFLD. Currently, no clinical data support this conclusion, and further verification is needed.
Abnormal accumulation of fat in liver cells is a prerequisite for the formation of NAFLD, which is caused by disorders of liver lipid metabolism. The homeostasis of hepatocyte lipid metabolism mainly depends on the dynamic balance of fatty acid uptake, fatty acid synthesis, and lipolysis processes. Glycerol and fatty acids are the raw materials required for synthesizing triglycerides. Glycerol is converted from dihydroxyacetone phosphate produced by glucose in the intestine. Fatty acids are primarily derived from three sources: ① Triglycerides in adipose tissue are decomposed into FFA, and some FFA participate in fat synthesis in the liver. The fat synthesized through this pathway accounts for 59% of the total fat in the liver. This process is primarily associated with regulating insulin signaling. Adipose tissue will abnormally decompose excess FFAs into the liver after insulin resistance, promoting NAFLD development. ② Glucose and fructose from carbohydrates in the food are digested and absorbed by the small intestine to form fatty acids through the adipogenesis pathway. The fatty acids are then transported to the liver for fat synthesis, which accounts for 26% of the total fat in the liver. ③ Excessive intake of saturated fatty acids in the diet will also affect the synthesis of liver fat, which accounts for 15% of the total fat in the liver. A. muciniphila can alleviate the abnormal decomposition of adipose tissue by reducing insulin resistance (Table 2). Additionally, supplementing pasteurized A. muciniphila reduces carbohydrate absorption, thereby reducing the production of glucose and fructose and effectively inhibiting the production of FFAs. The formation of NAFLD is also related to the timely consumption of fatty acids or fats. Supplementation with A. muciniphila increased the levels of L-aspartate in the gut-liver axis. L-Aspartate stimulated lipid oxidation and released energy by activating the hepatic LKB1-AMPK axis, thereby reducing liver fat accumulation. In conclusion, A. muciniphila can reduce the accumulation of fatty acids or fat in the liver through a variety of mechanisms, thereby alleviating NAFLD.
The strategy of using gut microbiota to target the improvement of metabolic diseases has been validated, based on the amount of accumulated experimental results and clinical data. In this review, obesity, T2DM, CVD and NAFLD were studied as typical representatives of metabolic diseases and elaborates the intricate relationship between them and A. muciniphila (Figure 2). A. muciniphila is a potential candidate strain for “next-generation probiotics” and is frequently mentioned in the pathological process of metabolic diseases. The abundance of A. muciniphila in the gut has a significant negative correlation with metabolic diseases, and variations in its abundance are recognized as a biomarker for the occurrence of metabolic diseases. Identifying the specific molecular mechanism or mode of action by which A. muciniphila alleviates metabolic syndrome is critical. Some common pathways have been identified in obesity. Pasteurized A. muciniphila reduces energy intake by reducing carbohydrate absorption and increases fecal energy excretion. A. muciniphila lysate upregulates the expression of SERPINA3G in adipocytes to inhibit adipogenesis. Obesity is the underlying cause of metabolic diseases. Therefore, when obesity is reduced, certain physiological indicators of the body will also change, including the improvement of intestinal barrier function, the correction of abnormal lipid metabolism, the reduction of blood cholesterol level, and the decrease of blood FFAs content, which directly reduces the risk of T2DM, CVD and NAFLD. In addition, A. muciniphila and its derivatives can specifically inhibit the onset of these three diseases. A. muciniphila-derived protein P9 binds to intercellular adhesion molecule 2 in intestinal L cells and promotes the secretion of GLP-1. P9 finally achieves the effect of indirect stimulation of insulin secretion. Pasteurized A. muciniphila reduces the ratio of TAM/TAMO in plasma and indirectly inhibits CVD. A. muciniphila activates the hepatic LKB1-AMPK axis by increasing the level of L-aspartic acid in the gut-liver axis and increases lipid oxidation and energy release. Thereby, it reduces fat accumulation in the liver and alleviates NAFLD. This review elaborates the probiotic effect of A. muciniphila on metabolic diseases and provides a theoretical basis for the clinical application of A. muciniphila as a new functional strain.
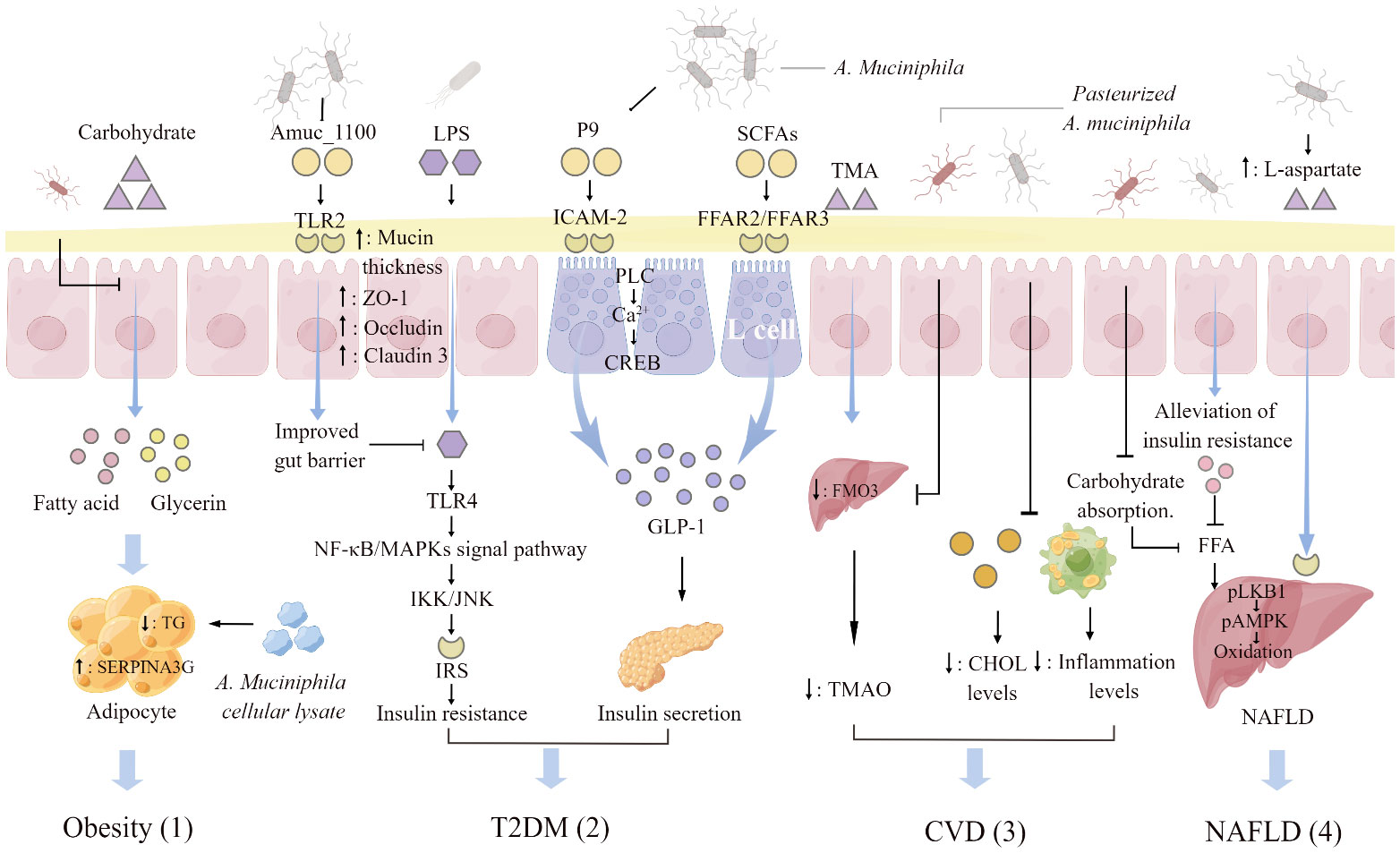
Figure 2 A. muciniphila regulating mechanisms associated with metabolic diseases. (1) Pasteurized A. muciniphila reduces glycerol and fatty acid levels and triglyceride (TG) production through inhibiting carbohydrate absorption. A. muciniphila cellular lysate upregulates serine protease inhibitor peptidase inhibitor clade 3G (SERPINA3G) expression in adipocytes and inhibits adipogenesis. (2) Activation of toll-like receptors 2 (TLR2) by Amuc _1100, an outer membrane protein of A. muciniphila, increases the thickness of the mucus layer and promotes the expression of tight junction proteins ZO-1, Occludin, and Claudin3. The enhancement of intestinal barrier function will hinder the insulin resistance induced by the activation of NF-KB/MAPKs signaling pathway caused by the combination of lipopolysaccharide (LPS) penetration and toll-like receptors 4 (TLR4). The glucagon-like protein P9 produced by A. muciniphila binds to the intercellular adhesion molecule 2 (ICAM-2) on the surface of L cells and activates phospholipase C (PLC), intracellular Ca2+ signaling, and CREB. P9 is involved in the secretion of GLP-1. Short- chain fatty acids (SCFAs) increased by A. muciniphila supplementation interact with free fatty acid receptor (FFAR) 2 and 3 on the surface of L cells to stimulate the secretion of GLP-1. (3) Pasteurized A. muciniphila inhibits the conversion of trimethylamine (TMA) to trimethylamine oxide (TMAO) by reducing the expression of hepatic flavin monooxygenase 3 (FMO3), and lowers the ratio of TAM/TAMO in plasma. A. muciniphila reduces the organism cholesterol (CHOL) and inflammation levels, indirectly reducing the risk of atherosclerosis and cardiovascular disease (CVD). (4) A. muciniphila indirectly decreases liver fat by regulating the level of free fatty acids (FFAs) in plasma. Supplementation with A. muciniphila increased the levels of L-aspartate in the gut-hepatic axis. L-Aspartate stimulate lipid oxidation and released energy by activating the hepatic LKB1-AMPK axis, thereby reducing liver fat accumulation. The figure was created using Figdraw.
A. muciniphila shows promise in improving metabolic diseases, with its abundance often inversely related to such disorders in both animal and human studies. However, the understanding of the specific biomolecules through which A. muciniphila influences metabolic diseases remains incomplete, a crucial aspect for its practical application. Currently, only a limited number of products containing heat-sterilized A. muciniphila are authorized for sale in Europe, facing significant marketization challenges. The main barriers to widespread use in clinical or food industries include: (1) Insufficient clinical data availability. (2) Need for further safety verification. (3) Challenges in cultivating A. muciniphila at high densities due to its anaerobic nature, leading to uncertainties in production feasibility while maintaining strain activity. While some clinical studies have demonstrated the probiotic effects of A. muciniphila and its safety in humans, limitations persist in terms of sample size, diversity, and demographics. Future research should focus on evaluating the physiological effects and safety of A. muciniphila on a larger scale, covering multiple dimensions comprehensively.
HN: Writing – original draft, Writing – review & editing. XX: Writing – review & editing. MZ: Writing – review & editing. DZ: Writing – review & editing. ZX: Writing – review & editing. TW: Writing – review & editing. RC: Writing – review & editing. DC: Writing – review & editing. FL: Writing – review & editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was funded by “Blueberry Pomace-Akkermansia muciniphila Synbiotics Regulatory Effects on Intestinal Microbiota” Fundamental Research Fund Project of Central Universities 2662019QD037 and “Agricultural Product Processing and Comprehensive Utilization” Team 1 Hubei Provincial Innovation Team Project (2021-2025)0120210150.
We thank the members of Laboratory 310, School of Food Science and Technology, Huazhong Agricultural University, for discussions on this manuscript.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
AUC, Area under the curve; cAMP, Cyclic adenosine monophosphate; DAG, Diacylglycerol; DEPC, Diethylpyrocarbonate; DPP-4, Dipeptidyl peptidase-4; DTT, Dithiothreitol; FBG, Fasting blood glucose; FFAR, Free fatty acid receptor; G-6-Pase, Glucose-6-phosphatase; GHbAlc, Glycated hemoglobin; GLP-1, Glucagon-like peptide-1; GPCR, G protein-coupled receptor; H-K-AKK, High-temperature killed A. muciniphila; HOMA-IR, Homeostatic model assessment of insulin resistance; IAM, Iodoacetamide; INS, Fasting insulin; IP3, Inositol-1,4,5-trisphosphate; ISI, Insulin sensitivity index; KID, Kinase-inducible domain; OGTT, Oral glucose tolerance test; P-AKK, Pasteurized A. muciniphila; PC3, Prohormone convertase 3; PEPCK, Phosphoenolpyruvate carboxykinase; PIP2, Phosphatidylinositol 4,5-bisphosphate; PKA, Protein kinase A; PLC, Phospholipase C; PP, Total protein of pasteurized A. muciniphila; RT-qPCR, Real-time fluorescence quantification PCR; SCFAs, Short- chain fatty acids; SDS, Sodium dodecyl sulfate; SDS-PAGE, Sodium dodecyl sulfate polyacrylamide gel electrophoresis; STZ, Streptozotocin; T2DM, Type 2 diabetes.
1. Xu Y, Wang N, Tan H-Y, Li S, Zhang C, Feng Y. Function of Akkermansia muciniphila in obesity: interactions with lipid metabolism, immune response and gut systems. Front Microbiol. (2020) 11:219. doi: 10.3389/fmicb.2020.00219
2. Corb Aron RA, Abid A, Vesa CM, Nechifor AC, Behl T, Ghitea TC, et al. Recognizing the benefits of pre-/probiotics in metabolic syndrome and type 2 diabetes mellitus considering the influence of akkermansia muciniphila as a key gut bacterium. Microorganisms. (2021) 9:618. doi: 10.3390/microorganisms9030618
3. Yan J, Sheng L, Li H. Akkermansia muciniphila: is it the Holy Grail for ameliorating metabolic diseases? Gut Microbes. (2021) 13:1984104. doi: 10.1080/19490976.2021.1984104
4. Shi Z, Lei H, Chen G, Yuan P, Cao Z, Ser H-L, et al. Impaired intestinal Akkermansia muciniphila and aryl hydrocarbon receptor ligands contribute to nonalcoholic fatty liver disease in mice. Msystems. (2021) 6(1):10–1128. doi: 10.1128/msystems.00985-00920
5. Derrien M, Vaughan EE, Plugge CM, de Vos WM. Akkermansia muciniphila gen. nov., sp. nov., a human intestinal mucin-degrading bacterium. Int J systematic evolutionary Microbiol. (2004) 54:1469–76. doi: 10.1099/ijs.0.02873-0
6. Plovier H, Everard A, Druart C, Depommier C, Van Hul M, Geurts L, et al. A purified membrane protein from Akkermansia muciniphila or the pasteurized bacterium improves metabolism in obese and diabetic mice. Nat Med. (2017) 23:107–13. doi: 10.1038/nm.4236
7. Van Passel MW, Kant R, Zoetendal EG, Plugge CM, Derrien M, Malfatti SA, et al. The genome of Akkermansia muciniphila, a dedicated intestinal mucin degrader, and its use in exploring intestinal metagenomes. PLoS One. (2011) 6:e16876. doi: 10.1371/journal.pone.0016876
8. Dubourg G, Lagier J-C, Armougom F, Robert C, Audoly G, Papazian L, et al. High-level colonisation of the human gut by Verrucomicrobia following broad-spectrum antibiotic treatment. Int J antimicrobial agents. (2013) 41:149–55. doi: 10.1016/j.ijantimicag.2012.10.012
9. Ouwerkerk JP, Aalvink S, Belzer C, de Vos WM. Akkermansia glycaniphila sp. nov., an anaerobic mucin-degrading bacterium isolated from reticulated python faeces. Int J systematic evolutionary Microbiol. (2016) 66:4614–20. doi: 10.1099/ijsem.0.001399
10. Rodriguez C, Taminiau B, Brévers B, Avesani V, Van Broeck J, Leroux A, et al. Faecal microbiota characterisation of horses using 16 rdna barcoded pyrosequencing, and carriage rate of clostridium difficile at hospital admission. BMC Microbiol. (2015) 15:1–14. doi: 10.1186/s12866-015-0514-5
11. Roeselers G, Mittge EK, Stephens WZ, Parichy DM, Cavanaugh CM, Guillemin K, et al. Evidence for a core gut microbiota in the zebrafish. ISME J. (2011) 5:1595–608. doi: 10.1038/ismej.2011.38
12. Zeng B, Han S, Wang P, Wen B, Jian W, Guo W, et al. The bacterial communities associated with fecal types and body weight of rex rabbits. Sci Rep. (2015) 5:9342. doi: 10.1038/srep09342
13. Ushida K, Segawa T, Tsuchida S, Murata K. Cecal bacterial communities in wild Japanese rock ptarmigans and captive Svalbard rock ptarmigans. J Veterinary Med Science. (2016) 78:251–7. doi: 10.1292/jvms.15-0313
14. Derakhshani H, De Buck J, Mortier R, Barkema HW, Krause DO, Khafipour E. The features of fecal and ileal mucosa-associated microbiota in dairy calves during early infection with Mycobacterium avium subspecies paratuberculosis. Front Microbiol. (2016) 7:426. doi: 10.3389/fmicb.2016.00426
15. Hildebrand F, Ebersbach T, Nielsen HB, Li X, Sonne SB, Bertalan M, et al. A comparative analysis of the intestinal metagenomes present in Guinea pigs (Cavia porcellus) and humans (Homo sapiens). BMC Genomics. (2012) 13:1–11. doi: 10.1186/1471-2164-13-514
16. Costello EK, Gordon JI, Secor SM, Knight R. Postprandial remodeling of the gut microbiota in Burmese pythons. ISME J. (2010) 4:1375–85. doi: 10.1038/ismej.2010.71
17. Kostopoulos I, Elzinga J, Ottman N, Klievink JT, Blijenberg B, Aalvink S, et al. Akkermansia muciniphila uses human milk oligosaccharides to thrive in the early life conditions in vitro. Sci Rep. (2020) 10:14330. doi: 10.1038/s41598-020-71113-8
18. Karcher N, Nigro E, Punčochář M, Blanco-Míguez A, Ciciani M, Manghi P, et al. Genomic diversity and ecology of human-associated Akkermansia species in the gut microbiome revealed by extensive metagenomic assembly. Genome Biol. (2021) 22:209. doi: 10.1186/s13059-021-02427-7
19. Guo X, Zhang J, Wu F, Zhang M, Yi M, Peng Y. Different subtype strains of Akkermansia muciniphila abundantly colonize in southern China. J Appl Microbiol. (2016) 120:452–9. doi: 10.1111/jam.13022
20. Guo X, Li S, Zhang J, Wu F, Li X, Wu D, et al. Genome sequencing of 39 Akkermansia muciniphila isolates reveals its population structure, genomic and functional diverisity, and global distribution in mammalian gut microbiotas. BMC Genomics. (2017) 18:1–12. doi: 10.1186/s12864-017-4195-3
21. Collado MC, Derrien M, Isolauri E, de Vos WM, Salminen S. Intestinal integrity and Akkermansia muciniphila, a mucin-degrading member of the intestinal microbiota present in infants, adults, and the elderly. Appl Environ Microbiol. (2007) 73:7767–70. doi: 10.1128/AEM.01477-07
22. Wu F, Guo X, Zhang M, Ou Z, Wu D, Deng L, et al. An Akkermansia muciniphila subtype alleviates high-fat diet-induced metabolic disorders and inhibits the neurodegenerative process in mice. Anaerobe. (2020) 61:102138. doi: 10.1016/j.anaerobe.2019.102138
23. Dao MC, Everard A, Aron-Wisnewsky J, Sokolovska N, Prifti E, Verger EO, et al. Akkermansia muciniphila and improved metabolic health during a dietary intervention in obesity: relationship with gut microbiome richness and ecology. Gut. (2016) 65:426–36. doi: 10.1136/gutjnl-2014-308778
24. Palleja A, Kashani A, Allin KH, Nielsen T, Zhang C, Li Y, et al. Roux-en-Y gastric bypass surgery of morbidly obese patients induces swift and persistent changes of the individual gut microbiota. Genome Med. (2016) 8:1–13. doi: 10.1186/s13073-016-0312-1
25. Zhang Z, Chen X, Loh YJ, Yang X, Zhang C. The effect of calorie intake, fasting, and dietary composition on metabolic health and gut microbiota in mice. BMC Biol. (2021) 19:1–14. doi: 10.1186/s12915-021-00987-5
26. Shi H, Zhang B, Abo-Hamzy T, Nelson JW, Ambati CSR, Petrosino JF, et al. Restructuring the gut microbiota by intermittent fasting lowers blood pressure. Circ Res. (2021) 128:1240–54. doi: 10.1161/CIRCRESAHA.120.318155
27. Liu J, Zhong Y, Luo XM, Ma Y, Liu J, Wang H. Intermittent fasting reshapes the gut microbiota and metabolome and reduces weight gain more effectively than melatonin in mice. Front Nutr. (2021) 8:784681. doi: 10.3389/fnut.2021.784681
28. Liu Z, Dai X, Zhang H, Shi R, Hui Y, Jin X, et al. Gut microbiota mediates intermittent-fasting alleviation of diabetes-induced cognitive impairment. Nat Commun. (2020) 11:855. doi: 10.1038/s41467-020-14676-4
29. Remely M, Hippe B, Geretschlaeger I, Stegmayer S, Hoefinger I, Haslberger A. Increased gut microbiota diversity and abundance of Faecalibacterium prausnitzii and Akkermansia after fasting: a pilot study. Wiener klinische Wochenschrift. (2015) 127:394–8. doi: 10.1007/s00508-015-0755-1
30. Jian C, Silvestre MP, Middleton D, Korpela K, Jalo E, Broderick D, et al. Gut microbiota predicts body fat change following a low-energy diet: A PREVIEW intervention study. Genome Med. (2022) 14:54. doi: 10.1186/s13073-022-01053-7
31. Basolo A, Hohenadel M, Ang QY, Piaggi P, Heinitz S, Walter M, et al. Effects of underfeeding and oral vancomycin on gut microbiome and nutrient absorption in humans. Nat Med. (2020) 26:589–98. doi: 10.1038/s41591-020-0801-z
32. Zhang L, Zhang T, Sun J, Huang Y, Liu T, Ye Z, et al. Calorie restriction ameliorates hyperglycemia, modulates the disordered gut microbiota, and mitigates metabolic endotoxemia and inflammation in type 2 diabetic rats. J Endocrinological Invest. (2023) 46:699–711. doi: 10.1007/s40618-022-01914-3
33. Alili R, Belda E, Fabre O, Pelloux V, Giordano N, Legrand R, et al. Characterization of the gut microbiota in individuals with overweight or obesity during a real-world weight loss dietary program: a focus on the bacteroides 2 enterotype. Biomedicines. (2021) 10:16. doi: 10.3390/biomedicines10010016
34. Tanca A, Abbondio M, Palomba A, Fraumene C, Marongiu F, Serra M, et al. Caloric restriction promotes functional changes involving short-chain fatty acid biosynthesis in the rat gut microbiota. Sci Rep. (2018) 8:14778. doi: 10.1038/s41598-018-33100-y
35. Zhou K. Strategies to promote abundance of Akkermansia muciniphila, an emerging probiotics in the gut, evidence from dietary intervention studies. J Funct foods. (2017) 33:194–201. doi: 10.1016/j.jff.2017.03.045
36. Everard A, Belzer C, Geurts L, Ouwerkerk JP, Druart C, Bindels LB, et al. Cross-talk between Akkermansia muciniphila and intestinal epithelium controls diet-induced obesity. Proc Natl Acad Sci. (2013) 110:9066–71. doi: 10.1073/pnas.1219451110
37. Axling U, Olsson C, Xu J, Fernandez C, Larsson S, Ström K, et al. Green tea powder and Lactobacillus plantarum affect gut microbiota, lipid metabolism and inflammation in high-fat fed C57BL/6J mice. Nutr Metab. (2012) 9:1–18. doi: 10.1186/1743-7075-9-105
38. Everard A, Lazarevic V, Derrien M, Girard M, Muccioli GG, Neyrinck AM, et al. Responses of gut microbiota and glucose and lipid metabolism to prebiotics in genetic obese and diet-induced leptin-resistant mice. Diabetes. (2011) 60:2775–86. doi: 10.2337/db11-0227
39. Halmos EP, Christophersen CT, Bird AR, Shepherd SJ, Gibson PR, Muir JG. Diets that differ in their FODMAP content alter the colonic luminal microenvironment. Gut. (2015) 64:93–100. doi: 10.1136/gutjnl-2014-307264
40. Roopchand DE, Carmody RN, Kuhn P, Moskal K, Rojas-Silva P, Turnbaugh PJ, et al. Dietary polyphenols promote growth of the gut bacterium Akkermansia muciniphila and attenuate high-fat diet–induced metabolic syndrome. Diabetes. (2015) 64:2847–58. doi: 10.2337/db14-1916
41. Zheng S, Huang K, Zhao C, Xu W, Sheng Y, Luo Y, et al. Procyanidin attenuates weight gain and modifies the gut microbiota in high fat diet induced obese mice. J Funct foods. (2018) 49:362–8. doi: 10.1016/j.jff.2018.09.007
42. Li Y, Li J, Su Q, Liu Y. Sinapine reduces non-alcoholic fatty liver disease in mice by modulating the composition of the gut microbiota. Food Funct. (2019) 10:3637–49. doi: 10.1039/C9FO00195F
43. Lima ACD, Cecatti C, Fidélix MP, Adorno MAT, Sakamoto IK, Cesar TB, et al. Effect of daily consumption of orange juice on the levels of blood glucose, lipids, and gut microbiota metabolites: controlled clinical trials. J medicinal Food. (2019) 22:202–10. doi: 10.1089/jmf.2018.0080
44. Cani PD, Depommier C, Derrien M, Everard A, de Vos WM. Akkermansia muciniphila: paradigm for next-generation beneficial microorganisms. Nat Rev Gastroenterol Hepatology. (2022) 19:625–37. doi: 10.1038/s41575-022-00631-9
45. Madden JA, Plummer SF, Tang J, Garaiova I, Plummer NT, Herbison M, et al. Effect of probiotics on preventing disruption of the intestinal microflora following antibiotic therapy: a double-blind, placebo-controlled pilot study. Int immunopharmacology. (2005) 5:1091–7. doi: 10.1016/j.intimp.2005.02.006
46. Hooker K, DiPiro J. Effect of antimicrobial therapy on bowel flora. Clin pharmacy. (1988) 7:878–88.
47. Nakashima T, Fujii K, Seki T, Aoyama M, Azuma A, Kawasome H. Novel gut microbiota modulator, which markedly increases Akkermansia muciniphila occupancy, ameliorates experimental colitis in rats. Digestive Dis Sci. (2022) 67:2899–911. doi: 10.1007/s10620-021-07131-x
48. Anderson R, Dalziel J, Gopal P, Bassett S, Ellis A, Roy N. The role of intestinal barrier function in early life in the development of colitis. Colitis. (2012), 1–30. doi: 10.5772/1555
49. Iqbal Z, Ahmed S, Tabassum N, Bhattacharya R, Bose D. Role of probiotics in prevention and treatment of enteric infections: A comprehensive review. 3 Biotech. (2021) 11:242. doi: 10.1007/s13205-021-02796-7
50. Gasaly N, De Vos P, Hermoso MA. Impact of bacterial metabolites on gut barrier function and host immunity: a focus on bacterial metabolism and its relevance for intestinal inflammation. Front Immunol. (2021) 12:658354. doi: 10.3389/fimmu.2021.658354
51. Wang M, Fu R-J, Xu D-Q, Chen Y-Y, Yue S-J, Zhang S, et al. Traditional Chinese Medicine: A promising strategy to regulate the imbalance of bacterial flora, impaired intestinal barrier and immune function attributed to ulcerative colitis through intestinal microecology. J Ethnopharmacology. (2023) 318:116879. doi: 10.1016/j.jep.2023.116879
52. Zimmet P, Alberti K, Shaw J. Global and societal implications of the diabetes epidemic. Nature. (2001) 414:782–7. doi: 10.1038/414782a
53. Consultation W. Obesity: preventing and managing the global epidemic. World Health Organ Tech Rep series. (2000) 894:1–253.
54. DiBaise JK, Foxx-Orenstein AE. Role of the gastroenterologist in managing obesity. Expert Rev Gastroenterol hepatology. (2013) 7:439–51. doi: 10.1586/17474124.2013.811061
55. Santacruz A, Collado MC, Garcia-Valdes L, Segura M, Martin-Lagos J, Anjos T, et al. Gut microbiota composition is associated with body weight, weight gain and biochemical parameters in pregnant women. Br J Nutr. (2010) 104:83–92. doi: 10.1017/S0007114510000176
56. Karlsson CL, Önnerfält J, Xu J, Molin G, Ahrné S, Thorngren-Jerneck K. The microbiota of the gut in preschool children with normal and excessive body weight. Obesity. (2012) 20:2257–61. doi: 10.1038/oby.2012.110
57. Teixeira T, Grześkowiak ŁM, Salminen S, Laitinen K, Bressan J, Peluzio M. Faecal levels of Bifidobacterium and Clostridium coccoides but not plasma lipopolysaccharide are inversely related to insulin and HOMA index in women. Clin Nutr. (2013) 32:1017–22. doi: 10.1016/j.clnu.2013.02.008
58. Liang C, Guo M, Liu T, Zhou X, Gong P, Lyu L, et al. Profiles of gut microbiota in children with obesity from Harbin, China and screening of strains with anti-obesity ability in vitro and in vivo. J Appl Microbiol. (2020) 129:728–37. doi: 10.1111/jam.14639
59. Escobar JS, Klotz B, Valdes BE, Agudelo GM. The gut microbiota of Colombians differs from that of Americans, Europeans and Asians. BMC Microbiol. (2014) 14:1–14. doi: 10.1186/s12866-014-0311-6
60. Schneeberger M, Everard A, Gómez-Valadés AG, Matamoros S, Ramírez S, Delzenne NM, et al. Akkermansia muciniphila inversely correlates with the onset of inflammation, altered adipose tissue metabolism and metabolic disorders during obesity in mice. Sci Rep. (2015) 5:16643. doi: 10.1038/srep16643
61. Hussain A, Yadav MK, Bose S, Wang J-H, Lim D, Song Y-K, et al. Daesiho-Tang is an effective herbal formulation in attenuation of obesity in mice through alteration of gene expression and modulation of intestinal microbiota. PLoS One. (2016) 11:e0165483. doi: 10.1371/journal.pone.0165483
62. Wang L, Wu Y, Zhuang L, Chen X, Min H, Song S, et al. Puerarin prevents high-fat diet-induced obesity by enriching Akkermansia muciniphila in the gut microbiota of mice. PLoS One. (2019) 14:e0218490. doi: 10.1371/journal.pone.0218490
63. Marvasti FE, Moshiri A, Taghavi MS, Riazi S, Taati M, Sadati SF, et al. The first report of differences in gut microbiota composition between obese and normal weight Iranian subjects. Iranian Biomed J. (2020) 24:148. doi: 10.29252/ibj.24.3.148
64. Wu T, Gao Y, Hao J, Geng J, Zhang J, Yin J, et al. Capsanthin extract prevents obesity, reduces serum TMAO levels and modulates the gut microbiota composition in high-fat-diet induced obese C57BL/6J mice. Food Res Int. (2020) 128:108774. doi: 10.1016/j.foodres.2019.108774
65. Medina-Vera I, Sanchez-Tapia M, Noriega-López L, Granados-Portillo O, Guevara-Cruz M, Flores-López A, et al. A dietary intervention with functional foods reduces metabolic endotoxaemia and attenuates biochemical abnormalities by modifying faecal microbiota in people with type 2 diabetes. Diabetes Metab. (2019) 45:122–31. doi: 10.1016/j.diabet.2018.09.004
66. Zhang J, Ni Y, Qian L, Fang Q, Zheng T, Zhang M, et al. Decreased abundance of Akkermansia muciniphila leads to the impairment of insulin secretion and glucose homeostasis in lean type 2 diabetes. Advanced Science. (2021) 8:2100536. doi: 10.1002/advs.202100536
67. Li X, Wang TX, Huang X, Li Y, Sun T, Zang S, et al. Targeting ferroptosis alleviates methionine-choline deficient (MCD)-diet induced NASH by suppressing liver lipotoxicity. Liver Int. (2020) 40:1378–94. doi: 10.1111/liv.14428
68. Du J, Zhang P, Luo J, Shen L, Zhang S, Gu H, et al. Dietary betaine prevents obesity through gut microbiota-drived microRNA-378a family. Gut Microbes. (2021) 13:1862612. doi: 10.1080/19490976.2020.1862612
69. Shin N-R, Lee J-C, Lee H-Y, Kim M-S, Whon TW, Lee M-S, et al. An increase in the Akkermansia spp. population induced by metformin treatment improves glucose homeostasis in diet-induced obese mice. Gut. (2014) 63:727–35. doi: 10.1136/gutjnl-2012-303839
70. Org E, Parks BW, Joo JWJ, Emert B, Schwartzman W, Kang EY, et al. Genetic and environmental control of host-gut microbiota interactions. Genome Res. (2015) 25:1558–69. doi: 10.1101/gr.194118.115
71. Shin J, Noh J-R, Chang D-H, Kim Y-H, Kim MH, Lee ES, et al. Elucidation of Akkermansia muciniphila probiotic traits driven by mucin depletion. Front Microbiol. (2019) 10:1137. doi: 10.3389/fmicb.2019.01137
72. Chelakkot C, Choi Y, Kim D-K, Park HT, Ghim J, Kwon Y, et al. Akkermansia muciniphila-derived extracellular vesicles influence gut permeability through the regulation of tight junctions. Exp Mol Med. (2018) 50:e450–0. doi: 10.1038/emm.2017.282
73. Depommier C, Everard A, Druart C, Plovier H, Van Hul M, Vieira-Silva S, et al. Supplementation with Akkermansia muciniphila in overweight and obese human volunteers: a proof-of-concept exploratory study. Nat Med. (2019) 25:1096–103. doi: 10.1038/s41591-019-0495-2
74. Li J, Lin S, Vanhoutte PM, Woo CW, Xu A. Akkermansia muciniphila protects against atherosclerosis by preventing metabolic endotoxemia-induced inflammation in Apoe–/– mice. Circulation. (2016) 133:2434–46. doi: 10.1161/CIRCULATIONAHA.115.019645
75. Kim S, Lee Y, Kim Y, Seo Y, Lee H, Ha J, et al. Akkermansia muciniphila prevents fatty liver disease, decreases serum triglycerides, and maintains gut homeostasis. Appl Environ Microbiol. (2020) 86:e03004–03019. doi: 10.1128/AEM.03004-19
76. Rao Y, Kuang Z, Li C, Guo S, Xu Y, Zhao D, et al. Gut Akkermansia muciniphila ameliorates metabolic dysfunction-associated fatty liver disease by regulating the metabolism of L-aspartate via gut-liver axis. Gut Microbes. (2021) 13:1927633. doi: 10.1080/19490976.2021.1927633
77. Higarza SG, Arboleya S, Arias JL, Gueimonde M, Arias N. Akkermansia muciniphila and environmental enrichment reverse cognitive impairment associated with high-fat high-cholesterol consumption in rats. Gut Microbes. (2021) 13:1880240. doi: 10.1080/19490976.2021.1880240
78. Ganesh BP, Klopfleisch R, Loh G, Blaut M. Commensal Akkermansia muciniphila exacerbates gut inflammation in Salmonella Typhimurium-infected gnotobiotic mice. PLoS One. (2013) 8:e74963. doi: 10.1371/journal.pone.0074963
79. Druart C, Plovier H, Van Hul M, Brient A, Phipps KR, de Vos WM, et al. Toxicological safety evaluation of pasteurized Akkermansia muciniphila. J Appl Toxicology. (2021) 41:276–90. doi: 10.1002/jat.4044
80. Depommier C, Van Hul M, Everard A, Delzenne NM, De Vos WM, Cani PD. Pasteurized Akkermansia muciniphila increases whole-body energy expenditure and fecal energy excretion in diet-induced obese mice. Gut Microbes. (2020) 11:1231–45. doi: 10.1080/19490976.2020.1737307
81. Lee JS, Song WS, Lim JW, Choi TR, Jo SH, Jeon HJ, et al. An integrative multiomics approach to characterize anti-adipogenic and anti-lipogenic effects of Akkermansia muciniphila in adipocytes. Biotechnol J. (2022) 17:2100397. doi: 10.1002/biot.202100397
82. Zhang X, Shen D, Fang Z, Jie Z, Qiu X, Zhang C, et al. Human gut microbiota changes reveal the progression of glucose intolerance. PLoS One. (2013) 8:e71108. doi: 10.1371/journal.pone.0071108
83. Régnier M, Rastelli M, Morissette A, Suriano F, Le Roy T, Pilon G, et al. Rhubarb supplementation prevents diet-induced obesity and diabetes in association with increased Akkermansia muciniphila in mice. Nutrients. (2020) 12:2932. doi: 10.3390/nu12102932
84. Qin J, Li Y, Cai Z, Li S, Zhu J, Zhang F, et al. A metagenome-wide association study of gut microbiota in type 2 diabetes. Nature. (2012) 490:55–60. doi: 10.1038/nature11450
85. Poitout V, Amyot J, Semache M, Zarrouki B, Hagman D, Fontés G. Glucolipotoxicity of the pancreatic beta cell. Biochim Biophys Acta (BBA)-Molecular Cell Biol Lipids. (2010) 1801:289–98. doi: 10.1016/j.bbalip.2009.08.006
86. Cnop M, Hannaert JC, Hoorens A, Eizirik DL, Pipeleers DG. Inverse relationship between cytotoxicity of free fatty acids in pancreatic islet cells and cellular triglyceride accumulation. Diabetes. (2001) 50:1771–7. doi: 10.2337/diabetes.50.8.1771
87. Zhou Y, Grill V. Long term exposure to fatty acids and ketones inhibits B-cell functions in human pancreatic islets of Langerhans. J Clin Endocrinol Metab. (1995) 80:1584–90. doi: 10.1210/jcem.80.5.7745004
88. Shen J, Wang S, Xia H, Han S, Wang Q, Wu Z, et al. Akkermansia muciniphila attenuated lipopolysaccharide-induced acute lung injury by modulating the gut microbiota and SCFAs in mice. Food Funct. (2023) 14:10401–17. doi: 10.1039/D3FO04051H
89. Müller M, Hernández MAG, Goossens GH, Reijnders D, Holst JJ, Jocken JW, et al. Circulating but not faecal short-chain fatty acids are related to insulin sensitivity, lipolysis and GLP-1 concentrations in humans. Sci Rep. (2019) 9:1–9. doi: 10.1038/s41598-019-48775-0
90. Yoon HS, Cho CH, Yun MS, Jang SJ, You HJ, J-h K, et al. Akkermansia muciniphila secretes a glucagon-like peptide-1-inducing protein that improves glucose homeostasis and ameliorates metabolic disease in mice. Nat Microbiol. (2021) 6:563–73. doi: 10.1038/s41564-021-00880-5
91. Fu Y, Liu B, Zhang N, Liu Z, Liang D, Li F, et al. Magnolol inhibits lipopolysaccharide-induced inflammatory response by interfering with TLR4 mediated NF-κB and MAPKs signaling pathways. J ethnopharmacology. (2013) 145:193–9. doi: 10.1016/j.jep.2012.10.051
92. Le Marchand-Brustel Y, Gual P, Gremeaux T, Gonzalez T, Barres R, Tanti J-F. Fatty acid-induced insulin resistance: role of insulin receptor substrate 1 serine phosphorylation in the retroregulation of insulin signalling. Biochem Soc Trans. (2003) 31:1152–6. doi: 10.1042/bst0311152
93. Martin-Gallausiaux C, Garcia-Weber D, Lashermes A, Larraufie P, Marinelli L, Teixeira V, et al. Akkermansia muciniphila upregulates genes involved in maintaining the intestinal barrier function via ADP-heptose-dependent activation of the ALPK1/TIFA pathway. Gut Microbes. (2022) 14:2110639. doi: 10.1080/19490976.2022.2110639
94. Qu S, Zheng Y, Huang Y, Feng Y, Xu K, Zhang W, et al. Excessive consumption of mucin by over-colonized Akkermansia muciniphila promotes intestinal barrier damage during Malignant intestinal environment. Front Microbiol. (2023) 14:1111911. doi: 10.3389/fmicb.2023.1111911
95. Ottman N, Reunanen J, Meijerink M, Pietilä TE, Kainulainen V, Klievink J, et al. Pili-like proteins of Akkermansia muciniphila modulate host immune responses and gut barrier function. PLoS One. (2017) 12:e0173004. doi: 10.1371/journal.pone.0173004
96. Zhou G, Myers R, Li Y, Chen Y, Shen X, Fenyk-Melody J, et al. Role of AMP-activated protein kinase in mechanism of metformin action. J Clin Invest. (2001) 108:1167–74. doi: 10.1172/JCI200113505
97. Shaw RJ, Lamia KA, Vasquez D, Koo S-H, Bardeesy N, DePinho RA, et al. The kinase LKB1 mediates glucose homeostasis in liver and therapeutic effects of metformin. Science. (2005) 310:1642–6. doi: 10.1126/science.1120781
98. Fullerton MD, Galic S, Marcinko K, Sikkema S, Pulinilkunnil T, Chen Z-P, et al. Single phosphorylation sites in Acc1 and Acc2 regulate lipid homeostasis and the insulin-sensitizing effects of metformin. Nat Med. (2013) 19:1649–54. doi: 10.1038/nm.3372
99. Foretz M, Hébrard S, Leclerc J, Zarrinpashneh E, Soty M, Mithieux G, et al. Metformin inhibits hepatic gluconeogenesis in mice independently of the LKB1/AMPK pathway via a decrease in hepatic energy state. J Clin Invest. (2010) 120:2355–69. doi: 10.1172/JCI40671
100. Madiraju AK, Erion DM, Rahimi Y, Zhang X-M, Braddock DT, Albright RA, et al. Metformin suppresses gluconeogenesis by inhibiting mitochondrial glycerophosphate dehydrogenase. Nature. (2014) 510:542–6. doi: 10.1038/nature13270
101. Miller RA, Chu Q, Xie J, Foretz M, Viollet B, Birnbaum MJ. Biguanides suppress hepatic glucagon signalling by decreasing production of cyclic AMP. Nature. (2013) 494:256–60. doi: 10.1038/nature11808
102. McCreight LJ, Bailey CJ, Pearson ER. Metformin and the gastrointestinal tract. Diabetologia. (2016) 59:426–35. doi: 10.1007/s00125-015-3844-9
103. Duca FA, Côté CD, Rasmussen BA, Zadeh-Tahmasebi M, Rutter GA, Filippi BM, et al. Metformin activates a duodenal Ampk–dependent pathway to lower hepatic glucose production in rats. Nat Med. (2015) 21:506–11. doi: 10.1038/nm.3787
104. Forslund K, Hildebrand F, Nielsen T, Falony G, Le Chatelier E, Sunagawa S, et al. Disentangling type 2 diabetes and metformin treatment signatures in the human gut microbiota. Nature. (2015) 528:262–6. doi: 10.1038/nature15766
105. De La Cuesta-Zuluaga J, Mueller NT, Corrales-Agudelo V, Velásquez-Mejía EP, Carmona JA, Abad JM, et al. Metformin is associated with higher relative abundance of mucin-degrading Akkermansia muciniphila and several short-chain fatty acid–producing microbiota in the gut. Diabetes Care. (2017) 40:54–62. doi: 10.2337/dc16-1324
106. Yue X, Wen S, Long-Kun D, Man Y, Chang S, Min Z, et al. Three important short-chain fatty acids (SCFAs) attenuate the inflammatory response induced by 5-FU and maintain the integrity of intestinal mucosal tight junction. BMC Immunol. (2022) 23:1–13. doi: 10.1186/s12865-022-00495-3
107. Farhadipour M, Depoortere I. The function of gastrointestinal hormones in obesity—implications for the regulation of energy intake. Nutrients. (2021) 13:1839. doi: 10.3390/nu13061839
108. Jones B, Bloom SR, Buenaventura T, Tomas A, Rutter GA. Control of insulin secretion by GLP-1. Peptides. (2018) 100:75–84. doi: 10.1016/j.peptides.2017.12.013
109. Lee H, Ko G. Effect of metformin on metabolic improvement and gut microbiota. Appl Environ Microbiol. (2014) 80:5935–43. doi: 10.1128/AEM.01357-14
110. Ke H, Li F, Deng W, Li Z, Wang S, Lv P, et al. Metformin exerts anti-inflammatory and mucus barrier protective effects by enriching Akkermansia muciniphila in mice with ulcerative colitis. Front Pharmacol. (2021) 12:726707. doi: 10.3389/fphar.2021.726707
111. Lüscher TF. Prevention: some important steps forward, but many unmet needs in a world with cardiovascular disease as the leading cause of death. Eur Heart J. (2016) 37:3179–81. doi: 10.1093/eurheartj/ehw566
112. Jiao J, Zhang Y, Han P, Zhai S. A preliminary study on the value of intestinal flora in predicting major adverse cardiovascular and cerebrovascular events in patients with refractory hypertension. Comput Math Methods Med. (2022) 2022. doi: 10.1155/2022/7723105
113. He X, Bai Y, Zhou H, Wu K. Akkermansia muciniphila alters gut microbiota and immune system to improve cardiovascular diseases in murine model. Front Microbiol. (2022) 13:906920. doi: 10.3389/fmicb.2022.906920
114. Luo Y, Zhang Y, Han X, Yuan Y, Zhou Y, Gao Y, et al. Akkermansia muciniphila prevents cold-related atrial fibrillation in rats by modulation of TMAO induced cardiac pyroptosis. EBioMedicine. (2022) 82. doi: 10.1016/j.ebiom.2022.104087
115. Herrington W, Lacey B, Sherliker P, Armitage J, Lewington S. Epidemiology of atherosclerosis and the potential to reduce the global burden of atherothrombotic disease. Circ Res. (2016) 118:535–46. doi: 10.1161/CIRCRESAHA.115.307611
116. Shen J, Tong X, Sud N, Khound R, Song Y, Maldonado-Gomez MX, et al. Low-density lipoprotein receptor signaling mediates the triglyceride-lowering action of Akkermansia muciniphila in genetic-induced hyperlipidemia. Arteriosclerosis thrombosis Vasc Biol. (2016) 36:1448–56. doi: 10.1161/ATVBAHA.116.307597
117. Rinella ME. Nonalcoholic fatty liver disease: A systematic review. Jama. (2015) 313:2263–73. doi: 10.1001/jama.2015.5370
118. Yilmaz Y, Eren F. Nonalcoholic steatohepatitis and gut microbiota: Future perspectives on probiotics in metabolic liver diseases. Turk J Gastroenterol. (2017) 28:327–8. doi: 10.5152/tjg
119. Ozkul C, Yalinay M, Karakan T, Yilmaz G. Determination of certain bacterial groups in gut microbiota and endotoxin levels in patients with nonalcoholic steatohepatitis. Turk J Gastroenterol. (2017) 28:361–9. doi: 10.5152/tjg
120. Juárez-Fernández M, Porras D, Petrov P, Román-Sagüillo S, García-Mediavilla MV, Soluyanova P, et al. The synbiotic combination of Akkermansia muciniphila and quercetin ameliorates early obesity and NAFLD through gut microbiota reshaping and bile acid metabolism modulation. Antioxidants. (2021) 10:2001. doi: 10.3390/antiox10122001
Keywords: Akkermansia muciniphila, metabolic diseases, obesity, gut microbiota, health
Citation: Niu H, Zhou M, Zogona D, Xing Z, Wu T, Chen R, Cui D, Liang F and Xu X (2024) Akkermansia muciniphila: a potential candidate for ameliorating metabolic diseases. Front. Immunol. 15:1370658. doi: 10.3389/fimmu.2024.1370658
Received: 15 January 2024; Accepted: 11 March 2024;
Published: 20 March 2024.
Edited by:
Subash Babu, International Centers for Excellence in Research (ICER), IndiaReviewed by:
Anuradha Rajamanickam, International Centers for Excellence in Research (ICER), IndiaCopyright © 2024 Niu, Zhou, Zogona, Xing, Wu, Chen, Cui, Liang and Xu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Dandan Cui, MzcwOTc1NjM1QHFxLmNvbQ==; Fengxia Liang, ZnhsaWFuZzVAaG90bWFpbC5jb20=; Xiaoyun Xu, eHV4aWFveXVuQG1haWwuaHphdS5lZHUuY24=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.