
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Immunol. , 09 May 2023
Sec. Microbial Immunology
Volume 14 - 2023 | https://doi.org/10.3389/fimmu.2023.1143526
 Yapeng Yang1,2
Yapeng Yang1,2 Jinhui He2
Jinhui He2 Yuqing Wang2
Yuqing Wang2 Lifeng Liang3
Lifeng Liang3 Zeyue Zhang2
Zeyue Zhang2 Xiang Tan2
Xiang Tan2 Shiyu Tao2
Shiyu Tao2 Zhifeng Wu2
Zhifeng Wu2 Miaomiao Dong2
Miaomiao Dong2 Jixia Zheng2
Jixia Zheng2 Hang Zhang2
Hang Zhang2 Shuaifei Feng2
Shuaifei Feng2 Wei Cheng2
Wei Cheng2 Qiyi Chen4
Qiyi Chen4 Hong Wei1,2*
Hong Wei1,2*Fecal microbiota transplantation (FMT) is an emerging and effective therapy for the treatment of inflammatory bowel disease (IBD). Previous studies have reported that compared with FMT, whole intestinal microbiota transplantation (WIMT) can more precisely replicate the community structure and reduce the inflammatory response of the host. However, it remains unclear whether WIMT is more effective in alleviating IBD. To examine the efficacy of WIMT and FMT in the intervention of IBD, GF (Germ-free) BALB/c mice were pre-colonized with whole intestinal microbiota or fecal microbiota before being treated with dextran sodium sulfate (DSS). As expected, the symptoms of colitis were alleviated by both WIMT and FMT, as demonstrated by the prevention of body weight loss and decreased the Disease activity index and histological scores in mice. However, WIMT’s anti-inflammatory effect was superior to that of FMT. In addition, the inflammatory markers myeloperoxidase (MPO) and eosinophil peroxidase were dramatically downregulated by WIMT and FMT. Furthermore, the use of two different types of donors facilitated the regulation of cytokine homeostasis in colitis mice; the level of the pro-inflammatory cytokine IL-1β in the WIMT group was significantly lower than that in the FMT group, while the level of the anti-inflammatory factor IL-10 was significantly higher than that in the FMT group. Both groups showed enhanced expression of occludin to protect the intestinal barrier in comparison with the DSS group, and the WIMT group demonstrated considerably increased levels of ZO-1. The sequencing results showed that the WIMT group was highly enriched in Bifidobacterium, whereas the FMT group was significantly enriched in Lactobacillus and Ochrobactrum. Correlation analysis revealed that Bifidobacterium was negatively correlated with TNF-α, whereas Ochrobactrum was positively correlated with MPO and negatively correlated with IL-10, which might be related to different efficacies. Functional prediction using PICRUSt2 revealed that the FMT group was considerably enriched in the L-arginine biosynthesis I and L-arginine biosynthesis IV pathway, whereas the WIMT group was enriched in the L-lysine fermentation to acetate and butanoate pathway. In conclusion, the symptoms of colitis were subsided to varying degrees by the two different types of donors, with the WIMT group being more effective than the FMT group. This study provides new information on clinical interventions for IBD.
Inflammatory bowel disease (IBD), which comprises Crohn’s disease (CD) and ulcerative colitis (UC) (1), is a chronic, fatal condition that primarily manifests as diarrhea, stomach pain, and rectal bleeding (2, 3). At present, IBD affects up to 0.5% of the population in the West, and this number is projected to continue to rise over the next ten years (2, 4). Additionally, China, a newly industrialized nation with a sizable population, may eventually see more cases of IBD than the West due to its move toward urbanization and westernization (2). Thus, IBD is a global illness (4).
Owing to advancements in next-generation sequencing methods, numerous variations in the gut microbiota’s composition and community have been reported in patients with IBD. The most common finding in IBD is a reduction in bacterial diversity, with a reduced abundance of Firmicutes, and an increased abundance of Proteobacteria, although some of the results linked to dysbiosis in IBD vary among studies due to changes in sample type, survey techniques, patient state, and pharmacological therapy (5–8). Probiotic therapy and other dysbiosis-correcting therapies, such as fecal microbiota transplantation (FMT), have shown promise for treating IBD (9). FMT is a therapeutic approach to ameliorate the abnormal microbial composition of the gut by delivering a patient with fecal microbiota from a healthy donor (9, 10). It has been emphasized as a treatment for correcting dysbiosis in IBD (9). FMT was originally used to treat UC in 1989 (11) and numerous clinical studies have demonstrated that it can dramatically reduce UC symptoms (12, 13). To increase the effectiveness of FMT intervention in UC, it is necessary to further investigate the active ingredients of fecal donors and the mechanism of action of FMT (13, 14). In addition, the composition of donor flora affects the efficacy of FMT in UC, indicating that variations in the composition of donor fecal flora are one of the factors affecting the therapeutic efficacy of FMT (15).
To date, most FMT have relied on stool samples from healthy donors (16). However, different gut segment microbes may also be involved in regulating host health (17). Less commonly, the topic of gut bacteria, both small and large intestinal microbes, has been investigated. By comparing the differences between FMT and whole intestinal microbiota transplantation (WIMT) in reshaping the community structure in germ-free (GF) mice, Li et al. found that WIMT better reproduced the donor microbiota structure and reduced the inflammatory response of the host compared to FMT (18). Given the link between microbiota and IBD, the effect of WIMT intervention in IBD is worth examining. It is possible to explore the connection between IBD and microbiota in GF animals because their microbial backgrounds are well understood (19). Therefore, to explore the differences in the efficacy of WIMT and FMT intervention in colitis mice, we used GF mice colonized with whole intestinal flora and fecal flora followed by dextran sodium sulfate (DSS) to induce the development of colitis in mice. This study provides basic data to support pertinent clinical applications.
Fresh feces and contents of whole intestinal segments (all contents of the jejunum, ileum, cecum, and colon) were collected from 8-week-old male SPF BALB/c mice (from the Experimental Animal Center of Huazhong Agricultural University) were collected in sterile fecal collector under anaerobic conditions (80% N2, 10% H2, 10% CO2), and the WIMT and FMT donor samples were homogenized at a ratio of 1:10 (m/v) to sterile saline glycerol buffer (15% glycerol concentration) and mixed well. After the samples were fully dissolved using coarse filtration with sterilized gauze, the bacterial solution was finally obtained by filtration through a 100 μm filter membrane (20).
Female GF BALB/c mice at 8-10 weeks were obtained from the Germ-Free Animal Platform of Huazhong Agricultural University. Mice were housed in a sterile environment (temperature 25 ± 2°C; relative humidity 45-60%; photoperiod 12h/d; light hours 06:30-18:30), and had free access to sterilized food and water. To study the effects of WIMT and FMT transplantation on the development of UC, the mice were divided into Control, DSS, WIMT, and FMT groups. The mice were instilled with 100 μL of bacterial solution or saline daily; the experimental design is shown in Figure 1. Body weight was recorded daily during the experiment to calculate the difference between the weight on the day of measurement and that on day 0 (21). At the end of the experiment, the feces of the mice were collected inside the isolator. The colon tissue, small intestinal contents (jejunal and ileal contents), and large intestinal contents (cecum and colon contents) were collected after the experimental mice were euthanized. Blood was immediately collected from the eyeball, and the separated serum was frozen at -80°C until analysis. All experimental methods were performed according to the Huazhong Agricultural University of Health Guide for the Care and Use of Laboratory Animals. The animal experiment ethics number for this study is HZAUMO-2023-0026.
The Disease Activity Index (DAI) includes weight loss score, fecal bleeding score, and stool traits, as shown in Table 1 (22, 23). Briefly, DAI was measured by weight change (no change = 0; 1-5% = 1; 5-10% = 2; 10-15% = 3; >15% = 4), fecal bleeding score (normal colored stool = 0; brown stool = 1; red stool = 2; bloody stool = 3; heavy bleeding = 4), and fecal traits (normal stool, good shape = 0; soft stool, soft stool adhering to the anus = 1-2; diarrhea, adherent anal = 3-4), which were determined by averaging the three scores (see Table 1).
Distal colon segments from each group of mice were fixed with 4% paraformaldehyde, paraffin-embedded, and cut into 4 μm-thick sections. Sections were stained with hematoxylin-eosin (H&E) and immunohistochemistry (IHC), and images were acquired under a microscope (Nikon Eclipse 80i, Japan). The H&E-stained sections were examined for the degree of inflammatory cell infiltration and tissue damage, and intestinal damage was assessed for the degree of infection, extent of infection, crypt damage, and extent of mucosal involvement (24) (see Table 2). The expression of ZO-1 (Proteintech Group, Inc. 21773-1-AP) and occludin (Proteintech Group, Inc. 27260-1-AP) was detected by immunohistochemistry according to the manufacturer’s instructions using Image Pro Plus 6.0 (Media Cybernetics, Inc.) for statistical analysis of mean optical density.
The kits were purchased from Shanghai Enzyme-linked Biotechnology Co. Ltd. (Shanghai, China). All assays were performed according to the manufacturer’s instructions, and IL-1β (ml063132), IL-6 (ml002293), IL-8 (ml001856), IL-17A (ml037864), TNF-α (ml002095), myeloperoxidase (MPO) (ml002070), eosinophil peroxidase (EPO) (ml769125), and serum diamine oxidase (DAO) (ml002070) concentrations were determined by ELISA. Tissue processing for ELISAs are as follows, 1g of tissue sample was weighed and 9ml of PBS (pH 7.2-7.4) was added to homogenise the sample. Centrifuge for about 20 minutes (2000-3000 rpm) and carefully collect the supernatant for testing. Briefly, the kits’ included diluent buffer was used to dilute both standards and samples. A microtitre plate with an antibody precoated in each well was then filled with 100 μL of the sample or standard in duplicate. Diluent buffer was used as a negative control. The plates were incubated for 2 h at 37°C. After incubation, 100 μL of biotin antibody was added to each well after removing the liquid and incubated for 1 h at 37°C. The wells were washed 3 times with 200 μL volume of wash buffer. Next, each well received 100 μL of horseradish peroxidase-avidin for 1 hour at 37°C. After a final wash, 90 μL of the supplied TMB substrate was added and incubated for 30 min in the dark at 37°C. 50 μL of the supplied stop solution was used to stop the reaction. Absorbance was measured at 450 nm using a plate reader (BioTek Instruments, Inc.), and the levels of cytokines in the samples was calculated from the standard curve.
The levels of DAO and D-lactic acid (D-LA) in serum were tested for permeability. D-LA (ml158174) was determined by spectrophotometry using a D-Lactic acid detection kit (Shanghai Enzyme-linked Biotechnology Co. Ltd.). Briefly, the visible spectrophotometer (Shanghai Enzyme-linked Biotechnology Co. Ltd.) is preheated for more than 30min, the wavelength is adjusted to 450nm and zeroed with distilled water. The reagents and samples were added sequentially to 1mL glass cuvette according to the reagent manufacturer’s instructions, mixed and immediately reacted in the dark at 37°C for 30min, and the absorbance value was read at 450nm. The levels of D-LA in the sample were calculated from the standard curve.
Samples were collected inside the isolator, immediately transported on ice to a −80°C refrigerator, and transported on dry ice for amplicon sequencing. Genomic DNA was extracted by the Cetyltrimethylammonium Bromide (CTAB) method and then amplified using specific primers with barcode (515F-806R for 16S V4 region) after assessing the purity and concentration of DNA. The PCR products were mixed in equal concentrations according to the concentration of the PCR product and then purified by electrophoresis using 1×TAE on a 2% agarose gel. Sequences with a primary band size between 400-450 bp were selected and the gel was cut to recover the target bands. Libraries were constructed using an Illumina TruSeq DNA PCR-Free Library Preparation Kit. The libraries were sequenced using NovaSeq 6000 after they were qualified using Qubit quantification and library testing. The analysis was carried out by splitting each sample from the downstream data based on barcode sequences and PCR amplification primer sequences, and the reads were spliced using FLASH software (V1.2.11, http://ccb.jhu.edu/software/FLASH/) after truncating the barcode and primer sequences to obtain Raw Tags. Raw Tags were then quality-controlled using the Fastp software to obtain high-quality Clean Tags. Finally, Clean Tags were compared to the database using Usearch software to detect chimeras and remove them (25) to obtain the final Effective Tags, and the DADA2 module or deblur in QIIME2 software (26) (https://qiime2.org/) was used for a module or deblur for noise reduction (27) and filtering out low-quality sequences to obtain the final Amplicon Sequence Variants (ASVs) and feature-table. The ASVs were then compared to the Silva database (V138-99) using the classify-sklearn module in R software to obtain species information for each ASV. Alpha diversity: R was used to calculate Shannon, Simpson, and Pielou indices and analyze the inter-group differences in alpha diversity. Beta diversity: Jaccard distances were calculated using R. The PCoA was plotted using the vegan package (versions 2.6-2) in R. Subsequently, the adonis function in R was used to analyze the significance of differences in community structure between groups. Functional annotation: Prediction of colony metabolic function using PICRUSt2. Distinctive and shared features and linear discriminant analysis effect size (LEfSe, p < 0.05, LDA > 2.0) were completed using Wekemo Bioincloud (https://www.bioincloud.tech).
Data were analyzed using GraphPad Prism 6 (GraphPad Software, LLC), and the Student’s t-test was used for statistical analysis between the two groups. A non-parametric test was used to analyse count data with Kruskal-Wallis test followed by Dunn’s multiple comparisons test. One-way ANOVA and Bonferroni’s multiple comparison test were performed on data from more than two groups, and the data are expressed as mean ± SEM. The differences were considered statistically significant at p < 0.05.
To investigate the effects of WIMT and FMT on colitis, mice with 3% DSS-induced colitis were treated with WIMT, FMT, and saline. The body weight of mice in the DSS group decreased significantly, and those of mice in the WIMT group decreased less than those of the mice in the FMT group. On the final day of the experiment, the body weights of mice in the FMT group were not statistically different compared with the DSS group, while the mice in the WIMT group were significantly heavier than those in the DSS group (p = 0.0056) (Figures 2A, B). WIMT and FMT intervention reduced DSS-induced DAI scores, with no significant difference on the last day DAI between the WIMT and FMT groups (Figures 2C, D). Mice in both groups with microbiota transplantation had lower weight of the spleen weight to body weight (Figure 2E). Histological staining showed that DSS treatment resulted in different degrees of histological damage in each group of mice (WIMT vs. DSS, p = 0.0002; FMT vs. DSS, p = 0.0163). Compared with the DSS group, donor fluids in both groups showed reduced inflammatory cell infiltration and mucosal damage in colonic tissue and reduced histological scores, with the lowest scores in the WIMT group, however, no significant difference was observed between the WIMT and FMT groups (Figures 2F, G). In summary, these results suggest that pre-colonization of both donor flora could alleviate DSS-induced colitis, with WIMT having a higher intervention efficacy than FMT.
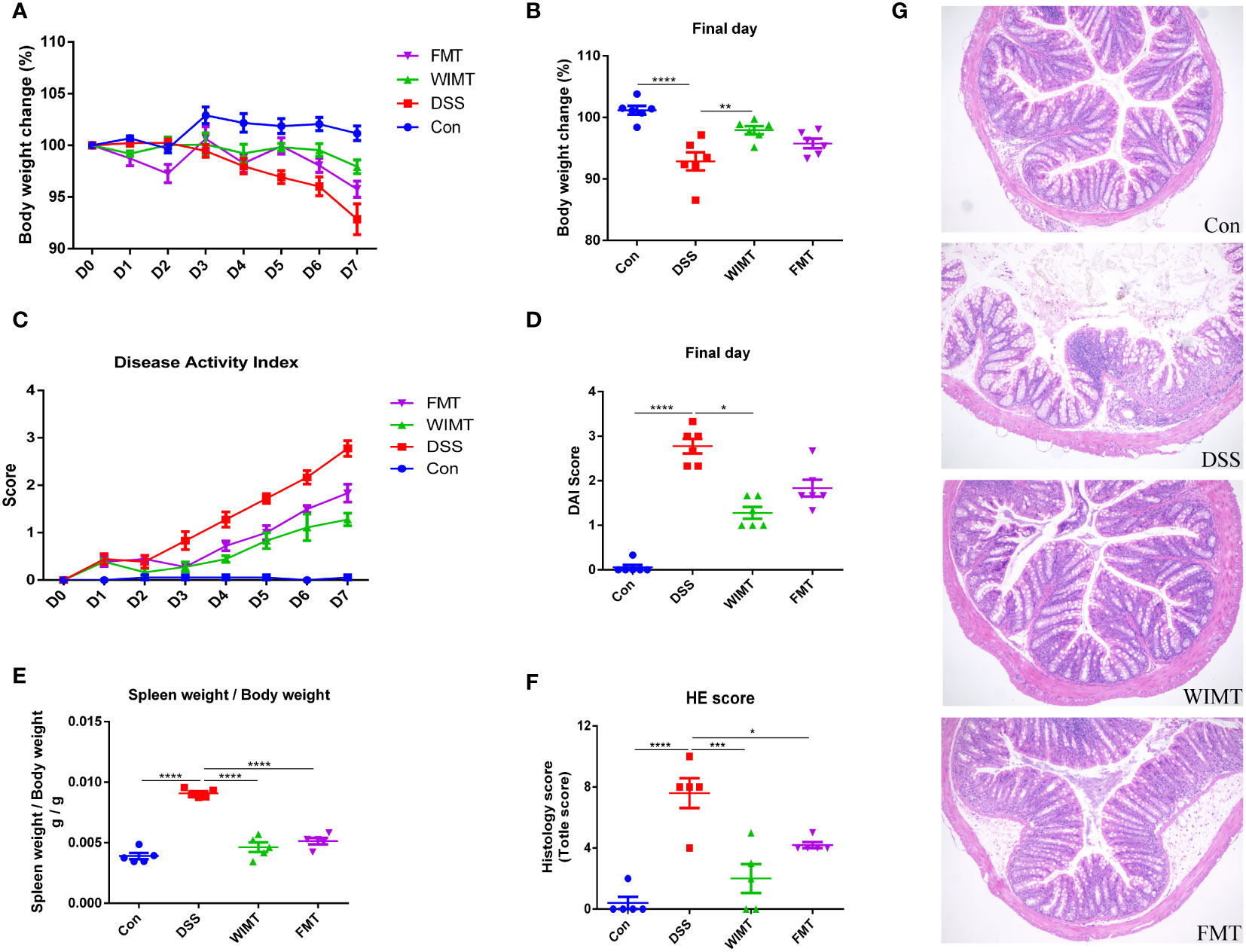
Figure 2 WIMT and FMT alleviated DSS-induced colitis to different extents. (A) Body weight change; (B) Body weight change on the final day; (C) DAI score; (D) DAI score on the final day; (E) Ratio of spleen weight to body weight; (F) Histological score; (G) H&E staining of colon tissue (100×). * p ≤ 0.05, ** p ≤ 0.01, *** p ≤ 0.001, **** p ≤ 0.0001, data are represented as mean ± SEM.
To explore the effects of the two donors flora on immune homeostasis and inflammatory markers of colitis, the levels of pro-inflammatory factors IL-1β, IL-6, IL-8, IL-17A, TNF-α, anti-inflammatory factor IL-10, and colitis markers MPO and EPO in colonic tissues were measured by ELISA. As shown in Figures 3A, B, WIMT and FMT interventions significantly reduced the levels of MPO (WIMT vs. DSS, p < 0.0001; FMT vs. DSS, p = 0.0008) and EPO (WIMT vs. DSS, p < 0.0001; FMT vs. DSS, p = 0.0001) in colonic tissues compared to those in the DSS group, and there was no significant difference between the WIMT and FMT groups. WIMT and FMT interventions significantly reduced the levels of pro-inflammatory factors IL-1β (WIMT vs. DSS, p < 0.0001; FMT vs. DSS, p = 0.0007), IL-6 (WIMT vs. DSS, p = 0.022; FMT vs. DSS, p = 0.0133), IL-8 (WIMT vs. DSS, p = 0.0002; FMT vs. DSS, p = 0.034), IL-17A (WIMT vs. DSS, p = 0.0003; FMT vs. DSS, p = 0.0011), and TNF-α (WIMT vs. DSS, p < 0.0001; FMT vs. DSS, p = 0.0034) in colonic tissues (Figures 3C-G) and increased IL-10 levels (WIMT vs. DSS, p < 0.0001; FMT vs. DSS, p = 0.0009) (Figure 3H). IL-1β levels were significantly lower in the WIMT group compared to the FMT group (p = 0.0345), while IL-10 levels were higher in the WIMT group (p = 0.0052). In summary, WIMT intervention alleviated the symptoms of colitis in mice by improving cytokine homeostasis.
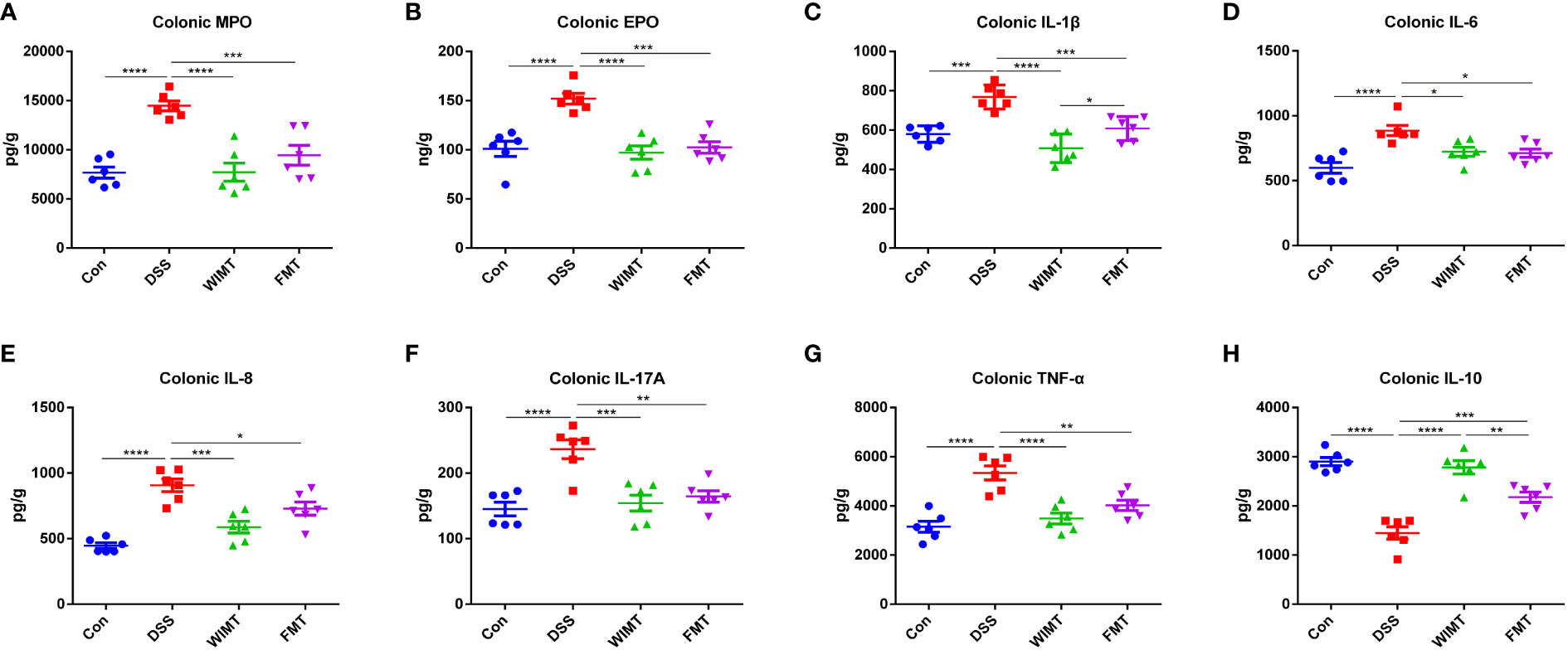
Figure 3 Effect of WIMT and FMT on the inflammatory response in DSS-induced colitis. (A) Colonic MPO; (B) Colonic EPO;(C) Colonic IL-1β; (D) Colonic IL-6; (E) Colonic IL-8; (F) Colonic TNF-α; (G) Colonic IL-17A; (H) Colonic IL-10. * p ≤ 0.05, ** p ≤ 0.01, *** p ≤ 0.001, **** p ≤ 0.0001, data are represented as mean ± SEM.
Since dysfunction of the intestinal epithelial barrier is associated with IBD, we hypothesized that both donor group would alleviate DSS-induced damage by protecting the intestinal barrier. We analyzed the average optical density (AOD) of tight junction proteins ZO-1 and occludin in four groups of mouse colonic tissues using IHC. As shown in Figures 4A-D, the AOD of colonic ZO-1 was significantly higher in the WIMT group than in the DSS group (p = 0.008), while there was no significant difference between the FMT and DSS groups. In addition, the AOD of occludin was significantly higher in both the WIMT and FMT groups than that in the DSS group (WIMT vs. DSS, p = 0.0029; FMT vs. DSS, p = 0.0031). To investigate the barrier mechanism underlying the difference in anti-colitis efficacy between the WIMT and FMT groups, we also evaluated serum DAO and D-LA levels linked with intestinal permeability, and there was no statistically significant difference between the two groups (Figures 4E, F). In summary, both donor group alleviated DSS-induced damage by maintaining intestinal barrier integrity.
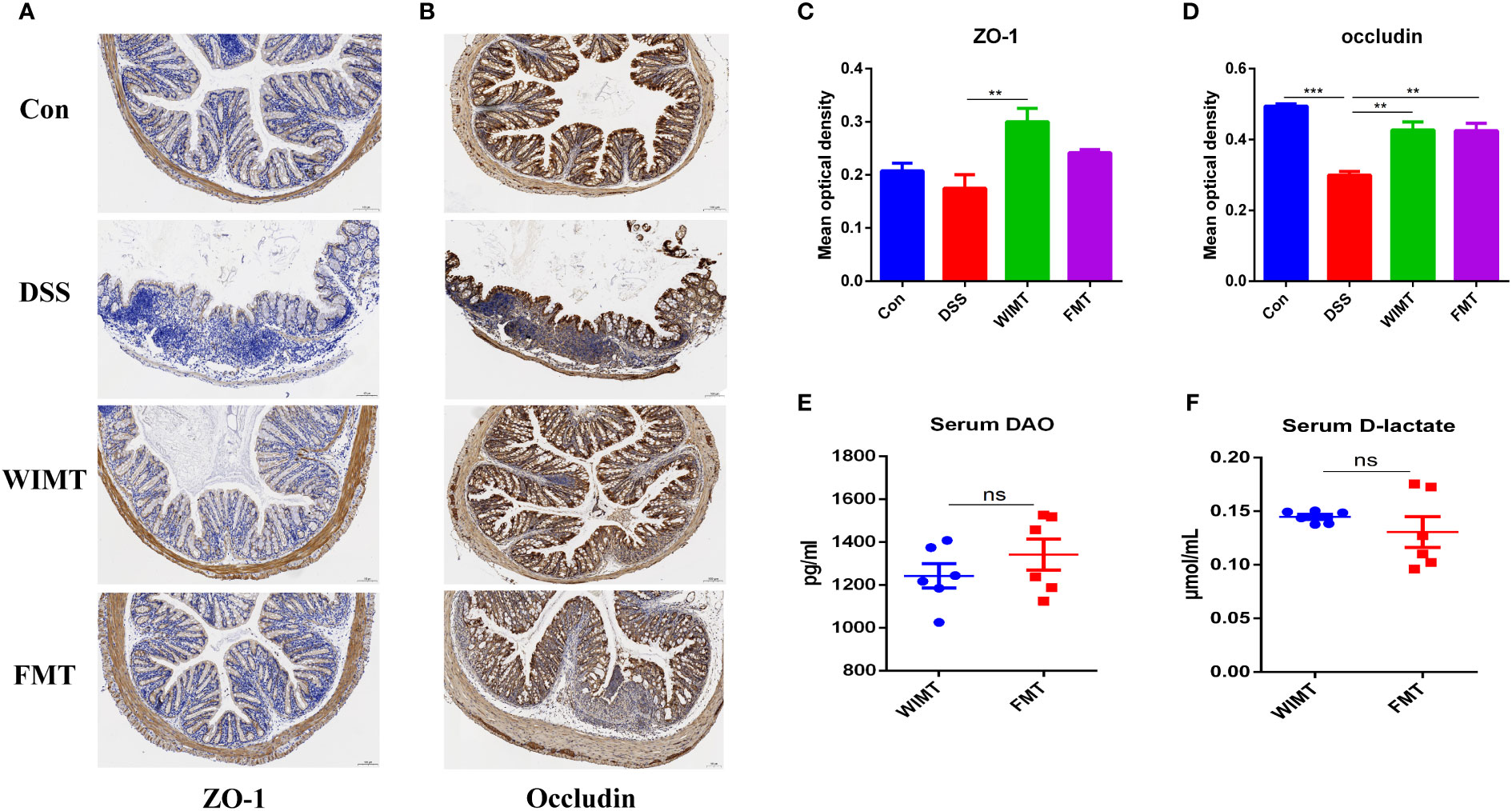
Figure 4 Effect of WIMT and FMT on the intestinal barrier in DSS-induced colitis. Immunohistochemistry for ZO-1 (A) and occludin (B) in each group (100μm, n = 3); (C, D) Average optical density. The changes of levels of serum DAO (E) and D-lactate (F). ** p ≤ 0.01, *** p ≤ 0.001, data are represented as mean ± SEM. ns, no significance difference.
Considering the relationship between microbiota and IBD, we analyzed the microbial diversity in the small intestine (jejunal and ileal contents), large intestine (cecum and colon contents), and feces in the WIMT and FMT groups using 16S rDNA. To assess the beta diversity of the community, we employed the Jaccard index. There was no significant difference between the small intestinal community structure of the WIMT (WSI) and the FMT groups (FSI) (Figure 5A). The large intestinal community structure of the WIMT group (WLI) was not significantly different from that of the FMT group (FLI) (Figure 5B). Furthermore, the fecal community structure of the WIMT group was not significantly different from that of the FMT group (Figure 5C). The Pielou, Shannon indices, and Simpson were used to evaluate alpha diversity (Figures 5D-F), and there was no significant difference between the two groups. These findings showed that differences in efficacy may not be related to the indices.
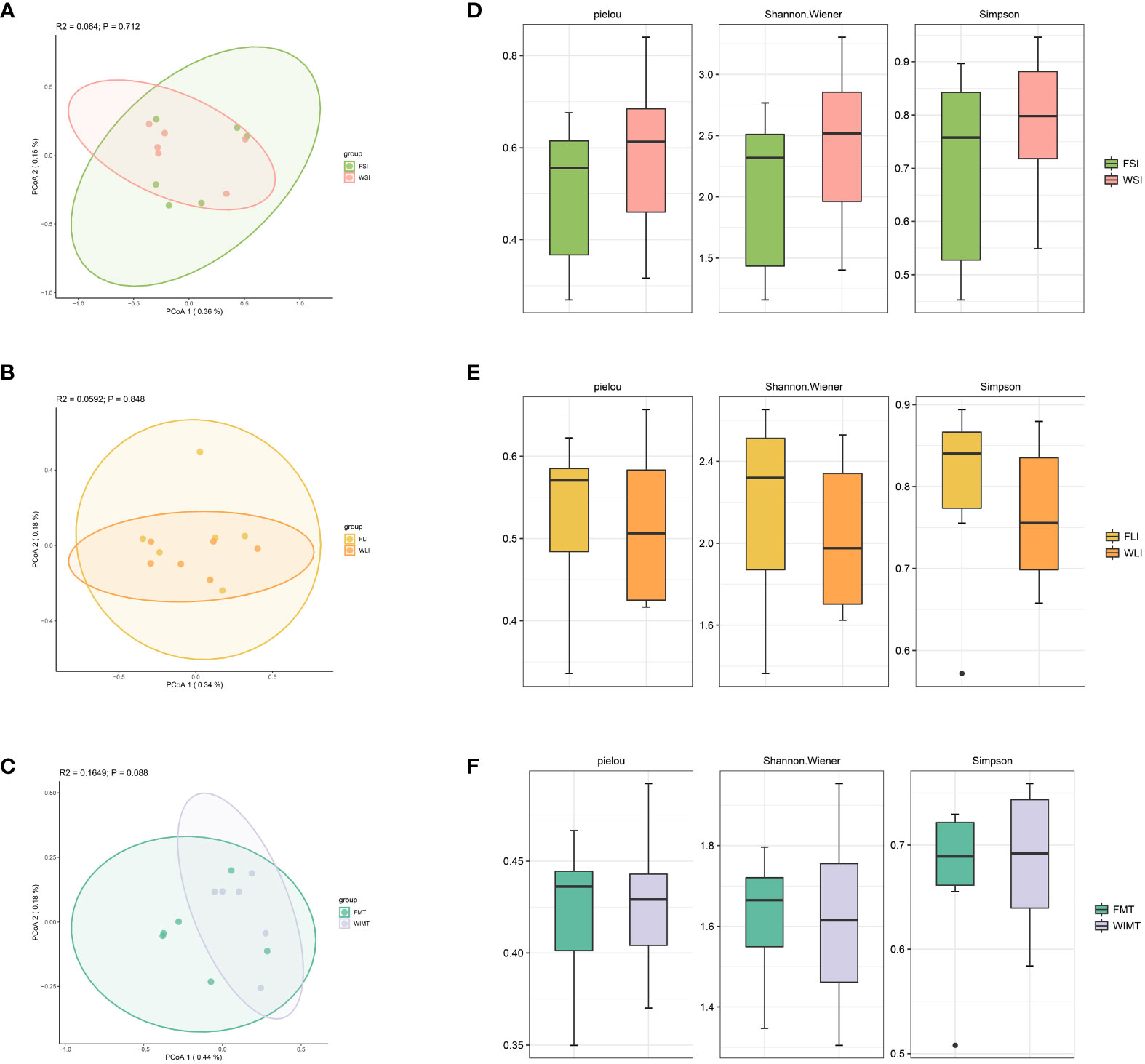
Figure 5 Effect of WIMT and FMT on the microbiota diversity in DSS-induced colitis. (A) PCoA analysis for FSI vs WSI groups; (B) PCoA analysis for FLI vs WLI groups;(C) PCoA analysis for FMT vs WMIT groups; (D) Alpha diversity of FSI and WSI groups; (E) Alpha diversity of FLI and WLI groups;(F) Alpha diversity of FMT and WIMT groups.
By examining the shared genera, we were able to explain the anti-inflammatory effects in both groups. A Venn diagram was plotted showing a total of 129 genera of small intestinal flora (Figure 6A, Supplement Table 1), 76 genera of large intestinal flora (Figure 6B, Supplement Table 2), and 60 genera of fecal flora (Figure 6C, Supplement Table 3) in the two groups of mice. The differences in microorganisms between the two groups were analyzed using LEfSe (LDA > 2) to explain the superior efficacy of the WIMT group. The results showed that Lachnospiraceae_NK4A136_group and Alloprevotella (enriched in feces, Figure 6D), Ochrobactrum (enriched in the small intestine, Figure 6E), and Lactobacillus (enriched in the large intestine, Figure 6F) were significantly enriched in the FMT group, while Bifidobacterium and Corynebacterium (enriched in feces, Figure 6D) were significantly enriched in the WIMT group. The correlation between various bacteria and cytokines is displayed on a heat map (Figure 6G). Bacteria Bifidobacterium enriched in the WIMT group was negatively correlated with pro-inflammatory factors TNF-α (p = 0.04264557), and Bacteria Ochrobactrum enriched in the FMT group was positively correlated with inflammatory biomarkers MPO (p = 0.011331713) and negatively correlated with anti-inflammatory factors and IL-10 (p = 0.041346). In conclusion, there were significant differences in the microbiota between the WIMT and FMT groups, and these differences were associated with the effectiveness of the intervention.
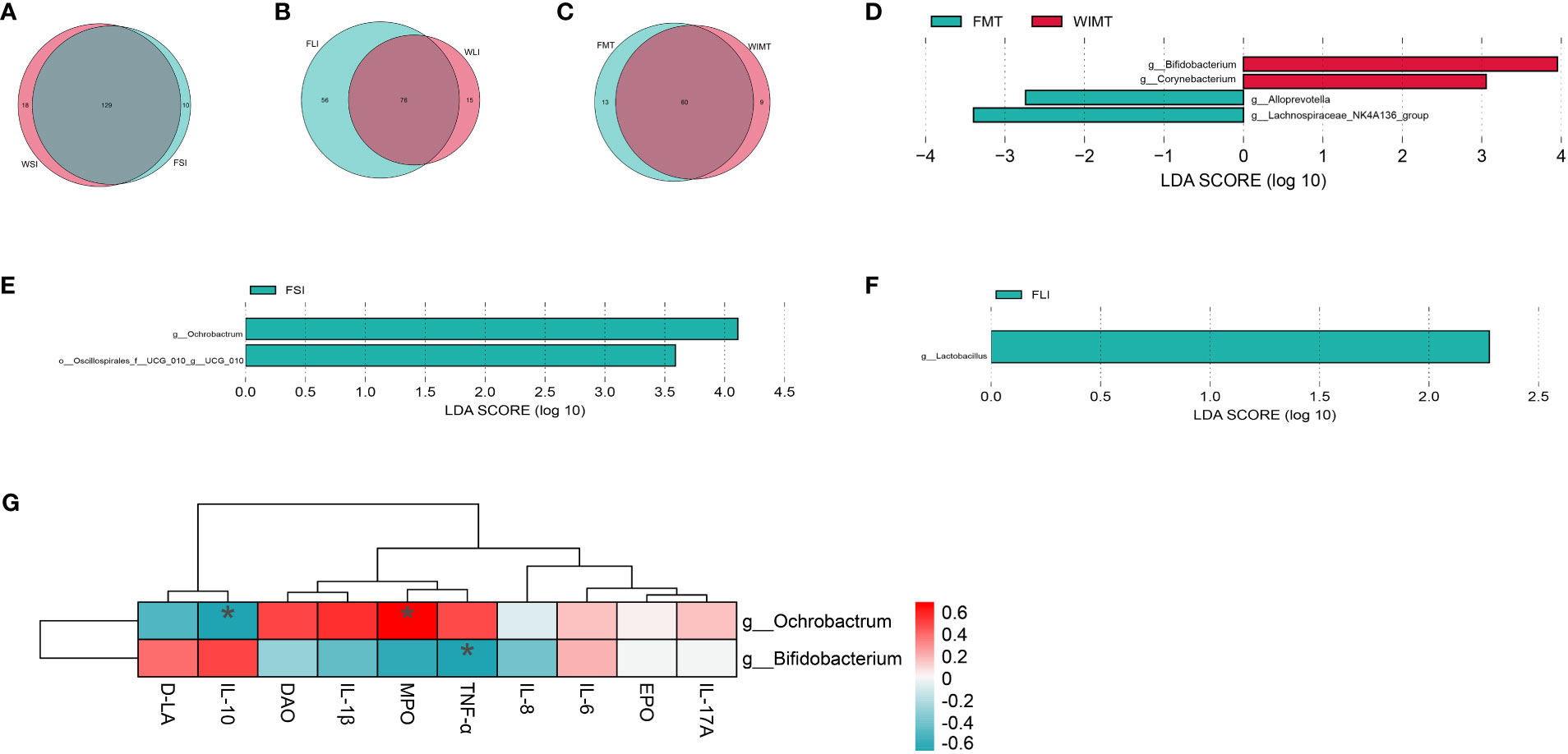
Figure 6 Differences in microbes of the WIMT and FMT groups. (A) Venn diagram for FSI and WSI groups; (B) Venn diagram for FLI and WLI groups;(C) Venn diagram for FMT and WMIT groups; (D) LEfSe analysis of WIMT and FMT groups; (E) LEfSe analysis of FSI and WSI groups; (F) LEfSe analysis of FLI and WLI groups; (G) Correlation analysis of differential bacteria with cytokines. * p ≤ 0.05.
To further clarify the mechanism of superior efficacy in the WIMT group at the functional level, we used PICRUSt2 to predict variations in microbially engaged metabolic pathways (MetaCyc database) in the small intestine, large intestine, and feces between the WIMT and FMT groups. The metabolic pathways, such as peptidoglycan biosynthesis II (staphylococci) (PWY-5265), L-arginine biosynthesis I (ARGSYN-PWY), and L-arginine biosynthesis IV (PWY-7400), were significantly enriched in the FMT group (Figures 7A, B, Supplement Table 4). In contrast, metabolic pathways, such as L-lysine fermentation to acetate and butanoate (P163-PWY), glycolysis V (P341-PWY), propylene glycol degradation (PWY-7013), and L-histidine degradation I (HISDEG-PWY), were significantly enriched in the WIMT group (Figures 7A, B, Supplement Table 4). The results indicated that the differences in efficacy between the WIMT and FMT groups were related to different metabolic pathways.
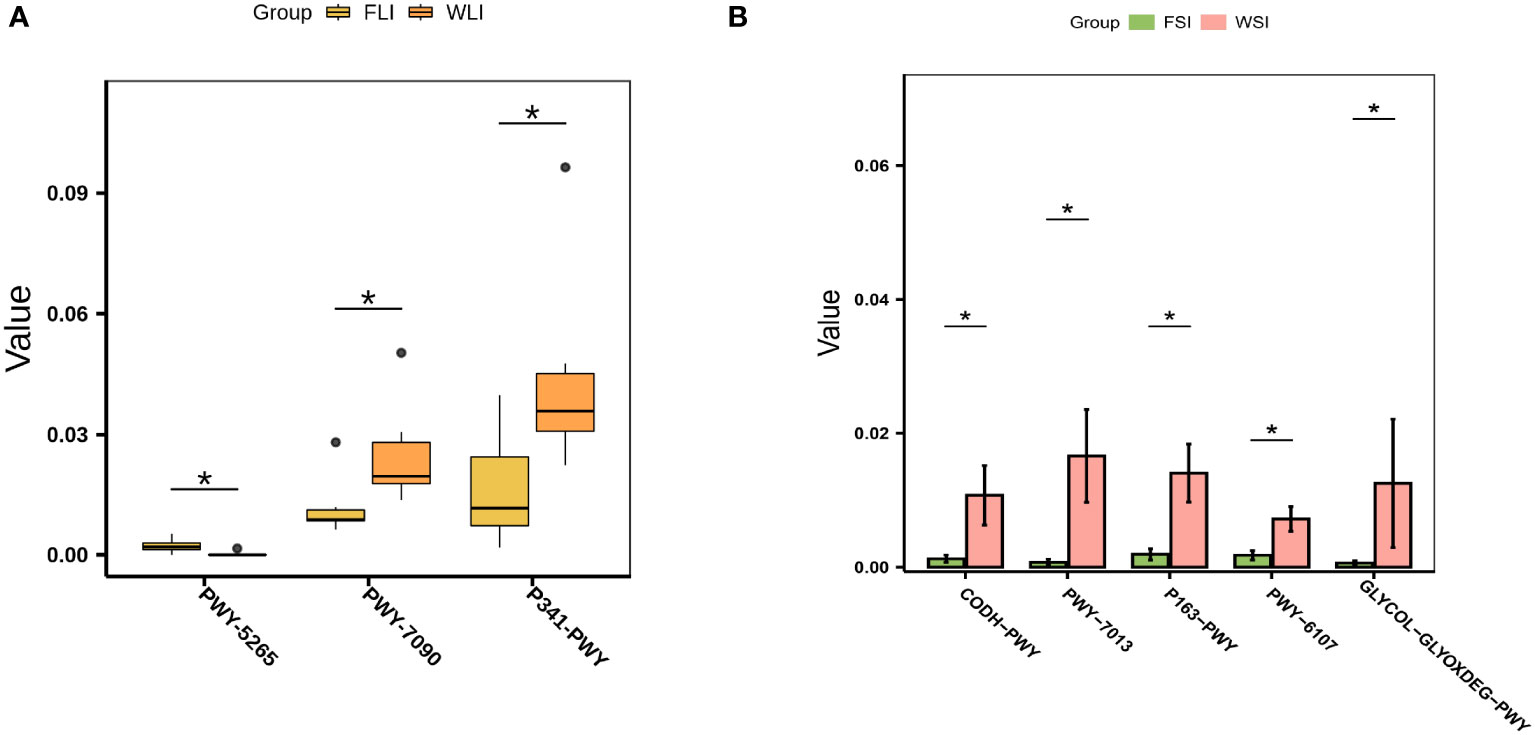
Figure 7 Effect of WIMT and FMT on the microbial functional in DSS-induced colitis. (A) Differential metabolic pathways between the FLI and WLI groups; (B) Differential metabolic pathways between FSI and WSI groups. * p ≤ 0.05.
IBD is a widespread condition that deteriorates the quality of life of many people (4). Several studies have reported alterations in the community and structure of the gut microbiota in patients (5–8). FMT has been utilized as an innovative and successful therapy to treat IBD by restoring the diversity and composition of gut microbiota (9). Previous research has shown that WIMT, as opposed to FMT, can more accurately reproduce the community structure and suppress the host’s inflammatory response (18). However, it is not clear whether WIMT is more effective in the intervention of IBD. In this study, the effects of WIMT and FMT on the susceptibility of DSS-induced colitis were examined. The trial findings demonstrated that by preventing weight loss and lowering the DAI and histological scores, both WIMT and FMT reduced the symptoms of colitis. Both groups of donors showed downregulated levels of inflammatory biomarkers MPO (28, 29) and EPO (30) and regulated homeostasis of colonic inflammatory factors. It was demonstrated that both groups of GF mice had decreased susceptibility to DSS and that the WIMT intervention efficacy was superior to that of the FMT group.
As with many other tissue injuries, IBD is characterized by distorted expression of inflammatory cytokines. Intestinal inflammation is regulated by cytokines IL-1β, IL-6, IL-8, TNF-α, and IL-10 (31–33). Increased levels of TNF-α, IL-1β, and IL-6 have been linked to intestinal dysfunction (34). By recruiting granulocytes and activating CD4+ T cells in IBD, IL-1β stimulates the production of pro-inflammatory molecules, such as IL-17A, IL-12, and IFN-γ, aggravating intestinal inflammation (35, 36). Secretion of the anti-inflammatory factor IL-10 ameliorates mucosal damage in IBD and protects lymphocytes, which inhibits IBD by suppressing the host autoimmune response (37, 38). In the present study, both WIMT and FMT significantly inhibited the expression of DSS-induced pro-inflammatory factors. The levels of IL-1β were significantly lower in the WIMT group than in the FMT group, and the levels of IL-10 were significantly higher in the WIMT group than in the FMT group, indicating that the WIMT group had better control over cytokine homeostasis. Intestinal epithelial barrier dysfunction is associated with IBD (39), and the epithelial cytoskeleton, which is composed of tight junction proteins such as ZO-1 and occludin, plays an important role in maintaining intestinal mucosal barrier function and regulating intestinal permeability (40–42). D-LA and DAO levels, which represent intestinal permeability, are significantly higher in patients with IBD (43). In this study, WIMT intervention significantly elevated ZO-1 and occludin expression levels in colonic tissues, and FMT intervention significantly elevated occludin expression levels in colonic tissues, and there was no significant difference in intestinal permeability between the WIMT and FMT groups. In conclusion, both WIMT and FMT could protect the intestinal barrier by regulating inflammatory factor homeostasis to lessen the damage caused by DSS.
It has been demonstrated that intestinal flora is essential for the development or remission of IBD (44). The microbiota sequencing data from this investigation revealed the presence of numerous shared genera in both groups of mice, such as Eubacterium_hallii_group in the small intestinal tract, Bifidobacterium in the large intestinal tract, and Parabacteroides in the feces. It has been reported that genera Eubacterium_hallii_group, Bifidobacterium, and Parabacteroides possess anti-inflammatory efficacy (45–47), and in the present study, these shared genera were associated with anti-inflammatory efficacy in both groups. Bifidobacterium, which improves the intestinal community structure and resists gastrointestinal inflammation (45), was considerably enriched in the WIMT group. The expression of tight junction proteins and the mucin family was upregulated by Bifidobacterium adolescentis, which belongs to the genus Bifidobacterium and regulates intestinal inflammation by reducing pro-inflammatory cytokines such as IL-6 and IL-1β, increasing the level of IL-10, and increasing Treg and Th2 cells in the lamina propria of the colon to inhibit the overgrowth of harmful bacteria (44). Bifidobacterium bifidum targets the Toll-like receptor 2 pathway in an NF-κB non-dependent manner, which enhances the intestinal epithelial tight junction barrier and prevents intestinal inflammation (48). Clinical research and animal experiments have demonstrated that Bifidobacterium longum can reduce the signs of chronic inflammation and colitis (49, 50). In the current study, the enrichment of Bifidobacterium in the WIMT group was significantly negatively correlated with the pro-inflammatory factor TNF-α, and the WIMT group in this study had superior efficacy compared to the FMT group as a result of Bifidobacterium enrichment.
Ochrobactrum, Lactobacillus, Alloprevotella, and Lachnospiraceae NK4A136_group were significantly enriched in the FMT group. Lactobacillus is a potential catalyst for the development of the human immune system (51). Together, prebiotics and Lactobacillus dramatically reduced the symptoms of UC in clinical investigations (52). Members of the genus Lactobacillus also function as probiotics. By controlling oxidative stress and immunological responses, Lactobacillus plantarum effectively protects against DSS-induced IBD in mice (53). Widespread in the intestines of healthy individuals, Lactobacillus reuteri regulates the intestinal immune system and can reduce inflammation via a number of processes (54). Butyrate can be produced by members of Lachnospiraceae (55), and it is not only a major source of energy for intestinal epithelial cells but also inhibits pro-inflammatory cytokine signaling pathways (56). Lactobacillus and Lachnospiraceae NK4A136, which were enriched in the FMT group, contributed to the anti-inflammatory efficacy of FMT. The abundance of Alloprevotella was found to be higher in the AOM/DSS-treated group than in the control group in a study by Wang et al. (57), raising the possibility that it may be related to the severity of colitis. According to a study by Walujkar et al., Ochrobactrum was substantially more abundant in the microbiota during the aggravated phase of UC than it in the remission phase, which helps identify the particular genera that dominate the microbiota during the disease (58). In the current study, the enriched Ochrobactrum in the FMT group was significantly positively correlated with the inflammatory marker MPO and significantly negatively correlated with the anti-inflammatory factor IL-10, which led to its poorer efficacy than that of WIMT.
Short-chain fatty acids (SCFAs; mainly acetate, propionate, and butyrate) produced by intestinal bacteria can modulate protective immunity and reduce tissue inflammation (59, 60). In this study, functional prediction analysis based on the MetaCyc database using PICRUSt2 revealed that glycolysis V (Pyrococcus, P341-PWY), propylene glycol degradation (PWY-7013), and lysine degradation to acetate and propionate (P163-PWY) pathways were significantly enriched in the WIMT group. The glycolytic V pathway produces pyruvate, which can be further converted to acetate in this metabolic pathway (61–63). Propylene glycol can be degraded by bacteria to produce propionate (64). Bacteria involved in lysine metabolism produce acetate and propionate in L-lysine fermentation through the acetate and butanoate pathways (65), and all three metabolic pathways enriched in the WIMT group may be involved in the production of SCFAs, which is related to its anti-inflammatory efficacy. Amino acids play a role in the prevention and treatment of IBD by regulating the physiological activity of intestinal epithelial cells and by protecting the intestinal barrier (66). The addition of arginine prevents LPS-induced oxidative damage and apoptosis (67). In this study, L-arginine biosynthesis I (ARGSYN-PWY) and L-arginine biosynthesis IV (PWY-7400) were significantly enriched in the FMT group, which could be associated with the FMT group’s ability to prevent inflammation and preserve the intestinal barrier.
There are some limitations of our study. Both WIMT and FMT were pre-colonized into mice and intervened continuously, thus reducing susceptibility to colitis. Further studies are needed to explore the differences in therapeutic efficacy between the two groups. In addition, the causal relationship between specific bacteria and IBD in animals or in in vitro experiments and the underlying molecular mechanisms need to be further investigated.
In conclusion, both WIMT and FMT could significantly reduce the susceptibility of mice to DSS-induced colitis by providing probiotics, protecting the intestinal mucosal barrier, and regulating the dynamic balance of cytokines. Notably, the mice in the WIMT group responded better to the intervention than those in the FMT group, which may be attributable to the enrichment of metabolic pathways involving SCFAs and Bifidobacterium in the WIMT group. This study provides new insights into microecological interventions for IBD.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation. The datasets presented in this study can be found in online repositories. Data is deposited in China National Microbiology Data Center (NMDC) with accession number NMDC10018297 (https://nmdc.cn/).
The animal study was reviewed and approved by Institutional Animal Care and Use Committee of the Huazhong Agricultural University.
YY, ST, and HW designed the experiment. YY, JH, YW, LL, ZZ, XT, HZ, ZW, MD, JZ, SF, and WC performed the animal trials, sample collection, and data analysis. YY drafted the manuscript. ST, QC, and HW revised the manuscript. All authors contributed to the article and approved the submitted version.
This work was supported by the National Key Research and Development Program of China (2021YFA0805904).
We thank QL for her guidance in writing the manuscript, and we thank QC for his constructive suggestions on the manuscript.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2023.1143526/full#supplementary-material
WIMT, whole intestinal microbiota transplantation; FMT, fecal microbiota transplantation; DSS, Dextran Sulfate Sodium; P341-PWY, glycolysis V (Pyrococcus); PWY-7013, propylene glycol degradation; P163-PWY, lysine degradation to acetate and propionate pathway; ARGSYN-PWY, L-arginine biosynthesis I); PWY-7400, L-arginine biosynthesis IV.
1. Ng SC, Shi HY, Hamidi N, Underwood FE, Tang W, Benchimol EI, et al. Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: a systematic review of population-based studies. Lancet (2017) 390(10114):2769–78. doi: 10.1016/S0140-6736(17)32448-0
2. Kaplan GG. The global burden of IBD: from 2015 to 2025. Nat Rev Gastroenterol Hepatol (2015) 12(12):720–7. doi: 10.1038/nrgastro.2015.150
3. Geremia A, Biancheri P, Allan P, Corazza GR, Di Sabatino A. Innate and adaptive immunity in inflammatory bowel disease. Autoimmun Rev (2014) 13(1):3–10. doi: 10.1016/j.autrev.2013.06.004
4. Molodecky NA, Soon IS, Rabi DM, Ghali WA, Ferris M, Chernoff G, et al. Increasing incidence and prevalence of the inflammatory bowel diseases with time, based on systematic review. Gastroenterology (2012) 142(1):46–54 e42; quiz e30. doi: 10.1053/j.gastro.2011.10.001
5. Frank DN, St Amand AL, Feldman RA, Boedeker EC, Harpaz N, Pace NR. Molecular-phylogenetic characterization of microbial community imbalances in human inflammatory bowel diseases. Proc Natl Acad Sci U S A (2007) 104(34):13780–5. doi: 10.1073/pnas.0706625104
6. Manichanh C, Rigottier-Gois L, Bonnaud E, Gloux K, Pelletier E, Frangeul L, et al. Reduced diversity of faecal microbiota in crohn's disease revealed by a metagenomic approach. Gut (2006) 55(2):205–11. doi: 10.1136/gut.2005.073817
7. Peterson DA, Frank DN, Pace NR, Gordon JI. Metagenomic approaches for defining the pathogenesis of inflammatory bowel diseases. Cell Host Microbe (2008) 3(6):417–27. doi: 10.1016/j.chom.2008.05.001
8. Scanlan PD, Shanahan F, O'Mahony C, Marchesi JR. Culture-independent analyses of temporal variation of the dominant fecal microbiota and targeted bacterial subgroups in crohn's disease. J Clin Microbiol (2006) 44(11):3980–8. doi: 10.1128/JCM.00312-06
9. Matsuoka K, Kanai T. The gut microbiota and inflammatory bowel disease. Semin Immunopathol (2015) 37(1):47–55. doi: 10.1007/s00281-014-0454-4
10. Zhang F, Cui B, He X, Nie Y, Wu K, Fan D, et al. Microbiota transplantation: concept, methodology and strategy for its modernization. Protein Cell (2018) 9(5):462–73. doi: 10.1007/s13238-018-0541-8
11. Bennet JD, Brinkman M. Treatment of ulcerative colitis by implantation of normal colonic flora. Lancet (1989) 1(8630):164. doi: 10.1016/s0140-6736(89)91183-5
12. Chen HT, Huang HL, Xu HM, Luo QL, He J, Li YQ, et al. Fecal microbiota transplantation ameliorates active ulcerative colitis. Exp Ther Med (2020) 19(4):2650–60. doi: 10.3892/etm.2020.8512
13. Lima SF, Gogokhia L, Viladomiu M, Chou L, Putzel G, Jin WB, et al. Transferable immunoglobulin a-coated odoribacter splanchnicus in responders to fecal microbiota transplantation for ulcerative colitis limits colonic inflammation. Gastroenterology (2022) 162(1):166–78. doi: 10.1053/j.gastro.2021.09.061
14. Megerlin F, Fouassier E, Lopert R, Bourlioux P. Faecal microbiota transplantation: a sui generis biological drug, not a tissue. Ann Pharm Fr (2014) 72(4):217–20. doi: 10.1016/j.pharma.2014.04.008
15. Ng SC, Kamm MA, Yeoh YK, Chan PKS, Zuo T, Tang W, et al. Scientific frontiers in faecal microbiota transplantation: joint document of Asia-pacific association of gastroenterology (APAGE) and Asia-pacific society for digestive endoscopy (APSDE). Gut (2020) 69(1):83–91. doi: 10.1136/gutjnl-2019-319407
16. Papanicolas LE, Gordon DL, Wesselingh SL, Rogers GB. Improving risk-benefit in faecal transplantation through microbiome screening. Trends Microbiol (2020) 28(5):331–9. doi: 10.1016/j.tim.2019.12.009
17. Leite G, Pimentel M, Barlow GM, Chang C, Hosseini A, Wang J, et al. Age and the aging process significantly alter the small bowel microbiome. Cell Rep (2021) 36(13):109765. doi: 10.1016/j.celrep.2021.109765
18. Li N, Zuo B, Huang S, Zeng B, Han D, Li T, et al. Spatial heterogeneity of bacterial colonization across different gut segments following inter-species microbiota transplantation. Microbiome (2020) 8(1):161. doi: 10.1186/s40168-020-00917-7
19. Li J, Wei H. Establishment of an efficient germ-free animal system to support functional microbiome research. Sci China Life Sci (2019) 62(10):1400–3. doi: 10.1007/s11427-019-9832-9
20. Fouladi F, Glenny EM, Bulik-Sullivan EC, Tsilimigras MCB, Sioda M, Thomas SA, et al. Sequence variant analysis reveals poor correlations in microbial taxonomic abundance between humans and mice after gnotobiotic transfer. ISME J (2020) 14(7):1809–20. doi: 10.1038/s41396-020-0645-z
21. Souza DG, Senchenkova EY, Russell J, Granger DN. MyD88 mediates the protective effects of probiotics against the arteriolar thrombosis and leukocyte recruitment associated with experimental colitis. Inflammation Bowel Dis (2015) 21(4):888–900. doi: 10.1097/MIB.0000000000000331
22. Nishiyama Y, Kataoka T, Yamato K, Taguchi T, Yamaoka K. Suppression of dextran sulfate sodium-induced colitis in mice by radon inhalation. Mediators Inflammation (2012) 2012:239617. doi: 10.1155/2012/239617
23. Murthy SN, Cooper HS, Shim H, Shah RS, Ibrahim SA, Sedergran DJ. Treatment of dextran sulfate sodium-induced murine colitis by intracolonic cyclosporin. Dig Dis Sci (1993) 38(9):1722–34. doi: 10.1007/BF01303184
24. Dieleman LA, Palmen MJ, Akol H, Bloemena E, Pena AS, Meuwissen SG, et al. Chronic experimental colitis induced by dextran sulphate sodium (DSS) is characterized by Th1 and Th2 cytokines. Clin Exp Immunol (1998) 114(3):385–91. doi: 10.1046/j.1365-2249.1998.00728.x
25. Haas BJ, Gevers D, Earl AM, Feldgarden M, Ward DV, Giannoukos G, et al. Chimeric 16S rRNA sequence formation and detection in Sanger and 454-pyrosequenced PCR amplicons. Genome Res (2011) 21(3):494–504. doi: 10.1101/gr.112730.110
26. Bolyen E, Rideout JR, Dillon MR, Bokulich NA, Abnet CC, Al-Ghalith GA, et al. Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat Biotechnol (2019) 37(8):852–7. doi: 10.1038/s41587-019-0209-9
27. Callahan BJ, McMurdie PJ, Rosen MJ, Han AW, Johnson AJ, Holmes SP. DADA2: high-resolution sample inference from illumina amplicon data. Nat Methods (2016) 13(7):581–3. doi: 10.1038/nmeth.3869
28. Klebanoff SJ, Coombs RW. Viricidal effect of polymorphonuclear leukocytes on human immunodeficiency virus-1. Role myeloperoxidase system. J Clin Invest (1992) 89(6):2014–7. doi: 10.1172/JCI115810
29. Chadwick VS, Schlup MM, Ferry DM, Chang AR, Butt TJ. Measurements of unsaturated vitamin B12-binding capacity and myeloperoxidase as indices of severity of acute inflammation in serial colonoscopy biopsy specimens from patients with inflammatory bowel disease. Scand J Gastroenterol (1990) 25(12):1196–204. doi: 10.3109/00365529008998554
30. Forbes E, Murase T, Yang M, Matthaei KI, Lee JJ, Lee NA, et al. Immunopathogenesis of experimental ulcerative colitis is mediated by eosinophil peroxidase. J Immunol (2004) 172(9):5664–75. doi: 10.4049/jimmunol.172.9.5664
31. Moschen AR, Tilg H, Raine T. IL-12, IL-23 and IL-17 in IBD: immunobiology and therapeutic targeting. Nat Rev Gastroenterol Hepatol (2019) 16(3):185–96. doi: 10.1038/s41575-018-0084-8
32. Walana W, Ye Y, Li M, Wang J, Wang B, Cheng JW, et al. IL-8 antagonist, CXCL8(3-72)K11R/G31P coupled with probiotic exhibit variably enhanced therapeutic potential in ameliorating ulcerative colitis. BioMed Pharmacother (2018) 103:253–61. doi: 10.1016/j.biopha.2018.04.008
33. Zhu W, Ren L, Zhang L, Qiao Q, Farooq MZ, Xu Q. The potential of food protein-derived bioactive peptides against chronic intestinal inflammation. Mediators Inflammation (2020) 2020:6817156. doi: 10.1155/2020/6817156
34. Zhang H, Hua R, Zhang B, Zhang X, Yang H, Zhou X. Serine alleviates dextran sulfate sodium-induced colitis and regulates the gut microbiota in mice. Front Microbiol (2018) 9:3062. doi: 10.3389/fmicb.2018.03062
35. Coccia M, Harrison OJ, Schiering C, Asquith MJ, Becher B, Powrie F, et al. IL-1beta mediates chronic intestinal inflammation by promoting the accumulation of IL-17A secreting innate lymphoid cells and CD4(+) Th17 cells. J Exp Med (2012) 209(9):1595–609. doi: 10.1084/jem.20111453
36. Veenbergen S, Li P, Raatgeep HC, Lindenbergh-Kortleve DJ, Simons-Oosterhuis Y, Farrel A, et al. IL-10 signaling in dendritic cells controls IL-1beta-mediated IFNgamma secretion by human CD4(+) T cells: relevance to inflammatory bowel disease. Mucosal Immunol (2019) 12(5):1201–11. doi: 10.1038/s41385-019-0194-9
37. Olszak T, Neves JF, Dowds CM, Baker K, Glickman J, Davidson NO, et al. Protective mucosal immunity mediated by epithelial CD1d and IL-10. Nature (2014) 509(7501):497–502. doi: 10.1038/nature13150
38. Shouval DS, Biswas A, Goettel JA, McCann K, Conaway E, Redhu NS, et al. Interleukin-10 receptor signaling in innate immune cells regulates mucosal immune tolerance and anti-inflammatory macrophage function. Immunity (2014) 40(5):706–19. doi: 10.1016/j.immuni.2014.03.011
39. Mehandru S, Colombel JF. The intestinal barrier, an arbitrator turned provocateur in IBD. Nat Rev Gastroenterol Hepatol (2021) 18(2):83–4. doi: 10.1038/s41575-020-00399-w
40. Salvo Romero E, Alonso Cotoner C, Pardo Camacho C, Casado Bedmar M, Vicario M. The intestinal barrier function and its involvement in digestive disease. Rev Esp Enferm Dig (2015) 107(11):686–96. doi: 10.17235/reed.2015.3846/2015
41. Wang Y, Tao H, Huang H, Xiao Y, Wu X, Li M, et al. The dietary supplement rhodiola crenulata extract alleviates dextran sulfate sodium-induced colitis in mice through anti-inflammation, mediating gut barrier integrity and reshaping the gut microbiome. Food Funct (2021) 12(7):3142–58. doi: 10.1039/d0fo03061a
42. Lin JC, Wu JQ, Wang F, Tang FY, Sun J, Xu B, et al. QingBai decoction regulates intestinal permeability of dextran sulphate sodium-induced colitis through the modulation of notch and NF-kappaB signalling. Cell Prolif (2019) 52(2):e12547. doi: 10.1111/cpr.12547
43. Song WB, Lv YH, Zhang ZS, Li YN, Xiao LP, Yu XP, et al. Soluble intercellular adhesion molecule-1, d-lactate and diamine oxidase in patients with inflammatory bowel disease. World J Gastroenterol (2009) 15(31):3916–9. doi: 10.3748/wjg.15.3916
44. Fan L, Qi Y, Qu S, Chen X, Li A, Hendi M, et al. B. adolescentis ameliorates chronic colitis by regulating Treg/Th2 response and gut microbiota remodeling. Gut Microbes (2021) 13(1):1–17. doi: 10.1080/19490976.2020.1826746
45. Alp G, Aslim B. Relationship between the resistance to bile salts and low pH with exopolysaccharide (EPS) production of bifidobacterium spp. isolated from infants feces and breast milk. Anaerobe (2010) 16(2):101–5. doi: 10.1016/j.anaerobe.2009.06.006
46. Hiippala K, Kainulainen V, Suutarinen M, Heini T, Bowers JR, Jasso-Selles D, et al. Isolation of anti-inflammatory and epithelium reinforcing bacteroides and parabacteroides spp. from a healthy fecal donor. Nutrients (2020) 12(4):935. doi: 10.3390/nu12040935
47. Almeida D, Machado D, Andrade JC, Mendo S, Gomes AM, Freitas AC. Evolving trends in next-generation probiotics: a 5W1H perspective. Crit Rev Food Sci Nutr (2020) 60(11):1783–96. doi: 10.1080/10408398.2019.1599812
48. Al-Sadi R, Dharmaprakash V, Nighot P, Guo S, Nighot M, Do T, et al. Bifidobacterium bifidum enhances the intestinal epithelial tight junction barrier and protects against intestinal inflammation by targeting the toll-like receptor-2 pathway in an NF-kappaB-Independent manner. Int J Mol Sci (2021) 22(15):8070. doi: 10.3390/ijms22158070
49. Singh S, Bhatia R, Khare P, Sharma S, Rajarammohan S, Bishnoi M, et al. Anti-inflammatory bifidobacterium strains prevent dextran sodium sulfate induced colitis and associated gut microbial dysbiosis in mice. Sci Rep (2020) 10(1):18597. doi: 10.1038/s41598-020-75702-5
50. Zhang M, Zhou L, Zhang S, Yang Y, Xu L, Hua Z, et al. Bifidobacterium longum affects the methylation level of forkhead box P3 promoter in 2, 4, 6-trinitrobenzenesulphonic acid induced colitis in rats. Microb Pathog (2017) 110:426–30. doi: 10.1016/j.micpath.2017.07.029
51. O'Callaghan J, O'Toole PW. Lactobacillus: host-microbe relationships. Curr Top Microbiol Immunol (2013) 358:119–54. doi: 10.1007/82_2011_187
52. Ganji-Arjenaki M, Rafieian-Kopaei M. Probiotics are a good choice in remission of inflammatory bowel diseases: a meta analysis and systematic review. J Cell Physiol (2018) 233(3):2091–103. doi: 10.1002/jcp.25911
53. Pan Y, Ning Y, Hu J, Wang Z, Chen X, Zhao X. The preventive effect of lactobacillus plantarum ZS62 on DSS-induced IBD by regulating oxidative stress and the immune response. Oxid Med Cell Longev (2021) 2021:9416794. doi: 10.1155/2021/9416794
54. Wang H, Zhou C, Huang J, Kuai X, Shao X. The potential therapeutic role of lactobacillus reuteri for treatment of inflammatory bowel disease. Am J Transl Res (2020) 12(5):1569–83.
55. Meehan CJ, Beiko RG. A phylogenomic view of ecological specialization in the lachnospiraceae, a family of digestive tract-associated bacteria. Genome Biol Evol (2014) 6(3):703–13. doi: 10.1093/gbe/evu050
56. Morgan XC, Tickle TL, Sokol H, Gevers D, Devaney KL, Ward DV, et al. Dysfunction of the intestinal microbiome in inflammatory bowel disease and treatment. Genome Biol (2012) 13(9):R79. doi: 10.1186/gb-2012-13-9-r79
57. Wang C, Li W, Wang H, Ma Y, Zhao X, Zhang X, et al. Saccharomyces boulardii alleviates ulcerative colitis carcinogenesis in mice by reducing TNF-alpha and IL-6 levels and functions and by rebalancing intestinal microbiota. BMC Microbiol (2019) 19(1):246. doi: 10.1186/s12866-019-1610-8
58. Walujkar SA, Kumbhare SV, Marathe NP, Patangia DV, Lawate PS, Bharadwaj RS, et al. Molecular profiling of mucosal tissue associated microbiota in patients manifesting acute exacerbations and remission stage of ulcerative colitis. World J Microbiol Biotechnol (2018) 34(6):76. doi: 10.1007/s11274-018-2449-0
59. Furusawa Y, Obata Y, Fukuda S, Endo TA, Nakato G, Takahashi D, et al. Commensal microbe-derived butyrate induces the differentiation of colonic regulatory T cells. Nature (2013) 504(7480):446–50. doi: 10.1038/nature12721
60. Kim CH, Park J, Kim M. Gut microbiota-derived short-chain fatty acids, T cells, and inflammation. Immune Netw (2014) 14(6):277–88. doi: 10.4110/in.2014.14.6.277
61. Mai X, Adams MW. Purification and characterization of two reversible and ADP-dependent acetyl coenzyme a synthetases from the hyperthermophilic archaeon pyrococcus furiosus. J Bacteriol (1996) 178(20):5897–903. doi: 10.1128/jb.178.20.5897-5903.1996
62. Glasemacher J, Bock AK, Schmid R, Schonheit P. Purification and properties of acetyl-CoA synthetase (ADP-forming), an archaeal enzyme of acetate formation and ATP synthesis, from the hyperthermophile pyrococcus furiosus. Eur J Biochem (1997) 244(2):561–7. doi: 10.1111/j.1432-1033.1997.00561.x
63. Ward DE, Kengen SW, van der Oost J, de Vos WM. Purification and characterization of the alanine aminotransferase from the hyperthermophilic archaeon pyrococcus furiosus and its role in alanine production. J Bacteriol (2000) 182(9):2559–66. doi: 10.1128/JB.182.9.2559-2566.2000
64. Xue J, Murrieta CM, Rule DC, Miller KW. Exogenous or l-rhamnose-derived 1,2-propanediol is metabolized via a pduD-dependent pathway in listeria innocua. Appl Environ Microbiol (2008) 74(22):7073–9. doi: 10.1128/AEM.01074-08
65. Barker HA, Kahn JM, Hedrick L. Pathway of lysine degradation in fusobacterium nucleatum. J Bacteriol (1982) 152(1):201–7. doi: 10.1128/jb.152.1.201-207.1982
66. Qin Q, Xu X, Wang X, Wu H, Zhu H, Hou Y, et al. Glutamate alleviates intestinal injury, maintains mTOR and suppresses TLR4 and NOD signaling pathways in weanling pigs challenged with lipopolysaccharide. Sci Rep (2018) 8(1):15124. doi: 10.1038/s41598-018-33345-7
Keywords: whole intestinal microbiota transplantation, fecal microbiota transplantation, inflammatory bowel disease, germ-free mice, 16S rDNA
Citation: Yang Y, He J, Wang Y, Liang L, Zhang Z, Tan X, Tao S, Wu Z, Dong M, Zheng J, Zhang H, Feng S, Cheng W, Chen Q and Wei H (2023) Whole intestinal microbiota transplantation is more effective than fecal microbiota transplantation in reducing the susceptibility of DSS-induced germ-free mice colitis. Front. Immunol. 14:1143526. doi: 10.3389/fimmu.2023.1143526
Received: 13 January 2023; Accepted: 11 April 2023;
Published: 09 May 2023.
Edited by:
Allen D. Smith, Agricultural Research Service (USDA), United StatesReviewed by:
Jie Yin, Hunan Agricultural University, ChinaCopyright © 2023 Yang, He, Wang, Liang, Zhang, Tan, Tao, Wu, Dong, Zheng, Zhang, Feng, Cheng, Chen and Wei. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hong Wei, d2VpaG9uZzYzNTI4QDE2My5jb20=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.