- 1Department of Traditional Chinese Medicine, Tangdu Hospital, Air Force Medical University (Fourth Military Medical University), Xi’an, China
- 2College of Health, Dongguan Polytechnic, Dongguan, China
- 3The Second Affiliated Hospital of Guangzhou University of Chinese Medicine, Guangzhou, China
- 4Department of Postgraduate Work, Xi’an Medical University, Xi’an, China
- 5Department of Neurosurgery, General Hospital of Northern Theater Command, Shenyang, China
- 6Department of Radiation Protection Medicine, Faculty of Preventive Medicine, Air Force Medical University (Fourth Military Medical University), Xi’an, China
- 7Shaanxi Key Laboratory of Free Radical and Medicine, Xi’an, China
- 8Key Laboratory of Integrated Traditional Chinese and Western Medicine Tumor Diagnosis and Treatment in Shaanxi Province, Xi’an, China
- 9Department of Gastroenterology, Tangdu Hospital, Air Force Medical University (Fourth Military Medical University), Xi’an, China
Gliomas are one of the most common primary malignant tumours of the central nervous system (CNS), of which glioblastomas (GBMs) are the most common and destructive type. The glioma tumour microenvironment (TME) has unique characteristics, such as hypoxia, the blood-brain barrier (BBB), reactive oxygen species (ROS) and tumour neovascularization. Therefore, the traditional treatment effect is limited. As cellular oxidative metabolites, ROS not only promote the occurrence and development of gliomas but also affect immune cells in the immune microenvironment. In contrast, either too high or too low ROS levels are detrimental to the survival of glioma cells, which indicates the threshold of ROS. Therefore, an in-depth understanding of the mechanisms of ROS production and scavenging, the threshold of ROS, and the role of ROS in the glioma TME can provide new methods and strategies for glioma treatment. Current methods to increase ROS include photodynamic therapy (PDT), sonodynamic therapy (SDT), and chemodynamic therapy (CDT), etc., and methods to eliminate ROS include the ingestion of antioxidants. Increasing/scavenging ROS is potentially applicable treatment, and further studies will help to provide more effective strategies for glioma treatment.
1 Introduction
Gliomas are the most common primary malignant tumours of the central nervous system (CNS), accounting for approximately 30% of all primary brain and CNS tumours and 80% of malignant brain tumours (1). According to the criteria set by the World Health Organization (WHO), the malignancy of gliomas is divided into grades I-IV, ranging from mild to severe. Glioblastomas (GBMs) are grade IV gliomas and are the most common type. Unfortunately, GBMs are also the most dangerous, with relapses being inevitable even after rigorous treatment (2). Due to the unique characteristics of the glioma tumour microenvironment (TME), such as hypoxia, the blood–brain barrier (BBB), reactive oxygen species (ROS), and tumour neovascularization, treatment often show poor efficacy (3–5). The standard treatment for GBMs is resection followed by radiotherapy and temozolomide (TMZ) chemotherapy, but the median survival of GBM patients is only 14.6 months (6). In addition, the humanized IgG1 monoclonal antibody bevacizumab is also commonly used in the clinical treatment of GBMs (7). According to the available studies, neither TMZ nor bevacizumab is sufficient to treat gliomas. TMZ causes alkylation of genomic DNA at the N7 and O6 sites of guanine and at the N3 site of adenine. When the alkylation lesion at the guanine O6 position is not repaired, it leads to mispairing during DNA replication, which triggers a break in the DNA strand and causes GBM cell death (8–10). However, O6-methylguanine-DNA methyltransferase (MGMT) exists in GBM cells, cleans the alkyl group produced by TMZ and repairs damaged DNA. The presence of MGMT is an important reason for the resistance of GBMs to TMZ (11). Bevacizumab targets a protein called vascular endothelial growth factor-A (VEGF-A) and slows tumour growth and proliferation by preventing tumour angiogenesis, thereby depriving GBM cells of nutrient uptake (7). However, due to tumour heterogeneity and insufficient pharmacokinetics, it is still difficult to prevent GBM recurrence with antiangiogenic therapy (12–14). Therefore, the search for new treatment methods for gliomas has become a hotspot of current research.
ROS are reactive substances produced by oxygen reduction, including hydrogen peroxide (H2O2), organic hydroperoxide (ROOH), singlet oxygen (1O2), ozone (O3), superoxide anion (O2˙‾), hydroxyl radical (OH·), and peroxyl radical (ROO·) (15), etc. Certain levels of ROS are required for cell survival and are involved in cell proliferation and differentiation (16), skeletal muscle contraction (17), immune response (18) and other processes. These physiological effects are based on the regulation of multiple signalling pathways by ROS, such as the nuclear factor-kappaB (NF-κB), phosphatidylinositol 3-kinase (PI3K)/protein kinase B (AKT), and mitogen-activated protein kinases (MAPKs) (19, 20). The normal function of cells also depends on the ROS threshold, which represents the critical point of intracellular ROS levels (15, 21). A level of ROS slightly below the threshold is helpful to maintain normal cell function. However, when ROS persistently accumulate abnormally beyond the threshold, they may cause irreversible oxidative damage to cells or even lead to cell death (21).
In gliomas, appropriate amounts of ROS can activate growth-related signalling pathways, induce DNA mutations, and promote invasion and metastasis (22–24). However, it has been shown that inducing ROS accumulation leads to glioma cell death (25, 26). In contrast, given the critical role of ROS in the cell, the depletion of ROS also makes it difficult for glioma cells to survive (27, 28). Therefore, controlling the level of ROS becomes a potential strategy for glioma treatment. According to the literature, methods to induce massive ROS production include photodynamic therapy (PDT) (29), sonodynamic therapy (SDT) (30) and chemodynamic therapy (CDT) (31). The main approach to ROS reduction is the application of various antioxidants (27, 28, 32). All these methods have the potential to be used to treat gliomas. Therefore, we need to better understand the mechanisms of ROS production and clearance in gliomas, as well as their role in the glioma TME. Meanwhile, the methods based on ROS generation/scavenging also contribute to the prevention and treatment of gliomas.
2 ROS production and antioxidant defence systems
ROS production is caused by exogenous environmental stimuli or endogenous metabolism. Exogenous ROS can be generated by environmental pollutants, such as heavy metals (33), ultraviolet radiation (34), asbestos (35), sulfur dioxide (36), and particulate matter with a diameter of less than 2.5 µm (PM2.5) (37). Endogenous ROS production is mainly dependent on the mitochondrial electron transport chain (ETC) (38) and NADPH oxidases (NOXs) (39). In some cases, peroxisomes (40) and endoplasmic reticulum membranes (41) have also been identified as ROS production sites.
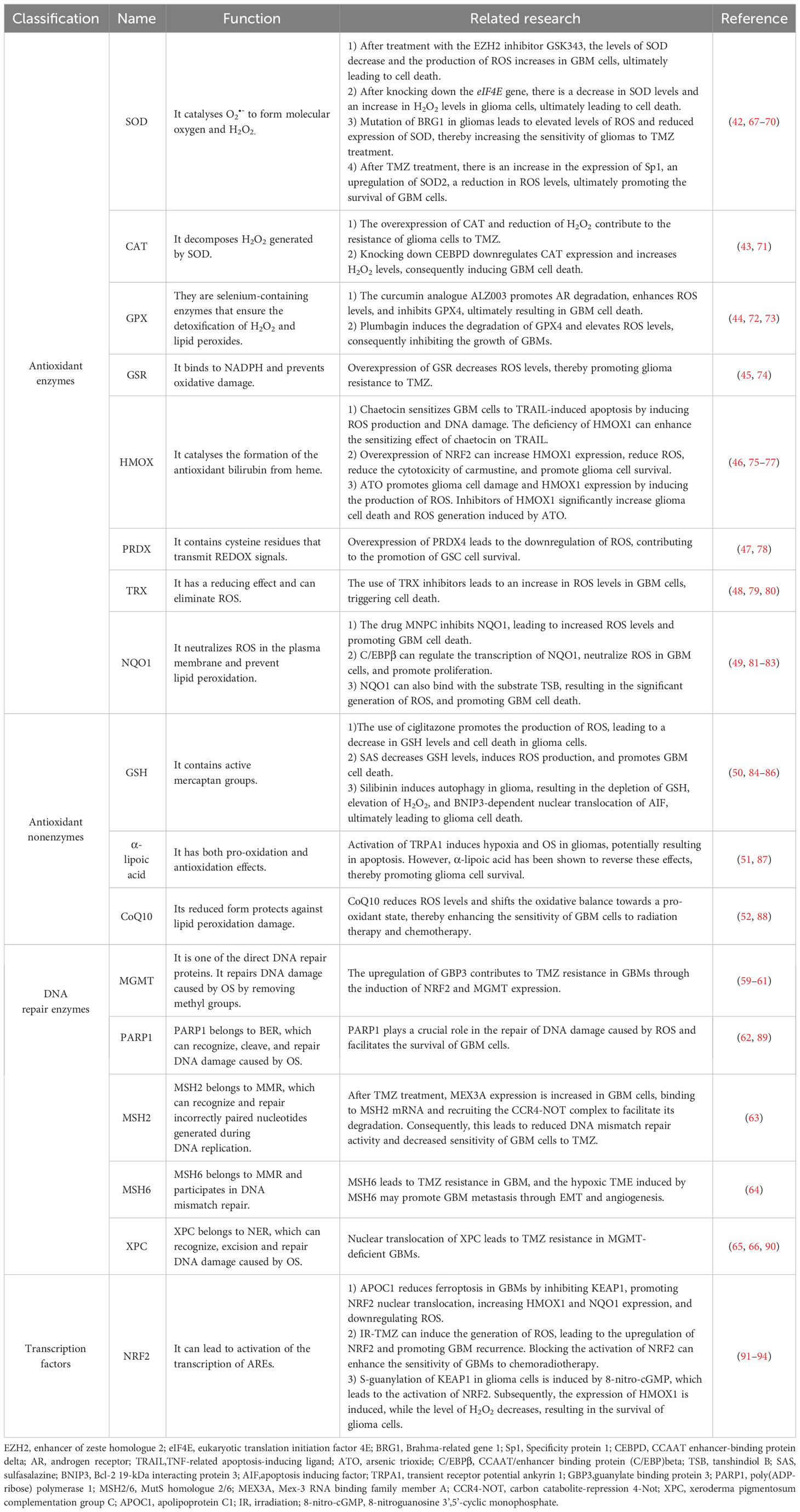
When ROS levels are elevated, glioma cells initiate their own antioxidant defence system in response to oxidative stress (OS). These antioxidant defence systems consist of a series of enzymes, such as superoxide dismutase (SOD) (42), catalase (CAT) (43), glutathione peroxidase (GPX) (44), glutathione reductase (GSR) (45), haem oxygenase (HMOX) (46), peroxiredoxin (PRDX) (47), thioredoxin (TRX) (48), and quinone oxidoreductase 1 (NQO1) (49). Nonenzymes include glutathione (GSH) (50), α-lipoic acid (51), and coenzyme Q10 (CoQ10) (52). Of note, nuclear factor erythroid 2-related factor 2 (NRF2) is a basic leucine zipper (bZIP) transcription factor that is an important controller of the activation of cellular antioxidant defence systems (53). Under normal conditions, Kelch-like ECH-associated protein 1 (KEAP1) can promote the polyubiquitination and degradation of NRF2 to maintain a certain level of NRF2. However, under OS, KEAP1 is oxidized, and NRF2 enters the nucleus and binds to the antioxidant response element (ARE) sequence, thereby activating the expression of the abovementioned series of antioxidant enzymes (54–58). In addition, after DNA damage caused by OS, DNA repair mechanisms in glioma cells are activated to repair damaged DNA and exert indirect antioxidant effects, such as direct repair (59–61), base excision repair (BER) (62), mismatch repair (MMR) (63, 64), and nucleotide excision repair (NER) (65, 66). A summary of the antioxidant defence systems in glioma cells is presented in Table 1.
3 The role of ROS in the glioma TME
The glioma TME plays an important role in the growth, invasion, recurrence and drug resistance of gliomas. Its major components include glioma cells, immune cells, signalling molecules, stromal cells, and extracellular matrix (ECM) (95). Immune cells include glioma-associated macrophages/microglia (GAMs), myeloid-derived suppressor cells (MDSCs), T cells, monocytes, neutrophils, dendritic cells (DCs) and natural killer (NK) cells. Signalling molecules include chemokines, cytokines, growth factors, and angiogenesis factors. Stromal cells include astrocytes and endothelial cells. The extracellular matrix (ECM) is a three-dimensional structure composed of fibrin, proteoglycans and other molecules that provides biochemical and structural support for surrounding cells and plays an important role in glioma invasion and metastasis (95, 96). Among them, as important regulatory molecules, the presence of ROS have a significant impact on the glioma TME. ROS not only affect the function of immune cells (Figure 1) but also participate in the process of glioma cell proliferation, invasion, metastasis and death. These studies will be discussed in this section.
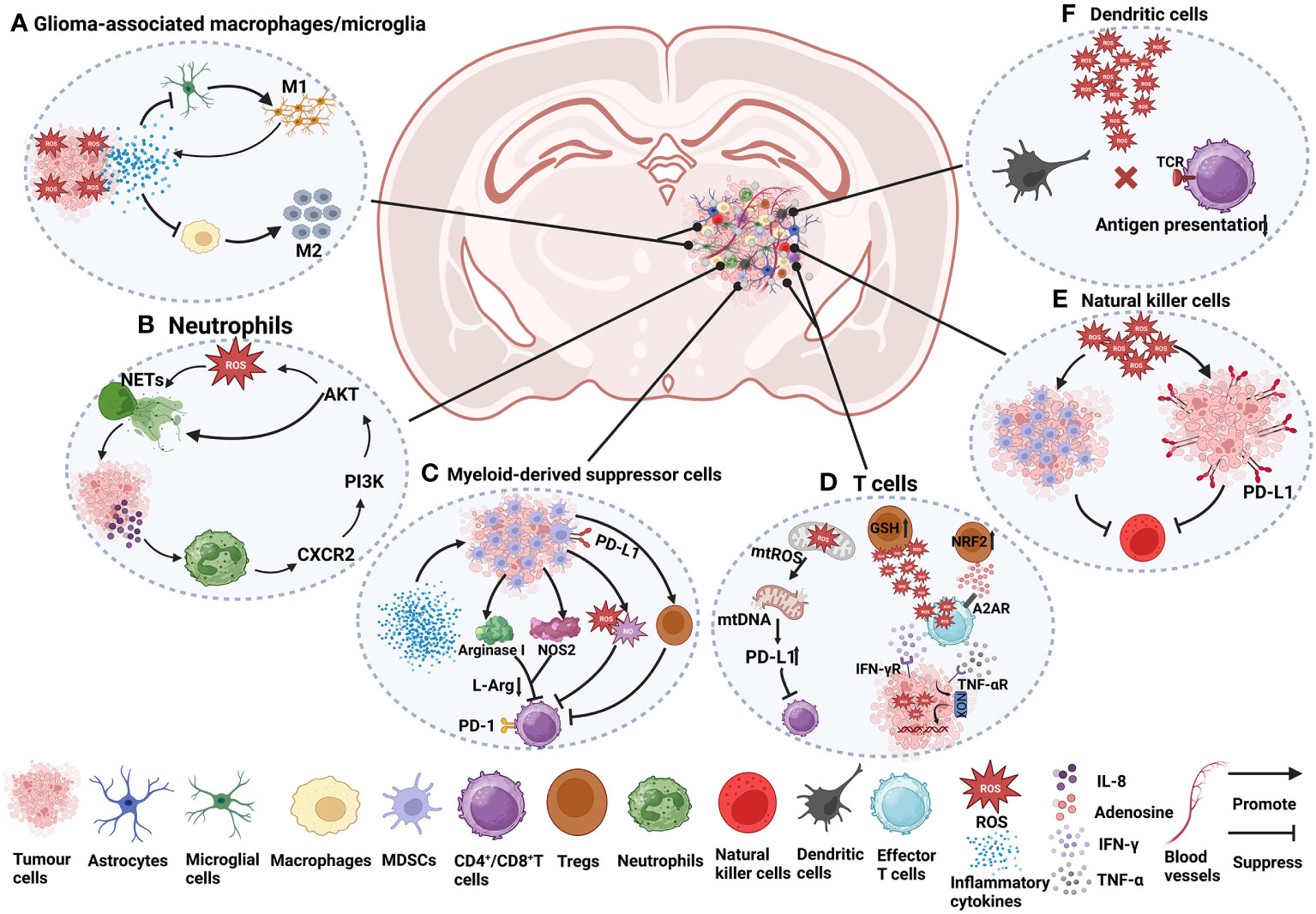
Figure 1 The role of ROS in immune cells in the glioma TME. (A) Under ROS stress, tumour cells secrete many cytokines, such as IL-4, IL-6, IL-10, and TGF-β, which cause macrophage immunosuppression and facilitate the recruitment of M2 tumour-associated macrophages (97–99). The activation of microglia is mainly manifested as the M1 type, accompanied by the release of a series of inflammatory factors (96, 100). (B) NETs induce glioma cells to secrete IL-8 to recruit neutrophils, promote the CXCR2/PI3K/AKT/ROS signalling axis, and finally promote the formation of NETs, forming a positive feedback pathway (101). (C) Cytokines and growth factors, such as granulocyte-macrophage colony stimulating factor (GM-CSF), granulocyte colony stimulating factor (G-CSF), IL-2, IL-1β, IL-6, and VEGF, can induce the aggregation of MDSCs in tumour hosts (102, 103). In the plasma of GBM patients, the level of arginase is often increased, which is related to the inhibitory function of MDSCs (102). Arginase I reduces L-arginine (L-Arg) levels (104), thereby inhibiting T-cell activation (103). Nitric oxide synthase 2 (NOS2) is another major catabolic enzyme for L-Arg metabolism in MDSCs (105). MDSCs also secrete NO and ROS, which induce T-cell inhibition (105). MDSCs also indirectly affect the activation of T cells by inducing Tregs (103). CD4+ effector memory T cells (CD4+ TEM) infiltrated by GBM strongly upregulate PD-1, and the corresponding ligand PD-L1 is expressed in MDSCs from tumours, which are involved in functional T-cell exhaustion (106). (D) The increase in mtROS causes mitochondrial DNA damage and upregulates the expression of PD-L1 to inhibit T-cell activation (97, 107). ROS produced by Tregs can suppress effector T cells (CD4+ and CD8+). Effector T cells can induce an increase in ROS in tumour cells through IFN-γ and TNF-α, which can damage tumour cell DNA and lead to tumour cell death. Tregs themselves are more resistant to oxidative stress due to the increased activity of the antioxidant system, for example, by increasing GSH and upregulating NRF2 (108). Adenosine produced by Tregs can also inhibit effector T-cell function in an A2AR-dependent manner (109). (E) ROS induce the proliferation of MDSCs and inhibit NK cell function (110). In addition, high levels of ROS promote PD-L1 expression on cancer cells, thereby inactivating NK cells (111). (F) High levels of ROS can disrupt antigen presentation between T cells and DCs, which in turn affects the recognition of tumour antigens by T cells (98, 108).
3.1 GAMs
Macrophages and microglia are important cell types in the immune system. Macrophages are primarily derived from bone marrow-resident haematopoietic stem cells (112). After entering the blood, mature mononuclear macrophages can settle in different tissues, such as the liver, lung, brain, lymph nodes and other organs and tissues, at which time they will become macrophages. Macrophages are involved in phagocytosis and clearance of pathogenic microorganisms, necrotic tissues, and secretion of a variety of inflammatory mediators involved in immune regulation and tissue repair (113–115). Microglia are induced by the colony-stimulating factor 1 receptor (CSF1R) and are generated from red myeloid progenitors of the yolk sac. They are self-renewing and reside in the CNS for a long time (116). Microglia play an important role in maintaining the homeostasis of the nervous system, including phagocytosis (117), promoting synapse formation (118), and supplying nutrients (119).
In the TME, macrophages can manifest as the M1 type (characterized by inflammatory and antitumour responses) or M2 type (involved in the repair of damaged tissues and anti-inflammation), but the TME tends to induce the differentiation of macrophages towards the M2 type (120–122). Anti-inflammatory factors released by tumours, such as interleukin (IL)-4, IL-10, and transforming growth factor-β (TGF-β), can promote the transformation of macrophages into the M2 type (123). M2 macrophages similarly release growth factors, such as vascular endothelial growth factor (VEGF), epidermal growth factor (EGF), and fibroblast growth factor (FGF), which promote tumour cell proliferation, invasion, and metastasis (124).
Glioma-infiltrated macrophages and microglia are collectively referred to as GAMs, which represent the largest population of cells infiltrating tumours, accounting for more than 1/3 of the total tumour mass (125, 126). GAMs play an important role in the glioma TME and promote tumour progression. First, anti-inflammatory factors such as IL-10 and TGF-β produced by GAMs inhibit the function of other immune cells in the TME and weaken the antitumour immune response (127, 128). Second, GAMs also secrete matrix metallopeptidase 2 (MMP2) and MMP9, which are able to breakdown matrix proteins, such as collagen and fibronectin, thereby enabling glioma cells to penetrate and invade the surrounding stromal tissues (129). Finally, GAMs also secrete proangiogenic molecules, such as VEGF and CXC motif chemokine ligand 2 (CXCL2), which have been shown to promote glioma growth and metastasis (129, 130).
In the glioma TME, ROS generally induce the generation of M2 GAMs (96, 131). ROS modulator 1 (Romo1), a membrane protein located on mitochondria, was found to regulate mitochondrial ROS (mtROS) production in GBMs (132). In GBM mouse models, overexpression of Romo1 induces ROS generation via mTORC1 signalling, which in turn promotes the polarization of bone marrow-derived macrophages (BMDMs) to the M2 type, resulting in a significant suppressive TME (133). In addition, a prognostic model of human GBMs and ROS showed that high expression of ROS-related genes such as HSPB1, LSP1 and PTX3 was closely associated with M2 macrophages and correlated with shorter survival of GBM patients. This suggests that ROS-related genes may be potential targets for GBM treatment. Therefore, inhibiting the polarization of macrophages towards M2 type and promoting the polarization towards M1 type may be beneficial for the treatment of GBMs (134). Besides, GAMs could survive in a high ROS environment mainly due to the action of antioxidant enzymes. A study of GBM tissues in humans and mice showed that the active antioxidant enzyme GPX1 was expressed at higher levels in GAMs than in GBM cells, resulting in GAMs being able to survive in a high ROS environment (135). It is known that GPX1 plays an important role in H2O2 detoxification (136). In summary, the antioxidant enzymes in GAMs protect them from ROS damage, which is necessary for the formation of M2 GAMs.
3.2 MDSCs
MDSCs, which are mainly differentiated from haematopoietic stem cells in the bone marrow, are a group of myeloid cells with heterogeneous and immature characteristics (137). In normal organisms, the levels of MDSCs in the peripheral blood tend to be very low (138). MDSCs have certain immunomodulatory effects, which can regulate the inflammatory response, inhibit overactivated immune cells, prevent excessive immune responses, and reduce tissue damage (105, 139).
Upon tumour stimulation, MDSCs are activated and released into peripheral blood and tissues. However, MDSCs often suppress the immune response and cause tumour escape (140, 141). The suppressive effect of MDSCs is mainly manifested by inhibiting the activity of other immune cells, including macrophages (142), CD4+ T cells (106), CD8+ T cells (143), NK cells (144), and DCs (145). First, MDSCs can highly express arginase-1 (ARG-1), which can convert arginine to uric acid and ornithine, thereby reducing the concentration of arginine in the internal environment (105). Arginine deficiency leads to limited activation of immune cells such as T cells (146) and NK cells (147), thereby impairing the immune response and promoting tumour development and metastasis. Second, MDSCs can secrete immunosuppressive cytokines, such as TGF-β and IL-10, which can inhibit the secretion of IL-12 by macrophages, thereby blocking the activity of cytotoxic T lymphocytes (CTLs) (148, 149). TGFβ-1 secreted by MDSCs also promotes the transformation of CD4+ T cells into immunosuppressive Tregs (150). Finally, MDSCs can also express immunosuppressive ligands, such as programmed death ligand-1 (PD-L1), which in turn suppresses T-cell priming and activation (151).
In gliomas, MDSCs comprise approximately 30% to 50% of the tumour entity (152). The increase in MDSCs is thought to be associated with glioma progression and immune escape (153). Generally, MDSCs can be divided into two main subsets based on their phenotype and function: monocytic (mMDSCs) and granulocyte/polymorphonuclear (gMDSCs) (154). Specifically, mMDSCs represent the major subset in the GBM TME. mMDSCs in the GBM TME of humans and mice expressed higher levels of adhesion molecules, such as integrin β1 and dipeptidyl peptidase 4 (DPP4), leading to enhanced cell adhesion and further promoting tumour migration and invasion (155). In addition, MDSCs can promote angiogenesis through the release of VEGF (156), as well as the release of cytokines such as IL-10, IL-6, and TGF-β under hypoxic conditions (157), thereby promoting glioma growth and invasion.
In the TME, MDSCs can survive in a high ROS environment because of their high expression of NRF2. On the one hand, NRF2 upregulated anti-OS genes in MDSCs and protected MDSCs from OS damage. On the other hand, NRF2 enhances the immunosuppressive activity of MDSCs by increasing their ability to produce ROS (158). In gliomas, ROS in MDSCs play an important role in maintaining the function of MDSCs (159). ROS can prevent the differentiation of MDSCs and promote the formation of an immunosuppressive TME (160). Specifically, ROS maintain the undifferentiated state of MDSCs by inhibiting the differentiation of MDSCs into mature immune cells such as macrophages and DCs (145, 161, 162). This undifferentiated state allows MDSCs to continue expressing immunosuppressive molecules such as TGF-β, IL-10 and PD-L1 (163). In addition to being able to impair the antigen presentation capacity of DCs (164–166), these immunosuppressive molecules can also inhibit the activity of T cells and induce the differentiation of T cells into Tregs (141, 167). Collectively, high levels of ROS play an important role in maintaining the undifferentiated state of MDSCs, which in turn mediates the immunosuppressive TME. Therefore, targeting MDSCs may become a promising therapeutic strategy.
3.3 T cells
T cells are members of the adaptive immune system that respond to antigens presented by antigen-presenting cells such as DCs and macrophages (168). T cells can be divided into CD4+ T cells and CD8+ T cells based on surface markers and function (169). CD4+ T cells have antigen receptors on their surface, which can recognize antigen fragments presented by MHC class II molecules on the surface of antigen-presenting cells and exert immune functions by activating other types of immune cells. CD8+ T cells generally refer to CTLs that can directly kill infected cells by MHC class I molecules (170). Regulatory T cells (Tregs) are a subset of CD4+ T cells that express the transcription factor forkhead box protein 3 (FOXP3) and play a role in inhibiting pathological immune responses and maintaining homeostasis in the body (171).
In tumours, CD4+ T cells mainly activate other immune cells, such as CD8+ T cells and NK cells, to enhance the immune response (172). CD4+ T cells can secrete cytokines, such as interferon-gamma (IFN-γ) and tumour necrosis factor-alpha (TNF-α), which directly kills tumour cells (173). CD8+ T cells carry specific T-cell antigen receptors (TCRs) that recognize and bind to tumour cells expressing specific antigens, thereby releasing cytotoxins, such as perforin and granzyme, to directly kill tumour cells (174). In addition, CD8+ T cells can also secrete cytokines, such as IFN-γ and TNF-α, which directly inhibit tumour cell growth and proliferation (175). Tregs play an indispensable role in maintaining the homeostasis of the immune system. Tregs suppress other immune cells and prevent excessive immune responses by producing inhibitory cytokines such as IL-10, IL-35, and TGF-β. However, overactive Tregs in turn limit the antitumour ability of immune cells (176).
In gliomas, T-cell dysfunction is often caused by the strong immune escape ability of glioma cells. Some studies have shown that human GBM cells are capable of producing the immunosuppressive factor TGF-β (177), which inhibits T-cell activation, thereby weakening the immune response (178). In addition, the human glioma TME has many immunosuppressive cells, such as M2 macrophages and Tregs, whose presence usually inhibits the activity and function of T cells and is associated with reduced overall survival of patients (179–181). Moreover, human glioma cells often express immune escape sites on the surface, such as PD-L1 and B7 homologue 3 (B7-H3), which can bind to immune checkpoint receptors, thereby inhibiting the activity and function of T cells (182).
ROS play an important role in regulating T-cell function and activity. Low levels of ROS can promote the activation and proliferation of T cells to enhance immune responses (183). However, higher levels of ROS can inhibit the secretion of cytokines by T cells and induce apoptosis (184). In the TME, excessive ROS may induce apoptosis of T cells, leading to decreased antitumour ability. For example, ROS produced by neutrophils or tumour cells can be transferred to T cells and cause OS, thereby causing hyporeactivity of T cells in cancer patients (185). In mouse glioma models, the administration of hyperbaric oxygen (HBO) can induce the generation of ROS in the thymus, which subsequently inhibits the generation of CD3+ T cells and promotes glioma growth in vivo (186). Therefore, high levels of ROS in the TME may lead to impaired T-cell function, which in turn enhances tumour escape. Targeting ROS in the TME to enhance the killing ability of T cells may be a potential therapeutic option.
3.4 Neutrophils
Neutrophils are derived from haematopoietic stem cells in the bone marrow through differentiation and maturation. When neutrophils mature, they enter the circulation and are distributed throughout the body through the blood (187, 188). Neutrophils are important immune cells of the body that are capable of engulfing and eliminating pathogens, such as bacteria and viruses (189, 190). In addition, when tissues are injured or infected, neutrophils rapidly migrate to the damaged site and release cytokines and chemokines to trigger local inflammation (191).
The role of neutrophils in tumours is complex. On the one hand, neutrophils are capable of killing tumour cells by releasing cytotoxic ROS (192) and by direct cell contact (193). On the other hand, neutrophils also promote tumour growth and metastasis by secreting immunosuppressive factors such as TGF-β, IL-6 and IL-8 (194) and interacting with circulating tumour cells (195). Furthermore, neutrophil extracellular traps play an important role in tumour progression. In the early stages of tumour invasion and metastasis, neutrophils can release neutrophil extracellular traps (NETs), which include DNA, tissue proteins and other substances. NETs can form channels suitable for tumour cell migration and protect tumour cells from immune system attack (196, 197).
Neutrophils, as mediators of inflammation, are early markers of GBM progression (198). Overall, neutrophils promote tumour growth, invasion, and angiogenesis. Neutrophils contribute to glioma infiltration by secreting elastase (199). Furthermore, neutrophils may also become resistant to antineoplastic therapy. In patients receiving anti-VEGF therapy, neutrophils contribute to glioma resistance to anti-VEGF therapy by increasing S100A4 expression and angiogenesis in glioma tissues (200). S100A4 is known to be a biomarker expressed in glioma stem-like cells (GSCs) that induces the tumorigenic activity of neutrophils (201). In addition, some studies have shown that the expression of MDSCs is increased in the peripheral blood of GBM patients, of which the neutrophilic MDSC subset accounts for the largest proportion, accounting for approximately 60% (102). Neutrophilic MDSCs derived from the peripheral blood of GBM patients can inhibit T-cell proliferation in vitro, which is related to the high expression of PD-L1 on effector memory CD4+ T cells (106).
Several studies have shown that ROS are important factors in promoting the formation of NETs (202–204). In chronic granulomas, NOXs are activated by protein kinase C (PKC) and produces ROS. These ROS can act as signalling molecules, causing neutrophils to release DNA and form a mesh-like structure, which then combines with the adhered granule proteins to form NETs (202). Furthermore, in primary mouse and human neutrophils, members of the MAPK family, such as c-Jun N-terminal kinases (JNKs) (203), extracellular signal-regulated kinases (ERKs) and p38 (204), can activate NOXs to generate ROS, which in turn induces the production of NETs. Similarly, ROS are similarly closely related to NETs in GBM TME. A study in human GBMs showed that NETs promote IL-8 secretion in GBMs by stimulating the NF-κB signalling pathway, which in turn stimulates endothelial cells to generate blood vessels to deliver essential nutrients and oxygen to the tumour site (101). When IL-8 binds to C-X-C motif receptor 2 (CXCR2) on neutrophils, it mediates the formation of NETs through the CXCR2/PI3K/AKT/ROS axis. This positive feedback loop stimulates the interaction between NETs and GBM cells and leads to profound changes in the TME (101). Recent studies in murine models of GBMs have additionally demonstrated that neutrophils promote the necrosis of GBM cells by transferring particles containing myeloperoxidase into these cells. This phenomenon induces OS, which is a result of the iron-dependent accumulation of lipid peroxides in GBM cells (205).
3.5 DCs
DCs, which differentiate from bone marrow haematopoietic stem cells through common DC progenitors (CDPs), play an important immunomodulatory role by presenting antigens (206). In tissues, DCs are usually naturally present and are considered to serve as a bridge connecting innate and adaptive immunity and are able to promote the transformation of innate to adaptive immune responses (207). Innate immunity refers to the immunity possessed by individuals at birth, which has a wide range of effects and is not triggered by specific antigens (208). Adaptive immunity is mainly the ability to respond to and adapt to a specific antigen or pathogen, which is achieved through T-cell-mediated cellular immunity and antibody-mediated humoral immunity (209).
Tumour formation is often accompanied by the expression of tumour antigens. Tumour antigens can be captured and processed by DCs and subsequently presented to naive T cells to induce their proliferation and differentiation into effector cells, such as CD8+ T cells, which subsequently kill the tumours (210). Furthermore, DCs can produce a variety of immune stimulating factors, such as cytokines and chemokines, which induce DCs and NK cells to reach inflammatory sites (211), as well as induce the activation of tumour-specific T cells (212).
Antigens released by glioma cells can be captured and processed by DCs, presented to T cells, and activate effector T-cell function (213). However, glioma cells can often evade immune surveillance by inhibiting the maturation of DCs. Studies have shown that tumour-conditioned medium (TCM) collected from the supernatant of human primary glioma cells can upregulate the expression of suppressor of cytokine signalling 1 (SOCS1) in DCs and then inhibit the NF-κB signalling pathway, thereby limiting the maturation of DCs. The subsequent suppression of T-cell activity, as well as IFN-γ secretion, results in immune escape of glioma cells (214). A previous study revealed that mouse gliomas have the ability to secrete cell factors including TGF-β and IL-10 (215). These factors are known to impede the maturation and functionality of DCs within the TME (216).
In addition, recent studies on the role of DCs in the progression of human GBMs have focused on the maintenance of DC homeostasis. Overexpression of NRF2 in DCs leads to the inhibition of DC maturation and subsequently reduces effector T-cell activation, which may be related to the decrease in ROS levels mediated by NRF2. In contrast, inhibition of NRF2 promotes the maturation of CD80+ and CD86+ DCs (217).
3.6 NK cells
NK cells belong to a type of lymphocyte that can eliminate tumour cells without specific antigens and are an important part of innate immunity (218). NK cells are derived from bone marrow haematopoietic stem cells and enter the circulation after maturation (219). Approximately 5-15% of lymphocytes in normal blood are NK cells (220).
NK cells have the ability to kill tumour cells. First, NK cells kill tumour cells by making direct contact with tumour cells that express specific ligands. There are a variety of activated receptors on the surface of NK cells, such as natural killer group 2 member D (NKG2D) and natural cytotoxicity receptors (NCRs; NKp46, NKp44 and NKp30) (221), etc. Among them, NKG2D is one of the most studied receptors and is able to recognize ligands on the surface of tumour cells, such as major histocompatibility complex class I polypeptide-related sequence A and B (MICA/B) and UL16-binding protein (ULBP). This recognition activates NK cells and prompts them to kill tumour cells (222). Second, NK cells can kill tumour cells through the antibody-dependent cellular cytotoxicity (ADCC) mechanism. When the antigens on the surface of tumour cells are labelled with specific antibodies, NK cells can bind to the specific antibodies through the CD16 (FcγRIIIa) receptor on their surface. Activated NK cells then release particles containing perforin and granzyme, which trigger apoptosis of antibody-labelled tumour cells (223, 224). Furthermore, NK cells can produce cytokines, such as IFN-γ and TNF-α, which can enter tumour cells and thus kill them (225). Moreover, IFN-γ released by NK cells can also inhibit tumour angiogenesis, thereby impeding tumour nutrient supply (226).
NK cells also have a killing effect on gliomas. First, NK cells can kill glioma cells by secreting perforin and granzyme B upon induction by IFN-β (227). Second, NK cells kill gliomas by specific activating receptors on their surface. When NKG2D and DNAX accessory molecule-1 (DNAM-1) on the surface of human NK cells bind to their ligands on the surface of GBM cells, they can trigger NK cell cytotoxicity and cause GBM cell death (228, 229). However, human GBM-derived TGF-β may lead to downregulation of NKG2D receptors on the surface of NK cells and contribute to GBM cell survival, suggesting that blocking TGF-β may be beneficial in the treatment of GBMs (230). Similarly, NK cells can also kill GBM cells through ADCC. Cetuximab is a monoclonal antibody that binds epidermal growth factor receptor (EGFR) on tumour cells (231). When administered, cetuximab binds to a human GBM surface antigen (EGFRvIII) and activates fragment crystallizable (Fc) receptors on NK cells, leading to NK cell-mediated cytotoxicity against GBM cells (232).
NK cells are often particularly sensitive to the cytotoxic effects of ROS, and their antitumour activity is often inhibited by ROS in the solid tumour TME, whereas antioxidant therapy may partially restore NK cell function (110, 233). Previous studies have shown that high levels of ROS in rats with fibrosarcoma can limit the adhesion of NK cells to similarly charged tumour cells by promoting the accumulation of anionic charges on their surface. This disadvantage can be prevented by antioxidant molecules such as CAT and SOD (234). Similarly, in vitro, the CD20 antibody rituximab triggered monocyte ROS production, which in turn inhibited the ADCC effect of NK cells on human primary leukaemia cells. However, antioxidant treatment (histamine dihydrochloride and diphenylene iodonium chloride) partially restored the ADCC effect of NK cells (235). At present, although some studies have suggested the inhibitory effect of ROS on NK cell activity in the TME, the study of ROS in NK cells in the glioma TME is still limited. Further studies will help to understand the effect of ROS on NK cell function in the glioma TME.
3.7 Glioma cells
The threshold of ROS is very important in cancer therapy. When ROS produced by tumour cells exceed a certain threshold and cannot be detoxified by antioxidants, it results in high levels of OS, which drives cancer cell death or cause them to become more sensitive to treatment. However, a low level of ROS in tumour cells contributes to their growth, proliferation, invasion and metastasis (236). Therefore, tumour cells need to maintain their ROS levels to maintain their survival and invasive abilities (237).
A study of tumour tissues and blood samples from glioma patients found that abnormal increases in ROS caused DNA damage in glioma cells, resulting in high expression of the DNA damage marker 8-oxo-deoxyguanosine (8-oxo-dG) and low expression of the epigenetic marker 5-methylcytosine (m5C). This is associated with increased malignancy of gliomas (22). In mouse models, glioma cells can overexpress aquaporin 8 (AQP8), which increases ROS levels, resulting in decreased expression of phosphatase and tensin homolog (PTEN) and increased expression of phosphorylated (p)-AKT, thereby promoting the growth and proliferation of gliomas (23). Moreover, the production of a significant amount of ROS induced by 12-O-tetradecanoylphorbol-13-acetate (TPA) can activate the MAPK pathway and cyclooxygenase-2 (COX-2)/prostaglandin E2 (PGE2) pathways, subsequently enhancing the in vitro migration and invasion capability of glioma cells (24). In contrast, high levels of ROS activate regulated glioma cell death programs, including apoptosis, necrosis, autophagy, ferroptosis, etc. For example, in vitro, salinomycin can activate p53, trigger the opening of mitochondrial permeability transition pore (mPTP), and induce the production and accumulation of mtROS, leading to the necrosis of glioma cells (25). The activation of transient receptor potential mucolipin 1 (TRPML1) inhibits autophagy in glioma cells in vitro, leading to ROS production and subsequent induction of apoptosis (238). Similarly, the increase in ROS induced by isoaaptamine also leads to apoptosis and autophagy in GBM cells in vitro (26). Furthermore, the heat shock protein 90 (HSP90) and dynamin-related protein 1 (DRP1) increase ROS production by regulating the expression of acyl-coenzyme A synthetase long-chain family member 4 (ACSL4) and mitochondrial morphology, leading to ferroptosis of gliomas in mice in vitro and in vivo (239).
Glioma stem-like cells (GSCs) are a subpopulation of GBM cells with stem cell characteristics. They have self-renewal, tumourigenicity and multidirectional differentiation potential and are closely related to the occurrence, development, treatment resistance and recurrence of GBMs (240). Many studies have confirmed that ROS are involved in the proliferation, self-renewal and differentiation of GSCs (241, 242). In a study conducted on human-derived GSCs, it was found that TGF-β upregulated the expression of the NOX4 gene, leading to the generation of ROS. Consequently, this ROS generation promoted GSC proliferation and maintained their stem cell state (241). Other studies have shown that serum stimulation in an in vitro environment is able to cause an increase in mitochondrial ROS within GSCs and modulate differentiation signalling pathways in GSCs. Interestingly, in the in vivo environment, increased ROS could greatly enhance glioma formation, which may be related to the activation of the NF-κB pathway by ROS (243). Compared with other tumour cells, GSCs have stronger antioxidant capacity (47). In vitro, the highly expressed antioxidant protein PRDX4 was able to mitigate OS in GSCs by reducing ROS generated by the protein folding process (47, 244). Furthermore, GSCs also inhibit mitochondrial respiration by increasing the expression of mitochondrial uncoupling protein 2 (UCP2), thereby alleviating OS caused by high levels of intracellular ROS and ensuring their own survival (245).
However, the understanding of the ROS threshold in cancer cells is still unclear. Measurement of the ROS threshold requires the consideration of multiple factors, including the concentration and type of ROS, the activity of intracellular antioxidant enzymes, and the type and physiological state of tumour cells (246–249). Therefore, more studies are needed to fully assess ROS thresholds and determine their impact on tumour cells. Overall, both ROS and thresholds play a crucial role in glioma cells. This provides a new research direction for ROS-based glioma therapy.
4 ROS-based glioma therapy
High levels of ROS are usually present in the glioma TME. On the one hand, these ROS are involved in the formation of a suppressive TME. On the other hand, they are involved in the process of glioma proliferation, invasion and migration. However, there is also a threshold for the levels of ROS in glioma cells. The induction of ROS production above the threshold can lead to an excessive OS response, causing DNA and protein damage and leading to glioma cell death (250). Conversely, depletion of ROS may also lead to the blocking of important signalling pathways involved in ROS, thereby promoting glioma death (27, 28, 32). Based on these findings, it is suggested that both methods of inducing ROS production and ROS scavenging have potential in the glioma treatment. These two therapeutic strategies may help to suppress glioma growth, enhance the immune response and improve the efficacy of other antitumour therapies.
4.1 Treatment to increase ROS levels
Excessive ROS can induce tumour death, so amplifying the effect of ROS may be a good way to kill tumours. For example, the use of PDT (251), SDT (252), CDT (253), can be beneficial therapies for GBMs. This part mainly summarizes the research progress of PDT, SDT and CDT in the treatment of gliomas, and discusses the application of nanodrug delivery platforms in them.
4.1.1 PDT
PDT is a technique that relies on ROS production to treat nononcological diseases as well as tumours. Its main components are excitation light, photosensitizers (PSs) and ROS (254). PSs are important components in determining the efficacy of PDT (255). Photoactivated PSs can produce cytotoxic ROS in the presence of oxygen, resulting in the killing of target cells (256, 257). To date, numerous PSs have been applied in the studies of gliomas, such as 5-aminolevulinic acid (5-ALA) (258), boronated porphyrin (BOPP) (259), talaporfin (260), and temoporfin (261). The wavelength of the light is also important. The optimal PDT wavelength is between 650 and 850 nm and should be consistent with the longest wavelength absorption band of the PSs, that is, the wavelength range corresponding to sufficient energy for maximum tissue penetration to result in sufficient ROS production (262). Notably, ROS produced by PSs, such as 1O2, O2˙‾, OH, OOH·and H2O2, are essential for killing tumours. The formation of O2˙‾ and free radicals is called a type I reaction, and the formation of 1O2 is called a type II reaction (263, 264).
PDT has been approved by the United States Food and Drug Administration (FDA) for the treatment of a variety of cancers, including skin cancer, oesophageal cancer, and lung cancer (265–267). PDT has been studied since the 1980s (268, 269), and has shown promising efficacy in many glioma preclinical studies (270–272). A bibliometric analysis of literature in the field of cancer PDT (CPDT) reveals that research on CPDT is showing a rapid growth trend over the past 20 years. Among them, nanotechnology-based PDT and enhanced PDT are the current research hotspots (273). However, PDT has still not been widely adopted due to its potential toxicity to healthy brain tissues, limited light penetration, and poor targeting (251, 274, 275).
In the past 10 years, three promising phase I/II clinical trials of PDT for glioma treatment have been conducted in adults and one has been conducted in minors. A total of four clinical trials were conducted for three drugs (photofrin, ALA, photobac®) (Table 2). Among them, NCT01682746 included 5 adolescent patients with brain tumours. The incidence of serious adverse events within 1 month of PDT treatment, the progression-free survival and the overall survival within 3 years were recorded, but no results of this clinical trial were reported. NCT03048240 included 10 adult patients with newly diagnosed GBM who were treated with 5-ALA fluorescence-guided surgery followed by intraoperative PDT (based on clinicaltrials.gov). At the interim analysis, the median progression-free survival (mPFS) was 17.1 months, and the median overall survival (mOS) was 23.1 months (276). This clinical trial result indicates that intraoperative PDT is a good option for treating recurrent gliomas.
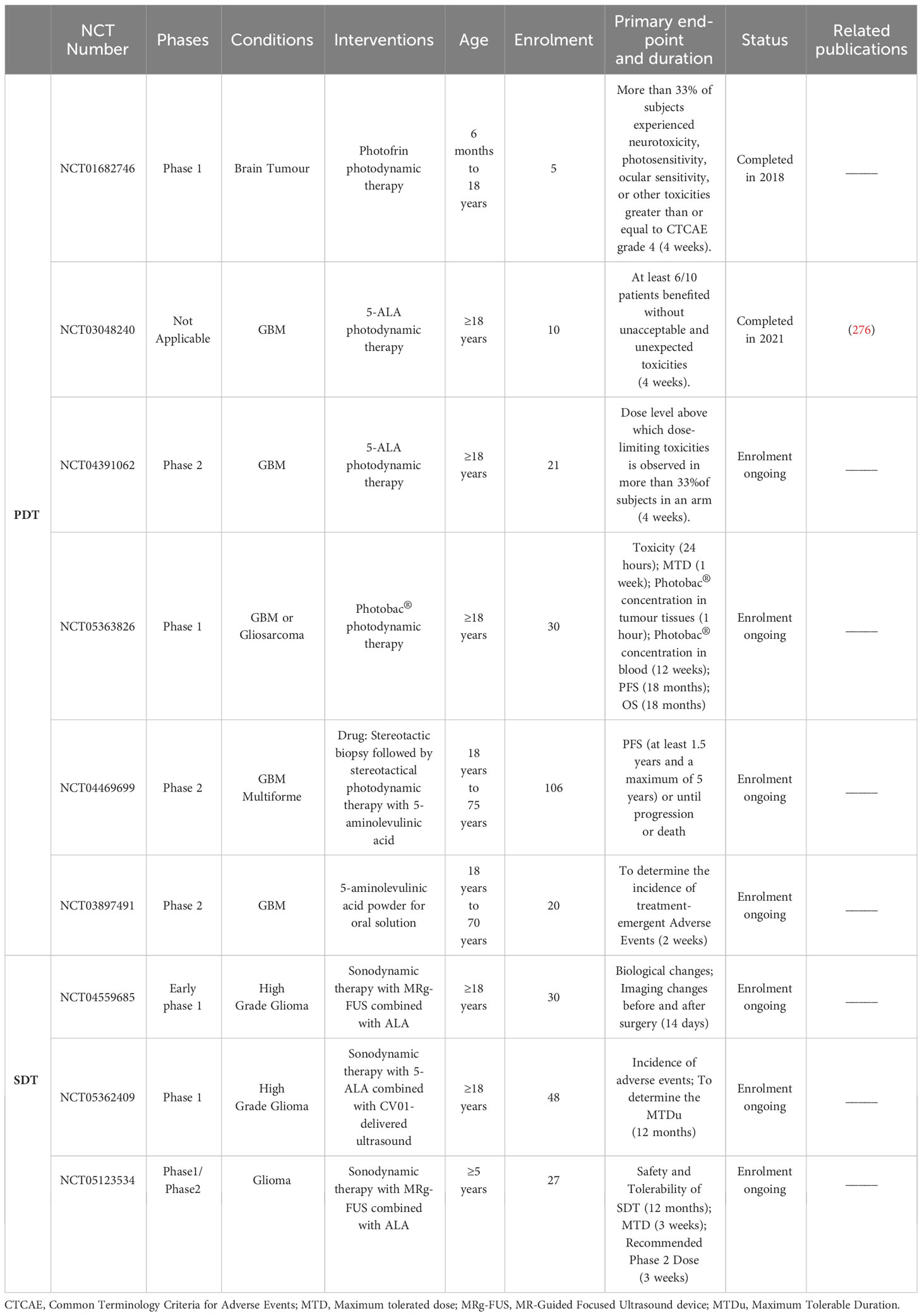
Table 2 Summary of completed/ongoing phase I/II clinical trials of ROS-generated PDT/SDT for glioma treatment by July 2023 (based on clinicaltrials.gov).
The tumour killing mechanisms of PDT are various, including inducing immunogenic cell death (ICD), destroying tumour blood vessels and inducing the release of inflammatory mediators in addition to the direct killing caused by high ROS. The combination of PDT and subsequent immune response induced by PDT is referred to as photodynamic immunotherapy (PDIT) (262, 277, 278). ICD refers to the death of tumour cells after PDT, which stimulates the immune system to produce a strong immune response by releasing damage-associated molecular patterns (DAMPs), cytokines, tumour-associated antigens (TAA) and other signalling molecules (279). These DAMPs can be recognized by the immune system and activate antitumour immune responses. DAMPs mainly include calreticulin (CALR), heat shock proteins 70/90 (HSP70/90), ATP, high-mobility group box-1 (HMGB1) nuclear protein, type I interferons (IFNs) and members of the IL-1 cytokine family, etc. In addition, ROS produced by PDT can destroy tumour blood vessels, limit tumour nutrient supply, and stimulate antitumour immune responses (262). Cytokines are able to trigger an inflammatory response that further enhances immune cell infiltration and activation (280, 281). The mechanism of PDIT in gliomas is illustrated in Figure 2.
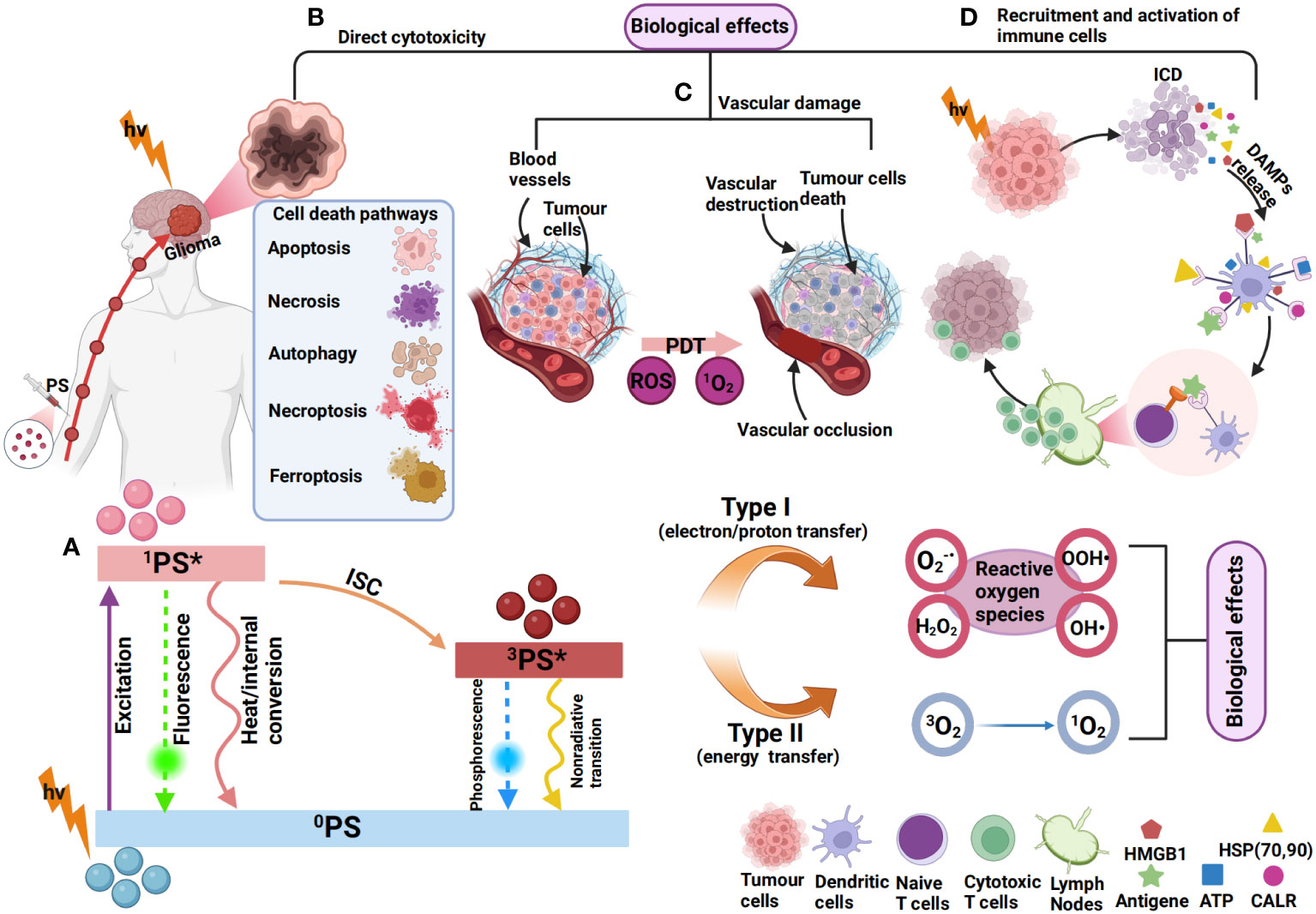
Figure 2 Mechanism of photodynamic immunotherapy for gliomas. (A) 0PS is activated to a singlet state (1PS*) after absorbing photons (hv). 1PS* can lose energy through internal conversion to heat and fluorescence. 1PS* can form a triplet state (3PS*) through the intersystem crossing process (ISC). 3PS* can be restored to 0PS by emitting phosphorescence and can also react with neighbouring molecules in two types of reactions (type I and type II). In type I reactions, 3PS* transfers an electron or a proton to form organic free radicals (O2-, OOH·, H2O2, OH·, etc.) that interact with cellular oxygen to produce cytotoxic ROS. In a type II reaction, the energy of 3PS* can be directly transferred to molecular oxygen (3O2) to form singlet oxygen (1O2). This results in various biological effects (264, 282, 283). (B) The most common types of cell death induced by PDT include apoptosis (284), autophagy (285), necrosis (286), necroptosis (287), and ferroptosis (270). (C) ROS produced by PDT can also cause vascular occlusion, leading to vascular damage, thereby affecting the blood supply of tumour cells (288). (D) PSs can induce immunogenic cell death (ICD), resulting in the exposure and release of DAMPs, such as ATP, HMGB1, CALR, HSP70/90, etc (262). The released DAMPs promote DC recruitment and maturation and present tumour antigens to T cells, leading to the activation of CD8+ T cells, which subsequently migrate in vivo to kill tumour cells (264).
4.1.2 SDT
Ultrasound (US) is a kind of mechanical vibration wave with strong tissue penetration ability that has been widely used in ultrasound imaging and ultrasound therapy. Among the US-derived techniques, SDT based on ROS production is a good strategy. Research on SDT began in the 1990s (289, 290). Based on the bibliometric analysis of SDT, studies have shown that since 2000, SDT has experienced rapid growth and has mainly focused on the fields of nanomaterials and cancer treatment, achieving significant results (291). The mechanism of SDT is to use low-frequency ultrasound to trigger sonosensitizers that accumulate at the tumour site, producing ROS and cavitation bubbles to kill the tumours. These ROS produce significant toxic effects on tumour cells in the 1 μm range (292, 293). The advantage of SDT is mainly that ultrasound can penetrate to a depth of 10 cm, which can kill tumours in deeper locations (294, 295). At present, most of the sonosensitizers used in reported SDT are photosensitizers or are derived from photosensitizers (296). However, SDT also has difficulty achieving the ideal tumour killing effect due to the presence of the BBB and the poor targeting effect of sonosensitizers such as porphyrins (297). Notably, TMZ can not only penetrate the BBB but also act as a sonosensitizer to induce necroptosis in GBMs. This provides new potential options for treating GBMs with SDT (293).
According to the literature, although SDT has been studied in gliomas for less than 20 years, it has shown promising efficacy in preclinical studies (30, 298, 299). However, due to the maturity of the technology and the factors of ultrasound equipment and other objective reasons, the research results of SDT are less than those of PDT, and clinical research is also in its infancy. At present, there are three clinical trials of SDT in gliomas under recruitment (based on clinicaltrials.gov), as shown in Table 2. More clinical trials are needed to verify the efficacy of SDT in the glioma treatment.
4.1.3 CDT
The concept of CDT was first proposed in 2016 by Bu, Shi et al. (300). CDT is dependent on transition metal ions in the TME to produce high levels of OH· through Fenton/Fenton-like reactions, resulting in tumour killing (301). The Fenton reaction refers to the complex chemical reaction of ferrous ions with H2O2, which eventually generates highly toxic OH· (302). Catalysts for Fenton-like reactions are usually other transition metals, such as copper (Cu) and manganese (Mn) (303, 304). Compared with PDT, the advantage of CDT is that it does not require laser irradiation, so it can avoid the limitations caused by light penetrating the tissues. Alternatively, the TME is characterized by acidity and H2O2 overexpression, which favours Fenton/Fenton-like reactions. However, when the pH at the tumour site is too high or H2O2 production within the tumour is insufficient, the Fenton/Fenton-like reaction will be insensitive, and CDT efficiency will be reduced (305). In general, CDT has the advantages of strong targeting, low adverse reactions, regulation of TME hypoxia, and low treatment cost, so it has great potential to be used in tumour therapy (301). In the glioma treatment, CDT is still in the preclinical stage, and no clinical trials have been carried out. However, it has been shown that CDT has good therapeutic efficacy and can exert more anti-glioma effects in combination with PDT (306) and photothermal therapy (PTT) (307).
4.1.4 Breaking through the BBB to enhance PDT/SDT/CDT
The BBB is a physical, chemical and biological barrier structure formed by capillary endothelial cells in the brain, surrounding astrocytes and muscle rings. The main function of the BBB is to maintain the stability of the brain environment, regulate the entry of nutrients, and prevent harmful substances from entering the brain through the blood (308, 309). Brain endothelial cells are composed of hydrophobic lipid bilayers with tight junctions. Therefore, drugs with large polarity and molecular weight often have difficulty passing the BBB (310). However, research has shown that human glioma cells can infiltrate through the perivascular space and extensively invade the brain away from the tumour mass. In this process, glioma cells displace the end feet of astrocytes, thereby disrupting the BBB, which may be beneficial for drug therapy (311, 312). However, effectively overcoming the limitations of the BBB remains a challenge. Currently, nanodrug delivery platforms (313), microbubble-enhanced focused ultrasound (MB-FUS) (314) and magnetic resonance-guided focused ultrasound (MRg-FUS) (315) are three promising approaches to break through the BBB.
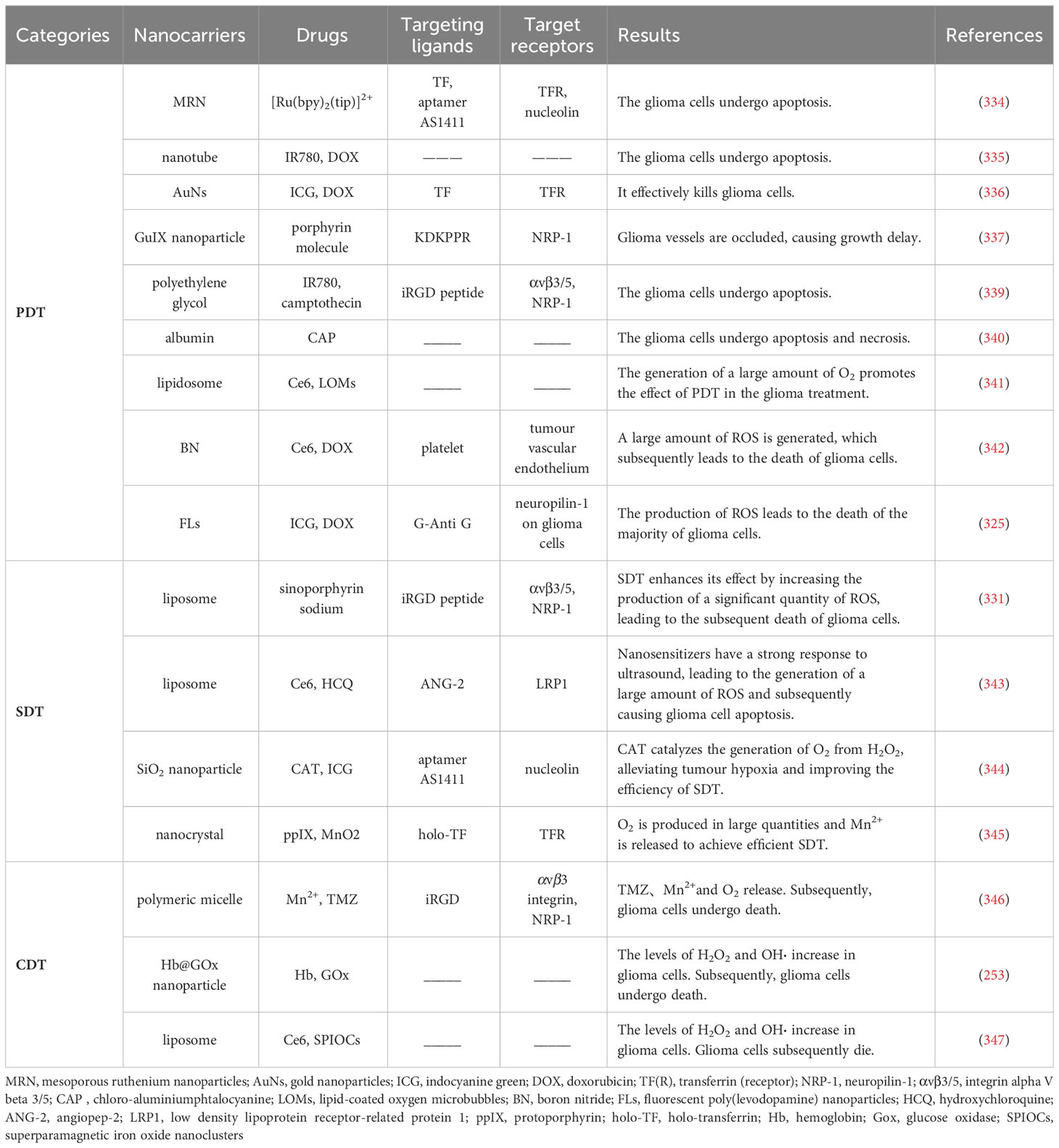
Nanotechnology is the study and application of particles or structures between 1 and 100 nm, where it can maximize drug transport and targeted delivery (316–318). Nanodrug delivery platforms are the application of nanotechnology in medicine. In general, nanodrug delivery platforms are usually composed of nanocores, nanocarriers, targeting ligands, drugs and surface modifications or may not completely contain these parts. Among them, the nanocore is the main component of the platform, mainly serving to support and stabilize the nanodrug delivery platform. It can be composed of materials such as gold (319), silicon (320), magnetic materials (321), etc. Nanocarriers refer to carriers that carry drugs on nanocores. An ideal nanocarrier can stably encapsulate drugs inside and release them at the appropriate time (322). Types of nanocarriers include gold nanoparticles, magnetic nanoparticles, carbon nanotubes, polymer micelles, and liposomes (323), etc. Targeting ligands attached to the nanocore include antibodies (324) and targeting peptides (325), which can precisely target the target. Nanocarriers can carry drugs, which include PSs such as chlorin e6 (Ce6) (326), immune checkpoint inhibitors such as nivolumab (327), and chemotherapy drugs such as doxorubicin (DOX) (328). Surface modification refers to the modification of the surface of the nanoplatform, such as polyethylene glycol (PEG), to enhance the hydropathy, stability, and biocompatibility of the nanoplatform and improve the retention time in vivo (329).
In general, a well-functioning nanodrug delivery platform can typically enhance the therapeutic effects of PDT (330), SDT (331), and CDT (332) for the treatment of glioma. Moreover, optimizing key components of nanodrug delivery platforms can be an effective strategy to break through the BBB. First, the targeting of the nanoplatform should be enhanced. Transferrin (TF), for example, targets a transferrin receptor (TFR) that is overexpressed on the surface of brain capillary endothelial cells and malignant brain tumours. Related studies have shown that TF-bound nanoplatforms can effectively cross the BBB and target gliomas (333, 334). Second, the development of new nanocarriers, such as nanotubes, gold nanoparticles, and magnetic nanoparticles, makes it easier for drugs to reach the glioma site (335–337). Furthermore, polymers such as polyethylene glycol (PEG) and polylactic-co-glycolic acid (PLGA) can be used to encapsulate the drug to allow crossing of the BBB. The advantages of such polymers are good biocompatibility, easy surface modification, etc., and the ability to control the rate of drug release (329, 338). When a nanodrug delivery platform is combined with PDT, SDT, and CDT, it will greatly increase the targeting ability of the three therapeutic strategies and the level of ROS production, leading to a “1 + 1 > 2” therapeutic effect. The summary of glioma-related research on the nanodrug delivery platforms combined with PDT, SDT, and CDT is shown in Table 3.
In addition to nanodrug delivery platforms, the use of MB-FUS (348–350) and MRg-FUS (315) to open the BBB for drug delivery both has great potential. Microbubbles are essentially small bubbles of biocompatible gases, such as nitrogen or perfluorocarbon, encapsulated in a lipid, protein, or polymer membrane (351). As blood with microbubbles flows through the brain, ultrasound waves are emitted precisely to target areas. The ultrasound stimulates microbubbles to oscillate violently and burst, producing a temporary, local pressure change that can temporarily open the tight junctions of the BBB and increase its permeability. In this way, drugs or macromolecules that cannot penetrate the BBB can enter the brain tissues, thus allowing for the effective treatment of gliomas (343, 352, 353). In recent studies, MB-FUS achieved BBB opening and increased drug aggregation in GBM regions to enhance antitumour effects (354–357). Notably, the method of opening the BBB using ultrasound microbubbles is reversible, does not damage neurons, and the BBB heals a few hours after exposure (358). Thus, this method has great potential for application. This technique is still in its early stages, and promising results have been demonstrated in clinical trials in glioma patients (359). Furthermore, as another method to open the BBB, MRg-FUS can accurately focus ultrasonic waves on the GBM region and provide real-time monitoring and guidance during therapy provided through magnetic resonance imaging (MRI). Ultrasound energy can raise the temperature of the BBB region, thus enhancing permeability. Consequently, platinum nanoparticles can more effectively penetrate the GBM tissues, thereby inhibiting the growth of GBM cells (315).
4.2 Treatment to scavenge/reduce ROS levels
Antioxidants are a class of compounds that inhibit oxidation by scavenging ROS and reducing OS, and they can help reduce or block oxidative reactions in cells (360). When OS occurs, antioxidants interact with ROS to capture and neutralize ROS, thereby protecting cells from oxidative damage (361). Common antioxidants include vitamin C, vitamin E, α-carotene, selenium, etc., which can be obtained through food intake or supplements (362). Furthermore, the use of antioxidants can inhibit tumorigenesis by preventing OS caused by various causes, and the mechanism is to repair damaged DNA and inhibit cancer occurrence, including gene mutations, oxidative chromosomal damage, and lipid peroxidation of cell membranes (131, 363).
There is considerable evidence that intake of antioxidants may help reduce the risk of gliomas (364). For example, CoQ10 can act as a ROS scavenger to increase the sensitivity of gliomas to TMZ, thereby inhibiting the invasion of glioma cells in vitro and in vivo. Mechanistically, CoQ10 can integrate into the mitochondrial membrane and reduce ROS production. It also reduced the expression of MMP9 and epithelial-mesenchymal transition (EMT) markers (28, 365). Naringenin is an antioxidant. Naringenin supplementation for 1 month can reduce lipid peroxidation and decrease the expression of PKC, NF-κB, cyclin D1(CCND1) and cyclin-dependent kinase 4 (CDK4), thereby inhibiting the proliferation of glioma cells in mouse models (27). Astaxanthin is a natural carotenoid, and adonixanthin is a product of its formation (366). Studies have confirmed that both have strong antioxidant capacity, which can cross the BBB and protect brain tissues from ischaemia or hemorrhage (367, 368). In mouse glioma models, astaxanthin and adonixanthin intake increased p38 phosphorylation in glioma cells, leading to cell cycle arrest. Furthermore, adonixanthin was able to reduce the expression of MMP2 and fibronectin downstream of ERK1/2 and AKT signalling pathways and inhibit invasion and metastasis in both in vitro and in vivo GBM models (369). Chrysin is a kind of flavonoid with antioxidant properties. The p38-MAPK pathway is activated in rat glioma cells treated with chrysin, resulting in the accumulation of p21 (WAF1/CIP1) protein, decreased activities of CDK2 and CDK4, and cell cycle arrest in G1 phase (32). Similarly, hypoxia-inducible factor-1alpha (HIF-1α) expression was blocked when the antioxidant melatonin was used, resulting in a significant inhibition of MMP2 and VEGF expression, thereby inhibiting GBM cell migration and invasion in vitro (370). Moreover, antioxidants quercetin (QE), baicalein (BE) and myricetin (ME) effectively downregulated ROS and MMP9 and inhibited glioma cell invasion/migration events in vitro (24).
However, some studies have shown that intake of antioxidants, such as carotenoids (371), vitamin E (372), and coffee (373), is not associated with the risk of developing gliomas. This may be related to factors such as bioavailability, dose, BBB permeability, and tumour heterogeneity (364). Therefore, the role of ROS scavenging using antioxidants in glioma therapy still needs to be confirmed by more studies.
5 Conclusion
ROS are products of cellular redox and play important functions in cells. Excessively high or low ROS levels are detrimental to cell survival. That is, there is a threshold for intracellular ROS, and when a large accumulation of ROS exceeds the threshold and cannot be neutralized by the antioxidant defence system, it leads to OS and thus cell death. For gliomas, there is also a threshold for ROS. Appropriate ROS levels can aid survival, but high levels of ROS can also lead to their own death. Therefore, ROS-based therapies are particularly important.
Currently, there are two common therapeutic approaches involving ROS in the therapy of glioma, which are increasing ROS levels to induce cell death or using antioxidants to inhibit progression. In terms of increasing ROS, PDT/SDT/CDT is the representative approach. With the development of modern nanotechnology, the corresponding drugs can better pass through the BBB. Preclinical studies have shown that PDT/SDT/CDT combined with nanotechnology shows potent antiglioma effects and has good potential for clinical application. Conversely, ROS reduction using antioxidants has also been shown to inhibit glioma initiation and progression. Nonetheless, both strategies have limitations. In addition to the unclear clinical efficacy of antioxidants for cancer treatment reported in the literature, there are also issues such as the uncertain toxicity and biosafety of nanomaterials (374, 375), and the uncertain stability and retention time of nanodrug delivery platforms (376). Therefore, in the future development of nanodrug delivery platforms targeting gliomas, it is necessary to enhance the targeting and stability of nanoparticles and improve the ability to cross the BBB and biosafety to provide effective treatment while reducing adverse reactions. At present, although there are still many obstacles to ROS-based therapy, ROS still have the potential to be widely used as a therapeutic target for gliomas.
Author contributions
Y-CY: Writing – original draft. YZ: Writing – original draft. S-JS: Visualization, Writing – original draft. C-JZ: Writing – review & editing. YB: Writing – review & editing. JW: Writing – review & editing. L-TM: Supervision, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. The preparation of this review was supported by the National Natural Science Foundation of China (No. 82101318 to YB), the Social Talent Fund Funding Program Project (2021SHRC044 to C-JZ), the Scientific Research Project Book of Traditional Chinese Medicine Administration of Shaanxi Province (SZY-KJCYC-2023-003 to C-JZ), and the Special Fund for the Cultivation and Enhancement of Military Traditional Chinese Medicine Service Capabilities (2021ZY021 to Jin Zheng).
Acknowledgments
We would like to express our heartfelt thanks to Dr. Jin Zheng, Director of TCM Department of Tangdu Hospital for her invaluable supportand guidance.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Anjum K, Shagufta BI, Abbas SQ, Patel S, Khan I, Shah SAA, et al. Current status and future therapeutic perspectives of glioblastoma multiforme (Gbm) therapy: A review. Biomed Pharmacother = Biomed Pharmacotherapie (2017) 92:681–9. doi: 10.1016/j.biopha.2017.05.125
2. Paw I, Carpenter RC, Watabe K, Debinski W, Lo HW. Mechanisms regulating glioma invasion. Cancer Lett (2015) 362(1):1–7. doi: 10.1016/j.canlet.2015.03.015
3. Barthel L, Hadamitzky M, Dammann P, Schedlowski M, Sure U, Thakur BK, et al. Glioma: molecular signature and crossroads with tumor microenvironment. Cancer metastasis Rev (2022) 41(1):53–75. doi: 10.1007/s10555-021-09997-9
4. Huang H, Zhang S, Li Y, Liu Z, Mi L, Cai Y, et al. Suppression of mitochondrial ros by prohibitin drives glioblastoma progression and therapeutic resistance. Nat Commun (2021) 12(1):3720. doi: 10.1038/s41467-021-24108-6
5. Ou A, Yung WKA, Majd N. Molecular mechanisms of treatment resistance in glioblastoma. Int J Mol Sci (2020) 22(1):315. doi: 10.3390/ijms22010351
6. Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. New Engl J Med (2005) 352(10):987–96. doi: 10.1056/NEJMoa043330
7. Diaz RJ, Ali S, Qadir MG, de la Fuente MI, Ivan ME, Komotar RJ. The role of bevacizumab in the treatment of glioblastoma. J neuro-oncol (2017) 133(3):455–67. doi: 10.1007/s11060-017-2477-x
8. Chien CH, Hsueh WT, Chuang JY, Chang KY. Dissecting the mechanism of temozolomide resistance and its association with the regulatory roles of intracellular reactive oxygen species in glioblastoma. J Biomed Sci (2021) 28(1):18. doi: 10.1186/s12929-021-00717-7
9. Thomas A, Tanaka M, Trepel J, Reinhold WC, Rajapakse VN, Pommier Y. Temozolomide in the era of precision medicine. Cancer Res (2017) 77(4):823–6. doi: 10.1158/0008-5472.Can-16-2983
10. Tomar MS, Kumar A, Srivastava C, Shrivastava A. Elucidating the mechanisms of temozolomide resistance in gliomas and the strategies to overcome the resistance. Biochim Biophys Acta Rev Cancer (2021) 1876(2):188616. doi: 10.1016/j.bbcan.2021.188616
11. Lee SY. Temozolomide resistance in glioblastoma multiforme. Genes Dis (2016) 3(3):198–210. doi: 10.1016/j.gendis.2016.04.007
12. Beal K, Abrey LE, Gutin PH. Antiangiogenic agents in the treatment of recurrent or newly diagnosed glioblastoma: analysis of single-agent and combined modality approaches. Radiat Oncol (London England) (2011) 6:2. doi: 10.1186/1748-717x-6-2
13. Fu M, Zhou Z, Huang X, Chen Z, Zhang L, Zhang J, et al. Use of bevacizumab in recurrent glioblastoma: A scoping review and evidence map. BMC Cancer (2023) 23(1):544. doi: 10.1186/s12885-023-11043-6
14. De Fazio S, Russo E, Ammendola M, Donato Di Paola E, De Sarro G. Efficacy and safety of bevacizumab in glioblastomas. Curr medicinal Chem (2012) 19(7):972–81. doi: 10.2174/092986712799320646
15. Sies H, Jones DP. Reactive oxygen species (Ros) as pleiotropic physiological signalling agents. Nat Rev Mol Cell Biol (2020) 21(7):363–83. doi: 10.1038/s41580-020-0230-3
16. Kumari S, Badana AK G, Mohan M, Shailender G, Malla R. Reactive oxygen species: A key constituent in cancer survival. biomark Insights (2018) 13:1177271918755391. doi: 10.1177/1177271918755391
17. Powers SK, Deminice R, Ozdemir M, Yoshihara T, Bomkamp MP, Hyatt H. Exercise-induced oxidative stress: friend or foe? J sport Health Sci (2020) 9(5):415–25. doi: 10.1016/j.jshs.2020.04.001
18. Eckert D, Rapp F, Tsedeke AT, Molendowska J, Lehn R, Langhans M, et al. Ros- and radiation source-dependent modulation of leukocyte adhesion to primary microvascular endothelial cells. Cells (2021) 11(1):72. doi: 10.3390/cells11010072
19. Roy J, Galano JM, Durand T, Le Guennec JY, Lee JC. Physiological role of reactive oxygen species as promoters of natural defenses. FASEB J (2017) 31(9):3729–45. doi: 10.1096/fj.201700170R
20. Gao Q. Oxidative stress and autophagy. Adv Exp Med Biol (2019) 1206:179–98. doi: 10.1007/978-981-15-0602-4_9
21. Poprac P, Jomova K, Simunkova M, Kollar V, Rhodes CJ, Valko M. Targeting free radicals in oxidative stress-related human diseases. Trends Pharmacol Sci (2017) 38(7):592–607. doi: 10.1016/j.tips.2017.04.005
22. Barciszewska AM, Giel-Pietraszuk M, Perrigue PM, Naskręt-Barciszewska M. Total DNA methylation changes reflect random oxidative DNA damage in gliomas. Cells (2019) 8(9):1065. doi: 10.3390/cells8091065
23. Hao Z, Huajun S, Zhen G, Yu X, Qian L, Ziling C, et al. Aqp8 promotes glioma proliferation and growth, possibly through the ros/pten/akt signaling pathway. BMC Cancer (2023) 23(1):516. doi: 10.1186/s12885-023-11025-8
24. Chiu WT, Shen SC, Chow JM, Lin CW, Shia LT, Chen YC. Contribution of reactive oxygen species to migration/invasion of human glioblastoma cells U87 via erk-dependent cox-2/pge(2) activation. Neurobiol Dis (2010) 37(1):118–29. doi: 10.1016/j.nbd.2009.09.015
25. Qin LS, Jia PF, Zhang ZQ, Zhang SM. Ros-P53-cyclophilin-D signaling mediates salinomycin-induced glioma cell necrosis. J Exp Clin Cancer Res: CR (2015) 34(1):57. doi: 10.1186/s13046-015-0174-1
26. Wen ZH, Kuo HM, Shih PC, Hsu LC, Chuang JM, Chen NF, et al. Isoaaptamine increases ros levels causing autophagy and mitochondria-mediated apoptosis in glioblastoma multiforme cells. Biomed Pharmacother = Biomed Pharmacotherapie (2023) 160:114359. doi: 10.1016/j.biopha.2023.114359
27. Sabarinathan D, Mahalakshmi P, Vanisree AJ. Naringenin, a flavanone inhibits the proliferation of cerebrally implanted C6 glioma cells in rats. Chemico-biological Interact (2011) 189(1-2):26–36. doi: 10.1016/j.cbi.2010.09.028
28. Burić SS, Podolski-Renić A, Dinić J, Stanković T, Jovanović M, Hadžić S, et al. Modulation of antioxidant potential with coenzyme Q10 suppressed invasion of temozolomide-resistant rat glioma in vitro and in vivo. Oxid Med Cell Longevity (2019) 2019:3061607. doi: 10.1155/2019/3061607
29. Hsia T, Small JL, Yekula A, Batool SM, Escobedo AK, Ekanayake E, et al. Systematic review of photodynamic therapy in gliomas. Cancers (2023) 15(15):3918. doi: 10.3390/cancers15153918
30. Wang X, Jia Y, Wang P, Liu Q, Zheng H. Current status and future perspectives of sonodynamic therapy in glioma treatment. Ultrasonics sonochemistry (2017) 37:592–9. doi: 10.1016/j.ultsonch.2017.02.020
31. Liu D, Dai X, Ye L, Wang H, Qian H, Cheng H, et al. Nanotechnology meets glioblastoma multiforme: emerging therapeutic strategies. Wiley Interdiscip Rev Nanomed Nanobiotechnol (2023) 15(1):e1838. doi: 10.1002/wnan.1838
32. Weng MS, Ho YS, Lin JK. Chrysin induces G1 phase cell cycle arrest in C6 glioma cells through inducing P21waf1/cip1 expression: involvement of P38 mitogen-activated protein kinase. Biochem Pharmacol (2005) 69(12):1815–27. doi: 10.1016/j.bcp.2005.03.011
33. Matés JM, Segura JA, Alonso FJ, Márquez J. Roles of dioxins and heavy metals in cancer and neurological diseases using ros-mediated mechanisms. Free Radical Biol Med (2010) 49(9):1328–41. doi: 10.1016/j.freeradbiomed.2010.07.028
34. Wang M, Charareh P, Lei X, Zhong JL. Autophagy: multiple mechanisms to protect skin from ultraviolet radiation-driven photoaging. Oxid Med Cell Longevity (2019) 2019:8135985. doi: 10.1155/2019/8135985
35. Poljšak B, Fink R. The protective role of antioxidants in the defence against ros/rns-mediated environmental pollution. Oxid Med Cell Longevity (2014) 2014:671539. doi: 10.1155/2014/671539
36. Zhang Y, Shen W, Zhang P, Chen L, Xiao C. Gsh-triggered release of sulfur dioxide gas to regulate redox balance for enhanced photodynamic therapy. Chem Commun (Cambridge England) (2020) 56(42):5645–8. doi: 10.1039/d0cc00470g
37. Liu K, Hua S, Song L. Pm2.5 exposure and asthma development: the key role of oxidative stress. Oxid Med Cell Longevity (2022) 2022:3618806. doi: 10.1155/2022/3618806
38. Nolfi-Donegan D, Braganza A, Shiva S. Mitochondrial electron transport chain: oxidative phosphorylation, oxidant production, and methods of measurement. Redox Biol (2020) 37:101674. doi: 10.1016/j.redox.2020.101674
39. Chocry M, Leloup L. The nadph oxidase family and its inhibitors. Antioxid Redox Signaling (2020) 33(5):332–53. doi: 10.1089/ars.2019.7915
40. He A, Dean JM, Lodhi IJ. Peroxisomes as cellular adaptors to metabolic and environmental stress. Trends Cell Biol (2021) 31(8):656–70. doi: 10.1016/j.tcb.2021.02.005
41. Wang Y, Qi H, Liu Y, Duan C, Liu X, Xia T, et al. The double-edged roles of ros in cancer prevention and therapy. Theranostics (2021) 11(10):4839–57. doi: 10.7150/thno.56747
42. Gowda P, Lathoria K, Umdor SB, Sen E. Brg1 mutation alters oxidative stress responses in glioblastoma. Neurochemistry Int (2021) 150:105189. doi: 10.1016/j.neuint.2021.105189
43. Lin HY, Lim SW, Hsu TI, Yang WB, Huang CC, Tsai YT, et al. Ccaat/enhancer-binding protein delta regulates glioblastoma survival through catalase-mediated hydrogen peroxide clearance. Oxid Med Cell Longevity (2022) 2022:4081380. doi: 10.1155/2022/4081380
44. Chen TC, Chuang JY, Ko CY, Kao TJ, Yang PY, Yu CH, et al. Ar ubiquitination induced by the curcumin analog suppresses growth of temozolomide-resistant glioblastoma through disrupting gpx4-mediated redox homeostasis. Redox Biol (2020) 30:101413. doi: 10.1016/j.redox.2019.101413
45. Zhu Z, Du S, Du Y, Ren J, Ying G, Yan Z. Glutathione reductase mediates drug resistance in glioblastoma cells by regulating redox homeostasis. J neurochemistry (2018) 144(1):93–104. doi: 10.1111/jnc.14250
46. Liu Y, Liang Y, Zheng T, Yang G, Zhang X, Sun Z, et al. Inhibition of heme oxygenase-1 enhances anti-cancer effects of arsenic trioxide on glioma cells. J neuro-oncol (2011) 104(2):449–58. doi: 10.1007/s11060-010-0513-1
47. Kim SH, Kwon CH, Nakano I. Detoxification of oxidative stress in glioma stem cells: mechanism, clinical relevance, and therapeutic development. J Neurosci Res (2014) 92(11):1419–24. doi: 10.1002/jnr.23431
48. Pires V, Bramatti I, Aschner M, Branco V, Carvalho C. Thioredoxin reductase inhibitors as potential antitumors: mercury compounds efficacy in glioma cells. Front Mol Biosci (2022) 9:889971. doi: 10.3389/fmolb.2022.889971
49. Lei K, Xia Y, Wang XC, Ahn EH, Jin L, Ye K. C/ebpβ Mediates nqo1 and gstp1 anti-oxidative reductases expression in glioblastoma, promoting brain tumor proliferation. Redox Biol (2020) 34:101578. doi: 10.1016/j.redox.2020.101578
50. Sleire L, Skeie BS, Netland IA, Førde HE, Dodoo E, Selheim F, et al. Drug repurposing: sulfasalazine sensitizes gliomas to gamma knife radiosurgery by blocking cystine uptake through system xc-, leading to glutathione depletion. Oncogene (2015) 34(49):5951–9. doi: 10.1038/onc.2015.60
51. Deveci HA, Akyuva Y, Nur G, Nazıroğlu M. Alpha lipoic acid attenuates hypoxia-induced apoptosis, inflammation and mitochondrial oxidative stress via inhibition of trpa1 channel in human glioblastoma cell line. Biomed Pharmacother = Biomed Pharmacotherapie (2019) 111:292–304. doi: 10.1016/j.biopha.2018.12.077
52. Frontiñán-Rubio J, Santiago-Mora RM, Nieva-Velasco CM, Ferrín G, Martínez-González A, Gómez MV, et al. Regulation of the oxidative balance with coenzyme Q10 sensitizes human glioblastoma cells to radiation and temozolomide. Radiother Oncol (2018) 128(2):236–44. doi: 10.1016/j.radonc.2018.04.033
53. Lu MC, Ji JA, Jiang ZY, You QD. The keap1-nrf2-are pathway as a potential preventive and therapeutic target: an update. Medicinal Res Rev (2016) 36(5):924–63. doi: 10.1002/med.21396
54. Broekgaarden M, Weijer R, van Gulik TM, Hamblin MR, Heger M. Tumor cell survival pathways activated by photodynamic therapy: A molecular basis for pharmacological inhibition strategies. Cancer metastasis Rev (2015) 34(4):643–90. doi: 10.1007/s10555-015-9588-7
55. Bellezza I, Giambanco I, Minelli A, Donato R. Nrf2-keap1 signaling in oxidative and reductive stress. Biochim Biophys Acta Mol Cell Res (2018) 1865(5):721–33. doi: 10.1016/j.bbamcr.2018.02.010
56. Zhan M, Wang H, Xu SW, Yang LH, Chen W, Zhao SX, et al. Variants in oxidative stress-related genes affect the chemosensitivity through nrf2-mediated signaling pathway in biliary tract cancer. EBioMedicine (2019) 48:143–60. doi: 10.1016/j.ebiom.2019.08.037
57. Chowdhury I, Fisher AB, Christofidou-Solomidou M, Gao L, Tao JQ, Sorokina EM, et al. Keratinocyte growth factor and glucocorticoid induction of human peroxiredoxin 6 gene expression occur by independent mechanisms that are synergistic. Antioxid Redox Signaling (2014) 20(3):391–402. doi: 10.1089/ars.2012.4634
58. Lv H, Zhu C, Wei W, Lv X, Yu Q, Deng X, et al. Enhanced keap1-nrf2/trx-1 axis by daphnetin protects against oxidative stress-driven hepatotoxicity via inhibiting ask1/jnk and txnip/nlrp3 inflammasome activation. Phytomed: Int J Phytother Phytopharmacol (2020) 71:153241. doi: 10.1016/j.phymed.2020.153241
59. Xu H, Jin J, Chen Y, Wu G, Zhu H, Wang Q, et al. Gbp3 promotes glioblastoma resistance to temozolomide by enhancing DNA damage repair. Oncogene (2022) 41(31):3876–85. doi: 10.1038/s41388-022-02397-5
60. Wick W, Weller M, van den Bent M, Sanson M, Weiler M, von Deimling A, et al. Mgmt testing–the challenges for biomarker-based glioma treatment. Nat Rev Neurol (2014) 10(7):372–85. doi: 10.1038/nrneurol.2014.100
61. Knijnenburg TA, Wang L, Zimmermann MT, Chambwe N, Gao GF, Cherniack AD, et al. Genomic and molecular landscape of DNA damage repair deficiency across the cancer genome atlas. Cell Rep (2018) 23(1):239–54.e6. doi: 10.1016/j.celrep.2018.03.076
62. Nitta M, Kozono D, Kennedy R, Stommel J, Ng K, Zinn PO, et al. Targeting egfr induced oxidative stress by parp1 inhibition in glioblastoma therapy. PloS One (2010) 5(5):e10767. doi: 10.1371/journal.pone.0010767
63. Gan T, Wang Y, Xie M, Wang Q, Zhao S, Wang P, et al. Mex3a impairs DNA mismatch repair signaling and mediates acquired temozolomide resistance in glioblastoma. Cancer Res (2022) 82(22):4234–46. doi: 10.1158/0008-5472.Can-22-2036
64. Chen Y, Liu P, Sun P, Jiang J, Zhu Y, Dong T, et al. Oncogenic msh6-cxcr4-tgfb1 feedback loop: A novel therapeutic target of photothermal therapy in glioblastoma multiforme. Theranostics (2019) 9(5):1453–73. doi: 10.7150/thno.29987
65. Ray A, Milum K, Battu A, Wani G, Wani AA. Ner initiation factors, ddb2 and xpc, regulate uv radiation response by recruiting atr and atm kinases to DNA damage sites. DNA Repair (2013) 12(4):273–83. doi: 10.1016/j.dnarep.2013.01.003
66. Yi GZ, Huang G, Guo M, Zhang X, Wang H, Deng S, et al. Acquired temozolomide resistance in mgmt-deficient glioblastoma cells is associated with regulation of DNA repair by dhc2. Brain: J Neurol (2019) 142(8):2352–66. doi: 10.1093/brain/awz202
67. Oyewole AO, Birch-Machin MA. Mitochondria-targeted antioxidants. FASEB J (2015) 29(12):4766–71. doi: 10.1096/fj.15-275404
68. Scuderi SA, Filippone A, Basilotta R, Mannino D, Casili G, Capra AP, et al. Gsk343, an inhibitor of enhancer of zeste homolog 2, reduces glioblastoma progression through inflammatory process modulation: focus on canonical and non-canonical nf-Kb/Iκbα Pathways. Int J Mol Sci (2022) 23(22):13915. doi: 10.3390/ijms232213915
69. Liang J, Yang Y, Li X, Cai G, Cao J, Zhang B. Expression of eif4e gene in glioma and its sensitivity to oxidative stress. Oxid Med Cell Longev (2022) 2022:5413035. doi: 10.1155/2022/5413035
70. Chang KY, Hsu TI, Hsu CC, Tsai SY, Liu JJ, Chou SW, et al. Specificity protein 1-modulated superoxide dismutase 2 enhances temozolomide resistance in glioblastoma, which is independent of O(6)-methylguanine-DNA methyltransferase. Redox Biol (2017) 13:655–64. doi: 10.1016/j.redox.2017.08.005
71. Flor S, Oliva CR, Ali MY, Coleman KL, Greenlee JD, Jones KA, et al. Catalase overexpression drives an aggressive phenotype in glioblastoma. Antioxid (Basel Switzerland) (2021) 10(12):1988. doi: 10.3390/antiox10121988
72. Pisoschi AM, Pop A. The role of antioxidants in the chemistry of oxidative stress: A review. Eur J Med Chem (2015) 97:55–74. doi: 10.1016/j.ejmech.2015.04.040
73. Zhan S, Lu L, Pan SS, Wei XQ, Miao RR, Liu XH, et al. Targeting nqo1/gpx4-mediated ferroptosis by plumbagin suppresses in vitro and in vivo glioma growth. Br J Cancer (2022) 127(2):364–76. doi: 10.1038/s41416-022-01800-y
74. Lv H, Zhen C, Liu J, Yang P, Hu L, Shang P. Unraveling the potential role of glutathione in multiple forms of cell death in cancer therapy. Oxid Med Cell Longevity (2019) 2019:3150145. doi: 10.1155/2019/3150145
75. Ozyerli-Goknar E, Sur-Erdem I, Seker F, Cingöz A, Kayabolen A, Kahya-Yesil Z, et al. The fungal metabolite chaetocin is a sensitizer for pro-apoptotic therapies in glioblastoma. Cell Death Dis (2019) 10(12):894. doi: 10.1038/s41419-019-2107-y
76. Dennery PA. Signaling function of heme oxygenase proteins. Antioxid Redox Signaling (2014) 20(11):1743–53. doi: 10.1089/ars.2013.5674
77. Sukumari-Ramesh S, Prasad N, Alleyne CH, Vender JR, Dhandapani KM. Overexpression of nrf2 attenuates carmustine-induced cytotoxicity in U87mg human glioma cells. BMC Cancer (2015) 15:118. doi: 10.1186/s12885-015-1134-z
78. West J, Roston T, David J, Allan K, Loberg M. Piecing together how peroxiredoxins maintain genomic stability. Antioxidants (2018) 7(12):177. doi: 10.3390/antiox7120177
79. Gao F, Zheng W, Gao L, Cai P, Liu R, Wang Y, et al. Au nanoclusters and photosensitizer dual loaded spatiotemporal controllable liposomal nanocomposites enhance tumor photodynamic therapy effect by inhibiting thioredoxin reductase. Advanced Healthc Mater (2017) 6(7):1601453. doi: 10.1002/adhm.201601453
80. Ahmadi R, Urig S, Hartmann M, Helmke BM, Koncarevic S, Allenberger B, et al. Antiglioma activity of 2,2’:6’,2”-terpyridineplatinum(Ii) complexes in a rat model–effects on cellular redox metabolism. Free Radic Biol Med (2006) 40(5):763–78. doi: 10.1016/j.freeradbiomed.2005.09.031
81. Lei K, Gu X, Alvarado AG, Du Y, Luo S, Ahn EH, et al. Discovery of a dual inhibitor of nqo1 and gstp1 for treating glioblastoma. J Hematol Oncol (2020) 13(1):141. doi: 10.1186/s13045-020-00979-y
82. Ross D, Siegel D. The diverse functionality of nqo1 and its roles in redox control. Redox Biol (2021) 41:101950. doi: 10.1016/j.redox.2021.101950
83. Zhong B, Yu J, Hou Y, Ai N, Ge W, Lu JJ, et al. A novel strategy for glioblastoma treatment by induction of noptosis, an nqo1-dependent necrosis. Free Radic Biol Med (2021) 166:104–15. doi: 10.1016/j.freeradbiomed.2021.02.014
84. Koehler CM, Beverly KN, Leverich EP. Redox pathways of the mitochondrion. Antioxid Redox Signaling (2006) 8(5-6):813–22. doi: 10.1089/ars.2006.8.813
85. Pérez-Ortiz JM, Tranque P, Vaquero CF, Domingo B, Molina F, Calvo S, et al. Glitazones differentially regulate primary astrocyte and glioma cell survival. Involvement of reactive oxygen species and peroxisome proliferator-activated receptor-gamma. J Biol Chem (2004) 279(10):8976–85. doi: 10.1074/jbc.M308518200
86. Wang C, He C, Lu S, Wang X, Wang L, Liang S, et al. Autophagy activated by silibinin contributes to glioma cell death via induction of oxidative stress-mediated bnip3-dependent nuclear translocation of aif. Cell Death Dis (2020) 11(8):630. doi: 10.1038/s41419-020-02866-3
87. Fasipe B, Faria A, Laher I. Potential for novel therapeutic uses of alpha lipoic acid. Curr medicinal Chem (2023) 30(35):3942–54. doi: 10.2174/0929867329666221006115329
88. Pisoschi AM, Pop A, Iordache F, Stanca L, Predoi G, Serban AI. Oxidative stress mitigation by antioxidants - an overview on their chemistry and influences on health status. Eur J medicinal Chem (2021) 209:112891. doi: 10.1016/j.ejmech.2020.112891
89. Visnes T, Benítez-Buelga C, Cázares-Körner A, Sanjiv K, Hanna BMF, Mortusewicz O, et al. Targeting ogg1 arrests cancer cell proliferation by inducing replication stress. Nucleic Acids Res (2020) 48(21):12234–51. doi: 10.1093/nar/gkaa1048
90. McAdam E, Brem R, Karran P. Oxidative stress-induced protein damage inhibits DNA repair and determines mutation risk and therapeutic efficacy. Mol Cancer Res: MCR (2016) 14(7):612–22. doi: 10.1158/1541-7786.Mcr-16-0053
91. Fabrizio FP, Sparaneo A, Trombetta D, Muscarella LA. Epigenetic versus genetic deregulation of the keap1/nrf2 axis in solid tumors: focus on methylation and noncoding rnas. Oxid Med Cell Longevity (2018) 2018:2492063. doi: 10.1155/2018/2492063
92. Zheng XJ, Chen WL, Yi J, Li W, Liu JY, Fu WQ, et al. Apolipoprotein C1 promotes glioblastoma tumorigenesis by reducing keap1/nrf2 and cbs-regulated ferroptosis. Acta Pharmacologica Sin (2022) 43(11):2977–92. doi: 10.1038/s41401-022-00917-3
93. Cong ZX, Wang HD, Zhou Y, Wang JW, Pan H, Zhang DD, et al. Temozolomide and irradiation combined treatment-induced nrf2 activation increases chemoradiation sensitivity in human glioblastoma cells. J Neurooncol (2014) 116(1):41–8. doi: 10.1007/s11060-013-1260-x
94. Fujii S, Sawa T, Ihara H, Tong KI, Ida T, Okamoto T, et al. The critical role of nitric oxide signaling, via protein S-guanylation and nitrated cyclic gmp, in the antioxidant adaptive response. J Biol Chem (2010) 285(31):23970–84. doi: 10.1074/jbc.M110.145441
95. Dapash M, Hou D, Castro B, Lee-Chang C, Lesniak MS. The interplay between glioblastoma and its microenvironment. Cells (2021) 10(9):2257. doi: 10.3390/cells10092257
96. Gieryng A, Pszczolkowska D, Walentynowicz KA, Rajan WD, Kaminska B. Immune microenvironment of gliomas. Lab Invest (2017) 97(5):498–518. doi: 10.1038/labinvest.2017.19
97. Kuo CL, Ponneri Babuharisankar A, Lin YC, Lien HW, Lo YK, Chou HY, et al. Mitochondrial oxidative stress in the tumor microenvironment and cancer immunoescape: foe or friend? J Biomed Sci (2022) 29(1):74. doi: 10.1186/s12929-022-00859-2
98. Kotsafti A, Scarpa M, Castagliuolo I, Scarpa M. Reactive oxygen species and antitumor immunity-from surveillance to evasion. Cancers (Basel) (2020) 12(7):1748. doi: 10.3390/cancers12071748
99. Wei J, Chen P, Gupta P, Ott M, Zamler D, Kassab C, et al. Immune biology of glioma-associated macrophages and microglia: functional and therapeutic implications. Neuro-oncology (2020) 22(2):180–94. doi: 10.1093/neuonc/noz212
100. Kaminska B, Mota M, Pizzi M. Signal transduction and epigenetic mechanisms in the control of microglia activation during neuroinflammation. Biochim Biophys Acta (2016) 1862(3):339–51. doi: 10.1016/j.bbadis.2015.10.026
101. Zha C, Meng X, Li L, Mi S, Qian D, Li Z, et al. Neutrophil extracellular traps mediate the crosstalk between glioma progression and the tumor microenvironment via the hmgb1/rage/il-8 axis. Cancer Biol Med (2020) 17(1):154–68. doi: 10.20892/j.issn.2095-3941.2019.0353
102. Raychaudhuri B, Rayman P, Ireland J, Ko J, Rini B, Borden EC, et al. Myeloid-derived suppressor cell accumulation and function in patients with newly diagnosed glioblastoma. Neuro-oncology (2011) 13(6):591–9. doi: 10.1093/neuonc/nor042
103. Ostrand-Rosenberg S, Sinha P. Myeloid-derived suppressor cells: linking inflammation and cancer. J Immunol (Baltimore Md: 1950) (2009) 182(8):4499–506. doi: 10.4049/jimmunol.0802740
104. Rodriguez PC, Quiceno DG, Ochoa AC. L-arginine availability regulates T-lymphocyte cell-cycle progression. Blood (2007) 109(4):1568–73. doi: 10.1182/blood-2006-06-031856
105. Gabrilovich DI, Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nat Rev Immunol (2009) 9(3):162–74. doi: 10.1038/nri2506
106. Dubinski D, Wölfer J, Hasselblatt M, Schneider-Hohendorf T, Bogdahn U, Stummer W, et al. Cd4+ T effector memory cell dysfunction is associated with the accumulation of granulocytic myeloid-derived suppressor cells in glioblastoma patients. Neuro-oncology (2016) 18(6):807–18. doi: 10.1093/neuonc/nov280
107. Tao B, Shi J, Shuai S, Zhou H, Zhang H, Li B, et al. Cyb561d2 up-regulation activates stat3 to induce immunosuppression and aggression in gliomas. J Trans Med (2021) 19(1):338. doi: 10.1186/s12967-021-02987-z
108. Aboelella NS, Brandle C, Kim T, Ding ZC, Zhou G. Oxidative stress in the tumor microenvironment and its relevance to cancer immunotherapy. Cancers (2021) 13(5):986. doi: 10.3390/cancers13050986
109. Ohta A, Sitkovsky M. Extracellular adenosine-mediated modulation of regulatory T cells. Front Immunol (2014) 5:304. doi: 10.3389/fimmu.2014.00304
110. Ma T, Renz BW, Ilmer M, Koch D, Yang Y, Werner J, et al. Myeloid-derived suppressor cells in solid tumors. Cells (2022) 11(2):310. doi: 10.3390/cells11020310
111. Wang M, Zhou Z, Wang X, Zhang C, Jiang X. Natural killer cell awakening: unleash cancer-immunity cycle against glioblastoma. Cell Death Dis (2022) 13(7):588. doi: 10.1038/s41419-022-05041-y
112. Chaplin DD. Overview of the immune response. J Allergy Clin Immunol (2010) 125(2 Suppl 2):S3–23. doi: 10.1016/j.jaci.2009.12.980
113. Gordon S, Taylor PR. Monocyte and macrophage heterogeneity. Nat Rev Immunol (2005) 5(12):953–64. doi: 10.1038/nri1733
114. Gracia-Hernandez M, Sotomayor EM, Villagra A. Targeting macrophages as a therapeutic option in coronavirus disease 2019. Front Pharmacol (2020) 11:577571. doi: 10.3389/fphar.2020.577571
115. Randolph GJ, Jakubzick C, Qu C. Antigen presentation by monocytes and monocyte-derived cells. Curr Opin Immunol (2008) 20(1):52–60. doi: 10.1016/j.coi.2007.10.010
116. Borst K, Dumas AA, Prinz M. Microglia: immune and non-immune functions. Immunity (2021) 54(10):2194–208. doi: 10.1016/j.immuni.2021.09.014
117. Brown GC, Neher JJ. Microglial phagocytosis of live neurons. Nat Rev Neurosci (2014) 15(4):209–16. doi: 10.1038/nrn3710
118. Nguyen PT, Dorman LC, Pan S, Vainchtein ID, Han RT, Nakao-Inoue H, et al. Microglial remodeling of the extracellular matrix promotes synapse plasticity. Cell (2020) 182(2):388–403.e15. doi: 10.1016/j.cell.2020.05.050
119. Nayak D, Roth TL, McGavern DB. Microglia development and function. Annu Rev Immunol (2014) 32:367–402. doi: 10.1146/annurev-immunol-032713-120240
120. Larionova I, Tuguzbaeva G, Ponomaryova A, Stakheyeva M, Cherdyntseva N, Pavlov V, et al. Tumor-associated macrophages in human breast, colorectal, lung, ovarian and prostate cancers. Front Oncol (2020) 10:566511. doi: 10.3389/fonc.2020.566511
121. Zeng F, Li G, Liu X, Zhang K, Huang H, Jiang T, et al. Plasminogen activator urokinase receptor implies immunosuppressive features and acts as an unfavorable prognostic biomarker in glioma. Oncologist (2021) 26(8):e1460–e9. doi: 10.1002/onco.13750
122. Shan K, Feng N, Cui J, Wang S, Qu H, Fu G, et al. Resolvin D1 and D2 inhibit tumour growth and inflammation via modulating macrophage polarization. J Cell Mol Med (2020) 24(14):8045–56. doi: 10.1111/jcmm.15436
123. Mantovani A, Sica A, Locati M. New vistas on macrophage differentiation and activation. Eur J Immunol (2007) 37(1):14–6. doi: 10.1002/eji.200636910
124. Lakshmi Narendra B, Eshvendar Reddy K, Shantikumar S, Ramakrishna S. Immune system: A double-edged sword in cancer. Inflammation Res (2013) 62(9):823–34. doi: 10.1007/s00011-013-0645-9
125. Chen Z, Hambardzumyan D. Immune microenvironment in glioblastoma subtypes. Front Immunol (2018) 9:1004. doi: 10.3389/fimmu.2018.01004
126. da Fonseca AC, Badie B. Microglia and macrophages in malignant gliomas: recent discoveries and implications for promising therapies. Clin Dev Immunol (2013) 2013:264124. doi: 10.1155/2013/264124
127. Chanmee T, Ontong P, Konno K, Itano N. Tumor-associated macrophages as major players in the tumor microenvironment. Cancers (2014) 6(3):1670–90. doi: 10.3390/cancers6031670
128. Noy R, Pollard JW. Tumor-associated macrophages: from mechanisms to therapy. Immunity (2014) 41(1):49–61. doi: 10.1016/j.immuni.2014.06.010
129. Zhou W, Yu X, Sun S, Zhang X, Yang W, Zhang J, et al. Increased expression of mmp-2 and mmp-9 indicates poor prognosis in glioma recurrence. Biomed Pharmacother = Biomed Pharmacotherapie (2019) 118:109369. doi: 10.1016/j.biopha.2019.109369
130. Brandenburg S, Müller A, Turkowski K, Radev YT, Rot S, Schmidt C, et al. Resident microglia rather than peripheral macrophages promote vascularization in brain tumors and are source of alternative pro-angiogenic factors. Acta Neuropathologica (2016) 131(3):365–78. doi: 10.1007/s00401-015-1529-6
131. Ramírez-Expósito MJ, Martínez-Martos JM. The delicate equilibrium between oxidants and antioxidants in brain glioma. Curr Neuropharmacol (2019) 17(4):342–51. doi: 10.2174/1570159x16666180302120925
132. Yu MO, Song NH, Park KJ, Park DH, Kim SH, Chae YS, et al. Romo1 is associated with ros production and cellular growth in human gliomas. J neuro-oncol (2015) 121(1):73–81. doi: 10.1007/s11060-014-1608-x
133. Sun G, Cao Y, Qian C, Wan Z, Zhu J, Guo J, et al. Romo1 is involved in the immune response of glioblastoma by regulating the function of macrophages. Aging (2020) 12(2):1114–27. doi: 10.18632/aging.102648
134. Kaushal P, Zhu J, Wan Z, Chen H, Ye J, Luo C. Prognosis and immune landscapes in glioblastoma based on gene-signature related to reactive-oxygen-species. Neuromolecular Med (2023) 25(1):102–19. doi: 10.1007/s12017-022-08719-w
135. Dokic I, Hartmann C, Herold-Mende C, Régnier-Vigouroux A. Glutathione peroxidase 1 activity dictates the sensitivity of glioblastoma cells to oxidative stress. Glia (2012) 60(11):1785–800. doi: 10.1002/glia.22397
136. Zhang Y, Handy DE, Loscalzo J. Adenosine-dependent induction of glutathione peroxidase 1 in human primary endothelial cells and protection against oxidative stress. Circ Res (2005) 96(8):831–7. doi: 10.1161/01.Res.0000164401.21929.Cf
137. Law AMK, Valdes-Mora F, Gallego-Ortega D. Myeloid-derived suppressor cells as a therapeutic target for cancer. Cells (2020) 9(3):561. doi: 10.3390/cells9030561
138. Peng M, Zhang Q, Liu Y, Guo X, Ju J, Xu L, et al. Apolipoprotein a-I mimetic peptide L-4f suppresses granulocytic-myeloid-derived suppressor cells in mouse pancreatic cancer. Front Pharmacol (2020) 11:576. doi: 10.3389/fphar.2020.00576
139. Veglia F, Perego M, Gabrilovich D. Myeloid-derived suppressor cells coming of age. Nat Immunol (2018) 19(2):108–19. doi: 10.1038/s41590-017-0022-x
140. Wang Y, Schafer CC, Hough KP, Tousif S, Duncan SR, Kearney JF, et al. Myeloid-derived suppressor cells impair B cell responses in lung cancer through il-7 and stat5. J Immunol (Baltimore Md: 1950) (2018) 201(1):278–95. doi: 10.4049/jimmunol.1701069
141. Huang B, Pan PY, Li Q, Sato AI, Levy DE, Bromberg J, et al. Gr-1+Cd115+ Immature myeloid suppressor cells mediate the development of tumor-induced T regulatory cells and T-cell anergy in tumor-bearing host. Cancer Res (2006) 66(2):1123–31. doi: 10.1158/0008-5472.Can-05-1299
142. Beury DW, Parker KH, Nyandjo M, Sinha P, Carter KA, Ostrand-Rosenberg S. Cross-talk among myeloid-derived suppressor cells, macrophages, and tumor cells impacts the inflammatory milieu of solid tumors. J Leukoc Biol (2014) 96(6):1109–18. doi: 10.1189/jlb.3A0414-210R
143. Zhao H, Teng D, Yang L, Xu X, Chen J, Jiang T, et al. Myeloid-derived itaconate suppresses cytotoxic cd8(+) T cells and promotes tumour growth. Nat Metab (2022) 4(12):1660–73. doi: 10.1038/s42255-022-00676-9
144. Hoechst B, Voigtlaender T, Ormandy L, Gamrekelashvili J, Zhao F, Wedemeyer H, et al. Myeloid derived suppressor cells inhibit natural killer cells in patients with hepatocellular carcinoma via the nkp30 receptor. Hepatol (Baltimore Md) (2009) 50(3):799–807. doi: 10.1002/hep.23054
145. Ostrand-Rosenberg S, Sinha P, Beury DW, Clements VK. Cross-talk between myeloid-derived suppressor cells (Mdsc), macrophages, and dendritic cells enhances tumor-induced immune suppression. Semin Cancer Biol (2012) 22(4):275–81. doi: 10.1016/j.semcancer.2012.01.011
146. García-Navas R, Munder M, Mollinedo F. Depletion of L-arginine induces autophagy as a cytoprotective response to endoplasmic reticulum stress in human T lymphocytes. Autophagy (2012) 8(11):1557–76. doi: 10.4161/auto.21315
147. Lamas B, Vergnaud-Gauduchon J, Goncalves-Mendes N, Perche O, Rossary A, Vasson MP, et al. Altered functions of natural killer cells in response to L-arginine availability. Cell Immunol (2012) 280(2):182–90. doi: 10.1016/j.cellimm.2012.11.018
148. Umansky V, Blattner C, Gebhardt C, Utikal J. The role of myeloid-derived suppressor cells (Mdsc) in cancer progression. Vaccines (2016) 4(4):36. doi: 10.3390/vaccines4040036
149. Su MT, Kumata S, Endo S, Okada Y, Takai T. Lilrb4 promotes tumor metastasis by regulating mdscs and inhibiting mir-1 family mirnas. Oncoimmunology (2022) 11(1):2060907. doi: 10.1080/2162402x.2022.2060907
150. Salminen A, Kaarniranta K, Kauppinen A. Immunosenescence: the potential role of myeloid-derived suppressor cells (Mdsc) in age-related immune deficiency. Cell Mol Life Sci (2019) 76(10):1901–18. doi: 10.1007/s00018-019-03048-x
151. Kim ES, Kim JE, Patel MA, Mangraviti A, Ruzevick J, Lim M. Immune checkpoint modulators: an emerging antiglioma armamentarium. J Immunol Res (2016) 2016:4683607. doi: 10.1155/2016/4683607
152. Salemizadeh Parizi M, Salemizadeh Parizi F, Abdolhosseini S, Vanaei S, Manzouri A, Ebrahimzadeh F. Myeloid-derived suppressor cells (Mdscs) in brain cancer: challenges and therapeutic strategies. Inflammopharmacology (2021) 29(6):1613–24. doi: 10.1007/s10787-021-00878-9
153. Kamran N, Kadiyala P, Saxena M, Candolfi M, Li Y, Moreno-Ayala MA, et al. Immunosuppressive myeloid cells’ Blockade in the glioma microenvironment enhances the efficacy of immune-stimulatory gene therapy. Mol Ther (2017) 25(1):232–48. doi: 10.1016/j.ymthe.2016.10.003
154. De Leo A, Ugolini A, Veglia F. Myeloid cells in glioblastoma microenvironment. Cells (2020) 10(1):18. doi: 10.3390/cells10010018
155. Bayik D, Bartels CF, Lovrenert K, Watson DC, Zhang D, Kay K, et al. Distinct cell adhesion signature defines glioblastoma myeloid-derived suppressor cell subsets. Cancer Res (2022) 82(22):4274–87. doi: 10.1158/0008-5472.Can-21-3840
156. Bronte V, Brandau S, Chen SH, Colombo MP, Frey AB, Greten TF, et al. Recommendations for myeloid-derived suppressor cell nomenclature and characterization standards. Nat Commun (2016) 7:12150. doi: 10.1038/ncomms12150
157. Noman MZ, Desantis G, Janji B, Hasmim M, Karray S, Dessen P, et al. Pd-L1 is a novel direct target of hif-1α, and its blockade under hypoxia enhanced mdsc-mediated T cell activation. J Exp Med (2014) 211(5):781–90. doi: 10.1084/jem.20131916
158. Beury DW, Carter KA, Nelson C, Sinha P, Hanson E, Nyandjo M, et al. Myeloid-derived suppressor cell survival and function are regulated by the transcription factor nrf2. J Immunol (Baltimore Md: 1950) (2016) 196(8):3470–8. doi: 10.4049/jimmunol.1501785
159. Grabowski MM, Sankey EW, Ryan KJ, Chongsathidkiet P, Lorrey SJ, Wilkinson DS, et al. Immune suppression in gliomas. J neuro-oncol (2021) 151(1):3–12. doi: 10.1007/s11060-020-03483-y
160. Ohl K, Tenbrock K. Reactive oxygen species as regulators of mdsc-mediated immune suppression. Front Immunol (2018) 9:2499. doi: 10.3389/fimmu.2018.02499
161. Kusmartsev S, Gabrilovich DI. Inhibition of myeloid cell differentiation in cancer: the role of reactive oxygen species. J Leukoc Biol (2003) 74(2):186–96. doi: 10.1189/jlb.0103010
162. Corzo CA, Cotter MJ, Cheng P, Cheng F, Kusmartsev S, Sotomayor E, et al. Mechanism regulating reactive oxygen species in tumor-induced myeloid-derived suppressor cells. J Immunol (Baltimore Md: 1950) (2009) 182(9):5693–701. doi: 10.4049/jimmunol.0900092
163. Irshad K, Srivastava C, Malik N, Arora M, Gupta Y, Goswami S, et al. Upregulation of atypical cadherin fat1 promotes an immunosuppressive tumor microenvironment via tgf-B. Front Immunol (2022) 13:813888. doi: 10.3389/fimmu.2022.813888
164. Shen T, Chen X, Chen Y, Xu Q, Lu F, Liu S. Increased pd-L1 expression and pd-L1/cd86 ratio on dendritic cells were associated with impaired dendritic cells function in hcv infection. J Med Virol (2010) 82(7):1152–9. doi: 10.1002/jmv.21809
165. Mittal SK, Cho KJ, Ishido S, Roche PA. Interleukin 10 (Il-10)-mediated immunosuppression: march-I induction regulates antigen presentation by macrophages but not dendritic cells. J Biol Chem (2015) 290(45):27158–67. doi: 10.1074/jbc.M115.682708
166. Li MO, Wan YY, Sanjabi S, Robertson AK, Flavell RA. Transforming growth factor-beta regulation of immune responses. Annu Rev Immunol (2006) 24:99–146. doi: 10.1146/annurev.immunol.24.021605.090737
167. Amarnath S, Mangus CW, Wang JC, Wei F, He A, Kapoor V, et al. The pdl1-pd1 axis converts human th1 cells into regulatory T cells. Sci Trans Med (2011) 3(111):111ra20. doi: 10.1126/scitranslmed.3003130
168. Muntjewerff EM, Meesters LD, van den Bogaart G. Antigen cross-presentation by macrophages. Front Immunol (2020) 11:1276. doi: 10.3389/fimmu.2020.01276
169. Spangler JB, Tomala J, Luca VC, Jude KM, Dong S, Ring AM, et al. Antibodies to interleukin-2 elicit selective T cell subset potentiation through distinct conformational mechanisms. Immunity (2015) 42(5):815–25. doi: 10.1016/j.immuni.2015.04.015
170. Waldman AD, Fritz JM, Lenardo MJ. A guide to cancer immunotherapy: from T cell basic science to clinical practice. Nat Rev Immunol (2020) 20(11):651–68. doi: 10.1038/s41577-020-0306-5
171. Savage PA, Klawon DEJ, Miller CH. Regulatory T cell development. Annu Rev Immunol (2020) 38:421–53. doi: 10.1146/annurev-immunol-100219-020937
172. Borst J, Ahrends T, Bąbała N, Melief CJM, Kastenmüller W. Cd4(+) T cell help in cancer immunology and immunotherapy. Nat Rev Immunol (2018) 18(10):635–47. doi: 10.1038/s41577-018-0044-0
173. Gähler A, Trufa DI, Chiriac MT, Tausche P, Hohenberger K, Brunst AK, et al. Glucose-restricted diet regulates the tumor immune microenvironment and prevents tumor growth in lung adenocarcinoma. Front Oncol (2022) 12:873293. doi: 10.3389/fonc.2022.873293
174. Kim KD, Bae S, Capece T, Nedelkovska H, de Rubio RG, Smrcka AV, et al. Targeted calcium influx boosts cytotoxic T lymphocyte function in the tumour microenvironment. Nat Commun (2017) 8:15365. doi: 10.1038/ncomms15365
175. Durgeau A, Virk Y, Corgnac S, Mami-Chouaib F. Recent advances in targeting cd8 T-cell immunity for more effective cancer immunotherapy. Front Immunol (2018) 9:14. doi: 10.3389/fimmu.2018.00014
176. Ni D, Tang T, Lu Y, Xu K, Shao Y, Saaoud F, et al. Canonical secretomes, innate immune caspase-1-, 4/11-gasdermin D non-canonical secretomes and exosomes may contribute to maintain treg-ness for treg immunosuppression, tissue repair and modulate anti-tumor immunity via ros pathways. Front Immunol (2021) 12:678201. doi: 10.3389/fimmu.2021.678201
177. Huang BR, Liu YS, Lai SW, Lin HJ, Shen CK, Yang LY, et al. Caix Regulates Gbm Motility and Tam Adhesion and Polarization through Egfr/Stat3 under Hypoxic Conditions. Int J Mol Sci (2020) 21(16):5838. doi: 10.3390/ijms21165838
178. Travis MA, Sheppard D. Tgf-B Activation and function in immunity. Annu Rev Immunol (2014) 32:51–82. doi: 10.1146/annurev-immunol-032713-120257
179. Ye Z, Zhang S, Cai J, Ye L, Gao L, Wang Y, et al. Development and validation of cuproptosis-associated prognostic signatures in who 2/3 glioma. Front Oncol (2022) 12:967159. doi: 10.3389/fonc.2022.967159
180. Ji H, Liu Z, Wang F, Sun H, Wang N, Liu Y, et al. Novel macrophage-related gene prognostic index for glioblastoma associated with M2 macrophages and T cell dysfunction. Front Immunol (2022) 13:941556. doi: 10.3389/fimmu.2022.941556
181. DiDomenico J, Lamano JB, Oyon D, Li Y, Veliceasa D, Kaur G, et al. The immune checkpoint protein pd-L1 induces and maintains regulatory T cells in glioblastoma. Oncoimmunology (2018) 7(7):e1448329. doi: 10.1080/2162402x.2018.1448329
182. Wang LC, Wang YL, He B, Zheng YJ, Yu HC, Liu ZY, et al. Expression and clinical significance of vista, B7-H3, and pd-L1 in glioma. Clin Immunol (Orlando Fla) (2022) 245:109178. doi: 10.1016/j.clim.2022.109178
183. FranChina DG, Dostert C, Brenner D. Reactive oxygen species: involvement in T cell signaling and metabolism. Trends Immunol (2018) 39(6):489–502. doi: 10.1016/j.it.2018.01.005
184. Malmberg KJ, Arulampalam V, Ichihara F, Petersson M, Seki K, Andersson T, et al. Inhibition of activated/memory (Cd45ro(+)) T cells by oxidative stress associated with block of nf-kappab activation. J Immunol (Baltimore Md: 1950) (2001) 167(5):2595–601. doi: 10.4049/jimmunol.167.5.2595
185. Cemerski S, Cantagrel A, Van Meerwijk JP, Romagnoli P. Reactive oxygen species differentially affect T cell receptor-signaling pathways. J Biol Chem (2002) 277(22):19585–93. doi: 10.1074/jbc.M111451200
186. Wang YG, Long J, Shao DC, Song H. Hyperbaric oxygen inhibits production of cd3+ T cells in the thymus and facilitates malignant glioma cell growth. J Int Med Res (2018) 46(7):2780–91. doi: 10.1177/0300060518767796
187. Hao L, Marshall AJ, Liu L. Bam32/dapp1-dependent neutrophil reactive oxygen species in wkymvm-induced microvascular hyperpermeability. Front Immunol (2020) 11:1028. doi: 10.3389/fimmu.2020.01028
188. Summers C, Rankin SM, Condliffe AM, Singh N, Peters AM, Chilvers ER. Neutrophil kinetics in health and disease. Trends Immunol (2010) 31(8):318–24. doi: 10.1016/j.it.2010.05.006
189. Stegelmeier AA, Chan L, Mehrani Y, Petrik JJ, Wootton SK, Bridle B, et al. Characterization of the impact of oncolytic vesicular stomatitis virus on the trafficking, phenotype, and antigen presentation potential of neutrophils and their ability to acquire a non-structural viral protein. Int J Mol Sci (2020) 21(17):6347. doi: 10.3390/ijms21176347
190. Qi X, Yu Y, Sun R, Huang J, Liu L, Yang Y, et al. Identification and characterization of neutrophil heterogeneity in sepsis. Crit Care (London England) (2021) 25(1):50. doi: 10.1186/s13054-021-03481-0
191. Kolaczkowska E, Kubes P. Neutrophil recruitment and function in health and inflammation. Nat Rev Immunol (2013) 13(3):159–75. doi: 10.1038/nri3399
192. Chang Y, Syahirah R, Wang X, Jin G, Torregrosa-Allen S, Elzey BD, et al. Engineering chimeric antigen receptor neutrophils from human pluripotent stem cells for targeted cancer immunotherapy. Cell Rep (2022) 40(3):111128. doi: 10.1016/j.celrep.2022.111128
193. Sionov RV, Fainsod-Levi T, Zelter T, Polyansky L, Pham CT, Granot Z. Neutrophil cathepsin G and tumor cell rage facilitate neutrophil anti-tumor cytotoxicity. Oncoimmunology (2019) 8(9):e1624129. doi: 10.1080/2162402x.2019.1624129
194. Masucci MT, Minopoli M, Carriero MV. Tumor associated neutrophils. Their role in tumorigenesis, metastasis, prognosis and therapy. Front Oncol (2019) 9:1146. doi: 10.3389/fonc.2019.01146
195. Szczerba BM, Castro-Giner F, Vetter M, Krol I, Gkountela S, Landin J, et al. Neutrophils escort circulating tumour cells to enable cell cycle progression. Nature (2019) 566(7745):553–7. doi: 10.1038/s41586-019-0915-y
196. Cools-Lartigue J, Spicer J, McDonald B, Gowing S, Chow S, Giannias B, et al. Neutrophil extracellular traps sequester circulating tumor cells and promote metastasis. J Clin Invest (2013) 123(8):3446–58. doi: 10.1172/jci67484
197. Brinkmann V, Reichard U, Goosmann C, Fauler B, Uhlemann Y, Weiss DS, et al. Neutrophil extracellular traps kill bacteria. Sci (New York NY) (2004) 303(5663):1532–5. doi: 10.1126/science.1092385
198. Rahbar A, Cederarv M, Wolmer-Solberg N, Tammik C, Stragliotto G, Peredo I, et al. Enhanced neutrophil activity is associated with shorter time to tumor progression in glioblastoma patients. Oncoimmunology (2016) 5(2):e1075693. doi: 10.1080/2162402x.2015.1075693
199. Iwatsuki K, Kumara E, Yoshimine T, Nakagawa H, Sato M, Hayakawa T. Elastase expression by infiltrating neutrophils in gliomas. Neurological Res (2000) 22(5):465–8. doi: 10.1080/01616412.2000.11740701
200. Liang J, Piao Y, Holmes L, Fuller GN, Henry V, Tiao N, et al. Neutrophils promote the Malignant glioma phenotype through S100a4. Clin Cancer Res (2014) 20(1):187–98. doi: 10.1158/1078-0432.Ccr-13-1279
201. Chow KH, Park HJ, George J, Yamamoto K, Gallup AD, Graber JH, et al. S100a4 is a biomarker and regulator of glioma stem cells that is critical for mesenchymal transition in glioblastoma. Cancer Res (2017) 77(19):5360–73. doi: 10.1158/0008-5472.Can-17-1294
202. Fuchs TA, Abed U, Goosmann C, Hurwitz R, Schulze I, Wahn V, et al. Novel cell death program leads to neutrophil extracellular traps. J Cell Biol (2007) 176(2):231–41. doi: 10.1083/jcb.200606027
203. Lv X, Liu Z, Xu L, Song E, Song Y. Tetrachlorobenzoquinone exhibits immunotoxicity by inducing neutrophil extracellular traps through a mechanism involving ros-jnk-nox2 positive feedback loop. Environ pollut (Barking Essex: 1987) (2021) 268(Pt B):115921. doi: 10.1016/j.envpol.2020.115921
204. Keshari RS, Verma A, Barthwal MK, Dikshit M. Reactive oxygen species-induced activation of erk and P38 mapk mediates pma-induced nets release from human neutrophils. J Cell Biochem (2013) 114(3):532–40. doi: 10.1002/jcb.24391
205. Yee PP, Wei Y, Kim SY, Lu T, Chih SY, Lawson C, et al. Neutrophil-induced ferroptosis promotes tumor necrosis in glioblastoma progression. Nat Commun (2020) 11(1):5424. doi: 10.1038/s41467-020-19193-y
206. Kurotaki D, Kawase W, Sasaki H, Nakabayashi J, Nishiyama A, Morse HC 3rd, et al. Epigenetic control of early dendritic cell lineage specification by the transcription factor irf8 in mice. Blood (2019) 133(17):1803–13. doi: 10.1182/blood-2018-06-857789
207. Dudek AM, Martin S, Garg AD, Agostinis P. Immature, semi-mature, and fully mature dendritic cells: toward a dc-cancer cells interface that augments anticancer immunity. Front Immunol (2013) 4:438. doi: 10.3389/fimmu.2013.00438
208. Demaria O, Cornen S, Daëron M, Morel Y, Medzhitov R, Vivier E. Harnessing innate immunity in cancer therapy. Nature (2019) 574(7776):45–56. doi: 10.1038/s41586-019-1593-5
209. Zhang S, Zeng Z, Liu Y, Huang J, Long J, Wang Y, et al. Prognostic landscape of tumor-infiltrating immune cells and immune-related genes in the tumor microenvironment of gastric cancer. Aging (2020) 12(18):17958–75. doi: 10.18632/aging.103519
210. Han JA, Kang YJ, Shin C, Ra JS, Shin HH, Hong SY, et al. Ferritin protein cage nanoparticles as versatile antigen delivery nanoplatforms for dendritic cell (Dc)-based vaccine development. Nanomed: Nanotechnol Biol Med (2014) 10(3):561–9. doi: 10.1016/j.nano.2013.11.003
211. Cooper MA, Fehniger TA, Fuchs A, Colonna M, Caligiuri MA. Nk cell and dc interactions. Trends Immunol (2004) 25(1):47–52. doi: 10.1016/j.it.2003.10.012
212. Kim JW, Kane JR, Panek WK, Young JS, Rashidi A, Yu D, et al. A dendritic cell-targeted adenoviral vector facilitates adaptive immune response against human glioma antigen (Cmv-ie) and prolongs survival in a human glioma tumor model. Neurotherapeutics: J Am Soc Exp Neurother (2018) 15(4):1127–38. doi: 10.1007/s13311-018-0650-3
213. Liu B, Ji Q, Cheng Y, Liu M, Zhang B, Mei Q, et al. Biomimetic gbm-targeted drug delivery system boosting ferroptosis for immunotherapy of orthotopic drug-resistant gbm. J Nanobiotechnol (2022) 20(1):161. doi: 10.1186/s12951-022-01360-6
214. He M, Chen X, Luo M, Ouyang L, Xie L, Huang Z, et al. Suppressor of cytokine signaling 1 inhibits the maturation of dendritic cells involving the nuclear factor kappa B signaling pathway in the glioma microenvironment. Clin Exp Immunol (2020) 202(1):47–59. doi: 10.1111/cei.13476
215. Kober C, Rohn S, Weibel S, Geissinger U, Chen NG, Szalay AA. Microglia and astrocytes attenuate the replication of the oncolytic vaccinia virus livp 1.1.1 in murine gl261 gliomas by acting as vaccinia virus traps. J Trans Med (2015) 13:216. doi: 10.1186/s12967-015-0586-x
216. Ugele I, Cárdenas-Conejo ZE, Hammon K, Wehrstein M, Bruss C, Peter K, et al. D-2-hydroxyglutarate and L-2-hydroxyglutarate inhibit il-12 secretion by human monocyte-derived dendritic cells. Int J Mol Sci (2019) 20(3):742. doi: 10.3390/ijms20030742
217. Wang J, Liu P, Xin S, Wang Z, Li J. Nrf2 suppresses the function of dendritic cells to facilitate the immune escape of glioma cells. Exp Cell Res (2017) 360(2):66–73. doi: 10.1016/j.yexcr.2017.07.031
218. Laskowski TJ, Biederstädt A, Rezvani K. Natural killer cells in antitumour adoptive cell immunotherapy. Nat Rev Cancer (2022) 22(10):557–75. doi: 10.1038/s41568-022-00491-0
219. Gillgrass A, Ashkar A. Stimulating natural killer cells to protect against cancer: recent developments. Expert Rev Clin Immunol (2011) 7(3):367–82. doi: 10.1586/eci.10.102
220. Campbell KS, Hasegawa J. Natural killer cell biology: an update and future directions. J Allergy Clin Immunol (2013) 132(3):536–44. doi: 10.1016/j.jaci.2013.07.006
221. Costa VV, Ye W, Chen Q, Teixeira MM, Preiser P, Ooi EE, et al. Dengue Virus-Infected Dendritic Cells, but Not Monocytes, Activate Natural Killer Cells through a Contact-Dependent Mechanism Involving Adhesion Molecules. mBio (2017) 8(4):e00741–17. doi: 10.1128/mBio.00741-17
222. Vantourout P, Willcox C, Turner A, Swanson CM, Haque Y, Sobolev O, et al. Immunological visibility: posttranscriptional regulation of human nkg2d ligands by the egf receptor pathway. Sci Trans Med (2014) 6(231):231ra49. doi: 10.1126/scitranslmed.3007579
223. Screpanti V, Wallin RP, Grandien A, Ljunggren HG. Impact of fasl-induced apoptosis in the elimination of tumor cells by nk cells. Mol Immunol (2005) 42(4):495–9. doi: 10.1016/j.molimm.2004.07.033
224. Antosova Z, Podzimkova N, Tomala J, Augustynkova K, Sajnerova K, Nedvedova E, et al. Sot101 induces nk cell cytotoxicity and potentiates antibody-dependent cell cytotoxicity and anti-tumor activity. Front Immunol (2022) 13:989895. doi: 10.3389/fimmu.2022.989895
225. Zhao J, Schlößer HA, Wang Z, Qin J, Li J, Popp F, et al. Tumor-derived extracellular vesicles inhibit natural killer cell function in pancreatic cancer. Cancers (2019) 11(6):874. doi: 10.3390/cancers11060874
226. Hayakawa Y, Takeda K, Yagita H, Smyth MJ, Van Kaer L, Okumura K, et al. Ifn-gamma-mediated inhibition of tumor angiogenesis by natural killer T-cell ligand, alpha-galactosylceramide. Blood (2002) 100(5):1728–33. doi: 10.1182/blood-2002-03-0789
227. Shah D, Comba A, Faisal SM, Kadiyala P, Baker GJ, Alghamri MS, et al. A novel mir1983-tlr7-ifnβ Circuit licenses nk cells to kill glioma cells, and is under the control of galectin-1. Oncoimmunology (2021) 10(1):1939601. doi: 10.1080/2162402x.2021.1939601
228. Huang L, Zhu P, Xia P, Fan Z. Wash has a critical role in nk cell cytotoxicity through lck-mediated phosphorylation. Cell Death Dis (2016) 7(7):e2301. doi: 10.1038/cddis.2016.212
229. Shida Y, Nakazawa T, Matsuda R, Morimoto T, Nishimura F, Nakamura M, et al. Ex vivo expanded and activated natural killer cells prolong the overall survival of mice with glioblastoma-like cell-derived tumors. Int J Mol Sci (2021) 22(18):9975. doi: 10.3390/ijms22189975
230. Crane CA, Han SJ, Barry JJ, Ahn BJ, Lanier LL, Parsa AT. Tgf-beta downregulates the activating receptor nkg2d on nk cells and cd8+ T cells in glioma patients. Neuro-oncology (2010) 12(1):7–13. doi: 10.1093/neuonc/nop009
231. Humblet Y. Cetuximab: an igg(1) monoclonal antibody for the treatment of epidermal growth factor receptor-expressing tumours. Expert Opin Pharmacother (2004) 5(7):1621–33. doi: 10.1517/14656566.5.7.1621
232. Avril T, Vauleon E, Hamlat A, Saikali S, Etcheverry A, Delmas C, et al. Human glioblastoma stem-like cells are more sensitive to allogeneic nk and T cell-mediated killing compared with serum-cultured glioblastoma cells. Brain Pathol (Zurich Switzerland) (2012) 22(2):159–74. doi: 10.1111/j.1750-3639.2011.00515.x
233. Whiteside TL. Nk cells in the tumor microenvironment and thioredoxin activity. J Clin Invest (2020) 130(10):5115–7. doi: 10.1172/jci141460
234. Nakamura K, Matsunaga K. Susceptibility of natural killer (Nk) cells to reactive oxygen species (Ros) and their restoration by the mimics of superoxide dismutase (Sod). Cancer Biother Radiopharmaceuticals (1998) 13(4):275–90. doi: 10.1089/cbr.1998.13.275
235. Werlenius O, Aurelius J, Hallner A, Akhiani AA, Simpanen M, Martner A, et al. Reactive oxygen species induced by therapeutic cd20 antibodies inhibit natural killer cell-mediated antibody-dependent cellular cytotoxicity against primary cll cells. Oncotarget (2016) 7(22):32046–53. doi: 10.18632/oncotarget.8769
236. Wang J, Yi J. Cancer cell killing via ros: to increase or decrease, that is the question. Cancer Biol Ther (2008) 7(12):1875–84. doi: 10.4161/cbt.7.12.7067
237. Sosa V, Moliné T, Somoza R, Paciucci R, Kondoh H, ME LL. Oxidative stress and cancer: an overview. Ageing Res Rev (2013) 12(1):376–90. doi: 10.1016/j.arr.2012.10.004
238. Liu Y, Wang X, Zhu W, Sui Z, Wei X, Zhang Y, et al. Trpml1-induced autophagy inhibition triggers mitochondrial mediated apoptosis. Cancer Lett (2022) 541:215752. doi: 10.1016/j.canlet.2022.215752
239. Miao Z, Tian W, Ye Y, Gu W, Bao Z, Xu L, et al. Hsp90 induces acsl4-dependent glioma ferroptosis via dephosphorylating ser637 at drp1. Cell Death Dis (2022) 13(6):548. doi: 10.1038/s41419-022-04997-1
240. Ji CC, Hu YY, Cheng G, Liang L, Gao B, Ren YP, et al. A ketogenic diet attenuates proliferation and stemness of glioma stem−Like cells by altering metabolism resulting in increased ros production. Int J Oncol (2020) 56(2):606–17. doi: 10.3892/ijo.2019.4942
241. García-Gómez P, Golán I, Dadras MS, Mezheyeuski A, Bellomo C, Tzavlaki K, et al. Nox4 regulates tgfβ-induced proliferation and self-renewal in glioblastoma stem cells. Mol Oncol (2022) 16(9):1891–912. doi: 10.1002/1878-0261.13200
242. Iranmanesh Y, Jiang B, Favour OC, Dou Z, Wu J, Li J, et al. Mitochondria’s role in the maintenance of cancer stem cells in glioblastoma. Front Oncol (2021) 11:582694. doi: 10.3389/fonc.2021.582694
243. Yuan S, Lu Y, Yang J, Chen G, Kim S, Feng L, et al. Metabolic activation of mitochondria in glioma stem cells promotes cancer development through a reactive oxygen species-mediated mechanism. Stem Cell Res Ther (2015) 6:198. doi: 10.1186/s13287-015-0174-2
244. Tavender TJ, Bulleid NJ. Peroxiredoxin iv protects cells from oxidative stress by removing H2o2 produced during disulphide formation. J Cell Sci (2010) 123(Pt 15):2672–9. doi: 10.1242/jcs.067843
245. Vallejo FA, Vanni S, Graham RM. Ucp2 as a potential biomarker for adjunctive metabolic therapies in tumor management. Front Oncol (2021) 11:640720. doi: 10.3389/fonc.2021.640720
246. Lu J, Tan M, Cai Q. The warburg effect in tumor progression: mitochondrial oxidative metabolism as an anti-metastasis mechanism. Cancer Lett (2015) 356(2 Pt A):156–64. doi: 10.1016/j.canlet.2014.04.001
247. Hyun DH. Insights into the New Cancer Therapy through Redox Homeostasis and Metabolic Shifts. Cancers (2020) 12(7):1822. doi: 10.3390/cancers12071822
248. Li P, Wu M, Wang J, Sui Y, Liu S, Shi D. Nac selectively inhibit cancer telomerase activity: A higher redox homeostasis threshold exists in cancer cells. Redox Biol (2016) 8:91–7. doi: 10.1016/j.redox.2015.12.001
249. Zucker SN, Fink EE, Bagati A, Mannava S, Bianchi-Smiraglia A, Bogner PN, et al. Nrf2 amplifies oxidative stress via induction of klf9. Mol Cell (2014) 53(6):916–28. doi: 10.1016/j.molcel.2014.01.033
250. Wang YF, Hu JY. Natural and synthetic compounds for glioma treatment based on ros-mediated strategy. Eur J Pharmacol (2023) 953:175537. doi: 10.1016/j.ejphar.2023.175537
251. Bhanja D, Wilding H, Baroz A, Trifoi M, Shenoy G, Slagle-Webb B, et al. Photodynamic therapy for glioblastoma: illuminating the path toward clinical applicability. Cancers (2023) 15(13):3427. doi: 10.3390/cancers15133427
252. Wan Q, Zou C, Hu D, Zhou J, Chen M, Tie C, et al. Imaging-guided focused ultrasound-induced thermal and sonodynamic effects of nanosonosensitizers for synergistic enhancement of glioblastoma therapy. Biomater Sci (2019) 7(7):3007–15. doi: 10.1039/c9bm00292h
253. Zheng T, Wang W, Ashley J, Zhang M, Feng X, Shen J, et al. Self-assembly protein superstructures as a powerful chemodynamic therapy nanoagent for glioblastoma treatment. Nano-micro Lett (2020) 12(1):151. doi: 10.1007/s40820-020-00490-6
254. Kwiatkowski S, Knap B, Przystupski D, Saczko J, Kędzierska E, Knap-Czop K, et al. Photodynamic therapy - mechanisms, photosensitizers and combinations. Biomed Pharmacother = Biomed Pharmacotherapie (2018) 106:1098–107. doi: 10.1016/j.biopha.2018.07.049
255. Yu Y, Jia H, Liu Y, Zhang L, Feng G, Tang BZ. Recent progress in type I aggregation-induced emission photosensitizers for photodynamic therapy. Mol (Basel Switzerland) (2022) 28(1):332. doi: 10.3390/molecules28010332
256. Semyachkina-Glushkovskaya O, Kurths J, Borisova E, Sokolovski S, Mantareva V, Angelov I, et al. Photodynamic opening of blood-brain barrier. Biomed Optics Express (2017) 8(11):5040–8. doi: 10.1364/boe.8.005040
257. Verger A, Brandhonneur N, Molard Y, Cordier S, Kowouvi K, Amela-Cortes M, et al. From molecules to nanovectors: current state of the art and applications of photosensitizers in photodynamic therapy. Int J Pharmaceutics (2021) 604:120763. doi: 10.1016/j.ijpharm.2021.120763
258. Mahmoudi K, Garvey KL, Bouras A, Cramer G, Stepp H, Jesu Raj JG, et al. 5-aminolevulinic acid photodynamic therapy for the treatment of high-grade gliomas. J neuro-oncol (2019) 141(3):595–607. doi: 10.1007/s11060-019-03103-4
259. Rosenthal MA, Kavar B, Hill JS, Morgan DJ, Nation RL, Stylli SS, et al. Phase I and pharmacokinetic study of photodynamic therapy for high-grade gliomas using a novel boronated porphyrin. J Clin Oncol (2001) 19(2):519–24. doi: 10.1200/jco.2001.19.2.519
260. Shimizu K, Nitta M, Komori T, Maruyama T, Yasuda T, Fujii Y, et al. Intraoperative photodynamic diagnosis using talaporfin sodium simultaneously applied for photodynamic therapy against Malignant glioma: A prospective clinical study. Front Neurol (2018) 9:24. doi: 10.3389/fneur.2018.00024
261. Mannino S, Molinari A, Sabatino G, Ciafrè SA, Colone M, Maira G, et al. Intratumoral vs systemic administration of meta-tetrahydroxyphenylchlorin for photodynamic therapy of malignant gliomas: assessment of uptake and spatial distribution in C6 rat glioma model. Int J Immunopathol Pharmacol (2008) 21(1):227–31. doi: 10.1177/039463200802100126
262. Donohoe C, Senge MO, Arnaut LG, Gomes-da-Silva LC. Cell death in photodynamic therapy: from oxidative stress to anti-tumor immunity. Biochim Biophys Acta Rev Cancer (2019) 1872(2):188308. doi: 10.1016/j.bbcan.2019.07.003
263. Chilakamarthi U, Giribabu L. Photodynamic therapy: past, present and future. Chem Rec (New York NY) (2017) 17(8):775–802. doi: 10.1002/tcr.201600121
264. Alzeibak R, Mishchenko TA, Shilyagina NY, Balalaeva IV, Vedunova MV, Krysko DV. Targeting immunogenic cancer cell death by photodynamic therapy: past, present and future. J Immunother Cancer (2021) 9(1):e001926. doi: 10.1136/jitc-2020-001926
265. Kawase Y, Iseki H. Parameter-finding studies of photodynamic therapy for approval in Japan and the USA. Photodiagnosis Photodyn Ther (2013) 10(4):434–45. doi: 10.1016/j.pdpdt.2013.03.001
266. Kessel D. Photodynamic therapy: A brief history. J Clin Med (2019) 8(10):1581. doi: 10.3390/jcm8101581
267. Champeau M, Vignoud S, Mortier L, Mordon S. Photodynamic therapy for skin cancer: how to enhance drug penetration? J Photochem Photobiol B Biol (2019) 197:111544. doi: 10.1016/j.jphotobiol.2019.111544
268. Kostron H, Swartz MR, Martuza RL. Photodynamic therapy is potentiated by co60 and intratumoral injection of hematoporphyrin derivative. J neuro-oncol (1988) 6(2):185–91. doi: 10.1007/bf02327395
269. Wilson BC, Muller PJ, Yanch JC. Instrumentation and light dosimetry for intra-operative photodynamic therapy (Pdt) of Malignant brain tumours. Phys Med Biol (1986) 31(2):125–33. doi: 10.1088/0031-9155/31/2/002
270. Turubanova VD, Balalaeva IV, Mishchenko TA, Catanzaro E, Alzeibak R, Peskova NN, et al. Immunogenic cell death induced by a new photodynamic therapy based on photosens and photodithazine. J Immunother Cancer (2019) 7(1):350. doi: 10.1186/s40425-019-0826-3
271. Omura N, Nonoguchi N, Fujishiro T, Park Y, Ikeda N, Kajimoto Y, et al. Ablation efficacy of 5-aminolevulinic acid-mediated photodynamic therapy on human glioma stem cells. Photodiagnosis Photodyn Ther (2023) 41:103119. doi: 10.1016/j.pdpdt.2022.103119
272. Zhang X, Guo M, Shen L, Hu S. Combination of photodynamic therapy and temozolomide on glioma in a rat C6 glioma model. Photodiagnosis Photodyn Ther (2014) 11(4):603–12. doi: 10.1016/j.pdpdt.2014.10.007
273. Cheng K, Guo Q, Shen Z, Yang W, Wang Y, Sun Z, et al. Bibliometric analysis of global research on cancer photodynamic therapy: focus on nano-related research. Front Pharmacol (2022) 13:927219. doi: 10.3389/fphar.2022.927219
274. Vanaclocha V, Sureda M, Azinovic I, Rebollo J, Cañón R, Sapena NS, et al. Photodynamic therapy in the treatment of brain tumours. A feasibility study. Photodiagnosis Photodyn Ther (2015) 12(3):422–7. doi: 10.1016/j.pdpdt.2015.05.007
275. Wang X, Wu M, Li H, Jiang J, Zhou S, Chen W, et al. Enhancing penetration ability of semiconducting polymer nanoparticles for sonodynamic therapy of large solid tumor. Advanced Sci (Weinheim Baden-Wurttemberg Germany) (2022) 9(6):e2104125. doi: 10.1002/advs.202104125
276. Vermandel M, Dupont C, Lecomte F, Leroy HA, Tuleasca C, Mordon S, et al. Standardized intraoperative 5-ala photodynamic therapy for newly diagnosed glioblastoma patients: A preliminary analysis of the indygo clinical trial. J neuro-oncol (2021) 152(3):501–14. doi: 10.1007/s11060-021-03718-6
277. Wei X, Song M, Jiang G, Liang M, Chen C, Yang Z, et al. Progress in advanced nanotherapeutics for enhanced photodynamic immunotherapy of tumor. Theranostics (2022) 12(12):5272–98. doi: 10.7150/thno.73566
278. Maeding N, Verwanger T, Krammer B. Boosting tumor-specific immunity using pdt. Cancers (2016) 8(10):91. doi: 10.3390/cancers8100091
279. Li D, Chen F, Cheng C, Li H, Wei X. Biodegradable materials with disulfide-bridged-framework confine photosensitizers for enhanced photo-immunotherapy. Int J Nanomed (2021) 16:8323–34. doi: 10.2147/ijn.S344679
280. Ahmed A, Tait SWG. Targeting immunogenic cell death in cancer. Mol Oncol (2020) 14(12):2994–3006. doi: 10.1002/1878-0261.12851
281. Mishchenko T, Balalaeva I, Gorokhova A, Vedunova M, Krysko DV. Which cell death modality wins the contest for photodynamic therapy of cancer? Cell Death Dis (2022) 13(5):455. doi: 10.1038/s41419-022-04851-4
282. Lan M, Zhao S, Liu W, Lee CS, Zhang W, Wang P. Photosensitizers for photodynamic therapy. Advanced Healthc Mater (2019) 8(13):e1900132. doi: 10.1002/adhm.201900132
283. Hu T, Wang Z, Shen W, Liang R, Yan D, Wei M. Recent advances in innovative strategies for enhanced cancer photodynamic therapy. Theranostics (2021) 11(7):3278–300. doi: 10.7150/thno.54227
284. Wang C, Chen X, Wu J, Liu H, Ji Z, Shi H, et al. Low-dose arsenic trioxide enhances 5-aminolevulinic acid-induced ppix accumulation and efficacy of photodynamic therapy in human glioma. J Photochem Photobiol B Biol (2013) 127:61–7. doi: 10.1016/j.jphotobiol.2013.06.001
285. Coupienne I, Bontems S, Dewaele M, Rubio N, Habraken Y, Fulda S, et al. Nf-kappab inhibition improves the sensitivity of human glioblastoma cells to 5-aminolevulinic acid-based photodynamic therapy. Biochem Pharmacol (2011) 81(5):606–16. doi: 10.1016/j.bcp.2010.12.015
286. Tetard MC, Vermandel M, Leroy HA, Leroux B, Maurage CA, Lejeune JP, et al. Interstitial 5-ala photodynamic therapy and glioblastoma: preclinical model development and preliminary results. Photodiagnosis Photodyn Ther (2016) 13:218–24. doi: 10.1016/j.pdpdt.2015.07.169
287. Miki Y, Akimoto J, Moritake K, Hironaka C, Fujiwara Y. Photodynamic therapy using talaporfin sodium induces concentration-dependent programmed necroptosis in human glioblastoma T98g cells. Lasers Med Sci (2015) 30(6):1739–45. doi: 10.1007/s10103-015-1783-9
288. Correia JH, Rodrigues JA, Pimenta S, Dong T, Yang Z. Photodynamic therapy review: principles, photosensitizers, applications, and future directions. Pharmaceutics (2021) 13(9):1332. doi: 10.3390/pharmaceutics13091332
289. Miyoshi N, Misík V, Fukuda M, Riesz P. Effect of gallium-porphyrin analogue atx-70 on nitroxide formation from a cyclic secondary amine by ultrasound: on the mechanism of sonodynamic activation. Radiat Res (1995) 143(2):194–202. doi: 10.2307/3579157
290. Miyoshi N, Misík V, Riesz P. Sonodynamic toxicity of gallium-porphyrin analogue atx-70 in human leukemia cells. Radiat Res (1997) 148(1):43–7. doi: 10.2307/3579537
291. Wu Z, Cheng K, Shen Z, Lu Y, Wang H, Wang G, et al. Mapping knowledge landscapes and emerging trends of sonodynamic therapy: A bibliometric and visualized study. Front Pharmacol (2022) 13:1048211. doi: 10.3389/fphar.2022.1048211
292. Lin X, Song J, Chen X, Yang H. Ultrasound-activated sensitizers and applications. Angewandte Chemie (International ed English) (2020) 59(34):14212–33. doi: 10.1002/anie.201906823
293. Wang F, Xu L, Wen B, Song S, Zhou Y, Wu H, et al. Ultrasound-excited temozolomide sonosensitization induces necroptosis in glioblastoma. Cancer Lett (2023) 554:216033. doi: 10.1016/j.canlet.2022.216033
294. Wan G, Chen X, Wang H, Hou S, Wang Q, Cheng Y, et al. Gene augmented nuclear-targeting sonodynamic therapy via nrf2 pathway-based redox balance adjustment boosts peptide-based anti-pd-L1 therapy on colorectal cancer. J Nanobiotechnol (2021) 19(1):347. doi: 10.1186/s12951-021-01094-x
295. Geng X, Chen Y, Chen Z, Wei X, Dai Y, Yuan Z. Oxygen-carrying biomimetic nanoplatform for sonodynamic killing of bacteria and treatment of infection diseases. Ultrasonics sonochemistry (2022) 84:105972. doi: 10.1016/j.ultsonch.2022.105972
296. Tung CH, Han MS, Kim Y, Qi J, O’Neill BE. Tumor ablation using low-intensity ultrasound and sound excitable drug. J Controlled Release: Off J Controlled Release Soc (2017) 258:67–72. doi: 10.1016/j.jconrel.2017.05.009
297. Guo QL, Dai XL, Yin MY, Cheng HW, Qian HS, Wang H, et al. Nanosensitizers for sonodynamic therapy for glioblastoma multiforme: current progress and future perspectives. Military Med Res (2022) 9(1):26. doi: 10.1186/s40779-022-00386-z
298. Bunevicius A, Pikis S, Padilla F, Prada F, Sheehan J. Sonodynamic therapy for gliomas. J neuro-oncol (2022) 156(1):1–10. doi: 10.1007/s11060-021-03807-6
299. Song D, Yue W, Li Z, Li J, Zhao J, Zhang N. Study of the mechanism of sonodynamic therapy in a rat glioma model. OncoTargets Ther (2014) 7:1801–10. doi: 10.2147/ott.S52426
300. Zhang C, Bu W, Ni D, Zhang S, Li Q, Yao Z, et al. Synthesis of iron nanometallic glasses and their application in cancer therapy by a localized fenton reaction. Angewandte Chemie (International ed English) (2016) 55(6):2101–6. doi: 10.1002/anie.201510031
301. Jia C, Guo Y, Wu FG. Chemodynamic therapy via fenton and fenton-like nanomaterials: strategies and recent advances. Small (Weinheim an der Bergstrasse Germany) (2022) 18(6):e2103868. doi: 10.1002/smll.202103868
302. Qian X, Zhang J, Gu Z, Chen Y. Nanocatalysts-augmented fenton chemical reaction for nanocatalytic tumor therapy. Biomaterials (2019) 211:1–13. doi: 10.1016/j.biomaterials.2019.04.023
303. Ma W, Zhang H, Li S, Wang Z, Wu X, Yan R, et al. A multifunctional nanoplatform based on fenton-like and russell reactions of cu, mn bimetallic ions synergistically enhanced ros stress for improved chemodynamic therapy. ACS Biomater Sci Eng (2022) 8(3):1354–66. doi: 10.1021/acsbiomaterials.1c01605
304. Li Y, Sun J, Sun SP. Mn(2+)-mediated homogeneous fenton-like reaction of fe(Iii)-nta complex for efficient degradation of organic contaminants under neutral conditions. J Hazard Mater (2016) 313:193–200. doi: 10.1016/j.jhazmat.2016.04.003
305. Qiao L, Yang H, Shao XX, Yin Q, Fu XJ, Wei Q. Research progress on nanoplatforms and nanotherapeutic strategies in treating glioma. Mol Pharmaceutics (2022) 19(7):1927–51. doi: 10.1021/acs.molpharmaceut.1c00856
306. Cao X, Li S, Chen W, Lu H, Ye L, Min Z, et al. Multifunctional hybrid hydrogel system enhanced the therapeutic efficacy of treatments for postoperative glioma. ACS Appl Mater Interfaces (2022) 14(24):27623–33. doi: 10.1021/acsami.2c05147
307. Cao Y, Jin L, Zhang S, Lv Z, Yin N, Zhang H, et al. Blood-brain barrier permeable and multi-stimuli responsive nanoplatform for orthotopic glioma inhibition by synergistic enhanced chemo-/chemodynamic/photothermal/starvation therapy. Eur J Pharm Sci (2023) 180:106319. doi: 10.1016/j.ejps.2022.106319
308. Abbott NJ, Rönnbäck L, Hansson E. Astrocyte-endothelial interactions at the blood-brain barrier. Nat Rev Neurosci (2006) 7(1):41–53. doi: 10.1038/nrn1824
309. Langen UH, Ayloo S, Gu C. Development and cell biology of the blood-brain barrier. Annu Rev Cell Dev Biol (2019) 35:591–613. doi: 10.1146/annurev-cellbio-100617-062608
310. Daneman R, Prat A. The blood-brain barrier. Cold Spring Harbor Perspect Biol (2015) 7(1):a020412. doi: 10.1101/cshperspect.a020412
311. Watkins S, Robel S, Kimbrough IF, Robert SM, Ellis-Davies G, Sontheimer H. Disruption of astrocyte-vascular coupling and the blood-brain barrier by invading glioma cells. Nat Commun (2014) 5:4196. doi: 10.1038/ncomms5196
312. Pacioni S, D’Alessandris QG, Buccarelli M, Boe A, Martini M, Larocca LM, et al. Brain invasion along perivascular spaces by glioma cells: relationship with blood-brain barrier. Cancers (2019) 12(1):18. doi: 10.3390/cancers12010018
313. Cui J, Wang X, Li J, Zhu A, Du Y, Zeng W, et al. Immune exosomes loading self-assembled nanomicelles traverse the blood-brain barrier for chemo-immunotherapy against glioblastoma. ACS Nano (2023) 17(2):1464–84. doi: 10.1021/acsnano.2c10219
314. Ting CY, Fan CH, Liu HL, Huang CY, Hsieh HY, Yen TC, et al. Concurrent blood-brain barrier opening and local drug delivery using drug-carrying microbubbles and focused ultrasound for brain glioma treatment. Biomaterials (2012) 33(2):704–12. doi: 10.1016/j.biomaterials.2011.09.096
315. Coluccia D, Figueiredo CA, Wu MY, Riemenschneider AN, Diaz R, Luck A, et al. Enhancing glioblastoma treatment using cisplatin-gold-nanoparticle conjugates and targeted delivery with magnetic resonance-guided focused ultrasound. Nanomed: Nanotechnol Biol Med (2018) 14(4):1137–48. doi: 10.1016/j.nano.2018.01.021
316. Zafar R, Zia KM, Tabasum S, Jabeen F, Noreen A, Zuber M. Polysaccharide based bionanocomposites, properties and applications: A review. Int J Biol Macromol (2016) 92:1012–24. doi: 10.1016/j.ijbiomac.2016.07.102
317. Pérez-Herrero E, Fernández-Medarde A. Advanced targeted therapies in cancer: drug nanocarriers, the future of chemotherapy. Eur J Pharmaceutics Biopharmaceutics (2015) 93:52–79. doi: 10.1016/j.ejpb.2015.03.018
318. Yetisgin AA, Cetinel S, Zuvin M, Kosar A, Kutlu O. Therapeutic nanoparticles and their targeted delivery applications. Mol (Basel Switzerland) (2020) 25(9):2193. doi: 10.3390/molecules25092193
319. Xu Q, Zhang H, Liu H, Han Y, Qiu W, Li Z. Inhibiting autophagy flux and DNA repair of tumor cells to boost radiotherapy of orthotopic glioblastoma. Biomaterials (2022) 280:121287. doi: 10.1016/j.biomaterials.2021.121287
320. Kang RH, Jang JE, Huh E, Kang SJ, Ahn DR, Kang JS, et al. A brain tumor-homing tetra-peptide delivers a nano-therapeutic for more effective treatment of a mouse model of glioblastoma. Nanoscale Horizons (2020) 5(8):1213–25. doi: 10.1039/d0nh00077a
321. Qi L, Liu J, Zhu H, Li Z, Lu K, Li T, et al. Inhibition of glioma proliferation and migration by magnetic nanoparticle mediated jam-2 silencing. J Mater Chem B (2014) 2(41):7168–75. doi: 10.1039/c4tb00954a
322. Teirlinck E, Barras A, Liu J, Fraire JC, Lajunen T, Xiong R, et al. Exploring light-sensitive nanocarriers for simultaneous triggered antibiotic release and disruption of biofilms upon generation of laser-induced vapor nanobubbles. Pharmaceutics (2019) 11(5):201. doi: 10.3390/pharmaceutics11050201
323. Chenthamara D, Subramaniam S, Ramakrishnan SG, Krishnaswamy S, Essa MM, Lin FH, et al. Therapeutic efficacy of nanoparticles and routes of administration. Biomater Res (2019) 23:20. doi: 10.1186/s40824-019-0166-x
324. Jamali Z, Khoobi M, Hejazi SM, Eivazi N, Abdolahpour S, Imanparast F, et al. Evaluation of targeted curcumin (Cur) loaded plga nanoparticles for in vitro photodynamic therapy on human glioblastoma cell line. Photodiagnosis Photodyn Ther (2018) 23:190–201. doi: 10.1016/j.pdpdt.2018.06.026
325. Dube T, Kumar N, Bishnoi M, Panda JJ. Dual blood-brain barrier-glioma targeting peptide-poly(Levodopamine) hybrid nanoplatforms as potential near infrared phototheranostic agents in glioblastoma. Bioconjugate Chem (2021) 32(9):2014–31. doi: 10.1021/acs.bioconjchem.1c00321
326. Lee CH, Lai PS, Lu YP, Chen HY, Chai CY, Tsai RK, et al. Real-time vascular imaging and photodynamic therapy efficacy with micelle-nanocarrier delivery of chlorin E6 to the microenvironment of melanoma. J Dermatol Sci (2015) 80(2):124–32. doi: 10.1016/j.jdermsci.2015.08.005
327. Hu N, Li W, Hong Y, Zeng Z, Zhang J, Wu X, et al. A pd1 targeted nano-delivery system based on epigenetic alterations of T cell responses in the treatment of gastric cancer. Mol Ther Oncolytics (2022) 24:148–59. doi: 10.1016/j.omto.2021.12.006
328. Zhao D, Liu N, Shi K, Wang X, Wu G. Preparation of a multifunctional verapamil-loaded nano-carrier based on a self-assembling pegylated prodrug. Colloids Surfaces B Biointerfaces (2015) 135:682–8. doi: 10.1016/j.colsurfb.2015.08.018
329. Yu T, Xu B, He L, Xia S, Chen Y, Zeng J, et al. Pigment epithelial-derived factor gene loaded novel cooh-peg-plga-cooh nanoparticles promoted tumor suppression by systemic administration. Int J Nanomed (2016) 11:743–59. doi: 10.2147/ijn.S97223
330. Kim HS, Lee DY. Nanomedicine in clinical photodynamic therapy for the treatment of brain tumors. Biomedicines (2022) 10(1):96. doi: 10.3390/biomedicines10010096
331. Sun Y, Wang H, Wang P, Zhang K, Geng X, Liu Q, et al. Tumor targeting dvdms-nanoliposomes for an enhanced sonodynamic therapy of gliomas. Biomater Sci (2019) 7(3):985–94. doi: 10.1039/c8bm01187g
332. Lin L, Wang S, Deng H, Yang W, Rao L, Tian R, et al. Endogenous labile iron pool-mediated free radical generation for cancer chemodynamic therapy. J Am Chem Soc (2020) 142(36):15320–30. doi: 10.1021/jacs.0c05604
333. Cui Y, Xu Q, Chow PK, Wang D, Wang CH. Transferrin-conjugated magnetic silica plga nanoparticles loaded with doxorubicin and paclitaxel for brain glioma treatment. Biomaterials (2013) 34(33):8511–20. doi: 10.1016/j.biomaterials.2013.07.075
334. Zhu X, Zhou H, Liu Y, Wen Y, Wei C, Yu Q, et al. Transferrin/aptamer conjugated mesoporous ruthenium nanosystem for redox-controlled and targeted chemo-photodynamic therapy of glioma. Acta biomaterialia (2018) 82:143–57. doi: 10.1016/j.actbio.2018.10.012
335. Cai X, Wang M, Mu P, Jian T, Liu D, Ding S, et al. Sequence-defined nanotubes assembled from ir780-conjugated peptoids for chemophototherapy of malignant glioma. Res (Washington DC) (2021) 2021:9861384. doi: 10.34133/2021/9861384
336. Dube T, Kompella UB, Panda JJ. Near infrared triggered chemo-ptt-pdt effect mediated by glioma directed twin functional-chimeric peptide-decorated gold nanoroses. J Photochem Photobiol B Biol (2022) 228:112407. doi: 10.1016/j.jphotobiol.2022.112407
337. Gries M, Thomas N, Daouk J, Rocchi P, Choulier L, Jubréaux J, et al. Multiscale selectivity and in vivo biodistribution of nrp-1-targeted theranostic aguix nanoparticles for pdt of glioblastoma. Int J Nanomed (2020) 15:8739–58. doi: 10.2147/ijn.S261352
338. Zhi K, Raji B, Nookala AR, Khan MM, Nguyen XH, Sakshi S, et al. Plga nanoparticle-based formulations to cross the blood-brain barrier for drug delivery: from R&D to cgmp. Pharmaceutics (2021) 13(4):500. doi: 10.3390/pharmaceutics13040500
339. Lu L, Zhao X, Fu T, Li K, He Y, Luo Z, et al. An irgd-conjugated prodrug micelle with blood-brain-barrier penetrability for anti-glioma therapy. Biomaterials (2020) 230:119666. doi: 10.1016/j.biomaterials.2019.119666
340. Davanzo NN, Pellosi DS, Franchi LP, Tedesco AC. Light source is critical to induce glioblastoma cell death by photodynamic therapy using chloro-aluminiumphtalocyanine albumin-based nanoparticles. Photodiagnosis Photodyn Ther (2017) 19:181–3. doi: 10.1016/j.pdpdt.2017.04.017
341. Song R, Hu D, Chung HY, Sheng Z, Yao S. Lipid-polymer bilaminar oxygen nanobubbles for enhanced photodynamic therapy of cancer. ACS Appl Mater Interfaces (2018) 10(43):36805–13. doi: 10.1021/acsami.8b15293
342. Xu HZ, Li TF, Ma Y, Li K, Zhang Q, Xu YH, et al. Targeted photodynamic therapy of glioblastoma mediated by platelets with photo-controlled release property. Biomaterials (2022) 290:121833. doi: 10.1016/j.biomaterials.2022.121833
343. Qu F, Wang P, Zhang K, Shi Y, Li Y, Li C, et al. Manipulation of mitophagy by “All-in-one” Nanosensitizer augments sonodynamic glioma therapy. Autophagy (2020) 16(8):1413–35. doi: 10.1080/15548627.2019.1687210
344. Wu T, Liu Y, Cao Y, Liu Z. Engineering macrophage exosome disguised biodegradable nanoplatform for enhanced sonodynamic therapy of glioblastoma. Advanced Mater (Deerfield Beach Fla) (2022) 34(15):e2110364. doi: 10.1002/adma.202110364
345. Liang K, Li Z, Luo Y, Zhang Q, Yin F, Xu L, et al. Intelligent nanocomposites with intrinsic blood-brain-barrier crossing ability designed for highly specific mr imaging and sonodynamic therapy of glioblastoma. Small (Weinheim an der Bergstrasse Germany) (2020) 16(8):e1906985. doi: 10.1002/smll.201906985
346. Tan J, Duan X, Zhang F, Ban X, Mao J, Cao M, et al. Theranostic nanomedicine for synergistic chemodynamic therapy and chemotherapy of orthotopic glioma. Advanced Sci (Weinheim Baden-Wurttemberg Germany) (2020) 7(24):2003036. doi: 10.1002/advs.202003036
347. Tang XL, Wang Z, Zhu YY, Xiao H, Xiao Y, Cui S, et al. Hypoxia-activated ros burst liposomes boosted by local mild hyperthermia for photo/chemodynamic therapy. J Controlled Release: Off J Controlled Release Soc (2020) 328:100–11. doi: 10.1016/j.jconrel.2020.08.035
348. Jung O, Thomas A, Burks SR, Dustin ML, Frank JA, Ferrer M, et al. Neuroinflammation associated with ultrasound-mediated permeabilization of the blood-brain barrier. Trends Neurosci (2022) 45(6):459–70. doi: 10.1016/j.tins.2022.03.003
349. Pandit R, Chen L, Götz J. The blood-brain barrier: physiology and strategies for drug delivery. Advanced Drug Delivery Rev (2020) 165-166:1–14. doi: 10.1016/j.addr.2019.11.009
350. Wu H, Tong L, Wang Y, Yan H, Sun Z. Bibliometric analysis of global research trends on ultrasound microbubble: A quickly developing field. Front Pharmacol (2021) 12:646626. doi: 10.3389/fphar.2021.646626
351. Lee H, Kim H, Han H, Lee M, Lee S, Yoo H, et al. Microbubbles used for contrast enhanced ultrasound and theragnosis: A review of principles to applications. Biomed Eng Lett (2017) 7(2):59–69. doi: 10.1007/s13534-017-0016-5
352. Chan MH, Chen W, Li CH, Fang CY, Chang YC, Wei DH, et al. An advanced in situ magnetic resonance imaging and ultrasonic theranostics nanocomposite platform: crossing the blood-brain barrier and improving the suppression of glioblastoma using iron-platinum nanoparticles in nanobubbles. ACS Appl Mater Interfaces (2021) 13(23):26759–69. doi: 10.1021/acsami.1c04990
353. Wu H, Zhou Y, Xu L, Tong L, Wang Y, Liu B, et al. Mapping knowledge structure and research frontiers of ultrasound-induced blood-brain barrier opening: A scientometric study. Front Neurosci (2021) 15:706105. doi: 10.3389/fnins.2021.706105
354. Yang Q, Zhou Y, Chen J, Huang N, Wang Z, Cheng Y. Gene therapy for drug-resistant glioblastoma via lipid-polymer hybrid nanoparticles combined with focused ultrasound. Int J Nanomed (2021) 16:185–99. doi: 10.2147/ijn.S286221
355. Wei HJ, Upadhyayula PS, Pouliopoulos AN, Englander ZK, Zhang X, Jan CI, et al. Focused ultrasound-mediated blood-brain barrier opening increases delivery and efficacy of etoposide for glioblastoma treatment. Int J Radiat Oncol Biol Phys (2021) 110(2):539–50. doi: 10.1016/j.ijrobp.2020.12.019
356. Liang S, Hu D, Li G, Gao D, Li F, Zheng H, et al. Nir-ii fluorescence visualization of ultrasound-induced blood-brain barrier opening for enhanced photothermal therapy against glioblastoma using indocyanine green microbubbles. Sci Bull (2022) 67(22):2316–26. doi: 10.1016/j.scib.2022.10.025
357. Porret E, Kereselidze D, Dauba A, Schweitzer-Chaput A, Jegot B, Selingue E, et al. Refining the delivery and therapeutic efficacy of cetuximab using focused ultrasound in a mouse model of glioblastoma: an (89)Zr-cetuximab immunopet study. Eur J Pharmaceutics Biopharmaceutics (2023) 182:141–51. doi: 10.1016/j.ejpb.2022.12.006
358. Hynynen K. Ultrasound for drug and gene delivery to the brain. Advanced Drug Delivery Rev (2008) 60(10):1209–17. doi: 10.1016/j.addr.2008.03.010
359. Carpentier A, Canney M, Vignot A, Reina V, Beccaria K, Horodyckid C, et al. Clinical trial of blood-brain barrier disruption by pulsed ultrasound. Sci Trans Med (2016) 8(343):343re2. doi: 10.1126/scitranslmed.aaf6086
360. Kim DO, Lee CY. Comprehensive study on vitamin C equivalent antioxidant capacity (Vceac) of various polyphenolics in scavenging a free radical and its structural relationship. Crit Rev Food Sci Nutr (2004) 44(4):253–73. doi: 10.1080/10408690490464960
361. Sökmen M, Akram Khan M. The antioxidant activity of some curcuminoids and chalcones. Inflammopharmacology (2016) 24(2-3):81–6. doi: 10.1007/s10787-016-0264-5
362. Lin HY, Fu Q, Kao YH, Tseng TS, Reiss K, Cameron JE, et al. Antioxidants associated with oncogenic human papillomavirus infection in women. J Infect Dis (2021) 224(9):1520–8. doi: 10.1093/infdis/jiab148
363. Qi X, Jha SK, Jha NK, Dewanjee S, Dey A, Deka R, et al. Antioxidants in brain tumors: current therapeutic significance and future prospects. Mol Cancer (2022) 21(1):204. doi: 10.1186/s12943-022-01668-9
364. Turkez H, Tozlu OO, Arslan ME, Mardinoglu A. Safety and efficacy assessments to take antioxidants in glioblastoma therapy: from in vitro experiences to animal and clinical studies. Neurochemistry Int (2021) 150:105168. doi: 10.1016/j.neuint.2021.105168
365. Fernández-Ayala DJ, Brea-Calvo G, López-Lluch G, Navas P. Coenzyme Q distribution in hl-60 human cells depends on the endomembrane system. Biochim Biophys Acta (2005) 1713(2):129–37. doi: 10.1016/j.bbamem.2005.05.010
366. Maoka T, Yasui H, Ohmori A, Tokuda H, Suzuki N, Osawa A, et al. Anti-oxidative, anti-tumor-promoting, and anti-carcinogenic activities of adonirubin and adonixanthin. J Oleo Sci (2013) 62(3):181–6. doi: 10.5650/jos.62.181
367. Pan L, Zhou Y, Li XF, Wan QJ, Yu LH. Preventive Treatment of Astaxanthin Provides Neuroprotection through Suppression of Reactive Oxygen Species and Activation of Antioxidant Defense Pathway after Stroke in Rats. Brain Res Bull (2017) 130:211–20. doi: 10.1016/j.brainresbull.2017.01.024
368. Nakamura S, Maoka T, Tsuji S, Hayashi M, Shimazawa M, Hara H. Central nervous system migration of astaxanthin and adonixanthin following their oral administration in cynomolgus monkeys. J Nutr Sci Vitaminol (2020) 66(5):488–94. doi: 10.3177/jnsv.66.488
369. Tsuji S, Nakamura S, Maoka T, Yamada T, Imai T, Ohba T, et al. Antitumour effects of astaxanthin and adonixanthin on glioblastoma. Mar Drugs (2020) 18(9):474. doi: 10.3390/md18090474
370. Zhang Y, Liu Q, Wang F, Ling EA, Liu S, Wang L, et al. Melatonin antagonizes hypoxia-mediated glioblastoma cell migration and invasion via inhibition of hif-1α. J Pineal Res (2013) 55(2):121–30. doi: 10.1111/jpi.12052
371. Holick CN, Giovannucci EL, Rosner B, Stampfer MJ, Michaud DS. Prospective study of intake of fruit, vegetables, and carotenoids and the risk of adult glioma. Am J Clin Nutr (2007) 85(3):877–86. doi: 10.1093/ajcn/85.3.877
372. Qin S, Wang M, Zhang T, Zhang S. Vitamin E intake is not associated with glioma risk: evidence from a meta-analysis. Neuroepidemiology (2014) 43(3-4):253–8. doi: 10.1159/000369345
373. Cote DJ, Bever AM, Wilson KM, Smith TR, Smith-Warner SA, Stampfer MJ. A prospective study of tea and coffee intake and risk of glioma. Int J Cancer (2020) 146(9):2442–9. doi: 10.1002/ijc.32574
374. Mohajeri M, Behnam B, Sahebkar A. Biomedical applications of carbon nanomaterials: drug and gene delivery potentials. J Cell Physiol (2018) 234(1):298–319. doi: 10.1002/jcp.26899
375. Yao Y, Zang Y, Qu J, Tang M, Zhang T. The toxicity of metallic nanoparticles on liver: the subcellular damages, mechanisms, and outcomes. Int J Nanomed (2019) 14:8787–804. doi: 10.2147/ijn.S212907
Keywords: glioma, ROS, tumor microenvironment, antioxidants, photodynamic therapy, sonodynamic therapy, chemodynamic therapy, nanodrug delivery platforms
Citation: Yang Y-C, Zhu Y, Sun S-J, Zhao C-J, Bai Y, Wang J and Ma L-T (2023) ROS regulation in gliomas: implications for treatment strategies. Front. Immunol. 14:1259797. doi: 10.3389/fimmu.2023.1259797
Received: 16 July 2023; Accepted: 30 October 2023;
Published: 07 December 2023.
Edited by:
Yu-Hang Zhang, Brigham and Women’s Hospital, United StatesReviewed by:
Haiyang Wu, Tianjin Medical University, ChinaRui Sun, Washington University in St. Louis, United States
Copyright © 2023 Yang, Zhu, Sun, Zhao, Bai, Wang and Ma. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Li-Tian Ma, malitian1234@163.com; Jin Wang, wangjinn@fmmu.edu.cn; Yang Bai, sydbaiyang@163.com
†These authors have contributed equally to this work and share first authorship
 Yu-Chen Yang
Yu-Chen Yang