- 1Department of Otolaryngology, Fujian Institute of Otorhinolaryngology, The First Affiliated Hospital of Fujian Medical University, Fuzhou, China
- 2National Regional Medical Center, Fujian Medical University, Fuzhou, China
In the conventional view, CD4+ regulatory T cell (Treg) represents a subset of lymphocytes that involve the perception and negative regulation of the immune response. CD4+Treg plays an important role in the maintenance of immune homeostasis and immune tolerance. However, recent studies have revealed that CD4+Treg do not suppress the immune response in some diseases, but promote inflammatory injury or inhibit tissue remodeling, suggesting the functional heterogeneity of CD4+Treg. Their involvement in tumor pathogenesis is more complex than previously understood. This article reviews the relevant research on the heterogeneity of CD4+Treg, subtype classification, and their relationship with tumor therapy.
1 Introduction
CD4+ regulatory T cell (Treg) was first identified by Sakaguchi et al., who discovered a population of CD4+CD25+T cells in the thymus of mice that could suppress the progression of autoimmune diseases (1). Traditionally, CD4+Treg has been believed can suppress the activities of T cells, B cells, natural killer (NK) cells, and other immune cells. Functional impairment of CD4+Treg is a significant contributing factor to the development of autoimmune diseases (2). Studies in the field of tumor microenvironment (TME) have shown that tumor cells exploit the immunosuppressive capacity of CD4+Treg in TME to evade immune surveillance and promote tumor progression (3). Therefore, targeting CD4+Treg in tumor therapy holds significant potential for development.
Given the crucial role of CD4+Treg in tumor progression, it is essential to gain a deeper understanding of the distinct functional subtypes of CD4+Treg. Recent advances in functional studies and single-cell sequencing have provided novel insights into the functional heterogeneity of CD4+Treg (4, 5) and identified new molecular targets for CD4+Treg in TME (6). This review aims to summarize the relevant research, including the functional heterogeneity and subtyping of CD4+Treg, and provide an overview of clinical trials targeting CD4+Treg.
2 Classification of CD4+Treg
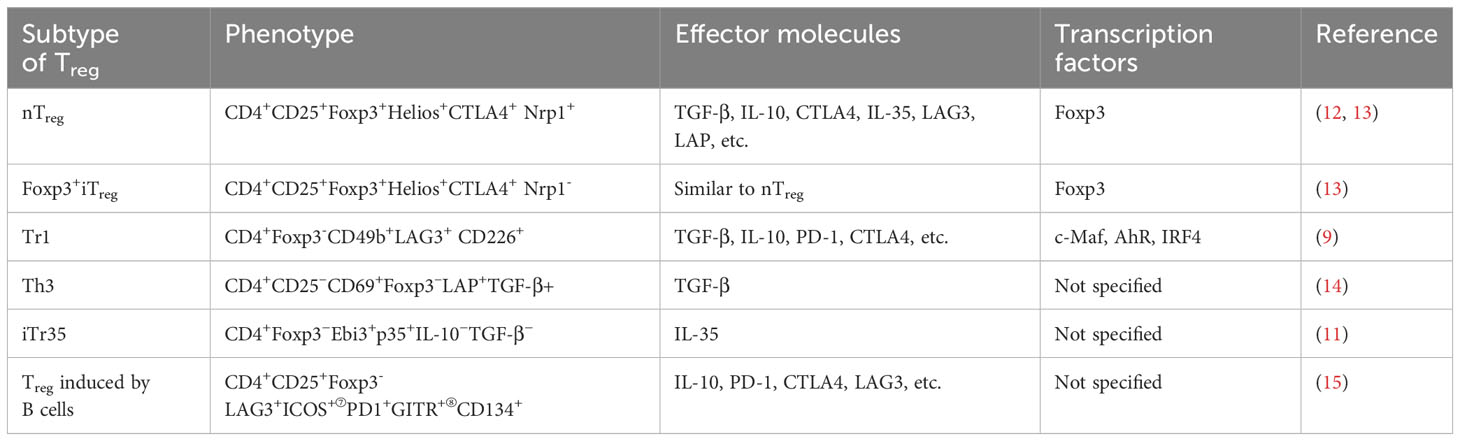
CD4+Treg can be classified into two main categories based on their cellular origins: natural-occurring Treg (nTreg) and peripheral Treg (pTreg). nTreg are generated in the thymus, the precursor cells of Treg mature into CD4+CD25+Foxp3+Treg following stimulation by IL-2/STAT5 and IL-4 signals (7). On the other hand, pTreg are derived from nTreg or initial CD4+T cells in the periphery under specific cytokine or molecular stimuli (8). Additionally, there is a subset of CD4+Treg known as induced Treg (iTreg), which can be generated in vitro experiments. These three types of cells constitute the classical classification of CD4+Treg. Subsequent research has further refined this classical classification based on Foxp3 expression. For example, iTreg can be further divided into Foxp3+iTreg and Foxp3-iTreg, with the latter including Type I Treg (Tr1) cells (9), T helper 3 (Th3) cells (10), and IL-35-induced CD4+Treg (iTr35) (11). Table 1 summarizes the common types of CD4+Treg and their corresponding phenotypes.
3 Heterogeneity and subtypes of CD4+ Treg
3.1 Subtypes of CD4+Treg
Previous studies on CD4+Treg have primarily focused on CD4+CD25+Foxp3+Treg. However, emerging evidence suggests functional heterogeneity within this population, and relying solely on the CD25+Foxp3+phenotype may lead to contradictory conclusions in studies investigating the same disease. Therefore, the establishment of a reliable subtyping strategy is crucial. Currently, two mainstream classification systems are widely used: functional subtypes proposed by the Sakaguchi et al. (16) and the Th-like Treg subtypes based on cytokine expression (17).
Sakaguchi et al. proposed a subdivision of CD4+Treg based on CD45RA expression in 2009, further categorizing them into three functional subsets: subset I consists of CD45RA+Foxp3lo/CD25lo resting Treg (rTreg); subset II comprises CD45RA-Foxp3hi/CD25hi effector Treg (eTreg), also known as activated Treg (aTreg); and subset III includes CD45RA-Foxp3lo/CD25lo non-Treg (16). Functionally, rTreg possess a certain degree of immunosuppressive capacity and express markers of naive cells such as CCR7 and CD62L. They can differentiate into eTreg upon antigen stimulation. Subset of eTreg exhibit stronger proliferation and immunosuppressive abilities but are more prone to apoptosis. Non-Treg, which were previously considered as cells secreting pro-inflammatory cytokines such as IFN-γ and IL-17 without immunosuppressive capabilities, were found to exhibit significant functional heterogeneity by Cuadrado et al. using mass spectrometry. CD127+ non-Treg and CD127- non-Treg displayed characteristics of conventional T cells and eTreg, respectively. Furthermore, the CD127- subset can be further subdivided based on CD49d and CCR4 expression, with CD127-CCR4+CD49d- cells showing high levels of IL-2 expression, while CD127-CCR4-CD49d+ cells exhibit elevated levels of IFN-γ and IL-17 (18).
In addition to the classification proposed by Sakaguchi et al., Halim et al. categorized CD4+Treg into four distinct subtypes, termed Th-like Treg, based on their cytokine secretion profiles and transcription factor expression in 2017. These subtypes include Th1-like Treg (CCR4+CCR6-CXCR3+, primarily secreting IFN-γ and TNF-α), Th2-like Treg (CCR4+CCR6-CXCR3-, primarily secreting IL-2, IL-4, IL-5, and IL-13), Th17-like Treg (CCR4+CCR6+CXCR3-, primarily secreting IL-17A/IL-17F), and Th1/17-like Treg (CCR4+CCR6+CXCR3+, secreting both IFN-γ and IL-17A without significant statistical differences compared to other subsets) (17). Each Th-like Treg subset exhibited transcription factor expression and cytokine secretion patterns similar to their corresponding Th cell counterparts. Furthermore, all Th-like Treg subtypes demonstrated immunosuppressive capabilities. However, this classification did not investigate the stability of Foxp3 expression in different subtypes, leading to inconsistent conclusions in various studies utilizing this classification system.
Besides to the aforementioned common classifications, the research conducted by Bluestone et al. revealed a stronger correlation between CD127 and Foxp3 expression levels compared to CD25. Furthermore, the suppressive function of the CD4+CD127lo/- subset was found to be superior to that of the CD4+CD25+ subset (19). Currently, the phenotype of CD4+CD25+CD127lo/- is widely used for isolation of CD4+Treg.
3.2 Foxp3 stability and the function of CD4+Treg
Current evidence suggests that stable expression of Foxp3 is closely associated with the immunoregulatory function of CD4+Treg. Therefore, the main factors regulating Foxp3 stability may be crucial for the heterogeneity of CD4+Treg function, such as local inflammatory cytokine stimulation or epigenetic regulation.
Under stimulation from the inflammatory microenvironment, the expression of Foxp3 in some cells may be affected, leading to the loss of negative immune regulatory function. Early in vitro studies have shown that CD4+CD25- T cells can transiently express CD25 and Foxp3 upon IL-2 or TGF-β stimulation, but these T cells do not possess immunosuppressive abilities (20). Recent research by Yi et al. has found that, under the stimulation of the inflammatory cytokine IL-6, TIGIT-positive CD4+Treg can stably express Foxp3 and maintain immunosuppressive function, while TIGIT-negative unstable cells lose Foxp3 expression and transition into an effector T cell phenotype (21). These results suggest that CD4+Treg with stable Foxp3 expression may possess immune regulatory capabilities, while cells with unstable Foxp3 expression may transition into other types of T cells under microenvironmental influences.
In recent years, the role of epigenetic regulation in Foxp3 expression has become a new research focus, with DNA methylation being the most common modification. The gene Foxp3 consists of 11 coding exons, 3 non-coding exons, and 10 introns. Within this gene region, there are 3 conserved non-coding sequences (CNS), with CNS2 containing numerous cytidine-phosphate-guanine (CpG) sites. These sites are methylated in effector T cells but exhibit demethylation in CD4+Treg cells (22), thus being referred to as the Treg-specific demethylated region (TSDR). Sakaguchi et al. previously discovered that there are differences in the methylation levels of the TSDR at the Foxp3 locus. TSDR in rTreg and eTreg exhibited demethylation, while TSDR in non-Treg had a higher degree of methylation, suggesting that Foxp3 expression is more stable in rTreg and eTreg (16). Additionally, a recent study found that the CD28-PKC-NF-κB signaling pathway inhibits demethylation in the CNS2 region of peripheral CD4+Treg, causing unstable Foxp3 expression in pTreg cells and subsequently affecting their immunosuppressive function (23). Furthermore, Ohkura et al. discovered a close relationship between single nucleotide polymorphisms in the epigenetic regulatory region of CD4+Treg and susceptibility to autoimmune diseases (24). Apart from CNS2, two other CNS regions have been shown to play important roles in stable Foxp3 expression and immune tolerance: CNS1, located in the promoter region of the Foxp3 gene, was found to be associated with pTreg differentiation and maintenance of fetal immune tolerance (25); and CNS3 mediates the clonal expansion of the CD4+Treg receptor repertoire, thereby controlling excessive immune responses of autoreactive T cells and maintaining immune homeostasis (26). Additionally, research by Kitagawa et al. found that the transcription factor Satb1 can bind to a CD4+Treg-specific super-enhancer and a newly discovered conserved non-coding region upstream of the Foxp3 promoter prior to CD4+Treg activation, promoting the development of CD4+Treg. This newly discovered region has been named CNS0 (27).
In addition to methylation, acetylation and histone modifications have also been shown to be associated with the stable expression of Foxp3. During the acetylation process, histone acetyltransferases (HATs) and histone deacetylases (HDACs) influence Foxp3 acetylation levels from different directions. Two studies by Li and Tao demonstrated that HAT TIP60 and class II HDACs (HDAC7 and HDAC9) can form complexes with Foxp3, regulating the immunosuppressive capacity of CD4+Treg (28, 29). Other studies further investigated the impact of acetylation on CD4+Treg function in specific diseases. Su et al. found that rheumatoid arthritis patients have a TIP60 functional deficiency, leading to instability of Foxp3 expression and impaired suppressive function of CD4+Treg (30). Jiang et al. discovered that the acetyltransferase p300/CBP-associated factor (PCAF) can acetylate Foxp3 and enhance its transcriptional activity, thereby promoting the suppressive function of CD4+Treg (31). These studies suggest that acetylation and deacetylation processes play a critical role in regulating the stability and function of Foxp3 in CD4+Treg. In the field of histone modifications, substantial progress has been made in understanding the role of Enhancer of zeste homolog 2 (EZH2). EZH2 is a catalytic enzyme of the polycomb repressive complex 2 (PRC2) responsible for the methylation of histone H3 lysine 27 (H3K27) at the Foxp3 locus, leading to the formation of H3K27me3 histone modification. Studies by DuPage et al. have demonstrated that Ezh2 is involved in stabilizing the expression of Foxp3 in a mice model, selective deletion of Ezh2 in CD4+Treg resulted in the development of autoimmune diseases and accompanied by reduced stability of CD4+Treg (32). This study suggests that Ezh2 may play a crucial role in maintaining Treg cell function. Goswami et al. found that selective deletion of Ezh2 in CD4+Treg delays tumor progression in mice, while the use of Ezh2 inhibitors in wild-type mice enhances the function of cytotoxic T lymphocytes (CTLs) and promotes the efficacy of anti-CTLA-4 antibodies, thus inhibiting tumor progression (33). These findings indicate that the histone modifications mediated by EZH2 may represent another important mechanism regulating the function of CD4+Treg.
4 Heterogeneity of CD4+Treg in the TME
Infiltration of CD4+Tregs in TME suppresses local anti-tumor immune responses and is associated with poor prognosis in various types of cancer. The heterogeneity of CD4+Treg offer diverse mechanisms that contribute to the modulation of anti-tumor immune responses and facilitate immune evasion. Therefore, accurate characterization of different CD4+Treg subsets in TME is crucial for gaining insights into the formation of the immunosuppressive TME as well as for improving patient prognosis.
The classification of Tregs subsets proposed by Sakaguchi et al. have been widely used to study the heterogeneity of CD4+Treg in TME. Saito et al., based on the classification, divided colorectal cancer into two types: Type A, characterized by predominant infiltration of eTreg and correlated with poor prognosis, and Type B, characterized by predominant infiltration of non-Treg and correlated with better prognosis (34). Similarly, studies on different types of tumors have consistently shown that eTreg are the major population in TME and correlated with poor prognosis (35, 36). Wang et al. found a high overlap between the phenotypes and TCR repertoire of tumor-infiltrating CD4+Treg and peripheral eTreg in patients with breast cancer. They proposed a cytokine signal index (CSI) based on the responsiveness of various peripheral CD4+Treg subsets to four cytokines, the CSI of eTreg could effectively predict patient prognosis and relapse (37).
Limited research focused on Th-like Treg in TME. Mizukami et al. have shown that tumor-infiltrating CD4+Treg express CCR4 in lymphoma and gastric cancer (38). Halim et al. demonstrated that CCR4+Treg in the TME predominantly exhibit Th2-like characteristics, they also discovered a significant elevation of CCR8 expression on Th2-like Treg (17), consistent with the single-cell sequencing results reported by De Simone (39). Van Damme et al. also confirmed that tumor-infiltrating CCR8+Treg was highly activated and immunosuppressive in both human and mouse tumors. Selective depletion of this subset resulted in increased responsiveness to immunotherapy (40). Downs-Canner et al. discovered that Th17 cells can differentiate into Th17-like Treg in ovarian cancer, thereby suppressing immune responses (41).
The emergence of single-cell RNA sequencing technology has advanced our understanding of the functional subgroups of CD4+Treg in tissue microenvironments, particularly in tumor-infiltrating sites. Several studies analyzed the immune cell infiltration in head and neck squamous cell carcinoma (HNSCC), non-small cell lung carcinoma (NSCLC), and breast cancer, respectively. Cillo et al. categorized CD4+Treg infiltrating HNSCC into six subsets, with subsets 2 and 4 representing early-stage CD4+Treg with upregulated IFN-related signaling pathways, while subsets 3 and 6 represented late-stage CD4+Treg with upregulated TNF-related signaling pathways (42). Guo et al. observed high expression of Foxp3, IL-2RA, and IKZF2 in tumor-infiltrating CD4+Treg, as well as increased expression of CTLA4, TIGIT, and TNFRSF9 in tumor-infiltrating CD4+Treg in NSCLC specimens. Further investigation revealed that the TNFRSF9+subset within tumor-infiltrating CD4+Treg exhibited potent immunosuppressive abilities (43). Azizi et al. identified five subsets of tumor-infiltrating CD4+Treg in samples of breast cancer and analyzed the expression of immune checkpoint-related genes within each subset. The results showed that all subsets exhibited high expression of CTLA4 and GITR, with three subsets showing elevated expression of TIGIT (6). The widespread adoption of single-cell sequencing technology enables a more comprehensive analysis to the expression of CD4+Treg-related genes in tissue microenvironment, facilitating the identification of biomarkers that effectively represent CD4+Treg functionality. However, these findings still require further validation through functional experiments.
5 Clinical trials targeting CD4+Treg in cancer
Targeting CD4+Treg has the potential to become an important approach in immunotherapy as tumor cells in TME can evade immune surveillance by recruiting immune-suppressive cells such as CD4+Treg. Considering the functional subgroups of CD4+Treg, the primary challenge lies in selectively targeting immunosuppressive subsets while minimizing the impact on other subpopulations, thus reducing the potential for undesirable autoimmune reactions. Early clinical trials primarily targeted CD25 as a marker for Treg, drugs such as denileukin and daclizumab have been reported to reduce the proportion of CD4+CD25+Treg and enhance the response rate to tumor vaccine (44). However, CD25 is not a specific marker to CD4+Treg, and the combination of CD25-targeted therapy with tumor vaccines can suppress the generation of tumor-specific T cells (45), limiting the application of CD25 antibodies.
CCR4 is predominantly expressed on the surface of eTreg (46), and previous studies indicated that blocking CCR4 can inhibit the accumulation of eTreg in TME (35). Therefore, targeting CCR4 has shown certain efficacy. Mogamulizumab, a monoclonal antibody targeting CCR4, has been approved by the FDA for the treatment of cutaneous T-cell lymphoma (47). A clinical trial combined mogamulizumab with PD-1 inhibitor (NCT02476123) found an objective response rate of 12% in various types of locally advanced/metastatic solid tumors. The combination significantly reduced the proportion of eTreg in both peripheral blood and tumor-infiltrating lymphocytes (TIL). Moreover, the combination therapy increased the proportion of CD8+T cells in TME regardless of tumor response, indicating the potential of CCR4 targeting therapy (48).
Inhibition of immune checkpoint receptors including CTLA-4, TIM-3, and LAG-3 had shown promising effects in clinical trials or pre-clinical studies. CTLA-4 is a co-inhibitory molecule expressed on the surface of CD4+Treg and negatively regulates T cell activation (49). Studies in patients with metastatic melanoma have shown that the CTLA-4 antibody ipilimumab can prolong overall survival, but it is also associated with severe adverse events in 10% to 15% of patients (50). Recent clinical trials have found that PD-1 antibody has a lower incidence of adverse events compared to ipilimumab(KEYNOTE-006, NCT01866319) (51), and combination of nivolumab and ipilimumab (CheckMate 067, NCT01844505) can further extend overall survival in patients with melanoma (52). The data from this trial suggest that CTLA-4 antibodies may potentially serve as adjunctive therapy to enhance the efficacy of PD-1 antibodies. TIM-3 is expressed on innate immune cells. Although TIM-3 is not a specific marker on the surface of CD4+Treg, recent studies have identified TIM-3+CD4+Treg within TME played a crucial inhibitory role by inducing exhaustion in effector T cells and TIM-3+CD4+Treg might be a potential therapeutic target (53). Combination therapy targeting TIM-3 and PD-1 mAb has already been tested in several solid tumor, and an ongoing Phase 1 clinical trial is currently evaluating the dosage and anti-tumor efficacy of a humanized anti-TIM-3 antibody in advanced solid tumors (NCT02817633) (54). LAG-3 is expressed on activated T cells including CD4+Treg (55) and LAG-3 can hinder the proliferation of effector T cells and dendritic cells, while promoting the differentiation of eTreg (56). Currently, there are two clinical trials investigating the efficacy of anti-LAG-3 alone or in combination with PD-L1 antibody in patients with advanced solid tumors (NCT01968109, NCT03156114) (57). OX40 is a costimulatory molecule expressed mostly on activated effector T cells and nTreg (58). Pre-clinical studies have demonstrated the immunosuppressive function of OX40+Treg and anti-OX40 could improve tumor control in mouse models (59, 60). A recent phase I clinical in HNSCC patients (NCT02274155) demonstrated that anti-OX40 administration was well tolerated and increased the infiltration of activated CD4+ and CD8+T cells (61). This data indicates the potential clinical utility of anti-OX-40, but further clinical trials are needed to confirm its efficacy.
Above clinical trials aim to reverse the immunosuppressive state of TME by inhibiting immunosuppressive cells such as CD4+Treg. Optimization of combination therapies that effectively target CD4+Treg while enhancing anti-tumor immune responses represents a promising avenue for the improved treatment of cancer. However, it is crucial to take into account the pathological subtype of cancer and adopt a targeted approach towards suppressive CD4+Treg, rather than indiscriminately depleting all Treg or other effector T cells. These considerations highlight the importance of further evaluation when developing novel therapeutic strategies in this context.
6 Conclusion
Although CD4+Treg represent a small proportion of lymphocytes, their immunoregulatory role in the TME is crucial. Effectively distinguishing the distinct functional heterogeneity of CD4+Treg subsets is a pressing challenge. Currently, the subtypes proposed by Sakaguchi et al. is the most widely used and exhibits good consistency in research conclusions. However, the subtypes of Th-like Treg reflects the plasticity of CD4+Treg in TME and still faces inconsistent findings across different studies. Furthermore, numerous clinical trials targeting CD4+Treg have been conducted, highlighting the pressing need to accurately inhibit specific functional Treg subsets involved in immunoregulation. In future research, rational subgroup analysis based on different surface markers is essential for a better understanding of the role of CD4+Treg in the TME.
Author contributions
HL: Conceptualization, Project administration, Writing – original draft. YX: Conceptualization, Formal analysis, Writing – review & editing. CL: Conceptualization, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This study was supported by grants from the National Natural Science Foundation of China (82271143 to YX), and the Startup Fund for Talents of Fujian Province (YJRC4120 to HL).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Itoh M, Takahashi T, Sakaguchi N, Kuniyasu Y, Shimizu J, Otsuka F, et al. Thymus and autoimmunity: production of CD25+CD4+ naturally anergic and suppressive T cells as a key function of the thymus in maintaining immunologic self-tolerance. J Immunol (1999) 162(9):5317–26. doi: 10.4049/jimmunol.162.9.5317
2. Sakaguchi S, Yamaguchi T, Nomura T, Ono M. Regulatory T cells and immune tolerance. Cell (2008) 133(5):775–87. doi: 10.1016/j.cell.2008.05.009
3. Fridman WH, Pages F, Sautes-Fridman C, Galon J. The immune contexture in human tumours: impact on clinical outcome. Nat Rev Cancer (2012) 12(4):298–306. doi: 10.1038/nrc3245
4. Guo C, Liu Q, Zong D, Zhang W, Zuo Z, Yu Q, et al. Single-cell transcriptome profiling and chromatin accessibility reveal an exhausted regulatory CD4+ T cell subset in systemic lupus erythematosus. Cell Rep (2022) 41(6):111606. doi: 10.1016/j.celrep.2022.111606
5. Miragaia RJ, Gomes T, Chomka A, Jardine L, Riedel A, Hegazy AN, et al. Single-cell transcriptomics of regulatory T cells reveals trajectories of tissue adaptation. Immunity (2019) 50(2):493–504.e7. doi: 10.1016/j.immuni.2019.01.001
6. Azizi E, Carr AJ, Plitas G, Cornish AE, Konopacki C, Prabhakaran S, et al. Single-cell map of diverse immune phenotypes in the breast tumor microenvironment. Cell (2018) 174(5):1293–308.e36. doi: 10.1016/j.cell.2018.05.060
7. Owen DL, Mahmud SA, Sjaastad LE, Williams JB, Spanier JA, Simeonov DR, et al. Thymic regulatory T cells arise via two distinct developmental programs. Nat Immunol (2019) 20(2):195–205. doi: 10.1038/s41590-018-0289-6
8. Chen W, Jin W, Hardegen N, Lei KJ, Li L, Marinos N, et al. Conversion of peripheral CD4+CD25- naive T cells to CD4+CD25+ regulatory T cells by TGF-beta induction of transcription factor Foxp3. J Exp Med (2003) 198(12):1875–86. doi: 10.1084/jem.20030152
9. Roncarolo MG, Gregori S, Bacchetta R, Battaglia M, Gagliani N. The biology of T regulatory type 1 cells and their therapeutic application in immune-mediated diseases. Immunity (2018) 49(6):1004–19. doi: 10.1016/j.immuni.2018.12.001
10. Chen Y, Kuchroo VK, Inobe J, Hafler DA, Weiner HL. Regulatory T cell clones induced by oral tolerance: suppression of autoimmune encephalomyelitis. Science (1994) 265(5176):1237–40. doi: 10.1126/science.7520605
11. Collison LW, Chaturvedi V, Henderson AL, Giacomin PR, Guy C, Bankoti J, et al. IL-35-mediated induction of a potent regulatory T cell population. Nat Immunol (2010) 11(12):1093–101. doi: 10.1038/ni.1952
12. Sugimoto N, Oida T, Hirota K, Nakamura K, Nomura T, Uchiyama T, et al. Foxp3-dependent and -independent molecules specific for CD25+CD4+ natural regulatory T cells revealed by DNA microarray analysis. Int Immunol (2006) 18(8):1197–209. doi: 10.1093/intimm/dxl060
13. Yadav M, Louvet C, Davini D, Gardner JM, Martinez-Llordella M, Bailey-Bucktrout S, et al. Neuropilin-1 distinguishes natural and inducible regulatory T cells among regulatory T cell subsets in vivo. J Exp Med (2012) 209(10):1713–S19. doi: 10.1084/jem.20120822
14. Weiner HL, da Cunha AP, Quintana F, Wu H. Oral tolerance. Immunol Rev (2011) 241(1):241–59. doi: 10.1111/j.1600-065X.2011.01017.x
15. Hsu L-H, Li K-P, Chu K-H, Chiang B-L. A B-1a cell subset induces Foxp3(-) T cells with regulatory activity through an IL-10-independent pathway. Cell Mol Immunol (2015) 12(3):354–65. doi: 10.1038/cmi.2014.56
16. Miyara M, Yoshioka Y, Kitoh A, Shima T, Wing K, Niwa A, et al. Functional delineation and differentiation dynamics of human CD4+ T cells expressing the FoxP3 transcription factor. Immunity (2009) 30(6):899–911. doi: 10.1016/j.immuni.2009.03.019
17. Halim L, Romano M, McGregor R, Correa I, Pavlidis P, Grageda N, et al. An atlas of human regulatory T helper-like cells reveals features of Th2-like tregs that support a tumorigenic environment. Cell Rep (2017) 20(3):757–70. doi: 10.1016/j.celrep.2017.06.079
18. Cuadrado E, van den Biggelaar M, de Kivit S, Chen YY, Slot M, Doubal I, et al. Proteomic analyses of human regulatory T cells reveal adaptations in signaling pathways that protect cellular identity. Immunity (2018) 48(5):1046–59.e6. doi: 10.1016/j.immuni.2018.04.008
19. Liu W, Putnam AL, Xu-Yu Z, Szot GL, Lee MR, Zhu S, et al. CD127 expression inversely correlates with FoxP3 and suppressive function of human CD4+ T reg cells. J Exp Med (2006) 203(7):1701–11. doi: 10.1084/jem.20060772
20. Wang J, Ioan-Facsinay A, van der Voort EI, Huizinga TW, Toes RE. Transient expression of FOXP3 in human activated nonregulatory CD4+ T cells. Eur J Immunol (2007) 37(1):129–38. doi: 10.1002/eji.200636435
21. Yi G, Zhao Y, Xie F, Zhu F, Wan Z, Wang J, et al. Single-cell RNA-seq unveils critical regulators of human FOXP3+ regulatory T cell stability. Sci Bull (Beijing) (2020) 65(13):1114–24. doi: 10.1016/j.scib.2020.01.002
22. Janson PC, Winerdal ME, Marits P, Thorn M, Ohlsson R, Winqvist O. FOXP3 promoter demethylation reveals the committed Treg population in humans. PloS One (2008) 3(2):e1612. doi: 10.1371/journal.pone.0001612
23. Mikami N, Kawakami R, Chen KY, Sugimoto A, Ohkura N, Sakaguchi S. Epigenetic conversion of conventional T cells into regulatory T cells by CD28 signal deprivation. Proc Natl Acad Sci USA (2020) 117:12258–68. doi: 10.1073/pnas.1922600117
24. Ohkura N, Yasumizu Y, Kitagawa Y, Tanaka A, Nakamura Y, Motooka D, et al. Regulatory T cell-specific epigenomic region variants are a key determinant of susceptibility to common autoimmune diseases. Immunity (2020) 52(6):1119–32.e4. doi: 10.1016/j.immuni.2020.04.006
25. Samstein RM, Josefowicz SZ, Arvey A, Treuting PM, Rudensky AY. Extrathymic generation of regulatory T cells in placental mammals mitigates maternal-fetal conflict. Cell (2012) 150(1):29–38. doi: 10.1016/j.cell.2012.05.031
26. Feng Y, van der Veeken J, Shugay M, Putintseva EV, Osmanbeyoglu HU, Dikiy S, et al. A mechanism for expansion of regulatory T-cell repertoire and its role in self-tolerance. Nature (2015) 528(7580):132–6. doi: 10.1038/nature16141
27. Kitagawa Y, Ohkura N, Kidani Y, Vandenbon A, Hirota K, Kawakami R, et al. Guidance of regulatory T cell development by Satb1-dependent super-enhancer establishment. Nat Immunol (2017) 18(2):173–83. doi: 10.1038/ni.3646
28. Li B, Samanta A, Song X, Iacono KT, Bembas K, Tao R, et al. FOXP3 interactions with histone acetyltransferase and class II histone deacetylases are required for repression. Proc Natl Acad Sci USA (2007) 104(11):4571–76. doi: 10.1073/pnas.0700298104
29. Tao R, de Zoeten EF, Ozkaynak E, Chen C, Wang L, Porrett PM, et al. Deacetylase inhibition promotes the generation and function of regulatory T cells. Nat Med (2007) 13(11):1299–307. doi: 10.1038/nm1652
30. Su Q, Jing J, Li W, Ma J, Zhang X, Wang Z, et al. Impaired Tip60-mediated Foxp3 acetylation attenuates regulatory T cell development in rheumatoid arthritis. J Autoimmun (2019) 100:27–39. doi: 10.1016/j.jaut.2019.02.007
31. Jiang H, Xin S, Yan Y, Lun Y, Yang X, Zhang J. Abnormal acetylation of FOXP3 regulated by SIRT-1 induces Treg functional deficiency in patients with abdominal aortic aneurysms. Atherosclerosis (2018) 271:182–92. doi: 10.1016/j.atherosclerosis.2018.02.001
32. DuPage M, Chopra G, Quiros J, Rosenthal WL, Morar MM, Holohan D, et al. The chromatin-modifying enzyme Ezh2 is critical for the maintenance of regulatory T cell identity after activation. Immunity (2015) 42(2):227–38. doi: 10.1016/j.immuni.2015.01.007
33. Goswami S, Apostolou I, Zhang J, Skepner J, Anandhan S, Zhang X, et al. Modulation of EZH2 expression in T cells improves efficacy of anti-CTLA-4 therapy. J Clin Invest (2018) 128(9):3813–8. doi: 10.1172/JCI99760
34. Saito T, Nishikawa H, Wada H, Nagano Y, Sugiyama D, Atarashi K, et al. Two FOXP3+CD4+ T cell subpopulations distinctly control the prognosis of colorectal cancers. Nat Med (2016) 22:679. doi: 10.1038/nm.4086
35. Sun W, Li WJ, Wei FQ, Wong TS, Lei WB, Zhu XL, et al. Blockade of MCP-1/CCR4 signaling-induced recruitment of activated regulatory cells evokes an antitumor immune response in head and neck squamous cell carcinoma. Oncotarget (2016) 7(25):37714–27. doi: 10.18632/oncotarget.9265
36. van Beek AA, Zhou G, Doukas M, Boor PPC, Noordam L, Mancham S, et al. GITR ligation enhances functionality of tumor-infiltrating T cells in hepatocellular carcinoma. Int J Cancer (2019) 145(4):1111–24. doi: 10.1002/ijc.32181
37. Wang L, Simons DL, Lu X, Tu TY, Solomon S, Wang R, et al. Connecting blood and intratumoral Treg cell activity in predicting future relapse in breast cancer. Nat Immunol (2019) 20(9):1220–30. doi: 10.1038/s41590-019-0429-7
38. Mizukami Y, Kono K, Kawaguchi Y, Akaike H, Kamimura K, Sugai H, et al. CCL17 and CCL22 chemokines within tumor microenvironment are related to accumulation of Foxp3+ regulatory T cells in gastric cancer. Int J Cancer (2008) 122(10):2286–93. doi: 10.1002/ijc.23392
39. De Simone M, Arrigoni A, Rossetti G, Gruarin P, Ranzani V, Politano C, et al. Transcriptional landscape of human tissue lymphocytes unveils uniqueness of tumor-infiltrating T regulatory cells. Immunity (2016) 45(5):1135–47. doi: 10.1016/j.immuni.2016.10.021
40. Van Damme H, Dombrecht B, Kiss M, Roose H, Allen E, Van Overmeire E, et al. Therapeutic depletion of CCR8(+) tumor-infiltrating regulatory T cells elicits antitumor immunity and synergizes with anti-PD-1 therapy. J immunotherapy Cancer (2021) 9(2):e001749. doi: 10.1136/jitc-2020-001749
41. Downs-Canner S, Berkey S, Delgoffe GM, Edwards RP, Curiel T, Odunsi K, et al. Suppressive IL-17A(+)Foxp3(+) and ex-Th17 IL-17A(neg)Foxp3(+) Treg cells are a source of tumour-associated Treg cells. Nat Commun (2017) 8:14649. doi: 10.1038/ncomms14649
42. Cillo AR, Kürten CHL, Tabib T, Qi Z, Onkar S, Wang T, et al. Immune landscape of viral- and carcinogen-driven head and neck cancer. Immunity (2020) 52(1):183–99.e9. doi: 10.1016/j.immuni.2019.11.014
43. Guo X, Zhang Y, Zheng L, Zheng C, Song J, Zhang Q, et al. Global characterization of T cells in non-small-cell lung cancer by single-cell sequencing. Nat Med (2018) 24(7):978–85. doi: 10.1038/s41591-018-0045-3
44. Rech AJ, Mick R, Martin S, Recio A, Aqui NA, Powell DJ Jr., et al. CD25 blockade depletes and selectively reprograms regulatory T cells in concert with immunotherapy in cancer patients. Sci Transl Med (2012) 4(134):134ra62. doi: 10.1126/scitranslmed.3003330
45. Jacobs JF, Punt CJ, Lesterhuis WJ, Sutmuller RP, Brouwer HM, Scharenborg NM, et al. Dendritic cell vaccination in combination with anti-CD25 monoclonal antibody treatment: a phase I/II study in metastatic melanoma patients. Clin Cancer Res (2010) 16(20):5067–78. doi: 10.1158/1078-0432.CCR-10-1757
46. Sugiyama D, Nishikawa H, Maeda Y, Nishioka M, Tanemura A, Katayama I, et al. Anti-CCR4 mAb selectively depletes effector-type FoxP3+CD4+ regulatory T cells, evoking antitumor immune responses in humans. Proc Natl Acad Sci USA (2013) 110(44):17945–50. doi: 10.1073/pnas.1316796110
47. Kasamon YL, Chen H, de Claro RA, Nie L, Ye J, Blumenthal GM, et al. FDA approval summary: mogamulizumab-kpkc for mycosis fungoides and sezary syndrome. Clin Cancer Res (2019) 25(24):7275-80. doi: 10.1158/1078-0432.CCR-19-2030
48. Doi T, Muro K, Ishii H, Kato T, Tsushima T, Takenoyama M, et al. A phase 1 study of the anti-CC chemokine receptor 4 antibody, mogamulizumab, in combination with nivolumab in patients with advanced or metastatic solid tumors. Clin Cancer Res (2019) 25(22):6614-22. doi: 10.1158/1078-0432.CCR-19-1090
49. Takahashi T, Tagami T, Yamazaki S, Uede T, Shimizu J, Sakaguchi N, et al. Immunologic self-tolerance maintained by CD25(+)CD4(+) regulatory T cells constitutively expressing cytotoxic T lymphocyte-associated antigen 4. J Exp Med (2000) 192(2):303–10. doi: 10.1084/jem.192.2.303
50. Hodi FS, O'Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, et al. Improved survival with ipilimumab in patients with metastatic melanoma. New Engl J Med (2010) 363(8):711–23. doi: 10.1056/NEJMoa1003466
51. Robert C, Ribas A, Schachter J, Arance A, Grob J-J, Mortier L, et al. Pembrolizumab versus ipilimumab in advanced melanoma (KEYNOTE-006): post-hoc 5-year results from an open-label, multicentre, randomised, controlled, phase 3 study. Lancet Oncol (2019) 20(9):1239–51. doi: 10.1016/S1470-2045(19)30388-2
52. Wolchok JD, Chiarion-Sileni V, Gonzalez R, Grob J-J, Rutkowski P, Lao CD, et al. . Long-Term Outcomes With Nivolumab Plus Ipilimumab or Nivolumab Alone Versus Ipilimumab in Patients With Advanced Melanoma. J Clin Oncol (2022) 40(2):127-137. doi: 10.1200/JCO.21.02229
53. Sakuishi K, Ngiow SF, Sullivan JM, Teng MW, Kuchroo VK, Smyth MJ, et al. TIM3(+)FOXP3(+) regulatory T cells are tissue-specific promoters of T-cell dysfunction in cancer. Oncoimmunology (2013) 2(4):e23849. doi: 10.4161/onci.23849
54. Kim HR, Gridelli C, Kapur D, Tufman A, Felip E, Velcheti V, et al. P1.11-01 cobolimab with dostarlimab and docetaxel in patients with advanced non-small cell lung cancer (NSCLC): COSTAR lung. J Thorac Oncol (2022) 17(Suppl 9):S109–S10. doi: 10.1016/j.jtho.2022.07.183
55. Camisaschi C, Casati C, Rini F, Perego M, De Filippo A, Triebel F, et al. LAG-3 expression defines a subset of CD4(+)CD25(high)Foxp3(+) regulatory T cells that are expanded at tumor sites. J Immunol (2010) 184(11):6545–51. doi: 10.4049/jimmunol.0903879
56. Maruhashi T, Okazaki IM, Sugiura D, Takahashi S, Maeda TK, Shimizu K, et al. LAG-3 inhibits the activation of CD4(+) T cells that recognize stable pMHCII through its conformation-dependent recognition of pMHCII. Nat Immunol (2018) 19(12):1415–26. doi: 10.1038/s41590-018-0217-9
57. Zettl M, Wurm M, Schaaf O, Mostböck S, Tirapu I, Apfler I, et al. Combination of two novel blocking antibodies, anti-PD-1 antibody ezabenlimab (BI 754091) and anti-LAG-3 antibody BI 754111, leads to increased immune cell responses. Oncoimmunology (2022) 11(1):2080328. doi: 10.1080/2162402X.2022.2080328
58. Yokouchi H, Yamazaki K, Chamoto K, Kikuchi E, Shinagawa N, Oizumi S, et al. Anti-OX40 monoclonal antibody therapy in combination with radiotherapy results in therapeutic antitumor immunity to murine lung cancer. Cancer Sci (2008) 99(2):361–7. doi: 10.1111/j.1349-7006.2007.00664.x
59. Lai C, August S, Albibas A, Behar R, Cho SY, Polak ME, et al. OX40+ Regulatory T cells in cutaneous squamous cell carcinoma suppress effector T-cell responses and associate with metastatic potential. Clin Cancer Res (2016) 22(16):4236–48. doi: 10.1158/1078-0432.CCR-15-2614
60. Niknam S, Barsoumian HB, Schoenhals JE, Jackson HL, Yanamandra N, Caetano MS, et al. Radiation followed by OX40 stimulation drives local and abscopal antitumor effects in an anti-PD1-resistant lung tumor model. Clin Cancer Res (2018) 24(22):5735–43. doi: 10.1158/1078-0432.CCR-17-3279
61. Duhen R, Ballesteros-Merino C, Frye AK, Tran E, Rajamanickam V, Chang SC, et al. Neoadjuvant anti-OX40 (MEDI6469) therapy in patients with head and neck squamous cell carcinoma activates and expands antigen-specific tumor-infiltrating T cells. Nat Commun (2021) 12(1):1047. doi: 10.1038/s41467-021-21383-1
Keywords: functional subtypes, tumor microenvironment, clinical trials, regulatory T cells, forkhead box protein 3
Citation: Lin H, Xu Y and Lin C (2024) Heterogeneity and subtypes of CD4+ regulatory T cells: implications for tumor therapy. Front. Immunol. 14:1291796. doi: 10.3389/fimmu.2023.1291796
Received: 10 September 2023; Accepted: 13 December 2023;
Published: 05 January 2024.
Edited by:
Mehdi Benamar, Harvard Medical School, United StatesReviewed by:
Haopeng Wang, ShanghaiTech University, ChinaCopyright © 2024 Lin, Xu and Lin. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yuanteng Xu, eHl0OTczQDE2My5jb20=; Chang Lin, bGluYzMwMUBzaW5hLmNvbQ==
 Hanqing Lin
Hanqing Lin Yuanteng Xu
Yuanteng Xu Chang Lin1,2*
Chang Lin1,2*