
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
MINI REVIEW article
Front. Immunol. , 24 November 2023
Sec. Cytokines and Soluble Mediators in Immunity
Volume 14 - 2023 | https://doi.org/10.3389/fimmu.2023.1295232
This article is part of the Research Topic Role of Carcinoembryonic Antigen-related Cell Adhesion Molecules in Pathogen Responses, Tumorigenicity, and Immune Modulation View all 7 articles
The Carcinoembryonic antigen-related cell adhesion molecule 1 (CEACAM1), also known as CD66a, is a member of the immunoglobulin superfamily. CEACAM1 was shown to be a prognostic marker in patients suffering from cancer. In this review, we summarize pre-clinical and clinical evidence linking CEACAM1 to tumorigenicity and cancer progression. Furthermore, we discuss potential CEACAM1-based mechanisms that may affect cancer biology.
The glycoprotein Carcinoembryonic antigen (CEA)-related cell adhesion molecule 1 (CEACAM1, also CD66a) is a member of the immunoglobulin superfamily. CEACAM1 is expressed in a broad range of different tissues and cell types like epithelial cells, vascular cells, immune cells as well as cancer cells (1–3). Based on its downregulation in early stages of colorectal cancer (CRC) detected in some of the earlier studies CEACAM1 was initially regarded as a potential tumor suppressor (4). However, cumulative evidence from pre-clinical as well as clinical data suggests a more complex influence of CEACAM1 on cancer biology. For instance CEACAM1 affects anti-cancer immune reactions by modulating the function of natural killer cells and T-cells (3). Furthermore, later clinical association studies indicate that CEACAM1 expression seems more likely to be associated with cancer progression and poor prognosis in most cancer entities. Therefore, the concept of CEACAM1 as a tumor suppressor needs to be revised. For that purpose, in this review we will I) summarize the clinical data on CEACAM1 and cancer progression and survival, II) give an overview of potential underlying mechanisms and III) highlight current therapeutic approaches and bottlenecks.
CEACAM1 is a member of the Carcinoembryonic antigen (CEA)-related cell adhesion molecule family. CEACAMs are either transmembrane proteins (CEACAM1, 3, and 4) or membrane-anchored G protein-coupled receptors (CEACAM 5, 6, 7, and 8). Their ectodomain consists of a variable number of IgGC2-related domains and a N-terminal IgV-related domain.
CEACAM1 comprises an extracellular, a transmembrane and a cytoplasmatic domain (5, 6). Due to alternative splicing 12 different variants exist in humans that differ in the composition of immunoglobulin (Ig)-like ectodomains as well as in the cytoplasmatic domain (7). The cytoplasmatic domain exists either in a long (CEACAM1-L, inclusion of exon 7) or a short (CEACAM1-S, exclusion of exon 7) version. Only CEACAM1-L possesses two immunoreceptor tyrosine-based inhibitory motifs (ITIMs) that when phosphorylated serve as a docking site for SRC homology 2 (SH2) domain-containing signaling proteins like SRC family kinases (SFKs) or cytoplasmic tyrosine phosphatases (SHPs). Thereby CEACAM1 can affect intracellular signaling in different ways (8–10). Besides formation of heterodimers with other CEACAM family members or serving as a pathogen receptor, CEACAM1 can either act as a monomer, a homodimer (i.e. two CEACAM1-L) or a heterodimer (CEACAM1-L and CEACAM1-S) (10–14). This is of particular interest because CEACAM1 monomers, homo- and heterodimers show different binding affinities for SFKs and SHPs (8). Thus, the expression ratio of CEACAM1-L to CEACAM1-S might also affect CEACAM1 signaling Unfortunately, so far only for breast cancer the L/S ratio of CEACAM1 was analyzed and found to be altered (15). Having such data for other cancer entities too might help to explain divergent results concerning cancer stage-related CEACAM1 expression and patient survival.
Several studies investigated the potential impact of CEACAM1 on cancer in human specimen. Due to an observed downregulation of CEACAM1 in early CRC, CEACAM1 was originally suggested to be a tumor suppressor (16, 17). However, these studies have important limitations. First, they were based only on a small number of samples. Second, the control tissue was taken from the same specimen right beside the cancerous tissue. Since CEACAM1 is upregulated under inflammatory conditions the peri-tumor milieu might have already altered basal CEACAM1 expression in those controls (18). Finally, in both studies only mRNA expression was analyzed lacking information on the presence of the functional protein. In contrast, a later analysis by Kang et al. revealed upregulation of CEACAM1 protein expression in colon adenocarcinoma compared to adenoma in a larger cohort (19). Therefore, the initial hypothesis of CEACAM1 downregulation in CRC must be revised. This is in line with Ieda et al. who have shown that CEACAM1-L dominance associates with shorter survival time in patients (20). The same group reported that CEACAM1-S located at the invasion front of the primary lesion is associated with poor prognosis of patients with colorectal liver metastasis (21).
Besides CRC, several studies indicate a potential role of CEACAM1 in multiple cancer entities. The majority of these studies shows enhanced CEACAM1 expression with progressive tumor stage and/or metastasis. Moreover, in most studies enhanced CEACAM1 expression correlates with shorter overall patient survival. However, from those clinical studies at present it cannot be excluded that CEACAM1 upregulation might be a side effect rather than a driver of cancer progression and metastasis. As mentioned above, this could be due to the cancer-associated pro-inflammatory milieu that might promote CEACAM1 expression (18, 22). Therefore, to analyze the relation of cause and effect between CEACAM1 and cancer and the underlying signaling in pre-clinical cancer models is a prerequisite for a later transition of experimental findings into clinical applications.
To enable a better overview of different cancer entities clinical studies are summarized in Table 1. For proper interpretation one has to keep in mind that CEACAM1 expression was analyzed by different methods (e.g., RNA expression, histological staining) using specimen of different origin (e.g., serum concentration, staining of tissue slides).
Despite its well-known association with cancer less is known about CEACAM1-dependent mechanisms that might affect cancer biology. To provide an overview we will summarize the current knowledge and discuss reasons that may account for the conflicting results reported in the literature regarding CEACAM1 and cancer cells. Figure 1 summarizes the role of CEACAM1 in tumor biology.
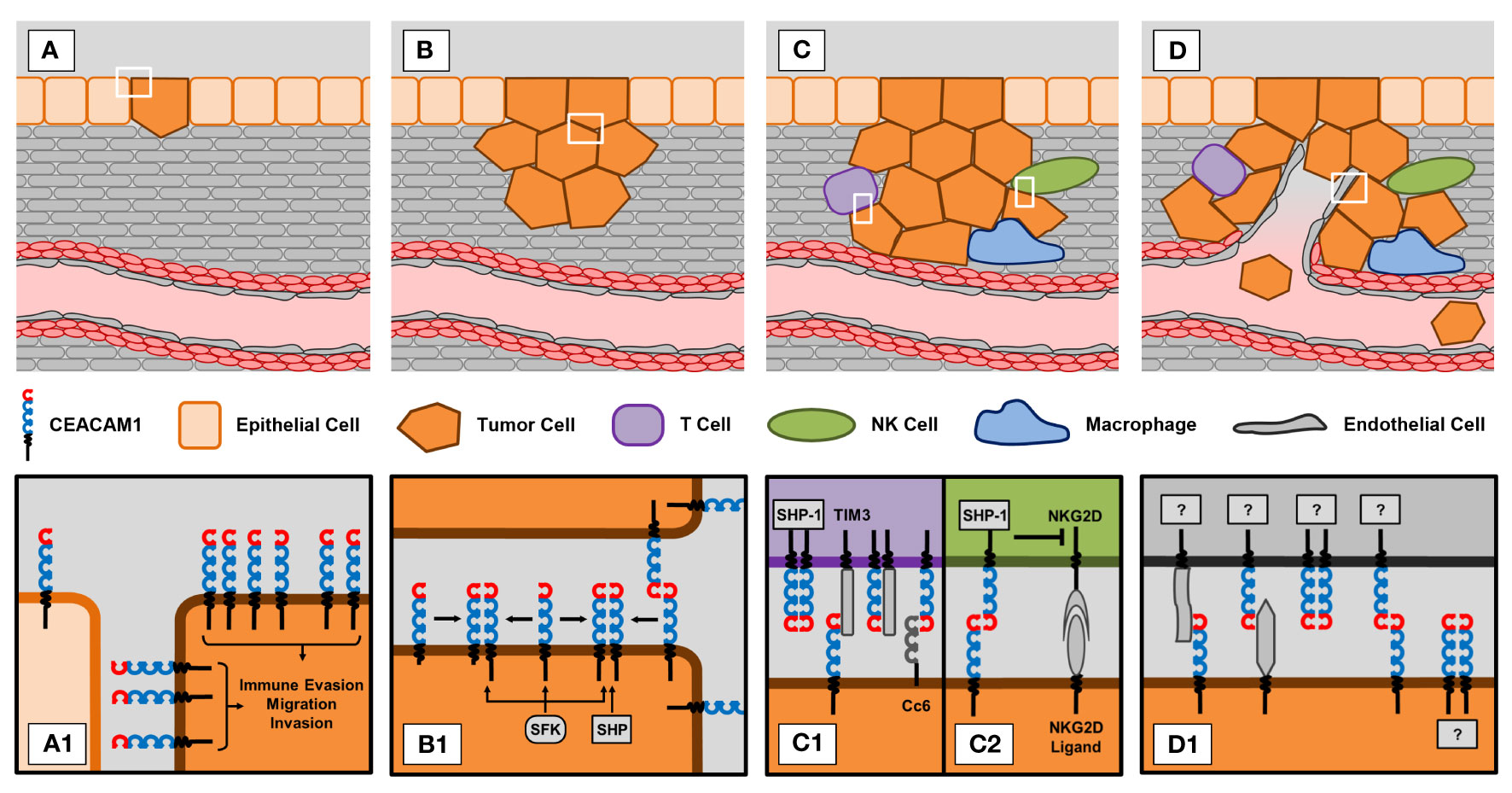
Figure 1 CEACAM1 in different stages of tumorigenesis and progression. (A) Epithelial-to-mesenchymal transition (EMT). (A1) Tumor cells show increased expression of CEACAM1 compared to normal epithelial cells. This upregulation of CEACAM1 is thought to promote processes that support tumor development, i.e. immune evasion, migration and invasion. (B) Proliferation of tumor cells. (B1) CEACAM1-dependent signaling greatly depends on the status of CEACAM1 molecules. Trans ligation of CEACAM1 molecules on neighboring cells also promotes cis ligation of CEACAM1 molecules expressed on the same cell. Depending on the expression ratio of long (L) and short (S) isoforms, the resulting dimers can consist of two CEACAM1-L molecules or of a combination of CEACAM1-L and CEACAM1-S. Whereas SHP phosphatases preferentially bind to CEACAM1-L homodimers, SFK kinases bind equally well to dimers and monomers. (C) Immune evasion. T cells and NK cells are capable of eliminating tumor cells. However, tumor cells have developed CEACAM1-dependent mechanisms to evade this immune response. (C1) Trans or cis ligation of CEACAM1 to TIM3 or binding of CEACAM6 (Cc6) to CEACAM1 on T cells abrogates anti-tumor activity of T cells. (C2) Similarly, trans ligation of CEACAM1 on tumor and NK cells blocks the cytotoxic effect of the activated NKG2D receptor expressed on NK cells. (D) Tumor vascularization. (D1) CEACAM1 is expressed on endothelial and tumor cells. Since CEACAM1 promotes angiogenesis, it is reasonable to speculate that interactions between endothelial and tumor cell CEACAM1 with or without involvement of other molecules might also affect tumor vascularization. However, this still needs to be investigated in more detail.
Leung et al. showed that azoxymethane-mediated induction of colon cancer was aggravated in Ceacam1-/- mice compared to WT mice suggesting an anti-tumorigenic effect of CEACAM1 expression (68). In line with that, CEACAM1 was also shown to repress epithelial to mesenchymal transition that is a critical step in oncogenesis (69).
Conflicting results have been reported regarding the effect of CEACAM1 on cancer cell proliferation that critically promotes tumor growth and size. Some studies reported inhibition of proliferation by CEACAM1. Overexpression of CEACAM1-L in MCF-7 breast cancer cells reduced EGF-stimulated cell growth (70). Similarly, CEACAM1 overexpression significantly suppressed proliferation of U266 and RPMI8266 multiple melanoma cells. Unfortunately, the underlying signaling mechanisms were not analyzed in this study (71). Singer et al. have also shown that overexpression of CEACAM1-L in A549 lung carcinoma cells decreased cell proliferation (72). Finally, Luebke et al. reported slightly increased proliferation rates due to the loss of CEACAM1 expression in human prostate cancer specimens again indicating an inhibitory effect of CEACAM1 on cancer cell proliferation (49).
In contrast, Han et al. found that siRNA-mediated CEACAM1 knockdown decreased proliferation in HT29 colon carcinoma cells. (73). Similarly, Ortenberg et al. have shown that overexpression of the L-form of CEACAM1 promotes proliferation of 526mel melanoma cells in a SOX2-dependent manner in vitro. Consequently, compared to normal 526mel cells, CEACAM1-overexpressing cells generated tumors with larger volumes when injected into mice (37).
The immune system is capable to eliminate cells that are foreign to the body or show genetic aberrations as found in tumor cells. Thereby the immune system is critical to prevent cancer. However, cancer cells can evade recognition and subsequent elimination by the immune system (74). This also applies to immune cell-based therapies e.g. by chimeric antigen receptor T cells (CAR T cells) (75) and results in unsatisfactory efficiency of these immune therapies in solid tumors (76). Therefore, novel approaches circumventing cancer cell-mediated inhibition of immune cells are required to unleash the full potential of anti-cancer immunotherapies. Since CEACAM1 affects the activation of T cells, B cells and NK cells in the context of cancer, it might be a promising target (2).
T cells play a critical role in adaptive immune responses. Although programmed cell death protein 1 (PD1) and cytotoxic T-lymphocyte-associated protein 4 (CTLA4) are well known immune checkpoints that guard against autoimmunity under physiological conditions, these immune checkpoints can also prevent the anti-cancer efficiency of endogenous or therapeutically applied exogenous modified T cells. Therefore, immune checkpoint inhibition is used clinically in anti-cancer therapies (75). However, not all patients benefit from immune checkpoint inhibition equally. This indicates additional mechanisms that are not covered by these treatments. In that context activation-induced expression of CEACAM1 has been shown to inhibit T cell function, particularly the long isoform (77–80). Upon dimerization CEACAM1-L recruits SHPs to the TCR/CD3 complex that dephosphorylate adjacent kinases or adaptor proteins thereby decreasing proliferation and activation of T cells (80–82). Contrary to this, CEACAM1-L was shown to decrease Fas-mediated apoptosis in T cells independent of ITIM phosphorylation via β-catenin signaling modulation (83). In the context of a colitis model, CEACAM1 was shown to inhibit the differentiation of naive T cells into Th1 but not Th2 cells (84). In contrast CEACAM1-S is rather suggested to promote activation of T cells (85, 86). This may be related to the absence of an ITIM motif in the short isoform (87).
Furthermore, the heterophilic cis interaction of CEACAM1-L with T cell-immunoglobulin and mucin-domain containing 3 (TIM3) results in T cell exhaustion and immune tolerance (88). A similar inhibitory effect was observed in CRC and neck squamous cell carcinoma by trans interaction of CEACAM1 on tumor cells with TIM3 on T cells (54, 89). Inhibition of CEACAM1 and TIM3 in CRC models synergistically stimulated the anti-tumor immune response. This is in line with observations in hematopoietic malignancies showing that blocking the CEACAM1-TIM3 interaction results in an attenuated NF-κB signaling (90). In a clinical study of patients with glioma the expression of CEACAM1 on T cells was negatively correlated with the Karnofsky score (91). This was attributed to an inhibitory effect of CEACAM1 on T cells. Similarly a recent study using clinical data and an in vivo CRC mouse model indicates that CEACAM1 marks a suppressive subset of intra-tumoral regulatory T cells (92).
In addition, trans interaction of CEACAM1 with CEACAM6 was reported in several entities of solid cancers. Interestingly, CEACAM6 inhibition enhanced the tumor-suppressive T cell function indicating a crosstalk of CEACAM1 with other CEA family members (93).
B cells are involved in anti-cancer immune responses by secretion of antibodies targeting tumor cell-specific antigens as well as recruitment and activation of other immune cells. The impact of CEACAM1 on B cells in cancer is still contradictory discussed (94). Khairnar et al. stated that CEACAM1 positively regulates B cell proliferation and survival via Syk-mediated NF-κB signaling (95). In contrast, others found that CEACAM1 co-localizes with the B cell receptor and prohibits cytokine production in a PI3K-dependent manner (96, 97). Further Greicius et al. showed that a CEACAM1-specific antibody induced the proliferation of mouse B cells in combination with IgM cross-linking (98). The underling signaling was not investigated. Interestingly, binding of Opa proteins of Neisseria gonorrhoeae to CEACAM1 induces apoptosis in B cells (99). Thereby CEACAM1 could lead to a decreased immune system activation. This was also shown for T cells in the context of Fusobacterium nucleatum. This is an anaerobic bacterium that is associated with several tumor entities and promotes tumorigenesis (100).
NK cells are cytotoxic lymphocytes of the innate immune system that contribute to immune surveillance in cancer. Cells express ligands of activating receptors located on NK cells (e.g. NKG2D). However, NK cell activation is prevented by ligation of major histocompatibility complex (MHC) class I molecules normally expressed on healthy cells to killer immunoglobulin-like receptors (KIRs) on NK cells. Since cancer cells often downregulate MHC I expression to evade recognition by T cells, this inhibitory signal is lost and the NK cell is activated (100). This results in the cytolysis of the malignant cells (101).
CEACAM1 is abundantly expressed on activated NK cells and functions as inhibitory co-receptor (101–104). Homophilic CEACAM1 interaction between NK cells and tumor cells blocks the initiation of cytolysis by NK cells via SHP1-dependent dephosphorylation of guanosine nucleotide exchange factor Vav1 (105). Furthermore, CEACAM1 expression on CRC cells decreases NK cell-mediated cytolysis by diminishing surface presentation of NKG2D ligands (106). This effect may be isoform-dependent. While CEACAM1-L decreases NK cell activity via downregulation of the NKG2D ligands MICA and ULBP2 by enhanced shedding, CEACAM1-3S increases the expression of NKG2D ligands (107). Clinical data show an upregulation of CEACAM1 in NK cells by hepatitis C virus (HCV) infection that was accompanied by reduced NK cell activity in vitro and in patients. Thereby CEACAM1 might facilitate chronification of HCV infection and transition to hepatocellular carcinoma (108).
Cancer stem cells (CSCs) are a special subtype of cancer cells, that are thought to multiply indefinitely and are resistant to chemotherapy. Epithelial cell adhesion molecule (EpCAM) is a well-established marker of CSCs (109). EpCAM expression in liver CSCs promotes resistance against NK cell-mediated cytotoxicity via upregulation of CEACAM1 (110).
Based on these findings, the impact of CEACAM1 inhibition on NK cell activity was investigated in pre-clinical studies. Antibody-mediated inhibition of CEACAM1 in head and neck squamous cell carcinoma cells enhances NK cell anti-cancer activity in vitro (111). Similar results were obtained in a non-small cell lung cancer in vivo xenograft mouse model (112). Interestingly first in vitro investigations also show efficiency of antibody-mediated inhibition of CEACAM5/CEACAM1 interaction to reduce CEACAM1-dependent NK cell inhibition in pancreatic and CRC cells (113). This indicates an interaction of different CEA family members in this process (101).
Generation of new blood vessels based on preexisting ones, called angiogenesis, promotes cancer growth and progression by providing supply with oxygen and nutrients with progressive cancer size. Therefore, targeting angiogenesis was proposed as anti-cancer therapy. However, in clinical practice anti-angiogenic treatment has not been as effective as expected (114). Recent evidence suggests that this might be due to a pro-angiogenic tumor environment that cannot be modulated by systemic drug application. Therefore, more localized interference with angiogenesis might provide better anti-tumor effects than current therapeutic strategies (115–117).
In this regard, CEACAM1 might be a putative target. CEACAM1 is expressed in microvessels within and in close proximity to tumors but not in larger blood vessels (118). This is of particular interest, since CEACAM1 mediates angiogenesis via enhanced VEGF/VEGFR-2 expression (18, 118). In line with this Gerstel et al. reported a crucial role of CEACAM1 for tumor angiogenesis. Using a murine mammary carcinogenesis model, they found that CEACAM1 deficiency results in vascular instability and alterations in ECM structure (119). Furthermore, CEACAM1 deficiency enhanced the permeability of tumor vasculature due to increased basal Akt kinase and endothelial nitric oxide synthase (eNOS) activities (120). This is in line with the concept that CEACAM1 is required for the establishment of the endothelial barrier (121, 122). Besides endothelial cells CEACAM1 expression in myeloid cells also promoted angiogenesis as shown by bone marrow transplantation experiments in mice (123). In contrast, in two subsequent studies Lu et al. reported that tumor angiogenesis mediated by myeloid cells is negatively regulated by CEACAM1 (124, 125). Furthermore, a study of Muturi et al. suggests that CEACAM1 expression in microvesicles derived from cancer cells might influence cancer signaling on other cells including endothelial cells (126).
Metastasis accounts for more than 90% of deaths from cancer (127). Migration and invasiveness of cancer cells are hallmarks of cancer metastasis that requires tumor cell dissemination from the primary tumor into different organs (128). Ebrahimnejad et al. showed that phosphorylation of CEACAM1-L at position Tyr488 enhances cell migration and matrix invasion of melanoma cells in an integrin β3-dependent manner (129). This is in line with older findings in endothelial cells demonstrating that CEACAM1 affects cell migration and integrin-dependent signaling (130). Forced expression of CEACAM1 in thyroid cancer cells promoted cell–matrix adhesion, migration and tumor invasiveness via upregulation of cyclin dependent kinase inhibitor 1A (p21) and diminished retinoblastoma protein (Rb) phosphorylation. Since this was accompanied by reduced tumor growth in xenografted mice it argues for a pro-metastatic effect of CEACAM1 rather than an effect on the primary tumor (59). Furthermore, CEACAM1 enhanced migration in CRC cells that was ascribed to increased N-cadherin expression (131). Similar, Ieda et al. reported that CEACAM1-L promotes invasion and migration in CRC cells (20). Based on association studies in human samples and a colony formation assay in soft-agar Yamaguchi et al. suggested that expression of CEACAM1-4S enhances the tumor-initiating property of colorectal cancer cells (21). However, this study lacks further analysis that is mandatory for such a statement. In contrast, Yang et al. reported that the short isoform of CEACAM1 decreased cell migration and invasiveness of breast cancer cells by affecting the balance between matrix metalloproteinase 2/tissue inhibitor of metalloproteinase 2 and E-/N-cadherin expression (132). For multiple myeloma cells, Xu et al. found a decrease in cell migration and invasion by CEACAM1 (71). Besides, a recent study suggests that macrophage-cancer cell interaction via CEACAM1 promotes cancer metastasis. This signaling is mediated by β-catenin that enhances metadherin expression on cancer cell surface. Metadherin in turn signals through CEACAM1 expressed on macrophages to produce the chemokine CCL3 (133).
Many cancer cells show deregulation of apoptosis that contributes to chemotherapy resistance (134). Therefore, anti-cancer agents have been developed that prime cancer cells for apoptosis (135). One of the first hints that CEACAM1 might support cellular survival by decreasing apoptosis came from studies investigating non-cancer cells like granulocytes and monocytes. Singer et al. showed that tyrosine phosphorylation of CEACAM1-L reduces apoptosis in granulocytes via an Erk1/2- and caspase-3-dependent pathway (136). This is in line with observations in endothelial cells (137). Later, Yu et al. showed that CEACAM1 decreases apoptosis of human monocytes via phosphatidylinositol 3-kinase- and Akt kinase-dependent survival signaling (138). In contrast, CEACAM1 was shown to promote apoptosis in a murine model of myocardial infarction. This was attributed to upregulation of mitochondrial Bax, increased cytosolic cytochrome C and cleaved caspase-3 (139).
Similar studies investigating the impact of CEACAM1 on apoptosis in cancer cells that include signaling pathway analysis are limited. Using siRNA-mediated knockdown of CEACAM1, Han et al. reported an anti-apoptotic effect of CEACAM1 in HT-29 CRC cells (73). In contrast, Nittka et al. showed that antibody-mediated crosslinking of CEACAM1 resulted in enhanced apoptosis in HT-29 CRC cells (4, 140). Unfortunately, in both studies the underlying CEACAM1-dependent signaling pathways were not investigated. Besides affecting apoptosis under basal conditions, CEACAM1-L was also shown to mediate chemoresistance to the widely used chemotherapeutic agent 5-fluorouracil (5-FU) in CRC cells, whereas CEACAM1-S did not. This suggests that long and short form of CEACAM1 might have opposite effects with regard to cell viability (141).
Similar to Nittka et al., Zaffran et al. observed enhanced apoptosis after treatment of Mel-14 melanoma cells with an anti-CEACAM1 antibody in vitro. This pro-apoptotic effect was attributed to an altered p53 expression and SHP1 phosphorylation (4, 142). However, in the same study in vivo application of this anti-CEACAM1 antibody had no effect on tumor size in a melanoma model (142). Since they were using the same melanoma cells for in vitro and in vivo experiments, this shows that the results of in vitro antibody treatments have to be interpreted with caution.
Of note, forced expression of the short isoform of CEACAM1 in MCF7 mammary carcinoma cells induced a more regular glandular morphology that was accompanied by apoptosis of the central cells within acini (143). However, this seems to be a more development-specific effect rather than a pro-apoptotic effect in general since peripheral acinar cells were not affected. In addition, a bioinformatics-based study analyzing cisplatin resistance in A549 lung carcinoma epithelial cells indicates that CEACAM1 promotes resistance suggesting an anti-apoptotic effect. Though experimental validation in cell culture is lacking yet (144).
Based on pre-clinical data CEACAM1-specific antibodies were proposed for anti-cancer therapy. In addition to their inhibitory effects on tumor cells themselves, anti-CEACAM1 antibodies were also reported to promote anti-tumor activity of several immune cell types (92, 111, 113, 145–147). Finally, since CEACAM1 expression in cancer tissue usually exceeds that in the surrounding healthy tissue by far, anti-CEACAM1 antibodies were suggested to be used in surgery to identify cancer tissues in order to enable complete cancer resection (148, 149).
The CEACAM1-specific antibody CM24, developed by cCAM Biotherapeutics Ltd., has shown efficacy against different types of cancer cells in vitro and in in vivo models of cancer (150, 151). Originally, the company announced initiation of a clinical phase 1 trial of CM24 in 2015 (152). In the same year Merck acquired cCAM Biotherapeutics (153). However, only one year later further development of CM24 was terminated by Merck due to new data: “During 2016, as a result of unfavorable efficacy data, the company determined that it would discontinue development of the pipeline program” (154, 155). Another company, Purple Biotech, plans to evaluate the same antibody CM24 in a phase I studies in the future (156, 157). Based on Merck’s unfavorable results, upcoming clinical studies using the CM24 antibody need to be regarded with caution.
However, the hitherto results do not prove the inefficacy of targeting CEACAM1 in anti-cancer therapy. Rather they suggest that this particular antibody might not be suitable for this purpose.
This is further illustrated by a report from McLeod et al. (158). They showed that another anti-CEACAM1 antibody (Cc1) facilitated binding of soluble CEACAM1 to CEACAM1 expressing cells instead of blocking CEACAM1-dependent signaling as expected. Furthermore, Nakajima et al. suggested that anti-CEACAM1 antibodies may activate CEACAM1-dependent signaling eventually by cross linking of CEACAM1 (78). Thus, antibodies intended to block CEACAM1 signaling in cancer cells probably may induce the opposite effect depending on antibody design. Similar concerns may apply to another anti-cancer strategy. Based on CEACAM1 upregulation, anti-CEACAM1 antibodies are suggested to mark cancer cells for immune cell-based killing. However, beside cancer cells a variety of cells express CEACAM1 depending on activation state and age (1, 2, 18, 159). Hence, this strategy may provoke serious side effects.
In conclusion, it is inevitable to extensively characterize anti-CEACAM1 antibodies that are intended to be used in clinical trials. Then it is reasonable to assume that novel anti-CECAM1 therapeutics might improve survival of cancer patient in the future (112).
Despite some conflicting findings, the vast majority of clinical studies supports the view that CEACAM1 promotes cancer progression and metastasis in humans. Regarding the interpretation of the results of clinical trials, some aspects have to be considered. First, specificity of anti-CEACAM1 antibodies might be compromised due to cross-reactivity with other CEACAM family members. Second, despite some evidence for possible adverse functions in cancer biology the impact of different CEACAM1 splice variants is still largely unknown.
One of the greatest unresolved issues is how CEACAM1 affects intra- and intercellular signaling since there is no systematic analysis of CEACAM1-dependent signaling in cancer yet. Closing this gap would be an important step to understand the mechanisms that underlie CEACAM1-mediated effects in cancer. This also might reveal other signaling pathways and targets that have not been linked to cancer so far and that could become even more promising targets in cancer therapy than CEACAM1 itself.
In addition, the accumulating evidence of CEACAM1’s repressing function on anti-cancer activity by the immune system makes it an attractive therapeutic target similar to established immune checkpoint inhibition. It is still speculative if such a combined targeted inhibition including CEACAM1 might overcome the limitations of immunotherapies against solid tumors.
LG: Conceptualization, Writing – original draft, Writing – review & editing. UR: Visualization, Writing – review & editing. GB: Writing – review & editing. VP: Writing – review & editing. SE: Writing – review & editing. FK: Conceptualization, Funding acquisition, Project administration, Supervision, Writing – original draft, Writing – review & editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by the Walter Schulz Foundation (Planegg, Germany) and the Cancer Research Advancement Foundation (Forschung hilft) at the University of Würzburg (Würzburg, Germany).
This work is dedicated to the memory of Professor Bernhard Singer (UKE Essen, Germany) who passed away too early. He was a committed leader and promoter of the entire CEACAM1 research field and a wonderful person and colleague.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Rueckschloss U, Kuerten S, Ergün S. The role of CEA-related cell adhesion molecule-1 (CEACAM1) in vascular homeostasis. Histochem Cell Biol (2016) 146:657–71. doi: 10.1007/s00418-016-1505-9
2. Kim WM, Huang Y-H, Gandhi A, Blumberg RS. CEACAM1 structure and function in immunity and its therapeutic implications. Semin Immunol (2019) 42:101296. doi: 10.1016/j.smim.2019.101296
3. Dankner M, Gray-Owen SD, Huang Y-H, Blumberg RS, Beauchemin N. CEACAM1 as a multi-purpose target for cancer immunotherapy. Oncoimmunology (2017) 6:e1328336. doi: 10.1080/2162402X.2017.1328336
4. Nittka S, Günther J, Ebisch C, Erbersdobler A, Neumaier M. The human tumor suppressor CEACAM1 modulates apoptosis and is implicated in early colorectal tumorigenesis. Oncogene (2004) 23:9306–13. doi: 10.1038/sj.onc.1208259
5. Belcher Dufrisne M, Swope N, Kieber M, Yang J-Y, Han J, Li J, et al. Human CEACAM1 N-domain dimerization is independent from glycan modifications. Structure (2022) 30:658–70.e5. doi: 10.1016/j.str.2022.02.003
6. Zhuo Y, Yang J-Y, Moremen KW, Prestegard JH. Glycosylation alters dimerization properties of a cell-surface signaling protein, carcinoembryonic antigen-related cell adhesion molecule 1 (CEACAM1). J Biol Chem (2016) 291:20085–95. doi: 10.1074/jbc.M116.740050
7. Beauchemin N, Draber P, Dveksler G, Gold P, Gray-Owen S, Grunert F, et al. Redefined nomenclature for members of the carcinoembryonic antigen family. Exp Cell Res (1999) 252:243–9. doi: 10.1006/excr.1999.4610
8. Müller MM, Klaile E, Vorontsova O, Singer BB, OBrink B. Homophilic adhesion and CEACAM1-S regulate dimerization of CEACAM1-L and recruitment of SHP-2 and c-Src. J Cell Biol (2009) 187:569–81. doi: 10.1083/jcb.200904150
9. Hu W, Bagramyan K, Bhatticharya S, Hong T, Tapia A, Wong P, et al. Phosphorylation of human CEACAM1-LF by PKA and GSK3β promotes its interaction with β-catenin. J Biol Chem (2021) 297:101305. doi: 10.1016/j.jbc.2021.101305
10. Gray-Owen SD, Blumberg RS. CEACAM1: contact-dependent control of immunity. Nat Rev Immunol (2006) 6:433–46. doi: 10.1038/nri1864
11. Behrens I-K, Busch B, Ishikawa-Ankerhold H, Palamides P, Shively JE, Stanners C, et al. The hopQ-CEACAM interaction controls CagA translocation, phosphorylation, and phagocytosis of helicobacter pylori in neutrophils. mBio (2020) 11:e03256–19. doi: 10.1128/mBio.03256-19
12. Catton EA, Bonsor DA, Herrera C, Stålhammar-Carlemalm M, Lyndin M, Turner CE, et al. Human CEACAM1 is targeted by a Streptococcus pyogenes adhesin implicated in puerperal sepsis pathogenesis. Nat Commun (2023) 14:2275. doi: 10.1038/s41467-023-37732-1
13. Nguyen QA, Schmitt L, Mejías-Luque R, Gerhard M. Effects of Helicobacter pylori adhesin HopQ binding to CEACAM receptors in the human stomach. Front Immunol (2023) 14:1113478. doi: 10.3389/fimmu.2023.1113478
14. Sheikh A, Fleckenstein JM. Interactions of pathogenic Escherichia coli with CEACAMs. Front Immunol (2023) 14:1120331. doi: 10.3389/fimmu.2023.1120331
15. Gaur S, Shively JE, Yen Y, Gaur RK. Altered splicing of CEACAM1 in breast cancer: identification of regulatory sequences that control splicing of CEACAM1 into long or short cytoplasmic domain isoforms. Mol Cancer (2008) 7:46. doi: 10.1186/1476-4598-7-46
16. Neumaier M, Paululat S, Chan A, Matthaes P, Wagener C. Biliary glycoprotein, a potential human cell adhesion molecule, is down-regulated in colorectal carcinomas. Proc Natl Acad Sci U S A (1993) 90:10744–8. doi: 10.1073/pnas.90.22.10744
17. Nollau P, Scheller H, Kona-Horstmann M, Rohde S, Hagenmüller F, Wagener C, et al. Expression of CD66a (human C-CAM) and other members of the carcinoembryonic antigen gene family of adhesion molecules in human colorectal adenomas. Cancer Res (1997) 57:2354–7.
18. Kleefeldt F, Bömmel H, Broede B, Thomsen M, Pfeiffer V, Wörsdörfer P, et al. Aging-related carcinoembryonic antigen-related cell adhesion molecule 1 signaling promotes vascular dysfunction. Aging Cell (2019) 18:e13025. doi: 10.1111/acel.13025
19. Kang W-Y, Chen W-T, Wu M-T, Chai C-Y. The expression of CD66a and possible roles in colorectal adenoma and adenocarcinoma. Int J Colorectal Dis (2007) 22:869–74. doi: 10.1007/s00384-006-0247-x
20. Ieda J, Yokoyama S, Tamura K, Takifuji K, Hotta T, Matsuda K, et al. Re-expression of CEACAM1 long cytoplasmic domain isoform is associated with invasion and migration of colorectal cancer. Int J Cancer (2011) 129:1351–61. doi: 10.1002/ijc.26072
21. Yamaguchi S, Yokoyama S, Ueno M, Hayami S, Mitani Y, Takeuchi A, et al. CEACAM1 is associated with recurrence after hepatectomy for colorectal liver metastasis. J Surg Res (2017) 220:353–62. doi: 10.1016/j.jss.2017.07.035
22. Ghavampour S, Kleefeldt F, Bömmel H, Volland J, Paus A, Horst A, et al. Endothelial barrier function is differentially regulated by CEACAM1-mediated signaling. FASEB J (2018) 32:5612–25. doi: 10.1096/fj.201800331R
23. Yang C, He P, Liu Y, He Y, Yang C, Du Y, et al. Down-regulation of CEACAM1 in breast cancer. Acta Biochim Biophys Sin (Shanghai) (2015) 47:788–94. doi: 10.1093/abbs/gmv075
24. Yang C, He P, Liu Y, He Y, Yang C, Du Y, et al. Assay of serum CEACAM1 as a potential biomarker for breast cancer. Clin Chim Acta (2015) 450:277–81. doi: 10.1016/j.cca.2015.09.005
25. Thöm I, Schult-Kronefeld O, Burkholder I, Schuch G, Andritzky B, Kastendieck H, et al. Expression of CEACAM-1 in pulmonary adenocarcinomas and their metastases. Anticancer Res (2009) 29:249–54.
26. Nolen BM, Lomakin A, Marrangoni A, Velikokhatnaya L, Prosser D, Lokshin AE. Urinary protein biomarkers in the early detection of lung cancer. Cancer Prev Res (Phila) (2014) 8:111–9. doi: 10.1158/1940-6207.CAPR-14-0210
27. Zhou M-q, Du Y, Liu Y-w, Wang Y-z, He Y-q, Yang C-x, et al. Clinical and experimental studies regarding the expression and diagnostic value of carcinoembryonic antigen-related cell adhesion molecule 1 in non-small-cell lung cancer. BMC Cancer (2013) 13:359. doi: 10.1186/1471-2407-13-359
28. Sienel W, Dango S, Woelfle U, Morresi-Hauf A, Wagener C, Brümmer J, et al. Elevated expression of carcinoembryonic antigen-related cell adhesion molecule 1 promotes progression of non-small cell lung cancer. Clin Cancer Res (2003) 9:2260–6.
29. Dango S, Sienel W, Schreiber M, Stremmel C, Kirschbaum A, Pantel K, et al. Elevated expression of carcinoembryonic antigen-related cell adhesion molecule 1 (CEACAM-1) is associated with increased angiogenic potential in non-small-cell lung cancer. Lung Cancer (2008) 60:426–33. doi: 10.1016/j.lungcan.2007.11.015
30. Laack E, Nikbakht H, Peters A, Kugler C, Jasiewicz Y, Edler L, et al. Expression of CEACAM1 in adenocarcinoma of the lung: a factor of independent prognostic significance. J Clin Oncol (2002) 20:4279–84. doi: 10.1200/JCO.2002.08.067
31. Thies A, Moll I, Berger J, Wagener C, Brümmer J, Schulze H-J, et al. CEACAM1 expression in cutaneous Malignant melanoma predicts the development of metastatic disease. J Clin Oncol (2002) 20:2530–6. doi: 10.1200/JCO.2002.05.033
32. Thies A, Berlin A, Brunner G, Schulze H-J, Moll I, Pfüller U, et al. Glycoconjugate profiling of primary melanoma and its sentinel node and distant metastases: implications for diagnosis and pathophysiology of metastases. Cancer Lett (2007) 248:68–80. doi: 10.1016/j.canlet.2006.05.020
33. Ortenberg R, Sapoznik S, Zippel D, Shapira-Frommer R, Itzhaki O, Kubi A, et al. Serum CEACAM1 elevation correlates with melanoma progression and failure to respond to adoptive cell transfer immunotherapy. J Immunol Res (2015) 2015:902137. doi: 10.1155/2015/902137
34. Markel G, Ortenberg R, Seidman R, Sapoznik S, Koren-Morag N, Besser MJ, et al. Systemic dysregulation of CEACAM1 in melanoma patients. Cancer Immunol Immunother (2010) 59:215–30. doi: 10.1007/s00262-009-0740-5
35. Gambichler T, Grothe S, Rotterdam S, Altmeyer P, Kreuter A. Protein expression of carcinoembryonic antigen cell adhesion molecules in benign and Malignant melanocytic skin lesions. Am J Clin Pathol (2009) 131:782–7. doi: 10.1309/AJCP24KXJVBZXENS
36. Khatib N, Pe'er J, Ortenberg R, Schachter J, Frenkel S, Markel G, et al. Carcinoembryonic antigen cell adhesion molecule-1 (CEACAM1) in posterior uveal melanoma: correlation with clinical and histological survival markers. Invest Ophthalmol Vis Sci (2011) 52:9368–72. doi: 10.1167/iovs.10-6006
37. Ortenberg R, Galore-Haskel G, Greenberg I, Zamlin B, Sapoznik S, Greenberg E, et al. CEACAM1 promotes melanoma cell growth through Sox-2. Neoplasia (2014) 16:451–60. doi: 10.1016/j.neo.2014.05.003
38. Sivan S, Suzan F, Rona O, Tamar H, Vivian B, Tamar P, et al. Serum CEACAM1 correlates with disease progression and survival in Malignant melanoma patients. Clin Dev Immunol (2012) 2012:290536. doi: 10.1155/2012/290536
39. Oliveira-Ferrer L, Tilki D, Ziegeler G, Hauschild J, Loges S, Irmak S, et al. Dual role of carcinoembryonic antigen-related cell adhesion molecule 1 in angiogenesis and invasion of human urinary bladder cancer. Cancer Res (2004) 64:8932–8. doi: 10.1158/0008-5472.CAN-04-0505
40. Tilki D, Singer BB, Shariat SF, Behrend A, Fernando M, Irmak S, et al. CEACAM1: a novel urinary marker for bladder cancer detection. Eur Urol (2010) 57:648–54. doi: 10.1016/j.eururo.2009.05.040
41. Igami K, Uchiumi T, Shiota M, Ueda S, Tsukahara S, Akimoto M, et al. Extracellular vesicles expressing CEACAM proteins in the urine of bladder cancer patients. Cancer Sci (2022) 113:3120–33. doi: 10.1111/cas.15438
42. Simeone DM, Ji B, Banerjee M, Arumugam T, Li D, Anderson MA, et al. CEACAM1, a novel serum biomarker for pancreatic cancer. Pancreas (2007) 34:436–43. doi: 10.1097/MPA.0b013e3180333ae3
43. Gong D-y, Fu H-x, Peng Y, You Y-q, Li Z-p. Diagnostic value of serum CEACAM1 in patients with pancreatic cancer. Nan Fang Yi Ke Da Xue Xue Bao (2011) 31:164–6.
44. Giulietti M, Occhipinti G, Principato G, Piva F. Weighted gene co-expression network analysis reveals key genes involved in pancreatic ductal adenocarcinoma development. Cell Oncol (Dordr) (2016) 39:379–88. doi: 10.1007/s13402-016-0283-7
45. Zińczuk J, Zaręba K, Romaniuk W, Kamińska D, Nizioł M, Baszun M, et al. Expression of chosen carcinoembryonic-related cell adhesion molecules in pancreatic intraepithelial neoplasia (PanIN) associated with chronic pancreatitis and pancreatic ductal adenocarcinoma (PDAC). Int J Med Sci (2019) 16:583–92. doi: 10.7150/ijms.32751
46. Nolen BM, Brand RE, Prosser D, Velikokhatnaya L, Allen PJ, Zeh HJ, et al. Prediagnostic serum biomarkers as early detection tools for pancreatic cancer in a large prospective cohort study. PLoS One (2014) 9:e94928. doi: 10.1371/journal.pone.0094928
47. Gebauer F, Wicklein D, Horst J, Sundermann P, Maar H, Streichert T, et al. Carcinoembryonic antigen-related cell adhesion molecules (CEACAM) 1, 5 and 6 as biomarkers in pancreatic cancer. PLoS One (2014) 9:e113023. doi: 10.1371/journal.pone.0113023
48. Albarran-Somoza B, Franco-Topete R, Delgado-Rizo V, Cerda-Camacho F, Acosta-Jimenez L, Lopez-Botet M, et al. CEACAM1 in cervical cancer and precursor lesions: association with human papillomavirus infection. J Histochem Cytochem (2006) 54:1393–9. doi: 10.1369/jhc.6A6921.2006
49. Luebke AM, Ricken W, Kluth M, Hube-Magg C, Schroeder C, Büscheck F, et al. Loss of the adhesion molecule CEACAM1 is associated with early biochemical recurrence in TMPRSS2:ERG fusion-positive prostate cancers. Int J Cancer (2020) 147:575–83. doi: 10.1002/ijc.32957
50. Busch C, Hanssen TA, Wagener C, OBrink B. Down-regulation of CEACAM1 in human prostate cancer: correlation with loss of cell polarity, increased proliferation rate, and Gleason grade 3 to 4 transition. Hum Pathol (2002) 33:290–8. doi: 10.1053/hupa.2002.32218
51. Phan D, Cheng C-J, Galfione M, Vakar-Lopez F, Tunstead J, Thompson NE, et al. Identification of Sp2 as a transcriptional repressor of carcinoembryonic antigen-related cell adhesion molecule 1 in tumorigenesis. Cancer Res (2004) 64:3072–8. doi: 10.1158/0008-5472.can-03-3730
52. Lucarini G, Zizzi A, Re M, Sayeed MA, Di Primio R, Rubini C. Prognostic implication of CEACAM1 expression in squamous cell carcinoma of the larynx: Pilot study. Head Neck (2019) 41:1615–21. doi: 10.1002/hed.25589
53. Wang F-F, Guan B-X, Yang J-Y, Wang H-T, Zhou C-J. CEACAM1 is overexpressed in oral tumors and related to tumorigenesis. Med Mol Morphol (2017) 50:42–51. doi: 10.1007/s00795-016-0147-2
54. Yang F, Zeng Z, Li J, Ren X, Wei F. TIM-3 and CEACAM1 are prognostic factors in head and neck squamous cell carcinoma. Front Mol Biosci (2021) 8:619765. doi: 10.3389/fmolb.2021.619765
55. Qian W, Huang P, Liang X, Chen Y, Guan B. High expression of carcinoembryonic antigen-associated cell adhesion molecule 1 is associated with microangiogenesis in esophageal squamous cell carcinoma. Transl Cancer Res (2020) 9:4762–9. doi: 10.21037/tcr-19-2039
56. Yu H, Yu J, Ren Y, Yang Y, Xiao X. Serum CEACAM1 level is associated with diagnosis and prognosis in patients with osteosarcoma. PLoS One (2016) 11:e0153601. doi: 10.1371/journal.pone.0153601
57. Di Meo A, Batruch I, Brown MD, Yang C, Finelli A, Jewett MA, et al. Searching for prognostic biomarkers for small renal masses in the urinary proteome. Int J Cancer (2020) 146:2315–25. doi: 10.1002/ijc.32650
58. Yang L, Liu Y, Zhang B, Yu M, Huang F, Zeng J, et al. CEACAM1 is a prognostic biomarker and correlated with immune cell infiltration in clear cell renal cell carcinoma. Dis Markers (2023) 2023:3606362. doi: 10.1155/2023/3606362
59. Liu W, Wei W, Winer D, Bamberger A-M, Bamberger C, Wagener C, et al. CEACAM1 impedes thyroid cancer growth but promotes invasiveness: a putative mechanism for early metastases. Oncogene (2007) 26:2747–58. doi: 10.1038/sj.onc.1210077
60. Ueshima C, Kataoka TR, Takei Y, Hirata M, Sugimoto A, Hirokawa M, et al. CEACAM1 long isoform has opposite effects on the growth of human mastocytosis and medullary thyroid carcinoma cells. Cancer Med (2017) 6:845–56. doi: 10.1002/cam4.1050
61. Takeuchi A, Yokoyama S, Nakamori M, Nakamura M, Ojima T, Yamaguchi S, et al. Loss of CEACAM1 is associated with poor prognosis and peritoneal dissemination of patients with gastric cancer. Sci Rep (2019) 9:12702. doi: 10.1038/s41598-019-49230-w
62. Zhou M, Jin Z, Liu Y, He Y, Du Y, Yang C, et al. Up-regulation of carcinoembryonic antigen-related cell adhesion molecule 1 in gastrointestinal cancer and its clinical relevance. Acta Biochim Biophys Sin (Shanghai) (2017) 49:737–43. doi: 10.1093/abbs/gmx060
63. Shi J-F, Xu S-X, He P, Xi Z-H. Expression of carcinoembryonic antigen-related cell adhesion molecule 1(CEACAM1) and its correlation with angiogenesis in gastric cancer. Pathol Res Pract (2014) 210:473–6. doi: 10.1016/j.prp.2014.03.014
64. Zhou C-J, Liu B, Zhu K-X, Zhang Q-H, Zhang T-G, Xu W-H, et al. The different expression of carcinoembryonic antigen-related cell adhesion molecule 1 (CEACAM1) and possible roles in gastric carcinomas. Pathol Res Pract (2009) 205:483–9. doi: 10.1016/j.prp.2009.01.006
65. Cruz PV, Wakai T, Shirai Y, Yokoyama N, Hatakeyama K. Loss of carcinoembryonic antigen-related cell adhesion molecule 1 expression is an adverse prognostic factor in hepatocellular carcinoma. Cancer (2005) 104:354–60. doi: 10.1002/cncr.21159
66. Zhu J, Yang Y, Ma C, Zhang G, Wang K, Hu S. CEACAM1 cytoplastic expression is closely related to tumor angiogenesis and poorer relapse-free survival after curative resection of hepatocellular carcinoma. World J Surg (2011) 35:2259–65. doi: 10.1007/s00268-011-1119-2
67. Kiriyama S, Yokoyama S, Ueno M, Hayami S, Ieda J, Yamamoto N, et al. CEACAM1 long cytoplasmic domain isoform is associated with invasion and recurrence of hepatocellular carcinoma. Ann Surg Oncol (2014) 21 Suppl 4:S505–14. doi: 10.1245/s10434-013-3460-1
68. Leung N, Turbide C, Olson M, Marcus V, Jothy S, Beauchemin N. Deletion of the carcinoembryonic antigen-related cell adhesion molecule 1 (Ceacam1) gene contributes to colon tumor progression in a murine model of carcinogenesis. Oncogene (2006) 25:5527–36. doi: 10.1038/sj.onc.1209541
69. Wegwitz F, Lenfert E, Gerstel D, von Ehrenstein L, Einhoff J, Schmidt G, et al. CEACAM1 controls the EMT switch in murine mammary carcinoma in vitro and in vivo. Oncotarget (2016) 7:63730–46. doi: 10.18632/oncotarget.11650
70. Abou-Rjaily GA, Lee SJ, May D, Al-Share QY, Deangelis AM, Ruch RJ, et al. CEACAM1 modulates epidermal growth factor receptor–mediated cell proliferation. J Clin Invest (2004) 114:944–52. doi: 10.1172/JCI21786
71. Xu J, Liu B, Ma S, Zhang J, Ji Y, Xu L, et al. Characterizing the tumor suppressor role of CEACAM1 in multiple myeloma. Cell Physiol Biochem (2018) 45:1631–40. doi: 10.1159/000487730
72. Singer BB, Scheffrahn I, Kammerer R, Suttorp N, Ergun S, Slevogt H. Deregulation of the CEACAM expression pattern causes undifferentiated cell growth in human lung adenocarcinoma cells. PLoS One (2010) 5:e8747. doi: 10.1371/journal.pone.0008747
73. Han Z−M, Huang H−M, Sun Y−W. Effect of CEACAM−1 knockdown in human colorectal cancer cells. Oncol Lett (2018) 16:1622–6. doi: 10.3892/ol.2018.8835
74. Galon J, Bruni D. Tumor immunology and tumor evolution: intertwined histories. Immunity (2020) 52:55–81. doi: 10.1016/j.immuni.2019.12.018
75. Waldman AD, Fritz JM, Lenardo MJ. A guide to cancer immunotherapy: from T cell basic science to clinical practice. Nat Rev Immunol (2020) 20:651–68. doi: 10.1038/s41577-020-0306-5
76. Marofi F, Motavalli R, Safonov VA, Thangavelu L, Yumashev AV, Alexander M, et al. CAR T cells in solid tumors: challenges and opportunities. Stem Cell Res Ther (2021) 12:81. doi: 10.1186/s13287-020-02128-1
77. Kammerer R, Hahn S, Singer BB, Luo JS, von Kleist S. Biliary glycoprotein (CD66a), a cell adhesion molecule of the immunoglobulin superfamily, on human lymphocytes: structure, expression and involvement in T cell activation. Eur J Immunol (1998) 28:3664–74. doi: 10.1002/(SICI)1521-4141(199811)28:11<3664:AID-IMMU3664>3.0.CO;2-D
78. Nakajima A, Iijima H, Neurath MF, Nagaishi T, Nieuwenhuis EE, Raychowdhury R, et al. Activation-induced expression of carcinoembryonic antigen-cell adhesion molecule 1 regulates mouse T lymphocyte function. J Immunol (2002) 168:1028–35. doi: 10.4049/jimmunol.168.3.1028
79. Moller MJ, Kammerer R, Grunert F, von Kleist S. Biliary glycoprotein (BGP) expression on T cells and on a natural-killer-cell sub-population. Int J Cancer (1996) 65:740–5. doi: 10.1002/(SICI)1097-0215(19960315)65:6<740:AID-IJC5>3.0.CO;2-Z
80. Morales VM, Christ A, Watt SM, Kim HS, Johnson KW, Utku N, et al. Regulation of human intestinal intraepithelial lymphocyte cytolytic function by biliary glycoprotein (CD66a). J Immunol (1999) 163:1363–70. doi: 10.4049/jimmunol.163.3.1363
81. Nagaishi T, Pao L, Lin S-H, Iijima H, Kaser A, Qiao S-W, et al. SHP1 phosphatase-dependent T cell inhibition by CEACAM1 adhesion molecule isoforms. Immunity (2006) 25:769–81. doi: 10.1016/j.immuni.2006.08.026
82. Chen Z, Chen L, Qiao S-W, Nagaishi T, Blumberg RS. Carcinoembryonic antigen-related cell adhesion molecule 1 inhibits proximal TCR signaling by targeting ZAP-70. J Immunol (2008) 180:6085–93. doi: 10.4049/jimmunol.180.9.6085
83. Li Y, Shively JE. CEACAM1 regulates Fas-mediated apoptosis in Jurkat T-cells via its interaction with β-catenin. Exp Cell Res (2013) 319:1061–72. doi: 10.1016/j.yexcr.2013.02.020
84. Iijima H, Neurath MF, Nagaishi T, Glickman JN, Nieuwenhuis EE, Nakajima A, et al. Specific regulation of T helper cell 1-mediated murine colitis by CEACAM1. J Exp Med (2004) 199:471–82. doi: 10.1084/jem.20030437
85. Chen D, Iijima H, Nagaishi T, Nakajima A, Russell S, Raychowdhury R, et al. Carcinoembryonic antigen-related cellular adhesion molecule 1 isoforms alternatively inhibit and costimulate human T cell function. J Immunol (2004) 172:3535–43. doi: 10.4049/jimmunol.172.6.3535
86. Donda A, Mori L, Shamshiev A, Carena I, Mottet C, Heim MH, et al. Locally inducible CD66a (CEACAM1) as an amplifier of the human intestinal T cell response. Eur J Immunol (2000) 30:2593–603. doi: 10.1002/1521-4141(200009)30:9<2593:AID-IMMU2593>3.0.CO;2-0
87. Helfrich I, Singer BB. Size matters: the functional role of the CEACAM1 isoform signature and its impact for NK cell-mediated killing in melanoma. Cancers (Basel) (2019) 11:356. doi: 10.3390/cancers11030356
88. Huang Y-H, Zhu C, Kondo Y, Anderson AC, Gandhi A, Russell A, et al. CEACAM1 regulates TIM-3-mediated tolerance and exhaustion. Nature (2015) 517:386–90. doi: 10.1038/nature13848
89. Zhang Y, Cai P, Li L, Shi L, Chang P, Liang T, et al. Co-expression of TIM-3 and CEACAM1 promotes T cell exhaustion in colorectal cancer patients. Int Immunopharmacol (2017) 43:210–8. doi: 10.1016/j.intimp.2016.12.024
90. Yu S, Ren X, Meng F, Guo X, Tao J, Zhang W, et al. TIM3/CEACAM1 pathway involves in myeloid-derived suppressor cells induced CD8+ T cells exhaustion and bone marrow inflammatory microenvironment in myelodysplastic syndrome. Immunology (2023) 168:273–89. doi: 10.1111/imm.13488
91. Li J, Liu X, Duan Y, Wang H, Su W, Wang Y, et al. Abnormal expression of circulating and tumor-infiltrating carcinoembryonic antigen-related cell adhesion molecule 1 in patients with glioma. Oncol Lett (2018) 15:3496–503. doi: 10.3892/ol.2018.7786
92. Jeon SH, Kang M, Jeon M, Chung Y, Kim AR, Lee YJ, et al. CEACAM1 marks highly suppressive intratumoral regulatory T cells for targeted depletion therapy. Clin Cancer Res (2023) 29:1794–806. doi: 10.1158/1078-0432.CCR-22-1843
93. Pinkert J, Boehm H-H, Trautwein M, Doecke W-D, Wessel F, Ge Y, et al. T cell-mediated elimination of cancer cells by blocking CEACAM6-CEACAM1 interaction. Oncoimmunology (2022) 11:2008110. doi: 10.1080/2162402X.2021.2008110
94. Zhang E, Ding C, Li S, Zhou X, Aikemu B, Fan X, et al. Roles and mechanisms of tumour-infiltrating B cells in human cancer: a new force in immunotherapy. biomark Res (2023) 11:28. doi: 10.1186/s40364-023-00460-1
95. Khairnar V, Duhan V, Maney SK, Honke N, Shaabani N, Pandyra AA, et al. CEACAM1 induces B-cell survival and is essential for protective antiviral antibody production. Nat Commun (2015) 6:6217. doi: 10.1038/ncomms7217
96. Lobo EO, Zhang Z, Shively JE. Pivotal Advance: CEACAM1 is a negative coreceptor for the B cell receptor and promotes CD19-mediated adhesion of B cells in a PI3K-dependent manner. J Leukoc Biol (2009) 86:205–18. doi: 10.1189/jlb.0109037
97. Tsugawa N, Yamada D, Watabe T, Onizawa M, Wang S, Nemoto Y, et al. CEACAM1 specifically suppresses B cell receptor signaling-mediated activation. Biochem Biophys Res Commun (2021) 535:99–105. doi: 10.1016/j.bbrc.2020.11.126
98. Greicius G, Severinson E, Beauchemin N, OBrink B, Singer BB. CEACAM1 is a potent regulator of B cell receptor complex-induced activation. J Leukoc Biol (2003) 74:126–34. doi: 10.1189/jlb.1202594
99. Pantelic M, Kim Y-J, Bolland S, Chen I, Shively J, Chen T. Neisseria gonorrhoeae kills carcinoembryonic antigen-related cellular adhesion molecule 1 (CD66a)-expressing human B cells and inhibits antibody production. Infect Immun (2005) 73:4171–9. doi: 10.1128/iai.73.7.4171-4179.2005
100. Galaski J, Shhadeh A, Umaña A, Yoo CC, Arpinati L, Isaacson B, et al. Fusobacterium nucleatum CbpF mediates inhibition of T cell function through CEACAM1 activation. Front Cell Infect Microbiol (2021) 11:692544. doi: 10.3389/fcimb.2021.692544
101. Stern N, Markel G, Arnon TI, Gruda R, Wong H, Gray-Owen SD, et al. Carcinoembryonic antigen (CEA) inhibits NK killing via interaction with CEA-related cell adhesion molecule 1. J Immunol (2005) 174:6692–701. doi: 10.4049/jimmunol.174.11.6692
102. Markel G, Lieberman N, Katz G, Arnon TI, Lotem M, Drize O, et al. CD66a interactions between human melanoma and NK cells: a novel class I MHC-independent inhibitory mechanism of cytotoxicity. J Immunol (2002) 168:2803–10. doi: 10.4049/jimmunol.168.6.2803
103. Markel G, Wolf D, Hanna J, Gazit R, Goldman-Wohl D, Lavy Y, et al. Pivotal role of CEACAM1 protein in the inhibition of activated decidual lymphocyte functions. J Clin Invest (2002) 110:943–53. doi: 10.1172/JCI15643
104. Thirion G, Feliu AA, Coutelier J-P. CD66a (CEACAM1) expression by mouse natural killer cells. Immunology (2008) 125:535–40. doi: 10.1111/j.1365-2567.2008.02867.x
105. Hosomi S, Chen Z, Baker K, Chen L, Huang Y-H, Olszak T, et al. CEACAM1 on activated NK cells inhibits NKG2D-mediated cytolytic function and signaling. Eur J Immunol (2013) 43:2473–83. doi: 10.1002/eji.201242676
106. Chen Z, Chen L, Baker K, Olszak T, Zeissig S, Huang Y-H, et al. CEACAM1 dampens antitumor immunity by down-regulating NKG2D ligand expression on tumor cells. J Exp Med (2011) 208:2633–40. doi: 10.1084/jem.20102575
107. Ullrich N, Heinemann A, Nilewski E, Scheffrahn I, Klode J, Scherag A, et al. CEACAM1-3S drives melanoma cells into NK cell-mediated cytolysis and enhances patient survival. Cancer Res (2015) 75:1897–907. doi: 10.1158/0008-5472.CAN-14-1752
108. Suda T, Tatsumi T, Nishio A, Kegasawa T, Yoshioka T, Yamada R, et al. CEACAM1 is associated with the suppression of natural killer cell function in patients with chronic hepatitis C. Hepatol Commun (2018) 2:1247–58. doi: 10.1002/hep4.1240
109. Liu Y, Wang Y, Sun S, Chen Z, Xiang S, Ding Z, et al. Understanding the versatile roles and applications of EpCAM in cancers: from bench to bedside. Exp Hematol Oncol (2022) 11:97. doi: 10.1186/s40164-022-00352-4
110. Park DJ, Sung PS, Kim J-H, Lee GW, Jang JW, Jung ES, et al. EpCAM-high liver cancer stem cells resist natural killer cell-mediated cytotoxicity by upregulating CEACAM1. J ImmunoTher Cancer (2020) 8:e000301. doi: 10.1136/jitc-2019-000301
111. Tam K, Lee Y, Schoppy D, Sunwoo JB. Abstract 33: CEACAM1 blockade increases NK cell cytotoxicity in head and neck squamous cell carcinoma. Clin Cancer Res (2017) 23:33. doi: 10.1158/1557-3265.AACRAHNS17-33
112. Lee EH, Lee J, Hur M, Park H-Y, Yum HI, Nam H, et al. 1199P - MG1124, a novel CEACAM1-targeted monoclonal antibody, has therapeutic potential as a combination partner of PD-1 inhibitors in NSCLC patients. Ann Oncol (2019) 30:v490. doi: 10.1093/annonc/mdz253.025
113. Fantini M, David JM, Annunziata CM, Morelli MP, Arlen PM, Tsang KY. The monoclonal antibody NEO-201 enhances natural killer cell cytotoxicity against tumor cells through blockade of the inhibitory CEACAM5/CEACAM1 immune checkpoint pathway. Cancer Biother Radiopharm (2020) 35:190–8. doi: 10.1089/cbr.2019.3141
114. Lugano R, Ramachandran M, Dimberg A. Tumor angiogenesis: causes, consequences, challenges and opportunities. Cell Mol Life Sci (2020) 77:1745–70. doi: 10.1007/s00018-019-03351-7
115. Kleefeldt F, Upcin B, Bömmel H, Schulz C, Eckner G, Allmanritter J, et al. Bone marrow-independent adventitial macrophage progenitor cells contribute to angiogenesis. Cell Death Dis (2022) 13:220. doi: 10.1038/s41419-022-04605-2
116. Upcin B, Henke E, Kleefeldt F, Hoffmann H, Rosenwald A, Irmak-Sav S, et al. Contribution of adventitia-derived stem and progenitor cells to new vessel formation in tumors. Cells (2021) 10:1719. doi: 10.3390/cells10071719
117. Alhudaithi SS, Almuqbil RM, Zhang H, Bielski ER, Du W, Sunbul FS, et al. Local targeting of lung-tumor-associated macrophages with pulmonary delivery of a CSF-1R inhibitor for the treatment of breast cancer lung metastases. Mol Pharm (2020) 17:4691–703. doi: 10.1021/acs.molpharmaceut.0c00983
118. Ergün S, Kilik N, Ziegeler G, Hansen A, Nollau P, Götze J, et al. CEA-related cell adhesion molecule 1: a potent angiogenic factor and a major effector of vascular endothelial growth factor. Mol Cell (2000) 5:311–20. doi: 10.1016/s1097-2765(00)80426-8
119. Gerstel D, Wegwitz F, Jannasch K, Ludewig P, Scheike K, Alves F, et al. CEACAM1 creates a pro-angiogenic tumor microenvironment that supports tumor vessel maturation. Oncogene (2011) 30:4275–88. doi: 10.1038/onc.2011.146
120. Nouvion A-L, Oubaha M, Leblanc S, Davis EC, Jastrow H, Kammerer R, et al. CEACAM1: a key regulator of vascular permeability. J Cell Sci (2010) 123:4221–30. doi: 10.1242/jcs.073635
121. Kleefeldt F, Rueckschloss U, Ergün S. CEACAM1 promotes vascular aging processes. Aging (Albany NY) (2020) 12:3121–3. doi: 10.18632/aging.102868
122. Bömmel H, Kleefeldt F, Zernecke A, Ghavampour S, Wagner N, Kuerten S, et al. Visualization of endothelial barrier damage prior to formation of atherosclerotic plaques. Histochem Cell Biol (2017) 148:117–27. doi: 10.1007/s00418-017-1562-8
123. Horst AK, Bickert T, Brewig N, Ludewig P, van Rooijen N, Schumacher U, et al. CEACAM1+ myeloid cells control angiogenesis in inflammation. Blood (2009) 113:6726–36. doi: 10.1182/blood-2008-10-184556
124. Lu R, Kujawski M, Pan H, Shively JE. Tumor angiogenesis mediated by myeloid cells is negatively regulated by CEACAM1. Cancer Res (2012) 72:2239–50. doi: 10.1158/0008-5472.CAN-11-3016
125. Samineni S, Zhang Z, Shively JE. Carcinoembryonic antigen-related cell adhesion molecule 1 negatively regulates granulocyte colony-stimulating factor production by breast tumor-associated macrophages that mediate tumor angiogenesis. Int J Cancer (2013) 133:394–407. doi: 10.1002/ijc.28036
126. Muturi HT, Dreesen JD, Nilewski E, Jastrow H, Giebel B, Ergun S, et al. Tumor and endothelial cell-derived microvesicles carry distinct CEACAMs and influence T-cell behavior. PloS One (2013) 8:e74654. doi: 10.1371/journal.pone.0074654
127. Dillekås H, Rogers MS, Straume O. Are 90% of deaths from cancer caused by metastases? Cancer Med (2019) 8:5574–6. doi: 10.1002/cam4.2474
128. Pijuan J, Barceló C, Moreno DF, Maiques O, Sisó P, Marti RM, et al. In vitro cell migration, invasion, and adhesion assays: from cell imaging to data analysis. Front Cell Dev Biol (2019) 7:107. doi: 10.3389/fcell.2019.00107
129. Ebrahimnejad A, Streichert T, Nollau P, Horst AK, Wagener C, Bamberger A-M, et al. CEACAM1 enhances invasion and migration of melanocytic and melanoma cells. Am J Pathol (2004) 165:1781–7. doi: 10.1016/S0002-9440(10)63433-5
130. Müller MM, Singer BB, Klaile E, OBrink B, Lucka L. Transmembrane CEACAM1 affects integrin-dependent signaling and regulates extracellular matrix protein-specific morphology and migration of endothelial cells. Blood (2005) 105:3925–34. doi: 10.1182/blood-2004-09-3618
131. Liu J, Di G, Wu C-T, Hu X, Duan H. CEACAM1 inhibits cell-matrix adhesion and promotes cell migration through regulating the expression of N-cadherin. Biochem Biophys Res Commun (2013) 430:598–603. doi: 10.1016/j.bbrc.2012.11.107
132. Yang C, Cao M, Liu Y, He Y, Yang C, Du Y, et al. Inhibition of cell invasion and migration by CEACAM1-4S in breast cancer. Oncol Lett (2017) 14:4758–66. doi: 10.3892/ol.2017.6791
133. To SK, Tang MK, Tong Y, Zhang J, Chan KK, Ip PP, et al. A selective β-catenin-metadherin/CEACAM1-CCL3 axis mediates metastatic heterogeneity upon tumor-macrophage interaction. Adv Sci (Weinh) (2022) 9:e2103230. doi: 10.1002/advs.202103230
134. Neophytou CM, Trougakos IP, Erin N, Papageorgis P. Apoptosis deregulation and the development of cancer multi-drug resistance. Cancers (Basel) (2021) 13:4363. doi: 10.3390/cancers13174363
135. Carneiro BA, El-Deiry WS. Targeting apoptosis in cancer therapy. Nat Rev Clin Oncol (2020) 17:395–417. doi: 10.1038/s41571-020-0341-y
136. Singer BB, Klaile E, Scheffrahn I, Müller MM, Kammerer R, Reutter W, et al. CEACAM1 (CD66a) mediates delay of spontaneous and Fas ligand-induced apoptosis in granulocytes. Eur J Immunol (2005) 35:1949–59. doi: 10.1002/eji.200425691
137. Kilic N, Oliveira-Ferrer L, Wurmbach J-H, Loges S, Chalajour F, Neshat-Vahid S, et al. Pro-angiogenic signaling by the endothelial presence of CEACAM1. J Biol Chem (2005) 280:2361–9. doi: 10.1074/jbc.M409407200
138. Yu Q, Chow EM, Wong H, Gu J, Mandelboim O, Gray-Owen SD, et al. CEACAM1 (CD66a) promotes human monocyte survival via a phosphatidylinositol 3-kinase- and AKT-dependent pathway. J Biol Chem (2006) 281:39179–93. doi: 10.1074/jbc.M608864200
139. Wang Y, Chen Y, Yan Y, Li X, Chen G, He N, et al. Loss of CEACAM1, a tumor-associated factor, attenuates post-infarction cardiac remodeling by inhibiting apoptosis. Sci Rep (2016) 6:21972. doi: 10.1038/srep21972
140. Nittka S, Böhm C, Zentgraf H, Neumaier M. The CEACAM1-mediated apoptosis pathway is activated by CEA and triggers dual cleavage of CEACAM1. Oncogene (2008) 27:3721–8. doi: 10.1038/sj.onc.1211033
141. Yamamoto N, Yokoyama S, Ieda J, Mitani Y, Yamaguchi S, Takifuji K, et al. CEACAM1 and hollow spheroid formation modulate the chemosensitivity of colorectal cancer to 5-fluorouracil. Cancer Chemother Pharmacol (2015) 75:421–30. doi: 10.1007/s00280-014-2662-y
142. Zaffran I, Landolina N, Gaur P, Rovis TL, Jonjic S, Mandelboim O, et al. Activation of CEACAM1 with an agonistic monoclonal antibody results in inhibition of melanoma cells. Cancer Gene Ther (2022) 29:1676–85. doi: 10.1038/s41417-022-00486-x
143. Kirshner J, Chen C-J, Liu P, Huang J, Shively JE. CEACAM1-4S, a cell-cell adhesion molecule, mediates apoptosis and reverts mammary carcinoma cells to a normal morphogenic phenotype in a 3D culture. Proc Natl Acad Sci U S A (2003) 100:521–6. doi: 10.1073/pnas.232711199
144. Khalaji A, Haddad S, Yazdani Y, Moslemi M, Alizadeh L, Baradaran B. A bioinformatics-based study on the Cisplatin-resistant lung cancer cells; what are the orchestrators of this phenom? Gene (2022) 834:146668. doi: 10.1016/j.gene.2022.146668
145. Dong J, Zhang Y, Wang B, Pu T, Shao L, Wang B, et al. Abstract 1867: Pre-clinical characterization of anti-CEACAM1 antibody. Cancer Res (2023) 83:1867. doi: 10.1158/1538-7445.AM2023-1867
146. Janicot M, Rupalla K, Schmidt A, Helfrich I, Singer BB. 1384 Novel, immune agonistic CEACAM1/5 antibody (YB-200) demonstrates anti-tumor efficacy and significantly increases B-cell response in syngeneic liver Hepa1-6 tumor microenvironment. J ImmunoTher Cancer (2022) 10:A1438. doi: 10.1136/jitc-2022-SITC2022.1384
147. Reuveni H, David HB, Rumble J, Meirson T, Schickler M. Abstract B029: CM24, a novel mAb against carcinoembryonic antigen-related cell adhesion molecule 1 (CEACAM1), suppresses Neutrophil Extracellular Trap (NET)-induced migration and metastasis of cancer cells. Cancer Res (2023) 83:B029–9. doi: 10.1158/1538-7445.METASTASIS22-B029
148. Hollandsworth HM, Amirfakhri S, Filemoni F, Schmitt V, Wennemuth G, Schmidt A, et al. Anti-carcinoembryonic antigen-related cell adhesion molecule antibody for fluorescence visualization of primary colon cancer and metastases in patient-derived orthotopic xenograft mouse models. Oncotarget (2020) 11:429–39. doi: 10.18632/oncotarget.27446
149. Hollandsworth HM, Schmitt V, Amirfakhri S, Filemoni F, Schmidt A, Landström M, et al. Fluorophore-conjugated Helicobacter pylori recombinant membrane protein (HopQ) labels primary colon cancer and metastases in orthotopic mouse models by binding CEA-related cell adhesion molecules. Transl Oncol (2020) 13:100857. doi: 10.1016/j.tranon.2020.100857
150. Ben-Moshe T, Sapir Y, Mandel I, Hakim M, Hashmueli S, Markel G. Inhibition of the novel immune checkpoint CEACAM1 enhances anti-tumor activity. Ann Oncol (2015) 26:ii23. doi: 10.1093/annonc/mdv093.2
151. Markel G, Sapir Y, Mandel I, Hakim M, Shaked R, Meilin E, et al. Inhibition of the novel immune checkpoint CEACAM1 to enhance anti-tumor immunological activity. J Clin Oncol (2016) 34:3044. doi: 10.1200/JCO.2016.34.15_suppl.3044
152. Fierce Biotech. cCAM Biotherapeutics Announces Initiation of Phase 1 Trial of CM-24, a Novel Immune Checkpoint Inhibitor for Cancer Immunotherapy (2015). Available at: https://www.fiercebiotech.com/biotech/ccam-biotherapeutics-announces-initiation-of-phase-1-trial-of-cm-24-a-novel-immune.
153. Merck. Merck Enhances Immuno-Oncology Portfolio with Acquisition of cCAM Biotherapeutics (2015). Available at: https://www.merck.com/news/merck-enhances-immuno-oncology-portfolio-with-acquisition-of-ccam-biotherapeutics/.
154. Securities and exchange commission of the United States. Annual Report Pursuant to Section 13 or 15(d) of the Securities Exchange Act of 1934; FORM 10-K: For the Fiscal Year Ended December 31, 2016 (2017). Available at: https://s21.q4cdn.com/488056881/files/doc_financials/2017/Q4/merck-q4-10k.pdf.
155. Carroll J. That $95M Merck gamble to acquire cCAM? It didn’t pay off (2017). Available at: https://endpts.com/that-95m-merck-gamble-to-acquire-ccam-it-didnt-pay-off/.
156. Shapira R, Weber JS, Geva R, Sznol M, Kluger HM, Wong DJL, et al. open-label, multicenter, single-dose escalation and multi-dose study of a monoclonal antibody targeting CEACAM1 in subjects with selected advanced or recurrent Malignancies. J Clin Oncol (2020) 38:3094. doi: 10.1200/JCO.2020.38.15_suppl.3094
157. Borazanci E, Al Hallak MN, Eder JP, Golan T, Pant S, Perets R, et al. 1027TiP A phase Ib study of CM24 in combination with nivolumab in adults with advanced solid tumors, followed by a phase IIa study of CM24 in combination with nivolumab in NSCLC, and in combination with nivolumab and nab-paclitaxel in pancreatic cancer. Ann Oncol (2021) 32:S861. doi: 10.1016/j.annonc.2021.08.1411
158. McLeod RL, Angagaw MH, Baral TN, Liu L, Moniz RJ, Laskey J, et al. Characterization of murine CEACAM1 in vivo reveals low expression on CD8+ T cells and no tumor growth modulating activity by anti-CEACAM1 mAb CC1. Oncotarget (2018) 9:34459–70. doi: 10.18632/oncotarget.26108
Keywords: CEACAM1, CEA, cancer, tumor, malignancy, metastasis, signaling
Citation: Götz L, Rueckschloss U, Balk G, Pfeiffer V, Ergün S and Kleefeldt F (2023) The role of carcinoembryonic antigen-related cell adhesion molecule 1 in cancer. Front. Immunol. 14:1295232. doi: 10.3389/fimmu.2023.1295232
Received: 15 September 2023; Accepted: 08 November 2023;
Published: 24 November 2023.
Edited by:
Vu Ngo, City of Hope, United StatesReviewed by:
Kwong Tsang, Precision Biologics, Inc., United StatesCopyright © 2023 Götz, Rueckschloss, Balk, Pfeiffer, Ergün and Kleefeldt. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Florian Kleefeldt, Zmxvcmlhbi5rbGVlZmVsZHRAdW5pLXd1ZXJ6YnVyZy5kZQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.