- 1Department of Cardiovascular Surgery, Union Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, China
- 2Department of Cardiology, The Central Hospital of Wuhan, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, China
Background: In observational and experimental studies, allergic diseases (AD) have been reported to be associated with some types of cardiovascular diseases (CVD), as both share common pathophysiological processes involving inflammation and metabolic disorders. However, the direction of the causal association between them remains unclear. This Mendelian randomization (MR) study aims to examine the bidirectional causality between AD and CVD.
Methods: We utilized publicly available genome-wide association study (GWAS) summary statistics data from European participants in the UK Biobank and the IEU Open GWAS database. Genetic variants associated with AD, asthma, and CVD were identified and used as instrumental variables to investigate the genetically causal association between them. MR analyses were performed using various analytical methods, including inverse variance weighted-fixed effects (IVW-FE), inverse variance weighted-multiplicative random effects (IVW-RE), MR-Egger, weighted median, weighted mode, and maximum likelihood. Sensitivity tests were conducted to assess the validity of the causality.
Results: The MR analysis with the IVW method revealed a genetically predicted association between AD and essential hypertension [odds ratio (OR)=0.9987, 95% confidence interval (CI): 0.9976-0.9998, P=0.024], as well as between asthma and atrial fibrillation (OR=1.001, 95% CI: 1.0004-1.0017, P=6.43E-05). In the reverse MR analyses, heart failure was associated with allergic diseases (OR=0.0045, 95% CI: 1.1890E-04 - 0.1695, P=0.004), while atherosclerosis (OR=8.7371E-08, 95% CI: 1.8794E-14 - 4.0617E-01, P=0.038) and aortic aneurysm and dissection (OR=1.7367E-07, 95% CI: 3.8390E-14 – 7.8567E-01, P=0.046) might be protective factors of asthma. However, after a Bonferroni correction, only the association between asthma and atrial fibrillation remained robust.
Conclusion: The MR study revealed that asthma is a predominant risk of atrial fibrillation in European individuals, consistent with most experimental and observational studies. Whether AD affects other CVD and the causality between them needs further investigation.
Introduction
The global prevalence of allergic diseases (AD) has increased dramatically, posing a considerable burden on global health (1). In some countries, nearly a quarter of the population is affected by AD (2). Epidemiological studies have identified risk factors for certain cardiovascular diseases (CVD), including inflammatory and immune responses, which are the primary pathophysiological processes of AD (3, 4). As a result, ADs have emerged as potential risk factors for CVD development, as reported by numerous experimental and observational studies (4–11). These studies revealed that CVD, including atrial fibrillation (5), atherosclerosis (4), heart attack (8), heart failure (8), hypertension (9, 10), stroke (6), and coronary heart disease (11) can be affected by asthma (5–7), atopic dermatitis (5, 8), and rhinitis (5, 9, 10). Nonetheless, the findings of these investigations have been inconclusive. In certain studies, AD has been identified as a potential risk factor for CVD (4–10). On the other hand, contrasting results have been proposed by other researchers (11).
Despite numerous observational studies examining the links between AD and CVD, the presence of confounding factors and unclear causal direction have led to biased conclusions. In an effort to minimize the impact of confounding variables and reverse causation, the Mendelian randomization (MR) approach was developed. In MR, the causal relationship between exposure and outcome are evaluated by instrumental variables (IVs). Genetic variants, which are distributed randomly during meiosis and remain unaltered after conception, are typically employed as IVs to enhance the validity of the findings. To ensure unbiased estimations, genetic variants can serve as IVs only if they fulfill the following criteria (12): First, the variants must exhibit a strong association with exposure. Second, the variants must be independent of any confounding factors related to the exposure-outcome connection. Finally, the variants should influence the outcome solely through the exposure pathway, not via any alternative biological routes. Moreover, MR can be extensively applied due to the availability of public genetic data.
Here, a bidirectional MR analysis was performed to investigate the causal relationship between AD and CVD, utilizing publicly accessible summary statistics.
Methods
Study design overview
First, the causal effects of AD on CVD were estimated, followed by an evaluation of the causal effects of CVD on AD (Figure 1). Genetic variants were required to meet three strict assumptions (12). Summary statistic datasets from recent genome-wide association studies (GWAS) of AD, asthma, and CVD were utilized.
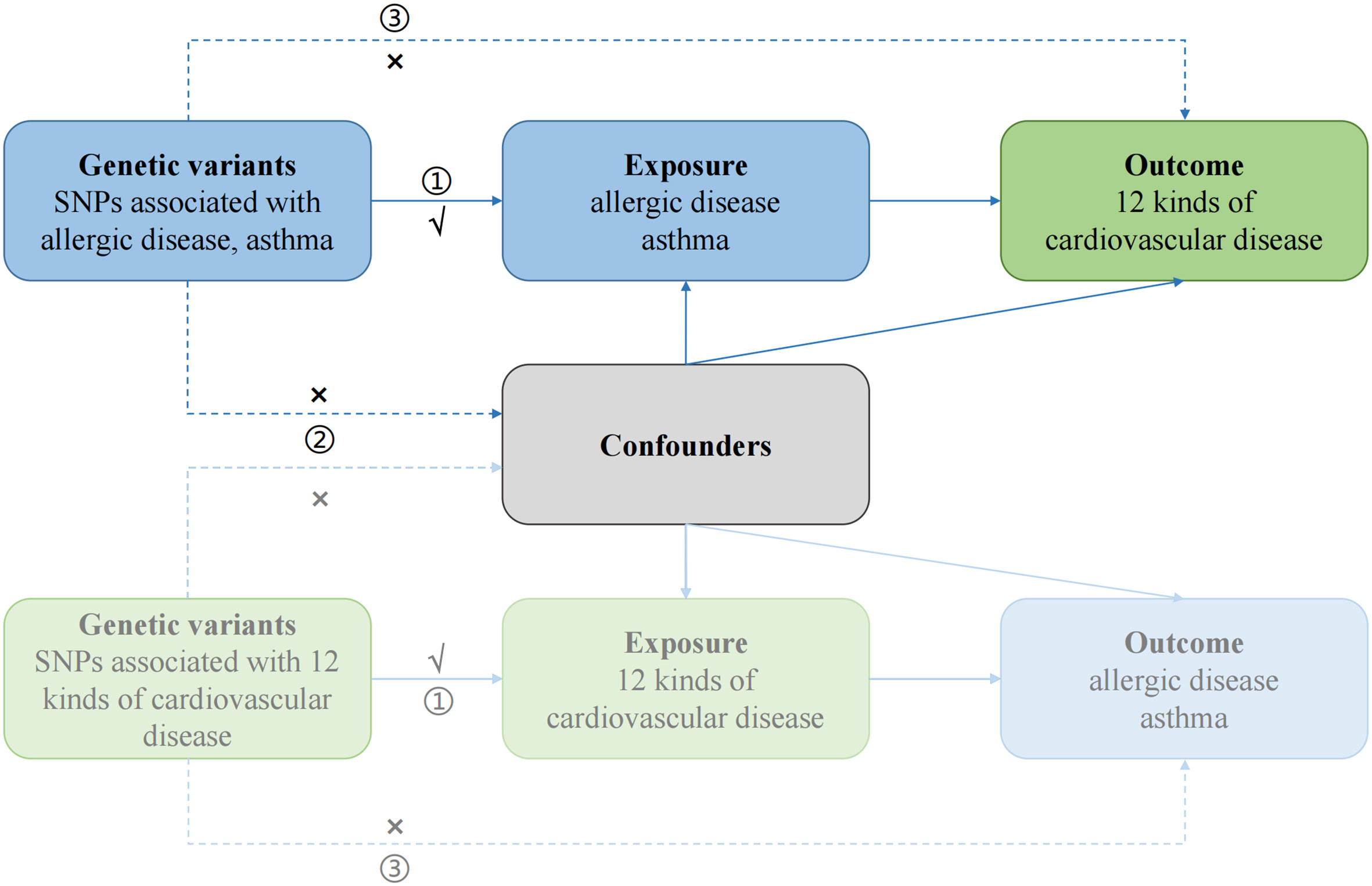
Figure 1 Study design of bidirectional Mendelian randomization study between AD and 12 kinds of CVD. The genetic variants used as instrumental variables meet the three conditions: ① the variants should be highly associated with exposure; ② the variants should be independent of confounding factors of the exposure-outcome association; and ③ the variants affect the outcome only via the exposure pathway and not through other biological pathways. The solid paths are significant; the dashed paths should not exist in our study.
Data sources and SNP selection of genetic instruments for AD
AD comprises asthma, hay fever, and eczema, which share many genetic variants that dysregulate the expression of immune-related genes and often coexist in the same individuals. The summary statistics for AD were obtained from the GWAS catalog (https://www.ebi.ac.uk/gwas/downloads/summary-statistics) or the IEU OpenGWAS project (https://gwas.mrcieu.ac.uk/) conducted by Ferreira et al. (13), which included 360,838 European individuals (180,129 cases vs 180,709 controls). Since many observational studies focused only on asthma (5–7) rather than all three ADs, we also estimated the causal effects of asthma on CVD. The summary statistics for asthma were obtained from the GWAS catalog or the IEU OpenGWAS project conducted by Demenais et al., including 127,669 individuals of European ancestry (19,954 cases vs 107,715 controls) (14). Detailed information on the conduction procedures and diagnostic criteria is described in the original publications. The sample characteristics of the study population are described in Supplementary Table S1.
Genetic variants associated with AD and asthma at genome-wide significance (P<5×10-8 and 5×10-6, respectively) were reported by the GWAS. Simultaneously, a linkage disequilibrium (LD) test was conducted to ensure the independence of clumped SNPs. The LD criteria were established as SNPs with r2 > 0.001 and a physical distance of kb< 10,000. SNPs with the lowest P values were retained. Subsequently, all 74 and 39 remaining SNPs related to AD and asthma, respectively, were examined in the Phenoscanner database (http://www.phenoscanner.medschl.cam.ac.uk/) to determine if these SNPs were associated with confounding factors or directly influenced the outcome (15) (P<5×10-8). We identified 10 SNPs associated with AD and six SNPs associated with asthma that were linked to confounders, as presented in Supplementary Table S2. After excluding these SNPs from the AD and asthma GWAS to mitigate potential pleiotropic effects, the remaining SNPs were utilized as IVs in the bidirectional MR analysis (refer to Supplementary Tables S3, S4).
Data sources and SNP selection of genetic instruments for CVD
The second round of GWAS results from the UK Biobank (UKB) (http://www.nealelab.is/uk-biobank) provided us with summary statistics on CVD, which encompassed heart arrhythmia, atrial fibrillation, supraventricular tachycardia, atherosclerosis, aortic aneurysm and dissection, stroke, peripheral vascular disease, cardiomyopathy, heart valve problems, heart failure, myocardial infarction, and essential hypertension. The UKB study is a prospective cohort study that gathered genetic and other data from over 500,000 individuals residing in the UK (16). These diseases were defined and identified by the UKB study based on self-reported status or ICD10 codes. Supplementary Table S1 summarizes the specifics of these various characteristics, including sample size, SNP count, phenotype code, and others.
We obtained the genome-wide significant variants (P< 5×10-5) associated with these CVDs, ensuring their independence (r2< 0.001, kb > 10,000). In a similar manner, we examined all SNPs using the Phenoscanner database and removed all SNPs linked to confounding variables, as shown in Supplementary Table S2. Details regarding the SNPs utilized as IVs are presented in Supplementary Tables S5–S16.
MR analysis
Following the removal of SNPs whose proxy SNPs were unavailable in the outcome GWAS, we harmonized the exposure and outcome data before evaluation of the association between AD or asthma and CVD. In the meantime, palindromic SNPs with intermediate allele frequency were excluded.
We employed the TwoSampleMR package (17) in R software (version 4.2.2) to conduct bidirectional MR analysis. The Wald ratio method was utilized to calculate the effect of each SNP, and a meta-analysis of the individual effect of each SNP was performed by inverse variance weighting (IVW) to generate the concluding beta estimate (beta outcome/beta exposure). The inverse variance weighted-fixed effects (IVW-FE) method was our primary analytical approach, as it is the most efficient and widely used. Since the outcome was binary (18), we converted it to the odds ratio (OR). To detect possible violations of IVs assumptions because of directional horizontal pleiotropy, namely the third assumption of SNP, we applied MR-Egger, which can effectively test the null causal hypothesis and serves as the basis method for horizontal pleiotropy in our analysis (19). The IVW method assumes that all SNPs are valid IVs, which can be supplemented by a weighted median (WM) model that provides a consistent effect even if half of the SNPs are pleiotropic (18). All three methods were applied to assess causal robustness of different assumptions, given the introduction of multiple genetic variants (18). Additionally, we employed maximum likelihood methods and weighted mode-based estimates to further analyze the association between exposure and outcome. The maximum likelihood method considers the sample overlap in two-sample MR and the uncertainty of the SNP-exposure association, which is disregarded in IVW (20). The weighted mode-based estimate is resilient to horizontal pleiotropy and exhibits lower type-I error rates than other methods, such as IVW, MR-Egger, WM, and simple median methods (21).
Heterogeneity based on IVW and MR-Egger methods was quantified using Cochran Q statistics and I2 statistics (22). We employed the MR pleiotropy residual sum and outlier (MR-PRESSO) test (23) to detect outlier SNPs (Nb Distribution = 10000, Significant Threshold = 0.05), which was conducted by the package “MR-PRESSO”, and the results are presented in Supplementary Table S17. Furthermore, a “leave-one-out” analysis was performed to determine if any single SNP was overly sensitive and disproportionately responsible for the outcome. F-statistics were used to evaluate the strength of SNPs to satisfy the first assumption (17). After determining the effect allele frequency (MAF) of the selected SNPs using the package “LDlinkR”, we calculated the F-statistic with the formula:
(N = sample size of the exposure, k = the number of selected SNPs, and R2 represents the phenotype variance induced by the SNPs.) When R2 is not available, we used the formula:
(β= the effect value of the genetic variant of the exposure, MAF = the effect allele frequency of selected SNPs, , SE = the standard error of the genetic variant of the exposure, and N = sample size of the exposure). All instruments exhibited F-statistics above the standard cutoff (>10), indicating the presence of sufficiently powerful instruments (24). Each R2 and F are shown in Supplementary Table S17.
Given the multiple testing, a p-value below 0.002 (0.05/24) was considered robust significance after a Bonferroni correction. A p-value between 0.002-0.05 was considered suggestive significance, and a p-value above 0.05 was considered no significance. All statistical analyses were two-sided.
Results
The causal effect of AD and asthma on CVD
In total, 64 and 33 LD-independent genetic variants were utilized as IVs for AD and asthma, respectively, following P value selection and LD clumping (refer to Supplementary Tables S3, S4). After removing SNPs that were unavailable in the outcome GWAS and distorted SNPs detected by MR-PRESSO and the “leave-one-out” test, the remaining SNPs were employed as IVs for AD and asthma. The “leave-one-out” test outcomes for the final SNPs are displayed in Supplementary Figures S1, S2. The F-statistics for the IVs of AD and asthma indicated that the IVs were robust instruments that reduced the bias of IV estimates.
We primarily employed the IVW-FE method to examine the genetic correlation between AD or asthma and CVD. Genetically predicted AD was linked to essential hypertension [odds ratio (OR)=0.9987, 95% confidence interval (CI): 0.9976-0.9998, P=0.024], potentially serving as a protective factor. However, this significance disappeared after applying the Bonferroni correction. Asthma was proposed as a risk factor for atrial fibrillation (OR=1.001, 95% CI: 1.0004-1.0017, P=6.43E-05), with robust significance after the Bonferroni correction, as depicted in Figure 2. The outcomes of other models are displayed in Supplementary Table S18. Sensitivity analyses were conducted to detect horizontal pleiotropy presence, confirming the IVW results’ reliability, as seen in Supplementary Table S19. Cochran’s Q test did not identify any heterogeneity based on IVW and MR-Egger tests, as shown in Supplementary Table S20. Reporting bias results, tested by MR-Egger and IVW, are presented in funnel plots (refer to Supplementary Figures S3, S4). The confounding factors’ impact on outcomes is illustrated in scatter plots (refer to Supplementary Figures S5, S6). Forest plots for each association pair for casualty are provided in Supplementary Figures S7, S8.
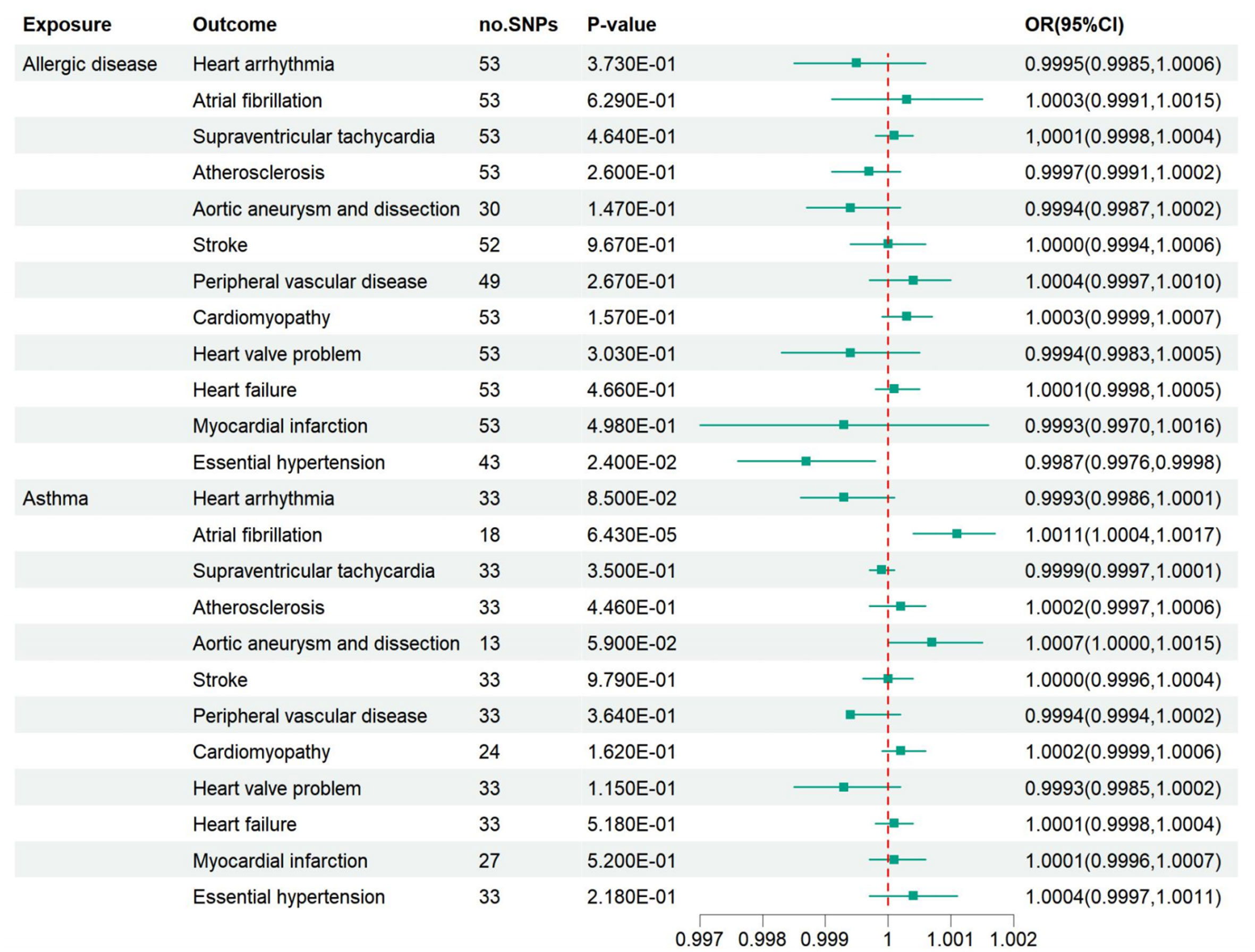
Figure 2 Associations of AD and asthma with 12 kinds of CVD. AD may be a protective factor against essential hypertension, and asthma is suggested to be a risk factor for atrial fibrillation. However, after a Bonferroni correction, there was only a robust association between asthma and atrial fibrillation. OR, odds ratio; CI, confidence interval.
The causal effect of CVD on AD and asthma
After LD clumping and searching in the Phenoscanner database, the remaining SNPs are shown in Supplementary Tables S5–S16. After excluding distortion and palindromic ambiguous SNPs, we used the remaining SNPs as final IVs to estimate the causal effect of CVD on AD and asthma. The “leave-one-out” test results and F-statistics are provided in Supplementary Figures S9, S10 and Supplementary Table S17. Based on the IVW-FE model, heart failure might be a protective factor against AD (OR=0.0045, 95% CI: 1.1890E-04 - 0.1695, P=0.004). Atherosclerosis (OR=8.7371E-08, 95% CI: 1.8794E-14 - 4.0617E-01, P=0.038) and aortic aneurysm and dissection (OR=1.7367E-07, 95% CI: 3.8390E-14 – 7.8567E-01, P=0.046) might be protective factors for asthma. However, no robust causal effect was detected between all 12 CVD and AD or asthma after the Bonferroni correction (Figure 3). The outcomes of other models are displayed in Supplementary Table S21. No horizontal pleiotropy or heterogeneity was suggested, as seen in Supplementary Tables S19, S20. Moreover, the funnel plots, scatter plots, and forest plots for each association pair for casualty are provided in Supplementary Figures S11–S16.
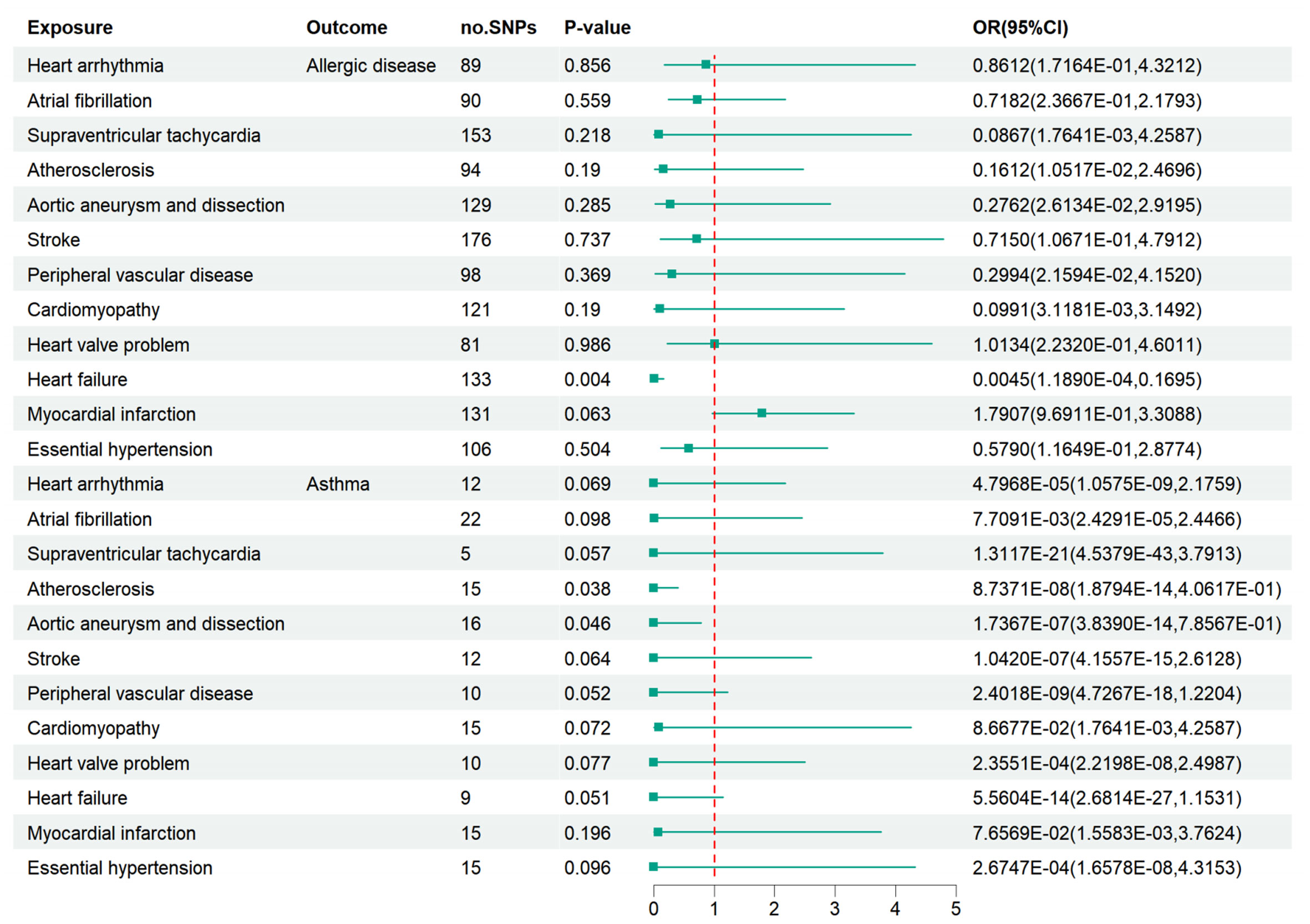
Figure 3 Associations of bioavailable AD and asthma with 12 kinds of CVD. Essential hypertension was suggested to be a protective factor against AD and asthma. Heart failure may be a suggestive protective factor of AD. Atherosclerosis and aortic aneurysm/dissection may be suggestively associated with asthma. However, there was no robust association between cardiovascular disease and AD or asthma after a Bonferroni correction.
Discussion
This bidirectional MR analysis offers evidence of a robust association between asthma and atrial fibrillation, even after applying the Bonferroni correction. AD exhibits a suggestive connection with essential hypertension. Conversely, heart failure, atherosclerosis, and aortic aneurysm and dissection might reduce the risk of AD and asthma. However, the reverse study did not show robust causality after the Bonferroni correction, indicating that there is only weak evidence to support that CVD causes AD.
To our knowledge, this is the first MR study to determine the bidirectional causal relationship between AD and CVD. A previous MR study established that asthma and atopic dermatitis are causal risk factors for heart failure (P=0.03 and 0.01) (25). However, the study could not demonstrate their causal link after the Bonferroni correction. Previous observational studies have produced conflicting conclusions. The presence of AD might be linked to an increased risk of CVD, including atrial fibrillation (5), atherosclerosis (4), heart attack (8), heart failure (8), hypertension (9, 10), stroke (6), and others. Moreover, the co-existence of allergic and CVD may increase the mortality of both diseases (6, 7). However, a decreased risk of CVD and all-cause mortality has also been reported in individuals with allergic rhinitis (11). Observational studies are typically affected by reverse causality or confounding factors, which could be avoided by an MR study.
Regarding the potential mechanism of these two types of diseases, experimental studies suggest that inflammatory and immune responses could be potent mechanisms (3, 4). AD, such as asthma, atopic dermatitis, and rheumatic arthritic, can induce chronic inflammation, which is associated with CVD (4–10). IgE and IgG are essential molecules in allergic reactions (26) associated with atherosclerosis (4). IgE-induced M1 macrophage polarization, foam cell formation, and vascular cell apoptosis contribute to plaque progression (26). Anti-IgE may serve as a protective factor for atherosclerosis, and treatment with an anti-IgE-neutralizing antibody could accelerate atherosclerotic lesion formation (27). High serum concentrations of IgG have been detected in ApoE-/- mice (28), and oxLDL-specific IgG has been observed in human plaques (29), using antibodies that may hinder the rapid regression of atherosclerotic lesions (30). Th2 cells are central to late-phase allergic inflammation, and a high Th2 cell frequency in circulation is associated with a reduced risk of myocardial infarction and stroke (31). Th2-related cytokine interleukin (IL)-4 may be a critical cytokine in early atherosclerosis progression (32), but IL-13 demonstrates a protective effect (33). Active mast cells can induce matrix degradation and apoptosis and recruit inflammatory cells to promote CVD progression (34–36). Apart from the immunological system’s pathophysiological processes, other conditions such as metabolism disorders (37, 38), central obesity (39), and typical lifestyle (40) could also contribute to the potential association between the two diseases. Some observational cohort studies have found a clinical connection between asthma and dyslipidemia, a significant risk factor for CVD (37, 38). Adults with eczema were more likely to experience sleep disturbances and smoke (40). However, our findings partially contradict these observational and experimental studies. We found that asthma is a risk factor for atrial fibrillation, but there was no causality between other AD and CVD. There may be a gap between the hypothesis, experimental studies, and the actual human situation.
This study has several limitations. First, the wide confidence interval around the effect estimate in the reverse study remains compatible with non-inferiority, limiting conclusions about the causal association between two diseases. Second, we used P<5×10-6 and 5×10-5 as the standard of genome-wide significance to select the variants related to asthma and the 12 kinds of CVD. The relatively small number of SNPs involved, especially in CVD and asthma, might make the SNPs less specific. Moreover, we did not stratify the causal association between AD and CVD by gender, age, BMI, and other factors. However, some studies suggest that these factors may affect the causality. Third, we were unable to detect an association between other AD and CVD due to the limited public genetic data. The complete data could only be obtained for participants of European descent, and we did not verify our conclusion in non-European populations. The instruments identified in European populations may not be suitable for non-European populations. Fourth, a small sample overlap between AD and CVD may lead to bias (41). Within large biobanks with sample sizes larger than 100,000, most two-sample MR methods can be safely used for one-sample MR, even with substantial confounding, particularly the IVW-FE, IVW-RM, weighted median, and weighted mode estimator, which are more robust to pleiotropy than one-sample MR methods (42). Since the UK Biobank is a large dataset with over 300,000 participants, and various compatible methods performing well in one-sample MR applied to our study, we believe our conclusions can be trusted. Furthermore, whole-genome arrays consist of common alleles only, which capture information on common but not rare alleles. However, sometimes low-frequency variants have larger effects than the common ones, although common alleles produce large effect sizes (43). More reliable instruments and larger samples are needed for more precise results.
Despite its limitations, the MR study had several advantages. First, we included variants across the phenotypes of AD and asthma from a recent meta-analysis. We extracted the IVs of the 12 kinds of CVD from the largest GWAS. Second, the bidirectional MR method reduced potential bias from confounding factors and reversed causality, ensuring a clear causal direction in our analysis. Third, we assessed the association between AD and a wide range of CVD, most of which were not previously examined based on genetic instruments. Fourth, it was easy to obtain extensive genetic data from the public genetic dataset using the MR method. The summary statistics can also be applied to individual-level data in terms of statistical power (44).
Conclusions
In summary, a robust association exists between asthma and atrial fibrillation, indicating that patients with asthma may have an increased risk of atrial fibrillation in European individuals. AD shows a suggestive association with essential hypertension, which attenuates significantly after applying the Bonferroni correction. In the reverse analysis, heart failure, atherosclerosis, and aortic aneurysm and dissection are negatively correlated with AD and asthma. However, the reverse study does not show robust causality after the Bonferroni correction, suggesting weak evidence. Whether AD exerts effects on CVD needs further investigation.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
Author contributions
SW chose the topic. ZW and PH identified genetic variants associated with exposure. SW, HL and PWY completed the subsequent data analysis and article writing. SC, JX and PY provided guidance and assistance throughout the process. All authors contributed to the article and approved the submitted version.
Acknowledgments
We are grateful to the participants in the UK Biobank and other cohorts and thank all the researchers who worked on the data collection.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2023.1175890/full#supplementary-material
Abbreviations
AD, Allergic diseases; CVD, Cardiovascular diseases; MR, Mendelian randomization; GWAS, Genome-wide association studies; AF, Atrial fibrillation; MI, Myocardial infarction.
References
1. Simpson CR, Newton J, Hippisley-Cox J, Sheikh A. Incidence and prevalence of multiple allergic disorders recorded in a national primary care database. J R Soc Med (2008) 101(11):558–63. doi: 10.1258/jrsm.2008.080196
2. Kang SY, Song WJ, Cho SH, Chang YS. Time trends of the prevalence of allergic diseases in Korea: a systematic literature review. Asia Pac Allergy (2018) 8(1):e8. doi: 10.5415/apallergy.2018.8.e8
3. Hung YH, Cheng CM, Lin WC, Bai YM, Su TP, Li CT, et al. Post-traumatic stress disorder and asthma risk: a nationwide longitudinal study. Psychiatry Res (2019) 276:25–30. doi: 10.1016/j.psychres.2019.04.014
4. Fernández-Gallego N, Castillo-González R, Méndez-Barbero N, López-Sanz C, Obeso D, Villaseñor A, et al. The impact of type 2 immunity and allergic diseases in atherosclerosis. Allergy (2022) 77(11):3249–66. doi: 10.1111/all.15426
5. Choi YJ, Choi EK, Han KD, Kwon S, Lee SY, Yang S, et al. Increased risk of atrial fibrillation in patients with atopic triad: a nationwide population-based study. J Allergy Clin Immunol Pract (2021) 9(9):3422–30. doi: 10.1016/j.jaip.2021.04.056
6. Strand LB, Tsai MK, Wen CP, Chang SS, Brumpton BM. Is having asthma associated with an increased risk of dying from cardiovascular disease? a prospective cohort study of 446 346 Taiwanese adults. BMJ Open (2018) 8(5):e019992. doi: 10.1136/bmjopen-2017-019992
7. Lemmetyinen RE, Karjalainen JV, But A, Renkonen RLO, Pekkanen JR, Toppila-Salmi SK, et al. Higher mortality of adults with asthma: a 15-year follow-up of a population-based cohort. Allergy (2018) 73(7):1479–88. doi: 10.1111/all.13431
8. Silverberg JI. Association between adult atopic dermatitis, cardiovascular disease, and increased heart attacks in three population-based studies. Allergy (2015) 70(10):1300–8. doi: 10.1111/all.12685
9. Kony S, Zureik M, Neukirch C, Leynaert B, Vervloet D, Neukirch F. Rhinitis is associated with increased systolic blood pressure in men: a population-based study. Am J Respir Crit Care Med (2003) 167(4):538–43. doi: 10.1164/rccm.200208-851OC
10. Heinrich J, Topp R, Brasche S. Rhinitis and blood pressure in adults. Am J Respir Crit Care Med (2003) 168(10):1243–5. doi: 10.1164/rccm.200304-525OC
11. Crans Yoon AM, Chiu V, Rana JS, Sheikh J. Association of allergic rhinitis, coronary heart disease, cerebrovascular disease, and all-cause mortality. Ann Allergy Asthma Immunol (2016) 117(4):359–64. doi: 10.1016/j.anai.2016.08.021
12. Hartwig FP, Davies NM, Hemani G, Davey Smith G. Two-sample mendelian randomization: avoiding the downsides of a powerful, widely applicable but potentially fallible technique. Int J Epidemiol. (2016) 45(6):1717–26. doi: 10.1093/ije/dyx028
13. Ferreira MA, Vonk JM, Baurecht H, Marenholz I, Tian C, Hoffman JD, et al. Shared genetic origin of asthma, hay fever and eczema elucidates allergic disease biology. Nat Genet (2017) 49(12):1752–7. doi: 10.1038/ng.3985
14. Demenais F, Margaritte-Jeannin P, Barnes KC, Cookson WOC, Altmüller J, Ang W, et al. Multiancestry association study identifies new asthma risk loci that colocalize with immune-cell enhancer marks. Nat Genet (2018) 50(1):42–53. doi: 10.1038/s41588-017-0014-7
15. Kamat MA, Blackshaw JA, Young R, Surendran P, Burgess S, Danesh J, et al. PhenoScanner V2: an expanded tool for searching human genotype-phenotype associations. Bioinformatics (2019) 35(22):4851–3. doi: 10.1093/bioinformatics/btz469
16. Sudlow C, Gallacher J, Allen N, Beral V, Burton P, Danesh J, et al. UK Biobank: an open access resource for identifying the causes of a wide range of complex diseases of middle and old age. PloS Med (2015) 12(3):e1001779. doi: 10.1371/journal.pmed.1001779
17. Hemani G, Zheng J, Elsworth B, Wade KH, Haberland V, Baird D, et al. The MR-base platform supports systematic causal inference across the human phenome. Elife (2018) 7:e34408(1–29). doi: 10.7554/eLife.34408
18. Burgess S, Bowden J, Fall T, Ingelsson E, Thompson SG. Sensitivity analyses for robust causal inference from mendelian randomization analyses with multiple genetic variants. Epidemiology (2017) 28(1):30–42. doi: 10.1097/ede.0000000000000559
19. Bowden J, Davey Smith G, Burgess S. Mendelian randomization with invalid instruments: effect estimation and bias detection through egger regression. Int J Epidemiol. (2015) 44(2):512–25. doi: 10.1093/ije/dyv080
20. Yavorska OO, Burgess S. MendelianRandomization: an r package for performing mendelian randomization analyses using summarized data. Int J Epidemiol. (2017) 46(6):1734–9. doi: 10.1093/ije/dyx034
21. Hartwig FP, Davey Smith G, Bowden J. Robust inference in summary data mendelian randomization via the zero modal pleiotropy assumption. Int J Epidemiol. (2017) 46(6):1985–98. doi: 10.1093/ije/dyx102
22. Bowden J, Del Greco MF, Minelli C, Zhao Q, Lawlor DA, Sheehan NA, et al. Improving the accuracy of two-sample summary-data mendelian randomization: moving beyond the NOME assumption. Int J Epidemiol. (2019) 48(3):728–42. doi: 10.1093/ije/dyy258
23. Verbanck M, Chen CY, Neale B, Do R. Detection of widespread horizontal pleiotropy in causal relationships inferred from mendelian randomization between complex traits and diseases. Nat Genet (2018) 50(5):693–8. doi: 10.1038/s41588-018-0099-7
24. Burgess S, Davey Smith G, Davies NM, Dudbridge F, Gill D, Glymour MM, et al. Guidelines for performing mendelian randomization investigations. Wellcome Open Res (2019) 4:186. doi: 10.12688/wellcomeopenres.15555.2
25. Guo YG, Zhang Y, Liu WL. The causal relationship between allergic diseases and heart failure: evidence from mendelian randomization study. PloS One (2022) 17(7):e0271985. doi: 10.1371/journal.pone.0271985
26. Shamji MH, Valenta R, Jardetzky T, Verhasselt V, Durham SR, Würtzen PA, et al. The role of allergen-specific IgE, IgG and IgA in allergic disease. Allergy (2021) 76(12):3627–41. doi: 10.1111/all.14908
27. Tsiantoulas D, Bot I, Ozsvar-Kozma M, Göderle L, Perkmann T, Hartvigsen K, et al. Increased plasma IgE accelerate atherosclerosis in secreted IgM deficiency. Circ Res (2017) 120(1):78–84. doi: 10.1161/circresaha.116.309606
28. Hutchinson MA, Park HS, Zanotti KJ, Alvarez-Gonzalez J, Zhang J, Zhang L, et al. Auto-antibody production during experimental atherosclerosis in ApoE(-/-) mice. Front Immunol (2021) 12:695220. doi: 10.3389/fimmu.2021.695220
29. Wang Y, Krémer V, Iannascoli B, Goff OR, Mancardi DA, Ramke L, et al. Specificity of mouse and human fcgamma receptors and their polymorphic variants for IgG subclasses of different species. Eur J Immunol (2022) 52(5):753–9. doi: 10.1002/eji.202149766
30. Schiopu A, Frendéus B, Jansson B, Söderberg I, Ljungcrantz I, Araya Z, et al. Recombinant antibodies to an oxidized low-density lipoprotein epitope induce rapid regression of atherosclerosis in apobec-1(-/-)/low-density lipoprotein receptor(-/-) mice. J Am Coll Cardiol (2007) 50(24):2313–8. doi: 10.1016/j.jacc.2007.07.081
31. Engelbertsen D, Andersson L, Ljungcrantz I, Wigren M, Hedblad B, Nilsson J, et al. T-Helper 2 immunity is associated with reduced risk of myocardial infarction and stroke. Arterioscler Thromb Vasc Biol (2013) 33(3):637–44. doi: 10.1161/atvbaha.112.300871
32. George J, Shoenfeld Y, Gilburd B, Afek A, Shaish A, Harats D. Requisite role for interleukin-4 in the acceleration of fatty streaks induced by heat shock protein 65 or mycobacterium tuberculosis. Circ Res (2000) 86(12):1203–10. doi: 10.1161/01.res.86.12.1203
33. Cardilo-Reis L, Gruber S, Schreier SM, Drechsler M, Papac-Milicevic N, Weber C, et al. Interleukin-13 protects from atherosclerosis and modulates plaque composition by skewing the macrophage phenotype. EMBO Mol Med (2012) 4(10):1072–86. doi: 10.1002/emmm.201201374
34. Bot I, Shi GP, Kovanen PT. Mast cells as effectors in atherosclerosis. Arterioscler Thromb Vasc Biol (2015) 35(2):265–71. doi: 10.1161/atvbaha.114.303570
35. Sun J, Sukhova GK, Wolters PJ, Yang M, Kitamoto S, Libby P, et al. Mast cells promote atherosclerosis by releasing proinflammatory cytokines. Nat Med (2007) 13(6):719–24. doi: 10.1038/nm1601
36. Heikkilä HM, Trosien J, Metso J, Jauhiainen M, Pentikäinen MO, Kovanen PT, et al. Mast cells promote atherosclerosis by inducing both an atherogenic lipid profile and vascular inflammation. J Cell Biochem (2010) 109(3):615–23. doi: 10.1002/jcb.22443
37. Liu L, Liu Y, Zhang X, Yuan YL, Chen ZH, Chen-Yu Hsu A, et al. Dyslipidemia is associated with worse asthma clinical outcomes: a prospective cohort study. J Allergy Clin Immunol Pract (2022). 863–872 doi: 10.1016/j.jaip.2022.11.037
38. Sheha DS, El-Korashi LA, AbdAllah AM, El-Begermy MM, Elmahdi AR. Dyslipidemia among allergic rhinitis patients: frequency and risk factors. World Allergy Organ J (2021) 14(3):100523. doi: 10.1016/j.waojou.2021.100523
39. Silverberg JI, Becker L, Kwasny M, Menter A, Cordoro KM, Paller AS. Central obesity and high blood pressure in pediatric patients with atopic dermatitis. JAMA Dermatol (2015) 151(2):144–52. doi: 10.1001/jamadermatol.2014.3059
40. Silverberg JI, Greenland P. Eczema and cardiovascular risk factors in 2 US adult population studies. J Allergy Clin Immunol (2015) 135(3):721–8. doi: 10.1016/j.jaci.2014.11.023
41. Burgess S, Davies NM, Thompson SG. Bias due to participant overlap in two-sample mendelian randomization. Genet Epidemiol. (2016) 40(7):597–608. doi: 10.1002/gepi.21998
42. Minelli C, Del Greco MF, van der Plaat DA, Bowden J, Sheehan NA, Thompson J. The use of two-sample methods for mendelian randomization analyses on single large datasets. Int J Epidemiol. (2021) 50(5):1651–9. doi: 10.1093/ije/dyab084
43. Swerdlow DI, Kuchenbaecker KB, Shah S, Sofat R, Holmes MV, White J, et al. Selecting instruments for mendelian randomization in the wake of genome-wide association studies. Int J Epidemiol. (2016) 45(5):1600–16. doi: 10.1093/ije/dyw088
Keywords: asthma, allergic disease, atrial fibrillation, cardiovascular disease, Mendelian randomization
Citation: Wang S, Liu H, Yang P, Wang Z, Hu P, Ye P, Xia J and Chen S (2023) Exploring the genetic association of allergic diseases with cardiovascular diseases: a bidirectional Mendelian randomization study. Front. Immunol. 14:1175890. doi: 10.3389/fimmu.2023.1175890
Received: 28 February 2023; Accepted: 19 May 2023;
Published: 02 June 2023.
Edited by:
Innocent Safeukui, University of Notre Dame, United StatesReviewed by:
Linshuoshuo Lyu, Yale University, United StatesEnjie Liu, First Affiliated Hospital of Zhengzhou University, China
Minglei Yang, Sun Yat-sen University, China
Copyright © 2023 Wang, Liu, Yang, Wang, Hu, Ye, Xia and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shu Chen, c2h1X2NoZW5AaHVzdC5lZHUuY24=; Jiahong Xia, amlhaG9uZy54aWFAaHVzdC5lZHUuY24=; Ping Ye, Ymx1ZTMxNEAxNjMuY29t
†These authors have contributed equally to this work and share first authorship
‡These authors have contributed equally to this work and share last authorship
 Shilin Wang
Shilin Wang Hao Liu
Hao Liu Peiwen Yang1
Peiwen Yang1 Jiahong Xia
Jiahong Xia