- 1Department of Visceral Surgery and Medicine, Inselspital, Bern University Hospital, University of Bern, Bern, Switzerland
- 2Department of BioMedical Research, University of Bern, Bern, Switzerland
- 3Department of Rheumatology and Immunology, University Hospital Bern, Bern, Switzerland
The rising global incidence of IgE-mediated allergic reactions poses a significant challenge to the quality of life of affected individuals and to healthcare systems, with current treatments being limited in effectiveness, safety, and disease-modifying capabilities. IgE acts by sensitizing the high-affinity IgE receptor FcεRI expressed by mast cells and basophils, tuning these cells for inflammatory degranulation in response to future allergen encounters. In recent years, IgG has emerged as an essential negative regulator of IgE-dependent allergic inflammation. Mechanistically, studies have proposed different pathways by which IgG can interfere with the activation of IgE-mediated inflammation. Here, we briefly summarize the major proposed mechanisms of action by which IgG controls the IgE-FcεRI inflammatory axis and how those mechanisms are currently applied as therapeutic interventions for IgE-mediated inflammation.
1 FcεRI sensitization and activation
Whether IgE switching occurs from IgM or IgG remains a debated topic and has been discussed elsewhere (1, 2). Here, we focus on how IgG controls the immediate allergic reaction once IgE is produced by B cells. The activation of inflammatory mast cell and basophil degranulation by IgE is a two-step process (3, 4). First, IgE needs to sensitize mast cells and basophils by binding to the high-affinity IgE receptor FcεRI. This interaction is remarkably stable and sensitization can thus persist for long periods, specifically in the more long-lived mast cells (5, 6). The second event is the encounter with an antigen that cross-links IgE bound to FcεRI leading to FcεRI intracellular signaling cascades and degranulation (7, 8). If improperly controlled, this reaction can have negative consequences such as in allergy, where harmless antigens (allergens) become activators of FcεRI. Currently, allergic diseases are posing massive challenges to global health and thus, strategies to inhibit the IgE : FcεRI inflammatory axis have been of high interest to clinical and pharmaceutical research (9, 10). In recent years, it has become clear that IgG antibodies may be important in both types of physiological FcεRI de-granulation control, the sensitization and the activation phase.
2 IgG specific for IgE in the prevention of FcεRI sensitization
We and others have shown that naturally occurring IgG anti-IgE autoantibodies are present to surprising levels in humans and mice. In both humans and mice, the antibodies can prevent basophil sensitization and FcεRI activation (11–14). Interestingly, the immunogenicity of self-IgE is regulated by a single highly conserved mannose glycosylation site on IgE, which is likewise preferentially recognized by the anti-IgE autoantibodies (12, 15). The same mannose glycan is crucial for both FcεRI binding and IgE sensitization (16, 17). It thus seems plausible that competition between anti-IgE autoantibodies and FcεRI for this single mannose glycosylation site explains how IgE-IgG ICs fail to activate FcεRI. Nevertheless, this aspect remains to be studied in further detail. We have shown another mechanism by which anti-IgE auto-antibodies act. The formation of IgG immune complexes with IgE (IgE-IgG-IC) results in preferential targeting of the low-affinity IgE receptor CD23 over FcεRI (12). CD23 is constitutively expressed in B cells but can be induced in a variety of other cells (18). We had previously shown that CD23 is essential in the clearance of IgE-allergen-IC but this is likewise the case for IgE-IgG-ICs (12, 19). Moreover, the immune response caused by IgE-IgG-ICs results in a boost of anti-IgE autoantibodies establishing a positive feedback loop that diminishes serum IgE levels further. Interestingly, studies have proposed maternal transfer of IgE-IgG-IC via the neonatal FcRn receptor (20, 21). It is important to note, that any dysregulation of those anti-IgE autoantibodies may lead to pathologies, for example they are thought to inadvertently cross-link FcεRI in Chronic Spontaneous Urticaria (CSU) or atopic dermatitis (AD) thus contributing to disease (22, 23). Overall, the role of natural anti-IgE antibodies in health and disease needs to be further investigated in future studies.
3 IgG specific for allergen in the inhibition of FcεRI activation
Once IgE has bound to FcεRI, allergen-specific IgG may still block allergic reactions by allergen-neutralization. IgG circulates the blood at very high concentrations compared to IgE and if enough specific IgG is present, is thus likely to form IgG-allergen ICs before FcεRI is encountered. Numerous studies have shown a role for blocking antibodies in preventing the initiation of the allergic cascade by impeding the binding of allergens to IgE (24–26). Due to their lower ability to activate complement and Fc-dependent effects, IgG4 antibodies have been considered specifically optimal blocking antibodies in allergy (27–30). However, neutralizing IgGs are not required to suppress FcεRI. Even if an IgG-allergen IC reaches the cell and is recognized by IgE, there is a second mechanism at work that suppresses the allergic response. Mast cells and basophils can express the inhibitory IgG receptor FcγRIIb, which contains an ITIM motif that activates the inositol phosphatase SHIP, a major negative immune regulator (31, 32). When FcεRI is cross-linked to FcγRIIb, the activation cascade of FcεRI is suppressed via SHIP (32–35). Notably, the inhibitory signal from FcγRIIb does not only act on individual allergen-IgG pairs but SHIP can spread around the cell membrane and dephosphorylate distant FcεRI and activated kinases (36). While it was often speculated that IgG4 is superior in suppressing the allergic reaction via FcγRIIb, it was recently shown that IgG1 and IgG4 appear to similarly suppress FcεRI activation via FcγRIIb (33, 37). Moreover, IgG affinity for the allergen seems less important for FcγRIIb engagement than for neutralizing antibodies (38). Finally, FcεRI : FcγRIIb complexes can be internalized and degraded, resulting in a de-sensitizing of effector cells, coupled with a reduction of IgE (39). In actuality, both neutralization and FcγRIIb engagement most likely occur simultaneously during polyclonal responses in vivo (40).
4 The role of IgG in allergy immunotherapy
Elevated levels of IgG antibodies have been observed in individuals with natural tolerance to allergens, suggesting a possibly inherent protective role of these antibodies (41). In mice, maternal IgG-allergen complexes transferred via breast milk and the neonatal receptor FcRn were shown to protect the offspring from allergy (42). On the other hand, IgG has been extensively studied in allergen-specific immunotherapy (AIT), a disease-modifying treatment for allergy, where increasing doses of specific allergens are administered to allergic individuals to build tolerance (43, 44). Successful AIT has been associated with elevated levels of allergen-specific IgG. Specifically, it is accepted that the induction of high IgG : IgE ratios in addition to regulatory T and B cells is a favorable and protective occurrence whereas IgE levels are initially increased with AIT before they are later reduced (30, 45–49). Moreover, high IgG levels have been associated with reduced risk of more severe, anaphylactic reactions (50). While in AIT, the IgG4 subclass is usually associated best with protection from allergy, this most likely reflects superior induction of IgG4 over IgG1 by most AIT-protocols and does not necessarily indicate an inferiority of IgG1 to neutralize the allergen or engage FcγRIIb. Recently, it was shown that IgG1 may suppress the allergic during earlier AIT responses whereas IgG4 gains importance in later stages (37, 49). Even though some mechanistic aspects remain elusive, current findings suggest AIT, the only current disease-modifying therapy for allergic diseases involves the induction of protective IgG antibodies.
5 Therapeutic approaches based on the anti-allergic properties of IgG
The IgG-mediated control of FcεRI sensitization and activation have been increasingly translated into clinical development and application. Omalizumab, a monoclonal anti-IgE antibody which blocks FcεRI sensitization has been developed a long time ago (51). Mechanistically, anti-IgE biologics act similar to natural anti-IgE autoantibodies by preventing or disrupting the FcεRI-IgE interaction and by lowering serum IgE levels. We have recently shown that omalizumab function, in identical fashion to natural anti-IgE autoantibodies, depends on the single IgE mannose glycosylation site (52). A number of other IgE-targeting approaches are explored, which have been reviewed by others (53–61). The development of allergen-specific IgG for the treatment of allergy is a more recent development and for the most part, clinical trials are still ongoing (62, 63). Two major strategies are currently being employed, both aiming at harnessing the protective role of allergen-specific IgG. Neutralizing monoclonal anti-allergen IgG antibodies have demonstrated efficacy in suppressing allergy in preclinical and clinical settings (64–72). While the results are generally positive, questions on their cost-effectiveness remain. Nevertheless, their ability to increase the safety of allergy immunotherapy and potentially boost beneficial immune responses is intriguing (63). A more cost-effective approach could be the induction of polyclonal IgG responses using immunization strategies that improve the current drawbacks of AIT including safety concerns, patient compliance, long treatment periods and varying efficacy. The challenge here is to modify the allergens in a way that reduces FcεRI cross-linking but enhances the induction of allergen-specific IgG. Others have reviewed the current landscape of vaccination strategies for improving AIT (73–79). We have focused on using allergens displayed on virus-like particles (VLP), which can reduce allergen reactivity while boosting IgG responses that increase protection via FcγRIIb (80, 81).
6 Discussion: future prospects and challenges
The overall importance of IgG in the control of IgE : FcεRI in basic and preclinical research is established. Ongoing and future research will define the relative importance of the here-presented individual pathways through which IgG confers protection against allergies (Figure 1). As research advances, it is expected that a more nuanced and contextual understanding of IgG in allergic reactions will be achieved. This will pave the way for innovative therapeutic strategies translating the protective potential of IgG into the clinics, thereby offering relief to millions of people suffering from allergies worldwide.
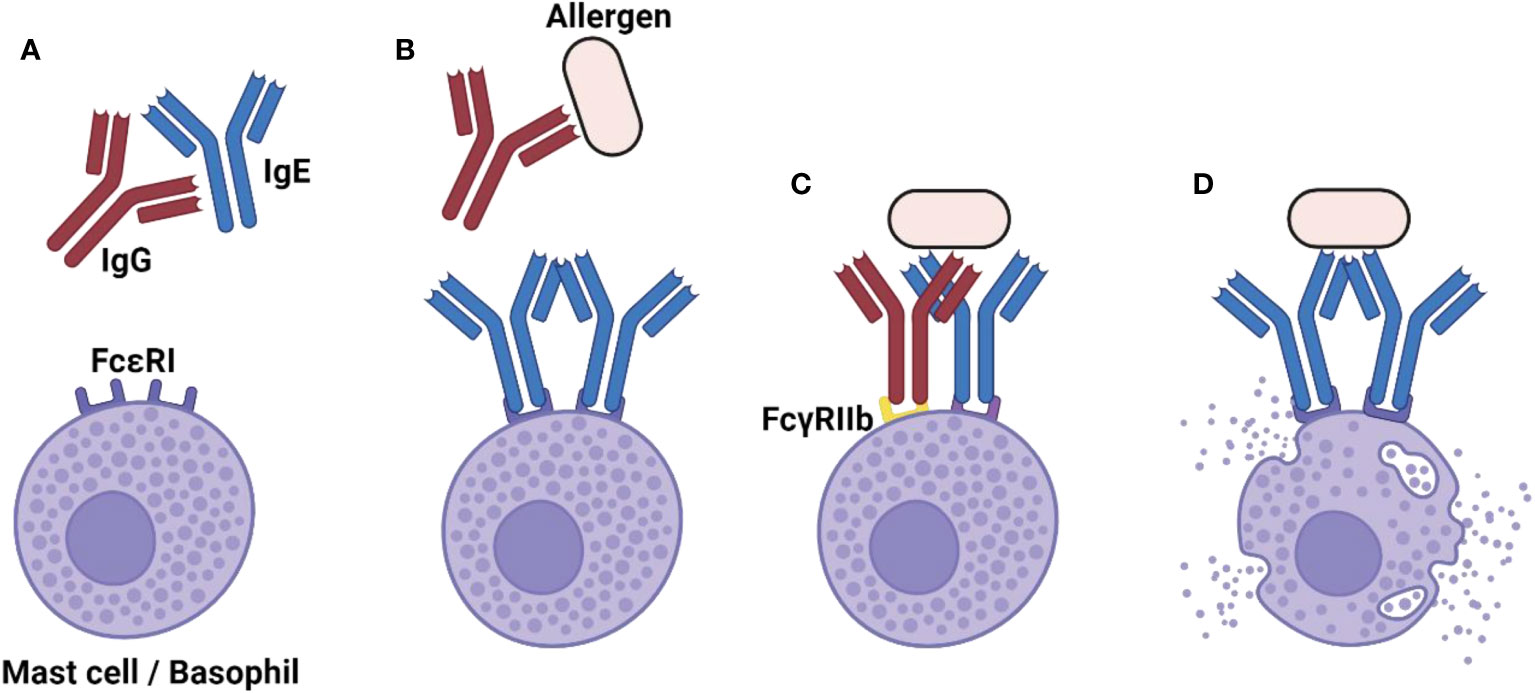
Figure 1 The diverse role of IgG in shutting down-the allergic reaction. (A) Naturally occurring IgG anti-IgE autoantibodies can limit the extent of FcεRI sensitization and thereby prevent the allergic reaction. (B) In the case that FcεRI sensitization has already occurred, neutralizing IgG anti-allergen antibodies can block allergen recognition by IgE, thus preventing FcεRI activation. (C) Even in absence of neutralizing IgG antibodies, the inhibitory FcγRIIb receptor suppresses the allergic reaction when it is cross-linked to FcεRI. (D) In the absence of IgG-mediated control, the allergic reaction can occur. Created with BioRender.com.
Author contributions
FS: Writing – original draft. MV: Writing – review & editing. MB: Writing – review & editing. PE: Writing – original draft.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This project was supported by funding from the following grants: SNF grant 310030_179165 to MV; SNF grant 310030_185114 to MB; SNF grant P2BEP3_188262 to PE.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Geha RS, Jabara HH, Brodeur SR. The regulation of immunoglobulin E class-switch recombination. Nat Rev Immunol (2003) 3:721–32. doi: 10.1038/nri1181
2. Saunders SP, Ma EGM, Aranda CJ, Curotto de Lafaille MA. Non-classical B cell memory of allergic igE responses. Front Immunol (2019) 10:715. doi: 10.3389/fimmu.2019.00715
3. Bulfone-Paus S, Nilsson G, Draber P, Blank U, Levi-Schaffer F. Positive and negative signals in mast cell activation. Trends Immunol (2017) 38:657–67. doi: 10.1016/j.it.2017.01.008
4. Gould HJ, Sutton BJ. IgE in allergy and asthma today. Nat Rev Immunol (2008) 8:205–17. doi: 10.1038/nri2273
5. Holdom MD, Davies AM, Nettleship JE, Bagby SC, Dhaliwal B, Girardi E, et al. Conformational changes in IgE contribute to its uniquely slow dissociation rate from receptor FcεRI. Nat Struct Mol Biol (2011) 18:571–6. doi: 10.1038/nsmb.2044
6. Galli SJ, Tsai M. IgE and mast cells in allergic disease. Nat Med (2012) 18:693–704. doi: 10.1038/nm.2755
7. Kinet J-P. The high-affinity IgE receptor (FcεRI): From Physiology to Pathology. Annu Rev Immunol (1999) 17:931–72. doi: 10.1146/annurev.immunol.17.1.931
8. Wilson BS, Pfeiffer JR, Oliver JM. Observing Fc epsilon RI signaling from the inside of the mast cell membrane. J Cell Biol (2000) 149:1131–42. doi: 10.1083/jcb.149.5.1131
9. Platts-Mills TAE. The allergy epidemics: 1870-2010. J Allergy Clin Immunol (2015) 136:3–13. doi: 10.1016/j.jaci.2015.03.048
10. Genuneit J, Seibold AM, Apfelbacher CJ, Konstantinou GN, Koplin JJ, La Grutta S, et al. Overview of systematic reviews in allergy epidemiology. Allergy (2017) 72:849–56. doi: 10.1111/all.13123
11. Engeroff P, Plattner K, Storni F, Thoms F, Frias Boligan K, Muerner L, et al. Glycan-specific IgG anti-IgE autoantibodies are protective against allergic anaphylaxis in a murine model. J Allergy Clin Immunol (2021) 147:1430–41. doi: 10.1016/j.jaci.2020.11.031
12. Plattner K, Gharailoo Z, Zinkhan S, Engeroff P, Bachmann MF, Vogel M. IgE glycans promote anti-IgE IgG autoantibodies that facilitate IgE serum clearance via Fc Receptors. Front Immunol (2022) 13:1069100. doi: 10.3389/fimmu.2022.1069100
13. Chan Y-C, Ramadani F, Santos AF, Pillai P, Ohm-Laursen L, Harper CE, et al. “Auto-anti-IgE”: Naturally occurring IgG anti-IgE antibodies may inhibit allergen-induced basophil activation. J Allergy Clin Immunol (2014) 134:1394–1401.e4. doi: 10.1016/j.jaci.2014.06.029
14. Jensen-Jarolim E, Vogel M, de Weck AL, Stadler BM. Anti-IgE autoantibodies mistaken for specific IgG. J Allergy Clin Immunol (1992) 89:31–43. doi: 10.1016/S0091-6749(05)80038-7
15. Plattner K, Bachmann MF, Vogel M. On the complexity of IgE: The role of structural flexibility and glycosylation for binding its receptors. Front Allergy (2023) 4:1117611. doi: 10.3389/falgy.2023.1117611
16. Shade K-TC, Platzer B, Washburn N, Mani V, Bartsch YC, Conroy M, et al. A single glycan on IgE is indispensable for initiation of anaphylaxis. J Exp Med (2015) 212:457–67. doi: 10.1084/jem.20142182
17. Shade K-T, Conroy ME, Anthony RM. IgE glycosylation in health and disease. Curr Top Microbiol Immunol (2019) 423:77–93. doi: 10.1007/82_2019_151
18. Engeroff P, Vogel M. The role of CD23 in the regulation of allergic responses. Allergy. (2021) 76:1981–9. doi: 10.1111/all.14724
19. Engeroff P, Caviezel F, Mueller D, Thoms F, Bachmann MF, Vogel M. CD23 provides a noninflammatory pathway for IgE-allergen complexes. J Allergy Clin Immunol (2020) 145:301–311.e4. doi: 10.1016/j.jaci.2019.07.045
20. Bundhoo A, Paveglio S, Rafti E, Dhongade A, Blumberg RS, Matson AP. Evidence that FcRn mediates the transplacental passage of maternal IgE in the form of IgG anti-IgE / IgE immune complexes. Clin Exp Allergy (2015) 45(6):1085–98. doi: 10.1111/cea.12508
21. Dwyer DF, Boyce JA. Neonatal mast cells and transplacental IgE transfer: A mechanism of disease inheritance or of passive infant barrier defense? J Allergy Clin Immunol (2021) 148:76–7. doi: 10.1016/j.jaci.2021.02.046
22. Kolkhir P, Giménez-Arnau AM, Kulthanan K, Peter J, Metz M, Maurer M. Urticaria. Nat Rev Dis Prim. (2022) 8:61. doi: 10.1038/s41572-022-00389-z
23. Poto R, Quinti I, Marone G, Taglialatela M, de Paulis A, Casolaro V, et al. IgG autoantibodies against igE from atopic dermatitis can induce the release of cytokines and proinflammatory mediators from basophils and mast cells. Front Immunol (2022) 13:880412. doi: 10.3389/fimmu.2022.880412
24. Flicker S, Valenta R. Renaissance of the blocking antibody concept in type I allergy. Int Arch Allergy Immunol (2003) 132:13–24. doi: 10.1159/000073260
25. Gadermaier E, James LK, Shamji MH, Blatt K, Fauland K, Zieglmayer P, et al. Epitope specificity determines cross-protection of a SIT-induced IgG4 antibody. Allergy. (2016) 71:36–46. doi: 10.1111/all.12710
26. Vizzardelli C, Gindl M, Roos S, Möbs C, Nagl B, Zimmann F, et al. Blocking antibodies induced by allergen-specific immunotherapy ameliorate allergic airway disease in a human/mouse chimeric model. Allergy. (2018) 73:851–61. doi: 10.1111/all.13363
27. Rispens T, Huijbers MG. The unique properties of IgG4 and its roles in health and disease. Nat Rev Immunol (2023) 23:763–78. doi: 10.1038/s41577-023-00871-z
28. Shamji MH, Kappen J, Abubakar-Waziri H, Zhang J, Steveling E, Watchman S, et al. Nasal allergen-neutralizing IgG4 antibodies block IgE-mediated responses: Novel biomarker of subcutaneous grass pollen immunotherapy. J Allergy Clin Immunol (2019) 143:1067–76. doi: 10.1016/j.jaci.2018.09.039
29. Vidarsson G, Dekkers G, Rispens T. IgG subclasses and allotypes: from structure to effector functions. Front Immunol (2014) 5:520. doi: 10.3389/fimmu.2014.00520
30. van der Neut Kolfschoten M, Schuurman J, Losen M, Bleeker WK, Martínez-Martínez P, Vermeulen E, et al. Anti-inflammatory activity of human IgG4 antibodies by dynamic Fab arm exchange. Science. (2007) 317:1554–7. doi: 10.1126/science.1144603
31. Ono M, Bolland S, Tempst P, Ravetch JV. Role of the inositol phosphatase SHIP in negative regulation of the immune system by the receptor Fc(gamma)RIIB. Nature. (1996) 383:263–6. doi: 10.1038/383263a0
32. Burton OT, Epp A, Fanny ME, Miller SJ, Stranks AJ, Teague JE, et al. Tissue-specific expression of the low-affinity igG receptor, fcγRIIb, on human mast cells. Front Immunol (2018) 9:1244. doi: 10.3389/fimmu.2018.01244
33. Bruhns P, Frémont S, Daëron M. Regulation of allergy by Fc receptors. Curr Opin Immunol (2005) 17:662–9. doi: 10.1016/j.coi.2005.09.012
34. Malbec O, Daëron M. The mast cell IgG receptors and their roles in tissue inflammation. Immunol Rev (2007) 217:206–21. doi: 10.1111/j.1600-065X.2007.00510.x
35. Kanagaratham C, El Ansari YS, Lewis OL, Oettgen HC. IgE and igG antibodies as regulators of mast cell and basophil functions in food allergy. Front Immunol (2020) 11:603050. doi: 10.3389/fimmu.2020.603050
36. Malbec O, Cassard L, Albanesi M, Jönsson F, Mancardi D, Chicanne G, et al. Trans-inhibition of activation and proliferation signals by Fc receptors in mast cells and basophils. Sci Signal (2016) 9(459):ra126. doi: 10.1126/scisignal.aag1401
37. Zinkhan S, Thoms F, Augusto G, Vogel M, Bachmann MF. On the role of allergen-specific IgG subclasses for blocking human basophil activation. Front Immunol (2022) 13:892631. doi: 10.3389/fimmu.2022.892631
38. Zha L, Leoratti FMS, He L, Mohsen MO, Cragg M, Storni F, et al. An unexpected protective role of low-affinity allergen-specific IgG through the inhibitory receptor FcγRIIb. J Allergy Clin Immunol (2018) 142:1529–1536.e6. doi: 10.1016/j.jaci.2017.09.054
39. Uermösi C, Zabel F, Manolova V, Bauer M, Beerli RR, Senti G, et al. IgG-mediated down-regulation of IgE bound to mast cells: a potential novel mechanism of allergen-specific desensitization. Allergy. (2014) 69:338–47. doi: 10.1111/all.12327
40. Strait RT, Morris SC, Finkelman FD. IgG-blocking antibodies inhibit IgE-mediated anaphylaxis in vivo through both antigen interception and FcγRIIb cross-linking. J Clin Invest. (2006) 116:833–41. doi: 10.1172/JCI25575
41. Savilahti EM, Rantanen V, Lin JS, Karinen S, Saarinen KM, Goldis M, et al. Early recovery from cow’s milk allergy is associated with decreasing IgE and increasing IgG4 binding to cow’s milk epitopes. J Allergy Clin Immunol (2010) 125:1315–1321.e9. doi: 10.1016/j.jaci.2010.03.025
42. Ohsaki A, Venturelli N, Buccigrosso TM, Osganian SK, Lee J, Blumberg RS, et al. Maternal IgG immune complexes induce food allergen–specific tolerance in offspring. J Exp Med (2017) 215:91–113. doi: 10.1084/jem.20171163
43. Jutel M, Agache I, Bonini S, Burks AW, Calderon M, Canonica W, et al. International consensus on allergy immunotherapy. J Allergy Clin Immunol (2015) 136:556–68. doi: 10.1016/j.jaci.2015.04.047
44. Durham SR, Shamji MH. Allergen immunotherapy: past, present and future. Nat Rev Immunol (2023) 23:317–28. doi: 10.1038/s41577-022-00786-1
45. Jutel M, Agache I, Bonini S, Burks AW, Calderon M, Canonica W, et al. International Consensus on Allergen Immunotherapy II: Mechanisms, standardization, and pharmacoeconomics. J Allergy Clin Immunol (2016) 137:358–68. doi: 10.1016/j.jaci.2015.12.1300
46. van de Veen W, Akdis M. Role of IgG4 in IgE-mediated allergic responses. J Allergy Clin Immunol (2016) 138:1434–5. doi: 10.1016/j.jaci.2016.07.022
47. Bachmann MF, Kündig TM. Allergen specific immunotherapy: is it vaccination against toxins after all? Allergy. (2017) 72:13–23. doi: 10.1111/all.12890
48. James LK, Bowen H, Calvert RA, Dodev TS, Shamji MH, Beavil AJ, et al. Allergen specificity of IgG 4-expressing B cells in patients with grass pollen allergy undergoing immunotherapy. J Allergy Clin Immunol (2012) 130:663–670.e3. doi: 10.1016/j.jaci.2012.04.006
49. Strobl MR, Demir H, Sánchez Acosta G, Drescher A, Kitzmüller C, Möbs C, et al. The role of IgG1 and IgG4 as dominant IgE-blocking antibodies shifts during allergen immunotherapy. J Allergy Clin Immunol (2023) 151:1371–1378.e5. doi: 10.1016/j.jaci.2023.01.005
50. Paolucci M, Wuillemin N, Köhli A, Ballmer-Weber B, Severin Y, Waeckerle-Men Y, et al. Multivariate allergen-specific analysis and profiling of serum antibodies from patients with peanut allergy. Clin Exp Allergy J Br Soc Allergy Clin Immunol (2023) 53:353–8. doi: 10.1111/cea.14262
51. Lanier B, Bridges T, Kulus M, Taylor AF, Berhane I, Vidaurre CF. Omalizumab for the treatment of exacerbations in children with inadequately controlled allergic (IgE-mediated) asthma. J Allergy Clin Immunol (2009) 124(6):1210–6. doi: 10.1016/j.jaci.2009.09.021
52. Plattner K, Augusto G, Muerner L, von Gunten S, Jörg L, Engeroff P, et al. IgE glycosylation is essential for the function of omalizumab. Allergy (2023) 78(9):2546–9. doi: 10.1111/all.15748
53. Gasser P, Tarchevskaya SS, Guntern P, Brigger D, Ruppli R, Zbären N, et al. The mechanistic and functional profile of the therapeutic anti-IgE antibody ligelizumab differs from omalizumab. Nat Commun (2020) 11(1):165. doi: 10.1038/s41467-019-13815-w
54. Kuo BS, Li CH, Chen JB, Shiung Y, Chu C, Lee C, et al. IgE-neutralizing UB-221 mAb, distinct from omalizumab and ligelizumab, exhibits CD23-mediated IgE downregulation and relieves urticaria symptoms. J Clin Invest (2022) 132(15): e157765. doi: 10.1172/JCI157765
55. Maurer M, Giménez-Arnau AM, Sussman G, Metz M, Baker DR, Bauer A, et al. Ligelizumab for chronic spontaneous urticaria. N Engl J Med (2019) 381:1321–32. doi: 10.1056/NEJMoa1900408
56. Gauvreau GM, Arm JP, Boulet L-PP, Leigh R, Cockcroft DW, Davis BE, et al. Efficacy and safety of multiple doses of QGE031 (ligelizumab) versus omalizumab and placebo in inhibiting allergen-induced early asthmatic responses. J Allergy Clin Immunol (2016) 138:1051–9. doi: 10.1016/j.jaci.2016.02.027
57. Staubach P, Alvaro-Lozano M, Sekerel BE, Maurer M, Ben-Shoshan M, Porter M, et al. Ligelizumab in adolescents with chronic spontaneous urticaria: Results of a dedicated phase 2b randomized clinical trial supported with pharmacometric analysis. Pediatr Allergy Immunol Off Publ. Eur Soc Pediatr Allergy Immunol (2023) 34:e13982. doi: 10.1111/pai.13982
58. Gasser P, Eggel A. Targeting IgE in allergic disease. Curr Opin Immunol (2018) 54:86–92. doi: 10.1016/j.coi.2018.05.015
59. Incorvaia C, Mauro M, Makri E, Leo G, Ridolo E. Two decades with omalizumab: what we still have to learn. Biologics (2018) 12:135–42. doi: 10.2147/BTT.S180846
60. Bauer RN, Manohar M, Singh AM, Jay DC, Nadeau KC. The future of biologics: Applications for food allergy. J Allergy Clin Immunol (2015) 135:312–23. doi: 10.1016/j.jaci.2014.12.1908
61. Jansson Å, Fuentes A, Magnusson A, Landström E, Öhman B, Kälvesten Å, et al. Efficient anti-IgE vaccination without anaphylactogenic properties. J Allergy Clin Immunol (2004) 113:S254. doi: 10.1016/j.jaci.2004.01.381
62. Landolina N, Levi-Schaffer F. Monoclonal antibodies: the new magic bullets for allergy: IUPHAR Review 17. Br J Pharmacol (2016) 173:793–803. doi: 10.1111/bph.13396
63. Pengo N, Wuillemin N, Bieli D, Gasser P. Anti-allergen monoclonal antibodies for the treatment of allergies. Allergo J Int (2023) 32:289–95. doi: 10.1007/s40629-023-00263-8
64. Paolucci M, Wuillemin N, Homère V, Bieli D, Köhli A, Ballmer-Weber B, et al. Targeting Ara h 2 with human-derived monoclonal antibodies prevents peanut-induced anaphylaxis in mice. Allergy. (2023) 78:1605–14. doi: 10.1111/all.15659
65. Orengo JM, Radin AR, Kamat V, Badithe A, Ben LH, Bennett BL, et al. Treating cat allergy with monoclonal IgG antibodies that bind allergen and prevent IgE engagement. Nat Commun (2018) 9:1421. doi: 10.1038/s41467-018-03636-8
66. Storni F, Cabral-Miranda G, Roesti E, Zha L, Engeroff P, Zeltins A, et al. A Single Monoclonal Antibody against the Peanut Allergen Ara h 2 Protects against Systemic and Local Peanut Allergy. Int Arch Allergy Immunol (2020) 181:334–41. doi: 10.1159/000505917
67. Kamal MA, Dingman R, Wang CQ, Lai C-H, Rajadhyaksha M, DeVeaux M, et al. REGN1908-1909 monoclonal antibodies block Fel d 1 in cat allergic subjects: Translational pharmacokinetics and pharmacodynamics. Clin Transl Sci (2021) 14:2440–9. doi: 10.1111/cts.13112
68. de Blay FJ, Gherasim A, Domis N, Meier P, Shawki F, Wang CQ, et al. REGN1908/1909 prevented cat allergen-induced early asthmatic responses in an environmental exposure unit. J Allergy Clin Immunol (2022) 150:1437–46. doi: 10.1016/j.jaci.2022.06.025
69. Atanasio A, Franklin MC, Kamat V, Hernandez AR, Badithe A, Ben L-H, et al. Targeting immunodominant Bet v 1 epitopes with monoclonal antibodies prevents the birch allergic response. J Allergy Clin Immunol (2022) 149:200–11. doi: 10.1016/j.jaci.2021.05.038
70. Gevaert P, De Craemer J, De Ruyck N, Rottey S, de Hoon J, Hellings PW, et al. Novel antibody cocktail targeting Bet v 1 rapidly and sustainably treats birch allergy symptoms in a phase 1 study. J Allergy Clin Immunol (2022) 149:189–99. doi: 10.1016/j.jaci.2021.05.039
71. Shamji MH, Singh I, Layhadi JA, Ito C, Karamani A, Kouser L, et al. Passive prophylactic administration with a single dose of anti-fel d 1 monoclonal antibodies REGN1908-1909 in cat allergen-induced allergic rhinitis: A randomized, double-blind, placebo-controlled clinical trial. Am J Respir Crit Care Med (2021) 204:23–33. doi: 10.1164/rccm.202011-4107OC
72. LaHood NA, Min J, Keswani T, Richardson CM, Amoako K, Zhou J, et al. Immunotherapy-induced neutralizing antibodies disrupt allergen binding and sustain allergen tolerance in peanut allergy. J Clin Invest (2023) 133(2):e164501. doi: 10.1172/JCI164501
73. Bachmann MF, Mohsen MO, Kramer MF, Heath MD. Vaccination against allergy: A paradigm shift? Trends Mol Med (2020) 26(4):357–68. doi: 10.1016/j.molmed.2020.01.007
74. Satitsuksanoa P, Głobińska A, Jansen K, van de Veen W, Akdis M. Modified allergens for immunotherapy. Curr Allergy Asthma Rep (2018) 18(2):9. doi: 10.1007/s11882-018-0766-x
75. Dorofeeva Y, Shilovskiy I, Tulaeva I, Focke-Tejkl M, Flicker S, Kudlay D, et al. Past, present, and future of allergen immunotherapy vaccines. Allergy. (2021) 76:131–49. doi: 10.1111/all.14300
76. Valenta R, Campana R, Focke-Tejkl M, Niederberger V. Vaccine development for allergen-specific immunotherapy based on recombinant allergens and synthetic allergen peptides: Lessons from the past and novel mechanisms of action for the future. J Allergy Clin Immunol (2016) 137:351–7. doi: 10.1016/j.jaci.2015.12.1299
77. Niederberger V, Marth K, Eckl-Dorna J, Focke-Tejkl M, Weber M, Hemmer W, et al. Skin test evaluation of a novel peptide carrier-based vaccine, BM32, in grass pollen-allergic patients. J Allergy Clin Immunol (2015) 136:1101–3.e8. doi: 10.1016/j.jaci.2015.03.034
78. Banerjee S, Weber M, Blatt K, Swoboda I, Focke-Tejkl M, Valent P, et al. Conversion of Der p 23, a new major house dust mite allergen, into a hypoallergenic vaccine. J Immunol (2014) 192:4867–75. doi: 10.4049/jimmunol.1400064
79. Mösges R, Zeyen C, Raskopf E, Acikel C, Sahin H, Allekotte S, et al. A randomized, double-blind, placebo-controlled trial with mannan-conjugated birch pollen allergoids. Allergy (2023). doi: 10.1111/all.15910
80. Engeroff P, Caviezel F, Storni F, Thoms F, Vogel M, Bachmann MF. Allergens displayed on virus-like particles are highly immunogenic but fail to activate human mast cells. Allergy. (2018) 73:341–9. doi: 10.1111/all.13268
Keywords: allergy, IgE, mast cells, basophils, degranulation, FcγRIIB, antibody, sensitization
Citation: Storni F, Vogel M, Bachmann MF and Engeroff P (2024) IgG in the control of FcεRI activation: a battle on multiple fronts. Front. Immunol. 14:1339171. doi: 10.3389/fimmu.2023.1339171
Received: 15 November 2023; Accepted: 11 December 2023;
Published: 11 January 2024.
Edited by:
Ferdaus Mohd Altaf Hossain, Sylhet Agricultural University, BangladeshReviewed by:
Wayne Robert Thomas, University of Western Australia, AustraliaCopyright © 2024 Storni, Vogel, Bachmann and Engeroff. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Paul Engeroff, cGF1bC5lbmdlcm9mZkB1bmliZS5jaA==
 Federico Storni
Federico Storni Monique Vogel2,3
Monique Vogel2,3 Paul Engeroff
Paul Engeroff